Highlights
► Mental imagery powerfully drives emotion. ► Mental imagery-based interventions may be useful for treating emotional disorders. ► The role, contents and neurocognitive subcomponents of mental imagery alter with age. ► We consider implications for understanding and treating emotional disorders.
Keywords: Mental imagery, Emotion, Emotional disorders, Childhood, Adolescence
Abstract
Mental imagery-based interventions are receiving increasing interest for the treatment of psychological disorders in adults. This is based on evidence that mental imagery potently influences the experience of emotion in non-clinical samples, and that a number of psychological disorders are marked by syndrome-specific, distressing abnormalities in mental imagery.
During childhood and adolescence, neurocognitive development impacting mental imagery processes may moderate its relationship with clinically-relevant emotional symptoms at a number of potential loci. Crucially, these changes could impact vulnerability to distressing mental imagery and the efficacy of mental imagery-based clinical interventions. This review synthesises evidence pertaining to developmental changes in the role and content of mental imagery, and in the cognitive sub-processes required to generate and sustain mental images. Subsequently, we discuss implications for understanding the developmental relationship between mental imagery, emotion and psychopathology.
Translational cognitive neuroscience research investigating the content, emotional impact and neurocognitive substrates of mental imagery across development may reveal insights into trajectories of vulnerability to symptoms of a number of psychological disorders. If proper consideration is given to developmental factors, techniques based on mental imagery may be valuable as part of a treatment armoury for child and adolescent clinical populations and those at risk of emotional disorders.
1. Introduction
Mental imagery is the experience of perception in the absence of concurrent sensory input (Kosslyn et al., 2006). Mental images can be held in iconic or short term memory immediately after cessation of the eliciting sensory input, or can be constructed from associative and episodic memory. Although often described as “seeing with the mind's eye”, mental imagery occurs in multiple sensory modalities (e.g. Anema et al., 2012, Daselaar et al., 2010, Girón et al., 2012). Indeed, it is suggested that every type of perception has a corresponding type of mental imagery (Moulton & Kosslyn, 2009). Given that a number of psychological disorders are marked by syndrome-specific, distressing abnormalities in mental imagery – and conversely that interventions targeting mental imagery can result in their attenuation – it is important to consider how mental imagery processes develop in tandem with typical and atypical experiences of emotion. An objective of this review is to synthesise developmental, cognitive neuroscience and clinical evidence pertaining to mental imagery and its influence on emotion across development, and to highlight potential implications for understanding and treating psychopathology.
From an experimental cognitive standpoint, our understanding of mental imagery is largely limited to unemotional forms of mental imagery. However, emotional mental imagery has been found to be increasingly important across of range of psychological disorders, from childhood to adulthood. Our review attempts to provide an initial (and we believe exciting) translational step in linking between cognitive neuroscience and psychopathology with a developmental perspective. Psychopathology stands to benefit from insights in cognitive neuroscience, yet examples where this has been successful remain surprisingly limited. By considering the pertinent clinical questions at which to direct further attention, cognitive neuroscience may be able to contribute to this endeavour by highlighting maturational changes in the functional neural underpinnings of clinically-relevant mental imagery phenomena.
In the field of cognitive neuroscience, it is understood that mental imagery generated on the basis of previous experience acts as a proactive filter for incoming perceptual information (Diekhof et al., 2011, Frith and Dolan, 1997). Thus, repeated exposure to a stimulus generates a predictive perceptual bias or template which facilitates subsequent apprehension of the stimulus. Mental imagery is said to provide a means of predicting the future, by simulating the potential consequences of anticipated events and actions based on past experience, i.e. “mental time-travel” (Frith and Dolan, 1997). According to Moulton and Kosslyn (2009), mental imagery performs an emulative function, where emulation is defined as a specific type of simulation in which the same mental processes that would operate in reality are recruited to produce the imaginal scenario.1 Indeed, the neurocognitive substrates of mental imagery overlap substantially with those involved in veridical perception (Kosslyn et al., 2006, Schacter et al., 2007). Another account still holds that mental imagery functions to represent information about behavioural goals, perhaps in the form of a mismatch between the current and desired state of reality (Conway et al., 2004).
In the field of clinical psychology research, it is noted that mental imagery is a powerful driver of emotion (Holmes et al., 2008c). Emotional disorders including post-traumatic stress disorder (PTSD), social anxiety/social phobia and depression are marked by syndrome-specific abnormalities in mental imagery content (Brewin et al., 2010, Holmes and Mathews, 2010). For example, the term “flashbacks” in PTSD is used to refer to memories from a traumatic event which appear as vivid (predominantly visual) mental images and intrude involuntarily into consciousness (Bourne et al., 2012). Patients with social phobia experience negative intrusive imagery of themselves performing undesirably (e.g. blushing bright red) in a social context, which in turn influences their social performance (Hirsch et al., 2003). Across disorders, abnormal mental imagery (e.g. negative involuntary flashbacks) is a source of emotional distress, and as such may represent a valid treatment target (Holmes and Mathews, 2010). A challenge for future research is to understand when – and why – vulnerabilities to abnormal mental imagery arise during childhood and adolescence, in terms of their relation to developmental changes in cognition and functional brain circuitry.
Treatment interventions aimed at reducing distressing mental imagery are increasingly being developed in clinical psychology practice (Hackmann et al., 2011). Approaches within cognitive behavioural therapy (CBT) such as real or imaginal exposure and imagery rescripting have been used to target intrusive images of trauma (flashbacks) in PTSD (Ehlers et al., 2005), to modify negative images of the self in social anxiety (Clark et al., 2006) and to reduce the influence of negative mental imagery in depression (Wheatley et al., 2007). More recently, treatment possibilities are emerging not from talking therapy but from an approach within the experimental psychopathology literature known as cognitive bias modification (CBM). For example, some CBM procedures aim to re-train interpretation bias (CBM-I; Hertel and Mathews, 2011) via computerised techniques. Negative interpretation bias, which is the tendency to interpret ambiguous stimuli in a negative manner, is thought to play a causal role in vulnerability to emotional disorders (Mathews and MacLeod, 2002). Mental imagery has now been incorporated within CBM-I procedures (Holmes et al., 2009, Lang et al., 2012). A number of recent studies using mental imagery-based CBM-I have shown that, in major depression and in sub-clinical samples with depressed mood, generating mental imagery of positive events can boost positive interpretations of ambiguous stimuli, reduce depressed mood and boost later performance on a behavioural task (Holmes et al., 2009, Lang et al., 2012, Pictet et al., 2011). Another, complementary candidate treatment intervention directly targets distressing mental imagery by disrupting image-based processing following viewing of emotional visual material, in order to reduce subsequent image-based intrusions. For example, in an experimental analogue model of PTSD, playing a visuospatial computer game (Tetris) after viewing a distressing film reduces the frequency of visual flashbacks – purportedly via competition for a common neurocognitive visual imagery resource (Holmes et al., 2010).
Vulnerability to adult psychological disorders can often be traced to childhood factors (Caspi et al., 1996, Gregory and Eley, 2007, Rutter, 1984). During adolescence, cognitive and neuroanatomical development, together with hormonal and socio-cultural changes, act to further canalise mental health trajectories (Patton and Viner, 2007). Arguably, the majority of emotional disorders marked by distressing mental imagery (Brewin et al., 2010, Holmes and Mathews, 2010) either have their mean age of onset in adolescence or young adulthood (Paus et al., 2008), or are traceable to emotional and cognitive vulnerabilities in childhood (Caspi et al., 1996, Gregory and Eley, 2007, Rutter, 1984). Given the purported role of mental imagery in these disorders, taking a developmental perspective on mental imagery may reveal insights into the causes and collaterals of adult symptomatology, as well as to resilience. Therefore, it could be advantageous to (a) conduct empirical studies with young populations to investigate the causal role of mental imagery in vulnerability to emotional problems and later onset psychopathology; and (b) identify clinical and at-risk child and adolescent populations for therapeutic and preventative mental imagery-based treatment interventions.
A prominent approach in developmental research (Morton and Frith, 1995) advocates the characterisation of phenomena at multiple levels of explanation, in order to elucidate causal mechanisms. Thus, it is important to distinguish between, and separately characterise, observations at the level of behaviour (e.g. distressing mental imagery), cognition (e.g. emotion regulation ability) and the brain (e.g. prefrontal cortex development), as a prerequisite to understanding their interrelationship across development. Below, we present evidence indicating development during childhood and adolescence in the role and content of mental imagery, and in the cognitive sub-processes required to generate, sustain and modify mental images. We also discuss the efficacy of mental imagery-based cognitive training paradigms in child and adolescent samples. Subsequently, we review evidence acquired from clinical and non-clinical adult samples indicating a causal impact of mental imagery on emotion, with discussion of potential underlying neurocognitive mechanisms. Finally, we suggest potential developmental changes in the relationship between mental imagery, emotion and psychopathology, with consideration for basic research on emotional disorders and for treatment interventions. Throughout, we argue that adopting a developmental stance on the “special relationship” (Holmes and Mathews, 2005) between mental imagery and emotion is vital for extending mental imagery-based treatment and preventative interventions to child and adolescent populations, and may reveal insights into trajectories of psychopathology and potentially their plasticity.
2. Development of mental imagery
2.1. Development of mental imagery role and content
From a very young age, children use mental imagery to assist performance in difficult or unfamiliar tasks (Keen, 2011, Mischel et al., 1989). This is consistent with the emulative function of mental imagery as proposed by Moulton and Kosslyn (2009; see also Schacter et al., 2007). According to this account, mental imagery is generated to discover the likely physical, emotional and social consequences of anticipated forthcoming events or alternative courses of action. It follows, then, that during development the role and contents of mental imagery should closely mirror the relevant skill domains that are being acquired.
Studies of prospective motor control suggest that infants of less than one year old use mental imagery of recently-viewed objects to reach for them in the dark (Clifton et al., 1991, Keen, 2011, McCarty et al., 2001). For example, in Clifton et al. (1991) infants viewed at a distance small (requiring one-handed grasp) versus large (requiring two-handed grasp) objects that were each associated with a distinctive sound, and subsequently were given the opportunity to reach for them in the dark. Infants aged 6 months old used the appropriate grasp, which implies the use of visual mental representations. In early childhood, performance on spatial reasoning puzzles is enhanced by the instruction to use mental imagery, with 3-year old children better able to predict where a marble would appear from a set of intertwining tubes if they are told to imagine its path (Joh et al., 2011). Children may also be able to use mental imagery to help with the important skill of delayed gratification. In preschool-aged children, the ability to resist eating a tempting treat is bolstered by the instruction to imagine it as a “picture” of a treat, rather than thinking of it as the treat itself (Mischel et al., 1989). With increasing age, children become more adept at choosing to adopt helpful image-based strategies, for example when presented with a set of alternative strategies (Mischel and Mischel, 1983).
In the social domain, “imaginary companions”, defined as vivid imaginary characters that are spoken to, played with and referred to throughout a normal day, are created and sustained by many children (Bouldin and Pratt, 1999) and may provide a child with opportunities to practice social interactions via active construction of fantasy scenarios (although it is unclear whether imaginary companions manifest as mental images per se, rather than as counterfactual propositions; Pylyshyn, 1973). While the direction of causality is difficult to determine, the presence of an imaginary companion at age 4–6 years is associated with enhanced ability to take into account an interactant's perspective during a referential communication task (Roby and Kidd, 2008). To our knowledge, the role and contents of naturally-occurring “social” mental imagery have not been systematically documented in older children. One hypothesis could be that beyond middle childhood, rather than giving rise to imaginary friends, mental imagery concerns and supports the emergence of personal and social identity, complex social understanding, and plans for the future (Adamson et al., 1999, Boyd, 2007).
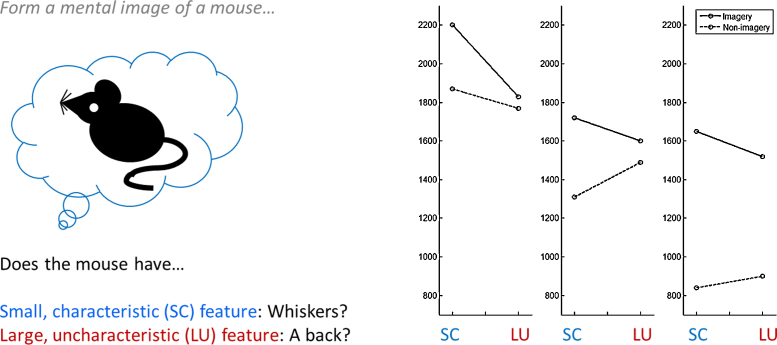
Consistent with the emulative account, there is evidence that the tendency to use mental imagery to accomplish a given cognitive task decreases with increasing age (and/or perhaps experience). In one study, 6 year olds, 10 year olds and young adults were asked to decide whether familiar animals had certain features, either with or without the instruction to visualise (Kosslyn, 1976; Fig. 1, left). Results showed shorter response times (RT) for large versus small visual features in the visual imagery condition across age, consistent with more rapid localisation of larger visual objects (Fig. 1, right). Overall, RTs were longer in the imagery versus non-imagery condition, and this difference widened with age due to a substantial decrease in RT in the non-imagery condition. Furthermore, only 10 year olds and adults were faster to evaluate highly semantically-characteristic versus uncharacteristic features of each animal in the non-imagery condition. The results were interpreted as indicating that older participants had readier access to a rapid strategy based on semantic associations in long-term memory; younger participants had not yet encoded this information in long-term memory, and thus invoked mental imagery to actively generate it (Kosslyn, 2005). Thus, mental imagery functions to emulate perception when an individual is not yet sufficiently expert to have the relevant semantic information to hand. An untested implication of this finding is that children's uncued mental imagery should also feature domains in which the individual has not yet attained a wide semantic knowledge base.
Fig. 1.
Decrease in reliance on mental imagery with age. In an initial non-imagery condition, 10 year olds and adults, but not 6 year olds, were faster to evaluate semantically-characteristic versus uncharacteristic features of animals (graph, dotted line). When subsequently instructed to use mental imagery (left), participants of all ages were faster to evaluate large versus small features (graph, solid line). This pattern of results suggests that 10 year olds and adults had access to a rapid strategy for evaluating the features based on semantic associations in long-term memory, whereas 6 year olds used mental imagery to actively generate the necessary information.
Adapted from Kosslyn, 1976, Kosslyn, 2005.
According to one school of thought in developmental psychology, young children naturally process stimuli in an imaginative, perceptually and emotionally rich mode of “absorption” (Harris, 2000). Evidence suggests that the emotional impact of imaginary scenarios (e.g. being chased by a monster) is similar when children (aged 4–6 years) are explicitly asked to “form a picture in their heads” to accompany this material, compared to when they are not explicitly asked to do so (Harris, 2000, chapter 4). Within the adult literature, it is understood that vivid mental imagery is an important component of absorption (Tellegen and Atkinson, 1974). It has been suggested that this “absorbed” mode of processing performs two related functions, in adults as well as in children (Harris, 2000). The first is to enable an individual to simulate the emotional consequences of alternate behavioural choices – consistent with the Kosslyn et al. emulative account. The second is to enable individuals to respond emotionally to verbal information conveyed by other individuals. From a developmental perspective, the latter may be particularly important for learning from verbal instruction (…Jack touched the stove and he got burned). One speculative suggestion is that a more absorbed mode of processing in childhood, relative to adulthood, could lead to deeper encoding of stressful events, with profound effects on an individual's sense of self and goals.
Evidence reviewed in this section highlights potential developmental changes in the specific content of mental imagery, and in its use within a given domain to generate de novo information, perhaps as a function of typical cognitive and social skills acquisition. We hypothesise that the role of mental imagery as defined broadly (e.g. emulation, mental time-travel) should not vary across development; however, as construed narrowly, the role of mental imagery in accomplishing particular behavioural goals (relevant for continued development and skill acquisition) may alter. This suggestion has a number of potential implications. First, the content of problematic mental imagery (e.g. a scary imaginary friend, negative images of the self) may alter developmentally. For example, the incidence and emotional impact of fear-related mental imagery is predicted to adhere to typical developmental trajectories of fear content: from fear of strangers, the dark and being alone in early childhood, to fear of failure and public criticism in later childhood and adolescence (Gullone and King, 1993, Gullone, 2000). This may have implications for understanding the developmental aetiology of certain psychological disorders (e.g. phobias, psychotic symptoms, social anxiety). Second, reliance on imagery-based (versus verbal linguistic) processing at younger ages suggests that in certain domains, clinical therapies which incorporate elements that target mental imagery (e.g. play, drawing or mental imagery itself) may be particularly suited to children's cognitive style (for fuller discussion, see Section 2.2). Third, more research is needed on the relationship between childhood mental imagery content and vulnerability to emotional distress. In the aftermath of the 9/11 terrorist attacks in the United States, London school children experienced intrusive fantasy mental imagery based on footage of the event (e.g. a family member jumping from a burning building) and associated PTSD symptoms (Holmes et al., 2007b). Understanding why this imagery was generated, and the mechanisms underlying its relationship with emotional symptoms, could inform treatment and the early identification of at-risk groups following stressful experiences.
2.2. Efficacy of mental imagery-based cognitive training paradigms in children and adolescents
A number of paradigms have been developed which harness mental imagery to modify phobias and cognitive biases in children and adolescents. In the “emotive imagery” technique (King et al., 1998), which is a variant of systematic desensitisation based on extinction (Wolpe, 1990), children identify with a favourite hero (e.g. Batman) and then undergo benign imaginal exposure to a feared stimulus (e.g. a spider). Evidence from single case series suggests this technique may reduce fear in children (King et al., 1989). Image rescripting incorporating imaginal exposure is used clinically in children to alleviate distress related to a number of conditions, including specific phobias (e.g. needle phobia; Raby and Edwards, 2011) and anticipation of recurrent nightmares (St-Onge et al., 2009).
Treatment packages focusing on changing cognitive vulnerability factors have also capitalised on techniques targeting imagery. In a randomised controlled trial, CBT with a mental imagery component (including imaginal reliving) was effective in alleviating symptoms of PTSD in individuals aged 8–18 years (Smith et al., 2007). Several recent CBM-I studies have instructed healthy adolescents to generate mental imagery to accompany standard CBM-I training procedures (Lau et al., 2012, Lothmann et al., 2011, Telman et al., 2012). Results indicate that participants trained to interpret material in a positively-valenced manner showed a tendency to select more positive interpretations than negative interpretations, whereas participants trained to interpret material in a negatively-valenced manner showed effects in the opposite direction. In two of these studies, participants in the positive CBM-I condition additionally showed a decrease in the self-reported impact of recent stressful life events and in their anxious responses to a laboratory stressor. Extending CBM-I with imagery to a sample of anxious adolescent patients, similar changes in interpretational style were also found (Fu et al., in press). Together, this evidence suggests that mental imagery-based CBT and CBM-I may be effective in boosting positive interpretations in child and adolescent samples.
A small number of studies in children and adolescents have begun to explicitly address the question of whether instructing participants to generate mental imagery enhances the efficacy of cognitive training interventions. In one study, an “imagery” condition modelled on the emotive imagery technique was reported to be less effective in reducing experimentally-induced threat bias in healthy participants aged 9–13 years relative to a “positive verbal information” condition (Muris et al., 2011). In this study, experimental threat bias was induced by verbally introducing participants to an imaginary animal with threatening features (e.g. sharp claws); In the positive verbal information condition participants were subsequently given alternate, benign verbal descriptors of the animal (e.g. soft little paws), whereas in the emotive imagery condition participants were instructed to imagine a popular cartoon character interacting with the threatening animal. Another study in healthy 10–12 year olds compared the impact of CBM-I with instructions to generate mental images versus verbal descriptions of training material, with greater effects on measures of mood and interpretation bias in the latter condition (Vassilopoulos et al., 2012). However, in both studies it is not clear how effective the “verbal” instructions were in inducing exclusively verbal versus image-based processing. Thus, with both studies, it is not clear that these results can be interpreted in terms of the effects of mental imagery versus verbal processing on cognitive training. Clearly, further studies are required to compare these alternative modes of delivery in child and adolescent populations.
In the next section, we review developmental evidence from tasks that examine the discrete cognitive sub-processes used to generate and sustain mental imagery. This evidence may lend insight into developmental changes in the ability to use mental imagery to modulate emotion, which may impact the choice and efficacy of treatment interventions.
2.3. Development of mental imagery sub-processes
A number of studies show age-associated improvement in performance on cued mental imagery tasks. Typically in these tasks, participants manipulate (e.g. generate, rotate) mental images of simple pre-learned stimuli (e.g. uppercase letters); RT and error measures are taken. Since these tasks are minimal, rather than naturalistic, they are appropriately controlled to address research questions concerning the basic cognitive components of mental imagery.
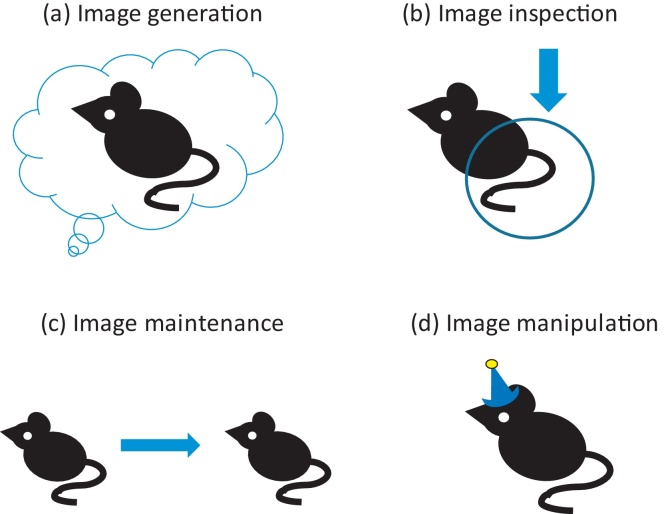
According to the Kosslyn et al. computational account of mental imagery (Kosslyn, 1996, Kosslyn et al., 2006), diverse mental imagery tasks are conceptualised as requiring one or more of the following cognitive sub-processes (see Fig. 2): (a) image generation, or the formation of an image within a “depictive buffer” shared by incoming sensory input; (b) image inspection, which involves shifting one's attention to a particular aspect of an image; (c) image maintenance, or the retention of an image such that it continues to be available for inspection; (d) image transformation, for example mentally rotating imagined objects (see also Pearson et al., 2013).
Fig. 2.
Mental imagery sub-processes. As described in the Kosslyn computational model, mental imagery tasks require one or more of the following cognitive sub-processes: (a) image generation, or the formation of an image within a depictive buffer shared by incoming sensory input; (b) image inspection, which involves shifting one's attention to a particular aspect of an image; (c) image maintenance, or the retention of an image such that it continues to be available for inspection; (d) image transformation, for example, rotating, shrinking or in other ways modifying the image.
Each of the four mental imagery sub-processes is said to be implemented by the combined action of several more basic, domain-general algorithmic neurocognitive components (for further details, see Kosslyn et al., 1984, Kosslyn et al., 2006). Since these are not restricted to mental imagery, any age-associated development in mental imagery sub-processes is likely to be underpinned by domain-general cognitive development, for example in information processing speed (Kail, 1991) and “executive functions” such as attentional control, response inhibition and working memory (Anderson et al., 2001, Luna et al., 2004, Verwoerd et al., 2009), and in their respective neural substrates (Dumontheil et al., 2010, Klingberg, 2006, Konrad et al., 2005, Luna and Sweeney, 2004).
2.3.1. Image generation
In neurocognitive terms, image generation (Fig. 2, top left) is thought to take place via prefrontal-based retrieval and integration of information from multimodal association cortex, for example spatial information from parietal cortex and object information from temporal cortex (De Borst et al., 2012, Farah et al., 1988, Herholz et al., 2012, Kosslyn et al., 2006). There is evidence that images are held on-line in a short-term depictive buffer, perhaps located in early sensory cortex (Daselaar et al., 2010, Farah and Smith, 1983, Ishai et al., 2000, Kosslyn et al., 2006; although see Bridge et al., 2012). Image generation can entail re-activation of perceptual information (…imagine your bed), or can result in new combinations of images (…imagine a bear sleeping in your bed). An empirical method for testing image generation ability is as follows. Participants are familiarised with uppercase letters formed by shading individual squares in a 4 × 5 grid. Subsequently, participants must decide whether a given letter cued auditorially, or by visual presentation of the equivalent lowercase letter, would cover two “X” marks presented on an unfilled grid. A 2–3 second decrease in RT for performing this image generation task relative to a perceptual control task is observed between the ages of 8, 14 and adulthood, with the most substantial development occurring between age 8 and 14 (Kosslyn et al., 1990). A prediction is that the development observed in this particular task is attributable to increasing speed or automaticity of associative memory retrieval (Wimmer and Howe, 2009), rather than to a change in some characteristic of the stored representations. For example, there is evidence that regions of the prefrontal cortex implicated in memory retrieval develop functionally and structurally throughout childhood and into adolescence (Dumontheil et al., 2008).
2.3.2. Image inspection
After an image has been generated, it is available for inspection (Fig. 2, top right), for example to perform reflective thinking (…where did I see that key?). It has been suggested that inspection of mental images operates via domain-general attentional mechanisms: attention can be shifted systematically across an image, or else features “pop out” (Kosslyn et al., 2006). Empirical studies have shown that responding based on visual inspection of mental images is slower when the virtual distance to be traversed is greater (Kosslyn, 1980). Between the ages of 8 and 14 years, there is a decrease in the time taken to respond based on scanning a mental image, with steeper decreases for larger scanning distances (Kosslyn et al., 1990). This is consistent with evidence showing development during middle childhood and early adolescence of attentional selection and the executive control of attention, and in their functional and structural neural substrates (Anderson et al., 2001, Klenberg et al., 2001, Konrad et al., 2005, Rueda et al., 2004).
2.3.3. Image maintenance
Mental images will be displaced from the shared depictive buffer by competing sensory input or subsequent mental images, unless they are actively maintained (Fig. 2, bottom left). Working memory and other executive functions are thought to support image maintenance (Borst et al., 2012, Kosslyn, 1996). There is evidence that these functions undergo protracted maturation during childhood and into adolescence (Luna et al., 2004), although an early study that investigated the development of visual mental imagery maintenance across age (age 5, 8, 14 and adult) found no evidence for development in image maintenance, per se (Kosslyn et al., 1990). Neuroimaging evidence suggests protracted development within fronto-parietal networks that implement working memory (Klingberg, 2006, Olesen et al., 2003). Furthermore, it has been shown that estimates of the capacity of working memory for maintaining multiple items are highly sensitive to the empirical protocol used (Bays et al., 2010). If some other index of image maintenance were adopted, for example estimates of precision from the working memory literature (Bays and Husain, 2008), it is possible that evidence may be obtained for development of mental imagery maintenance throughout childhood and into adolescence (Burnett Heyes et al., 2012).
2.3.4. Image manipulation
Mental images can be manipulated in a multitude of ways; for example, by stretching, rotating or distorting imaginal visual objects (Fig. 2, bottom right). This manipulation relies upon sub-processes such as working memory, response inhibition and selective attention (Prime and Jolicoeur, 2010). A common method for investigating image manipulation is to use mental rotation tasks (Shepard and Cooper, 1982, Shepard and Metzler, 1971). Typically in these tasks, participants decide whether pairs of visual objects are rotated, or rotated and mirror-reversed versions of one another. Results from developmental studies show a decrease with age (e.g. 8–14 years) in the RT and error cost of rotating objects through larger distances (Kosslyn et al., 1990, Marmor, 1977). For example, Kosslyn et al. (1990) reported mean adult RT for 180° rotation of approximately 3 s (versus 2 s for 0°); the equivalent in 8 year olds was 7 s (3.5 s). This development may be underpinned by maturation of working memory, response inhibition and selective attention, and their neural substrates (Klingberg, 2006, Konrad et al., 2005, Luna and Sweeney, 2004). An outstanding empirical question is the extent to which development in the speed and efficiency of image manipulation in cued, artificial imagery tasks has impact for the efficacy of more naturalistic – including therapeutic – image manipulation paradigms (Holmes et al., 2007a, St-Onge et al., 2009, Verwoerd et al., 2009).
Evidence reviewed in this section highlights protracted development in the ability to generate, inspect, maintain and manipulate mental images, most likely underpinned by continuing neurocognitive development in attentional control, response inhibition and working memory. An implication of this development is that children and adolescents may require support to master experimental and clinical (e.g. CBM-I) paradigms that require effortful control over image-based emotional content. On the flip side, another untested implication is that, since children and adolescents may not be cognitively equipped to achieve full control over mental imagery, they may be more vulnerable to the deleterious impact of distressing mental images that “come to mind unbidden” (e.g. scary monsters, flashbacks to a traumatic event). This offers an additional incentive to conduct empirical research on image-based clinical interventions for intrusive emotional imagery in child and adolescent populations. In the next section, we consider in more detail the relationship between mental imagery and emotion.
3. Mental imagery and emotion
Mental imagery is a potent driver of emotion. Indirect evidence to this effect is shown by studies in clinical adult populations investigating the content of mental imagery and its role in the maintenance of emotional symptoms (Holmes and Mathews, 2010). Direct experimental evidence of a causal relationship between mental imagery and emotion has been obtained using laboratory-based mental imagery paradigms (Holmes et al., 2008c). Below, we give examples of these two sources of evidence before describing the proposed mechanistic neurocognitive influence of mental imagery on emotion.
3.1. Mental imagery in emotional disorders
As discussed earlier, there is evidence that mental imagery contributes to emotional symptoms across a range of psychological disorders (Brewin et al., 2010, Holmes and Mathews, 2010). In PTSD, image-based memories of a previous trauma-eliciting event cause intense distress in the present moment (Brewin, 2001, Ehlers and Clark, 2000). In social anxiety, intrusive mental images of undesirable social outcomes (e.g. “seeing” oneself blushing crimson) cause distress and somatic symptoms during social encounters (Hackmann et al., 2000). Holding in mind a positive (rather than negative) image of the self alleviates this distress, suggesting a temporally-causal role (Hirsch et al., 2003). There is evidence for a paucity of positive imagery for the future in depressed mood and major depression, and this is hypothesised to contribute to depressed symptoms (Holmes et al., 2008b, Morina et al., 2011). In bipolar disorder, vivid prospective mental imagery of statistically unlikely personal outcomes is thought to amplify both dysphoric and hypomanic states (Hales et al., 2011, Holmes et al., 2008a). The extent to which mental imagery plays a causal role in emotional symptoms of other psychological disorders in which abnormal mental imagery has been observed, for example agoraphobia (Day et al., 2004), obsessive–compulsive disorder (Speckens et al., 2007) and body dysmorphic disorder (Osman et al., 2004), remains to be established. At present, there is a dearth of empirical research investigating the association between mental imagery and emotional disorders in childhood and adolescence.
3.2. Laboratory-based mental imagery paradigms
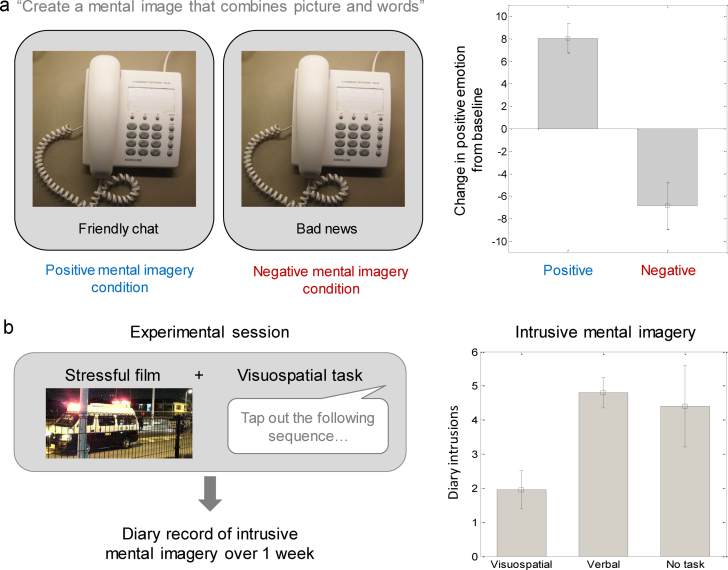
A paradigm that has been developed to evaluate the causal influence of mental imagery on emotion instructs participants to generate mental imagery based on stimuli with negative, positive or neutral valence – either short auditory or written scenarios, or pictures paired with disambiguating phrases (Fig. 3(a), left) – followed by measures of emotion (Holmes et al., 2006, Holmes et al., 2008c). A number of studies using these paradigms have shown greater changes in emotion when healthy adult participants form mental images as opposed to verbal descriptors of the same emotional material (Holmes et al., 2009, Holmes et al., 2006, Holmes et al., 2008c). In addition, increases in self-reported positive mood and decreases in self-reported negative mood are observed when healthy and dysphoric adult participants generate mental images of positively-valenced (versus negative or neutral) material (Fig. 3(a); Holmes et al., 2009, Holmes et al., 2006, Pictet et al., 2011). Changes are also observed in more implicit measures of mood and/or cognition, such as pleasantness ratings of ambiguous photographs, positive interpretations of homophones, and latency to complete a fishing game (the latter purportedly reflecting motivation/persistence; Pictet et al., 2011). As reviewed in Section 2.2, CBM-I paradigms with imagery instructions have shown consistent effects in clinical and non-clinical adolescent samples.
Fig. 3.
Impact of mental imagery on emotion. (a) Participants construct a mental image based on experimental stimuli with positive or negative valence (left). Adults (right) show an increase in positive mood following image-based processing of positive material, and a decrease in positive mood following image-based processing of negative material (Pictet et al., 2011). This emotional effect is attenuated by verbal processing of the material (Holmes et al., 2009, Holmes et al., 2006). (b) Involuntary memories of a stressful film are reduced by performing a visuospatial task that interferes with mental imagery, and are unaffected by performing a verbal task. (Deeprose et al., 2012)
Another method for investigating the causal influence of mental imagery on emotion is to present emotional (e.g. distressing, euphoric) film clips and test effects on the frequency of subsequent involuntary emotional images of the film (analogue flashbacks) over the next week. Experimental manipulations can be introduced that enable, versus compete with, mental imagery-based processing (e.g. completing a verbal “pub quiz” versus playing a demanding visuospatial game post film (Fig. 3(b), left; Holmes et al., 2010)). Disrupting the ability to engage in image-based processing following viewing of both negative and positive emotional movies reduces subsequent intrusive visual imagery (Davies et al., 2012, Deeprose et al., 2012). That is, flashbacks to distressing film clips are more frequent when viewing occurs shortly prior to a manipulation that enables, versus competes with, image-based processing (Fig. 3(b), right; Deeprose et al., 2012). To our knowledge, this paradigm has not been tested in children or adolescents, and ethical issues would need to be considered before doing so.
3.3. Mechanisms mediating the impact of mental imagery on emotion
A number of potential neurocognitive mechanisms have been proposed to account for the influence of mental imagery on emotion, although the degree to which these mechanisms are distinct from one another is unclear (Holmes and Mathews, 2010). Such distinctions are important, as they could potentially have implications for understanding developmental differences in peak onset of emotional disorders marked by abnormal mental imagery (Paus et al., 2008) and for the choice of clinical treatment strategies for children of different ages (Barrett, 2000).
3.3.1. Overlap with sensory perception
Mental imagery and on-line sensory perception recruit partially overlapping neurocognitive substrates. Functional neuroimaging studies show that visual perception and visual mental imagery recruit visual cortex, and auditory perception and auditory mental imagery recruit auditory cortex (Herholz et al., 2012, Kosslyn et al., 1993). Mental imagery of an object or event is said to emulate veridical perception of the same, triggering downstream emotional responses in an “as if” manner (Moulton and Kosslyn, 2009). This mechanism may account for increases in positive mood when healthy participants generate mental imagery based on positive stimulus material: Imagining a sunny walk along the beach activates visual and other sensory cortical components that would respond to the actual experience, with downstream effects on semantic and emotion-processing components.
3.3.2. “Direct” or involuntary route?
In PTSD, an apparently innocuous environmental cue can trigger vividly sensory mental imagery flashbacks that have strong emotional and autonomic consequences (e.g. racing heart), without the experience of causative cognitive processing (Orr et al., 1993, Reynolds and Brewin, 1998). This has led to the suggestion that involuntary mental imagery can make “direct” contact with modular emotion-processing (e.g. limbic) brain systems, using evolutionarily ancient neural circuitry that bypasses the cortical pathways that can mediate awareness (Holmes and Mathews, 2010, LeDoux, 2000). Recently, the existence and functional significance of the neural circuitry hypothesised to implement “direct” contact in humans has been re-evaluated (Pessoa and Adolphs, 2010). As an alternative possibility, the richly detailed “flashbulb” imagery attributed to this route may result from a relatively automatic, associative form of episodic remembering triggered by external cues (Berntsen and Hall, 2004).
A small number of functional neuroimaging studies have examined brain activation during the re-living of flashbacks in adults and adolescents diagnosed with PTSD (Lanius et al., 2001, Lanius et al., 2003, Shin, 2004, Whalley et al., 2013, Yang et al., 2004). In one fMRI study, activity was compared in adults with PTSD during flashbacks versus recall of traumatic episodic memories that did not have flashback characteristics (Whalley et al., 2013). Relative to non-flashback recall, flashbacks were associated with increased activity in sensory and motor cortical regions, including the mid-occipital cortex, insula, precentral gyrus and supplementary motor area, and decreased activity in the midbrain, parahippocampal gyrus and precuneus/posterior cingulate cortex. In a recent analogue study that investigated the neural correlates of viewing scenes that later recurred as a non-clinical analogue of flashbacks, healthy adult participants viewed distressing film clips during fMRI and used a diary to record intrusions during one week follow-up (Bourne et al., 2012). Viewing of scenes that later recurred as flashbacks and were recorded in the diary was associated with widespread increases in activity in the amygdala, bilateral middle temporal gyrus, rostral anterior cingulate cortex, bilateral middle temporal gyrus, ventral occipital cortex and left inferior frontal gyrus relative to viewing equally distressing scenes that did not recur. This study provides the first prospective evidence that the brain behaves differently whilst experiencing emotional scenes that will subsequently recur as flashbacks, relative to those which do not “flash back”.
3.3.3. Autobiographical memory reactivation
The third mechanism by which mental imagery is hypothesised to influence emotion is via contact with autobiographical memories (Holmes and Mathews, 2010). As such, this mechanism may not be wholly distinct from those described above. Functional neuroimaging studies show that recall of autobiographical memories is associated with interactions between the medial temporal lobe (e.g. hippocampus) and regions of the prefrontal cortex (Cabeza and St Jacques, 2007). Recall of emotional autobiographical memories in particular is associated with amygdalar-hippocampal interactions, and recall of vividly sensory autobiographical memories is associated with activity in sensory cortex (Cabeza and St Jacques, 2007). The neurocognitive mechanisms of mental imagery overlap substantially with those for autobiographical memory retrieval (Schacter et al., 2007). Mental imagery of past, prospective and fantasy events is thought to involve reactivating and recombining fragmentary memory representations (Conway and Pleydell-Pearce, 2000, Schacter et al., 2007). Therefore, in reactivating previous memory fragments – either involuntarily or deliberately (e.g. to construct a fantasy scenario; to generate mental imagery based on experimental stimulus material) – the emotional experiences arising from the original event may be re-experienced.
3.3.4. Modulating the emotional impact of mental imagery
To the extent that mental imagery and on-line sensory perception recruit overlapping neurocognitive substrates, wider research on emotion perception and its regulation can also be brought to bear on understanding the emotional impact of mental imagery. By one view, the emotional impact of particular stimulus content depends upon the interplay between bottom-up, “early appraisal” mechanisms operating via limbic structures such as the amygdala, and top-down (e.g. prefrontal) regulatory mechanisms which act to modify emotional responses. As an example of a clinical application of such a distinction, it is suggested that cognitive interventions (e.g. CBT) train individuals to modify the emotional content of potentially threatening material via prefrontal modulation of limbic and other sensory association cortex (Browning et al., 2010), whereas pharmacological treatment with antidepressant medication attenuates the initial amygdalar “appraisal” response (DeRubeis et al., 2008). A recent fMRI study in which healthy participants aged 10–22 years engaged in cognitive reappraisal of emotional stimulus material showed an age-related increase in activation of prefrontal cognitive control-related regions which paralleled improvement in a behavioural index of reappraisal ability (McRae et al., 2012; see also Lau et al., 2011a, Lau et al., 2011b). Elsewhere, it has been suggested that the perception and regulation of emotion are perhaps not best viewed as functionally distinct, but as interdependent (Barrett and Bar, 2009) and as overlapping with habitual perceptual processes and executive functions such as selective attention (Ochsner and Gross, 2005, Todd et al., 2012).
4. Implications for understanding and treating early emotional disorders
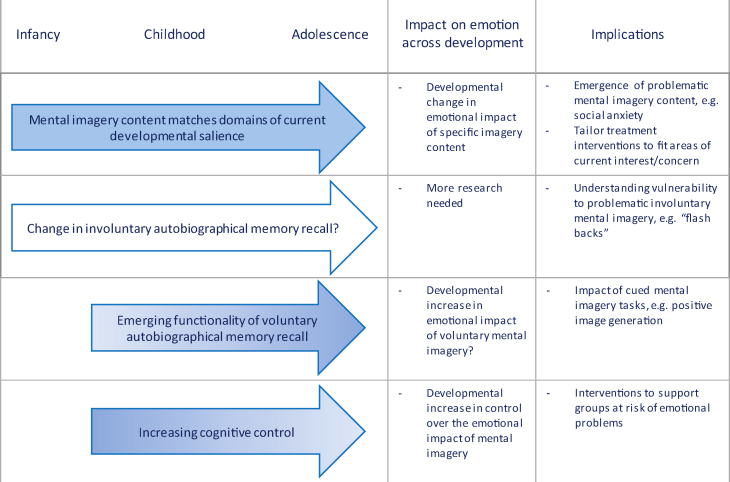
Evidence reviewed in Section 2.1 suggests that for a given cognitive, procedural or epistemic domain, children rely more heavily upon image-based processing relative to adults, and that this fulfils an important developmental function in terms of skill acquisition. Certainly, there is evidence that children's mental imagery can be vivid and compelling (Harris, 2000). This suggests two possibilities. First, identifying the existence of problematic imagery may provide a useful treatment target. Second, treatment techniques that incorporate mental imagery may be suited to children's cognitive style. As such, mental imagery-based clinical therapies – which are currently under-represented in child and adolescent evidence-based clinical practice – may help provide a powerful adjunct or alternative to verbal-based clinical therapies in areas of current developmental or individual salience or concern (Fig. 4).
Fig. 4.
Developmental trajectories of mental imagery and emotion. Potential implications for understanding vulnerability to emotional disorders and for clinical interventions are suggested.
Adolescence, for example, is marked by increasing peer-orientedness and concern with how one appears to others (Gullone and King, 1993, Steinberg, 2008), as well as by a developmentally heightened risk for social anxiety disorder (Wittchen and Fehm, 2003). Social anxiety disorder is characterised by intrusive negative images of the self which impact negatively on performance in social encounters (Hirsch et al., 2003). Therefore, interventions aimed at boosting positive mental imagery of social situations could be preventative in adolescent or pre-adolescent individuals at risk for social anxiety (Holmes et al., 2009), or could be harnessed to help break maintenance cycles (Spence et al., 1999).
Evidence reviewed in Section 2.3 suggests that, relative to adults, children and adolescents may be less equipped to achieve cognitive control over emotional mental imagery. For example, with increasing age, children become more competent at adopting strategies to protect against the emotional impact of make-believe scenarios, such as reminding themselves that a “monster” is “not real” (Harris, 2000). Therefore, training in strategies to modify intrusive negative imagery may have utility in a variety of situations – from coping with monsters, to addressing problematic images of the self, and dealing with negative intrusive imagery in the aftermath of a stressful event (Holmes et al., 2007a). Of course, we are suggesting such possibilities based on a cognitive science perspective on the development of mental imagery and its relationship to emotion. Any clinical treatment innovation would need to proceed within an appropriate translational framework.
Autobiographical memory becomes progressively more detailed and contextualised at recall throughout childhood and into early adolescence, and this is thought to at least partly reflect maturation of prefrontal-based retrieval processes (Bauer et al., 2007, Cooper et al., 2011, Ghetti and Bunge, 2012, Tulving, 1995). An untested prediction of this finding is that, with increasing age in middle childhood and early adolescence, increased effectiveness of deliberate memory recall may paradoxically lead to an increased emotional response to cued mental imagery tasks specifically (Fig. 4). Another potential implication is that understanding developmental changes in the neurocognitive mechanisms which underlie encoding and retrieval could have implications for understanding flashback formation, and potential age differences in vulnerability to PTSD symptoms (Green et al., 1991).
A speculative suggestion from the literature is that neuroanatomical maturation within prefrontal cortex, leading to an enhanced ability to bring to mind past or current complex goals and alternative outcomes, contributes to the increased risk for major depression during the second versus first decade of life (Davey et al., 2008). Given the proposed relationship between mental imagery and behavioural goals (Conway et al., 2004), it could be interesting to investigate the relationship between adolescent mental imagery for the future, depressive symptoms, and reductions in goal-directed behaviour (Cléry-Melin et al., 2011) – in particular, whether boosting positive mental imagery for the future could be beneficial in adolescents at risk of depressive illness.
In social anxiety disorder, the onset of symptoms can commonly be traced to a single stressful social incident which forms the basis of subsequent intrusive imagery (Hackmann et al., 2000). Similar findings have been reported in agoraphobia (Day et al., 2004), obsessive–compulsive disorder (Speckens et al., 2007) and of course PTSD (Berntsen and Rubin, 2007). It would be valuable to understand in mechanistic cognitive-developmental terms why certain memories – many of which are traceable to childhood and adolescence – rather than others become problematic (Berntsen and Rubin, 2007).
Distressing or unhelpful mental imagery is a feature of many emotional disorders (Brewin et al., 2010, Holmes and Mathews, 2010). Arguably, the majority of these disorders either have their mean age of onset in adolescence or young adulthood (Paus et al., 2008), or are traceable to emotional and cognitive vulnerabilities in childhood (Caspi et al., 1996, Gregory and Eley, 2007, Rutter, 1984). An important joint goal for cognitive neuroscience and clinical psychology research is to understand in neurocognitive mechanistic terms the developmentally-unfolding relationship between these vulnerabilities, the incidence of distressing mental imagery, and the maintenance or emergence of symptoms. Potentially, there may be optimal developmental time windows during which mental imagery-based treatment interventions have maximum impact.
5. Summary
Mental imagery-based treatment interventions are receiving increasing attention in the treatment of psychological disorders such as PTSD, social anxiety and depression in adults. Here, we present a developmental framework for understanding mental imagery-based components of these disorders, and for extending research on mental imagery-based treatments to child and adolescent populations. Treatment approaches and outcomes for children lag behind those for adults, and innovation is warranted. We suggest that developmental cognitive neuroscience may be able to contribute a piece in this puzzle.
In children and in adults, mental imagery functions to emulate real events, highlighting their potential consequences. We have shown evidence that children use mental imagery to boost performance of difficult or unfamiliar tasks, and that for a given cognitive task, the tendency to spontaneously use mental imagery (relative to verbal or semantic processing) decreases with age. At the same time, the cognitive sub-processes that support the deliberate generation, manipulation and maintenance of mental images undergo protracted development throughout childhood and adolescence, underpinned by the maturation of executive and processing capabilities.
A burgeoning empirical literature suggests that mental imagery powerfully drives the experience of emotion in clinical disorders and in laboratory-based empirical tasks. This has two implications for developmental research and practice. First, studying the relationship between mental imagery and emotion in child and adolescent populations may uniquely reveal insights into neurocognitive mechanisms of vulnerability to certain psychological symptoms. Secondly, if proper consideration is given to developmental factors, mental imagery-based treatment interventions may be effective in preventing and addressing emotional disorders. Evidence-based mental imagery therapies are currently under-exploited in child and adolescent clinical populations, but may be well-suited to younger individuals’ cognitive style.
We do not suggest that mental imagery underlies the full constellation of symptoms across emotional disorders. However, given mounting evidence for a role of mental imagery in psychopathology, and the potential tractability of mental imagery as a treatment target, understanding the underlying mechanisms from a developmental standpoint is of both clinical and theoretical importance.
Conflicts of Interest
The authors declare no conflict of interest.
Acknowledgments
SBH is supported by a British Academy postdoctoral fellowship. JYL is supported by the ESRC, British Academy, Nuffield Foundation and Brain and Behavior Foundation. EAH is supported by a Wellcome Trust Clinical Fellowship (WT088217), the Medical Research Council, the Lupina Foundation, and the National Institute for Health Research (NIHR) Oxford Biomedical Research Centre based at Oxford University Hospitals Trust. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health.
We are grateful to Simon Blackwell, Ian Davies, Lalitha Iyadurai and Belinda Platt for comments on the manuscript.
Footnotes
In contradistinction, other types of simulation would merely mimic the content of the imaginal scenario.
References
- Adamson L., Hartman S.G., Lyxell B. Adolescent identity—a qualitative approach: self-concept, existential questions and adult contacts. Scandinavian Journal of Psychology. 1999;40(1):21–31. doi: 10.1111/1467-9450.00094. [DOI] [PubMed] [Google Scholar]
- Anderson V.A., Anderson P., Northam E., Jacobs R., Catroppa C. Development of executive functions through late childhood and adolescence in an australian sample. Developmental Neuropsychology. 2001;20(1):385–406. doi: 10.1207/S15326942DN2001_5. [DOI] [PubMed] [Google Scholar]
- Anema H.A., De Haan A.M., Gebuis T., Dijkerman H.C. Thinking about touch facilitates tactile but not auditory processing. Experimental Brain Research. Experimentelle Hirnforschung. Experimentation Cerebrale. 2012 doi: 10.1007/s00221-012-3020-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett Treatment of childhood anxiety: developmental aspects. Clinical Psychology Review. 2000;20(4):479–494. doi: 10.1016/s0272-7358(99)00038-0. [DOI] [PubMed] [Google Scholar]
- Barrett Bar. See it with feeling: affective predictions during object perception. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 2009;364(1521):1325–1334. doi: 10.1098/rstb.2008.0312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer P.J., Burch M.M., Scholin S.E., Güler O.E. Using cue words to investigate the distribution of autobiographical memories in childhood. Psychological Science. 2007;18(10):910–916. doi: 10.1111/j.1467-9280.2007.01999.x. [DOI] [PubMed] [Google Scholar]
- Bays P.M., Husain M. Dynamic shifts of limited working memory resources in human vision. Science (New York, NY) 2008;321(5890):851–854. doi: 10.1126/science.1158023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bays, P. M., Wu, E. Y., & Husain, M., 2010. Working memory capacity reflects precision of storage not the number of items stored. SfN abstract HHH46, 197.1.
- Berntsen D., Hall N.M. The episodic nature of involuntary autobiographical memories. Memory & Cognition. 2004;32(5):789–803. doi: 10.3758/bf03195869. [DOI] [PubMed] [Google Scholar]
- Berntsen D., Rubin D.C. When a trauma becomes a key to identity: enhanced integration of trauma memories predicts posttraumatic stress disorder symptoms. Applied Cognitive Psychology. 2007;21(4):417–431. [Google Scholar]
- Borst G., Ganis G., Thompson W.L., Kosslyn S.M. Representations in mental imagery and working memory: evidence from different types of visual masks. Memory & Cognition. 2012;40(2):204–217. doi: 10.3758/s13421-011-0143-7. [DOI] [PubMed] [Google Scholar]
- Bouldin P., Pratt C. Characteristics of preschool and school-age children with imaginary companions. The Journal of Genetic Psychology. 1999;160(4):397–410. doi: 10.1080/00221329909595553. [DOI] [PubMed] [Google Scholar]
- Bourne C., Mackay C.E., Holmes E.A. The neural basis of flashback formation: the impact of viewing trauma. Psychological Medicine, FirstView. 2012:1–12. doi: 10.1017/S0033291712002358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd D. Why youth social network sites: the role of networked publics in teenage social life. The John D. and Catherine T. MacArthur Foundation Series on Digital Media and Learning. 2007:119–142. [Google Scholar]
- Brewin, April 2001. A cognitive neuroscience account of posttraumatic stress disorder and its treatment. BEHAV RES THER. Retrieved June 28, 2012, from http://eprints.ucl.ac.uk/11533/. [DOI] [PubMed]
- Brewin C.R., Gregory J.D., Lipton M., Burgess N. Intrusive images in psychological disorders: characteristics, neural mechanisms, and treatment implications. Psychological Review. 2010;117(1):210–232. doi: 10.1037/a0018113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridge H., Harrold S., Holmes E.A., Stokes M., Kennard C. Vivid visual mental imagery in the absence of the primary visual cortex. Journal of Neurology. 2012;259(6):1062–1070. doi: 10.1007/s00415-011-6299-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browning M., Holmes E.A., Murphy S.E., Goodwin G.M., Harmer C.J. Lateral prefrontal cortex mediates the cognitive modification of attentional bias. Biological Psychiatry. 2010;67(10):919–925. doi: 10.1016/j.biopsych.2009.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnett Heyes S., Zokaei N., Van der Staaij I., Bays P.M., Husain M. Development of visual working memory precision in childhood. Developmental Science. 2012 doi: 10.1111/j.1467-7687.2012.01148.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R., St Jacques P. Functional neuroimaging of autobiographical memory. Trends in Cognitive Sciences. 2007;11(5):219–227. doi: 10.1016/j.tics.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Caspi A., Moffitt T.E., Newman D.L., Silva P.A. Behavioral observations at age 3 years predict adult psychiatric disorders. Longitudinal evidence from a birth cohort. Archives of General Psychiatry. 1996;53(11):1033–1039. doi: 10.1001/archpsyc.1996.01830110071009. [DOI] [PubMed] [Google Scholar]
- Clark D.M., Ehlers A., Hackmann A., McManus F., Fennell M., Grey N. Cognitive therapy versus exposure and applied relaxation in social phobia: a randomized controlled trial. Journal of Consulting and Clinical Psychology. 2006;74(3):568–578. doi: 10.1037/0022-006X.74.3.568. [DOI] [PubMed] [Google Scholar]
- Cléry-Melin M.-L., Schmidt L., Lafargue G., Baup N., Fossati P., Pessiglione M. Why don’t you try harder? An investigation of effort production in major depression. PLoS ONE. 2011;6(8):e23178. doi: 10.1371/journal.pone.0023178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clifton R.K., Rochat P., Litovsky R.Y., Perris E.E. Object representation guides infants’ reaching in the dark. Journal of Experimental Psychology. Human Perception and Performance. 1991;17(2):323–329. doi: 10.1037//0096-1523.17.2.323. [DOI] [PubMed] [Google Scholar]
- Conway M.A., Pleydell-Pearce C.W. The construction of autobiographical memories in the self-memory system. Psychological Review. 2000;107(2):261–288. doi: 10.1037/0033-295x.107.2.261. [DOI] [PubMed] [Google Scholar]
- Conway, Meares K., Standart S. Images and goals. Memory. 2004;12(4):525–531. doi: 10.1080/09658210444000151. [DOI] [PubMed] [Google Scholar]
- Cooper J.M., Vargha-Khadem F., Gadian D.G., Maguire E.A. The effect of hippocampal damage in children on recalling the past and imagining new experiences. Neuropsychologia. 2011;49(7):1843–1850. doi: 10.1016/j.neuropsychologia.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daselaar S.M., Porat Y., Huijbers W., Pennartz C.M.A. Modality-specific and modality-independent components of the human imagery system. NeuroImage. 2010;52(2):677–685. doi: 10.1016/j.neuroimage.2010.04.239. [DOI] [PubMed] [Google Scholar]
- Davey C.G., Yücel M., Allen N.B. The emergence of depression in adolescence: development of the prefrontal cortex and the representation of reward. Neuroscience and Biobehavioral Reviews. 2008;32(1):1–19. doi: 10.1016/j.neubiorev.2007.04.016. [DOI] [PubMed] [Google Scholar]
- Davies C., Malik A., Pictet A., Blackwell S.E., Holmes E.A. Involuntary memories after a positive film are dampened by a visuospatial task: unhelpful in depression but helpful in mania? Clinical Psychology & Psychotherapy. 2012 doi: 10.1002/cpp.1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day S.J., Holmes E.A., Hackmann A. Occurrence of imagery and its link with early memories in agoraphobia. Memory (Hove, England) 2004;12(4):416–427. doi: 10.1080/09658210444000034. [DOI] [PubMed] [Google Scholar]
- De Borst A.W., Sack A.T., Jansma B.M., Esposito F., De Martino F., Valente G. Integration of ‘what’ and ‘where’ in frontal cortex during visual imagery of scenes. NeuroImage. 2012;60(1):47–58. doi: 10.1016/j.neuroimage.2011.12.005. [DOI] [PubMed] [Google Scholar]
- Deeprose C., Zhang S., Dejong H., Dalgleish T., Holmes E.A. Imagery in the aftermath of viewing a traumatic film: using cognitive tasks to modulate the development of involuntary memory. Journal of Behavior Therapy and Experimental Psychiatry. 2012;43(2):758–764. doi: 10.1016/j.jbtep.2011.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeRubeis R.J., Siegle G.J., Hollon S.D. Cognitive therapy versus medication for depression: treatment outcomes and neural mechanisms. Nature Reviews. Neuroscience. 2008;9(10):788–796. doi: 10.1038/nrn2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diekhof E.K., Kipshagen H.E., Falkai P., Dechent P., Baudewig J., Gruber O. The power of imagination—How anticipatory mental imagery alters perceptual processing of fearful facial expressions. NeuroImage. 2011;54(2):1703–1714. doi: 10.1016/j.neuroimage.2010.08.034. [DOI] [PubMed] [Google Scholar]
- Dumontheil I., Burgess P.W., Blakemore S.-J. Development of rostral prefrontal cortex and cognitive and behavioural disorders. Developmental Medicine and Child Neurology. 2008;50(3):168–181. doi: 10.1111/j.1469-8749.2008.02026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumontheil I., Hassan B., Gilbert S.J., Blakemore S.-J. Development of the selection and manipulation of self-generated thoughts in adolescence. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2010;30(22):7664–7671. doi: 10.1523/JNEUROSCI.1375-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers A., Clark D.M., Hackmann A., McManus F., Fennell M. Cognitive therapy for post-traumatic stress disorder: development and evaluation. Behaviour Research and Therapy. 2005;43(4):413–431. doi: 10.1016/j.brat.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Ehlers, Clark A cognitive model of posttraumatic stress disorder. Behaviour Research and Therapy. 2000;38(4):319–345. doi: 10.1016/s0005-7967(99)00123-0. [DOI] [PubMed] [Google Scholar]
- Farah M.J., Hammond K.M., Levine D.N., Calvanio R. Visual and spatial mental imagery: dissociable systems of representation. Cognitive Psychology. 1988;20(4):439–462. doi: 10.1016/0010-0285(88)90012-6. [DOI] [PubMed] [Google Scholar]
- Farah M., Smith A. Perceptual interference and facilitation with auditory imagery. Attention, Perception, & Psychophysics. 1983;33(5):475–478. doi: 10.3758/bf03202899. [DOI] [PubMed] [Google Scholar]
- Frith C., Dolan R.J. Brain mechanisms associated with top-down processes in perception. Philosophical Transactions of the Royal Society of London, Series B: Biological Sciences. 1997;352(1358):1221–1230. doi: 10.1098/rstb.1997.0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu, X., Du, Y., Au, S., Lau, J.Y.F. Reducing negative interpretations in adolescents with anxiety disorders: a preliminary study investigating the effects of a single session of cognitive bias modification training. Developmental Cognitive Neuroscience, in press. [DOI] [PMC free article] [PubMed]
- Ghetti S., Bunge S.A. Neural changes underlying the development of episodic memory during middle childhood. Developmental Cognitive Neuroscience. 2012;2(4):381–395. doi: 10.1016/j.dcn.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girón E.C., McIsaac T., Nilsen D. Effects of kinesthetic versus visual imagery practice on two technical dance movements: a pilot study. Journal of Dance Medicine & Science: Official Publication of the International Association for Dance Medicine & Science. 2012;16(1):36–38. [PubMed] [Google Scholar]
- Green B.L., Korol M., Grace M.C., Vary M.G., Leonard A.C., Gleser G.C., Smitson-Cohen S. Children and disaster: age, gender, and parental effects on PTSD symptoms. Journal of the American Academy of Child and Adolescent Psychiatry. 1991;30(6):945–951. doi: 10.1097/00004583-199111000-00012. [DOI] [PubMed] [Google Scholar]
- Gregory A., Eley T. Genetic influences on anxiety in children: what we’ve learned and where we’re heading. Clinical Child and Family Psychology Review. 2007;10(3):199–212. doi: 10.1007/s10567-007-0022-8. [DOI] [PubMed] [Google Scholar]
- Gullone The development of normal fear: a century of research. Clinical Psychology Review. 2000;20(4):429–451. doi: 10.1016/s0272-7358(99)00034-3. [DOI] [PubMed] [Google Scholar]
- Gullone E., King N.J. The fears of youth in the 1990: contemporary normative data. The Journal of Genetic Psychology. 1993;154(2):137–153. doi: 10.1080/00221325.1993.9914728. [DOI] [PubMed] [Google Scholar]
- Hackmann, Bennett-Levy, Holmes, editors. Oxford Guide to Imagery in Cognitive Therapy. 1st ed. OUP Oxford; UK: 2011. [Google Scholar]
- Hackmann, Clark D.M., McManus F. Recurrent images and early memories in social phobia. Behaviour Research and Therapy. 2000;38(6):601–610. doi: 10.1016/s0005-7967(99)00161-8. [DOI] [PubMed] [Google Scholar]
- Hales S.A., Deeprose C., Goodwin G.M., Holmes E.A. Cognitions in bipolar affective disorder and unipolar depression: imagining suicide. Bipolar Disorders. 2011;13(7–8):651–661. doi: 10.1111/j.1399-5618.2011.00954.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris P.L. vol. xii. Blackwell Publishing; Malden: 2000. (The Work of the Imagination). [Google Scholar]
- Herholz S.C., Halpern A.R., Zatorre R.J. Neuronal correlates of perception, imagery, and memory for familiar tunes. Journal of Cognitive Neuroscience. 2012 doi: 10.1162/jocn_a_00216. [DOI] [PubMed] [Google Scholar]
- Hertel P.T., Mathews A. Cognitive bias modification past perspectives, current findings, and future applications. Perspectives on Psychological Science. 2011;6(6):521–536. doi: 10.1177/1745691611421205. [DOI] [PubMed] [Google Scholar]
- Hirsch C.R., Clark D.M., Mathews A., Williams R. Self-images play a causal role in social phobia. Behaviour Research and Therapy. 2003;41(8):909–921. doi: 10.1016/s0005-7967(02)00103-1. [DOI] [PubMed] [Google Scholar]
- Holmes E.A., Arntz A., Smucker M.R. Imagery rescripting in cognitive behaviour therapy: images, treatment techniques and outcomes. Journal of Behavior Therapy and Experimental Psychiatry. 2007;38(4):297–305. doi: 10.1016/j.jbtep.2007.10.007. [DOI] [PubMed] [Google Scholar]
- Holmes E.A., Creswell C., O’Connor T.G. Posttraumatic stress symptoms in London school children following September 11, 2001: an exploratory investigation of peri-traumatic reactions and intrusive imagery. Journal of Behavior Therapy and Experimental Psychiatry. 2007;38(4):474–490. doi: 10.1016/j.jbtep.2007.10.003. [DOI] [PubMed] [Google Scholar]
- Holmes E.A., Geddes J.R., Colom F., Goodwin G.M. Mental imagery as an emotional amplifier: application to bipolar disorder. Behaviour Research and Therapy. 2008;46(12):1251–1258. doi: 10.1016/j.brat.2008.09.005. [DOI] [PubMed] [Google Scholar]
- Holmes E.A., James E.L., Kilford E.J., Deeprose C. Key steps in developing a cognitive vaccine against traumatic flashbacks: visuospatial Tetris versus verbal Pub Quiz. PLoS ONE. 2010;5(11):e13706. doi: 10.1371/journal.pone.0013706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes E.A., Lang T.J., Moulds M.L., Steele A.M. Prospective and positive mental imagery deficits in dysphoria. Behaviour Research and Therapy. 2008;46(8):976–981. doi: 10.1016/j.brat.2008.04.009. [DOI] [PubMed] [Google Scholar]
- Holmes E.A., Lang T.J., Shah D.M. Developing interpretation bias modification as a ‘cognitive vaccine’ for depressed mood: imagining positive events makes you feel better than thinking about them verbally. Journal of Abnormal Psychology. 2009;118(1):76–88. doi: 10.1037/a0012590. [DOI] [PubMed] [Google Scholar]
- Holmes E.A., Mathews A. Mental imagery and emotion: a special relationship? Emotion (Washington, DC) 2005;5(4):489–497. doi: 10.1037/1528-3542.5.4.489. [DOI] [PubMed] [Google Scholar]
- Holmes E.A., Mathews A. Mental imagery in emotion and emotional disorders. Clinical Psychology Review. 2010;30(3):349–362. doi: 10.1016/j.cpr.2010.01.001. [DOI] [PubMed] [Google Scholar]
- Holmes E.A., Mathews A., Dalgleish T., Mackintosh B. Positive interpretation training: effects of mental imagery versus verbal training on positive mood. Behavior Therapy. 2006;37(3):237–247. doi: 10.1016/j.beth.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Holmes E.A., Mathews A., Mackintosh B., Dalgleish T. The causal effect of mental imagery on emotion assessed using picture–word cues. Emotion (Washington, DC) 2008;8(3):395–409. doi: 10.1037/1528-3542.8.3.395. [DOI] [PubMed] [Google Scholar]
- Ishai A., Ungerleider L.G., Haxby J.V. Distributed neural systems for the generation of visual images. Neuron. 2000;28(3):979–990. doi: 10.1016/s0896-6273(00)00168-9. [DOI] [PubMed] [Google Scholar]
- Joh A.S., Jaswal V.K., Keen R. Imagining a way out of the gravity bias: preschoolers can visualize the solution to a spatial problem. Child Development. 2011;82(3):744–750. doi: 10.1111/j.1467-8624.2011.01584.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kail R. Developmental change in speed of processing during childhood and adolescence. Psychological Bulletin. 1991;109(3):490–501. doi: 10.1037/0033-2909.109.3.490. [DOI] [PubMed] [Google Scholar]
- Keen R. The development of problem solving in young children: a critical cognitive skill. Annual Review of Psychology. 2011;62(1):1–21. doi: 10.1146/annurev.psych.031809.130730. [DOI] [PubMed] [Google Scholar]
- King, Cranstoun, Josephs Emotive imagery and children's night-time fears: a multiple baseline design evaluation. Journal of Behavior Therapy and Experimental Psychiatry. 1989;20(2):125–135. doi: 10.1016/0005-7916(89)90045-1. [DOI] [PubMed] [Google Scholar]
- King N.J., Molloy G.N., Heyne D., Murphy G.C., Ollendick T.H. Emotive imagery treatment for childhood phobias: a credible and empirically validated intervention? Behavioural and Cognitive Psychotherapy. 1998;26(02):103–113. [Google Scholar]
- Klenberg L., Korkman M., Lahti-Nuuttila P. Differential development of attention and executive functions in 3- to 12-year-old finnish children. Developmental Neuropsychology. 2001;20(1):407–428. doi: 10.1207/S15326942DN2001_6. [DOI] [PubMed] [Google Scholar]
- Klingberg T. Development of a superior frontal-intraparietal network for visuo-spatial working memory. Neuropsychologia. 2006;44(11):2171–2177. doi: 10.1016/j.neuropsychologia.2005.11.019. [DOI] [PubMed] [Google Scholar]
- Konrad K., Neufang S., Thiel C.M., Specht K., Hanisch C., Fan J. Development of attentional networks: an fMRI study with children and adults. NeuroImage. 2005;28(2):429–439. doi: 10.1016/j.neuroimage.2005.06.065. [DOI] [PubMed] [Google Scholar]
- Kosslyn Reflective thinking and mental imagery a perspective on the development of posttraumatic stress disorder. Development and Psychopathology. 2005;17(03):851–863. doi: 10.1017/S0954579405050406. [DOI] [PubMed] [Google Scholar]
- Kosslyn S.M., Margolis J.A., Barrett A.M., Goldknopf E.J., Daly P.F. Age differences in imagery abilities. Child Development. 1990;61(4):995–1010. [PubMed] [Google Scholar]
- Kosslyn, Stephen M., Alpert N.M., Thompson W.L., Maljkovic V., Weise S.B., Chabris C.F. Visual mental imagery activates topographically organized visual cortex: PET investigations. Journal of Cognitive Neuroscience. 1993;5(3):263–287. doi: 10.1162/jocn.1993.5.3.263. [DOI] [PubMed] [Google Scholar]
- Kosslyn, Stephen M., Brunn J., Cave K.R., Wallach R.W. Individual differences in mental imagery ability: a computational analysis. Cognition. 1984;18(1–3):195–243. doi: 10.1016/0010-0277(84)90025-8. [DOI] [PubMed] [Google Scholar]
- Kosslyn S.M. Using imagery to retrieve semantic information: a developmental study. Child Development. 1976;47(2):434–444. [Google Scholar]
- Kosslyn S.M. Harvard University Press; Cambridge, MA, USA: 1980. Image and Mind. [Google Scholar]
- Kosslyn S.M. MIT Press; Cambridge, MA, USA: 1996. Image and Brain: The Resolution of the Imagery Debate. [Google Scholar]
- Kosslyn S.M., Thompson W.L., Ganis G. Oxford University Press; Oxford, UK: 2006. The Case for Mental Imagery. [Google Scholar]
- Lang T.J., Blackwell S.E., Harmer C.J., Davison P., Holmes E.A. Cognitive bias modification using mental imagery for depression: developing a novel computerized intervention to change negative thinking styles. European Journal of Personality. 2012;26(2):145–157. doi: 10.1002/per.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanius R.A., Williamson P.C., Densmore M., Boksman K., Gupta M.A., Neufeld R.W. Neural correlates of traumatic memories in posttraumatic stress disorder: a functional MRI investigation. American Journal of Psychiatry. 2001;158(11):1920–1922. doi: 10.1176/appi.ajp.158.11.1920. [DOI] [PubMed] [Google Scholar]
- Lanius R.A., Williamson P.C., Hopper J., Densmore M., Boksman K., Gupta M.A. Recall of emotional states in posttraumatic stress disorder: an fMRI investigation. Biological Psychiatry. 2003;53(3):204–210. doi: 10.1016/s0006-3223(02)01466-x. [DOI] [PubMed] [Google Scholar]
- Lau J.Y.F., Molyneaux E., Telman M.D., Belli S. The plasticity of adolescent cognitions: data from a novel cognitive bias modification training task. Child Psychiatry and Human Development. 2011;42(6):679–693. doi: 10.1007/s10578-011-0244-3. [DOI] [PubMed] [Google Scholar]
- Lau J.Y., Britton J.C., Nelson E.E., Angold A., Ernst M., Goldwin M. Distinct neural signatures of threat learning in adolescents and adults. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(11):4500–4505. doi: 10.1073/pnas.1005494108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau J.Y., Belli S.R., Chopra R.B. Cognitive bias modification training in adolescents reduces anxiety to a psychological challenge. Clinical Child Psychology and Psychiatry. 2012 doi: 10.1177/1359104512455183. [DOI] [PubMed] [Google Scholar]
- LeDoux J.E. Emotion circuits in the brain. Annual Review of Neuroscience. 2000;23(1):155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Lothmann C., Holmes E.A., Chan S.W.Y., Lau J.Y.F. Cognitive bias modification training in adolescents: effects on interpretation biases and mood. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 2011;52(1):24–32. doi: 10.1111/j.1469-7610.2010.02286.x. [DOI] [PubMed] [Google Scholar]
- Luna B., Garver K.E., Urban T.A., Lazar N.A., Sweeney J.A. Maturation of cognitive processes from late childhood to adulthood. Child Development. 2004;75(5):1357–1372. doi: 10.1111/j.1467-8624.2004.00745.x. [DOI] [PubMed] [Google Scholar]
- Luna B., Sweeney J.A. The emergence of collaborative brain function: fMRI studies of the development of response inhibition. Annals of the New York Academy of Sciences. 2004;1021(1):296–309. doi: 10.1196/annals.1308.035. [DOI] [PubMed] [Google Scholar]
- Marmor G.S. Mental rotation and number conservation: are they related? Developmental Psychology. 1977;13(4):320–325. [Google Scholar]
- Mathews A., MacLeod C. Induced processing biases have causal effects on anxiety. Cognition & Emotion. 2002;16(3):331–354. [Google Scholar]
- McCarty M.E., Clifton R.K., Ashmead D.H., Lee P., Goubet N. How infants use vision for grasping objects. Child Development. 2001;72(4):973–987. doi: 10.1111/1467-8624.00329. [DOI] [PubMed] [Google Scholar]
- McRae K., Gross J.J., Weber J., Robertson E.R., Sokol-Hessner P., Ray R.D. The development of emotion regulation: an fMRI Study of cognitive reappraisal in children, adolescents and young adults. Social Cognitive and Affective Neuroscience. 2012;7(1):11–22. doi: 10.1093/scan/nsr093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mischel Mischel W. The development of children's knowledge of self-control strategies. Child Development. 1983;54(3):603–619. [Google Scholar]
- Mischel W., Shoda Y., Rodriguez M.I. Delay of gratification in children. Science (New York, NY) 1989;244(4907):933–938. doi: 10.1126/science.2658056. [DOI] [PubMed] [Google Scholar]
- Morina N., Deeprose C., Pusowski C., Schmid M., Holmes E.A. Prospective mental imagery in patients with major depressive disorder or anxiety disorders. Journal of Anxiety Disorders. 2011;25(8):1032–1037. doi: 10.1016/j.janxdis.2011.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton J., Frith U. Developmental Psychopathology, Vol. 1.: Theory and Methods. Wiley; New York: 1995. Causal modelling: a structural approach to developmental psychology. [Google Scholar]
- Moulton S.T., Kosslyn S.M. Imagining predictions: mental imagery as mental emulation. Philosophical Transactions of the Royal Society of London, Series B, Biological Sciences. 2009;364(1521):1273–1280. doi: 10.1098/rstb.2008.0314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muris P., Huijding J., Mayer B., Van As W., Van Alem S. Reduction of verbally learned fear in children: a comparison between positive information, imagery, and a control condition. Journal of Behavior Therapy and Experimental Psychiatry. 2011;42(2):139–144. doi: 10.1016/j.jbtep.2010.11.006. [DOI] [PubMed] [Google Scholar]
- Ochsner K.N., Gross J.J. The cognitive control of emotion. Trends in Cognitive Sciences. 2005;9(5):242–249. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Olesen P.J., Nagy Z., Westerberg H., Klingberg T. Combined analysis of DTI and fMRI data reveals a joint maturation of white and grey matter in a fronto-parietal network. Cognitive Brain Research. 2003;18(1):48–57. doi: 10.1016/j.cogbrainres.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Orr S.P., Pitman R.K., Lasko N.B., Herz L.R. Psychophysiological assessment of posttraumatic stress disorder imagery in World War II and Korean combat veterans. Journal of Abnormal Psychology. 1993;102(1):152–159. doi: 10.1037//0021-843x.102.1.152. [DOI] [PubMed] [Google Scholar]
- Osman S., Cooper M., Hackmann A., Veale D. Spontaneously occurring images and early memories in people with body dysmorphic disorder. Memory (Hove, England) 2004;12(4):428–436. doi: 10.1080/09658210444000043. [DOI] [PubMed] [Google Scholar]
- Patton G.C., Viner R. Pubertal transitions in health. The Lancet. 2007;369(9567):1130–1139. doi: 10.1016/S0140-6736(07)60366-3. [DOI] [PubMed] [Google Scholar]
- Paus T., Keshavan M., Giedd J.N. Why do many psychiatric disorders emerge during adolescence? Nature Reviews Neuroscience. 2008;9(12):947–957. doi: 10.1038/nrn2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson D.G., Deeprose C., Wallace-Hadrill S.M.A., Burnett Heyes S., Holmes E.A. Assessing mental imagery in clinical psychology: a review of imagery measures and a guiding framework. Clinical Psychology Review. 2013;33(1):1–23. doi: 10.1016/j.cpr.2012.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa L., Adolphs R. Emotion processing and the amygdala: from a ‘low road’ to ‘many roads’ of evaluating biological significance. Nature Reviews. Neuroscience. 2010;11(11):773–783. doi: 10.1038/nrn2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pictet A., Coughtrey A.E., Mathews A., Holmes E.A. Fishing for happiness: the effects of generating positive imagery on mood and behaviour. Behaviour Research and Therapy. 2011;49(12):885–891. doi: 10.1016/j.brat.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prime D.J., Jolicoeur P. Mental rotation requires visual short-term memory: evidence from human electric cortical activity. Journal of Cognitive Neuroscience. 2010;22(11):2437–2446. doi: 10.1162/jocn.2009.21337. [DOI] [PubMed] [Google Scholar]
- Pylyshyn Z.W. What the mind's eye tells the mind's brain: a critique of mental imagery. Psychological Bulletin. 1973;80(1):1–24. [Google Scholar]
- Raby C., Edwards D. Unrecognized hospital trauma as a source of complex psychiatric symptoms: a systematic case study with implications for children's rights and evidence-based practice. Psychotherapy Research. 2011;21(5):541–553. doi: 10.1080/10503307.2011.587470. [DOI] [PubMed] [Google Scholar]
- Reynolds M., Brewin C.R. Intrusive memories in depression and posttraumatic stress disorder. Behaviour Research and Therapy. 1998;37(3):201–215. doi: 10.1016/s0005-7967(98)00132-6. [DOI] [PubMed] [Google Scholar]
- Roby A.C., Kidd E. The referential communication skills of children with imaginary companions. Developmental Science. 2008;11(4):531–540. doi: 10.1111/j.1467-7687.2008.00699.x. [DOI] [PubMed] [Google Scholar]
- Rueda M.R., Fan J., McCandliss B.D., Halparin J.D., Gruber D.B., Lercari L.P., Posner M.I. Development of attentional networks in childhood. Neuropsychologia. 2004;42(8):1029–1040. doi: 10.1016/j.neuropsychologia.2003.12.012. [DOI] [PubMed] [Google Scholar]
- Rutter M. Psychopathology and development: I. Childhood antecedents of adult psychiatric disorder. The Australian and New Zealand Journal of Psychiatry. 1984;18(3):225–234. doi: 10.3109/00048678409161295. [DOI] [PubMed] [Google Scholar]
- Schacter D.L., Addis D.R., Buckner R.L. Remembering the past to imagine the future: the prospective brain. Nature Reviews Neuroscience. 2007;8(9):657–661. doi: 10.1038/nrn2213. [DOI] [PubMed] [Google Scholar]
- Shepard R.N., Cooper L.A. MIT Press; Cambridge, MA: 1982. Mental Images and their Transformations. [Google Scholar]
- Shepard R.N., Metzler J. Mental Rotation of Three-Dimensional Objects. Science. 1971;171(3972):701–703. doi: 10.1126/science.171.3972.701. [DOI] [PubMed] [Google Scholar]
- Shin O.S. Regional cerebral blood flow in the amygdala and medial prefrontalcortex during traumatic imagery in male and female vietnam veterans with ptsd. Archives of General Psychiatry. 2004;61(2):168–176. doi: 10.1001/archpsyc.61.2.168. [DOI] [PubMed] [Google Scholar]
- Smith P., Yule W., Perrin S., Tranah T., Dalgleish T., Clark D.M. Cognitive-behavioral therapy for PTSD in children and adolescents: a preliminary randomized controlled trial. Journal of the American Academy of Child and Adolescent Psychiatry. 2007;46(8):1051–1061. doi: 10.1097/CHI.0b013e318067e288. [DOI] [PubMed] [Google Scholar]
- Speckens A.E.M., Hackmann A., Ehlers A., Cuthbert B. Imagery special issue: intrusive images and memories of earlier adverse events in patients with obsessive compulsive disorder. Journal of Behavior Therapy and Experimental Psychiatry. 2007;38(4):411–422. doi: 10.1016/j.jbtep.2007.09.004. [DOI] [PubMed] [Google Scholar]
- Spence S.H., Donovan C., Brechman-Toussaint M. Social skills, social outcomes, and cognitive features of childhood social phobia. Journal of Abnormal Psychology. 1999;108(2):211–221. doi: 10.1037//0021-843x.108.2.211. [DOI] [PubMed] [Google Scholar]
- Steinberg L. A social neuroscience perspective on adolescent risk-taking. Developmental Review: DR. 2008;28(1):78–106. doi: 10.1016/j.dr.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St-Onge M., Mercier P., De Koninck J. Imagery rehearsal therapy for frequent nightmares in children. Behavioral Sleep Medicine. 2009;7(2):81–98. doi: 10.1080/15402000902762360. [DOI] [PubMed] [Google Scholar]
- Tellegen A., Atkinson G. Openness to absorbing and self-altering experiences (‘absorption’), a trait related to hypnotic susceptibility. Journal of Abnormal Psychology. 1974;83(3):268–277. doi: 10.1037/h0036681. [DOI] [PubMed] [Google Scholar]
- Telman, M., Lau, J., Holmes, E., 2012 Modifying adolescent interpretation biases through cognitive training: effects on negative affect and stress appraisals, Clinical Child Psychology and Psychiatry 1359104512455183. [DOI] [PMC free article] [PubMed]
- Todd R.M., Cunningham W.A., Anderson A.K., Thompson E. Affect-biased attention as emotion regulation. Trends in Cognitive Sciences. 2012;16(7):365–372. doi: 10.1016/j.tics.2012.06.003. [DOI] [PubMed] [Google Scholar]
- Tulving E. Organization of memory – Quo vadis? In: Gazzaniga M.S., editor. The Cognitive Neurosciences. MIT Press; Cambridge, MA: 1995. pp. 839–847. [Google Scholar]
- Vassilopoulos S.P., Blackwell S.E., Moberly N.J., Karahaliou E. Comparing imagery and verbal instructions for the experimental modification of interpretation and judgmental bias in children. Journal of Behavior Therapy and Experimental Psychiatry. 2012;43(1):594–601. doi: 10.1016/j.jbtep.2011.08.004. [DOI] [PubMed] [Google Scholar]
- Verwoerd J., Wessel I., De Jong P.J. Individual differences in experiencing intrusive memories: the role of the ability to resist proactive interference. Journal of Behavior Therapy and Experimental Psychiatry. 2009;40(2):189–201. doi: 10.1016/j.jbtep.2008.08.002. [DOI] [PubMed] [Google Scholar]
- Whalley M.G., Kroes M.C.W., Huntley Z., Rugg M.D., Davis S.W., Brewin C.R. An fMRI investigation of posttraumatic flashbacks. Brain and Cognition. 2013;81(1):151–159. doi: 10.1016/j.bandc.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheatley J., Brewin C.R., Patel T., Hackmann A., Wells A., Fisher P., Myers S. ‘I’ll believe it when I can see it’: imagery rescripting of intrusive sensory memories in depression. Journal of Behavior Therapy and Experimental Psychiatry. 2007;38(4):371–385. doi: 10.1016/j.jbtep.2007.08.005. [DOI] [PubMed] [Google Scholar]
- Wimmer M.C., Howe M.L. The development of automatic associative processes and children's false memories. Journal of Experimental Child Psychology. 2009;104(4):447–465. doi: 10.1016/j.jecp.2009.07.006. [DOI] [PubMed] [Google Scholar]
- Wittchen H.-U., Fehm L. Epidemiology and natural course of social fears and social phobia. Acta psychiatrica Scandinavica. Supplementum. 2003;417:4–18. doi: 10.1034/j.1600-0447.108.s417.1.x. [DOI] [PubMed] [Google Scholar]
- Wolpe J. Pergamon Press; Tarrytown, NY: 1990. The Practice of Behavior Therapy. [Google Scholar]
- Yang P., Wu M.-T., Hsu C.-C., Ker J.-H. Evidence of early neurobiological alternations in adolescents with posttraumatic stress disorder: a functional MRI study. Neuroscience Letters. 2004;370(1):13–18. doi: 10.1016/j.neulet.2004.07.033. [DOI] [PubMed] [Google Scholar]