Abstract
Executive functions (EF) reached full maturity during the transition from adolescence to adulthood. Human studies provide important information about adolescent developmental trajectories; however, little remains known about the neural circuits underlying the acquisition of mature EF. Ethical and technical considerations with human subjects limit opportunities to design experimental studies that allows for an in-depth understanding of developmental changes in neural circuits that regulate cognitive maturation. Preclinical models can offer solutions to this problem. Unfortunately, current rodent models of adolescent development have inherent flaws that limit their translational value. For instance, females are often omitted from studies, preventing the assessment of potential sex-specific developmental trajectories. Furthermore, it remains unclear whether cognitive developmental changes in rodents are similar to those observed in humans. Here, we tested adolescent and adult male and female mice in a neurocognitive battery of assays. Based on this approach, we assessed mice performances within distinct subdomains of EF, and observed similarities with human developmental trajectories. Furthermore, the sex-specific cognitive changes we observed were paralleled by molecular and neural activity changes demonstrating that our approach can be used in future research to assess the contribution of precise neural circuits to adolescent cognitive maturation.
Keywords: Executive functions, Adolescence, Prefrontal cortex, Striatum, Mice
1. Introduction
Executive function (EF) is used as an umbrella term that comprises a wide range of cognitive processes, including for instance attention, cognitive flexibility, working memory, and problem solving. EF develops throughout adolescence to reach full maturity in adulthood (Best and Miller, 2010, Satterthwaite et al., 2013). Human studies of EF development throughout the postnatal period are largely based on assessment of brain activity while performing a cognitive assay (e.g. Casey et al., 2005). While this information provides important clues about how developing EF become organized, its interpretation is limited as changes in brain activity could be either a cause or an effect of EF maturation. In addition, such approaches provide limited information about the precise neural circuits and neural cell populations that might underlie EF development. Obtaining this information is of major importance not only to gain a better understanding of postnatal brain development, but also because impairments in EF are a major cause of disability in neurodevelopmental disorders, including schizophrenia, autism, and attention-deficit/hyperactivity disorder (ADHD). Finding effective treatments for these cognitive deficits must remain a priority despite moderate success thus far. Ethical and technical considerations inherent to the use of human subjects preclude us from obtaining an in-depth understanding of the neural circuits underlying the acquisition of mature EF. Furthermore, prior to launching clinical trials in human populations, preclinical studies are a necessary and obligatory first step. Unfortunately, preclinical studies in rodent models of EF deficits rarely translate into effective therapies in human populations. This raises the urgency for developing improved and more reliable preclinical models that can be confidently translated to human studies. To our opinion, current preclinical rodent-based studies that aim at studying EF development and deficits have two main weaknesses: first, they tend to use single behavioral endpoints as a proxy for performances within specific subdomains of EF (i.e. performance in the extra-dimensional set shifting task as a measure of cognitive flexibility deficits – Denis Goetghebeur and Dias, 2014; or performance in the Y-maze as a measure of working memory – Milenkovic et al., 2014), rather than attempting to integrate data from multiple assays to obtain a more accurate representation of performances in distinct subdomains of EF, as often performed in human population (Weinberger et al., 2016); then, they often target only male subjects, while sex differences in postnatal development have been reported in humans (i.e. Gur et al., 2012). The primary goal of the current study is to address both of these concerns by (1) using a battery of cognitive assays associated with unique statistical analyses to ensure that each subdomain of EF considered here is the result of multiple behavioral endpoints, and (2) including both male and female mice to describe potential sex-specific developmental trajectories of EF. Ultimately, we expect our data will provide evidence of the maturation of EF in mice during the transition from adolescence to adulthood, similarly to what is observed in humans. Confirming a similar pattern of postnatal development of EF between mice and humans is a necessary step to later address other important questions related to the development EF and how deficits might arise, and then translate these preclinical findings to clinical studies.
In addition to these two main goals, we also aim to determine the extent to which changes in fronto-striatal circuits during the transition from adolescence to adulthood correlate with the acquisition of mature EF. The postnatal refinement of EF is paralleled by important changes at the neural circuit level, particularly within the prefrontal cortex (PFC) (i.e. Hoftman and Lewis, 2011) and the striatal regions (i.e. Galván, 2010, Sturman and Moghaddam, 2012), suggesting a potential mechanistic relationship between adolescent fronto-striatal remodeling and acquisition of adult EF. In adolescence, the PFC experiences a gain of inhibitory GABAergic transmission (Hoftman and Lewis, 2011) characterized by a large increase in expression of parvalbumin (PV), a calcium-binding protein expressed in specific GABAergic interneurons that tightly regulate the activity of pyramidal cells. The acquisition of prefrontal inhibition during adolescence promotes the formation of gamma band oscillation (Lewis et al., 2012), which is necessary for cognitive performances that rely on the PFC, such as EF (Senkowski and Gallinat, 2015). Interestingly, the PFC of subjects diagnosed with a neurodevelopmental disorder, such as schizophrenia or autism, display an immature GABAergic system characterized by a low level of PV expression and by reduced levels of GAD67 (the rate limiting enzyme necessary for the synthesis of GABA) within PV interneurons (Hoftman and Lewis, 2011, Hashemi et al., 2017). There is also an increasing amount of evidence demonstrating that connections from the frontal cortex to the dorsal striatum contribute significantly to aspects of EF (Godefroy, 2003). Interestingly, abnormal striatal activity has been associated with abnormal attention and impulsivity (two aspects of EF) in patients diagnosed with ADHD (Oldehinkel et al., 2016). Similarly, exposure to stress during adolescence is recognized to affect fronto-striatal circuitry leading to impaired decision-making and choice behavior (see Galván and Rahdar, 2013 for a review). However, there remains a lack of research into the potential relationship between healthy adolescent development of the PFC and striatum and the acquisition of adult EF. The novel mouse model developed here aims to address this point.
2. Material and methods
2.1. Animals
Adolescent (postnatal-day [PND] 28–60) and adult (PND > 65) C57bl6/J male and female mice were used. Adolescent mice were bred in our colony from breeding pairs originally ordered from Jackson Laboratory (Maine, US). In-house breeding was necessary for the adolescent group since by the time they would have been shipped from the vendor and habituated to our colony, they would have been too old to start the adolescent testing. Adult mice were directly ordered from Jackson Laboratory. Because housing conditions in our colony are different from those in Jackson Laboratory facilities (i.e. different cage size), adult mice were allowed at least 1 week of habituation to our colony room prior to testing. Once in our colony, mice were group-housed per sex (2–5 mice per cage – unless specified otherwise) and maintained on a 12-h reverse light-dark cycle with access to food and water ad libitum. In adult females, the estrus cycle was recorded by vaginal swabbing at the end of each testing day to determine whether the stage of the estrus cycle influenced data. All procedures were approved by The Ohio State University Office of Responsible Research Practices and conformed to the U.S. National Institutes of Health Guide for the Care and Use of Laboratory Animals. Efforts were made to minimize the number of mice used and their discomfort. N = 7–11 mice per age group per sex per behavioral assay were used, and N = 4 mice per age group per sex were used for the Western Blot and cFos counting.
2.2. Behavioral assays of EF
Three different cognitive tasks were used to assess various aspects of EF: (1) problem solving abilities in the puzzle box test, (2) cognitive flexibility in the attentional set shifting task (ASST), and (3) working memory in the T-maze delayed alternation working memory task. All tests were conducted during the dark phase of the light-dark cycle, under red light, and all mice were habituated to be handled by an experimenter once per day for at least 3 days prior to the beginning of testing (unless otherwise specified). At the beginning of each testing day, all animals were habituated to the testing room for one hour.
2.2.1. Puzzle box
Beginning on PND30 for the adolescent group and PND70 for the adult group, male and female mice were tested in the puzzle box as previously described by Ben Abdallah et al. (2011). The arena is a rectangular acrylic white box divided into compartments by a removable barrier: a large (58 × 28 cm) start zone exposed to bright overhead lights, and a small (15 × 28 cm) enclosed shaded zone (goal box). An underpass (2 cm deep × 4 cm wide × 4 cm long) below the removable barrier allows mice to move from one compartment to the other. The test takes place over 3 days, with 3 trials per day during which the underpass is obstructed necessitating the mice to develop strategies to pass into the goal box. Aside from the motivation to move from an aversive large and brightly-lit area to a confined and protected area, place preference was established by restricting access to food the night before the first testing day and by placing a reward (1/4 piece of General Mills Honey Nut Cheerios) in the goal box. The first day of the test corresponds to training during which the underpass is unobstructed and mice learn to move from the brightly-lit start area to the dark chamber over 3 trials (T1-T3). During the second day, the 1st trial (T4) is similar to the last trial of day 1 (T3). During trials T5 and T6 of day 2, the underpass is filled with clean sawdust requiring mice to dig through to the goal box (burrowing puzzle). During the third day, the 1st trial (T7) is similar to the last trial of day 2 (T6). During trials T8 and T9 of day 3, the underpass is obstructed by a cardboard plug which needs to be pulled by the mouse to permit entry to the goal box (plug puzzle). For each trial, the latency to enter the covered compartment was recorded. This sequence of trials gives access to various cognitive functions: problem solving ability (T5 and T8), short-term memory (T3, T6, T9), and long-term memory (T4 and T7).
2.2.2. ASST
Beginning on PND28 for the adolescent group and PND60 for the adult group, male and female mice were tested in the ASST as previously described by Heisler et al. (2015). Mice were singly housed and food deprived to maintain 85% of free-feeding body weight. The ASST apparatus consists of a 30.48 × 20.32 × 17.78-cm opaque white acrylic chamber divided by a removable gate into a start area (12.7 × 20.32-cm) and a testing area (17.78 × 20.32-cm). In the testing area, the left and right sides are divided by a 5-inch wall. Non-porous plastic ramekins (3.81 cm depth, 8.89 cm diameter) are placed to each side of this wall and are filled with various digging medium cues. 2 μl of essential oil was added to absorbent paper and taped to the front of the pot to serve as odor cues. Our modified ASST procedure spans 12 days broken down into five phases: handling (days 1–4), food restriction (days 5–12), acclimation to testing arena (days 8–9), training (day 10), and testing (days 11–12). All phases of the ASST were performed during the dark active cycle under red light. During the first four days of the procedure, mice were habituated to handling by the experimenter twice per day and body weights were recorded. Prior to food restriction, mice were left group housed with siblings of the same sex (2–5 per cage). On day 5, mice were singly housed and placed on a restricted diet to maintain 85–90% of normal body weight. For the duration of the test, mice were fed ∼2 g of food and two average-sized pieces of food reward (Kellogg’s Rice Krispies cereal) evenly distributed between two ramekins placed in their cage. During food restriction, mice were transported to the testing room and allowed to acclimate for 1 h per day. On days 8 and 9, mice were introduced to the testing apparatus, scattered with soiled bedding from their home cage, and allowed to freely explore for one hour. Every five minutes, a food reward was given in two familiar ramekins placed in the testing area in order to train the mice to associate the ramekins with a food reward. On day 10, mice were trained to dig in the ramekins to locate the food reward. The training consisted of several less than 3-min trials where the mice were allowed to retrieve food rewards from both ramekins in the testing area. Following each successful trial, beginning with empty ramekins, small amounts of clean bedding were gradually added to bury the cereal. Between each trial, mice were coaxed back into the holding area until the reward was reset and the starting gate removed. When a mouse reliably demonstrated the ability to dig in a full ramekin to find the food reward, training was complete.
Set shifting was tested following training and consisted of two test sessions conducted over two consecutive days. On the first day of testing (Day 11), animals were tested in the following tasks: simple discrimination (SD), compound discrimination (CD), reversal of the CD (R1), and intra-dimensional shift (IDS1). On the second day of testing (Day 12), animals were tested in a second and third intra-dimensional shift (IDS2 and IDS3), reversal of IDS3 (IDS3R), and the extra-dimensional shift (EDS). Individuals only progressed from one discrimination task to the next after establishing a cognitive set defined as eight consecutive correct responses. Prior to testing, each animal was semi-randomly assigned to either a media or scent group (i.e. the relevant dimension predictive of reward) so that half of the animals from each condition were in each group. In addition, all media and scent pairings were randomized between animals. The number of trials to criterion, the average latency to make a choice and the number of errors for each task were scored.
2.2.3. T-maze
Beginning on PND29 for the adolescent group and PND65 for the adult group, male and female mice were tested in the T-maze delayed alternation working memory task as previously described by Izquierdo et al. (2006). Mice were singly housed and food deprived to maintain 85% of free-feeding body weight. The apparatus consists of an acrylic black T-shape arena. The runway arm is 41.5 × 6.5 cm while the goal arms are 34 × 6.5 cm. Food rewards (Kellogg’s Rice Krispies cereal) are placed in opaque dishes placed at the end of each goal arm, to avoid mice to see the reward before making a choice. A partition is positioned in the runway arm so as to form a start box. Extra-maze cues are positioned at the end of each goal arm and were kept constant throughout the test. During the first two days of the test, mice are acclimated to the apparatus littered with ∼15 pieces of cereal and allowed to eat the rewards for a 10-min period per day. Training starts on the 3rd day: each session (one per day) is composed of 11 trials. During the 1st trial, both goal arms are baited with a reward. The mouse is free to choose either arm. The arm not chosen is blocked to prevent the mouse from consuming the other reward. After consuming the reward, the mouse is gently pushed back into the start box where it remained for a period of 5 s (ITI = 5 s) while the goal arms were wiped with 70% ethanol to prevent the mouse from using scent cues to make a choice. For each of the consecutive 10 trials, the same procedure is followed with the exception that only one arm is baited (i.e. the arm that was not chosen during the previous trial). A visit to the same goal arm as the previous trial is scored as incorrect, while a visit in the opposite arm is scored as correct. A mouse moves to the delayed alternation testing phase once it reaches the criterion of 80% correct choices (8 out of 10 trials) across two consecutive sessions. During the delayed alternation task, a same procedure as that used during training is followed during training, the sole difference being that the inter-trial interval (ITI) is increased to 15 s. Criterion is met once a mouse averages 75% correct across two consecutive sessions. The total number of sessions needed to reach the criterion of success during the training and delayed alternation phases is recorded.
2.3. Data analysis of EF
Data were analyzed using the software Prism 5.01 (GraphPad Software Inc., CA, USA) unless otherwise specified. For a first series of analyses, males and females were analyzed separately since our primary goal is to demonstrate changes in EF performances by age, and not necessarily by sex. Each dependent variable of each test was first analyzed independently from each other with age (adolescent vs. adult) as the independent variable. For the puzzle box, the various cognitive functions (i.e. problem solving abilities, short-term memory, and long-term memory) were analyzed separately using a repeated measure ANOVA, with trial as the repeated variable. For the ASST, the latency to choice was analyzed using a repeated measure ANOVA to determine a general effect of age throughout the ASST testing, with task as the repeated variable. The number of trials to reach criterion and the number of errors were analyzed in SPSS (NY, USA) using a repeated measure MANOVA, since both variables are not independent. The set-shifting measures (IDS and EDS tasks) and the reversal measures (R and IDS3-R tasks) were then analyzed separately due to the known contribution of different brain structures (the medial PFC and the orbitofrontal cortex, respectively – Bissonette et al., 2008) to these functions. For the T-maze, the average number of sessions needed to reach the criterion of success was analyzed using a Student t-test. For adult females, the effect of the estrus cycle stage on the performance within each test was first analyzed using a one-way ANOVA for each dependent variable. No effect of the estrus cycle was found, so data of adult females were pooled into one group.
For the second series of analyses we used a z-score analysis approach as described previously by Guilloux et al. (2011) to obtain a robust measure of subdomains of EF across complementary tasks. The following subdomains of EF were included in this analysis: problem solving abilities (including trials T5 and T8) and short term memory (including trials T3, T6 and T9) tested in the puzzle box, set-shifting (including IDS1 and EDS) and reversal (including R and IDS3R) measures tested in the ASST (including the number of trials to reach criterion and the number of errors for each z-score calculation), and the working memory performances tested in the T-maze. The z-score for each subdomain was calculated according to the following equation:
where X represent the individual data for the observed parameter, μ and σ represent the mean and standard deviation for the control group, respectively. The male adult group was used as control and was given a Z-score of “0” for reference. To assess for both age and sex effects, two-way ANOVA were used to analyze these data. Tukey’s posthoc analyses were conducted when appropriate.
2.4. Molecular analyses in brain tissues
Molecular analyses were performed on brains of adolescent and adult mice tested in the assays described above. For c-Fos immunohistochemistry analysis, brains were collected 90 min after the T-maze task. Animals were anaesthetized with isoflurane and perfused transcardially with 0.1 M phosphate-buffered saline (pH 7.4) followed by fresh 4% paraformaldehyde (PFA). Brains were removed and post-fixed overnight in PFA at 4 °C, placed in 30% sucrose until they sank, then frozen on dry ice and sectioned at 50 μm using a cryostat. All brain sections containing the PFC and the striatum were collected so as to obtain 3 sets of each region. Sections were stored in cryoprotectant solution at −20 °C until immunohistochemistry on free-floating sections was performed. c-Fos expression was assessed via single DAB-immunohistochemistry using a rabbit anti-c-Fos primary antibody (1:1000; sc-52; SantaCruz Biotechnology, Inc, TX, USA) as previously described (Shepard et al., 2016). The quantitative analysis of c-Fos in the PFC (dorsal PFC − dPFC: cingulate and prelimbic cortices; ventral PFC – vPFC: infralimbic cortex) and the dorsal striatum was achieved using the unbiased stereology method by an experimenter blind to the age and sex of the mice using the optical fractionator method, with assistance from the StereoInvestigator software from MBF Bioscience (Williston, VT, USA). For Western Blot analysis of prefrontal GABAergic and catecholaminergic, and striatal dopaminergic maturation, brains were collected 30 min after the puzzle box task, and immediately frozen on dry ice and kept in −80 °C until processed as described previously (Coutellier et al., 2014). Antibodies against parvalbumin (PV – ABCam #ab11427; 1:500), GAD67 (ThermoFisher Scientific #PA5-21397; 1:5000) and tyrosine hydroxylase (TH – Novus Biologicals #NB300-109; 1:1000) were used for PFC samples, while TH antibody was used for striatum samples. Β-actin was used as the reference gene (ABCam #ab8227–1:1000). Blot were analyzed using Image J software (Rasband, 1997–2016; National Institutes of HealthRasband, 1997Rasband, 1997–2016; National Institutes of Health)
2.5. Data analysis of molecular endpoints
The number of cFos expressing cells in the PFC (prelimbic and infralimbic cortices) and striatum, and the average signal intensity of PV, GAD67, and TH were analyzed using two-way ANOVAs with sex and age as independent variables, followed by Tukey’s posthoc analyses when appropriate using the software Prism 5.01 (GraphPad Software Inc., CA, USA). Correlations between protein levels or the number of cFos-expressing cells in the dPFC, vPFC, and dorsal striatum, and behavioral endpoints were also assessed using the Pearson’s correlation coefficient. Because of the sex differences observed at the behavioral levels, correlations analyses were conducted separately for males and females.
3. Results
3.1. Puzzle box performances
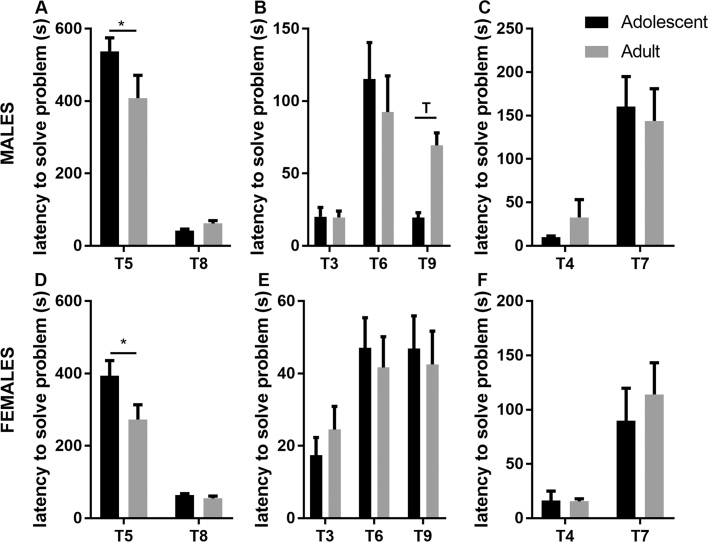
In males, no difference for T1 and T2 between the adolescent and adult groups was found showing similar habituation to the apparatus and ability and motivation to use the underpass to move from the large brightly-lit compartment to the small covered goal box (p > 0.05). Analysis of problem solving abilities in T5 and T8 showed a significant trial effects (p < 0.0001–T5 latency to solve the problem > T8) and a significant interaction between trial and age (p = 0.046) (Fig. 1A). Bonferroni’s posthoc analysis indicated that adolescent male mice took longer to solve the burrowing puzzle (T5) than adult mice (p = 0.03), while no age effect was found regarding the plug puzzle (T8–p > 0.05). Analysis of short-term memory in T3, T6, T9 showed a significant trial effect (p < 0.0001–T6 latency to solve the problem > T3 and T9) and a trend toward a significant interaction between trial and age (p = 0.051). Bonferroni posthoc tests indicated that adult mice tended to take longer to solve the problem than adolescent mice in T9 (p = 0.077) (Fig. 1B). Analysis of long-term memory (T4 and T7) indicated a significant trial effect (p < 0.0001–T4 latency to solve the problem < T7) but no age or interaction effect (Fig. 1C).
Fig. 1.
Adolescent male and female mice show impaired problem-solving abilities in the puzzle box test. (A and D) Adolescent mice require a longer time to move from the lit compartment to the dark chamber during trail 5 and 8 (T5 and T8) of the test. (B and E) Short-term memory (trials 3, 6, 9–T3, T6, T9) and (C and F) long-term memory (trials 4 and 7–T4 and T7) are not different between adolescent and adult mice. *p < 0.05; Tp < 0.08. Male adolescent N = 10; Male adult N = 9; Female adolescent N = 8; Female adult N = 11.
In females, one animal was removed from the analysis as it failed trial 1, suggesting a lack of motivation to perform the task. No difference for T1 and T2 between the adolescent and adult groups was found (p > 0.05). Analysis of problem solving abilities in T5 and T8 showed a significant trial effect (p < 0.0001–T5 latency to solve the problem > T8), a significant age effect (p = 0.041) and a trend for a significant interaction (p = 0.085) (Fig. 1D). Bonferroni’s posthoc analysis showed that adult mice solved the burrowing puzzle T5 faster than adolescent mice (p = 0.015). Analysis of short term memory in T3, T6 and T9 indicated a significant trial effect (p = 0.002–mice performed faster in T3 latency to solve problem < T6 and T9) (Fig. 1E). Analysis of long-term memory (T4 and T7) showed a significant trial effect (p = 0.0012–T4 latency to solve the problem < T7) but no effect of age and no interaction (Fig. 1F). Shorter latency to solve the problem in T3 and T4, vs. T6/T9 and T7, respectively is however not surprising since during these two trials there is no physical barriers that need to be removed by the mouse to use the underpass.
3.2. ASST performances
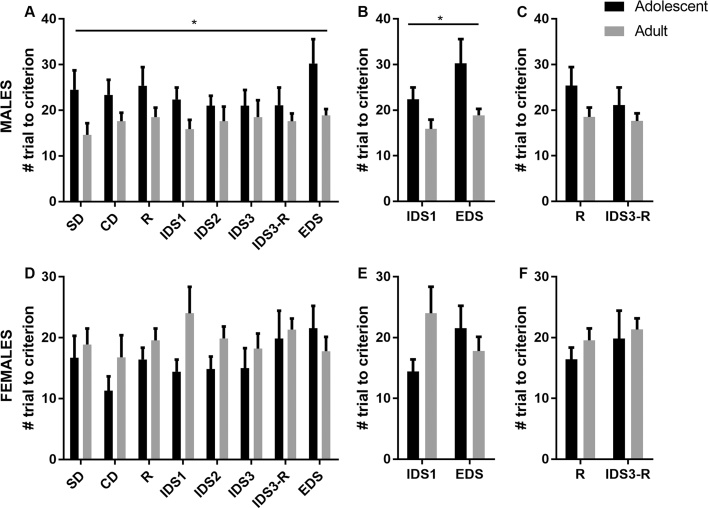
In males, the repeated measure MANOVA indicated a main effect of age (p = 0.04), which was observed for both dependent variables (number of trials to reach criterion: p = 0.021 – Fig. 2A; number of errors: p = 0.013 – Table 1A). No significant task or task-by-age effect was found (p > 0.05). Altogether, adolescent males required more trials to reach the criterion. When assessing set-shifting abilities (IDS1 and EDS tasks) a significant age effect was still observed (p = 0.013), which was true for both the number of trials to reach criterion (p = 0.012 – Fig. 2B) and the number of errors (p = 0.003 – Table 1A). Analysis of reversal abilities (R and IDS3-R tasks – Fig. 2C and Table 1A) did not show any significant age or task effect (p > 0.1). Finally, analysis of the latency to choice (Table 1B) indicated significant task and age effects (p = 0.009 and p = 0.03, respectively). Adolescent males had a shorter latency to make a choice when compared with adult males throughout the ASST task. Tukey’s posthoc analyses regarding the task effect indicate that all mice displayed a shorter latency to choice during the 2 reversal tasks (R and IDS3-R) when compare to SD (p = 0.05 and p = 0.008, respectively). The analysis of the latency to choice during the set-shifting tasks or the reversal tasks did not reveal any effect of age or task (p > 0.1).
Fig. 2.
Adolescent male mice performed poorly in the ASST in comparison to adult mice. (A) Adolescent males require more trials to reach the criterion of completion throughout the ASST; (B) this is particularly true for tasks involving set-shifting, and not those of reversal (C). (D, E and F) In females, no age effect was found. *p < 0.05. Male adolescent N = 8; Male adult N = 8; Female adolescent N = 7; Female adult N = 9.
Table 1A.
Number of errors recorded during each task of the ASST. Adolescent male mice made significantly more error than adult male mice (*p = 0.003) while no age effect was found in females.
| Task | Adolescent Males* | Adult Males | Adolescent Females | Adult Females |
|---|---|---|---|---|
| SD | 5.25 ± 1.69 | 3.33 ± 1.11 | 3.29 ± 1.15 | 4.56 ± 1.26 |
| CD | 5.87 ± 1.48 | 5.67 ± 1.76 | 1.71 ± 1.13 | 3.56 ± 1.44 |
| R | 8.65 ± 2.1 | 4.75 ± 0.75 | 5.29 ± 1.57 | 5.89 ± 0.82 |
| IDS1 | 7.5 ± 1.3 | 3.5 ± 0.73 | 3.14 ± 0.86 | 7.22 ± 1.88 |
| IDS2 | 5.12 ± 0.77 | 3.5 ± 0.96 | 2.71 ± 0.97 | 5.0 ± 0.85 |
| IDS3 | 5.5 ± 1.15 | 3.0 ± 0.78 | 2.71 ± 1.17 | 3.89 ± 1.02 |
| IDS3-R | 6.0 ± 1.24 | 5.25 ± 0.64 | 9.14 ± 4.34 | 5.78 ± 0.55 |
| EDS | 8.87 ± 1.87 | 5.75 ± 0.5 | 6.0 ± 1.72 | 4.0 ± 0.69 |
Table 1B.
Latency to choice (in seconds) recorded during each task of the ASST. Adolescent male mice had a shorter latency to choice than adult male mice (*p = 0.03). In females, adolescent mice had a higher latency to choice than adult (***p = 0.001).
| Task | Adolescent Males* | Adult Males | Adolescent Females*** | Adult Females |
|---|---|---|---|---|
| SD | 11.35 ± 1.22 | 15.92 ± 3.70 | 16.15 ± 2.16 | 11.13 ± 0.83 |
| CD | 9.66 ± 1.37 | 10.66 ± 1.16 | 12.66 ± 1.96 | 12.8 ± 2.39 |
| R | 7.18 ± 1.37 | 7.42 ± 0.45 | 11.85 ± 2.54 | 10.06 ± 1.18 |
| IDS1 | 9.46 ± 1.51 | 9.86 ± 0.89 | 15.30 ± 2.51 | 11.61 ± 2.30 |
| IDS2 | 7.1 ± 1.19 | 11.0 ± 2.59 | 15.96 ± 2.91 | 9.09 ± 1.60 |
| IDS3 | 6.6 ± 0.80 | 8.61 ± 1.43 | 12.05 ± 2.36 | 7.09 ± 0.96 |
| IDS3-R | 5.28 ± 0.85 | 7.41 ± 1.14 | 11.65 ± 2.87 | 6.20 ± 0.90 |
| EDS | 8.08 ± 2.25 | 7.39 ± 0.91 | 10.83 ± 2.65 | 8.80 ± 1.35 |
In females, the repeated measure MANOVA did not reveal a significant effect of age (p > 0.05). This was true for both dependent variables (number of trials to reach criterion: p = 0.21 – Fig2D; number of errors: p = 0.52 – Table 1A). An almost significant trend was found regarding a task effect (p = 0.058). further analyses indicated that the number of errors was affected by the task (Table 1A), where females made less errors in the CD task vs. IDS3-R task (p = 0.02). When assessing set-shifting abilities (IDS and EDS tasks) or reversal abilities (R and IDS3-R tasks), no significant effect of age, task or interaction was noticed (Fig. 2E and 2F, respectively). Finally, the analysis of the latency to choice (Table 1B) revealed a significant age effect (p = 0.001), adolescent females having a higher latency to choice than adult females throughout the ASST task. The analysis of the latency to choice during the set-shifting tasks or the reversal tasks indicated that, while there was no age effect in set-shifting tasks, there is a significant age effect in the reversal tasks (p = 0.03), particularly in IDS3-R (p = 0.09).
3.3. T-maze performances
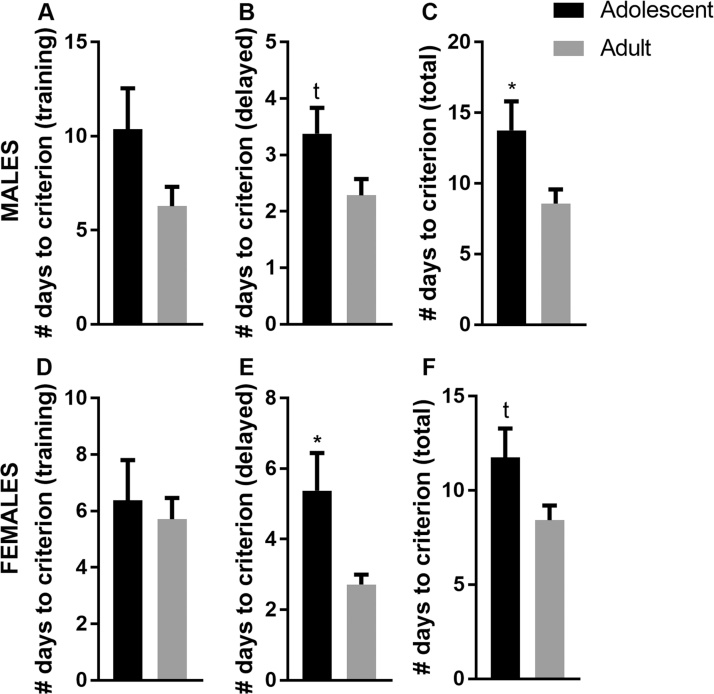
In males, the number of training days required to reach criterion was not significantly different between adolescent and adult mice (p > 0.1 – Fig. 3A). The number of days necessary to reach criterion in the delayed alternation phase of the task tended to be higher in adolescent mice when compared to adult mice (p = 0.074 – Fig. 3B). Altogether, the total number of days needed to complete the task was significantly higher in the adolescent group than in the adult group (p = 0.049 – Fig. 3C). In females, adolescent and adult mice required a similar amount of days to reach the criterion during the training phase of the task (p > 0.1 – Fig. 3D). However, in the delayed alternation phase of the task, adolescent females needed significantly more days to reach criterion than adult females (p = 0.042 – Fig. 3E). Altogether the adolescent female mice tended to need more days to complete the task (p = 0.089 – Fig. 3F).
Fig. 3.
Adolescent male and female mice show impaired working memory in the T-maze delayed alternation test. (A and D) Male and female adolescent and adult mice require the same number of days to reach the criterion of completion during the training phase of the task; (B and E) however, when a working memory load is introduced in the form of a 15-s delay, both male and female adolescent mice need more days to reach the criterion of success than adult mice. (C and F) Overall, adult mice required less days to complete the task than adolescent mice. *p < 0.05; tp < 0.09. Male adolescent N = 8; Male adult N = 7; Female adolescent N = 8; Female adult N = 7.
3.4. Z-score analysis of subdomains of EF and analysis of sex differences
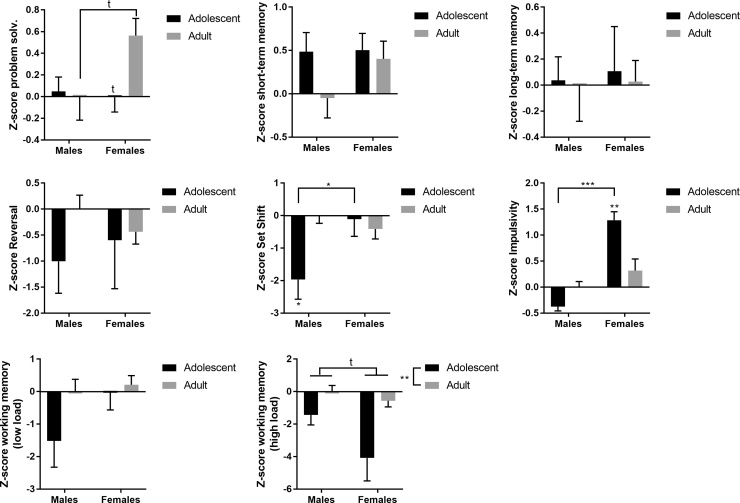
The Z-score analysis allowed us to provide integrative data across different variables recorded within one test (i.e. number of trials to reach criterion and number of errors in the ASST), or across trials within one assay that assess a similar cognitive function (i.e. T5 and T8 in the puzzle box both assess problem solving abilities). Results are presented in Fig. 4. In the puzzle box, the Z-score analysis of short-term and long-term memory did not reveal significant age or sex effects. However, the analysis of the z-score for problem solving showed an almost significant interaction between age and sex (p = 0.06). Tukey’s multiple comparisons indicated that the z-score of adolescent females tended to be lower than that of their adult counterparts (p = 0.09), and that adult females performed better than adult males in this task (p = 0.08).
Fig. 4.
Z-score analysis of the various subdomains of EF tested in the puzzle box (top row), attentional set shifting task (middle row), and the t-maze test (bottom row). Asterisks placed on top of a black bar indicate a difference between adolescent and adult within a sex group. *** p < 0.001; **p < 0.01; *p < 0.05; tp < 0.1.
In the ASST, no significant effect of age or sex was found regarding the z-score for reversal. However, a significant interaction was found for the z-score for set shifting (p = 0.01). Posthoc Tukey’s analyses showed that adolescent males set shifting abilities were lower than that of adult males (p = 0.02) and than that of adolescent females (p = 0.03). A z-score for impulsivity was calculated using the average latency to choice across all ASST trials. A significant sex effect was found (p < 0.0001) as well as a significant interaction (p = 0.0004). Posthoc analyses indicated that adolescent males are more impulsive (lower latency to choice) than adolescent females (p < 0.0001), and that adolescent females are less impulsive (longer latency to choice) than adult ones (p = 0.001).
In the T-maze test, z-score analysis of performances during the training phase did not indicate any age or sex effect. When tested for high-load working memory by adding a longer delay, we noticed a significant age effect (p = 0.008), adult mice performing better than adolescent mice, as well as a trend for a sex effect (p = 0.07), females performing worse than males.
3.5. Western Blot analysis of prefrontal PV and GAD67, and striatal TH
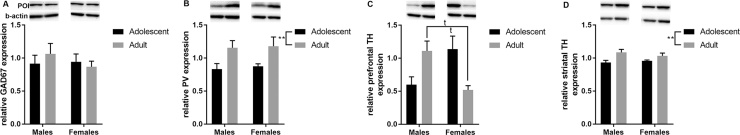
Patterns of developmental changes within the prefrontal cortex and striatum were very similar for males and females, as indicated by the absence of sex effects (Fig. 5). However, a significant interaction between age and sex was found for TH levels in the PFC (p = 0.005 – Fig. 5C). Tukey’s posthoc analyses indicated that adult females tended to have lower levels of prefrontal TH when compared to adult males (p = 0.09) and to adolescent females (p = 0.07). Both expression levels of prefrontal PV and striatal TH varied with age (main age effect p = 0.008 and p = 0.007, respectively). Adolescent mice have lower PV and TH expression when compared to adults (Fig. 5B and D). Finally, no age or sex effect was found for prefrontal GAD67 expression (Fig. 5A).
Fig. 5.
The relative expression of various proteins within the PFC and the striatum differ between adolescent and adult mice. (A) The level of GAD67 in the PFC does not differ between adolescent and adult mice; however, (B and D) adolescent mice have lower levels of parvalbumin (PV) in the PFC and lower level of tyrosine hydroxylase (TH) in the striatum when compared to adult values. (C) Finally, TH levels in the PFC show sex-specific developmental changes: adolescent females have higher levels of prefrontal TH when compared to adult mice. Also, adult females have less prefrontal TH than adult males. POI: protein of interest; **p < 0.01; tp < 0.1. N = 4 per age group, per sex.
Correlation analyses between levels of PV and TH in the PFC and striatum, and the various Z-scores calculated based on the performances in the puzzle box show that only in females, the level of prefrontal TH expression is inversely correlated with the z-score for short-term memory (R = −0.820; p = 0.024). No other correlation was found significant (p > 0.05).
3.6. cFos analysis in the PFC and dorsal striatum
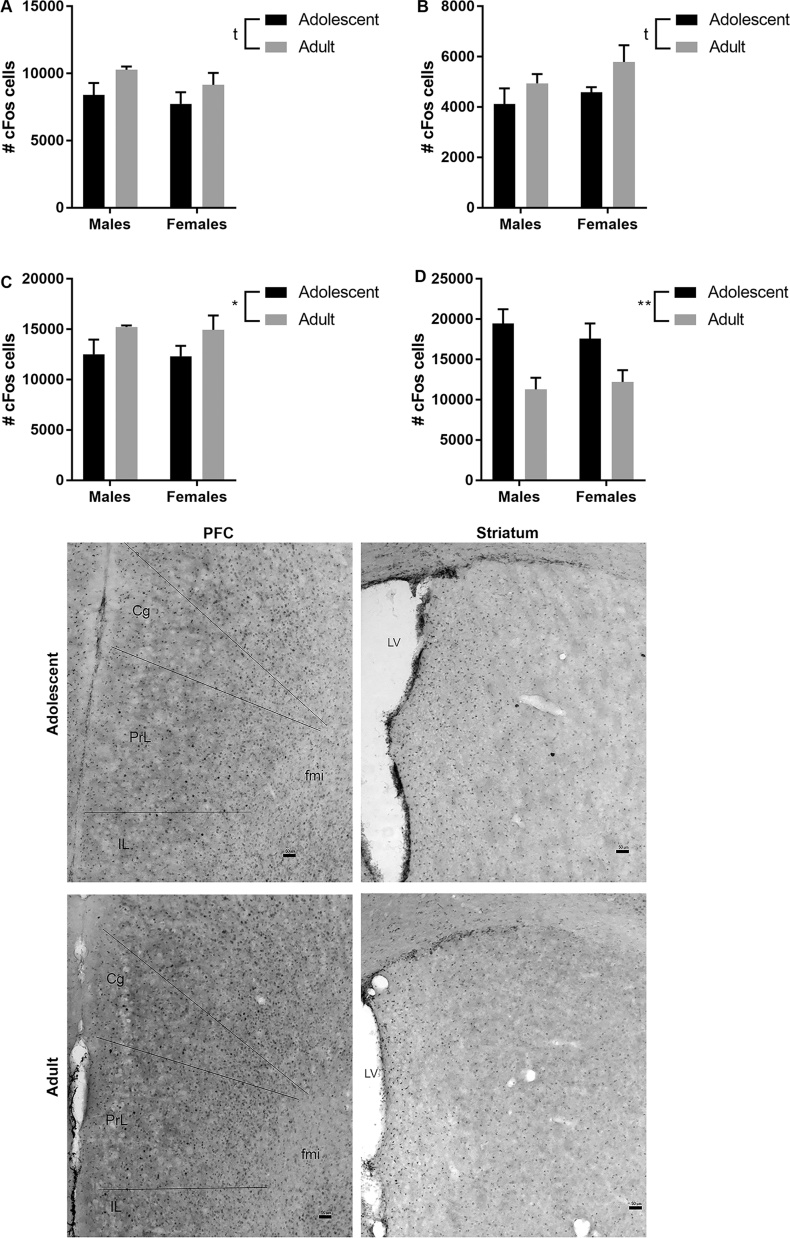
Analysis of cFos expression in the dPFC and vPFC showed a trend toward an age effect for both subregions of the PFC (dPFC: p = 0.07; vPFC: p = 0.07 – Fig. 6A and B), with adolescent mice having lower cFos than adults. This is supported when both subregions were combined to obtain cFos expression in the entire medial PFC (age effect: p = 0.05 – Fig. 6C). Similarly, a significant age effect for cFos expression in the striatum was found (p = 0.001) with higher cFos expression in adolescent mice. No sex difference was noticed (Fig. 6D).
Fig. 6.
Neural activity in the PFC and dorsal striatum of male and female adolescent mice differ from that of adult mice during a task of working memory (T-maze task). The number of cfos expressing cells in the dPFC (A), vPFC (B), and total PFC (C) is lower in adolescent mice when compared to adult mice, while the reverse is true regarding the dorsal striatum (D). Representative pictures of cFos staining in the PFC and dorsal striatum were taken at lower magnification (×5). Cg: cingulate cortex; PrL: prelimbic cortex; IL: infralimbic cortex; fmi: anterior forceps of corpus callosum; LV: lateral ventricle; bar scale = 50 μm. **p < 0.01; tp = 0.07. N = 4 per age group, per sex.
In males, correlation analyses indicate a significant inverse correlation between the total number of days needed to complete the task and the number of cFos-positive cells in the dPFC. Similarly, the z-score of the overall performances in the t-maze was positively correlated with cFos expression in the dPFC indicating that high prefrontal cFos expression coincides with better performances in the t-maze. In females, performances in the t-maze tended to be inversely correlated with cFos expression in the dPFC and vPFC. More importantly, the z-scores of delayed alternation and of the overall performances in the t-maze were inversely correlated with cFos expression in the dorsal striatum (Table 2).
Table 2.
Pearson’s correlation between the number of cFos-expressing cells in the dPFC, vPFC and dorsal striatum, and the total amount of days necessary to complete the t-maze task or the corresponding z-score for performances in the t-maze task of working memory in male and female mice. R = Pearson coefficient; **p < 0.01; *p < 0.05; tp < 0.08.
| dPFC | vPFC | Dorsal Striatum | ||
|---|---|---|---|---|
| Males | Total amount of days | R = −0.822* | R = −0.498 | R = 0.271 |
| Z-score training | R = 0.605 | R = 0.324 | R = −0.058 | |
| Z-score delay | R = 0.563 | R = 0.629 | R = −0.401 | |
| Overall z-score | R = 0.903** | R = 0.712t | R = −0.328 | |
| Females | Total amount of days | R = −0.658t | R = −0.669t | R = 0.476 |
| Z-score training | R = 0.645 | R = 0.426 | R = 0.688 | |
| Z-score delay | R = −0.004 | R = 0.165 | R = −0.844** | |
| Overall z-score | R = 0.214 | R = 0.405 | R = −0.786* | |
4. Discussion
Utilizing a battery of neurocognitive assays, the goal of this study was to determine whether adolescent development of EF follows a similar pattern in mice to that observed in humans. We also aimed to highlight developmental changes within the fronto-striatal circuits which are known in humans to undergo changes during the adolescent period. While others have previously tested developmental changes in single aspects of EF in rodents (i.e. Newman and McGaughy, 2011, Johnson and Wilbrecht, 2011, Koss et al., 2011), to our knowledge, our study not only is the first to present a thorough analysis of the development of EF in mice during adolescence using multiple assays to reflect different subdomains of EF, but offers an original approach using z-score analyses to obtain more robust measures of performances in defined EF subdomains across complementary parameters. Similar to human for whom performances in the executive domain improve significantly during the adolescent period (i.e. Gur et al., 2012), we found that adolescent mice performed more poorly in some aspects of EF than adult mice. This cognitive maturation from adolescence to adulthood is paralleled by molecular changes within the PFC and striatum. Furthermore, our study includes both male and female subjects, highlighting for the first time interesting sex differences in developmental trajectories. Altogether, our findings reinforce the appropriateness of utilizing mouse models to study the neurobiological and behavioral events that occur during adolescence, to unravel the neural and molecular mechanisms that underlie the acquisition of adult EF, and to potentially generate preclinical data for the development of therapeutics for the treatment of EF deficits.
The data presented here provide a description of the developmental changes that affect several subdomains of EF, and reveal very specific patterns of development that depend not only on the subdomain considered, but also on the subject’s sex. For instance, we observed that high-load working memory (in the form of a 15-s delayed alternation in the T-maze) is not yet fully developed in adolescence in both males and females, which replicate previous findings (Koss et al., 2011). Similarly, cognitive flexibility and impulsivity (in the form of latency to make a decision in the ASST) are still undeveloped in adolescent males; however, adolescent females perform similarly to adults in these two subdomains of EF. This suggests that while maturation of cognitive flexibility, in the form of set shifting, and impulsivity has already taken place in females at the time adolescent testing took place (PND40), this is not the case for males. This concurs with similar work in mice demonstrating that cognitive flexibility matures during puberty in females (PND30) (Piekarski et al., 2017), and with work in humans revealing poorer regulation of attention in adolescent boys than girls (Gur et al., 2012). However, recent researches in mouse models and humans revealed that juvenile and adolescent subjects are more flexible than adults (Johnson and Wilbrecht, 2011, Gopnik et al., 2017). These findings have been interpreted as a way to cope with the ever-changing environment that developing individuals face, in comparison to an environment that is more stable, and thus require less flexibility and adaptation in adult life. The discrepancy between these findings and ours originate most likely from differences in the type of cognitive flexibility assessed. We used the ASST and more specifically the EDS task as a measure of flexibility. This approach allowed us to measure the formation of an attentional set and the ability to shift attention. On the other hand, Johnson and Wilbrecht used the 4-choice digging task, which assess decision-making and requires behavioral inhibition, while Gopnik et al. observed better flexibility in adolescence specifically in the social domain. Altogether, this indicates that “cognitive flexibility” is likely to be a very general term that underlie multiple subdomains that are regulated by distinct brain circuits with their own specific developmental trajectories.
Another sex-specific developmental pattern was observed regarding problem solving abilities. These abilities seem to be more developed in adolescent male mice than in adolescent females. Adolescent females performed poorly when compared to their adult counterparts, while no age difference was observed in males. However, this effect seems to be explained by the very high performance of adult females in this task when compared to both adolescent females and adult males. This finding is in contradiction with previous works in which males were found to either outperform (Galsworthy et al., 2005) or perform equally (Milenkovic et al., 2014) to females in adulthood. Dissimilarities in findings might be explained by genetic differences as Galsworthy et al. used outbred strains of mice, while Milenkovic et al. used transgenic mice. It would be interesting to further assess genetic differences in developmental trajectories of problem solving, and other domains of cognitive abilities, to obtain a better understanding of postnatal changes in EF. In any case, our findings indicate the processes that underlie the maturation of these specific subdomains of EF mature at different rate in males and females. Interestingly, performances in the other cognitive domains tested here, including long-term memory (trials 4 and 7 of the puzzle box), reversal learning in the ASST, and low-load working memory (5 s ITI in the T-maze task) were not influenced by age, suggesting that they are already fully developed at the time mice were tested (PND35-45). It is likely that the brain regions (i.e. hippocampus) or neural circuits that regulate these specific cognitive skills have already matured by this age.
While our results are likely due to age differences and difference in brain maturation, we cannot ignore that aspects of our approach might include some contributing factors. First, because of the food restriction that is required for each assay, mice were single-housed. Social isolation in adolescence (a sensitive period of development) might impact brain development more readily than social isolation in adulthood when the brain is less plastic. Indeed, others found that social isolation starting mid-adolescence results in cognitive deficits in adulthood as tested in the AST (Lander et al., 2017). However, these deficits were observed after a period of 30-days of social isolation, which covers the entire adolescent period. With our approach, cognitive testing in adolescence occurs within a couple of days of the beginning of the social isolation period, which might be insufficient to contribute to severe cognitive deficits as observed here in adolescent mice. Then, differences in housing conditions during developmental periods between the adolescent and adult mice might also confound some of our results. Adolescent mice were bred in our colony, while adult mice were purchased directly from the vendor. However, we have made every effort to raise and maintain mice to be tested in adolescence in conditions very similar to those faced by the adult group in the vendor colony, including temperature and humidity, and access to food and water ad libitum. Finally, our findings should be confirmed through the testing of additional cohorts of mice since in some instances our sample size might have been too small to detect age differences in cognitive performances when sex differences were present.
Of importance to this study is the known contribution of the medial PFC to the regulation of EF. The medial PFC includes the anterior cingulate cortex and the prelimbic and infralimbic cortices, and corresponds to the human Broadman's area 46. GABAergic and dopaminergic transmission in the PFC have both been implicated in EF. Gamma activity in the medial PFC, regulated primarily by PV interneurons, has been associated with attentional processing, working memory, and cognitive flexibility in rodents, non-human primates, and humans (Gregoriou et al., 2014, Honkanen et al., 2015, Murray et al., 2015, Kim et al., 2016) while dopaminergic prefrontal circuits have been associated with rule coding and goal-directed behaviors (Ott et al., 2014). Previous work in rats, non-human primates, and humans (Caballero and Tseng, 2016, Hoftman and Lewis, 2011, Catts et al., 2013), as well as our current findings in mice, reliably demonstrates that the level of PV expression in the PFC increases during the transition from adolescence to adulthood. This gain in prefrontal inhibitory transmission could contribute to the maturation of cognitive flexibility, working memory, and attention. Our correlation analysis between prefrontal PV levels and performances in the puzzle box does not support this idea. However, our methodology allowed us to correlate protein levels with performances assessed solely in the puzzle box (problem solving and short-term memory); it thus remains to be determined whether other subdomains of EF are more correlated with changes in prefrontal GABAergic transmission. Interestingly, the EF subdomains that we found to be unaffected by age, such as reversal learning or low-demand working memory, are not directly regulated by the medial PFC, as demonstrated by lesion studies; rather, these subdomains are regulated by the orbitofrontal cortex or hippocampus (Birrell and Brown, 2000, McAlonan and Brown, 2003, Ben Abdallah et al., 2011, Rossi et al., 2012), which can have an earlier developmental trajectory than that of the medial PFC. Our data also indicate significant changes within prefrontal TH as indicated by an increase and decrease of TH expression from adolescence to adulthood in male and female mice, respectively. A similar developmental trajectory in male rats has been recently reported (Willing et al., 2017); however, the reduced level of prefrontal TH expression in adult females vs. adolescent ones was unexpected. While the assessment of TH does not allow us to distinguish between dopaminergic and norepinephrinergic transmission, interestingly, prefrontal TH levels in females were the only molecular endpoint to be significantly correlated with performances in the puzzle box task, where low levels of TH (as found in adult) coincide with better short-term memory. Our data support the idea of sex-specific neural circuits regulating short-term memory as previously suggested by imaging studies in humans (Speck et al., 2000).
Our data further support the importance of a maturing medial PFC to the acquisition of adult EF abilities; we provide evidence for differential cFos expression, as a marker of neuronal activity, in the medial PFC of adolescent and adult mice performing the T-maze task, and affirm a correlation between cFos expression and cognitive performance, as assessed by the number of days required to complete the t-maze task, and by the z-scores calculated in the t-maze. The lower level of cFos expression in the mPFC of adolescent mice is correlated with poorer performances (more days required to reach criterion) in the working memory task. Interestingly, decreased prefrontal activity has also been associated with neurocognitive impairments in patients with schizophrenia (Barch et al., 2001). Altogether, our findings support other studies in animals and humans showing that normal prefrontal activity is important in the regulation of several subdomains of EF (Zhou et al., 2016, Cheng et al., 2017, Kindler et al., 2017). Future studies should further examine this aspect, especially since correlational relationships do not necessarily imply causation; it would be important to determine if lower activity of the medial PFC during adolescence, potentially due to an immature PV system, is directly responsible for adolescents’ poorer performances in EF tasks.
Neuroimaging studies have implicated the involvement of the striatum in executive processes that require planning and set-shifting (Rogers et al., 2000, Lewis et al., 2004). Like the PFC, the striatum, specifically the dopaminergic striatal system, undergoes major restructuration during the adolescent period (Matthews et al., 2013, Hammerslag and Gulley, 2016). Our data support these previous findings in rodents, as indicated by the increase in striatal TH expression from adolescence to adulthood in both male and female mice. This developmental pattern of striatal TH in mice and in rats (as described by Matthews et al., 2013) seems to be in contradiction with human findings. Haycock et al. (2003) described an increase in striatal TH from birth until two-years of age, followed by a slight decrease and steady-state in adulthood. To our knowledge, this study is the only one providing a developmental description of TH levels in human striatum, and thus needs confirmation. However, the discrepancy between rodents and humans can rely on the methodology followed to collect brains: in our study brains have been collected after a cognitive task which could have influence TH levels since TH expression and dopamine synthesis are activity-dependent. Furthermore, we observed that neural activity as measured by cFos expression within the striatum of adolescent mice performing the T-maze task was higher than that of adult mice. It is unclear why this higher level of cFos expression is observed. One interesting possibility would be an interaction between striatal and prefrontal neuronal activity. Indeed, a study in schizophrenic subjects displaying EF deficits indicated an inverse correlation between neural activity of the PFC and striatal dopaminergic function (Meyer-Lindenberg et al., 2002). Finally, we noted that this age-specific activity of the dorsal striatum correlates inversely with performances in the T-maze task only in females. This indicates that the high level of cFos expressed in the dorsal striatum of adolescent females coincides with lower z-scores, and thus with lower performances in working memory, particularly working memory with high load (since only the z-score for the delayed alternation was correlated with cFos expression). Why a similar inverse correlation was not found in male mice remains unclear. It might be that different neural circuits regulate more strongly working memory in males than in females. Indeed, the dorsal striatum has not been directly implicated in the regulation of working memory, but rather in decision making and goal-directed behaviors (Barnes et al., 2005, Graybiel, 2008). It would be interesting to measure neural activity in the dorsal striatum after exposure to other EF tasks.
5. Conclusion
In this study, we used multiple cognitive assays in mice to study in a sex-specific manner the cognitive changes that occur during the transition from adolescence to adulthood. We believe this method has the potential for high translational value and we hope that it will be used in future studies to obtain a better understanding of the adolescent brain and behavior. A main limitation of the current approach resides in the fact that due to the short adolescent period of mice and the length of each behavioral assays, it is difficult to test a single animal in multiple tests. The inclusion of other assays, such as the self-paced 5-choice serial reaction time task (SP-5C – Remmelink et al., 2017) which last 5 days, could allow for multiple testing: one mouse could be assessed for its problem solving abilities and short and long-term memory in the Puzzle-box over 3 days, and for its impulsivity and attention in the SP-5C over 5 days. Despite some differences between our findings in mice and previously published findings in humans regarding developmental trajectories of EF and associated brain regions, important similarities were found, particularly as they relate to sex differences, and correlations between the activity of specific brain regions and cognitive performances. Preclinical rodent-based models could therefore be used to understand the developmental changes in neural circuits that contribute to behavioral refinements during the adolescent period, and to assess sex differences and hormonal influences on developmental trajectories. Indeed, causative relationships between the maturation of precise neural circuits and the behavioral modifications that occur in the cognitive domain during adolescence in males and females have not yet been clearly established.
Conflict of Interest
None.
References
- Barch D.M., Carter C.S., Braver T.S., Sabb F.W., MacDonald A., 3rd., Noll D.C., Cohen J.D. Selective deficits in prefrontal cortex function in medication-naive patients with schizophrenia. Arch. Gen. Psychiatry. 2001;58(3):280–288. doi: 10.1001/archpsyc.58.3.280. [DOI] [PubMed] [Google Scholar]
- Barnes T.D., Kubota Y., Hu D., Jin D.Z., Graybiel A.M. Activity of striatal neurons reflects dynamic encoding and recoding of procedural memories. Nature. 2005;437:1158–1161. doi: 10.1038/nature04053. [DOI] [PubMed] [Google Scholar]
- Ben Abdallah N.M., Fuss J., Trusel M., Galsworthy M.J., Bobsin K., Colacicco G., Deacon R.M., Riva M.A., Kellendonk C., Sprengel R., Lipp H.P., Gass P. The puzzle box as a simple and efficient behavioral test for exploring impairments of general cognition and executive functions in mouse models of schizophrenia. Exp. Neurol. 2011;227(1):42–52. doi: 10.1016/j.expneurol.2010.09.008. [DOI] [PubMed] [Google Scholar]
- Best J.R., Miller P.H. A developmental perspective on executive function. Child Dev. 2010;81(6):1641–1660. doi: 10.1111/j.1467-8624.2010.01499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birrell J.M., Brown V.J. Medial frontal cortex mediates perceptual attentional set shifting in the rat. J. Neurosci. 2000;20(11):4320–4324. doi: 10.1523/JNEUROSCI.20-11-04320.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissonette G.B., Martins G.J., Franz T.M., Harper E.S., Schoenbaum G., Powell E.M. Double dissociation of the effects of medial and orbital prefrontal cortical lesions on attentional and affective shifts in mice. J. Neurosci. 2008;28(44):11124–11130. doi: 10.1523/JNEUROSCI.2820-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caballero A., Tseng K.Y. GABAergic function as a limiting factor for prefrontal maturation during adolescence. Trends Neurosci. 2016;39(7):441–448. doi: 10.1016/j.tins.2016.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey B.J., Galvan A., Hare T.A. Changes in cerebral functional organization during cognitive development. Curr. Opin. Neurobiol. 2005;15(2):239–244. doi: 10.1016/j.conb.2005.03.012. [DOI] [PubMed] [Google Scholar]
- Catts V.S., Fung S.J., Long L.E., Joshi D., Vercammen A., Allen K.M., Fillman S.G., Rothmond D.A., Sinclair D., Tiwari Y., Tsai S.Y., Weickert T.W., Weickert Shannon. Rethinking schizophrenia in the context of normal neurodevelopment. Front. Cell. Neurosci. 2013;7(60) doi: 10.3389/fncel.2013.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng J., Liu A., Shi M.Y., Yan Z. Disrupted glutamatergic transmission in prefrontal cortex contributes to behavioral abnormality in an animal model of ADHD. Neuropsychopharmacology. 2017;42(10):2096–2104. doi: 10.1038/npp.2017.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutellier L., Ardestani P.M., Shamloo M. β1-adrenergic receptor activation enhances memory in Alzheimer's disease model. Ann. Clin. Transl. Neurol. 2014;1(5):348–360. doi: 10.1002/acn3.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denis Goetghebeur P.J., Dias R. The attentional set-shifting test paradigm in rats for the screening of novel pro-cognitive compounds with relevance for cognitive deficits in schizophrenia. Curr. Pharm. Des. 2014;20(31):5060–5068. doi: 10.2174/1381612819666131216114909. [DOI] [PubMed] [Google Scholar]
- Galsworthy M.J., Paya-Cano J.L., Liu L., Monleón S., Gregoryan G., Fernandes C., Schalkwyk L.C., Plomin R. Assessing reliability, heritability and general cognitive ability in a battery of cognitive tasks for laboratory mice. Behav. Genet. 2005;35(5):675–692. doi: 10.1007/s10519-005-3423-9. [DOI] [PubMed] [Google Scholar]
- Galván A. Adolescent development of the reward system. Front. Hum. Neurosci. 2010;4:6. doi: 10.3389/neuro.09.006.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galván A., Rahdar A. The neurobiological effects of stress on adolescent decision making. Neuroscience. 2013;249:223–231. doi: 10.1016/j.neuroscience.2012.09.074. [DOI] [PubMed] [Google Scholar]
- Godefroy O. Frontal syndrome and disorders of executive functions. J. Neurol. 2003;250(1):1–6. doi: 10.1007/s00415-003-0918-2. [DOI] [PubMed] [Google Scholar]
- Gopnik A., O'Grady S., Lucas C.G., Griffiths T.L., Wente A., Bridgers S., Aboody R., Fung H., Dahl R.E. Changes in cognitive flexibility and hypothesis search across human life history from childhood to adolescence to adulthood. Proc. Natl. Acad. Sci. U. S. A. 2017:201700811. doi: 10.1073/pnas.1700811114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graybiel A.M. Habits, rituals, and the evaluative brain. Annu. Rev. Neurosci. 2008;31:359–387. doi: 10.1146/annurev.neuro.29.051605.112851. [DOI] [PubMed] [Google Scholar]
- Gregoriou G.G., Rossi A.F., Ungerleider L.G., Desimone R. Lesions of prefrontal cortex reduce attentional modulation of neuronal responses and synchrony in V4. Nat. Neurosci. 2014;17(7):1003–1011. doi: 10.1038/nn.3742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilloux J.P., Seney M., Edgar N., Sibille E. Integrated behavioral z-scoring increases the sensitivity and reliability of behavioral phenotyping in mice: relevance to emotionality and sex. J. Neurosci. Methods. 2011;197(1):21–31. doi: 10.1016/j.jneumeth.2011.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gur R.C., Richard J., Calkins M.E., Chiavacci R., Hansen J.A., Bilker W.B., Loughead J., Connolly J.J., Qiu H., Mentch F.D., Abou-Sleiman P.M., Hakonarson H., Gur R.E. Age group and sex differences in performance on a computerized neurocognitive battery in children age 8–21. Neuropsychology. 2012;26(2):251–265. doi: 10.1037/a0026712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammerslag L.R., Gulley J.M. Sex differences in behavior and neural development and their role in adolescent vulnerability to substance use. Behav. Brain Res. 2016;298(Pt A):15–26. doi: 10.1016/j.bbr.2015.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashemi E., Ariza J., Rogers H., Noctor S.C., Martínez-Cerdeño V. The number of parvalbumin-expressing interneurons is decreased in the medial prefrontal cortex in autism. Cereb. Cortex. 2017;27(3):1931–1943. doi: 10.1093/cercor/bhw021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haycock J.W., Becker L., Ang L., Furukawa Y., Hornykiewicz O., Kish S.J. Marked disparity between age-related changes in dopamine and other presynaptic dopaminergic markers in human striatum. J. Neurochem. 2003;87(3):574–585. doi: 10.1046/j.1471-4159.2003.02017.x. [DOI] [PubMed] [Google Scholar]
- Heisler J.M., Morales J., Donegan J.J., Jett J.D., Redus L., O'Connor J.C. The attentional set shifting task: a measure of cognitive flexibility in mice. J. Vis. Exp. 2015:96. doi: 10.3791/51944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoftman G.D., Lewis D.A. Postnatal developmental trajectories of neural circuits in the primate prefrontal cortex: identifying sensitive periods for vulnerability to schizophrenia. Schizophr. Bull. 2011;37(3):493–503. doi: 10.1093/schbul/sbr029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honkanen R., Rouhinen S., Wang S.H., Palva J.M., Palva S. Gamma oscillations underlie the maintenance of feature-specific information and the contents of visual working memory. Cereb. Cortex. 2015;25(10):3788–3801. doi: 10.1093/cercor/bhu263. [DOI] [PubMed] [Google Scholar]
- Izquierdo A., Wiedholz L.M., Millstein R.A., Yang R.J., Bussey T.J., Saksida L.M., Holmes A. Genetic and dopaminergic modulation of reversal learning in a touchscreen-based operant procedure for mice. Behav. Brain Res. 2006;171(2):181–188. doi: 10.1016/j.bbr.2006.03.029. [DOI] [PubMed] [Google Scholar]
- Johnson C., Wilbrecht L. Juvenile mice show greater flexibility in multiple choice reversal learning than adults. Dev. Cogn. Neurosci. 2011;1(4):540–551. doi: 10.1016/j.dcn.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H., Ährlund-Richter S., Wang X., Deisseroth K., Carlén M. Prefrontal parvalbumin neurons in control of attention. Cell. 2016;164(1-2):208–218. doi: 10.1016/j.cell.2015.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kindler J., Schultze-Lutter F., Hauf M., Dierks T., Federspiel A., Walther S., Schimmelmann B.G., Hubl D. Increased striatal and reduced prefrontal cerebral blood flow in clinical high risk for psychosis. Schizophr. Bull. 2017 doi: 10.1093/schbul/sbx070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koss W.A., Franklin A.D., Juraska J.M. Delayed alternation in adolescent and adult male and female rats. Dev. Psychobiol. 2011;53(7):724–731. doi: 10.1002/dev.20543. [DOI] [PubMed] [Google Scholar]
- Lander S.S., Linder-Shacham D., Gaisler-Salomon I. Differential effects of social isolation in adolescent and adult mice on behavior and cortical gene expression. Behav. Brain Res. 2017;316:245–254. doi: 10.1016/j.bbr.2016.09.005. [DOI] [PubMed] [Google Scholar]
- Lewis S.J., Dove A., Robbins T.W., Barker R.A., Owen A.M. Striatal contributions to working memory: a functional magnetic resonance imaging study in humans. Eur. J. Neurosci. 2004;19(3):755–760. doi: 10.1111/j.1460-9568.2004.03108.x. [DOI] [PubMed] [Google Scholar]
- Lewis D.A., Curley A.A., Glausier J.R., Volk D.W. Cortical parvalbumin interneurons and cognitive dysfunction in schizophrenia. Trends Neurosci. 2012;35(1):57–67. doi: 10.1016/j.tins.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews M., Bondi C., Torres G., Moghaddam B. Reduced presynaptic dopamine activity in adolescent dorsal striatum. Neuropsychopharmacology. 2013;38(7):1344–1351. doi: 10.1038/npp.2013.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAlonan K., Brown V.J. Orbital prefrontal cortex mediates reversal learning and not attentional set shifting in the rat. Behav. Brain Res. 2003;146(1):97–103. doi: 10.1016/j.bbr.2003.09.019. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A., Miletich R.S., Kohn P.D., Esposito G., Carson R.E., Quarantelli M., Weinberger D.R., Berman K.F. Reduced prefrontal activity predicts exaggerated striatal dopaminergic function in schizophrenia. Nat. Neurosci. 2002;5(3):267–271. doi: 10.1038/nn804. [DOI] [PubMed] [Google Scholar]
- Milenkovic M., Mielnik C.A., Ramsey A.J. NMDA receptor-deficient mice display sexual dimorphism in the onset and severity of behavioural abnormalities. Genes Brain Behav. 2014;13(8):850–862. doi: 10.1111/gbb.12183. [DOI] [PubMed] [Google Scholar]
- Murray A.J., Woloszynowska-Fraser M.U., Ansel-Bollepalli L., Cole K.L., Foggetti A., Crouch B., Riedel G., Wulff P. Parvalbumin-positive interneurons of the prefrontal cortex support working memory and cognitive flexibility. Sci. Rep. 2015;5:16778. doi: 10.1038/srep16778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman L.A., McGaughy J. Adolescent rats show cognitive rigidity in a test of attentional set shifting. Dev. Psychobiol. 2011;53(4):391–401. doi: 10.1002/dev.20537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldehinkel M., Beckmann C.F., Pruim R.H., van Oort E.S., Franke B., Hartman C.A., Hoekstra P.J., Oosterlaan J., Heslenfeld D., Buitelaar J.K., Mennes M. Attention-Deficit/Hyperactivity Disorder symptoms coincide with altered striatal connectivity. Biol. Psychiatry Cogn. Neurosci. Neuroimaging. 2016;1(4):353–363. doi: 10.1016/j.bpsc.2016.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ott T., Jacob S.N., Nieder A. Dopamine receptors differentially enhance rule coding in primate prefrontal cortex neurons. Neuron. 2014;84(6):1317–1328. doi: 10.1016/j.neuron.2014.11.012. [DOI] [PubMed] [Google Scholar]
- Piekarski D.J., Boivin J.R., Wilbrecht L. Ovarian hormones organize the maturation of inhibitory neurotransmission in the frontal cortex at puberty onset in female mice. Curr. Biol. 2017;27(12):1735–1745. doi: 10.1016/j.cub.2017.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasband, W.S., ImageJ, U.S. National Institutes of Health, Bethesda, Maryland, USA, https://imagej.nih.gov/ij/, 1997–2016.
- Remmelink E., Chau U., Smit A.B., Verhage M., Loos M. A one-week 5-choice serial reaction time task to measure impulsivity and attention in adult and adolescent mice. Sci. Rep. 2017;7:42519. doi: 10.1038/srep42519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers R.D., Andrews T.C., Grasby P.M., Brooks D.J., Robbins T.W. Contrasting cortical and subcortical activations produced by attentional-set shifting and reversal learning in humans. J. Cogn. Neurosci. 2000;12(1):142–162. doi: 10.1162/089892900561931. [DOI] [PubMed] [Google Scholar]
- Rossi M.A., Hayrapetyan V.Y., Maimon B., Mak K., Je H.S., Yin H.H. Prefrontal cortical mechanisms underlying delayed alternation in mice. J. Neurophysiol. 2012;108(4):1211–1222. doi: 10.1152/jn.01060.2011. [DOI] [PubMed] [Google Scholar]
- Satterthwaite T.D., Wolf D.H., Erus G., Ruparel K., Elliott M.A., Gennatas E.D. Functional maturation of the executive system during adolescence. J. Neurosci. 2013;33(41):16249–16261. doi: 10.1523/JNEUROSCI.2345-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senkowski D., Gallinat J. Dysfunctional prefrontal gamma-band oscillations reflect working memory and other cognitive deficits in schizophrenia. Biol. Psychiatry. 2015;77(12):1010–1019. doi: 10.1016/j.biopsych.2015.02.034. [DOI] [PubMed] [Google Scholar]
- Shepard R., Page C.E., Coutellier L. Sensitivity of the prefrontal GABAergic system to chronic stress in male and female mice: relevance for sex differences in stress-related disorders. Neuroscience. 2016;332:1–12. doi: 10.1016/j.neuroscience.2016.06.038. [DOI] [PubMed] [Google Scholar]
- Speck O., Ernst T., Braun J., Koch C., Miller E., Chang L. Gender differences in the functional organization of the brain for working memory. Neuroreport. 2000;11(11):2581–2585. doi: 10.1097/00001756-200008030-00046. [DOI] [PubMed] [Google Scholar]
- Sturman D.A., Moghaddam B. Striatum processes reward differently in adolescents versus adults. Proc. Natl. Acad. Sci. U. S. A. 2012;109(5):1719–1724. doi: 10.1073/pnas.1114137109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger R., Yi J., Calkins M., Guri Y., McDonald-McGinn D.M., Emanuel B.S., Zackai E.H., Ruparel K., Carmel M., Michaelovsky E., Weizman A., Gur R.C., Gur R.E., Gothelf D. Neurocognitive profile in psychotic versus nonpsychotic individuals with 22q11.2 deletion syndrome. Eur. Neuropsychopharmacol. 2016;26(10):1610–1618. doi: 10.1016/j.euroneuro.2016.08.003. [DOI] [PubMed] [Google Scholar]
- Willing J., Cortes L.R., Brodsky J.M., Kim T., Juraska J.M. Innervation of the medial prefrontal cortex by tyrosine hydroxylase immunoreactive fibers during adolescence in male and female rats. Dev. Psychobiol. 2017;59(5):583–589. doi: 10.1002/dev.21525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X., Zhu D., Qi X.L., Li S., King S.G., Salinas E., Stanford T.R., Constantinidis C. Neural correlates of working memory development in adolescent primates. Nat. Commun. 2016;7:13423. doi: 10.1038/ncomms13423. [DOI] [PMC free article] [PubMed] [Google Scholar]