Highlights
-
•
Cognitive and ERP assessments were performed following exercise and seated rest.
-
•
The sample was bifurcated according to baseline inhibitory control performance.
-
•
Selective increases in accuracy and P3 amplitude were observed for lower-performers.
-
•
Generalized P3 latency and N2 amplitude changes were observed after exercise.
Keywords: Executive function, Flanker, Higher-performers, Lower-performers, Physical activity
Abstract
The present study examined the effects of moderate-intensity aerobic exercise on aspects of cognitive control in two groups of children categorized by higher- and lower-task performance. Event-related brain potentials (ERPs) were collected in 40 preadolescent children during a modified flanker task following 20 min of treadmill walking and seated rest on separate occasions. Participants were bifurcated into two groups based on task performance following the resting session. Findings revealed that following exercise, higher-performers maintained accuracy and exhibited no change in P3 amplitude compared to seated rest. Lower-performers demonstrated a differential effect, such that accuracy measures improved, and P3 amplitude increased following exercise. Lastly, both groups displayed smaller N2 amplitude and shorter P3 latency following exercise, suggesting an overall facilitation in response conflict and the speed of stimulus classification. The current findings replicate prior research reporting the beneficial influence of acute aerobic exercise on cognitive performance in children. However, children with lower inhibitory control capacity may benefit the most from single bouts of exercise. These data are among the first to demonstrate the differential effect of physical activity on individuals who vary in inhibitory control, and further support the role of aerobic exercise for brain health during development.
1. Introduction
Despite evidence that physical activity participation is associated with improved cognitive function (Hillman et al., 2008), academic performance (Castelli et al., 2007, Chomitz et al., 2009), and overall health (Butte et al., 2007) in children, there remains an overwhelming decline in the amount of time dedicated to physical activity across the school day (Andersen et al., 1998, Center for Education Policy, 2007). Such trends co-occur with the increasing prevalence of sedentary behaviors in industrialized society (Ng and Popkin, 2012) and are accompanied by increasing rates of obesity, type-2 diabetes, and other metabolic disorders (Centers for Disease Control and Prevention, 2012). To counteract these developments, organizations have begun to advocate acute activity breaks in the classroom (National Association for Sport and Physical Education, 2008), and in recent years the number of investigations surrounding the impact of active teaching lessons has steadily risen (e.g., Donnelly et al., 2009, Kibbe et al., 2011). In addition, studies focusing on single bouts of physical activity indicate that increasing the amount of time spent physically active may foster transient cognitive benefits (Sibley and Etnier, 2003, Tomporowski, 2003), which have important implications for scholastic performance.
One such aspect of cognition that has received much attention in recent years is cognitive control (i.e., top-down goal directed behavior), which has been found amenable to interventions consisting of a single bout of moderate physical activity (Best, 2012, Drollette et al., 2012, Hillman et al., 2009, Pontifex et al., 2013). However, few studies have ventured beyond demonstrating a link between the effects of physical activity on cognition by delving deeper into questions regarding how, and in what context, this beneficial relationship exists. Accordingly, the aim of the present investigation was to explore individual differences in cognitive control capacity and whether a single bout of physical activity may modulate individual differences in this aspect of cognition. Given the importance of cognitive control for scholastic performance (Diamond and Lee, 2011, Gathercole et al., 2008, Hillman et al., 2012), this research has implications for how physical activity may be implemented during the school day among children who demonstrate variability in cognitive behaviors.
The term ‘cognitive control’ refers to operations responsible for motivated actions and self-regulation that assist in selecting, scheduling, maintaining, and coordinating the computational processes that underlie perception, memory, and action (Norman and Shallice, 1986, Rogers and Monsell, 1995). Within the domain of cognitive control lie interrelated processes of inhibition, working memory, and cognitive flexibility (Diamond, 2006, Miyake et al., 2000), which have been examined using neuroimaging techniques such as event-related brain potentials (ERPs). The high temporal resolution of ERPs offers a comprehensive means for assessing aspects of the information processing stream that comprise cognitive control or other related processes as they unfold. The present study utilized ERP techniques during a modified version of the Eriksen flanker task (Eriksen and Eriksen, 1974) to assess inhibitiory control, one aspect of cognitive control. Common ERP components that reflect aspects of information processing involved in inhibitory control include the N2 (Schmitt et al., 2000) and P3 (Donchin, 1981), which occur following the presentation of a stimulus. During the flanker task, modulation of the N2 component is thought to represent the need to upregulate control over incorrect response preparation (Folstein and Van Petten, 2008), with the amplitude associated with the ability to monitor response conflict (i.e., larger amplitude is associated with increased conflict; Schmitt et al., 2000). A second component that is modulated by alterations in the stimulus environment is the P3 component. The amplitude of this component is thought to reflect attentional resource allocation during stimulus engagement (i.e., larger amplitude is associated with greater allocation of resources; Polich, 2007), and the latency is theorized to represent an index of stimulus classification and evaluation speed, independent of response selection and action (i.e., shorter latency is associated with faster processing speed; Duncan-Johnson, 1981, Verleger, 1997).
Previous acute exercise research, utilizing ERP techniques has demonstrated better task performance, which was accompanied by increased P3 amplitude only for trials requiring the upregulation of inhibitory control following exercise (Hillman et al., 2009, Pontifex et al., 2013). The findings suggested that acute exercise selectively modulates cognitive control during tasks that necessitate greater amounts of inhibition. The present study sought to further tease apart this relationship using these previously published investigations by examining combined cohorts of preadolescent children who differ in their inhibitory control ability. That is, a great deal of research has investigated individual differences in cognitive control capacity (see Engle and Kane, 2004 for review) with children of lower capacity demonstrating increased behavioral issues in the classroom, inattentiveness, and high levels of distractibility (Alloway et al., 2009). Further, previous research in adults has examined individual differences in working memory following a single bout of exercise (Sibley and Beilock, 2007). The results indicated that following the cessation of walking the lowest performing group increased performance above baseline measures, with no significant change observed for the higher-performing groups (Sibley and Beilock, 2007). This finding suggests that the acute exercise effects were selective to individuals with poor cognitive performance at baseline. Thus, a logical extension of these findings would be to determine whether single bouts of physical activity have influence upon children whom differ along other aspects of cognitive (i.e., inhibitory) control.
Accordingly, exercise-induced modulation in task performance and neuroelectric function were examined in preadolescent children who differed in their inhibitory control capacity. To date, no such research has explored whether single bouts of physical activity differentially modulate cognitive control in children who vary in their capacity to perform such functions. However, based on previous research (Engle and Kane, 2004, Sibley and Beilock, 2007), it was hypothesized that only the lower-performing children would exhibit greater response accuracy following exercise. That is, higher-performing children were expected to display a markedly smaller change in performance. Further, predictions surrounding the P3 component were expected to mirror prior findings in children and young adults indicating a disproportionately larger change during task conditions necessitating the upregulation of inhibitory control (Hillman et al., 2003, Hillman et al., 2009, Pontifex et al., 2013). It was also expected that larger change in P3 amplitude would be observed for the lower-performing children following the single bout of exercise; thus reflecting a neural mechanism for the observed disproportionate behavioral change.
2. Methods
2.1. Participants
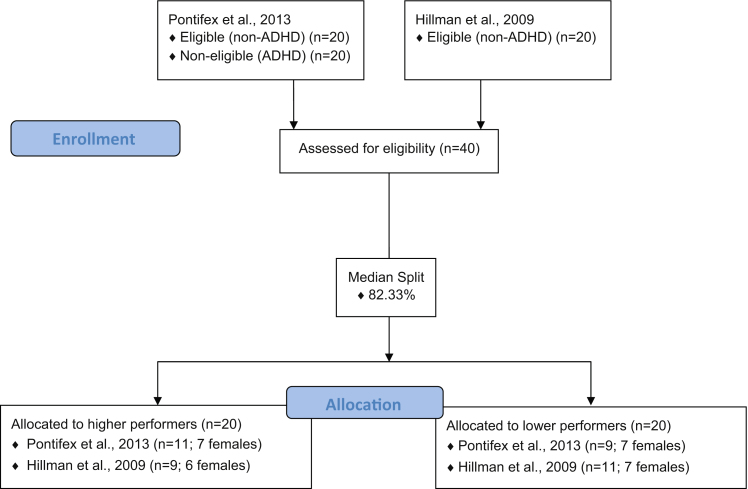
Data for the current investigation were combined from two equal subsets of children engaged in identical protocols involving acute exercise and cognitive assessment (Hillman et al., 2009, Pontifex et al., 2013; see Fig. 1).1 Table 1 provides demographic information for the study sample. A total of 40 (27 females) healthy 8–10 years old children from the east-central Illinois region were included in the current investigation. Participants and their legal guardian signed informed assent and consent waivers approved by the Institutional Review Board (IRB) of the University of Illinois at Urbana-Champaign. Guardians were asked to complete a health history and demographics questionnaire, as well as other documentation indicating that their child was free of neurological diseases, attentional disorders (indexed by the ADHD Rating Scale IV; DuPaul et al., 1998), physical disabilities that could be exacerbated by exercise participation (Physical Activity Readiness Questionnaire [PAR-Q]; Thomas et al., 1992), and that participants had normal or corrected-to-normal vision. Additionally, pubertal timing data were acquired using the Tanner Staging System (Tanner, 1962, Taylor et al., 2001), which was completed by the guardian in cooperation with the participant. All participants were prepubescent (i.e., a score ≤2 on a 5 point scale) at the time of testing. Socioeconomic status (SES) was calculated using a trichotomous index based on: (1) highest level of education obtained by the mother and father, (2) number of parents who worked full time, and (3) participation in a free or reduced-price lunch program at school (Birnbaum et al., 2002). In accordance with a priori hypotheses, the sample population was bifurcated into two groups (higher-performers and lower-performers) based on flanker task performance for incongruent trials (i.e., the condition requiring the greatest amount of inhibitory control) during the seated rest condition. Accuracy was selected to represent inhibitory control, rather than reaction time, due to the differential control in response speed in children compared to adults. That is, children demonstrate impulsive response selection resulting in maintenance of reaction time across trial types regardless of increasing cognitive control demand (Christakou et al., 2009, Davidson et al., 2006). Adults, however, slow response selection in order to maintain response accuracy during difficult trial types. Thus, accuracy, compared to reaction time, more accurately reflects inhibitory control in children (Davidson et al., 2006). Accordingly, a median split identified higher-performers as those that scored at or above 82.33%, and lower-performers as those that scored below 82.33% on incongruent accuracy measures during the seated rest (i.e., baseline) condition (see Table 2).
Fig. 1.
Enrollment flow chart demonstrating the origin of higher- and lower-performing participants collected from two separate published studies (Pontifex et al., 2013, Hillman et al., 2009).
Table 1.
Mean (SE) values for higher- and lower-performer's demographic information and fitness data.
| Measure | All participants | Higher performers | Lower performers |
|---|---|---|---|
| n | 40 (27 females) | 20 (13 females) | 20 (14 females) |
| Age (years) | 9.7 (0.7) | 9.8 (0.1) | 9.6 (0.2) |
| SES | 2.3 (0.1) | 2.4 (0.1) | 2.3 (0.1) |
| Tanner | 1.4 (0.4) | 1.5 (0.1) | 1.4 (0.1) |
| BMI (kg/m2) | 19.2 (0.8) | 20.3 (1.3) | 18.2 (1.0) |
| K-BIT (IQ) | 119.7 (1.8) | 118.8 (2.1) | 120.6 (3.1) |
| VO2max (ml/kg/min) | 40.3 (1.1) | 40.1 (1.6) | 40.5 (1.6) |
| VO2max (percentile) | 22 (3.8) | 20.6 (5.7) | 23.4 (5.3) |
| Walking mean HR (bpm) | 128.3 (1.2) | 127.1 (1.4) | 129.1 (1.9) |
| HRmax (bpm) | 190.8 (1.9) | 190.2 (2.1) | 191.3 (3.4) |
Note: SES is classified as “low” (score below 2), “moderate” (score between 2 and 3), and “high” (score greater than 3); Tanner scores ≤2 indicate that children were prepubescent at the time of testing; BMI is body mass index; walking mean HR is the average heart rate during the acute walking period; HRmax is the maximum HR achieved during cardiorespiratory fitness (VO2max) assessment.
Table 2.
Mean (SE) values for neuroelectric and behavioral measures as a function of performance and session.
| Higher performers |
Lower performers |
|||
|---|---|---|---|---|
| Measure | Walking | Seated rest | Walking | Seated rest |
| RT (ms) congruent | 507 (14) | 500 (15) | 504 (15) | 477 (16) |
| RT (ms) incongruent | 541 (13) | 534 (18) | 552 (15) | 535 (18) |
| Accuracy (%) congruent | 93.7 (1.1) | 94.2 (1.0) | 93.6 (1.0) | 87.5 (1.3) |
| Accuracy (%) incongruent | 89.2 (1.6) | 90.2 (1.2) | 87.1 (1.7) | 73.8 (1.4) |
| RT interference | 34.1 (5.5) | 34.2 (7.5) | 47.6 (5.5) | 57.8 (7.5) |
| Accuracy interference | 4.5 (1.0) | 3.9 (1.2) | 6.5 (1.3) | 13.7 (1.3) |
| N2 amplitude (μV) | −1.8 (0.9) | −5.3 (1.6) | −3.3 (0.9) | −5.9 (1.6) |
| N2 latency (ms) | 249 (6.9) | 246 (8.6) | 228 (6.8) | 232 (8.6) |
| P3 amplitude (μV) | 8.5 (1.0) | 8.0 (0.9) | 9.4 (1.0) | 5.2 (0.9) |
| P3 latency (ms) | 383 (9.1) | 411 (9.8) | 404 (9.2) | 421 (9.8) |
Note: For ERP data (N2 and P3), all mean (SE) values are collapsed across congruency conditions and respective midline electrode sites; RT is mean reaction time.
2.2. Flanker
All participants completed a modified version of the Eriksen flanker task (Eriksen and Eriksen, 1974) to assess aspects of cognitive control including inhibition. All stimuli were presented focally on a computer screen at a distance of approximately 1 m using Neuroscan Stim software (Compumedics, Charlotte, NC). Participants were instructed to attend to the center target stimulus amid four identical flanking stimuli that were either congruent (i.e., facing the same direction) or incongruent (i.e., facing the opposite direction; Drollette et al., 2012, Hillman et al., 2009, Pontifex et al., 2013), and respond as quickly and accurately as possible with a thumb press in accordance with the directionality of the target using a response pad. Two blocks of 100 trials were presented with equiprobable congruency and directionality. Differences between the combined protocols included stimulus type (3 cm tall goldfish on a blue background vs. 2.5 cm tall white arrows on a black background), duration (200 ms vs. 120 ms), and interstimulus interval (fixed 1700 ms vs. variable 1100 ms, 1300 ms, and 1500 ms).2
2.3. Neuroelectric assessment
Electroencephalographic (EEG) activity was recorded from 64 electrode sites arranged according to the international 10-10 System (Chatrian et al., 1985) using a Neuroscan Quick-Cap (Compumedics, Charlotte, NC). Prior to EEG recordings all electrodes maintained an impedance <10 kΩ. Online, continuous data were referenced to a midline electrode placed at midpoint between Cz and CPz with AFz electrode site serving as the ground. Additional electrodes were placed above and below the left orbit and outer canthus of each eye to monitor electrooculograhic (EOG) activity. Continuous data were digitized at a sampling rate of 500 Hz, amplified 500 times with a DC to 70 Hz filter, and a 60-Hz notch filter was applied using a Neuroscan SynAmps2 amplifier. Offline, continuous data were referenced to averaged mastoids (M1, M2) rather than an average reference of all sensors because the 64 channel electrode array utilized in the present investigation did not cover a sufficient surface area of the entire head (i.e., no sensors were placed in the ventral region below the FPz-T7-Oz-T8 equator) to warrant appropriate mathmatical justification for use of this method. Further, data were corrected for EOG artifacts using a spatial filter (Compumedics Neuroscan, 2003). Trials were rejected if a response error occurred or an identified artifact exceeded ±75 μV. ERPs were acquired for flanker trials using stimulus-locked epochs for correct trials from −100 to 1000 ms, which were baseline corrected using the −100 to 0 ms prestimulus period and filtered using a zero-phase shift low-pass filter at 30 Hz (24 dB/oct). The latency and amplitude for each ERP component was quantified using the local peak amplitude and corresponding latency within a 200–400 ms latency window for the N2, and a 300–600 ms latency window for the P3.
2.4. Cardiorespiratory fitness assessment
Maximal oxygen consumption (VO2max) was collected by a computerized indirect calorimetry system (ParvoMedics True Max 2400, Sandy, UT) with measures of heart rate (HR), average respiratory exchange ratio (RER), and oxygen uptake assessed every 20 s. VO2max was expressed as milliliters per kilogram of body weight per minute (ml/kg/min). A Polar HR monitor (model A1: Polar Electro, Finland) was fitted to each participant prior to assessment, which took place on a motor-driven treadmill (Life Fitness 93T Classic; Brunswick Corporation, Schiller Park, IL) and followed a modified Balke protocol (American College of Sports Medicine, 2010). Every 2 min, workload (treadmill incline) was increased by 2.5%, and ratings of perceived exertion (RPE) were measured using the pictographic children's OMNI Scale (Utter et al., 2002), which ranges from a score of 0, “not tired at all”, to 10, “very, very tired”. All participants were provided a 2 min walking warm-up prior to running speed being held constant throughout the remainder of the assessment until volitional exhaustion. Criterion for achieving VO2max included achieving two or more of the following: (a) a plateau in oxygen consumption evidenced by an increase in workload with a corresponding increase of <2 ml/kg/min, (b) a peak HR ≥ 185 bpm (American College of Sports Medicine, 2010) and an HR plateau (Freedson and Goodman, 1993), (c) RER ≥ 1.0 (Bar-Or, 1983), and/or (d) RPE on the children's OMNI scale ≥8 (Utter et al., 2002).
2.5. Procedure
The protocol used a within-subjects design in which all participants completed two separate cognitive testing sessions in the lab at approximately the same time of day on separate days (8.5 ± 8.4 days between sessions). These testing sessions were conducted following 20 min of either moderate intensity treadmill walking at an intensity of 60–70% of maximal HR, or quiet rest while seated in a chair that was safely placed on the same treadmill. All preliminary paperwork (i.e., informed consent/assent, demographics, and prescreening measures) were completed by the guardian and participant. Trained laboratory staff also administered the Edinburgh Handedness Inventory (Oldfield, 1971) to determine hand dominance, as well as the Kaufman Brief Intelligence Test (K-BIT; Kaufman and Kaufman, 1990) to assess intelligence quotient. VO2max assessment was always performed on a separate testing day or following the resting cognitive session to avoid any confounding effects of acute physical activity. As such, participants were instructed to avoid all moderate to vigorous physical activity throughout the day prior to testing while trying to adhere as closely as possible to their normal routine. Session order was randomized and counterbalanced such that half of the participants received the exercise session prior to their rest day, and vice versa. At the start of each session participants were fitted with a Polar HR monitor to measure resting HR, followed by HR and RPE recordings every 2 min. Following each session (i.e., exercise or seated rest), participants were outfitted with an electrode cap in preparation for EEG recordings during flanker task performance. During this time (∼22.5 ± 3.4 min), participants’ HR was allowed to return to within 10% of baseline to avoid any general arousal effects on ERP measures stemming from exercise participation. Once cap preparation was completed, participants were seated in a quiet testing chamber, provided task instructions, and sufficient practice trials prior to performing the task. Upon completion of the study, participants were briefed on the purpose of the study and received $10/h remuneration.
2.6. Statistical analysis
Statistical procedures were conducted using SPSS v.19 (SPSS, Chicago, IL). Repeated-measures ANOVAs were performed with higher- and lower-performers as the between-subjects factor. Findings are reported using the Greenhouse–Geisser correction statistic for violations of sphericity. The family-wise alpha level was set at 0.05, and Bonferroni corrected t-tests were used for post hoc comparisons and reported using mean and standard error (SE). Further reporting included partial η2 and estimated effect size for main effects and interactions. Flanker accuracy percentage and mean reaction time (RT) were separately analyzed using 2 (performance: high performers, low performers) × 2 (session: rest, exercise) × 2 (congruency: congruent trials, incongruent trials) models. Additionally, analysis of flanker accuracy and RT interference was performed separately using a 2 (performance) × 2 (session) model. Further, the ERP analysis was performed using peak amplitude and latency of the N2 and P3 components. N2 amplitude and latency were entered into separate 2 (performance) × 2 (session) × 2 (congruency) × 3 (site: Fz, FCz, Cz) models. P3 analysis was similar to the N2 analysis, save for the inclusion of 7 sites: Fz, FCz, Cz, CPz, Pz, POz, Oz. Lastly, given the nature of bifurcating groups according to task peformance, significant behavioral results were expected between higher- and lower-performers during the rest session, which served as a manipulation check.
3. Results
Table 2 provides mean (SE) values of neuroelectric and behavioral measures as a function of performance and session. All significant results are represented in Table 3 for behavioral task performance and Table 4 for neuroelectric measures. Initial analyses were performed to determine confounding variables related to session order. The omnibus analyses for flanker behavior performance as well as the ERP components of N2 and P3 revealed no interaction, F's(1, 36) ≤ 2.7, p ≥ 0.11, thus all further analyses were collapsed across session order.
Table 3.
Summary of statistical analyses for behavioral performance.
| Effect | F | df1/df2 | p | η2 |
|---|---|---|---|---|
| Flanker mean RT | ||||
| Congruency | 120.7 | 1, 38 | <0.001 | 0.76 |
| Performance × congruency | 6.0 | 1, 38 | 0.019 | 0.14 |
| Flanker accuracy | ||||
| Performance | 21.3 | 1, 38 | <0.001 | 0.36 |
| Session | 23.4 | 1, 38 | <0.001 | 0.38 |
| Congruency | 132.8 | 1, 38 | <0.001 | 0.78 |
| Performance × session | 32.7 | 1, 38 | <0.001 | 0.46 |
| Performance × congruency | 26.1 | 1, 38 | <0.001 | 0.41 |
| Congruency × session | 6.7 | 1, 38 | 0.013 | 0.15 |
| Performance × congruency × session | 9.9 | 1, 38 | 0.003 | 0.21 |
| Flanker accuracy interference | ||||
| Performance | 26.1 | 1, 38 | <0.001 | 0.41 |
| Session | 6.7 | 1, 38 | 0.013 | 0.15 |
| Performance × session | 9.8 | 1, 38 | 0.003 | 0.21 |
Note: Only significant (p < 0.05) effects are reported.
Table 4.
Summary of statistical analyses for N2 & P3 neuroelectric measures.
| Effect | F | df1/df2 | p | η2 |
|---|---|---|---|---|
| N2 ERP amplitude | ||||
| Session | 10.4 | 1, 38 | 0.003 | 0.21 |
| Site | 6.6 | 1.3, 47.9 | 0.009 | 0.15 |
| P3 ERP amplitude | ||||
| Session | 9.7 | 1, 38 | 0.004 | 0.20 |
| Site | 6.4 | 1.9, 74 | 0.003 | 0.14 |
| Performance × session | 6.3 | 1, 38 | 0.016 | 0.14 |
| P3 ERP latency | ||||
| Session | 10.0 | 1, 38 | 0.003 | 0.21 |
| Site | 4.3 | 3.3, 124 | 0.005 | 0.10 |
Note: Only significant (p < 0.05) effects are reported.
3.1. Task performance
3.1.1. Reaction time
The omnibus analysis for flanker mean RT revealed a main effect of congruency (see Table 3), F(1, 38) = 120.7, p < 0.001, η2 = 0.76, that was superseded by a performance × congruency interaction, F(1, 38) = 6.0, p = 0.019, η2 = 0.14. Post hoc tests indicated shorter RT for congruent trials for higher- (503.8 ± 12.3 ms) and lower-performers (490.6 ± 13.7 ms) compared to incongruent trials (higher-performers: 537.9 ± 13.3 ms, lower-performers: 543.3 ± 13.8 ms), t's(19) ≥ 8.1, p's < 0.001. No further significant main effects or interactions were observed for mean RT, F's(1, 38) ≤ 1.8, p ≥ 0.187, η2 ≤ 0.05.
3.1.2. Accuracy
The omnibus analysis for flanker accuracy revealed main effects of all factors (see Table 3), F's(1, 38) ≥ 6.7, p ≤ 0.013, η2 ≥ 0.15, that was superseded by a performance × congruency × session interaction, F(1, 38) = 9.9, p = 0.003, η2 = 0.21. Post hoc tests indicated greater mean accuracy for only lower-performers following exercise (congruent: 93.6 ± 1.0%, incongruent: 87.1 ± 1.7%) compared to following seated rest (congruent: 87.5 ± 1.3%, incongruent: 73.8 ± 1.4%) for both trial types, t's(19) ≥ 5.0, p < 0.001. Flanker accuracy for higher-performers did not modulate following exercise, t's(19) ≤ 0.7, p ≥ 0.52. In addition, comparison of trial types within sessions revealed greater mean accuracy for higher-performers (congruent: 94.2 ± 1.0%, incongruent: 90.2 ± 1.2%) compared to lower-performers (congruent: 87.5 ± 1.3%, incongruent: 73.8 ± 1.4%) for both trial types (with incongruent trials serving as a manipulation check due to the bifurcation of groups according to performance during the rest session), and only following seated rest, t's(38) ≥ 4.1, p < 0.001, indicating that following exercise lower-performers improved performance to a level comparable to higher-performers (see Table 2).
3.1.3. Interference
A secondary analysis for flanker mean RT interference (incongruent–congruent) revealed no significant main effects or interactions involving performance or session, F's(1, 38) ≤ 0.9, p ≥ 0.342, η2 ≤ 0.02. However, for task performance (congruent–incongruent), an interference effect of session was observed, F(1, 38) = 7.1, p = 0.011, η2 = 0.16, that was superseded by a performance × session interaction, F(1, 38) = 9.9, p = 0.003, η2 = 0.21. Decomposition of the interaction indicated greater mean interference for lower-performers following seated rest (13.7 ± 1.3%) compared to following exercise (6.5 ± 1.3%), t(19) = 3.4, p = 0.003, and compared to higher-performers (3.9 ± 1.2%) following seated rest (see Table 2), t(38) = 5.5, p < 0.001, indicating that following exercise lower-performers decrease accuracy interference to a level consonant with higher-performers. Further, higher-performers, unlike lower-performers, did not modulate interference accuracy across sessions, t(19) = 0.4, p = 0.66 (see Table 2).
3.2. Neuroelectric measures
3.2.1. N2 amplitude
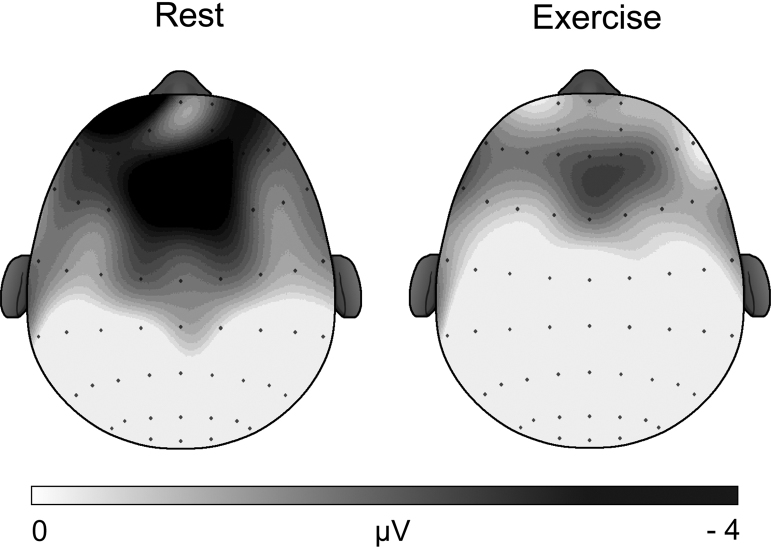
The omnibus analysis for N2 amplitude revealed a main effect of session, F(1, 38) = 10.4, p = 0.003, η2 = 0.21, indicating smaller mean N2 amplitude for all participants following exercise (−2.6 ± 0.7 μV) compared to following seated rest (−5.6 ± 1.1 μV; see Fig. 2, Fig. 3). Further, analysis revealed a main effect of site, F(1.3, 47.9) = 6.6, p = 0.009, η2 = 0.15, with post hoc tests indicating Fz and FCz exhibiting significantly larger amplitude (Fz: −4.7 ± 0.9 μV, FCz: −4.7 ± 1.0 μV) relative to Cz (−2.8 ± 0.7 μV), t's(39) ≥ 2.6, p's ≤ 0.013. No other main effects or interactions were observed for N2 amplitude or latency (see Table 4).
Fig. 2.
Topographic plots of N2 amplitude collapsed across congruency and performance.
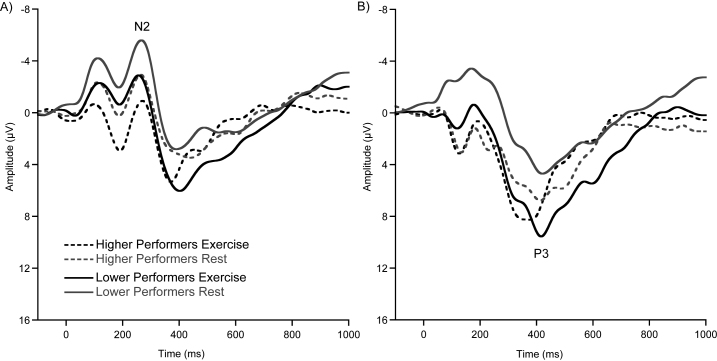
Fig. 3.
Stimulus-locked grand-average waveform from (A) the FCz electrode site and (B) the Pz electrode site, collapsed across congruency conditions for higher- and lower-performers.
3.2.2. P3 amplitude
The omnibus analysis for P3 amplitude revealed a main effect of session, F(1, 38) = 9.7, p = 0.004, η2 = 0.20, that was superseded by an interaction of performance × session, F(1, 38) = 6.3, p = 0.016, η2 = 0.14. Post hoc tests indicated smaller P3 amplitude for lower-performers following seated rest (5.2 ± 0.8 μV) compared to after exercise (9.4 ± 1.1 μV), t(19) = 4.3, p ≤ 0.001, and compared to higher-performers following seated rest (8.0 ± 0.9 μV), t(38) = 2.3, p = 0.025 (see Fig. 3, Fig. 4). In accordance with behavior results, higher-performers’ P3 amplitude did not significantly modulate following exercise, t(19) = 0.4, p = 0.67. Further analysis revealed a main effect of site, F(1.9, 74) = 6.4, p = 0.003, η2 = 0.14, with post hoc tests indicating Pz as the site of maximal amplitude (9.2 ± 0.7 μV) compared to FCz (6.7 ± 0.7 μV) and Fz, (5.5 ± 0.6 μV), t's(39) ≥ 3.2, p's ≤ 0.002. Further analysis revealed numerically larger, but nonsignificant, amplitude at Pz (9.2 ± 0.7 μV) compared to POz (8.5 ± 0.8 μV), CPz (8.2 ± 0.6 μV), Oz (8.3 ± 1.0 μV), and Cz (8.0 ± 0.7 μV), t's(39) ≥ 1.4, p's ≤ 0.159. No other main effects or interactions were observed for P3 amplitude (see Table 4).
Fig. 4.
Topographic plots of P3 amplitude collapsed across congruency for higher- and lower-performers.
3.2.3. P3 latency
The omnibus analysis for P3 latency revealed a main effect of session, F(1, 38) = 10, p = 0.003, η2 = 0.21, indicating shorter P3 latency for all participants following exercise (394 ± 6.5 ms) compared to following seated rest (416 ± 6.8 ms; see Fig. 3b). Further analysis revealed a main effect of site, F(3.3, 124) = 4.3, p = 0.005, η2 = 0.10, with post hoc tests indicating Oz as the site with the shortest latency (393.2 ± 6.3 ms) compared to Cz (412.3 ± 6.0 ms) and CPz (412.0 ± 7.1 ms), t's(39) ≥ 2.8, p's ≤ 0.007. No further effects or interactions were observed for P3 latency (see Table 4).
4. Discussion
Overall, findings revealed that a single, acute bout of moderate aerobic exercise facilitates cognitive performance, with more widespread effects observed for the lower-performing group. Specifically, lower-performers demonstrated improvements in response accuracy and interference measures to a level comparable to that of higher-performers following the cessation of exercise. By contrast, no behavioral modulation was observed for the higher-performing group. Examination of neuroelectric measures indicated that P3 amplitude modulation supported the behavioral findings, such that lower-performers exhibited larger P3 amplitude (equivalent to that of higher-performers) following the cessation of the exercise bout; an effect that was not realized during the seated rest condition. In addition, shorter P3 latency and decreased N2 amplitude was observed across both groups following exercise, suggesting faster cognitive processing speed and a reduction in response inhibition associated with conflict monitoring following the cessation of exercise. Taken together, such findings suggest that single bouts of moderate intensity exercise may have a disproportionate benefit to children characterized by lower inhibitory control capacity.
4.1. Task performance
Novel to the present investigation is that improvements in task performance were only manifest in lower-performing children. That is, lower-performing children exhibited better performance in flanker accuracy after exercise to a level that was equivocal to that of the higher-performing group. Interestingly, no modulation of reaction time was observed for both groups, indicating that the observed increase in task performance among lower-performers was related to improvements in underlying cognitive control strategy rather than a trade-off with response speed. Further, such findings in task performance suggested that previous acute exercise effects (Drollette et al., 2012, Hillman et al., 2009, Pontifex et al., 2013) may be driven, in part, by groups of children characterized by lower cognitive control and self-regulatory behavior.
Evaluation of prior research provides evidence for this assertion. For example, Mahar et al. (2006) reported that increasing physical activity opportunities in the classroom improved on-task behavior to a greater extent for children who demonstrated poor on-task behavior at baseline. Further, Pontifex et al. (2013) examined acute exercise modulation among children with and without attention-deficit/hyperactivity disorder (ADHD), a psychological outcome characterized by deficits in self-regulatory behavior and inappropriate levels of inattentiveness. Although both groups improved performance on the flanker task following exercise, only the ADHD group exhibited an increase in a neuroelectric component (i.e., error-related negativity, ERN) that has been linked to the upregulation of processes involved in action monitoring. Importantly, this increase in the ERN potential was accompanied by an increase in task performance following errors only in the ADHD group. Such a pattern of results suggests that individual's exhibiting deficits in inhibitory control (i.e., ADHD) benefit from short bouts of exercise through the upregulation or effective allocation of resources directed at self-regulation.
Additional evidence may be drawn from Sibley and Beilock (2007) who assessed individual differences in working memory capacity among college-aged students. They found that the lowest performing group increased performance above baseline measures, with no significant change observed for the higher-performing groups. Taken together, individuals characterized by deficits in aspects of cognitive control may be more amenable to the beneficial effects associated with single bouts of moderate physical activity. As such, the current approach of identifying groups of individuals according to cognitive control ability may be an effective means for targeting specific populations that would benefit the most from physical activity interventions. Further, while physical activity has a number of acute benefits, it is not clear whether those benefits include transient changes in cognitive control for higher-performing individuals. Research should continue to pursue an individual differences approach to better understand this relationship across individuals who differ in their level of cognitive control ability.
Additionally, our combined re-analysis of flanker task performance verifies previous results (Pontifex et al., 2013) and replicates other work (Drollette et al., 2012) demonstrating improvements post-exercise for congruent and incongruent trial types. Although a general enhancement for lower-performers was observed, it should be noted that Hillman et al. (2009) reported selective modulation for incongruent trials with only a positive, non-significant, trend for congruent trials. Such findings, in addition to neuroelectric results (Hillman et al., 2003, Hillman et al., 2009, Kamijo et al., 2007, Pontifex et al., 2013), demonstrate that acute exercise selectively modulates trial types that necessitate greater amounts of inhibitory control. The present study suggests a similar conclusion. That is, while both trial types were significant, incongruent trial accuracy improved nearly 13% compared to 6% for congruent trials following exercise. Secondary analyses of the interference effect, a measure of added conflict due to the presence of incongruent flanking stimuli versus congruent flanking stimuli, revealed that lower-performs improved their accuracy by nearly 7% following exercise with no modulation observed among higher-performers. Collectively, not only do acute exercise benefits appear selective for individuals characterized by deficits in aspects of cognitive control capacity, these robust findings provide additional support for the general, yet selective modulation of inhibitory control. However, given the paucity of research with children and variations in exercise intensity, duration, modality, and cognitive performance trajectory post exercise, further investigation is necessary to better understand these selective interactions.
4.2. ERPs
Consonant with our a priori hypothesis, lower-performers exhibited robust modulation in P3 amplitude following a single bout of exercise, whereas higher-performers demonstrated no significant change. Interestingly, P3 amplitude modulation for both groups coincided with behavioral performance. Based on an inhibitory hypothesis of the P3 in which the amplitude reflects modulation of attentional resource allocation (Polich, 2007), the present findings suggest a link between the neural mechanisms associated with inhibitory control and the observed behavioral changes following acute exercise. This relation observed in our re-analysis confirms the robust association found previously in separate studies (Hillman et al., 2009, Pontifex et al., 2013) and replicates the association observed in young adults (Hillman et al., 2003), in that all studies observed larger P3 amplitude following an acute bout of exercise in accordance with improvements in response accuracy. Collectively, exercise has acute, transient effects that may serve to facilitate behavioral and neuroelectrical indices reflecting an upregulation in the amount of attentional control during environmental interaction, with the current investigation providing additional evidence that such effects may be selective to individuals with lower cognitive control ability. Further, it should be noted that Pontifex et al. (2013) observed a general enhancement in P3 amplitude for ADHD children and healthy controls. Although speculative, it may be that the healthy children, who were absent of an ADHD diagnosis, exhibited a wide spectrum of cognitive control behavior, since individual difference measures were not collected. Therefore, in accordance with the present findings, modulation of P3 amplitude would be expected among those who demonstrate poorer cognitive control ability, thus potentially facilitating P3 amplitude modulation in not only the group characterized by attentional deficits (i.e., ADHD children), but also the control group. However, further research is needed to better understand individual differences in cognitive control and the influence of acute exercise on P3 amplitude.
Conversely, N2 amplitude and P3 latency were modulated following exercise for both higher- and lower-performing groups. Although behavioral improvements were only observed for the lower-performing group, the additional ERP modulation suggests differential efficiency in neuroelectric processing between groups. That is, P3 latency represents the time required to detect and process a stimulus in the environment (Kutas et al., 1977) with shorter latencies indicative of improved mental performance (Polich et al., 1983). Such findings suggest that acute bouts of exercise necessitate faster mental processing speed across all groups of children regardless of cognitive control differences. In addition, a reduction in N2 amplitude occurs when there is a need for reducing subsequent response conflict activation on task-relevant information (Corbetta and Shulman, 2002, Egner and Hirsch, 2005), with the present results suggesting that following the cessation of exercise children are better able to reduce response conflict necessitated by the distracting flanking stimuli during the flanker task. Interestingly, N2 modulation has not been reported in association with acute exercise in past studies, and thus hypotheses were not formulated a priori. However, previously published ERP waveforms (Hillman et al., 2009) appear to demonstrate N2 modulation trending in a similar manner as to the current investigation, but statistical analysis were not reported. Alternatively, this investigation is the first to observe a significant effect of acute exercise on the modulation of the N2 component. Thus, modulation of N2 amplitude and P3 latency suggest that acute bouts of exercise necessitate a reduction in response conflict and shorter cognitive processing speed across all groups of children regardless of individual differences in cognitive control capacity. Such results further implicate the benefit of acute bouts of physical activity among children who demonstrate not only lower-inhibitory control capacity but also for children who maintain higher levels of performance. Although differential ERP modulation between groups is evident with selectively larger benefits for lower-performers, the collective results suggest that both groups benefit.
Multiple mechanisms have been proposed to account for changes in cognitive performance following exercise including general arousal (Kamijo et al., 2004), increases in oxidative cerebral blood flow (Querido and Sheel, 2007), and upregulation of neuronal proliferation and cell survival (e.g., brain-derived neurotrophic factor, insulin like growth factor 1, 5-HT; van Praag et al., 1999, Vaynman and Gomez-Pinilla, 2005). In addition, evaluation of transcranial direct current stimulation (tDCS) may provide new insight for proposed mechanisms based on the resemblance of the current selective ERP modulation among higher- and lower-performers and previous tDCS investigations. That is, prior tDCS research has demonstrated that through a process of artificial depolarization and hyperpolarization, utilizing a weak electrical current conducted through specific brain regions, neural cortical activation is enhanced with concurrent improvements in cognitive control performance (see Nitsche et al., 2008, for review; Ohn et al., 2008, Tseng et al., 2012). Of particular interest is the work by Tseng et al. (2012) who evaluated effects of tDCS on ERP components among high- and low-performers based on working memory task performance. Results indicated that following 15 min of tDCS, only the low-performers improved accuracy with concurrent modulation of separate ERP components related to visual attention (N2pc; Luck and Hillyard, 1994) and memory maintenance (SPCN or CDA; Jolicoeur et al., 2008) compared to sham tDCS among the same participants on a different day. Thus, the observed changes in cognitive control and accompanying neuroelectric modulation are nearly identical with the directionality of the present results, suggesting that the pathways responsible for organic exercise-induced cognitive regulation are similar to that induced artificially by tDCS, with further evidence indicating that both methods are selective to those characterized by poorer cognitive control capacity. Although a detailed discussion of the observed relationship is beyond the scope of the present investigation, such observations may provide direction for future research to effectively elucidate the underlying mechanisms associated with cognitive changes in relation to acute exercise.
Despite the observed effects following a single bout of exercise, certain limitations should be noted. First, higher- and lower-performing groups were bifurcated based on incongruent trial performance during the rest condition. An alternative approach might have been the use of interference scores since they provide a measure of the upregulation of control to manage interference during the incongruent, relative to the congruent, condition. However the current results demonstrate interference accuracy differences between groups at baseline, suggesting that incongruent trials and interference measures are consonant in delineating group differences in inhibitory control. Further, future research may benefit by incorporating an independent baseline measure of inhibitory control to parse groups. That is, without an independent measure the change in performance for the lower-performing group may reflect the regression toward the mean rather than the effect of acute exercise, as proposed herein. However, the overall pattern of ERP results and behavior performance observed for the higher-performing group contradict this possibility. Specifically, higher-performers maintained greater accuracy across the exercise and rest conditions rather than decreasing performance following exercise. Additionally, the P3 amplitude trends for both higher- and lower-performing groups parallel the behavior results and provide independent evidence of exercise-related modulation in performance. Collectively, these findings do not follow the trends associated with this statistical phenomenon and further suggest that the observed effects across groups are due to exercise.
Second, an assessment of affect or cognitive engagement was not measured during resting or exercise. Thus, although speculative, the observed behavior performance (only for lower-performers) may be associated with lassitude as a function of 20 min of quiet resting. However, given that such a relationship was not observed in the higher-performers, this is unlikely. Lastly, the null results for flanker performance among higher-performers may be limited by a ceiling effect. That is, better performance post-exercise may not have been realized for the higher-performers due to peak performance during the rest and exercise condition. Such limitations present challenges for future research given that higher-performers are expected to perform at optimal levels. Regardless, task difficulty may be an important aspect to consider in future investigations of individual differences, acute exercise, and cognition.
In conclusion, single bouts of moderate intensity exercise may be an effective means for modulating neural activity associated with the efficient allocation of attentional resources coupled with reductions in conflict monitoring. The current findings further suggest that such a relationship may be enhanced to a greater extent among groups of children characterized by lower levels of cognitive control ability. The results not only provide support for the necessity of short physical activity breaks during the school day (National Association for Sport and Physical Education, 2008), but also indicate the importance of using physical activity as a means of regulating attention in the classroom among children who need it most. Such findings are important given that the ability of children to regulate their behavior through cognitive control strategies varies substantially with more than 17% of healthy kindergarten age children entering school unable to effectively pay attention and modulate inappropriate behaviors (McClelland et al., 2000). Therefore, single bouts of physical activity throughout the school day may serve as an effective tool for increasing attentiveness in the classroom, thus leading to a more effective educational environment that promotes better learning in youth.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgment
This research was supported by grants from the National Institute of Child Health and Human Development (HD055352) to Charles Hillman.
Footnotes
Only the non-ADHD control children were sampled from Pontifex et al. (2013). Thus, all participants in the present study were free of an ADHD diagnosis (see Fig. 1 for enrollment and group assignment).
Due to the slight difference in stimulus type, duration, and inter-trial interval used across flanker tasks in the two studies, statistical analyses were performed to determine confounding variables related to study sample. The omnibus analyses, which included Study as a between-subjects factor, yielded non-significant differences for flanker task performance and ERP assessment. Specifically, no interactions were observed for behavior, F's(1, 36) ≤ 0.6, p ≥ 0.46, η2 ≤ 0.02, or ERP components, F's(1, 36) ≤ 2.2, p ≥ 0.14, η2 ≤ 0.06. Thus, all analyses were collapsed across study sample. Further, the two samples did not differ on any demographic factor, t's(38) ≤ 1.5, p's ≥ 0.14. Finally, following group assignments, higher- and lower-performers were similarly distributed across samples (see Fig. 1).
Contributor Information
Eric S. Drollette, Email: drollet1@illinois.edu.
Mark R. Scudder, Email: mscudde2@illinois.edu.
Lauren B. Raine, Email: lraine2@illinois.edu.
R. Davis Moore, Email: rdmoore@illinois.edu.
Brian J. Saliba, Email: saliba1@illinois.edu.
Matthew B. Pontifex, Email: pontifex@msu.edu.
Charles H. Hillman, Email: chhillma@illinois.edu.
References
- Alloway T.P., Gathercole S.E., Kirkwood H., Elliott J. The cognitive and behavioral characteristics of children with low working memory. Child Dev. 2009;80(2):606–621. doi: 10.1111/j.1467-8624.2009.01282.x. [DOI] [PubMed] [Google Scholar]
- American College of Sports Medicine . 8th ed. Lippincott Williams & Wilkins; New York: 2010. ACSM's Guidelines for Exercise Testing and Prescription. [Google Scholar]
- Andersen R.E., Crespo C.J., Bartlett S.J., Cheskin L.J., Pratt M. Relationship of physical activity and television watching with body weight and level of fatness among children: results from the Third National Health and Nutrition Examination Survey. J. Am. Med. Assoc. 1998;279:938–942. doi: 10.1001/jama.279.12.938. [DOI] [PubMed] [Google Scholar]
- Bar-Or O. Springer-Verlag; New York: 1983. Pediatric Sports Medicine for the Practitioner: From Physiologic Principles to Clinical Applications. [Google Scholar]
- Best J.R. Exergaming immediately enhances children's executive function. Dev. Psychol. 2012;48:1501–1510. doi: 10.1037/a0026648. [DOI] [PubMed] [Google Scholar]
- Birnbaum A.S., Lytle L.A., Murray D.M., Story M., Perry C.L., Boutelle K.N. Survey development for assessing correlates of young adolescents’ eating. Am. J. Health Behav. 2002;26:284–295. doi: 10.5993/ajhb.26.4.5. [DOI] [PubMed] [Google Scholar]
- Butte N.F., Christiansen E., Sorenson T.I. Energy imbalance underlying the development of childhood obesity. Obesity (Silver Spring) 2007;15:3056–3066. doi: 10.1038/oby.2007.364. [DOI] [PubMed] [Google Scholar]
- Castelli D.M., Hillman C.H., Buck S.M., Erwin H.E. Physical fitness and academic achievement in third- and fifth-grade students. J. Sport Exerc. Psychol. 2007;29:239–252. doi: 10.1123/jsep.29.2.239. [DOI] [PubMed] [Google Scholar]
- Center for Education Policy . Center for Education Policy; Washington, DC: 2007. Choices, Changes, and Challenges: Curriculum and Instruction in the NCLB Era. [Google Scholar]
- Centers for Disease Control and Prevention Trends in the prevalence of extreme obesity among US preschool-aged children living in low-income families, 1998–2010. J. Am. Med. Assoc. 2012;308(24):2563–2565. doi: 10.1001/jama.2012.108099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatrian G.E., Lettich E., Nelson P.L. Ten percent electrode system for topographic studies of spontaneous and evoked EEG activity. Am. J. EEG Technol. 1985;25:83–92. [Google Scholar]
- Chomitz V.R., Slining M.M., McGowan R.J., Mitchell S.E., Dawson G.F., Hacker K.A. Is there a relationship between physical fitness and academic achievement? Positive results from public school children in the northeastern United States. J. School Health. 2009;79:30–37. doi: 10.1111/j.1746-1561.2008.00371.x. [DOI] [PubMed] [Google Scholar]
- Christakou A., Halari R., Smith A.B., Ifkovits E., Brammer M., Rubia K. Sex-dependent age modulation of frontostriatal and temporo-parietal activation during cognitive control. NeuroImage. 2009;48:223–236. doi: 10.1016/j.neuroimage.2009.06.070. [DOI] [PubMed] [Google Scholar]
- Compumedics Neuroscan . Compumedics Neuroscan; El Paso, TX.: 2003. Offline Analysis of Acquired Data (SCAN 4.3—Vol. II, EDIT 4.3) [Software Manual] [Google Scholar]
- Corbetta M., Shulman G.L. Control of goal-directed and stimulus-driven attention in the brain. Nat. Rev. Neurosci. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Davidson M.C., Amso D., Anderson L.C., Diamond A. Development of cognitive control and executive functions from 4 to 13 years: evidence from manipulations of memory, inhibition, and task switching. Neuropsychologia. 2006;44:2037–2078. doi: 10.1016/j.neuropsychologia.2006.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond A. The early development of executive functions. In: Bialystok E., Craik F.I., editors. Lifespan Cognition: Mechanisms of Change. Oxford University Press; New York: 2006. pp. 70–95. [Google Scholar]
- Diamond A., Lee K. Interventions shown to aid executive function development in children 4 to 12 years old. Science. 2011;333:959–964. doi: 10.1126/science.1204529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donchin E. Surprise!… Surprise? Psychophysiology. 1981;18:493–513. doi: 10.1111/j.1469-8986.1981.tb01815.x. [DOI] [PubMed] [Google Scholar]
- Donnelly J.E., Greene J.L., Gibson C.A., Smith B.K., Washburn R.A., Sullivan D.K. Physical activity across the curriculum (PAAC): a randomized controlled trial to promote physical activity and diminish overweight and obesity in elementary school children. Prev. Med. 2009;49:336–341. doi: 10.1016/j.ypmed.2009.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drollette E.S., Shishido T., Pontifex M.B., Hillman C.H. Maintenance of cognitive control during and after walking in preadolescent children. Med. Sci. Sports Exerc. 2012;10:2017–2024. doi: 10.1249/MSS.0b013e318258bcd5. [DOI] [PubMed] [Google Scholar]
- Duncan-Johnson C.C. P3 latency: a new metric of information processing. Psychophysiology. 1981;18:207–215. doi: 10.1111/j.1469-8986.1981.tb03020.x. [DOI] [PubMed] [Google Scholar]
- DuPaul G.J., Power T.J., Anastopoulos A.D., Reid R. Guilford Press; New York: 1998. ADHD Rating Scale-IV: Checklists, Norms, and Clinical Interpretation. [Google Scholar]
- Egner T., Hirsch J. Cognitive control mechanisms resolve conflict through cortical amplification of task-relevant information. Nat. Neurosci. 2005;8:1784–1790. doi: 10.1038/nn1594. [DOI] [PubMed] [Google Scholar]
- Engle R.W., Kane M.J. Executive attention, working memory capacity, and a two-factor theory of cognitive control. In: Ross B., editor. vol. 44. Elsevier; New York: 2004. pp. 145–199. (The Psychology of Learning and Motivation). [Google Scholar]
- Eriksen B.A., Eriksen C.W. Effects of noise letters upon the identification of a target letter in a nonsearch task. Percept. Psychophys. 1974;16:143–149. [Google Scholar]
- Folstein J.R., Van Petten C. Influence of cognitive control and mismatch on the N2 component of the ERP: a review. Psychophysiology. 2008;45:152–170. doi: 10.1111/j.1469-8986.2007.00602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedson P.S., Goodman T.L. Measurement of oxygen consumption. In: Rowland T.W., editor. Pediatric Laboratory Exercise Testing: Clinical Guidelines. Human Kinetics; Champaign, IL: 1993. pp. 91–113. [Google Scholar]
- Gathercole S.E., Alloway T.P., Kirkwood H.J., Elliot J.G., Holmes J., Hilton K.A. Attentional and executive function behaviors in children with poor working memory. Learn. Individ. Differ. 2008;18:214–223. [Google Scholar]
- Hillman C.H., Erickson K.I., Kramer A.F. Be smart, exercise your heart: exercise effects on brain and cognition. Exerc. Brain Cogn. 2008;9:58–65. doi: 10.1038/nrn2298. [DOI] [PubMed] [Google Scholar]
- Hillman C.H., Pontifex M.B., Motl M.B., O‘Leary K.C., Johnson C.R., Scudder M.R. From ERP's to academics. Dev. Cogn. Neurosci. 2012;2S:S90–S98. doi: 10.1016/j.dcn.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillman C.H., Pontifex M.B., Raine L.B., Castelli D.M., Hall E.E., Kramer A.F. The effect of acute treadmill walking on cognitive control and academic achievement in preadolescent children. Neuroscience. 2009;159:1044–1054. doi: 10.1016/j.neuroscience.2009.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillman C.H., Snook E.M., Jerome G.J. Acute cardiovascular exercise and executive control function. Int. J. Psychophysiol. 2003;48:307–314. doi: 10.1016/s0167-8760(03)00080-1. [DOI] [PubMed] [Google Scholar]
- Jolicoeur P., Brisson B., Robitaille N. Dissociation of the N2pc and sustained posterior contralateral negativity in a choice response task. Brain Res. 2008;1215:160–172. doi: 10.1016/j.brainres.2008.03.059. [DOI] [PubMed] [Google Scholar]
- Kamijo K., Nishihira Y., Hatta A., Kaneda T., Wasaka T., Kida T. Differential influences of exercise intensity on information processing in the central nervous system. Eur. J. Appl. Physiol. 2004;92:305–311. doi: 10.1007/s00421-004-1097-2. [DOI] [PubMed] [Google Scholar]
- Kamijo K., Nishihira Y., Higashiura T., Kuroiwa K. The interactive effect of exercise intensity and task difficulty on human cognitive processing. Int. J. Psychophysiol. 2007;65:114–121. doi: 10.1016/j.ijpsycho.2007.04.001. [DOI] [PubMed] [Google Scholar]
- Kaufman A.S., Kaufman N.L. American Guidance Service; Circle Pines, MN: 1990. Kaufman Brief Intelligence Test Manual. [Google Scholar]
- Kibbe D.L., Hackett J., Hurley M., McFarland A., Schubert K.G., Schultz A. Ten years of TAKE 10!: integrating physical activity with academic concepts in elementary school classrooms. Prev. Med. 2011;52:S43–S50. doi: 10.1016/j.ypmed.2011.01.025. [DOI] [PubMed] [Google Scholar]
- Kutas M., McCarthy G., Donchin E. Augmenting mental chronometry: the P300 as a measure of stimulus evaluation. Science. 1977;197:792–795. doi: 10.1126/science.887923. [DOI] [PubMed] [Google Scholar]
- Luck S.J., Hillyard S.A. Effects of spatial cuing on luminance detectability: psychophysical and electrophysiological evidence for early selection. J. Exp. Psychol. Hum. Percept. Perform. 1994;20:1000–1014. doi: 10.1037//0096-1523.20.4.887. [DOI] [PubMed] [Google Scholar]
- Mahar M., Murphy S., Rowe D., Golden J., Shields A., Raedeke T. Effects of a classroom-based program on physical activity and on-task behavior. Med. Sci. Sports Exerc. 2006;38:2086–2094. doi: 10.1249/01.mss.0000235359.16685.a3. [DOI] [PubMed] [Google Scholar]
- McClelland M.M., Morrison F.J., Holmes D.L. Children at risk for early academic problems: the role of learning-related social skills. Early Child. Res. Q. 2000;15(3):307–329. [Google Scholar]
- Miyake A., Friedman N.P., Emerson M.J., Witzki A.H., Howerter A. The unity and diversity of executive functions and their contributions to complex Frontal Lobe tasks: a latent variable analysis. Cogn. Psychol. 2000;41:49–100. doi: 10.1006/cogp.1999.0734. [DOI] [PubMed] [Google Scholar]
- National Association for Sport and Physical Education . National Association for Sport and Physical Education; Reston, VA: 2008. Comprehensive School Physical Activity Programs [Position Statement] [Google Scholar]
- Ng S.W., Popkin B.M. Time use and physical activity: a shift away from movement across the globe. Obes. Rev. 2012;13:659–680. doi: 10.1111/j.1467-789X.2011.00982.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitsche M.A., Cohen L.G., Wassermann E.M., Priori A., Lang N., Antal A. Transcranial direct current stimulation: state of the art 2008. Brain Stimul. 2008;1:206–223. doi: 10.1016/j.brs.2008.06.004. [DOI] [PubMed] [Google Scholar]
- Norman D.A., Shallice T. Attention to action: willed and automatic control of behavior. In: Davidson R.J., Schwartz G.E., Shapiro D., editors. vol. 4. Plenum Press; New York: 1986. pp. 1–18. (Consciousness and Self-Regulation: Advances in Research and Theory). [Google Scholar]
- Ohn S.H., Park C., Yoo W., Ko M., Choi K.P., Kim G. Time-dependent effect of transcranial direct current stimulation on the enhancement of working memory. Cogn. Neurosci. Neuropsychol. 2008;19(1):43–47. doi: 10.1097/WNR.0b013e3282f2adfd. [DOI] [PubMed] [Google Scholar]
- Oldfield R.C. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Polich J. Updating p300: an integrative theory of p3a and p3b. Clin. Neurophysiol. 2007;118:2128–2148. doi: 10.1016/j.clinph.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polich J., Howard L., Starr A. P300 latency correlates with digit span. Psychophysiology. 1983;20:665–669. doi: 10.1111/j.1469-8986.1983.tb00936.x. [DOI] [PubMed] [Google Scholar]
- Pontifex M.B., Saliba B.J., Raine L.B., Picchietti D.L., Hillman C.H. Exercise improves behavioral, neurocognitive, and scholastic performance in children with attention-deficit/hyperactivity disorder. J. Pediatr. 2013;162:543–551. doi: 10.1016/j.jpeds.2012.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Querido J.S., Sheel A.W. Regulation of cerebral blood flow during exercise. Sports Med. 2007;37:765–785. doi: 10.2165/00007256-200737090-00002. [DOI] [PubMed] [Google Scholar]
- Rogers R.D., Monsell S. Cost of a predictable switch between simple cognitive tasks. J. Exp. Psychol. Gen. 1995;124:207–231. [Google Scholar]
- Schmitt B.M., Münte T.F., Kutas M. Electrophysiological estimates of the time course of semantic and phonological encoding during implicit picture naming. Psychophysiology. 2000;37:473–484. [PubMed] [Google Scholar]
- Sibley B.A., Beilock S.L. Exercise and working memory: an individual differences investigation. J. Sport Exerc. Psychol. 2007;29:783–791. doi: 10.1123/jsep.29.6.783. [DOI] [PubMed] [Google Scholar]
- Sibley B.A., Etnier J.L. The relationship between physical activity and cognition in children: a meta-analysis. Pediatr. Exerc. Sci. 2003;15:243–256. [Google Scholar]
- Tanner J.M. Blackwell Scientific Publications; Oxford: 1962. Growth at Adolescence: With a General Consideration of the Effects of Hereditary and Environmental Factors Upon Growth and Maturation from Birth to Maturity. [Google Scholar]
- Taylor S.J.C., Whincup P.H., Hindmarsh P.C., Lampe F., Odoki K., Cook D.G. Performance of a new pubertal self-assessment questionnaire: a preliminary study. Pediatr. Perinat. Epidemiol. 2001;15:88–94. doi: 10.1046/j.1365-3016.2001.00317.x. [DOI] [PubMed] [Google Scholar]
- Thomas S., Reading J., Shephard R.J. Revision of the Physical Activity Readiness Questionnaire (PAR-Q) Can. J. Sport Sci. 1992;17:338–345. [PubMed] [Google Scholar]
- Tomporowski P.D. Cognitive and behavioral responses to acute exercise in youths: a review. Pediatr. Exerc. Sci. 2003;15:348–359. [Google Scholar]
- Tseng P., Hsu T., Chang C., Tzeng O.L., Hung D.L., Muggleton N.G. Unleashing potential: transcranial direct current stimulation over the right posterior parietal cortex improves change detection in low-performing individuals. J. Neurosci. 2012;32(31):10554–10561. doi: 10.1523/JNEUROSCI.0362-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utter A.C., Roberson R.J., Nieman D.C., Kang J. Children's OMNI scale of perceived exertion: walking/running evaluation. Med. Sci. Sports Exerc. 2002;34:139–144. doi: 10.1097/00005768-200201000-00021. [DOI] [PubMed] [Google Scholar]
- van Praag H., Kempermann G., Gage F.H. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat. Neurosci. 1999;2:266–270. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- Vaynman S., Gomez-Pinilla F. License to run: exercise impacts functional plasticity in the intact and injured central nervous system by using neurotrophins. Neurorehabil. Neural Repair. 2005;19:283–295. doi: 10.1177/1545968305280753. [DOI] [PubMed] [Google Scholar]
- Verleger R. On the utility of P3 latency as an index of mental chronometry. Psychophysiology. 1997;34:131–156. doi: 10.1111/j.1469-8986.1997.tb02125.x. [DOI] [PubMed] [Google Scholar]