Highlights
-
•
Previous work shows cerebellar Crus I supports imitation via interaction with pSTS.
-
•
Teens with (ASD) and without (TD) autism completed an imitation task in the scanner.
-
•
Teens with ASD recruited Crus I less strongly during imitation than TD teens.
-
•
PPI strength between pSTS and Crus I predicted mentalizing in teens with ASD.
Keywords: Autism spectrum disorders, Cerebellum, Imitation, Effective connectivity, Mentalizing, Superior temporal sulcus
Abstract
Posterior superior temporal sulcus (pSTS) is specialized for interpreting perceived human actions, and disruptions to its function occur in autism spectrum disorder (ASD). Here we consider the role of Crus I of neocerebellum in supporting pSTS function. Research has associated Crus I activity with imitation and biological motion perception, and neocerebellum is theorized to coordinate activity among cerebral sites more generally. Moreover, cerebellar abnormalities have been associated with ASD. We hypothesized that disordered Crus I–pSTS interactions could predict social deficits in ASD. 15 high functioning adolescents with ASD and 15 same-age comparison youth participated in an fMRI imitation paradigm; ratings of mentalizing ability were collected via parent report. We predicted that stronger Crus I–pSTS interactions would be associated with better mentalizing ability. Consistent with these hypotheses, stronger psychophysiological interactions between Crus I and right pSTS were associated with greater mentalizing ability among adolescents with ASD. Whole-brain analyses also indicated that typically developing youth recruited right inferior frontal gyrus, left pSTS, medial occipital regions, and precuneus more strongly during imitation than did youth with ASD. Overall, these results indicate that variability in neocerebellar interactions with key cortical social brain sites may help explain individual differences in social perceptual outcomes in ASD.
1. Introduction
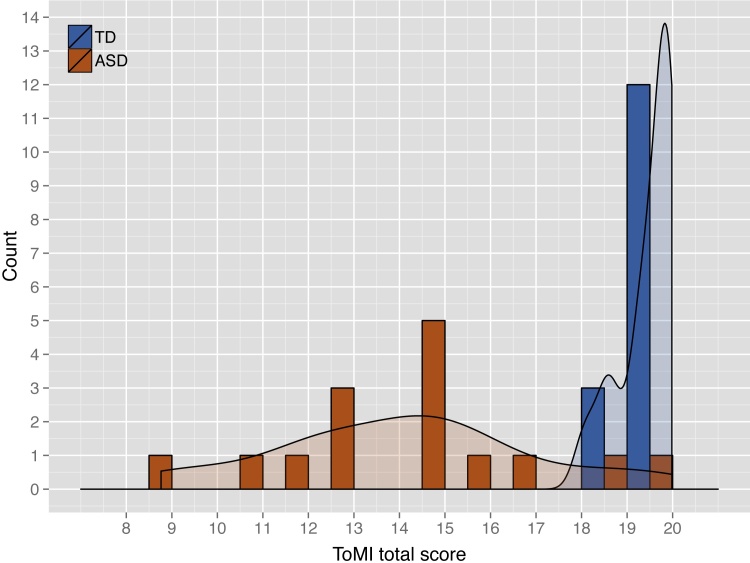
Autism spectrum disorders (ASD) are characterized by pervasive impairments in the domains of social-communicative skills and restricted/repetitive behavior (APA, 2000). Heterogeneity in ASD constitutes a challenge to understanding the etiology of the disorder (Jones and Klin, 2009, Betancur, 2011, Pelphrey et al., 2011). For example, deficits in theory of mind, or mentalizing – making inferences about others’ minds – are widespread in ASD (Baron-Cohen, 2001). However, performance on mentalizing assessments is not universally poor in this population (see, e.g., the wide distributions of theory of mind scores among participants with ASD in Dissanayake and Macintosh (2003), or Fig. 1 of this report). Linking such mentalizing variability to differences in neural function may help explain some of the heterogeneity in ASDs. We propose that a region of posterior–lateral cerebellum, Crus I, may contribute to social perception via interaction with posterior superior temporal sulcus (pSTS), an interaction which holds the potential to predict individual variability in social outcomes in ASD.
Fig. 1.
Frequency histogram depicting distribution of ToMI-T total scores across sample of adolescents with ASD versus TD adolescents. For the purposes of visualization, scores are rounded to the nearest whole number, and density curves are overlaid.
A likely target of Crus I influence on cortex-mediated social function is the pSTS. The pSTS is a key brain site for the perception and interpretation of human actions (Pelphrey and Carter, 2008a, Pelphrey and Morris, 2006). Its relative specialization for this particular lower-level function leads to its flexible recruitment in a variety of social engagement processes that require meaning to be extracted from motion cues, including imitation (Decety et al., 2002, Iacoboni et al., 2001, Williams et al., 2007) and mentalizing (Brunet et al., 2000, Frith and Frith, 2003, Gallagher and Frith, 2003). Posterior STS is by no means the sole neural substrate of these abilities, but rather is thought to interact with other constituent processes as an interpreter of human action, to give rise to these complex social skills. In the case of imitation, pSTS interacts with regions supporting motor, perceptive (occipital sites), attentive (superior parietal lobule), and simulative skills (inferior parietal lobule, inferior frontal gyrus) (Caspers et al., 2010, Molenberghs et al., 2009). In the case of mentalizing, pSTS interacts with brain sites associated with generating and maintaining social scripts (temporal pole), representing thoughts (temporo-parietal junction), understanding emotions (anterior cingulate cortex), and “decoupling” the contents of others’ minds from reality (medial prefrontal cortex) (Frith and Frith, 2003, Gallagher and Frith, 2003, Saxe, 2010). The complexity of the networks required to support these social skills suggests that, in addition to the strength of local engagement of pSTS, connectivity between pSTS and other key brain sites may also be crucial to social function. While individual differences in structure (Hadjikhani et al., 2006) and function (Gendry Meresse et al., 2005) along pSTS correlate with severity of social deficits in ASD, atypical functional connectivity between pSTS and other cortical sites also predicts social impairment (Shih et al., 2011).
While atypical pSTS connectivity within the cortex has been documented in ASD, a potentially important relationship between pSTS and the posterior–lateral hemispheres of the cerebellum – the neocerebellum – has not yet been explored. Conventional notions of cerebellar function focused on its role in motor and vestibular processes. However, more recent theories of cerebellar function have attempted to account for this structure's involvement in a range of cognitive and affective functions (Stoodley and Schmahmann, 2009, Stoodley and Schmahmann, 2010), as well as its widespread connectivity with cerebral regions outside of motor cortex, including linkages to frontal, parietal, limbic/paralimbic, and temporal regions (Sasaki et al., 1975, Schmahmann and Pandya, 1991, Kelly and Strick, 2003, Salmi et al., 2010). Given the relative homogeneity of the cellular structure of the cerebellum (Eccles et al., 1967, Bloedel, 1992), paired with the highly specific, varying patterns of connectivity across its multiple lobules (Buckner et al., 2011), a number of theorists have suggested that the cerebellum performs a uniform operation across its extent, whose functional effects differ depending on the patterns of connectivity at a particular cerebellar locus (Bloedel, 1992, Ito, 1993, Ramnani, 2006). This operation may involve generating predictive inputs, monitoring responses, and providing adaptive feedback regarding the cerebral process subserved, leading to greater efficiency and routinization of the function, and freeing up resources in the cerebral region served to address events requiring a more flexible response (Ito, 1993, Ramnani, 2006, Schmahmann, 1991).
Recent meta-analysis indicates that neocerebellum is recruited during a variety of social cognitive tasks (Van Overwalle et al., 2014). In particular, Crus I was reliably activated during observation of body part movement as well as across a range of tasks involving mentalizing functions. This Crus I social cognitive involvement may be related to interactions with pSTS. Probabilistic tractography in humans suggests an anatomical loop between left Crus I and right pSTS (Sokolov et al., 2014). Functionally, these sites appear to interact during a number of social perceptual processes. Psychophysiological interaction analysis (PPI) of imitation-related brain activity has identified connections between bilateral Crus I and right pSTS that are specifically recruited during imitation (Jack et al., 2011), and dynamic causal modeling of brain response to human locomotion also indicates interactions between left Crus I and right pSTS (Sokolov et al., 2012). Further, interactions between left Crus I–II and right pSTS are apparent in healthy adults viewing Heider and Simmel (1944) stimuli, that is, geometric shapes whose movements invite inferences of animacy and social–emotional content (Jack and Pelphrey, 2014). Collectively, these findings suggest that Crus I may facilitate a range of pSTS functions relevant to social perception. Given evidence of cellular (Bailey et al., 1998, Kemper and Bauman, 2002, Ritvo et al., 1986, Vargas et al., 2005, Whitney et al., 2009), molecular (Fatemi et al., 2001, Lee et al., 2002, Purcell et al., 2001, Sajdel-Sulkowska et al., 2009, Yip et al., 2009), and functional (Allen et al., 2004, Allen and Courchesne, 2003, Mostofsky et al., 2009, Haist et al., 2005) cerebellar abnormalities in ASD, coupled with evidence of disruptions to pSTS, we hypothesized that atypical connectivity between Crus I and right pSTS would predict social perceptual deficits in ASD.
In the current project, we assessed these hypotheses in the context of an imitation task previously demonstrated to index Crus I–RpSTS interactions among neurotypical individuals (Jack et al., 2011). We reasoned that if Crus I facilitates pSTS activity across a variety of social perception tasks, then assessing the interaction between these two sites in one social context might allow us to make inferences about the robustness of this interaction in other contexts as well. Specifically, we predicted that stronger Crus I–RpSTS interactions during an imitation paradigm would predict better mentalizing skill. While imitation and mentalizing differ with regards to a number of their underlying constituent processes, they overlap in terms of recruitment of pSTS, and in requiring not just the perception of human action, but also the coordination of a response guided by information about those actions. Furthermore, biological motion processing (instantiated by pSTS function) has been theorized to be a foundational ability for the development of a fully fledged theory of mind, both generally (Frith and Frith, 2003), and in the specific case of deficits associated with autism (Pelphrey and Carter, 2008a). We anticipated that a significant portion of the individual variability in higher-level social outcomes among youth with ASD, including in mentalizing abilities, could be explained as a function of temporocerebellar connectivity evident in lower-level social perception processes.
A secondary aim of this project was to describe the patterns of brain activity that characterize imitation in both typically developing adolescents and those with ASD at a more general group level. We focus on the non-motor aspects of imitation (e.g., biological motion perception, attentional control, simulation) by contrasting brain activity evoked during imitation of simple manual sequences based on a human model versus execution of the same sequences based on visuospatial cues.
2. Materials and methods
2.1. Participants
Participants were 15 youth with ASD (13 males; aged 12–17) and 15 typically developing (TD) youth (11 males; aged 12–17). See Table 1 for demographic information. The local Institutional Review Board approved this project. Parents provided written informed consent and youth provided assent. Families received a small amount of money for participating.
Table 1.
ASD and comparison group differences in demographic variables.
| ASD (n = 15) | Comparison (n = 15) | Difference (p) | |
|---|---|---|---|
| Age (years) | 14.20 (1.61) | 13.80 (1.70) | .514 |
| Male (n) | 13 | 11 | .361 |
| White (n) | 13 | 15 | .343 |
| Medicated (n) | 12 | 1 | <.001 |
| Full Scale IQ | 110.53 (14.98) | 112.27 (7.99) | .696 |
| Verbal IQ | 109.33 (17.33) | 114.33 (10.17) | .343 |
| Performance IQ | 110.33 (14.90) | 107.60 (10.49) | .566 |
| Laterality quotient | 57.08 (62.39) | 50.23 (48.34) | .739 |
| Parent education level | 5.40 (.74) | 5.27 (.80) | .638 |
| Household income ($) | 95,071 (52,580) | 121,846 (47,586) | .179 |
Note: Except where otherwise indicated, numbers in table are raw group means with standard deviations in parentheses. T-tests were used to calculate group differences for continuous variables, and chi-square for discrete variables. Parental education was reported on the following scale: 1: eighth grade or less; 2: some high school; 3: high school graduate; 4: some college; 5: college graduate; 6: graduate degree.
Families of youth with ASD were recruited via newsletter and broadcast email through regional groups offering services and support to the autism community. Parents reported that their children had received diagnoses on the autism spectrum (autism, Asperger's disorder, or pervasive developmental disorder not otherwise specified) from a specialist, generally a clinical psychologist (n = 8), developmental pediatrician (n = 3) or other M.D. (n = 2).1 The Social Communication Questionnaire (SCQ) was then used as an initial screener for the presence of ASD (Rutter et al., 2003) with a cut-point of 12 to improve sensitivity (Corsello et al., 2007). The Autism Diagnostic Observation Schedule (ADOS) (Lord et al., 2000) was then administered by examiners certified to ADOS research reliability standards. All participants in the ASD group met criteria for either an “autism” or “autism spectrum” classification according to the ADOS algorithm (Table 2). Parents also completed the Social Responsiveness Scale (SRS) (Constantino et al., 2003). Most youth in the ASD group had SRS total scores in the “severe” range of social impairment (n = 12), with fewer in the “mild to moderate” range (n = 2). One individual received a total SRS t-score of 57, which falls below the “mild to moderate” classification, but within the range of scores which “mildly affected” individuals with ASD may occasionally obtain (Constantino et al., 2003). No participants in the comparison group surpassed cutoffs on the SCQ or in their SRS total score. See Table 2 for details of ASD and comparison group differences on social–behavioral measures.
Table 2.
ASD and comparison group differences in social–behavioral variables.
| ASD (n = 15) | Comparison (n = 15) | Difference (p) | |
|---|---|---|---|
| SCQa | 23.67 (5.91) | 1.77 (1.96) | <.001 |
| SRS Totala | 83.47 (12.53) | 42.60 (5.90) | <.001 |
| Social awareness | 71.47 (11.38) | 40.93 (7.43) | <.001 |
| Social cognitiona | 77.87 (14.78) | 42.73 (6.56) | <.001 |
| Social communicationa | 79.87 (12.92) | 41.27 (5.75) | <.001 |
| Social motivation | 80.13 (13.41) | 49.20 (10.92) | <.001 |
| Autistic mannerisms | 85.47 (9.21) | 44.00 (7.46) | <.001 |
| SRS Social Impairment Classification | |||
| “Severe” range (n) | 12 | 0 | |
| “Mild to Moderate” range (n) | 2 | 0 | |
| “Normal” range (n) | 1b | 15 | |
| ToMI Totala | 14.06 (2.87) | 19.45 (0.59) | <.001 |
| Earlya | 14.52 (2.63) | 19.55 (0.48) | <.001 |
| Basica | 15.79 (2.34) | 19.75 (0.30) | <.001 |
| Advanceda | 11.58 (4.13) | 19.08 (1.14) | <.001 |
| ADOS Algorithm Total | 13.00 (3.19) | – | – |
| Communication | 3.73 (1.49) | – | – |
| Social interaction | 8.60 (1.77) | – | – |
| Stereotyped/restricted | 1.60 (1.12) | – | – |
| ADOS Algorithm Classification | |||
| Autism (n) | 13 | – | |
| Autism spectrum (n) | 2 | – | |
Note: Except where otherwise indicated, numbers in table are raw group means with standard deviations in parentheses.
Equal variances not assumed.
SRS total t score = 57, within the 55–59 range of scores which youth with “very mild, ‘high-functioning”’ ASD may occasionally obtain (56).
Exclusionary criteria for both groups were full scale IQ (FSIQ) below 70, history of head trauma, seizure, stroke, or neurosurgery, current supervision by a neurologist, or use of an antipsychotic or mood stabilizer. Use of a selective serotonin reuptake inhibitor, or a stimulant preparation typically used in the treatment of attention deficit/hyperactivity disorder, were not exclusionary criteria for either group, given the high rates of treatment for comorbid attentional and internalizing problems among individuals with ASD (Joshi et al., 2010), as well as our desire to have a representative but not “super-normal” comparison group (Hinshaw, 2002). Medication use was significantly more frequent among the group with ASD (Table 1).
IQ scores were obtained using Wechsler Abbreviated Scale of Intelligence (WASI) (Wechsler, 1999); there were no significant between-group differences in FSIQ or in performance or verbal IQ subscale scores. Hand preference was assessed using the Edinburgh Handedness Index, which yields a continuous measure of participants’ laterality, with positive scores indicating a more rightward preference (Oldfield, 1971) (Table 1).
2.2. Theory of Mind Inventory
Parents completed the Theory of Mind Inventory (ToMI), a questionnaire assessing parents’ perception of their children's mentalizing skills (Hutchins et al., 2008). Scores on this measure range from 0 to 20, with higher scores indicating greater mastery of a skill or set of skills. This measure yields a Total score (ToMI-T: 48 items; α = .99) as well as Early, Basic, and Advanced subscale scores. The Early subscale is comprised of items thought to assess mentalizing abilities that typically develop in infancy and toddlerhood, such as the ability to recognize basic emotions, engage in social referencing, and understand the difference between intended and accidental actions (ToMI-Early: 7 items; α = .90). The Basic subscale is made up of items thought to assess mentalizing abilities that develop in the preschool years, such as false belief understanding, understanding pretense, and understanding that others’ feelings, desires, and beliefs guide their behavior (ToMI-Basic: 19 items; α = .96). The Advanced subscale appears to tap into somewhat later-developing and more sophisticated mentalizing abilities such as understanding sarcasm, figures of speech, and false smiles (ToMI-Adv: 16 items; α = .98).
2.3. Experimental design
During three functional scans, participants engaged in the same imitation paradigm used in Jack et al. (2011). The paradigm consisted of an observation (OBS) condition in which participants passively viewed a human model executing a randomized sequence of four finger presses on a key pad (Supplementary Video 1); an imitation (IMI) condition in which participants imitated the model using an MR-compatible button box (Supplementary Video 1); and an execute (EXE) condition in which participants also executed finger presses but did so based on visuospatial cues (Supplementary Video 2). The primary contrast of interest, IMI > EXE, allowed us to isolate non-motor aspects of imitation that involved response to human action cues. Each trial lasted between 7 and 9 s with interstimulus intervals ranging from 8 to 12 s. This slow event-related design was intended to facilitate a more statistically powerful PPI analysis, based on the considerations in Gitelman et al. (2003). Trials were randomized within 7.46- to 7.75-min runs containing 18 trials (six per condition) for a total of 54 trials. Latency and accuracy of responses were recorded; only error-free trials were included in data analysis.
2.4. Imaging parameters
Scanning was performed on a Siemens 3 Tesla MAGNETOM Trio high speed imaging device equipped with a 12-channel head-coil. 176 T1 weighted images were acquired using Siemens’ MPRAGE (magnetization-prepared rapid-acquired gradient echoes) pulse sequence (TR = 1900 ms; TE = 2.53 ms; FOV = 250 mm; voxel size = 1 mm × 1 mm × 1 mm) and used for coregistration with the functional data. Whole brain functional images were acquired using a T2*-weighted Echo Planar (EPI) sequence sensitive to blood oxygenation level dependent (BOLD) contrast (TR, 2000 ms; TE, 40 ms; voxel size, 3.0 mm × 3.0 mm × 4.2 mm; flip angle = 90°). Twenty-eight transverse slices were acquired, and runs consisted of the acquisition of 237 successive brain volumes.
2.5. Data analysis
2.5.1. FMRI preprocessing
FMRI data processing was conducted using FEAT (FMRI Expert Analysis Tool) Version 6.00, part of FSL (FMRIB's Software Library, www.fmrib.ox.ac.uk/fsl). Head motion was detected by center of mass measurements implemented using automated scripts developed for quality assurance purposes. The criterion for exclusion from data analysis was a deviation from the center of mass in any dimension >3 mm; however, no participant met this criterion. After quality assurance procedures, the following pre-statistics processing was applied: motion correction using MCFLIRT (Jenkinson et al., 2002), non-brain removal using BET (Smith, 2002), spatial smoothing using a Gaussian kernel of full-width half-maximum 5 mm, grand-mean intensity normalization of the entire 4D dataset by a single multiplicative factor, and highpass temporal filtering (Gaussian-weighted least-squares straight line fitting, with sigma = 50.0 s). Linear registration to the high resolution structural and the Montreal Neurologic Institute (MNI) Template standard space images was carried out using FLIRT (Jenkinson et al., 2002, Jenkinson and Smith, 2001); registration from the structural to standard space was then further refined using FNIRT non-linear registration (Andersson et al., 2007).
For each individual dataset, independent component analysis-based data exploration was carried out using MELODIC (Multivariate Exploratory Linear Optimized Decomposition into Independent Components) (Beckmann and Smith, 2004) in order to investigate the possible presence of unexpected artifacts. After manual review to identify obvious scanner- or movement-related artifacts, chosen noise components were removed, producing a filtered and de-noised dataset for use in subsequent analyses.
2.5.2. Assessment of group differences in local brain activity
First-level analysis (i.e., within-subject analysis of individual experimental runs) of the functional data was conducted using FEAT, with time-series statistical analysis executed using FILM (FMRIB's Improved Linear Model) with local autocorrelation correction (Woolrich et al., 2001). FSL's fsl_motion_outliers tool was used on non-motion-corrected functional data to detect time-points corrupted by large motion using the DVARS metric described in Power et al. (2012). A confound matrix was generated identifying time-points for which the root mean squared intensity difference from volume N to volume N + 1 was greater than the 75th percentile plus 1.5 times the interquartile range. This matrix was used to regress out corrupt time-points at first level. Standard motion parameters (six regressors representing translations and rotations in the x, y, and z dimensions) were also included in the first level model. Second-level analysis (i.e., within-subject analysis across all runs) was carried out using a fixed effects model, by forcing the random effects variance to zero in FLAME (FMRIB's Local Analysis of Mixed Effects) (Beckmann et al., 2003, Woolrich et al., 2004, Woolrich, 2008). Third-level analysis (i.e., between-subjects analysis) was carried out using FLAME stage 1 and stage 2 with automatic outlier detection and de-weighting (Woolrich, 2008). Z (Gaussianized T/F) statistic images were thresholded using clusters determined by Z > 2.3 and a (corrected) cluster significance threshold of p = .05 (Worsley, 2001). De-meaned age, laterality quotient, and full-scale IQ were included at third level as nuisance regressors. Variances were calculated separately for the two groups. The IMI > EXE contrast was of primary theoretical interest, targeting non-motor aspects of imitation by presumably subtracting out motor-related activity; IMI > baseline between-group differences were also of interest. Other contrasts (IMI > OBS, OBS > IMI, OBS > EXE, and group differences in OBS > baseline, EXE > baseline) are reported in the Supplement. BOLD activity within these contrasts and in the main task conditions was also compared between groups.
2.5.3. PPI analysis
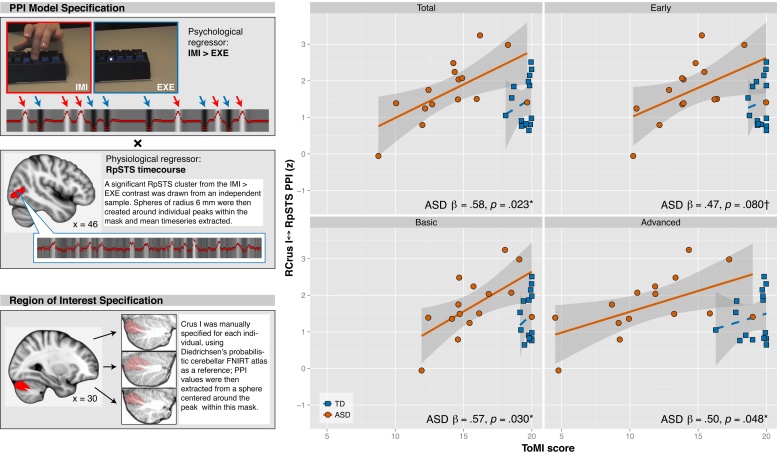
PPI analysis was carried out according to the guidelines in O’Reilly et al. (2012). First level analysis was carried out in FEAT (Woolrich et al., 2001), with time-series statistical analysis carried out using FILM with local autocorrelation correction and cluster thresholding with correction for multiple comparisons at Z > 2.3 and adjusted p = .05. Motion outliers and motion parameters were included as nuisance regressors in the first-level model. The psychological regressor of interest (IMI > EXE) was convolved with a double-gamma hemodynamic response function (HRF). The physiological regressor was created using an RpSTS seed region defined from an independent sample of neurotypical individuals who completed the same paradigm (Jack et al., 2011). Specifically, within this independent sample, the significant RpSTS cluster from the IMI > EXE contrast was thresholded at Z > 4 to create a mask. This mask was then applied to each individual in the present sample, and the peak voxel within the mask was identified. Finally, a sphere of radius 6 mm was created centered around this subject-specific peak, and the average timecourse was extracted from this spherical region of interest (ROI). We chose this approach as a compromise between the signal washout that can occur when averaging over a large brain region and the possibility that the analysis could be dominated by one outlier voxel when assessing the peak voxel alone. The process by which the psychological and physiological regressors were formed is illustrated in the left panel of Fig. 4. Temporal filtering was applied to the psychological regressor and a temporal derivative added. The PPI regressor was the interaction term between the psychological and physiological regressors, with the psychological regressor zero-centered about the minimum and maximum values and the physiological regressor de-meaned. Two regressors of no interest – (IMI + EXE) and (OBS) – were convolved with a double-gamma HRF with temporal filtering applied and a temporal derivative added. All convolutions were applied prior to forming the interaction term; thereafter no further convolution was applied.
Fig. 4.
Left panel: illustration of the process by which the PPI regressor was created. At top, example clips from the IMI (red) and EXE (blue) stimuli, with a representation of the IMI > EXE psychological regressor below. At middle, the physiological regressor is illustrated; a sagittal view of the MNI brain indicates the RpSTS mask (red) within which individual IMI > EXE peaks were identified, with an example sphere centered around a peak in blue. At bottom, a sagittal view of the right Crus I region of interest as specified in Diedrichsen's probabilistic cerebellar FNIRT atlas, and three illustrative subject-specific masks created from this reference image. Right panel: associations between ToMI scores and average PPI value between right pSTS seed region and right Crus I, with fit lines by group and 95% confidence interval around the estimate. Annotations are the standardized beta weights and p-values indicating estimates of the relationship between RCrus I–RpSTS and ToMI score for youth with ASD, derived from the full model (Table 6). Associations between ToMI scores and the left Crus I-right pSTS PPI value demonstrated a similar, but weaker pattern. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Second level analysis was carried out using a fixed-effects model within FLAME (Beckmann et al., 2003, Woolrich et al., 2004, Woolrich, 2008). At second level, the Z-statistic value of the PPI regressor for each participant was extracted from right and left Crus I (RCrus I–RpSTS, LCrus I–RpSTS). These cerebellar ROIs were manually created to match each individual's lobular structure, using Diedrichsen's probabilistic cerebellar FNIRT atlas (Diedrichsen et al., 2009) as a reference (see left panel of Fig. 4); thereafter, spheres of 4 mm were created around individuals’ PPI peaks within their subject-specific masks, and the average within these spheres was calculated. Linear regression models were created that predicted ToMI scores from PPI values, controlling for FSIQ, laterality, and age, with the predictor and all covariates de-meaned. To compare the predictive value of connectivity versus regional RpSTS activity alone, we reran these models substituting each subject's average Z-statistic value within the RpSTS seed during IMI > EXE (RpSTSIMI>EXE) for the PPI score.
Third-level PPI analysis was carried out using FLAME stage 1 and stage 2 with automatic outlier detection and de-weighting, and age, laterality, and FSIQ included as covariates. Z statistic images were thresholded using clusters determined by Z > 2.3 and a (corrected) cluster significance threshold of p = .05 (Worsley, 2001). Variances were calculated separately for both groups. The group mean of the PPI regressor at third level was evaluated individually for both groups, as well as mean differences in activation between groups.
3. Results
3.1. Descriptive statistics
Measures of task performance in the scanner, including response latency, error rate, and number of valid trials obtained for analysis, did not significantly differ between groups (Table 3). Average relative root mean squared (RMS) head movement did not differ between groups and was generally low; there was a small but statistically significant difference between groups in average absolute RMS movement (ASD M = 0.11 mm, SD = 0.02 mm; TD M = 0.09 mm, SD = 0.02 mm). Groups did not differ in terms of the number of volumes identified as motion outliers (Table 3). On average, youth with ASD performed more poorly on all social–behavioral measures (Table 2).
Table 3.
ASD and comparison group differences in head motion and task performance variables.
| ASD (n = 15) | Comparison (n = 15) | Difference (p) | |
|---|---|---|---|
| Root mean squared (RMS) movement (mm) | |||
| Relative (volume to volume difference) | 0.09 (0.03) | 0.07 (0.03) | .120 |
| Absolute (relative to reference volume) | 0.11 (0.02) | 0.09 (0.02) | .017 |
| Motion outliers | |||
| # motion outliers across all runsa | 50.40 (14.79) | 45.00 (25.10) | .480 |
| Range in total # outliers across all runs | 29–75 | 7–94 | – |
| Task errors per run | 5.20 (5.32) | 3.11 (2.84) | .190 |
| Response latency (sec) | 1.72 (0.03) | 1.71 (0.01) | .593 |
| Latency: IMI trials | 1.88 (0.05) | 1.87 (0.05) | .683 |
| Latency: EXE trials | 1.68 (0.06) | 1.68 (0.02) | .892 |
| Valid OBS trials | 15.40 (3.11) | 16.00 (2.54) | .567 |
| Valid IMI trials | 19.27 (1.94) | 19.13 (2.39) | .868 |
| Valid EXE trials | 17.60 (1.12) | 17.80 (0.41) | .522 |
Equal variances not assumed.
Between-group variance in ToMI-T scores differed (Levene's test F(1, 28) = 13.11, p = .001), with TD youth displaying lower variance (Fig. 1; Table 2) and an extremely restricted range. This pattern was also evident across subscale scores. Consequently, for models in which ToMI scores were used as the outcome variable, analyses were run for the subsamples separately, and the models for TD youth had standard errors corrected using the HC3 heteroscedasticity consistent covariance matrix recommended for small sample sizes (Long and Ervin, 2000).
3.2. FMRI data
3.2.1. Local functional specialization
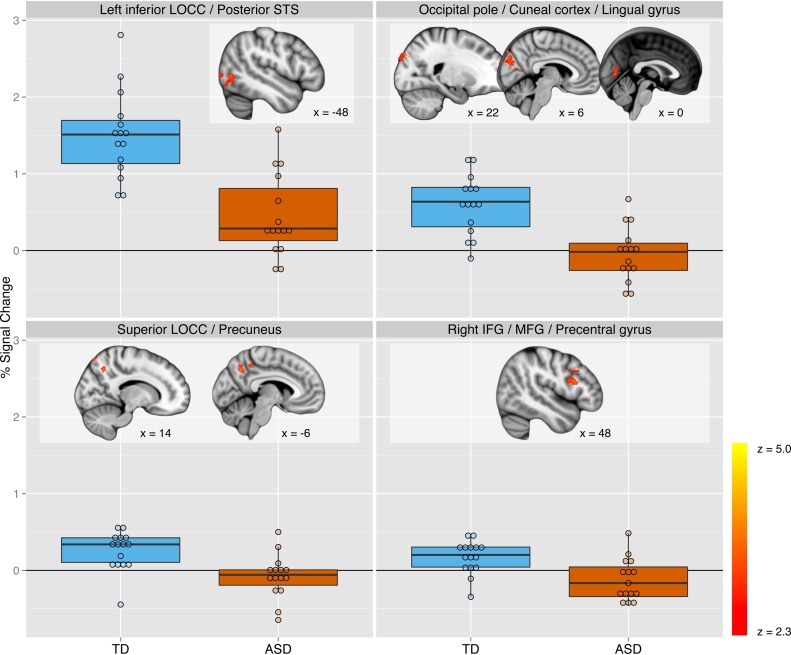
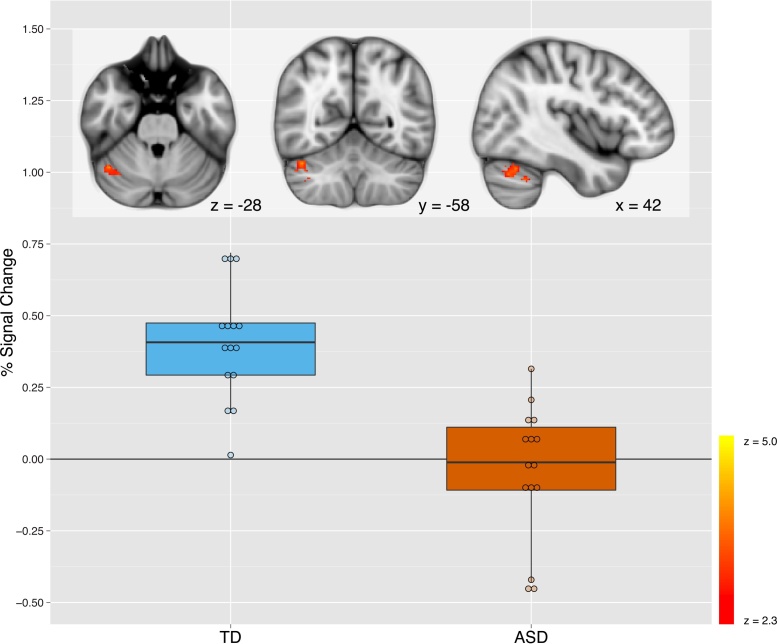
Brain sites sensitive to the IMI > EXE contrast were generally consistent with previous research (Caspers et al., 2010) for both groups (Table 4). TD youth demonstrated significantly higher response than youth with ASD to the IMI > EXE contrast throughout occipital regions, including clusters encompassing occipital pole (OCP), lingual gyrus, and cuneal cortex; superior lateral occipital cortex (LOCC) and precuneus (PCu); and left inferior LOCC into pSTS. TD youth also showed higher response to this contrast in a cluster that extended from the pars opercularis of the inferior frontal gyrus (IFG) into middle frontal gyrus and precentral gyrus (Table 5; Fig. 2). During IMI trials relative to baseline, TD youth demonstrated significantly greater activity than did youth with ASD throughout the cerebellum, with clusters located in right CrusI and left lobule VI (Table 5; Fig. 3). See Supplement for results from non-targeted contrasts (IMI > OBS, OBS > IMI, OBS > EXE contrasts, group differences in OBS and EXE).
Table 4.
Cluster maxima and local peaks identified in the IMI > EXE task contrast for each group.
| Anatomical Region | TD |
ASD |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Hem | x | y | z | Z | Hem | x | y | z | Z | |
| Temporal | ||||||||||
| Posterior superior temporal sulcus | R | 46 | −70 | −6 | 16.84 | R | 60 | −34 | 10 | 4.39 |
| Planum temporale | – | – | – | – | – | R | 52 | −30 | 16 | 5.32 |
| Occipital | ||||||||||
| Occipital fusiform | R | 20 | −78 | −16 | 16.22 | R | 20 | −76 | −14 | 9.45 |
| Inferior lateral occipital cortex | L | −50 | −76 | −6 | 15.09 | – | – | – | – | – |
| Inferior lateral occipital cortex | R | 46 | −78 | −10 | 13.44 | R | 52 | −64 | −12 | 8.37 |
| Superior lateral occipital cortex | L | −18 | −86 | 30 | 12.45 | L | −24 | −86 | 14 | 7.91 |
| Lingual gyrus | – | – | – | – | – | R | 8 | −84 | −10 | 8.30 |
| Parietal | ||||||||||
| Superior parietal lobule | L | −34 | −52 | 56 | 4.78 | L | −32 | −48 | 52 | 6.77 |
| Parietal operculum | – | – | – | – | – | R | 50 | −32 | 22 | 6.56 |
| Parietal operculum | – | – | – | – | – | L | −48 | −38 | 24 | 4.25 |
| Anterior supramarginal gyrus | – | – | – | – | – | L | −64 | −26 | 30 | 3.46 |
| Frontal | ||||||||||
| Precentral gyrus | R | 42 | −2 | 54 | 5.54 | R | 38 | −6 | 42 | 4.47 |
| Precentral gyrus | L | −56 | −2 | 42 | 4.50 | – | – | – | – | – |
| Inferior frontal gyrus (pars op.) | R | 40 | 12 | 18 | 4.80 | – | – | – | – | – |
| Subcortical | ||||||||||
| Thalamus | R | 16 | −30 | −4 | 8.40 | – | – | – | – | – |
| Thalamus | L | −18 | −30 | −4 | 7.83 | – | – | – | – | – |
| Brain stem | R | 6 | −28 | −14 | 3.29 | – | – | – | – | – |
Note: MNI coordinates are reported. Hem, hemisphere; R, right; L, left; Z, Z-statistic; pars op.: pars opercularis.
Table 5.
Significant ASD versus comparison group differences in target imitation analyses.
| Anatomical region | Hem | x | y | z | Z | k |
|---|---|---|---|---|---|---|
| IMI>EXETD>ASD | ||||||
| Occipital pole | R | 22 | −88 | 38 | 4.46 | 612 |
| Lingual gyrus | – | 0 | −76 | 4 | 3.50 | – |
| Cuneal cortex | – | 6 | −86 | 42 | 3.49 | – |
| Sup. lateral occipital cortex | R | 14 | −74 | 60 | 3.89 | 428 |
| Precuneus | L | −6 | −62 | 40 | 3.82 | – |
| Inf. lateral occipital cortex | L | −48 | −74 | −12 | 3.29 | 321 |
| Posterior STS | L | −48 | −70 | 2 | 3.22 | – |
| Inf. frontal gyrus (pars op.) | R | 48 | 18 | 18 | 3.62 | 319 |
| Middle frontal gyrus | R | 50 | 18 | 34 | 3.35 | – |
| Precentral gyrus | R | 52 | 12 | 32 | 3.26 | – |
| IMITD>ASD | ||||||
| Cerebellar VI | L | −20 | −64 | −32 | 3.97 | 702 |
| Vermis VIIIa | – | −6 | −64 | −34 | 3.54 | – |
| Vermis IX | – | 2 | −58 | −32 | 3.46 | – |
| Cerebellar V | L | −14 | −56 | −24 | 3.41 | – |
| Crus I | R | 42 | −58 | −28 | 3.89 | 547 |
| VIIb | R | 42 | −56 | −52 | 3.69 | – |
| Crus II | R | 42 | −58 | −46 | 3.65 | – |
Note: Coordinates reported are in MNI space. Hem, hemisphere; R, right; L, left; Z, Z-statistic; k, voxel extent; Inf, inferior; Sup, superior; STS, superior temporal sulcus.
Fig. 2.
As a group, TD youth demonstrated significantly greater activity than youth with ASD to the IMI > EXE contrast in a variety of occipital and temporo-occipital regions, as well as in right IFG. Boxplots summarize the distribution of brain response (expressed as percent signal change) in these regions for each group; overlaid dotplots indicate individual IMI > EXE values. Inset brain images depict sagittal views of the significant clusters, with the MNI coordinate of the slice indicated.
Fig. 3.
During IMI trials, TD compared to ASD youth showed significantly higher activity bilaterally across cerebellum, including in a cluster with a peak in right Crus I. These boxplots depict the distribution of brain response (expressed as percent signal change) within this cluster, masked to highlight activity exclusively within right Crus I as defined by Diedrichsen's probabilistic cerebellar FNIRT atlas. Overlaid dotplots indicate individual IMI values. Inset brain images (radiological orientation) depict this region of right Crus I, with MNI coordinates indicated.
3.2.2. Group differences in PPI values
No significant within-group main effects or between-group differences were identified at level three of PPI analysis.
3.2.3. Associations between PPI values and mentalizing skill
Among youth with ASD, higher PPI values between Crus I and RpSTS predicted significantly greater ToMI scores, and these effects were stronger in right Crus I (Table 6). Specifically, PPI value with RpSTS in right Crus I was significantly associated with ToMI-T score. Broken down by ToMI subscale, RCrus I–RpSTS was marginally associated with Early, and significantly associated with Basic and Advanced subscale scores (Fig. 4). The PPI value with RpSTS in left Crus I was marginally associated with ToMI-T score; broken down by ToMI subscale, LCrus I–RpSTS was significantly associated with Early but no other subscale scores (Supplemental Figure 1). Crus I–RpSTS PPI values were not associated with ToMI-T scores among TD youth, either in right (β = .10, p = .611) or left (β = −.19, p = .651) Crus I.
Table 6.
Linear models predicting ASD group ToMI Total and subscale scores from PPI values between right pSTS and Crus I.
| Left Crus I |
Right Crus I |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| β | B | SE | t | p | β | B | SE | t | p | |
| ToMI Total | ||||||||||
| (Intercept) | – | 14.00 | 0.66 | 21.36 | <.001* | – | 13.69 | 0.60 | 23.00 | <.001* |
| Age | .42 | 0.74 | 0.51 | 1.46 | .175 | .36 | 0.64 | 0.46 | 1.40 | .191 |
| Laterality | −.30 | −0.01 | 0.01 | −1.08 | .307 | −.29 | −0.01 | 0.01 | −1.19 | .263 |
| Full scale IQ | .26 | 0.05 | 0.06 | 0.88 | .401 | .27 | 0.05 | 0.05 | 1.06 | .315 |
| RpSTS–CrusI | .47 | 1.72 | 0.89 | 1.92 | .083† | .58 | 2.00 | 0.74 | 2.69 | .023* |
| Adj. R2 = .23, F = 2.04, p = .165 | Adj. R2 = .39, F = 3.20, p = .062† | |||||||||
| ToMI Early | ||||||||||
| (Intercept) | – | 14.45 | 0.53 | 27.12 | <.001* | – | 14.22 | 0.61 | 23.29 | <.001* |
| Age | .44 | 0.72 | 0.41 | 1.75 | .111 | .43 | 0.70 | 0.47 | 1.48 | .170 |
| Laterality | −.38 | −0.02 | 0.01 | −1.53 | .157 | −.35 | −0.01 | 0.01 | −1.26 | .235 |
| Full scale IQ | .13 | 0.02 | 0.05 | 0.52 | .616 | .21 | 0.04 | 0.05 | 0.75 | .471 |
| RpSTS–CrusI | .59 | 1.98 | 0.73 | 2.73 | .021* | .47 | 1.48 | 0.76 | 1.95 | .080† |
| Adj. R2 = .40, F = 3.30, p = .057† | Adj. R2 = .24, F = 2.08, p = .159 | |||||||||
| ToMI Basic | ||||||||||
| (Intercept) | – | 15.80 | 0.58 | 27.15 | <.001* | – | 15.55 | 0.51 | 30.41 | <.001* |
| Age | .21 | 0.30 | 0.45 | 0.67 | .517 | .14 | 0.21 | 0.39 | 0.53 | .608 |
| Laterality | −.18 | −0.01 | 0.01 | −0.59 | .568 | −.18 | −0.01 | 0.01 | −0.69 | .504 |
| Full scale IQ | .33 | 0.05 | 0.05 | 1.04 | .321 | .32 | 0.05 | 0.04 | 1.20 | .258 |
| RpSTS–CrusI | .40 | 1.18 | .79 | 1.49 | .168 | .57 | 1.62 | 0.64 | 2.53 | .030* |
| Adj. R2 = .09, F = 1.33, p = .325 | Adj. R2 = .32, F = 2.65, p = .097† | |||||||||
| ToMI Advanced | ||||||||||
| (Intercept) | – | 11.45 | 0.95 | 12.08 | <.001* | – | 11.06 | 0.89 | 12.37 | <.001* |
| Age | .51 | 1.31 | 0.74 | 1.78 | .106 | .46 | 1.19 | 0.69 | 1.72 | .115 |
| Laterality | −.34 | −0.02 | 0.02 | −1.21 | .254 | −.33 | −0.02 | 0.02 | −1.29 | .227 |
| Full scale IQ | .24 | 0.07 | 0.08 | 0.83 | .425 | .26 | 0.07 | 0.07 | 0.97 | .353 |
| RpSTS–CrusI | .42 | 2.21 | 1.29 | 1.71 | .118 | .50 | 2.51 | 1.12 | 2.25 | .048* |
| Adj. R2 = .22, F = 2.01, p = .170 | Adj. R2 = .33, F = 2.75, p = .089† | |||||||||
Note: Degrees of freedom on all F statistics: (4, 10).
p < .05.
p < .10.
When substituted into models in place of PPI values, greater RpSTSIMI>EXE activity was marginally associated with lower ToMI-T scores for youth with ASD (β = −.55, p = .055). RpSTSIMI>EXE activity was not associated with these scores for comparison youth (β = .10, p = .809).
4. Discussion
We examined how coordinated activity between neocerebellum and pSTS occurring during the perception and use of human action cues (specifically, in the context of imitation) was related to mentalizing outcomes for adolescents with and without ASD. As predicted, greater Crus I–RpSTS connectivity was associated with better parent-reported mentalizing skill among youth with ASD, with effects stronger in right than left Crus I. Additionally, patterns of imitation-related activity were congruent with previous research, and while somewhat similar across both youth with ASD and comparison youth, typically developing youth did demonstrate stronger response in right IFG, medial occipital regions, precuneus, and left postero-inferior temporo-occipital regions.
4.1. Associations between Crus I–RpSTS connectivity and mentalizing skill
Crus I–RpSTS interactions during perception and use of information about others’ actions were positively associated with mentalizing outcomes, but only among youth with ASD. Associations between parent-reported mentalizing skill and connectivity with pSTS were stronger in right Crus I, with the PPI between RpSTS and right Crus I significantly predicting the overall ToMI score. When broken down by subscale, the Basic and Advanced subscales were significantly related to the RCrus I–RpSTS PPI value, and the Early subscale was marginally related. In left Crus I, connectivity with RpSTS was primarily associated with the Early subscale of the ToMI, with non-significant associations with the Basic and Advanced subscales and a marginal association with the full measure. Given the expectation that youth would attend to the movements of another and use these movements to guide action sequences of their own, the Crus I–RpSTS coordinations we observed likely index efficient processing of human action cues in the context of a need to utilize these cues to drive behavior. Neocerebellar activity may facilitate continued RpSTS responding as well as interactions between RpSTS and other sites whose function relies upon information about socially meaningful human actions (Jack et al., 2011, Sokolov et al., 2012).
If Crus I–RpSTS interactions are primarily important when a response is needed to a biological motion cue, then the stronger effects in right versus left Crus I might be related to the fact that we would expect communication to be more intense between the active (right) hand and the ipsilateral hemisphere of the cerebellum. However, it is important to note that these effects cannot be exclusively motoric. First, our task condition contrast controlled for motor confounds. Second, considerable evidence indicates that Crus I is not a sensorimotor region of the cerebellum. Lesion studies in humans find that damage to lobule VII of cerebellum, which includes Crus I, is associated with minimal motor impairment, suggesting that this region is not necessary for motor function (Stoodley and Schmahmann, 2009). Moreover, both animal and human work indicates that tracts link this region to premotor, prefrontal, and posterior parietal cortices, but not to motor regions (Kelly and Strick, 2003, Salmi et al., 2010).
Given the likely basic social function of these Crus I–RpSTS interactions, it is intuitive that this connectivity metric should be associated with those early mentalizing skills which rely strongly on appropriate responding to basic cues of social meaning (e.g., facial expressions, gaze shifts, pointing) and using those cues to shape one's own behavior. However, our results indicate that Crus I–RpSTS connectivity also predicts advanced mentalizing abilities. While some aspects of advanced mentalizing, like understanding figures of speech, may be mediated in a more cognitive fashion (Frith and Frith, 2003), many advanced processes depend upon being able to rapidly and appropriately respond to subtle human action cues embedded in complex interactions. For example, the ability to differentiate between good-natured and malicious teasing requires use of information about others’ vocal tone, facial affect, and body language. Alternatively or in addition, greater routinization (Ito, 1993, Ramnani, 2006, Schmahmann, 1991) of biological motion perception in pSTS, via predictive inputs and adaptive feedback from Crus I, may free up temporal and prefrontal resources for higher level mentalizing processes that require greater flexibility and abstraction. Overall, these findings are consistent with previous work indicating that pSTS connectivity (Shih et al., 2011) is impacted in ASD, and that individual differences in pSTS can be related to social functioning (Hadjikhani et al., 2006, Gendry Meresse et al., 2005, Shih et al., 2011). These results also complement work indicating functional (Allen et al., 2004, Allen and Courchesne, 2003, Mostofsky et al., 2009, Haist et al., 2005) and functional connectivity (Mostofsky et al., 2009) disruptions in the cerebellum. However, this project extends previous findings by illuminating the role that interactions between RpSTS and neocerebellum may play in supporting social functioning among individuals with ASD.
Degree of RpSTS recruitment alone did not have the same predictive relationship with mentalizing outcomes as did Crus I–RpSTS effective connectivity. Rather, higher RpSTSIMI>EXE activity was associated with lower parent-reported mentalizing scores. This pattern ran counter to our tendency to think of social brain activity that is greater in magnitude or extent as “better.” Perhaps in this case, given the relative simplicity of the task and the number of trials to which participants were exposed, lower RpSTSIMI>EXE activity, averaged across all trials, indicated more rapid learning and adaptation to the task. A higher average RpSTSIMI>EXE value across all trials, on the other hand, might indicate greater load and/or a less efficient process. Given that we did not predict this outcome a priori, these thoughts are speculative, and further investigation is needed.
Overall, a key feature of these findings is the importance of neocerebellar involvement when behavior must be informed by others’ cues. While RpSTS shows specialization for perception of human motion, interactions with Crus I might provide the rapid predictive guidance necessary for fluent and efficient use of this information during a dynamic social situation.
4.2. Local functional specialization for non-motor aspects of imitation
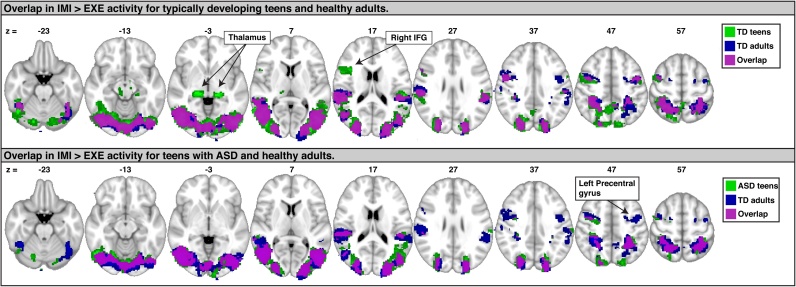
In previous work with neurotypical adults using an identical paradigm, significant IMI > EXE related activity was found in bilateral pSTS, SPL, IPL, and premotor cortex (Jack et al., 2011). Results in the present sample, both among typically developing teens and those with ASD, were largely similar to those found in the neurotypical adults. Fig. 5 demonstrates the extensive overlap in IMI > EXE activity among the two groups investigated in the current study, as well as the neurotypical adults from our previous investigation (Jack et al., 2011). In particular the peak (in MNI coordinates) of RpSTS activity was relatively similar among typically developing adolescents (46, −70, −6), adults (50, −70, 0), and previous work (Pelphrey et al., 2005a) focused on identifying the locus of specifically hand-related biological motion perception (49, −68, 0–4). The peak for adolescents with ASD was comparatively more anterior (60, −34, 10), but the broader extent of significant activity to the contrast overlapped with that of the other groups (significant activity was found in the ASD sample also at 46, −70, −6; Z = 4.22). Overall, this suggests that non-motor aspects of imitation recruit largely similar processes in both adolescents and adults, and between both typically developing teens and high-functioning teens with ASD.
Fig. 5.
A series of sagittal slices in MNI space (radiological orientation) illustrates the degree of overlap (magenta) between activity associated with the IMI > EXE contrast in an independent sample of neurotypical adults (blue) who participated in the same paradigm (35) and either typically developing teens (green, top) or teens with ASD (green, bottom). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
However, there are a few notable differences between youth with ASD and TD youth. TD teens showed significantly higher activity in pars opercularis of right IFG than did adolescents with ASD, with extension into dorsal premotor regions including middle frontal gyrus. Pars opercularis of IFG has previously been identified as associated with imitation of simple manual gestures (Molnar-Szakacs et al., 2005, Iacoboni et al., 1999, Grèzes et al., 2003), and meta-analysis of multiple imitation studies indicate that this region of IFG, in combination with dorsolateral premotor cortex, is associated with motor imitation (Van Overwalle and Baetens, 2009). In typically developing individuals, repetitive transcranial magnetic stimulation to this region disrupts execution of imitative finger presses (Heiser et al., 2003). The implications of IFG activity during imitation have been controversial both in the typically developing population and in the specific case of ASD, with disagreements arising between those who would attribute IFG activity to mirror neuron function (Iacoboni, 2005) versus processes such as attention to the timing aspects of stimuli (Makuuchi, 2005). In one meta-analysis of studies of neurotypical individuals, imitation-related activity was found reliably in dorsal premotor cortex but not IFG specifically (Molenberghs et al., 2009). In the autism literature, reduced or absent inferior frontal activation relative to controls during orofacial imitation has been found previously and attributed to deficits in the mirror neuron system (Nishitani et al., 2004, Dapretto et al., 2006). However, in other work on manual imitation contrasting individuals with ASD versus typically developing controls, control subjects failed to show the expected imitation-related activity in IFG (Williams et al., 2006). In the same study, subjects with ASD showed significantly greater activity in dorsal premotor regions (middle frontal gyrus and precentral gyrus) to an imitation > execution contrast than control subjects. This finding is in direct opposition to our finding of reduced activity in our ASD sample in these regions, perhaps as a result of a different execution condition (finger press executed in response to a cross overlaid on either a picture of a hand or a gray rectangle). Thus, the role of these regions in imitation among youth with ASD remains somewhat ambiguous.
Compared to youth with ASD, typically developing adolescents also demonstrated a more robust IMI > EXE response in left inferior lateral occipital cortex/left pSTS, in medial occipital regions including occipital pole, cuneus, and lingual gyrus, and in a region of superior lateral occipital cortex that extended into precuneus. This difference in left pSTS is congruent with previous work documenting structural (Hadjikhani et al., 2006, Boddaert et al., 2004) and functional (Pelphrey et al., 2005b, Kaiser et al., 2010, Pelphrey and Carter, 2008b) differences in STS in individuals with ASD. In previous work related to the perception of biological motion, both in individuals with ASD and in typically developing individuals, effects are generally stronger in right than left pSTS. However, among healthy adults, differentiating intended versus unintended motion (i.e., arm motion initiated voluntarily by the performer versus arm motion initiated with mechanical assistance) appears to be lateralized to left, rather than right pSTS (Morris et al., 2008). Consequently, the left-lateralization of the difference between TD youth and youth with ASD could indicate that, as a group, youth with ASD are less robustly attributing intention and agency to the observed movements. The stronger recruitment of precuneus among TD youth is also provocative. Previous work in neurotypical individuals indicates that this region is involved in shifting attention (Le et al., 1998), visually tracking moving targets (Culham et al., 1998), and motor imagery (Hanakawa et al., 2003, Ogiso et al., 2000), as well as mentalizing (Van Overwalle and Baetens, 2009, Van Veluw and Chance, 2014) and supporting representation of the self and a first-person sense of agency in action (Cavanna and Trimble, 2006, Vogeley and Fink, 2003). Given these functions, we can speculate on several possible functions of greater precuneus recruitment during the IMI > EXE contrast among typical youth. This finding could indicate that TD youth were engaging in more motor imagery during the imitation trials, imagining mapping the movements of the observed hand onto their own as a strategy for motor execution. This interpretation might suggest that youth with ASD had a strategy more similar across both the imitation and execution conditions, perhaps focusing in both cases more on the to-be-pressed key than the dynamic information provided by the observed hand movements during IMI trials. Alternatively, TD youth might engage in more automatic mentalizing than youth with ASD when exposed to even such a basic human stimulus.
4.3. Conclusions
Together with evidence from neurotypical samples (Jack et al., 2011, Sokolov et al., 2012), these findings demonstrate the importance of interactions between Crus I of the neocerebellum and pSTS in supporting social function. Including the neocerebellum in models of neural connectivity may be able to provide us with richer information about social processing. Key social brain regions do not operate in isolation; rather, their actions must be well-coordinated with each other and updated rapidly to reflect the changing environment. The neocerebellum, with its unique sensitivity to the timing properties of stimuli and its diverse connective pathways, may help to facilitate the activity of the pSTS as it processes information about meaningful human movements in the context of dynamic social interactions. In this way, without being functionally specialized for social processes per se, the neocerebellum, and specifically Crus I, could be conceptualized as a part of the social brain by virtue of the role it plays in supporting other more classically social regions.
Funding
This work was supported by a Pathway to Independence Grant from the National Institute of Mental Health (R00-MH079617 to J.P.M.) and by research funds from the University of Virginia. A portion of the neuroimaging analysis was conducted through the Yale University Biomedical High Performance Computing Center, which is supported by Biomedical Research Support Shared Instrumentation Grants from the National Center for Research Resources (S10 RR019895 and S10 RR029676-01). The funding sources had no involvement in study design; collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the article for publication.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Acknowledgments
The authors wish to thank Meghan Cronk for assistance with participant recruitment, Matthew D. Lerner for assistance with diagnostic confirmation, Jaime Castle-Shifflet and Jon Christopher for assistance with MR data collection, Maggie Kistner and Chaney Detmer-Lillard for assistance with data coding and entry, Nicholas J. Carriero and Robert D. Bjornson for assistance with computing cluster access, and John Bonvillian, Jason Druzgal, Angeline Lillard, and Amori Y. Mikami for advice and feedback regarding study design. Special thanks to Danielle Z. Bolling for extensive comments on the final manuscript. The authors also wish to express their gratitude to the generous families and youth who participated in this study.
Footnotes
Available online 14 August 2014
Two families did not provide complete information regarding this diagnosis (date and place of evaluation, type of specialist involved). Their children received qualifying scores on the ADOS and other measures (Participant 1 – SCQ: 26; SRS-T: 79; ADOS algorithm total: 10; Participant 2 – SCQ: 17; SRS-T: 78; ADOS algorithm total: 15).
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.dcn.2014.08.001.
Appendix A. Supplementary data
The following are the supplementary data to this article:
Associations between ToMI scores and average PPI value between right pSTS seed region and left Crus I, with fit lines by group and 95% confidence interval around the estimate. Annotations are the standardized beta weights and p-values indicating estimates of the relationship between LCrus I–RpSTS and ToMI score for youth with ASD, derived from the full model (Table 6).
References
- Allen G., Courchesne E. Differential effects of developmental cerebellar abnormality on cognitive and motor functions in the cerebellum: an fMRI study of autism. Am. J. Psychiatry. 2003;160:262–273. doi: 10.1176/appi.ajp.160.2.262. [DOI] [PubMed] [Google Scholar]
- Allen G., Müller R.-A., Courchesne E. Cerebellar function in autism: functional magnetic resonance image activation during a simple motor task. Biol. Psychiatry. 2004;56:269–278. doi: 10.1016/j.biopsych.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Andersson J.L.R., Jenkinson M., Smith S.M. 2007. Non-linear Registration, aka Spatial Normalisation. [Google Scholar]
- American Psychiatric Association . 4th ed. American Psychiatric Association; Washington, DC: 2000. Diagnostic and Statistical Manual of Mental Disorders. [Google Scholar]
- Bailey A., Luthert P., Dean A., Harding B., Janota I., Montgomery M. A clinicopathological study of autism. Brain. 1998;121:889–905. doi: 10.1093/brain/121.5.889. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S. Theory of mind and autism: a review. In: Glidden L.M., editor. vol. 23. Elsevier; 2001. pp. 169–184. (International Review of Research in Mental Retardation). [Google Scholar]
- Beckmann C.F., Smith S.M. Probabilistic independent component analysis for functional magnetic resonance imaging. IEEE Trans. Med. Imaging. 2004;23:137–152. doi: 10.1109/TMI.2003.822821. [DOI] [PubMed] [Google Scholar]
- Beckmann C.F., Jenkinson M., Smith S.M. General multilevel linear modeling for group analysis in FMRI. Neuroimage. 2003;20:1052–1063. doi: 10.1016/S1053-8119(03)00435-X. [DOI] [PubMed] [Google Scholar]
- Betancur C. Etiological heterogeneity in autism spectrum disorders: more than 100 genetic and genomic disorders and still counting. Brain Res. 2011;1380:42–77. doi: 10.1016/j.brainres.2010.11.078. [DOI] [PubMed] [Google Scholar]
- Bloedel J.R. Functional heterogeneity with structural homogeneity: how does the cerebellum operate? Behav Brain Sci. 1992;15:666–678. [Google Scholar]
- Boddaert N., Chabane N., Gervais H., Good C.D., Bourgeois M., Plumet M.-H. Superior temporal sulcus anatomical abnormalities in childhood autism: a voxel-based morphometry MRI study. Neuroimage. 2004;23:364–369. doi: 10.1016/j.neuroimage.2004.06.016. [DOI] [PubMed] [Google Scholar]
- Brunet E., Sarfati Y., Hardy-Baylé M.C., Decety J. A PET investigation of the attribution of intentions with a nonverbal task. Neuroimage. 2000;11:157–166. doi: 10.1006/nimg.1999.0525. [DOI] [PubMed] [Google Scholar]
- Buckner R.L., Krienen F.M., Castellanos A., Diaz J.C., Yeo B.T.T. The organization of the human cerebellum estimated by intrinsic functional connectivity. J. Neurophysiol. 2011;106:2322–2345. doi: 10.1152/jn.00339.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspers S., Zilles K., Laird A.R., Eickhoff S.B. ALE meta-analysis of action observation and imitation in the human brain. Neuroimage. 2010;50:1148–1167. doi: 10.1016/j.neuroimage.2009.12.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanna A.E., Trimble M.R. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 2006;129:564–583. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- Constantino J.N., Davis S.A., Todd R.D., Schindler M.K., Gross M.M., Brophy S.L. Validation of a brief quantitative measure of autistic traits: comparison of the social responsiveness scale with the autism diagnostic interview-revised. J. Autism Dev. Disord. 2003;33:427–433. doi: 10.1023/a:1025014929212. [DOI] [PubMed] [Google Scholar]
- Corsello C., Hus V., Pickles A., Risi S., Cook E.H., Leventhal B.L., Lord C. Between a ROC and a hard place: decision making and making decisions about using the SCQ. J. Child Psychol. Psychiatry. 2007;48:932–940. doi: 10.1111/j.1469-7610.2007.01762.x. [DOI] [PubMed] [Google Scholar]
- Culham J.C., Brandt S.A., Cavanagh P., Kanwisher N.G., Dale A.M., Tootell R.B. Cortical fMRI activation produced by attentive tracking of moving targets. J. Neurophysiol. 1998;80:2657–2670. doi: 10.1152/jn.1998.80.5.2657. [DOI] [PubMed] [Google Scholar]
- Dapretto M., Davies M.S., Pfeifer J.H., Scott A.A., Sigman M., Bookheimer S.Y., Iacoboni M. Understanding emotions in others: mirror neuron dysfunction in children with autism spectrum disorders. Nat. Neurosci. 2006;9:28–30. doi: 10.1038/nn1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decety J., Chaminade T., Grèzes J., Meltzoff A.N. A PET exploration of the neural mechanisms involved in reciprocal imitation. Neuroimage. 2002;15:265–272. doi: 10.1006/nimg.2001.0938. [DOI] [PubMed] [Google Scholar]
- Diedrichsen J., Balsters J.H., Flavell J., Cussans E., Ramnani N. A probabilistic MR atlas of the human cerebellum. Neuroimage. 2009;46:39–46. doi: 10.1016/j.neuroimage.2009.01.045. [DOI] [PubMed] [Google Scholar]
- Dissanayake C., Macintosh K. Mind reading and social functioning in children with autistic disorder and Asperger's disorder. In: Repacholi B., Slaughter V., editors. Individual Differences in Theory of Mind: Implications for Typical and Atypical Development. Psychology Press; New York: 2003. pp. 213–239. [Google Scholar]
- Eccles J.C., Ito M., Szentágothai J. Springer; Berlin/Heidelberg: 1967. The Cerebellum as a Neuronal Machine. [Google Scholar]
- Fatemi S.H., Stary J.M., Halt A.R., Realmuto G.R. Dysregulation of Reelin and Bcl-2 proteins in autistic cerebellum. J. Autism Dev. Disord. 2001;31:529–535. doi: 10.1023/a:1013234708757. [DOI] [PubMed] [Google Scholar]
- Frith U., Frith C.D. Development and neurophysiology of mentalizing. Philos. Trans. R. Soc. Lond. B: Biol. Sci. 2003;358:459–473. doi: 10.1098/rstb.2002.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher H.L., Frith C.D. Functional imaging of “theory of mind”. Trends Cogn. Sci. 2003;7:77–83. doi: 10.1016/s1364-6613(02)00025-6. [DOI] [PubMed] [Google Scholar]
- Gendry Meresse I., Zilbovicius M., Boddaert N., Robel L., Philippe A., Sfaello I. Autism severity and temporal lobe functional abnormalities. Ann. Neurol. 2005;58:466–469. doi: 10.1002/ana.20597. [DOI] [PubMed] [Google Scholar]
- Gitelman D.R., Penny W.D., Ashburner J., Friston K.J. Modeling regional and psychophysiologic interactions in fMRI: the importance of hemodynamic deconvolution. Neuroimage. 2003;19:200–207. doi: 10.1016/s1053-8119(03)00058-2. [DOI] [PubMed] [Google Scholar]
- Grèzes J., Armony J.L., Rowe J., Passingham R.E. Activations related to “mirror” and “canonical” neurones in the human brain: an fMRI study. Neuroimage. 2003;18:928–937. doi: 10.1016/s1053-8119(03)00042-9. [DOI] [PubMed] [Google Scholar]
- Hadjikhani N., Joseph R.M., Snyder J., Tager-Flusberg H. Anatomical differences in the mirror neuron system and social cognition network in autism. Cereb. Cortex. 2006;16:1276–1282. doi: 10.1093/cercor/bhj069. [DOI] [PubMed] [Google Scholar]
- Haist F., Adamo M., Westerfield M., Courchesne E., Townsend J. The functional neuroanatomy of spatial attention in autism spectrum disorder. Dev. Neuropsychol. 2005;27:425–458. doi: 10.1207/s15326942dn2703_7. [DOI] [PubMed] [Google Scholar]
- Hanakawa T., Immisch I., Toma K., Dimyan M.A., Van Gelderen P., Hallett M. Functional properties of brain areas associated with motor execution and imagery. J. Neurophysiol. 2003;89:989–1002. doi: 10.1152/jn.00132.2002. [DOI] [PubMed] [Google Scholar]
- Heider F., Simmel M. An experimental study of apparent behavior. Am. J. Psychiatry. 1944;57:243–259. [Google Scholar]
- Heiser M., Iacoboni M., Maeda F., Marcus J., Mazziotta J.C. The essential role of Broca's area in imitation. Eur. J. Neurosci. 2003;17:1123–1128. doi: 10.1046/j.1460-9568.2003.02530.x. [DOI] [PubMed] [Google Scholar]
- Hinshaw S.P. Preadolescent girls with attention-deficit/hyperactivity disorder: I. Background characteristics, comorbidity, cognitive and social functioning, and parenting practices. J. Consult. Clin. Psychol. 2002;70:1086–1098. doi: 10.1037//0022-006x.70.5.1086. [DOI] [PubMed] [Google Scholar]
- Hutchins T.L., Bonazinga L.A., Prelock P.A., Taylor R.S. Beyond false beliefs: the development and psychometric evaluation of the perceptions of children's theory of mind measure-experimental version (PCToMM-E) J. Autism Dev. Disord. 2008;38:143–155. doi: 10.1007/s10803-007-0377-1. [DOI] [PubMed] [Google Scholar]
- Iacoboni M. Neural mechanisms of imitation. Curr. Opin. Neurobiol. 2005;15:632–637. doi: 10.1016/j.conb.2005.10.010. [DOI] [PubMed] [Google Scholar]
- Iacoboni M., Woods R.P., Brass M., Bekkering H., Mazziotta J.C., Rizzolatti G. Cortical mechanisms of human imitation. Science. 1999;80(286):2526–2528. doi: 10.1126/science.286.5449.2526. [DOI] [PubMed] [Google Scholar]
- Iacoboni M., Koski L.M., Brass M., Bekkering H., Woods R.P., Dubeau M.-C. Reafferent copies of imitated actions in the right superior temporal cortex. Proc. Natl. Acad. Sci. U. S. A. 2001;98:13995–13999. doi: 10.1073/pnas.241474598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M. Movement and thought: identical control mechanisms by the cerebellum. Trends Neurosci. 1993;16:444–447. doi: 10.1016/0166-2236(93)90073-u. [DOI] [PubMed] [Google Scholar]
- Jack A., Pelphrey K.A. Neural correlates of animacy attribution include neocerebellum in healthy adults. Cereb. Cortex. 2014 doi: 10.1093/cercor/bhu146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack A., Englander Z.A., Morris J.P. Subcortical contributions to effective connectivity in brain networks supporting imitation. Neuropsychologia. 2011;49:3689–3698. doi: 10.1016/j.neuropsychologia.2011.09.024. [DOI] [PubMed] [Google Scholar]
- Jenkinson M., Smith S.M. A global optimisation method for robust affine registration of brain images. Med. Image Anal. 2001;5:143–156. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Jenkinson M., Bannister P.R., Brady J.M., Smith S.M. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Jones W., Klin A. Heterogeneity and homogeneity across the autism spectrum: the role of development. J. Am. Acad. Child Adolesc. Psychiatry. 2009;48:471–473. doi: 10.1097/CHI.0b013e31819f6c0d. [DOI] [PubMed] [Google Scholar]
- Joshi G., Petty C., Wozniak J., Henin A., Fried R., Galdo M. The heavy burden of psychiatric comorbidity in youth with autism spectrum disorders: a large comparative study of a psychiatrically referred population. J. Autism Dev. Disord. 2010;40:1361–1370. doi: 10.1007/s10803-010-0996-9. [DOI] [PubMed] [Google Scholar]
- Kaiser M.D., Hudac C.M., Shultz S., Lee S.M., Cheung C., Berken A.M. Neural signatures of autism. Proc. Natl. Acad. Sci. U. S. A. 2010;107:21223–21228. doi: 10.1073/pnas.1010412107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly R.M., Strick P.L. Cerebellar loops with motor cortex and prefrontal cortex of a nonhuman primate. J. Neurosci. 2003;23:8432–8444. doi: 10.1523/JNEUROSCI.23-23-08432.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemper T.L., Bauman M.L. Neuropathology of infantile autism. Mol. Psychiatry. 2002;7:S12–S13. doi: 10.1038/sj.mp.4001165. [DOI] [PubMed] [Google Scholar]
- Le T.H., Pardo J.V., Hu X. 4 T-fMRI study of nonspatial shifting of selective attention: cerebellar and parietal contributions. J. Neurophysiol. 1998;79:1535–1548. doi: 10.1152/jn.1998.79.3.1535. [DOI] [PubMed] [Google Scholar]
- Lee M., Martin-Ruiz C., Graham A., Court J., Jaros E., Perry R. Nicotinic receptor abnormalities in the cerebellar cortex in autism. Brain. 2002;125:1483–1495. doi: 10.1093/brain/awf160. [DOI] [PubMed] [Google Scholar]
- Long J.S., Ervin L.H. Using heteroscedasticity consistent standard errors in the linear regression model. Am. Stat. 2000;54:217–224. [Google Scholar]
- Lord C., Risi S., Lambrecht L., Cook E.H., Leventhal B.L., DiLavore P.C. The Autism Diagnostic Observation Schedule-Generic: a standard measure of social and communication deficits associated with the spectrum of autism. J. Autism Dev. Disord. 2000;30:205–223. [PubMed] [Google Scholar]
- Makuuchi M. Is Broca's area crucial for imitation? Cereb. Cortex. 2005;15:563–570. doi: 10.1093/cercor/bhh157. [DOI] [PubMed] [Google Scholar]
- Molenberghs P., Cunnington R., Mattingley J.B. Is the mirror neuron system involved in imitation? A short review and meta-analysis. Neurosci. Biobehav. Rev. 2009;33:975–980. doi: 10.1016/j.neubiorev.2009.03.010. [DOI] [PubMed] [Google Scholar]
- Molnar-Szakacs I., Iacoboni M., Koski L.M., Mazziotta J.C. Functional segregation within pars opercularis of the inferior frontal gyrus: evidence from fMRI studies of imitation and action observation. Cereb. Cortex. 2005;15:986–994. doi: 10.1093/cercor/bhh199. [DOI] [PubMed] [Google Scholar]
- Morris J.P., Pelphrey K.A., McCarthy G. Perceived causality influences brain activity evoked by biological motion. Soc. Neurosci. 2008;3:16–25. doi: 10.1080/17470910701476686. [DOI] [PubMed] [Google Scholar]
- Mostofsky S.H., Powell S.K., Simmonds D.J., Goldberg M.C., Caffo B., Pekar J.J. Decreased connectivity and cerebellar activity in autism during motor task performance. Brain. 2009;132:2413–2425. doi: 10.1093/brain/awp088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishitani N., Avikainen S., Hari R. Abnormal imitation-related cortical activation sequences in Asperger's syndrome. Ann. Neurol. 2004;55:558–562. doi: 10.1002/ana.20031. [DOI] [PubMed] [Google Scholar]
- O’Reilly J.X., Woolrich M.W., Behrens T.E.J., Smith S.M., Johansen-Berg H. Tools of the trade: psychophysiological interactions and functional connectivity. Soc. Cogn. Affect. Neurosci. 2012;7:604–609. doi: 10.1093/scan/nss055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogiso T., Kobayashi K., Sugishita M. The precuneus in motor imagery: a magnetoencephalographic study. Neuroreport. 2000;11:1345–1349. doi: 10.1097/00001756-200004270-00039. [DOI] [PubMed] [Google Scholar]
- Oldfield R.C. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Pelphrey K.A., Carter E.J. Brain mechanisms for social perception: lessons from autism and typical development. Ann. N. Y. Acad. Sci. 2008;1145:283–299. doi: 10.1196/annals.1416.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelphrey K.a., Carter E.J. Charting the typical and atypical development of the social brain. Dev. Psychopathol. 2008;20:1081–1102. doi: 10.1017/S0954579408000515. [DOI] [PubMed] [Google Scholar]
- Pelphrey K.A., Morris J.P. Brain mechanisms for interpreting the actions of others from biological-motion cues. Curr. Dir. Psychol. Sci. 2006;15:136–140. doi: 10.1111/j.0963-7214.2006.00423.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelphrey K.A., Morris J.P., Michelich C.R., Allison T., McCarthy G. Functional anatomy of biological motion perception in posterior temporal cortex: an fMRI study of eye, mouth and hand movements. Cereb. Cortex. 2005;15:1866–1876. doi: 10.1093/cercor/bhi064. [DOI] [PubMed] [Google Scholar]
- Pelphrey K.A., Morris J.P., McCarthy G. Neural basis of eye gaze processing deficits in autism. Brain. 2005;128:1038–1048. doi: 10.1093/brain/awh404. [DOI] [PubMed] [Google Scholar]
- Pelphrey K.A., Shultz S., Hudac C.M., Vander Wyk B.C. Constraining heterogeneity: the social brain and its development in autism spectrum disorder. J. Child Psychol. Psychiatry. 2011;52:631–644. doi: 10.1111/j.1469-7610.2010.02349.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power J.D., Barnes K.A., Snyder A.Z., Schlaggar B.L., Petersen S.E. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012;59:2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell A.E., Jeon O.H., Zimmerman A.W., Blue M.E., Pevsner J. Postmortem brain abnormalities of the glutamate neurotransmitter system in autism. Neurology. 2001;57:1618–1628. doi: 10.1212/wnl.57.9.1618. [DOI] [PubMed] [Google Scholar]
- Ramnani N. The primate cortico-cerebellar system: anatomy and function. Nat. Rev. Neurosci. 2006;7:511–522. doi: 10.1038/nrn1953. [DOI] [PubMed] [Google Scholar]
- Ritvo E.R., Freeman B.J., Scheibel A.B., Duong T., Robinson H., Guthrie D., Ritvo A. Lower Purkinje cell counts in the cerebella of four autistic subjects: initial findings of the UCLA-NSAC Autopsy Research Report. Am. J. Psychiatry. 1986;143:862–866. doi: 10.1176/ajp.143.7.862. [DOI] [PubMed] [Google Scholar]
- Rutter M., Bailey A., Lord C. Westeren Psychological Services; Los Angeles, CA: 2003. SCQ: The Social Communication Questionnaire – Manual. [Google Scholar]
- Sajdel-Sulkowska E.M., Xu M., Koibuchi N. Increase in cerebellar neurotrophin-3 and oxidative stress markers in autism. Cerebellum. 2009;8:366–372. doi: 10.1007/s12311-009-0105-9. [DOI] [PubMed] [Google Scholar]
- Salmi J., Pallesen K.J., Neuvonen T., Brattico E., Korvenoja A., Salonen O., Carlson S. Cognitive and motor loops of the human cerebro-cerebellar system. J. Cogn. Neurosci. 2010;22:2663–2676. doi: 10.1162/jocn.2009.21382. [DOI] [PubMed] [Google Scholar]
- Sasaki K., Oka H., Matsuda Y., Shimono T., Mizuno N. Electrophysiological studies of the projections from the parietal association area to the cerebellar cortex. Exp. Brain Res. 1975;23:91–102. doi: 10.1007/BF00238732. [DOI] [PubMed] [Google Scholar]
- Saxe R. The right temporo-parietal junction: a specific brain region for thinking about thoughts. In: Leslie A., German T., editors. Handbook of Theory Mind. Taylor & Francis Group; 2010. [Google Scholar]
- Schmahmann J.D. An emerging concept: the cerebellar contribution to higher function. Arch. Neurol. 1991;48:1178–1187. doi: 10.1001/archneur.1991.00530230086029. [DOI] [PubMed] [Google Scholar]
- Schmahmann J.D., Pandya D.N. Projections to the basis pontis from the superior temporal sulcus and superior temporal region in the rhesus monkey. J. Comp. Neurol. 1991;308:224–248. doi: 10.1002/cne.903080209. [DOI] [PubMed] [Google Scholar]
- Shih P., Keehn B., Oram J.K., Leyden K.M., Keown C.L., Müller R.-A. Functional differentiation of posterior superior temporal sulcus in autism: a functional connectivity magnetic resonance imaging study. Biol. Psychiatry. 2011;70:270–277. doi: 10.1016/j.biopsych.2011.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S.M. Fast robust automated brain extraction. Hum. Brain Mapp. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokolov A.A., Erb M., Gharabaghi A., Grodd W., Tatagiba M.S., Pavlova M.A. Biological motion processing: the left cerebellum communicates with the right superior temporal sulcus. Neuroimage. 2012;59:2824–2830. doi: 10.1016/j.neuroimage.2011.08.039. [DOI] [PubMed] [Google Scholar]
- Sokolov A.A., Erb M., Grodd W., Pavlova M.A. Structural loop between the cerebellum and the superior temporal sulcus: evidence from diffusion tensor imaging. Cereb. Cortex. 2014;24:626–632. doi: 10.1093/cercor/bhs346. [DOI] [PubMed] [Google Scholar]
- Stoodley C.J., Schmahmann J.D. Functional topography in the human cerebellum: a meta-analysis of neuroimaging studies. Neuroimage. 2009;44:489–501. doi: 10.1016/j.neuroimage.2008.08.039. [DOI] [PubMed] [Google Scholar]
- Stoodley C.J., Schmahmann J.D. Evidence for topographic organization in the cerebellum of motor control versus cognitive and affective processing. Cortex. 2010;46:831–844. doi: 10.1016/j.cortex.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Overwalle F., Baetens K. Understanding others’ actions and goals by mirror and mentalizing systems: a meta-analysis. Neuroimage. 2009;48:564–584. doi: 10.1016/j.neuroimage.2009.06.009. [DOI] [PubMed] [Google Scholar]
- Van Overwalle F., Baetens K., Mariën P., Vandekerckhove M. Social cognition and the cerebellum: a meta-analysis of over 350 fMRI studies. Neuroimage. 2014;86:554–572. doi: 10.1016/j.neuroimage.2013.09.033. [DOI] [PubMed] [Google Scholar]
- Van Veluw S.J., Chance S.A. Differentiating between self and others: an ALE meta-analysis of fMRI studies of self-recognition and theory of mind. Brain Imaging Behav. 2014;8:24–38. doi: 10.1007/s11682-013-9266-8. [DOI] [PubMed] [Google Scholar]
- Vargas D.L., Nascimbene C., Krishnan C., Zimmerman A.W., Pardo C.A. Neuroglial activation and neuroinflammation in the brain of patients with autism. Ann. Neurol. 2005;57:67–81. doi: 10.1002/ana.20315. [DOI] [PubMed] [Google Scholar]
- Vogeley K., Fink G.R. Neural correlates of the first-person-perspective. Trends Cogn. Sci. 2003;7:38–42. doi: 10.1016/s1364-6613(02)00003-7. [DOI] [PubMed] [Google Scholar]
- Wechsler D. 1999. WASI Manual. San Antonio, TX. [Google Scholar]
- Whitney E.R., Kemper T.L., Rosene D.L., Bauman M.L., Blatt G.J. Density of cerebellar basket and stellate cells in autism: evidence for a late developmental loss of Purkinje cells. J. Neurosci. Res. 2009;87:2245–2254. doi: 10.1002/jnr.22056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J.H.G., Waiter G.D., Gilchrist A., Perrett D.I., Murray A.D., Whiten A. Neural mechanisms of imitation and “mirror neuron” functioning in autistic spectrum disorder. Neuropsychologia. 2006;44:610–621. doi: 10.1016/j.neuropsychologia.2005.06.010. [DOI] [PubMed] [Google Scholar]
- Williams J.H.G., Whiten A., Waiter G.D., Pechey S., Perrett D.I. Cortical and subcortical mechanisms at the core of imitation. Soc. Neurosci. 2007;2:66–78. doi: 10.1080/17470910701268059. [DOI] [PubMed] [Google Scholar]
- Woolrich M.W. Robust group analysis using outlier inference. Neuroimage. 2008;41:286–301. doi: 10.1016/j.neuroimage.2008.02.042. [DOI] [PubMed] [Google Scholar]
- Woolrich M.W., Ripley B.D., Brady J.M., Smith S.M. Temporal autocorrelation in univariate linear modeling of fMRI data. Neuroimage. 2001;14:1370–1386. doi: 10.1006/nimg.2001.0931. [DOI] [PubMed] [Google Scholar]
- Woolrich M.W., Behrens T.E.J., Beckmann C.F., Jenkinson M., Smith S.M. Multilevel linear modelling for fMRI group analysis using Bayesian inference. Neuroimage. 2004;21:1732–1747. doi: 10.1016/j.neuroimage.2003.12.023. [DOI] [PubMed] [Google Scholar]
- Worsley K.J. Statistical analysis of activation images. In: Jezzard P., Matthews P.M., Smith S.M., editors. Functional MRI: An Introduction to Methods. Oxford University Press; New York: 2001. pp. 251–270. [Google Scholar]
- Yip J., Soghomonian J.J., Blatt G.J. Decreased GAD65 mRNA levels in select subpopulations of neurons in the cerebellar dentate nuclei in autism: an in situ hybridization study. Autism Res. 2009;2:50–59. doi: 10.1002/aur.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Associations between ToMI scores and average PPI value between right pSTS seed region and left Crus I, with fit lines by group and 95% confidence interval around the estimate. Annotations are the standardized beta weights and p-values indicating estimates of the relationship between LCrus I–RpSTS and ToMI score for youth with ASD, derived from the full model (Table 6).