Abstract
In specific language impairment (SLI), there is a delay in the child’s oral language skills when compared with nonverbal cognitive abilities. The problems typically relate to phonological and morphological processing and word learning. This article reviews studies which have used mismatch negativity (MMN) in investigating low-level neural auditory dysfunctions in this disorder. With MMN, it is possible to tap the accuracy of neural sound discrimination and sensory memory functions. These studies have found smaller response amplitudes and longer latencies for speech and non-speech sound changes in children with SLI than in typically developing children, suggesting impaired and slow auditory discrimination in SLI. Furthermore, they suggest shortened sensory memory duration and vulnerability of the sensory memory to masking effects. Importantly, some studies reported associations between MMN parameters and language test measures. In addition, it was found that language intervention can influence the abnormal MMN in children with SLI, enhancing its amplitude. These results suggest that the MMN can shed light on the neural basis of various auditory and memory impairments in SLI, which are likely to influence speech perception.
Keywords: Specific language impairment, Auditory processing, Mismatch negativity (MMN)
1. Introduction
Specific Language Impairment (SLI) is a common developmental disorder, affecting about 3–10% of children (Tomblin et al., 1997), which has a serious impact on the child’s psychosocial and educational outcome (Conti-Ramsden et al., 2013, Johnson et al., 2010). SLI is diagnosed if the child’s oral language is delayed compared to other, nonverbal cognitive abilities and there is no other apparent neurological or sensory explanation. On average, children with SLI learn new words more slowly, have difficulties in understanding complex sentences, and often the language they produce is poorer than in typically developing peers (Leonard, 2014).
SLI affects many cognitive functions, particularly phonological (Ramus et al., 2013) and morphological (Bishop, 2014) processing, verbal short-term memory (Archibald and Gathercole, 2006), and implicit learning of sequences of information (Lum et al., 2014). However, SLI, which is highly heritable (Kang and Drayna, 2011), is most likely a heterogeneous multidimensional disorder with several partly independent risk factors. For example, some cognitive components underlying SLI might affect language abilities directly, whereas some other risk factors may have an indirect impact leading to poor language acquisition of language skills. In adolescents and adults the separation of different causal factors might be difficult if not impossible, promoting the importance to study children as early as possible.
Event-related potentials (ERPs) offer a means to investigate certain deficits, such as poor speech sound discrimination, associated with SLI. ERPs reveal the time course of sound processing from neural sound encoding (sound-elicited deflections) to discrimination, followed by an attention switch to intrusive sounds (Näätänen, 1992). The high temporal resolution of ERPs enables one to determine which processes (e.g., encoding or discrimination of sounds, or formation or retention of sound memory traces) and processing stages (e.g., early automatic vs. later attentive) are abnormal. With the mismatch negativity (MMN), which reflects the early, “low-level”, stages of auditory information processing at the subcortical and cortical levels (Escera et al., 2014), one can study these deficits even in children and infants. This is clearly beneficial since infant perception and cognition are altogether challenging to assess and in children low motivation or ability to perform experimental tasks can influence the results.
The current review discusses how the MMN has been and can be used to investigate and predict auditory, language, and memory dysfunctions in SLI. To this end we first introduce MMN (particularly those aspects of the MMN that are relevant for investigating SLI). Thereafter, we present an overview of the studies using the MMN in investigating the neural basis of SLI, and suggest future directions for research in order to deepen the understanding of the neural basis of SLI.
1.1. The mismatch negativity in investigating auditory cognition
1.1.1. An overview of MMN
MMN is a negative displacement in the sound-elicited ERP, the waveform of which includes the P1-N1-P2 complex in adults (Näätänen, 1992) and the P1-N2-N4 complex in children under the age of 11 (Ponton et al., 2000). The MMN, peaking at 150–200 ms from change onset in adults, is elicited by any discriminable change or violation of recent auditory regularities (for reviews, see Näätänen et al., 2007, Winkler, 2007). Apart from the acoustic differences between sounds, the MMN amplitude is also influenced by auditory linguistic experience and also underlying auditory discrimination abilities (see Kujala and Näätänen, 2010, for a review). The main generators of the MMN originate in the supratemporal auditory cortices and frontal regions (for reviews, see Alho, 1995, Kujala, 2007). It has been argued that the MMN elicitation is based on the formation of a memory representation, which lasts about 4–15 s (Cowan et al., 1993, Mäntysalo and Näätänen, 1987, Ulanovsky et al., 2004). An alternative interpretation was postulated (May and Tiitinen, 2010), suggesting that the MMN results just because the neurons responding to standard stimuli have a higher degree of refractoriness than those responding to deviant stimuli. However, with certain experimental manipulations the contribution of exogenous responses can be avoided and a genuine MMN can be obtained (Näätänen et al., 2011).
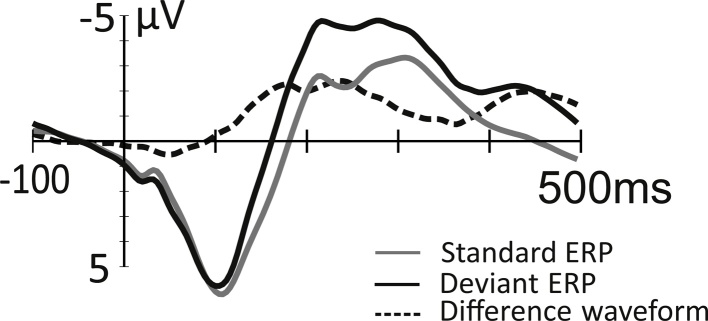
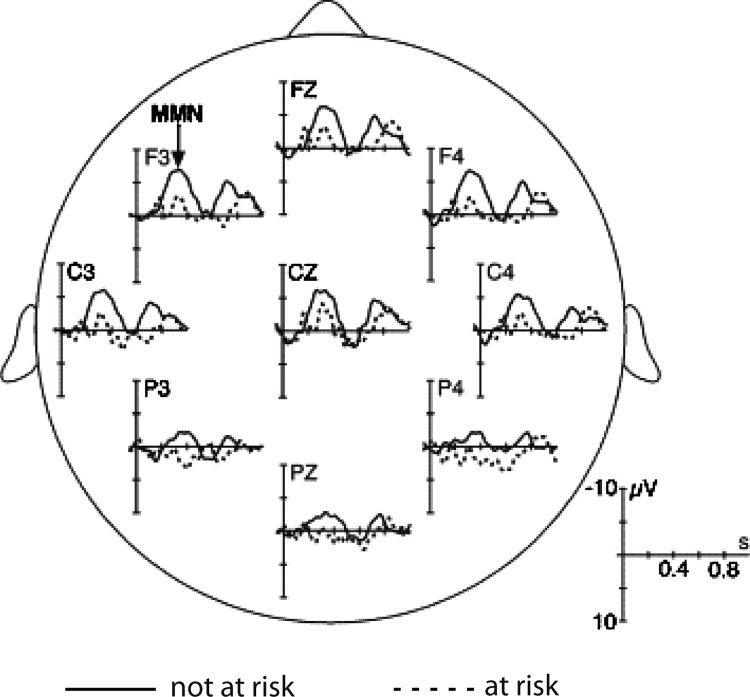
Neural discrimination responses are ontogenetically very early, being elicited even in fetuses (Huotilainen et al., 2005). Change-elicited responses are usually positively-displaced in infants, being therefore called the mismatch response (MMR). An adult-like negativity starts to emerge at the age of 3–6 months, but in this development there is wide inter-individual variation (Trainor et al., 2003). By the school age, this response typically shows the negative polarity of the MMN (Fig. 1), although a positive MMR sometimes coincides with this response (Maurer et al., 2003, Shafer et al., 2011, Lee et al., 2012). The MMR/MMN peak latency decreases as children mature (e.g., Glass et al., 2008, Morr et al., 2002, Shafer et al., 2000).
Fig. 1.
Average ERPs to speech sounds (syllable/pi:/or/ke:/; grey line) and vowel changes in the syllables (to syllable/pe:/or/ki:/, respectively; black line) in six-year old typically developing children (N = 63). The difference wave (ERP to the standard stimulus subtracted from ERP to the deviant stimulus) is shown with a dashed line. The MMN can be seen at around 150–250 ms and the LDN at around 400–500 ms from stimulus onset.
Figure adapted from Kuuluvainen et al. (2016).
The MMN is often followed by a late discriminative negativity (LDN) in children (Korpilahti et al., 2001, Maurer et al., 2003), the amplitude of which behaves differently from that of the MMN, the LDN being smaller for larger stimulus changes (Bishop et al., 2011, Hommet et al., 2009, Liu et al., 2014). The LDN might reflect additional processing of sounds that are difficult to discriminate (Bishop et al., 2011, Hommet et al., 2009, Liu et al., 2014), or the establishment of children’s neural phonological representations (Kuuluvainen et al., 2016, Liu et al., 2014). The LDN amplitude decreases with age (Bishop et al., 2011, Liu et al., 2014), and is usually not elicited in adults (see, however, Barry et al., 2009).
In the next sections, we will briefly describe how the MMN can be used to investigate memory, discrimination, and language functions, which are compromised in SLI. Thereafter, findings obtained with MMN/LDN in investigating these functions in SLI will be discussed.
1.1.2. Sensory memory
A prolongation of the presentation interval of sounds abolishes MMN, since the trace of each sound fades away when the next sound enters the auditory system (Näätänen, 1992). The MMN was found to be abolished with stimulus offset-to-onset intervals of 4 and 8 s. (for example, Mäntysalo and Näätänen, 1987), but some MMN studies have suggested longer sensory-memory lifetimes (e.g., Böttcher-Gandor and Ullsperger, 1992, Sams et al., 1993, Winkler et al., 2002). By using different stimulus presentation rates one can compare the duration of the sensory memory trace between different participant groups.
The formation of memory traces can be studied with backward-masking paradigms, in which a masking stimulus is presented after sounds, which should interfere with the formation of the sound memory trace (Hawkins and Presson, 1986). The masking effect, prohibiting the discrimination of the sounds, occurs if the interval between the sound and the masker is sufficiently short (Hawkins and Presson, 1986). In a study using 25-ms long tones and 55-ms long maskers, deviant tones neither elicited an MMN nor were behaviorally discriminated from the standard tones when a masking sound occurred 20–50 ms after the tone offset (Winkler and Näätänen, 1992). However, a prolongation of this interval to 150 ms resulted in successful sound discrimination and MMN elicitation. The memory-erasing effect of a masking stimulus, which quickly follows the test sounds, might reflect the integration of successive sound events to meaningful entities (Bregman, 1990).
1.1.3. Sound discrimination
The MMN amplitude is large and latency short if the deviant-standard difference is large, whereas the amplitude diminishes and latency gets longer if this difference becomes small (Novitski et al., 2004, Sams et al., 1985, Tiitinen et al., 1994). Furthermore, easily discriminable stimulus differences also elicit large and early MMN responses, whereas differences which are harder to discriminate result in small-amplitude MMNs with a longer latency (Kujala and Näätänen, 2010). Due to this association, the MMN can also be used as a measure of learning and plasticity. Indeed, its amplitude becomes larger for stimuli used in discrimination training (Kujala and Näätänen, 2010).
1.1.4. Speech vs. nonspeech processing
The MMNs elicited by nonspeech or unfamiliar sounds are larger over the right than the left scalp areas (Giard et al., 1995, Paavilainen et al., 1991) and have stronger generator sources in the right than left hemisphere (Kuuluvainen et al., 2014, Shtyrov et al., 2000, Tervaniemi et al., 2000). MMNs to speech sounds belonging to the individual’s native language, in turn, are relatively more lateralized to the left hemisphere (Kuuluvainen et al., 2014, Näätänen et al., 1997, Shtyrov et al., 2000). These partly separate neural substrates for speech vs. nonspeech processing enable one to disentangle dysfunctions of speech vs. nonspeech processing.
1.2. The literature and methods of the current review
The literature search was carried out from Web of Science, Pubmed, Google scholar, and Ovid Medline, by combining search terms as follows: (MMN OR mismatch negativity OR MMNm OR MMR OR mismatch response) AND (SLI OR language impairment). Only articles published in English by 23.3.2017 were included. This search resulted in 26 articles, from which we had to exclude papers including insufficient information on methodology or data, or data which clearly indicate methodological flaws (altogether 4 articles). For example, studies failing to show responses insignificantly differing from zero even in the healthy control group were excluded. Furthermore, a study reporting an MMN amplitude of 16 microvolts was excluded, since this magnitude is clearly an outlier compared to the average of around −2 microvolts found in the other studies of the current review. In addition, we did not include publications lacking information on EEG recording (for example, place of reference), data processing (for example, artefact removal) or quantification (for example, how the amplitudes were measured). Furthermore, studies including participants having obvious co-morbidities (e.g., dyslexia or attention deficit in the sample of children with SLI) were excluded. However, because various co-morbidities are associated with SLI and particularly the older studies did not always sufficiently pay attention to assessing and reporting them, it is possible that data of some of the studies included in this review are influenced by co-morbidities.
The majority of MMN studies on SLI have investigated acoustic or speech discrimination. To summarize and compare the results of those studies, discussed in Sections 2.1–2.3, we carried out a forest-plot analysis. Forest plot is a common method to visualize systematic effects over several studies (Lewis and Clarke, 2001). In the current review, forest plots were used to visualize amplitude differences between the groups in microvolts. Only studies reporting means and standard deviations were included in the analysis. The visualization shows the amount, direction, and the confidence interval of the difference between the groups (in this case, between the language impaired and control groups), and the data point size is scaled according to the effect size of the individual study. In the current review, the weighted overall difference over all the individual studies is shown at the bottom of the plot (random-effects model). Analyses and visualizations were conducted with metafor package (Viechtbauer, 2010; package version 1.9.8) of R (R-Core-Team, 2016; version 3.3.1).
2. Sound discrimination in SLI
2.1. Non-speech sound frequency discrimination in SLI
Frequency is an important cue in speech processing, particularly for vowel identity (in terms of spectral cues of formants) and consonant identity (formant transitions). In addition, it serves prosodic functions and in Tone languages (e.g., Mandarin) lexical functions. Therefore, it is not surprising that most of the MMN studies on children with SLI have investigated sound frequency discrimination ability. In the first study using MMN for investigating children with SLI Korpilahti and Lang (1994) determined whether frequency (500 Hz vs. 553 Hz) discrimination is impaired in SLI (which they called dysphasia, which was an alternative label at that time). They found that the MMN for the frequency change was diminished in children with SLI. Interestingly, MMN latency correlated with age only in the typically developing control group, which suggests that the maturation of the auditory cortex might be abnormal in children with SLI. Children in this study were 10 years old, but similarly diminished MMN amplitudes with the same paradigm were also found in 5-year old children with SLI (Holopainen et al., 1997). Consistent with these results, Holopainen et al. (1998) found in their 5–9 year old SLI group a diminished MMN for the same frequency change, but additionally the MMN latency was delayed. Furthermore, the diminished MMN amplitude was associated with children’s language skills.
The effect of different stimulus parameters was studied further with 1020, 1050 and 1100 Hz deviants using a 1000 Hz tone as the standard stimulus and employing stimulus-onset asynchronies (SOA) of 270 ms and 470 ms (Ahmmed et al., 2008). The results showed attenuated MMN amplitudes in a SLI group, but in the longer SOA condition and for the largest 5% and 10% deviants only, possibly because the 2% deviant might have been too difficult to discriminate even for the control group, thus not differentiating the groups.
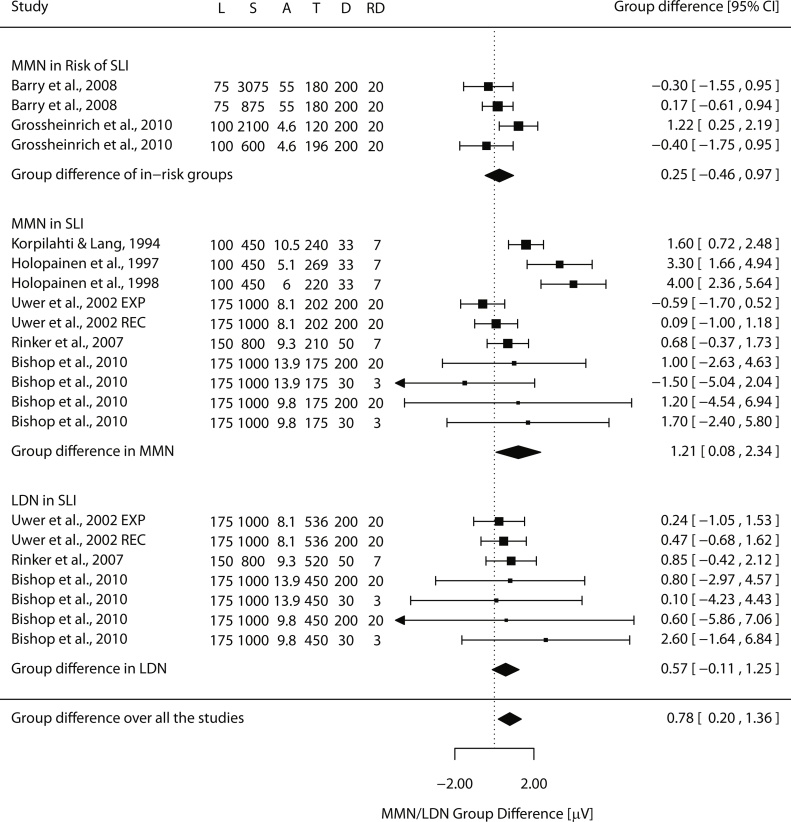
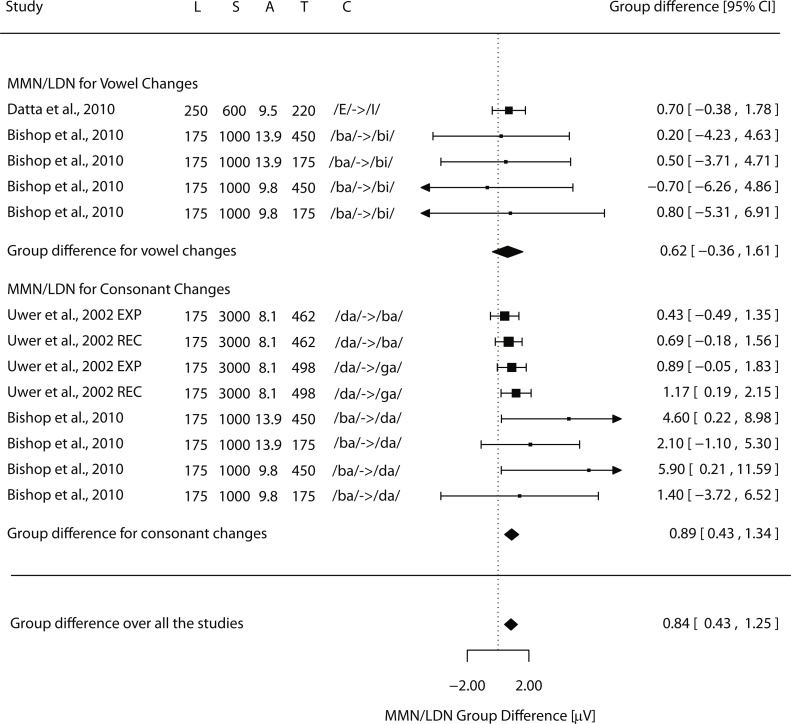
The studies described above found MMN group differences within a time window of 150–275 ms after the sound onset. However, group differences have also been investigated in a later time window of 350–600 ms, presumably including the LDN. Rinker et al. (2007) found significant MMNs, elicited by a change from 700 Hz to 750 Hz, for both groups in the earlier time window, but in the LDN window, the response was significant only in the typically developing group. Furthermore, the scalp topographies of the responses were significantly more right-ward lateralized in children with SLI than in the control children in both time windows. In line with these findings, Bishop et al. (2010) found that only the LDN component was significantly diminished in children with SLI, but for the small deviants (3% change from 1000 Hz to 1030 Hz) only. Consistent with this, Uwer et al. (2002) found no MMN group difference to a large frequency change from 1000 Hz to 1200 Hz, which might not be sufficiently challenging to differentiate the groups (see Fig. 2).
Fig. 2.
Forest plot of tone frequency discrimination studies showing the difference of MMN/LDN amplitudes between the groups in μVs. The black square indicates the mean difference between the groups in each study (in μV; positive values indicate diminished MMN/LDN responses in SLI) and its size is scaled based on the effect size (a larger square for a bigger effect size). The dotted vertical line represents zero microvolt difference between the groups and horizontal lines at each square show associated confidence intervals (the arrow indicating that the line should continue). Black diamonds represent the results of the meta-analyses (random-effects model, RE) and their lateral tips show the corresponding confidence interval. L = length of tone stimuli, S = SOA, A = the mean age of participants in years, T = the mean time point of the analyzed MMN/LDN in ms, D = the difference between the standard and the deviant tones in absolute Hz, and RD = relative difference in percentage.
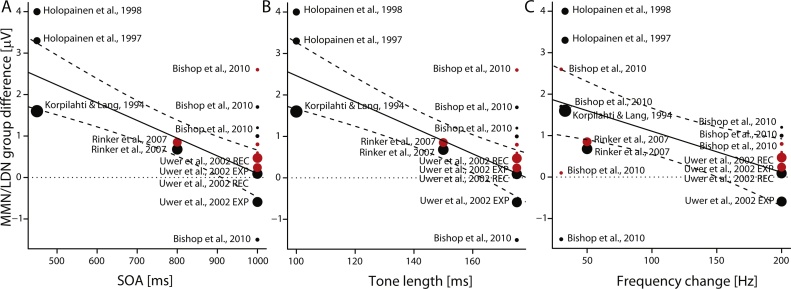
Fig. 2, Fig. 3 summarize the results concerning MMN/LDN findings on frequency discrimination in children with or at risk for SLI. According to Fig. 2, the MMN/LDN amplitude is on average diminished in individuals with or at risk for SLI. Fig. 3 suggests that more prominent differences between SLI and control groups are found when the SOA (Fig. 3A) and sound duration (Fig. 3B) are short and the frequency difference between the deviant and standard stimulus is small (Fig. 3C). However, since these three experimental parameters are strongly associated with each other, one cannot tell which of them has the most important influence.
Fig. 3.
The MMN/LDN difference between language impaired and control groups in tone frequency discrimination studies shown as a function of A) SOA, B) the length of the tones, and C) the absolute frequency difference between the standard and deviant tones. The size of the ball-shaped marker indicates the effect size of the study and the colour indicates the analysis time window (black = MMN, t < 275 ms; red = LDN, t > 275 ms).
2.2. Non-speech sound duration discrimination in SLI
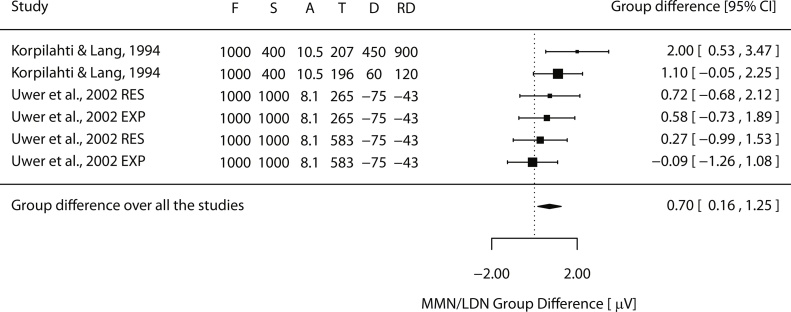
The MMNs to tone duration changes have been investigated in children with SLI to our knowledge only in two studies (Fig. 4). Korpilahti and Lang (1994) compared MMNs elicited by 110-ms and 500-ms long deviants against 50-ms long standard tones, reporting significantly smaller MMN in the SLI than control group for the large change only. Uwer et al. (2002), in turn, compared MMNs to tone duration changes in children with receptive or expressive SLI and those without SLI, finding insignificant MMN differences between the three groups. They used a duration change from 175 ms to 100 ms, which is much smaller than the one that yielded group differences in the study of Korpilahti and Lang. These studies also differ in the stimulus presentation intervals (400-ms stimulus offset-to-onset interval vs. 1000-ms stimulus onset-to-onset interval, respectively) used, the direction of the change (increment vs. decrement, respectively) and the analysis time window (window around the mean vs. individual peak, respectively), which makes it difficult to derive clear conclusions based on these studies.
Fig. 4.
Forest plot of tone duration discrimination studies showing the difference of MMN/LDN amplitude between the groups in μVs. The black square indicates the mean difference between the groups in each study (in μV; positive values indicate diminished MMN/LDN responses in SLI) and its size is scaled based on the effect size (a larger square for a bigger effect size). The dotted vertical line represents zero microvolt difference between the groups and horizontal lines at each square show associated confidence intervals (the arrow indicating that the line should continue). Black diamonds represent the results of the meta-analyses (random-effects model, RE) and their lateral tips show the corresponding confidence interval. F = frequency of tone stimuli, S = SOA, A = the mean age of participants in years, T = the mean time point of the analyzed MMN/LDN in ms, D = the difference between the standard and the deviant tones in absolute Hz, and RD = relative difference in percentage.
Overall, the results reported on the discrimination of non-speech sound frequency and duration differences in children with SLI suggest that at least some of them have dysfunctions in low-level auditory discrimination not limited to speech processing. This difficulty is the most apparent when the stimulus presentation rate is rapid (SOA < 500 ms; see also Bishop, 2007) and, in studies on MMNs elicited by frequency changes, also when the absolute frequency difference between the standard and deviant is small (<100 Hz).
2.3. Speech sound discrimination in SLI
Phonemes are an important basic unit of speech processing. Neural representations of phonemes are acquired during the first year of life when the infant’s speech system becomes tuned to the native language (e.g., Kuhl, 2004, for a review). Quick and accurate mapping of phonemes is crucial for fluent language processing. Furthermore, developmental language and learning problems have a strong association with impaired low-level phoneme processing and poor phonological representations (for reviews, see, e.g., Kujala, 2007, Ramus, 2014, Snowling et al., 2000).
Absent or diminished MMNs/LDNs have been reported in SLI for speech sound changes (Datta et al., 2010, Shafer et al., 2011, Shafer et al., 2005) or phoneme changes in syllables (Bishop et al., 2010, Uwer et al., 2002) or short words (Davids et al., 2011, Tuomainen, 2015). In addition, there are associations between these neural measures and perceptual, cognitive, or language-skill tests, as suggested by the studies discussed below.
A series of studies (Datta et al., 2010, Shafer et al., 2011, Shafer et al., 2005) examined vowel discrimination in SLI by recording MMN/LDN and behavioural responses and determined whether the impairment in vowel processing in children with SLI depends on vowel length. They used/ε/(like in “bet”) vowel as the standard and/I/(like in “bit”) vowel as the deviant and two lengths of the vowels, 50 ms (Shafer et al., 2005) and 250 ms (Datta et al., 2010). In addition to a passive oddball condition, they used an active condition with auditory tone targets and additional discrimination tests. They found that while children with SLI had normal-sized MMN responses to longer vowels (Datta et al., 2010), they were diminished to shorter vowels (Shafer et al., 2005). Significant group differences were obtained for the MMN but not for the later LDN window, in which both groups had a significant response (Shafer et al., 2005). Furthermore, the LDN to long vowels was predominant on the right scalp areas in children with SLI, whereas in typically developing children it was more left lateralized (Datta et al., 2010). Attending to auditory or visual modality did not influence MMN/LDN amplitude differences between the groups (Datta et al., 2010, Shafer et al., 2005). In the discrimination tests, children with SLI were able to detect the vowel targets equally well as typically developing children, but in the vowel identification task they performed more poorly (Datta et al., 2010, Shafer et al., 2011, Shafer et al., 2005).
These results suggest, firstly, that shorter sounds are harder to neurally discriminate than longer sounds in SLI (Fig. 5), consistent with results obtained with non-speech stimuli (Fig. 2, Fig. 3, Fig. 4), as discussed above. Secondly, attention did not influence these results suggesting that neither of the groups benefitted more than the other one from attending to the stimuli. Thirdly, the vowel-discrimination impairment of children with SLI was evident also at the perceptual level, as reflected in poor performance in a vowel identification test. This was interpreted to reflect deficient matching of acoustic input with phonological representations or problems in the maintenance of the stimuli in memory sufficiently long for accomplishing the matching task (Datta et al., 2010).
Fig. 5.
Forest plot of speech-sound discrimination studies showing the difference of MMN/LDN amplitude between the groups in μVs. The black square indicates the mean difference between the groups in each study (in μV; positive values indicate diminished MMN/LDN responses in SLI) and its size is scaled based on the effect size (a larger square for a bigger effect size). The dotted vertical line represents zero microvolt difference between the groups and horizontal lines at each square show associated confidence intervals (the arrow indicating that the line should continue). Black diamonds represent the results of the meta-analyses (random-effects model, RE) and their lateral tips show the corresponding confidence interval. L = length of speech stimuli, S = SOA, A = the mean age of participants in years, T = the mean point of the analyzed MMN/LDN in ms, C = the specific speech sound contrast (standard −> deviant).
Diminished change-elicited responses were also found in children with SLI for consonant changes in syllables (Fig. 5) and these responses were associated with nonword repetition. A study including both vowel and consonant changes in syllables (/ba/standard with/da/and/bi/deviants) found diminished LDNs for the consonant but not for the vowel changes in children with SLI (Bishop et al., 2010). Frequency analysis of this component (4–7 Hz in 300–600 ms time range) showed a significant correlation in the SLI group, with a more diminished LDN response being associated with poorer nonword repetition performance. Consistent with these results, adults who were poor in nonword repetition had smaller late change-elicited responses than those who were good in nonword repetition (Barry et al., 2009). These are important findings, because poor nonword repetition performance was proposed to be one of the most reliable markers of SLI (Bishop et al., 1996).
Some studies failed to show group differences of MMNs elicited by speech sound changes between children with and without SLI. For example, a study with two consonant and two vowel changes in syllables found no significant differences between the groups in the MMNm, the magnetic counterpart of the MMN (Pihko et al., 2008). The reasons for this might be a poor MMNm signal in some participants, yielding no reliable dipoles in the analysis, and a small number of participants (n = 11 in each group).
In addition to diminished MMN/LDN amplitudes, delayed MMN latencies were found to be associated with SLI. For example, Roberts et al. (2012) recorded MMNm in the auditory cortex to vowel (/u/vs./a/) and tone (300 Hz vs. 700 Hz) changes in children with or without SLI. Their general MMNm index (an average of MMNm to speech and tone sounds in both hemispheres) indicated a significantly delayed response in the SLI group, which was remarkably large, about 55 ms. Furthermore, the classification accuracy of the MMNm between the groups was 89.1%, the sensitivity for SLI being 84% and specificity 92%.
These results imply that neural speech sound discrimination is deficient in children with SLI in multiple ways, including diminished MMN amplitudes, delayed latencies, and atypical scalp distributions. These results suggest poor and slow speech sound discrimination accuracy and diminished contribution of the language-dominant left hemisphere to these processes. Moreover, evidence was found for a connection between the MMN/LDN and language skills.
2.4. Sensory memory trace in SLI
Efficient functioning of sensory memory is essential for supporting auditory and speech perception. The individual sounds of speech come in a rapid succession, which requires segmentation of the input and identification of the sounds. Rapid auditory inputs are susceptible to masking effects from preceding and succeeding sounds which negatively impacts perception (Massaro et al., 1976), which degrade the detection of the identities of the sounds. In addition, the maintenance of the heard input in the sensory memory is mandatory in order to identify and remember words, grammar units, and their relationships. Therefore, it is important to determine the sensory memory integrity in language deficits.
There is evidence of elevated masking in the auditory system in SLI. For example, Marler et al. (2002) showed elevated sound detection thresholds in children with SLI, particularly during backward masking, and found neural correlates of masking effects with the MMN. In the MMN experiment, their standard stimulus was 108 dB (SPL) and the deviant one 88 dB (SPL) in intensity, both 10 ms in duration, whereas the masking sound, which immediately followed the stimuli, was a 150-ms long narrow-band noise masker. Stimuli were presented in 500-ms and 1000-ms SOA conditions, the results of which were combined since they did not differ. The MMN amplitude was found to be diminished and latency delayed in children with SLI. Unfortunately, there was no control condition without a masking stimulus, which would be necessary to confirm the specific effects of the masker on the MMN. However, the combined MMN and psychophysics results support the hypothesis of elevated neural masking effects in SLI.
Corroborating results were found in a behavioural study in which children had to detect tones while there was a masking noise either concurrently, before, or after these tones (Wright et al., 1997). It was found that tones had to be presented with a higher intensity to children with SLI to be detected. Noise presented after the tones, causing backward masking effects, was the most detrimental for these children yielding no performance overlap in the backward masking condition between the groups. The results suggesting vulnerability to masking might contribute at least partially to the speech processing difficulties of children with SLI, since the sounds occurring at a rapid pace in speech are likely to mask each other.
However, a study by Bishop et al. (1999) could not demonstrate significant differences between language impaired and unimpaired children in behavioural detection of sounds in backward masking conditions. This discrepancy might result from differences in the stimuli in these three studies. For example, both in the study of Wright et al. (1997) and Marler et al. (2002) the stimulus was a 10-ms sound, whereas it was a more salient 20-ms sound in the study of Bishop et al. (1999). Also the number of participants in these studies was low, 8–11 per each group, which might have influenced the findings. Particularly, a low number of child participants may yield unreliable results in tests requiring motivation and ability to attend. This problem could be even more pronounced in children with developmental disorders, since they might have poor cognitive abilities. MMN recordings might be of help in overcoming these problems.
Also sensory memory maintenance might be impaired in SLI, as suggested by studies using various SOAs in recording MMN. For example, at the age of 5 years, MMNs were recorded from children, who were classified as early or late talkers at the age of around 2 years (Grossheinrich et al., 2010), with a paradigm enabling the investigation of sensory memory duration in a short recording time (Grau et al., 1998). In a short SOA condition, the stimuli, of which 87,5% were standard 1000 Hz tones and 12,5% deviant 1200 Hz tones, were presented with a SOA of 500 ms. In a long-SOA condition, stimuli were presented with this SOA in trains of 4 tones, the inter-train offset-to-onset interval being 2 s, the trains starting either with a standard or randomly with a deviant stimulus. No MMN amplitude differences were found in the short-SOA condition. However, a group difference was found in the condition with long inter-train intervals, with the MMN being diminished in the group of late talkers, suggesting a more rapid decay of the memory trace in these individuals. A similar result of MMN diminution when the interval was long but not when it was short was also found for parents of children with SLI (Barry et al., 2008).
These results suggest abnormally low tolerance to masking effects of sounds and shortened duration of sensory memory in SLI. These deficits could potentially influence, on the one hand, correct identification of speech sounds and, on the other, integrating information over time, which is necessary for memorizing past input to understand speech, particularly longer words. This suggestion is supported by results showing that MMNs are not elicited by changes within words in adults who were parents of children with SLI and poor in nonword repetition (Barry et al., 2009).
3. Associations between sound discrimination and language development in children at risk for SLI
Since SLI has a strong genetic component (for a review, see e.g., Newbury and Monaco, 2010), some of its neural indices should exist even in early childhood. For example, the infant counterpart of the MMN, the MMR, was recorded in 2-month old infants with or without a familial background of SLI for a vowel duration change in syllable/ba/(Friedrich et al., 2004). The MMR was found to be significantly delayed in the infant group at risk for SLI. In another study, infants with a familial history of language problems were followed up with ERP recordings at 3, 9, 12, 16, 24, 36 and 48 months, and the relationship of these ERPs with language skills was determined at the ages of 3 and 4 years (Choudhury and Benasich, 2011). The stimuli were pairs of 100 Hz tones with within-pair intervals of 70 ms or 300 ms. In deviant pairs, the second tone was 300 Hz instead of 100 Hz in frequency. A diminished MMR, especially in the left hemisphere, was found in infants at risk for SLI at ages of 6, 9, 12, and 36 months, but only in the condition with the short interval. In addition, both the amplitude and latency of a negative component preceding the positive MMR (called “the N2” by the authors) to the deviant stimulus in this condition (at 6, 9 and 12 months) predicted language test performance at the ages of 3 and 4 years so that a larger amplitude and a shorter latency were associated with a better performance in different language tests.
Another study had stimuli which consisted of syllable patterns/ba:ba/and/baba:/, the deviances including both the vowel length change in the first syllable and the onset and intensity (stress) changes of the second syllable (Weber et al., 2005). MMNs were recorded at the age of 5 months and the estimate of expressive language skills was assessed by a parental inventory at the ages of one and two years. Subgroups were formed from these production estimates and MMN of these subgroups was compared. The MMN (this time negative) for the stress pattern change was found to be smaller at the left hemisphere sites in children who later had weaker expressive language skills (see Fig. 6). Furthermore, the MMN amplitude correlated with the later language-related/word production scores, with larger MMN amplitudes for stress pattern changes during infancy being associated with better language production skills 7 months later (Weber et al., 2005).
Fig. 6.
The MMN reflects poor word-stress discrimination in infants with SLI. MMNs (the responses to the standard stimuli subtracted from those to the deviant stimuli) to changes in the stress of a word in 5-month old infants with (dashed line) or without (continuous line) a risk for SLI.
Figure adopted from Weber et al. (2005).
Grossheinrich et al. (2010), in turn, determined language skills at the age of 2 years, and recorded MMNs for frequency deviants at the age of 5 years. As already discussed in this review, they found diminished MMNs in those 5-year old children who were late talkers at the age of 2 years specifically in a condition requiring the retention of the tone memory trace for 2 s but not when the stimuli appeared with 500-ms intervals. Moreover, the MMN amplitude was associated with a neuropsychological test measuring memory for the order of words (“the word order subtest”), in which the child hears a list of words and has to point pictures of the spoken items in their presentation order.
The results discussed above show MMR/MMN/LDN amplitude reduction particularly over the left scalp areas and delayed latency in infants at familial risk for language deficits. Furthermore, these results suggest that the neural deficits associated with SLI can be detected even in infancy and indicate an association between the MMN/MMR and language measures in children.
4. Effects of intervention on SLI and MMN
In order to understand the neural mechanisms associated with SLI, it is also helpful to determine which neural processes change when language problems are alleviated by intervention. To our knowledge, there are very few studies which have looked at the effects of intervention at the neural level of individuals with SLI. Pihko et al. (2007) determined with magnetoencephalography (MEG) recordings the influence of language intervention on SLI. There were two matched groups of 5-year-old children with SLI, one of them carrying out language exercises (speech, phoneme, and articulation discrimination training, rapid processing, linguistic and phonological awareness training) and the other physical exercises in 20-30-min group training sessions 3 times a week for 8 weeks.
Auditory-cortex responses were recorded before and after the intervention period for two stimulus sets: standard/da/, deviants/ga/and/ba/, and standard/su/, deviants/sy/and/so/. It was found that language exercise enhanced both obligatory responses to the syllables and the MMNm to the/sy/deviant in the left hemisphere. In addition, it improved discrimination of those syllables which originally were the hardest to discriminate (/da/-/ba/and/su/-/so/pairs).
5. Concluding discussion and future directions
The results discussed in this review consistently show deficits in low-level neural processing in SLI. Impairments were reported in cortical consonant and vowel discrimination as well as tone frequency and duration discrimination, as reflected by diminished MMN/LDN amplitudes and delayed latencies (Fig. 2, Fig. 3, Fig. 4, Fig. 5). Additionally, the central nervous system of children with SLI appears to suffer more than normal from masking effects caused by successive sounds and sensory memory retention problems when sounds occur with long intervals. Furthermore, results showing atypical response amplitude distributions on the scalp suggest partly distinct neural sources in auditory processing in children with than without SLI. More recently, several studies have determined associations between the MMN/LDN measures and language/cognitive functions, and used follow-up designs to assess whether early signs of abnormal neural dysfunction can predict future language problems. These studies found important associations between these different measures, which suggests a strong connection, perhaps even a causal one, between the MMN/LDN responses and SLI.
The results obtained with the MMN/LDN elicited by speech and non-speech sounds in participants with SLI fairly systematically suggest poor neural discrimination of various sound types (Fig. 2, Fig. 3, Fig. 4, Fig. 5), which is compatible with the literature suggesting poor phonological functions in SLI (Ramus et al., 2013). Yet, some studies failed to find significant MMN/LDN differences between the SLI and control groups in some stimulus conditions (e.g., Ahmmed et al., 2008, Uwer et al., 2002). It is likely that some stimulus parameters are more sensitive than others in tapping auditory problems in SLI. For example, the strongest evidence for abnormal non-speech frequency and duration discrimination in SLI was found when stimuli were presented with a rapid SOA (Fig. 2, Fig. 3, Fig. 4; see also Bishop, 2007). Furthermore, a very small difference between the standard and deviant stimulus may result in a small MMN/LDN response also in the control group (“floor effect”; e.g., Ahmmed et al., 2008) and a very large one in strong MMN/LDN amplitudes even in the SLI group (“ceiling effect”; e.g., Uwer et al., 2002) abolishing significant group differences. Furthermore, some studies found group differences with brief but not with longer stimuli (Shafer et al., 2005), perhaps because brief sounds might not form an equally strong memory trace in children with than without SLI. Therefore, these parameters should be systematically varied to find those that best distinguish the groups and illuminate the types of neural deficits that are associated with SLI and may underlie the language dysfunctions.
The low-level auditory dysfunction in SLI appears to be associated with, on the one hand, problems in distinguishing between sound types, such as stimuli with different frequencies, durations, or speech sound identities and, on the other, with sensory memory problems. Group differences have been found with a relatively rapid SOA (200–600 ms; Datta et al., 2010, Korpilahti and Lang, 1994), as discussed above, which should not pose high demands on memory-trace formation or maintenance. Studies comparing MMNs obtained in various SOA conditions support this notion, showing no MMN group differences to large frequency changes in short SOA conditions but diminished MMNs in participants with or at risk for language impairment in long SOA conditions (e.g., Barry et al., 2008, Grossheinrich et al., 2010), suggesting difficulties in maintaining the memory trace of the stimulus. This kind of faster than normal sensory memory trace decay might impair sound representation formation, which could result, e.g., in poor phonological representations in infancy. It also might affect the representation formation during listening, influencing speech perception.
The auditory system both has to maintain memory representations of heard sounds over hundreds of milliseconds to seconds and detect the identities of sounds rapidly succeeding each other. For example, when listening to speech one has to detect phonemes while they are heard in a very rapid succession, whereby the consecutive sounds may impose masking effects on one another. In children with SLI the diminished MMNs/LDNs to consonant changes in CV syllables (e.g., Bishop et al., 2010, Uwer et al., 2002) could be caused by a difficulty in discriminating the differences in rapid spectrotemporal changes included in consonants but also by masking effects caused by the adjacent sounds, or both. Indeed, such backward masking effect was found to be elevated in SLI in behavioral (Wright et al., 1997) and MMN experiments (Marler et al., 2002).
All these problems discussed above may potentially contribute to the difficulties the children with SLI encounter, since for fluent speech, fast and accurate ability in making distinctions between different speech sounds is needed (e.g., Strange, 2011, for a review). Weak speech-sound memory traces make speech processing less automatic, which might hamper the more attention-demanding language processes, such as grammar. It is tempting to speculate that these multiple challenges in low-level auditory processing in SLI discussed above could influence higher-order language functions, such as morphology and comprehension. There is currently insufficient evidence for this conclusion, but available data from a number of studies are encouraging, and suggest the need for further studies to determine how the impaired low-level functions are associated with language deficits in SLI.
Research on SLI and other developmental disorders influenced by multiple genes has the challenge of showing a true causality between the neural abnormalities and the actual functional difficulties influencing everyday life. For example, the sound-discrimination deficit evidenced by many studies might simply co-occur with all the other symptoms typical for SLI, having no actual causal role in language processing. This was not sufficiently acknowledged by earlier studies aiming at, for instance, testing the theory of the rapid auditory temporal processing in SLI (see Bishop, 2007, for a review), according to which problems in detecting auditory cues underlies language deficits (Tallal, 2004). Indeed, many studies using the MMN suggested impaired discrimination of auditory cues in these disorders (Bishop, 2007), but they were unable to demonstrate a causal relationship between the auditory deficits and subsequent language impairment.
Some more recent studies not merely reported perceptual impairments but determined whether, for example, the neural indices have a significant correlation with language tests, giving further insight to this potential connection. For instance, significant associations have been reported for diminished or delayed MMNs in children at risk for SLI and later language abilities (e.g., Choudhury and Benasich, 2011, Weber et al., 2005), suggesting that there indeed is a connection between abnormal low-level neural processes and SLI. In addition, language-intervention induced enhancements in MMNm were shown concurrently with improvement in speech-sound discrimination tests (Pihko et al., 2007).
Next, we would like to suggest some directions for future research. With MMN studies it might be possible to disentangle which low-level impairments are associated with the actual problems in using language efficiently. For example, it could be hypothesized that deficiency in discriminating phonemes is associated with problems in learning new words, which is a common problem in SLI (Kan and Windsor, 2010), since weak representations of sounds could be expected to result in poor word-form memory traces. Furthermore, understanding complex sentences should be associated with sensory-memory duration since retention of items (words, morphological units, etc.) in memory is vital for grasping the meaning of sentences. Besides determining the associations between low-level impairments and various components in language processing, causal relationships between these could be tested by investigating the influence of targeted interventions. For example, if discrimination training of temporal cues results in improved phonological skills and enhancement of the MMN in individuals with SLI, one could claim that a deficit in discriminating temporal cues underlies phonological dysfunctions in SLI.
Another relatively under researched area in MMN studies on SLI is whether the MMN diminution in SLI results from a deficit in true sensory-memory or neural adaptation-related processes (May and Tiitinen, 2010, Näätänen et al., 2011). Disentangling the contribution of the discrimination vs. adaptation/dishabituation processes on auditory functions in SLI would further illuminate the nature of neural dysfunctions in this disorder.
To achieve a more comprehensive picture on SLI with the help of MMN, one should include a large number of participants, optimized MMN paradigms enabling, for instance, the assessment of both discrimination and memory functions in a short time, and large language and cognitive test batteries. This would help to identify subtypes of SLI, which could serve as a basis for designing targeted interventions. Furthermore, it is important to select proper experimental parameters for the MMN recordings, ensuring both sufficient differences between stimuli to minimize possible floor effects, while at the same time not choosing stimulus differences that are so great they result in ceiling effects. Furthermore, the influence of SOA should be taken into account, since it has a distinct effect on the MMN recorded from participants with than without SLI (Grossheinrich et al., 2010, Barry et al., 2008). In addition, reliable individual data, including sufficient trials, should be acquired to achieve reliable results from correlation analyses, since the MMN is a very small brain signal (see, for example, Kujala et al., 2007, for a review on methodological issues in studies using the MMN). Furthermore, by using modern neuroimaging tools, such as MEG, one could determine the dynamics of the two temporal-lobe MMN sources and separate speech-specific from other auditory dysfunctions.
Conflict of Interest
None.
Acknowledgements
This work was supported by the Academy of Finland (grant numbers: 276414 and 288435) and Jane and Aatos Erkko Foundation.
References
- Ahmmed A.U., Clarke E.M., Adams C. Mismatch negativity and frequency representational width in children with specific language impairment. Dev. Med. Child Neurol. 2008;50:938–944. doi: 10.1111/j.1469-8749.2008.03093.x. [DOI] [PubMed] [Google Scholar]
- Alho K. Cerebral generators of mismatch negativity (MMN) and its magnetic counterpart (MMNm) elicited by sound changes. Ear Hear. 1995;16:38–51. doi: 10.1097/00003446-199502000-00004. [DOI] [PubMed] [Google Scholar]
- Archibald L.M.D., Gathercole S.E. Short-term and working memory in specific language impairment. Int. J. Lang. Commun. Disord. 2006;41:675–693. doi: 10.1080/13682820500442602. [DOI] [PubMed] [Google Scholar]
- Böttcher-Gandor C., Ullsperger P. Mismatch negativity in event-related potentials to auditory stimuli as a function of varying interstimulus interval. Psychophysiology. 1992;29:546–550. doi: 10.1111/j.1469-8986.1992.tb02028.x. [DOI] [PubMed] [Google Scholar]
- Barry J.G., Hardiman M.J., Line E., White K.B., Yasin I., Bishop D.V.M. Duration of auditory sensory memory in parents of children with SLI: A mismatch negativity study. Brain Lang. 2008;104:75–88. doi: 10.1016/j.bandl.2007.02.006. [DOI] [PubMed] [Google Scholar]
- Barry J.G., Hardiman M.J., Bishop D.V.M. Mismatch response to polysyllabic nonwords: a neurophysiological signature of language learning capacity. PLoS One. 2009;4:e6270. doi: 10.1371/journal.pone.0006270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop D.V., North T., Donlan C. Nonword repetition as a behavioural marker for inherited language impairment: evidence from a twin study. J. Child Psychol. Psychiatry. 1996;37:391–403. doi: 10.1111/j.1469-7610.1996.tb01420.x. [DOI] [PubMed] [Google Scholar]
- Bishop D.V., Carlyon R.P., Deeks J.M., Bishop S.J. Auditory temporal processing impairment: neither necessary nor sufficient for causing language impairment in children. J. Speech Lang. Hear. Res. 1999;42:1295–1310. doi: 10.1044/jslhr.4206.1295. [DOI] [PubMed] [Google Scholar]
- Bishop D.V.M., Hardiman M.J., Barry J.G. Lower-frequency event-related desynchronization: a signature of late mismatch responses to sounds, which is reduced or absent in children with specific language impairment. J. Neurosci. 2010;30:15578–15584. doi: 10.1523/JNEUROSCI.2217-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop D.V.M., Hardiman M.J., Barry J.G. Is auditory discrimination mature by middle childhood? A study using time-frequency analysis of mismatch responses from 7 years to adulthood. Dev. Sci. 2011;14:402–416. doi: 10.1111/j.1467-7687.2010.00990.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop D.V.M. Using mismatch negativity to study central auditory processing in developmental language and literacy impairments: where are we, and where should we be going? Psychol. Bull. 2007;133:651–672. doi: 10.1037/0033-2909.133.4.651. [DOI] [PubMed] [Google Scholar]
- Bishop D.V.M. Problems with tense marking in children with specific language impairment: not how but when. Philos Trans R Soc L. B Biol Sci. 2014;369:20120401. doi: 10.1098/rstb.2012.0401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bregman A. The Perceptual Organization of Sound, Bradford, Cambridge, MA. MIT, Press; Cambridge, MA: 1990. Auditory Scene Analysis. [Google Scholar]
- Choudhury N., Benasich A.A. Maturation of auditory evoked potentials from 6 to 48 months: prediction to 3 and 4 year language and cognitive abilities. Clin. Neurophysiol. 2011;122:320–338. doi: 10.1016/j.clinph.2010.05.035. [DOI] [PubMed] [Google Scholar]
- Conti-Ramsden G., Mok P.L.H., Pickles A., Durkin K. Adolescents with a history of specific language impairment (SLI): Strengths and difficulties in social, emotional and behavioral functioning. Res. Dev. Disabil. 2013;34:4161–4169. doi: 10.1016/j.ridd.2013.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan N., Winkler I., Teder W., Näätänen R. Memory prerequisites of mismatch negativity in the auditory event-related potential (ERP) J. Exp. Psychol. Learn. Mem. Cogn. 1993;19:909–921. doi: 10.1037//0278-7393.19.4.909. [DOI] [PubMed] [Google Scholar]
- Datta H., Shafer V.L., Morr M.L., Kurtzberg D., Schwartz R.G. Electrophysiological indices of discrimination of long-duration, phonetically similar vowels in children with typical and atypical language development. J. Speech Lang. Hear. Res. 2010;53:757–777. doi: 10.1044/1092-4388(2009/08-0123). [DOI] [PubMed] [Google Scholar]
- Davids N., Segers E., van den Brink D., Mitterer H., van Balkom H., Hagoort P., Verhoeven L. The nature of auditory discrimination problems in children with specific language impairment: an MMN study. Neuropsychologia. 2011;49:19–28. doi: 10.1016/j.neuropsychologia.2010.11.001. [DOI] [PubMed] [Google Scholar]
- Escera C., Leung S., Grimm S. Deviance detection based on regularity encoding along the auditory hierarchy: electrophysiological evidence in humans. Brain Topogr. 2014;27:527–538. doi: 10.1007/s10548-013-0328-4. [DOI] [PubMed] [Google Scholar]
- Friedrich M., Weber C., Friederici A.D. Electrophysiological evidence for delayed mismatch response in infants at-risk for specific language impairment. Psychophysiology. 2004;41:772–782. doi: 10.1111/j.1469-8986.2004.00202.x. [DOI] [PubMed] [Google Scholar]
- Giard M.H., Lavikahen J., Reinikainen K., Perrin F., Bertrand O., Pernier J., Näätänen R. Separate representation of stimulus frequency, intensity, and duration in auditory sensory memory: an event-related potential and dipole-model analysis. J. Cogn. Neurosci. 1995;7:133–143. doi: 10.1162/jocn.1995.7.2.133. [DOI] [PubMed] [Google Scholar]
- Glass E., Sachse S., von Suchodoletz W. Development of auditory sensory memory from 2 to 6 years: an MMN study. J. Neural Transm. 2008;115:1221–1229. doi: 10.1007/s00702-008-0088-6. [DOI] [PubMed] [Google Scholar]
- Grau C., Escera C., Yago E., Polo M.D. Mismatch negativity and auditory sensory memory evaluation: a new faster paradigm. Neuroreport. 1998;9:2451–2456. doi: 10.1097/00001756-199808030-00005. [DOI] [PubMed] [Google Scholar]
- Grossheinrich N., Kademann S., Bruder J., Bartling J., Von Suchodoletz W. Auditory sensory memory and language abilities in former late talkers: a mismatch negativity study. Psychophysiology. 2010;47:822–830. doi: 10.1111/j.1469-8986.2010.00996.x. [DOI] [PubMed] [Google Scholar]
- Hawkins H.L., Presson J. Auditory information processing. In: Boff K.R., Kaufman L., Thomas J.P., editors. Handbook of Perception and Human Performance, Vol. 2: Cognitive Processes and Performance. John Wiley & Sons; Oxford, England: 1986. pp. 1–64. [Google Scholar]
- Holopainen I.E., Korpilahti P., Juottonen K., Lang H., Sillanpaa M. Attenuated auditory event-related potential (mismatch negativity) in children with developmental dysphasia. Neuropediatrics. 1997;28:253–256. doi: 10.1055/s-2007-973709. [DOI] [PubMed] [Google Scholar]
- Holopainen I.E., Korpilahti P., Juottonen K., Lang H., Sillanpaa M. Abnormal frequency mismatch negativity in mentally retarded children and in children with developmental dysphasia. J. Child Neurol. 1998;13:178–183. doi: 10.1177/088307389801300406. [DOI] [PubMed] [Google Scholar]
- Hommet C., Vidal J., Roux S., Blanc R., Barthez M.A., De Becque B., Barthelemy C., Bruneau N., Gomot M. Topography of syllable change-detection electrophysiological indices in children and adults with reading disabilities. Neuropsychologia. 2009;47:761–770. doi: 10.1016/j.neuropsychologia.2008.12.010. [DOI] [PubMed] [Google Scholar]
- Huotilainen M., Kujala A., Hotakainen M., Parkkonen L., Taulu S., Simola J., Nenonen J., Karjalainen M., Näätänen R. Short-term memory functions of the human fetus recorded with magnetoencephalography. Neuroreport. 2005;16:81–84. doi: 10.1097/00001756-200501190-00019. (00001756-200501190-00019) [DOI] [PubMed] [Google Scholar]
- Johnson C.J., Beitchman J.H., Brownlie E.B. Twenty-year follow-up of children with and without speech-language impairments: family, educational, occupational, and quality of life outcomes. Am. J. Speech Lang. Pathol. 2010;19:51–65. doi: 10.1044/1058-0360(2009/08-0083). [DOI] [PubMed] [Google Scholar]
- Kan P.F., Windsor J. Word learning in children with primary language impairment: a meta-analysis. J. Speech Lang. Hear. Res. 2010;53:739–756. doi: 10.1044/1092-4388(2009/08-0248). [DOI] [PubMed] [Google Scholar]
- Kang C., Drayna D. Genetics of speech and language disorders. Annu. Rev. Genomics Hum. Genet. 2011;12:145–164. doi: 10.1146/annurev-genom-090810-183119. [DOI] [PubMed] [Google Scholar]
- Korpilahti P., Lang H.A. Auditory ERP components and mismatch negativity in dysphasic children. Electroencephalogr. Clin. Neurophysiol. 1994;91:256–264. doi: 10.1016/0013-4694(94)90189-9. [DOI] [PubMed] [Google Scholar]
- Korpilahti P., Krause C.M., Holopainen I., Lang A.H. Early and late mismatch negativity elicited by words and speech-like stimuli in children. Brain Lang. 2001;76:332–339. doi: 10.1006/brln.2000.2426. [DOI] [PubMed] [Google Scholar]
- Kuhl P.K. Early language acquisition: cracking the speech code. Nat. Rev. Neurosci. 2004;5:831–843. doi: 10.1038/nrn1533. [DOI] [PubMed] [Google Scholar]
- Kujala T., Näätänen R. The adaptive brain: a neurophysiological perspective. Prog. Neurobiol. 2010 doi: 10.1016/j.pneurobio.2010.01.006. [DOI] [PubMed] [Google Scholar]
- Kujala T. The role of early auditory discrimination deficits in language disorders. J. Psychophysiol. 2007;21:239–250. [Google Scholar]
- Kujala T., Tervaniemi M., Schröger E. The mismatch negativity in cognitive and clinical neuroscience: theoretical and methodological considerations. Biol. Psychol. 2007;74:1–19. doi: 10.1016/j.biopsycho.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Kuuluvainen S., Nevalainen P., Sorokin A., Mittag M., Partanen E., Putkinen V., Seppänen M., Kähkönen S., Kujala T. The neural basis of sublexical speech and corresponding nonspeech processing: a combined EEG-MEG study. Brain Lang. 2014;130:19–32. doi: 10.1016/j.bandl.2014.01.008. [DOI] [PubMed] [Google Scholar]
- Kuuluvainen S., Alku P., Makkonen T., Lipsanen J., Kujala T. Cortical speech and non-speech discrimination in relation to cognitive measures in preschool children. Eur. J. Neurosci. 2016;43:738–750. doi: 10.1111/ejn.13141. [DOI] [PubMed] [Google Scholar]
- Lee C.-Y., Yen H., Yeh P., Lin W.-H., Cheng Y.-Y., Tzeng Y.-L., Wu H.-C. Mismatch responses to lexical tone, initial consonant, and vowel in Mandarin-speaking preschoolers. Neuropsychologia. 2012;50:3228–3239. doi: 10.1016/j.neuropsychologia.2012.08.025. [DOI] [PubMed] [Google Scholar]
- Leonard L. second ed. The MIT Press; Cambridge, MA: 2014. Children with Specific Language Impairment. [Google Scholar]
- Lewis S., Clarke M. Forest plots: trying to see the wood and the trees. BMJ. 2001;322:1479–1480. doi: 10.1136/bmj.322.7300.1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H.M., Chen Y.C., Tsao F.M. Developmental changes in mismatch responses to mandarin consonants and lexical tones from early to middle childhood. PLoS One. 2014;9 doi: 10.1371/journal.pone.0095587. (ARTN e95587) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lum J.A., Conti-Ramsden G., Morgan A.T., Ullman M.T. Procedural learning deficits in specific language impairment (SLI): a meta-analysis of serial reaction time task performance. Cortex. 2014;51:1–10. doi: 10.1016/j.cortex.2013.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mäntysalo S., Näätänen R. The duration of a neuronal trace of an auditory stimulus as indicated by event-related potentials. Biol. Psychol. 1987;24:183–195. doi: 10.1016/0301-0511(87)90001-9. [DOI] [PubMed] [Google Scholar]
- Marler J.A., Champlin C.A., Gillam R.B. Auditory memory for backward masking signals in children with language impairment. Psychophysiology. 2002;39:767–780. doi: 10.1111/1469-8986.3960767. [DOI] [PubMed] [Google Scholar]
- Massaro D.W., Cohen M.M., Idson W.L. Recognition masking of auditory lateralization and pitch judgments. J. Acoust. Soc. Am. 1976;59:434–441. doi: 10.1121/1.380887. [DOI] [PubMed] [Google Scholar]
- Maurer U., Bucher K., Brem S., Brandeis D. Altered responses to tone and phoneme mismatch in kindergartners at familial dyslexia risk. Neuroreport. 2003;14:2245–2250. doi: 10.1097/00001756-200312020-00022. [DOI] [PubMed] [Google Scholar]
- May P.J.C., Tiitinen H. Mismatch negativity (MMN), the deviance-elicited auditory deflection, explained. Psychophysiology. 2010;47:66–122. doi: 10.1111/j.1469-8986.2009.00856.x. [DOI] [PubMed] [Google Scholar]
- Morr M.L., Shafer V.L., Kreuzer J.A., Kurtzberg D. Maturation of mismatch negativity in typically developing infants and preschool children. Ear Hear. 2002;23:118–136. doi: 10.1097/00003446-200204000-00005. [DOI] [PubMed] [Google Scholar]
- Näätänen R., Lehtokoski A., Lennes M., Cheour M., Huotilainen M., Iivonen A., Vainio M., Alku P., Ilmoniemi R.J., Luuk A., Allik J., Sinkkonen J., Alho K. Language-specific phoneme representations revealed by electric and magnetic brain responses. Nature. 1997;385:432–434. doi: 10.1038/385432a0. [DOI] [PubMed] [Google Scholar]
- Näätänen R., Paavilainen P., Rinne T., Alho K. The mismatch negativity (MMN) in basic research of central auditory processing: a review. Clin. Neurophysiol. 2007;118:2544–2590. doi: 10.1016/j.clinph.2007.04.026. [DOI] [PubMed] [Google Scholar]
- Näätänen R., Kujala T., Winkler I. Auditory processing that leads to conscious perception: a unique window to central auditory processing opened by the mismatch negativity and related responses. Psychophysiology. 2011;48:4–22. doi: 10.1111/j.1469-8986.2010.01114.x. [DOI] [PubMed] [Google Scholar]
- Näätänen R. Psychology Press; 1992. Attention and Brain Function. [Google Scholar]
- Newbury D.F., Monaco A.P. Genetic advances in the study of speech and language disorders. Neuron. 2010;68:309–320. doi: 10.1016/j.neuron.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novitski N., Tervaniemi M., Huotilainen M., Näätänen R. Frequency discrimination at different frequency levels as indexed by electrophysiological and behavioral measures. Cogn. Brain Res. 2004;20:26–36. doi: 10.1016/j.cogbrainres.2003.12.011. [DOI] [PubMed] [Google Scholar]
- Paavilainen P., Alho K., Reinikainen K., Sams M., Näätänen R. Right hemisphere dominance of different mismatch negativities. Electroencephalogr. Clin. Neurophysiol. 1991;78:466–479. doi: 10.1016/0013-4694(91)90064-b. [DOI] [PubMed] [Google Scholar]
- Pihko E., Mickos A., Kujala T., Pihlgren A., Westman M., Alku P., Byring R., Korkman M. Group intervention changes brain activity in bilingual language-impaired children. Cereb. Cortex. 2007;17:849–858. doi: 10.1093/cercor/bhk037. [DOI] [PubMed] [Google Scholar]
- Pihko E., Kujala T., Mickos A., Alku P., Byring R., Korkman M. Language impairment is reflected in auditory evoked fields. Int. J. Psychophysiol. 2008;68:161–169. doi: 10.1016/j.ijpsycho.2007.10.016. [DOI] [PubMed] [Google Scholar]
- Ponton C.W., Eggermont J.J., Kwong B., Don M. Maturation of human central auditory system activity: evidence from multi-channel evoked potentials. Clin. Neurophysiol. 2000;111:220–236. doi: 10.1016/s1388-2457(99)00236-9. [DOI] [PubMed] [Google Scholar]
- Ramus F., Marshall C.R., Rosen S., van der Lely H.K. Phonological deficits in specific language impairment and developmental dyslexia: towards a multidimensional model. Brain. 2013;136:630–645. doi: 10.1093/brain/aws356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramus F. Neuroimaging sheds new light on the phonological deficit in dyslexia. Trends Cogn. Sci. 2014;18:274–275. doi: 10.1016/j.tics.2014.01.009. [DOI] [PubMed] [Google Scholar]
- Rinker T., Kohls G., Richter C., Maas V., Schulz E., Schecker M. Abnormal frequency discrimination in children with SLI as indexed by mismatch negativity (MMN) Neurosci. Lett. 2007;413:99–104. doi: 10.1016/j.neulet.2006.11.033. [DOI] [PubMed] [Google Scholar]
- Roberts T.P.L., Heiken K., Kahn S.Y., Qasmieh S., Blaskey L., Solot C., Parker W.A., Verma R., Edgar J.C. Delayed magnetic mismatch negativity field, but not auditory M100 response, in specific language impairment. Neuroreport. 2012;23:463–468. doi: 10.1097/WNR.0b013e32835202b6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sams M., Paavilainen P., Alho K., Näätänen R. Auditory frequency discrimination and event-related potentials. Electroencephalogr. Clin. Neurophysiol. Evoked Potentials. 1985;62:437–448. doi: 10.1016/0168-5597(85)90054-1. [DOI] [PubMed] [Google Scholar]
- Sams M., Hari R., Rif J., Knuutila J. The human auditory sensory memory trace persists about 10 sec: neuromagnetic evidence. J. Cogn. Neurosci. 1993;5:363–370. doi: 10.1162/jocn.1993.5.3.363. [DOI] [PubMed] [Google Scholar]
- Shafer V.L., Morr M.L., Kreuzer J.A., Kurtzberg D. Maturation of mismatch negativity in school-age children. Ear Hear. 2000;21:242–251. doi: 10.1097/00003446-200006000-00008. [DOI] [PubMed] [Google Scholar]
- Shafer V.L., Morr M.L., Datta H., Kurtzberg D., Schwartz R.G. Neurophysiological indexes of speech processing deficits in children with specific language impairment. J. Cogn. Neurosci. 2005;17:1168–1180. doi: 10.1162/0898929054475217. [DOI] [PubMed] [Google Scholar]
- Shafer V.L., Schwartz R.G., Martin B. Evidence of deficient central speech processing in children with specific language impairment: the T-complex. Clin. Neurophysiol. 2011;122:1137–1155. doi: 10.1016/j.clinph.2010.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shtyrov Y., Kujala T., Palva S., Ilmoniemi R.J., Naatanen R. Discrimination of speech and of complex nonspeech sounds of different temporal structure in the left and right cerebral hemispheres. Neuroimage. 2000;12:657–663. doi: 10.1006/nimg.2000.0646. [DOI] [PubMed] [Google Scholar]
- Snowling M., Bishop D.V.M., Stothard S.E. Is preschool language impairment a risk factor for dyslexia in adolescence? J. Child Psychol. Psychiatry Allied Discip. 2000;41:587–600. doi: 10.1111/1469-7610.00651. [DOI] [PubMed] [Google Scholar]
- Strange W. Automatic selective perception (ASP) of first and second language speech: a working model. J. Phon. 2011;39:456–466. [Google Scholar]
- Tallal P. Improving language and literacy is a matter of time. Nat. Rev. Neurosci. 2004;5:721–728. doi: 10.1038/nrn1499. [DOI] [PubMed] [Google Scholar]
- Tervaniemi M., Medvedev S.V., Alho K., Pakhomov S.V., Roudas M.S., Van Zuijen T.L., Näätänen R. Lateralized automatic auditory processing of phonetic versus musical information: a PET study. Hum. Brain Mapp. 2000;10:74–79. doi: 10.1002/(SICI)1097-0193(200006)10:2<74::AID-HBM30>3.0.CO;2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiitinen H., May P., Reinikainen K., Näätänen R. Attentive novelty detection in humans is governed by pre-attentive sensory memory. Nature. 1994 doi: 10.1038/372090a0. [DOI] [PubMed] [Google Scholar]
- Tomblin J.B., Records N.L., Buckwalter P., Zhang X.Y., Smith E., O’Brien M. Prevalence of specific language impairment in kindergarten children. J. Speech Lang. Hear. Res. 1997;40:1245–1260. doi: 10.1044/jslhr.4006.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trainor L., McFadden M., Hodgson L., Darragh L., Barlow J., Matsos L., Sonnadara R. Changes in auditory cortex and the development of mismatch negativity between 2 and 6 months of age. Int. J. Psychophysiol. 2003:5–15. doi: 10.1016/s0167-8760(03)00148-x. [DOI] [PubMed] [Google Scholar]
- Tuomainen O.T. Auditory short-term memory trace formation for nonspeech and speech in SLI and dyslexia as indexed by the N100 and mismatch negativity electrophysiological responses. Neuroreport. 2015;26:374–379. doi: 10.1097/WNR.0000000000000357. [DOI] [PubMed] [Google Scholar]
- Ulanovsky N., Las L., Farkas D., Nelken I. Multiple time scales of adaptation in auditory cortex neurons. J. Neurosci. 2004;24:10440–10450. doi: 10.1523/JNEUROSCI.1905-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uwer R., Albrecht R., von Suchodoletz W. Automatic processing of tones and speech stimuli in children with specific language impairment. Dev. Med. Child Neurol. 2002;44:527–532. doi: 10.1017/s001216220100250x. [DOI] [PubMed] [Google Scholar]
- Viechtbauer W. Conducting Meta-Analyses in R with the metafor Package. J. Stat. Software. 2010;36:1–48. [Google Scholar]
- Weber C., Hahne A., Friedrich M., Friederici A.D. Reduced stress pattern discrimination in 5-month-olds as a marker of risk for later language impairment: neurophysiologial evidence. Cogn. Brain Res. 2005;25:180–187. doi: 10.1016/j.cogbrainres.2005.05.007. [DOI] [PubMed] [Google Scholar]
- Winkler I., Näätänen R. Event-related potentials in auditory backward recognition masking: a new way to study the neurophysiological basis of sensory memory in humans. Neurosci. Lett. 1992;140:239–242. doi: 10.1016/0304-3940(92)90111-j. [DOI] [PubMed] [Google Scholar]
- Winkler I., Korzyukov O., Gumenyuk V., Cowan N., Linkenkaer-Hansen K., Ilmoniemi d.R.J., Alho K., Näätänen R. Temporary and longer term retention of acoustic information. Psychophysiology. 2002;39:530–534. doi: 10.1017/s0048577201393186. (10.1017.S0048577201393186) [DOI] [PubMed] [Google Scholar]
- Winkler I. Interpreting the mismatch negativity. J. Psychophysiol. 2007;21:147–163. [Google Scholar]
- Wright B.A., Lombardino L.J., King W.M., Puranik C.S., Leonard C.M., Merzenich M.M. Deficits in auditory temporal and spectral resolution in language-impaired children. Nature. 1997;387:176–178. doi: 10.1038/387176a0. [DOI] [PubMed] [Google Scholar]