Highlights
-
•
Acute exercise enhances P300 amplitude in children with ADHD and healthy peers.
-
•
Exercise-induced benefits for inhibition are influenced by exercise modality.
-
•
Children with ADHD benefit most from aerobic exercise.
-
•
Aerobic and coordinative exercise had similar effects in healthy controls.
Keywords: P300, Executive function, Cognitive performance, Flanker task, Development, Healthy children
Abstract
The current body of evidence suggests that an aerobic exercise session has a beneficial effect on inhibitory control, whereas the impact of coordinative exercise on this executive function has not yet been examined in children with ADHD. Therefore, the present study aims to investigate the acute effects of aerobic and coordinative exercise on behavioral performance and the allocation of attentional resources in an inhibitory control task.
Using a cross-over design, children with ADHD-combined type and healthy comparisons completed a Flanker task before and after 20 min moderately-intense cycling exercise, coordinative exercise and an inactive control condition. During the task, stimulus-locked event-related potentials were recorded with electroencephalography.
Both groups showed an increase of P300 amplitude and decrease of reaction time after exercise compared to the control condition. Investigating the effect of exercise modality, aerobic exercise led to greater increases of P300 amplitude and reductions in reaction time than coordinative exercise in children with ADHD.
The findings suggest that a single exercise bout improves inhibitory control and the allocation of attentional resources. There were some indications that an aerobic exercise session seems to be more efficient than coordinative exercise in reducing the inhibitory control deficits that persist in children with ADHD.
1. Introduction
Attention-Deficit/Hyperactivity Disorder (ADHD) is considered to be the most common neurodevelopmental disorder among children, with a global prevalence rate of 6% (Polanczyk et al., 2014). The behavioral symptoms of this neuropsychiatric disorder comprise a developmentally inappropriate pattern of inattention and impulsiveness/hyperactivity with an initial manifestation before children have reached 12 years of age (American Psychiatric Association, 2013). In 60% of all cases those symptoms persist into adulthood (Sibley et al., 2016), affecting people with ADHD at different stages throughout life. Based on investigations of brain structure and function, volume reductions and abnormal activation patterns of the fronto-parietal network have been discussed as neurobiological mechanisms underlying ADHD dysfunctions (Cortese et al., 2012, Rubia et al., 2014). Meta-analytical findings have revealed that the behavioral consequences of such functional abnormalities are deficits in inhibitory control, vigilance and working memory (Willcutt et al., 2005). As failures in inhibitory control have long been regarded as the core cognitive deficit in ADHD (Lipszyc and Schachar, 2010), many researchers have used neurophysiological indices to study possible underlying mechanisms. In this respect, impaired inhibitory control in children with ADHD has been related to a reduced allocation of attentional resources, delayed processing and evaluation of stimuli as well as failures to implement action monitoring (Doehnert et al., 2010, Kratz et al., 2011, Liotti et al., 2010). Regarding evidence-based medicine, meta-analytical findings have shown that treatment with methylphenidate (MPH) has only a small, but positive effect on inhibitory control (Tamminga et al., 2016). This limited efficacy highlights the need for additional complementary or alternative interventions for managing cognitive deficits in ADHD.
Interestingly, physical exercise has been found to elicit acute benefits for overall cognitive performance, particularly when the duration of the exercise session is longer than 10 min (Chang et al., 2012a). Focusing on moderately-intense exercise, a recent meta-analysis showed that aerobic exercise (defined as the rhythmical contraction and relaxation of large muscle groups over a prolonged time) had a moderate effect on different executive functions, including inhibitory control, in healthy children (Ludyga et al., 2016). Likewise there is accumulating evidence pointing to beneficial effects of acute aerobic exercise in children with ADHD (Berwid and Halperin, 2012). According to Ludyga et al. (2017), experimental studies in children with ADHD have consistently shown improvements of inhibitory control following aerobic exercise at moderate intensity, whereas results were heterogeneous for other executive functions.
Going beyond behavioral measures, researchers have used event-related potentials (ERPs) to examine cognitive operations that contribute to improved inhibitory control following aerobic exercise. Based on a review of studies with healthy children, Hillman et al. (2011) found that acute improvements of inhibitory control after aerobic exercise are associated with an increased amplitude of the P300 component. According to Polich (2007), P300 amplitude is proportional to the resources allocated towards the suppression of superfluous neuronal activity to facilitate processing of target stimulus events. Consequently, the exercise-induced increase of P300 amplitude observed across several experimental studies with healthy populations (Hillman et al., 2011) is considered to reflect an improved inhibition of extraneous neuronal activity. Some studies also reported decreased P300 latency following aerobic exercise (Hillman et al., 2003, Kamijo et al., 2007), which supports an increase in stimulus classification speed (Polich, 2007). Investigating acute effects of moderately-intense aerobic in children with ADHD and their healthy peers, Pontifex et al. (2013) found increases of P300 amplitude and decreases of P300 latency during a task tapping inhibitory control in both groups. In line with this findings, previous studies have also reported that children with ADHD improved behavioral performance in this cognitive domain after a single session of aerobic exercise (Chang et al., 2012b, Piepmeier et al., 2015).
Based on an examination of individual differences, Drollette et al. (2014) further showed that children with low inhibitory control elicited larger P300 amplitudes after moderate aerobic exercise than children with high inhibitory control. Given that children with ADHD show deficits in inhibitory control, this is a first indication that acute aerobic exercise may elicit greater benefits in children with ADHD compared to healthy comparisons. This is suggested to be due to a greater reserve for cognitive enhancements in individuals with low inhibitory control (Drollette et al., 2014, Sibley and Beilock, 2007). Additionally, greater benefits can be expected in children with ADHD as the exercise-induced increase of arousal (Kamijo et al., 2004, Kamijo et al., 2007) may counteract the task-related hypo-activation that is evident ADHD (Cortese et al., 2012). Further insights into the differential effects of aerobic exercise in children with ADHD and healthy comparisons are necessary to draw conclusions on whether exercise normalizes and/or enhances the inhibitory aspect of executive control and the allocation of attentional resources.
Whereas the majority of studies have focused on the acute effects of aerobic exercise on executive functions, other modalities have received far less attention. Evidence, albeit limited, suggests that exercises requiring higher cognitive engagement, such as coordinative exercise, elicits a greater enhancement of executive functions than simpler exercises characterized by lower cognitive demands (Best, 2010, Tomporowski et al., 2015). Coordinative exercises are characterized by complex motor movements that demand multiple degrees of freedom and interaction with body parts and/or objects for goal-directed behaviors (Newell, 1985, Pesce, 2012). The execution of such motor tasks results in a co-activation of the cerebellum and the prefrontal cortex (Ito, 2008). In turn, increased activity of the prefrontal cortex has been linked with improved inhibitory control in previous studies (Endo et al., 2013, Yanagisawa et al., 2010). Consequently, it is reasonable to suggest that coordinative exercise transiently benefits cognitive performance. Although some experimental studies have already reported improved inhibitory control and attention after such cognitively-engaging exercise in healthy children (Jäger et al., 2014, Schmidt et al., 2015), it remains to be elucidated whether or not children with ADHD can expect similar benefits. Moreover, P300 amplitude and latency have not yet been investigated as potential mechanisms that contribute to enhanced inhibitory control following coordinative exercise. Further research is necessary to determine if aerobic exercise or coordinative exercise is more efficient for the promotion of inhibitory control in children with ADHD and healthy peers.
The present study aimed to investigate the acute effects of aerobic exercise and coordinative exercise on inhibitory control and the allocation of attentional resources in children with ADHD and healthy comparisons. As previous findings have shown superior benefits in individuals with low executive function (Drollette et al., 2014, Sibley and Beilock, 2007), we expected that a single exercise session would improve inhibitory control and the allocation of attentional resources (Hypothesis 1a), with greater benefits elicited in children with ADHD relative to healthy comparisons (Hypothesis 1b). Based on previous reviews (Best, 2010, Tomporowski et al., 2015), it is further hypothesised that coordinative exercise benefits behavioral performance on the inhibitory control task and the allocation of attentional resources to a greater extent than aerobic exercise (Hypothesis 2).
2. Material and methods
2.1. Participants
For an experimental study, children with ADHD (n = 7 f/11 m) were recruited from local pediatricians and the University of Basel Children's Hospital. Additionally, a healthy control group (n = 8 f/10 m) was recruited from local schools. Participants were deemed eligible if they were between 11 and 16 years of age, had corrected-to or normal vision and right hand dominance based on the Edinburgh Handedness Inventory (Oldfield, 1971). In addition to those criteria, children with ADHD had to be diagnosed with ADHD-combined type according to the DSM-IV and to be under therapy with MPH. These criteria were chosen to reduce the risk that possible benefits elicited by exercise are confounded by differences in the ADHD type and treatment. The diagnosis of ADHD and the exclusion of Autism as comorbid condition was based on multiple assessments (anamnesis including a structured interview, standardized questionnaires, assessment of comorbidities) by neuropediatricians and pediatricians specialized on ADHD. Participants with an acute or chronic disease, which is classified as a contraindication for exercise according to ACSM standards and/or which impairs the practicability of the scheduled exercise session or any injury or disease affecting the functionality of the right hand were excluded. The characteristics of the participants that were included in the final data analysis are displayed in Table 1. Prior to testing, all participants as well as their legal guardians provided informed written consent. Study procedures were in line with the Declaration of Helsinki and ethical approval was granted by the local ethics committee.
Table 1.
Participants’ characteristics.
| ADHD (n = 5 f/11 m) |
Controls (n = 8 f/10 m) |
Student’s T-test |
||||
|---|---|---|---|---|---|---|
| M | SD | M | SD | T | p | |
| Age in y | 12.8 | 1.8 | 13.5 | 1.3 | −1.26 | 0.217 |
| Height in cm | 160.6 | 10.9 | 165.2 | 10.9 | −1.24 | 0.224 |
| Body mass in kg | 54.1 | 16.1 | 55.4 | 12.5 | −0.27 | 0.791 |
| Body mass index in kg m−2 | 20.8 | 5.0 | 20.2 | 3.3 | 0.44 | 0.644 |
| PWC170 in W kg−1 | 2.38 | 0.69 | 2.50 | 0.49 | −0.59 | 0.557 |
| DSM-IV inattention | 63.6 | 6.4 | 47.1 | 11.1 | 5.24 | <0.001 |
| DSM-IV hyperactivity | 68.1 | 6.6 | 50.2 | 8.0 | 7.04 | <0.001 |
| DSM-IV combined | 67.1 | 5.6 | 47.9 | 10.6 | 6.46 | <0.001 |
Note: ADHD = Attention deficit/hyperactivity disorder; DSM IV = Diagnostic and Statistical Manual for Psychiatric Disorders, 4th edition; DSM-IV clinical symptoms were assessed using the Conners 3 Scales − Parent Version; reported are T-values, corrected for sex.
2.2. Procedures
The present study comprised four laboratory visits. At the first visit, participants were familiarized with the laboratory and completed a health check-up, including electrocardiography, measurement of blood pressure and pulmonary function. In the meantime, legal guardians were asked to report the medical history of the participating child and to fill in the validated German adaptation of the Conners 3 Scales − Parent Version (Lidzba et al., 2013). Afterwards, participants completed a practice block of a modified Flanker task (Eriksen and Eriksen, 1974) and a submaximal exercise test (PWC 170) on a cycle ergometer (Bland et al., 2012). This required participants to cycle at 60–70 rpm, while the initial workload (20 W for <50 kg; 30 W for >50 kg) was increased every two minutes. The power output for the subsequent stage was based on the heart rate in the final ten seconds of each stage. The test was terminated when the participant finished a stage with a heart rate of ≥165 bpm.
Seven to 14 days after the screening visit, experimental sessions were scheduled. Using a cross-over design, participants completed a modified Flanker task before and ten minutes after aerobic exercise, coordinative exercise and watching a video, on three separate days respectively, at the same time of the day (±2 h). During the cognitive task, electroencephalographic activity was recorded continuously. The order of the sessions was randomized and counterbalanced across participants. In the aerobic exercise condition, participants were cycling at moderate intensity over 20 min on an ergometer (E200 P, COSMED, Italy). In accordance with Norton et al. (2010), 65–70% of the maximum heart rate was used as heart rate target for moderate intensity exercise. The maximal heart rate was estimated using the formula 208-0.7*(age), which is recommended for children aged seven to 17 years (Mahon et al., 2010). To ensure that participants remained in their individual heart rate targets, power output was adjusted at regular intervals if necessary.
In the 20 min coordinative exercise session, children performed exercises demanding object control skills and bilateral coordination of lower and upper extremities. For example, exercises included balancing on an exercise ball, one leg stands on a balance board while catching and throwing a ball, quickly switching between different variations of jumping jacks, stepping through colored rings in a predefined order as well as keeping balloons in the air using right or left hand or leg based on the color of the balloon. The difficulty of each exercise was adjusted to individual coordinative skills, so that participants were able to perform the exercise without mistakes over at least 30 s.
In the control condition, participants were seated in front of a monitor and watched a 20 min documentary on exercise behavior in adults. Similar to previous studies investigating acute effects of exercise on cognitive performance, the video was not suggested to be cognitively-demanding (Piepmeier et al., 2015, Stroth et al., 2009). All sessions were performed in the laboratory, so that no transition from one room to another one was required. In each condition, heart rate was continuously recorded using heart rate monitors (V800, Polar Electro, Finnland). Additionally, participants were asked to report perceived exertion (RPE) in 5 min intervals during aerobic and coordinative exercise.
2.3. Inhibitory control task
Information processing and inhibitory control were assessed using a modified Flanker task, which was administered with E-Prime Software (Psychology Software Tools, USA). The task requires participants to respond to a centrally presented target stimulus (in this case a fish) amid lateral flanking stimuli. In congruent trials five fish were facing the same direction (either left or right), whereas in incongruent trials the direction of the centrally presented fish was different from flanking fish. During the task, participants were required to respond by pressing a button corresponding to the direction of the target stimulus. Prior to testing, participants were instructed by the investigator. Additionally, instructions were displayed on the screen to make sure that they understood the task. Following a practice round with 20 trials, participants completed four blocks with 40 trials each. The test blocks were interspersed by a 30 s recovery period. The order of the trials was randomized and congruency (congruent, incongruent) as well as directionality (left, right) of the stimuli was equiprobable. Black fish (vertical visual angle: 1.8°; distance between fish: 1.4°) were presented focally for 200 ms on a white background with a response window of 1000 ms. The inter-stimulus interval varied randomly between 1700 and 2100 ms. Cognitive performance was assessed by calculating the mean reaction time for correct responses as well as mean accuracy in both congruent and incongruent trials.
2.4. EEG recording and processing
Using a 128-channel HydroCel Geodesic Sensor Net (EGI, USA) electroencephalographic activity was recorded continuously during the modified flanker task at an environmental temperature of 20 °C. Between the pre- and post-exercise assessments, the EEG net remained on the participant’s head. As recommended for high input-impedance amplifiers (Ferree et al., 2001), scalp electrode impedances were controlled and maintained below 50 K Ω at both measurement time points. During the acquisition, EEG data was amplified by a Net Amps 300 (EGI, USA) system, referenced to the vertex channel (Cz), low-pass filtered at 100 Hz and digitized at a sampling rate of 250 Hz. Offline processing was performed using the NetStation software package (EGI, USA). Based on the timing delay of the video system that was measured with a photosensor, the event codes were shifted to account for this delay. A finite response (FIR) bandpass filter was applied on the collected data, so that a frequency range of 0.5–30 Hz remained for analysis. Filtered data was inspected for eye movements and blinks by using an automatic detection algorithm on VEOG and HEOG channels. Continuous EEG data was then re-referenced to average mastoids and segmented relative to the stimulus onset (−200 ms to 600 ms). Of the resulting segments only congruent and incongruent trials with correct responses were used for further analysis. Segments were marked as bad if they contained blinks or eye movements, ten or more channels exceeded a voltage threshold of 180 μV or a transition threshold of 100 μV. The remaining segments were visually inspected for artifacts. At least 25 good segments in both pre- and post-exercise were required for a dataset to be included for further analyses. The total amount of segments (control condition: 40 ± 4 at pre; 41 ± 6 at post; aerobic exercise: 40 ± 5 at pre; 37 ± 7 at post; coordinative exercise: 39 ± 5 at pre; 37 ± 6 at post) was not different between groups and conditions. Following baseline correction using the interval from −200 ms to stimulus onset, artifact-free segments were averaged.
For the analysis of the P300 component of ERPs, amplitude and latency measures were performed in a time window of 250–600 ms after the stimulus onset. The selection of the time window was based on previous lab experience and a review of the literature. Adaptive mean was calculated as the average amplitude of 20 ms prior to and 20 ms after the peak, as this measure has been shown to be an efficient estimator of the true ERP signal particularly for individual-subject latency variability (Clayson et al., 2013). P300 latency was assessed using the fractional latency metric, defining 70% of the peak amplitude as relative criterion for the onset (Kiesel et al., 2008). Latency measures were obtained from Pz and amplitude measures were averaged for the parietal region (P1, P2, P3, P4 and Pz).
2.5. Statistics
As recommended by Faul et al. (2007), sample size was calculated a priori using G*Power 3.1. Based on effect sizes reported in previous studies (Hillman et al., 2009, Pontifex et al., 2013) and an alpha level set to 0.05, the initial power analysis indicated that ten participants per group were required to reach 85% statistical power. The statistical analysis of collected data was performed with SPSS 25.0 (IBM Statistics, USA) for Windows. In advance, Gaussian distribution of the data was verified with the Shapiro Wilk Test. Possible differences in submaximal power output, age, BMI between healthy children and children with ADHD were investigated using Student’s T-Test. This test was also applied to compare mean heart rate and RPE between the exercise conditions. For these statistical comparisons, the level of significance was set to p ≤ 0.05.
For overall analyses, a 2 (time: pre, post) × 3 (condition: aerobic exercise, coordinative exercise, control condition) × 2 (congruency: congruent, incongruent) × 2 (group: ADHD, controls) ANOVA was applied to assess the acute effects of exercise on task performance and P300. Main effects and interactions were reported. Subsequently, pre-planned contrasts were calculated based on hypotheses. Firstly, the effects of exercise (average of aerobic and coordinative exercise) were compared with the control condition by assessing the interaction of time and condition (Hypothesis 1a). Subsequently, the 3-way interaction of time, condition and group was calculated to examine whether or not exercise elicits greater benefits for inhibitory control and associated cognitive processes (Hypothesis 1b). Secondly, the effects of aerobic exercise were compared with coordinative exercise within the ADHD and control group to examine whether or not coordinative exercise elicits greater benefits in one of the groups (Hypothesis 2). For these statistical analyses (overall and hypotheses tests), the level of significance was adjusted for multiple comparisons on task performance (reaction time and accuracy) and P300 measures (amplitude and latency), so that effects were considered statistically significant at p ≤ 0.025.
3. Results
Of 36 eligible participants, two children from the ADHD group discontinued the study prematurely due to illness. Additionally, one healthy child had to be excluded from the analysis of ERPs, because data was contaminated by blinks. During the aerobic exercise session, participants’ mean heart rate was not different from the predefined heart rate target (139.4 ± 2.1 vs. 139.1 ± 0.8 bpm), T(33) = 0.7, p = 0.474. Furthermore, the mean heart rate during aerobic exercise was higher than the mean heart rate during coordinative exercise (139.4 ± 2.1 vs. 131.3 ± 11.6 bpm), T(33) = 4.2, p < 0.001. In contrast, mean RPE was not different between the exercise conditions (13.0 ± 1.3 vs. 12.7 ± 1.1), T(33) = 1.2, p = 0.238. When participants were watching the video, the heart rate was not different from the resting state (70.7 ± 8.9 vs. 71.9 ± 10.7 bpm), T(33) = 1.8, p = 0.088.
3.1. Overall analyses
Based on the omnibus analyses, there was a main effect of congruency on reaction time, F(1,32) = 131.3, p < 0.001, η2 = 0.80, and accuracy, F(1,33) = 86.1, p < 0.001, η2 = 0.73, showing shorter reaction time (334.4 ± 64.9 vs. 360.9 ± 71.9 ms) and higher accuracy (93.8 ± 0.05 vs. 83.8 ± 0.08%) for congruent compared to incongruent trials. For reaction time, main effects of group F(1,32) = 8.9, p = 0.005, η2 = 0.22, and time were found, F(1,32) = 25.1, p < 0.001, η2 = 0.44, indicating shorter reaction times in healthy comparisons relative to children with ADHD (318.1 ± 39.2 ms vs. 380.8 ± 79.1 ms) and at post-test compared to pre-test (354.9 ± 70.2 ms vs. 340.4 ± 67.2 ms). Additionally, there was an interaction of time and condition for reaction time, F(2,31) = 9.8, p = 0.001, η2 = 0.39, superseded by a time x condition x group interaction, F(2,31) = 4.3, p = 0.023, η2 = 0.22. No further significant interactions were observed for reaction time and accuracy (Table 2).
Table 2.
Main effects and interactions obtained from the omnibus analyses.
| F | p | Eta2 | 1-Beta | ||
|---|---|---|---|---|---|
| Reaction time in ms | Time | 25.1 | <0.001 | 0.44 | 0.99 |
| Condition | 0.1 | 0.897 | <0.01 | 0.06 | |
| Group | 8.9 | 0.005 | 0.22 | 0.82 | |
| Congruency | 131.3 | <0.001 | 0.80 | 1.00 | |
| Time x condition | 11.8 | <0.001 | 0.27 | 0.99 | |
| Time x group | 1.6 | 0.217 | 0.05 | 0.23 | |
| Condition x group | 0.3 | 0.759 | 0.01 | 0.09 | |
| Time x condition x congruency | 0.3 | 0.764 | 0.02 | 0.09 | |
| Accuracy in% | Time | 0.9 | 0.349 | 0.03 | 0.15 |
| Condition | <0.1 | 0.977 | <0.01 | 0.05 | |
| Group | 2.6 | 0.119 | 0.07 | 0.34 | |
| Congruency | 86.1 | <0.001 | 0.73 | 1.00 | |
| Time x condition | 1.7 | 0.202 | 0.10 | 0.33 | |
| Time x group | <0.1 | 0.852 | <0.01 | 0.05 | |
| Condition x group | 0.3 | 0.754 | 0.02 | 0.09 | |
| Time x condition x congruency | 0.5 | 0.587 | 0.03 | 0.13 | |
| P300 amplitude in μV | Time | 24.3 | <0.001 | 0.44 | 0.99 |
| Condition | 0.7 | 0.485 | 0.05 | 0.16 | |
| Group | <0.1 | 0.837 | <0.01 | 0.06 | |
| Congruency | 47.9 | <0.001 | 0.61 | 1.00 | |
| Time x condition | 15.5 | <0.001 | 0.33 | 0.99 | |
| Time x group | <0.1 | 0.999 | <0.01 | 0.05 | |
| Condition x group | 1.7 | 0.194 | 0.05 | 0.32 | |
| Time x condition x congruency | 1.5 | 0.228 | 0.09 | 0.30 | |
| P300 latency in ms | Time | 3.7 | 0.063 | 0.11 | 0.46 |
| Condition | 2.2 | 0.124 | 0.07 | 0.43 | |
| Group | 6.5 | 0.016 | 0.17 | 0.70 | |
| Congruency | 24.9 | <0.001 | 0.45 | 0.99 | |
| Time x condition | 1.9 | 0.148 | 0.06 | 0.28 | |
| Time x group | 0.8 | 0.364 | 0.03 | 0.15 | |
| Condition x group | 0.7 | 0.479 | 0.02 | 0.17 | |
| Time x condition x congruency | 0.7 | 0.477 | 0.02 | 0.17 | |
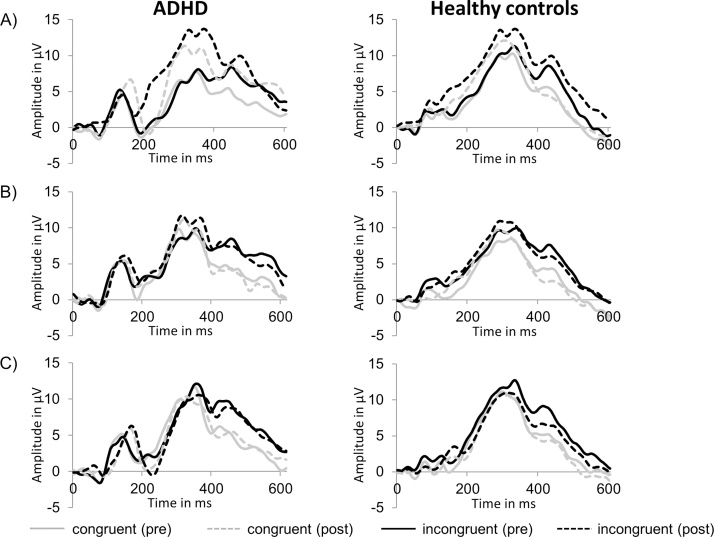
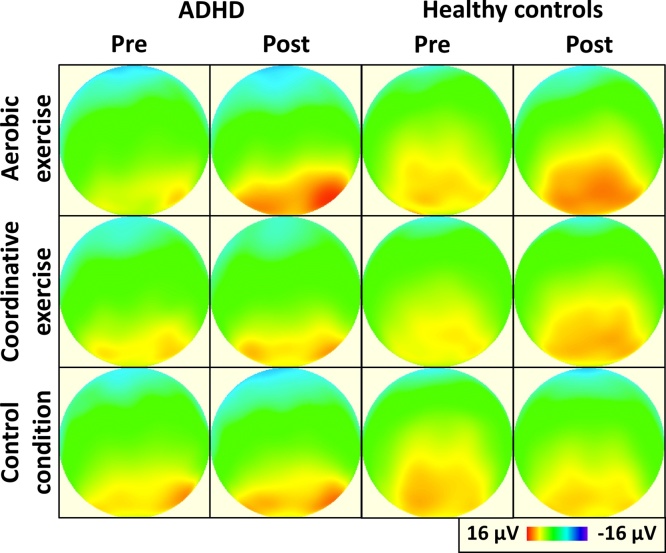
With regard to ERPs, there was a main effect of congruency for P300 amplitude, F(1,31) = 47.9, p < 0.001, η2 = 0.61, and latency, F(1,31) = 24.8, p < 0.001, η2 = 0.45, indicating lower amplitude (11.8 ± 4.9 vs. 13.1 ± 5.3 μV) and shorter latency (264.2 ± 56.8 vs. 282.9 ± 60.9 ms) for congruent compared to incongruent trials. Additionally, a main effect of time on P300 amplitude was found, F(1,31) = 24.3, p < 0.001, η2 = 0.44, so that participants had a higher P300 amplitude at post-test compared to pre-test (13.0 ± 5.2 vs. 11.9 ± 5.0 μV). For P300 latency, a main effect of group, F(1,31) = 6.5, p = 0.016, η2 = 0.17, indicated that healthy comparisons had shorter latencies than the ADHD group (250.6 ± 41.4 vs. 298.0 ± 63.6 ms). Moreover, a time by condition interaction was observed for P300 amplitude, F(2,30) = 15.9, p < 0.001, η2 = 0.52, but not for P300 latency. No further significant interactions were observed for amplitude and latency measures of the P300 component. Grand-averaged ERP waveforms for each group and session are provided in Fig. 1 and the topographic distribution of the P300 amplitude across the scalp is displayed in Fig. 2.
Fig. 1.
Grand-averaged event-related potentials at Pz before and after A) aerobic exercise, B) coordinative exercise and C) watching a video. Note: ADHD = Attention-Deficit/Hyperactivity Disorder.
Fig. 2.
Topographic plots of P300 amplitude before and after the experimental sessions in children with ADHD and healthy comparisons. Note: ADHD = Attention-Deficit/Hyperactivity Disorder; plots present the peak amplitude of stimulus-locked ERPs collapsed across congruent and incongruent trials.
3.2. Acute effects of exercise compared to the control condition
Contrasting general effects of exercise (independent of modality) against the control condition, there was a significant interaction of time and condition for reaction time, F(1,32) = 17.4, p < 0.001, η2 = 0.35, indicating a greater decrease of reaction time following exercise compared to watching a video in both groups (Table 3). No such interaction was observed for accuracy.
Table 3.
Reaction time for congruent and incongruent trials of the Flanker task before and after the experimental sessions in children with ADHD and healthy controls.
| ADHD (n = 16) |
Controls (n = 18) |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Pre |
Post |
Pre |
Post |
||||||
| M | SD | M | SD | M | SD | M | SD | ||
| Reaction time on congruent trials in ms | Aerobic exercise | 390.1 | 99.4 | 354.1 | 78.5 | 309.8 | 37.3 | 301.1 | 41.0 |
| Coordinative exercise | 369.8 | 72.3 | 358.3 | 76.8 | 313.1 | 39.8 | 296.9 | 35.5 | |
| Control condition | 363.2 | 82.2 | 365.5 | 78.9 | 306.4 | 35.2 | 306.0 | 41.4 | |
| Reaction time on incongruent trials in ms | Aerobic exercise | 417.9 | 107.3 | 375.9 | 81.9 | 338.0 | 50.6 | 321.1 | 48.6 |
| Coordinative exercise | 407.6 | 84.7 | 385.8 | 90.5 | 339.2 | 45.4 | 320.4 | 39.1 | |
| Control condition | 391.6 | 74.8 | 390.3 | 89.9 | 335.1 | 53.9 | 330.1 | 50.5 | |
Note: ADHD = Attention Deficit/Hyperactivity Disorder.
For P300 amplitude, there was an interaction of time and condition, F(1,31) = 20.5, p < 0.001, η2 = 0.39, showing that P300 amplitude was increased to a greater extent after exercise compared to the control condition (Table 4). In contrast, changes in P300 latency from pre- to post-test were not significantly different between the exercise and control conditions. Furthermore, based on the first contrast, there was no significant interaction of time, condition and group for any of the dependent variables (see supplement for further details).
Table 4.
P300 latency and amplitude before and after the experimental sessions in children with ADHD and healthy controls.
| ADHD (n = 16) |
Controls (n = 17) |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Pre |
Post |
Pre |
Post |
||||||
| M | SD | M | SD | M | SD | M | SD | ||
| P300 adaptive mean amplitude in μV | Aerobic exercise | 10.7 | 3.5 | 13.8 | 3.8 | 11.9 | 5.9 | 14.3 | 6.7 |
| Coordinative exercise | 13.1 | 6.3 | 13.5 | 5.5 | 11.2 | 6.8 | 12.8 | 7.1 | |
| Control condition | 12.4 | 3.6 | 12.4 | 3.6 | 12.0 | 6.0 | 11.6 | 6.2 | |
| P300 latency (70% peak amplitude) in ms | Aerobic exercise | 320.8 | 76.9 | 300.8 | 70.8 | 254.8 | 54.6 | 249.9 | 63.1 |
| Coordinative exercise | 303.6 | 89.5 | 298.1 | 79.6 | 266.6 | 48.4 | 241.2 | 35.3 | |
| Control condition | 275.6 | 57.0 | 289.4 | 72.0 | 247.1 | 43.7 | 244.1 | 38.6 | |
Note: ADHD = Attention Deficit/Hyperactivity Disorder; Data are collapsed across congruent and incongruent trials.
3.3. Acute effects of aerobic exercise compared to coordinative exercise
Contrasting the effects of aerobic exercise against the effects of coordinative exercise, there was an interaction of time, condition and group for reaction time, F(1,32) = 7.4, p = 0.01, η2 = 0.19, but not for accuracy. Further examination revealed a time by condition interaction for reaction time in children with ADHD, F(1,32) = 6.1, p = 0.025, η2 = 0.26, indicating that aerobic exercise led to a greater reduction of reaction time than coordinative exercise (Table 3). In healthy comparisons, exercise-induced changes in reaction time were not significantly different between the two exercise modalities.
With regard to P300 measures, no interaction of time, condition and group was found for latency and amplitude. As the interaction approached significance for P300 amplitude, F(1,31) = 3.8, p = 0.06, η2 = 0.11, the time by condition interaction was further examined within groups for exploratory purposes. In children with ADHD, a greater increase of P300 amplitude following aerobic exercise compared to coordinative exercise (Table 4) was supported by a time by condition interaction, F(1,31) = 10.3, p = 0.006, η2 = 0.41. In contrast, the pre- to post-test change of P300 amplitude was not significantly different between the aerobic and coordinative exercise condition in healthy comparisons (see supplement for further details).
4. Discussion
The findings of the present study revealed that in comparison to an inactive control condition, a single exercise session elicited benefits for behavioral performance and the allocation of attentional resources in children with ADHD and healthy comparisons. This improvement was indicated by increased P300 amplitude and decreased reaction time on congruent and incongruent trials of the Flanker task. Although there were some indications that the magnitude of the effects on behavioral outcomes is influenced by the ADHD status, the findings provide no clear evidence that individuals with deficits in executive function can expect greater exercise-induced improvements in this cognitive domain (Drollette et al., 2014, Sibley and Beilock, 2007). Thus, the first hypothesis was only partly supported, because exercise improved the allocation of attentional resources to a similar extent in both children with ADHD and healthy comparisons. When the influence of exercise modality was assessed, children with ADHD showed greater improvements of task performance and a tendency towards a higher increase of the P300 amplitude following aerobic exercise compared to coordinative exercise. In contrast, there was no evidence that modality influences the exercise-induced benefits for inhibitory control in healthy children. Consequently, the second hypothesis assuming that coordinative exercise would be more beneficial than aerobic exercise had to be rejected.
The present findings are in line with previous experimental studies showing improved reaction time on inhibition tasks in children with ADHD following a single exercise session (Chang et al., 2012b, Piepmeier et al., 2015). In contrast to time-dependent measures, accuracy on the Flanker task was not influenced by the exercise bout. This argues against a speed-accuracy tradeoff, so that cognitive enhancements seem to be due to different physiological mechanisms rather than a change from prevention to promotion focus or vice versa (Förster et al., 2003). As response time involves speed of stimulus classification, stimulus evaluation, response selection, and motor preparation (Doucet and Stelmack, 1999, Polich, 2007), a single exercise session seems to benefit one or more of these processes.
Previous studies provide evidence for a decreased latency and increased amplitude of the P300 component of ERPs following exercise in both children with ADHD (Pontifex et al., 2013) and healthy comparisons (Hillman et al., 2009). The P300 latency is sensitive to task processing demands and proportional to stimulus evaluation timing (Polich, 2007), so that an exercise-induced decrease indicates faster cognitive processing. Similar to previous studies, ADHD-related deficits in classification speed were evidenced by a longer P300 latency (Fallgatter et al., 2004, Monastra, 2008), but exercise did not reduce the time required to detect and evaluate a target stimulus in the present study. However, a single exercise session increased P300 amplitude in children with ADHD and healthy comparisons. These specific changes mirror the exercise-induced speeding of response times in both groups, so that higher performance on the Flanker task is suggested to be partly due to improved resource allocation. This is further supported by Ramchurn et al. (2014), who have found that amplitude measures of the P300 component rather than latencies are associated with slower or faster responding. The present results are consistent with previous studies, which have linked increased P300 amplitude with beneficial effects of aerobic exercise on inhibitory control in children with and without ADHD (Hillman et al., 2009, Hillman et al., 2011, Pontifex et al., 2013). However, there is a possibility that changes in other components of ERPs, which have not been assessed, have also contributed to an increased inhibitory control.
Different mechanisms have been considered to account for cognitive enhancements following moderate aerobic exercise. In this respect, favorable changes in cerebral metabolism, blood flow and release of catecholamines in particular have been suggested to create a nutritive environment within the brain (Kashihara et al., 2009, Secher et al., 2008). Furthermore, aerobic exercise has been found to elicit transient changes in cortical activation (Ludyga et al., 2015), whereby higher activity of the prefrontal cortex is related to increased inhibitory control (Yanagisawa et al., 2010). Therefore, it seems likely that aerobic exercise compared to coordinative exercise led to greater improvements of reaction time in children with ADHD by reducing the task-related hypo-arousal associated with ADHD (Cortese et al., 2012). In contrast, both exercise modalities had similar impacts on inhibitory control in healthy comparisons. Although previous studies have also found beneficial effects of exercise with higher cognitive demands on this executive function component (Jäger et al., 2014, Schmidt et al., 2015), the underlying mechanisms for different behavioral responses between children with ADHD and healthy comparisons remain unclear. However, the reticular-activating hypofrontality model of acute exercise might offer a possible explanation (Dietrich and Audiffren, 2011). Based on this model, exercise triggers an activation of different neurotransmitter systems that enhance cognitive control, but disengages executive functions when motor execution demands the allocation of additional resources. As the activity of the motor cortex increases as a function of complexity of the movement (Leff et al., 2011), coordinative exercise might prevent enhancements of inhibitory control in children with ADHD due to a limited availability of resources that are necessary for performing an executive function task. This is supported by Rubia et al. (1999), who have found that ADHD is associated with hypofrontality during higher-order motor control.
For the interpretation of the present findings, some limitations have to be taken into account. From a methodological perspective, it is likely that findings of the present study were influenced by carry-over effects due to the cross-over design. To reduce these effects, participants were familiarized with the assessments 7–14 days prior to the experiment and sessions were scheduled with at least fivedays between conditions to ensure a sufficient wash-out period. With regard to the EEG assessments, it is possible that sweating artifacts have affected data quality as the EEG remained on the participant’s head during the exercise sessions. However, it should be noted that the exercise sessions were moderately-intense and lasted 20 min only. As the environmental temperature was held constant at 20°, participants were not expected to sweat a lot. Furthermore, it cannot be ruled out that the choice of the control condition has influenced the present findings. However, comparing exercise-induced improvements with possible changes after watching a video seem to be more ecologically valid than comparisons with seated rest. This is due to the fact that children and adolescents rarely have the chance to rest in settings, in which such improvements would be highly advantageous (e.g. educational settings). Another limitation related to the control condition was that children with ADHD tended to have higher P300 amplitudes before watching the video compared to pre-exercise assessments. Therefore, it might be assumed that there was a greater reserve for improvements in inhibitory control and associated cognitive processes prior to the exercise sessions. Knowing that ADHD is associated with a high intra-individual variability of reaction time (Tamm et al., 2012), this pattern is considered to be due to random day-to-day variability. As effects of exercise were assessed in children with ADHD-combined type undergoing treatment with methylphenidate, it remains unclear whether or not a single aerobic session elicits similar benefits for inhibitory control in other types of ADHD or children undergoing other forms of therapy (e.g. neurofeedback, behavioral therapy). Additionally, it cannot be ruled out that greater benefits of aerobic exercise for inhibitory control in ADHD are confounded by methylphenidate treatment. However, a previous study has shown that moderate aerobic exercise elicits similar improvements of behavioral symptoms and attention in users and non-users of methylphenidate (Medina et al., 2010). Moreover, the present results do not allow conclusions on whether or not gender and/or intelligence moderate the beneficial effects of exercise for behavioral performance and the allocation of attentional resources. A preliminary examination of the interaction of gender, time and condition yielded no significant results for the dependent variables. With regard to intelligence, it is less likely that this variable has influenced the present results, because the number of participants with a higher educational level was equal between groups. Furthermore, the present findings do not permit any conclusions about the durability of the observed effects. Due to the high practical relevance, future studies should therefore examine the time course of the effects of aerobic and coordinative exercise on inhibitory control in children with and without ADHD.
In conclusion, acute exercise leads to transient improvements of inhibitory control and the allocation of attentional resources in both children with ADHD and healthy peers. In comparison to coordinative exercise, an aerobic exercise session seems to be more efficient to temporarily reduce the inhibitory control deficit that persists in children with ADHD. Therefore, children should be encouraged to strategically use a single exercise session to prepare for situations demanding high inhibitory control, such as examinations and learning phases.
Conflict of Interest
None.
Footnotes
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.dcn.2017.10.007.
Appendix A. Supplementary data
The following are Supplementary data to this article:
References
- American Psychiatric Association . 5th ed. American Psychiatric Association; Washington, D.C: 2013. Diagnostic and Statistical Manual of Mental Disorders: DSM-5. [Google Scholar]
- Berwid O.G., Halperin J.M. Emerging support for a role of exercise in attention-deficit/hyperactivity disorder intervention planning. Curr. Psychiatry Rep. 2012;14(5):543–551. doi: 10.1007/s11920-012-0297-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Best J.R. Effects of physical activity on children’s executive function: contributions of experimental research on aerobic exercise. Dev. Rev. 2010;30(4):331–351. doi: 10.1016/j.dr.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bland J., Pfeiffer K., Eisenmann J.C. The PWC170: comparison of different stage lengths in 11–16 year olds. Eur. J. Appl. Physiol. 2012;112(5):1955–1961. doi: 10.1007/s00421-011-2157-z. [DOI] [PubMed] [Google Scholar]
- Chang Y.K., Labban J.D., Gapin J.I., Etnier J.L. The effects of acute exercise on cognitive performance: a meta-analysis. Brain Res. 2012;1453:87–101. doi: 10.1016/j.brainres.2012.02.068. [DOI] [PubMed] [Google Scholar]
- Chang Y.-K., Liu S., Yu H.-H., Lee Y.-H. Effect of acute exercise on executive function in children with attention deficit hyperactivity disorder. Arch. Clin. Neuropsychol. 2012;27(2):225–237. doi: 10.1093/arclin/acr094. [DOI] [PubMed] [Google Scholar]
- Clayson P.E., Baldwin S.A., Larson M.J. How does noise affect amplitude and latency measurement of event-related potentials (ERPs)? A methodological critique and simulation study. Psychophysiology. 2013;50(2):174–186. doi: 10.1111/psyp.12001. [DOI] [PubMed] [Google Scholar]
- Cortese S., Kelly C., Chabernaud C., Proal E., Di Martino A., Milham M.P., Castellanos F.X. Toward systems neuroscience of ADHD: a meta-analysis of 55 fMRI studies. Am. J. Psychiatry. 2012;169(10):1038–1055. doi: 10.1176/appi.ajp.2012.11101521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich A., Audiffren M. The reticular-activating hypofrontality (RAH) model of acute exercise. Neurosci. Biobehav. Rev. 2011;35(6):1305–1325. doi: 10.1016/j.neubiorev.2011.02.001. [DOI] [PubMed] [Google Scholar]
- Doehnert M., Brandeis D., Imhof K., Drechsler R., Steinhausen H.-C. Mapping attention-deficit/hyperactivity disorder from childhood to adolescence–no neurophysiologic evidence for a developmental lag of attention but some for inhibition. Biol. Psychiatry. 2010;67(7):608–616. doi: 10.1016/j.biopsych.2009.07.038. [DOI] [PubMed] [Google Scholar]
- Doucet C., Stelmack R.M. The effect of response execution on P3 latency, reaction time, and movement time. Psychophysiology. 1999;36(3):351–363. doi: 10.1017/s0048577299980563. [DOI] [PubMed] [Google Scholar]
- Drollette E.S., Scudder M.R., Raine L.B., Moore R.D., Saliba B.J., Pontifex M.B., Hillman C.H. Acute exercise facilitates brain function and cognition in children who need it most: an ERP study of individual differences in inhibitory control capacity. Dev. Cognit. Neurosci. 2014;7:53–64. doi: 10.1016/j.dcn.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo K., Matsukawa K., Liang N., Nakatsuka C., Tsuchimochi H., Okamura H., Hamaoka T. Dynamic exercise improves cognitive function in association with increased prefrontal oxygenation. J. Physiol. Sci. 2013;63(4):287–298. doi: 10.1007/s12576-013-0267-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksen B.A., Eriksen C.W. Effects of noise letters upon the identification of a target letter in a nonsearch task. Percept. Psychophys. 1974;16(1):143–149. [Google Scholar]
- Förster J., Higgins E.T., Bianco A.T. Speed/accuracy decisions in task performance: built-in trade-off or separate strategic concerns? Organ. Behav. Hum. Decis. Process. 2003;90(1):148–164. [Google Scholar]
- Fallgatter A.J., Ehlis A.-C., Seifert J., Strik W.K., Scheuerpflug P., Zillessen K.E., Warnke A. Altered response control and anterior cingulate function in attention-deficit/hyperactivity disorder boys. Clin. Neurophysiol. 2004;115(4):973–981. doi: 10.1016/j.clinph.2003.11.036. [DOI] [PubMed] [Google Scholar]
- Faul F., Erdfelder E., Lang A.-G., Buchner A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods. 2007;39(2):175–191. doi: 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- Ferree T.C., Luu P., Russell G.S., Tucker D.M. Scalp electrode impedance, infection risk, and EEG data quality. Clin. Neurophysiol. 2001;112(3):536–544. doi: 10.1016/s1388-2457(00)00533-2. [DOI] [PubMed] [Google Scholar]
- Hillman C.H., Snook E.M., Jerome G.J. Acute cardiovascular exercise and executive control function. Int. J. Psychophysiol. 2003;48(3):307–314. doi: 10.1016/s0167-8760(03)00080-1. [DOI] [PubMed] [Google Scholar]
- Hillman C.H., Pontifex M.B., Raine L.B., Castelli D.M., Hall E.E., Kramer A.F. The effect of acute treadmill walking on cognitive control and academic achievement in preadolescent children. Neuroscience. 2009;159(3):1044–1054. doi: 10.1016/j.neuroscience.2009.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillman C.H., Kamijo K., Scudder M. A review of chronic and acute physical activity participation on neuroelectric measures of brain health and cognition during childhood. Prev. Med. 2011:S21–S28. doi: 10.1016/j.ypmed.2011.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M. Control of mental activities by internal models in the cerebellum. Nat. Rev. Neurosci. 2008;9(4):304–313. doi: 10.1038/nrn2332. [DOI] [PubMed] [Google Scholar]
- Jäger K., Schmidt M., Conzelmann A., Roebers C.M. Cognitive and physiological effects of an acute physical activity intervention in elementary school children. Front. Psychol. 2014;5:1473. doi: 10.3389/fpsyg.2014.01473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamijo K., Nishihira Y., Hatta A., Kaneda T., Kida T., Higashiura T., Kuroiwa K. Changes in arousal level by differential exercise intensity. Clin. Neurophysiol. 2004;115(12):2693–2698. doi: 10.1016/j.clinph.2004.06.016. [DOI] [PubMed] [Google Scholar]
- Kamijo K., Nishihira Y., Higashiura T., Kuroiwa K. The interactive effect of exercise intensity and task difficulty on human cognitive processing. Int. J. Psychophysiol. 2007;65(2):114–121. doi: 10.1016/j.ijpsycho.2007.04.001. [DOI] [PubMed] [Google Scholar]
- Kashihara K., Maruyama T., Murota M., Nakahara Y. Positive effects of acute and moderate physical exercise on cognitive function. J. Physiol. Anthropol. 2009;28(4):155–164. doi: 10.2114/jpa2.28.155. [DOI] [PubMed] [Google Scholar]
- Kiesel A., Miller J., Jolicoeur P., Brisson B. Measurement of ERP latency differences: a comparison of single-participant and jackknife-based scoring methods. Psychophysiology. 2008;45(2):250–274. doi: 10.1111/j.1469-8986.2007.00618.x. [DOI] [PubMed] [Google Scholar]
- Kratz O., Studer P., Malcherek S., Erbe K., Moll G.H., Heinrich H. Attentional processes in children with ADHD: An event-related potential study using the attention network test. Int. J. Psychophysiol. 2011;81(2):82–90. doi: 10.1016/j.ijpsycho.2011.05.008. [DOI] [PubMed] [Google Scholar]
- Leff D.R., Orihuela-Espina F., Elwell C.E., Athanasiou T., Delpy D.T., Darzi A.W., Yang G.-Z. Assessment of the cerebral cortex during motor task behaviours in adults: a systematic review of functional near infrared spectroscopy (fNIRS) studies. Neuroimage. 2011;54(4):2922–2936. doi: 10.1016/j.neuroimage.2010.10.058. [DOI] [PubMed] [Google Scholar]
- Lidzba K., Chritiansen H., Drechsler R., von Keith Conners. 3rd edition. Bern: Huber; 2013. Conners Skalen zu Aufmerksamkeit und Verhalten-3. Deutschsprachige Adaptation der Conners. [Google Scholar]
- Liotti M., Pliszka S.R., Higgins K., Perez R., Semrud-Clikeman M. Evidence for specificity of ERP abnormalities during response inhibition in ADHD children: a comparison with reading disorder children without ADHD. Brain Cogn. 2010;72(2):228–237. doi: 10.1016/j.bandc.2009.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipszyc J., Schachar R. Inhibitory control and psychopathology: a meta-analysis of studies using the stop signal task. J. Int. Neuropsychol. Soc. 2010;16(6):1064–1076. doi: 10.1017/S1355617710000895. [DOI] [PubMed] [Google Scholar]
- Ludyga S., Hottenrott K., Gronwald T. Effects of different training loads and environmental conditions on EEG activity. Deutsche Zeitschrift für Sportmedizin. 2015;2015(05):113–120. [Google Scholar]
- Ludyga S., Gerber M., Brand S., Holsboer-Trachsler E., Puhse U. Acute effects of moderate aerobic exercise on specific aspects of executive function in different age and fitness groups: a meta-analysis. Psychophysiology. 2016;53(11):1611–1626. doi: 10.1111/psyp.12736. [DOI] [PubMed] [Google Scholar]
- Ludyga S., Brand S., Gerber M., Pühse U. Exercise as neuro-enhancer in children with ADHD − cognitive and behavioral effects. In: Meeusen R., Schaefer S., Tomporowski P.D., Bailey R., editors. Physical Activity and Educational Achievement: Insights from Exercise Neuroscience. Routlegde; 2017. [Google Scholar]
- Mahon A.D., Marjerrison A.D., Lee J.D., Woodruff M.E., Hanna L.e. Evaluating the prediction of maximal heart rate in children and adolescents. Res. Q. Exerc. Sport. 2010;81(4):466–471. doi: 10.1080/02701367.2010.10599707. [DOI] [PubMed] [Google Scholar]
- Medina J.A., Netto T.L.B., Muszkat M., Medina A.C., Botter D., Orbetelli R., Miranda M.C. Exercise impact on sustained attention of ADHD children, methylphenidate effects. Attention Deficit Hyperactivity Disord. 2010;2(1):49–58. doi: 10.1007/s12402-009-0018-y. [DOI] [PubMed] [Google Scholar]
- Monastra V.J. Quantitative electroencephalography and attention-deficit/hyperactivity disorder: implications for clinical practice. Curr. Psychiatry Rep. 2008;10(5):432–438. doi: 10.1007/s11920-008-0069-3. [DOI] [PubMed] [Google Scholar]
- Newell K.M. vol. 27. Elsevier; 1985. pp. 295–317. (Coordination, Control and Skill. In Advances in Psychology. Differing Perspectives in Motor Learning, Memory, and Control). [Google Scholar]
- Norton K., Norton L., Sadgrove D. Position statement on physical activity and exercise intensity terminology. J. Sci. Med. Sport. 2010;13(5):496–502. doi: 10.1016/j.jsams.2009.09.008. [DOI] [PubMed] [Google Scholar]
- Oldfield R.C. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9(1):97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Pesce C. Shifting the focus from quantitative to qualitative exercise characteristics in exercise and cognition research. J. Sport Exerc. Psychol. 2012;34(6):766–786. doi: 10.1123/jsep.34.6.766. [DOI] [PubMed] [Google Scholar]
- Piepmeier A.T., Shih C.-H., Whedon M., Williams L.M., Davis M.E., Henning D.A., Etnier J.L. The effect of acute exercise on cognitive performance in children with and without ADHD. J. Sport Health Sci. 2015;4(1):97–104. [Google Scholar]
- Polanczyk G.V., Willcutt E.G., Salum G.A., Kieling C., Rohde L.A. ADHD prevalence estimates across three decades: an updated systematic review and meta-regression analysis. Int. J. Epidemiol. 2014;43(2):434–442. doi: 10.1093/ije/dyt261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polich J. Updating P300: an integrative theory of P3a and P3b. Clin. Neurophysiol. 2007;118(10):2128–2148. doi: 10.1016/j.clinph.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontifex M.B., Saliba B.J., Raine L.B., Picchietti D.L., Hillman C.H. Exercise improves behavioral, neurocognitive, and scholastic performance in children with ADHD. J. Pediatr. 2013;162(3):543–551. doi: 10.1016/j.jpeds.2012.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramchurn A., Fockert J.W., de Mason L., Darling S., Bunce D. Intraindividual reaction time variability affects P300 amplitude rather than latency. Front. Hum. Neurosci. 2014;8 doi: 10.3389/fnhum.2014.00557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubia K., Overmeyer S., Taylor E., Brammer M., Williams S.C., Simmons A., Bullmore E.T. Hypofrontality in attention deficit hyperactivity disorder during higher-order motor control: a study with functional MRI. Am. J. Psychiatry. 1999;156(6):891–896. doi: 10.1176/ajp.156.6.891. [DOI] [PubMed] [Google Scholar]
- Rubia K., Alegria A., Brinson H. Imaging the ADHD brain: disorder-specificity, medication effects and clinical translation. Expert Rev. Neurother. 2014;14(5):519–538. doi: 10.1586/14737175.2014.907526. [DOI] [PubMed] [Google Scholar]
- Schmidt M., Egger F., Conzelmann A. Delayed positive effects of acute coordinative exercise on children’s attention. Percept. Mot. Skills. 2015;121(2):431–446. doi: 10.2466/22.06.PMS.121c22x1. [DOI] [PubMed] [Google Scholar]
- Secher N.H., Seifert T., van Lieshout J.J. Cerebral blood flow and metabolism during exercise: implications for fatigue. J. Appl. Physiol. 2008;104(1):306–314. doi: 10.1152/japplphysiol.00853.2007. [DOI] [PubMed] [Google Scholar]
- Sibley B.A., Beilock S.L. Exercise and working memory: an individual differences investigation. J. Sport Exercise Psychol. 2007;29(6):783–791. doi: 10.1123/jsep.29.6.783. [DOI] [PubMed] [Google Scholar]
- Sibley M.H., Swanson J.M., Le Arnold Hechtman L.T., Owens E.B., Stehli A., Pelham W.E. Defining ADHD symptom persistence in adulthood: optimizing sensitivity and specificity. J. Child Psychol. Psychiatry Allied Disciplines [Epub ahead of print] 2016 doi: 10.1111/jcpp.12620. (Advance online publication) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroth S., Kubesch S., Dieterle K., Ruchsow M., Heim R., Kiefer M. Physical fitness, but not acute exercise modulates event-related potential indices for executive control in healthy adolescents. Brain Res. 2009;1269:114–124. doi: 10.1016/j.brainres.2009.02.073. [DOI] [PubMed] [Google Scholar]
- Tamm L., Narad M.E., Antonini T.N., O'Brien K.M., Hawk L.W., Epstein J.N. Reaction time variability in ADHD: a review. Neurotherapeutics. 2012;9(3):500–508. doi: 10.1007/s13311-012-0138-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamminga H.G., Reneman L., Huizenga H.M., Geurts H.M. Effects of methylphenidate on executive functioning in attention-deficit/hyperactivity disorder across the lifespan: a meta-regression analysis. Psychol. Med. 2016;46(9):1791–1807. doi: 10.1017/S0033291716000350. [DOI] [PubMed] [Google Scholar]
- Tomporowski P.D., McCullick B., Pendleton D.M., Pesce C. Exercise and children's cognition: the role of exercise characteristics and a place for metacognition. J. Sport Health Sci. 2015;4(1):47–55. [Google Scholar]
- Willcutt E.G., Doyle A.E., Nigg J.T., Faraone S.V., Pennington B.F. Validity of the executive function theory of attention-deficit/hyperactivity disorder: a meta-Analytic review. Biol. Psychiatry. 2005;57(11):1336–1346. doi: 10.1016/j.biopsych.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Yanagisawa H., Dan I., Tsuzuki D., Kato M., Okamoto M., Kyutoku Y., Soya H. Acute moderate exercise elicits increased dorsolateral prefrontal activation and improves cognitive performance with Stroop test. Neuroimage. 2010;50(4):1702–1710. doi: 10.1016/j.neuroimage.2009.12.023. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.