Highlights
► We investigate action observation related brain activity using MEG. ► We examine differences between Asperger and typically developed children. ► Stronger modulation of Mu-power at frontal and central sites in control children. ► Stronger premotor and weaker prefrontal sources in typical than Asperger children. ► Sources correlate strongly with social competence but not intellectual skills.
Keywords: Action observation, Asperger's syndrome, Magnetencephalography, Mu-rhythm, Social skills
Abstract
Children with Asperger's syndrome show deficits in social functioning while their intellectual and language development is intact suggesting a specific dysfunction in mechanisms mediating social cognition. An action observation/execution matching system might be one such mechanism. Recent studies indeed showed that electrophysiological modulation of the “Mu-rhythm” in the 10–12 Hz range is weaker when individuals with Asperger's syndrome observe actions performed by others compared to controls. However, electrophysiological studies typically fall short in revealing the neural generators of this activity. To fill this gap we assessed magnetoencephalographic Mu-modulations in Asperger's and typically developed children, while observing grasping movements. Mu-power increased at frontal and central sensors during movement observation. This modulation was stronger in typical than in Asperger children. Source localization revealed stronger sources in premotor cortex, the intraparietal lobule (IPL) and the mid-occipito-temporal gyrus (MOTG) and weaker sources in prefrontal cortex in typical participants compared to Asperger. Activity in premotor regions, IPL and MOTG correlated positively with social competence, whereas prefrontal Mu-sources correlated negatively with social competence. No correlation with intellectual ability was found at any of these sites. These findings localize abnormal Mu-activity in the brain of Asperger children providing evidence which associates motor-system abnormalities with social-function deficits.
1. Introduction
Asperger's syndrome belongs to the autistic spectrum disorders (ASD). It is characterized by impaired social interaction and repetitive or stereotyped behavior (American Psychiatric Association, 2000). In contrast to low-functioning autistic individuals, people with Asperger's do not usually show delayed language or cognitive development.
Several authors suggested that social deficits in ASD are linked to impairments in representing other people's mental states evident, for example, in ‘theory-of-mind’ tasks (Baron-Cohen et al., 1985), identifying facial expressions (Lindner and Rosén, 2006), or inferring mental states from nonverbal social cues (David et al., 2010). A possible neural foundation of the ability to understand other's mental states lies in the activation of brain regions crucial for the representation of the own experience of such states. One influential theory posits that this ability is based on simulating observed actions of others and estimating the actor's intentions or state-of-mind on the basis of one's own intentions or state-of-mind associated with the simulated act (Davies and Stone, 1995, Carruthers and Smith, 1996). Tentative biological support for this simulation theory has been provided by studies showing activation of the same motor representations during action execution and observation (di Pellegrino et al., 1992, Gallese et al., 1996). Various physiological manifestations of observation-action matching have been reported. For instance modulations of electroencephalographic (EEG) and neuromagnetic (MEG) oscillations in the alpha range (8–12 Hz) which, when recorded over somatosensory regions are labeled Mu-rhythms, are modulated during own motor execution and while observing actions performed by others (e.g. Hari et al., 1998). Further, Mu-rhythm is sensitive to goal-directedness (Muthukumaraswamy et al., 2004) as are also mirror neurons (Gallese et al., 1996). However, although Mukamel et al. (2010) recently demonstrated the existence of premotor neurons (but also in other areas) that responded both during action execution and observation using single cell recordings in humans, this still does not necessarily mean that these are the neurons that generate the EEG Mu-rhythm.
Nevertheless, in the EEG Mu-suppression during action observation is an often reported pattern (Hari et al., 1998; for a review see Pineda, 2005, Caetano et al., 2007). In fact, the full sequence of neurophysiological events during action execution or observation consists of an initial sharp Mu-suppression, followed by an extended period of power increase, the so-called Mu rebound (Neuper et al., 2006) which is thought to reflect inhibitory control (Pfurtscheller and Neuper, 1997). Schuch et al. (2010) showed that power decrease and increase of Mu are related: the stronger the observation-evoked decrease, the stronger the following rebound. In other words, both decrease and increase reflect the reactivity of the sensorimotor system. Also, at least in adults, modulations in the beta frequency range appear to exhibit similar suppression and rebound properties as the Mu-rhythm proper (Hari, 2006).
However, motor simulation theory in a strong version, implying that action understanding primarily grounded in the sensory motor systems, has been criticized on logical grounds (Hickok, 2009) and some failures to produce empirical evidence for such a mechanism have fueled skepticism (Lingnau et al., 2009). In particular, motor system activations during action observation have been suggested to arise as a consequence of action understanding rather than representing the process itself (see for example Mahon and Caramazza, 2008, Lingnau and Petris, 2012).
Still, recent studies suggest that Mu-modulation might be involved in mediating social skills and that it might be abnormal in ASD: Mu-modulation is stronger while seeing socially relevant events (Oberman et al., 2007) and following the administration of oxytocin (Perry et al., 2010a), which is sometimes regarded as a “social hormone”. Furthermore, ASD patients who exhibit social difficulties have been reported to show reduced EEG Mu-modulation during observation of a stranger's grasping acts (Oberman et al., 2008), although a previous MEG study produced no evidence for abnormal brain oscillatory responses during action viewing in ASD (Avikainen et al., 1999), leading to the suggestion that ASD individuals are impaired at imitation of motor acts (Avikainen et al., 1999, Nishitani et al., 2004), but not in the neural correlates of action observation. On the other hand, a more recent MEG study found reduced observation-induced post-movement beta rebound in ASD versus typical adults (Honaga et al., 2010).
Evidence on an association between Mu-rhythm modulation and external measures of social skills in general and in Asperger's syndrome in particular is equivocal. Such an association has been demonstrated for cerebral blood flow indicators of motor simulation in ASD (Dapretto et al., 2006). For the EEG, Bernier et al. (2007) report, in both adults with ASD and controls, correlations of around .5 between Mu-modulation over motor cortex (surrounding electrodes C3 and C4) during movement observation and imitation, and various questionnaire indices of behavioral imitation skills. Assessment of social skills is not reported. Investigating typically developing children and children with ASD, Raymaekers et al. (2009) tested the relationship between Mu-modulations over motor cortex (C3, Cz, C4) and intelligence, symptom severity, and age. They found in ASD a negative correlation between intelligence and Mu-suppression as well as between age and Mu-suppression, but no relationship with symptom severity. Investigating undergraduates Perry et al. (2010b) found smaller Mu-suppression over motor cortex (C3, Cz, C4) in individuals with higher empathy questionnaire scores.
Thus, correlation with external measures does not unambiguously corroborate the relevance of Mu-rhythms to human social cognition or the notion that stronger Mu-modulations are associated with better social skills and conversely, reduced Mu-responsiveness is associated with social impairment in ASD. One reason for these ambiguities may be that different brain regions contribute to the generation and modulation of the Mu rhythm, which may not all be equally relevant to this system's role in social cognition. Recent EEG studies with their focus on Mu-rhythm modulation over central sites typically fall short in revealing the generator structure of this activity.
Here, using MEG, we localize the cortical sources of ASD/control differences in Mu-modulation and, thereby, further specify the functional neuroanatomy of the human Mu-rhythms, providing information regarding relevant deviations characteristic to ASD. We measured action-observation-related changes focusing on the 10–12 Hz MEG Mu-rhythm component in Asperger's syndrome and typically developed children and localized the respective cortical sources of this activity. In a second, independent step, we examined whether social skills in healthy children or social deficits in ASD are specifically related to observation-elicited modulation of Mu, correlating the sources of Mu-rhythm related activity across the entire source space and regardless of potential group differences with measures of social competence or social problems.
2. Methods
2.1. Participants
Children diagnosed with Asperger's syndrome and their parents were contacted via the Konstanz (Germany) school district's autism spokesperson and two mental health care institutions. To qualify for the study, participants had to fulfill the following criteria: not younger than ten years of age, no additional psychological disorders, school performance within an average range, and no epilepsy. Asperger's syndrome diagnosis was established by a licensed psychiatrist according to DSM-IV diagnostic criteria for Asperger's syndrome (299.8). The children in the control group were recruited from an existing database of volunteers. Nine children with Asperger's syndrome (1 female, 8 male) and nine typically developed children (3 female, 6 male) participated in the study. The Asperger's children had a mean age of 13.39 years and the control children of 12.31. The numerical differences in age and gender distribution were not significant. At the time of testing, children from both groups were regularly enrolled in Germany's public school system. The children received payment (5 € per hour) and a small gift, while parents were financially compensated for travel expenses. All participants had normal or corrected to normal vision. The parents signed an informed consent form explaining the study and detailing participants’ rights according to the Declaration of Helsinki (http://www.wma.net/en/30publications/10policies/b3/index.html).
2.2. Questionnaires and psychological tests
In order to gain information about the participants’ behavior and cognitive abilities, questionnaires and psychological tests were given to the participants and their parents: parents filled in the German version of the Children Behavior Check List (Achenbach, 1991), which assesses three domains of competence (Activities, Social, School) and eight behavioral impairments (Social Withdrawal, Somatic Complaints, Anxious/Depressed, Social Problems, Thought Problems, Attention Problems, Delinquent Behavior, Aggressive Behavior). Depending on their age, intellectual abilities were either assessed using Raven's Colored Progressive Matrices (CPM, <12 years, Becker et al., 1980) or Raven's Standard Progressive Matrices (SPM, >12 years, Heller et al., 1998). In addition, the participants completed the subtests Hand Imitation and Numbers Recall measuring sequential processing focusing on short-term memory from the Kaufman Assessment Battery for Children (German version, Mechler and Preuß, 2001), a battery that assesses nonverbal intellectual abilities.
2.3. Task, materials and design
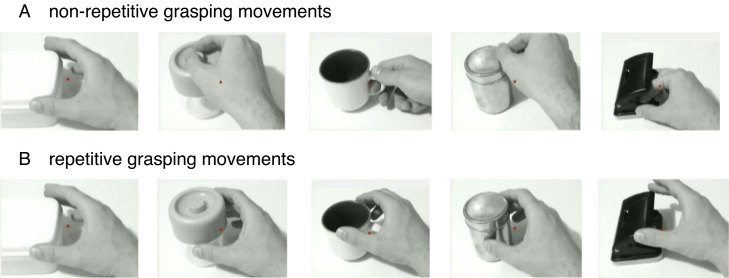
We adopted and slightly modified the experimental procedures used by Shmuelof and Zohary (2006) and Perry and Bentin (2009). The stimuli consisted of 14 continuous black and white 40 s video clips presenting different hand movements sequentially. Six videos showed a right hand reaching to a small object (e.g. box, cups, scissors) from the right side, grasping the object and releasing it. One grasping movement lasted between 900 and 1500 ms resulting in 31–33 movements per video clip. Left hand clips were generated by flipping the right-hand movements horizontally (see Shmuelof and Zohary, 2006). Half of the videos showed always the same (repetitive) grasping movements and the other half different (non-repetitive) ones (see Fig. 1). These stimuli were previously chosen in order to investigate adaptation processes (Shmuelof and Zohary, 2006, Perry and Bentin, 2009), which however in this experiment did not yield significant results and therefore are not discussed further.
Fig. 1.
Illustration of the grasping movements used in the non-repetitive (A) and repetitive (B) condition.
In addition to the twelve hand movement videos, a 40 s baseline condition for each side displaying non-biological motion was presented. The baseline condition consisted of a ball moving from side to side, once from the left (baseline for the left hand movements) and once from the right (baseline for the right hand movements), again with one movement lasting between 900 and 1500 ms. A red fixation dot was centered on each video which changed its color to blue for one second between one and four times during each video. Participants were asked to fixate the dot and to count the number of times the color changed. The baseline task aimed to engage the participants’ attention in a controlled manner, and ensure maintenance of central fixation.
The order of the video-clips was randomized and each one was followed by a short break (self-terminated by the participant, usually, about 15–20 s) during which the participants could relax their eyes. The experiment was controlled using PRESENTATION software (Neurobehavioral Systems®, Albany, NY, USA) and projected to the ceiling in the MEG chamber by means of a mirror system.
2.4. Data acquisition
Neuromagnetic brain activity was recorded during movement observation with a 148-channel magnetometer system (MAGNES 2500 WH, 4D Neuroimaging, San Diego, USA), installed in a magnetically shielded chamber (Vakuumschmelze, Hanau), while participants lay in a supine position. The MEG was sampled at a rate of 678.17 Hz and an online band-pass filtered from 0.1 Hz to 200 Hz. Prior to the experiment, five index points on the subjects head (nasion, inion, left and right ear canal, and CZ) and the subject's head shape were digitized with a Polhemus 3 Space® Fasttrack. The participant's head position relative to the pick-up coils of the MEG was measured before and after the recording.
2.5. Data analysis
2.5.1. Behavioral data
Group differences on the K-ABC, Raven CPM/SPM, and CBCL were calculated using independent t-tests. For the Raven test, raw scores were transformed to T-scores to ensure comparable scaling of the CPM and SPM versions of the test before calculating group statistics.
2.5.2. Sensor data
The MEG data were analyzed with FieldTrip, an open-source Matlab-toolbox (http://www.ru.nl/fcdonders/fieldtrip, Oostenveld et al., 2011). The data of each of the 14 videos were cut into 20 non-overlapping segments of 2 s. Segments with strong non-physiological artifacts (e.g. channel jumps) were excluded. Data were down-sampled to 300 Hz and an independent component analysis (ICA) was carried out. Based on the ICA, components associated with ocular and cardiac activity were identified and removed from the entire raw data set. Segments including any remaining artifacts were manually excluded from the subsequent analyses. For each 2-s segment, the power for frequencies between 1 and 30 Hz was computed using a fast Fourier transform (FFT) with a Hanning window.
For further analyses, the ratio of the power during the experimental conditions relative to the power during the baseline conditions were calculated separately for each side ([power(biological movement) − power(ball movement)]/power(ball movement)). To control for the influence of this chosen baseline procedure (as also used in Perry and Bentin, 2009), we also analyzed the sensor power effects using another baseline correction (power(non-repetitive movement) − power(repetitive movement))/power(repetitive movement). This led to a very similar activity pattern and we therefore stayed with the method used by Perry and Bentin (2010). The reported results refer to these baseline-corrected measures of Mu-power modulation. Distribution of the power for the different frequencies corresponding to the different conditions in the present sample revealed strongest modulations in the Mu-range between 10 and 12 Hz (see Fig. 1), leading us to focus analyses on this frequency band. As the term Mu-rhythm is associated with the 8–12 Hz frequency range over premotor or sensorimotor regions (e.g. Hari and Salmelin, 1997), we will label the MEG in the 10–12 Hz range as high-Mu if it was recorded over fronto-central sensors or in premotor/prefrontal sources; otherwise we will use the term high-alpha (cf., Petsche et al., 1997).
2.5.3. Source analysis
A multi-taper frequency analysis based on Slepian sequences with a smoothing of 2 Hz was applied in order to derive the sensor level cross-spectral density matrix between 10 and 12 Hz. The previously assessed individual head shapes were used to create pseudo-individual MRI images, by an affine transformation of the “headshape” of an MNI-template onto the individual headshape points. In order to accomplish group statistics and for illustrative purposes, these pseudo-individual MRI images were normalized onto a standard MNI brain (http://www.bic.mni.mcgill.ca/brainweb) using SPM2 (http://www.fil.ion.ucl.ac.uk/spm). Dynamic imaging of coherent sources (DICS, Gross et al., 2001), a frequency-domain adaptive spatial filtering algorithm, was used to identify the sources of the frequency of interest (10–12 Hz). This algorithm has proven to be particularly powerful when localizing oscillatory sources (Liljeström et al., 2005).
T-tests were applied to the source data to quantify significant differences from baseline within each participant group using dependent samples t-tests. Differences between the groups were calculated for repetitive and non-repetitive movements using independent sample t-tests. For all comparisons we used an alpha level of p < .01.
2.5.4. Correlation analysis
Voxel-wise correlations without any a priori localization assumptions were calculated between sources of cortical high-Mu and the behavioral scores that differentiated best between the two groups of children. Differences were present primarily in scales reflecting core social skill deficits in Asperger's (see Table 1). From the CBCL one competence score (social competence) and one impairment score (social problems) were chosen for the correlation analyses with high-Mu source activities. Clusters of significant correlations with a type-I error level of .01 or less were identified and contiguous voxels were collapsed into anatomical regions. For regions exhibiting strong linear correlations, mean activity was calculated over all voxels belonging to the region and correlated with the behavioral scores for illustrative purposes. We devised a permutation test to explore the influence of pure group differences on these correlations, by shuffling behavioral scores and brain activity data within each group and calculating correlations over both groups combined. This was repeated 5000 times thereby creating a distribution of correlation scores. From this distribution we were capable to derive the probability of our empirically observed correlation under the assumption that it was caused by a potential group difference alone.
Table 1.
Questionnaire mean scores and statistical group differences for the CBCL, CPM/SPM, and the K-ABC. Standard errors are in brackets.
| Control children | Asperger's children | t-Test | |
|---|---|---|---|
| CBCL | |||
| Competence scales | |||
| Activities | 53.11 (1.65) | 49.75 (2.33) | n.s. |
| Social competence | 49.67 (1.49) | 36.13 (2.60) | t(15) = 4.65, p < .001 |
| School performance | 52.89 (0.86) | 45.88 (3.26) | t(15) = 2.19, p < .05 |
| Syndrome scales | |||
| Social withdrawal | 51.67 (0.80) | 70 (2.84) | t(15) = −6.54, p < .001 |
| Somatic complaints | 51.78 (0.92) | 60 (3.64) | t(15) = −2.31, p < .05 |
| Anxious/depressed | 53.44 (2.02) | 61.38 (4.31) | n.s. |
| Social problems | 52.56 (1.30) | 76.38 (3.55) | t(15) = −6.60, p < .001 |
| Thought problems | 50 (0) | 67.38 (3.14) | t(15) = −5.90, p < .001 |
| Attention problems | 52.78 (1.00) | 69.25(3.55) | t(15) = −4.71, p < .001 |
| Delinquent behavior | 51.78 (1.19) | 59.78 (2.09) | t(15) = −3.42, p < .01 |
| Aggressive behavior | 53 (1.73) | 60.5 (2.98) | t(15) = −2.24, p < .05 |
| CPM/SPM | 48.67 (3.37) | 45.78 (2.47) | n.s. |
| K-ABC | |||
| Hand imitation | 56.0 (2.8) | 57.67 (4.29) | n.s. |
| Numbers recall | 57.67 (3.27) | 54.67 (2.92) | n.s. |
3. Results
3.1. Questionnaires and psychological tests
Table 1 displays the mean values for the questionnaire and test data. The two groups did not differ regarding CPM/SPM and K-ABC scores. However, Asperger's children scored significantly lower on social competence scales and significantly higher on all social impairment scales of the CBCL. Due to experimenter error, the CBCL score was missing for one child in the Asperger's group.
3.2. MEG
3.2.1. Sensor data
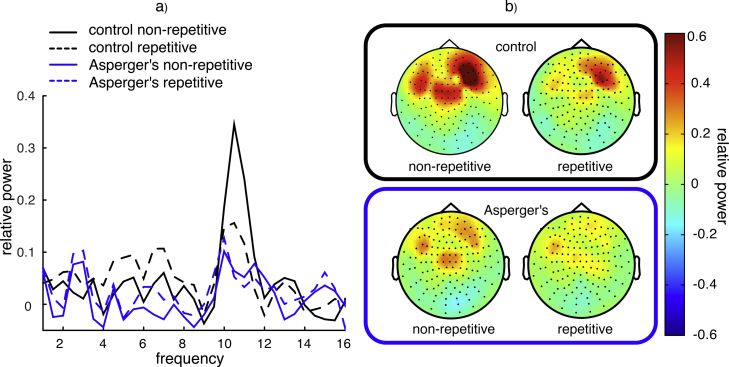
The FFT spectra averaged over all sensors for the two groups separately are displayed in Fig. 2A for the repetitive and non-repetitive biological movement condition. In contrast to recent EEG studies, we found elevation of high-Mu power while observing biological movement. This power increase in the 10–12 Hz range was conspicuous and topographically distinct especially in the non-repetitive condition and particularly in the data recorded at frontal and central sensors in typically developed children, (see Fig. 2B). In contrast, in both groups there was only a little change in high-Mu power in response to repetitive stimulation. Overall the group difference in power was larger for the non-repetitive than for the repetitive condition (see Fig. 2A).
Fig. 2.
(A) Frequency distribution over all 148 MEG sensors for repetitive and non-repetitive movements separately for control (top right) and Asperger's children (bottom right). (B) Topographical illustration of the 10–12 Hz frequency range.
3.2.2. Source localization of group differences
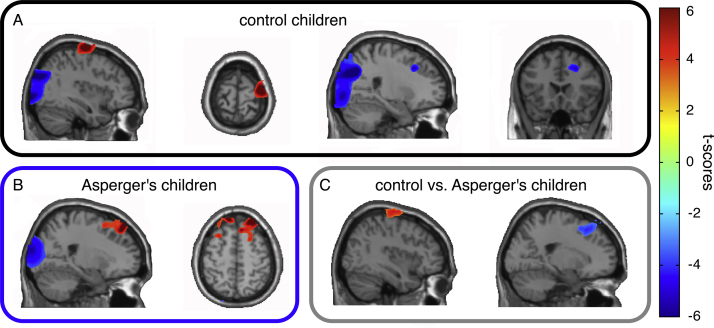
Group comparisons of MEG source activity revealed that for the non-repetitive movements, the Asperger's syndrome and the control groups differed significantly in the pattern of high-Mu-modulation while no differences were found between the two groups in response to repetitive movements. Therefore, the source analysis focused on the non-repetitive movements condition. Within-group dependent t-statistics on source activity in the control group revealed enhancement in high-Mu power while observing biological movements relative to baseline in the right premotor cortex (Brodmann area, BA 6, Talairach coordinates: X: 39, Y: −9, Z: 70) and a concurrent reduction in high-Mu power in right middle prefrontal cortex (BA 8, X: 32, Y: 28, Z: 38; see Fig. 3A). In contrast, Asperger's children lacked the premotor enhancement and showed bilaterally increased activity in the prefrontal cortex (BA 8, right: X: 24, Y: 45, Z: 48, left: X: −9, Y: 50, Z: 48; see Fig. 3B). In addition, in both groups, sources in the occipital cortex showed decreased activity in the high-alpha power. Between-group comparisons using independent t-statistics showed that for non-repetitive movements the right premotor (BA 6, X: 39, Y: −8, Z: 66) source of Mu was stronger in the control than in the Asperger group, whereas the right prefrontal (BA 8, X: 28, Y: 30, Z: 45) source was weaker in the control than in the Asperger group. Two additional regions with differential high-alpha activity were found in both groups: the right middle occipital–temporal gyrus (MOTG, BA 19/37, X: 58, Y: −67, Z: 6) and the right inferior parietal lobe (IPL, BA 40, X: 49, Y: −52, Z: 36) where non-repetitive stimulation elicited more activity than repetitive stimulation. There were no group differences in occipital sources of alpha (Fig. 3C).
Fig. 3.
Sources of significant differences (t-values) between the non-repetitive condition compared to baseline in control and Asperger's children and for control versus Asperger's children during the non-repetitive condition. (A) Increased 10–12 Hz activity is localized in right premotor areas (BA 6). Decreased 10–12 Hz activity is localized in occipital regions and right middle frontal gyrus (BA 8). (B) Decreased 10–12 Hz activity is localized in occipital regions and increased activity in prefrontal areas (BA 8). (C) Increased 10–12 Hz activity for the control relative to the Asperger's children is localized in right premotor areas (BA 6). Decreased 10–12 Hz activity for the control relative to the Asperger's children is localized in right middle/superior frontal gyrus (BA 8).
3.2.3. Correlation with social skill scores
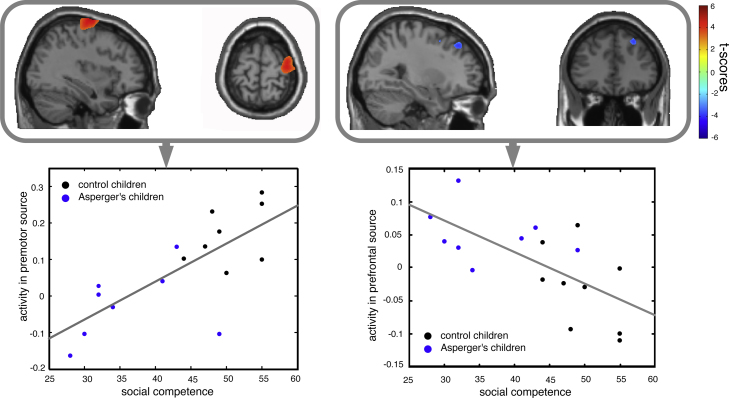
The independently conducted, not regionally constrained, correlations between the sources of the 10–12 Hz MEG activity and the scores of social competence and social skill impairments across groups corroborated and extended the group comparisons: although the correlations included the entire source space, the social competence and social skills impairment scales correlated significantly only with sources overlapping or in close vicinity to the regions identified previously in the between-group comparisons, namely the right premotor, right middle frontal gyrus, right IPL, and the right MOTG. These correlations indicated that the more socially competent an individual was, the stronger was the source of high-Mu activity during observation of non-repetitive movements in right premotor regions (BA 6), right IPL (BA 40), and right MOTG (BA 19/37, all positive correlations; Fig. 4, Fig. 5) and the less high-Mu activity occurred in right prefrontal regions (BA 8, negative correlation; Fig. 4, right). The same or at least largely overlapping regions (see Table 2) were correlated with social skill impairments but, obviously, in the reverse direction: the more an individual tended to have social problems, the less high-Mu activity was generated in right premotor regions (negative correlation) while more high-Mu activity was generated in right prefrontal regions (positive correlation).
Fig. 4.
Left: Mu-activity in premotor cortex (BA 6, X: 42, Y: −5, Z: 65) is strongly correlated with social competence scale. The scatterplot in the lower panel indicates an r = .74 with p < .01 for this region (average over voxels). Right: An inverse correlation was obtained for prefrontal cortex (BA 8, X: 30, Y: 39, Z: 39). The scatterplot in the lower panel indicates an r = −.66 with p < .01 for this region (averaged over voxels). Note the close similarity of the regions identified in the correlation analysis and those identified in the group comparison (Fig. 2C) and the almost perfect separation of the groups in the scatterplots.
Fig. 5.
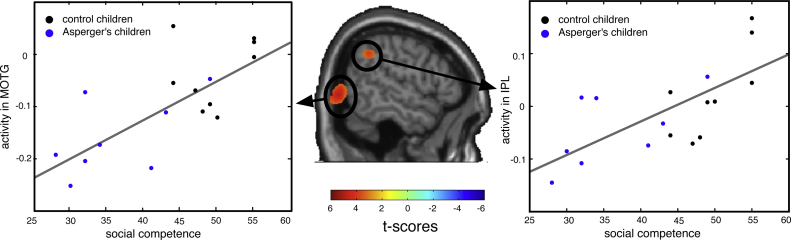
Left: High-alpha activity in MOTG (BA 19/37, X: 52, Y: −75, Z: 3) is strongly correlated with the social competence scale. The scatterplot in the lower panel indicates an r = .73 with p < .01 for this region (average over voxels). Right: Correlation of high-alpha activity in the IPL (BA 40, X: 65, Y: −41, Z: 36) with social competence. The scatterplot in the lower panel indicates an r = −.70 with p < .01 for this region (averaged over voxels).
Table 2.
Talairach coordinates for 10–12 Hz sources correlating with social competence and social problems and respective correlation coefficients as well as the correlations between these sources and measures of intellectual abilities (CPM/SPM). Middle occipital-temporal gyrus (MOTG), inferior parietal lobe (IPL).
| Social competence |
Social problems |
|||||
|---|---|---|---|---|---|---|
| X, Y, Z | r | CPM/SPM r | X, Y, Z | r | CPM/SPM r | |
| Premotor right (BA6) | 42, −5, 65 | .74*** | −.16 | 41, −15, 69 | −.66** | −.16 |
| Prefrontal right (BA8) | 30, 39, 39 | −.66** | −.26 | 28, 39, 46 | .69** | −.32 |
| IPL right (BA 40) | 65, −41, 36 | .70** | −.17 | 46, −56, 34 | −.68** | −.003 |
| MOTG right (BA 19/37) | 52, −75, 3 | .73*** | −.07 | 39, −67, 6 | −.63** | −.004 |
p < .01.
p < .001.
BA 6 and BA 8 correlated selectively with socially relevant scores (CBCL scales, in particular social competence and social problems) while measures of intellectual abilities did not show any relationship with sources of Mu-activity in these regions. Please see Table 2 for further details.
Permutation tests revealed that it was unlikely that only the group differences between the two groups solely accounted for the correlations. For example, for the relationship between social competence and the premotor area 92.24% of the correlation distribution, were below the here yielded correlation of 0.74. The mean of this distribution was 0.57 which reflects the strength of correlation that could be expected if only the group differences accounted for the relationship. As can be seen in Fig. 4 there is data of one Asperger's child influencing the dampening the correlations due to high scores in social competence. In order to investigate this influence further, the same calculations as above were conducted excluding this one person. It occurs then that 99.72% of the correlation distribution are below the one found here. This suggests that there is a relationship between social competence and premotor activity which is not solely accounted for by group differences.
4. Discussion
This study investigated neuromagnetic (MEG) brain activity during action observation and assed its relationship to social skills in Asperger's compared to healthy control children.
Stronger Mu-rhythm modulations were found in typically developing children than in children with Asperger's, in particular in response to non-repetitive hand movement stimulation. Going beyond previous electrophysiology studies, the cortical sources of these group differences were revealed and localized in premotor and prefrontal areas, in the inferior parietal lobe, and around the occipito-temporal junction. Using measures of cerebral blood flow, similar regions have been previously implicated in aspects of social cognition (e.g. Calvo-Merino et al., 2005, Lawrence et al., 2006). Further, the association of Mu generating regions with measures of social cognition was examined. Remarkably, virtually identical regions emerged as critical for the implementation of social cognition skills from independent correlations of Mu-source activity with social competence and social-skills impairment scores. This correspondence provides strong evidence that the brain regions showing differences between typically developing children and children with Asperger's syndrome in the extent of Mu-rhythm modulation during action observation are indeed involved in social cognition. Previous EEG and MEG studies suggested that changes in the power of Mu-rhythms reflect the activation of a system of action representations shared by the observer and the actor in adults (Hari et al., 1998, Muthukumaraswamy et al., 2004, Caetano et al., 2007), as well as children (Bernier et al., 2007). This system is presumed to facilitate the understanding of social cues (Lepage and Théoret, 2006, Oberman et al., 2007, Cheng et al., 2008, Oberman et al., 2008), although previous correlative evidence with measures of social cognition, broadly defined, was ambiguous (Bernier et al., 2007, Raymaekers et al., 2009, Perry et al., 2010b).
The present results specify differences in the functional neuroanatomy of movement observation as reflected in Mu-activity in typically developing children and children with Asperger's syndrome. Such differences are in line with considerations on the role of Mu-rhythms in mediating social cognition and the social problems in ASD. Results corroborate EEG findings by (e.g. Bernier et al., 2007, Oberman et al., 2008), but deviate from a previous MEG study by Avikainen et al. (1999) who found no ASD-typical difference. Present findings localize group differences to premotor and prefrontal areas as well as IPL and MOTG but not primary visual areas, suggesting abnormalities in motor control, but not basic perception. In concert with the correlations found between the activity of these sources and social skills, the current data provide support for the involvement of a motor resonance (mirror) system in social behavior and indicate a relationship between a dysfunction of this system in children with ASD and their characteristic impairments in social skills.
It is noteworthy that, in contrast to other studies, we found an increase of 10–12 Hz MEG activity at fronto-central sensors while the participants observed hand movements relative to baseline, rather than a decrease.
This pattern may be due to the temporal dynamics of Mu-decrease and subsequent spontaneous increase (rebound). In general, the increased Mu-power is assumed to reflect inhibitory control as for example proposed by Pfurtscheller and Neuper (1997) who reported that during movements Mu decreased in relevant motor areas but interestingly increased in unrelated motor areas. Furthermore, Schuch et al. (2010) showed that power decrease and increase of Mu are related: the stronger the observation evoked decrease was the stronger the following rebound. In other words, both decrease and increase reflect the reactivity of the sensorimotor system. If now the rebound phase dominates across the extracted FFT window, a net increase in activity will be observed. This was, for instance observed in a recent report investigating modulations of 20 Hz activity following movement observation (Honaga et al., 2010) which is sometimes regarded as a subcomponent of Mu-activity and often exhibits similar suppression and rebound patterns as Mu. Source analyses revealed reduced beta rebound in premotor and medial prefrontal cortex in adult ASD patients after movement observation but not following movement execution compared to control participants. Given that in the current study no peaks occurred in the beta range (20–25 HZ) and that the post-movement beta rebound has been suggested to be age-dependent (Gaetz et al., 2010), we did not investigate beta activity. It is possible that different temporal profiles of Mu-suppression and subsequent rebound have contributed to the pattern of our results: i.e. an extended rebound period will be weighted relatively more by the spectral analysis than the brief periods of suppression. Due to the present stimulus presentation (videos with multiple, sequential movements differing in duration), examination of this idea using stimulus-locked time–frequency analysis is challenging and should be preferably investigated in another study especially dedicated to this question. Nevertheless, we tried to investigate this issue further by applying time–frequency analysis. Unfortunately, the number of trials decreased dramatically for this analysis (6 trials of 40 s per condition) and given the jitters in timing within experimental and baseline conditions (duration between 900 and 1500 ms for a single movement) we could neither find a decrease across trials nor on single trial level. Mukamel et al. (2010) characterizing the response profiles of individual cells in supplementary motor area (SMA) during action execution and observation, found in proximity of each other cells that responded with excitation during both action execution and observation, cells that responded to both with inhibition and cells that responded to one with excitation and the other with inhibition. Given that Perry and Bentin (2009) in their EEG study report post-movement observation Mu-reduction using a virtually identical stimulation and spectral analysis protocol as presently used, the possibility arises that Mu-suppression and subsequent power increase are differentially contributed to by radial (EEG) and tangential (MEG) sources. This is open to further research. In any case, the present study confirms Honaga et al.’s (2010) previous reports of differences between individuals with Asperger's and control participants in recruitment of premotor cortical regions during action observation caused by beta generator enhancement and extends them to high-Mu generators and children.
Furthermore, ASD children showed stronger Mu-generator activity than normal in response to biological movement observation in a more anterior region, in the middle prefrontal cortex (BA 8). Group differences here might point to differential motor inhibition in Asperger's and control children during the observation task (Brass et al., 2001, Rubia et al., 2001). Brass and colleagues showed that the middle prefrontal cortex is sensitive to inhibition of imitative response tendencies. Concerning ASD, activity in a subregion of the middle prefrontal cortex, assessed in a visual response inhibition task, correlated with more severe measures of repetitive behavior (Agam et al., 2010) thereby supporting this region's relevance for motor inhibition and the impaired functioning in ASD.
Alternatively, Lingnau and Petris (2012) report more frontal activation during action understanding for more difficult tasks even in student volunteers. Possibly, parsing non-repetitive movements is, as part of the clinical problems, subjectively more difficult for ASD children than for typically developed ones, causing them to rely more on anterior brain regions, whereas typically developing children can rely primarily on premotor regions.
Overall, the pattern of event-related Mu-generation in the anterior regions is reversed, and activity is significantly reduced in children with Asperger's syndrome. Of note, differences between Asperger's and control children were found only in response to non-repetitive hand movements. This might indicate dampened, but not completely absent, Mu-rhythm reactivity in Asperger's. In line with diagnostic criteria, Asperger's children appear to be unimpaired regarding general intellectual development as indicated by CPM/SPM test scores as well as in hand imitation abilities (assessed by the K-ABC battery), which presumably stand at the basis of or, at least, are an important component of the human mirror neuron system (hMNS e.g. Buccino et al., 2001, Iacoboni and Dapretto, 2006). While this outcome is consistent with studies showing residual imitation skills in ASD (Bird et al., 2007, Hamilton et al., 2007), others studies found impaired imitation (e.g. Oberman et al., 2008), suggesting that the whole architecture of motor cognition is developmentally impaired in children with autism (Cattaneo et al., 2007, Gallese et al., 2009). Haswell et al. (2009) hold that children with ASD do not recruit premotor areas during the observation of motor acts because they form stronger than normal associations between self-generated motor commands and proprioception which implies that they depend more on intrinsic coordinates of motion and less on extrinsic coordinates which are represented in premotor areas among others. In line with the latter view, it is possible that whereas in the control group the significantly reduced modulation of Mu-rhythms in the repetitive relative to the non-repetitive conditions reflect adaptation of the motor activity while observing repetitive movements, in the Asperger group this mechanism was not at all activated. However, a recent study reported intact motor adaptation in autistic patients compared to control participants (Dinstein et al., 2010).
In addition to pointing to a specific perceptual-motor deficiency in Asperger's syndrome, the present study links social skill impairments, which characterize these individuals, to perceptual-motor deficiency. This is in line with previous investigations of behavior and motor performance in ASD. For example, ASD patients show more impairments in motor control processes compared to control children (Jansiewicz et al., 2006) and social scores and the ability to perform sequential, complex, and nonstereotyped movements correlate in ASD patients and control participants (Dziuk et al., 2007) even when basic motor skills were accounted for.
Here, we directly demonstrate that social skills are related to the perceptual motor resonance neural system (also known as hMNS) in typically developed individuals as well. This is evident in the correlations found between the level of Mu-modulation and social skills which were calculated across all the participants and were not restricted to specific predefined regions as identified via the group comparisons. This analysis revealed that the same premotor and prefrontal areas which showed between-groups differences in Mu-modulation were also most strongly correlated which the CBCL scores. Importantly our permutation test makes the possibility that these associations are driven by group differences alone highly unlikely. Higher social competence and reduced social-skills impairment were associated with enhanced Mu-activity in premotor (BA 6) regions while the opposite pattern occurred for prefrontal areas (BA 8). Hence, the present data augments the validity of previous functional magnetic resonance imaging studies that found correlations between premotor activity during observation of emotional facial expressions in healthy controls as well as ASD and their social skills (Dapretto et al., 2006), but where the relationship with a specific neural signal is left open. Our study also corroborates previous EEG Mu-modulation studies in which the link between motor cognition and social cognition in healthy individuals has been theoretically implied, but not directly demonstrated (e.g. Oberman et al., 2007, Cheng et al., 2008, Perry et al., 2010b, Perry et al., 2011, Centelles et al., 2011).
In addition to frontal sources, differential high-Mu activity between groups and corresponding correlation with social skills and deficits was found also in the right IPL and the right MOTG. The IPL has been associated with the MNS in humans (e.g. Chong et al., 2008) as well as in the monkey (Fogassi et al., 2005). More posterior parietal regions have also been shown to respond to both observed and executed actions (Buccino et al., 2001). Specifically related to our findings are studies showing increased activity in the MOTG during observation of meaningful hand actions compared to stationary hands (e.g. Grezes et al., 1998, Peigneux et al., 2000). Posterior parietal areas have also been shown to be relevant for social impairment in ASD (Amaral et al., 2008) and both, IPL and MOTG, together with precentral areas, are important to distinguish between actions produced by oneself from those produced by others (Ruby and Decety, 2001). Thus, our findings along with the above mentioned ones support the notion of a relationship between observation related motor activity and social skills. However, especially the MOTG activity might suggest an alternative explanation. Within their ‘grounded by interaction’ hypothesis Mahon and Caramazza (2008) proposed that for conceptual understanding motor activation (simulation) alone is not enough but an interaction with cognitive processes is required. This idea is supported by a study of Lingnau and Petris (2012) who showed that the BOLD signal in response to point-light displays is modulated by the difficulty of understanding an action in frontal compared to temporal areas (MOTG). They propose that a first step of analysis to understand an action takes place within the MOTG, a region of the ventral stream where memory representations are stored and information from different modalities are bound together. A subsequent step especially needed for actions which are more complex and difficult to understand then occurs in frontal areas. The conclusion from this hypothesis is that action understanding is not reached by motor simulation alone but also by activation of memory representations and integration of different modalities. For the present study, this would mean that although we find strong correlations with social skills in premotor areas, this effect might originate in the ventral stream, the MOTG, and spreads from there to frontal areas. From the present data, it is not possible to disentangle motor simulation and non-motor-simulation theories.
In conclusion, the present MEG study demonstrated a strong relationship between action observation, Mu-activity, and social skills. Activations localized to premotor and prefrontal sources as well as parietal and occipital-temporal sources. These sources not only revealed differences between control and Asperger's children but were also strongly correlated with social skills. These perception-motor activation abnormalities in Asperger's children may, therefore, account for their characteristic deficits in social behavior. Future studies with a focus on temporal aspects might show whether these abnormalities primarily originate in the motor domain or result from interactions with other more ‘cognitive’ regions.
Conflict of interest
None.
Acknowledgements
We thank Tessa Maniura and Ursula Lommen for help in data acquisition. We further thank Dr. Isabella Paul and Prof. Peter Gessler for help in patient recruitment. We especially wish to acknowledge the contribution made by Shlomo Bentin, who sadly passed away before the work could be published. The work was supported by the Deutsche Forschungsgemeinschaft (KI1286/4-1) and the Zukunftskolleg of the University of Konstanz.
References
- Achenbach T.M. Department of Psychiatry, University of Vermont; 1991. Manual for the Child Behavior Checklist/4-18 and 1991 Profile. [Google Scholar]
- Agam Y., Joseph R., Barton J., Manoach D. Reduced cognitive control of response inhibition by the anterior cingulate cortex in autism spectrum disorders. NeuroImage. 2010;52:336–347. doi: 10.1016/j.neuroimage.2010.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaral D.G., Schumann C.M., Nordahl C.W. Neuroanatomy of autism. Trends in Neuroscience. 2008;31:137–145. doi: 10.1016/j.tins.2007.12.005. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association . APA; Washington, DC: 2000. Diagnostic and Statistical Manual of Mental Disorders. [Google Scholar]
- Avikainen S., Kulomäki T., Hari R. Normal movement reading in Asperger subjects. Neuroreport. 1999;10:3467. doi: 10.1097/00001756-199911260-00001. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S., Leslie A.M., Frith U. Does the autistic child have a theory of mind? Cognition. 1985;21:37–46. doi: 10.1016/0010-0277(85)90022-8. [DOI] [PubMed] [Google Scholar]
- Becker P., Schaller S., Schmidtke A. Beltz Test Gesellschaft; Weinheim: 1980. Coloured Progressive Matrices-Manual. [Google Scholar]
- Bernier R., Dawson G., Webb S., Murias M. EEG mu rhythm and imitation impairments in individuals with autism spectrum disorder. Brain and Cognition. 2007;64:228–237. doi: 10.1016/j.bandc.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird G., Leighton J., Press C., Heyes C. Intact automatic imitation of human and robot actions in autism spectrum disorders. Proceedings of the Royal Society of London. Series B: Biological Sciences. 2007;274:3027. doi: 10.1098/rspb.2007.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brass M., Zysset S., Von Cramon D.Y. The inhibition of imitative response tendencies. NeuroImage. 2001;14:1416–1423. doi: 10.1006/nimg.2001.0944. [DOI] [PubMed] [Google Scholar]
- Buccino G., Binkofski F., Fink G.R., Fadiga L., Fogassi L., Gallese V., Seitz R.J., Zilles K., Rizzolatti G., Freund H.J. Action observation activates premotor and parietal areas in a somatotopic manner: an fMRI study. European Journal of Neuroscience. 2001;13:400–404. [PubMed] [Google Scholar]
- Caetano G., Jousmaki V., Hari R. Actor's and observer's primary motor cortices stabilize similarly after seen or heard motor actions. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:9058–9062. doi: 10.1073/pnas.0702453104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvo-Merino B., Glaser D.E., Grèzes J., Passingham R.E., Haggard P. Action observation and acquired motor skills: an fMRI study with expert dancers. Cerebral Cortex. 2005;15:1243–1249. doi: 10.1093/cercor/bhi007. [DOI] [PubMed] [Google Scholar]
- Carruthers P., Smith P.K. Cambridge University Press; Cambridge: 1996. Theories of Theories of Mind. [Google Scholar]
- Cattaneo L., Fabbri-Destro M., Boria S., Pieraccini C., Monti A., Cossu G., Rizzolatti G. Impairment of actions chains in autism and its possible role in intention understanding. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:17825. doi: 10.1073/pnas.0706273104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centelles L., Assaiante C., Nazarian B., Anton J.L. Recruitment of both the mirror and the mentalizing networks when observing social interactions depicted by point-lights: a neuroimaging study. PLoS One. 2011;6(1):e15749. doi: 10.1371/journal.pone.0015749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y., Lee P.L., Yang C.Y., Lin C.P., Hung D., Decety J. Gender differences in the mu rhythm of the human mirror-neuron system. PLoS One. 2008;3:2113. doi: 10.1371/journal.pone.0002113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong T.T.J., Cunnington R., Williams M.A., Kanwisher N., Mattingley J.B. fMRI adaptation reveals mirror neurons in human inferior parietal cortex. Current Biology. 2008;18:1576–1580. doi: 10.1016/j.cub.2008.08.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dapretto M., Davies M., Pfeifer J., Scott A., Sigman M., Bookheimer S., Iacoboni M. Understanding emotions in others: mirror neuron dysfunction in children with autism spectrum disorders. Nature Neuroscience. 2006;9:28–30. doi: 10.1038/nn1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David N., Aumann C., Bewernick B., Santos N., Lehnhardt F., Vogeley K. Investigation of mentalizing and visuospatial perspective taking for self and other in Asperger syndrome. Journal of Autism and Developmental Disorders. 2010;40:290–299. doi: 10.1007/s10803-009-0867-4. [DOI] [PubMed] [Google Scholar]
- Davies M., Stone T. Blackwell; Oxford: 1995. Mental Simulation: Evaluations and Applications. [Google Scholar]
- Dinstein I., Thomas C., Humphreys K., Behrmann M., Heeger D.J. Normal movement selectivity in autism. Neuron. 2010;13:461–469. doi: 10.1016/j.neuron.2010.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- di Pellegrino, Fadiga, Fogassi, Gallese, Rizzolatti Understanding motor events: a neurophysiological study. Experimental Brain Research. 1992;91:176–180. doi: 10.1007/BF00230027. [DOI] [PubMed] [Google Scholar]
- Dziuk M., Larson J., Apostu A., Mahone E., Denckla M., Mostofsky S. Dyspraxia in autism: association with motor, social, and communicative deficits. Developmental Medicine & Child Neurology. 2007;49:734–739. doi: 10.1111/j.1469-8749.2007.00734.x. [DOI] [PubMed] [Google Scholar]
- Fogassi L., Ferrari P.F., Gesierich B., Rozzi S., Chersi F., Rizzolatti G. Parietal lobe: from action organization to intention understanding. Science. 2005;308:662–667. doi: 10.1126/science.1106138. [DOI] [PubMed] [Google Scholar]
- Gaetz W., MacDonald M., Cheyne D., Snead O.C. Neuromagnetic imaging of movement-related cortical oscillations in children and adults: age predicts post-movement beta rebound. NeuroImage. 2010;51:792–807. doi: 10.1016/j.neuroimage.2010.01.077. [DOI] [PubMed] [Google Scholar]
- Gallese, Fadiga, Fogassi, Rizzolatti Action recognition in the premotor cortex. Brain. 1996;119:593–609. doi: 10.1093/brain/119.2.593. [DOI] [PubMed] [Google Scholar]
- Gallese V., Rochat M., Cossu G., Sinigaglia C. Motor cognition and its role in the phylogeny and ontogeny of action understanding. Developmental Psychology. 2009;45:103. doi: 10.1037/a0014436. [DOI] [PubMed] [Google Scholar]
- Grezes J., Costes N., Decety J. Top-down effect of strategy on the perception of human biological motion: a PET investigation. Perception and Action: Recent Advances in Cognitive Neuropsychology. 1998;15:553. doi: 10.1080/026432998381023. [DOI] [PubMed] [Google Scholar]
- Gross J., Kujala J., Hämäläinen M., Timmermann L., Schnitzler A., Salmelin R. Dynamic imaging of coherent sources: studying neural interactions in the human brain. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:694. doi: 10.1073/pnas.98.2.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton A., Brindley R., Frith U. Imitation and action understanding in autistic spectrum disorders: how valid is the hypothesis of a deficit in the mirror neuron system? Neuropsychologia. 2007;45:1859–1868. doi: 10.1016/j.neuropsychologia.2006.11.022. [DOI] [PubMed] [Google Scholar]
- Hari R. Action–perception connection and the cortical mu rhythm. Progress in Brain Research. 2006;159:253–260. doi: 10.1016/S0079-6123(06)59017-X. [DOI] [PubMed] [Google Scholar]
- Hari R., Salmelin R. Human cortical oscillations: a neuromagnetic view through the skull. Trends in Neuroscience. 1997;20:44–49. doi: 10.1016/S0166-2236(96)10065-5. [DOI] [PubMed] [Google Scholar]
- Hari R., Forss N., Avikainen S., Kirveskari E., Salenius S., Rizzolatti G. Activation of human primary motor cortex during action observation: a neuromagnetic study. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:15061–15065. doi: 10.1073/pnas.95.25.15061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haswell C.C., Izawa J., Dowell L.R., Mostofsky S.H., Shadmehr R. Representation of internal models of action in the autistic brain. Nature Neuroscience. 2009;12:970–972. doi: 10.1038/nn.2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller K.A., Kratzmeier H., Lengfelder A. Beltz-Test; Goettingen, Germany: 1998. Standard Progressive Matrices: Matrizen-Test-Manual, Band 1. [Google Scholar]
- Hickok Eight problems for the mirror neuron theory of action understanding in monkeys and humans. Journal of Cognitive Neuroscience. 2009;21:1229–1243. doi: 10.1162/jocn.2009.21189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honaga E., Ishii R., Kurimoto R., Canuet L.K.I., Takahasi H., Nakahachi T., Iwase M., Mizuta I., Yoshimine T., Takeda M. Post-movement beta rebound abnormality as indicator of mirror neuron system dysfunction in autistic spectrum disorder: an MEG study. Neuroscience Letters. 2010;478:141–145. doi: 10.1016/j.neulet.2010.05.004. [DOI] [PubMed] [Google Scholar]
- Iacoboni M., Dapretto M. The mirror neuron system and the consequences of its dysfunction. Nature Reviews. Neuroscience. 2006;7:942–951. doi: 10.1038/nrn2024. [DOI] [PubMed] [Google Scholar]
- Jansiewicz E., Goldberg M., Newschaffer C., Denckla M., Landa R., Mostofsky S. Motor signs distinguish children with high functioning autism and Asperger's syndrome from controls. Journal of Autism and Developmental Disorders. 2006;36:613–621. doi: 10.1007/s10803-006-0109-y. [DOI] [PubMed] [Google Scholar]
- Lawrence E.J., Shaw P., Giampietro V.P., Surguladze S., Brammer M.J., David A.S. The role of ‘shared representations’ in social perception and empathy: an fMRI study. NeuroImage. 2006;29:1173–1184. doi: 10.1016/j.neuroimage.2005.09.001. [DOI] [PubMed] [Google Scholar]
- Lepage J.F., Théoret H. EEG evidence for the presence of an action observation–execution matching system in children. European Journal of Neuroscience. 2006;23:2505–2510. doi: 10.1111/j.1460-9568.2006.04769.x. [DOI] [PubMed] [Google Scholar]
- Liljeström M., Kujala J., Jensen O., Salmelin R. Neuromagnetic localization of rhythmic activity in the human brain: a comparison of three methods. NeuroImage. 2005;25:734–745. doi: 10.1016/j.neuroimage.2004.11.034. [DOI] [PubMed] [Google Scholar]
- Lindner J., Rosén L. Decoding of emotion through facial expression, prosody and verbal content in children and adolescents with Asperger's syndrome. Journal of Autism and Developmental Disorders. 2006;36:769–777. doi: 10.1007/s10803-006-0105-2. [DOI] [PubMed] [Google Scholar]
- Lingnau A., Gesierich B., Caramazza A. Asymmetric fMRI adaptation reveals no evidence for mirror neurons in humans. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:9925–9993. doi: 10.1073/pnas.0902262106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingnau A., Petris S. Action understanding within and outside the motor system: the role of task difficulty. Cerebral Cortex. 2012 doi: 10.1093/cercor/bhs112. [DOI] [PubMed] [Google Scholar]
- Mahon B.Z., Caramazza A. A critical look at the embodied cognition hypothesis and a new proposal for grounding conceptual content. Journal of Physiology Paris. 2008;102:59–70. doi: 10.1016/j.jphysparis.2008.03.004. [DOI] [PubMed] [Google Scholar]
- Mechler P., Preuß U. 2001. Kaufman Assessment Battery for Children, dt. Version (K-ABC) [Google Scholar]
- Mukamel R., Ekstrom A., Kaplan J., Iacoboni M., Fried I. Single neuron responses in humans during execution and observation of actions. Current Biology. 2010;20:750–756. doi: 10.1016/j.cub.2010.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthukumaraswamy S., Johnson B.W., McNair N.A. Mu rhythm modulation during observation of an object-directed grasp. Brain Research. Cognitive Brain Research. 2004;19:195–201. doi: 10.1016/j.cogbrainres.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Neuper C., Wörtz M., Pfurtscheller G. ERD/ERS patterns reflecting sensorimotor activation and deactivation. Progress in Brain Research. 2006;159:211–222. doi: 10.1016/S0079-6123(06)59014-4. [DOI] [PubMed] [Google Scholar]
- Nishitani N., Avikainen S., Hari R. Abnormal imitation-related cortical activation sequences in Asperger's syndrome. Annals of Neurology. 2004;55:558–562. doi: 10.1002/ana.20031. [DOI] [PubMed] [Google Scholar]
- Oberman L.M., Pineda J.A., Ramachandran V.S. The human mirror neuron system: a link between action observation and social skills. Social Cognitive and Affective Neuroscience. 2007;2:62–66. doi: 10.1093/scan/nsl022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberman L.M., Ramachandran V.S., Pineda J.A. Modulation of mu suppression in children with autism spectrum disorders in response to familiar or unfamiliar stimuli: the mirror neuron hypothesis. Neuropsychologia. 2008;46:1558–1565. doi: 10.1016/j.neuropsychologia.2008.01.010. [DOI] [PubMed] [Google Scholar]
- Oostenveld R., Fries P., Maris E., Schoffelen J.M. FieldTrip: open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Computational Intelligence and Neuroscience. 2011;2011:1–9. doi: 10.1155/2011/156869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peigneux P., Salmon E., Van Der Linden M., Garraux G., Aerts J., Delfiore G., Degueldre C., Luxen A., Orban G., Franck G. The role of lateral occipitotemporal junction and area MT/V5 in the visual analysis of upper-limb postures. NeuroImage. 2000;11:644–655. doi: 10.1006/nimg.2000.0578. [DOI] [PubMed] [Google Scholar]
- Perry A., Bentin S., Shalev I., Israel S., Uzefovsky F., Bar-On D., Ebstein R.P. Intranasal oxytocin modulates EEG mu/alpha and beta rhythms during perception of biological motion. Psychoneuroendocrinology. 2010;35:1446–1453. doi: 10.1016/j.psyneuen.2010.04.011. [DOI] [PubMed] [Google Scholar]
- Perry A., Troje N.F., Bentin S. Exploring motor system contributions to the perception of social information: evidence from EEG activity in the mu/alpha frequency range. Social Neuroscience. 2010:1–13. doi: 10.1080/17470910903395767. [DOI] [PubMed] [Google Scholar]
- Perry A., Stein L., Bentin S. Motor and attentional mechanisms involved in social interaction – evidence from mu and alpha EEG suppression. NeuroImage. 2011;58:895–904. doi: 10.1016/j.neuroimage.2011.06.060. [DOI] [PubMed] [Google Scholar]
- Perry A., Bentin S. Mirror activity in the human brain while observing hand movements: a comparison between EEG desynchronization in the mu-range and previous fMRI results. Brain Research. 2009;1282:126–132. doi: 10.1016/j.brainres.2009.05.059. [DOI] [PubMed] [Google Scholar]
- Petsche H., Kaplan S., Von Stein A., Filz O. The possible meaning of the upper and lower alpha frequency ranges for cognitive and creative tasks. International Journal of Psychophysiology. 1997;26:77–97. doi: 10.1016/s0167-8760(97)00757-5. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G., Neuper C. Motor imagery activates primary sensorimotor area in humans. Neuroscience Letters. 1997;239:65–68. doi: 10.1016/s0304-3940(97)00889-6. [DOI] [PubMed] [Google Scholar]
- Pineda J.A. The functional significance of mu rhythms: translating seeing and hearing into doing. Brain Research. Brain Research Reviews. 2005;50:57–68. doi: 10.1016/j.brainresrev.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Raymaekers R., Wiersema J.R., Roeyers H. EEG study of the mirror neuron system in children with high functioning autism. Brain Research. 2009;1304:113–121. doi: 10.1016/j.brainres.2009.09.068. [DOI] [PubMed] [Google Scholar]
- Rubia K., Russell T., Overmeyer S., Brammer M.J., Bullmore E.T., Sharma T., Simmons A., Williams S.C.R., Giampietro V., Andrew C.M. Mapping motor inhibition: conjunctive brain activations across different versions of go/no-go and stop tasks. NeuroImage. 2001;13:250–261. doi: 10.1006/nimg.2000.0685. [DOI] [PubMed] [Google Scholar]
- Ruby P., Decety J. Effect of subjective perspective taking during simulation of action: a PET investigation of agency. Nature Neuroscience. 2001;4:546–550. doi: 10.1038/87510. [DOI] [PubMed] [Google Scholar]
- Schuch S., Bayliss A.P., Klein C., Tipper S.P. Attention modulates motor system activation during action observation: evidence for inhibitory rebound. Experimental Brain Research. 2010;205:235–249. doi: 10.1007/s00221-010-2358-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shmuelof L., Zohary E. A mirror representation of others’ actions in the human anterior parietal cortex. Journal of Neuroscience. 2006;26:9736–9742. doi: 10.1523/JNEUROSCI.1836-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]