Highlights
► Children and adults administered attentional shifting task while scanned at 4 T. ► Cognitive control network identified by means of independent components analysis. ► Age-related differences in functional connectivity in the identified network assessed. ► Adults showed stronger connectivity between lateral prefrontal, anterior cingulate, and inferior parietal cortex. ► Children showed stronger connectivity between frontal pole and insula.
Keywords: Development, Cognitive flexibility, fMRI, Brain networks, ICA
Abstract
The Dimensional Change Card Sort (DCCS) is a standard procedure for assessing executive functioning early in development. In the task, participants switch from sorting cards one way (e.g., by color) to sorting them a different way (e.g., by shape). Traditional accounts associate age-related changes in DCCS performance with circumscribed changes in lateral prefrontal cortex (lPFC) functioning, but evidence of age-related differences in the modulation of lPFC activity by switching is mixed. The current study therefore tested for possible age-related differences in functional connectivity of lPFC with regions that comprise a larger cognitive control network. Functional magnetic resonance imaging (fMRI) data collected from children and adults performing the DCCS were analyzed by means of independent components analysis (ICA). The analysis revealed several important age-related differences in functional connectivity of lPFC. In particular, lPFC was more strongly connected with the anterior cingulate, inferior parietal cortex, and the ventral tegmental area in adults than in children. Theoretical implications are discussed.
The Dimensional Change Card Sort (DCCS; Zelazo, 2006) is a standard procedure for assessing executive functioning early in development. In the task, participants sort bivalent cards (e.g., red trucks) one way (e.g., by color) and then are instructed to switch and sort the same cards a new way (e.g., by shape). Exercising flexibility of this kind is particularly difficult for children. Three- and 4-year-olds typically perseverate by repeatedly sorting by old rules after being instructed to switch and sort by new rules, and err in this way despite apparent knowledge of the correct response (Zelazo, 2006). Later in development, most children correctly switch, but show larger switch-related behavioral costs relative to adolescents and adults (Cepeda et al., 2001, Crone et al., 2006, Davidson et al., 2006, Huizinga and van der Molen, 2011, Lehto et al., 2003, Waxer and Morton, 2011b, Weed et al., 2008).
Age-related changes in DCCS performance have traditionally been associated with the functional development of the lateral prefrontal cortex (Bunge and Zelazo, 2006, Dempster, 1992, Diamond, 2002, Kirkham et al., 2003, Morton and Munakata, 2002). First, like mental flexibility specifically and cognitive control generally, dorso- and ventrolateral prefrontal cortex follow a protracted developmental trajectory, showing continued change in synaptogenesis (Huttenlocher and Dabholkar, 1997), gray matter density (Sowell et al., 2001), cortical thickness (Shaw et al., 2008), and myelination (Nagy et al., 2004) into early adulthood. Second, lesions to lPFC lead to inflexibility reminiscent of that observed in young children. For example, lesions to lPFC, but not other cortical areas, lead to increased perseveration in the Wisconsin Card Sorting Task (Berg, 1948), an inferential rule use task similar in its demands to the DCCS. And third, switching tasks, including the DCCS (Moriguchi and Hiraki, 2009, Moriguchi and Hiraki, 2011), are commonly associated with activity in lPFC (Barber and Carter, 2005, Cole and Schneider, 2007). Taken together then, there is support for the idea that the development of flexible rule use in the DCCS is associated with age-related changes in lPFC activity.
There is, however, also countervailing evidence that speaks against this basic association (Morton et al., 2009, Wendelken et al., 2012). In one study, children and adults were administered a modified version of the DCCS as changes in blood oxygenation were measured by means of fMRI (Morton et al., 2009). Although all participants showed greater activity in dorso- and ventrolateral PFC in conditions that required rule-switching as compared to conditions that did not require rule-switching, age-related differences in brain activity were confined to the dorsal premotor and superior parietal cortices, regions that fall well-outside lPFC proper. In a second fMRI study, children and adults switched between rules based on color and spatial orientation (Wendelken et al., 2012). Rule-switching was associated with greater activity in dlPFC as compared to rule repetition, but the effect of rule switching on dlPFC activity was equivalent for both groups, as reflected in a statistically non-significant interaction of Switching and Age in dlPFC activity. One possibility, suggested by Wendelken et al. (2012), is that there are age-related changes in the temporal dynamics of activation in dlPFC such that rule updating in dlPFC occurs more slowly in children than in adults.
A related possibility that we investigated in the present study is that there are age-related changes in lPFC's functional integration with a larger cognitive control network. Despite a predominant focus on lPFC in theoretical (Dempster, 1992, Diamond, 2002, Kirkham et al., 2003) and empirical (Moriguchi and Hiraki, 2009, Moriguchi and Hiraki, 2011) work on the development of dimensional switching, recent evidence suggests that, in the context of higher-order mental operations, lPFC does not function independently, but forms part of a larger cognitive control network. First, switching generally (Barber and Carter, 2005, Cole and Schneider, 2007, Liston et al., 2006, Wendelken et al., 2012), and DCCS performance specifically (Morton et al., 2009), is associated with activity in many regions beyond lPFC, including the anterior cingulate cortex, dorsal premotor cortex, inferior frontal junction, inferior and superior parietal cortex, the caudate nucleus, and the thalamus. These regions co-activate across a broad range of executive tasks (Duncan, 2010, Duncan and Owen, 2000), are densely interconnected by white matter fiber tracts (Olesen et al., 2003), have intrinsically correlated signal timecourses (Cole and Schneider, 2007, Seeley et al., 2007), and rapidly exchange information in the context of attentionally demanding tasks (Buschman and Miller, 2007). Functional connectivity of lPFC with these regions is associated with individual differences in higher-order cognitive functioning (Danielmeier et al., 2011, Langen et al., 2012, Mulder et al., 2011, Nagy et al., 2004, van den Bos et al., 2012), including switching (Cole and Schneider, 2007), and changes considerably in early development (Allen et al., 2011, Fair et al., 2007, Kelly et al., 2008, Langen et al., 2012, Mulder et al., 2011, Stevens et al., 2009).
The present investigation therefore tested whether DCCS performance is associated with age-related differences in the functional connectivity of lateral prefrontal cortex with a larger cognitive control network. To test this possibility, fMRI data acquired from children and adults performing the DCCS (Morton et al., 2009) were re-analyzed using spatial independent component analysis (or spatial ICA; Calhoun et al., 2009). Spatial ICA is a statistical procedure for revealing hidden sources underlying a set of observations such that the revealed sources are maximally independent. Applied to the analysis of an fMRI volumetric time-series, the procedure assumes that each volume of the series is a mixture of a finite number of spatially independent sources. ICA then blindly decomposes or un-mixes the observed data to reveal a set of spatial components, each with an associated timecourse. Objective selection of theoretically meaningful components (e.g., components that show an effect of switching or are otherwise associated with cognitive control) can be achieved by using predictors from a standard fMRI design matrix to predict variance in component timecourses in the context of a GLM, spatially correlating component topographies with a network template, or both. Isolating a network of interest in this way carries several advantages. First, because artifacts like those associated with subject motion (Power et al., 2012) or biological rhythms have unique spatio-temporal profiles, ICA can isolate and assign these artifacts to separate components, leaving remaining components relatively free of these unwelcome sources of variance. Second, through the use of objective component selection procedures, components that are directly associated with dimensional switching can be identified. Group differences in connectivity and activation of this network can then be revealed by means of group contrasts on the spatial and temporal components respectively. Finally, because ICA is computed on all voxels comprising a volumetric time series, the resulting characterization of network organization is not biased by an a priori selection of regions of interest.
Therefore, the current study used spatial ICA to test for age-related differences in the functional connectivity of networks selected on the basis of their association with DCCS performance. Children and adults performed the DCCS as T2*-weighted images were acquired by means of a 4-Tesla MRI scanner (Morton et al., 2009). Resulting images were decomposed into a set of 20 maximally independent spatial components by means of spatial ICA. Switch-related components were then identified by means of an objective component selection procedure. Of interest was whether functional connectivity in selected task-related executive networks would differ across adults and children.
On the basis of previous analyses, we expected lPFC and its associated network to show greater activity during switch blocks than repeat blocks, but that the magnitude of this effect would be equivalent across children and adults. At the same time, we predicted that functional connectivity between lPFC and other cognitive control regions including the anterior cingulate cortex, parietal cortex, and subcortical structures, would be stronger in adults than in children.
1. Method
1.1. Participants
As reported in Morton et al. (2009), participants included 14 children (7 females, Mage = 12.1, SD = 0.3) and 13 adults (3 females, Mage = 23.9, SD = 2.7). Children were recruited from the Child Development Participation Pool at the University of Western Ontario, and were primarily of middle and upper-middle class backgrounds. Adult participants were primarily graduate students of middle and upper-middle class backgrounds. All participants were right-handed and were screened for previous neurological or psychiatric conditions. Adult participants provided written consent to their participation; parents of child participants provided written consent to their children's participation. All aspects of the study were conducted in accordance with the Declaration of Helsinki.
1.2. MRI data acquisition
Data were collected on a 4-T Siemens whole-body MRI scanner (Siemens, Erlangen, Germany). A transmit-only receive-only (TORO) cylindrical birdcage radio frequency (RF) head coil (Barberi et al., 2000) was used for signal transmission and detection. A series of T1-weighted anatomical scans in the sagittal plane were used for localization and alignment of the imaging planes for functional scans. Twenty-five 3-mm thick functional planes were collected at a slightly oblique angle, covering the superior cortical surface down to a plane extending from the frontal pole to the top of the cerebellum. Magnetic field homogeneity was optimized over functional runs using a constrained three-dimensional phase shimming procedure (Klassen and Menon, 2004). T2*-weighted images were collected with an interleaved, four-segment, optimized spiral imaging acquisition protocol (TR = 3000 ms, TE = 15 ms, flip angle = 40°, matrix size 64 × 64, field of view (FOV) 22.0 cm × 22.0 cm, voxel resolution 3.44 mm × 3.44 mm × 3.00 mm). Motion was minimized using a wooden cradle and foam packing of the area surrounding the head.
1.3. Experimental design
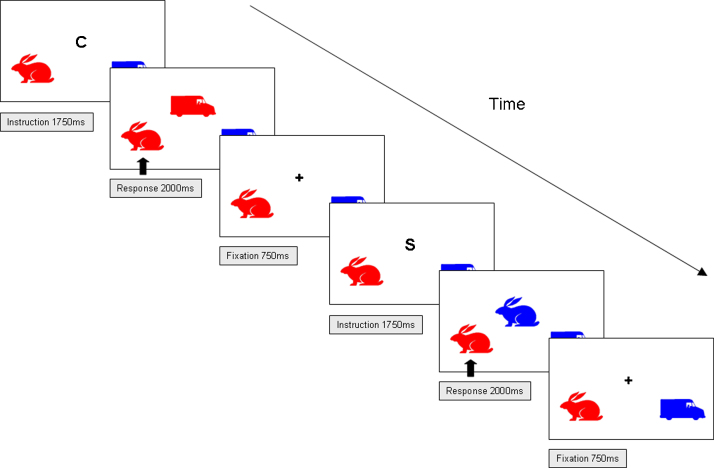
Participants were administered a computerized, repeated-trials variant of the DCCS ((Morton et al., 2009); see Fig. 1). A blue truck and a red rabbit served as target stimuli and were present in the bottom left and right corners of the screen respectively throughout the task. Trials began with a centrally presented cue (1750 ms) that indicated the sorting rule for that trial (‘C’ for color; ‘S’ for shape), followed by a centrally presented test stimulus (2000 ms). Test stimuli included a red truck and a blue rabbit and matched each target on a single dimension. Participants sorted the test stimuli by means of a button-press according to the conditional rule: “If color, then if blue, press left, if red press right; but if shape, then if truck, press left, if rabbit press right.” All participants responded using the index and middle fingers of their right hand. Trials were administered in the form of a block design. Thirty-six second (12 volume) blocks contained eight 3750 ms (1750 ms cue + 2000 ms response period) trials each separated by a 750 ms inter-trial interval. Switch blocks contained four (50%) switch trials administered randomly within each block and repeat blocks consisted of eight (100%) repeat trials. Individual blocks were separated by 18-s (6 volume) rest periods during which participants remained focused on a fixation cross. To avoid ambiguity in the classification of trials (i.e., as either switches or repeats), the sorting rule on the first trial of each block was the same as the sorting rule on the last trial of the previous block and these trials were classified as repeats. The task was administered in 10 separate 234 s (78-volume) runs that each contained two switch and two repeat blocks, with block order counterbalanced across runs.
Fig. 1.
An illustration of two representative trials from the modified Dimensional Change Card Sort task used in the present study. Trials began with an instruction cue indicating the rule on that trial, followed by the presentation of a stimulus to which participants responded, followed by a fixation point. On switch trials, the rule was different than on the previous trial; on repeat trials, the rule was the same as on the previous trial. Individual trials were administered in the form of a block design.
1.4. fMRI data preprocessing
Prior to preprocessing, motion along 3 directions of translation and around 3 axes of rotation were estimated for each run. Motion was constrained to a maximum of 3 mm over the entire run. To mitigate the untoward influence of sudden movements (for discussion, see Power et al., 2012), we were more aggressive about excluding runs with isolated abrupt movements than was true in our previous analysis (Morton et al., 2009), dropping any run with a sudden movement of between 1 mm and 3 mm. As well, in our previous analysis, runs with abrupt movements were retained, but volumes preceding or following abrupt movements retained in instances in which the movements occurred at the end or the beginning of the runs respectively. In the current analysis, these runs were excluded entirely. The resulting data set consisted of 104 usable runs from 11 adults, and 94 usable runs from 12 children, reduced from our original report owing to the use of stricter motion criteria. Data were preprocessed using SPM2 (FIL, UCL, London, UK). Data were motion-corrected by aligning each volume of each run to the first volume of the first functional run collected following the acquisition of the T1-weighted anatomical scan. Functional scans were then warped into Montreal Neurological Institute stereotactic space (MNI, Montreal, Canada) and smoothed using an 8 mm full-width at half-maximum Gaussian smoothing kernel.
1.5. Independent component analysis
A single group ICA was conducted on all subjects’ functional data using the Group ICA of fMRI toolbox for MATLAB (GIFT, v2.0d – MIND Research Network, Albuquerque, United States). A two-step data reduction procedure reduced preprocessed volumes to a set of 20 components, with the first step consisting of a subject-level PCA and the second step consisting of a group PCA on temporally concatenated data. ICA was then performed on the resulting 20 components by means of the Infomax algorithm. The reliability of the resulting decomposition was tested by means of the ICASSO procedure, in which the ICA is iterated 100 times with random initial weights, and the clustering structure of the obtained components is visualized in signal space (Himberg et al., 2004). Following the recommendations of Erhardt et al. (Erhardt et al., 2011), subject-specific timecourses and spatial maps were then back-reconstructed from the group components using GICA3. Timecourses were then intensity-normalized and linearly detrended.
1.6. Component selection
As the goal was to test for age-related differences in functional connectivity within a cognitive control network activated by the DCCS, analysis focused on those components that spatially overlapped with an “executive” or cognitive control network (see Fig. 2; Seeley et al., 2007), and also showed greater activity during switch compared to repeat blocks. To identify components that spatially overlapped with the cognitive control network (CCN), all 20 extracted components were spatially correlated with a template of the cognitive control network (Seeley et al., 2007) and that was comprised of ventro- and dorsolateral PFC, anterior cingulate cortex, dorsal striatum, and the intraparietal sulcus (see Fig. 2). Any component that spatially correlated with the CCN template was then further tested to determine whether the component also exhibited switch-related activity. This was achieved by regressing separate switch and repeat predictors from a standard fMRI design matrix on the timecourses of these components. Predictors were created by convolving a boxcar function representing the onsets and offsets of each condition with a standard sum of two gammas hemodynamic response function. To test for an effect of switching, we tested whether beta coefficient estimates were significantly greater for the switch than for the repeat predictor in the context of a one-way analysis of variance (ANOVA) using GIFT's statistics on beta weights toolbox.
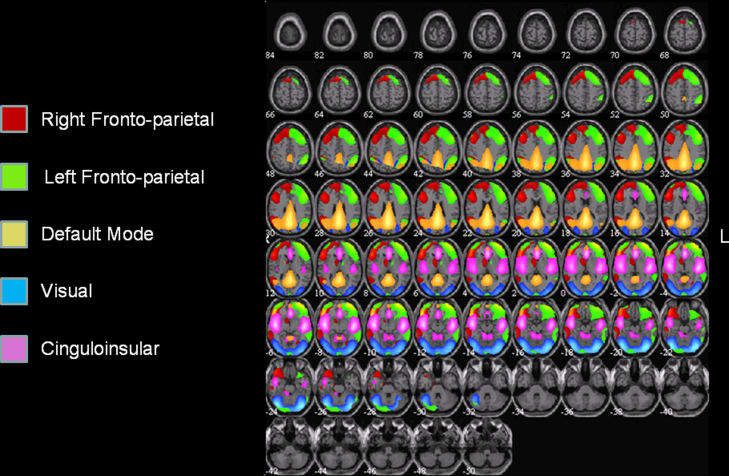
Fig. 2.
A composite view of five group components yielded by the ICA decomposition. Group components were converted to Z-scores and then mapped for the purpose of visualization at a threshold of Z > 1.
1.7. Testing age-related differences in functional connectivity
Individual participant representations of selected executive control components were t-normalized, resulting in a whole brain t-map of voxel-level contributions to the component for each individual participant. Child and adult maps were then compared by means of a voxelwise two-sample t-test. Resulting t-maps (adults < children, children > adults) were corrected for multiple comparisons such that the family-wise error rate was kept at or below p = .05. Voxels that showed statistically greater values for adults compared to children were said to show greater functional connectivity to the network defined by the component for adults compared to children, insofar as these voxels were more strongly related to the component in adults than in children. Voxels that showed statistically greater values for children compared to adults were said to show greater functional connectivity to the network defined by the component for children compared to adults, insofar as these voxels were more strongly related to the component in children than in adults.
2. Results
2.1. Behavior
Children's and adult's response latency and accuracy on switch and repeat trials (see Table 1) was compared by means of a 2-factor mixed Analysis of Variance (ANOVA), with Trial Type (switch, repeat) and Group (children, adults) as within- and between-subjects factors respectively. There was effect of Trial Type on response time, F (1, 21) = 34.2, p < .001, and accuracy F (1, 21) = 10.8, p < .01, with responses on switch trials significantly slower and more error prone than responses on repeat trials. Importantly though, there was no effect of Age and no interaction of Trial Type and Age on response time. The interaction of Trial Type and Age on accuracy, significant in our previous report, trended toward but did not achieve statistical significance in the current analysis. Both groups therefore performed the task comparably.
Table 1.
Behavioral data.
| Mean response time (SE) |
Mean accuracy (SE) |
|||
|---|---|---|---|---|
| Switch trials | Repeat trials | Switch trials | Repeat trials | |
| Adults (n = 11) | 745.5 (51.3) | 705.1 (47.6) | .96 (.02) | .97 (.01) |
| Children (n = 12) | 748.5 (52.4) | 715.2 (48.1) | .91 (.01) | .96 (.01) |
2.2. fMRI: ICA
Group ICA decomposed preprocessed fMRI data into 20 maximally independent components. Fig. 2 provides a composite view of five such components, including right and left fronto-parietal components, a cinguloinsular component, a default mode network component, and a visual component. Results of the ICASSO procedure (Himberg et al., 2004) confirmed the stability of the decomposition, with highly similar components extracted in 96–99% of the 100 iterations of the ICA run with different random seeds.
2.3. fMRI: component selection
2.3.1. Spatial correlation
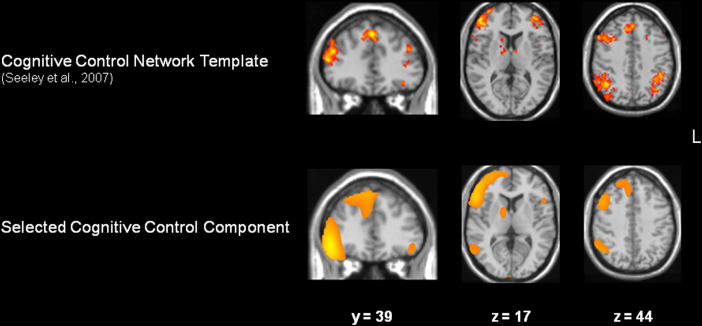
Of the 20 components extracted in the initial ICA decomposition, 2 spatially correlated with the executive network template: the right fronto-parietal component, r = .23, p < .05 (see Fig. 3), and the left fronto-parietal component, r = .23, p < .05. Correlation coefficients for the remaining 18 components were .08 or lower.
Fig. 3.
Cognitive control network template (from Seeley et al., 2007) and the selected right fronto-parietal component. Spatial correlation confirmed the strong correspondence of the two maps (p < .05), which was especially evident in lateral prefrontal, anterior cingulate/medial prefrontal cortex, dorsal striatum, and parietal cortex.
2.3.2. Timecourse analysis
The two fronto-parietal components selected by means of spatial correlation were then submitted to timecourse analysis. A two-way mixed ANOVA comparing mean beta coefficients for switch and repeat predictors across adults and children revealed greater activity during the switch than the repeat condition in the right fronto-parietal component, F (1, 22) = 5.75, p < .05, and no interaction of Condition (switch versus repeat) and Group (adults vs children). Beta coefficients for switch and repeat predictors did not differ for the left fronto-parietal component.
2.3.3. Summary
A two-step selection procedure identified a right fronto-parietal network as one that both spatially matched a template of the cognitive control network (Seeley et al., 2007) and showed greater activity during switch compared to repeat blocks. This component became the focus of analyses aimed at identifying age-related differences in functional connectivity (see Fig. 3).
2.4. fMRI: age-related differences in functional connectivity
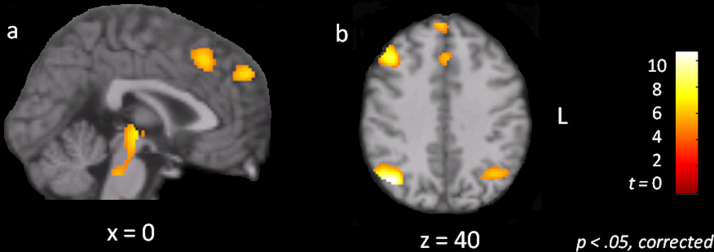
Fig. 4 is a map of the contrast adults > children, and reveals regions more strongly connected to the cognitive control network (CCN) for adults compared to children. These regions included bilateral dorsolateral prefrontal cortex, right inferior frontal gyrus, anterior cingulate/medial prefrontal cortex, bilateral inferior parietal cortex, and the ventral tegmental area (a full list of regions is presented in Table 2). Fig. 5 is a map of the contrast children > adults, and reveals regions more strongly connected to the CCN for children compared to adults. These regions included the anterior extent of the superior and middle frontal gyri bilaterally, bilateral frontal pole, right anterior insula, and left posterior temporal cortex (a full list of regions is presented in Table 3).
Fig. 4.
Statistical parametric maps showing voxels more strongly connected to the cognitive control network in adults as compared to children, p < .05, corrected. (a) Sagittal slice showing group differences in the anterior cingulate cortex, the medial superior frontal gyrus, pulvinar, and ventral tegmental area. (b) Axial slice showing group differences in right dorsolateral prefrontal cortex, anterior cingulate cortex, medial superior frontal gyrus, and inferior parietal cortex bilaterally.
Table 2.
Summary of regions showing stronger functional connectivity to the cognitive control network in adults compared to children.
| Region | BA | Hemisphere | X | Y | Z | K | Max t |
|---|---|---|---|---|---|---|---|
| Inferior parietal cortex | 7, 40 | R | 40 | −68 | 40 | 1142 | 10.6 |
| L | −42 | −62 | 44 | 221 | 6.7 | ||
| −20 | −74 | 20 | 46 | 4.9 | |||
| Inferior frontal gyrus | 45, 47 | R | 36 | 24 | −14 | 214 | 9.1 |
| 44 | 50 | −12 | 205 | 6.5 | |||
| 56 | 22 | 2 | 184 | 5.8 | |||
| L | −27 | 20 | −4 | 140 | 8.0 | ||
| Middle frontal gyrus | 9, 46 | R | 42 | 28 | 40 | 345 | 8.1 |
| L | −42 | 26 | 28 | 371 | 6.0 | ||
| Putamen | L | −26 | 5 | 0 | 161 | 7.1 | |
| Medial superior frontal gyrus | 6 | L/R | 0 | 56 | 36 | 163 | 6.6 |
| Anterior cingulate | 24, 32 | L | −2 | 28 | 44 | 183 | 6.4 |
| Ventral tegmental area | R | 4 | −24 | −20 | 30 | 5.5 | |
| Superior frontal gyrus | 6 | R | 12 | 38 | 36 | 47 | 5.4 |
| Pulvinar | R | 12 | −30 | −10 | 107 | 5.4 |
X, Y, and Z are the MNI coordinates of the peak voxel in each region; K is cluster size, in 2 mm × 2 mm × 2 mm voxels; max t is the value of the t-statistic as measured at the peak voxel.
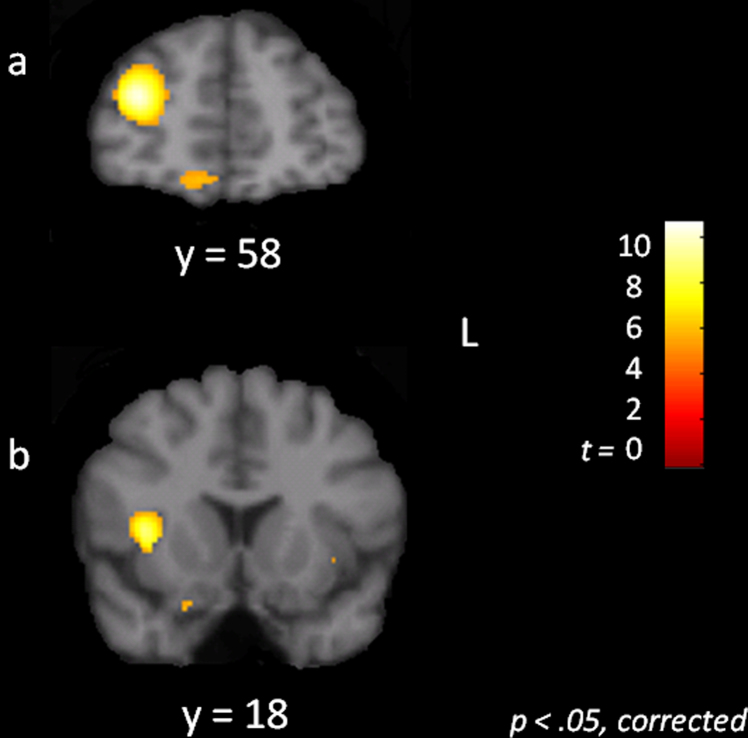
Fig. 5.
Statistical parametric maps showing voxels more strongly connected to the cognitive control network in children as compared to adults, p < .05, corrected. There was no evidence of stronger lPFC connectivity with the cognitive control network in children compared to adults. (a) Coronal slice showing group differences in the anterior extent of the inferior and middle frontal gyri/frontal pole. (b) Coronal slice showing group differences in the right anterior insula.
Table 3.
Summary of regions showing stronger functional connectivity to the cognitive control network in children compared to adults.
| Region | BA | Hemisphere | X | Y | Z | K | Max t |
|---|---|---|---|---|---|---|---|
| Frontal pole | 10, 11 | R | 32 | 58 | 20 | 2182 | 9.3 |
| 14 | 68 | 20 | 7.9 | ||||
| L | −18 | 66 | 10 | 66 | 5.8 | ||
| Anterior insula | R | 36 | 18 | 8 | 807 | 7.7 | |
| Inferior frontal gyrus | 45, 47 | L | −40 | 20 | −2 | 10 | 4.8 |
| Superior temporal sulcus | 22, 38, 41 | R | 40 | −38 | −2 | 586 | 8.1 |
| Middle temporal gyrus | 21, 39 | R | 46 | −52 | −14 | 4.6 | |
| R | 48 | −18 | −14 | 35 | 5.3 | ||
| L | −38 | −40 | 4 | 159 | 6.0 | ||
| Fusiform gyrus | 19, 37 | R | 4 | −74 | −22 | 255 | 5.9 |
| L | −25 | −68 | −18 | 301 | 5.8 | ||
| L | −8 | −76 | −18 | 5.6 | |||
| Superior frontal sulcus | 6 | R | 24 | 8 | 62 | 169 | 5.7 |
| Precuneus | 7, 31 | R | 2 | −46 | 44 | 72 | 5.5 |
X, Y, and Z are the MNI coordinates of the peak voxel in each region; K is cluster size, in 2 mm × 2 mm × 2 mm voxels; max t is the value of the t-statistic as measured at the peak voxel.
3. Discussion
To investigate the association of lPFC connectivity and DCCS performance across development, fMRI images collected from children and adults performing the DCCS were decomposed into a set of 20 maximally independent components by means of spatial ICA. From this set, a functionally relevant component that included lPFC was identified and compared across groups. As in our previous analysis, regions comprising this component were modulated by switching similarly for adults and children. Interestingly, a comparison of lPFC connectivity with regions comprising this component revealed age-related differences that were consistent with our predictions. In particular, lPFC in adults was more strongly connected to the anterior cingulate/medial prefrontal cortex, parietal cortex, the thalamus, and the ventral tegmental area than it was in children. By contrast, anterior frontal cortex and insula were more strongly connected to this network in children than in adults.
Age-related changes in DCCS performance specifically and cognitive control more generally have traditionally been associated with circumscribed changes in lateral prefrontal cortex function (Dempster, 1992, Diamond, 2002, Kirkham et al., 2003, Moriguchi and Hiraki, 2009, Moriguchi and Hiraki, 2011). Indeed, both individual and developmental differences in DCCS performance have been linked, for example, to variation in ventrolateral PFC activity (Moriguchi and Hiraki, 2009, Moriguchi and Hiraki, 2011). At the same time, fMRI investigations of older children and adults have found that switching modulates lPFC activity similarly for children and adults (Morton et al., 2009, Wendelken et al., 2012), but is associated with temporal dynamics of activation in dlPFC that differ for children and adults. In particular, rule updating in dlPFC appears to occur more slowly in children than it does in adults. Interestingly, signals for updating actively maintained lPFC representations originate in dopamine neurons within the ventral tegmental area and basal ganglia (O’Reilly, 2006, O’Reilly and Frank, 2006), the same regions that, in the current analysis, were more strongly connected to lPFC in adults than in children. The current findings therefore offer a potentially interesting complement to evidence that lPFC is slower to update in children than in adults (Wendelken et al., 2012). To the extent that functional connectivity between the lPFC and VTA is weaker, signals from the VTA to lPFC may act more slowly to update maintained rule representations within lPFC.
Aspects of the present findings also converge with studies of age-related change in the structural and functional connections of lPFC with other brain regions, and their association with developing higher-order cognition. Our finding that functional connectivity between lPFC and parietal cortex was stronger in adults than in children, for example, is consistent with reports that fronto-parietal connectivity increases both in strength and structural integrity early in development (Nagy et al., 2004), leading potentially to age-related gains in working memory capacity and moment-to-moment adjustments in control (Fair et al., 2007). We also found evidence of age-related increases in functional connectivity of lPFC and several subcortical regions, including the midbrain (i.e., ventral tegmental area), dorsal striatum (i.e., putamen), and the thalamus. lPFC connections with these regions form one of a series of striatal–thalamocortical loops (Alexander et al., 1986) that develop slowly (Sowell et al., 1999) and play a critical role in cognitive and behavioral self-regulation (Casey et al., 2007, Frank, 2005, Langen et al., 2012, Mulder et al., 2011). Finally, we observed stronger connectivity between lPFC and anterior cingulate cortex in adults than in children. Although lPFC is extensively connected to and frequently co-activates with the ACC, several studies have reported age-related decreases in functional connectivity strength between these two regions (Fair et al., 2007, Kelly et al., 2008). The basis of this discrepancy is unclear. Although motion estimates for adults and children were indistinguishable in the current study, motion artifacts are often more pronounced in children's than in adults data, and can inflate functional connectivity estimates of short-range connections, such as connections between lPFC and ACC (Power et al., 2012).
We also found evidence of age-related decreases in functional connectivity of frontopolar, insular, and temporal cortex with the larger cognitive control network. It is possible these findings converge with earlier studies of the development of cognitive control networks. Using seed-based measures of functional connectivity, these earlier studies found that fronto-parietal networks emerge over development with the integration of lateral prefrontal, inferior parietal, and cingulate cortices, and a corresponding segregation of anterior PFC and insular cortex (Fair et al., 2007, Tononi et al., 1994). However, some caution is probably warranted, given that age-related differences in seed-based measures of functional connectivity can emerge if there are small differences in the structure of motion artifacts between participants of different ages (Power et al., 2012). At a minimum, evidence of both age-related increases and decreases in functional connectivity over development point to the possibility that children and adults approach tasks such as the DCCS in qualitatively different ways.
Taken together, the current findings challenge prevailing maturational accounts that link the development of cognitive flexibility to circumscribed age-related changes in lPFC function and that assume the capacity for switching resides entirely within the confines of lPFC. Although lPFC was clearly involved in switching, as reflected by greater lPFC activity during switch than repeat blocks, so too were a number of cognitive control regions well outside of lPFC. Moreover, age-related differences associated with rule switching were most evident in the functional connectivity of lPFC rather than its profile of activation. As such, the current findings suggest that rule-switching and its development are linked to changes in the functional organization of a larger cognitive control network rather than the functional maturation of lPFC alone.
Linking rule-switching and its development to a larger network does not imply that constituent regions of the control network make identical contributions to switching. Instead, there is good evidence that lPFC (and the dorsal region in particular) is specialized for the active maintenance of attention guiding rules (Fuster et al., 2000, Miller and Cohen, 2001), and that related cognitive operations, such as updating, shielding, and strengthening active representations, emerge through the functional interaction of lPFC with other parts of the cognitive control network. These include interactions with the: VTA/BG, potentially important for updating active representations (O’Reilly and Frank, 2006); parietal cortex, potentially important for shielding active representations in working memory (Nagy et al., 2004); and anterior cingulate cortex, potentially important for strengthening active representations (Botvinick et al., 2001, Debener et al., 2005, Kerns et al., 2004, Ridderinkhof et al., 2004). Complex mental operations like switching build on all of these basic operations, given that active representations of current sorting rules are maintained (Morton and Munakata, 2002) and even strengthened (Waxer and Morton, 2011a) on repeat trials, but updated on switch trials (Wendelken et al., 2012). Thus, higher-order cognitive operations, such as switching in the DCCS, are likely emergent products of rapid bidirectional interactions among many functionally specialized brain regions rather than irreducible operations linked to activity in one circumscribed area alone.
One important avenue of future research therefore would be to cleave global performance in complex tasks such as the DCCS into constituent subprocesses, and relate these subprocesses to subcomponents of the larger cognitive control network. There is evidence, for example, that cue representation and conflict processing make independent contributions to overall variability in DCCS performance, and develop at different rates (Waxer and Morton, 2011b). And in task-switching paradigms, active preparation and task-set inertia also dissociate over development, with task-set inertia but not active preparation showing age-related change (Cepeda et al., 2001). Indeed, evidence from adult neuroimaging studies suggest some functional specialization within the cognitive control network during task switching, with dlPFC supporting active maintenance processes (akin to cue maintenance/active preparation), and ACC/dlPFC involved in response preparation (Cole and Schneider, 2007). It is conceivable then that subprocesses underlying DCCS and task-switching performance might follow distinct developmental trajectories because these subprocesses are associated with distinct sub-components of a larger cognitive control network. Testing such speculations is beyond the scope of the present study, given that such processes were aggregated in time owing to the use of a blocked design, but are deserving of future research attention.
3.1. Caveats and future directions
There are a number of important caveats to the present analysis. First, although the analysis was based on an adequate sampling of data from each participant (close to 800 volumes per subject), there was a relatively small number of participants in the final sample. Thus, the findings certainly deserve replication with a larger data set. Second, our objective component selection procedure focused the analysis on a cognitive control component that was activated by the DCCS. Although we used this component selection criterion to ensure that any age differences in functional connectivity were observed in a network that was relevant for DCCS performance, it raises the possibility that the selected network was merely a shared timecourse introduced by the task itself. If so, then the present analysis would be largely convergent with our previously reported GLM analysis (Morton et al., 2009). While we acknowledge this concern, it does not, in our opinion, apply to the current analysis. First, there is abundant evidence in the literature that the cognitive control component we selected for age comparison is readily observed whether ICA is applied to task or resting state data (Calhoun et al., 2008). Indeed, the template we used to select the cognitive control component was a fronto-parietal component decomposed from resting data (Seeley et al., 2007). Thus, it seems unlikely that the cognitive control network examined in this study was merely a set of voxels that shared a timecourse induced by the task. Second, the age-related differences we report pertain to the spatial distribution rather than task-induced changes in the timecourse of the selected component. As in our original analysis (Morton et al., 2009), there was an effect of switching, but no interaction of switching and group in the timecourse of this component. Thus, the current results are not in our opinion, redundant with the previous findings, but suggest age-related differences in functional connectivity within a network that activates to the DCCS similarly across children and adults.
One final consideration is that group spatial ICA assumes all individuals in a sample share a common set of spatial components, or sources. And although group ICA is robust against variability in spatial sources, and can re-construct individual spatial sources with a high degree of accuracy if there is sufficient overlap in spatial sources across the group (Allen et al., 2012), substantial inter-individual differences in spatial sources will undermine the fidelity of the group decomposition and the interpretability of the resulting subject-level components (Allen et al., 2012). While an important concern, certainly in the context of a comparison of children and adults, the quality and stability of the current decomposition suggests that age-related variability in the structure of underlying sources was not sufficient to compromise the fidelity of the group decomposition. Indeed, the cognitive control component selected by our component selection procedure showed good overlap with the template (see Fig. 3), suggesting our group decomposition had good fidelity.
In sum, the current findings provide preliminary evidence that DCCS performance is associated with activity in a cognitive control network, and that this network undergoes changes in functional organization between late childhood and early adulthood. Future research should investigate whether similar changes extend to other tasks and/cognitive control processes beyond the DCCS and dimensional shifts of attention.
Conflict of interest statement
The authors have no conflict of interest to declare.
Acknowledgement
This research was made possible by an NSERC Discovery grant to JBM.
References
- Alexander G.E., DeLong M.R., Strick P.L. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annual Review of Neuroscience. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- Allen E.A., Erhardt E.B., Damaraju E., Gruner W., Segall J.M., Silva R.F. A baseline for the multivariate comparison of resting-state networks. Frontiers in Systems Neuroscience. 2011;5:2. doi: 10.3389/fnsys.2011.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen E.A., Erhardt E.B., Wei Y., Eichele T., Calhoun V.D. Capturing inter-subject variability with group independent component analysis of fMRI data: a simulation study. Neuroimage. 2012;59(4):4141–4159. doi: 10.1016/j.neuroimage.2011.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber A.D., Carter C.S. Cognitive control involved in overcoming prepotent response tendencies and switching between tasks. Cerebral Cortex. 2005;15(7):899–912. doi: 10.1093/cercor/bhh189. [DOI] [PubMed] [Google Scholar]
- Barberi E.A., Gati J.S., Rutt B.K., Menon R.S. A transmit-only/receive-only (TORO) RF system for high-field MRI/MRS applications. Magnetic Resonance in Medicine. 2000;43(2):284–289. doi: 10.1002/(sici)1522-2594(200002)43:2<284::aid-mrm16>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Berg E.A. A simple objective technique for measuring flexibility in thinking. Journal of General Psychology. 1948;39:15–22. doi: 10.1080/00221309.1948.9918159. [DOI] [PubMed] [Google Scholar]
- Botvinick M.M., Braver T.S., Barch D.M., Carter C.S., Cohen J.D. Conflict monitoring and cognitive control. Psychological Review. 2001;108(3):624–652. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- Bunge S.A., Zelazo P.D. A brain-based account of the development of rule use in childhood. Current Directions in Psychological Science. 2006;15(3):118–121. [Google Scholar]
- Buschman T.J., Miller E.K. Top-down versus bottom-up control of attention in the prefrontal and posterior parietal cortices. Science. 2007;315(5820):1860–1862. doi: 10.1126/science.1138071. [DOI] [PubMed] [Google Scholar]
- Calhoun V.D., Kiehl K.A., Pearlson G.D. Modulation of temporally coherent brain networks estimated using ICA at rest and during cognitive tasks. Human Brain Mapping. 2008;29(7):828–838. doi: 10.1002/hbm.20581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun V.D., Liu J., Adali T. A review of group ICA for fMRI data and ICA for joint inference of imaging, genetic, and ERP data. Neuroimage. 2009;45(1 Suppl.):S163–S172. doi: 10.1016/j.neuroimage.2008.10.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey B.J., Epstein J.N., Buhle J., Liston C., Davidson M.C., Tonev S.T. Frontostriatal connectivity and its role in cognitive control in parent–child dyads with ADHD. American Journal of Psychiatry. 2007;164(11):1729–1736. doi: 10.1176/appi.ajp.2007.06101754. [DOI] [PubMed] [Google Scholar]
- Cepeda N.J., Kramer A.F., Gonzalez de Sather J.C. Changes in executive control across the life span: examination of task-switching performance. Developmental Psychology. 2001;37(5):715–730. [PubMed] [Google Scholar]
- Cole M.W., Schneider W. The cognitive control network: integrated cortical regions with dissociable functions. Neuroimage. 2007;37(1):343–360. doi: 10.1016/j.neuroimage.2007.03.071. [DOI] [PubMed] [Google Scholar]
- Crone E.A., Bunge S.A., van der Molen M.W., Ridderinkhof K.R. Switching between tasks and responses: a developmental study. Developmental Science. 2006;9(3):278–287. doi: 10.1111/j.1467-7687.2006.00490.x. [DOI] [PubMed] [Google Scholar]
- Danielmeier C., Eichele T., Forstmann B.U., Tittgemeyer M., Ullsperger M. Posterior medial frontal cortex activity predicts post-error adaptations in task-related visual and motor areas. Journal of Neuroscience. 2011;31(5):1780–1789. doi: 10.1523/JNEUROSCI.4299-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson M.C., Amso D., Anderson L.C., Diamond A. Development of cognitive control and executive functions from 4 to 13 years: evidence from manipulations of memory, inhibition, and task switching. Neuropsychologia. 2006;44(11):2037–2078. doi: 10.1016/j.neuropsychologia.2006.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debener S., Ullsperger M., Siegel M., Fiehler K., von Cramon D.Y., Engel A.K. Trial-by-trial coupling of concurrent electroencephalogram and functional magnetic resonance imaging identifies the dynamics of performance monitoring. Journal of Neuroscience. 2005;25(50):11730–11737. doi: 10.1523/JNEUROSCI.3286-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempster F.N. The rise and fall of the inhibitory mechanism: toward a unified theory of cognitive development and aging. Developmental Review. 1992;12:45–75. [Google Scholar]
- Diamond A. Normal development of prefrontal cortex from birth to young adulthood: cognitive functions, anatomy and biochemistry. In: Knight S.A., Stuss D.T., editors. Principles of Frontal Lobe Function. Oxford University Press; New York: 2002. [Google Scholar]
- Duncan J. The multiple-demand (MD) system of the primate brain: mental programs for intelligent behaviour. Trends in Cognitive Sciences (Regular Edition) 2010;14(4):172–179. doi: 10.1016/j.tics.2010.01.004. [DOI] [PubMed] [Google Scholar]
- Duncan J., Owen A.M. Common regions of the human frontal lobe recruited by diverse cognitive demands. Trends in Neurosciences. 2000;23(10):475–483. doi: 10.1016/s0166-2236(00)01633-7. [DOI] [PubMed] [Google Scholar]
- Erhardt E.B., Rachakonda S., Bedrick E.J., Allen E.A., Adali T., Calhoun V.D. Comparison of multi-subject ICA methods for analysis of fMRI data. Human Brain Mapping. 2011;32(12):2075–2095. doi: 10.1002/hbm.21170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair D.A., Dosenbach N.U.F., Church J.A., Cohen A.L., Brahmbhatt S., Miezin F.M. Development of distinct control networks through segregation and integration. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(33):13507–13512. [Google Scholar]
- Frank M.J. Dynamic dopamine modulation in the basal ganglia: a neurocomputational account of cognitive deficits in medicated and nonmedicated parkinsonism. Journal of Cognitive Neuroscience. 2005;17(1):51–72. doi: 10.1162/0898929052880093. [DOI] [PubMed] [Google Scholar]
- Fuster J.M., Bodner M., Kroger J.K. Cross-modal and cross-temporal association in neurons of frontal cortex. Nature. 2000;405(6784):347–351. doi: 10.1038/35012613. [DOI] [PubMed] [Google Scholar]
- Himberg J., Hyvärinen A., Esposito F. Validating the independent components of neuroimaging time series via clustering and visualization. Neuroimage. 2004;22(3):1214–1222. doi: 10.1016/j.neuroimage.2004.03.027. [DOI] [PubMed] [Google Scholar]
- Huizinga M., van der Molen M.W. Task switching and shifting between stopping and going: developmental change in between-trial control adjustments. Journal of Experimental Child Psychology. 2011;108(3):484–503. doi: 10.1016/j.jecp.2010.10.004. [DOI] [PubMed] [Google Scholar]
- Huttenlocher P.R., Dabholkar A.S. Regional differences in synaptogenesis in human cerebral cortex. Journal of Comparative Neurology. 1997;387:167–178. doi: 10.1002/(sici)1096-9861(19971020)387:2<167::aid-cne1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Kelly A., Di Martino A., Uddin L., Shehzad Z., Gee D., Reiss P. Development of anterior cingulate functional connectivity from late childhood to early adulthood. Cerebral Cortex. 2008;19(3):640–657. doi: 10.1093/cercor/bhn117. [DOI] [PubMed] [Google Scholar]
- Kerns J.G., Cohen J.D., MacDonald A.W., Cho R.Y., Stenger V.A., Carter C.S. Anterior cingulate conflict monitoring and adjustments in control. Science. 2004;303(5660):1023–1026. doi: 10.1126/science.1089910. [DOI] [PubMed] [Google Scholar]
- Kirkham N.Z., Cruess L., Diamond A. Helping children apply their knowledge to their behavior on a dimension-switching task. Developmental Science. 2003;6(5):449–476. [Google Scholar]
- Klassen L.M., Menon R.S. Robust automated shimming technique using arbitrary mapping acquisition parameters (RASTAMAP) Magnetic Resonance in Medicine. 2004;51(5):881–887. doi: 10.1002/mrm.20094. [DOI] [PubMed] [Google Scholar]
- Langen M., Leemans A., Johnston P., Ecker C., Daly E., Murphy C.M. Fronto-striatal circuitry and inhibitory control in autism: findings from diffusion tensor imaging tractography. Cortex. 2012;48(2):183–193. doi: 10.1016/j.cortex.2011.05.018. [DOI] [PubMed] [Google Scholar]
- Lehto J., Juujarvi P., Kooistra L., Pulkkinen L. Dimensions of executive functioning: evidence from children. British Journal of Developmental Psychology. 2003;21:59–80. [Google Scholar]
- Liston C., Matalon S., Hare T.A., Davidson M.C., Casey B.J. Anterior cingulate and posterior parietal cortices are sensitive to dissociable forms of conflict in a task-switching paradigm. Neuron. 2006;50(4):643–653. doi: 10.1016/j.neuron.2006.04.015. [DOI] [PubMed] [Google Scholar]
- Miller E.K., Cohen J.D. An integrative theory of prefrontal cortex function. Annual Review of Neuroscience. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Moriguchi Y., Hiraki K. Neural origin of cognitive shifting in young children. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(14):6017–6021. doi: 10.1073/pnas.0809747106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriguchi Y., Hiraki K. Longitudinal development of prefrontal function during early childhood. Developmental Cognitive Neuroscience. 2011;1(2):153–162. doi: 10.1016/j.dcn.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton J.B., Bosma R., Ansari D. Age-related changes in brain activation associated with dimensional shifts of attention: an fMRI study. Neuroimage. 2009;46(1):249–256. doi: 10.1016/j.neuroimage.2009.01.037. [DOI] [PubMed] [Google Scholar]
- Morton J.B., Munakata Y. Active versus latent representations: a neural network model of perseveration, dissociation, and decalage. Developmental Psychobiology. 2002;40(3):255–265. doi: 10.1002/dev.10033. [DOI] [PubMed] [Google Scholar]
- Mulder M.J., van Belle J., van Engeland H., Durston S. Functional connectivity between cognitive control regions is sensitive to familial risk for ADHD. Human Brain Mapping. 2011;32(9):1511–1518. doi: 10.1002/hbm.21141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy Z., Westerberg H., Klingberg T. Maturation of white matter is associated with the development of cognitive functions during childhood. Journal of Cognitive Neuroscience. 2004;16(7):1227–1233. doi: 10.1162/0898929041920441. [DOI] [PubMed] [Google Scholar]
- O’Reilly R.C. Biologically based computational models of high-level cognition. Science. 2006;314(5796):91–94. doi: 10.1126/science.1127242. [DOI] [PubMed] [Google Scholar]
- O’Reilly R.C., Frank M.J. Making working memory work: a computational model of learning in the prefrontal cortex and basal ganglia. Neural Computation. 2006;18(2):283–328. doi: 10.1162/089976606775093909. [DOI] [PubMed] [Google Scholar]
- Olesen P.J., Nagy Z., Westerberg H., Klingberg T. Combined analysis of DTI and fMRI data reveals a joint maturation of white and grey matter in a fronto-parietal network. Brain Research. Cognitive Brain Research. 2003;18(1):48–57. doi: 10.1016/j.cogbrainres.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Power J.D., Barnes K.A., Snyder A.Z., Schlaggar B.L., Petersen S.E. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012;59(3):2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridderinkhof K.R., Ullsperger M., Crone E.A., Nieuwenhuis S. The role of the medial frontal cortex in cognitive control. Science. 2004;306(5695):443–447. doi: 10.1126/science.1100301. [DOI] [PubMed] [Google Scholar]
- Seeley W.W., Menon V., Schatzberg A.F., Keller J., Glover G.H., Kenna H. Dissociable intrinsic connectivity networks for salience processing and executive control. Journal of Neuroscience. 2007;27(9):2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P., Kabani N.J., Lerch J.P., Eckstrand K., Lenroot R., Gogtay N. Neurodevelopmental trajectories of the human cerebral cortex. Journal of Neuroscience. 2008;28(14):3586–3594. doi: 10.1523/JNEUROSCI.5309-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell E.R., Thompson P.M., Holmes C.J., Jernigan T.L., Toga A.W. In vivo evidence for post-adolescent brain maturation in frontal and striatal regions. Nature Neuroscience. 1999;2(10):859–861. doi: 10.1038/13154. [DOI] [PubMed] [Google Scholar]
- Sowell E.R., Thompson P.M., Tessner K.D., Toga A.W. Mapping continued brain growth and gray matter density reduction in dorsal frontal cortex: Inverse relationships during postadolescent brain maturation. Journal of Neuroscience. 2001;21(22):8819–8829. doi: 10.1523/JNEUROSCI.21-22-08819.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens M.C., Skudlarski P., Pearlson G.D., Calhoun V.D. Age-related cognitive gains are mediated by the effects of white matter development on brain network integration. Neuroimage. 2009;48(4):738–746. doi: 10.1016/j.neuroimage.2009.06.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tononi G., Sporns O., Edelman G. A measure for brain complexity: relating functional segregation and integration in the nervous system. Proceedings of the National Academy of Sciences of the United States of America. 1994;91:5033–5037. doi: 10.1073/pnas.91.11.5033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Bos W., Cohen M.X., Kahnt T., Crone E.A. Striatum-medial prefrontal cortex connectivity predicts developmental changes in reinforcement learning. Cerebral Cortex. 2012;22(6):1247–1255. doi: 10.1093/cercor/bhr198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waxer M., Morton J.B. The development of future-oriented control: an electrophysiological investigation. Neuroimage. 2011;56(3):1648–1654. doi: 10.1016/j.neuroimage.2011.02.001. [DOI] [PubMed] [Google Scholar]
- Waxer M., Morton J.B. Multiple processes underlying dimensional change card sort performance: a developmental electrophysiological investigation. Journal of Cognitive Neuroscience. 2011;23(11):3267–3279. doi: 10.1162/jocn_a_00038. [DOI] [PubMed] [Google Scholar]
- Weed M.R., Bryant R., Perry S. Cognitive development in macaques: attentional set-shifting in juvenile and adult rhesus monkeys. Neuroscience. 2008;157(1):22–28. doi: 10.1016/j.neuroscience.2008.08.047. [DOI] [PubMed] [Google Scholar]
- Wendelken C., Munakata Y., Baym C., Souza M., Bunge S.A. Flexible rule use: common neural substrates in children and adults. Developmental Cognitive Neuroscience. 2012;2:329–339. doi: 10.1016/j.dcn.2012.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelazo P.D. The Dimensional Change Card Sort (DCCS): a method of assessing executive function in children. Nature Protocols. 2006;1(1):297–301. doi: 10.1038/nprot.2006.46. [DOI] [PubMed] [Google Scholar]