Highlights
-
•
Systematic investigation of subcomponents of reading (prelexical, orthographic, phonological, and lexico-semantic).
-
•
Region of interest analysis based on a meta-analytic (in the present version meta is written in italic) map of the core reading system in children.
-
•
Task comparison showed important role of occipito-temporal and frontal systems in children.
-
•
Results favor the idea of an interactive reading network in children.
Keywords: Prelexical, Orthographic, Phonological, Lexico-semantic, Children, fMRI
Abstract
The reading system can be broken down into four basic subcomponents in charge of prelexical, orthographic, phonological, and lexico-semantic processes. These processes need to jointly work together to become a fluent and efficient reader. Using functional magnetic resonance imaging (fMRI), we systematically analyzed differences in neural activation patterns of these four basic subcomponents in children (N = 41, 9–13 years) using tasks specifically tapping each component (letter identification, orthographic decision, phonological decision, and semantic categorization). Regions of interest (ROI) were selected based on a meta-analysis of child reading and included the left ventral occipito-temporal cortex (vOT), left posterior parietal cortex (PPC), left inferior frontal gyrus (IFG), and bilateral supplementary motor area (SMA). Compared to a visual baseline task, enhanced activation in vOT and IFG was observed for all tasks with very little differences between tasks. Activity in the dorsal PPC system was confined to prelexical and phonological processing. Activity in the SMA was found in orthographic, phonological, and lexico-semantic tasks. Our results are consistent with the idea of an early engagement of the vOT accompanied by executive control functions in the frontal system, including the bilateral SMA.
1. Introduction
Learning to read is one of the most important educational milestones in a child's development. Becoming an efficient and fluent reader requires the development of four basic subcomponents: prelexical, orthographic, phonological, and lexico-semantic processing (e.g., Jacobs and Ziegler, 2015). In skilled readers, a large number of highly intertwined brain regions work in concert (e.g., Pollack et al., 2015, Price, 2012) to achieve the ultimate goal: comprehension (Jacobs, 2015). Generally, it is assumed that reading is supported by three principal interactive cortical systems differing in structure, connectivity, and functionality: ventral occipito-temporal (vOT), dorsal occipito-parietal, and inferior frontal systems (Carreiras et al., 2014, Price, 2012). While these systems are well described in skilled adult readers, less research has been devoted to normally developing children. For instance, the specific role of the ventral system in reading development and the interplay between ventral and dorsal systems are still discussed (Boros et al., 2016, Paulesu et al., 2014, Pugh et al., 2000, Pugh et al., 2010, van der Mark et al., 2011). The aim of the present study was to break down the complex reading process into its central subcomponents and to systematically investigate the neural response patterns associated with each subcomponent.
1.1. Subcomponents of reading and their neurofunctional bases
The basic subcomponents of reading have often been linked to separable neural networks (Backes et al., 2002, Hofmann and Jacobs, 2014, Taylor et al., 2013, Welcome and Joanisse, 2012). The first crucial step of reading is letter identification, which has been associated with the occipital cortex (Boros et al., 2016, Dehaene et al., 2015). After low-level but nonetheless highly specialized analysis, processing proceeds along different streams. Whole-word forms are computed in the ventral system (Seghier et al., 2008), which comprises the vOT, inferior temporal gyrus (ITG; Kronbichler et al., 2004), middle temporal gyrus (MTG; Noonan et al., 2013) and projects onto the inferior frontal gyrus (IFG). In contrast, grapheme-to-phoneme conversion and phonological analysis are performed by the dorsal system (e.g., Braun et al., 2015a) comprising the superior temporal gyrus (STG) and posterior parietal cortex (PPC). Both processing streams project onto the frontal system, comprising the IFG and insula, among others, which have been implicated in the integration of information and semantic retrieval (Binder et al., 2009, Heim et al., 2005, Kuhlmann et al., 2016), as well as executive processing and decision-making (Bitan et al., 2005, Rowe et al., 2005).
1.2. Reading acquisition
During the early stages of reading acquisition, children have to map letters onto pre-existing phonological representations typically using a serial grapheme-to-phoneme reading strategy (Goswami et al., 2001, Grainger and Ziegler, 2011, Ziegler et al., 2014). According to the dual-route theory of reading, the initially slow and laborious phonological decoding is gradually replaced by parallel letter processing leading to the formation of two types of location-invariant sublexical encoding processes (Grainger et al., 2012, Grainger and Ziegler, 2011). The so-called coarse-grained coding allows for the direct mapping of letters onto whole-word representations, whereas the fine-grained coding supports position-sensitive phonological decoding. At the neuronal level, rapid parallel coarse-grained orthographic coding has been associated with the ventral system, whereas fine-grained phonologically-based coding has been associated with the dorsal system (Boros et al., 2016, van der Mark et al., 2009, Vinckier et al., 2007).
Results from neuroimaging studies of reading in children are still partly inconsistent and incomplete (cf., Houdé et al., 2010). This might be due to the fact that these studies often examine only specific subcomponents of reading (e.g., Bach et al., 2010, Bitan et al., 2007a, Bitan et al., 2007b) or focus on group differences comparing different populations (e.g., Backes et al., 2002, Cao et al., 2006). In their meta-analysis, Martin et al. (2015) aggregated the results of 20 studies investigating different reading-related processes in children and identified a widespread core network of reading in children including the left vOT, PPC, IFG, and the bilateral supplementary motor area (SMA). The results of the meta-analysis are in favor of an early engagement of the ventral system in children. Moreover, high convergence of activation across studies in children was found in the STG emphasizing the importance of phonology-based processes in reading acquisition (Jobard et al., 2003, Pugh et al., 2000, Pugh et al., 2010, Turkeltaub et al., 2003). Reading-related activation in the PPC was interpreted as part of a fronto-parietal control system (Corbetta and Shulman, 2002, Fair et al., 2008, Spreng et al., 2010). Activation in IFG has been suggested to play an important role in linguistic as well as nonlinguistic processes in reading acquisition (Booth et al., 2002, Booth et al., 2004, Shaywitz et al., 2002). Finally, the largest cluster was localized in bilateral SMA. The SMA has not only been associated with phonological rehearsal and covert articulation (Price, 2012) but also with goal-directed behavior and executive control (Bonini et al., 2014, Hertrich et al., 2016). Although the meta-analysis of Martin et al. (2015) gives a good idea about the core reading network in children, the evidence is aggregated across different studies using a variety of tasks that differ in format (passive versus active tasks) and demands. Thus, additional studies are needed to investigate the neural correlates of each of the central subcomponent within the same children using highly comparable tasks that only differ in their linguistic computations.
1.3. Present study
To systematically investigate the neural correlates of the basic subcomponents of the reading system in children, we designed four comparable tasks each tapping one of the component processes of reading (for similar approaches in adults and children, see Backes et al., 2002, Shaywitz et al., 1998, Welcome and Joanisse, 2012). These tasks were letter identification in consonant strings, orthographic decision, phonological decision, and semantic categorization. The goal was to investigate the degree of differentiation and specialization of each subcomponent in a sample of typically developing German children. We hypothesized that prelexical processing might primarily engage posterior parts of the vOT system. Moreover, we were interested in finding out whether orthographic and phonological processing would show distinguishable patterns of neural activation (i.e., ventral versus dorsal system) and to what extent both processes activate the IFG. Finally, we expected both ventral and dorsal systems to be engaged in lexico-semantic processing complemented by frontal systems. Given the strong involvement of STG and bilateral SMA in children (Martin et al., 2015), we hypothesized these regions to be active during all component processes except for prelexical processing.
2. Materials and methods
2.1. Participants
Fifty-six German-speaking children (9–13 years; M = 11.8; 22 female) participated in the experiment. All participants were right-handed and had normal or corrected-to-normal vision. None of the children had a history of reading difficulties or language impairment, neurological diseases, psychiatric disorders or head injuries. Two parents reported suspicion of dyslexia within their family, but the respective children showed age-appropriate performance in the standardized reading tests as did the other children. Only one child obtained an strinkingly low nonverbal IQ score. Excluding this child from the analysis did not change the results. This participant was therefore kept in the analyses. Data of six children could not be analyzed due to technical problems during the functional magnetic resonance imaging (fMRI) sessions, movements of two children exceeded the a-priori set criterion of maximum head movement (>± 5 mm/degrees translation or rotation; Addis, 2004). Seven additional children were excluded based on in-scanner accuracy values. They had error rates higher than 40% in the visual control baseline task or during letter identification (for detailed description of tasks, see 2.3 Tasks and stimuli). This resulted in a final fMRI-sample of 41 children (9–13 years, M = 11.9, 18 female). Age was normally distributed within the fMRI-sample. One child was 9 years old, 5 were 10 years old, 17 were 11 years old, 11 were 12 years old, and 7 were 13 years old (for sample characteristics see Table 1). Children were recruited through newspaper advertisements, prior studies conducted at the university and advertisements in Berlin schools. All parents and children gave written informed consent to participate in the study. Subjects received financial compensation for their participation. Experimental procedures were approved by the Ethics Committee of the German Association for Psychology.
Table 1.
Descriptive statistics on age, grade, and standardized behavioral measures (for better comparison children are split into three age groups).
| young | middle | old | ||||
|---|---|---|---|---|---|---|
| N = 6 |
N = 17 |
N = 18 |
||||
| Mean | (SD) | Mean | (SD) | Mean | (SD) | |
| Sample characteristics | ||||||
| Age | 10.4 | (.55) | 11.5 | (.32) | 12.9 | (.55) |
| Age range | 9.7–10.9 | 11.0–11.9 | 12.0–13.7 | |||
| Gradea | 5 | (.75) | 6 | (.5) | 7 | (.7) |
| Standardized behavioral measures | ||||||
| SLRT-II word reading (PR) | 51.7 | (16) | 56.8 | (29) | 57 | (34) |
| SLRT-II pseudoword reading (PR) | 49.8 | (17) | 47.8 | (25) | 51.7 | (30) |
| SLS sentence reading (T) | 14.5 | (8) | 11.9 | (8) | 19.2 | (10) |
| CFT vocabulary (T) | 59.7 | (9) | 61.4 | (7) | 53.2 | (7) |
| HAWIK-IV block test | 11.5 | (39) | 12.1 | (3) | 11.7 | (2) |
Note. SLRT-II: Salzbuger Lese-Rechtschreibtest; SLS: Salzburger Lese-Screening; CFT: Wortschatztest des Grundintelligenztest Skala; HAWIK-IV: Hamburger-Wechsler-Intelligenztest für Kinder; SD = standard deviation; PR = percentile rank; T = age normed t-value.
Median is reported.
2.2. Psychometric measures
All children completed a battery of reading and language tests. Nonverbal intelligence was assessed with the block test of the German adaptation of the Wechsler Intelligence Scale for Children (HAWIK-IV; Hamburger-Wechsler-Intelligenztest für Kinder − IV; Petermann and Petermann, 2007). Vocabulary was tested with a computer-based version of the Culture Fair Test (CFT; Wortschatztest der Grundintelligenztest Skala – 2. Revision; Weiß, 2008). Word reading was tested using two subtests of the SLRT-II (Lese- und Rechtschreibtest II − Weiterentwicklung des Salzbuger Lese- und Rechtschreibtest; Moll and Landerl, 2010) in which children had to read lists of words and pseudowords. Global reading ability was assessed using a computer-based screening (SLS; Salzburger Lese-Screening für die Klassenstufen 5–8; Auer et al., 2005) in which short sentences had to be judged on their semantic content. Children showed the full scale of age-appropriate reading ability, i.e. above average, average, and below average (see Table 1). For further statistical analyses, internally studentized residuals were computed for the scores in vocabulary, word, pseudoword and sentence reading to control confounding effects of nonverbal IQ, age and grade of school.
2.3. Tasks and stimuli
During the fMRI session, participants were given five decision tasks. While one constituted a visual control baseline, each of the remaining four tasks specifically targeted one of the four basic subcomponents of reading: prelexical, orthographic, phonological, and lexico-semantic processing.
Letter Identification (LETTER). Position-specific prelexical processing was assessed with the LETTER task (Ziegler et al., 2008). Children had to decide whether a consonant string contained the predefined target letter “r” (e.g., dbnrl vs. non-target, dlptd).
Orthographic Decision (ORTHO). To investigate orthographic processing, children had to judge whether a letter string was a correctly spelled German word (target: e.g. Ecke; corner) or a German pseudohomophone (non-target: e.g. Hauß [haʊs], correct German spelling: Haus; house). Pseudohomophones are pseudowords that sound like an existing word. The only way to successfully solve this task is to access orthographic information at the level of the orthographic lexicon.
Phonological Decision (PHONO). Phonological processing was evaluated by presenting pseudowords that were either homophonic to a German word (pseudohomophone) or not (target: e.g., Waal [wa:l], correct German spelling: Wal; whale, vs. non-target: e.g., Buhn [bu:n]). Children had to decide whether the visually presented word sounded like a real word. The only way to solve this task is to compute the phonology of the string and check whether the computed phonology corresponds to that of a real word (i.e., phonological lexicon).
Semantic Categorization (SEMCAT). To assess lexico-semantic processing, children had to decide whether a presented word was a living (target: e.g., Hase; rabbit) or a non-living object (non-target: e.g., Tisch; table).
Visual Control Baseline (CTRL). Non-linguistic visual feature processing was assessed as a perceptual baseline. Here, children had to decide whether tilted slashes pointed towards the same direction or not (target: /////vs. non-target: ///\/).
Each task consisted of 80 items (40 targets, 40 non-targets). Words and basewords of pseudohomophones and pseudowords were matched for word length (4–5 letters), bigram frequency, word frequency, normalized lemma frequency of (base) word, and orthographic neighborhood density (Coltheart et al., 1977). Pseudowords and pseudohomophones were created by exchanging one letter of an existing German noun. The 40 pairs of letter strings were carefully matched for bigram frequency, number of corpus-sized letters, letters with ascenders and descenders, respectively. Matching was based on the dlex database (dlexDB: www.dlexdb.de; Heister et al., 2011). For a more detailed description of tasks and stimuli, compare Froehlich et al. (2016).
2.4. fMRI paradigm
Prior to the fMRI session, children performed a short training outside the scanner to become familiar with the five decision tasks. The main experiment was divided into four runs each comprising the five tasks. Task order was counter-balanced across runs to control for sequence effects and were presented in blocks containing 20 trials each. At the beginning of each block, a cue screen was presented for 4000 ms informing the child about the upcoming task. This was followed by a jittered fixation cross with a mean duration of 6000 ms (range 3000–9000 ms). Each trial consisted of a fixation cross presented for 1000 ms followed by the presentation of a stimulus for 2000 ms. To allow the blood oxygen level dependent (BOLD) signal to return to baseline, a fixation cross was presented for 15 s at the end of each block. The order of trials, blocks, and runs was pseudo-randomized across children. Stimuli were presented in black at the center of a gray screen on dual display goggles (VisuaStim, MR Research, USA) using Python 2.7 (Python Software Foundation, https://www.python.org/). Earplugs and headphones were provided to attenuate scanner noise. Children were encouraged to make their decision as accurately and quickly as possible. They responded with a target/non-target button press on each trial using their middle and index finger of the left hand. Each run took about seven minutes and was followed by a short break to improve compliance and maintain attention of the children. The entire scanning session lasted for about 40 min.
2.5. fMRI data acquisition and preprocessing
Imaging was performed with a 3.0 T Siemens Magnetom Tim Trio scanner (Siemens, Erlangen, Germany) equipped with a 12-channel head coil at the Center for Cognitive Neuroscience Berlin (CCNB). In each of the four runs, 233 whole-brain functional T2*weighted echoplanar (EPI) pulse sequences (TE: 30 ms, TR: 2000 ms, 70° Flip Angle, 37 slices, matrix: 64 × 64, field of view (FOV): 192 mm; 3 × 3 × 3 mm3 voxel size, 50% interslice gap) were acquired resulting in a total of 932 axial volumes. Additionally, a T1-weighted matched-bandwidth high-resolution anatomical scan with same slice prescription as EPI was acquired (176 sagittal sections, 2 × 2 × 2 mm3 voxel size, matrix: 256 × 256).
MRI data were preprocessed and analyzed using the SPM12 software package (Wellcome Department of Imaging Neuroscience, University College London, UK, 2014). Brain regions are reported according to the Montreal Neurological Institute (MNI) space brain atlas provided by the Anatomical Automatic Labeling toolbox (AAL; Tzourio-Mazoyer et al., 2002). Images were corrected for EPI distortion and slice acquisition time. To correct for head movements, functional images were spatially realigned to the mean volume by means of rigid body transformation. Resliced images were then coregistered to the structural T1 scan. T1-weighted images were segmented according to six tissue probability maps for native space components (gray matter, white matter, cerebrospinal fluid, skull, soft, and non-brain tissue). The nonlinear Fast Diffeomorphic Anatomical Image Registration Algorithm (DARTEL; Ashburner, 2007) was used to create a study-specific template. We subsequently estimated the transformation from this study-group specific template to MNI space. According to Kang et al. (2003), measurement error for children above the age of six years is negligible when using a standard adult stereotactic space. Functional images were finally resampled to a resolution of 3 × 3 × 3 mm3 and spatially smoothed with an 8 mm (FWHM) Gaussian kernel.
2.6. Statistical analyses
2.6.1. Analysis of in-scanner task performance
Mean response times (RT) and accuracy rates of the five decision tasks were analyzed using linear mixed-effects and logistic mixed-effects regression with task as fixed factor and subject and item as crossed random factors, respectively (Baayen et al., 2008, Barr, 2013). Analyses were run in R version 3.3.0 (R Core Team, 2017) using the lme4-package (Bates et al., 2014) and the car-package (Fox and Weisberg, 2011).
2.6.2. Whole-brain analysis
After preprocessing, data were analyzed time-locked to block onset in the context of the General Linear Model (GLM) as implemented in SPM12. Statistical parametric maps were generated for each subject using a linear combination of the functions derived by convolving the standard hemodynamic response function with the actual time series of the decision tasks. To control for confounds induced by movement artifacts or signal drift due to scanner properties, the six realignment parameters generated during the preprocessing pipeline were entered in the design matrix as regressors of no interest. We also modelled the cues as an additional regressor of no interest. This resulted in a GLM including twelve regressors per run for each subject. Individual contrast images were computed for each reading task (LETTER, ORTHO, PHONO, and SEMCAT) versus the visual control baseline (CTRL). Both correct and incorrect responses were included in the analysis. For group analysis, we used a flexible factorial design for random effects. For all contrasts, we used a threshold of p< 0.001, uncorrected for multiple comparison at voxel level, and a familywise-error corrected (FWE) threshold of p< 0.05 at peak level with an extent of 50 voxels (Genovese et al., 2002).
2.6.3. Region of interest analysis
Regions of interest (ROI) were defined on the basis of the meta-analysis by Martin et al. (2015). The meta-analysis encompassed 20 studies investigating reading-related neural activity in children 7–12 years old (total number of children: N = 395). Studies were included if they met the following criteria: healthy participants, reading-related tasks using visual stimuli, 3-D coordinates of single contrasts against a low-level baseline, reported in Talairach or MNI-space and alphabetic writing systems. To compute statistical parametric maps of effect sizes, Anisotropic Effect-Size Signed Differential Mapping was performed for each original study. In a second step, random effects GLM was used to combine the individual effect size maps. In a last step, the authors evaluated the robustness of the meta-analytic map using a whole-brain voxel-based jackknife sensitivity analysis. This procedure resulted in a reading-related activation map that included four main clusters: a bilateral cluster around the SMA (k = 3.426), one cluster in the left IFG (k = 2.809) with local maxima in the middle frontal gyrus, pars opercularis, pars triangularis and precentral gyrus (PRG). A further cluster was identified in the left vOT (k = 3.433) with peaks in ITG, MTG, and STG. The fourth cluster was located in the left PPC (k = 128) with a local maximum in the superior parietal lobe (SPL). These peaks were used in the present study as ROIs to investigate the activation patterns of the four reading-related decision tasks contrasted against CTRL. To account for multiple testing, the task-specific activation in the four ROIs, i.e. vOT, PPC, IFG, SMA, was thresholded with p< 0.001 uncorrected on voxel level and FWE corrected with p< 0.05 on peak level (k ≥ 15).
2.6.4. Task comparison: activation patterns across regions and tasks
To further examine the nature of the four subcomponents of reading, we extracted the beta values of the four ROIs for each task condition separately using the MarsBaR toolbox (MarsBaR 0.44; Brett et al., 2002). Beta values are reported in arbitrary units proportional to BOLD signal. Activations were first averaged across voxels and subsequently across subjects for each task and entered into a linear-mixed model with ROI, task and age as predictive factors. To control for in-scanner task performance (mean accuracy), we computed internally studentized residuals and used those for further analyses. Subjects were added to the model as random effect because of the repeated-measures design. The model was evaluated using the maximum likelihood method (“nlme”-package; Pinheiro, 2016) as implemented in R. Significance of the effects was tested using Type III Wald chi-square tests (“car”-package; Fox and Weisberg, 2011). Tukey corrected post-hoc tests (“multcomp”-package, Hothorn et al., 2014) were conducted to explore differences in studentized residuals of the beta values among ROIs and tasks. To explore interindividual differences in task-specific neural activity in the four ROIs, we computed pairwise correlations with age as well as out of scanner reading tests (studentized residuals). Significance testing was adjusted for multiple comparison using Holm-Bonferroni correction (“psych”-package”; Revelle, 2017).
3. Results
3.1. In-scanner task performance
Accuracy rates and mean RTs are summarized in Table 2 and detailed results of the linear-mixed models are provided in the Supplementary Material (Tables S1 and S2). Linear-mixed effects regression of mean RTs showed a significant main effect of task (χ2(4) = 448.6, p < 0.001). We conducted planned comparisons to further investigate RT patterns across tasks: Shortest RTs were observed for LETTER (b = −294.7, SE = 16.1, t = −18.3). Mean RTs for SEMCAT (b = −169.2, SE = 14.0, t = −12.1) were shorter than for ORTHO (b = −96.9, SE = 11.6, t = −8.34) and PHONO. In turn, longer RTs were obtained for PHONO (b = −77.2, SE = 11.8, t = −6.56) compared to ORTHO. According to Baayen et al. (2008), absolute t-values above 2 can be considered to be significant given the large size of the data set. The logistic mixed-effect model for accuracy yielded a main effect of task (χ2(4) = 140.5, p < 0.001). Planned comparisons showed highest accuracy rates for LETTER (b = 1.65, SE = 0.19, z = 8.83, p < 0.001). Children made fewer errors in SEMCAT (b = 1.27, SE = 0.13, z = 9.62, p < 0.001) than in both ORTHO (b = 0.43, SE = 0.10, z = 4.55, p < 0.001) and PHONO.
Table 2.
Accuracies (in percent) and mean response times (RTs; in ms) for all five decision tasks.
| Accuracies | (SD) | RTs | (SD) | |
|---|---|---|---|---|
| Visual control baseline | 95.58 | (21) | 735 | (208) |
| Letter identification | 93.32 | (25) | 935 | (252) |
| Orthographic decision | 75.95 | (43) | 1103 | (332) |
| Phonological decision | 73.57 | (44) | 1275 | (313) |
| Semantic categorization | 84.09 | (37) | 1048 | (299) |
Note. SD = standard deviation, RT = response time.
In summary, analyses of RTs and accuracy rates suggest that PHONO was the most demanding task, followed by ORTHO. Both tasks need a significant amount of decoding ability for successful task completion. Children responded faster and made fewer errors in SEMCAT than in ORTHO and PHONO suggesting that the lexico-semantic task was relatively easy. As expected, shortest RTs and lowest error rates were observed for LETTER, indicating that prelexical processing was already highly automatized in our sample of children. These differences in performance reflect the degree to which the subcomponents of reading vary in their computational complexity.
3.2. Whole-brain analysis of the subcomponents of reading
As can be seen in Figure 1, which plots BOLD responses in each stimulus condition against CTRL, all tasks activated the classical reading network comprised of dorsal, ventral, and frontal areas in a highly similar way. Even letter identification in consonant strings showed small clusters in temporal, parietal and frontal foci. As expected, all tasks showed activity in ventral and dorsal primary visual areas. A list of all activated brain regions and their coordinates is provided in the Appendix A (Table A1).
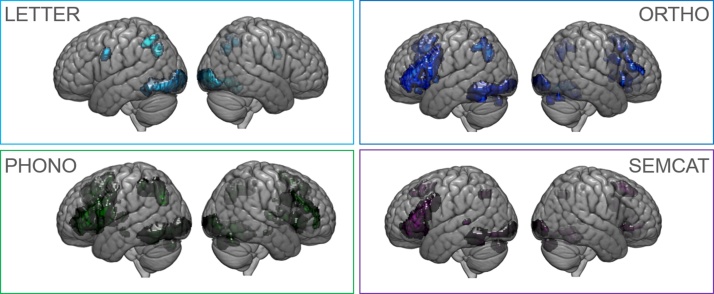
Fig. 1.
Whole brain activation maps of the four reading tasks in the left hemisphere are depicted. Letter identification (A: LETTER in light blue), orthographic decision (B: ORTHO in dark blue), phonological decision (C: PHONO in green), and semantic categorization (D: SEMCAT in purple). Thresholds: p < 0.05 FWE corrected on peak level, k ≥ 50 voxels. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.3. Region of interest analysis
For ROI analysis, we contrasted the activation elicited by the reading-related tasks against the CTRL task in the four core regions of the meta-analytic map of reading-related activation in children (Martin et al., 2015). The neural activation patterns associated with the four basic subcomponents of reading are displayed in Figure 2 and Table 3, Table 4, Table 5, Table 6.
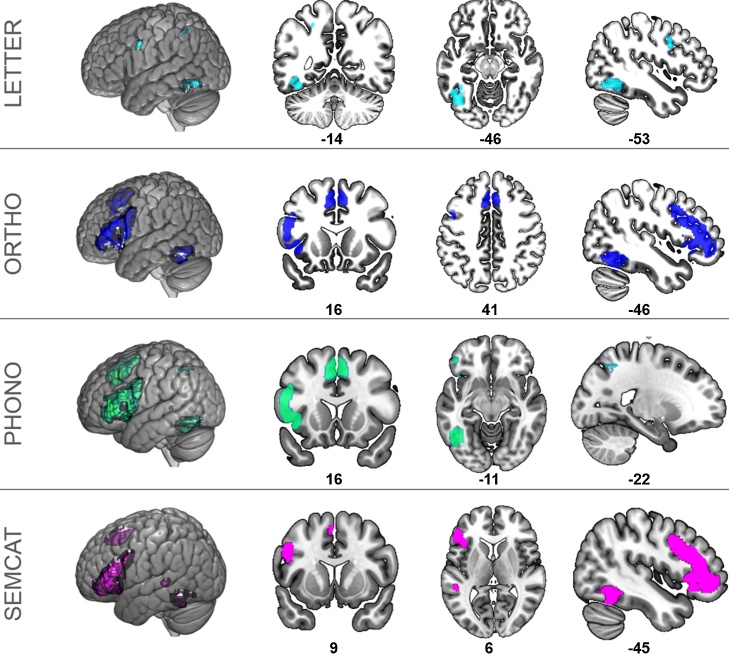
Fig. 2.
Surface rendering and selected slices (axial, coronal, sagittal) of neural activation for each reading task versus visual control baseline task for the four regions of interest (ROIs) based on the meta-analysis of reading in children (Martin et al., 2015) with a threshold of p < 0.05 FWE corrected on peak level, k ≥ 15 voxels. Contrast maps of the reading tasks are color coded. LETTER: letter identification (light blue); ORTHO: orthographic decision (dark blue); PHONO: phonological decision (green); SEMCAT: semantic categorization (purple). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Table 3.
Overview of neural activity associated with the letter identification versus visual control baseline task in the four regions of interest.
| Anatomical Location |
MNIa |
Size | Peak | ||
|---|---|---|---|---|---|
| x | y | z | k | T | |
| Occipito-Temporal (left hemisphere) | |||||
| Inferior Occipital Gyrus | −39 | −66 | −11 | 669 | 8.04 |
| Inferior Temporal Gyrus | −44 | −69 | −11 | 8.01 | |
| Fusiform Gyrus | −41 | −53 | −18 | 6.38 | |
| Fusiform Gyrus | −47 | −54 | −15 | 6.27 | |
| Inferior Temporal Gyrus | −45 | −53 | −11 | 5.87 | |
| Parietal (left hemisphere) | |||||
| Superior Parietal Lobe | −24 | −53 | 48 | 32 | 6.46 |
| Superior Parietal Lobe | −27 | −48 | 47 | 5.75 | |
| Superior Parietal Lobe | −24 | −59 | 50 | 5.46 | |
| Frontal (left hemisphere) | |||||
| Precentral Gyrus | −45 | 3 | 33 | 83 | 6.75 |
| Supplementary Motor Area (bilateral) | |||||
| – | |||||
Note. Neural activity masked by meta-analytic map of reading-related activation in children (Martin et al., 2015). Clusters are presented with a threshold of p < 0.001 uncorrected, p < 0.05 FWE corrected, and a cluster threshold of k > 15. Clusters in italic are local maxima in the superordinate cluster.
MNI coordinates of cluster center of mass.
Table 4.
Overview of neural activity associated with the orthographic decision task versus visual control baseline task in the four regions of interest.
| Anatomical Location |
MNIa |
Size | Peak | ||
|---|---|---|---|---|---|
| x | y | z | k | T | |
| Occipito-Temporal (left hemisphere) | |||||
| Inferior Temporal Gyrus | −48 | −54 | −15 | 1492 | 10.29 |
| Inferior Temporal Gyrus | −47 | −51 | −11 | 9.83 | |
| Inferior Temporal Gyrus | −47 | −62 | −11 | 9.27 | |
| Inferior Occipital Gyrus | −42 | −66 | −11 | 8.92 | |
| Parietal (left hemisphere) | |||||
| – | |||||
| Frontal (left hemisphere) | |||||
| Precentral Gyrus | −44 | 6 | 32 | 4432 | 12.31 |
| Insula | −36 | 21 | 2 | 10.41 | |
| Pars Triangularis | −45 | 27 | 20 | 9.70 | |
| Pars Triangularis | −47 | 32 | 12 | 9.39 | |
| Pars Opercularis | −50 | 9 | 14 | 8.89 | |
| Pars Triangularis | −48 | 45 | 0 | 7.55 | |
| Middle Frontal Gyrus | −50 | 23 | 27 | 7.38 | |
| Pars Triangularis | −47 | 44 | −5 | 7.33 | |
| Pars Triangularis | −41 | 41 | 2 | 7.02 | |
| Supplementary Motor Area (bilateral) | |||||
| LH SMA | −5 | 15 | 51 | 2445 | 9.83 |
| LH Superior Frontal Gyrus | −3 | 27 | 45 | 9.19 | |
| RH SMA | 8 | 17 | 50 | 7.31 | |
| LH Superior Frontal Gyrus | −6 | 30 | 32 | 6.43 | |
| RH Superior Frontal Gyrus | 9 | 26 | 36 | 5.66 | |
| RH Superior Frontal Gyrus | 6 | 29 | 33 | 5.49 | |
Note. Neural activity masked by meta-analytic map of reading-related activation in children (Martin et al., 2015). Clusters are presented with a threshold of p < 0.001 uncorrected, p < 0.05 FWE corrected, and a cluster threshold of k > 15. Clusters in italic are local maxima in the superordinate cluster.
MNI coordinates of cluster center of mass; SMA = Supplementary Motor Area; LH = left hemisphere; RH = right hemisphere.
Table 5.
Overview of neural activity associated with the phonological decision versus visual control baseline task in the four regions of interest.
| Anatomical Location |
MNIa |
Size | Peak | ||
|---|---|---|---|---|---|
| x | y | z | k | T | |
| Occipito-Temporal (left hemisphere) | |||||
| Inferior Temporal Gyrus | −47 | −54 | −15 | 1586 | 11.65 |
| Inferior Occipital Gyrus | −44 | −69 | −11 | 10.11 | |
| Fusiform Gyrus | −38 | −75 | −18 | 5.72 | |
| Middle Temporal Gyrus | −53 | −36 | 6 | 18 | 5.75 |
| Parietal (left hemisphere) | |||||
| Inferior Parietal Lobe | −27 | −48 | 45 | 78 | 8.36 |
| Superior Parietal Lobe | −24 | −53 | 48 | 8.13 | |
| Superior Parietal Lobe | −24 | −59 | 50 | 7.42 | |
| Superior Parietal Lobe | −26 | −66 | 51 | 6.55 | |
| Frontal (left hemisphere) | |||||
| Precentral Gyrus | −44 | 6 | 33 | 5248 | 14.06 |
| Insula | −36 | 21 | 0 | 13.03 | |
| Pars Triangularis | −47 | 27 | 20 | 11.58 | |
| Pars Opercularis | −51 | 12 | 12 | 11.17 | |
| Pars Triangularis | −48 | 32 | 12 | 10.54 | |
| Pars Triangularis | −48 | 35 | 5 | 9.25 | |
| Pars Triangularis | −48 | 44 | 0 | 9.20 | |
| Pars Orbitalis | −39 | 32 | −3 | 6.64 | |
| Supplementary Motor Area (bilateral) | |||||
| LH SMA | −5 | 17 | 51 | 3281 | 12.74 |
| LH Superior Frontal Gyrus | −3 | 27 | 47 | 10.37 | |
| LH Mid Cingulate Cortex | −6 | 29 | 32 | 7.67 | |
| LH SMA | −8 | 24 | 33 | 7.29 | |
| RH Superior Frontal Gyrus | 6 | 30 | 32 | 6.65 | |
Note. Neural activity masked by meta-analytic map of reading-related activation in children (Martin et al., 2015). Clusters are presented with a threshold of p < 0.001 uncorrected, p < 0.05 FWE corrected, and a cluster threshold of k > 15. Clusters in italic are local maxima in the superordinate cluster.
MNI coordinates of cluster center of mass; SMA = Supplementary Motor Area; LH = left hemisphere; RH = right hemisphere.
Table 6.
Overview of neural activity associated with semantic categorization versus visual control baseline task in the four regions of interest.
| Anatomical Location |
MNIa |
Size | Peak | ||
|---|---|---|---|---|---|
| x | y | z | k | T | |
| Occipito-Temporal (left hemisphere) | |||||
| Fusiform Gyrus | −42 | −53 | −18 | 961 | 8.48 |
| Inferior Temporal Gyrus | −47 | −50 | −11 | 8.29 | |
| Inferior Temporal Gyrus | −39 | −47 | −14 | 6.57 | |
| Inferior Temporal Gyrus | −39 | −66 | −11 | 6.05 | |
| Inferior Occipital Gyrus | −41 | −72 | −12 | 6.76 | |
| Middle Temporal Gyrus | −51 | −38 | 6 | 77 | 6.63 |
| Parietal (left hemisphere) | |||||
| – | |||||
| Frontal (left hemisphere) | |||||
| Precentral Gyrus | −44 | 8 | 30 | 3851 | 10.27 |
| Pars Triangularis | −51 | 30 | 18 | 9.79 | |
| Middle Frontal Gyrus | −47 | 27 | 20 | 9.73 | |
| Pars Triangularis | −50 | 32 | 14 | 9.59 | |
| Pars Triangularis | −51 | 23 | 29 | 9.46 | |
| Pars Opercularis | −44 | 23 | 29 | 9.38 | |
| Middle Frontal Gyrus | −53 | 18 | 29 | 8.98 | |
| Pars Triangularis | −45 | 45 | −3 | 8.47 | |
| Pars Orbitalis | −44 | 24 | −3 | 8.25 | |
| Pars Opercularis | −51 | 15 | 14 | 6.00 | |
| Supplementary Motor Area (bilateral) | |||||
| LH SMA | −5 | 20 | 53 | 1025 | 12.74 |
Note. Neural activity masked by meta-analytic map of reading-related activation in children (Martin et al., 2015). Clusters are presented with a threshold of p < 0.001 uncorrected, p < 0.05 FWE corrected, and a cluster threshold of k > 15. Clusters in italic are local maxima in the superordinate cluster.
MNI coordinates of cluster center of mass; SMA = Supplementary Motor Area; LH = left hemisphere.
LETTER showed a large cluster in the vOT with local maxima in inferior occipital gyrus (IOG), fusiform gyrus (FG), and ITG. A small cluster was identified in the SPL, and PRG as part of the IFG.
ORTHO was associated with significant neural activity in all clusters of the meta-analytic child reading map except for the PPC. Peak activity in the vOT (including ITG and FG) was located anterior to LETTER. We further identified a large cluster in the IFG and bilateral SMA.
PHONO showed significant activation in the vOT including IOG, ITG and FG as well as an additional peak in MTG. PHONO was further associated with activity in PPC, IFG, and bilateral SMA.
SEMCAT yielded neural activity in the vOT (including MTG), IFG, and bilateral SMA.
3.4. Task comparison: activation patterns in the core reading system of children
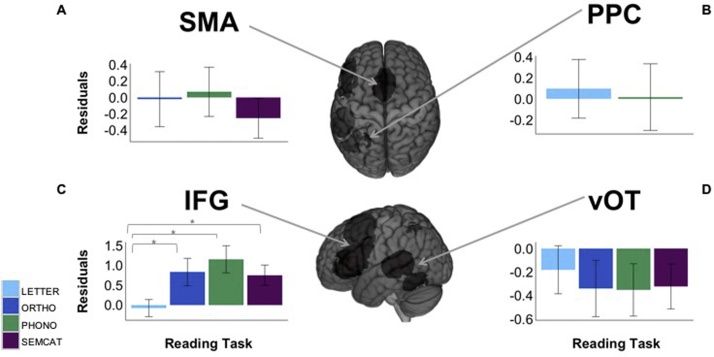
To analyze differences among neural signatures of the reading network in children, we computed a linear-mixed model with ROI and task as within-subject predictor variables to investigate their influence on BOLD signal (studentized residuals of beta values controlled for task performance). Results showed a significant main effect of ROI (χ2(3) = 16.78, p < 0.001). Pairwise comparisons (Tukey-corrected post-hoc t-tests) revealed significantly lower beta values in vOT, PPC and bilateral SMA compared to IFG (all p’s < 0.001). We also found a significant main effect of task (χ2 (3) = 10.90). Post-hoc comparisons showed that this effect was driven by PHONO leading to significant more activity than LETTER (b = 0.36, t = 3.29, p < 0.01) and SEMCAT (b = 0.37, t = 3.37, p < 0.01). Likewise, we observed a significant interaction of ROI and task (χ2(9) = 142.3, p < 0.001). The activation patterns of the different conditions across tasks are presented in Figure 3. In vOT, all four reading tasks led to similar neural activity (all p’s > 0.05) compared to CTRL. In PPC, only LETTER and PHONO obtained significant neural activity compared to CTRL, but no difference was found between these two tasks (both p’s > 0.05). In IFG, all four tasks were associated with significant neural activity as compared to CTRL. Yet, LETTER showed less activity in IFG than ORTHO, PHONO, and SEMCAT (all p’s < 0.05), which did not differ from each other (all p’s > 0.05). The bilateral SMA was associated with significant neural activity for ORTHO, PHONO, and SEMCAT. All three tasks yielded similar neural activity (all p’s > 0.05; for detailed analyses and results see Supplementary Material, Table S3). When controlling for multiple comparison we did not obtain significant correlations between task-specific BOLD signal in the four ROIs and age or out-of- scanner reading tests (all p’s > 0.05; cf. Supplementary Material, Fig. S1). These results indicate that processing of all four central subcomponents is already quite automatized and stable in the core reading system (as represented by the ROIs) of normally developing children aging 9–13 years. Consequently, we did not observe any developmental effects nor interindividual differences with respect to general performance in the given reading tasks.
Fig. 3.
A–D Bar plots of mean BOLD response (studentized residuals of beta values in arbitrary units; y-axis) for each reading task versus visual control baseline task (x-axis) for each of the four core regions of the meta-analytic contrast map of reading-related activity in children (Martin et al., 2015). Bar plots of the reading tasks are color coded. LETTER: letter identification (light blue); ORTHO: orthographic decision (dark blue); PHONO: phonological decision (green); SEMCAT: semantic categorization (purple). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
4. Discussion
In the present study, we investigated neural response patterns of all four central subcomponents of reading, i.e. prelexical, orthographic, phonological, and lexico-semantic processing in a large sample of normally developing German-speaking children aged nine to 13 years. We systematically examined how the core regions of the widespread reading system of children (cf. Martin et al., 2015) are linked to each of the component processes and observed both significant overlap as well as partly distinct neural response patterns. In particular, prelexical processing was not entirely confined to the posterior vOT but was associated with additional activity in the SPL and even the frontal system (PRG). Consistent with our predictions, orthographic processing was associated with neural activity in the vOT, including the FG and ITG, accompanied by activity in the IFG. Notably, phonological processing was the only component process eliciting activity in all four core regions of the child reading system. Compared to orthographic processing, we observed additional activity in the MTG and PPC as well as more distributed activity in the IFG. Similar to phonological processing, the MTG was involved in semantic processing. The bilateral SMA showed pronounced involvement in all subcomponent tasks except for prelexical processing.
4.1. Occipito-temporal cortex
Reading-induced modifications in vOT responsiveness occur during the earliest phases of reading acquisition (Brem et al., 2010, Monzalvo et al., 2012). Neurons in the vOT are thought to be specifically tuned to prelexical encoding of script (Cohen et al., 2000, Cohen et al., 2002, Dehaene and Cohen, 2011, Dehaene et al., 2015). Yet, whether the so-called visual word form area (VWFA) is exclusively devoted to prelexical visual word processing is still a matter of debate (cf. Price and Devlin, 2003, Price and Devlin, 2011, Zhao et al., 2016). In their cohort of kindergarten children, Brem et al. (2010) found peak activation in the FG located posterior to the coordinates of the VWFA reported in adults (Binder et al., 2006, Cohen et al., 2002, Vinckier et al., 2007). In the present study, we identified peak activation in the vOT close to the standard coordinates of the VWFA (Binder et al., 2006; MNI coordinates −42 –57 −12) for all four subprocesses.
While the ITG has been generally ascribed to orthographic processing of letter strings (but see: Price and Devlin, 2003, Price and Devlin, 2011), the anterior parts might be involved in phonological analysis (Bitan et al., 2005, Newman and Joanisse, 2011), semantic operations (Cao et al., 2006, Booth et al., 2007, Richlan et al., 2009, Sandak et al., 2004) and multimodal integration (Cohen et al., 2004). Importantly, the MTG was recently identified as a key region for the access to stored lexico-semantic representations and whole-word phonology (Braun et al., 2015a, b). Our results suggest that these findings generalize to children, since the MTG was strongly and specifically involved in phonological and lexico-semantic processing. To sum up, we observed a posterior-to-anterior gradient of increasingly specific activity: While all subprocesses were implicated in the ITG and FG, only phonological and lexico-semantic processing yielded additional activity in the MTG.
Contrary to our hypothesis, none of the subcomponent tasks was associated with activity in the STG. This may seem surprising, since the STG has consistently been implicated in phonological processing in children (Jobard et al., 2003, Pugh et al., 2000). However, these results as well as those included in Martin et al.’s (2015) meta-analysis were largely based on studies using rhyme judgment, which are subject to orthographic strategies (e.g., Seidenberg and Tanenhaus, 1979). In contrast, in our phonological task, children had to compute phonological codes sublexically and match those with lexical phonology. These rather demanding operations may involve brain regions other than the STG.
Indeed, we identified the MTG, IPL and the IFG (including the PRG) to be linked to phonological decision. These regions have reliably been ascribed to phonological processing (Braun et al., 2009, Price, 2012). Within the IFG, we observed significant neural activity in the PRG. Interestingly, Bitan et al. (2007b) reported an age-related decrease in STG activation accompanied by an age-related increase in activation in the PRG. It seems, that children of the present study predominantly engaged regions dedicated to abstract phonological segmentation and covert articulation to solve the phonological task. This may point towards a diminished role of regions that are predominantly recruited during the initial steps of reading acquisition and are mainly attributed to sensory-phonology based reading strategies (Bitan et al., 2007a, Jobard et al., 2003).
4.2. Posterior parietal cortex
The PPC system can be divided into superior and inferior parts. The inferior part is thought to be involved in phonological processing and conversion from orthography to phonology, among others (e.g., Booth et al., 2002, Jobard et al., 2003, Price, 2012). The increasing importance of whole-word processing in the ventral system during reading acquisition is thought to go hand in hand with a decreasing role of phonology based processes (Grainger et al., 2012) associated with the dorsal system (Booth et al., 2002, Paulesu et al., 2014, van der Mark et al., 2011). In line with these model-based and neurofunctional findings, neural activity in the IPL was confined to phonological processing. To make a correct decision, children had to ignore irrelevant spelling and focus on the phonological structure. Likewise, previous research has identified the IPL to be sensitive to the conflict between phonological and orthographic information (Booth et al., 2002, Booth et al., 2004, Cao et al., 2006). Furthermore, the PPC is thought to correspond to a fronto-parietal control network (Bitan et al., 2006, Bressler et al., 2008, Corbetta et al., 2002). Thus, neural activity in both the PPC and IFG during phonological decision might reflect the mapping of orthographic onto phonological information and a subsequent spelling-check (Braun et al., 2015a, Braun et al., 2015b, Ziegler et al., 2001) to verify the accuracy of the decision.
For both, prelexical and phonological processing, we observed enhanced activity in the SPL that has been associated with auditory and visual attention (Bitan et al., 2005, Bitan et al., 2007a, Gottlieb, 2007), as well as goal-directed behavior (Cabeza et al., 2008, Corbetta and Shulman, 2002). To decide whether a letter string involves a predefined target letter, children have to shift their visual attention in a serial manner across the string. Likewise, serial grapheme-to-phoneme analysis is necessary to distinguish between pseudowords and pseudohomophones as required in the phonological decision task. Accordingly, these tasks place high demands on visuo-spatial processing, which might be associated with the observed SPL activation.
4.3. Inferior frontal gyrus
Anatomically and functionally distinguishable parts of the IFG have been associated with numerous linguistic processes including grapheme-to-phoneme conversion (Fiebach et al., 2002), explicit lexical search (Heim et al., 2005), phonological recoding (Taylor et al., 2013), semantic retrieval and integration (Hagoort, 2013) and orthographic choice (Montant et al., 2011). Besides, the IFG has also been linked to cognitive control, behavioral monitoring and top-down modulation (Bitan et al., 2006, Fiebach et al., 2007, Graves et al., 2010). In the present study, we observed strong involvement of the left IFG for orthographic, phonological, and lexico-semantic processing. Semantic retrieval is not necessarily required for solving the orthographic task, since word form information is sufficient to make a lexical decision on words versus pseudohomophones (Cohen et al., 2000, Cohen et al., 2002, Dehaene and Cohen, 2011, Grainger and Jacobs, 1996, Jacobs et al., 1998). Nonetheless, we observed neural activity in response to this task in the IFG. Thus, children might have accessed lexico-semantic information to select among competing items in the orthographic task to confirm their lexical decision (Braun et al., 2009, Grainger et al., 2012), which is in line with recent computational, behavioral and electrophysiological evidence for top-down connections from semantic to orthographic layers (Hofmann and Jacobs, 2014, Stuellein et al., 2016).
4.4. Supplementary motor area
We found that the bilateral SMA was strongly involved in orthographic, phonological, and lexico-semantic processing in children. The SMA has been implicated in articulatory recoding and speech motor control (Hertrich et al., 2016, Price, 2012) but has also been identified as an important region within the attentional-control network. If its primary role were the maintenance of attention, goal-directed behavior, and action monitoring (Bonini et al., 2014), our data would indeed suggest the need for enhanced action monitoring and error processing in reading-related tasks in children.
5. Conclusion
Our attempt to “neurofunctionally dissect” the widespread core reading system of children into its basic subcomponents showed both similarities and differences across the four component processes. The most striking finding is that the vOT was not only in charge of low-level orthographic processing but appeared to be involved in all four components (cf. Schurz et al., 2010). Similarly, the IFG was not only activated by lexico-semantic categorization (Glezer et al., 2016, Montant et al., 2011) and this was true when differences in within-scanner performance were taken into account to partial out potential differences in task demands. Thus, the present data support the idea of an interactive network account of visual word processing. Whether those regions addressing all subcomponents of reading (i.e., vOT, IFG) may serve as potential hubs within such an interactive network needs further investigation.
Conflict of interest
None.
Author contributions
EF, JZ, HH, AJ contributed to the conception and design of the study. JL analyzed the data with advice from EF, CM, and HH. JL, JZ, MB and AJ drafted the manuscript. All authors contributed to the final version of the paper.
Funding
This work was supported by the German Federal Ministry of Education and Research (Bundesministerium für Bildung und Forschung, BMBF) with grants FKZ 01GJ1010A and 01GJ1404.
Acknowledgments
We would like to thank Anna Martin, Fabio Richlan and their team for providing the child-reading contrast map of their meta-analysis for the present study. We would further like to thank all research assistants of the Department of Experimental and Neurocognitive Psychology at the Freie Universität Berlin and the members of the CCNB for their help. Parts of the data were previously analyzed within the scope of a bachelor thesis.
Footnotes
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.dcn.2017.07.002.
Contributor Information
Johanna Liebig, Email: johanna.liebig@fu-berlin.de.
Eva Froehlich, Email: eva.froehlich@fu-berlin.de.
Carmen Morawetz, Email: carmen.morawetz@fu-berlin.de.
Mario Braun, Email: Mario.Braun@sbg.ac.at.
Arthur M. Jacobs, Email: ajacobs@zedat.fu-berlin.de.
Hauke R. Heekeren, Email: hauke.heekeren@fu-berlin.de.
Johannes C. Ziegler, Email: Johannes.Ziegler@univ-amu.fr.
Appendix A
Table A1.
Summary of activations for letter identification (LETTER), orthographic decision (ORTHO), phonological decision (PHONO), and semantic categorization (SEMCAT) relative to baseline activation during visual control baseline task (CTRL).
| Contrast | Anatomical Location | MNIa |
Size |
Peak |
||||
|---|---|---|---|---|---|---|---|---|
| x | y | z | k | T | ||||
| LETTER | Occipital | R Inf. Occip. Gyrus | 26 | −96 | −4 | 1648 | 14.11 | |
| 36 | −90 | −6 | 11.05 | |||||
| R Inf. Occip. Gyrus | −28 | −94 | −8 | 3235 | 13.69 | |||
| −40 | −69 | −10 | 8.66 | |||||
| Temporal | L Fusiform Gyrus | −40 | −52 | −18 | 6.38 | |||
| L Mid. Occip. Gyrus | −22 | −64 | 36 | 808 | 6.96 | |||
| Parietal | L Sup. Parietal Lobe | −26 | −54 | 48 | 6.71 | |||
| Frontal | L Precentral | −42 | 2 | 32 | 438 | 7.47 | ||
| ORTHO | Occipital | L Inf. Occip. Gyrus | 27 | −94 | −4 | 1219 | 11.43 | |
| R Inf. Occip. Gyrus | −32 | −90 | −8 | 3640 | 10.46 | |||
| Temporal | L Inf. Temp. Gyrus | −48 | −54 | −15 | 10.29 | |||
| L Inf. Temp. Gyrus | −46 | −62 | −10 | 9.27 | ||||
| Parietal | L Sup. Parietal Lobe | −22 | −66 | 42 | 1033 | 7.68 | ||
| L Inf. Parietal Lobe | −26 | −54 | 46 | 6.79 | ||||
| L Sup. Parietal Lobe | −30 | −60 | 56 | 5.95 | ||||
| Frontal | L Precentral | −39 | 4 | 30 | 8441 | 13.5 | ||
| L Insula | −32 | 21 | 3 | 11.39 | ||||
| L Pars Triangularis | −45 | 27 | 20 | 9.7 | ||||
| R Pars Triangularis | 46 | 32 | 18 | 510 | 7.51 | |||
| R Mid. Front. Gyrus | 48 | 48 | 12 | 5.9 | ||||
| R Pars Triangularis | 52 | 30 | 27 | 5.28 | ||||
| L Ant. Cingulate | −4 | 6 | 27 | 67 | 6.88 | |||
| R Pars Opercularis | 44 | 10 | 27 | 360 | 6.66 | |||
| R Insula | 33 | 24 | −2 | 1126 | 10.14 | |||
| R Pars Opercularis | 46 | 16 | 10 | 5.19 | ||||
| L Suppl. Motor Area | −4 | 15 | 51 | 2877 | 9.83 | |||
| L Sup. Front. Gyrus | −3 | 27 | 45 | 9.19 | ||||
| R Suppl. Motor Area | 8 | 16 | 50 | 7.31 | ||||
| Subcortical | R Cerebelum | 9 | −81 | −26 | 360 | 7.84 | ||
| L Cerebelum | −6 | −81 | −26 | 115 | 6.42 | |||
| R Cerebelum | 33 | −68 | −26 | 220 | 6.39 | |||
| L Pallidum | −18 | 4 | 2 | 89 | 6.29 | |||
| PHONO | Occipital | R Inf. Occip. Gyrus | 27 | −94 | −3 | 1397 | 12.96 | |
| L Inf. Occip. Gyrus | −33 | −90 | −8 | 4614 | 12.32 | |||
| Temporal | L Inf. Temp. Gyrus | −46 | −54 | −15 | 11.65 | |||
| L Fusiform Gyrus | −42 | −70 | −10 | 10.6 | ||||
| Occipital | L Sup. Occip. Cortex | −22 | −68 | 36 | 2869 | 8.84 | ||
| Parietal | L Inf. Parietal Lobe | −26 | −52 | 45 | 8.8 | |||
| L Sup. Parietal Lobe | −27 | −58 | 51 | 8.09 | ||||
| Frontal | L Precentral | −39 | 4 | 30 | 11088 | 15.64 | ||
| L Insula | −30 | 21 | 0 | 14.84 | ||||
| L Pars Triangularis | −46 | 27 | 20 | 11.58 | ||||
| L Suppl. Motor Area | −4 | 16 | 51 | 4174 | 12.74 | |||
| L Mid. Sup. Frontal | −3 | 27 | 46 | 10.37 | ||||
| L Ant. Cingulate | −8 | 28 | 30 | 8.33 | ||||
| R Insula | 33 | 24 | −2 | 1823 | 12.27 | |||
| R Pars Opercularis | 46 | 16 | 9 | 5.76 | ||||
| R Mid. Frontal | 46 | 32 | 20 | 2201 | 8.66 | |||
| R Pars Opercularis | 42 | 9 | 28 | 8.57 | ||||
| R Pars Triangularis | 56 | 21 | 28 | 7.45 | ||||
| L Ant. Cingulate | −4 | 6 | 27 | 75 | 7.41 | |||
| R Ant. Cingulate | 8 | 3 | 28 | 70 | 7.07 | |||
| R Mid. Cingulate | 8 | −9 | 30 | 5.53 | ||||
| Subcortical | R Cerebelum | 9 | −80 | −26 | 933 | 10.39 | ||
| L Cerebelum | −8 | −81 | −27 | 7.72 | ||||
| R Cerebelum | 34 | −68 | −26 | 756 | 7.81 | |||
| L Thalamus | −8 | −15 | 10 | 364 | 7.53 | |||
| L Pallidum | −15 | 6 | 3 | 769 | 7.34 | |||
| L Caudate | −14 | −2 | 20 | 7.06 | ||||
| L Hippocampus | −9 | −14 | −10 | 123 | 6.05 | |||
| R Pallidum | 12 | 2 | −2 | 56 | 5.89 | |||
| R Thalamus | 6 | −9 | 3 | 5.55 | ||||
| SEMCAT | Occipital | R Inf. Occip. Gyrus | 26 | −96 | −4 | 946 | 11.18 | |
| R Inf. Occip. Gyrus | 38 | −92 | −6 | 7.68 | ||||
| L Mid. Occip. Gyrus | −27 | −98 | −8 | 2345 | 10.23 | |||
| L Inf. Occip. Gyrus | −33 | −90 | −8 | 9.5 | ||||
| Temporal | L Fusiform Gyrus | −42 | −52 | −18 | 8.48 | |||
| L Mid. Temp. Gyrus | −51 | −38 | 6 | 82 | 6.63 | |||
| Parietal | L Inf. Parietal Lobe | −30 | −62 | 42 | 255 | 6.23 | ||
| Frontal | L Precentral | −39 | 6 | 30 | 6695 | 11.2 | ||
| L Pars Triangularis | −51 | 30 | 18 | 9.79 | ||||
| L Pars Triangularis | −51 | 22 | 28 | 9.46 | ||||
| L Suppl. Motor Area | −4 | 20 | 52 | 1045 | 8.81 | |||
| R Insula | 33 | 22 | −4 | 310 | 7.41 | |||
| R Pars Orbitalis | 36 | 32 | −6 | 5.99 | ||||
| Subcortical | R Cerebelum | 9 | −82 | −26 | 647 | 9.77 | ||
| L Cerebelum | −8 | −81 | −27 | 69 | 6.22 | |||
Note. Clusters are presented with a threshold of p < 0.001 uncorrected, p < 0.05 FWE corrected on peak level, and a cluster threshold of k ≥ 50. Clusters in italic are local maxima in the superordinate global maximum.
MNI coordinates of cluster center of mass.
Appendix B. Supplementary data
The following is Supplementary data to this article:
References
- Addis D.R. 2004. Analysis of Functional Magnetic Resonance Imaging Data Using SPM2: Preprocessing.http://www.memorylab.org/Files/SPM2_PreProc.pdf Retrieved from. [Google Scholar]
- Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage. 2007;38(1):95–113. doi: 10.1016/j.neuroimage.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Auer M., Gruber G., Mayringer H., Wimmer H. Verlag Hans Huber; Bern: 2005. SLS 5–8. Salzburger Lese-Screening für die Klassenstufen; pp. 5–8. [Google Scholar]
- Baayen R.H., Davidson D.J., Bates D.M. Mixed-effects modeling with crossed random effects for subjects and items. J. Mem. Lang. 2008;59(4):390–412. [Google Scholar]
- Bach S., Brandeis D., Hofstetter C., Martin E., Richardson U., Brem S. Early emergence of deviant frontal fMRI activity for phonological processes in poor beginning readers. Neuroimage. 2010;53(2):682–693. doi: 10.1016/j.neuroimage.2010.06.039. [DOI] [PubMed] [Google Scholar]
- Backes W., Vuurman E., Wennekes R., Spronk P., Wuisman M., van Engelshoven J., Jolles J. Atypical brain activation of reading processes in children with developmental dyslexia. J. Child Neurol. 2002;17(12):867–871. doi: 10.1177/08830738020170121601. [DOI] [PubMed] [Google Scholar]
- Barr D.J. Random effects structure for testing interactions in linear mixed-effects models. Front. Psychol. 2013;4:328. doi: 10.3389/fpsyg.2013.00328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder J.R., Medler D.A., Westbury C.F., Liebenthal E., Buchanan L. Tuning of the human left fusiform gyrus to sublexical orthographic structure. Neuroimage. 2006;33(2):739–748. doi: 10.1016/j.neuroimage.2006.06.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder J.R., Desai R.H., Graves W.W., Conant L.L. Where is the semantic system? A critical review and meta-analysis of 120 functional neuroimaging studies. Cereb. Cortex. 2009;19(12):2767–2796. doi: 10.1093/cercor/bhp055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitan T., Booth J.R., Choy J., Burman D.D., Gitelman D.R., Mesulam M.M. Shifts of effective connectivity within a language network during rhyming and spelling. J. Neurosci. 2005;25(22):5397–5403. doi: 10.1523/JNEUROSCI.0864-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitan T., Burman D.D., Lu D., Cone N.E., Gitelman D.R., Mesulam M.M., Booth J.R. Weaker top–down modulation from the left inferior frontal gyrus in children. Neuroimage. 2006;33(3):991–998. doi: 10.1016/j.neuroimage.2006.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitan T., Burman D.D., Chou T.L., Lu D., Cone N.E., Cao F., Booth J.R. The interaction between orthographic and phonological information in children: an fMRI study. Hum. Brain Mapp. 2007;28(9):880–891. doi: 10.1002/hbm.20313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitan T., Cheon J., Lu D., Burman D.D., Gitelman D.R., Mesulam M.M., Booth J.R. Developmental changes in activation and effective connectivity in phonological processing. Neuroimage. 2007;38(3):564–575. doi: 10.1016/j.neuroimage.2007.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonini F., Burle B., Liégeois-Chauvel C., Régis J., Chauvel P., Vidal F. Action monitoring and medial frontal cortex: leading role of supplementary motor area. Science. 2014;343(6173):888–891. doi: 10.1126/science.1247412. [DOI] [PubMed] [Google Scholar]
- Booth J.R., Burman D.D., Meyer J.R., Gitelman D.R., Parrish T.B., Mesulam M.M. Functional anatomy of intra-and cross-modal lexical tasks. Neuroimage. 2002;16(1):7–22. doi: 10.1006/nimg.2002.1081. [DOI] [PubMed] [Google Scholar]
- Booth J.R., Burman D.D., Meyer J.R., Gitelman D.R., Parrish T.B., Mesulam M.M. Development of brain mechanisms for processing orthographic and phonologic representations. J. Cogn. Neurosci. 2004;16(7):1234–1249. doi: 10.1162/0898929041920496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth J.R., Cho S., Burman D.D., Bitan T. Neural correlates of mapping from phonology to orthography in children performing an auditory spelling task. Dev. Sci. 2007;10(4):441–451. doi: 10.1111/j.1467-7687.2007.00598.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boros M., Anton J.L., Pech-Georgel C., Grainger J., Szwed M., Ziegler J.C. Orthographic processing deficits in developmental dyslexia: beyond the ventral visual stream. Neuroimage. 2016;128:316–327. doi: 10.1016/j.neuroimage.2016.01.014. [DOI] [PubMed] [Google Scholar]
- Braun M., Hutzler F., Ziegler J.C., Dambacher M., Jacobs A.M. Pseudohomophone effects provide evidence of early lexico-phonological processing in visual word recognition. Hum. Brain Mapp. 2009;30(7):1977–1989. doi: 10.1002/hbm.20643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun M., Hutzler F., Münte T.F., Rotte M., Dambacher M., Richlan F., Jacobs A.M. The neural bases of the pseudohomophone effect: phonological constraints on lexico-semantic access in reading. Neuroscience. 2015;295:151–163. doi: 10.1016/j.neuroscience.2015.03.035. [DOI] [PubMed] [Google Scholar]
- Braun M., Jacobs A.M., Richlan F., Hawelka S., Hutzler F., Kronbichler M. Many neighbors are not silent. fMRI evidence for global lexical activity in visual word recognition. Front. Hum. Neurosci. 2015:9. doi: 10.3389/fnhum.2015.00423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brem S., Bach S., Kucian K., Kujala J.V., Guttorm T.K., Martin E., Lyytinen H., Brandeis D., Richardson U. Brain sensitivity to print emerges when children learn letter-speech sound correspondences. Proc. Natl. Acad. Sci. 2010;107(17):7939–7944. doi: 10.1073/pnas.0904402107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bressler S.L., Tang W., Sylvester C.M., Shulman G.L., Corbetta M. Top-down control of human visual cortex by frontal and parietal cortex in anticipatory visual spatial attention. J. Neurosci. 2008;28(40):10056–10061. doi: 10.1523/JNEUROSCI.1776-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brett M., Anton J.L., Valabregue R., Poline J.B. MarsBaR: region of interest analysis using an SPM toolbox. Human Brain Mapping Annual Meeting. 2002 [Google Scholar]
- Cabeza R., Ciaramelli E., Olson I.R., Moscovitch M. The parietal cortex and episodic memory: an attentional account. Nat. Rev. Neurosci. 2008;9(8):613–625. doi: 10.1038/nrn2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao F., Bitan T., Chou T.L., Burman D.D., Booth J.R. Deficient orthographic and phonological representations in children with dyslexia revealed by brain activation patterns. J. Child Psychol. Psychiatry. 2006;47(10):1041–1050. doi: 10.1111/j.1469-7610.2006.01684.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carreiras M., Armstrong B.C., Perea M., Frost R. The what, when, where, and how of visual word recognition. Trends Cogn. Sci. 2014;18(2):90–98. doi: 10.1016/j.tics.2013.11.005. [DOI] [PubMed] [Google Scholar]
- Cohen L., Dehaene S., Naccache L., Lehéricy S., Dehaene-Lambertz G., Hénaff M.A., Michel F. The visual word form area. Brain. 2000;123(2):291–307. doi: 10.1093/brain/123.2.291. [DOI] [PubMed] [Google Scholar]
- Cohen L., Lehéricy S., Chochon F., Lemer C., Rivaud S., Dehaene S. Language-specific tuning of visual cortex? Functional properties of the Visual Word Form Area. Brain. 2002;125(5):1054–1069. doi: 10.1093/brain/awf094. [DOI] [PubMed] [Google Scholar]
- Cohen L., Jobert A., Le Bihan D., Dehaene S. Distinct unimodal and multimodal regions for word processing in the left temporal cortex. Neuroimage. 2004;23(4):1256–1270. doi: 10.1016/j.neuroimage.2004.07.052. [DOI] [PubMed] [Google Scholar]
- Coltheart M., Davelaar E., Jonasson T., Besner D. Access to the internal lexicon. In: Dornic S., editor. Attention and Performance VI: Proceedings of the Sixth International Symposium on Attention and Performance. Erlbaum; Hillsdale, NJ: 1977. pp. 535–555. [Google Scholar]
- Corbetta M., Shulman G.L. Control of goal-directed and stimulus-driven attention in the brain. Nat. Rev. Neurosci. 2002;3(3):201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Corbetta M., Kincade J.M., Shulman G.L. Neural systems for visual orienting and their relationships to spatial working memory. J. Cogn. Neurosci. 2002;14(3):508–523. doi: 10.1162/089892902317362029. [DOI] [PubMed] [Google Scholar]
- Dehaene S., Cohen L. The unique role of the visual word form area in reading. Trends Cogn. Sci. 2011;15(6):254–262. doi: 10.1016/j.tics.2011.04.003. [DOI] [PubMed] [Google Scholar]
- Dehaene S., Cohen L., Morais J., Kolinsky R. Illiterate to literate: behavioural and cerebral changes induced by reading acquisition. Nat. Rev. Neurosci. 2015;16(4):234–244. doi: 10.1038/nrn3924. [DOI] [PubMed] [Google Scholar]
- Fair D.A., Cohen A.L., Dosenbach N.U., Church J.A., Miezin F.M., Barch D.M., Schlaggar B.L. The maturing architecture of the brain's default network. Proc. Natl. Acad. Sci. 2008;105(10):4028–4032. doi: 10.1073/pnas.0800376105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiebach C.J., Friederici A.D., Müller K., Von Cramon D.Y. fMRI evidence for dual routes to the mental lexicon in visual word recognition. J. Cogn. Neurosci. 2002;14(1):11–23. doi: 10.1162/089892902317205285. [DOI] [PubMed] [Google Scholar]
- Fiebach C.J., Ricker B., Friederici A.D., Jacobs A.M. Inhibition and facilitation in visual word recognition: prefrontal contribution to the orthographic neighborhood size effect. Neuroimage. 2007;36(3):901–911. doi: 10.1016/j.neuroimage.2007.04.004. [DOI] [PubMed] [Google Scholar]
- Froehlich E., Liebig J., Ziegler J.C., Braun M., Lindenberger U., Heekeren H.R., Jacobs A.M. Drifting through basic subprocesses of reading: a hierarchical diffusion model analysis of age effects on visual word recognition. Front. Psychol. 2016:7. doi: 10.3389/fpsyg.2016.01863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genovese C.R., Lazar N.A., Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage. 2002;15(4):870–878. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- Glezer L.S., Eden G., Jiang X., Luetje M., Napoliello E., Kim J., Riesenhuber M. Uncovering phonological and orthographic selectivity across the reading network using fMRI-RA. Neuroimage. 2016;138:248–256. doi: 10.1016/j.neuroimage.2016.05.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goswami U., Ziegler J.C., Dalton L., Schneider W. Pseudohomophone effects and phonological recoding procedures in reading development in English and German. J. Mem. Lang. 2001;45(4):648–664. [Google Scholar]
- Gottlieb J. From thought to action: the parietal cortex as a bridge between perception, action, and cognition. Neuron. 2007;53(1):9–16. doi: 10.1016/j.neuron.2006.12.009. [DOI] [PubMed] [Google Scholar]
- Grainger J., Jacobs A.M. Orthographic processing in visual word recognition: a multiple read-out model. Psychol. Rev. 1996;103(3):518. doi: 10.1037/0033-295x.103.3.518. [DOI] [PubMed] [Google Scholar]
- Grainger J., Ziegler J. A dual-route approach to orthographic processing. Front. Psychol. 2011;2(54) doi: 10.3389/fpsyg.2011.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grainger J., Lété B., Bertand D., Dufau S., Ziegler J.C. Evidence for multiple routes in learning to read. Cognition. 2012;123(2):280–292. doi: 10.1016/j.cognition.2012.01.003. [DOI] [PubMed] [Google Scholar]
- Graves W.W., Desai R., Humphries C., Seidenberg M.S., Binder J.R. Neural systems for reading aloud: a multiparametric approach. Cereb. Cortex. 2010;20(8):1799–1815. doi: 10.1093/cercor/bhp245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagoort P. MUC (memory, unification, control) and beyond. Front. Psychol. 2013;4(416) doi: 10.3389/fpsyg.2013.00416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim S., Alter K., Ischebeck A.K., Amunts K., Eickhoff S.B., Mohlberg H., Zilles K., von Cramon D.Y., Friederici A.D. The role of the left Brodmann's areas 44 and 45 in reading words and pseudowords. Cogn. Brain Res. 2005;25(3):982–993. doi: 10.1016/j.cogbrainres.2005.09.022. [DOI] [PubMed] [Google Scholar]
- Heister J., Würzner K.M., Bubenzer J., Pohl E., Hanneforth T., Geyken A., Kliegl R. dlexDB-eine lexikalische Datenbank für die psychologische und linguistische Forschung. Psychologische Rundschau. 2011;62:10–20. [Google Scholar]
- Hertrich I., Dietrich S., Ackermann H. The role of the supplementary motor area for speech and language processing. Neurosci. Biobehav. Rev. 2016;68:602–610. doi: 10.1016/j.neubiorev.2016.06.030. [DOI] [PubMed] [Google Scholar]
- Hofmann M.J., Jacobs A.M. Interactive activation and competition models and semantic context: from behavioral to brain data. Neurosci. Biobehav. Rev. 2014;46:85–104. doi: 10.1016/j.neubiorev.2014.06.011. [DOI] [PubMed] [Google Scholar]
- Houdé O., Rossi S., Lubin A., Joliot M. Mapping numerical processing, reading, and executive functions in the developing brain: an fMRI meta-analysis of 52 studies including 842 children. Dev. Sci. 2010;13(6):876–885. doi: 10.1111/j.1467-7687.2009.00938.x. [DOI] [PubMed] [Google Scholar]
- Jacobs A.M., Ziegler J.C. Neurocognitive psychology of visual word recognition. In: Wright J.D., editor. second ed. vol 25. Elsevier; Oxford: 2015. pp. 214–219. (International Encyclopedia of the Social & Behavioral Sciences). [Google Scholar]
- Jacobs A.M., Rey A., Ziegler J.C., Grainger J. MROM-p: an interactive activation, multiple readout model of orthographic and phonological processes in visual word recognition. In: Grainger J., Jacobs A.M., editors. Localist Connectionist Approaches to Human Cognition. Erlbaum; Mahwah, NJ: 1998. pp. 147–187. [Google Scholar]
- Jacobs A.M. Neurocognitive poetics: methods and models for investigating the neuronal and cognitive-affective bases of literature reception. Front. Hum. Neurosci. 2015;9:186. doi: 10.3389/fnhum.2015.00186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jobard G., Crivello F., Tzourio-Mazoyer N. Evaluation of the dual route theory of reading: a metanalysis of 35 neuroimaging studies. Neuroimage. 2003;20(2):693–712. doi: 10.1016/S1053-8119(03)00343-4. [DOI] [PubMed] [Google Scholar]
- Kang H.C., Burgund E.D., Lugar H.M., Petersen S.E., Schlaggar B.L. Comparison of functional activation foci in children and adults using a common stereotactic space. Neuroimage. 2003;19(1):16–28. doi: 10.1016/s1053-8119(03)00038-7. [DOI] [PubMed] [Google Scholar]
- Kronbichler M., Hutzler F., Wimmer H., Mair A., Staffen W., Ladurner G. The visual word form area and the frequency with which words are encountered: evidence from a parametric fMRI study. Neuroimage. 2004;21(3):946–953. doi: 10.1016/j.neuroimage.2003.10.021. [DOI] [PubMed] [Google Scholar]
- Kuhlmann M., Hofmann M.J., Briesemeister B.B., Jacobs A.M. Mixing positive and negative valence: affective-semantic integration of bivalent words. Sci. Rep. 2016;6 doi: 10.1038/srep30718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin A., Schurz M., Kronbichler M., Richlan F. Reading in the brain of children and adults: a meta-analysis of 40 functional magnetic resonance imaging studies. Hum. Brain Mapp. 2015;36(5):1963–1981. doi: 10.1002/hbm.22749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moll K., Landerl K. Huber; Bern: 2010. SLRT-II: Lese-und Rechtschreibtest; Weiterentwicklung des Salzburger Lese-und Rechtschreibtests. [Google Scholar]
- Montant M., Schön D., Anton J.-L., Ziegler J.C. Orthographic contamination of Broca's area. Front. Psychol. 2011;2:378. doi: 10.3389/fpsyg.2011.00378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monzalvo K., Fluss J., Billard C., Dehaene S., Dehaene-Lambertz G. Cortical networks for vision and language in dyslexic and normal children of variable socio-economic status. Neuroimage. 2012;61(1):258–274. doi: 10.1016/j.neuroimage.2012.02.035. [DOI] [PubMed] [Google Scholar]
- Newman R.L., Joanisse M.F. Modulation of brain regions involved in word recognition by homophonous stimuli: an fMRI study. Brain Res. 2011;1367:250–264. doi: 10.1016/j.brainres.2010.09.089. [DOI] [PubMed] [Google Scholar]
- Noonan K.A., Jefferies E., Visser M., Ralph M.A.L. Going beyond inferior prefrontal involvement in semantic control: evidence for the additional contribution of dorsal angular gyrus and posterior middle temporal cortex. J. Cogn. Neurosci. 2013;25(11):1824–1850. doi: 10.1162/jocn_a_00442. [DOI] [PubMed] [Google Scholar]
- Paulesu E., Danelli L., Berlingeri M. Reading the dyslexic brain: multiple dysfunctional routes revealed by a new meta-analysis of PET and fMRI activation studies. Front. Hum. Neurosci. 2014;8(830) doi: 10.3389/fnhum.2014.00830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petermann F., Petermann U. Huber; Bern: 2007. Hambug-Wechsler-Intelligenztest für Kinder IV (HAWIK-IV) [Google Scholar]
- Pollack C., Luk G., Christodoulou J.A. A meta-analysis of functional reading systems in typically developing and struggling readers across different alphabetic languages. Front. Psychol. 2015;6(191) doi: 10.3389/fpsyg.2015.00191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price C.J., Devlin J.T. The myth of the visual word form area. Neuroimage. 2003;19(3):473–481. doi: 10.1016/s1053-8119(03)00084-3. [DOI] [PubMed] [Google Scholar]
- Price C.J., Devlin J.T. The Interactive Account of ventral occipitotemporal contributions to reading. Trends Cogn. Sci. 2011;15(6):246–253. doi: 10.1016/j.tics.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price C.J. A review and synthesis of the first 20years of PET and fMRI studies of heard speech, spoken language and reading. Neuroimage. 2012;62(2):816–847. doi: 10.1016/j.neuroimage.2012.04.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugh K.R., Mencl W.E., Jenner A.R., Katz L., Frost S.J., Lee J.R., Shaywitz S.E., Shaywitz B.A. Functional neuroimaging studies of reading and reading disability (developmental dyslexia) Ment. Retard. Dev. Disabil Res. Rev. 2000;6(3):207–213. doi: 10.1002/1098-2779(2000)6:3<207::AID-MRDD8>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Pugh K.R., Frost S.J., Sandak R., Landi N., Moore D., Della Porta G., Rueckl J.G., Mencl W.E. Mapping the word reading circuitry in skilled and disabled readers. In: Cornelissen P.L., Hansen P.C., Kringelbach M.L., Pugh K.R., editors. The Neural Basis of Reading. Oxford University Press; New York: 2010. pp. 281–305. [Google Scholar]
- Richlan F., Kronbichler M., Wimmer H. Functional abnormalities in the dyslexic brain: a quantitative meta-analysis of neuroimaging studies. Hum. Brain Mapp. 2009;30(10):3299–3308. doi: 10.1002/hbm.20752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team . R Foundation for Statistical Computing; Vienna, Austria: 2017. R: A Language and Environment for Statistical Computing. Retrieved from: https://www.R-project.org. [Google Scholar]
- Rowe J.B., Stephan K.E., Friston K., Frackowiak R.S., Passingham R.E. The prefrontal cortex shows context-specific changes in effective connectivity to motor or visual cortex during the selection of action or colour. Cereb. Cortex. 2005;15(1):85–95. doi: 10.1093/cercor/bhh111. [DOI] [PubMed] [Google Scholar]
- Sandak R., Mencl W.E., Frost S.J., Pugh K.R. The neurobiological basis of skilled and impaired reading: recent findings and new directions. Sci. Studi. Read. 2004;8(3):273–292. [Google Scholar]
- Schurz M., Sturm D., Richlan F., Kronbichler M., Ladurner G., Wimmer H. A dual-route perspective on brain activation in response to visual words: evidence for a length by lexicality interaction in the visual word form area (VWFA) Neuroimage. 2010;49(3):2649–2661. doi: 10.1016/j.neuroimage.2009.10.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seghier M.L., Lee H.L., Schofield T., Ellis C.L., Price C.J. Inter-subject variability in the use of two different neuronal networks for reading aloud familiar words. Neuroimage. 2008;42(3):1226–1236. doi: 10.1016/j.neuroimage.2008.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidenberg M.S., Tanenhaus M.K. Orthographic effects on rhyme monitoring. J. Exp. Psychol.: Hum. Learn. Mem. 1979;5(6):546–554. [Google Scholar]
- Shaywitz S.E., Shaywitz B.A., Pugh K.R., Fulbright R.K., Constable R.T., Mencl W.E., Katz L. Functional disruption in the organization of the brain for reading in dyslexia. Proc. Natl. Acad. Sci. 1998;95(5):2636–2641. doi: 10.1073/pnas.95.5.2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaywitz B.A., Shaywitz S.E., Pugh K.R., Mencl W.E., Fulbright R.K., Skudlarski P., Constable R.T., Marchione K.E., Fletcher J.M., Lyon G.R., Gore J.C. Disruption of posterior brain systems for reading in children with developmental dyslexia. Biol. Psychiatry. 2002;52(2):101–110. doi: 10.1016/s0006-3223(02)01365-3. [DOI] [PubMed] [Google Scholar]
- Spreng R.N., Stevens W.D., Chamberlain J.P., Gilmore A.W., Schacter D.L. Default network activity, coupled with the frontoparietal control network, supports goal-directed cognition. Neuroimage. 2010;53(1):303–317. doi: 10.1016/j.neuroimage.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuellein N., Radach R.R., Jacobs A.M., Hofmann M.J. No one way ticket from orthography to semantics in recognition memory: N400 and P200 effects of associations. Brain Res. 2016;1639:88–98. doi: 10.1016/j.brainres.2016.02.029. [DOI] [PubMed] [Google Scholar]
- Taylor J.S.H., Rastle K., Davis M.H. Can cognitive models explain brain activation during word and pseudoword reading? A meta-analysis of 36 neuroimaging studies. Psychol. Bull. 2013;139(4):766–791. doi: 10.1037/a0030266. [DOI] [PubMed] [Google Scholar]
- Turkeltaub P.E., Gareau L., Flowers D.L., Zeffiro T.A., Eden G.F. Development of neural mechanisms for reading. Nat. Neurosci. 2003;6(7):767–773. doi: 10.1038/nn1065. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N., Landeau B., Papathanassiou D., Crivello F., Etard O., Delcroix N., Mazoyer B., Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15(1):273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- van der Mark S., Bucher K., Maurer U., Schulz E., Brem S., Buckelmüller, Kronbichler M., Loenneker T., Klaver P., Matrin E., Brandeis D. Children with dyslexia lack multiple specializations along the visual word-form (VWF) system. Neuroimage. 2009;47(4):1940–1949. doi: 10.1016/j.neuroimage.2009.05.021. [DOI] [PubMed] [Google Scholar]
- van der Mark S., Klaver P., Bucher K., Maurer U., Schulz E., Brem S., Martin E., Brandeis D. The left occipitotemporal system in reading: disruption of focal fMRI connectivity to left inferior frontal and inferior parietal language areas in children with dyslexia. Neuroimage. 2011;54(3):2426–2436. doi: 10.1016/j.neuroimage.2010.10.002. [DOI] [PubMed] [Google Scholar]
- Vinckier F., Dehaene S., Jobert A., Dubus J.P., Sigman M., Cohen L. Hierarchical coding of letter strings in the ventral stream: dissecting the inner organization of the visual word-form system. Neuron. 2007;55(1):143–156. doi: 10.1016/j.neuron.2007.05.031. [DOI] [PubMed] [Google Scholar]
- Weiß R.H. Hogrefe; Göttingen: 2008. CFT 20-R mit WS/ZF-R: Grundintelligenztest Skala 2–Revision (CFT 20-R) mit Wortschatztest und Zahlenfolgetest – Revision. [Google Scholar]
- Welcome S.E., Joanisse M.F. Individual differences in skilled adult readers reveal dissociable patterns of neural activity associated with component processes of reading. Brain Lang. 2012;120(3):360–371. doi: 10.1016/j.bandl.2011.12.011. [DOI] [PubMed] [Google Scholar]
- Zhao L., Chen C., Shao L., Wang Y., Xiao X., Chen C., Yang J., Zevin J., Xue G. Orthographic and phonological representations in the fusiform cortex. Cereb. Cortex. 2016 doi: 10.1093/cercor/bhw300. [DOI] [PubMed] [Google Scholar]
- Ziegler J.C., Perry C., Jacobs A.M., Braun M. Identical words are read differently in different languages. Psychol. Sci. 2001;12(5):379–384. doi: 10.1111/1467-9280.00370. [DOI] [PubMed] [Google Scholar]
- Ziegler J.C., Castel C., Pech-Georgel C., George F., Alario F.X., Perry C. Developmental dyslexia and the dual route model of reading: simulating individual differences and subtypes. Cognition. 2008;107(1):151–178. doi: 10.1016/j.cognition.2007.09.004. [DOI] [PubMed] [Google Scholar]
- Ziegler J.C., Perry C., Zorzi M. Modelling reading development through phonological decoding and self-teaching: implications for dyslexia. Phil. Trans. R. Soc. B : Biol. Sci. 2014;369(1634) doi: 10.1098/rstb.2012.0397. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.