Abstract
In language, the relative order of words in sentences carries important grammatical functions. However, the developmental origins and the neural correlates of the ability to track word order are to date poorly understood. The current study therefore investigates the origins of infants’ ability to learn about the sequential order of words, using near-infrared spectroscopy (NIRS) with newborn infants. We have conducted two experiments: one in which a word order change was implemented in 4-word sequences recorded with a list intonation (as if each word was a separate item in a list; list prosody condition, Experiment 1) and one in which the same 4-word sequences were recorded with a well-formed utterance-level prosodic contour (utterance prosody condition, Experiment 2). We found that newborns could detect the violation of the word order in the list prosody condition, but not in the utterance prosody condition. These results suggest that while newborns are already sensitive to word order in linguistic sequences, prosody appears to be a stronger cue than word order for the identification of linguistic units at birth.
Keywords: Word order, Newborn infants, Prosody, Near-infrared spectroscopy
1. Introduction
The relative order of words in sentences carries important grammatical functions. Exactly which functions are expressed through word order differs across the languages of the world. In English, word order encodes the basic syntactic categories Subject, Verb and Object (e.g. The girl saw the boy. vs. The boy saw the girl.). In Turkish, Basque or Hungarian, by contrast, these are indicated by case marking (e.g. Hungarian: A lány látta a fiút. vs. A fiú látta a lányt.), while word order is typically used for semantic/pragmatic functions (Hungarian: A lány látta a fiút − neutral sentence, equivalent to ‘The girl saw the boy’; but A fiút látta a lány − focus sentence, with prosodic prominence on the word ‘fiút’, equivalent to ‘It is the boy (and not someone else) that the girl saw.’).
Learning word order is therefore essential for the acquisition of language. This ability is well attested in older infants and children (e.g. Braine, 1963, Brown, 1973, Gervain et al., 2008b, Gervain and Werker, 2013, Höhle et al., 2001, Mandel et al., 1996, Marcus et al., 1999), but its developmental origins are not well understood. Even less is known about its neural correlates in development. The current study therefore investigates newborn infants’ ability to learn about word order, using near-infrared spectroscopy (NIRS).
1.1. Infants’ knowledge of the word order of their native languages
From their earliest productions, toddlers appear to respect the basic word order regularities of their native language (Brown, 1973, Clahsen and Muysken, 1986, Meisel, 1986). English-learning children, for instance, place adjectives before nouns (e.g. big train, red book), animate possessors before possesses (Mommy lunch) and objects after verbs (hit ball, all examples from (Brown et al., 1970)). Children acquiring languages in which word order is more flexible than in English, and grammatical functions are more typically indicated by case marking, also acquire the most frequent, most commonly used word order patterns of their native language at the same age as English-exposed infants, and quickly become sensitive to the complementary roles of word order and case marking (for Japanese, Hakuta, 1982; for Hungarian, MacWhinney et al., 1985; for Serbo-Croation, Turkish and Italian, Slobin and Bever, 1982).
Theoretical explanations diverge as to whether this is the result of full-blown and possibly innate abstract syntactic knowledge (e.g. Brown, 1973, Guasti, 2002, Pinker, 1984) or whether children initially possess more simple sentence frames or schemas, mostly based on input statistics (e.g. Akhtar, 1999, Slobin and Bever, 1982, Tomasello, 2000). Nevertheless the fact remains that the basic word order of young children’s production already follows the word order regularities of their mother tongue.
In perception, this knowledge is detectable even earlier (Bernard and Gervain, 2012, Gervain et al., 2008b, Gervain and Werker, 2013, Höhle et al., 2001, Shi, 2014, Weissenborn et al., 1996). Indeed, as early as 8 months of age, infants show a preference for the relative order of function words (e.g. pronouns, prepositions, articles etc.) and content words (e.g. nouns, verbs, adjectives etc.) in their native language. Thus, Italian (Gervain et al., 2008b), English (Gervain and Werker, 2013) and French (Bernard and Gervain, 2012) 8-month-olds prefer the functor-initial order, typical of these languages (e.g. on the table, where “on” and “the” are functors, “table” is a content word), while their Japanese peers show a preference for the functor-final order (Gervain et al., 2008b), characteristic of Japanese (Tokyo ni Tokyo to “to/in Tokyo”). Even more interestingly, bilinguals exposed to a functor-initial and a functor-final language, e.g. English and Japanese, can use the different prosodic patterns associated with these two word order patterns respectively as cues to distinguish between the two orders. Thus prosody is an important cue for word order learning early in development. As another example, before the end of their second birthday, German-learning infants recognize the syntactic contexts that obligatorily require the verb to occupy the sentence-final position (Hans singt, weil er glücklich ist. Hans signs because he happy is “Hans is singing, because he is happy.”).
1.2. Infants’ ability to learn about word order in artificial grammars
The above results indicate that infants and young children very quickly become familiar with the word order of their native languages. This suggests that the general ability to learn word order is present very early in development. This has been confirmed in a large number of artificial grammar learning studies with young infants (e.g. Benavides-Varela and Mehler, 2015, Gomez and Gerken, 2000, Marcus et al., 1999). Artificial grammars are miniature “languages” implementing one or a small number of rules of interest over a predefined lexicon (typically of nonce words). The earliest evidence for the ability to learn word order in an artificial grammar comes from a study in which 2-month-old English-learning infants have been shown to track the order of words in 4-word sequences (Mandel et al., 1996). Specifically, when habituated to the utterance “Cats would jump benches”, infants dishabituated to the utterance “Cats jump wood benches” after a 2-min silent delay, as shown by an increase in their sucking rate in a high-amplitude sucking procedure. Both utterances were prosodically and syntactically well-formed, and contained words made up of the same phonemes. They only differed in the order of the two middle words. If, however, infants were habituated to two, syntactically and prosodically well-formed sentence fragments containing the same 4 words, i.e. “Cats jump. Wood benches.”, they did not dishabituate to the same word order change, i.e. “Cats would. Jump benches.” The authors concluded that 2-month-old infants are already able to keep track of the sequential order of words, and preferentially compute this within prosodically and syntactically well-formed utterances.
1.3. The role of prosody
Previous results suggest that prosody plays a very important role in young infants’ perception of speech, in general (e.g. Christophe et al., 2003, Cooper and Aslin, 1990, Morgan and Demuth, 1996, Nazzi et al., 1998b, Ramus et al., 2000), and in learning about word order, in particular (Gervain and Werker, 2013, Mandel et al., 1996). Indeed, prosody is the first property of speech that infants have experience with, as in utero maternal tissues filter the speech signal, suppressing individual sounds, but transmitting prosody. Prosody may facilitate speech perception and language acquisition in at least three ways. First, prosodic contours, the acoustic correlates of which even very young infants are sensitive to (Nazzi et al., 1998b, Woodward and Aslin, 1990), demarcate chunks in the otherwise continuous speech signal that correspond to linguistically meaningful units, such as utterances or phrases, within which linguistic and statistical computations apply. This is well illustrated by 2-month-olds’ ability to detect a change in word order inside a prosodic (and syntactic) unit, but not across a prosodic boundary (Mandel et al., 1996). Prosodic boundaries have similarly been shown to block word form candidates during statistically-based speech segmentation (Shukla et al., 2011). Second, prosody, more specifically the location and acoustic realization of prosodic prominence, correlates with lexical and morphosyntactic properties in many languages, and can thus serve as perceptual cues to these phenomena (the prosodic bootstrapping hypothesis, (Morgan and Demuth, 1996)). Thus, 7–8-month-old infants can use the typical lexical stress pattern of their native language to segment out new words from the speech stream. English-learning infants readily extract stress-initial or trochaic word forms such as “doctor” or “candle” from continuous passages, but fail with words such as “guitar” having a stress-final or iambic pattern, less common in English (Jusczyk et al., 1993). At approximately the same age, infants also start using the correlation between properties of phrasal prominence and the basic word order of a language to break into the morphosyntax of their native language (Bernard and Gervain, 2012, Gervain and Werker, 2013).
Third, newborns and young infants are able to use prosody to discriminate between function words and content words (Shi et al., 2006, Shi et al., 1999, Shi and Werker, 2001, Shi and Werker, 2003). Indeed, even newborns can discriminate a list of functors from a list of content words based on the prosodic differences between the two word classes. Functors are prosodically and phonologically minimal, e.g. they are often monosyllabic, they do not carry lexical stress, they typically have a simpler syllabic structure than content words etc. This ability may be particularly useful for newborns and young infants to track the relative order of functors and content words in their native language, which is a fundamental aspect of word order.
1.4. The neural correlates of learning word order
Little is known about the neural structures underlying young learners’ ability to learn word order, and to our knowledge, no study to date has investigated the neural correlates of this ability at the beginning of life.
In adults, most syntactic, semantic and pragmatic functions signaled by word order are processed in the superior temporal cortex and in the inferior frontal gyrus, most notably Broca’s area/Brodmann areas 44 and 45, responsible for sequence learning and higher-order structure building (Friederici, 2011, Friederici, 2012, Musso et al., 2003).
While less is known about the neural structures supporting language acquisition throughout development, the existing MRI, EEG and NIRS studies (Dehaene-Lambertz, 2000, Friederici, 2005, Gervain et al., 2011, Jasinska and Petitto, 2013, Minagawa-Kawai et al., 2011) suggest that the functional specialization of the brain for speech and language is already present from birth and involves similar structures as in adults, i.e. the classical language areas cited above. Indeed, speech in the native language has been found to be processed in the left (Dehaene-Lambertz et al., 2002, Pena et al., 2003, Sato et al., 2012) or in the bilateral (May et al., 2011) temporal and fronto-temporal areas already at birth and at 3 months of age. Thus whether the lateralization of speech processing typical in adults (prosody predominantly processed in the right hemisphere, other aspects in the left hemisphere) is already found at birth remains an open question. Furthermore, an increasingly strong lateralization has been found for the processing of different speech units, phonemes lateralizing to the left, prosodic features lateralizing to the right, as language processing becomes less universal and acoustically based and increasingly attuned to the native language towards the end of the first year of life (Cristia et al., 2014, Minagawa-Kawai et al., 2011, Sato et al., 2009).
While no imaging studies have looked at the neural correlates of word order processing in infants, existing NIRS studies on newborns’ (Gervain et al., 2008a, Gervain et al., 2011) and older infants’ (Wagner et al., 2011) ability to encode the sequential position of syllable repetitions provides indirect evidence. Gervain et al. (2011) has found that newborns are able to discriminate between artificial language sequences with an ABB (e.g. “mubaba”, “penana” etc.) and an AAB structure (e.g. “babamu”, “nanape” etc.), suggesting that they are able to encode the relative position of the repeated A and the unrepeated B syllables. Furthermore, Ferry et al. (2015) reported that the newborn brain detects changes of syllable positions within words, particularly when syllables are located at the edges (“simebutalefo” → “fomebutalesi”). In both studies, detecting a change in relative position has been found to activate the bilateral temporal cortices, as well as the left inferior frontal cortex. Discrimination between the two structures tested in Gervain et al. (2011), most relevant for tracking word order, has been found in the left inferior frontal cortex.
1.5. The current study
In the current study, we have asked whether the ability to track the sequential order of words may be observed even earlier in development than previously reported, i.e. at birth. This is of considerable theoretical importance, since abilities that are acquired through experience may not be present early on in development, whereas genetically endowed, core abilities may be operational from the very beginning of development. Revealing the ability to learn the order of words in linguistic sequences at birth, and how this ability interacts with prosody, would thus imply that infants may start learning about their native grammar earlier than believed before.
We used optical brain imaging (near-infrared spectroscopy, NIRS) as our method as it is well suited even for newborns, and because very little is known about the neural correlates of sequence and word order learning at birth. Yet, identifying the neural correlates of the ability to learn word order can provide insight into its nature.
As discussed above, the ability to track word order has been reported at about the same age in infants exposed to different word orders across languages. This suggests that the ability to learn word order does not depend on the infants’ particular linguistic experience, but relies instead on learning abilities common across all languages. Here we hypothesized that, provided some simplified conditions, precursors of this ability should already be found in infants at birth.
Even though no previous study has directly evaluated the neurobiological correlates of word order tracking in newborns, we expect to find them in the classical language areas that pick up syntactic violations in adults (Friederici, 2005), including the left temporal and frontal areas.
In the light of the results on the role of prosody, we have conducted two experiments: one in which a word order change was implemented in 4-word sequences recorded with a list intonation (as if each word was a separate item in a list; list prosody condition, Experiment 1) and one in which the same 4-word sequences were recorded with a well-formed utterance-level prosodic contour (utterance prosody condition, Experiment 2). We reasoned that if prosody makes it easier for newborns to track word order, as it does for 2-month-olds (Mandel et al., 1996), newborns should be able to detect a change in word order in at least the utterance prosody condition. However, given that newborns prenatally only have experience with prosody, but not with other aspects of language, they might simply use prosody to identify the native speech signal and do not yet perform computations within its linguistic units. In this case, they might find it easier to track word order in the list prosody condition.
Our 4-word sequences were always made up of two function and two content words. Thus, in addition to a well-formed intonational contour (or the lack of it), the prosodic differences between function words and content words, which newborns are known to be sensitive to (Shi et al., 1999), might also provide relevant cues for infants to track word order in our task − as well as in the actual language input.
2. Experiment 1
In this experiment, we sought to establish whether newborns are able to detect a change in the sequential order of four words. We used NIRS to record newborns brain responses to identical repetitions of the 4-word sequences as well as to presentations where two of the four words were interchanged. This allowed us to determine whether newborns can detect the word order violations, and if so, what neural mechanisms subserve this ability.
2.1. Materials and methods
2.1.1. Participants
Thirty-one full-term, healthy French-exposed neonates were tested at the maternity ward of the Robert Debré Hospital in Paris. Of these, 11 were not included in the final analysis, because their data files got lost (2), they did not finish the experiment due to fussiness and crying (6) or because they didn’t provide enough analyzable data due to movement artifacts and/or dense hair (3). Infants were not retained for analysis if more than 66% of their data was rejected. The remaining 20 babies (8 girls, age range 1-3 days, mean age: 2.0 days, mean weight 3498 g, weight range: 2850–4390 g, Apgar at 10 min after birth: 10, normal otoacoustic emissions test) were included in the final analysis. All parents gave informed consent prior to participation. The study was approved by the CERES ethics committee of the Université Paris Descartes.
2.1.2. Material
Our stimuli consisted of 4-word-long sequences. The words were selected from the French subcorpora of the CHILDES database (MacWhinney, 2000) and were reproduced by a female native speaker of French with child-directed intonation. We used 50 actual French words. Half of them were function words (e.g. que ‘what’, ta ‘your’, un ‘a/an’, dans ‘in’), the other half content words (e.g. biberon ‘baby bottle’, maison ‘house’, papa ‘dad’, regarde ‘look’). The words were combined into 4-word sequences following one of the four possible orders of two function (F) and two content (C) words, i.e. F-F-C-C, C-C-F-F, F-C-F-C or C-F-C-F. While the words were actual French words, the sequences (and their subparts) did not constitute grammatical French sentences or phrases. This was done in order to create sequences that mimic the distribution of words found in natural languages, but that contain word combinations that are unfamiliar to the infants so as to avoid that some sequences be better learned during the experiment because they have occurred in the prenatal or short postnatal experience of the participants.
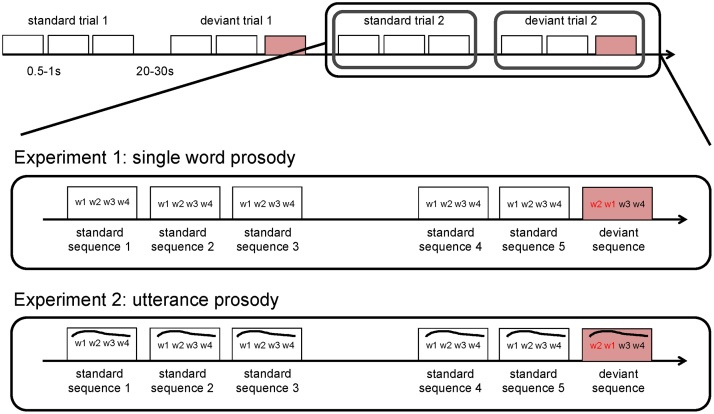
From these 50 words, we have created 96 different sequences (e.g. et appelle de allez ‘and call of go’). Each sequence was repeated three times in an identical manner in a standard block (Fig. 1). The repetitions within the block were separated by silences varying in duration between 0.9-1.1 s. Words within sequences were separated by 50 msec pauses. A standard block was always followed by a deviant block, whereby the sequence was first repeated twice as in the standard block, and a third time with a change in its word order (e.g. appelle et de allez). The change involved switching the position of one or more words such that the F-F-C-C or C-C-F-F sequences became F-C-F-C or C-F-C-F sequences and the other way around.
Fig. 1.
The design of Experiments 1 and 2.
In total, we had 24 [standard block − deviant block] pairs (Fig. 1). Blocks, whether standard or deviant, lasted on average 5.8 s (range 4.1–7.2 s), and were separated by silences of jittered duration between 20 and 30 s long. The order of the block pairs was pseudo-randomized and counterbalanced across participants. Importantly, sequences that were used as standard for one infant appeared as deviant for another infant and vice versa.
2.1.3. Procedure
Infants were tested in a dimly lit, quiet room of the maternity ward of the Robert Debré Hospital, Paris, France, while lying in their cribs in a state of quiet rest or sleep. At least one parent was present throughout the testing session.
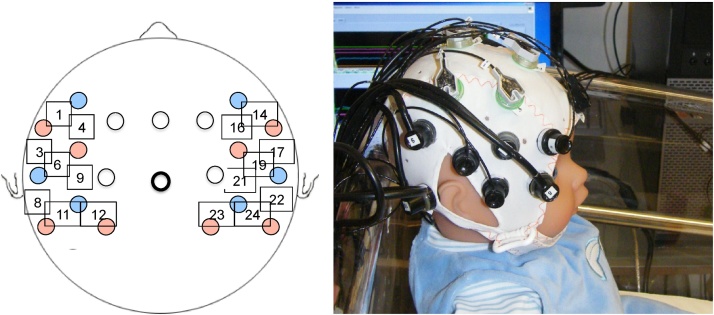
We used a NIRx NIRScout 8–16 system with a source-detector separation of 3 cm, using pulsated LED lights at 760 nm and 850 nm. The optical probes were inserted into an elastic cap (EasyCap, BrainProducts GmbH, Germany), which was placed on the newborns’ head following surface landmarks (vertex, tragus, ears) as shown in Fig. 2. No localization analysis could be performed for the current dataset, as photographs and distance measures of the probe placement were not available for all infants. However, in a previous study from our lab (Abboub et al., 2016), using the same configuration, cap and probe placement, the NIRS channels used here were found to be positioned over the following brain areas: inferior-middle frontal area (LH: channels 1, 4, 6; RH: 14, 16, 19), the inferior frontal area (LH: channel 3; RH: channel 17), the supramarginal gyrus (LH: channels 9, 12; RH: channels 21, 23), as well as the angular and superior temporal gyri (LH: channels 8, 11; RH: channels 22, 24). These locations were defined by mapping the measures and photographed locations onto the surface of the 3D MRI reconstruction of the newborn head and then projecting these onto the closest underlying cortical areas on a neonate MRI1.5T template using the MNI stereotaxic atlas (Shi et al., 2011). Since photographs of the probe placement for individual participants were not available and because we used average brain atlases and not individual brain scans of participants, the above-described localizations are accurate on average at the group level, but may be imprecise for any individual participant. As indicated in Fig. 2, electroencephalography (EEG) was also recorded in this study simultaneously with NIRS. The EEG data will be reported in a separate publication elsewhere.
Fig. 2.
Probe placement as shown on a schematic head and on a newborn doll model.
Stimuli were administered through loudspeakers positioned at a distance of 1.5 m from the newborns’ head, at an angle of 30°, and elevated to the height of the infants’ crib. A portable computer running E-Prime delivered the stimuli and sent time stamps to the NIRS machine. The machine sampled at a frequency of 10.417 Hz.
2.1.4. Data analysis
Changes in the concentration of oxygenated hemoglobin (oxyHb) and deoxygenated hemoglobin (deoxyHb) were calculated from the absorption of near-infrared light as metabolic indicators of neural activity. OxyHb and deoxyHb were entered into the data analysis.
To eliminate high-frequency noises (heartbeat, etc.) and overall trends, the data were band-pass filtered between 0.01 and 1 Hz. Movement artifacts, defined as concentration changes larger than 0.1 mmol × mm over 0.2 msec, were removed by rejecting block–channel pairs where artifacts occurred. For the nonrejected blocks, a baseline was linearly fitted between the means of the 5 s preceding the onset of the block and the 5 s starting 24 s after the onset of the block. For each block, data were averaged over a 15-s time window starting from the onset of stimulation.
We statistically analyzed the data by conducting channel-wise t-tests for standard and deviant blocks with respect to baseline as well as directly comparing the two block types. To use a more data-driven approach, we also performed a cluster-based permutation test (Maris and Oostenveld, 2007) comparing the two conditions. This analysis allows us to confirm the t-test results and to define regions of interest (ROIs) in a non-arbitrary, data-driven, yet anatomically informed fashion. Such an analysis has been successfully used in the past with infant NIRS data in a number of studies (Ferry et al., 2015, Mahmoudzadeh et al., 2013), and offers an attractive alternative to an ad hoc selection of ROIs when no prior studies are available to guide ROI selection, which is our case. As an additional advantage, the permutation analysis avoids the problem of losing statistical power due to an overly strict correction for multiple comparisons, which often plagues infant NIRS data.
The general idea behind permutation-based statistical testing is that if an effect observed in the data is real and not spurious, then it should disappear once the data is shuffled, i.e. permuted, randomly a large number of times. If among the datasets generated by random permutations, we only obtain the empirically observed pattern of results a small number of times (e.g. in fewer than 5% of the permuted datasets, corresponding to the conventional significance level of alpha = 0.05), then we can be confident that we did not observe it spuriously. If, by contrast, it appears in more than 5% of the permuted datasets, then it is most probably a spurious pattern, and not a significant effect. We used cluster-based permutation tests, which take into account spatial and/or temporal adjacency, clustering together samples that show a significant effect, if they are adjacent in space or time. Specifically, to conduct the permutation test, we first ran paired-sample t-tests between the standard and deviant conditions for each pair of data points within the time series of the hemodynamic response in each channel. Then all temporally and spatially adjacent data points with a t-value greater than a standard threshold (t = 2, which corresponds to the conventional alpha = 0.05 p value and was chosen on the basis of the existing literature, Maris and Oostenveld, 2007, Ferry et al., 2015) were grouped together into cluster candidates. Two pairs of samples were considered temporally adjacent if they were consecutive and spatially adjacent if the two channels in which they appeared were in the same anatomical area as defined by the localization analysis in Abboub et al. (2016), and were at a distance of 3 cm from one another in our headgear. Once cluster candidates were formed, we calculated a t-value for each of them, i.e. a cluster-level t-value, by summing the t-values of every data point included in the cluster candidate. We then identified the cluster candidate with the largest t-value for each hemisphere. We then needed to test whether the t-value of this winning cluster candidate is significantly different from chance. This was done using random permutations of the dataset: the data was randomly labeled as belonging to one or the other experimental condition. The same t-test statistic as before was computed for all pairs of data points in each random permutation. Cluster candidates were then formed as before. The proportion of random permutations that produce a cluster-level statistic greater than the actually observed one provides the p value of the test. In all, 1000 permutations under the null hypothesis were conducted.
To confirm and generalize the patterns observed in the channel-wise analyses, we also ran an omnibus within-subject ANOVA with factors Block Type (Standard/Deviant) and Hemisphere (LH/RH) over a region of interest (ROI) comprising channels 3, 6, 8, 9 and 11 in the LH and channels 17, 19, 21, 22 and 24 in the RH. These ROIs were chosen on the basis of the permutation test results (see also below).
In order to test for learning and neural habituation effects, we also analyzed the time course of the responses. Indeed, previous work (Benavides-Varela et al., 2012, Bouchon et al., 2015, Gervain et al., 2008a) indicates that the perceived variability vs. redundancy in the stimuli leads to observable habituation and dishabituation (repetition suppression and enhancement) effects. This is particularly relevant for our experimental design, as it measures the detection of a deviant stimulus after a series of repetitions of a standard one. Since our paradigm involves identical repetitions of the same stimuli, we will be using the term ‘repetition suppression’ synonymously with the more general term ‘neural habituation’. If infants cannot discriminate between the deviant and the standard, then they perceive both block types as consisting of identical repetitions of the same stimuli, and we would therefore observe strong repetition suppression or habituation effects. To capture any such effects, we conducted a within-subject ANOVA with factors Time (first 2 blocks/last 2 blocks), Block Type (Standard/Deviant) and Hemisphere (LH/RH). The first two blocks are blocks 1–2, the last two blocks are blocks 23–24.
All analyses were conducted with both oxyHb and deoxyHb.
2.2. Results
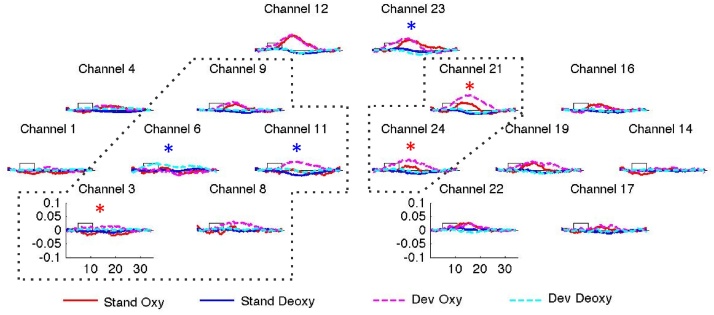
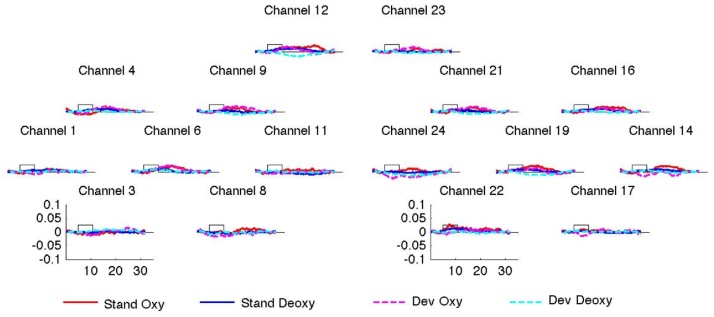
The grand average results of Experiment 1 are shown in Fig. 3 averaged together for all blocks belong to the same type (standard or deviant) across all infants.
Fig. 3.
The grand average results of Experiment 1. The x axis represents time in sec. The y axis shows concentration change in mmol × mm. The curves indicate grand average responses for standard (oxyHb: continuous red line, deoxyHb: continuous blue line) and deviant blocks (oxyHb: dashed pink line, deoxyHb: dashed turquoise line). The rectangle along the x axis indicates time of stimulation. Asterisks indicate channels with a statistically significant advantage for the deviant over the standard blocks in oxyHb (red asterisks) and deoxyHb (blue asterisks) concentration change. The ROIs obtained through the permutation test in the left hemisphere (LH) and the right hemisphere (RH) respectively are encircled using dotted lines. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
The results of the channel-by-channel t-tests are reported in Table 1. The permutation analysis (Fig. 3) yielded a statistically significant cluster comprising channels 3, 6, 8, 9 and 11 in the LH (p = 0.01) and channels 21 and 24 in the RH (p = 0.014), converging with the results of the t-tests comparing the two block types. In both clusters, activation was stronger in response to the deviant than to the standard blocks. We took the cluster for the LH as the basis for defining the ROIs for the subsequent ANOVAs. Thus the ROI in the LH corresponded exactly to the cluster, and for the sake as symmetry, for the RH we took the corresponding channels, i.e. a ROI including, but larger than the significant cluster for the RH. It would have also been possible to use ROIs strictly following the significant clusters, but such asymmetrical analyses are not standardly used in NIRS methodology.1
Table 1.
Summary of the channel-by-channel comparisons in Experiment 1. Asterisks mark channels in which the cluster-based permutation analysis yielded an advantage for deviant blocks over standard blocks.
| comparison | significant channels | t-value | p-value (uncorrected) |
|---|---|---|---|
| standard vs. baseline (oxyHb: st > bl) |
channel 12 | t(19) = 2.76 | p = 0.0143 |
| standard vs. baseline (deoxyHb: st < bl) | channel 11 | t(19) = 2.689 | p = 0.0149 |
| deviant vs. baseline (oxyHb: dev > bl) |
channel 8 | t(19) = 2.34 | p = 0.031 |
| channel 12 | t(19) = 2.75 | p = 0.015 | |
| channel 19 | t(19) = 2.16 | p = 0.044 | |
| channel 21 | t(19) = 4.15 | p = 0.0005 | |
| channel 23 | t(19) = 2.59 | p = 0.021 | |
| channel 24 | t(19) = 2.22 | p = 0.040 | |
| deviant vs. baseline (deoxyHb: dev < bl) |
channel 6 | t(19) = 2.27 | p = 0.038 |
| channel 11 | t(19) = 2.17 | p = 0.044 | |
| channel 23 | t(19) = 2.15 | p = 0.047 | |
| standard vs. deviant (oxyHb: dev > st) |
channel 3* | t(19) = 2.24 | p = 0.037 |
| channel 21* | t(19) = 2.73 | p = 0.013 | |
| channel 24* | t(19) = 2.22 | p = 0.040 | |
| standard vs. deviant (deoxyHb: dev < st) | channel 6 | t(19) = 2.27 | p = 0.038 |
| channel 11 | t(19) = 2.17 | p = 0.044 | |
| channel 23 | t(19) = 2.15 | p = 0.047 |
The ANOVA with the within-subject factors Block Type (Standard/Deviant) and Hemisphere (LH/RH) over oxyHb concentration changes yielded a main effect of Block Type (F(1,19) = 5.187, p = 0.0345), as deviant blocks evoked a stronger response than standard blocks. The same ANOVA over deoxyHb concentration changes yielded a significant Block Type X Hemisphere interaction (F(1,19) = 4.99, p = 0.0377) due to a greater, i.e. more negative deoxy response to the Deviant blocks in the RH than in the LH (Scheffe post hoc test p = 0.008), as well as to a smaller deoxy response to the Deviant than to the Standard blocks in the LH (Scheffe post hoc test p = 0.003).
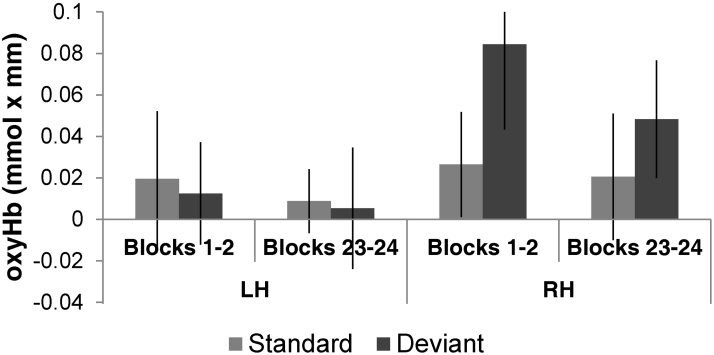
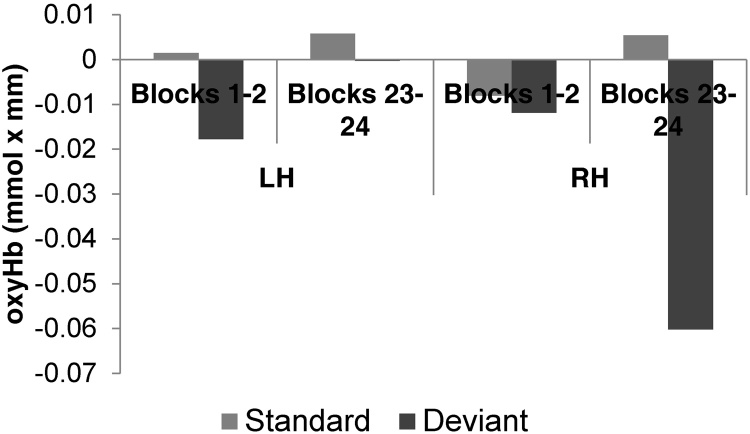
Testing for potential habituation effects during the time course of the experiment, the ANOVA with Time (first 2 blocks/last 2 blocks), Block Type (Standard/Deviant) and Hemisphere (LH/RH) over oxyHb (Fig. 4) and deoxy concentration changes yielded no significant effects or interactions, despite the numerical differences between conditions (Fig. 4). This is most probably due to the large variability in the dataset, as here we only average together two blocks per condition.
Fig. 4.
The average responses to standard (light grey) and deviant (dark grey) blocks in the LH and RH in the first two (blocks 1–2) and the last two (blocks 23–24) blocks of Experiment 1, illustrating potential habituation effects throughout the time course of the experiment.
2.3. Discussion
We have consistently observed greater activation to the deviant than to the standard blocks in all of the analyses. Comparisons with respect to baseline show that more channels responded to the deviant than to the standard blocks. Direct comparisons of the two block types further confirm an advantage for deviant over the standard blocks both in the channel-wise comparisons and in the ANOVAs. This advantage for the deviant blocks, violating newborns’ prior expectation regarding word order, is in line with a mismatch, surprise or dishabituation response, which is typically greater in amplitude than the response to the standard or repeated stimuli. The advantage was observed in both hemispheres, although the channels showing it were not fully analogous. In the LH, more of the inferior frontal and superior temporal channels activated, whereas in the RH, the activation was rather localized in the supramarginal and superior temporal channels, a subset of the channels involved in the LH. The activation observed in the LH confirmed our predictions, as it was localized in previously identified, classical language areas (Friederici 2005), in which even newborns have been shown to process information about the sequential order of linguistic stimuli (Gervain et al., 2008a, Gervain et al., 2011). The greater involvement of the LH thus meshes well with previous results. Since no previous study investigated the neural correlates of word order violations in newborns or young infants, the activation observed in the RH is less straightforward to interpret. One possibility is that sequence and order learning is more bilateral at birth than later in life, as is the case for the discrimination of certain phonological contrasts (e.g. phonemes, pitch accent etc.; Sato et al., 2009, Minagawa-Kawai et al., 2011). Further research is needed to clarify the exact function of the observed response in the RH.
We note that there were both overlaps and differences in the channels showing a significant oxyHb and a significant deoxyHb response. We interpret either as a signature of neural activation. Ideally, a channel should show significant changes in both hemoglobin species. However, infant data is often noisy and highly variable, resulting in a non-significant result for one hemoglobin species even when the other is significant. This is pattern of results is not uncommon in developmental studies (Gervain et al., 2011, Lloyd-Fox et al., 2010).
Taken together, these results provide firm evidence that newborns are able to track the order of words in linguistic sequences, and can detect a violation, if this order is changed. Furthermore, in the LH, this detection ability is localized in the classical language areas, most probably involving Broca’s area, as predicted.
In the current experiment, words within a sequence were recorded separately. Therefore, sequences did not form a single (utterance-like) prosodic unit, i.e. an intonational phrase (Nespor and Vogel, 1986). The order of the individual words was the only information newborns needed to process and learn at the level of the entire sequence to notice the violation. While this design establishes newborns’ ability to track the sequential order of words, it differs greatly from infants’ real experience with speech, whereby sequences of multiple words constitute not only larger grammatical, but also prosodic units. Indeed, newborns have sophisticated abilities processing different aspects of speech prosody, including universal abilities to process and discriminate prosodic features they have never heard before (e.g. Nazzi et al., 1998a, Ramus et al., 2000), as well as evidence of prenatal learning about the prosody of the language heard in utero (e.g. Mampe et al., 2009, Moon et al., 1993).
In Experiment 2, we therefore sought to explore how newborns process word order when words are embedded in a well-formed intonational phrase characteristic of their native language. As outlined in the Introduction, one possibility is that prosody helps newborns track word order, as was found in 2-month-olds (Mandel et al., 1996), who readily detected word order violations within a single utterance-level prosodic unit, but not across a prosodic boundary. Alternatively, newborns might only focus on the well-formedness of the prosody, and fail to detect word order violations, because prosodic contours are what they are most familiar with in their native language, and/or because the well-formedness of the prosodic contour might mask or override the word order violation.
3. Experiment 2
In the current experiment, we have tested word order violations in the same 4-word sequences as before, with the only difference that now the sequences were recorded as single, well-formed intonational phrases.
3.1. Material and methods
3.1.1. Participants
Twenty-one full-term, healthy French-exposed neonates, who did not participate in Experiment 1, were tested at the maternity ward of the Robert Debré Hospital in Paris. Of these, 1 was not included in the final analysis, because she didn’t provide enough analyzable data due to movement artifacts and/or dense hair. Infants were not retained for analysis if more than 50% data was rejected in both conditions. The remaining 20 babies (10 girls, age range 14 days, mean age: 2.1 day, mean weight 3394 g, weight range: 2235–4535 g Apgar at 10 min after birth: 10 and normal otoacoustic emissions test) were included in the final analysis. All parents gave informed consent prior to participation. The study was approved by the CERES ethics committee of the Université Paris Descartes.
3.1.2. Material
The same 4-word sequences were used as in Experiment 1, except that sequences were recorded as a whole. A professional female native speaker (an actress) was instructed to pronounce the sequences with a natural declarative sentence intonation. All sequences were recorded in a similar fashion in a single session intermixed with meaningful filler sentences that served to help the speaker maintain a natural prosody. Since the sequences are not grammatical French utterances, we made sure that adult French native speakers perceived the prosody of each sequence as natural.
As we used natural recordings and not synthesized speech, the deviant sequence differed from its standard not only in word order, but also in prosody. This difference was small, as the speaker was instructed to read each sequence with the same (well-formed, declarative sentence) intonation as much as possible. Indeed, acoustic analyses of deviant and standard sequences showed no significant differences in the mean duration t(94) = .45, p = .65, maximum f0 peak t(94) = 1.58, p = .11, minimum f0 peak t(94)=.08, p = .93, mean pitch t(94) = 0.66, p = .51, pitch variability t(94) = .33, p = .74 and intensity t(94) = .46, p = .65 Additionally, as in Experiment 1, sequences that served as standards for one infant were used as deviants for another infant, thus for the participant group as a whole, specificities of the prosodic properties of individual sequences could not affect the results.
3.1.3. Procedure
The procedure was identical to the one used in Experiment 1.
3.1.4. Data analysis
The data was analyzed in the same way as in Experiment 1.
3.2. Results
The grand average results of Experiment 2 are shown in Fig. 5 averaged together for all blocks belong to the same type (standard or deviant) across all infants.
Fig. 5.
The grand average results of Experiment 2. Plotting conventions are the same as in Fig. 3.
The results of the channel-by-channel t-tests are reported in Table 2. The permutation test revealed a significant cluster consisting of channel 3 in the LH (p = 0.029), whereby activation in the deviant blocks was stronger than in the standard blocks, and no significant cluster in the RH. In the absence of significant clusters emerging from the permutation test, we defined the ROIs for the subsequent ANOVAs as in Experiment 1. Running the Block Type (standard/deviant) x Hemisphere (LH/RH) ANOVA with the results of the permutation test, i.e. with no ROI in the RH and thus no factor Hemisphere, would have reduced it to a one-way ANOVA with Block Type as the only factor over the ROI consisting of the single significant channel of the permutation test. This simply amounts to running the single channel comparison between the two conditions again. We thus preferred instead to run the ANOVA with the ROIs of Exp 1. Otherwise, no Block Type (standard/deviant) x Hemisphere (LH/RH) ANOVA would have been possible.
Table 2.
Summary of the channel-by-channel comparisons in Experiment 2.
| comparison | significant channels | t-value | p-value (uncorrected) |
|---|---|---|---|
| standard vs. baseline (oxyHb: st > bl) |
– | – | – |
| standard vs. baseline (deoxyHb: st < bl) | channel 4 | t(19) = 3.09 | p = 0.006 |
| channel 14 | t(19) = 2.69 | p = 0.014 | |
| channel 16 | t(19) = 3.01 | p = 0.007 | |
| deviant vs. baseline (oxyHb: dev > bl) |
– | – | – |
| deviant vs. baseline (deoxyHb: dev < bl) |
– | – | – |
| standard vs. deviant (oxyHb: dev > st) |
– | – | – |
| standard vs. deviant (deoxyHb: dev < st) | channel 12 | t(19) = 2.48 | p = 0.023 |
The within-subject ANOVA with factors Block Type (standard/deviant) and Hemisphere (LH/RH) over oxyHb concentration changes revealed no significant main effect or interaction. A similar ANOVA over deoxyHb concentration changes yielded a marginal Block Type X Hemisphere interaction (F(1.19) = 4.329, p = 0.0512), as the deviant blocks tended to evoke a greater decrease in deoxyHb concentrations than standard blocks in the RH only (Scheffe post hoc p = 0.009).
The ANOVA testing for neural habituation effects with Time (first 2 blocks/last 2 blocks), Block Type (Standard/Deviant) and Hemisphere (LH/RH) over oxyHb concentration changes revealed a significant main effect of Time (F(1,182) = 6.39, p = 0.013; Fig. 6), as responses were overall larger in the first 2 than in the last 2 blocks. Furthermore, the main effect of Block Type was also significant (F(1.18) = 4.88, p = 0.029), as responses were more negative, i.e. there was a greater deactivation, to Deviant as compared to Standard blocks. Additionally, the Time x Hemisphere interaction was also significant (F(1.19) = 6.20, p = 0.0145), as this deactivation from the initial to the final blocks was present in the RH (Scheffe post hoc p = 0.0006), but not in the LH. A similar ANOVA over deoxyHb concentrations yielded.no significant effects or interaction.
Fig. 6.
The average responses to standard (light grey) and deviant (dark grey) blocks in the LH and RH in the first two (blocks 1–2) and the last two (blocks 23–24) blocks of Experiment 2, showing a habituation effect.
3.3. Discussion
In the grand average results, we have observed rather weak responses to both standard and deviant blocks, and a similarly small advantage for deviant blocks in the direct comparison of the two block types. The time course analysis revealed, however, that these weak responses are not due to infants’ failure to process or encode the stimuli. Rather, they are due to a strong repetition suppression response, i.e. to neural habituation, over the course of the experiment. The hemodynamic responses decreased significantly over time.
We argue that this decreasing response is indeed a repetition suppression or, more generally, a habituation effect, which arises as a result of repeated presentations of what the newborn brain perceives as identical stimuli. The change in word order is not detected, and while the prosodic contours of the different sequences are slightly different, they are all well-formed declarative utterances. The repeated presentation of these similar prosodic contours is what gives rise to the suppression effect. Indeed, as discussed earlier, repeated stimuli have been shown to produce such an effect in the newborn NIRS response (Bouchon et al., 2015, Nordt et al., 2016), similarly to neural habituation effects observed in EEG and MRI data in older infants and in adults (e.g. Gotts et al., 2012, Henson and Rugg, 2003). For instance, newborns’ NIRS responses have been found to decrease, showing a repetition suppression effect, when the stimuli contained several repetitions or had little variability (Bouchon et al., 2015).
The slight difference between the two block types that the time course analysis has revealed as well as its right lateralization further support this hypothesis. Deviant blocks might have evoked a stronger suppression effect simply because they constitute the 4th-6th repetition of a given sequence, as compared to the 1st-3rd repetitions, and are thus even more redundant than the standard blocks. The fact that this suppression effect is mainly observed in the right hemisphere, in the supramarginal and superior temporal areas, where language prosody has been found to be processed even in young infants in NIRS studies (Friederici, 2002, Homae et al., 2006), further implies that it is indeed the redundant repetitions of the prosodic pattern that trigger suppression.
4. General discussion and conclusion
We have conducted two NIRS studies testing whether newborn infants can detect a change in the order of words in 4-word-long nonsense sequences. We have found that sequences violating the word order of a previously presented sequence evoke a greater hemodynamic response in the left inferior frontal and superior temporal as well as in the right supramarginal and superior temporal areas, but only if the words in the sequences are presented prosodically independently, and not embedded in a full utterance-level prosodic contour.
While prosody was found to guide 2-month-olds ability to learn word order, as they readily detected a change inside a prosodic unit, but not across prosodic boundaries (Mandel et al., 1996), newborns seemed to be unable to track word order inside utterance-level prosodic units. Why is this so? Several, not mutually exclusive explanations can be proposed. First, it is possible that prenatal experience plays an important role. Speech as heard in utero is low-pass filtered by maternal tissues. As a result, individual sounds and words are largely suppressed, but prosody, both melody and rhythm, are preserved. As a result, newborns have considerable experience with prosody, showing evidence both in perception (Moon et al., 1993, Moon et al., 2013) and in the production of their communicative cries (Mampe et al., 2009) that they are familiar with at least some properties of their native prosody. It might thus be the case that recognizing these familiar prosodic patterns is what their speech perception system focuses on, allowing newborns to zoom in on the sounds in their environment that constitute the most appropriate input for language acquisition. In this respect, it will be interesting to test in the future whether newborns can track word order within ill-formed and hence unfamiliar prosodic contours or inside prosodic contours from unfamiliar languages. Such experiments will allow us to assess how prenatal experience lays the foundations of subsequent language development. Second, it might be argued that prosody and word order together provide too much information for newborns to handle. Simultaneously processing two cues, at two different linguistic levels may overload newborns’ language processing or related cognitive (e.g. memory etc.) abilities. While we know that newborns can identify acoustic cues that signal at least some word boundaries (Christophe et al., 1994), it may still be hard for them to locate all of the word boundaries within a larger prosodic unit, e.g. because co-articulation or suprasegmental phonological phenomena might mask some of them. There is indeed a difference in this respect between the stimuli used in our two experiments. While the words in Experiment 1 were not concatenated with pauses longer than those naturally appearing in the stimuli of Experiment 2, they definitely lacked co-articulation between words, which might have made it easier for infants to identify the individual words and hence to track their relative order. This could have further facilitated it for infants to individuate the prosodically shorter, weaker, more minimal functors from the more prominent content words (Shi et al., 2006), and use this as an additional cue to track the relative orders of these two categories of words. The predictions of this explanation will also need to be tested in future research manipulating the segmentation cues available to infants in the stimuli.
Irrespectively of the role of prosody, these are the first results that establish the ability to track word order in newborn infants. Furthermore, they suggest that classical language areas such as the left inferior frontal and superior temporal regions are involved. These results mesh well with previous findings showing that the neural specialization for speech and language processing is to a large extent already in place very early in development (e.g. Dehaene-Lambertz et al., 2002, Mahmoudzadeh et al., 2013, Pena et al., 2003). They are also compatible with recent studies suggesting that the ability to track order of elements in speech (i.e. syllables), although present in young infants, might be constrained by cognitive biases (Benavides-Varela and Mehler 2015) and modulated by subtle prosodic cues (Ferry et al., 2015).
In sum, we have found evidence that a precursor of the ability to learn the sequential order of words is already present at birth, paving the ground for language acquisition.
Conflict of interest
None.
Acknowledgements
This work was supported by an Emergence(s) grant from the City of Paris, an ANR grant nr. ANR-15-CE37-0009-01, a HFSP Young Investigator Grant nr. RGY-2014-073, the Labex EFL grant of the ANR progam “Investissements d’Avenir” (reference: ANR-10-LABX-0083), as well as the Marie Curie ITN grant “PredictAble” to JG. We thank Maria Clemencia Ortiz Barajas for her help with the permutation analysis. We are grateful to the personnel of the maternity of the Robert Debré Hospital, Paris, for their support and help with recruitment and testing.
Footnotes
We have selected the larger ROI, found in the LH as the basis for this symmetrical analysis. It would have also been possible to use the smaller ROI, found in the RH. This analysis had indeed been performed, and found to give a similar of pattern of results as the analysis with the larger ROI. We have opted to use the larger ROI as it corresponds more closely to the ROIs used in previous NIRS studies investigating speech and language processing in young infants.
One infant did not have sufficient non-rejected data in blocks 1, 2, 23 and 24 and did not therefore contribute to this analysis.
References
- Abboub N., Nazzi T., Gervain J. Prosodic grouping at birth. Brain Lang. 2016;162:46–59. doi: 10.1016/j.bandl.2016.08.002. [DOI] [PubMed] [Google Scholar]
- Akhtar N. Acquiring basic word order: evidence for data-driven learning of syntactic structure. J. Child Lang. 1999;26(02):339–356. doi: 10.1017/s030500099900375x. [DOI] [PubMed] [Google Scholar]
- Benavides-Varela S., Mehler J. Verbal positional memory in 7-month-Olds. Child Dev. 2015;86(1):209–223. doi: 10.1111/cdev.12291. [DOI] [PubMed] [Google Scholar]
- Benavides-Varela S., Hochmann J.-R., Macagno F., Nespor M., Mehler J. Newborn’s brain activity signals the origin of word memories. Proc. Natl. Acad. Sci. 2012;109(44):17908–17913. doi: 10.1073/pnas.1205413109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard C., Gervain J. Prosodic cues to word order: what level of representation? Front. Lang. Sci. 2012;3:451. doi: 10.3389/fpsyg.2012.00451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchon C., Nazzi T., Gervain J. Hemispheric asymmetries in repetition enhancement and suppression effects in the newborn brain. PLoS One. 2015;10(10):e0140160. doi: 10.1371/journal.pone.0140160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braine M.D. On learning the grammatical order of words. Psychol. Rev. 1963;70(4):323–348. doi: 10.1037/h0047696. [DOI] [PubMed] [Google Scholar]
- Brown R. Free Press; New York, NY: 1970. Psycholinguistics: Selected Papers by Roger Brown. [Google Scholar]
- Brown R.W. Harvard University Press; Cambridge, Mass: 1973. A First Language; The Early Stages. [Google Scholar]
- Christophe A., Dupoux E., Bertoncini J., Mehler J. Do infants perceive word boundaries? An empirical study of the bootstrapping of lexical acquisition. J. Acoust. Soc. Am. 1994;95(3):1570–1580. doi: 10.1121/1.408544. [DOI] [PubMed] [Google Scholar]
- Christophe A., Nespor M., Guasti M.T., Van Ooyen B. Prosodic structure and syntactic acquisition: the case of the head-direction parameter. Dev. Sci. 2003;6(2):211–220. [Google Scholar]
- Clahsen H., Muysken P. The availability of universal grammar to adult and child learners-a study of the acquisition of German word order. Second Lang. Res. 1986;2(2):93–119. [Google Scholar]
- Cooper R.P., Aslin R.N. Preference for infant-directed speech in the first month after birth. Child Dev. 1990;61(5):1584–1595. [PubMed] [Google Scholar]
- Cristia A., Minagawa-Kawai Y., Egorova N., Gervain J., Filippin L., Cabrol D., Dupoux E. Neural correlates of infant accent discrimination: an fNIRS study. Dev. Sci. 2014;7(4):628–635. doi: 10.1111/desc.12160. [DOI] [PubMed] [Google Scholar]
- Dehaene-Lambertz G., Dehaene S., Hertz-Pannier L. Functional neuroimaging of speech perception in infants. Science. 2002;298(5600):2013–2015. doi: 10.1126/science.1077066. [DOI] [PubMed] [Google Scholar]
- Dehaene-Lambertz G. Cerebral specialization for speech and non-speech stimuli in infants. J. Cogn. Neurosci. 2000;12(3):449–460. doi: 10.1162/089892900562264. [DOI] [PubMed] [Google Scholar]
- Ferry A.L., Fló A., Brusini P., Cattarossi L., Macagno F., Nespor M., Mehler J. On the edge of language acquisition: inherent constraints on encoding multisyllabic sequences in the neonate brain. Dev. Sci. 2015;19(3):488–503. doi: 10.1111/desc.12323. [DOI] [PubMed] [Google Scholar]
- Friederici A.D. Towards a neural basis of auditory sentence processing. Trends Cogn. Sci. 2002;6(2):78–84. doi: 10.1016/s1364-6613(00)01839-8. [DOI] [PubMed] [Google Scholar]
- Friederici A.D. Neurophysiological markers of early language acquisition: from syllables to sentences. Trends Cogn. Sci. 2005;9(10):481–488. doi: 10.1016/j.tics.2005.08.008. [DOI] [PubMed] [Google Scholar]
- Friederici A.D. The brain basis of language processing: from structure to function. Physiol. Rev. 2011;91(4):1357–1392. doi: 10.1152/physrev.00006.2011. [DOI] [PubMed] [Google Scholar]
- Friederici A.D. The cortical language circuit: from auditory perception to sentence comprehension. Trends Cogn. Sci. 2012 doi: 10.1016/j.tics.2012.04.001. [DOI] [PubMed] [Google Scholar]
- Gervain J., Werker J.F. Prosody cues word order in 7-month-old bilingual infants. Nat. Commun. 2013;4:1490. doi: 10.1038/ncomms2430. [DOI] [PubMed] [Google Scholar]
- Gervain J., Macagno F., Cogoi S., Pena M., Mehler J. The neonate brain detects speech structure. Proc. Natl. Acad. Sci. U. S. A. 2008;105(37):14222–14227. doi: 10.1073/pnas.0806530105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gervain J., Nespor M., Mazuka R., Horie R., Mehler J. Bootstrapping word order in prelexical infants: a Japanese-Italian cross-linguistic study. Cognit. Psychol. 2008;57(1):56–74. doi: 10.1016/j.cogpsych.2007.12.001. [DOI] [PubMed] [Google Scholar]
- Gervain J., Mehler J., Werker J.F., Nelson C.A., Csibra G., Lloyd-Fox S., Aslin R.N. Near-infrared spectroscopy: a report from the McDonnell infant methodology consortium. Dev. Cogn. Neurosci. 2011;1(1):22–46. doi: 10.1016/j.dcn.2010.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez R.L., Gerken L. Infant artificial language learning and language acquisition. Trends Cogn. Sci. 2000;4(5):178–186. doi: 10.1016/s1364-6613(00)01467-4. [DOI] [PubMed] [Google Scholar]
- Gotts S.J., Chow C.C., Martin A. Repetition priming and repetition suppression: a case for enhanced efficiency through neural synchronization. Cogn. Neurosci. 2012;3(3–4):227–237. doi: 10.1080/17588928.2012.670617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guasti M.T. MIT Press; Cambridge, Mass: 2002. Language Acquisition: The Growth of Grammar. [Google Scholar]
- Höhle B., Weissenborn J., Schmitz M., Ischebeck A. Approaches to Bootstrapping: Phonological, Lexical, Syntactic and Neurophysiological Aspects of Early Language Acquisition. John Benjamins; Amsterdam: 2001. Discovering word order regularities: the role of prosodic information for early parameter setting. [Google Scholar]
- Hakuta K. Interaction between particles and word order in the comprehension and production of simple sentences in Japanese children. Dev. Psychol. 1982;18(1):62. [Google Scholar]
- Henson R.N.A., Rugg M.D. Neural response suppression, haemodynamic repetition effects, and behavioural priming. Neuropsychologia. 2003;41(3):263–270. doi: 10.1016/s0028-3932(02)00159-8. [DOI] [PubMed] [Google Scholar]
- Homae F., Watanabe H., Nakano T., Asakawa K., Taga G. The right hemisphere of sleeping infant perceives sentential prosody. Neurosci. Res. 2006;54(4):276–280. doi: 10.1016/j.neures.2005.12.006. [DOI] [PubMed] [Google Scholar]
- Jasinska K., Petitto L.A. How age of bilingual exposure can change the neural systems for language in the developing brain: a functional near infrared spectroscopy investigation of syntactic processing in monolingual and bilingual children. Dev. Cogn. Neurosci. 2013;6:87–101. doi: 10.1016/j.dcn.2013.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jusczyk P.W., Cutler A., Redanz N.J. Infants’ preference for the predominant stress patterns of English words. Child Dev. 1993;64:675–687. [PubMed] [Google Scholar]
- Lloyd-Fox S., Blasi A., Elwell C. Illuminating the developing brain: the past, present and future of functional near infrared spectroscopy. Neurosci. Biobehav. Rev. 2010;24(3):269–284. doi: 10.1016/j.neubiorev.2009.07.008. [DOI] [PubMed] [Google Scholar]
- MacWhinney B., Pléh C., Bates E. The development of sentence interpretation in Hungarian. Cognit. Psychol. 1985;17(2):178–209. [Google Scholar]
- MacWhinney B. 3rd. Lawrence Erlbaum; Mahwah, NJ: 2000. (The CHILDES Project: Tools for Analyzing Talk). [Google Scholar]
- Mahmoudzadeh M., Dehaene-Lambertz G., Fournier M., Kongolo G., Goudjil S., Dubois J., Wallois F. Syllabic discrimination in premature human infants prior to complete formation of cortical layers. Proc. Natl. Acad. Sci. 2013;110(12):4846–4851. doi: 10.1073/pnas.1212220110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mampe B., Friederici A.D., Christophe A., Wermke K.1. Newborns’ cry melody is shaped by their native language. Curr. Biol. 2009;19(23):1994–1997. doi: 10.1016/j.cub.2009.09.064. [DOI] [PubMed] [Google Scholar]
- Mandel D.R., KemlerNelson D.G., Jusczyk P.W. Infants remember the order of words in a spoken sentence. Cogn. Dev. 1996;11:181–196. [Google Scholar]
- Marcus G.F., Vijayan S., Rao S.B., Vishton P.M. Rule learning by seven-month-old infants. Science. 1999;283(5398):77–80. doi: 10.1126/science.283.5398.77. [DOI] [PubMed] [Google Scholar]
- Maris E., Oostenveld R. Nonparametric statistical testing of EEG-and MEG-data. J. Neurosci. Methods. 2007;164(1):177–190. doi: 10.1016/j.jneumeth.2007.03.024. [DOI] [PubMed] [Google Scholar]
- May L., Byers-Heinlein K., Gervain J., Werker J.F. Language and the newborn brain: does prenatal language experience shape the neonate neural response to speech? Front. Lang. Sci. 2011;2:222. doi: 10.3389/fpsyg.2011.00222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meisel J.M. Word order and case marking in early child language. Evidence from simultaneous acquisition of two first languages: French and German. Linguistics. 1986;24(1):123–184. [Google Scholar]
- Minagawa-Kawai Y., Cristia A., Dupoux E. Cerebral lateralization and early speech acquisition: a developmental scenario. Dev. Cogn. Neurosci. 2011 doi: 10.1016/j.dcn.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon C., Cooper R.P., Fifer W.P. Two-day-olds prefer their native language. Infant Behav. Dev. 1993;16(4):495–500. [Google Scholar]
- Moon C., Lagercrantz H., Kuhl P.K. Language experienced in utero affects vowel perception after birth: a two-country study. Acta Paediatr. 2013;102(2):156–160. doi: 10.1111/apa.12098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan J.L., Demuth K. Lawrence Erlbaum Associates, Inc.; Hillsdale, NJ, England: 1996. Signal to Syntax: Bootstrapping from Speech to Grammar in Early Acquisition. [Google Scholar]
- Musso M., Moro A., Glauche V., Rijntjes M., Reichenbach J., Buchel C., Weiller C. Broca’s area and the language instinct. Nat. Neurosci. 2003;6(7):774–781. doi: 10.1038/nn1077. [DOI] [PubMed] [Google Scholar]
- Nazzi T., Bertoncini J., Mehler J. Language discrimination by newborns: toward an understanding of the role of rhythm. J. Exp. Psychol. Hum. Percept. Perform. 1998;24(3):756–766. doi: 10.1037//0096-1523.24.3.756. [DOI] [PubMed] [Google Scholar]
- Nazzi T., Floccia C., Bertoncini J. Discrimination of pitch contours by neonates. Infant Behav. Dev. 1998;21(4):779–784. [Google Scholar]
- Nespor M., Vogel I. Vol. 28. Foris; Dordrecht, Holland; Riverton, N.J., U.S.A: 1986. (Prosodic Phonology). [Google Scholar]
- Nordt M., Hoehl S., Weigelt S. The use of repetition suppression paradigms in developmental cognitive neuroscience. Cortex. 2016 doi: 10.1016/j.cortex.2016.04.002. [DOI] [PubMed] [Google Scholar]
- Pena M., Maki A., Kovacic D., Dehaene-Lambertz G., Koizumi H., Bouquet F., Mehler J. Sounds and silence: an optical topography study of language recognition at birth. PNAS. 2003;100(20):11702–11705. doi: 10.1073/pnas.1934290100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinker S. Vol. 7. Harvard University Press; Cambridge, Mass: 1984. (Language Learnability and Language Development). [Google Scholar]
- Ramus F., Hauser M.D., Miller C., Morris D., Mehler J. Language discrimination by human newborns and by cotton-top tamarin monkeys. Science. 2000;288(5464):349–351. doi: 10.1126/science.288.5464.349. [DOI] [PubMed] [Google Scholar]
- Sato Y., Sogabe Y., Mazuka R. Development of hemispheric specialization for lexical pitch-accent in japanese infants. J. Cogn. Neurosci. 2009 doi: 10.1162/jocn.2009.21377. [DOI] [PubMed] [Google Scholar]
- Sato H., Hirabayashi Y., Tsubokura H., Kanai M., Ashida T., Konishi I., Uchida-Ota M., Konishi Y., Maki A. Cerebral hemodynamics in newborn infants exposed to speech sounds: a whole-head optical topography study. Hum. Brain Map. 2012;33(9):2092–2103. doi: 10.1002/hbm.21350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi R., Werker J.F. Six-month-old infants’ preference for lexical words. Psychol. Sci. 2001;12(1):71–76. doi: 10.1111/1467-9280.00312. [DOI] [PubMed] [Google Scholar]
- Shi R., Werker J.F. The basis of preference for lexical words in 6-month-old infants. Dev. Sci. 2003;6(5):484–488. [Google Scholar]
- Shi R., Werker J.F., Morgan J.L. Newborn infants’ sensitivity to perceptual cues to lexical and grammatical words. Cognition. 1999;72(2):B11–B21. doi: 10.1016/s0010-0277(99)00047-5. [DOI] [PubMed] [Google Scholar]
- Shi R., Cutler A., Werker J., Cruickshank M. Frequency and form as determinants of functor sensitivity in English-acquiring infants. J. Acoust. Soc. Am. 2006;119(6):EL61–7. doi: 10.1121/1.2198947. [DOI] [PubMed] [Google Scholar]
- Shi F., Yap P.-T., Wu G., Jia H., Gilmore J.H., Lin W., Shen D. Infant brain atlases from neonates to 1-and 2-year-olds. PLoS One. 2011;6(4):e18746. doi: 10.1371/journal.pone.0018746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi R. Functional morphemes and early language acquisition. Child Dev. Perspect. 2014;8(1):6–11. [Google Scholar]
- Shukla M., White K.S., Aslin R.N. Prosody guides the rapid mapping of auditory word forms onto visual objects in 6-mo-old infants. Proc. Natl. Acad. Sci. 2011;108(15):6038. doi: 10.1073/pnas.1017617108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slobin D.I., Bever T.G. Children use canonical sentence schemas: a crosslinguistic study of word order and inflections. Cognition. 1982;12(3):229–265. doi: 10.1016/0010-0277(82)90033-6. [DOI] [PubMed] [Google Scholar]
- Tomasello M. Do young children have adult syntactic competence? Cognition. 2000;74(3):209–253. doi: 10.1016/s0010-0277(99)00069-4. [DOI] [PubMed] [Google Scholar]
- Wagner J.B., Fox S.E., Tager-Flusberg H., Nelson C.A. Neural processing of repetition and non-repetition grammars in 7- and 9-month-old infants. Front. Psychol. 2011;2:168. doi: 10.3389/fpsyg.2011.00168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissenborn J., Höhle B., Kiefer D., Cavar D. Children’s sensitivity to word-order violations in german: evidence for very early parameter-Setting. Stringfellow A., editor. Boston University Conference on Language Development. 1996;Vol. 22 [Google Scholar]
- Woodward J.Z., Aslin R.N. Segmentation cues in maternal speech to infants. International Conference on Infant Studies; 1990. [Google Scholar]