Highlights
-
•
The error-related negativity (ERN) component indexes performance monitoring.
-
•
Review of studies on age-related differences in the ERN in children and adolescents.
-
•
Review of evidence on the neural basis of the ERN in adults.
-
•
Discussion of how developmental changes in the ERN might relate to brain maturation.
Keywords: Development, Cingulate, Error processing, Event-related potentials, Multimodal imaging, Prefrontal cortex
Abstract
To realize our goals we continuously adapt our behavior according to internal or external feedback. Errors provide an important source for such feedback and elicit a scalp electrical potential referred to as the error-related negativity (ERN), which is a useful marker for studying typical and atypical development of cognitive control mechanisms involved in performance monitoring. In this review, we survey the available studies on age-related differences in the ERN in children and adolescents. The majority of the studies show that the ERN increases in strength throughout childhood and adolescence, suggesting continued maturation of the neural systems for performance monitoring, but there are still many unresolved questions. We further review recent research in adults that has provided important insights into the neural underpinnings of the ERN and performance monitoring, implicating distributed neural systems than include the dorsal anterior and posterior cingulate cortex, the lateral prefrontal cortex, insula, basal ganglia, thalamus and white matter connections between these regions. Finally, we discuss the possible roles of structural and functional maturation of these brain regions in the development of the ERN. Overall, we argue that future work should use multimodal approaches to give a better understanding of the neurocognitive development of performance monitoring.
1. Introduction
A critical function of our cognitive system is the ability to monitor and evaluate the outcomes and consequences of behavior and adapts subsequent behavior accordingly in order to realize goals. To accomplish this, we rely on some form of feedback on our actions, and when the feedback is self-generated, we refer to this ability as “self-monitoring” (Segalowitz and Dywan, 2009). Electrophysiological methods provide important evidence about the neurocognitive systems associated with this form of performance monitoring because “action slips” – typically fast and impulsive errors, based on insufficient processing of relevant stimuli (van Veen and Carter, 2006) – are known to elicit a negative electrical potential called error-related negativity (ERN) or error negativity (Ne) (Falkenstein et al., 1991, Gehring et al., 1993). Studies indicate that the ERN increases in strength during childhood and adolescence, presumably reflecting development of cognitive control functions and assumed to be caused, in part, by the structural and functional maturation of the brain. Here, we provide a critical account and review of studies on age-related differences in the ERN in children and adolescents. We then review studies on the neural basis of the ERN, and finally discuss how the maturation of distinct neural networks might underlie developmental changes in the ERN and performance monitoring.
The ERN is a sharp response-locked event-related potential (EPR) often evoked by commission of errors in speeded response tasks, that peaks 50–100 ms following the erroneous response, and has a maximum at frontocentral midline scalp recording sites (Bush et al., 2000, Hajcak, 2012, Simons, 2010) (Fig. 1). The ERN signal is believed to lead to remedial or compensatory action such as error correction (Rodriguez-Fornells et al., 2002) or the long-known slowing down of performance immediately after an incorrect response; post-error slowing (PES) (Rabbitt, 1966). Several theories on the functional significance of the ERN have been proposed, including the conflict monitoring theory (Botvinick et al., 2001, Botvinick et al., 2004, Carter and van Veen, 2007, Yeung et al., 2004) and the reinforcement learning theory (Holroyd and Coles, 2002, Holroyd et al., 2005), suggesting that ERN reflects either the detection and processing of cognitive conflict or an evaluative function signifying “worse than expected events”, respectively. Across the diverging theoretical perspectives, there is, however, general consensus that the ERN indexes modality nonspecific cognitive control mechanisms involved in performance monitoring (Taylor et al., 2007, van Veen and Carter, 2006). Additionally, there is also evidence that the ERN is related to motivation and affect (Hajcak, 2012, Segalowitz and Dywan, 2009). In children, this seems to be the case for instance for social evaluation, as larger ERNs have been reported in children being observed by a friend compared to children performing a task alone (Kim et al., 2005).
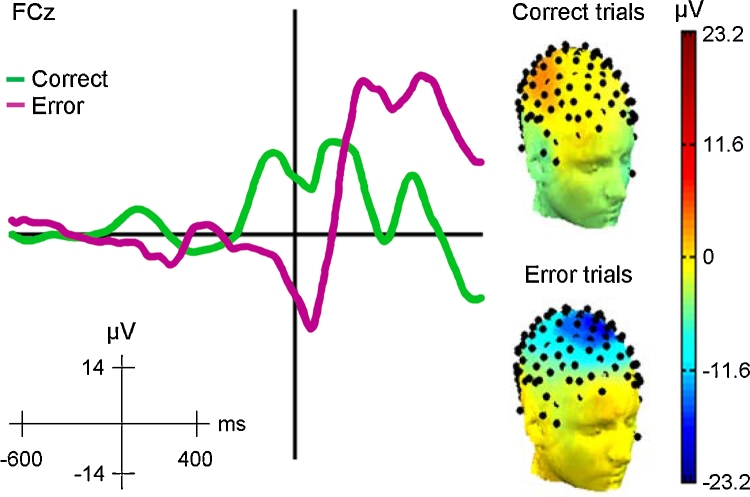
Fig. 1.
The error-related negativity. Left panel, Grand average response-locked ERPs from a speeded arrow flanker task from 76 participants 8–19 years old for correct incongruent trials (green) and error incongruent trials (purple). Right panel, 3D topographical maps of scalp potentials at 50 ms after responses for correct and error trials from a representative participant. Unpublished data.
As we will review in detail below, a growing number of studies document developmental changes in the ERN throughout childhood and adolescence, and converging evidence from a range of different methods indicate that medial frontal brain regions, particularly the dorsal (caudal) anterior cingulate cortex (ACC) and the posterior cingulate cortex (PCC), are critically involved in the generation of the ERN. Research further suggests that the ERN is substantially influenced by genetic factors and that it may be of utility as a biological marker in studies of risk for certain psychiatric disorders (Olvet and Hajcak, 2008, Ullsperger, 2010). A twin study found that 47% of the variance in the ERN amplitude in adolescents was accounted for by genetic factors (Anokhin et al., 2008), while studies making use of polymorphisms of candidate genes affecting neurotransmission point to involvement of dopamine and serotonin in particular (Biehl et al., 2011, Fallgatter et al., 2004, Holmes et al., 2010, Kramer et al., 2007, Meyer et al., 2012a, Mueller et al., 2011). Since the neural sources of the ERN are relatively well described, and several specific genetic contributions have been indicated, this electrophysiological marker of performance monitoring constitutes a very promising model system for studying gene – brain structure – brain function – behavior relationships in typical and atypical development.
The ERN is generally followed by the error positivity (Pe), a positive-going slower wave which appears after 200–400 ms with a slightly more posterior scalp distribution (Falkenstein et al., 2000, Overbeek et al., 2005). Less is known about the functional significance of the Pe, and studies indicate that it is more invariant across development than the ERN, with Pe amplitudes in childhood similar to those of adults (Davies et al., 2004b, Wiersema et al., 2007). For the sake of brevity, neither the Pe nor other possibly associated ERP components, such as the correct-related negativity (CRN) (Ford, 1999, Vidal et al., 2003), the feedback-related negativity (FRN) (Gehring and Willoughby, 2002, Miltner et al., 1997) or the stimulus-locked N2 (Wessel et al., 2012), will be discussed further in this review. Unless otherwise stated, the reviewed studies did thus not include trial-by-trial feedback.
In the first part, we provide an overview of available studies on age-related differences in the ERN in typically and atypically developing children and adolescents and discuss some important common limitations. The second part of the review deals with studies on the neural correlates of the ERN, where most available studies have been performed on adults. In the third and final part we discuss how the structural and functional maturation of specific brain regions might support developmental changes in the ERN and performance monitoring.
2. Development of the ERN
2.1. ERN development in children and adolescents
A number of cross-sectional studies have now examined age-related differences in the ERN between typically developing children, adolescents and adults (Table 1). The majority of the available studies focus on developmental changes in the ERN amplitude, while the latency, scalp distribution and general morphology appear to be much more similar across age (see Wiersema et al. (2007) for a discussion about latency differences). In the first reports on the development of the ERN, Davies et al., 2004a, Davies et al., 2004b used a standard letter flanker task in a large sample between 7 and 18 years of age and observed inconsistent ERN responses in children 7–12 years old and a steadily increasing ERN throughout adolescence (Fig. 2). Specifically, there was quadratic age relationship, indicating an initial minor drop in the ERN amplitude, with a subsequent rise in adolescence. Further, an age × sex interaction was also found, indicating an earlier adolescent increase in ERN in girls than in boys. Later work by partly the same group has replicated that children (10 years) and adolescents (15–16 years), respectively, display smaller ERNs than young adults (Santesso and Segalowitz, 2008, Santesso et al., 2006b). Development of the ERN in early adolescence has also been shown by Ladouceur et al., 2004, Ladouceur et al., 2007 using an arrow version of the flanker task. In brief, the results showed the ERN amplitude to be greater in adults and older adolescents (14–19 years) than in older children and young adolescents (9–14 years). Moreover, the ERN amplitude was related to PES in all age groups, while a relationship with task performance was found only in the adult group, suggesting protracted development of the functional significance of the ERN (Ladouceur et al., 2007). Protracted development of the ERN is also indicated by a more recent study that found the ERN to be larger in adults than in children, but no difference between younger (6–9 years) and older children (10–12 years) (van Meel et al., 2012).
Table 1.
Summary of studies on the ERN and age-related differences in typically developing children and adolescents.
| Study | Task | n | Age-range (years) | Effect of age on the ERN amplitude | Other findings |
|---|---|---|---|---|---|
| Arbel and Donchin (2011) | Letter flanker | 17 | 8–10 | NaN | ERN with typical morphology and spatiotemporal distribution in children |
| Brooker et al. (2011) | Child ANT | 15 | 4–8 | n.s. | ERN detectable in young children |
| Davies et al., 2004a, Davies et al., 2004b | Letter flanker | 124 | 7–18 | Increase with age through adolescence | Inconsistent ERN in children |
| Eppinger et al. (2009) | Probabilistic learning task | 17/18 | 10–12/19–24 | n.s. when performance levels were equated | |
| Hajcak et al. (2008) | Modified Simon task | 18 | 8–16 | Increase with age | Children and adolescents with OCD showed larger ERN, but a smaller ERN-age correlation |
| Hanna et al. (2012) | Arrow flanker | 44 | 10–18 | Increase with age | Children and adolescents with OCD showed larger ERN, but no correlation between ERN and age |
| Hogan et al. (2005) | 2-/4-Choice response task | 23 | 12–22 | Increase with age for complex task condition | No age effect for simpler conditions |
| Kim et al. (2007) | Go/No-go | 5/4/13 | 7–8/9–11/21–25 | Young children < old children | No difference between either child group and adults |
| Ladouceur et al. (2004) | Arrow flanker | 5/6 | 9–14/14–17 | Young adolescents < old adolescents | Larger ERN in trials with PES in both groups |
| Ladouceur et al. (2012) | Arrow flanker | 14 | 7–15 | Increase with age | Children and adolescents with depression showed smaller ERN and no correlation between ERN and age |
| Ladouceur et al. (2007) | Arrow flanker | 15/15/16 | 9–14/14–19/19–50 | Young adolescents < old adolescents/adults | Similar source models for all three groups |
| Meyer et al. (2012b) | Arrow flanker | 55 | 8–13 | Marginal increase with age in late childhood | Age moderated the relationship between ERN and anxiety |
| Richardson et al. (2011) | Modified flanker | 36/41 | 7/9 | n.s. | Larger ERN associated with lower intraindividual variability in RT |
| Santesso and Segalowitz (2008) | Letter flanker/Go/No-go | 35/39 | 15–16/18–20 | Adolescents < adults | Similar source models for both groups for both tasks |
| Santesso et al. (2006b) | Letter flanker | 39/29 | 10/18–30 | Children < adults | |
| Torpey et al. (2012) | Go/No-go | 328 | 5–7 | Increase with age in young children (ΔERN) | Larger ΔERN associated with higher task accuracy |
| Torpey et al. (2009) | Go/No-go | 18 | 5–7 | NaN | ERN detectable in young children |
| van Meel et al. (2012) | Modified arrow flanker | 23/24/16 | 6–9/10–12/18–26 | Young children/old children < adults | |
| Wiersema et al. (2007) | Go/No-go | 13/13/13 | 7–8/13–14/23–24 | Children < adolescents/adults |
Increase: more negative amplitude. ΔERN: the difference between error and correct trials.
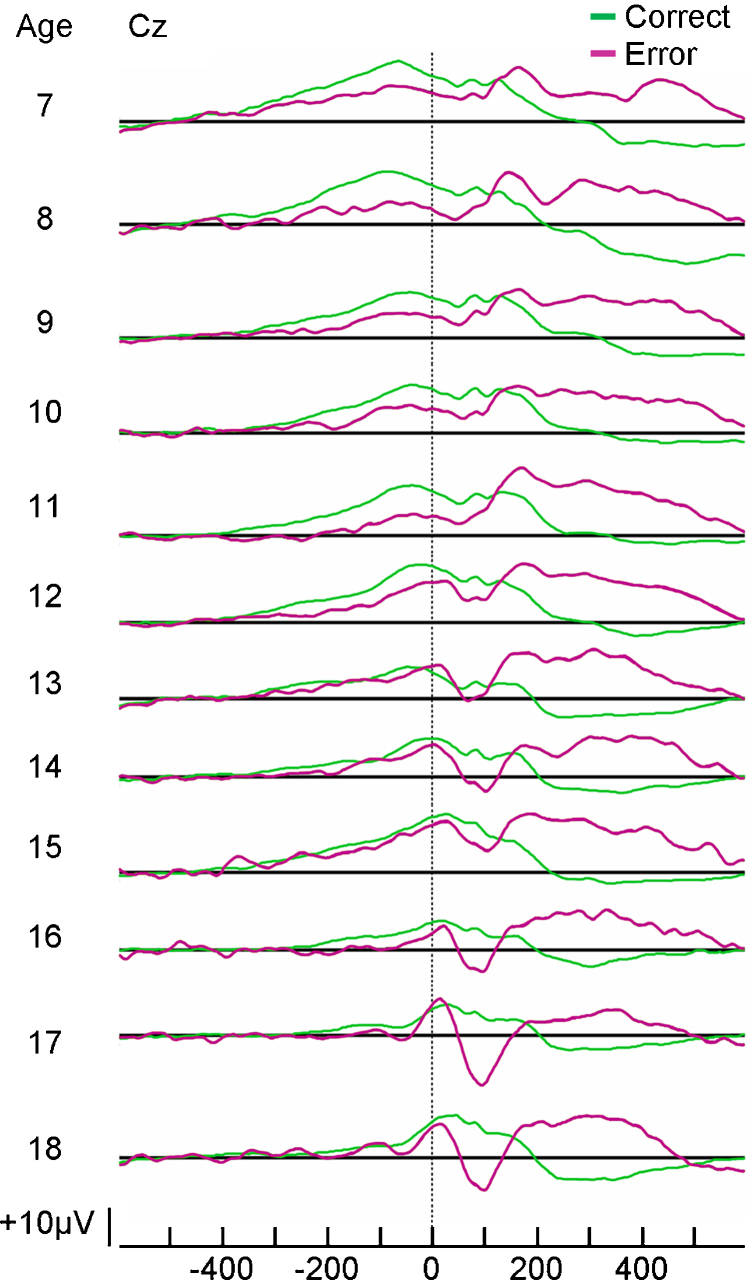
Fig. 2.
Developmental changes in the ERN. Grand average response-locked ERPs across age for correct trials (green) and error trials (purple) based on a sample of 124 participants 7–18 years old.
Source: Adapted with permission from Davies et al. (2004b).
Age-related increases in the ERN do however seem to depend on task complexity. In an important study by Hogan et al. (2005) with participants 12–22 years, a correlation between age and the ERN amplitude was found only for a more complex choice response task condition, and not for simpler conditions. This might indicate that the neural networks underpinning the ERN are present and functional in early adolescence, but appear less mature when challenged by increasing task difficulty. The conclusion that the developmental trajectory of the ERN amplitude relies upon on task complexity is also indirectly supported by two studies that employed a different paradigm: the Go/No-go task. Both studies found reduced ERN amplitudes only for groups of children 7–8 years, while adult-like ERNs were observed already in the early teens (Kim et al., 2007, Wiersema et al., 2007) (but see Santesso and Segalowitz (2008) that also used a Go/No-go task). Further studies directly comparing age-effects across task paradigms and difficulty conditions are needed.
Taken together, these studies indicate that the ERN generally increases with age from childhood and throughout adolescence (i.e. more negative amplitude with higher age), suggesting continued maturation of the neural systems for performance monitoring. It should however also be noted that other studies have found no (Eppinger et al., 2009, Richardson et al., 2011) or only marginally significant (Meyer et al., 2012b) effects of age on the ERN. Furthermore, the age at which the ERN amplitude reaches an adult level remains unclear and the disparate findings across studies likely depend upon multiple factors, including task complexity. Direct comparisons across studies are however difficult due to differences in recording systems, processing and analysis. This includes different ways of defining the ERN, e.g. as simple peak amplitude, the difference between error and correct trials (ΔERN), peak-to-peak difference within error trials or area measures, as well as the use of different time-windows for baseline correction and after responses.
Age-related changes in the ERN occur in the context of marked changes in task performance, with children generally having higher error rates, longer response times and larger intraindividual variability (Tamnes et al., 2012). Lower error rates have been shown to correlate with stronger ERN amplitudes in both adults and children (Torpey et al., 2012, Westlye et al., 2009), and apparent developmental increases in the ERN could thus be a product of the decreasing error rate itself in that the subjective significance of each error may be greater when few errors are made. However, several of the above reviewed studies also controlled for the number of included error trials, and still found significant age-related differences in the ERN amplitude (Davies et al., 2004b, Wiersema et al., 2007). There are also several other interpretive difficulties that to lesser degrees have been addressed. Development may for instance alter the cognitive strategies used, the affective response the participant has to the task, the degree of latency jitter or the signal-to-noise ratio, all of which could in principle contribute to apparent age-related differences in the ERN (Segalowitz et al., 2010). Vigilance concerning these dilemmas in analysis and interpretation in future studies is warranted.
2.2. ERN in younger children
More recent studies indicate that by using tasks that have been developed or modified for age appropriateness rather than applying typical adult task, the ERN can in fact also be observed in even younger children (Brooker et al., 2011, Torpey et al., 2009, Torpey et al., 2012) (Fig. 3). Torpey et al. (2009) demonstrated that erroneous responses elicited the ERN in children aged 5–7 years old; however, the ERN amplitude was not moderated by trial value as expected based on studies on adults. Using the child version of the Attention Network Test (ANT), Brooker et al. (2011) found that the ERN was discernible in children 4–8 years old, although the amplitude was somewhat smaller than typically seen in adults. Age did however not predict the ERN difference score within this age-range.
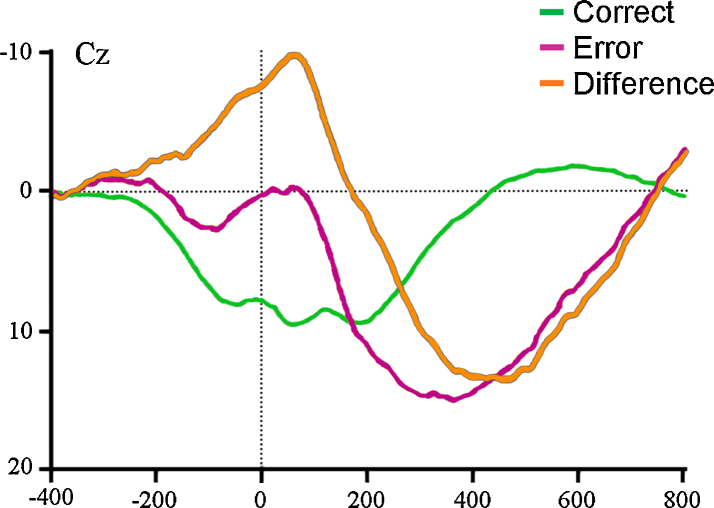
Fig. 3.
ERN in younger children. Grand average response-locked ERPs from 328 children 5–7 years old for correct trials (green), error trials (purple), and the error minus correct difference waveform (orange). Negative is plotted upward.
Source: Adapted with permission from Torpey et al. (2012).
Recently, Torpey et al. (2012) published the to date decidedly largest ERN study on typically developing children which included 328 participants 5–7 years old. As in their smaller earlier study with the same Go/No-go task, the ERN was clearly observable and temporally and spatially similar to ERNs described in older children and adults. Further, even with a narrow age-range of less than three years, higher age was associated with a larger ΔERN. Larger ΔERN was also positively associated with several measures of task performance, also after controlling for age, suggesting that the enhancement of the ERN over development is reflected in more efficient performance monitoring of errors. The results of these studies raises the intriguing question of how early in development the ERN can be elicited given that the task is simple enough and age-appropriate. The youngest age at which the ERN can be reliability observed, is clearly still not known.
2.3. Atypical ERN development
Links between the ERN and various psychiatric disorders and personality traits in adults have been well documented (Olvet and Hajcak, 2008, Weinberg et al., 2012). Approximately as many as half of all adult mental disorders begin during childhood or adolescence (Jones, 2013), and it is therefore potentially important to examine the associations with the ERN in a developmental perspective. Moreover, as discussed below, the cingulate cortices are probably involved in the generation of the ERN and these areas are central in regulatory processes which appear disturbed in a number of childhood psychiatric conditions (Walhovd et al., 2012). Importantly, opposite effects on the ERN amplitude are indicated for different groups of disorders and traits. Increased ERNs have been shown among children and adolescents with obsessive compulsive disorder (OCD) or symptoms (Hajcak et al., 2008, Hanna et al., 2012, Santesso et al., 2006a), anxiety (Ladouceur et al., 2006, Meyer et al., 2012b) and a history of behavioral inhibition (McDermott et al., 2009). Notably, Meyer et al. (2012b) found that the ERN related to anxiety only in older children (11–13 years), indicating that the relationship may change as a function of age. Conversely, reduced ERN amplitudes have been found in children with poor social behavior (Santesso et al., 2005), depression (Ladouceur et al., 2012), history of temperamental negative emotionality or maternal anxiety disorder (Torpey et al., 2013) and schizophrenia symptoms (Laurens et al., 2010). For children diagnosed with attention deficit hyperactivity disorder, the results are more equivocal, as some studies have found reduced ERN amplitudes (Albrecht et al., 2008, Liotti et al., 2005, van Meel et al., 2007), while others have found no difference or even increased amplitudes (Burgio-Murphy et al., 2007, Jonkman et al., 2007, Wiersema et al., 2005).
Altered ERN amplitude may be a useful marker for psychopathology in children and adolescents, but it is clearly not a specific index for any one or few mental disorders or traits. Olvet and Hajcak (2008) instead suggested that the higher-order categories of internalizing and externalizing disorders might be characterized by hyperactive and hypoactive performance monitoring, respectively. Still, some results concerning i.e. depression and negative emotionality (Ladouceur et al., 2012, Torpey et al., 2013) are inconsistent with this model. Also, psychotic disorders such as schizophrenia do not fit will within this framework (Olvet and Hajcak, 2008).
Furthermore, only a few studies have investigated the effects of age on the ERN in psychopathology beginning early in life. Hajcak et al. (2008) found a smaller correlation between age and the ERN amplitude in children and adolescents with OCD (8–16 years) compared to healthy controls, although the difference was not significant. Smaller or absent age-effects on the ERN have also intriguingly been found in another study on pediatric OCD (10–18 years) (Hanna et al., 2012), and also for children and adolescents with depression (7–15 years) (Ladouceur et al., 2012).
2.4. Limitations and future directions
A growing number of studies have investigated age-related differences in performance monitoring as indexed by the ERN in children and adolescents, which substantially have increased our knowledge about performance processing in the developing brain. Still, several of the studies share common limitations and there are many unresolved questions. First, many of the studies included samples with few participants and all except two studies included fewer than 80 subjects (see Table 1). Second, several of the studies either include participants in discrete age-groups or create relatively arbitrary groups for statistical analyses. This limits the possible conclusions that can be drawn across studies and the latter may also result in an unnecessary loss of statistical power compared to considering age as a continuous variable. Third, with the exception of the quadratic age-model reported by Davies et al. (2004b), there have been no attempts to delineate the possibly non-linear developmental trajectory of the ERN. Fourth, considering the possible role of puberty in brain development (Blakemore et al., 2010) and given that sex differences have been indicated in age-related differences in the ERN (Davies et al., 2004b), future studies should further investigate sex differences in ERN development and the impact of puberty. Fifth, analyses have been limited to time-domain averaging (Mouraux and Iannetti, 2008). Finally, all current studies used cross-sectional designs. Further work with large samples with wide and continuous age-ranges, and preferably longitudinal designs, are needed to better understand both typical and atypical development of the ERN and performance monitoring. Future studies should also investigate ERN development across multiple tasks that vary in complexity, as it is likely that task difficulty plays a role in observed age-related differences.
3. Neural basis of the ERN
To discuss how developmental changes in the ERN might be related to structural and functional maturation of specific brain regions, we first review evidence on its neural correlates. Identification of neural generators of ERP components is a complex endeavor because no single technique is able to overcome all methodological and conceptual difficulties alone (Linden, 2005), and we therefore review evidence from a multitude of methods.
3.1. Source localization studies
Several high-density electroencephalography (EEG) source localization studies of the ERN have been performed in adults (e.g. (Dehaene et al., 1994, Herrmann et al., 2004, Mathewson et al., 2005, van Veen and Carter, 2002, Vocat et al., 2008)) and some in developmental populations (Ladouceur et al., 2007, Santesso and Segalowitz, 2008). There have also been a few magnetoencephalography (MEG) source localization studies (Keil et al., 2010, Miltner et al., 2003). Agam et al. (2011) systematically summarized and compared the source coordinates from 15 of these studies. The results showed that the mean ERN source locus was in the dorsal ACC (Fig. 4). However, the loci varied considerably across studies and several sources also fell in the PCC. Agam et al. (2011) also estimated the source of the ERN from their own combined EEG and MEG data, and localized the source to the PCC. Moreover, the PCC waveform peaked significantly earlier than the dorsal ACC waveform and also best reflected the timing of the ERN. One of the two available EEG source localization studies in developmental populations identified an ERN source in the vicinity of the dorsal ACC for both a late adolescent and an adult group (Ladouceur et al., 2007), while the second study identified slightly different sources for the flanker task and the Go/No-go task, with a more posterior source for the former (Santesso and Segalowitz, 2008). Thus, it seems that the cingulate cortex, possibly both dorsal anterior and posterior sections, generates the ERN.
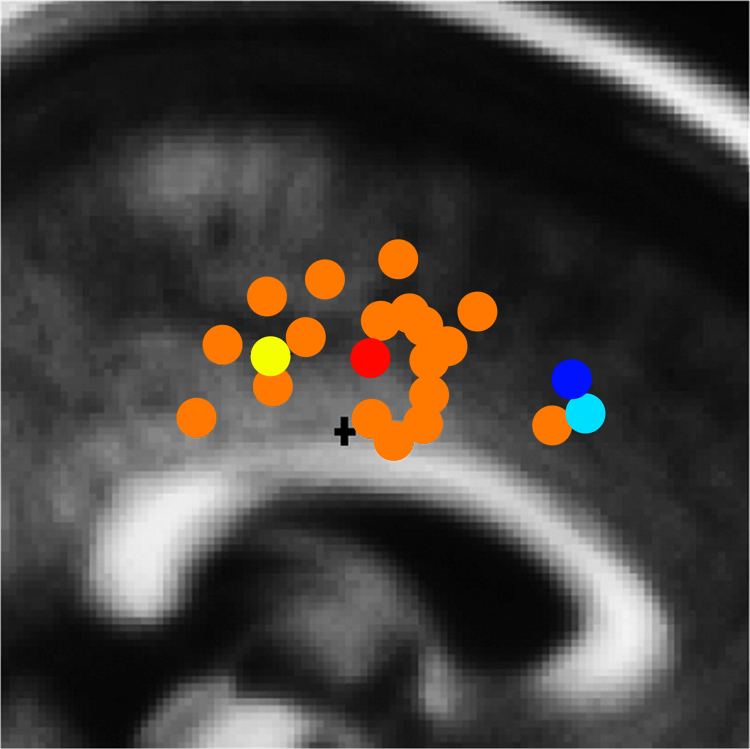
Fig. 4.
A summary of ERN source localizations and error-related fMRI activations. Yellow: peak location of the ERN source estimate in Agam et al. by combined electroencephalography and magnetoencephalography data in 30 adult participants. Orange: one or more ERN source locations from 15 studies reviewed in Agam et al. Red: the mean coordinates of the 15 ERN source studies. Cyan: peak location of error-related fMRI activation in Agam et al. Blue: mean coordinates of error-related fMRI activation based on a meta-analysis of 13 studies (Ridderinkhof et al., 2004). The plus sign indicates the dorsal ACC–PCC boundary.
Source: Adapted with permission from Agam et al. (2011).
Interestingly, intracranial recordings in epilepsy surgery candidates (Brazdil et al., 2002, Brazdil et al., 2005) and both single-unit and local-field potential recording in macaque monkeys (Godlove et al., 2011, Ito et al., 2003) have also showed error-related activity in the medial frontal cortex and the ACC. However, the regional specificity of these findings remains to be further characterized. For instance, a recent study recording local-field potentials in patients with idiopathic dystonia showed an early modulation of the ERN in the pallidum (Herrojo Ruiz et al., 2013).
3.2. Functional neuroimaging
Functional magnetic resonance imaging (fMRI) studies have identified a reliable correlate of errors: activation of the dorsal ACC for erroneous compared with correct responses (Beckmann et al., 2009, Carter et al., 1998, Critchley et al., 2005, Hester et al., 2004, Kiehl et al., 2000, Mathalon et al., 2003, Menon et al., 2001, Ridderinkhof et al., 2004). In a particularly elegant study, Debener et al. (2005) further demonstrated that the single-trial ERN predicted concurrent fMRI activity in the rostral cingulate zone and was related to ensuing behavioral adjustments (PES). In the multimodal study by Agam et al. (2011), it was found that errors elicited robust bilateral dorsal ACC activation, and also that the ERN marginally correlated with activation of both the dorsal ACC and PCC and that these two regions showed coordinated activity based on functional connectivity. However, as the source of the ERN was located to the PCC, they concluded that the findings were inconsistent with the common view of fMRI activation of the dorsal ACC as the hemodynamic reflection of the ERN, and instead suggested that the PCC generates the ERN and communicates with the dorsal ACC to subserve error processing. Huster et al. (2011) found that single trial ERPs for error-trials correlated with the time-courses of independent components derived from fMRI-data localized to the anterior midcingulate cortex (caudal ACC), the pre-supplementary motor area, the insula and parts of the basal ganglia, thus implicating a number of brain regions in performance monitoring. Finally, two other groups recently employed joint independent component analysis to couple electrophysiological and hemodynamic data and describe the spatiotemporal dynamics of the processing of performance errors; both indicating central, but far from exclusive, roles for the dorsal ACC and lateral prefrontal cortex (Donamayor et al., 2012, Edwards et al., 2012). More work is needed e.g. on the interplay between specific divisions of the cingulate cortex and between the ACC and the lateral prefrontal cortex during performance monitoring.
3.3. Quantitative structural neuroimaging
At least two studies have also investigated brain structure correlates of the ERN in adults. Beste et al. (2008) found a reduced ERN in adult patients with Huntington's disease as compared with presymptomatic gene-mutation carriers, and that ERN amplitude was correlated with gray matter (GM) volume in the right medial frontal gyrus in the symptomatic patients. For the presymptomatic patients, there were no significant correlations with brain volumes; however this group consisted of only 12 subjects. In a large sample of healthy adults, Westlye et al. (2009) combined electrophysiology and diffusion tensor imaging (DTI) and found that fractional anisotropy (FA) in the left posterior cingulum bundle correlated with the ERN, so that more negative ERN amplitudes were associated with higher FA, likely reflecting higher white matter (WM) integrity and structural connectivity. These results indicate that properties of the fibers in the cingulate gyrus have effects related to cingulate function, for example, as measured by ERN amplitude. Further, the ERN predicted response accuracy, but there were no relationships between FA and performance, indicating that electrophysiological measures may constitute intermediate explanatory variable connecting DTI indices of WM organization, synchronization of large cell assemblies, and behavior (Westlye et al., 2009).
The performance monitoring system also involves behavioral adjustments after commission of an error, such as error correction and heightened controlled cognition, resulting in PES. Intriguingly, Agam et al. (2011) showed that faster error correction was associated with increased microstructural integrity (FA) of the posterior cingulum bundle. Further, Fjell et al. (2012b) found the PES to be positively correlated to WM volume in several frontal regions and to WM integrity (as indexed by lower mean and axial diffusivity) in multiple tracts (see also Danielmeier et al. (2011)). Structural imaging studies thus also implicate the cingulate cortex, as well as the cingulum tracts, in action monitoring, but also a more distributed neural network.
3.4. Lesion studies
Questions about the necessity of specific brain regions for the ERN signal has led to investigations of patients with lesions, either temporarily induced or accidental. Attenuation of the ERN, along with a reduction in error-corrective behavior has been observed after application of transcranial magnetic stimulation (TMS) over the medial frontal cortex, while not after lateral frontal stimulation (Rollnik et al., 2004). In studies of rare patients with lesions of the medial prefrontal cortex, including the ACC, the ERN has also been found to be affected (Stemmer et al., 2004, Swick and Turken, 2002). A recent study did however observe the ERN in two patients with unilateral lesions to the ACC and surrounding tissue, indicating that unilateral damage is not necessarily associated with abolishment of the ERN (Løvstad et al., 2012). Interpretative caution is however generally called for in single case studies.
A dynamic relationship between medial frontal activity associated with performance monitoring and the lateral prefrontal cortex is indicated, as patients with lateral prefrontal lesions also show ERN deficits (Gehring and Knight, 2000, Ullsperger and von Cramon, 2006, Ullsperger et al., 2002). Evidence from patients with discrete frontal WM lesions, but with intact medial and lateral frontal cortex, further suggest that the connections between these areas are critical for the generation and propagation of the ERN (Hogan et al., 2006). Additionally, patients with focal thalamic lesions also show diminished ERNs and post-error adjustments (Peterburs et al., 2011, Seifert et al., 2011).
3.5. Neural networks for performance monitoring
Several lines of evidence, including source localization EEG and MEG studies, intracranial neurophysiological recordings, fMRI studies, structural imaging and lesion studies, point to a ERN neural generator(s) localized in the posterior medial frontal cortex, most likely in the dorsal ACC or the PCC. However, distributed neural networks are likely involved in modulating the ERN and in performance monitoring, including lateral prefrontal regions, insula, basal ganglia structures, thalamus and WM fiber connection between these regions (Taylor et al., 2007). The structural and functional maturation of these neural networks presumably underlie developmental changes in performance monitoring abilities and the ERN. Thus, we now turn to a description of the developmental brain changes that may support development of the ERN through childhood and adolescence.
4. Brain maturation and development of the ERN
4.1. Principles of brain maturation
Throughout childhood and adolescence, the brain undergoes a multifaceted and dynamic maturational process. Structural magnetic resonance imaging (MRI) studies document that while the first years of life are characterized by GM increases (Gilmore et al., 2012, Knickmeyer et al., 2008), older children and adolescents mainly show cortical GM decreases, increasing WM volumes and heterogeneous changes in subcortical structures (Brown et al., 2012, Koolschijn and Crone, 2013, Lenroot et al., 2007, Raznahan et al., 2011, Sowell et al., 2004, Sullivan et al., 2011, Tamnes et al., 2013, Tiemeier et al., 2010, van Soelen et al., 2012, White et al., 2010, Østby et al., 2009). Additionally, DTI studies indicate prolonged development of structural connectivity in the form of increases in FA and overall diffusivity decreases (Bava et al., 2010, Giorgio et al., 2010, Lebel and Beaulieu, 2011, Peters et al., 2012, Tamnes et al., 2010, Westlye et al., 2010). Importantly, the rates and timing of these changes vary regionally in the brain. Cortical maturation in general progresses in a posterior-to-anterior order with relatively late maturation of prefrontal regions (Gogtay et al., 2004, Shaw et al., 2008, Tamnes et al., 2013) and DTI studies suggest especially protracted development of fronto-temporal connections (Colby et al., 2011, Lebel et al., 2012, Tamnes et al., 2010). Brain functional development seems to involve progressively increased activations in task-relevant brain regions that mediate cognitive control functions (Rubia, 2013) or a strengthening of top-down modulatory influences (Bitan et al., 2006, Hwang et al., 2010). In terms of functional connectivity, it is also believed that networks of brain activity show both integration and segregation with increasing age (Fair et al., 2007, Uddin et al., 2010).
The specific biological processes causing these changes remain relatively poorly understood, as estimates of their extent and time course mostly rely upon extrapolation from very limited postmortem material and from data acquired in other species (Brown and Jernigan, 2012). Increases in WM volumes and apparent cortical reductions in adolescence are likely influenced by increased caliber and myelination of axons coursing within or near the lower cortical layers (Benes, 1989, Benes et al., 1994, Yakovlev and Lecours, 1967). Additionally, dendritic and axonal growth and synaptogenesis are followed by regressive changes in the form of simplification or elimination of neuronal processes and synapses (Bourgeois and Rakic, 1993, Huttenlocher and Dabholkar, 1997, Petanjek et al., 2011). These, as well as multiple other biological processes, likely contribute to increased processing specialization and efficiency, and may underlie developmental changes in performance monitoring abilities, but it is not known how they affect the ERN signal.
4.2. Maturation of performance monitoring networks
Based on the above review of the neural correlates of the ERN and given the regional heterogeneity of brain maturation, it is plausible that developmental changes in the ERN reflect structural and functional changes in specific brain regions, including the dorsal ACC and PCC, lateral prefrontal cortices, insula, basal ganglia structures, thalamus and WM tracts connecting these regions. However, this remains speculative, since only one cross-sectional study so far has investigated these relationships (Liu et al., 2013).
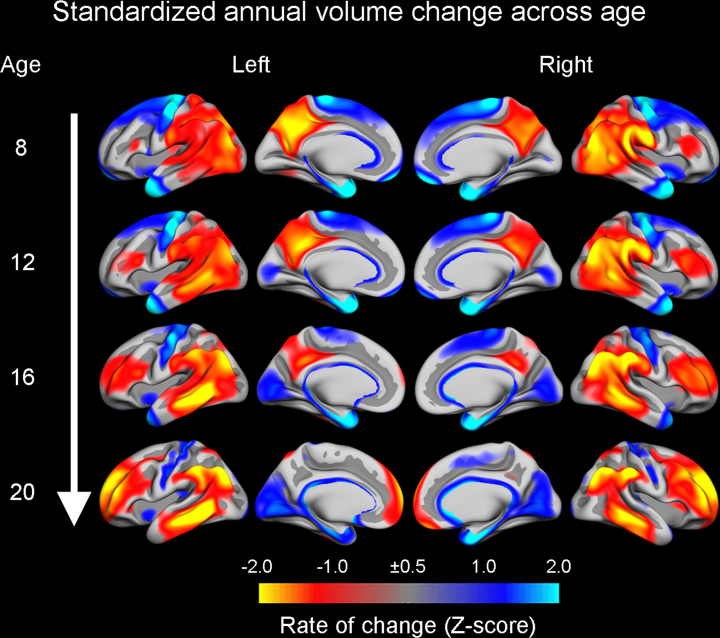
To delineate regional differences in the temporal patterns of cortical maturation, we estimated relative annual cortical volume changes per year from late childhood to young adulthood (8–22 years) (Tamnes et al., 2013). Although we found significant longitudinal volume reductions across almost the entire cortical surface, the ACC and the anterior part of the PCC only showed average or smaller than average rates of volume reduction consistently across the age-range. In contrast, a gradual relative increase in rates of volume reduction was observed in the lateral prefrontal cortices (Fig. 5). Further, the rate of change was generally higher in the cerebral cortex than in the subcortical structures, including basal ganglia structures and the thalamus. Recently, Liu et al. (2013) examined the ERN and GM volumes in 20 youth with OCD (10–19 years) and 20 age-matched controls. Intriguingly, larger ERN amplitude was found to be correlated with lower GM density in the left lateral orbitofrontal cortex across both groups, while an association driven by the OCD group was observed between larger ΔERN amplitude and lower GM density in the posterior medial frontal cortex. Additionally, there was a group difference in the association between the ERN and GM density in the right insula. These results thus provide preliminary evidence that variability in the pace of structural maturation of lateral prefrontal cortical regions underlie typical developmental changes in the ERN in adolescence.
Fig. 5.
Standardized cortical change across age in children and adolescents. To illustrate relatively higher and relatively lower rates of change at different ages, longitudinal annual percentage volume change estimates were z-transformed across the surface for each hemisphere (n = 85, 8–22 years). Red–yellow areas indicate the largest relative cortical reductions at different ages, while blue–cyan areas indicate smaller relative reductions.
Source: Adapted with permission from Tamnes et al. (2013).
The development of distributed neural networks is also critically dependent upon maturation of major WM tracts connecting different cortical and subcortical regions. Interestingly, DTI studies show that the cingulum bundles mature later than most other major tracts (Lebel and Beaulieu, 2011, Lebel et al., 2012). Further studies directly testing the relationships between developmental change in the ERN and brain maturation are however needed to establish whether the especially protracted maturation of lateral prefrontal cortices and the cingulum tracts in fact are important for late developmental changes in the ERN. Regarding the cingulate cortex, which did not show relative increasing rates of volume change in our data from participants 8–22 years (Tamnes et al., 2013), it is intriguing to note that surface area of the dorsal ACC has been found to be related to cognitive control performance in younger children (4–12 years), but not in adolescents (12–21 years) (Fjell et al., 2012a).
There is also some evidence that functional maturation of the dorsal ACC and adjacent cortices facilitates developmental improvements in performance monitoring. It has for instance been found that adults show increased brain activation compared to children and adolescents in the ACC/posterior medial frontal cortex during errors, even when equating performance (Fitzgerald et al., 2010, Rubia et al., 2007), although one study did not observe such a difference in the ACC, but in a number of other brain regions (Braet et al., 2009). Another fMRI study with participants 8–27 years found that the dorsal ACC showed greater activity for error trials than for correct trial on an antisaccade task and that the activity difference increased from childhood to adulthood (Velanova et al., 2008). Interestingly, it has also been indicated that pediatric OCD patients compared to controls show a stronger age-related increase in error-related ACC activation (Huyser et al., 2011). Studies of external feedback processing also suggest that the dorsal ACC and the lateral prefrontal cortex are functionally late developed and do not show fully mature activation patterns before late in adolescents, although longitudinal analyses failed to show significant change in activation over time (Crone et al., 2008, Koolschijn et al., 2011). Moreover, a functional connectivity study indicates that in children, the dorsal ACC region may be relatively disconnected from a cinguloopercular control network identified in adults, but more closely connected to a frontoparietal network (Fair et al., 2007). Together, these results give some evidence that the performance of children and adolescents receive less support from internal or external feedback signaling from the dorsal ACC and that functional changes in this system underlie improvements in performance monitoring capacity during development.
5. Conclusions
A number of studies document age-related increases in the ERN throughout childhood and adolescence, likely reflecting development of cognitive control mechanisms involved in performance monitoring. Further studies are, however, needed to investigate the presence of the ERN in young children, to delineate the typical and atypical developmental trajectories of the ERN, and to better understand how these trajectories interact with e.g. task complexity and possible changes in cognitive strategies used, affective responses, latency jitter and the signal-to-noise ratio. Nonetheless, the relative low cost of data collection, together with its superior temporal resolution, make electrophysiological techniques very valuable for examining development of neurocognitive processes (Taylor and Baldeweg, 2002).
Several lines of evidence, mainly from studies on adults, show that the ERN most likely is generated in the dorsal ACC and the PCC, but that distributed neural networks also are involved. There are indications that the functional maturation of the dorsal ACC and adjacent cortices facilitates developmental improvements in performance monitoring and that the protracted structural maturation of the lateral prefrontal cortex and the cingulum tracts also may be important for late developmental changes in the ERN. Further multimodal imaging studies are however crucial to test how developmental changes in the ERN relate to structural and functional brain maturation.
Conflict of interest
The authors have no conflict of interest to report.
Acknowledgements
This work was supported by grants from the Norwegian Research Council (K.B.W., A.M.F.), the European Research Council (K.B.W., A.M.F.), the Department of Psychology, University of Oslo (C.K.T., K.B.W. and A.M.F.), and the U.S.-Norway Fulbright Foundation (C.K.T.).
References
- Agam Y., Hamalainen M.S., Lee A.K., Dyckman K.A., Friedman J.S., Isom M., Makris N., Manoach D.S. Multimodal neuroimaging dissociates hemodynamic and electrophysiological correlates of error processing. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:17556–17561. doi: 10.1073/pnas.1103475108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albrecht B., Brandeis D., Uebel H., Heinrich H., Mueller U.C., Hasselhorn M., Steinhausen H.C., Rothenberger A., Banaschewski T. Action monitoring in boys with attention-deficit/hyperactivity disorder, their nonaffected siblings, and normal control subjects: evidence for an endophenotype. Biological Psychiatry. 2008;64:615–625. doi: 10.1016/j.biopsych.2007.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anokhin A.P., Golosheykin S., Heath A.C. Heritability of frontal brain function related to action monitoring. Psychophysiology. 2008;45:524–534. doi: 10.1111/j.1469-8986.2008.00664.x. [DOI] [PubMed] [Google Scholar]
- Arbel Y., Donchin E. When a child errs: the ERN and the Pe complex in children. Psychophysiology. 2011;48:55–63. doi: 10.1111/j.1469-8986.2010.01042.x. [DOI] [PubMed] [Google Scholar]
- Bava S., Thayer R., Jacobus J., Ward M., Jernigan T.L., Tapert S.F. Longitudinal characterization of white matter maturation during adolescence. Brain Research. 2010;1327:38–46. doi: 10.1016/j.brainres.2010.02.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann M., Johansen-Berg H., Rushworth M.F. Connectivity-based parcellation of human cingulate cortex and its relation to functional specialization. Journal of Neuroscience. 2009;29:1175–1190. doi: 10.1523/JNEUROSCI.3328-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benes F.M. Myelination of cortical-hippocampal relays during late adolescence. Schizophrenia Bulletin. 1989;15:585–593. doi: 10.1093/schbul/15.4.585. [DOI] [PubMed] [Google Scholar]
- Benes F.M., Turtle M., Khan Y., Farol P. Myelination of a key relay zone in the hippocampal formation occurs in the human brain during childhood, adolescence, and adulthood. Archives of General Psychiatry. 1994;51:477–484. doi: 10.1001/archpsyc.1994.03950060041004. [DOI] [PubMed] [Google Scholar]
- Beste C., Saft C., Konrad C., Andrich J., Habbel A., Schepers I., Jansen A., Pfleiderer B., Falkenstein M. Levels of error processing in Huntington's disease: a combined study using event-related potentials and voxel-based morphometry. Human Brain Mapping. 2008;29:121–130. doi: 10.1002/hbm.20374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biehl S.C., Dresler T., Reif A., Scheuerpflug P., Deckert J., Herrmann M.J. Dopamine transporter (DAT1) and dopamine receptor D4 (DRD4) genotypes differentially impact on electrophysiological correlates of error processing. PLoS ONE. 2011;6:e28396. doi: 10.1371/journal.pone.0028396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitan T., Burman D.D., Lu D., Cone N.E., Gitelman D.R., Mesulam M.M., Booth J.R. Weaker top-down modulation from the left inferior frontal gyrus in children. NeuroImage. 2006;33:991–998. doi: 10.1016/j.neuroimage.2006.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore S.J., Burnett S., Dahl R.E. The role of puberty in the developing adolescent brain. Human Brain Mapping. 2010;31:926–933. doi: 10.1002/hbm.21052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botvinick M.M., Braver T.S., Barch D.M., Carter C.S., Cohen J.D. Conflict monitoring and cognitive control. Psychological Review. 2001;108:624–652. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- Botvinick M.M., Cohen J.D., Carter C.S. Conflict monitoring and anterior cingulate cortex: an update. Trends in Cognitive Sciences. 2004;8:539–546. doi: 10.1016/j.tics.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Bourgeois J.P., Rakic P. Changes of synaptic density in the primary visual cortex of the macaque monkey from fetal to adult stage. Journal of Neuroscience. 1993;13:2801–2820. doi: 10.1523/JNEUROSCI.13-07-02801.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braet W., Johnson K.A., Tobin C.T., Acheson R., Bellgrove M.A., Robertson I.H., Garavan H. Functional developmental changes underlying response inhibition and error-detection processes. Neuropsychologia. 2009;47:3143–3151. doi: 10.1016/j.neuropsychologia.2009.07.018. [DOI] [PubMed] [Google Scholar]
- Brazdil M., Roman R., Daniel P., Rektor I. Intracerebral error-related negativity in a simple Go/NoGo task. Journal of Psychophysiology. 2005;19:244–255. [Google Scholar]
- Brazdil M., Roman R., Falkenstein M., Daniel P., Jurak P., Rektor I. Error processing – evidence from intracerebral ERP recordings. Experimental Brain Research. 2002;146(4):460–466. doi: 10.1007/s00221-002-1201-y. [DOI] [PubMed] [Google Scholar]
- Brooker R.J., Buss K.A., Dennis T.A. Error-monitoring brain activity is associated with affective behaviors in young children. Developmental Cognitive Neuroscience. 2011;1:141–151. doi: 10.1016/j.dcn.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown T.T., Jernigan T.L. Brain development during the preschool years. Neuropsychology Review. 2012;22:313–333. doi: 10.1007/s11065-012-9214-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown T.T. Neuroanatomical assessment of biological maturity. Current Biology. 2012;22:1693–1698. doi: 10.1016/j.cub.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgio-Murphy A., Klorman R., Shaywitz S.E., Fletcher J.M., Marchione K.E., Holahan J., Stuebing K.K., Thatcher J.E., Shaywitz B.A. Error-related event-related potentials in children with attention-deficit hyperactivity disorder, oppositional defiant disorder, reading disorder, and math disorder. Biological Psychology. 2007;75:75–86. doi: 10.1016/j.biopsycho.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush G., Luu P., Posner M.I. Cognitive and emotional influences in anterior cingulate cortex. Trends in Cognitive Sciences. 2000;4:215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Carter C.S., Braver T.S., Barch D.M., Botvinick M.M., Noll D., Cohen J.D. Anterior cingulate cortex, error detection, and the online monitoring of performance. Science. 1998;280:747–749. doi: 10.1126/science.280.5364.747. [DOI] [PubMed] [Google Scholar]
- Carter C.S., van Veen V. Anterior cingulate cortex and conflict detection: an update of theory and data. Cognitive, Affective & Behavioral Neuroscience. 2007;7:367–379. doi: 10.3758/cabn.7.4.367. [DOI] [PubMed] [Google Scholar]
- Colby J.B., Van Horn J.D., Sowell E.R. Quantitative in vivo evidence for broad regional gradients in the timing of white matter maturation during adolescence. NeuroImage. 2011;54:25–31. doi: 10.1016/j.neuroimage.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley H.D., Tang J., Glaser D., Butterworth B., Dolan R.J. Anterior cingulate activity during error and autonomic response. NeuroImage. 2005;27:885–895. doi: 10.1016/j.neuroimage.2005.05.047. [DOI] [PubMed] [Google Scholar]
- Crone E.A., Zanolie K., Van Leijenhorst L., Westenberg P.M., Rombouts S.A. Neural mechanisms supporting flexible performance adjustment during development. Cognitive, Affective & Behavioral Neuroscience. 2008;8:165–177. doi: 10.3758/cabn.8.2.165. [DOI] [PubMed] [Google Scholar]
- Danielmeier C., Eichele T., Forstmann B.U., Tittgemeyer M., Ullsperger M. Posterior medial frontal cortex activity predicts post-error adaptations in task-related visual and motor areas. Journal of Neuroscience. 2011;31:1780–1789. doi: 10.1523/JNEUROSCI.4299-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies P.L., Segalowitz S.J., Gavin W.J. Development of error-monitoring event-related potentials in adolescents. Annals of the New York Academy of Sciences. 2004;1021:324–328. doi: 10.1196/annals.1308.039. [DOI] [PubMed] [Google Scholar]
- Davies P.L., Segalowitz S.J., Gavin W.J. Development of response-monitoring ERPs in 7- to 25-year-olds. Developmental Neuropsychology. 2004;25:355–376. doi: 10.1207/s15326942dn2503_6. [DOI] [PubMed] [Google Scholar]
- Debener S., Ullsperger M., Siegel M., Fiehler K., von Cramon D.Y., Engel A.K. Trial-by-trial coupling of concurrent electroencephalogram and functional magnetic resonance imaging identifies the dynamics of performance monitoring. Journal of Neuroscience. 2005;25:11730–11737. doi: 10.1523/JNEUROSCI.3286-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehaene S., Posner M.I., Tucker D.M. Localization of a neural system for error detection and compensation. Psychological Science. 1994;5:303–305. [Google Scholar]
- Donamayor N., Heilbronner U., Munte T.F. Coupling electrophysiological and hemodynamic responses to errors. Human Brain Mapping. 2012;33:1621–1633. doi: 10.1002/hbm.21305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards B.G., Calhoun V.D., Kiehl K.A. Joint ICA of ERP and fMRI during error-monitoring. NeuroImage. 2012;59:1896–1903. doi: 10.1016/j.neuroimage.2011.08.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eppinger B., Mock B., Kray J. Developmental differences in learning and error processing: evidence from ERPs. Psychophysiology. 2009;46:1043–1053. doi: 10.1111/j.1469-8986.2009.00838.x. [DOI] [PubMed] [Google Scholar]
- Fair D.A., Dosenbach N.U., Church J.A., Cohen A.L., Brahmbhatt S., Miezin F.M., Barch D.M., Raichle M.E., Petersen S.E., Schlaggar B.L. Development of distinct control networks through segregation and integration. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:13507–13512. [Google Scholar]
- Falkenstein M., Hohnsbein J., Hoormann J., Blanke L. Effects of crossmodal divided attention on late ERP components. II. Error processing in choice reaction tasks. Electroencephalography and Clinical Neurophysiology. 1991;78:447–455. doi: 10.1016/0013-4694(91)90062-9. [DOI] [PubMed] [Google Scholar]
- Falkenstein M., Hoormann J., Christ S., Hohnsbein J. ERP components on reaction errors and their functional significance: a tutorial. Biological Psychology. 2000;51:87–107. doi: 10.1016/s0301-0511(99)00031-9. [DOI] [PubMed] [Google Scholar]
- Fallgatter A.J., Herrmann M.J., Roemmler J., Ehlis A.C., Wagener A., Heidrich A., Ortega G., Zeng Y., Lesch K.P. Allelic variation of serotonin transporter function modulates the brain electrical response for error processing. Neuropsychopharmacology. 2004;29:1506–1511. doi: 10.1038/sj.npp.1300409. [DOI] [PubMed] [Google Scholar]
- Fitzgerald K.D., Perkins S.C., Angstadt M., Johnson T., Stern E.R., Welsh R.C., Taylor S.F. The development of performance-monitoring function in the posterior medial frontal cortex. NeuroImage. 2010;49:3463–3473. doi: 10.1016/j.neuroimage.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fjell A.M. Multimodal imaging of the self-regulating developing brain. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:19620–19625. doi: 10.1073/pnas.1208243109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fjell A.M., Westlye L.T., Amlien I.K., Walhovd K.B. A multi-modal investigation of behavioral adjustment: post-error slowing is associated with white matter characteristics. NeuroImage. 2012;61:195–205. doi: 10.1016/j.neuroimage.2012.03.007. [DOI] [PubMed] [Google Scholar]
- Ford J.M. Schizophrenia: the broken P300 and beyond. Psychophysiology. 1999;36:667–682. [PubMed] [Google Scholar]
- Gehring W.J., Goss B., Coles M.G.H., Meyer D.E., Donchin E. A neural system for error detection and compensation. Psychological Science. 1993;4:385–390. [Google Scholar]
- Gehring W.J., Knight R.T. Prefrontal-cingulate interactions in action monitoring. Nature Neuroscience. 2000;3:516–520. doi: 10.1038/74899. [DOI] [PubMed] [Google Scholar]
- Gehring W.J., Willoughby A.R. The medial frontal cortex and the rapid processing of monetary gains and losses. Science. 2002;295:2279–2282. doi: 10.1126/science.1066893. [DOI] [PubMed] [Google Scholar]
- Gilmore J.H., Shi F., Woolson S.L., Knickmeyer R.C., Short S.J., Lin W., Zhu H., Hamer R.M., Styner M., Shen D. Longitudinal development of cortical and subcortical gray matter from birth to 2 years. Cerebral Cortex. 2012;22:2478–2485. doi: 10.1093/cercor/bhr327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giorgio A., Watkins K.E., Chadwick M., James S., Winmill L., Douaud G., De Stefano N., Matthews P.M., Smith S.M., Johansen-Berg H., James A.C. Longitudinal changes in grey and white matter during adolescence. NeuroImage. 2010;49:94–103. doi: 10.1016/j.neuroimage.2009.08.003. [DOI] [PubMed] [Google Scholar]
- Godlove D.C., Emeric E.E., Segovis C.M., Young M.S., Schall J.D., Woodman G.F. Event-related potentials elicited by errors during the stop-signal task. I. Macaque monkeys. Journal of Neuroscience. 2011;31:15640–15649. doi: 10.1523/JNEUROSCI.3349-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogtay N., Giedd J.N., Lusk L., Hayashi K.M., Greenstein D., Vaituzis A.C., Nugent T.F., 3rd, Herman D.H., Clasen L.S., Toga A.W., Rapoport J.L., Thompson P.M. Dynamic mapping of human cortical development during childhood through early adulthood. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajcak G. What we’ve learned from mistakes: Insights from error-related brain activity. Current Directions in Psychological Science. 2012;21:101–106. [Google Scholar]
- Hajcak G., Franklin M.E., Foa E.B., Simons R.F. Increased error-related brain activity in pediatric obsessive-compulsive disorder before and after treatment. American Journal of Psychiatry. 2008;165:116–123. doi: 10.1176/appi.ajp.2007.07010143. [DOI] [PubMed] [Google Scholar]
- Hanna G.L., Carrasco M., Harbin S.M., Nienhuis J.K., LaRosa C.E., Chen P., Fitzgerald K.D., Gehring W.J. Error-related negativity and tic history in pediatric obsessive-compulsive disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 2012;51:902–910. doi: 10.1016/j.jaac.2012.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann M.J., Rommler J., Ehlis A.C., Heidrich A., Fallgatter A.J. Source localization (LORETA) of the error-related-negativity (ERN/Ne) and positivity (Pe) Cognitive Brain Research. 2004;20:294–299. doi: 10.1016/j.cogbrainres.2004.02.013. [DOI] [PubMed] [Google Scholar]
- Herrojo Ruiz M., Huebl J., Schonecker T., Kupsch A., Yarrow K., Krauss J.K., Schneider G.H., Kuhn A.A. Involvement of human internal globus pallidus in the early modulation of cortical error-related activity. Cerebral Cortex. 2013 doi: 10.1093/cercor/bht002. (in press) [DOI] [PubMed] [Google Scholar]
- Hester R., Fassbender C., Garavan H. Individual differences in error processing: a review and reanalysis of three event-related fMRI studies using the GO/NOGO task. Cerebral Cortex. 2004;14:986–994. doi: 10.1093/cercor/bhh059. [DOI] [PubMed] [Google Scholar]
- Hogan A.M., Vargha-Khadem F., Kirkham F.J., Baldeweg T. Maturation of action monitoring from adolescence to adulthood: an ERP study. Developmental Science. 2005;8:525–534. doi: 10.1111/j.1467-7687.2005.00444.x. [DOI] [PubMed] [Google Scholar]
- Hogan A.M., Vargha-Khadem F., Saunders D.E., Kirkham F.J., Baldeweg T. Impact of frontal white matter lesions on performance monitoring: ERP evidence for cortical disconnection. Brain. 2006;129:2177–2188. doi: 10.1093/brain/awl160. [DOI] [PubMed] [Google Scholar]
- Holmes A.J., Bogdan R., Pizzagalli D.A. Serotonin transporter genotype and action monitoring dysfunction: a possible substrate underlying increased vulnerability to depression. Neuropsychopharmacology. 2010;35:1186–1197. doi: 10.1038/npp.2009.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holroyd C.B., Coles M.G. The neural basis of human error processing: reinforcement learning, dopamine, and the error-related negativity. Psychological Review. 2002;109:679–709. doi: 10.1037/0033-295X.109.4.679. [DOI] [PubMed] [Google Scholar]
- Holroyd C.B., Yeung N., Coles M.G., Cohen J.D. A mechanism for error detection in speeded response time tasks. Journal of Experimental Psychology: General. 2005;134:163–191. doi: 10.1037/0096-3445.134.2.163. [DOI] [PubMed] [Google Scholar]
- Huster R.J., Eichele T., Enriquez-Geppert S., Wollbrink A., Kugel H., Konrad C., Pantev C. Multimodal imaging of functional networks and event-related potentials in performance monitoring. NeuroImage. 2011;56:1588–1597. doi: 10.1016/j.neuroimage.2011.03.039. [DOI] [PubMed] [Google Scholar]
- Huttenlocher P.R., Dabholkar A.S. Regional differences in synaptogenesis in human cerebral cortex. Journal of Comparative Neurology. 1997;387:167–178. doi: 10.1002/(sici)1096-9861(19971020)387:2<167::aid-cne1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Huyser C., Veltman D.J., Wolters L.H., de Haan E., Boer F. Developmental aspects of error and high-conflict-related brain activity in pediatric obsessive-compulsive disorder: a fMRI study with a Flanker task before and after CBT. Journal of Child Psychology and Psychiatry and Allied Disciplines. 2011;52:1251–1260. doi: 10.1111/j.1469-7610.2011.02439.x. [DOI] [PubMed] [Google Scholar]
- Hwang K., Velanova K., Luna B. Strengthening of top-down frontal cognitive control networks underlying the development of inhibitory control: a functional magnetic resonance imaging effective connectivity study. Journal of Neuroscience. 2010;30:15535–15545. doi: 10.1523/JNEUROSCI.2825-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito S., Stuphorn V., Brown J.W., Schall J.D. Performance monitoring by the anterior cingulate cortex during saccade countermanding. Science. 2003;302:120–122. doi: 10.1126/science.1087847. [DOI] [PubMed] [Google Scholar]
- Jones P.B. Adult mental health disorders and their age at onset. British Journal of Psychiatry. 2013;54:s5–s10. doi: 10.1192/bjp.bp.112.119164. [DOI] [PubMed] [Google Scholar]
- Jonkman L.M., van Melis J.J., Kemner C., Markus C.R. Methylphenidate improves deficient error evaluation in children with ADHD: an event-related brain potential study. Biological Psychology. 2007;76:217–229. doi: 10.1016/j.biopsycho.2007.08.004. [DOI] [PubMed] [Google Scholar]
- Keil J., Weisz N., Paul-Jordanov I., Wienbruch C. Localization of the magnetic equivalent of the ERN and induced oscillatory brain activity. NeuroImage. 2010;51:404–411. doi: 10.1016/j.neuroimage.2010.02.003. [DOI] [PubMed] [Google Scholar]
- Kiehl K.A., Liddle P.F., Hopfinger J.B. Error processing and the rostral anterior cingulate: an event-related fMRI study. Psychophysiology. 2000;37:216–223. [PubMed] [Google Scholar]
- Kim E.Y., Iwaki N., Imashioya H., Uno H., Fujita T. Error-related negativity in a visual go/no-go task: children vs. adults. Developmental Neuropsychology. 2007;31:181–191. doi: 10.1080/87565640701190775. [DOI] [PubMed] [Google Scholar]
- Kim E.Y., Iwaki N., Uno H., Fujita T. Error-related negativity in children: effect of an observer. Developmental Neuropsychology. 2005;28:871–883. doi: 10.1207/s15326942dn2803_7. [DOI] [PubMed] [Google Scholar]
- Knickmeyer R.C., Gouttard S., Kang C., Evans D., Wilber K., Smith J.K., Hamer R.M., Lin W., Gerig G., Gilmore J.H. A structural MRI study of human brain development from birth to 2 years. Journal of Neuroscience. 2008;28:12176–12182. doi: 10.1523/JNEUROSCI.3479-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koolschijn P.C., Crone E.A. Sex differences and structural brain maturation from childhood to early adulthood. Developmental Cognitive Neuroscience. 2013;5:106–118. doi: 10.1016/j.dcn.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koolschijn P.C., Schel M.A., de Rooij M., Rombouts S.A., Crone E.A. A three-year longitudinal functional magnetic resonance imaging study of performance monitoring and test-retest reliability from childhood to early adulthood. Journal of Neuroscience. 2011;31:4204–4212. doi: 10.1523/JNEUROSCI.6415-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer U.M., Cunillera T., Camara E., Marco-Pallares J., Cucurell D., Nager W., Bauer P., Schule R., Schols L., Rodriguez-Fornells A., Munte T.F. The impact of catechol-O-methyltransferase and dopamine D4 receptor genotypes on neurophysiological markers of performance monitoring. Journal of Neuroscience. 2007;27:14190–14198. doi: 10.1523/JNEUROSCI.4229-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladouceur C.D., Dahl R.E., Birmaher B., Axelson D.A., Ryan N.D. Increased error-related negativity (ERN) in childhood anxiety disorders: ERP and source localization. Journal of Child Psychology and Psychiatry and Allied Disciplines. 2006;47:1073–1082. doi: 10.1111/j.1469-7610.2006.01654.x. [DOI] [PubMed] [Google Scholar]
- Ladouceur C.D., Dahl R.E., Carter C.S. ERP correlates of action monitoring in adolescence. Annals of the New York Academy of Sciences. 2004;1021:329–336. doi: 10.1196/annals.1308.040. [DOI] [PubMed] [Google Scholar]
- Ladouceur C.D., Dahl R.E., Carter C.S. Development of action monitoring through adolescence into adulthood: ERP and source localization. Developmental Science. 2007;10:874–891. doi: 10.1111/j.1467-7687.2007.00639.x. [DOI] [PubMed] [Google Scholar]
- Ladouceur C.D., Slifka J.S., Dahl R.E., Birmaher B., Axelson D.A., Ryan N.D. Altered error-related brain activity in youth with major depression. Developmental Cognitive Neuroscience. 2012;2:351–362. doi: 10.1016/j.dcn.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurens K.R., Hodgins S., Mould G.L., West S.A., Schoenberg P.L., Murray R.M., Taylor E.A. Error-related processing dysfunction in children aged 9 to 12 years presenting putative antecedents of schizophrenia. Biological Psychiatry. 2010;67:238–245. doi: 10.1016/j.biopsych.2009.07.030. [DOI] [PubMed] [Google Scholar]
- Lebel C., Beaulieu C. Longitudinal development of human brain wiring continues from childhood into adulthood. Journal of Neuroscience. 2011;31:10937–10947. doi: 10.1523/JNEUROSCI.5302-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel C., Gee M., Camicioli R., Wieler M., Martin W., Beaulieu C. Diffusion tensor imaging of white matter tract evolution over the lifespan. NeuroImage. 2012;60:340–352. doi: 10.1016/j.neuroimage.2011.11.094. [DOI] [PubMed] [Google Scholar]
- Lenroot R.K., Gogtay N., Greenstein D.K., Wells E.M., Wallace G.L., Clasen L.S., Blumenthal J.D., Lerch J., Zijdenbos A.P., Evans A.C., Thompson P.M., Giedd J.N. Sexual dimorphism of brain developmental trajectories during childhood and adolescence. NeuroImage. 2007;36:1065–1073. doi: 10.1016/j.neuroimage.2007.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden D.E.J. The P300: where in the brain is it produced and what does it tell us? Neuroscientist. 2005;11:563–576. doi: 10.1177/1073858405280524. [DOI] [PubMed] [Google Scholar]
- Liotti M., Pliszka S.R., Perez R., Kothmann D., Woldorff M.G. Abnormal brain activity related to performance monitoring and error detection in children with ADHD. Cortex. 2005;41:377–388. doi: 10.1016/s0010-9452(08)70274-0. [DOI] [PubMed] [Google Scholar]
- Liu Y., Hanna G.L., Carrasco M., Gehring W.J., Fitzgerald K.D. Altered relationship between electrophysiological response to errors and gray matter volumes in an extended network for error-processing in pediatric obsessive-compulsive disorder. Human Brain Mapping. 2013 doi: 10.1002/hbm.22240. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Løvstad M., Funderud I., Meling T., Kramer U.M., Voytek B., Due-Tønnessen P., Endestad T., Lindgren M., Knight R.T., Solbakk A.K. Anterior cingulate cortex and cognitive control: neuropsychological and electrophysiological findings in two patients with lesions to dorsomedial prefrontal cortex. Brain and Cognition. 2012;80:237–249. doi: 10.1016/j.bandc.2012.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathalon D.H., Whitfield S.L., Ford J.M. Anatomy of an error: ERP and fMRI. Biological Psychology. 2003;64:119–141. doi: 10.1016/s0301-0511(03)00105-4. [DOI] [PubMed] [Google Scholar]
- Mathewson K.J., Dywan J., Segalowitz S.J. Brain bases of error-related ERPs as influenced by age and task. Biological Psychology. 2005;70:88–104. doi: 10.1016/j.biopsycho.2004.12.005. [DOI] [PubMed] [Google Scholar]
- McDermott J.M., Perez-Edgar K., Henderson H.A., Chronis-Tuscano A., Pine D.S., Fox N.A. A history of childhood behavioral inhibition and enhanced response monitoring in adolescence are linked to clinical anxiety. Biological Psychiatry. 2009;65:445–448. doi: 10.1016/j.biopsych.2008.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V., Adleman N.E., White C.D., Glover G.H., Reiss A.L. Error-related brain activation during a Go/NoGo response inhibition task. Human Brain Mapping. 2001;12:131–143. doi: 10.1002/1097-0193(200103)12:3<131::AID-HBM1010>3.0.CO;2-C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer A., Klein D.N., Torpey D.C., Kujawa A.J., Hayden E.P., Sheikh H.I., Singh S.M., Hajcak G. Additive effects of the dopamine D2 receptor and dopamine transporter genes on the error-related negativity in young children. Genes, Brain and Behavior. 2012;11:695–703. doi: 10.1111/j.1601-183X.2012.00812.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer A., Weinberg A., Klein D.N., Hajcak G. The development of the error-related negativity (ERN) and its relationship with anxiety: Evidence from 8 to 13 year-olds. Developmental Cognitive Neuroscience. 2012;2:152–161. doi: 10.1016/j.dcn.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miltner W.H.R., Braun C.H., Coles M.G.H. Event-related brain potentials following incorrect feedback in a time-estimation task: Evidence for a “generic” neural system for error detection. Journal of Cognitive Neuroscience. 1997;9:788–798. doi: 10.1162/jocn.1997.9.6.788. [DOI] [PubMed] [Google Scholar]
- Miltner W.H.R., Lemke U., Weiss T., Holroyd C., Scheffers M.K., Coles M.G. Implementation of error-processing in the human anterior cingulate cortex: a source analysis of the magnetic equivalent of the error-related negativity. Biological Psychology. 2003;64:157–166. doi: 10.1016/s0301-0511(03)00107-8. [DOI] [PubMed] [Google Scholar]
- Mouraux A., Iannetti G.D. Across-trial averaging of event-related EEG responses and beyond. Magnetic Resonance Imaging. 2008;26:1041–1054. doi: 10.1016/j.mri.2008.01.011. [DOI] [PubMed] [Google Scholar]
- Mueller E.M., Makeig S., Stemmler G., Hennig J., Wacker J. Dopamine effects on human error processing depend on catechol-O-methyltransferase VAL158MET genotype. Journal of Neuroscience. 2011;31:15818–15825. doi: 10.1523/JNEUROSCI.2103-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olvet D.M., Hajcak G. The error-related negativity (ERN) and psychopathology: toward an endophenotype. Clinical Psychology Review. 2008;28:1343–1354. doi: 10.1016/j.cpr.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overbeek T.J.M., Nieuwenhuis S., Ridderinkhof K.R. Dissociable components of error processing: On the functional significance of te Pe vis-à-vis the ERN/Ne. Journal of Psychophysiology. 2005;19:319–329. [Google Scholar]
- Petanjek Z., Judas M., Simic G., Rasin M.R., Uylings H.B., Rakic P., Kostovic I. Extraordinary neoteny of synaptic spines in the human prefrontal cortex. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:13281–13286. doi: 10.1073/pnas.1105108108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterburs J., Pergola G., Koch B., Schwarz M., Hoffmann K.P., Daum I., Bellebaum C. Altered error processing following vascular thalamic damage: evidence from an antisaccade task. PLoS ONE. 2011;6:e21517. doi: 10.1371/journal.pone.0021517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters B.D., Szeszko P.R., Radua J., Ikuta T., Gruner P., DeRosse P., Zhang J.P., Giorgio A., Qiu D., Tapert S.F., Brauer J., Asato M.R., Khong P.L., James A.C., Gallego J.A., Malhotra A.K. White matter development in adolescence: diffusion tensor imaging and meta-analytic results. Schizophrenia Bulletin. 2012;38:1308–1317. doi: 10.1093/schbul/sbs054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabbitt P.M.A. Errors and error correction in choice-response tasks. Journal of Experimental Psychology. 1966;71:264–272. doi: 10.1037/h0022853. [DOI] [PubMed] [Google Scholar]
- Raznahan A., Shaw P., Lalonde F., Stockman M., Wallace G.L., Greenstein D., Clasen L., Gogtay N., Giedd J.N. How does your cortex grow? Journal of Neuroscience. 2011;31:7174–7177. doi: 10.1523/JNEUROSCI.0054-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson C., Anderson M., Reid C.L., Fox A.M. Neural indicators of error processing and intraindividual variability in reaction time in 7 and 9 year-olds. Developmental Psychobiology. 2011;53:256–265. doi: 10.1002/dev.20518. [DOI] [PubMed] [Google Scholar]
- Ridderinkhof K.R., Ullsperger M., Crone E.A., Nieuwenhuis S. The role of the medial frontal cortex in cognitive control. Science. 2004;306:443–447. doi: 10.1126/science.1100301. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Fornells A., Kurzbuch A.R., Munte T.F. Time course of error detection and correction in humans: neurophysiological evidence. Journal of Neuroscience. 2002;22:9990–9996. doi: 10.1523/JNEUROSCI.22-22-09990.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollnik J.D., Schroder C., Rodriguez-Fornells A., Kurzbuch A.R., Dauper J., Moller J., Munte T.F. Functional lesions and human action monitoring: combining repetitive transcranial magnetic stimulation and event-related brain potentials. Clinical Neurophysiology. 2004;115:145–153. doi: 10.1016/j.clinph.2003.05.001. [DOI] [PubMed] [Google Scholar]
- Rubia K. Functional brain imaging across development. European Child and Adolescent Psychiatry. 2013 doi: 10.1007/s00787-012-0291-8. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubia K., Smith A.B., Taylor E., Brammer M. Linear age-correlated functional development of right inferior fronto-striato-cerebellar networks during response inhibition and anterior cingulate during error-related processes. Human Brain Mapping. 2007;28:1163–1177. doi: 10.1002/hbm.20347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santesso D.L., Segalowitz S.J. Developmental differences in error-related ERPs in middle- to late-adolescent males. Developmental Psychology. 2008;44:205–217. doi: 10.1037/0012-1649.44.1.205. [DOI] [PubMed] [Google Scholar]
- Santesso D.L., Segalowitz S.J., Schmidt L.A. ERP correlates of error monitoring in 10-year olds are related to socialization. Biological Psychology. 2005;70:79–87. doi: 10.1016/j.biopsycho.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Santesso D.L., Segalowitz S.J., Schmidt L.A. Error-related electrocortical responses are enhanced in children with obsessive-compulsive behaviors. Developmental Neuropsychology. 2006;29:431–445. doi: 10.1207/s15326942dn2903_3. [DOI] [PubMed] [Google Scholar]
- Santesso D.L., Segalowitz S.J., Schmidt L.A. Error-related electrocortical responses in 10-year-old children and young adults. Developmental Science. 2006;9:473–481. doi: 10.1111/j.1467-7687.2006.00514.x. [DOI] [PubMed] [Google Scholar]
- Segalowitz S.J., Dywan J. Individual differences and developmental change in the ERN response: implications for models of ACC function. Psychological Research. 2009;73:857–870. doi: 10.1007/s00426-008-0193-z. [DOI] [PubMed] [Google Scholar]
- Segalowitz S.J., Santesso D.L., Jetha M.K. Electrophysiological changes during adolescence: a review. Brain and Cognition. 2010;72:86–100. doi: 10.1016/j.bandc.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Seifert S., von Cramon D.Y., Imperati D., Tittgemeyer M., Ullsperger M. Thalamocingulate interactions in performance monitoring. Journal of Neuroscience. 2011;31:3375–3383. doi: 10.1523/JNEUROSCI.6242-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P., Kabani N.J., Lerch J.P., Eckstrand K., Lenroot R., Gogtay N., Greenstein D., Clasen L., Evans A., Rapoport J.L., Giedd J.N., Wise S.P. Neurodevelopmental trajectories of the human cerebral cortex. Journal of Neuroscience. 2008;28:3586–3594. doi: 10.1523/JNEUROSCI.5309-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons R.F. The way of our errors: theme and variations. Psychophysiology. 2010;47:1–14. doi: 10.1111/j.1469-8986.2009.00929.x. [DOI] [PubMed] [Google Scholar]
- Sowell E.R., Thompson P.M., Leonard C.M., Welcome S.E., Kan E., Toga A.W. Longitudinal mapping of cortical thickness and brain growth in normal children. Journal of Neuroscience. 2004;24:8223–8231. doi: 10.1523/JNEUROSCI.1798-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stemmer B., Segalowitz S.J., Witzke W., Schonle P.W. Error detection in patients with lesions to the medial prefrontal cortex: an ERP study. Neuropsychologia. 2004;42:118–130. doi: 10.1016/s0028-3932(03)00121-0. [DOI] [PubMed] [Google Scholar]
- Sullivan E.V., Pfefferbaum A., Rohlfing T., Baker F.C., Padilla M.L., Colrain I.M. Developmental change in regional brain structure over 7 months in early adolescence: comparison of approaches for longitudinal atlas-based parcellation. NeuroImage. 2011;57:214–224. doi: 10.1016/j.neuroimage.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swick D., Turken A.U. Dissociation between conflict detection and error monitoring in the human anterior cingulate cortex. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:16354–16359. doi: 10.1073/pnas.252521499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamnes C.K., Fjell A.M., Westlye L.T., Østby Y., Walhovd K.B. Becoming consistent: developmental reductions in intraindividual variability in reaction time are related to white matter integrity. Journal of Neuroscience. 2012;32(3):972–982. doi: 10.1523/JNEUROSCI.4779-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamnes C.K., Walhovd K.B., Dale A.M., Østby Y., Grydeland H., Richardson G., Westlye L.T., Roddey J.C., Hagler D.J., Jr., Due-Tønnessen P., Holland D., Fjell A.M. The Alzheimer's disease neuroimaging initiative. Brain development and aging: overlapping and unique patterns of change. NeuroImage. 2013;68:63–74. doi: 10.1016/j.neuroimage.2012.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamnes C.K., Østby Y., Fjell A.M., Westlye L.T., Due-Tønnessen P., Walhovd K.B. Brain maturation in adolescence and young adulthood: regional age-related changes in cortical thickness and white matter volume and microstructure. Cerebral Cortex. 2010;20:534–548. doi: 10.1093/cercor/bhp118. [DOI] [PubMed] [Google Scholar]
- Taylor M.J., Baldeweg T. Application of EEG ERP and intracranial recordings to the investigation of cognitive functions in children. Developmental Science. 2002;5:318–334. [Google Scholar]
- Taylor S.F., Stern E.R., Gehring W.J. Neural systems for error monitoring: recent findings and theoretical perspectives. Neuroscientist. 2007;13:160–172. doi: 10.1177/1073858406298184. [DOI] [PubMed] [Google Scholar]
- Tiemeier H., Lenroot R.K., Greenstein D.K., Tran L., Pierson R., Giedd J.N. Cerebellum development during childhood and adolescence: a longitudinal morphometric MRI study. NeuroImage. 2010;49:63–70. doi: 10.1016/j.neuroimage.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torpey D.C., Hajcak G., Kim J., Kujawa A., Klein D.N. Electrocortical and behavioral measures of response monitoring in young children during a Go/No-Go task. Developmental Psychobiology. 2012;54:139–150. doi: 10.1002/dev.20590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torpey D.C., Hajcak G., Kim J., Kujawa A.J., Dyson M.W., Olino T.M., Klein D.N. Error-related brain activity in young children: associations with parental anxiety and child temperamental negative emotionality. Journal of Child Psychology and Psychiatry. 2013 doi: 10.1111/jcpp.12041. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torpey D.C., Hajcak G., Klein D.N. An examination of error-related brain activity and its modulation by error value in young children. Developmental Neuropsychology. 2009;34:749–761. doi: 10.1080/87565640903265103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin L.Q., Supekar K., Menon V. Typical and atypical development of functional human brain networks: insights from resting-state FMRI. Frontiers in Systems Neuroscience. 2010;4:21. doi: 10.3389/fnsys.2010.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullsperger M. Genetic association studies of performance monitoring and learning from feedback: the role of dopamine and serotonin. Neuroscience and Biobehavioral Reviews. 2010;34:649–659. doi: 10.1016/j.neubiorev.2009.06.009. [DOI] [PubMed] [Google Scholar]
- Ullsperger M., von Cramon D.Y. The role of intact frontostriatal circuits in error processing. Journal of Cognitive Neuroscience. 2006;18:651–664. doi: 10.1162/jocn.2006.18.4.651. [DOI] [PubMed] [Google Scholar]
- Ullsperger M., von Cramon D.Y., Muller N.G. Interactions of focal cortical lesions with error processing: evidence from event-related brain potentials. Neuropsychology. 2002;16:548–561. doi: 10.1037//0894-4105.16.4.548. [DOI] [PubMed] [Google Scholar]
- van Meel C.S., Heslenfeld D.J., Oosterlaan J., Sergeant J.A. Adaptive control deficits in attention-deficit/hyperactivity disorder (ADHD): the role of error processing. Psychiatry Research. 2007;151:211–220. doi: 10.1016/j.psychres.2006.05.011. [DOI] [PubMed] [Google Scholar]
- van Meel C.S., Heslenfeld D.J., Rommelse N.N., Oosterlaan J., Sergeant J.A. Developmental trajectories of neural mechanisms supporting conflict and error processing in middle childhood. Developmental Neuropsychology. 2012;37:358–378. doi: 10.1080/87565641.2011.653062. [DOI] [PubMed] [Google Scholar]
- van Soelen I.L., Brouwer R.M., van Baal G.C., Schnack H.G., Peper J.S., Collins D.L., Evans A.C., Kahn R.S., Boomsma D.I., Hulshoff Pol H.E. Genetic influences on thinning of the cerebral cortex during development. NeuroImage. 2012;59:3871–3880. doi: 10.1016/j.neuroimage.2011.11.044. [DOI] [PubMed] [Google Scholar]
- van Veen V., Carter C.S. The timing of action-monitoring processes in the anterior cingulate cortex. Journal of Cognitive Neuroscience. 2002;14:593–602. doi: 10.1162/08989290260045837. [DOI] [PubMed] [Google Scholar]
- van Veen V., Carter C.S. Error detection, correction, and prevention in the brain: a brief review of data and theories. Clinical EEG and Neuroscience. 2006;37:330–335. doi: 10.1177/155005940603700411. [DOI] [PubMed] [Google Scholar]
- Velanova K., Wheeler M.E., Luna B. Maturational changes in anterior cingulate and frontoparietal recruitment support the development of error processing and inhibitory control. Cerebral Cortex. 2008;18:2505–2522. doi: 10.1093/cercor/bhn012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal F., Burle B., Bonnet M., Grapperon J., Hasbroucq T. Error negativity on correct trials: a reexamination of available data. Biological Psychology. 2003;64:265–282. doi: 10.1016/s0301-0511(03)00097-8. [DOI] [PubMed] [Google Scholar]
- Vocat R., Pourtois G., Vuilleumier P. Unavoidable errors: a spatio-temporal analysis of time-course and neural sources of evoked potentials associated with error processing in a speeded task. Neuropsychologia. 2008;46:2545–2555. doi: 10.1016/j.neuropsychologia.2008.04.006. [DOI] [PubMed] [Google Scholar]
- Walhovd K.B., Tamnes C.K., Østby Y., Due-Tønnessen P., Fjell A.M. Normal variation in behavioral adjustment relates to regional differences in cortical thickness in children. European Child and Adolescent Psychiatry. 2012;21:133–140. doi: 10.1007/s00787-012-0241-5. [DOI] [PubMed] [Google Scholar]
- Weinberg A., Klein D.N., Hajcak G. Increased error-related brain activity distinguishes generalized anxiety disorder with and without comorbid major depressive disorder. J. Journal of Abnormal Psychology. 2012;121:885–896. doi: 10.1037/a0028270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessel J.R., Danielmeier C., Morton J.B., Ullsperger M. Surprise and error: common neuronal architecture for the processing of errors and novelty. Journal of Neuroscience. 2012;32:7528–7537. doi: 10.1523/JNEUROSCI.6352-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westlye L.T., Walhovd K.B., Bjørnerud A., Due-Tønnessen P., Fjell A.M. Error-related negativity is mediated by fractional anisotropy in the posterior cingulate gyrus–a study combining diffusion tensor imaging and electrophysiology in healthy adults. Cerebral Cortex. 2009;19:293–304. doi: 10.1093/cercor/bhn084. [DOI] [PubMed] [Google Scholar]
- Westlye L.T., Walhovd K.B., Dale A.M., Bjørnerud A., Due-Tønnessen P., Engvig A., Grydeland H., Tamnes C.K., Østby Y., Fjell A.M. Life-span changes of the human brain white matter: diffusion tensor imaging (DTI) and volumetry. Cerebral Cortex. 2010;20:2055–2068. doi: 10.1093/cercor/bhp280. [DOI] [PubMed] [Google Scholar]
- White T., Su S., Schmidt M., Kao C.Y., Sapiro G. The development of gyrification in childhood and adolescence. Brain and Cognition. 2010;72:36–45. doi: 10.1016/j.bandc.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiersema J.R., van der Meere J.J., Roeyers H. ERP correlates of impaired error monitoring in children with ADHD. Journal of Neural Transmission. 2005;112:1417–1430. doi: 10.1007/s00702-005-0276-6. [DOI] [PubMed] [Google Scholar]
- Wiersema J.R., van der Meere J.J., Roeyers H. Developmental changes in error monitoring: an event-related potential study. Neuropsychologia. 2007;45:1649–1657. doi: 10.1016/j.neuropsychologia.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Yakovlev P.I., Lecours A.R. The myelogenetic cycle of regional maturation of the brain. In: Minkowski A., editor. Regional Development of the Brain Early in Life. Blackwell Scientific Publications Inc.; Boston, MA: 1967. pp. 3–70. [Google Scholar]
- Yeung N., Botvinick M.M., Cohen J.D. The neural basis of error detection: conflict monitoring and the error-related negativity. Psychological Review. 2004;111:931–959. doi: 10.1037/0033-295x.111.4.939. [DOI] [PubMed] [Google Scholar]
- Østby Y., Tamnes C.K., Fjell A.M., Westlye L.T., Due-Tønnessen P., Walhovd K.B. Heterogeneity in subcortical brain development: A structural magnetic resonance imaging study of brain maturation from 8 to 30 years. Journal of Neuroscience. 2009;29:11772–11782. doi: 10.1523/JNEUROSCI.1242-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]