Graphical abstract
Keywords: Attention-deficit hyperactivity disorder (ADHD), Event-related potentials (ERP), Reward, Cue P3, Cue-CNV, Target-P3
Abstract
Objective
Neural hypo-sensitivity to cues predicting positive reinforcement has been observed in ADHD using the Monetary Incentive Delay (MID) task. Here we report the first study using an electrophysiological analogue of this task to distinguish between (i) cue related anticipation of reinforcement and downstream effects on (ii) target engagement and (iii) performance in a clinical sample of adolescents with ADHD and controls.
Methods
Thirty-one controls and 32 adolescents with ADHD aged 10–16 years performed the electrophysiological (e)-MID task − in which preparatory cues signal whether a response to an upcoming target will be reinforced or not − under three conditions; positive reinforcement, negative reinforcement (response cost) and no consequence (neutral). We extracted values for both cue-related potentials known to be, both, associated with response preparation and modulated by reinforcement (Cue P3 and Cue CNV) and target-related potentials (target P3) and compared these between ADHD and controls.
Results
ADHD and controls did not differ on cue-related components on neutral trials. Against expectation, adolescents with ADHD displayed Cue P3 and Cue CNV reinforcement-related enhancement (versus neutral trials) compared to controls. ADHD individuals displayed smaller target P3 amplitudes and slower and more variable performance − but effects were not modulated by reinforcement contingencies. When age, IQ and conduct problems were controlled effects were marginally significant but the pattern of results did not change.
Discussion
ADHD was associated with hypersensitivity to positive (and marginally negative) reinforcement reflected on components often thought to be associated with response preparation − however these did not translate into improved attention to targets. In the case of ADHD, upregulated CNV may be a specific marker of hyper-arousal rather than an enhancement of anticipatory attention to upcoming targets. Future studies should examine the effects of age, IQ and conduct problems on reinforcement sensitivity in ADHD.
1. Introduction
Attention-deficit/hyperactivity disorder (ADHD) is a complex neurodevelopmental disorder, which affects about 5% of children and adolescents (Polanczyk et al., 2007). It is associated with deficits in a range of neuropsychological processes and related neural circuits (Faraone et al., 2015) which are hypothesized to interact to impair decision-making (Sonuga-Barke et al., 2016). A number of neuro-biological models have proposed that the core of these difficulties is a hypodopamineric state that disrupts the individual’s response to reinforcement in the brain’s reward centres (i.e., ventral striatum; orbito-frontal cortex) thought to lead to a reduced sensitivity to cues of upcoming reinforcement and therefore to disrupted learning (Sagvolden et al., 2005, Sonuga-Barke and Fairchild, 2012, Tripp and Wickens, 2008). Behavioural and electrophysiological evidence for this view is mixed (Luman et al., 2005). Some have found that individuals with ADHD, compared to unaffected controls, show a hypersensitivity to the introduction of contingent reinforcement (Fosco et al., 2015). Others have reported an ADHD-related insensitivity to the imposition of reinforcement (van Meel et al., 2011) and to changing contingencies (Alsop et al., 2016). Effects may be dependent on incentive type (Demurie et al., 2011, Umemoto et al., 2014) and reinforcement valence − with a recent study showing greater sensitivity to punishment (Furukawa et al., 2017) and delay (Marco et al., 2009, Scheres et al., 2014, Yu et al., 2015) in ADHD. The evidence from functional Magnetic Resonance Imaging (fMRI) studies appears more consistent. Building on early work by Scheres et al. (2007), Plichta and Scheres, (2014) reported a meta-analysis of studies using the monetary incentive delay (MID) task −in which preparatory cues signal whether a response to a simple reaction task target will be reinforced or not − contingent on performance (Knutson et al., 2001, Knutson et al., 2000, Liu et al., 2011, Lutz and Widmer, 2014). This confirmed a consistent pattern of diminished activation in the ventral striatum, a key hub in the brain’s reward circuit, to cues predicting future reinforcement.
Because of its strong spatial resolution, fMRI is ideal for examining the general predictions of dopamine dysfunction models of ADHD − reduced activation in reward hubs in response to reinforcement cues. However, because of its limited temporal resolution, it is unable to draw more fine-grained distinctions in the neural underpinnings of the cognitive processes linking hypo-sensitivity to reinforcement in ADHD with poor performance on tasks and ultimately to disrupted learning. For instance, one plausible hypothesis is that in ADHD there is a failure of attentional upregulation following cues of impending contingent reinforcement, which then impedes effective target processing by reducing attentional preparation. In the current paper we tested this hypothesis directly using an electrophysiological version of the MID (e-MID; Broyd et al., 2012). Like the MID, the e-MID task consists of consecutive cue, target, and feedback stimuli, where the type of feedback received (positive or negative) depends on the speed of the behavioural response to the target. Anticipatory cues indicate whether the individual can win or lose money in the upcoming trial, depending on their response speed to the target, and how much they can win or lose. Crucially, for the goals of the current paper, this task effectively differentiates the specific brain potentials associated with initial reinforcement cues from later engagement with targets (Broyd et al., 2012).
Two components of cue-related brain potentials are modifiable by the reinforcement-related information they convey. The first component, which is characterised by a positivity over centro-parietal areas, emerges between 300 and 600 ms post-stimulus, is termed Cue P3 (Broyd et al., 2012, Goldstein et al., 2008, Novak and Foti, 2015, Pfabigan and Tran, 2015) or late positivity (Schupp et al., 2004). It reflects allocation of attention to cue (Broyd et al., 2012, Novak and Foti, 2015, Polich and Kok, 1995) and is modulated by the value of both monetary and social rewards (Flores et al., 2015, Goldstein et al., 2006a, Goldstein et al., 2006b) and by variations in the emotional significance of stimuli (Schupp et al., 2004). The second is the Contingent Negative Variation (CNV). The CNV is a slow negative potential emerging at fronto- central electrode sites, preceding behavioural responses. It is related to anticipatory motor response preparation in thalamo-cortico-striatal networks (Brunia et al., 2012, Walter et al., 1964). Some studies have shown that the CNV is modulated by the anticipation of affective or motivationally significant stimuli (Baas et al., 2002, Novak and Foti, 2015). Other studies have failed to replicate this effect (Goldstein et al., 2006a, Goldstein et al., 2006b, Pfabigan et al., 2014). Setting it apart from the P3, the CNV is suggested to indicate the cognitive transition towards preparing responses as modulated by the motivational salience of the upcoming stimuli (Novak and Foti, 2015). In support of this functional distinction between Cue P3 and CNV, combined ERP and fMRI data provided evidence that they are differentially correlated with brain activity. Highlighting its relevance to prior fMRI studies − the Cue P3 co-varies with Blood-Oxygen-Level Dependent (BOLD) signals in the ventral striatum during reward anticipation (Pfabigan et al., 2014), while the CNV co-varies with reward- related BOLD activity in a network including the thalamus, ventral striatum, and supplementary motor areas (Plichta et al., 2013).
In ADHD, on non-incentivized trials, both Cue P3 (Albrecht et al., 2013, Brandeis et al., 2002, van Leeuwen et al., 1998) and CNV (Albrecht et al., 2013, Banaschewski et al., 2003; van Leeuwen et al., 1998) are attenuated, suggesting impaired cue orientation and response preparation. Little is known about the effects of incentives and motivational valence on Cue P3 and CNV in ADHD. Banaschewski and colleagues (2008) found no evidence of cue-type effects (signaling Go, No-go or neutral trials) on a continuous performance task (CPT-AX). Benikos & Johnstone (2009) demonstrated reduced CNV amplitude to cues signaling fast, as opposed to medium and slow event rate trials. Heinrich and colleagues (2014) found no differential effects of reward and non-reward related cues on either cue component.
Regarding target processing, there is evidence to suggest that target-evoked centro-parietal positivity, similar to the cue-evoked P3, is a robust neural marker of task-relevant and motivated attention following the appearance of a target. The P3 component, elicited approximately 300–600 ms post-stimulus, is related to stimulus evaluation and categorization processes (review by Polich, 2007). Its amplitude is sensitive to the motivational context of the target stimuli both in healthy adolescents (mean age 15 years) and adults using the e-MID paradigm (Broyd et al., 2012) and in adolescents aged 14–17 with and without ADHD utilizing a version of the go/no-go paradigm (Groom et al., 2010). In addition, reduced overall target-P3 amplitudes have consistently been reported in ADHD compared to controls (Groom et al., 2010, Rodriguez and Baylis, 2007, Szuromi et al., 2011). In ADHD the overall lower target-P3 amplitude is suggested to reflect a deficit in the attention to task required for target detection. Accordingly, a couple of fMRI studies demonstrated reduced activation in brain areas sub-serving top-down attentional processes and target detection in children and adolescents with ADHD (Cao et al., 2008, Tamm et al., 2006).
In the current paper, we test the hypothesis that hypo-responsiveness to reinforcement in ADHD leads to deficient cue-induced, pre-target upregulation of attentional resources that will be reflected in down-stream attenuation of target engagement and so poorer performance. Our predictions are that; (i) reinforcement (compared to a neutral condition) will increase Cue P3, Cue-CNV and Target P3 amplitudes; (ii) these effects will be attenuated in ADHD and as a consequence (iii) less improvement on reinforced trials will be seen for ADHD than control individuals. The effects of positive versus negative reinforcement will be explored − but based on the current literature a prediction with regard to this is difficult to make.
2. Methods
2.1. Participants
Thirty-one typically developing adolescents (11 girls) and 32 adolescents with ADHD (5 girls), aged between 10 and 16 years were recruited into the study (see Table 1 and sections 2.2. and 2.4 on exclusion criteria). Informed written consent was obtained from parent(s). Written assent was obtained from adolescents. Adolescents with ADHD were recruited from local child and adolescent mental health clinics and all had a clinical diagnosis of ADHD. Controls were recruited from local mainstream schools.
Table 1.
Sample characteristics.
| ADHD (n = 20) |
Controls (n = 26) |
Comparison |
||||
|---|---|---|---|---|---|---|
| Mean | S.D. | Mean | S.D. | t | p value | |
| Child age (years) | 11.60 | 1.60 | 13.00 | 1.50 | 3.00 | 0.004 |
| Full Scale IQ | 96.70 | 12.40 | 109.6 | 11.70 | 3.50 | <0.01 |
| SDQ Self-report | ||||||
| Hyperactivity | 6.10 | 2.10 | 2.80 | 2.10 | −4.87 | <0.001 |
| Conduct Problems | 4.90 | 1.70 | 1.30 | 1.00 | −8.80 | <0.001 |
| SDQ Teacher-report | ||||||
| Hyperactivity | 6.80 | 2.20 | 1.50 | 1.90 | −6.27 | <0.001 |
| Conduct problems | 2.80 | 2.40 | 0.15 | 0.55 | −3.90 | <0.01 |
| SDQ Parent-report | ||||||
| Hyperactivity | 8.95 | 1.46 | 2.30 | 2.30 | −11.0 | <0.001 |
| Conduct Problems | 5.90 | 2.20 | 1.35 | 2.00 | −7.00 | <0.001 |
| ADHD Parent-report | ||||||
| Inattention | 21.20 | 4.20 | 5.70 | 4.70 | −11.50 | <0.001 |
| Hyperactivity-Impulsivity | 21.50 | 3.70 | 5.50 | 4.50 | −12.95 | <0.001 |
Note: ADHD Parent-report as measured by DISC-IV. For teacher-SDQ, data were available from 13 controls and 12 adolescents with ADHD. For all other measures, data were available from 26 controls (8 girls) and 20 adolescents with ADHD (2 girls).
2.2. Materials, inclusion and exclusion criteria
Participants all undertook a comprehensive clinical research assessment as part of the South Hampshire ADHD Register (SHARe). This included the ADHD section of the parent version of the Diagnostic Interview Schedule for Children-NIMH (DISC-IV; Shaffer et al., 1993). The self-report, parent and teacher versions of the Strengths and Difficulties Questionnaire (SDQ; Goodman, 1997) were also administered. Full scale IQ was assessed using the Wechsler Intelligence Scale for Children (WISC-IV; Wechsler, 2004). Adolescents with ADHD were only included if they met criteria for ADHD on the DISC-IV The DISC-IV covers the manifestation of ADHD symptoms in multiple settings including both home and school. Only participants with both inattention and hyperactivity (combined type) presentations were included. Control adolescents completed the same measures as the participants with ADHD apart from the DISC-IV. They also only completed the Block Design and Vocabulary subtests of the WISC-IV. For both groups, WISC–IV Full Scale IQ was estimated based on the scores of the Block Design and Vocabulary subtests (Sattler, 2001). General exclusion criteria were IQ < 75 and diagnosis of autism spectrum disorder or a neurological condition. In addition, one control child was excluded as they scored above borderline thresholds on the hyperactivity subscales of the SDQ-parent report. All participants with ADHD scored above borderline thresholds on the hyperactivity subscales of the SDQ-parent report and SDQ-teacher report. For teacher report-SDQ, data were available from 13 controls and 12 adolescents with ADHD. Due to missing data from teachers, only parent report-SDQ data were included in subsequent analyses. One child with ADHD dropped out during testing and one was excluded due to a technical error. Seven adolescents with ADHD also had a DISC-IV Conduct Disorder (CD) diagnosis based on the DISC and 11 were taking stimulant medication. These participants were asked to withdraw their medication 24 h prior to testing (5 half- lives). The two groups were not matched on IQ, age and gender at the time of recruitment. Participant characteristics are presented in Table 1. The study was approved by the University of Southampton Ethics Committee and the National Health Service (NHS) Research Ethics Committee.
2.3. Experimental paradigm and procedure
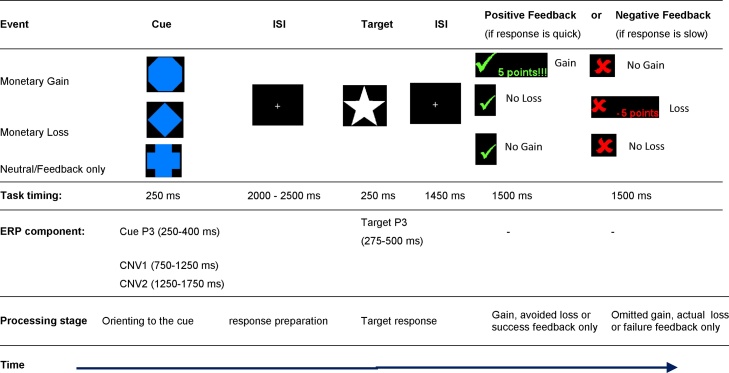
Participants performed an electrophysiological analogue (Broyd et al., 2012) of the Monetary Incentive Delay task (MID; Knutson et al., 2000) under three conditions − positive reinforcement (monetary gain for responses that met the RT criteria), negative reinforcement (monetary loss for responses that failed to meet that criteria) and neutral (no monetary consequence of RT performance). At the start of each trial, one of three possible blue cues (indicating the condition) was presented on a computer screen (250 ms). After an interval of between 2000 and 2500 ms (relative to the start of the trial) a white star target was presented (250 ms). Participants were instructed to respond to the target as quickly as possible with the thumb of their dominant hand via a button box. All conditions included some feedback.
Feedback was provided 1450 ms following the offset of the target stimulus with a green tick for ‘fast enough’ and a red cross for ‘too slow’ responses. This task included an adaptive algorithm which tracked each participant’s response on a trial by trial basis and adjusted the response window for a ‘fast enough’ response so that all participants received positive feedback, based on their own performance, on 66% of trials. This also ensured that all participants gained the same amount money (each participant received £10). The three cue types indicating the condition were presented with equal probability and in random order: In the Neutral/feedback-only condition (signaled by the blue cross cue) participants received feedback about the speed of their response but they could neither win nor lose money. In the Gain/feedback + points gain condition (signaled by a blue octagon cue) participants received positive feedback following a fast response and gained 5 points (i.e., 20 pence) and negative feedback following a slow response and no points or money were gained. In the Loss/feedback + points loss condition, (signaled by a blue diamond cue), participants received positive feedback following a fast response and avoided the loss of 5 points (i.e., 20 pence), and negative feedback following a slow response and lost 5 points (i.e., 20 pence). Participants were told that they would receive their total cash winnings at the end of the last task block. A practice block of 30 trials was completed prior to the experimental blocks to allow participants to learn the association between each cue and experimental condition. Participants completed three experimental blocks of 60 trials with a break between each block. Participants were not paid for participation but their travel expenses were reimbursed. In addition, adolescents received the monetary rewards they gained at the end of the experimental task. A schematic representation of the task is presented in Fig. 1.
Fig. 1.
Timing and ERP components of the electrophysiological-Monetary Incentive Delay (e-MID) task.
2.4. EEG recording and pre-processing
We used an electrode cap (Easycap, Herrsching, Germany) containing 52 equidistantly spaced silver/silver chloride (Ag/AgCl) electrodes. EEG data was recorded using Neuroscan Synamps2 70 channel EEG system, DC-coupled recording equipment. The data were sampled at 500 Hz with a low pass filter at 70 Hz and referenced to an electrode on the nose. This reference was kept throughout the analyses to keep its use consistent with previous studies (Broyd et al., 2012). A ground electrode was fitted midway between the electrode at the vertex and frontal sites. Vertical electro-oculogram (vEOG) was recorded from four electrodes: two bipolar electrodes were placed directly beneath the left and right eyes and affixed with tape, while the two electrodes placed above the right and left eye were included within the electrode cap. Impedances were kept below 5 kΩ.
The data were high pass filtered at 0.2 Hz and lowpass filtered at 15 Hz offline. Data pre-processing was done in Neuroscan (Scan 4.5). For cue-locked ERP analyses, epochs were locked to the onset of the cue stimuli and were extracted from −200 to +1800 ms. Baselines were calculated in the −200 to 0 ms relative to the onset of the cue stimuli. For target-locked ERP analyses, epochs were locked to the onset of the target stimuli and were extracted from −200 to +800 ms. Baselines were calculated in the −200 to 0 ms period relative to the onset of the target stimuli. Epochs locked to the feedback were also extracted − however, due to the low number of clean epochs per subject, the feedback trials were omitted from further analysis. Epochs containing data points above or below ±150 μV, or with eye-movements as determined based on vEOG channels, were rejected. An ocular artifact reduction procedure (Semlitsch et al., 1986) based on left eye vEOG activity was used to remove blink artifacts and other eye-movements from the ERP data. Individual ERP averages were based on a minimum of 20 trials of a total of 60 epochs for each condition (gain, loss, neutral). Based on these criteria, the cue CNV analysis included 20 adolescents with ADHD and 24 controls, the Cue P3 analysis included 20 adolescents with ADHD and 26 controls, and the Target P3 analyses included 24 adolescents with ADHD and 24 controls. The number of artifact-free trials for the two groups was as follows: Cue: Controls: Gain: M = 39.00, SD = 11.14, Loss: M = 40.70, SD = 9.87, Neutral: M = 37.00, SD = 11.42, ADHD: Gain: M = 28.45, SD = 7.60, Loss: M = 28.50, SD = 7.70, Neutral: 27.05, SD = 7.08. Target: Controls: Gain: M = 53.10, SD = 6.70, Loss: M = 52.50, SD = 6.90, Neutral: 52.56, SD: 7.80, ADHD: Gain: M = 43.74, SD = 10.30, Loss: 44.80, SD = 10.24, Neutral: M = 45.25, SD = 9.10. There was no difference in the number of artifact-free trials between conditions for cue (p > 0.08) and target (p > 0.43). There were fewer cue and target artifact-free epochs for adolescents with ADHD than controls (p < 0.01). There were also fewer artifact-free trials for younger compared to older adolescents (see Supplement 1). However, mean amplitude is not biased by the mean number of trials (Luck, 2010).
A baseline-to-peak mean amplitude method was used to calculate the ERP components. This consisted of measuring the average amplitude over a time window that included the component of interest (Handy, 2005). The average amplitude method is recommended due to its insensitivity to latency variability (Luck, 2005). Peaks were confirmed by visual inspection and clearly visible in all individual waveforms. We identified the ERP components using the control data, then examined the sensitivity of these components to the incentive conditions and then based on the control groups’ ERPs examined the ADHD group’s ERPs. Following the cue stimulus, a clear cue P3 component could be observed and was quantified from 250 to 400 ms at centroparietal sites 1, 4, 5, 6, 12, 13 and 14 (see Supplement 3 for electrode map) in line with previous research using the same task in adolescents (Broyd et al., 2012). The CNV was quantified in two time windows (CNV1: 750–1250 ms and CNV2: 1250–1750 ms) at frontocentral sites 1, 2, 3, 4, 5, 6, 7, 8 and 18. These time windows were chosen for consistency with our previous research using the same task in adolescents (Broyd et al., 2012). These time windows were themselves based on previous research (Goldstein et al., 2006a, Goldstein et al., 2006b). The target P3 was quantified from 275 to 500 ms at centroparietal sites 1, 4, 5, 6, 12, 13 and 14 in line with previous research (Broyd et al., 2012).
2.5. Data analysis
Repeated measures ANOVAs tested the effects of group as a between-subjects factor and condition (gain, loss, neutral) as a within-subjects factor on mean reaction time (MRT), SD of RT, Cue P3, CNV and target P3. We conducted planned comparisons of the reinforcement conditions (gain, loss) against neutral and then exploratory comparisons of gain versus loss conditions. Only significant results are reported. RT data were trimmed to remove responses, which were faster than 150 milliseconds and exceeded ± 2.5 SD around the mean response time. For consistency the behavioural analyses included the same participants as the ERP analyses- twenty-six controls (18 girls) and 20 CEHD (2 girls) adolescents’ data were included after excluding outliers. For the CNV analysis as indicated by prior research a second within-subject variable, time window was added to compare the effects for CNV1 and CNV2. Where groups significantly differed on age and IQ these variables were added as covariates. Pearson’s correlations examined the relationship between ERPs and performance. Finally, Pearson’s correlations examined the relationship between ERPs and child age, IQ and conduct problems.
3. Results
3.1. Behavioural performance
Table 2 presents RT and SD of RT data for each group and condition. When RT was the dependent variable there was an overall main effect of condition (F (2, 88) = 13.98, p < 0.001). Adolescents were faster when they could gain points (F (1, 44) = 33.15, p < 0.001) and could avoid losing points (F (1, 44) = 9.18, p < 0.01) compared to the neutral condition. There was also a trend for adolescents to be faster when they could gain points compared to avoiding losing points but this effect did not reach significance (F (1, 44) = 3.14, p = 0.08). The effect of group was significant − adolescents with ADHD were slower than controls (F (1, 44) = 7.40, p < 0.01). The Group × Condition interaction was not significant (F (2, 88) = 0.71, p=0.50). For the SD of RT there was a significant main effect of condition (2, 88) = 9.80, p < 0.001). Adolescents had larger SD of RT for the neutral condition compared to the gain (F (1, 44) = 15.47, p < 0.001) and loss (F (1, 44) = 4.45, p < 0.05) condition. They also had larger SD of RT for loss compared to the gain (F (1, 44) = 7.03, p < 0.05). The effect of group was significant − adolescents with ADHD had larger SD of RT than controls (F (1, 44) = 4.50, p = 0.040). The Group × Condition interaction was not significant (F (2, 88) = 1.08, p = 0.34). For the MRT after controlling for age and IQ only the effect of group remained significant. For the MRT after controlling for conduct problems only the effect of condition remained significant. For the SD of RT, controlling for age reduced the group effect to non-significant levels (p = 0.07). For the SD of RT, after controlling for IQ and conduct problems, the effects were non-significant (p > 0.05).
Table 2.
Mean reaction time (MRT) and SD of reaction time in the two groups.
| ADHD |
Controls |
|||
|---|---|---|---|---|
| Mean | S.D. | Mean | S.D. | |
| MRT (ms) | ||||
| Gain | 346.10 | 57.60 | 301.0 | 41.0 |
| Loss | 350.90 | 60.07 | 310.0 | 43.30 |
| Neutral | 363.05 | 64.90 | 328.35 | 48.60 |
| SD of RT (ms) | ||||
| Gain | 87.95 | 45.67 | 66.46 | 31.16 |
| Loss | 105.80 | 41.03 | 74.40 | 35.67 |
| Neutral | 108.75 | 45.26 | 92.80 | 48.60 |
3.2. Electrophysiological
Grand mean averages are displayed in Fig. 2 alongside bar charts showing the group and condition effects on Cue P3, Cue-CNV1 and Cue-CNV2 and Target P3. Topographic maps for all components are presented in Fig. 2, Fig. 3. Independent-samples t-tests examined differences in ERP mean amplitude values between males and females. Results showed no significant differences between males and females for the targeted ERP components in controls [t (24) < 1.48, p > . 12], ADHD group [t (18) < −2.00, p > .05], or overall sample [t (44) < −2.50, p > .05]. Pearson’s correlations showed no significant associations between ERPs and age, IQ or conduct problems (all ps > .05).
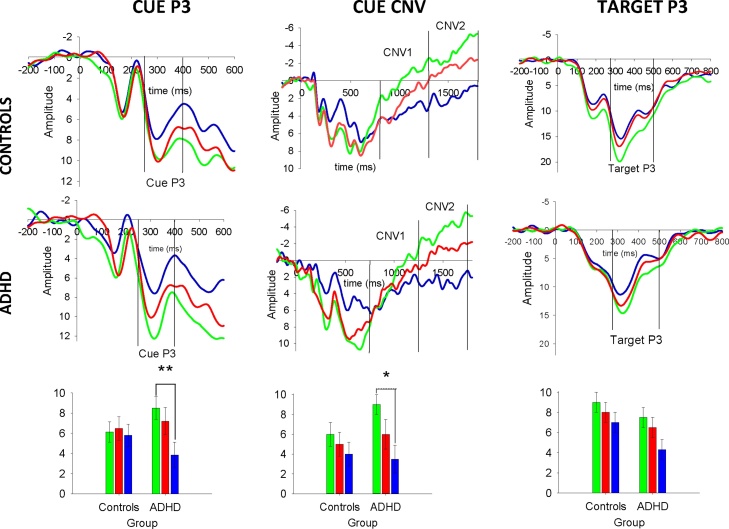
Fig. 2.
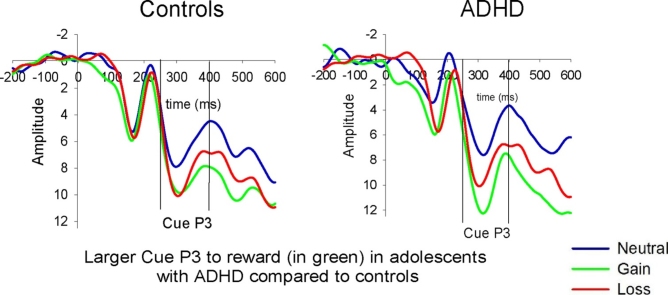
Grand mean averages and bar graphs with error bars for the Cue P3, CNV2 and Target P3 per condition in the ADHD and controls group. Amplitude is shown in μV on the y axis and time in ms on the x-axis. The bar graphs plot the ERPs in an adjusted positive scale for the CNV to capture the amount of the amplitude change. Error bars represent standard error of the mean. Group x Condition interactions with enhanced amplitudes to the gain vs. neutral in ADHD relative to controls for the Cue P3 (p <0.01) and CNV2 (p < 0.05) Gain Loss Neutral.
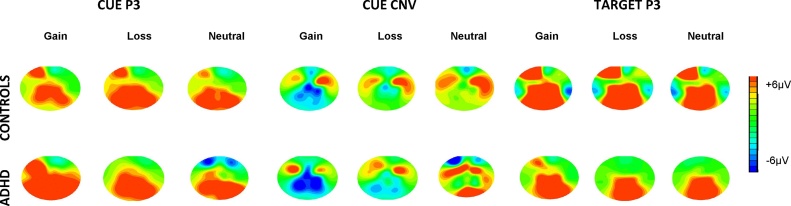
Fig. 3.
Topographic maps for the mean voltage distribution per condition and group for the Cue P3, Cue CNV2 and Target P3. The figure shows enhanced Cue P3 and CNV2 to gain in ADHD compared to controls and reduced overall Target P3 amplitudes in ADHD compared to controls. Scalp values represent the ends of the colour scale in μV for the Cue P3, Cue CNV2 and Target P3. Dark blue = negativity, Dark red = positivity.
3.2.1. Cue P3
The group main effect was not significant (F (1, 40) = 0.07, p = 0.80). There was a main effect of condition (F (2, 80) = 6.45, p =0.003) with larger P3 amplitudes for gain (F (1, 40) = 9.80, p =0.003) and loss (F (1, 40) = 7.09, p =0.010) compared to the neutral condition. There was no significant difference between gain and loss conditions (F (1, 40) = 0.55, p =0.46). There was a significant group x condition interaction effect on cue P3 amplitudes (F (2, 80) = 4.36, p =0.016). The two groups did not differ in the neutral condition (F (1, 41) = 1.31, p =0.25). When examining the simple main effect of condition on the Cue P3 for each group, results showed that amplitudes were significantly greater in the gain compared to the neutral condition for individuals with ADHD (F (1, 21) = 9.45, p =0.006) but not for controls (F (1, 29) = 2.70, p =0.10). These effects were marginally significant when comparing loss with the neutral condition. In particular, Cue P3 amplitudes were marginally greater in the loss compared to the neutral condition for individuals with ADHD (F (1, 21) = 4.46, p =0.05) and a tendency was observed with controls but this effect did not reach significance (F (1, 29) = 3.20, p =0.08). Adding age, IQ and conduct problems to the model reduced the effect related to the gain compared to neutral in the ADHD group only to non-significant levels (F (1, 34) < 2.70, p > .10).
3.2.2. Cue CNV
There was no main effect of group (F (1, 44) = 0.13, p = 0.70). There was a main effect of condition (F (2, 88) = 25.12, p .001 < 0.001) with larger amplitudes for the gain (F (1, 44) = 50.09, p .001 < 0.001) and loss (F (1, 44) = 6.08, p =0.018) than neutral condition and smaller CNVs for loss compared to gain (F (1, 44) = 21.53, p .001 < 0.001). There was a significant condition x time window x group interaction (F (2, 88) = 3.83, p =0.025). We investigated this by examining the effects of group and condition for CNV1 and CNV2 separately. For CNV1 there was no significant interaction between group and condition (F (2, 88) = 0.80, p =0.92). For CNV2 there was a significant group x condition interaction (F (2, 88) = 3.32, p =0.040). There was no difference in CNV2 amplitude between the groups in the neutral condition (F (1, 45) = 0.06, p =0.80), while there was a larger difference in amplitude between neutral and gain conditions for adolescents with ADHD than controls (F (1, 42) = 7.90, p =0.007); When comparing the two groups for each condition separately in an One-Way ANOVA the gain condition CNV2 amplitudes were higher in the ADHD group compared to the control group (F (1, 42) = 4.20, p =0.04). The two groups did not differ in CNV2 amplitudes to the loss condition (F (1, 42) = 0.22, p =0.64). Adding IQ did not change the results. Adding age and conduct problems reduced this effect to non-significant levels (F (1, 40) < 3.30, p > .070).
3.2.3. Target P3
There was a significant effect of condition (F (2, 92) = 5.60, p .01 < 0.01) - P3 amplitudes were larger for gain (F (1, 46) = 7.94, p =0.007) and marginally larger for loss (F (1, 46) = 3.96, p =0.050) compared to the neutral condition. There was an effect of group (F (1, 46) = 4.36, p =0.040) with adolescents with ADHD having lower amplitudes across all conditions (see also Supplement 2). There was no interaction between group x condition (F (2, 92) = 0.11, p > .70). Controlling for age, IQ and conduct problems did not reduce the effect of group non-significant levels (ps < 0.05).
3.3. Correlations between ERPs and performance
Pearson’s correlations examined the relationship between ERPs (Cue P3, CNV, and Target P3) and performance (mean reaction time −MRT and SD of RT) in the whole sample. Results showed that target P3 amplitudes to the neutral condition were negatively correlated with MRT for gain (r = −0.35, p = 0.034) and loss (r = −0.36, p=0.030) and SD of RT for loss (r = −0.50, p=0.001). Target P3 to loss was negatively correlated with the SD of RT for gain (r = −0.35 p = 0.030). After controlling for age, IQ and conduct problems, the only association which remained significant was that between target P3 to neutral and SD of RT for loss. There we no significant correlations between performance measures and Cue P3 (all ps > .12) or CNV (all ps > 0.18).
4. Discussion
This is the first study to utilize the e-MID to decompose the electrophysiological brain responses to positive and negative reinforcement during anticipatory and target stages of reinforcement processing in a clinical sample of adolescents with ADHD and controls. There were a number of findings of note.
First, the targeted components (Cue P3, CNV and target-P3) were sensitive to the experimental manipulation. In particular, the e-MID produced predictable effects of reinforcement across the groups with the Cue P3 component amplitude greater in response to cues of monetary gain and loss, confirming the sensitivity of this component to the anticipation of incentives consistent with previous research using the e-MID task (Broyd et al., 2012). Similarly, we found larger CNV-component amplitudes to gain and loss compared to the neutral condition, suggesting enhanced anticipatory brain activity to incentives. It is interesting to note that previous work using the same task in adolescents of the same age as this study (Broyd et al., 2012) did not find significant effects of gain and loss on the amplitude of the CNV component. However, consistent with this previous study we confirmed that the target-P3 component was sensitive to both gain and loss, suggesting enhanced attentional control to motivationally significant task-relevant stimuli.
Second, individuals with ADHD and controls did not differ in terms of cue-related components in neutral conditions. Our results therefore stand in contrast with earlier research showing reduced allocation of attention (Polich and Kok, 1995) and motivational resources (Groom et al., 2010) as reflected by the cue P3, in adolescents with ADHD compared to controls.
Third, against predictions and fMRI findings (Fosco et al., 2015, Scheres et al., 2007, Ströhle et al., 2008) there was no evidence of ADHD related hyposensitivity to cues predicting upcoming reinforcement (either positive or negative). Instead, individuals with ADHD showed larger increase in neural response in terms of P3 and CNVs, in the gain condition compared to controls. This finding stands in contrast to much prior fMRI work showing reinforcement hypo-sensitivity in ADHD (Plichta and Scheres, 2014, Sonuga-Barke et al., 2016). In contrast to this, but in line with our findings, a number of recent studies report that individuals with ADHD are unusually sensitive to reinforcement cues. A recent fMRI MID study in a large sample of adolescents with ADHD (n = 150) found that relative to controls, adolescents with ADHD showed increased responses in the anterior cingulate, anterior frontal cortex, and cerebellum during reward anticipation (von Rhein et al., 2015). Also of relevance, a number of recent studies have shown hypersensitivity of the brain reward system in adolescents with increased risk-taking and behavioural impulsivity (Casey, 2015, Casey et al., 2016). Similar work has shown neural hyper-sensitivity to emotional (angry, happy) compared to neutral vocal stimuli (as reflected by elevated early components −e.g. N100) in 6–11-year-old children with ADHD compared to controls (Chronaki et al., 2015). The above findings are consistent with behavioural work showing hyper-responsiveness to social rewards in 8–13-year olds with ADHD relative to controls (Kohls et al., 2009).
Fourth, a reinforcement effect (positive and marginally negative compared neutral) was the strongest effect and positive compared to negative reinforcement differences, especially in terms of interactions with group, were less common. Recent studies have suggested that individuals with ADHD might display increased neural response to positive compared to negative stimuli. This is consistent with earlier work showing larger ventral-striatum and pregenual anterior cingulate cortex (ACC) activation in adolescents with externalising disorders (ADHD and CD) compared to controls in response to notifications of positive (monetary reward) versus negative (failure to win reward) stimuli (Bjork et al., 2010). More recent work has shown increased responses to positive −but not negative- relative to neutral images in temporal regions during an affective Stroop task in 13-year-olds with ADHD (Hwang et al., 2015). Other studies have shown hyper-sensitivity to punishment in ADHD. For example, individuals with ADHD showed increased activity in bilateral amygdalae and left anterior insula compared to controls during loss versus gain anticipation (Wilbertz et al., 2015) and larger feedback-related negativity (FRN) amplitudes to losses in guessing and gambling tasks (van Meel et al., 2015).
Fifth, individuals with ADHD displayed deficient target-related activations (i.e., P3) in the neutral condition. This was predicted and is consistent with the prior literature supporting the idea of impaired attention to targets in ADHD (Broyd et al., 2012, Groom et al., 2010, Rodriguez and Baylis, 2007, Szuromi et al., 2011). They also displayed RT and RT variability related deficits. In neither case was there any evidence that such deficits, either in terms of neural or behavioural response to targets in ADHD were ameliorated by adding incentives (either positive or negative). This effect is inconsistent with previous ERP (Groom et al., 2010) and fMRI (Cao et al., 2008) studies.
When taken together with the cue-related hypersensitivity to reinforcement seen in the individuals with ADHD, this finding highlights the dissociability of cue and target related processes and their relative impact on performance. The positive effects of incentives on cue-related components thought to reflect different stages of cued response preparation did not translate into downstream positive effects of target related attention or performance in the “gain” condition. It is particularly striking in this regard that the positive effects of reinforcement were observed most clearly on the CNV2 a component understood to specifically reflect anticipation of the target stimulus and engagement of effortful processes associated with the required response, rather than the CNV1, reflecting more primitive less consciously controlled alerting properties of the cue. Our findings complement recent research demonstrating distinct neural activation patterns while processing anticipatory relative to outcome related rewards (Silverman et al., 2015).
There are a number of possible interpretations of this intriguing finding. First, it is possible that individuals with ADHD have problems translating improved preparation and response readiness into better target related attention − perhaps because of difficulties maintaining a positive response set between cue and target − although in the current study the period between cue and target was always short. A second, possibility is that, at least in the case of individuals with ADHD, elevation of the cue P3-CNV complex does not equate to improved response preparation − in which case it would be no surprise that attention and performance did not improve when performance was positively reinforced. One perspective that provides a framework for understanding such effects is the state regulation model of ADHD dysfunction (Sergeant, 2005, van der Meere, 2005). This model postulates that individuals with ADHD have difficulty adjusting their state to deal with changing demands within their environment. One prediction of this model is that individuals with ADHD have difficulty modulating states of either hyper- or hypo-arousal. Adding reinforcement increases arousal. From this perspective the upregulation of cue-related components reflect a failure to regulate a hyper-aroused state induced by cues of future incentives rather than a sign of enhanced preparation for the processing of upcoming stimuli. If this were the case then one might expect that performance would in fact be disrupted more in the reinforcement condition for individuals with ADHD, compared to controls. This does not seem to be the case.
It is worth considering the role of age, IQ and conduct problems in the above effects. Research has supported developmental effects on performance and ERPs in cognitive tasks (Mathes et al., 2016). Adolescence is characterised by marked maturational changes in the brain (Segalowitz et al., 2010) and functional changes associated with subregions of the prefrontal cortex (Dumontheil et al., 2010). A recent study showed that while adults learned from both reward and punishment, adolescents learned from reward but were less likely to learn from punishment in a reinforcement learning paradigm (Palminteri et al., 2016). In comparison to adolescents and adults, children showed larger Cue P3 amplitudes but smaller ERPs reflecting response anticipation and response suppression (Hämmerer et al., 2010). Modulation of the P3b component by novel targets was evident in children, adolescents and adults, but it decreased in amplitude with age (Rojan-Benjunea et al., 2015). P300 amplitude in a visual oddball paradigm considerably reduced across adolescence (review by Segalowitz et al., 2010). Such reductions have been suggested to reflect greater response inhibition (Groom and Cragg, 2015). In addition, the P300 event-related potential has been consistently associated with externalising problems, including conduct problems (Banaschewski et al., 2003, Bertoletti et al., 2014). Research has shown that adolescent boys with conduct problems (e.g., rules violations) failed to exhibit the normal maturational increase in P300 amplitudes in auditory tasks (Bauer and Hesselbrock, 2003). Similarly, adolescents with conduct problems showed reduced fronto-central P300 amplitudes and prolonged P300 latency in an auditory oddball paradigm (Kim et al., 2001). These abnormalities were argued to reflect inefficient deployment of neural resources in processing cognitive task-relevant information (Gao and Raine, 2009). The present study has a number of limitations. First, the sample size was relatively small and effects reported in this study should be explored further with larger samples. Although gender differences in ERP amplitudes were not observed in the present sample, effects should be explored further with gender matched samples in future research. Second, the ADHD and control groups were not matched on age, IQ or conduct problems. When these covariates were added the effects were reduced to non-significant levels for the Cue P3 and the Cue CNV, although the pattern of results did not change. Future studies should be powered and designed to rule out the effects of IQ and co-morbidity with conduct problems on reinforcement sensitivity. Third, we were not able to test the impact of reward type (i.e., social vs. non-social, Kohls et al., 2009). This leaves open the possibility that our results were due to adolescents with ADHD valuing rewards in a qualitatively different way from controls (Morsink et al., 2017). Fourth, the task was not optimized to study feedback-related processes because of insufficient numbers of artifact-free trials. In the present task, all conditions included some feedback and this may have been considered in itself as a reinforcer. In addition, the task can be further developed to adjust for task demands to the individual participant’s level and reduce possible effects of group differences in task performance on ERP measures (Groom et al., 2010, Rosch and Hawk, 2013). Our study partly supported the predicted effects of condition and group on performance, although the reliance on RT performance and the use of tracking algorithm limited the behavioural results. Future studies should include a broader range of performance measures. It should also be noted that results from the correlation analyses between the ERPs and performance should be interpreted with caution given their exploratory nature and the multiple tests conducted. Finally, we had to exclude a number of cases because of movement and other artifacts. This is perhaps inevitable when working with adolescents with ADHD but it is possible that the more severe cases were lost to analysis.
In summary, we demonstrate, for the first time, reward-related modulation of cue-related brain potentials using the e-MID task in a clinical sample of adolescents with ADHD. Individuals with ADHD appeared hyper- rather than hypo-sensitive to anticipatory reinforcement cues (reflected by elevated Cue P3 and CNV components). However, this did not translate into downstream effects on improved target related attention (as measured by target P3) or performance. The possibility that individuals with ADHD fail to exploit the upregulation of CNV during response preparation because of a failure to regulate their hyper-aroused state in the face of predicted reinforcement needs to be explored in future studies. The e-MID task can be utilized as a valuable complement to fMRI paradigms in clinical populations of adolescents with ADHD.
Conflict of Interest
None.
Acknowledgements
The research was supported by the Institute for Disorders of Impulse and Attention (IDIA) at the University of Southampton. The South Hampshire ADHD register (SHARe) was supported by an unrestricted grant from Shire Pharmaceuticals and a grant from Solent NHS Trust. We thank the parents and adolescents who participated in this research. We acknowledge the contribution to the assessment of ADHD patients of Christine Cornforth for her role in the creation of the original South Hampshire ADHD Register and for data collection, along with contribution of Emma Lee, Mark Knowles, Wai Chen, Darko Turic, Georgia Chronaki, Sarah Williams, Margaret Thompson, Andrew Sibley, Brenda Meyer, Michael Rolt, Harriet Jefferson, Fiona, McEwan, Cathy Laver-Bradbury and Anan El Masry.
References
- Albrecht B., Brandeis D., Uebel H., Valko L., Heinrich H., Drechsler R., Banaschewski T. Familiality of neural preparation and response control in childhood attention deficit-hyperactivity disorder. Psychol. Med. 2013;4(9):1997–2011. doi: 10.1017/S003329171200270X. [DOI] [PubMed] [Google Scholar]
- Alsop B., Furukawa E., Sowerby P., Jensen S., Moffat C., Tripp G. Behavioral sensitivity to changing reinforcement contingencies in attention-deficit hyperactivity disorder. J. Child Psychol. Psychiatry. 2016;57(8):947–956. doi: 10.1111/jcpp.12561. [DOI] [PubMed] [Google Scholar]
- Baas J.M.P., Kenemans J.L., Böcker K.B.E., Verbaten M.N. Threat-induced cortical processing and startle potentiation. Neuroreport. 2002;13(1):133–137. doi: 10.1097/00001756-200201210-00031. [DOI] [PubMed] [Google Scholar]
- Banaschewski T., Brandeis D., Heinrich H., Albrecht B., Brunner E., Rothenberger A. Association of ADHD and conduct disorder?brain electrical evidence for the existence of a distinct subtype. J. Child Psychol. Psychiatry. 2003;3:356–376. doi: 10.1111/1469-7610.00127. [DOI] [PubMed] [Google Scholar]
- Bauer L.O., Hesselbrock V.M. Brain maturation and subtypes of conduct disorder: interactive effects on p300 amplitude and topography in male adolescents. J. Am. Acad. Child Adolesc. Psychiatry. 2003;42(1):106–115. doi: 10.1097/00004583-200301000-00017. [DOI] [PubMed] [Google Scholar]
- Bertoletti E., Michelini G., Moruzzi S., Ferrer G., Ferini-Strambi L., Stazi M.A. A general population twin study of conduct problems and the auditory P300 waveform. J. Abnorm. Child Psychol. 2014;42:861–869. doi: 10.1007/s10802-013-9836-7. [DOI] [PubMed] [Google Scholar]
- Bjork J.M., Smith A.R., Chen G., Hommer D.W. Adolescents, adults and rewards: comparing motivational neurocircuitry recruitment using fMRI. PLoS One. 2010;5(7):e11440. doi: 10.1371/journal.pone.0011440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandeis D., Banaschewski T., Baving L., Georgiewa P., Blanz B., Schmidt M.H., Scheuerpflug P. Multicenter P300 brain mapping of impaired attention to cues in hyperkinetic children. J. Am. Acad. Child Adolescent Psychiatry. 2002;41(8):990–998. doi: 10.1097/00004583-200208000-00018. [DOI] [PubMed] [Google Scholar]
- Broyd S.J., Richards H.J., Helps S.K., Chronaki G., Bamford S., Sonuga-Barke E.J.S. An electrophysiological monetary incentive delay (e-MID) task: a way to decompose the different components of neural response to positive and negative monetary reinforcement. J. Neurosci. Methods. 2012;209(1):40–49. doi: 10.1016/j.jneumeth.2012.05.015. [DOI] [PubMed] [Google Scholar]
- Brunia C., van Boxtel G., Böcker K. Oxford University Press Oxford; 2012. Negative Slow Waves as Indices of Anticipation: The Bereitschaftspotential, the Contingent Negative Variation, and the Stimulus-preceding Negativity The Oxford Handbook of Event-related Potential Components; pp. 189–207. [Google Scholar]
- Cao Q., Zangb Y., Zhub C., Caoa X., Suna L., Zhouc X., Wang Y. Alerting deficits in children with attention deficit/hyperactivity disorder: event-related fMRI evidence. Brain Res. 2008;1219:159–168. doi: 10.1016/j.brainres.2008.04.028. [DOI] [PubMed] [Google Scholar]
- Casey B.J., Galván A., Somerville L.H. Beyond simple models of adolescence to an integrated circuit-based account: a commentary. Dev. Cognitive Neurosci. 2016;17:128–130. doi: 10.1016/j.dcn.2015.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey B.J. Beyond simple models of self-control to circuit-based accounts of adolescent behavior. Annu. Rev. Psychol. 2015:66. doi: 10.1146/annurev-psych-010814-015156. [DOI] [PubMed] [Google Scholar]
- Chronaki G., Benikos N., Fairchild G., Sonuga-Barke E.J.S. Atypical neural responses to vocal anger in attention-deficit/hyperactivity disorder. J. Child Psychol. Psychiatry. 2015;56(4):477–487. doi: 10.1111/jcpp.12312. [DOI] [PubMed] [Google Scholar]
- Demurie E., Roeyers H., Baeyens D., Sonuga-Barke E. Common alterations in sensitivity to type but not amount of reward in ADHD and autism spectrum disorders. J. Child Psychol. Psychiatry. 2011;52(11):1164–1173. doi: 10.1111/j.1469-7610.2010.02374.x. [DOI] [PubMed] [Google Scholar]
- Dumontheil I., Houlton R., Christoff K., Blakemore S.J. Development of relational reasoning during adolescence. Dev. Sci. 2010;13(6):F15–24. doi: 10.1111/j.1467-7687.2010.01014.x. [DOI] [PubMed] [Google Scholar]
- Faraone S.V., Asherson P., Banaschewski T., Biederman J., Buitelaar J.K., Ramos-Quiroga J.A., Franke B. Attention-deficit/hyperactivity disorder. Nature Rev. Dis. Primers. 2015:15020. doi: 10.1038/nrdp.2015.20. [DOI] [PubMed] [Google Scholar]
- Flores A., Münte T.F., Doñamayor N. Event-related EEG responses to anticipation and delivery of monetary and social reward. Biol. Psychol. 2015;109:10–19. doi: 10.1016/j.biopsycho.2015.04.005. [DOI] [PubMed] [Google Scholar]
- Fosco W.D., Hawk L.W., Rosch K.S., Bubnik M.G. Evaluating cognitive and motivational accounts of greater reinforcement effects among children with attention-deficit/hyperactivity disorder. Behav. Brain Func. 2015;11(1):1–9. doi: 10.1186/s12993-015-0065-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa E., Alsop B., Sowerby P., Jensen S., Tripp G. Evidence for increased behavioral control by punishment in children with attention-deficit hyperactivity disorder. J. Child Psychol. Psychiatry. 2017;58(3):248–257. doi: 10.1111/jcpp.12635. [DOI] [PubMed] [Google Scholar]
- Gao Y., Raine A. P3 event-related potential impairments in antisocial and psychopathic individuals: a meta-analysis. Biol. Psychol. 2009;82(3):199–210. doi: 10.1016/j.biopsycho.2009.06.006. [DOI] [PubMed] [Google Scholar]
- Goldstein R.Z., Cottone L.A., Jia Z., Maloney T., Volkow N.D., Squires N.K. The effect of graded monetary reward on cognitive event-related potentials and behavior in young healthy adults. Int. J. Psychophysiol. 2006;62(2):272–279. doi: 10.1016/j.ijpsycho.2006.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein R.Z., Cottone L.A., Jia Z., Maloney T., Volkow N.D., Squires N.K. The effect of graded monetary reward on cognitive event-related potentials and behavior in young healthy adults. Int. J. Psychophysiol. 2006;62:272–279. doi: 10.1016/j.ijpsycho.2006.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein R.Z., Parvaz M.A., Maloney T., Alia-Klein N., Woicik P.A., Telang F., Volkow N.D. Compromised sensitivity to monetary reward in current cocaine users: an ERP study. Psychophysiology. 2008;45(5):705–713. doi: 10.1111/j.1469-8986.2008.00670.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman R. The strengths and difficulties questionnaire: a research note. J. Child Psychol. Psychiatry. 1997;38(5):581–586. doi: 10.1111/j.1469-7610.1997.tb01545.x. [DOI] [PubMed] [Google Scholar]
- Groom M.J., Cragg L. Differential modulation of the N2 and P3 event-related potentials by response conflict and inhibition. Brain Cognition. 2015;97:1–9. doi: 10.1016/j.bandc.2015.04.004. [DOI] [PubMed] [Google Scholar]
- Groom M.J., Cahill J.D., Bates A.T., Jackson G.M., Calton T.G., Liddle P.F., Hollis C. Electrophysiological indices of abnormal error-processing in adolescents with attention deficit hyperactivity disorder (ADHD) J. Child Psychol. Psychiatry. 2010;51(1):66–76. doi: 10.1111/j.1469-7610.2009.02128.x. [DOI] [PubMed] [Google Scholar]
- Hämmerer D., Li S.C., Müller V., Lindenberger U. An electrophysiological study of response conflict processing across the lifespan: assessing the roles of conflict monitoring, cue utilization, response anticipation, and response suppression. Neuropsychologia. 2010;48:3305–3316. doi: 10.1016/j.neuropsychologia.2010.07.014. [DOI] [PubMed] [Google Scholar]
- Handy T.C. A Methods Handbook. MIT Press; Cambridge, MA: 2005. Basic principles of ERP quantification. In handy, T. C. event-Related potentials; pp. 33–55. [Google Scholar]
- Hwang S., White S.F., Nolan Z.T., Craig Williams W., Sinclair S., Blair R.J.R. Executive attention control and emotional responding in attention-deficit/hyperactivity disorder — A functional MRI study. NeuroImage Clin. 2015;9:545–554. doi: 10.1016/j.nicl.2015.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M.S., Kim J.J., Kwon J.S. Frontal P300 decrement and executive dysfunction in adolescents with conduct problems. Child Psychiatry Hum. Dev. 2001;2:93–a106. doi: 10.1023/a:1012299822274. [DOI] [PubMed] [Google Scholar]
- Knutson B., Westdorp A., Kaiser E., Hommer D. FMRI visualization of brain activity during a monetary incentive delay task. Neuroimage. 2000;12(1):20–27. doi: 10.1006/nimg.2000.0593. [DOI] [PubMed] [Google Scholar]
- Knutson B., Fong G.W., Adams C.M., Varner J.L., Hommer D. Dissociation of reward anticipation and outcome with event-related fMRI. Neuroreport. 2001;12(17):3683–3687. doi: 10.1097/00001756-200112040-00016. [DOI] [PubMed] [Google Scholar]
- Kohls G., Peltzer J., Herpertz-Dahlmann B., Konrad K. Differential effects of social and non-social reward on response inhibition in children and adolescents. Dev. Sci. 2009;12(4):614–625. doi: 10.1111/j.1467-7687.2009.00816.x. [DOI] [PubMed] [Google Scholar]
- Liu X., Hairston J., Schrier M., Fan J. Common and distinct networks underlying reward valence and processing stages: a meta-analysis of functional neuroimaging studies. Neurosci. Biobehav. Rev. 2011;35(5):1219–1236. doi: 10.1016/j.neubiorev.2010.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luck S.J. Averaging, artifact rejection and artifact correction. In: Luck S.J., editor. An Introduction to the Event-Related Potential Technique. MIT Press; Cambridge, MA: 2005. pp. 131–174. [Google Scholar]
- Luck S.J. 2010. Is It Legitimate to Compare Conditions with Different Numbers of Trials? (In U.-D.) [Google Scholar]
- Luman M., Oosterlaan J., Sergeant J.A. The impact of reinforcement contingencies on AD/HD: A review and theoretical appraisal. Clin. Psychol. Rev. 2005;25(2):183–213. doi: 10.1016/j.cpr.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Lutz K., Widmer M. What can the monetary incentive delay task tell us about the neural processing of reward and punishment? Neurosci. Neuroecon. 2014;3:33–45. [Google Scholar]
- Marco R., Miranda A., Schlotz W., Melia A., Mulligan A., Müller U., Sonuga-Barke E.J.S. Delay and reward choice in ADHD: An experimental test of the role of delay aversion. Neuropsychology. 2009;23(3):367–380. doi: 10.1037/a0014914. [DOI] [PubMed] [Google Scholar]
- Mathes B., Khalaidovski K., Wienke A.S., Schmiedt-Fehr C., Basar-Eroglu C. Maturation of the P3 and concurrent oscillatory processes during adolescence. Clin. Neurophysiol. 2016;7:2599–2609. doi: 10.1016/j.clinph.2016.04.019. [DOI] [PubMed] [Google Scholar]
- Morsink S., Sonuga-Barke E., Mies G., Glorie N., Lemiere J., Van der Oord S., Danckaerts M. What motivates individuals with ADHD? A qualitative analysis from the adolescent’s point of view. Eur. Child Adolescent Psychiatry. 2017:1–10. doi: 10.1007/s00787-017-0961-7. [DOI] [PubMed] [Google Scholar]
- Novak K.D., Foti D. Teasing apart the anticipatory and consummatory processing of monetary incentives: an event-related potential study of reward dynamics. Psychophysiology. 2015;52(11):1470–1482. doi: 10.1111/psyp.12504. [DOI] [PubMed] [Google Scholar]
- Palminteri S., Kilford E.J., Coricelli G., Blakemore S.-J. The computational development of reinforcement learning during adolescence. PLoS Comput. Biol. 2016;12(6):e1004953. doi: 10.1371/journal.pcbi.1004953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfabigan D.M., Tran U.S. Editorial: behavioral and physiological bases of attentional biases: paradigms, participants, and stimuli. Front. Psychol. 2015;6:686. doi: 10.3389/fpsyg.2015.00686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfabigan D.M., Seidel E.-M., Sladky R., Hahn A., Paul K., Grahl A., Lamm C. P300 amplitude variation is related to ventral striatum BOLD response during gain and loss anticipation: an EEG and fMRI experiment. NeuroImage. 2014;96(100):12–21. doi: 10.1016/j.neuroimage.2014.03.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plichta M.M., Scheres A. Ventral?striatal responsiveness during reward anticipation in ADHD and its relation to trait impulsivity in the healthy population: a meta-analytic review of the fMRI literature. Neurosci. Biobehav. Rev. 2014;38:125–134. doi: 10.1016/j.neubiorev.2013.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plichta M.M., Wolf I., Hohmann S., Baumeister S., Boecker R., Schwarz A.J., Brandeis D. Simultaneous EEG and fMRI reveals a causally connected subcortical-Cortical network during reward anticipation. J. Neurosci. 2013;33(36):14526–14533. doi: 10.1523/JNEUROSCI.0631-13.2013. (0631-13.2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polanczyk G., Silva de Lima M., Lessa Horta B., Biederman J., Rohde L.A. The worldwide prevalence of ADHD: a systematic review and metaregression analysis. Am. J. Psychiatry. 2007;6:942–948. doi: 10.1176/ajp.2007.164.6.942. [DOI] [PubMed] [Google Scholar]
- Polich J. Updating P300: An Integrative Theory of P3a and P3b. Clin. Neurophysiology: Official J. Int. Fed. Clin. Neurophysiol. 2007;118(10):2128–2148. doi: 10.1016/j.clinph.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polich J., Kok A. Cognitive and biological determinants of P300: an integrative review. Biol. Psychol. 1995;41(2):103–146. doi: 10.1016/0301-0511(95)05130-9. [DOI] [PubMed] [Google Scholar]
- Rodriguez P.D., Baylis G.C. Activation of brain attention systems in individuals with symptoms of ADHD. Behav. Neurol. 2007;18(2):115–130. doi: 10.1155/2007/865717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojan-Benjunea M.A., Sauqué-Poggio A.M., Barriga-Paulino C.I., Rodríguez-Martínez E.I., Gómez C.M. Development of behavioral parameters and ERPs in a novel-target visual detection paradigm in children, adolescents and young adults. Behav. Brain Func. 2015;22:11. doi: 10.1186/s12993-015-0067-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosch K.S., Hawk L.W. The effects of performance-based rewards on neurophysiological correlates of stimulus, error, and feedback processing in children with ADHD. Psychophysiology. 2013;50(11) doi: 10.1111/psyp.12127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagvolden T., Johansen E.B., Aase H., Russell V.A. A dynamic developmental theory of attention-deficit/hyperactivity disorder (adhd) predominantly hyperactive/impulsive and combined subtypes. Behav. Brain Sci. 2005;28:397–468. doi: 10.1017/S0140525X05000075. [DOI] [PubMed] [Google Scholar]
- Sattler J.M. 4th ed. ed. CA: Author; San Diego: 2001. Assessment of Children: Cognitive Applications. [Google Scholar]
- Scheres A., Milham M.P., Knutson B., Castellanos F.X. Ventral striatal hyporesponsiveness during reward anticipation in attention-Deficit/Hyperactivity disorder. Biol. Psychiatry. 2007;61(5):720–724. doi: 10.1016/j.biopsych.2006.04.042. [DOI] [PubMed] [Google Scholar]
- Scheres A., Tontsch C., Thoeny A.L., Sumiya M. Temporal reward discounting in children, adolescents, and emerging adults during an experiential task. Front. Psychol. 2014;5:711. doi: 10.3389/fpsyg.2014.00711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schupp H.T., Öhman A., Junghöfer M., Weike A.I., Stockburger J., Hamm A.O. The facilitated processing of threatening faces: an ERP analysis. Emotion. 2004;4(2):189–200. doi: 10.1037/1528-3542.4.2.189. [DOI] [PubMed] [Google Scholar]
- Segalowitz S.J., Santesso D.L., Jetha M.K. Electrophysiological changes during adolescence: a review. Brain and Cognition. 2010;72(1):86–100. doi: 10.1016/j.bandc.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Semlitsch H.V., Anderer P., Schuster P., Presslich O. A solution for reliable and valid reduction of ocular artifacts applied to the P300 ERP. Psychophysiology. 1986;23(6):695–703. doi: 10.1111/j.1469-8986.1986.tb00696.x. [DOI] [PubMed] [Google Scholar]
- Sergeant J.A. Modeling attention-Deficit/Hyperactivity disorder: a critical appraisal of the cognitive-Energetic model. Biol. Psychiatry. 2005;57(11):1248–1255. doi: 10.1016/j.biopsych.2004.09.010. [DOI] [PubMed] [Google Scholar]
- Shaffer D., Schwab-Stone M., Fisher P., Cohen P., Placentini J., Davies M., Regier D. The diagnostic interview schedule for children-Revised version (DISC-R): I. preparation, field testing, interrater reliability, and acceptability. J. Am. Acad. Child Adolescent Psychiatry. 1993;32(3):643–650. doi: 10.1097/00004583-199305000-00023. [DOI] [PubMed] [Google Scholar]
- Silverman M.H., Jedd K., Luciana M. Neural networks involved in adolescent reward processing: an activation likelihood estimation meta-analysis of functional neuroimaging studies. NeuroImage. 2015;122:427–439. doi: 10.1016/j.neuroimage.2015.07.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonuga-Barke E.J.S., Fairchild G. Neuroeconomics of attention-deficit/Hyperactivity disorder: differential influences of medial, dorsal, and ventral prefrontal brain networks on suboptimal decision making? Biol. Psychiatry. 2012;72(2):126–133. doi: 10.1016/j.biopsych.2012.04.004. [DOI] [PubMed] [Google Scholar]
- Sonuga-Barke E.J.S., Cortese S., Fairchild G., Stringaris A. Annual Research Review: transdiagnostic neuroscience of child and adolescent mental disorders −differentiating decision making in attention-deficit/hyperactivity disorder, conduct disorder, depression, and anxiety. J. Child Psychol. Psychiatry. 2016;57(3):321–349. doi: 10.1111/jcpp.12496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ströhle A., Stoy M., Wrase J., Schwarzer S., Schlagenhauf F., Huss M., Heinz A. Reward anticipation and outcomes in adult males with attention-deficit/hyperactivity disorder. NeuroImage. 2008;39(3):966–972. doi: 10.1016/j.neuroimage.2007.09.044. [DOI] [PubMed] [Google Scholar]
- Szuromi B., Czobor P., Komlósi S., Bitter I. P300 deficits in adults with attention deficit hyperactivity disorder: a meta-analysis. Psychol. Med. 2011;41(7):1529–1538. doi: 10.1017/S0033291710001996. [DOI] [PubMed] [Google Scholar]
- Tamm L., Menon V., Reiss A.L. Parietal attentional system aberrations during target detection in adolescents with attention deficit hyperactivity disorder: event-Related fMRI evidence. Am. J. Psychiatry. 2006;163(6):1033–1043. doi: 10.1176/ajp.2006.163.6.1033. [DOI] [PubMed] [Google Scholar]
- Tripp G., Wickens J.R. Research Review: dopamine transfer deficit: a neurobiological theory of altered reinforcement mechanisms in ADHD. J. Child Psychol. Psychiatry. 2008;49(7):691–704. doi: 10.1111/j.1469-7610.2007.01851.x. [DOI] [PubMed] [Google Scholar]
- Umemoto A., Lukie C.N., Kerns K.A., Müller U., Holroyd C.B. Impaired reward processing by anterior cingulate cortex in children with attention deficit hyperactivity disorder. Cog. Affec. Behav. Neurosci. 2014;14(2):698–714. doi: 10.3758/s13415-014-0298-3. [DOI] [PubMed] [Google Scholar]
- Walter W.G., Cooper R., Aldridge V.J., McCallum W.C., Winter A.L. Contingent negative variation: an electric sign of sensori-Motor association and expectancy in the human brain. Nature. 1964;203(4943):380–384. doi: 10.1038/203380a0. [DOI] [PubMed] [Google Scholar]
- Wechsler D. 4th ed. Pearson Assessment; London: 2004. The Wechsler Intelligence Scale for Children. [Google Scholar]
- Wilbertz G., Delgado M.R., Van Elst L.T., Maier S., Philipsen A., Blechert J. Neural response during anticipation of monetary loss is elevated in adult attention deficit hyperactivity disorder. World J. Biol. Psychiatry. 2015:1–11. doi: 10.3109/15622975.2015.1112032. [DOI] [PubMed] [Google Scholar]
- van Leeuwen T.H., Steinhausen H.C., Overtoom C.C.E., Pascual-Marqui R.D., van’t Klooster B., Rothenberger A., Brandeis D. The continuous performance test revisited with neuroelectric mapping: impaired orienting in children with attention deficits. Behav. Brain Res. 1998;1:97–110. doi: 10.1016/s0166-4328(97)00173-3. [DOI] [PubMed] [Google Scholar]
- van Meel C.S., Heslenfeld D.J., Oosterlaan J., Luman M., Sergeant J.A. ERPs associated with monitoring and evaluation of monetary reward and punishment in children with ADHD. J. Child Psychol. Psychiatry. 2011;52(9):942–953. doi: 10.1111/j.1469-7610.2010.02352.x. [DOI] [PubMed] [Google Scholar]
- van Meel C.S., Oosterlaan J., Henlenfeld D., Sergeant J.A. Telling good from bad news: ADHD differentially affects processing of posiitve and negaative feedback during guessing. Neuropsychologia. 2015;43(13):1946–1954. doi: 10.1016/j.neuropsychologia.2005.03.018. [DOI] [PubMed] [Google Scholar]
- van der Meere J. State regulation and attention-deficit/hyperactivity disorder. In: Gozal D., Molfese D.L., editors. Attention Deficit Hyperactivity Disorder: From Genes to Patients. NJ Humana Press Inc.; Totowa: 2005. pp. 413–433. [Google Scholar]
- von Rhein D., Cools R., Zwiers M.P., van der Schaaf M., Franke B., Luman M., Buitelaar J. Increased neural responses to reward in adolescents and young adults with attention-Deficit/Hyperactivity disorder and their unaffected siblings. J. Am. Acad. Child Adolescent Psychiatry. 2015;54(5):394–402. doi: 10.1016/j.jaac.2015.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X., Sonuga-Barke E., Liu X. Preference for smaller sooner over larger later rewards in ADHD: contribution of delay duration and paradigm type. J. Atten. Disord. 2015 doi: 10.1177/1087054715570390. [DOI] [PubMed] [Google Scholar]