Highlights
-
•
Studied fear acquisition and extinction in offspring of anxious and depressed mothers.
-
•
Offspring of anxious mothers showed larger reactivity during fear extinction.
-
•
Offspring of depressed mothers showed attenuated reactivity during fear acquisition.
-
•
Suggests distinct premorbid risk markers due to maternal anxiety and depression.
Keywords: Children, Anxiety, Depression, High risk, Conditioning, Extinction, Skin conductance
Abstract
Maternal anxiety and depression are significant risk factors for the development of these disorders in offspring. The pathways through which risk is conferred remain unclear. This study examined fear acquisition and extinction in 26 children at high risk for emotional disorders by virtue of maternal psychopathology (n = 14 with a mother with a principal anxiety disorder and n = 12 with a mother with a principal unipolar depressive disorder) and 31 low risk controls using a discriminative Pavlovian conditioning procedure. Participants, aged between 7 and 14 years, completed 16 trials of discriminative conditioning of two geometric figures, with (CS+) and without (CS−) an aversive tone (US), followed by 8 extinction trials (4 × CS+, 4 × CS−). In the context of comparable discriminative conditioning, children of anxious mothers showed larger skin conductance responses during extinction to the CS+ compared to the CS−, and to both CSs from the first to the second block of extinction trials, in comparison with low risk controls. Compared to low risk controls, children of depressed mothers showed smaller skin conductance responses to the CS+ than the CS− during acquisition. These findings suggest distinct psychophysiological premorbid risk markers in offspring of anxious and depressed mothers.
1. Introduction
Anxiety and depression are two of the most common mental health problems affecting children, with 10–20% of school-aged children experiencing emotional disorders during their young lives (Mathews et al., 2011). These disorders cause life-long impairment (Bittner et al., 2007), and are costly to families (Bodden et al., 2008) and national health care systems (Andrews et al., 2004). Parental anxiety and/or depressive disorders are significant risk factors for the development of these disorders in offspring (e.g., Goodman and Gotlib, 1999, Hammen et al., 1990, Rapee et al., 2009, Weissman et al., 1987). Parental depressive disorders are associated with a threefold increase in an individual's risk for developing a depressive episode during adolescence (Hammen, 1997, Williamson et al., 2004). Maternal depression is associated with an earlier onset and more severe course of depression in offspring (Lieb et al., 2002; for a recent review see Gotlib et al., 2006). Similarly, offspring of parents with anxiety disorders are at 3.5 (range 1.3–13.3) times greater risk for anxiety disorders than are offspring of control parents (e.g., Merikangas et al., 1999). Therefore, the investigation of offspring of parents with anxiety and depressive disorders is a powerful strategy to identify premorbid risk markers and early signs of expressions of these conditions.
Learning models emphasise that anxiety develops through the association of a conditioned stimulus (CS+) and an aversive unconditional stimulus (US), and conversely, that anxiety extinguishes through repeated presentation of the CS+ in the absence of the US, which is the underlying theoretical framework of exposure therapy (e.g., Bouton et al., 2001, Craske et al., 2009, Davis et al., 2000, Fid, 2006, Grillon, 2002, Mineka and Öhman, 2002, Mineka and Zinbarg, 2006, Rachman, 1977; see Vervliet et al., 2012 or Boschen et al., 2009 for reviews). A recent meta-analysis (i.e., Lissek et al., 2005) concluded that within discriminative conditioning studies that require both excitatory responding to a CS+ paired with a US and inhibitory responding to a CS− presented alone, both anxious adults and control adults display comparable levels of differential conditioning, as reflected by larger skin conductance and subjective responses to the CS+ than the CS−. However, anxious adults show overall elevated responses to both CSs compared with control adults, and maintain modestly higher levels of conditioned responding during extinction trials (although this group difference is strongest in CS+ only conditioning procedures) (Lissek et al., 2005).
Basic science research has found similar evidence of larger responding to the CS+ as well as overall elevated responses to both CSs in anxious compared to non-anxious children using discriminative conditioning and extinction experiments (e.g., Craske et al., 2008, Lau et al., 2008, Liberman et al., 2006, Waters et al., 2009). These findings have been interpreted within an associative framework that emphasises elevated fear responding to excitatory cues of threat (i.e., CS+) and impaired response inhibition to signals of safety (CS− and extinction trials) in the pathogenesis of anxiety disorders (e.g., Davis et al., 2000; see Lissek et al., 2005). Another associative account for the findings is overgeneralisation from the CS+ to the CS− due to failure to discriminate the stimulus features that distinguish threat from safety cues (see Lissek et al., 2005, for a review). Non-associative explanations of elevated responding to both CS+ and CS− primarily focus on sensitisation, or elevated responsiveness to the US and other novel stimuli due to elevated anxious state, and habituation, or decreased responding over repeated presentations of specific stimuli (Lissek et al., 2005).
To date, there is one published study to the authors’ knowledge that examines aversive conditioning and extinction in high risk children by virtue of parental anxiety disorders. Craske et al. (2008) found that in the context of similar levels of differential conditioning, as indexed by larger skin conductance responses (SCRs) and subjective evaluations of the CS+ compared to the CS−, high risk children exhibited larger SCRs to the timing of the US on CS+ and CS− trials during acquisition, and larger orienting SCRs to both CSs during extinction trials, in comparison with low risk controls. There were no significant differences in subjective arousal and valence (unpleasantness) ratings between the groups. Overall, findings indicated that children at high risk for anxiety due to parental anxiety displayed larger psychophysiological responses to stimuli signalling threat (CS+) that generalise to cues signalling safety (CS−) and are slower to extinguish in comparison with low risk controls.
Anxiety and depression share numerous risk factors, high rates of comorbidity, and treatment approaches (see Craske and Waters, 2005). However, the specificity of aetiological processes to these disorders is not well understood and an enhanced understanding of common and specific underlying mechanisms would improve knowledge on the pathophysiology of these disorders. To the authors’ knowledge, there are no published studies to date that examine differences in aversive conditioning and extinction in offspring of anxious versus depressed and healthy parents. Therefore, the specificity of these learning-based processes as mechanisms by which risk due to parental anxiety versus depression is conferred to offspring remains unclear. However, recent reviews of neurophysiological studies suggest there may be distinct neurophysiological indicators of depression (e.g., Vaidyanathan et al., 2012). For example, findings from startle eye blink modulation experiments suggest that depression is associated with decreased fear-potentiated startle, and a flattened affect-startle reflex pattern compared to never-depressed healthy controls (e.g., Allen et al., 1999, Brown et al., 1998, Kaviani et al., 2004, McTeague et al., 2009; see also Vaidyanathan et al., 2012). On the other hand, first and second generation offspring of depressed parents compared to low risk offspring showed increased startle reactivity throughout fear-potentiation protocols, similar to that found in anxious adults and offspring of parents with anxiety disorders (e.g., Grillon et al., 2005). In terms of skin conductance measures, evidence suggests that depression, especially endogenous depression, may be associated with lower skin conductance levels and more patients who are SCR non-responders to unpleasant auditory stimuli compared to healthy controls (Lader and Wing, 1964, Mirkin and Coppen, 1980). Varied findings may be due to differing components of depression (e.g., endogenous versus negative affect versus anhedonia), variation in symptom severity, and the methodology and psychophysiological measures employed (see Vaidyanathan et al., 2012 for a review). Nevertheless, these findings primarily point to depression being associated with attenuated psychophysiological responding to negative/stressful stimuli, or the anticipation thereof, and failure to show appropriate reactivity to pleasant stimuli. Together, these findings suggest that depressed individuals may be relatively unaffected by external stimulation (Mirkin and Coppen, 1980), perhaps through the breadth and incessant nature of depressive disorders taking their toll on the human defensive system, thereby reducing psychophysiological reactivity to salient emotional cues (McTeague et al., 2012). Thus, of interest in the present study was whether attenuated psychophysiological responding is a premorbid risk marker that develops during the acquisition of aversive learning in the offspring of depressed parents relative to low risk offspring of never depressed parents.
Based on theoretical accounts of anxiety and depressive disorders (Bouton et al., 2001, Craske et al., 2009, Davis et al., 2000, Fid, 2006, Goodman and Gotlib, 1999, Grillon, 2002, Hammen, 1991, Mineka and Öhman, 2002, Mineka and Zinbarg, 2006, Rachman, 1977), the significant comorbidity between them (see Craske and Waters, 2005), and the risk that these disorders pose to offspring of affected parents (see Rapee et al., 2009, Hammen et al., 1990, Weissman et al., 1987), the current study examined aversive Pavlovian conditioning and extinction in the offspring of anxious and depressed mothers compared to mothers without a history of psychopathology. Based on previous evidence (i.e., Craske et al., 2008), it was hypothesised that in the context of similar levels of discriminative conditioning, children of anxious mothers would show larger SCRs to both CSs during acquisition and extinction, in comparison with low risk controls and offspring of depressed mothers. In contrast, if diminished psychophysiological reactivity to aversive cues that is characteristic of depression (e.g., Mirkin and Coppen, 1980, Allen et al., 1999, Kaviani et al., 2004, McTeague et al., 2009) is a risk marker for the transmission of depression to offspring, then it was hypothesised that children of depressed mothers would show smaller SCRs to the CS+ compared to the CS− during acquisition and relative to low risk children and offspring of anxious mothers. Moreover, if they remain unresponsive to external stimulation, they were expected to display attenuated responding throughout extinction in comparison with the other groups.
2. Method
2.1. Participants
One hundred and nineteen parent–child dyads were initially assessed to participate in this study which was approved by the Griffith University Human Research Ethics Committee. They were recruited through community advertisements, primary school and university notices and newsletters, local newspapers, GPs, and community mental health clinics as part of a larger study on risk factors for the development of emotional disorders in children. Initial exclusion criteria included (a) the child having a psychiatric disorder, including an anxiety or mood disorder, chronic medical condition, intellectual impairment, pervasive developmental disorder, bipolar disorder, oppositional defiant disorder or psychosis, (b) the mother having a past or current chronic medical condition, intellectual impairment, bipolar disorder, psychosis or any psychiatric disorder other than anxiety and unipolar depression, and (c) if the participating parent was not the child's biological mother. Of the 119 dyads assessed, 28 were excluded due to the child meeting criteria for an anxiety disorder (they were referred for treatment); 3 were excluded due to incomplete diagnostic assessment data, 3 were excluded due to the child's biological mother being unable to participate (2 mothers due to divorce; 1 mother was deceased), 8 were excluded because families failed to attend the laboratory session, 11 children withdrew from or declined to complete the conditioning and extinction experiment, and 9 children had unusable skin conductance data either due to technical problems (n = 4) or too much missing data due to movement artefacts (n = 5).
Thus, the final sample of 55 children included 31 low-risk children (mothers and children without psychiatric disorder) and 26 high-risk children, of which 14 had mothers with a principal lifetime diagnosis of an anxiety disorder and 12 had mothers with a principal lifetime diagnosis of a depressive disorder, with their children having no psychiatric disorder. Table 1 summarises the principal (i.e., most severe) lifetime diagnoses of mothers in the ANX and DEP groups. Five children were prescribed Ventolin for asthma. No children were on medication at the time of assessment. Demographic comparisons are reported in Table 2.
Table 1.
Principal diagnoses of mothers in the ANX and DEP groups.
| Principal diagnosis | Current diagnosis (N = 9) |
Past diagnosis (N = 17) |
||
|---|---|---|---|---|
| n | Mean severity | n | Mean severity | |
| ANX (n = 14) | 9 | 6.2 | 5 | 7.5 |
| Generalised anxiety | 3 | 6 | 1 | 8 |
| Obsessive–compulsive | 0 | – | 2 | 8 |
| Social phobia | 2 | 6 | 0 | – |
| Specific phobia | 2 | 7 | 0 | – |
| Panic | 1 | 6 | 0 | – |
| Separation anxiety | 0 | – | 1 | 6 |
| PTSD | 1 | 6 | 1 | 8 |
| DEP (n = 12) | 0 | 0 | 12 | 6.72 |
| Major depression | 0 | – | 11 | 6.45 |
| Depressive disorder NOS | 0 | – | 1 | 7 |
Note. Mean severity = 0 (no interference) to 8 (very severely interfering).
Table 2.
Descriptive demographic and symptom measures as a function of group.
| Measure | CON (n = 31) | ANX (n = 14) | DEP (n = 12) |
|---|---|---|---|
| Age | 9.56 (1.48) | 9.77 (1.33) | 9.90 (1.47) |
| Gender (M:F) | 14:17 | 7:7 | 4:8 |
| Country born (% Australia) | 85 | 86 | 82 |
| Mother marital status (% married)* | 96 | 79 | 58 |
| Parent age | |||
| Mother | 42.1 (4.7) | 41.8 (5.4) | 42.3 (4.9) |
| Father | 44.3 (6.9) | 45.6 (6.6) | 43.4 (6.1) |
| Parent SESa | |||
| Mother | 4.12 (1.31) | 4.96 (1.06) | 4.06 (0.70) |
| Father | 3.94 (0.96) | 4.04 (0.88) | 3.76 (0.91) |
| Mother STAI trait* | 30.03 (7.27) | 37.29 (8.94) | 38.00 (7.60) |
| SCAS-P total | 12.48 (8.84) | 12.07 (5.75) | 10.33 (5.55) |
| SCAS-C total | 22.58 (13.87) | 26.07 (13.51) | 21.33 (14.76) |
| CES-DC total | 11.77 (7.37) | 11.14 (4.61) | 10.67 (6.27) |
| CS−US contingency aware (%) | 76% | 57% | 83% |
| Child subjective anxiety | |||
| Pre-Acq | 1.74 (1.76) | 1.50 (1.56) | 1.00 (1.35) |
| Post-Acq | 1.84 (1.92) | 1.64 (1.55) | 1.50 (1.51) |
| Post-Ext | 0.74 (1.34) | 0.64 (0.93) | 0.50 (0.80) |
Significant difference between low-risk and high-risk groups.
Occupation prestige assessed with Daniel Prestige Scale (Daniel, 1983) (range: 1 = high; 7 = low).
2.2. Measures
2.2.1. Maternal diagnostic status
The Anxiety Disorders Interview Schedule for DSM-IV, Lifetime Version (ADIS-IV-L; Brown et al., 1994) is a semi-structured interview that assesses current episodes of DSM-IV anxiety, mood and substance use disorders in addition to past (i.e., lifetime) episodes of these disorders. Clinical postgraduate students who had undergone specialised training in administering the ADIS-IV-L conducted the interviews. Mothers were asked to respond to a series of questions relating to their experience of symptoms of various psychological disorders at present and in the past. If mothers endorsed enough symptoms, they rated the degree of interference caused by the symptoms for both current and past diagnoses. A scale from 0 to 8 was used where 0 represented no interference and 8 represented very severe interference. Criteria for a disorder were met if a prescribed number of symptoms were endorsed and a clinician severity rating (CSR) of four or greater was assigned based on symptoms, distress and interference (Brown et al., 2001). The ADIS-IV-L has demonstrated sound psychometric properties, with good inter-rater reliability (Brown et al., 2001). The ADIS-IV-L was administered in person or over the telephone. The lifetime diagnosis (past or present) with the highest clinician severity rating was considered to be the principal (i.e., most severe) diagnosis. All ADIS-IV-L diagnoses were reviewed in supervision and 20% of all interviews were audiotaped and coded by an independent rater for reliability purposes. Inter-rater reliability was excellent (e.g., principal diagnosis κ = .89; second diagnosis κ = .82).
2.2.2. Child diagnostic status
The Anxiety Disorders Interview Schedule for DSM-IV, Parent version (ADIS-P; Silverman and Albano, 1996) was used to assess the presence/absence of psychiatric disorders in children. Children were considered to have an anxiety disorder if they met DSM-IV criteria with a clinical severity rating (CSR) of four or higher (scale 0–8), for at least their principal anxiety diagnosis (i.e., most severe at presentation). The ADIS-P was administered over the telephone. The telephone version of the ADIS is as reliable as face-to-face administration (Lyneham and Rapee, 2005). The ADIS has demonstrated excellent reliability and strong concurrent validity with other measures of childhood anxiety (Silverman et al., 2001, Wood et al., 2002). The ADIS-P was administered by postgraduate clinical students trained by clinical psychologists experienced in anxiety assessment and ADIS administration and reviewed in supervision. Twenty percent of interviews were audio-taped and coded by an independent rater blind to children's diagnostic status. Inter-rater reliability showed excellent agreement for both disorders present and absent (e.g., disorders present: principal diagnosis κ = .84; second diagnosis κ = .83; third diagnosis κ = .85).
2.2.3. Maternal symptom measure
The Trait Scale of the State-Trait Anxiety Inventory (STAI Trait; Spielberger et al., 1970) is a 20 item self-report measure that was completed by mothers to assess their anxiety proneness and their tendency to respond with anxiety to perceived threats in the environment. Each of the items is assessed on a four point scale with respondents indicating the extent to which the statement applies to them generally, ranging from 1 (almost never) to 4 (almost always). The STAI has good psychometric properties (e.g., Spielberger et al., 1983, Spielberger, 1989).
2.2.4. Child symptom measures
The Spence Children's Anxiety Scale, Parent and Child version (SCAS-P and SCAS-C; Spence, 1998). The SCAS-P (39-item parent report measure) and SCAS-C (45-item child self-report measure; 6 positive filler items), both contain 4-point response scales (0 = never true to 3 = always true), yield total scores reflecting symptom severity, and possess sound psychometric properties. Mean SCAS-P total scores of 14.2 and 31.8, and mean SCAS-C total scores of 18.8 and 32.2 are reported for non-clinical and clinically anxious children, respectively (Nauta et al., 2004, Spence, 1998).
The Centre for Epidemiologic Studies Depression Scale for Children (CES-DC; Weissman et al., 2006) is a 20-item self-report inventory to measure depressive symptoms in children aged 7–17 years. Children are asked to respond on a four-point scale (0 = not at all to 3 = a lot) indicating how frequently the items have happened to them in the past week (e.g., I was bothered by things that usually don’t bother me). Scores range from 0 to 60 with total score of 15 or higher considered to be clinically significant. The CES-DC has adequate reliability and validity (Faulstich et al., 1986).
2.3. Conditioning and extinction task
2.3.1. Electrophysiological materials and equipment
The US was an unpleasant tone; 1-s, 1000-Hz pure tone set at 100 dB and delivered through Sony stereophonic headphones (see Waters et al., 2009 for data on intensity and unpleasantness ratings). The CSs were geometric shapes, a pastel pink trapezoid (CS+) and a pastel cream triangle (CS−), an oval and octagon were used as control shapes. The CS shapes were presented for 8 s, either to the left or right of a central fixation cross. The geometric shapes were presented equal number of trials, on a Dell 19″ colour monitor at a distance of approximately 1 m and a visual angle that averaged 9.6 degrees.
Skin conductance responses (SCR) were recorded during the conditioning task using Ag/AgCI electrodes placed on the middle and index fingers of the participant's non-dominant hand as well as a ground electrode placed in the centre of the forehead. SCR data was acquired using a Grass Technologies amplifier system (Model 15RXI) and were digitised and sampled online using the LabVIEW programming software (Version 7; National Instruments Corporation, 2003) which was installed on a Dell Precision workstation computer. SCR data was DC amplified at 2000 Hz.
2.3.2. Verbal ratings
Participants rated the CS geometric shapes comprising a trapezoid and a triangle, prior to and after acquisition, and following extinction. Ratings were made using a self-assessment mannequin (SAM), which illustrated cartoon like figures, providing subjective ratings of their valence (unpleasant − pleasant) and arousal (calm – worked up) of the four shapes. Arousal was rated using a one tailed likert scale ranging from 1 (calm) to 9 (very aroused), whereas valence was rated using a two tailed likert scale ranging from 1 (very pleasant) to 5 (neutral) to 9 (very unpleasant) (Center for the Study of Emotion and Attention, 1999). Children also rated their subjective level of anxiety prior to and after acquisition, and following extinction, using a one tailed likert scale ranging from 0 (not at all) to 10 (very anxious).
2.3.3. Contingency awareness
Upon completion of the acquisition phase, children were asked whether they noticed if the US tone, which was delivered through the headphones, coincided with any of the shapes presented. If the child responded with “Yes”, they were then asked to identify which shape was presented with the tone and responses were recorded verbatim. Contingency awareness was considered to be achieved if the child identified that the tone was paired with the correct shape.
2.4. Procedure
When mothers contacted the research team in response to study advertisements, information about the study was provided and information and parent consent forms were emailed or posted to interested families. Upon return of these forms, the participating mother was screened over the telephone about their own current and past anxiety and depression and that of their participating child. The psychopathology of children was assessed next using the ADIS-P-IV (Silverman and Albano, 1996). If the child did not meet criteria for any psychological disorder, the mother was then assessed with the ADIS-IV-L (Brown et al., 1994) regarding their own current and past psychiatric status. This was conducted either via the telephone (N = 27) or at the university clinic (N = 61). Mothers were asked whether the biological father had ever had anxiety or depression or had been treated for either of these disorders. Only families in which the biological father was reported not to have any psychiatric disorders were included.
Children of mothers meeting criteria for a principal lifetime anxiety disorder were assigned to the ANX group, while children of mothers meeting criteria for a principal lifetime unipolar depressive disorder were allocated to the DEP group, while children of mothers with no lifetime psychiatric diagnoses were allocated to the CON group.
2.4.1. Experimental assessment of children and mothers
Experimental sessions were carried out in a research laboratory during which a research assistant supervised children at all times. The experimental session began with an orienting period during which mothers and children were familiarised with the laboratory. The tasks to be completed were explained to both the child and mother and children were informed that they were able to withdraw at any time. After children's assent to participate in the experiment was obtained, mothers moved to another room where they completed questionnaires. Electrode recording devices were attached to the child and they were seated alone in an experimental room within the laboratory, interconnected with a closed-circuit camera.
Prior to the Pavlovian conditioning and extinction task, children were asked to rate their subjective anxiety and valence and arousal for the CSs using the SAM. They were then instructed to pay attention to the shapes presented one at a time on the computer screen and loud tones presented through headphones.
Acquisition phase. The procedure was identical to that used by Waters et al. (2009) and Craske et al. (2008). During the acquisition phase, children received 16 conditioning trials (eight CS+ and eight CS−) presented in random order with the caveat that no more than two trials of either the CS+ or CS− were presented sequentially, and that the first two trials were a CS+ and a CS−. The CS+ and CS− were presented on either side of a fixation cross which remained on the screen from CS offset to onset to help maintain children's focus. The CSs were presented for 8 s. On CS+ trials, US onset was at 7 s and offset at 8 s with the CS+ offset and the US was paired with the CS+ on 100% of CS+ trials. The CS− was always presented alone. The inter-trial interval (from CS onset to CS offset) varied from 20 to 30 s (mean 25 s). After the acquisition trials, participants’ contingency awareness was assessed and they were asked to rerate their subjective anxiety and the four geometric shapes using the SAM. They were then instructed to leave the headphones on and continue paying attention for the next phase of the experiment.
Extinction phase. The extinction phase consisted of eight trials: four CS+ trials without the US pairing and four CS− trials. The trials were presented in random order with no more than two sequential presentations of either the CS+ or CS−. The CSs were presented an equal number of times to the left and right of the central fixation cross. After the extinction trials, subjective anxiety levels and the SAM ratings of the four geometric shapes were completed again.
Afterwards, the electrodes were removed and the research assistant read aloud the items from the child questionnaires and recorded the child's answers verbatim to prevent variation in children's reading ability from affecting their responses. During this time, mothers who had not completed the ADIS-IV-L over the telephone did so with a clinical postgraduate student trained in ADIS-IV-L administration. The experimental session lasted 1.5–2 h.
2.5. Statistical analyses, data screening and response definitions
2.5.1. Skin conductance data
SCR data were inspected trial-by-trial for artefacts associated with technical interference, excessive drowsiness, movement, or behaviours such as coughing, sneezing, deep sighs or when the child was not looking at the CS+/− presented on the screen, as determined via observation via the closed-circuit camera. Observations were made through closed-circuit television and additional channels of recording, including horizontal EOG (for eye movements and gaze shifts). Trials in which artefacts occurred were scored as missing. Five participants were excluded due to missing data, defined as more than half of the trials for either CS missing during the acquisition and extinction phase. SCR data were scored blind to children's diagnostic status and group membership.
The magnitude of the phasic SCR elicited during each CS was scored within three latency windows as the distance between the trough and apex of the curve, expressed in microsiemens (μS). First interval responses were those that began 1–4 s following onset of the CS and reflects initial orienting to the signal value of the CS that is enhanced during CSs paired with a US (Öhman, 1983). Second interval responses reflect anticipation of the US (Öhman, 1983, Prokasy and Ebel, 1967), and began 4–7 s following CS onset. Third interval responses began 7–11 s following CS onset (Prokasy and Kumpfer, 1973) and provide a means to examine responses to the US on CS+ trials and the effects of US omission for CS− trials across the groups. All SCR data were transformed using a square root transformation to normalise the distributions (Venables and Christie, 1980), which is in line with previous research utilising skin conductance responses (e.g., Waters et al., 2009). Data were not range-corrected to allow comparisons with previous research (e.g., Craske et al., 2008, Liberman et al., 2006, Waters et al., 2009). However, supplementary analyses using range-corrected values revealed identical main and interaction effects.
For the purpose of data analysis, the trials from each CS were collapsed into four blocks of two trials for acquisition and two blocks of two trials for extinction. The three latency window magnitudes were analysed using separate linear mixed models analysis of variance (ANOVA) for repeated measurements with Satterthwaite's approximation for degrees of freedom. Therefore, separate 3 (Group: ANX, DEP, CON) × 2 (CS: CS+, CS−) × 4 (Block: first, second, third, fourth) mixed models ANOVA were carried out for FIRs, SIRs and TIRs during acquisition, and 3 (Group: ANX, DEP, CON) × 2 (CS: CS+, CS−) × 4 (Block: first, second) mixed models ANOVA were carried out for FIRs, SIRs and TIRs during extinction. Bonferroni corrections were used in follow up comparisons to control for the accumulation of alpha error due to multiple comparisons.
2.5.2. Verbal ratings
Valence and arousal ratings of the CS+ and CS− were analysed using a 2 (CS: CS+, CS−) × 3 (Phase: pre-acquisition, post-acquisition, post-extinction) × 3 (Group: CON, DEP, ANX) repeated-measures analysis of variance (ANOVA). Children's subjective anxiety ratings were analysed using a 3 (Phase: pre-acquisition, post-acquisition, post-extinction) × 3 (Group: CON, DEP, ANX) repeated-measures ANOVA. Children's contingency awareness of the relationship between the CS+ and US was analysed using chi-square analysis. Bonferroni corrections were used in follow up comparisons to control for the accumulation of alpha error due to multiple comparisons.
3. Results
3.1. Group comparisons
3.1.1. Demographics
Compared to the CON group, there were no significant differences in children's gender, both χ2 < .99, n.s., or country of birth, both χ2 < .50, in the ANX or DEP groups. There were no significant group differences in children's age, F(2, 57) = 0.80, p = .91, mothers or fathers age, both F < .33, n.s, or mothers or fathers occupational prestige, both F < 2.10, n.s. Marital status differed significantly between the CON and DEP groups, χ2 (1, N = 46) = 6.66, p = .02 (Fisher's exact text), with more divorced mothers in the DEP than the CON group, but not between the CON and ANX groups, χ2 < 1.44, n.s. (see Table 2).1
3.1.2. Mothers diagnoses and symptom measures
The principal lifetime diagnosis (i.e., most severe) of mothers in the DEP and ANX groups differed according to whether the diagnosis was current or past, χ2 (1, N = 26) = 11.78, p = .001 (see Table 1).2 Nine of the 14 mothers in the ANX group had a current principal diagnosis while the 12 mothers in the DEP group had a past principal diagnosis. However, mothers did not differ in the severity of their principal diagnosis, t = .68, n.s., and with the exception of one mother with past childhood separation anxiety disorder, mothers in both groups experienced their principal diagnosis (or episodes thereof) during their child's lifetime. Depressed and anxious mothers had significantly higher scores on the STAI Trait scale, F(2, 57) 7.10, p = .002, compared to the CON group (both p < .014) but did not differ significantly from each other (p > .05).
3.1.3. Child symptom measures
There were no significant differences between children in the CON, DEP and ANX groups on either SCAS-P total scores, SCAS-C total scores, or CES-DC total scores, all F's < .86, n.s. (see Table 2).
3.2. Children's subjective ratings
Compared to the CON group (23 aware:8 unaware), there were no significant differences in contingency awareness of the ANX group (8 aware:6 unaware), or the DEP group (10 aware:2 unaware), both χ2 < 1.80, n.s. (see Table 2).3 Differences in contingency awareness of the ANX and DEP group were also not significant, χ2 < 2.08, n.s.
A 3 (Group) × 3 (Phase) repeated-measures ANOVA of subjective anxiety ratings revealed a significant main effect of Phase, F(1, 108) = 10.18, p = .001, ηp2 = .16. The Group main effect and the interaction were not significant, both F's < .54, n.s. The phase main effect was due to significantly lower anxiety ratings after extinction compared to before acquisition (p = .006) and after acquisition (p < .001); ratings in the latter phases did not differ significantly (p = .87).
A 3 (Group) × 3 (Phase) × 2 (CS) repeated-measures ANOVA of CS valence ratings revealed a significant main effect of CS, F(1, 54) = 11.93, p = .004, ηp2 = .18, Phase, F(2, 92.08) = 6.25, p = .003, ηp2 = .10, and a significant CS × Phase interaction, F(2, 103.54) = 3.28, p = .045, ηp2 = .057 (see Table 3). There were no significant differences in the ratings of the CSs at pre-acquisition (t(56) = .06, n.s). However, the CS+ was rated as significantly more unpleasant than the CS− after acquisition (t(56) = 3.76, p = .007) but not extinction (t(56) = .69, n.s). All groups rated the CS+ as more unpleasant after acquisition compared to before acquisition (t(56) = 3.82, p < .001) and compared to after extinction (t(56) = 2.17, p = .034). There were no significant changes in valence ratings of the CS− across phases (all p > .40).
Table 3.
Mean subjective anxiety and CS valence and arousal ratings (+SD) as a function of experimental phase and group.
| Pre-Acq |
Post-Acq |
Post-Ext |
||||
|---|---|---|---|---|---|---|
| CS+ | CS− | CS+ | CS− | CS+ | CS− | |
| CS arousal | ||||||
| CON | 3.48 (2.18) | 3.35 (2.15) | 3.90 (2.62) | 2.29 (2.28) | 2.61 (2.09) | 2.35 (2.27) |
| ANX | 1.85 (1.70) | 2.14 (2.17) | 3.14 (2.53) | 2.00 (2.18) | 2.17 (1.99) | 1.86 (1.87) |
| DEP | 1.67 (1.77) | 1.50 (0.90) | 3.33 (2.93) | 1.33 (1.15) | 2.53 (2.22) | 1.17 (0.58) |
| Mean | 2.33 (1.88) | 2.33 (1.74) | 3.46 (2.69) | 1.87 (1.87) | 2.44 (2.10) | 1.79 (1.57) |
| CS valence | ||||||
| CON | 3.32 (1.62) | 3.45 (2.41) | 4.67 (2.42) | 3.13 (2.47) | 3.77 (2.81) | 4.03 (2.62) |
| ANX | 3.71 (1.85) | 2.85 (1.66) | 5.00 (2.09) | 3.57 (1.83) | 4.43 (2.65) | 3.29 (1.73) |
| DEP | 4.00 (1.80) | 3.17 (2.17) | 4.67 (2.24) | 3.50 (2.43) | 4.03 (2.56) | 4.00 (2.34) |
| Mean | 3.68 (1.76) | 3.16 (2.08) | 4.78 (2.25) | 3.40 (2.24) | 4.07 (2.67) | 3.77 (2.23) |
Note. CS arousal ratings: 1 = calm to 9 = very aroused; CS valence ratings: 1 = very pleasant to 9 = very unpleasant.
A 3 (Group) × 3 (Phase) × 2 (CS) repeated-measures ANOVA of CS arousal ratings revealed a significant main effect of CS, F(1, 54) = 13.47, p < .001, ηp2 = .20, Phase, F(2, 108) = 4.90, p = .009, ηp2 = .083, and a significant CS × Phase interaction, F(2, 108) = 8.10, p < .001, ηp2 = .15 (see Table 3). There were no significant differences in the ratings of the CSs at pre-acquisition (t(56) = .01, n.s). However, the CS+ was rated as significantly more arousing than the CS− after acquisition (t(56) = 4.46, p < .001) and after extinction (t(56) = 2.35, p = .022). All groups rated the CS+ as more arousing after acquisition compared to before acquisition (t(56) = 4.46, p = .001) and compared to after extinction (t(56) = 3.65, p = .001). There were no significant changes in arousal ratings of the CS− across phases (all p > .59) (see Table 3).
3.3. Children's skin conductance responses
3.3.1. Acquisition
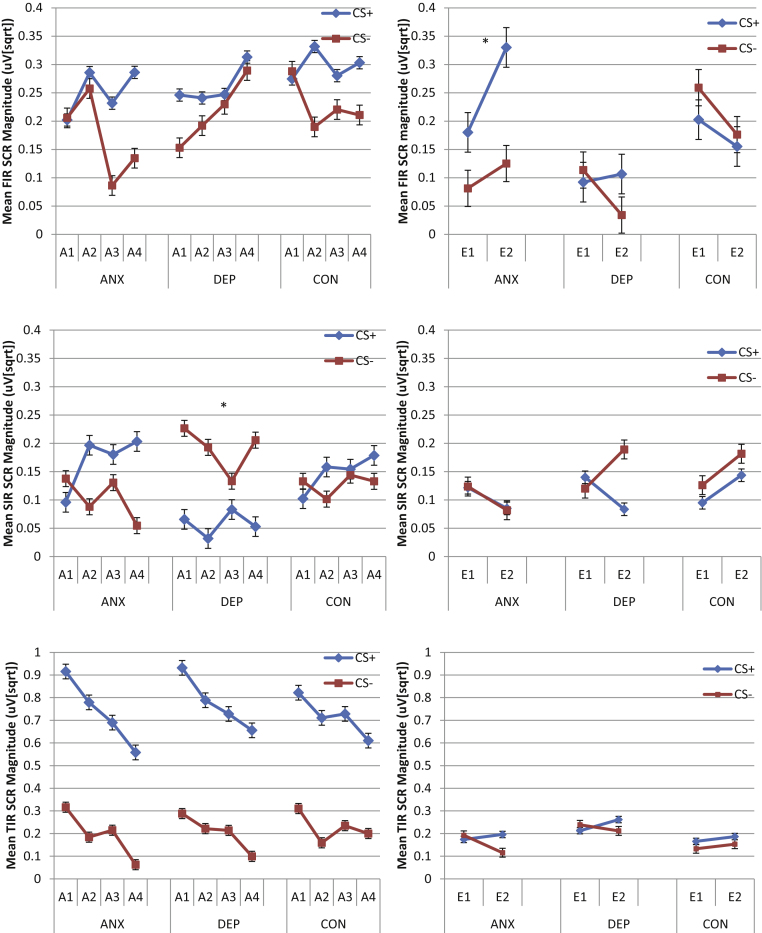
The 3 (Group: ANX, DEP, CON) × 2 (CS: CS+, CS−) × 4 (Block: first, second, third, fourth) mixed models ANOVA of FIRs confirmed the acquisition of associative learning by a significant main effect of CS, F(1, 261.13) = 5.43, p = .02. As seen in Fig. 1, follow-up comparisons confirmed that FIRs were larger overall to the CS+ (M = .28, SE = .02) than the CS− (M = .21, SE = .02). There were no significant main or interacting effects of Group, all F's < 1.05, n.s. (see Fig. 1, upper left panel).
Fig. 1.
Mean first interval (upper panel), second interval (middle panel), and third interval (lower panel) skin conductance responses to the CS+ and the CS− during each block of acquisition (A1–A4) trials (left panels) and extinction trials (E1–E2) (right panels) as a function of group (* indicates significant effects involving group).
The 3 (Group: ANX, DEP, CON) × 2 (CS: CS+, CS−) × 4 (Block: first, second, third, fourth) mixed models ANOVA of SIRs found a significant CS × Group interaction, F(2, 296.05) = 5.62, p = .004. As seen in Fig. 1 (middle left panel), follow up comparisons revealed that participants in the DEP group showed significantly smaller SIRs to the CS+ (M = .06, SE = .03) compared to the CS− (M = .19, SE = .03), p = .004. Furthermore, the DEP group showed significantly smaller SIRs to the CS+ compared to the ANX group (M = .17, SE = .03; p = .04), and CON group (M = .15, SE = .02; p = .04) which did not differ significantly from each other (p > .76). There were no significant differences between groups in responding to the CS− (all p > .05).
A 3 (Group: ANX, DEP, CON) × 2 (CS: CS+, CS−) × 4 (Block: first, second, third, fourth) mixed models analysis of variance (ANOVA) was conducted to examine TIRs (see Fig. 1, lower left panel). There was a significant main effect of CS, F(1, 259.28) = 253.66, p < .001 and a significant main effect of Block, F(3, 793.02) = 14.81, p < .001. As seen in Fig. 1, TIRs were larger overall to the CS+ (M = .74, SE = .03) than the CS− (M = .21, SE = .03). Furthermore, responding was significantly larger in the first (M = .60, SE = .03) compared to the second block (M = .47, SE = .03; p = .001), third block (M = .47, SE = .03; p = .002) and fourth block (M = .37, SE = .03; p < .001). TIRs were significantly larger in the second and third block compared to the fourth block (both p > .018).
3.3.2. Extinction
A 3 (Group: ANX, DEP, CON) × 2 (CS: CS+, CS−) × 2 (Block: first, second) mixed models analysis of variance (ANOVA) was conducted to examine FIRs (see Fig. 1, upper right panel). A significant main effect of Group was found, F(2, 105.74) = 3.08, p = .05, as well as a significant two-way interaction between Block and Group, F(1, 411.41) = 3.80, p = .023 and between CS and Group, F(1, 248.87) = 3.02, p = .05. The Block × Group interaction reflected that the ANX group had significantly larger FIRs in the second compared to the first block (p = .05), whereas SCRs in the CON group reduced from the first to the second block (p = .04), and there were no significant effects of block in the DEP group (p = .44). Furthermore, in the second block, the FIRs of the ANX group were significantly larger compared to the DEP group (p = .02) but not the CON group (p = .43). Differences between the CON and DEP groups were also not significant (p = .08). The CS × Group interaction reflected that the ANX group had significant larger FIRs to the CS+ than the CS− (p = .018) whereas CS differences were not significant for the DEP or CON groups (p's > .054). There also were no significant Group differences at either level of CS (all p > .052). There were no significant effects for SIRs or TIRs, all F's < 2.10, n.s. (see Fig. 1, middle and lower right panels).
3.4. Supplementary analyses
Additional analyse were performed to compare the number of SCR FIR and SIR non-responders to the CS+ during acquisition, defined as less than two SCRs across the 8 CS+ trials (i.e., one or no SCRs). There were no significant differences between the CON and DEP groups in the number of FIR non-responders (DEP = 2/12; CON = 8/31), χ2 < .41, n.s. However, there were significantly more SIR non-responders in the DEP group compared to the CON group (DEP = 9/12; CON = 8/31), χ2 < 4.56, p = .045 (Fisher's Exact Test). There were no significant group differences in FIR or SIR non-responders between the ANX and CON groups (FIR: ANX = 2/14; SIR: ANX = 7/14), all χ2 < .75, n.s.
Correlations between all dependent and independent variables are presented in Table 4, Table 5. In addition, correlations between parent and child symptom measures revealed two significant associations: SCAS-C total scores were significantly correlated with SCAS-P total scores, r(57) = .27, p = .047, and with CES-DC total scores, r(57) = .50, p < .001.
Table 4.
Correlations between skin conductance measures and symptom measures (N = 57).
| Measure | Mother |
Child |
||
|---|---|---|---|---|
| STAI-T | SCAS-P | SCAS-C | CES-DC | |
| Acq CS− FIR | −.15 | .18 | .01 | −.22 |
| Acq CS+ FIR | −.22 | .06 | −.02 | −.16 |
| Ext CS− FIR | −.17 | .09 | −.12 | −.18 |
| Ext CS+ FIR | −.13 | .07 | −.09 | −.24 |
| Acq CS− SIR | .09 | .02 | −.07 | −.25a |
| Acq CS+ SIR | −.09 | −.05 | .10 | −.25a |
| Ext CS− SIR | −.02 | −.08 | −.06 | −.19 |
| Ext CS+ SIR | −.25a | −.10 | −.14 | −.22 |
| Acq CS− TIR | −.11 | .07 | .02 | −.12 |
| Acq CS+ TIR | −.06 | −.05 | −.07 | −.28* |
| Ext CS− TIR | −.13 | .15 | −.16 | −.14 |
| Ext CS+ TIR | −.24 | .15 | −.12 | −.24 |
p < .05.
p < .06.
Table 5.
Correlations between CS valence and arousal measures and symptom measures (N = 57).
| Measure | Mother |
Child |
||
|---|---|---|---|---|
| STAI-T | SCAS-P | SCAS-C | CES-DC | |
| Pre-Acq CS− valence | −.16 | .13 | .12 | .02 |
| Pre-Acq CS+ valence | .03 | .17 | .11 | .24 |
| Post-Acq CS− valence | −.04 | .19 | .08 | .36** |
| Post-Acq CS+ valence | −.14 | .06 | −.19 | .23 |
| Post-Ext CS− valence | −.01 | −.04 | −.13 | .19 |
| Post-Ext CS+ valence | −.13 | .06 | −.14 | .20 |
| Pre-Acq CS− arousal | −.13 | .10 | .19 | .21 |
| Pre-Acq CS+ arousal | −.10 | .07 | .17 | .20 |
| Post-Acq CS− arousal | −.14 | −.15 | .11 | .27* |
| Post-Acq CS+ arousal | −.24 | .02 | .08 | .34** |
| Post-Ext CS− arousal | −.17 | −.14 | .01 | .22 |
| Post-Ext CS+ arousal | −.12 | −.16 | .03 | .18 |
Note. CS valence ratings: 1 = very pleasant to 9 = very unpleasant; CS arousal ratings: 1 = calm to 9 = very aroused.
p < .05.
p < .001.
4. Discussion
The present study found that in the context of comparable discriminative conditioning, subjective valence and arousal responses and levels of contingency awareness, children of anxious mothers showed larger skin conductance conditioned responses during extinction to the CS+ compared to the CS−, and to both CSs from the first to the second block of trials, in comparison with low risk offspring. These findings partially replicate those of Craske et al. (2008), who also found no group differences in subjective measures or contingency awareness but did find that offspring of anxious parents exhibited larger skin conductance conditioned responses during extinction that were undifferentiated by CS type. However, inconsistent with Craske et al. (2008), we did not find that high risk offspring of anxious parents had larger third interval skin conductance conditioned responses during acquisition compared to low risk offspring. These findings could suggest that excitatory responding to aversive stimuli (i.e., the tone US) is more variable amongst high risk offspring of anxious parents, whereas deficits in learning to inhibit psychophysiological responses when new learning should take place (CS− no US association) (e.g., Davis et al., 2000) may be a more reliable candidate risk marker that distinguishes high from low risk offspring. Indeed, the present findings accord with those from clinical studies finding that anxious children display larger skin conductance conditioned responses during extinction trials to the CS+ compared to the CS− (Liberman et al., 2006, Waters et al., 2009) or collapsed across both CSs (Craske et al., 2008). Still unknown, however, is whether these impairments in inhibiting psychophysiological responses following new information that a stimulus is now safe actually underlies the elevated risk for anxiety in children of anxious parents. It will be important for future studies to extend these findings by using longitudinal designs to assess the onset of anxiety disorders as an outcome measure.
The other major finding in this study was that, compared to low risk controls, children of depressed mothers showed smaller skin conductance responses to the CS+ than the CS− during acquisition. This is the first study to the authors’ knowledge to study aversive conditioning and extinction in children of depressed mothers. Skin conductance responses of smaller magnitudes on CS+ trials were evident in second interval responses, suggesting that offspring of depressed mothers were largely unresponsive during anticipation of the US, rather than in actual reactivity to the US (i.e., no group differences in TIRs) or in initial orienting to the CS+ (although inspection of Fig. 1 suggests a lack of discrimination in FIRs in the DEP group, the overall analysis revealed no significant group differences).
Previous research has shown that depression is associated with decreased fear-potentiated startle and less pleasure-inhibited startle, contributing to a generally flattened affect-startle pattern (see Vaidyanathan et al., 2012). Thus, one explanation for the present results is that the DEP group showed impaired inhibition of skin conductance responses to the safety of the CS−. However, this is not supported by the results because there were no significant group differences in second interval response magnitudes on CS− trials. Also, inspection of the means in Fig. 1 for the DEP group indicates a substantial decrease in the magnitudes of SIRs (M = .055) compared to FIRs (M = .27) for CS+ trials but not CS− trials (FIR M = .21; SIR M = .19). Moreover, the supplementary analyses confirmed that the reduction in magnitude of SIRs to the CS+ in the DEP group was due to significantly more SCR non-responders compared to the CON group (i.e., children who had only one or no SIRs across the 8 CS+ trials). Therefore, a more likely explanation is that offspring of depressed mothers were less responsive psychophysiologically to external stimuli signalling threat (i.e., CS+ trials) and that this manifested in the anticipation of forthcoming threat (i.e., SIRs) (Öhman, 1983, Prokasy and Ebel, 1967). Thus, the present findings extend previous evidence of broad psychophysiological dysregulation in depressive disorders (e.g., Burke et al., 2005, Rao et al., 2008, Waugh et al., 2012, Young et al., 2000) to include dysregulation of skin conductance reactivity in offspring of depressed mothers.
The mechanism/s underlying impaired inhibition of skin conductance responses following new information signalling safety in offspring of anxious mothers (i.e., retarded extinction), and diminished skin conductance responses during anticipation of a pending aversive event in offspring of depressed mothers are not clear. The interaction between genetic (Gregory and Eley, 2007), neurobiological (Mirkin and Coppen, 1980), and temperament factors, such as negative affect (Zinbarg et al., 2010), as well as learning-based mechanisms such as exposure to maternal maladaptive cognitions, behaviour and affect (see Goodman and Gotlib, 1999 and Rapee et al., 2009 for reviews) are likely to interact to influence offspring psychophysiological dysregulation during initial aversive learning in offspring of depressed mothers and safety learning in offspring of anxious mothers. Studies with larger samples that include multiple measures across time are required to clarify the relative contribution of these risk markers to disorder onset in high risk offspring.
The present findings suggest distinctions between offspring of anxious and depressed parents in the psychophysiological measure of skin conductance responses whereby offspring of anxious mothers show increased reactivity during extinction of aversive learning while offspring of depressed mothers show diminished responding during anticipation of a pending aversive event during conditioning. However, it is notable that offspring of anxious and depressed parents have similarly shown increased startle eye blink reactivity throughout fear-potentiation protocols (e.g., Grillon et al., 1998, Grillon et al., 2005). Taken together, these findings could suggest both common and specific patterns of psychophysiological dysregulation among offspring of depressed and anxious parents. Longitudinal research with multiple measures from offspring of anxious and depressed parents will help elucidate common and specific risk factors for the onset of emotional disorders.
It is important to note that dysregulation may well extend to evaluative measures such as subjective appraisals of the CSs and US expectancy ratings had these responses been assessed trial-by-trial as was the case for skin conductance responses. Thus, the inconsistencies between the subjective ratings and trial-by-trial skin conductance responses should not be interpreted as these two measures indexing underlying mechanisms that are differentially sensitive to risk due to maternal anxiety and depression. Although subjective ratings have more consistently been interpreted as reflective of affective learning, the claim that skin conductance responses are reflective of expectancy learning has been questioned (e.g., Lovibond and Shanks, 2002, Öhman and Soares, 1998, Wiens and Öhman, 2002). Similar debate exists about whether the proposed measures of affective learning and expectancy learning reflect on qualitatively different learning mechanisms (e.g., Baeyens et al., 1995, De Houwer et al., 2001) or are manifestations of a single underlying mechanism (e.g., Field and Davey, 1997, Lipp and Purkis, 2005, Shanks and Dickinson, 1990). Therefore, the manner in which measures have been collected, i.e., trial-by-trial or pre- to post-phase tests, may be critical to the absence of results in the subjective versus skin conductance measures. Relatedly, even though post-acquisition phase contingency awareness levels did not differ significantly between the groups, nor did contingency awareness affect the results, only 72% of children averaged across the whole sample could correctly identify the CS+-US association after acquisition. Although this compares favourably to a previous study (i.e., Waters et al., 2009) in which only 55% of healthy controls achieved contingency awareness by post-acquisition, future studies should assess both skin conductance responses and subjective ratings using both trial-by-trial and pre- to post-phase procedures.
The present study should be considered in relation to other limitations. Parent–child dyads were primarily recruited through the community rather than mental health clinics and the sample sizes of the DEP and ANX groups were small, which limits the generalisability of findings. Although timing of diagnosis (i.e., current; past) did not affect outcomes, the groups unexpectedly differed in the number of mothers with a current versus past principal diagnosis. Also, although all mothers except for one experienced their disorder or episodes thereof during their child's lifetime, it would be valuable in future research to examine the effects of when mothers experienced these disorders/episodes during their child's lifetime. Also, although it would have been informative to study offspring of mothers with a single diagnosis of either anxiety or depression, this was not feasible due to high rates of comorbidity. Nevertheless, the approach used here facilitates comparison with other high risk studies (e.g., Craske et al., 2008) and clinical studies (e.g., Liberman et al., 2006, Waters et al., 2009), in which inclusion was based on either parent or child principal diagnosis. Moreover, differences in acquisition and extinction phases were found between offspring of mothers with principal anxiety versus depressive disorders despite comorbidity on subsidiary diagnoses, indicating that the relationship between type of maternal principal disorder and aversive conditioning and extinction in offspring deserves further investigation. In terms of methodology, it would be valuable in future studies to include more trials per CS condition during acquisition and extinction phases, and to assess physiological, subjective and contingency awareness measures trial-by-trial as well as pre- to post-phase. It will also be important to follow children longitudinally to determine whether differences in aversive learning and extinction between offspring of anxious and depressed mothers predict long-term psychopathology.
In summary, the present study found that offspring of anxious mothers showed retarded extinction of learned fear responses, as indexed by skin conductance responses, thereby replicating prior research (Craske et al., 2008). By contrast, offspring of depressed mothers showed diminished reactivity during acquisition of fear learning. This appears to be a novel finding that is broadly consistent with previous evidence of diminished skin conductance responsiveness, reduced fear-potentiated startle and affective startle modulation in adults with depression (Mirkin and Coppen, 1980, Lader and Wing, 1964, Allen et al., 1999, Brown et al., 1998, Kaviani et al., 2004, McTeague et al., 2009). These findings suggest there may be distinct psychophysiological premorbid risk markers by virtue of maternal anxiety and depression. Future research using multiple measures with long-term follow-up of the offspring of anxious and depressed mothers will help confirm whether these risk markers play a role in the onset of emotional disorders.
Conflict of interest
The authors declare no financial interest or conflict of interest.
Acknowledgement
This research was supported by Australian Research Council Grant DP1095536 awarded to Dr Allison Waters.
Footnotes
Preliminary analyses with marital status as a covariate revealed no significant effects of the covariate.
Preliminary analyses of all dependent measures as a function of past versus current principal diagnoses or with past versus current principal diagnoses as a covariate in the main analyses revealed no significant effects of the timing of principal diagnosis.
Preliminary analyses of all dependent measures with and without unaware children included revealed the same results. Thus, all children were retained in the analyses.
References
- Allen N.B., Trinder J., Brennan C. Affective startle modulation in clinical depression: preliminary findings. Biological Psychiatry. 1999;46(4):542–550. doi: 10.1016/s0006-3223(99)00025-6. [DOI] [PubMed] [Google Scholar]
- Andrews G., Issakidis C., Sanderson K., Corry J., Lapsley H. Utilising survey data to inform public policy: comparison of the cost effectiveness of treatment of ten mental disorders. British Journal of Psychiatry. 2004;184:526–533. doi: 10.1192/bjp.184.6.526. [DOI] [PubMed] [Google Scholar]
- Baeyens F., Eelen P., Crombez G. Pavlovian associations are forever: on classical conditioning and extinction. Journal of Psychophysiology. 1995;9:127–141. [Google Scholar]
- Bittner A., Egger H., Erkanli A., Costello J., Foley D., Angold A. What do childhood anxiety disorders predict? Journal of Child Psychology and Psychiatry. 2007;48(12):1174–1183. doi: 10.1111/j.1469-7610.2007.01812.x. [DOI] [PubMed] [Google Scholar]
- Bodden D.H.M., Dirksen C.D., Bogels S.M. Societal burden of clinically anxious youth referred for treatment: a cost-of-illness study. Journal of Abnormal Child Psychology. 2008;36(4):487–497. doi: 10.1007/s10802-007-9194-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boschen M.J., Neumann D.L., Waters A.M. Relapse of successfully treated anxiety and fear: theoretical issues and recommendations for clinical practice. Australian and New Zealand Journal of Psychiatry. 2009;43(2):89–100. doi: 10.1080/00048670802607154. [DOI] [PubMed] [Google Scholar]
- Bouton M.E., Mineka S., Barlow D.H. A contemporary learning theory perspective on the etiology of panic disorder. Psychological Review. 2001;108:4–32. doi: 10.1037/0033-295x.108.1.4. [DOI] [PubMed] [Google Scholar]
- Brown T.A., Chorpita B.F., Barlow D.H. Structural relationships among dimensions of the DSM-IV anxiety and mood disorders and dimensions of negative affect, positive affect, and autonomic arousal. Journal of Abnormal Psychology. 1998;107(2):179–192. doi: 10.1037//0021-843x.107.2.179. [DOI] [PubMed] [Google Scholar]
- Brown T.A., DiNardo P.A., Barlow D.H. Graywind; Albany, NY: 1994. The Anxiety Disorders Interview Schedule for DSM-IV. [Google Scholar]
- Brown T.A., DiNardo P.A., Lehman C.L., Campbell L.A. Reliability of DSM-IV anxiety and mood disorders: implications for the classification of emotional disorders. Journal of Abnormal Psychology. 2001;110(1):49–58. doi: 10.1037//0021-843x.110.1.49. [DOI] [PubMed] [Google Scholar]
- Burke H.M., Davis M.C., Otte C., Mohr D.C. Depression and cortisol responses to psychological stress: a meta-analysis. Psychoneuroendocrinology. 2005;30(9):846–856. doi: 10.1016/j.psyneuen.2005.02.010. [DOI] [PubMed] [Google Scholar]
- Center for the Study of Emotion Attention . Author, University of Florida; Gainesville, FL: 1999. The International Affective Picture System: Digitized Photographs. [Google Scholar]
- Craske M.G., Rauch S.L., Ursano R., Prenoveau J., Pine D.S., Zinbarg R.E. What is an anxiety disorder? Depression and Anxiety. 2009;26(12):1066–1085. doi: 10.1002/da.20633. [DOI] [PubMed] [Google Scholar]
- Craske M.G., Waters A.M. Panic disorder, phobias, and generalized anxiety disorder. Nolen-Hoeksema S., Cannon T., Widiger T., Baker T., Luthar S., Mineka S., Munoz R., Salmon D., editors. Annual Review of Clinical Psychology. 2005;1:197–225. doi: 10.1146/annurev.clinpsy.1.102803.143857. [DOI] [PubMed] [Google Scholar]
- Craske M.G., Waters A.M., Bergman R.L., Naliboff B., Lipp O.V., Negoro H., Ornitz E.M. Is aversive learning a marker of risk for anxiety disorders in children? Behaviour Research and Therapy. 2008;46(8):954–967. doi: 10.1016/j.brat.2008.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M., Falls W.A., Gewirtz J. Neural systems involved in fear inhibition: extinction and conditioned inhibition. In: Myslobodsky M., Weiner I., editors. Contemporary Issues in Modeling Psychopathology. Kluwer; Boston: 2000. pp. 113–142. [Google Scholar]
- De Houwer J., Thomas S., Baeyens F. Associative learning of likes and dislikes: a review of 25 years of research on human evaluative conditioning. Psychological Bulletin. 2001;127:852–869. doi: 10.1037/0033-2909.127.6.853. [DOI] [PubMed] [Google Scholar]
- Faulstich M.E., Carey M.P., Ruggiero L., Enyart P., Gresham F. Assessment of depression in childhood and adolescence: an evaluation of the Center for Epidemiological Studies Depression Scale for Children (CES-DC) American Journal of Psychiatry. 1986;143(8):1024–1027. doi: 10.1176/ajp.143.8.1024. [DOI] [PubMed] [Google Scholar]
- Field A.P. I don’t like it because it eats sprouts: conditioning preferences in children. Behaviour Research and Therapy. 2006;44(3):439–455. doi: 10.1016/j.brat.2005.03.006. [DOI] [PubMed] [Google Scholar]
- Field A.P., Davey G.C.L. Conceptual conditioning: evidence for an artifactual account of evaluative learning. Learning and Motivation. 1997;28:446–464. [Google Scholar]
- Goodman S.H., Gotlib I.H. Risk for psychopathology in the children of depressed mothers: a developmental model for understanding mechanisms of transmission. Psychological Review. 1999;106(3):458–490. doi: 10.1037/0033-295x.106.3.458. [DOI] [PubMed] [Google Scholar]
- Gotlib I.H., Joorman J., Minor K.L., Cooney R.E. Cognitive and biological functioning in children at risk for depression. In: Canli T., editor. Biology of Personality and Individual Differences. Guilford Press; New York: 2006. pp. 353–398. [Google Scholar]
- Gregory A.M., Eley T.C. Genetic influences on anxiety in children: what we’ve learned where we’re heading. Clinical Child Family Psychology. 2007;10(3):199–212. doi: 10.1007/s10567-007-0022-8. [DOI] [PubMed] [Google Scholar]
- Grillon C. Startle reactivity and anxiety disorders: aversive conditioning, context, and neurobiology. Biological Psychiatry. 2002;52(10):958–975. doi: 10.1016/s0006-3223(02)01665-7. [DOI] [PubMed] [Google Scholar]
- Grillon C., Dierker L., Merikangas K.R. Fear-potentiated startle in adolescent offspring of parents with anxiety disorders. Biological Psychiatry. 1998;44:990–997. doi: 10.1016/s0006-3223(98)00188-7. [DOI] [PubMed] [Google Scholar]
- Grillon C., Warner V., Hille J., Merikangas K.R., Bruder G.E., Tenke C.E., Weissman M.M. Families at high and low risk for depression: a three-generation startle study. Biological Psychiatry. 2005;57(9):953–960. doi: 10.1016/j.biopsych.2005.01.045. [DOI] [PubMed] [Google Scholar]
- Hammen C. Springer-Verlag; New York: 1991. Depression Runs in Families: The Social Context of Risk and Resilience in Children of Depressed Mothers. [Google Scholar]
- Hammen C. Children of depressed parents: the stress context. In: Wolchik S.A., Sandler I.N., editors. Handbook of Children's Coping: Linking Theory and Intervention. Perseus Publishing; New York: 1997. pp. 131–157. [Google Scholar]
- Hammen C., Burge D., Stansbury K. Relationship of mother and child variables to child outcomes in a high risk sample: a causal modeling analysis. Developmental Psychology. 1990;26:24–30. [Google Scholar]
- Kaviani H., Gray J.A., Checkley S.A., Raven P.W., Wilson G.D., Kumari V. Affective modulation of the startle response in depression: influence of the severity of depression, anhedonia, and anxiety. Journal of Affective Disorders. 2004;83(1):21–31. doi: 10.1016/j.jad.2004.04.007. [DOI] [PubMed] [Google Scholar]
- Lader M.H., Wing L. Habituation of the psycho-galvanic reflex in patients with anxiety states and in normal subjects. Journal of Neurological and Neurosurgical Psychiatry. 1964;27:210–218. doi: 10.1136/jnnp.27.3.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau J., Lissek S., Nelson E., Lee Y., Roberson-Nay R., Poeth K., Pine D.S. Fear conditioning in adolescents with anxiety disorders: results from a novel experimental paradigm. Journal of American Academy of Child and Adolescent Psychiatry. 2008;47(1):94–102. doi: 10.1097/chi.0b01e31815a5f01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberman L., Lipp O.V., Spence S.H., March S. Evidence of retarded extinction of aversive learning in anxious children. Behaviour Research and Therapy. 2006;44(10):1491–1502. doi: 10.1016/j.brat.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Lieb R., Isensee B., Hofler M., Pfister H., Wittchen H.U. Parental major depression and the risk of depression and other mental disorders in offspring: a prospective-longitudinal community study. Archives of General Psychiatry. 2002;59(4):365–374. doi: 10.1001/archpsyc.59.4.365. [DOI] [PubMed] [Google Scholar]
- Lipp O.V., Purkis H.M. No support for dual process accounts of human affective learning in simple Pavlovian conditioning. Cognition and Emotion. 2005;19(2):269–282. doi: 10.1080/02699930441000319. [DOI] [PubMed] [Google Scholar]
- Lissek S., Powers A., McClure E., Phelps E., Woldehawariat G., Grillon C., Pine D.S. Classical fear conditioning in the anxiety disorders: a meta-analysis. Behaviour Research and Therapy. 2005;43(11):1391–1424. doi: 10.1016/j.brat.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Lovibond P.F., Shanks D.R. The role of awareness in Pavlovian conditioning: empirical evidence and theoretical implications. Journal of Experimental Psychology: Animal Behavior processes. 2002;28(1):3–26. [PubMed] [Google Scholar]
- Lyneham H.J., Rapee R.M. Agreement between telephone and in-person delivery of a structured interview for anxiety disorders in children. Journal of the American Academy of Child and Adolescent Psychiatry. 2005;44(3):274–283. doi: 10.1097/00004583-200503000-00012. [DOI] [PubMed] [Google Scholar]
- Mathews R.R.S., Hall W.D., Vos T., Patton G.C., Degenhardt L. What are the major drivers of prevalent disability burden in young Australians? Medical Journal of Australia. 2011;194(5):232–235. doi: 10.5694/j.1326-5377.2011.tb02951.x. [DOI] [PubMed] [Google Scholar]
- McTeague L.M., Lang P.J., Wangelin B.C., Laplante M.-C., Bradley M.M. Defensive mobilization in specific phobia: Fear specificity, negative affectivity, and diagnostic prominence. Biological Psychiatry. 2012;72:8–11. doi: 10.1016/j.biopsych.2012.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McTeague L.M., Lang P.J., Laplante M.C., Cuthbert B.N., Strauss C.C., Bradley M.M. Fearful imagery in social phobia: generalization, comorbidity, and physiological reactivity. Biological Psychiatry. 2009;65(5):374–382. doi: 10.1016/j.biopsych.2008.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merikangas K.R., Avenevoli S., Dierker L., Grillon C. Vulnerability factors among children at risk for anxiety disorders. Society of Biological Psychiatry. 1999;99:172–179. doi: 10.1016/s0006-3223(99)00172-9. [DOI] [PubMed] [Google Scholar]
- Mineka S., Öhman A. Phobias and preparedness: the selective, automatic, and encapsulated nature of fear. Biological Psychiatry. 2002;52(10):927–937. doi: 10.1016/s0006-3223(02)01669-4. [DOI] [PubMed] [Google Scholar]
- Mineka S., Zinbarg R. A contemporary learning theory perspective on the etiology of anxiety disorders: it's not what you thought it was. The. American Psychologist. 2006;61(1):10–26. doi: 10.1037/0003-066X.61.1.10. [DOI] [PubMed] [Google Scholar]
- Mirkin A.M., Coppen A. Electrodermal activity in depression: clinical and biochemical correlates. British Journal of Psychiatry. 1980;137:93–97. doi: 10.1192/bjp.137.1.93. [DOI] [PubMed] [Google Scholar]
- Nauta M.H., Scholing A., Rapee R.M., Abbott M., Spence S.H., Waters A. A parent-report measure of children's anxiety: psychometric properties and comparison with child-report in a clinic and normal sample. Behaviour Research and Therapy. 2004;42(7):813–839. doi: 10.1016/S0005-7967(03)00200-6. [DOI] [PubMed] [Google Scholar]
- Öhman A. Evaluating evaluative conditioning: some comments on “Cognition, evaluations, and conditioning: Rules of sequence and rules of consequence” by Levy and Martin. Advances in Behavioral Research and Therapy. 1983;4:213–218. [Google Scholar]
- Öhman A., Soares J.J.F. Emotional conditioning to masked stimuli: expectancies for aversive outcomes following nonrecognized fear-relevant stimuli. Journal of Experimental Psychology: General. 1998;127(1):69–82. doi: 10.1037//0096-3445.127.1.69. [DOI] [PubMed] [Google Scholar]
- Prokasy W.F., Ebel H.C. Three components of the classically conditioned GSR in human subjects. Journal of Experimental Psychology. 1967;73:247–256. [Google Scholar]
- Prokasy W.F., Kumpfer K.L. Classical conditioning. In: Prokasy W.F., Raskin D.C., editors. Electrodermal activity in psychological research. Academic Press; San Diego, CA: 1973. pp. 157–202. [Google Scholar]
- Rachman S. The conditioning theory of fear acquisition: a critical examination. Behaviour Research and Therapy. 1977;15:375–387. doi: 10.1016/0005-7967(77)90041-9. [DOI] [PubMed] [Google Scholar]
- Rao U., Hammen C., Ortiz L.R., Chen L.A., Poland R.E. Effects of early and recent adverse experiences on adrenal response to psychosocial stress in depressed adolescents. Biological Psychiatry. 2008;64(6):521–526. doi: 10.1016/j.biopsych.2008.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapee R.M., Schniering C.A., Hudson J.L. Anxiety disorders during childhood and adolescence: origins and treatment. Annual Review of Clinical Psychology. 2009;5:311–341. doi: 10.1146/annurev.clinpsy.032408.153628. [DOI] [PubMed] [Google Scholar]
- Shanks D.R., Dickinson A. Contingency awareness in evaluative conditioning: a comment on Baeyens, Eelen and van den Bergh. Cognition and Emotion. 1990;4:19–30. [Google Scholar]
- Silverman W.K., Albano A.M. Graywind; Albany, NY: 1996. The Anxiety Disorders Interview Schedule for DSM-IV, Child and Parent Versions. [Google Scholar]
- Silverman W.K., Saavedra L.M., Pina A.A. Test–retest reliability of anxiety symptoms and diagnoses with the Anxiety Disorders Interview Schedule for DSM-IV: child and parent versions. Journal of American Academy of Child and Adolescent Psychiatry. 2001;40(8):937–944. doi: 10.1097/00004583-200108000-00016. [DOI] [PubMed] [Google Scholar]
- Spielberger C.D. 2nd ed. Consulting Psychologists Press; Palo Alto, CA: 1989. State-Trait Anxiety Inventory: A Comprehensive Bibliography. [Google Scholar]
- Spielberger C.D., Gorsuch R.L., Lushene R.E. Consulting Psychologists Press; Palo Alto, CA: 1970. STAI: Manual for the State-Trait Anxiety Inventory. [Google Scholar]
- Spielberger C.D., Gorsuch R.L., Lushene R., Vagg P.R., Jacobs G.A. Consulting Psychologists Press; Palo Alto, CA: 1983. Manual for the State-Trait Anxiety Inventory. [Google Scholar]
- Spence S.H. A measure of anxiety symptoms among children. Behaviour Research and Therapy. 1998;36(5):545–566. doi: 10.1016/s0005-7967(98)00034-5. [DOI] [PubMed] [Google Scholar]
- Vaidyanathan U., Nelson L.D., Patrick C.J. Clarifying domains of internalizing psychopathology using neurophysiology. Psychological Medicine. 2012;42:447–459. doi: 10.1017/S0033291711001528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venables P.H., Christie M.J. Electrodermal activity. In: Martion I., Venables P.H., editors. Techniques in Psychophysiology. Wiley; New York: 1980. pp. 3–67. [Google Scholar]
- Vervliet B., Craske M.G., Hermans D. Fear extinction and relapse: state of the art. Annual Review of Clinical Psychology. 2012;9:215–248. doi: 10.1146/annurev-clinpsy-050212-185542. [DOI] [PubMed] [Google Scholar]
- Waters A.M., Henry J., Neumann D.L. Aversive Pavlovian conditioning in childhood anxiety disorders: impaired response inhibition and resistance to extinction. Journal of Abnormal Psychology. 2009;118(2):311–321. doi: 10.1037/a0015635. [DOI] [PubMed] [Google Scholar]
- Waugh C.E., Muhtadie L., Thompson R.J., Joormann J., Gotlib I.H. Affective and physiological responses to stress in girls at elevated risk for depression. Development and Psychopathology. 2012;24(2):661–675. doi: 10.1017/S0954579412000235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman M.M., Gammon G.D., John K., Merikangas K.R., Warner V., Prusoff B.A., Sholomskas D. Children of depressed parents Increased psychopathology and early onset of major depression. Archives of General Psychiatry. 1987;44(10):847–853. doi: 10.1001/archpsyc.1987.01800220009002. [DOI] [PubMed] [Google Scholar]
- Weissman M.M., Wickramaratne P., Nomura Y., Warner Y., Pilowsky D., Verdeli H. Offspring of depressed parents: 20 years later. American Journal of Psychiatry. 2006;163(6):1001–1008. doi: 10.1176/ajp.2006.163.6.1001. [DOI] [PubMed] [Google Scholar]
- Wiens S., Öhman A. Unawareness is more than a chance event: Comment on Lovibond and Shanks. Journal of Experimental Psychology: Animal Behavior Processes. 2002;28(1):27–31. [PubMed] [Google Scholar]
- Williamson D.E., Birmaher B., Axelson D.A., Ryan N.D., Dahl R.E. First episode of depression in children at low and high familial risk for depression. Journal of the American Academy of Child and Adolescent Psychiatry. 2004;43(3):291–297. doi: 10.1097/00004583-200403000-00010. [DOI] [PubMed] [Google Scholar]
- Wood J., Piacentini J.C., Bergman R.L., McCracken J., Barrios V. Concurrent validity of the anxiety disorders section of the anxiety disorders interview schedule for DSM-IV: child and parent versions. Journal of Clinical Child and Adolescent Psychology. 2002;31(3):335–342. doi: 10.1207/S15374424JCCP3103_05. [DOI] [PubMed] [Google Scholar]
- Young E.A., Lopez J.F., Murphy-Weinberg V., Watson S.J., Akil H. Hormonal evidence for altered responsiveness to social stress in major depression. Neuropsychopharmacology. 2000;23(4):411–418. doi: 10.1016/S0893-133X(00)00129-9. [DOI] [PubMed] [Google Scholar]
- Zinbarg R.E., Mineka S., Craske M.G., Griffith J.W., Sutton J., Rose R.D., Nazarian M., Mor N., Waters A.M. The Northwestern-UCLA Youth Emotion Project: associations of cognitive vulnerabilities, neuroticism and gender with past diagnoses of emotional disorders in adolescents. Behaviour Research and Therapy. 2010;48:347–358. doi: 10.1016/j.brat.2009.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]