Abstract
Background
Abnormalities in habituation have been documented in Autism Spectrum Disorder (ASD) and Williams syndrome (WS). Such abnormalities have been proposed to underlie the distinctive social and non-social difficulties that define ASD, including sensory features and repetitive behaviours, and the distinctive social phenotype characterizing WS.
Methods
We measured habituation in 39 preschoolers with ASD, 20 peers with WS and 19 typically developing (TD) children using an eye-tracking protocol that measured participants’ duration of attention in response to a repeating stimulus and a novel stimulus presented side by side across multiple trials.
Results
Participants in the TD group and the WS group decreased their attention toward the repeating stimulus and increased their attention to the novel stimulus over time. Conversely, the ASD group showed a similar attentional response to the novel and repeating stimuli. Habituation was correlated with social functioning in the WS but not in the ASD group. Contrary to predictions, slower habituation in ASD was associated with lower severity of repetitive behaviours.
Conclusions
Habituation appears to be intact in WS and impaired in ASD. More research is needed to clarify the nature of the syndrome-specific patterns of correlations between habituation and social and non-social functioning in these neurodevelopmental disorders.
Keywords: Habituation, Learning, Eye-tracking, Repetitive behaviours, Social cognition, Autism, Williams syndrome
1. Introduction
Individuals with Autism Spectrum Disorder (ASD) often show prolonged attention to visual repetition, such as watching the spin-cycle of a washing machine (Kanner, 1943, Baron-Cohen, 2006), and reduced attention to social stimuli (Klin et al., 2015). Both phenomena, which reflect the pathognomonic social impairments and behavioural rigidity that defines ASD, have been linked to potential abnormalities in habituation (Green et al., 2015, Guiraud et al., 2011, Ramaswami, 2014).
Habituation is defined by the progressive decrement in response to a stimulus when it is repeated (Thorpe, 1966, Schmid et al., 2015). From infancy onwards, repeated exposure to an event make children less interested and responsive to it over time. This process reflects a strategic allocation of processing resources away from what is “already known” in favour of “what is not known yet”, thus facilitating learning and adaptive response to changes in the environment (Groves and Thompson, 1970, Lloyd et al., 2014). Habituation in infancy is thought to reflect information processing efficiency and it is predictive of cognitive functioning later in life (Bornstein and Sigman, 1986, Colombo, 1993).
It has been proposed that lack of habituation to sensory inputs might result in an exaggerated perception of changes in the environment in children with ASD. This in turn would lead to sensory overstimulation, distress and the perception of the environment as highly unpredictable, with repetitive behaviours serving as a way of constraining and controlling this unpredictability by imposing structure (Dawson and Lewy, 1989, Uljarevic, 2013). This account is consistent with the original descriptions of Leo Kanner (1943), and more detailed theoretical accounts put forward by other scholars (e.g. Hutt et al., 1964, Ornitz and Rivto, 1968, Kinsbourne, 1980), who suggested the potential role of repetitive behaviours as a means of warding of anxiety caused by inefficient habituation to sensory stimuli (see also Gomot et al., 2008, Leekam et al., 2011).
Some empirical research appears to be consistent with this framework. For example, James and Barry (1980) documented lack of habituation in response to the repeated observation of visual stimuli (white circles on a black background) in ASD. More recently, Perry et al. (2007) documented that adults with ASD were slower compared to typical peers in dampening their response to repeated stimuli in a startle response paradigm, and Guiraud et al. (2011) reported reduced habituation to repeated sounds in infants at high risk for autism. However, counterevidence exists, including reports of normative habituation in ASD, and a lack of clear associations between habituation and repetitive behaviours (Baranek et al., 2006, Rogers and Ozonoff, 2005, McCormick et al., 2014). Some of the inconsistent findings might reflect heterogeneity in habituation profiles within the ASD population. Indeed, research has suggested the existence of subgroups, characterized by different habituation response to sensory stimuli within the ASD population. For example, Schoen et al. (2008) identified two subgroups within a sample of 40 children with ASD (age range: 5–15 years), one characterized by slower latency and faster habituation and the other by faster latency and slower habituation in response to sensory stimuli. Similar findings were reported by Hirstein et al. (2001). Additionally, Green et al. (2015) reported decreased neural habituation in sensory cortices and the amygdala in a subgroup of individuals with ASD who were characterized by sensory over-responsiveness at the behavioural level.
Abnormalities in habituation have been also linked to the social deficits characterizing ASD. Webb et al. (2010) documented slowed visual habituation to faces in toddlers with severe symptoms of ASD, but not in those whose symptoms were mild. Moreover, the slower habituation rate was correlated with poor social and communication skills. Similarly, other studies reported reduced neural habituation (lack of decrease in amygdala responsiveness) in response to faces in children and adults with ASD (Kleinhans et al., 2009, Swartz et al., 2013, Wiggins et al., 2014). Atypical habituation was associated with severity of social impairment, and modulated by both the properties of the stimuli (emotions displayed in the face stimuli) and the participants’ characteristics. Based on this body of research, it has been proposed that lack of habituation is causally related to the core social and non-social symptoms of autism, with social symptoms reflecting failure to habituate and consequent hyper-responsivity to social stimuli, and repetitive behaviours emerging as a coping strategy in response to over-responsivity to sensory inputs (Guiraud et al., 2011, Ramaswami, 2014, Sinha et al., 2014, Wiggins et al., 2014).
Somewhat paradoxically, a lack of habituation has also been proposed to be causally related to the behavioural features of William syndrome (WS), a condition characterized by symptoms that are remarkably different from those that define ASD. WS is a rare neurodevelopmental disorder (estimated prevalence of 1:7500 to 1:20,000; Stromme et al., 2002) presenting with impaired visuospatial abilities and social-pragmatic skills alongside an increased drive for social engagement. While WS and ASD present with similar difficulties in “reading” the meaning of people’s gaze and facial expressions (Tager-Flusberg and Skwerer, 2013), individuals with WS, in sharp contrast with those with ASD, show unusually intense interest toward social stimuli, in particular toward faces and emotional displays, as well as increased motivation for social interaction (Hocking, 2017, Riby and Hancock, 2008, Riby and Hancock, 2009, Riby et al., 2013, Dodd and Porter, 2010, Doherty-Sneddon et al., 2009). Recently, it has been proposed that the increased social motivation characterizing WS might reflect a failure to habituate to faces, which causes social stimuli to appear unusually novel and interesting (Järvinen et al., 2012, Järvinen et al., 2013). Empirical findings supporting this notion include evidence of slow habituation in electrodermal activity response to social (and non-social) stimuli in adults with WS (Järvinen et al., 2012). However, no other studies have examined habituation in young children with WS.
In summary, a lack of habituation has been linked to both the sensory abnormalities and impaired social abilities in ASD, and the increased social motivation that characterizes the behavioural profile in WS. To resolve the logical inconsistency of a specific factor (reduced habituation) leading to opposing behavioural profiles (decreased social orienting/motivation in ASD and increased social orienting/motivation in WS), cross-syndrome research focused on habituation in ASD and WS is needed.
The current study addresses this issue by examining habituation in preschoolers with ASD, age- and IQ-matched children with WS, and a comparison group of typically developing (TD) children. In particular, we investigated whether (a) visual habituation to repeated stimuli differed across children with ASD, WS and TD; and (b) whether habituation rate was associated with social and non-social features within each group. On the basis of the literature discussed above, we predicted abnormal habituation across both the ASD and the WS groups. We also expected associations between habituation and both social features and repetitive behaviours in ASD, in keeping with previous research. Due to the lack of previous research, no specific hypotheses were proposed regarding the associations between habituation and social features in young children with WS.
2. Methods
2.1. Participants
The participants were 39 preschoolers with Autism Spectrum Disorder (ASD; mean age = 44.1 months), 20 children with William syndrome (WS; mean age = 50.8 months) and 19 typically developing (TD) children (mean age = 49.4 months). Participants with ASD were recruited through the Victorian Autism Specific Early Learning and Care Centre, an autism intervention program located at the La Trobe University Community Children’s Centre. Participants in the WS group were recruited through the Williams Syndrome Family Support Group (Victoria) and the Williams Syndrome Association Australia. The TD children were recruited through a childcare setting affiliated with Macquarie University.
The diagnoses of ASD were previously made by community-based health care professionals and confirmed for the study using the Autism Diagnostic Observation Schedule-Second edition (ADOS-2) (Lord et al., 2012) administered by a clinician with demonstrated reliability in the use of this measure. Exclusion criteria for the ASD group included the presence of uncorrected hearing or vision impairment, and the presence of a major medical problem. All participants with WS had their diagnosis confirmed with the positive fluorescent in situ hybridisation (FISH) test and displayed the typical ∼1.6 Mb heterozygous microdeletion at 7q11.23. The TD children had no reported neurodevelopmental disorders. The ASD and WS groups did not differ on language, cognitive level, motor skills or overall adaptive behaviour (Table 1). However, as expected, children with WS had higher scores on the Socialization subscale of the VABS compared to the children with ASD. Both ASD and WS groups had significantly lower scores on each measure compared to children in the TD group.
Table 1.
Participant characteristics.
| ASD (N = 39) | WS (N = 20) | TD (N = 19) |
T testvalue p-value ASD vs WS |
T testvalue p-value ASD vs TD |
T testvalue p-value WS vs TD |
|
|---|---|---|---|---|---|---|
| Age (months): M (SD) | 44.16 (11.56) | 50.80 (16.65) | 49.45 (11.49) | 0.12 | 0.11 | 0.77 |
| Gender: M, F | 37, 2 | 10, 10 | 14, 5 | – | – | – |
| MSEL Total DQ M (SD) | 62.08 (26.79) | 56.89 (15.20) | 105.29 (14.31) | 0.43 | <0.001 | <0.001 |
| MSEL Verbal DQ M (SD) | 56.09 (28.77) | 56.44 (17.45) | 104.24 (16.42) | 0.96 | <0.001 | <0.001 |
| MSEL NonVerbal DQ M (SD) | 68.07 (26.42) | 57.34 (14.50) | 104.95 (16.64) | 0.10 | <0.001 | <0.001 |
| VABS Communication M (SD) | 72.11 (19.17) | 71.60 (11.31) | 106.35 (9.62) | 0.91 | <0.001 | <0.001 |
| VABS Daily Living Skills M (SD) | 74.56 (27.02) | 70.65 (11.67) | 102.12 (12.93) | 0.54 | <0.001 | <0.001 |
| VABS Socialization M (SD) | 72.92 (14.28) | 81.75 (12.57) | 106.88 (14.81) | 0.02 | <0.001 | <0.001 |
| VABS Motor skills M (SD) | 76.58 (18.95) | 69.65 (10.88) | 103.53 (9.88) | 0.14 | <0.001 | <0.001 |
| VABS ABC score M (SD) | 71.00 (19.27) | 70.35 (10.20) | 104.18 (11.21) | 0.88 | <0.001 | <0.001 |
| ADOS-2 Social Affect M (SD) | 13.29 (4.59) | – | – | |||
| ADOS-2 Repetitive Behaviours: M (SD) | 7.48 (1.80) | – | – |
ASD = Autism Spectrum Disorder; WS = Williams Syndrome; TD = Typically Developing; MSEL = Mullen Scales of Early Learning; VABS = Vineland Adaptive Behavior Scales, Second Edition; ADOS-2 = Autism Diagnostic Observation Schedule-Second Edition.
2.2. Measures
Participants’ cognitive level was measured with the Mullen Scales of Early Learning (MSEL) (Mullen, 1995). Developmental quotient (DQ) scores were calculated according to the formula: DQ = age equivalent scores/chronological age X 100, and averaged to create an overall DQ, a verbal DQ (encompassing the receptive and expressive language subscales) and a non-verbal DQ (encompassing the visual reception and fine motor subscales). Two participants with WS were administered the Differential Ability Scales second edition (DAS-II) (Elliott, 2007) as a measure of intellectual ability as they were too old to be administered the MSEL (maximum age 68 months). Research has shown good convergent validity of the MSEL and the DAS-II (Bishop et al., 2011). Additionally, participants’ adaptive behaviour was assessed using the Vineland Adaptive Behavior Scales (VABS) (Sparrow et al., 2005).
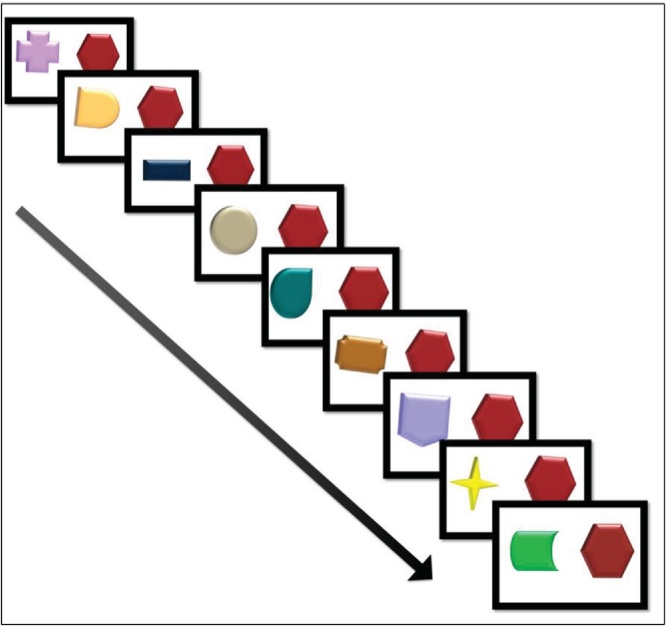
The habituation protocol measured the fixation duration to a repeating stimulus and to a novel stimulus presented side by side on a computer screen across nine trials presented in a fixed random order. In each trial, two shapes appear simultaneously on the left and right sides of the computer screen, both describing a 360° spin rotation, one each to the left and right of the child’s midline. One shape was different on every trial (the novel stimulus), while the other remained unchanged across trials (the repeating stimulus). All stimuli were against a white background. There were two fixed random orders, so that for half participants the repeating stimulus was on right side of the screen and for the other half it was on the left side of the screen. The duration of each video was 27 s, 3 s for each of the 9 trials. The stimuli comprised non-social, emotionally neutral stimuli to avoid the risk that characteristics other than novelty/repetition would elicit between-group differences in baseline salience attributed to the stimuli (Fig. 1).
Fig. 1.
Eye-tracking stimuli. In each trial, two shapes appear simultaneously on the left and right sides of the computer screen. One shape was different on every trial (the novel stimulus), while the other remained unchanged across trials (the repeating stimulus). The duration of each trial was 3 s.
2.3. Procedure
The study was approved by the La Trobe University Human Ethics Committee and informed consent was obtained from the children’s parents. The children were tested in a quiet room in one of three University or early intervention settings, depending on where the child was recruited. Three children with WS were administered the standardized tests and the experimental task in their home due to travelling difficulties on the part of their families. The experiment presented here were part of a larger study examining social and non-social learning in young children with ASD and WS.
Participants were seated in a comfortable chair, 60 cm from the computer monitor in front of a small table. No explicit direction was given. Videos were presented on a Tobii T120 binocular eye-tracker monitor with an imbedded camera (120-Hz, 1280 × 1024 pixels resolution, average precision of 0.5 of visual angle). During observation of the video-stimuli, participants’ eye-movements were recorded using the eye-tracker system and analysed using frame-by-frame defined areas of interest using Tobii Studio analysis software. Fixation criteria were set to Tobii Studio defaults of a 30-pixel dispersion threshold for 100 ms. The two regions of interest included in the analyses were the novel stimulus and the repeating stimulus.
The rate of change (slope) of attention duration to the Novel and to the Repeating stimuli over trials was computed to measure habituation. To show habituation to the repeating stimulus, participants’ attention to the novel stimulus should increase while their attention to the repeating stimulus should decrease over trials (Fantz, 1964, Colombo and Mitchell, 2009). Furthermore, the number of trials to habituation was calculated as an additional index of habituation, based on the conventional algorithm indicating two consecutive fixations to the repeated stimuli at a decrement of 50% or more from the mean of the two longest previous fixations within the session as the criterion for habituation (Colombo et al., 1987, Colombo et al., 2012).
Eye-tracking calibration was controlled by Tobii Studio software. A five-point calibration and validation procedure was used, with calibrations being signalled as “valid” by the software when all five points showed good fit in the computed mapping for both eyes. The procedure was repeated until the five points were properly calibrated for each eye.
3. Results
We first measured participants’ total amount of fixations to the screen (using fixation count as the metric), in order to rule out group differences in attentional engagement with the task. Results of a one-way between-subjects ANOVA showed no effect of Group [F (2, 76) = 0.07; p = 0.92].
Next, participants’ rate of change in total fixation duration to the Novel and Repeating stimuli over trials were subjected to a 3 (Group) X 2 (Condition − Novel, Repeating) ANOVA. There was a main effect of Condition [F (2, 74) = 12.49, p =0.001, η2p = 0.14], no main effect of Group [F (2, 74) = 1.12, p =0.33, η2p = 0.02], and a significant Group X Condition interaction [F (2, 74) = 4.61, p =0.01. η2p = 0.11]. Follow-up pair-wise comparisons showed that while participants in the WS and TD decreased their attention to repeating stimulus and increased their attention to the novel stimulus over time (WS: adjusted p [Bonferroni] = 0.001, η2p = 0.13; TD: adjusted p [Bonferroni] = 0.02, η2p = 0.07), this was not the case in the ASD group (adjusted p [Bonferroni] = 0.89, η2p = 0.00) who showed a decrease in attention to both stimuli over time.
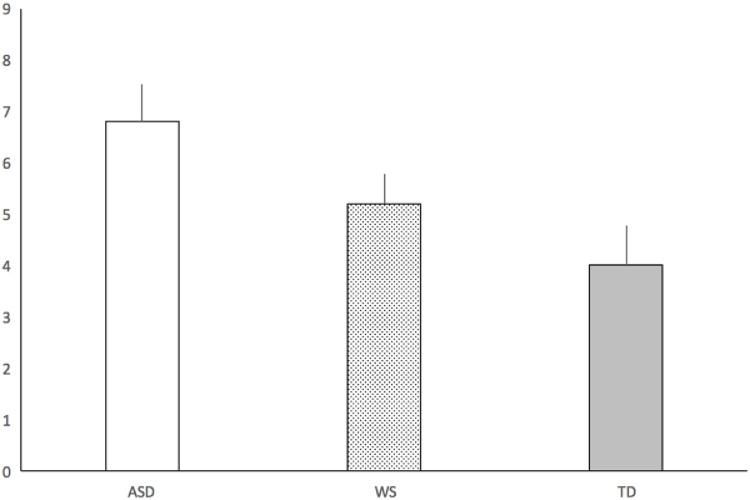
The number of trials to habituation was subjected to a one-way ANOVA, which revealed a main effect of Group [F (2, 74) = 3.56, p =0.33, η2p = 0.09]. Pair-wise comparisons showed that participants in the TD group needed fewer trials to habituate to the repeating stimulus compared to participants in the ASD group (adjusted p [Bonferroni] <0.05, η2p = 0.09). Conversely, there was no difference in number of trials to habituation between the TD and the WS groups (adjusted p [Bonferroni] = 1.00, η2p = 0.00) or between the ASD and WS groups (adjusted p [Bonferroni] = 0.41, η2p = 0.04). In average, the number of trials to habituation was 6.8 (SD = 4.5) in the ASD group, 5.2 (SD = 2.5) in the WS group, and 4 (SD = 3.3) in the TD group (see Fig. 2).
Fig. 2.
Average number of trials to reach habituation.
X-axis represents trials. Error bars represent standard errors. ASD = Autism Spectrum Disorder; WS = Williams Syndrome; TD = Typically Developing.
Associations between habituation indexes and relevant participant characteristics were examined using Pearson Product Moment Correlation Coefficients. We found no correlations between habituation indexes and age and DQ in any of the groups. In the WS group, there was a negative association between number of trials to habituation and each subscale of the VABS Socialization Scales (Interpersonal Relationship, r = −0.46, p < 0.05; Social Play, r = −0.44, p = 0.06; Social Coping r = −0.45, p < 0.05), which indicates that slower habituation related to poorer social functioning in WS.
In the ASD group, there was a negative correlation between number of trials to habituation and repetitive behaviours, as measured though the ADOS Restricted and Repetitive Behaviors (RRBs) calibrated score (r = −0.36, p < 0.05). To further explore the link between habituation and RBBs, we conducted a one-way ANOVA examining whether children with ASD with high levels of RRBs (N = 9), defined as scoring in the top 20% of score distribution (Lyall et al., 2014, Uljarević et al., 2016), differed from the rest of the sample (n = 30) in terms of the number of trials to habituation. This analysis confirmed that participants with ASD in the high RRB group habituated more rapidly to the repeating stimuli compared to participants with ASD who had low repetitive behaviours [F(1, 30) = 6.167, p = 0.019, Cohen’s d = 1.14].
4. Discussion
The current study employed a novel eye-tracking paradigm to examine visual attention in response to novel versus repeating stimuli in preschoolers with ASD, those with WS, and TD children. Although children with WS and TD decreased their attention toward a repeating stimulus and increased their attention to a novel stimulus over time, children with ASD showed a decrease in attention to both stimuli over time. Our findings showed that children with WS who habituated more rapidly to the repeating stimuli had better social skills compared to those showing slower habituation. Contrary to expectations, our findings showed that slow habituation in ASD was associated with lower severity of RRBs. These findings are discussed below.
4.1. Habituation in ASD
Children in the ASD group attended less to the repeated stimuli over time, similar to the WS and TD groups. However, our findings showed decreased attention not only to the repeated stimuli over time but also to the novel stimuli in ASD − suggesting a decreased responsivity to novelty versus repetition in this population (Vivanti et al., 2017). In addition, they needed more trials to habituate to the repetitive stimuli. Our findings do not support, however, models linking atypical habituation to repetitive behaviours and social impairments in ASD, as slow habituation was unrelated to social functioning as measured by the ADOS-2 and the VABS. Importantly, previous research in the area that has linked reduced habituation to severity of ASD features has been predominately focused on habituation to social stimuli (e.g., Kleinhans et al., 2009, Swartz et al., 2013, Webb et al., 2010, Wiggins et al., 2014). It is possible that the non-social stimuli used in the current study, by ‘detaching’ habituation from social processing, captured a dimension of habituation that does not contribute to the severity of ASD symptoms. Somewhat paradoxically, we found that children with slower habituation had fewer RRBs in our ASD sample. Given the inconsistency of this pattern with existing theoretical frameworks and the paucity of research focused on this issue, further research is needed to explore this counterintuitive association.
4.2. Habituation in WS
Our findings showed similar visual habituation between preschoolers with WS and those with TD, contrary to previous studies which have reported slower habituation in WS (Järvinen et al., 2012). The current findings suggested that WS and TD children decreased their attention toward the repeating stimulus and increased their attention to the novel stimulus over time to a similar degree, and there were no significant differences in the number of trials needed to habituate. Methodological differences between the current study and the Järvinen et al. study may explain these contrasting results. In particular, the Järvinen et al. study involved a much older sample, and stimuli designed to elicit emotional responses, while the repeating and novel stimuli in the present study were emotionally neutral. Additionally, the Järvinen et al. study focused on electrodermal activity habituation, while our study measured visual habituation. While the two phenomena are thought to reflect the same underlying construct of decreased responsivity in response to repeated exposure of the same stimulation (Turk-Browne et al., 2008), it is possible that differences in measurement techniques contributed to the conflicting findings.
Despite the lack of significant differences between the WS and the TD groups across habituation measures, we found that those children with WS who required more trials to habituate showed poorer social functioning. This association suggests that children who are slower in registering repetition versus novelty may struggle more with processing social information; however, the correlational nature of this finding does not allow us to establish the causal direction of this association, and further research is needed to explore this issue.
Overall, results from our cross-syndrome study provide several new insights on the role played by habituation in the social and non-social features of ASD and WS. First, while the atypical response to repetition in the ASD group documented here is intuitively consistent with several autistic features, including a drive towards constancy, sameness and predictability, and “aversion” to unpredictable and chaotic social situations, our correlational data provide little support for the view that a habituation impairment underlies the symptoms of ASD. Additionally, differences in habituation observed in the current study do not appear to reflect a fundamental habituation impairment. Importantly, children with ASD were able to parse stimuli according to a “novelty” versus “repetition” principle; although they required more trials to “get bored” of repetition compared to the other groups, their attention was longer in response to the first presentation of the stimulus and became shorter as the same stimulus was repeatedly presented. However, when observing novel and repeating stimuli side by side in the computer screen, children with ASD did not show an attentional preference for the novel stimulus. This phenomenon might reflect a diminished propensity, rather than an absolute inability, to allocate attentional resources toward novel information when both novel and repeated information are available. This reduced “novelty bias”, coupled with slower habituation, might play a role in the learning difficulties characterizing ASD (Vivanti and Rogers, 2014, Vivanti et al., 2016, Vivanti et al., 2017). Habituation reflects an adaptive filter mechanism that facilitates allocation of cognitive resources to novel “not-yet-learned” information, and therefore provides children with a powerful means to incorporate new knowledge into their behavioural repertoire; this mechanism also prevents “already learned” information from saturating processing capacity. Early emerging differences in this strategic allocation of attention might impact early learning experiences, as selective attention and adaptive action in response to environmental inputs play a major role in shaping developmental trajectories. Failing to pay special attention to stimuli that were never seen/heard before compared to familiar ones might disproportionally affect learning in the social domain, as people are more likely to be a source of novel information compared to objects. Therefore, it is possible that the differences in the habituation task documented here, rather than being a fundamental impairment that causes the symptoms of ASD, reflect a subtle alteration in the “novelty preference” bias which can affect learning experiences during critical developmental stages.
Similarly, our results do not support the view of a fundamental habituation deficit in children with WS. Rather, our correlational data indicate that efficiency of habituation in this syndrome is relevant to social functioning. Social information processing requires rapid detection of patterns and quick, adaptive responses to constantly changing scenarios (Engel et al., 2001). It is possible that, despite the motivation to engage with social information that characterizes children with WS, individual differences in processing novelty versus repetition affect their ability to efficiently navigate the ceaseless flow of stimuli in everyday social situations. This view is consistent with research indicating that individuals with WS often show normative or enhanced performance in simple social tasks (e.g., imitation of emotional expressions; Fidler et al., 2007), but struggle with complex social information processing (e.g., complex social judgements on trustworthiness; Tager-Flusberg and Skwerer, 2013). Importantly, while the current findings point to the potential relevance of habituation to behavioural features in ASD and WS, longitudinal studies will be needed to examine the nature, correlates and developmental consequences of divergent habituation profiles across these neurodevelopmental disorders.
This study has a number of limitations that warrant mention. Firstly, the fact the participants with ASD were paying less attention to the novel stimuli over time could reflect a lack of interest in the task rather than reduced habituation. However, a number of factors mitigate against this interpretation. First, we used a relatively small number of trials in order to maximize engagement with the task, and to avoid attrition due to inattention in young children with ASD and WS. Secondly, we calculated the number of fixations to the screen as a measure of attentional engagement with the task, and found similar number of fixations across groups (although the duration of those fixations decreased in children with ASD). Therefore, our findings that children with ASD differ from those with WS (who were closely matched in the terms of cognitive functioning) in their attentional response to the novel stimuli is unlikely to simply reflect less engagement with the task.
Another limitation is that, due to the observational nature of the protocol, the ADOS-2 is limited in its ability to assess restricted and rigid patterns of behaviours/insistence on sameness. Additionally, as different types of repetitive behaviours have been suggested to differ in terms of neural underpinnings (Langen et al., 2011), behavioural correlates, triggers and functions (Leekam et al., 2011), developmental trajectories (Evans et al., 1997) as well as patterns of familiarity and genetic underpinnings (Silverman et al., 2002, Uljarević et al., 2016), future research should focus on measures that capture the multi-dimensional nature of repetitive behaviours. Furthermore, it cannot be excluded that despite our preventative efforts, characteristics other than the repetitive versus novel nature of the stimuli influenced the children’s gaze patterns. However, if the two classes of stimuli elicited different gaze patterns due to some unmeasured stimulus properties, all the groups would be subject to the same bias.
Despite these limitations, our cross-syndrome approach and the novel experimental paradigm used to examine habituation can be viewed as strengths of the current study, and provided us with the opportunity to address a number of gaps and limitations in previous research. In particular, habituation was examined for the first time in a homogeneous sample of preschoolers with WS, allowing us to investigate this process during early development. We also measured visual habituation using eye-tracking in a passive view paradigm—an approach that offers more precision in capturing attentional patterns compared to behavioural coding techniques. It is also one that is considered to be optimally suited for research with young children with neurodevelopmental disorders who experience difficulties with following verbal instructions and handling complex social and cognitive demands, such as in children with ASD and WS (Venker and Kover, 2015). Furthermore, the use of non-social and arousal-neutral stimuli allowed us to eliminate the potential confounding factor introduced by the differences in social-emotional processing that distinguish children with ASD and WS. We therefore contend that our paradigm provided an unbiased test of response to novelty versus repetition compared to the use of social and emotionally-relevant stimuli that has been commonly employed in previous studies. Future studies should build on this paradigm by manipulating relevant stimulus properties including the reward value and cognitive load.
In conclusion, atypical habituation has been proposed to underlie both the social and non-social difficulties (including atypical sensory responsivity) that define children with ASD, as well as the distinctive social phenotype in WS. Our findings indicate that habituation appears to be normative in WS and impaired in ASD. Furthermore, habituation appeared to be associated with social and non-social functioning according to syndrome-specific patterns that were inconsistent with predictions from current theoretical perspectives. Future studies are needed to further examine the role of habituation in the social and non-social areas of impairment that characterize children with ASD and WS.
Conflict of interest
None.
Acknowledgements
We would like to acknowledge the children and parents involved in the study, the Victorian Autism Specific Early Learning and Care Centre Team who facilitated recruitment and testing of the ASD sample, the Williams Syndrome Family Support Group (Victoria) and the Williams Syndrome Association Australia. We would also like to acknowledge the valuable contribution of A/Prof Melanie Porter, Stephanie Sievers, Anna Atkinson, Jessica Reeve, Simone Griffith, Jacqueline Maya and Cathriona Clarke.
Contributor Information
Giacomo Vivanti, Email: giacomo.vivanti@drexel.edu.
Darren R. Hocking, Email: D.Hocking@latrobe.edu.au.
Peter A.J. Fanning, Email: p.fanning@latrobe.edu.au.
Valentina Postorino, Email: valentina.postorino86@gmail.com.
Luigi Mazzone, Email: gigimazzone@yahoo.it.
Cheryl Dissanayake, Email: c.dissanayake@latrobe.edu.au.
References
- Baranek G.T., David F.J., Poe M.D., Stone W.L., Watson L.R. Sensory experiences questionnaire. J. Child Psychol. Psychiatry. 2006;47(6):591–601. doi: 10.1111/j.1469-7610.2005.01546.x. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S. Two new theories of autism: hyper-systemising and assortative mating. Arch. Dis. Child. 2006;91(1):2–5. doi: 10.1136/adc.2005.075846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop S.L., Guthrie W., Coffing M., Lord C. Convergent validity of the Mullen Scales of Early Learning and the differential ability scales in children with autism spectrum disorders. Am. J. Intell. Dev. Disabil. 2011;116(5):331–343. doi: 10.1352/1944-7558-116.5.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein M.H., Sigman M.D. Continuity in mental development from infancy. Child Dev. 1986:251–274. doi: 10.1111/j.1467-8624.1986.tb00025.x. [DOI] [PubMed] [Google Scholar]
- Colombo J., Mitchell D.W. Infant visual habituation. Neurobiol. Learn. Mem. 2009;92(2):225–234. doi: 10.1016/j.nlm.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo J., Mitchell D.W., O'Brien M., Horowitz F.D. The stability of visual habituation during the first year of life. Child Dev. 1987:474–487. [PubMed] [Google Scholar]
- Colombo J., Brez C.C., Curtindale L.M. Infant perception and cognition. Handb. Psychol. 2012;6:61–89. [Google Scholar]
- Colombo J. Vol. 5. Sage publications; 1993. (Infant Cognition: Predicting Later Intellectual Functioning). [Google Scholar]
- Dawson G., Lewy A. Arousal, attention and the socioemotional impairments of individuals with autism. In: Dawson G., editor. Autism: Nature, Diagnosis, and Treatment. Guilford; New York: 1989. pp. 49–74. [Google Scholar]
- Dodd H.F., Porter M.A. I see happy people: attention bias towards happy but not angry facial expressions in Williams syndrome. Cognit. Neuropsychiatry. 2010;15(6):549–567. doi: 10.1080/13546801003737157. [DOI] [PubMed] [Google Scholar]
- Doherty-Sneddon G., Riby D.M., Calderwood L., Ainsworth L. Stuck on you: face-to-face arousal and gaze aversion in Williams syndrome. Cognit. Neuropsychiatry. 2009;14(6):510–523. doi: 10.1080/13546800903043336. [DOI] [PubMed] [Google Scholar]
- Elliott C. 2nd ed. Harcourt Assessment; San Antonio, TX: 2007. Differential Ability Scale. [Google Scholar]
- Engel A.K., Fries P., Singer W. Dynamic predictions: oscillations and synchrony in top–down processing. Nat. Rev. Neurosci. 2001;2(10):704–716. doi: 10.1038/35094565. [DOI] [PubMed] [Google Scholar]
- Evans D.W., Leckman J.F., Carter A., Reznick J.S., Henshaw D., King R.A., Pauls D. Ritual: habit and perfectionism: the prevalence and development of compulsive-like behavior in normal young children. Child Dev. 1997;68:58–68. [PubMed] [Google Scholar]
- Fantz R.L. Visual experience in infants: decreased attention to familiar patterns relative to novel ones. Science. 1964;146(3644):668–670. doi: 10.1126/science.146.3644.668. [DOI] [PubMed] [Google Scholar]
- Fidler D.J., Hepburn S.L., Most D.E., Philofsky A., Rogers S.J. Emotional responsivity in young children with Williams syndrome. Am. J. Ment. Retard. 2007;112(3):194–206. doi: 10.1352/0895-8017(2007)112[194:ERIYCW]2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomot M., Belmonte M.K., Bullmore E.T., Bernard F.A., Baron-Cohen S. Brain hyper-reactivity to auditory novel targets in children with high-functioning autism. Brain. 2008;131(9):2479–2488. doi: 10.1093/brain/awn172. [DOI] [PubMed] [Google Scholar]
- Green S.A., Hernandez L., Tottenham N., Krasileva K., Bookheimer S.Y., Dapretto M. Neurobiology of sensory overresponsivity in youth with autism spectrum disorders. JAMA Psychiatry. 2015;72(8):778–786. doi: 10.1001/jamapsychiatry.2015.0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groves P.M., Thompson R.F. Habituation: a dual-process theory. Psychol. Rev. 1970;77(5):419. doi: 10.1037/h0029810. [DOI] [PubMed] [Google Scholar]
- Guiraud J.A., Kushnerenko E., Tomalski P., Davies K., Ribeiro H., Johnson M.H., BASIS Team Differential habituation to repeated sounds in infants at high risk for autism. Neuroreport. 2011;22(16):845–849. doi: 10.1097/WNR.0b013e32834c0bec. [DOI] [PubMed] [Google Scholar]
- Hirstein W., Iversen P., Ramachandran V.S. Autonomic responses of autistic children to people and objects. Proceedings of the Royal Society of London B: Biological. Sciences. 2001;268(1479):1883–1888. doi: 10.1098/rspb.2001.1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hocking D. Williams syndrome. In: Rinehart N., Bradshaw J.L., Enticott P., editors. Developmental Disorders of the Brain. Psychology Press New York : Taylor Francis Group; 2017. [Google Scholar]
- Hutt C., Hutt S.J., Lee D., Ounsted C. Arousal and childhood autism. Nature. 1964;204:908–909. doi: 10.1038/204908a0. [DOI] [PubMed] [Google Scholar]
- Järvinen A.M., Dering B., Neumann D., Ng R., Crivelli D., Grichanik M., Korenberg J.R., Bellugi U. Sensitivity of the autonomic nervous system to visual and auditory affect across social and non-social domains in Williams syndrome. Front. Psychol. 2012:343. doi: 10.3389/fpsyg.2012.00343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Järvinen A., Korenberg J.R., Bellugi U. The social phenotype of Williams syndrome. Curr. Opin. Neurobiol. 2013;23(3):414–422. doi: 10.1016/j.conb.2012.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James A.L., Barry R.J. Respiratory and vascular responses to simple visual stimuli in autistics, retardates and normals. Psychophysiology. 1980;17:541–547. doi: 10.1111/j.1469-8986.1980.tb02294.x. [DOI] [PubMed] [Google Scholar]
- Kanner L. Autistic disturbances of affective contact. Nerv. Child. 1943;2:217–250. [PubMed] [Google Scholar]
- Kinsbourne M. Do repetitive movement patterns in children and animals serve a dearousing function? Dev. Behav. Pediatr. 1980;1:39–42. [PubMed] [Google Scholar]
- Kleinhans N.M., Johnson L.C., Richards T., Mahurin R., Greenson J., Dawson G., Aylward E. Reduced neural habituation in the amygdala and social impairments in autism spectrum disorders. Am. J. Psychiatry. 2009;166(4):467–475. doi: 10.1176/appi.ajp.2008.07101681. [DOI] [PubMed] [Google Scholar]
- Klin A., Shultz S., Jones W. Social visual engagement in infants and toddlers with autism: early developmental transitions and a model of pathogenesis. Neurosci. Biobehav. Rev. 2015;50:189–203. doi: 10.1016/j.neubiorev.2014.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langen M., Durston S., Kas M.J.H., Van Engeland H., Staal W.G. The neurobiology of repetitive behavior: ... and men. Neurosci. Biobehav. Rev. 2011;35:356–365. doi: 10.1016/j.neubiorev.2010.02.005. [DOI] [PubMed] [Google Scholar]
- Leekam S.R., Prior M.R., Uljarevic M. Restricted and repetitive behaviors in autism spectrum disorders: a review of research in the last decade. Psychol. Bull. 2011;137(4):562–593. doi: 10.1037/a0023341. [DOI] [PubMed] [Google Scholar]
- Lloyd D.R., Medina D.J., Hawk L.W., Fosco W.D., Richards J.B. Habituation of reinforcer effectiveness. Front. Integr. Neurosci. 2014;7:107. doi: 10.3389/fnint.2013.00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C., Rutter M., DiLavore P.C., Risi S., Gotham K., Bishop S. Western Psychological Services; Los Angeles, CA: 2012. Autism Diagnostic Observation Schedule −2. [Google Scholar]
- Lyall K., Constantino J.N., Weisskopf M.G., Roberts A.L., Ascherio A., Santangelo S.L. Parental social responsiveness and risk of autism spectrum disorder in offspring. JAMA Psychiatry. 2014;71(8):936–942. doi: 10.1001/jamapsychiatry.2014.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick C., Hessl D., Macari S.L., Ozonoff S., Green C., Rogers S.J. Electrodermal and behavioral responses of children with autism spectrum disorders to sensory and repetitive stimuli. Autism Res. 2014;7(4):468–480. doi: 10.1002/aur.1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen E.M. Circle Pinesmerican Guidance Service; Circle Pines: 1995. Mullen Scales of Early Learning. [Google Scholar]
- Ornitz E.M., Rivto E. Perceptual inconstancy in early infantile autism. Arch. Gen. Psychiatry. 1968;18:76–98. doi: 10.1001/archpsyc.1968.01740010078010. [DOI] [PubMed] [Google Scholar]
- Perry W., Minassian A., Lopez B., Maron L., Lincoln A. Sensorimotor gating deficits in adults with autism. Biol. Psychiatry. 2007;61(4):482–486. doi: 10.1016/j.biopsych.2005.09.025. [DOI] [PubMed] [Google Scholar]
- Ramaswami M. Network plasticity in adaptive filtering and behavioral habituation. Neuron. 2014;82(6):1216–1229. doi: 10.1016/j.neuron.2014.04.035. [DOI] [PubMed] [Google Scholar]
- Riby D.M., Hancock P.J.B. Viewing it differently: social scene perception in Williams syndrome and autism. Neuropsychologia. 2008;46:2855–2860. doi: 10.1016/j.neuropsychologia.2008.05.003. [DOI] [PubMed] [Google Scholar]
- Riby D.M., Hancock P.J.B. Looking at movies and cartoons: eye-tracking evidence from Williams syndrome and autism. J. Intellect. Disabil. Res. 2009;53 doi: 10.1111/j.1365-2788.2008.01142.x. [DOI] [PubMed] [Google Scholar]
- Riby D.M., Hancock P.J., Jones N., Hanley M. Spontaneous and cued gaze-following in autism and Williams syndrome. J. Neurodev. Disord. 2013;5(1):1. doi: 10.1186/1866-1955-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers S.J., Ozonoff S. Annotation: what do we know about sensory dysfunction in autism? A critical review of the empirical evidence. J. Child Psychol. Psychiatry. 2005;46(12):1255–1268. doi: 10.1111/j.1469-7610.2005.01431.x. [DOI] [PubMed] [Google Scholar]
- Schmid S., Wilson D.A., Rankin C.H. Habituation mechanisms and their impact on cognitive function. Front. Integr. Neurosci. 2015:5. doi: 10.3389/fnint.2014.00097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoen S.A., Miller L.J., Brett-Green B., Hepburn S.L. Psychophysiology of children with autism spectrum disorder. Res. Autism Spectrum Disord. 2008;2(3):417–429. [Google Scholar]
- Silverman J.M., Smith C.J., Schmeidler J., Hollander E., Lawlor B.A., Fitzgerald M., Buxbaum J.D., Delaney K., Galvin P. Symptom domains in autism and related conditions: evidence for familiality. Am. J. Med. Genet. 2002;114(1):64–73. doi: 10.1002/ajmg.10048. [DOI] [PubMed] [Google Scholar]
- Sinha P., Kjelgaard M.M., Gandhi T.K., Tsourides K., Cardinaux A.L., Pantazis D., Diamond S.P., Held R.M. Autism as a disorder of prediction. Proc. Natl. Acad. Sci. 2014;111(42):15220–15221. doi: 10.1073/pnas.1416797111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparrow S., Balla D., Cicchetti D. 2nd ed. American Guidance Service; Circle Pines, MN: 2005. Vineland Adaptive Behavior Scales. [Google Scholar]
- Stromme P., Bjornstad P.G., Ramstad K. Prevalence estimation of Williams syndrome. J. Child Neurol. 2002;17(4):269–271. doi: 10.1177/088307380201700406. [DOI] [PubMed] [Google Scholar]
- Swartz J.R., Wiggins J.L., Carrasco M., Lord C., Monk C.S. Amygdala habituation and prefrontal functional connectivity in youth with autism spectrum disorders. J. Am. Acad. Child Adolesc. Psychiatry. 2013;52(1):84–93. doi: 10.1016/j.jaac.2012.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tager-Flusberg H., Skwerer D. Social engagement does not lead to social cognition: evidence from williams syndrome. In: Banaji M., Gelman S., editors. Navigating the Social World. Oxford Press; 2013. [Google Scholar]
- Thorpe W.H. Harvard University Press; Cambridge, MA: 1966. Learning and Instinct in Animals. [Google Scholar]
- Turk-Browne N.B., Scholl B.J., Chun M.M. Babies and brains: habituation in infant cognition and functional neuroimaging. Front. Hum. Neurosci. 2008;2:16. doi: 10.3389/neuro.09.016.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uljarević M., Evans D.W., Alvares G.A., Whitehouse A.O.J. Relationship between restricted and repetitive behaviours in children with autism spectrum disorder and their parents. Mol. Autism. 2016;7(1):29. doi: 10.1186/s13229-016-0091-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uljarevic M. Cardiff University; 2013. Repetitive Behaviours, Anxiety and Sensory Problems in Children with Autism and Correlates of Anxiety in Their Parents. (PhD Thesis) [Google Scholar]
- Venker C.E., Kover S.T. An open conversation on using eye-gaze methods in studies of neurodevelopmental disorders. J. Speech Lang. Hear. Res. 2015;58(6):1719–1732. doi: 10.1044/2015_JSLHR-L-14-0304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivanti G., Rogers S.J. Autism and the mirror neuron system. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2014;369(1644) doi: 10.1098/rstb.2013.0184. (20130184) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivanti G., Hocking D., Fanning P., Dissanayake C. Social affiliation motives modulate spontaneous learning in Williams Syndrome but not in Autism. Mol. Autism. 2016 doi: 10.1186/s13229-016-0101-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivanti, G., Dawson, G., Rogers, S.J. (2017) Early Learning and Autism. In: Vivanti, G., Duncan, E., Dawson, G., Rogers, S.J. (Eds.), Implementing the Group-Based Early Start Denver Model for Preschoolers with Autism. Springer, New York.
- Webb S.J., Jones E.J., Merkle K., Namkung J., Toth K., Greenson J., Murias M., Dawson G. Toddlers with elevated autism symptoms show slowed habituation to faces. Child Neuropsychol. 2010;16(3):255–278. doi: 10.1080/09297041003601454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiggins J.L., Swartz J.R., Martin D.M., Lord C., Monk C.S. Serotonin transporter genotype impacts amygdala habituation in youth with autism spectrum disorders. Soc. Cogn. Affect. Neurosci. 2014;9(6):832–838. doi: 10.1093/scan/nst039. [DOI] [PMC free article] [PubMed] [Google Scholar]