Abstract
Background
Anxiety disorders are the most common form of psychopathology and often begin early in development. Therefore, there is interest in identifying neural biomarkers that characterize pathways leading to anxiety disorders early in the course of development. A substantial amount of work focuses on the error-related negativity (ERN) as a biomarker of anxiety. While two previous reviews have focused on the relationship of the ERN and anxiety in adults, no previous review has focused on this issue in children and adolescents.
Results and conclusions
Overall, 22 studies were included in the current review. A number of patterns emerged, including: 1.) The ERN is enhanced in clinically anxious children at all ages (6–18 years old), regardless of the task used to measure the ERN. 2.) Studies focusing on anxiety symptoms and temperamental fear suggest that the relationship between the ERN and normative anxiety may change across development. 3.) The ERN can predict the onset of anxiety disorders across different developmental periods. 4.) The ERN relates to other markers of risk for anxiety (e.g., aversive startle potentiation) in children and adolescents.
Abbreviations: ERN, error-related negativity
Keywords: Biomarker, Anxiety, Error-related negativity, ERN, Developmental psychopathology
1. Introduction
Anxiety disorders are the most common form of psychopathology and often cause chronic impairment across the lifespan (Kessler et al., 2005). A substantial amount of research suggests both adults and children are affected by clinical anxiety, and that adult anxiety most commonly begins early in development (Beesdo et al., 2009, Last et al., 1996, Pine et al., 1998, Wittchen et al., 2000). In light of the fact that anxiety disorders tend to begin early in life and often result in chronic impairment, characterizing developmental pathways that lead to anxious versus healthy trajectories may improve prevention and intervention strategies.
In an effort to map healthy versus anxious trajectories, research has begun examining the development of core neural systems that underlie clinical anxiety (Pine, 2007). Identifying neural biomarkers that co-occur with anxiety, as well as those that precede the onset of anxiety, may enhance our understanding of the etiopathogenesis of clinical anxiety, in addition to improving our ability to implement preventative strategies − before symptoms become impairing. Recent evidence suggests that treatment earlier in the course of the development of anxiety disorders results in better long-term functioning (Mancebo et al., 2014) − therefore, early identification may be particularly important to improve our ability to implement preventative strategies. Additionally, identifying early biomarkers of risk may provide novel targets of treatment for cognitive, behavioral, or pharmacological approaches.
A substantial amount of work has focused on the error-related negativity as a biomarker of anxiety in adults (Meyer, 2016). The ERN is a sharp negative-going peak in the event-related potential waveform at fronto-central sites and is elicited when people make errors of commission on simple laboratory-based reaction time tasks1 (see Fig. 1). The ERN has been shown to be increased in anxious adult populations in over 40 studies to date (Moser et al., 2013). Disorders characterized by anxious apprehension (i.e., cognitive symptoms of anxiety) as opposed to anxious arousal (i.e., acute fear response) seem to be particularly related to an increased ERN − e.g., obsessive-compulsive disorder (OCD; Endrass et al., 2008, Gehring et al., 2000, Weinberg et al., 2015) and generalized anxiety disorder (GAD; Weinberg et al., 2012a, Weinberg et al., 2012b, Weinberg et al., 2010, Xiao et al., 2011). Two recent reviews suggest that the ERN’s relationship to anxiety is robust (Cavanagh and Shackman, 2015, Moser et al., 2013), finding an overall effect size of approximately r = 0.35. However, both of these reviews focus on adult populations. Despite the importance of utilizing neural markers to characterize early developmental trajectories, and evidence that there are important changes in the ERN and its relationship with anxiety across development (Davies et al., 2004, Meyer et al., 2012, Tamnes et al., 2013), no previous review has focused on this issue in children and adolescents. The object of the current review is to consolidate previous work on the ERN and anxiety within a developmental theoretical framework focusing on the normative shift in fear and anxiety that occurs during the transition from childhood to adolescence − and to delineate normative versus clinical trajectories of anxiety.
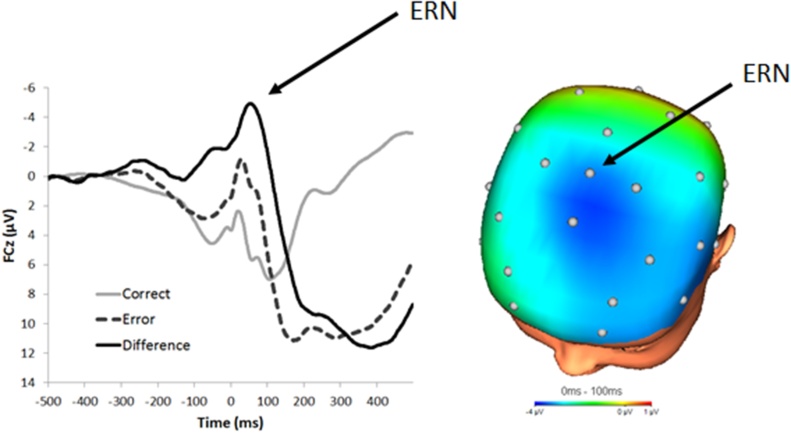
Fig. 1.
Response-locked ERP waveforms at FCz during a flankers task in 150 females between the ages of 8 and 14 years old. On the right, a topographical map depicting the difference between error and correct responses in the time range of the ERN (0–100 ms).
1.1. The ERN in the context of a developmental framework
The ERN is thought to be generated in the anterior cingulate cortex (ACC), a region of the brain where information about pain, threat, and punishment is integrated to change behavior (Shackman et al., 2011). We have conceptualized errors as a specific type of threat − indeed, errors do prompt a cascade of physiological responses consistent with defensive responding (e.g., skin conductance response, heart rate deceleration, potentiated startle reflex, pupil dilation, corrugator muscle contraction; Weinberg et al., 2012a, Weinberg et al., 2012b). Unlike many other aversive stimuli, errors are self-generated. Therefore, we view variability in the ERN to reflect the degree to which internally generated threat (i.e., errors) is experienced as aversive and salient to an individual. This is consistent with work linking the ERN to increased monitoring of one’s own behavior (Weinberg et al., 2016).
Recent models advocate the importance of considering developmental changes in the neural mechanisms underlying three core systems: 1.) reward/approach, 2.) harm-avoidance, and 3.) regulation (Casey et al., 2008, Ernst et al., 2006). While these models do emphasize development changes in harm-avoidance and fear, they treat this construct as a unitary phenomenon. However, a wealth of evidence suggests that as children grow older, anxiety tends to transition from fear of external threat (e.g., the dark, animals, insects, weather) to self-conscious shyness and worry about behavioral competence and social evaluation (i.e., internal threat) (Copeland et al., 2014, Crozier and Burnham, 1990, Gullone, 2000, Spence et al., 2001, Vasey et al., 1994). These findings suggest that fearfulness may not unilaterally increase or decrease during development, but rather change its manifestation across time.
In the current review, we review the ERN using a developmental framework related to this phenomenological shift in normative fear. Considering that the ERN may index a particular type of threat sensitivity, this neural marker may be useful in tracing the changing manifestations of anxiety across development. The ERN may be useful in tracking this normative developmental shift from external to internal threat sensitivity that we hypothesize occurs between the ages of 7–9 years old. Additionally, the ERN may also differentially track clinical anxiety early in development.
1.2. An alternative perspective: the ERN and cognitive control
It should also be noted that an alternative view suggests that the ERN indexes individual variation in cognitive control (Moser et al., 2013). From this perspective, the increased ERN magnitude observed in anxious individuals is thought to reflect decreased or inefficient cognitive control due to worry. One implication of this model is that individuals low in cognitive control should be characterized by an increased ERN. However, there is some evidence that individuals low in working memory are characterized by a decreased ERN (Coleman et al., 2017, Miller et al., 2012). In fact, two studies suggests that increasing working-memory load reduces the ERN (Klawohn et al., 2016, Maier and Steinhauser, 2017) − which is opposite of what this model would predict. To date, there are no studies examining the relationship between individual differences in the ERN, cognitive control, and anxiety. Given the paucity of work in this area, the current review focuses on a developmental framework related to fear and anxiety and does not examine cognitive control. Future work should examine whether deficits in cognitive control mediate the relationship between the ERN and anxiety.
1.3. Stability and change in the ERN across development
A critical, yet often ignored, requirement for the study of individual differences is the examination of the psychometric properties of neural measures. This is particularly important in developmental work insofar as psychometric properties could account for developmental effects (i.e., apparent age-related differences in ERN might reflect psychometric changes). Psychometric work on the ERN has recently been extended from adults to children and adolescents, finding reasonable trait-like stability in the ERN (Pontifex et al., 2010, Segalowitz et al., 2010). Specifically, we have examined the reliability and stability of the ERN in children and adolescents initially aged 8–13 years-old, over the course of two years (Meyer et al., 2014a, Meyer et al., 2015a). These data suggest impressive test-retest reliability (Cronbach’s alpha = 0.51) across time and good internal consistency. Specifically, the internal consistency of the ERN at both assessments exceeded 0.80, suggesting that this component has high internal consistency across different stages of development. And further, within adolescents, the ERN elicited by two different tasks (flankers and Go/no-go) were moderately correlated (r = 0.47) indicating convergent validity of the ERN. Taken together, these data suggest that the ERN is a reliable and stable measure of error processing in children and adolescents.
While the ERN has been found to be a psychometrically sound measure in children and adolescents, important changes in error-processing occur across development (Tamnes et al., 2013). For example, test-retest reliability can be high if the entire sample is changing at the same rate. Indeed, using a letter flanker task in a large sample between the ages of 7 and 18 years old, Davies et al. (2004) observed an increase in ERN magnitude across development. Additionally, they observed a quadratic relationship between ERN and age, with an initial dip in ERN amplitude around the time of pubertal onset, and subsequent rise until reaching adult-like levels around age 18. Furthermore, Davies et al. (2004) observed an interaction between this trajectory and gender, indicating that the ERN began to increase sooner for girls than for boys − suggesting a link to pubertal onset. Other work from this same group has suggested that children and adolescents (between the ages of 10 and 16) displayed a reduced ERN compared to adults (Santesso and Segalowitz, 2008). Since then, fourteen studies have found an increasing ERN across development (for a review, see: Tamnes et al., 2013).
In developmental populations, studies utilizing source-localization suggest the ERN may be generated in the dorsal ACC or posterior cingulate cortex (PCC; Buzzell et al., 2017, Ladouceur et al., 2007, Santesso and Segalowitz, 2008).2 Consistent with findings suggesting the ERN increases with age, diffusion tensor imaging (DTI) studies have found that the cingulum bundle (a white matter tract that underlies the ACC) matures later than most of the other tracts (Lebel and Beaulieu, 2011, Lebel et al., 2012). Further, one resting state functional connectivity study suggests that in children, the dorsal ACC is relatively disconnected from a cinguloopercular control network that has previously been identified in adults (Fair et al., 2007). Additionally, an fMRI study including participants between the ages of 8 and 27 years old found that error-related dorsal ACC activity increased with age (Velanova et al., 2008) and recent work suggests that additional error-related activity within the insula, orbiotofrontal cortex, and inferior frontal gyrus increased with age as well (Buzzell et al., 2017). Collectively, these studies suggest that error-related neural activity undergoes normative changes across development. Considering these changes, it is important to explore this biomarker at different developmental stages in relation to anxiety.
1.4. The ERN in children and adolescents with anxiety disorders
Although the ERN does increase with age, it can be measured early in development (Torpey et al., 2012). We identified seven studies that examined the magnitude of the ERN in children and adolescents with clinical anxiety disorders measured via diagnostic interviews (see Table 1; Carrasco et al., 2013, Hajcak et al., 2008, Hanna et al., 2012, Kujawa et al., 2016, Ladouceur et al., 2006, Meyer et al., 2016a, Meyer et al., 2016b, Meyer et al., 2016c, Meyer et al., 2013a, Meyer et al., 2013b).3 Similar to studies in adult populations, these studies universally found that the ERN is increased in clinically anxious children. Three of the studies examined the ERN in children with OCD (Carrasco et al., 2013, Hajcak et al., 2008, Hanna et al., 2012), one study focused on children and adolescents with GAD and social anxiety disorder (Kujawa et al., 2016), and the other three examined a heterogeneous sample of children with pediatric anxiety disorders (Ladouceur et al., 2006, Meyer et al., 2016a, Meyer et al., 2016b, Meyer et al., 2016c, Meyer et al., 2013a, Meyer et al., 2013b). An increased ERN appears to be associated with clinical OCD (Carrasco et al., 2013, Hajcak et al., 2008, Hanna et al., 2012) and social anxiety disorder (Kujawa et al., 2016) in young individuals; however, the specificity of an enhanced ERN to any other anxiety disorder is unclear. Due to the limited amount of children with any one type of anxiety disorder in the other three studies, it was impossible to examine specificity. Future studies could explore to what extent the ERN may be related to specific disorders in developmental populations.
Table 1.
Includes studies that examined the error-related negativity (ERN) in relation to anxiety disorders (top) and anxiety symptoms (bottom) in children and adolescent populations. Information included in table columns: first author and year of publication, task used to measure the ERN, questionnaire or interview used to measure anxiety, age range of participants, main findings regarding the ERN and development, main finding regarding the ERN and anxiety.
| Task | Sample (N) | Anxiety Measure | Age | Developmental Analyses | Results | |
|---|---|---|---|---|---|---|
| Anxiety Disorders: | ||||||
| Carrasco et al. (2013) | Arrow flanker | 40 OCD | K-SADS-PL | 10–17 yrs | ↑ERN = ↑Age | ↑ERN in Pediatric OCD |
| 40 HC | In patients: ERN ≠ symptoms | |||||
| In controls: ↑ERN = ↑OCD symptoms | ||||||
| Hajcak et al. (2008) | Simon | 18 OCD | Y-BOCS | 8–17 yrs | ↑ERN = ↑Age | ↑ERN in Pediatric OCD |
| 18 HC | ERN ≠ symptoms | |||||
| Hanna et al. (2012) | Arrow flanker | 44 OCD | K-SADS-PL | 10–19 yrs | In controls: ↑ERN = ↑Age | ↑ERN in Pediatric OCD |
| 44 HC | In patients: ERN ≠ Age | ERN ≠ symptoms | ||||
| Ladouceuer et al. (2006) | Arrow flanker | 12 ANX | K-SADS-PL, SCARED | 8–14 yrs | none | ↑ERN in Pediatric Anxiety Disorders |
| 13 HC | ERN ≠ symptoms | |||||
| Meyer et al. (2013a) | Go/no-go | 48 ANX | PAPA | 6 yrs | ↑ERN = ↑Age | ↑ERN in Pediatric Anxiety Disorders |
| 48 HC | ||||||
| Meyer et al. (2017) | Arrow flanker | 25 ANX | K-SADS-PL | 9 yrs | None | ↑ERN in Pediatric Anxiety Disorders |
| 130 HC | ||||||
| Kujawa et al. (2016) | Arrow flanker | 35 HC | K-SADS-PL | 8–26yrs | ↑ERN = ↑Age | ↑ERN in Social Anxiety Disorders |
| 18 SAD | No ERN difference in GAD | |||||
| 20 GAD | ||||||
| Anxiety Symptoms: | ||||||
| Meyer et al. (2012) | Arrow flanker | 55 | Parent SCARED | 8–13 yrs | ERN x Age = Anxiety symptoms | Older children: ↑ERN = ↑ anxiety symptoms |
| Younger children: ↓ERN = ↑ anxiety symptoms | ||||||
| Santesso et al. (2006) | Letter flanker | 37 | CBCL − OC | 10 yrs | None | ↑ERN = ↑ OC anxiety symptoms |
| Weinberg et al. (2016) | Arrow flanker | 515 | IDAS − checking | 13–15 yrs | ERN x Age = Checking symptoms | Older children: ↑ERN = ↑ checking symptoms |
| (specific to anxiety and not depression) | ||||||
| Bress et al. (2015) | Arrow flanker | 25 | Parent SCARED | 11–13 yrs | None | ↑ERN = ↑ anxiety symptoms |
| (specific to anxiety and not depression) | ||||||
| Lo et al., (2016) | Go/no-go | 139 | Parent RCADS | 5–8 yrs | No relationship between age and ERN | ↓ERN = ↑ separation anxiety symptoms |
It is important to note that while the ERN was increased in clinically anxious children, dimensional anxiety symptoms were unrelated to the ERN magnitude in all six of these studies. Additionally, in Carrasco et al. (2013), results suggested that among children and adolescents, the ERN magnitude was related to OCD symptoms in individuals without clinical OCD, but among children in the clinical range, symptom severity did not relate to the ERN.
While the majority of these studies were completed in middle to late childhood and adolescence (between the ages of 8 and 17 yrs), one study did find that young children (6 yrs old) with anxiety disorders were characterized by an increased ERN (Meyer, 2013). This finding suggests that the ERN may be a useful neural marker of clinical anxiety early in development. Future studies should examine the ERN in even younger clinically anxious children. And, while some studies did include developmental analyses; none of the studies examined whether the strength of the relationship between the ERN and clinical anxiety changed across development. It will be important for future research to explore whether the ERN is a better marker of clinical anxiety at different stages of development.
Consistent with the developmental studies discussed above, four of the studies listed in Table 1 found that the magnitude of the ERN increased with age across the entire sample (Carrasco et al., 2013, Hajcak et al., 2008, Hanna et al., 2012, Meyer et al., 2013a, Meyer et al., 2013b). Only one study examined whether the developmental increase in the ERN varied by diagnostic status − finding that the ERN increased with age in controls, but not patients with OCD (Hanna et al., 2012). Future studies in clinical populations should explore the possibility that the ERN has pre-maturely undergone developmental changes and reached “adult-like” magnitude early in the course of development.
It is also notable that the ERN was found to be increased in clinically anxious children using a variety of tasks to measure the ERN. Results were similar using an arrow flanker (Carrasco et al., 2013, Hanna et al., 2012, Ladouceur et al., 2006, Meyer et al., 2017a), Simon (Hajcak et al., 2008), and Go/no-go task (Meyer et al., 2013a, Meyer et al., 2013b), and after controlling for accuracy (Carrasco et al., 2013, Hanna et al., 2012, Meyer et al., 2013a, Meyer et al., 2013b). Additionally, the magnitude of the ERN increased with age when using an arrow flanker, Simon, and Go/no-go task as well. These results suggest that the ERN/clinical anxiety and ERN/development relationship is robust in children, regardless of the task used to elicit mistakes. However, it should be noted that some studies do not control for behavior (reaction time or accuracy) when examining the ERN/anxiety relationship and no study has formally evaluated the possibility of task-related or performance-related differences underlying the ERN/anxiety or ERN/development relationship in children and adolescents.
1.5. The ERN and anxiety symptoms in children and adolescents
We identified five studies that examined the relationship between anxiety symptoms and the ERN in non-clinical developmental populations (see Table 1; Bress et al., 2015, Lo et al., 2016, Meyer et al., 2012, Santesso et al., 2006, Weinberg et al., 2016).4 These studies examined the ERN/anxiety relationship amongst children and adolescents between the ages of 5 and 15 years old who did not have any anxiety disorder. Two of these studies found that increased anxiety symptoms related to an increased ERN magnitude in late childhood (between the ages of 10 and 13 years old; Bress et al., 2015, Santesso et al., 2006). One study specifically explored the relationship between the ERN and obsessive-compulsive symptoms derived from the CBCL (Santesso et al., 2006) and the other study examined an overall anxiety symptoms score derived from the SCARED (Bress et al., 2015).
Two of the four studies examined the influence of development on the ERN/anxiety relationship (Meyer et al., 2012, Weinberg et al., 2016). In a developmental sample spanning the ages of 8–13, Meyer et al. found that the relationship between the ERN and anxiety symptoms changed across development (Meyer et al., 2012). Among older children, a larger ERN was related to increased anxiety symptoms. Although the relationship was only significant at a trend level, in younger children, increased anxiety symptoms were related to a blunted ERN. Additionally, Lo et al. (2016) found that young children (between the ages of 5 and 8 years old), characterized by increased separation anxiety symptoms, displayed an attenuated ERN.
Additionally, in a sample of adolescent females, we found a similar pattern (Weinberg et al., 2016). Results suggested that the relationship between the ERN and anxiety symptoms (checking behavior specifically) changed across development: amongst older girls, a larger ERN was related to anxiety symptoms; however, this relationship was not significant among younger girls. Collectively, these studies suggest that the strength of the relationship between normative variation in anxiety symptoms and the ERN may increase in magnitude as children age.
We have begun to conceptualize this pattern in relation to work on normative developmental changes in anxiety. As children grow older, anxiety tends to transition from fear of concrete, external threat to more abstract, self-conscious shyness and worry about behavioral competence and social evaluation (Gullone, 2000). In this way, younger children with increased levels of normative anxiety are characterized by anxious arousal (i.e., fear) and concern with external threat. These children may be more preoccupied by the lab environment (e.g., the dark room, the experimenter, separation from the parent) than their performance on the task, and thereby display a decreased ERN. However, older children characterized by increased levels of normative anxiety may have begun to be concerned with social evaluation and behavioral competence − and thereby display an increased response to errors.
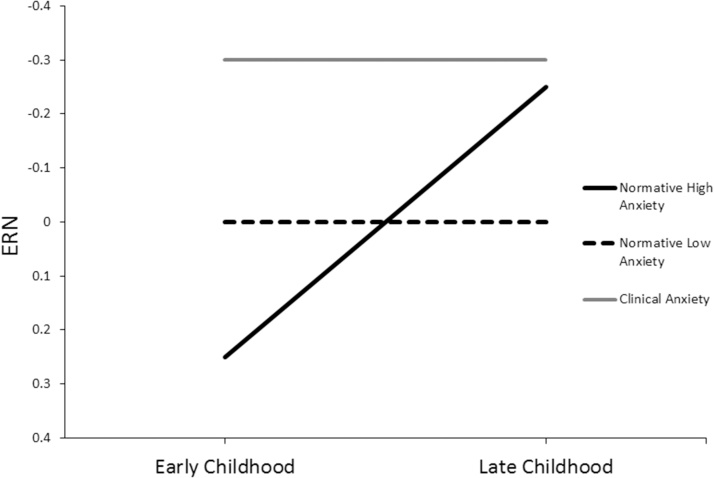
For illustration purposes, Fig. 2 depicts a model wherein the relationship between the ERN and anxiety changes within individuals during the transition between early and later childhood. In this model, as fears related to performance increase among children characterized by normative anxiety, an increase in the ERN is observed. However, among children low in anxiety, no developmental increases in the ERN is are expected to occur.
Fig. 2.
Model depicting the theoretical moderation of trait anxiety on the relationship between the ERN and age during the transition between early and late childhood. Based on studies in the current review, we expect that increased anxiety symptoms within the normative range will relate to a decreased ERN in early childhood, when children tend to be more fearful of external stimuli (e.g., the dark), and that increased anxiety symptoms within the normative range will relate to an increased ERN in later childhood, when children tend to be more fearful of internally generated stimuli (e.g., their performance on a task). Based on studies in the current review, this pattern of results has been observed both between and within-subjects, suggesting that being high in trait anxiety may result in a significant increase in ERN magnitude from early to late childhood. Additionally, this model puts forth the proposition that children with clinical levels of anxiety will display an increases ERN regardless of developmental stage.
Additionally, in this model, children characterized by clinical levels of anxiety, display an increased ERN at all developmental time points. Using this developmental framework, children with clinical anxiety do not undergo the normative developmental increases in the ERN or anxiety symptoms; rather, they may have already undergone these changes, achieving adult-like levels prematurely. This is consistent with the findings previously discussed wherein young children characterized by clinical levels of anxiety display an increased ERN. Moreover, dimensional anxiety symptoms do not relate to the ERN magnitude in clinical populations and in one study, developmental increases in the ERN were not observed in children with clinical diagnoses (Hanna et al., 2012).
Although further work is still needed to clarify these trajectories, it is clear that the relationship between the ERN and normative variability in anxiety may differ across development. In light of the potential utility of using the ERN as a neurobehavioral risk marker to predict the onset and course of anxiety, it is important to further characterize the relationship between normative levels of anxiety and error processing in children and adolescents while simultaneously considering the potential moderating role of development.
Two of the studies included examine the specificity of the relationship between the ERN and anxiety symptoms. For example, Bress et al. (2015) found that the ERN related to anxiety, but not depression symptoms in children and adolescents. In a study by Weinberg et al. (2016), results suggested that within the same individuals, while anxiety symptoms related to an increased ERN, depression symptoms related to a blunted ERN. In this study, the relationship between the ERN and depression symptoms was not moderated by age. This is consistent with other work suggesting that the ERN is decreased in children with major depressive disorder (Ladouceur et al., 2012), and in children at risk for depression (i.e., children with a maternal history of chronic depression; Meyer et al., 2016a, Meyer et al., 2016b, Meyer et al., 2016c). Taken together, these results suggest that an increased ERN may be a biomarker for anxiety, a relatively decreased ERN may be a biomarker for depression.
Furthermore, findings from Weinberg et al. (2016) suggested that an increased ERN was specifically related to the IDAS anxiety symptom scale indexing checking symptoms, even when controlling for all other anxiety symptom scales. Checking reflects the tendency to engage in self-monitoring of one’s own behavior to reduce anxiety (e.g., repeatedly checking to make sure one turned off the light). It light of these findings, it appears that the ERN is specific to anxiety versus depression, and can also be linked to a well-defined transdiagnostic construct with behavioral and clinical significance (i.e., checking).
1.6. The ERN and child temperament
In this context, temperament is used to refer to early-emerging patterns of the expression of emotion and behavior that are thought to be relatively stable across time, and rooted in biological systems. We identified six studies that examined the relationship between the ERN and temperament (see Table 2; Brooker et al., 2011, Lahat et al., 2014, McDermott et al., 2009; Meyer, Hajcak et al., Under Review; Moser et al., 2015, Torpey et al., 2013). These studies focused on temperamental fear and behavioral inhibition. Behavioral inhibition is defined by wariness, fear, and low exploration in novel situations and is thought to predict anxious trajectories (Kagan, 1997) and temperamental fear is defined by stable and consistent expression of fear in multiple contexts (Goldsmith and Campos, 1990). In the majority of these studies, child temperament was measured relatively early in development (between 1 and 3 years of age) through laboratory observational measures, and the ERN was measured at various developmental stages.
Table 2.
Includes studies that examined the error-related negativity (ERN) in relation to temperament (fear or behavioral inhibition) in children and adolescent populations. Information included in table columns: first author and year of publication, task used to measure the ERN, questionnaire, interview, or lab-based assessment used to measure anxiety or temperament, age range of participants, main findings regarding the ERN and development, main finding regarding the ERN and temperament.
| Task | Sample (N) | Anxiety Measure | Age | Developmental Analyses | Results | |
|---|---|---|---|---|---|---|
| Temperament: | ||||||
| McDermott et al. (2009) | Letter flanker | 113 | BI, K-SADS-PL | BI = 14 and 24 months, 4 and 7 yrs, ERN = 15 yrs | ↑early BI = ↑ adolescent ERN | BI x ERN = adolescent Anxiety Disorders |
| within-subjects | ||||||
| Lahat et al. (2014) | Fish flanker task | 291 | BI, K-SADS-PL | BI = 24 and 36 months, ERN = 7 yrs | ↑early BI = ↑ child ERN | BI x ERN = Social Phobia Disorder |
| within-subjects | ||||||
| Torpey et al. (2013) | Go/No-Go | 413 | Temperamental Fear | Fear = 3 yrs, ERN = 6 yrs | ↑early Fear = ↓ Age 6 ERN | ↑early Fear = ↓ Age 6 ERN, independent of maternal history of anxiety |
| Meyer et al. Invited Resubmission | Go/No-Go, Arrow flanker | 271 | Temperamental Fear | Fear = 3 yrs, ERN = 6 and 9 yrs | ↑early Fear = ↓ Age 6 ERN | ↑early Fear children characterized by developmental trajectory: ↓ERN to ↑ERN |
| ↑early Fear = ↑Age 9 ERN | ||||||
| within-subjects | ||||||
| Moser et al. (2015) | Fish flanker task | 13 | Temperamental Fear | 5–6 yrs | none | ↑ Fear = ↓ ERN |
| Brooker et al. (2014) | modified flanker task (ANT) | 41 | Temperamental Fear | Fear = 2 yrs, ERN = 4 yrs | none | ERN was only observed in ↑ fear group |
The pattern of results from studies focusing on temperament are consistent with the findings discussed above regarding anxiety symptoms. Early behavioral inhibition and temperamental fear are related to an increased ERN in older children and adolescents. For example, McDermott et al. (2009) found that behavioral inhibition measured at 14 and 24 months and 4 and 7 years related to an increased ERN when participants were 15 years of age. Similarly, Lahat et al. (2014) found that behavioral inhibition assessed at 24 and 36 months of age related to an increased ERN when children were 7 years old. Consistent with these findings, we found that temperamental fear assessed at 3 years of age related to an increased ERN when children were 9 years old (Meyer, Hajcak et al., Under Review). Taken together, these prospective-longitudinal studies suggest that toddlers characterized by increased behavioral inhibition or temperamental fear are more likely to be characterized by an increased ERN magnitude in middle to late childhood and adolescence.
Consistent with the pattern observed regarding child anxiety symptoms, the relationship between child temperament and the ERN appears to shift during development. First reported in Torpey et al. (2013), temperamental fear measured at age 3 related to a decreased ERN when measured in young children (6 years old). In a follow-up study, we examined the ERN in this sample of children three years later (Meyer, Hajcak et al., Under Review). Indeed, the same children characterized by high temperamental fear who had a decreased ERN at the age 6 assessment were characterized by an increased ERN at the age 9 assessment. This study extended previous work by investigating the developmental trajectory of the ERN/fear relationship within-subjects.
Moreover, another study examining temperamental fear and child ERN magnitude in this same age range (5–6 years old) replicated this pattern of results (Moser et al., 2015). Children characterized by high fear (assessed via behavioral observations in the lab) also displayed a blunted ERN. We have conceptualized this developmental shift in the same manner as discussed above in relation to anxiety symptoms. Children characterized by increased temperamental fear are more likely to be afraid of external stimuli (e.g., the dark room during the lab visit, being separated from their parent, etc.) during early childhood and may therefore be less invested in their behavioral performance consequences during the ERN assessment. As these children grow older, their fears may become increasingly linked to behavioral performance and thereby relate to an increased ERN.
1.7. The ERN: predicting the onset of anxiety
In addition to being able to index current disease state, a neural biomarker may also be useful in detecting who is at risk for developing clinical anxiety. Identifying neural biomarkers that not only correlate with anxiety, but can predict the subsequent onset of anxiety disorders across development is critical to furthering our understanding of the etiopathogenesis of anxiety, as well as for increasing the efficacy of early intervention strategies. We identified four studies that examine the extent to which the ERN can predict the onset of anxiety disorders in children and adolescents (see Table 3; Lahat et al., 2014, McDermott et al., 2009, Meyer et al., 2016a, Meyer et al., 2016b, Meyer et al., 2016c).
Table 3.
Includes studies that examined the error-related negativity (ERN) in relation to prediction of anxiety disorders (top) and other risk factors of anxiety (bottom) in children and adolescent populations. Information included in table columns: first author and year of publication, task used to measure the ERN, questionnaire or interview used to measure anxiety, age range of participants, main findings regarding the ERN and development, main finding regarding the ERN and anxiety. ** denotes that these studies were previously included in Tables 1 and 2.
| Task | Sample (N) | Anxiety Measure | Age | Developmental Analyses | Results | |
|---|---|---|---|---|---|---|
| Prediction: | ||||||
| Meyer et al. (2015b) | Go/No-Go | 236 Total | K-SADS-PL; CBCL | ERN = 6yrs, Anxiety Disorders = 9yrs | ↑ Age 6 ERN = ↑ Anxiety Disorders at Age 9 | ↑ Age 6 ERN = ↑ Anxiety Disorders at Age 9, even when controlling for baseline anxiety symptoms and maternal history of anxiety |
| 26 ANX | ||||||
| **McDermott et al. (2009) | Letter flanker | 113 | BI, K-SADS-PL | BI = 14 and 24 months, 4 and 7 yrs, ERN = 15yrs | ↑early BI = ↑ adolescent ERN | BI x ERN = adolescent Anxiety Disorders |
| within-subjects | ||||||
| **Lahat et al. (2014) | Fish flanker task | 291 | BI, K-SADS-PL | BI = 24 and 36 months, ERN = 7 yrs | ↑early BI = ↑ child ERN | BI x ERN = Social Phobia Disorder |
| within-subjects | ||||||
| Meyer et al. (2017) | Go/No-Go | 223 | CBCL | Fear = 3 yrs, ERN = 6 yrs, Anxiety symptoms = 9 yrs old | ↑ Age 6 ERN x Age 3 Fear x Hurricane stressors = ↑ in post-hurricane anxiety symptoms | ↑ Age 6 ERN = increased vulnerability to hurricane-related increases in anxiety symptoms |
| Associations with other risk factors: | ||||||
| **Carrasco et al. (2013) | Arrow flanker | 40 HC | K-SADS-PL | 10–17 yrs | ↑ERN = ↑Age | ↑ERN in healthy siblings of children with OCD |
| 19 siblings | ||||||
| **Meyer et al. (2017) | Arrow flanker | 155 | K-SADS-PL; fear-potentiated startle | 9 yrs | None | ↑ERN = ↑ fear-potentiated startle |
| Jackson et al. (2017) | Arrow flanker | 54 | BIS, startle habituation | 8–14 yrs | ↑ERN = ↑pubertal status | ↑ERN = ↓ startle habituation to CS+ |
| ↑ERN = ↑ BIS | ||||||
| **Torpey et al. (2013) | Go/No-Go | 413 Total | Maternal History of Anxiety | 6 yrs | ↑ERN = ↑Age | Maternal Anxiety = ↓ERN in child |
| **Hajcak et al. (2008) | Simon | 18 OCD | Y-BOCS | 8–17 yrs | ↑ERN = ↑Age | ↑ERN in children who no longer met criteria for OCD (post treatment) |
| 18 HC | ||||||
| Lo et al. (2015) | Go/No-Go and fish flanker task | 90 | Startle, resting parietal asymmetry | 3–7 yrs | ↑ERN = ↑Age | ↓ERN = ↑ startle |
| ↓ERN = ↑ alpha asymmetry | ||||||
Results were consistent across all four studies: an increased ERN early in life related to an increased risk for anxiety disorders later in development. One study found that an increased ERN magnitude predicted the onset of new anxiety disorders while controlling for baseline anxiety symptoms, as well as maternal history of anxiety (Meyer et al., 2015b). In this study, the ERN was measured in healthy children when they were 6 years old and anxiety disorders were assessed three years later via diagnostic interview with the parent and child, when children were 9 years old (Meyer et al., 2015b). Consistent with the developmental model previously discussed (see Fig. 2), children with a current anxiety disorder or children who will go on to develop one, are characterized by an early-emerging increased ERN.
Two studies examined the interaction of early behavioral inhibition and the ERN magnitude in predicting anxiety disorders later in development (Lahat et al., 2014, McDermott et al., 2009). McDermott et al. (2009) found that ERN magnitude moderated the association between early behavioral inhibition and adolescent anxiety disorders, such that children high in behavioral inhibition and characterized by an increased ERN were particularly vulnerable to the onset of anxiety disorders in adolescence. It should be noted, that in this study, the ERN and anxiety disorders were measured at the same time point. Similarly, in a study by Lahat et al. (2014), the association between early behavioral inhibition and middle childhood anxiety disorders (9 years old) was moderated by ERN magnitude (measured at Age 7), such that children high in behavioral inhibition and characterized by an enhanced ERN were more likely to experience social phobia. While some children high in behavioral inhibition will go on to develop anxiety disorders, most will not (Buss and Kiel, 2013, Lahat et al., 2011). These studies suggest that the ERN may be useful in delineating clinical from non-clinical trajectories amongst children high in behavioral inhibition, thereby facilitating early identification and intervention efforts.
A recent study extended these findings by examining the interaction between the ERN and temperamental fear in predicting stress-mediated increases in anxiety symptoms following a natural disaster (i.e., Hurrican Sandy; Meyer et al., 2016a, Meyer et al., 2016b, Meyer et al., 2016c). Results suggested that children who were high in fear at age 3 and experienced increased hurricane stressors were characterized by a subsequent increase in anxiety symptoms − but only when they were also characterized by an increased ERN at age 6. This pattern of results is consistent with previous work finding that early temperament and the ERN interact to predict risk for anxiety. Additionally, these findings support a diathesis-stress model, suggesting that the combination of early temperamental fear and an increased ERN together elevate the risk for increases in anxiety following environmental stress.
One prediction that follows from the developmental model put forth in this review (see Fig. 2) would be that young children characterized by increased fear and a large ERN would be particularly at risk for clinical anxiety − given that normatively anxious young children are characterized by a decreased ERN. Results from the studies discussed above support this notion − early temperamental fear or behavioral inhibition interact with the ERN to predict risk for anxiety later in development.
1.8. The ERN and other risk factors for anxiety
Given that the ERN has been proposed as an index of threat sensitivity and is thought to index risk for anxiety disorders, it is important to examine the ERN in relation to other risk factors in children and adolescents. We identified six such studies– and risk factors ranged from heredity to aversive startle potentiation (Carrasco et al., 2013, Hajcak et al., 2008, Jackson et al., 2017, Lo et al., 2015, Torpey et al., 2013). The majority of these studies found that increased ERN magnitude related to risk for anxiety.
Two studies examined the ERN in children at risk for OCD (Carrasco et al., 2013, Hajcak et al., 2008) − finding evidence for an association between risk and an increased ERN in children. For example, in a sample of children and adolescents between the ages of 10 and 17 years old, the ERN was found to be increased in healthy, unaffected siblings of children with OCD (Carrasco et al., 2013). Additionally, a study by Hajcak et al. (2008) found evidence for an increased ERN in children after successful treatment for OCD. These children continued to display an increased ERN, even though their symptoms no longer met diagnostic criteria for OCD. Together, these studies suggest that children at risk for OCD may be characterized by an increased ERN.
Three studies found that the ERN related to altered startle responding in children and adolescents (Jackson et al., 2017, Lo et al., 2016, Meyer et al., 2016a, Meyer et al., 2016b, Meyer et al., 2016c). The human startle response is a widely studied and well-validated measure of defensive activation (Lang, 1994, Lang et al., 1990) and can be measured in humans by the magnitude of eye muscle contraction in response to a loud acoustic probe. Many studies suggest that the magnitude of the startle response is potentiated when participants are viewing threatening stimuli (Bradley et al., 2006, Lang et al., 2000). In Meyer et al. (2016c), 9-year-old children characterized by a large ERN also exhibited greater potentiation of the startle response while viewing unpleasant images, but not while viewing neutral or pleasant images. These results suggest that aversive potentiation of the startle reflex and the ERN magnitude may index overlapping individual variation in threat sensitivity in children.
The second study examined startle habituation during a fear-learning task in relation to the ERN magnitude (Jackson et al., 2017). In a group of females between the ages of 8 and 14 years old, participants completed a learning task wherein faces were paired with an aversive scream. During this task, startle probes were administered to measure threat reactivity and as expected, startle responding was potentiated to the faces paired with the scream (CS + ) and habituated across the task. An increased ERN magnitude related to decreased startle habituation to the CS+ and also to an increased score on a self-report measure of the behavioral inhibition system − both risk factors for anxiety disorders (Amodio et al., 2008, Turner et al., 2005). However, it should be noted that Lo et al., found that the ERN related to a smaller startle response in a sample of 3–7 year old children (Lo et al., 2015). In this study, the negative condition startle magnitude was not calculated relative to an ITI or the positive condition − so interpretation of these findings are difficult. Taken together, results from studies that integrate startle reactivity and the ERN in children and adolescents suggest that the magnitude of the ERN may track defensive reactivity as indexed by the startle response. Future studies should explore to what extent using these measures in conjunction may increase our ability to identify and predict anxious developmental trajectories.
One study suggested that maternal history of anxiety related to a blunted ERN in young children (Torpey et al., 2013). Consistent with the developmental pattern discussed previously, it is possible that anxious or anxiety-prone young children (these children were 6 years old) may not yet be characterized by increased concern regarding their own behavior and may therefore display decreased response monitoring due to fears related to external stimuli in the testing environment. Future studies could explore this possibility by examining to what extent a history of maternal anxiety relates to the ERN magnitude in older children and adolescents. It is also possible that the association between maternal anxiety and a reduced ERN in young children is related to the fact that maternal anxiety is a risk factor for the development of a wide variety of psychopathology, including externalizing disorders and depression (Beidel and Turner, 1997, Bijl et al., 2002, Merikangas et al., 1998), which have been associated with a blunted ERN (Hall et al., 2007, Ladouceur et al., 2012).
2. Conclusions and future directions
Overall, results from the studies reviewed confirm that the ERN is associated with anxiety in children and adolescents. Children and adolescents with clinical anxiety disorders were consistently characterized by an increased ERN, across different developmental stages and using different tasks to elicit the ERN. However, normative variation in anxiety symptoms and temperamental behavioral inhibition and fear displayed a relationship with the ERN that changed from early to late childhood. While the ERN appears to be blunted in anxious young children, the ERN is increased in anxious older children and adolescents. We conceptualized this pattern in the context of the changing expression of normative fear from externally focused stimuli in early childhood to internally generated threat in later childhood. Moreover, younger children with clinical anxiety may have already begun to monitor for behavioral competence and are more sensitive to internal threat, thereby displaying an early-emerging increased ERN.
Results from the current review are consistent with the developmental framework outlined above. The studies reviewed indicate that the relationship between the ERN and normative anxiety symptoms may change during the transition from early to late childhood. We hypothesize that this change is related to the changing phenomenology of fear during this time. However, future work is needed to confirm this proposition. For example, self-report measures that assess specific fears may be used to examine these trajectories. It is possible that anxious young children characterized by a decreased ERN would report fears related to the dark or strangers, whereas these same children may report fears related to evaluation and performance later in childhood that would co-occur with an increase in the ERN. Future work could also utilize other neural measures to investigate this possibility − for example, measuring neural reactivity to different types of threatening images to examine whether children become more or less reactive to specific types of threats and whether this tracks the normative development of the ERN.
It is also important to consider potential neurodevelopmental factors that may underlie the developmental shift in the ERN/anxiety relationship. One factor to consider is the differential development of the rostral and dorsal ACC. For example, one study found that rostral ACC activity varied as a function of age, whereas dorsal ACC activity was only found in adults (Velanova et al., 2008), suggesting that the ratio of dorsal to rostral ACC may change across development. Given some evidence that hypoactive rostral ACC activity is associated with anxiety (Adleman et al., 2002, Cunningham et al., 2002), this changing ratio of dorsal to rostral ACC activity may relate to the developmental shift in the ERN/anxiety relationship. Additionally, findings from a recent study suggest that while error-related activity in the PCC increases linearly across development, so does activity in the insula and orbiotofrontal cortex − both of which have been associated with interceptive processing (Bechara et al., 2000, Buzzell et al., 2017, Critchley et al., 2004). It is possible that developmental changes in these neural networks underlie the developmental shift observed in the ERN/anxiety relationship. Future studies utilizing fMRI are needed to investigate these possibilities.
Results from the current review also suggested that the relationship between the ERN and anxiety demonstrates specificity in developmental populations. For example, the ERN appears to be blunted in depressed children and in children at risk for depression. Furthermore, when both anxiety and depression symptoms are entered simultaneously predicting the ERN, they have opposing effects, such that depression symptoms relate to a decreased ERN and anxiety symptoms relate to an increased ERN (Weinberg et al., 2016). While the relationship between anxiety symptoms and the ERN seem to shift across development, in this study, the relationship between depression symptoms and the ERN does not. Future studies are needed to confirm this pattern. Additionally, when all anxiety symptoms are compared, checking behavior appears to have the most robust relationship with the ERN in adolescents − suggesting the ERN may index a transdiagnostic phenotype related to checking.
The ERN magnitude is related to a variety of risk markers for anxiety in children and adolescents − including aversive startle potentiation. Future work should explore to what extent the ERN relates to other correlates of anxiety (e.g., pupil dilation, skin conductance, heart rate, corrugator activity, attentional bias to threat, etc.) with the aim of creating a biosignature of risk factors that may best predict the onset of anxiety disorders across development. Using a latent variable approach − creating a measure of the overlapping variance between multiple measures of threat sensitivity — may improve our predictive ability. In light of the fact that the ERN relates to other psychophysiological measures of threat sensitivity in children, it may be useful in the context of a psychoneurometric approach (Yancey et al., 2016). This approach entails combining multiple physiological indicators as well as scale measures to form a composite psychoneurometric index of a trait in order to improve conceptualization and operationalization of psychopathology.
It should be noted that one limitation of the current review is that many of the studies included were derived from only a few laboratories. Future studies should be conducted across multiple laboratories. Additionally, given the tasks utilized by the studies included in the current review, it is not possible to determine whether anxious or older children are: 1.) better at detecting they made an error versus 2.) are more reactive to having made an error.
An important future direction is examining to what extent the ERN can be leveraged as a diagnostic tool in clinic or school settings. The low cost of collecting EEG data, combined with the relatively low level of training required to administer the EEG assessments makes this a feasible measure for applied settings. Additionally, collecting EEG data from a child or adolescent can take less than 15 min, making this measure both affordable and time-efficient (Bress et al., 2015). Future work is needed to determine developmentally appropriate clinical cutoff scores and norms, as well as standardized tasks, for this assessment to be integrated into clinic and school settings. For example, the current model suggests that early childhood may be the optimal time to use the ERN to identify children at-risk for anxiety − such that young children high in anxiety symptoms and displaying an increased ERN may be particularly at risk. Future work is needed to explore this possibility.
In addition to using the ERN as a diagnostic tool in developmental populations, it may also serve as a target of treatment. Given the fact that the ERN is elevated early in the course of development, before anxiety symptoms become impairing, it is critical to identify factors that may modify the ERN early in life − so as to prevent the onset of clinical anxiety. Indeed, although ERN magnitude seems to be moderately heritable (Anokhin et al., 2008), a large portion of variance is unaccounted for by genetic influences (between 40 and 60%) and is better accounted for by environmental factors. Work in the lab indicates that the ERN is increased when errors are punished (Riesel et al., 2012), and this effect persists after punishment ends. Thus, learning-related experiences surrounding error commission seem to impact the ERN. Extending these findings, we recently found that harsh or punishing parenting styles are related to an increased ERN in young children, and the magnitude of the ERN mediated the relationship between harsh parenting styles and anxiety disorders in children (Meyer et al., 2014). The relationship between the ERN and harsh parenting has also been found in even younger children (Brooker and Buss, 2014). Future studies should determine if intervention strategies focusing on parenting styles may impact children’s ERNs and thereby risk for anxiety.
Recent work has also begun to examine the extent to which behavioral and cognitive interventions may impact the ERN in children and adolescents. In one study, participants who completed an attention bias modification (ABM) program designed to train participants to disengage their attention away from threatening stimuli and increase their attention towards neutral or positive stimuli, displayed a reduced ERN (Nelson et al., 2015). However, it should be noted that this study did not include pre/post measures of the ERN. Ongoing research as part of a large, longitudinal study is currently examining whether multiple sessions of ABM training can reduce the ERN and risk for anxiety disorders in adolescent females. Future work should examine novel methods of targeting the ERN early in development and examine to what extent reducing the ERN can modulate risk for anxiety in children and adolescents.
Conflict of Interest
None.
Footnotes
The ERN is commonly measured using: the flankers task (participants press a button depending on the direction of the center arrow, while ignoring the direction of the flanking arrows), the go/no-go task (participants must withhold a response on “no-go” trials), and the Stroop task (participants must press a button depending on the color of a word, while ignoring the text). For a comparison of tasks, see: Meyer et al., 2013a, Meyer et al., 2013b; Riesel et al. (2013).
However, it should be noted that the ACC has been observed to be involved in processes other than error processing (Bush et al., 2000, Devinsky et al., 1995, MacDonald et al., 2000, Vogt et al., 1992).
Clinical diagnoses were assessed using the following interviews: the schedule for affective disorders and schizophrenia for school-age children (i.e., K-SADS-PL; Kaufman et al., 1997) and the preschool age psychiatric assessment (i.e., PAPA; Egger et al., 1999).
Anxiety symptoms were measured with the following assessments: the screen for child anxiety related emotional disorders (i.e., SCARED; Birmaher et al., 1997), the child behavior checklist (i.e., CBCL; Achenbach and Edelbrock, 1981), the inventory of depression and anxiety symptoms (i.e., IDAS; Watson et al., 2012), the revised child and anxiety and depression scales (i.e., RCADS; Chorpita et al., 2005), and the Yale-Brown obsessive-compulsive scale (i.e., Y-BOCS; Goodman et al., 1989).
References
- Achenbach T.M., Edelbrock C. 1981. Child Behavior Checklist. (Burlington, Vt) [Google Scholar]
- Adleman N.E., Menon V., Blasey C.M., White C.D., Warsofsky I.S., Glover G.H., Reiss A.L. A Developmental fMRI study of the stroop color-word task. Neuroimage. 2002;16(1):61–75. doi: 10.1006/nimg.2001.1046. [DOI] [PubMed] [Google Scholar]
- Amodio D.M., Master S.L., Yee C.M., Taylor S.E. Neurocognitive components of the behavioral inhibition and activation systems: implications for theories of self-regulation. Psychophysiology. 2008;45(1):11–19. doi: 10.1111/j.1469-8986.2007.00609.x. [DOI] [PubMed] [Google Scholar]
- Anokhin A.P., Golosheykin S., Heath A.C. Heritability of frontal brain function related to action monitoring. Psychophysiology. 2008;45(4):524–534. doi: 10.1111/j.1469-8986.2008.00664.x. [DOI] [PubMed] [Google Scholar]
- Bechara A., Damasio H., Damasio A.R. Emotion, decision making and the orbitofrontal cortex. Cereb. Cortex. 2000;10(3):295–307. doi: 10.1093/cercor/10.3.295. [DOI] [PubMed] [Google Scholar]
- Beesdo K., Knappe S., Pine D.S. Anxiety and anxiety disorders in children and adolescents: developmental issues and implications for DSM-V. Psychiatr. Clin. N. Am. 2009;32(3):483–524. doi: 10.1016/j.psc.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beidel D.C., Turner S.M. At risk for anxiety: I. psychopathology in the offspring of anxious parents. J. Am. Acad. Child Adolesc. Psychiatry. 1997;36(7):918–924. doi: 10.1097/00004583-199707000-00013. [DOI] [PubMed] [Google Scholar]
- Bijl R.V., Cuijpers P., Smit F. Psychiatric disorders in adult children of parents with a history of psychopathology. Soc. Psychiatry Psychiatr. Epidemiol. 2002;37(1):7–12. doi: 10.1007/s127-002-8208-8. [DOI] [PubMed] [Google Scholar]
- Birmaher B., Khetarpal S., Brent D., Cully M., Balach L., Kaufman J., Neer S.M. The screen for child anxiety related emotional disorders (SCARED): scale construction and psychometric characteristics. J. Am. Acad. Child Adolesc. Psychiatry. 1997;36(4):545–553. doi: 10.1097/00004583-199704000-00018. [DOI] [PubMed] [Google Scholar]
- Bradley M.M., Codispoti M., Lang P.J. A multi-process account of startle modulation during affective perception. Psychophysiology. 2006;43(5):486–497. doi: 10.1111/j.1469-8986.2006.00412.x. [DOI] [PubMed] [Google Scholar]
- Bress J.N., Meyer A., Hajcak G. Differentiating anxiety and depression in children and adolescents: evidence from event-related brain potentials. J. Clin. Child Adolesc. Psychol. 2015;44(2):238–249. doi: 10.1080/15374416.2013.814544. [DOI] [PubMed] [Google Scholar]
- Brooker R.J., Buss K.A. Harsh parenting and fearfulness in toddlerhood interact to predict amplitudes of preschool error-related negativity. Dev. Cognit. Neurosci. 2014;9:148–159. doi: 10.1016/j.dcn.2014.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooker R.J., Buss K.A., Dennis T.A. Error-monitoring brain activity is associated with affective behaviors in young children. Dev. Cognit. Neurosci. 2011;1(2):141–152. doi: 10.1016/j.dcn.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush G., Luu P., Posner M.I. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn. Sci. 2000;4(6):215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Buss K.A., Kiel E.J. Springer; 2013. Temperamental Risk Factors for Pediatric Anxiety Disorders Pediatric Anxiety Disorders; pp. 47–68. [Google Scholar]
- Buzzell G.A., Richards J.E., White L.K., Barker T.V., Pine D.S., Fox N.A. Development of the error-monitoring system from ages 9–35: unique insight provided by MRI-constrained source localization of EEG. Neuroimage. 2017;157:13–26. doi: 10.1016/j.neuroimage.2017.05.045. http://www.sciencedirect.com/science/article/pii/S1053811917304445 ISSN 1053-8119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasco M., Harbin S.M., Nienhuis J.K., Fitzgerald K.D., Gehring W.J., Hanna G.L. Increased error-Related brain activity in youth with obsessive-Compulsive disorder and unaffected siblingseased error-Related brain activity In youth with obsessive-Compulsive disorder and unaffected siblings. Depress. Anxiety. 2013;30(1):39–46. doi: 10.1002/da.22035. [DOI] [PubMed] [Google Scholar]
- Casey B., Jones R.M., Hare T.A. The adolescent brain. Ann. N. Y. Acad. Sci. 2008;1124(1):111–126. doi: 10.1196/annals.1440.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh J.F., Shackman A.J. Frontal midline theta reflects anxiety and cognitive control: meta-analytic evidence. J. Physiol.-Paris. 2015;109(1):3–15. doi: 10.1016/j.jphysparis.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chorpita B.F., Moffitt C.E., Gray J. Psychometric properties of the Revised Child Anxiety and Depression Scale in a clinical sample. Behav. Res. Ther. 2005;43(3):309–322. doi: 10.1016/j.brat.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Coleman J.R., Watson J.M., Strayer D.L. Working memory capacity and task goals modulate error-related ERPs. Psychophysiology. 2017:1–14. doi: 10.1111/psyp.12805. [DOI] [PubMed] [Google Scholar]
- Copeland W.E., Angold A., Shanahan L., Costello E.J. Longitudinal patterns of anxiety from childhood to adulthood: the great smoky mountains study. J. Am. Acad. Child Adolesc. Psychiatry. 2014;53(1):21–33. doi: 10.1016/j.jaac.2013.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley H.D., Wiens S., Rotshtein P., Öhman A., Dolan R.J. Neural systems supporting interoceptive awareness. Nat. Neurosci. 2004;7(2):189–195. doi: 10.1038/nn1176. [DOI] [PubMed] [Google Scholar]
- Crozier W.R., Burnham M. Age-related differences in children's understanding of shyness. Br. J. Dev. Psychol. 1990;8(2):179–185. [Google Scholar]
- Cunningham M.G., Bhattacharyya S., Benes F.M. Amygdalo-cortical sprouting continues into early adulthood: implications for the development of normal and abnormal function during adolescence. J. Comp. Neurol. 2002;453(2):116–130. doi: 10.1002/cne.10376. [DOI] [PubMed] [Google Scholar]
- Davies P.L., Segalowitz S.J., Gavin W.J. Development of response-monitoring ERPs in 7- to 25-year-olds. Dev. Neuropsychol. 2004;25(3):355–376. doi: 10.1207/s15326942dn2503_6. [DOI] [PubMed] [Google Scholar]
- Devinsky O., Morrell M.J., Vogt B.A. Contributions of anterior cingulate cortex to behaviour. Brain. 1995;118(1):279–306. doi: 10.1093/brain/118.1.279. [DOI] [PubMed] [Google Scholar]
- Egger, Ascher B., Angold A. Center for Developmental Epidemiology, Department of Psychiatry and Behavioral Sciences, Duke University Medical Center; 1999. The Preschool Age Psychiatric Assessment: Version 1.1. (Unpublished Interview Schedule) [Google Scholar]
- Endrass T., Klawohn J., Schuster F., Kathmann N. Overactive performance monitoring in obsessive-compulsive disorder: ERP evidence from correct and erroneous reactions. Neuropsychologia. 2008;46(7):1877–1887. doi: 10.1016/j.neuropsychologia.2007.12.001. [DOI] [PubMed] [Google Scholar]
- Ernst M., Pine D.S., Hardin M. Triadic model of the neurobiology of motivated behavior in adolescence. Psychol. Med. 2006;36(03):299–312. doi: 10.1017/S0033291705005891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair D.A., Dosenbach N.U., Church J.A., Cohen A.L., Brahmbhatt S., Miezin F.M., Schlaggar B.L. Development of distinct control networks through segregation and integration. Proc. Natl. Acad. Sci. 2007;104(33):13507–13512. [Google Scholar]
- Gehring W.J., Himle J., Nisenson L.G. Action-Monitoring dysfunction in obsessive-Compulsive disorder. Psychol. Sci. 2000;11(1):1–6. doi: 10.1111/1467-9280.00206. [DOI] [PubMed] [Google Scholar]
- Goldsmith H.H., Campos J.J. The structure of temperamental fear and pleasure in infants: a psychometric perspective. Child Dev. 1990;61(6):1944–1964. [PubMed] [Google Scholar]
- Goodman W.K., Price L.H., Rasmussen S.A., Mazure C., Fleischmann R.L., Hill C.L., Charney D.S. The Yale-Brown obsessive compulsive scale: i. Development, use, and reliability. Arch. Gen. Psychiatry. 1989;46(11):1006–1011. doi: 10.1001/archpsyc.1989.01810110048007. [DOI] [PubMed] [Google Scholar]
- Gullone E. The development of normal fear: a century of research. Clin. Psychol. Rev. 2000;20(4):429–451. doi: 10.1016/s0272-7358(99)00034-3. [DOI] [PubMed] [Google Scholar]
- Hajcak G., Franklin M.E., Foa E.B., Simons R.F. Increased error-Related brain activity in pediatric obsessive-Compulsive disorder before and after treatment. Am. J. Psychiatry. 2008;165(1):116–123. doi: 10.1176/appi.ajp.2007.07010143. [DOI] [PubMed] [Google Scholar]
- Hall J.R., Bernat E.M., Patrick C.J. Externalizing psychopathology and the error-related negativity. Psychol. Sci. 2007;18(4):326–333. doi: 10.1111/j.1467-9280.2007.01899.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna G.L., Carrasco M., Harbin S.M., Nienhuis J.K., LaRosa C.E., Chen P., Gehring W.J. Error-Related negativity and tic history in pediatric obsessive-Compulsive disorder. J. Am. Acad. Child Adolesc. Psychiatry. 2012 doi: 10.1016/j.jaac.2012.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson F., Nelson B.D., Meyer A., Hajcak G. Pubertal Development, BIS, and ERN Impact Startle Habituation During Fear Conditioning in 8–15 Year-Old Girls. Dev. Psychobiol. 2017;59(4):436–448. doi: 10.1002/dev.21506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagan J. Temperament and the reactions to unfamiliarity. Child Dev. 1997;68(1):139–143. [PubMed] [Google Scholar]
- Kaufman J., Birmaher B., Brent D., Rao U., Flynn C., Moreci P., Ryan N. Schedule for affective disorders and schizophrenia for school-age children-present and lifetime version (K-SADS-PL): initial reliability and validity data. J. Am. Acad. Child Adolesc. Psychiatry. 1997;36(7):980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Kessler R.C., Berglund P., Demler O., Jin R., Merikangas K.R., Walters E.E. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch. Gen. Psychiatry. 2005;62(6):593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- Klawohn J., Endrass T., Preuss J., Riesel A., Kathmann N. Modulation of hyperactive error signals in obsessive–compulsive disorder by dual-task demands. J. Abnorm. Psychol. 2016;125(2):292. doi: 10.1037/abn0000134. [DOI] [PubMed] [Google Scholar]
- Kujawa A., Weinberg A., Bunford N., Fitzgerald K.D., Hanna G.L., Monk C.S., Phan K.L. Error-related brain activity in youth and young adults before and after treatment for generalized or social anxiety disorder. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2016;71:162–168. doi: 10.1016/j.pnpbp.2016.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladouceur C.D., Dahl R.E., Birmaher B., Axelson D.A., Ryan N.D. Increased error-related negativity (ERN) in childhood anxiety disorders: ERP and source localization. J. Child Psychol. Psychiatry. 2006;47(10):1073–1082. doi: 10.1111/j.1469-7610.2006.01654.x. [DOI] [PubMed] [Google Scholar]
- Ladouceur C.D., Dahl R.E., Carter C.S. Development of action monitoring through adolescence into adulthood: ERP and source localization. Dev. Sci. 2007;10(6):874–891. doi: 10.1111/j.1467-7687.2007.00639.x. [DOI] [PubMed] [Google Scholar]
- Ladouceur C.D., Slifka J.S., Dahl R.E., Birmaher B., Axelson D.A., Ryan N.D. Altered error-related brain activity in youth with major depression. Dev. Cognit. Neurosci. 2012;2(3):351–362. doi: 10.1016/j.dcn.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahat A., Hong M., Fox N.A. Behavioural inhibition: is it a risk factor for anxiety? Int. Rev. Psychiatry. 2011;23(3):248–257. doi: 10.3109/09540261.2011.590468. [DOI] [PubMed] [Google Scholar]
- Lahat A., Lamm C., Chronis-Tuscano A., Pine D.S., Henderson H.A., Fox N.A. Early behavioral inhibition and increased error monitoring predict later social phobia symptoms in childhood. J. Am. Acad. Child Adolesc. Psychiatry. 2014 doi: 10.1016/j.jaac.2013.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang P.J., Bradley M.M., Cuthbert B.N. Emotion, attention, and the startle reflex. Psychol. Rev. 1990;97(3):377. [PubMed] [Google Scholar]
- Lang P.J., Davis M., Öhman A. Fear and anxiety: animal models and human cognitive psychophysiology. J. Affect. Disord. 2000;61(3):137–159. doi: 10.1016/s0165-0327(00)00343-8. [DOI] [PubMed] [Google Scholar]
- Lang P.J. The motivational organization of emotion: affect-reflex connections. Emotions: Essays Emotion Theory. 1994:61–93. [Google Scholar]
- Last C.G., Perrin S., Hersen M., Kazdin A.E. A prospective study of childhood anxiety disorders. J. Am. Acad. Child Adolesc. Psychiatry. 1996;35(11):1502–1510. doi: 10.1097/00004583-199611000-00019. [DOI] [PubMed] [Google Scholar]
- Lebel C., Beaulieu C. Longitudinal development of human brain wiring continues from childhood into adulthood. J. Neurosci. 2011;31(30):10937–10947. doi: 10.1523/JNEUROSCI.5302-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel C., Gee M., Camicioli R., Wieler M., Martin W., Beaulieu C. Diffusion tensor imaging of white matter tract evolution over the lifespan. Neuroimage. 2012;60(1):340–352. doi: 10.1016/j.neuroimage.2011.11.094. [DOI] [PubMed] [Google Scholar]
- Lo S.L., Schroder H.S., Moran T.P., Durbin C.E., Moser J.S. Neurophysiological evidence of an association between cognitive control and defensive reactivity processes in young children. Dev. Cognit. Neurosci. 2015;15:35–47. doi: 10.1016/j.dcn.2015.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo S.L., Schroder H.S., Fisher M.E., Durbin C.E., Fitzgerald K.D., Danovitch J.H., Moser J.S. Associations between disorder-Specific symptoms of anxiety and error-Monitoring brain activity in young children. J. Abnormal Child Psychol. 2016:1–10. doi: 10.1007/s10802-016-0247-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald A.W., Cohen J.D., Stenger V.A., Carter C.S. Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science. 2000;288(5472):1835–1838. doi: 10.1126/science.288.5472.1835. [DOI] [PubMed] [Google Scholar]
- Maier M.E., Steinhauser M. Working memory load impairs the evaluation of behavioral errors in the medial frontal cortex. Psychophysiology. 2017:1–11. doi: 10.1111/psyp.12899. [DOI] [PubMed] [Google Scholar]
- Mancebo M.C., Boisseau C.L., Garnaat S., Eisen J.L., Greenberg B., Sibrava N.J., Rasmussen S.A. Long-term course of pediatric obsessive-Compulsive disorder: three years of prospective follow-up. Compr. Psychiatry. 2014;55(7):1498–1504. doi: 10.1016/j.comppsych.2014.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott J.M., Perez-Edgar K., Henderson H.A., Chronis-Tuscano A., Pine D.S., Fox N.A. A history of childhood behavioral inhibition and enhanced response monitoring in adolescence are linked to clinical anxiety. Biol. Psychiatry. 2009;65(5):445–448. doi: 10.1016/j.biopsych.2008.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merikangas K.R., Dierker L.C., Szatmari P. Psychopathology among offspring of parents with substance abuse and/or anxiety disorders: a high-risk study. J. Child Psychol. Psychiatry Allied Disciplines. 1998;39(5):711–720. [PubMed] [Google Scholar]
- Meyer A., Weinberg A., Klein D.N., Hajcak G. The development of the error-related negativity (ERN) and its relationship with anxiety: evidence from 8 to 13 year-olds. Dev. Cognit. Neurosci. 2012;2(1):152–161. doi: 10.1016/j.dcn.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer A., Hajcak G., Torpey D.C., Kujawa A., Kim J., Bufferd S., Klein D.N. Increased error-related brain activity in six-year-old children with clinical anxiety. J. Abnorm. Child Psychol. 2013;41(8):1257–1266. doi: 10.1007/s10802-013-9762-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer A., Riesel A., Proudfit G.H. Reliability of the ERN across multiple tasks as a function of increasing errors. Psychophysiology. 2013;50(12):1220–1225. doi: 10.1111/psyp.12132. [DOI] [PubMed] [Google Scholar]
- Meyer A., Bress J., Proudfit G.H. Psychometric properties of the error-Related negativity in children and adolescents. Psychophysiology. 2014;51(7):602–610. doi: 10.1111/psyp.12208. [DOI] [PubMed] [Google Scholar]
- Meyer A., Proudfit G.H., Bufferd S.J., Kujawa A.J., Laptook R.S., Torpey D.C., Klein D.N. Self-reported and observed punitive parenting prospectively predicts increased error-related negativity in six-year-old children. J. Abnorm. Child Psychol. 2015;43(5):821–829. doi: 10.1007/s10802-014-9918-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer A., Hajcak G., Torpey-Newman D.C., Kujawa A., Klein D.N. Enhanced error-related brain activity in children predicts the onset of anxiety disorders between the ages of 6 and 9. J. Abnorm. Psychol. 2015;124(2):266. doi: 10.1037/abn0000044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer A., Bress J.N., Hajcak G., Gibb B.E. Maternal depression is related to reduced error-Related brain activity in child and adolescent offspring. J. Clin. Child Adolesc. Psychol. 2016:1–12. doi: 10.1080/15374416.2016.1138405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer A., Danielson C.K., Danzig A.P., Bhatia V., Bromet E., Carlson G., Klein D. Neural reactivity to mistakes and temperamental fearfulness prospectively predict the impact of Hurricane Sandy stressors on internalizing symptoms in children. JAACAP. 2016 [Google Scholar]
- Meyer A., Glenn C.R., Kujawa A., Klein D., Hajcak G. Error-related brain activity is related to aversive potentiation of the startle response in children, but only the ERN is associated with anxiety disorders. Emotion. 2016;17(3):487. doi: 10.1037/emo0000243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer A., Hajcak G., Glenn C.R., Kujawa A.J., Klein D.N. Error-related brain activity is related to aversive potentiation of the startle response in children, but only the ERN is associated with anxiety disorders. Emotion. 2017;17(3):487. doi: 10.1037/emo0000243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer A., Hajcak G., Torpey-Newman D.C., Kujawa A., Olino T., Dyson M.W., Klein D. Early temperamental fearfulness and the developmental trajectory of error-related brain activity. Dev. Psychobiol. 2017 doi: 10.1002/dev.21605. (Invited Resbumission) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer A. Developing psychiatric biomarkers: a review focusing on the error-Related negativity as a biomarker for anxiety. Curr. Treat. Options Psychiatry. 2016:1–9. [Google Scholar]
- Miller A.E., Watson J.M., Strayer D.L. Individual differences in working memory capacity predict action monitoring and the error-related negativity. J. Exp. Psychol. 2012;38(3):757. doi: 10.1037/a0026595. [DOI] [PubMed] [Google Scholar]
- Moser J.S., Moran T.P., Schroder H.S., Donnellan M.B., Yeung N. On the relationship between anxiety and error monitoring: a meta-analysis and conceptual framework. Front. Hum. Neurosci. 2013;7 doi: 10.3389/fnhum.2013.00466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser J.S., Durbin C.E., Patrick C.J., Schmidt N.B. Combining neural and behavioral indicators in the assessment of internalizing psychopathology in children and adolescents. J. Clin. Child Adolesc. Psychol. 2015;44(2):329–340. doi: 10.1080/15374416.2013.865191. [DOI] [PubMed] [Google Scholar]
- Nelson B.D., Jackson F., Amir N., Hajcak G. Single-session attention bias modification and error-related brain activity. Cognit. Affective Behav. Neurosci. 2015:1–11. doi: 10.3758/s13415-015-0365-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pine D.S., Cohen P., Gurley D., Brook J., Ma Y. The risk for early-adulthood anxiety and depressive disorders in adolescents with anxiety and depressive disorders. Arch. Gen. Psychiatry. 1998;55(1):56–64. doi: 10.1001/archpsyc.55.1.56. [DOI] [PubMed] [Google Scholar]
- Pine D.S. Research review: a neuroscience framework for pediatric anxiety disorders. J. Child Psychol. Psychiatry. 2007;48(7):631–648. doi: 10.1111/j.1469-7610.2007.01751.x. [DOI] [PubMed] [Google Scholar]
- Pontifex M.B., Scudder M.R., Brown M.L., O'Leary K.C., Wu C.-T., Themanson J.R., Hillman C.H. On the number of trials necessary for stabilization of error-related brain activity across the life span. Psychophysiology. 2010;47(4):767–773. doi: 10.1111/j.1469-8986.2010.00974.x. [DOI] [PubMed] [Google Scholar]
- Riesel A., Weinberg A., Endrass T., Kathmann N., Hajcak G. Punishment has a lasting impact on error-related brain activity. Psychophysiology. 2012;49(2):239–247. doi: 10.1111/j.1469-8986.2011.01298.x. [DOI] [PubMed] [Google Scholar]
- Riesel A., Weinberg A., Endrass T., Meyer A., Hajcak G. The ERN is the ERN is the ERN? Convergent validity of error-related brain activity across different tasks. Biol. Psychol. 2013 doi: 10.1016/j.biopsycho.2013.04.007. [DOI] [PubMed] [Google Scholar]
- Santesso D.L., Segalowitz S.J. Developmental differences in error-related ERPs in middle-to late-adolescent males. Dev. Psychol. 2008;44(1):205. doi: 10.1037/0012-1649.44.1.205. [DOI] [PubMed] [Google Scholar]
- Santesso D.L., Segalowitz S.J., Schmidt L.A. Error-related electrocortical responses are enhanced in children with obsessive-compulsive behaviors. Dev. Neuropsychol. 2006;29(3):431–445. doi: 10.1207/s15326942dn2903_3. [DOI] [PubMed] [Google Scholar]
- Segalowitz S.J., Santesso D.L., Murphy T.I., Homan D., Chantziantoniou D.K., Khan S. Retest reliability of medial frontal negativities during performance monitoring. Psychophysiology. 2010;47(2):260–270. doi: 10.1111/j.1469-8986.2009.00942.x. [DOI] [PubMed] [Google Scholar]
- Shackman A.J., Salomons T.V., Slagter H.A., Fox A.S., Winter J.J., Davidson R.J. The integration of negative affect, pain and cognitive control in the cingulate cortex. Nat. Rev. Neurosci. 2011;12(3):154–167. doi: 10.1038/nrn2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spence S.H., Rapee R., McDonald C., Ingram M. The structure of anxiety symptoms among preschoolers. Behav. Res. Ther. 2001;39(11):1293–1316. doi: 10.1016/s0005-7967(00)00098-x. [DOI] [PubMed] [Google Scholar]
- Tamnes C.K., Walhovd K.B., Torstveit M., Sells V.T., Fjell A.M. Performance monitoring in children and adolescents: a review of developmental changes in the error-related negativity and brain maturation. Dev. Cognit. Neurosci. 2013;6(0):1–13. doi: 10.1016/j.dcn.2013.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torpey D.C., Hajcak G., Kim J., Kujawa A., Klein D.N. Electrocortical and behavioral measures of response monitoring in young children during a Go/No-Go task. Dev. Psychobiol. 2012;54(2):139–150. doi: 10.1002/dev.20590. (n/a-n/a) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torpey D.C., Hajcak G., Kim J., Kujawa A.J., Dyson M.W., Olino T.M., Klein D.N. Error-related brain activity in young children: associations with parental anxiety and child temperamental negative emotionality. J. Child Psychol. Psychiatry. 2013;54(8):854–862. doi: 10.1111/jcpp.12041. (no-no) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner S.M., Beidel D.C., Roberson-Nay R. Offspring of anxious parents: reactivity, habituation, and anxiety-proneness. Behav. Res. Ther. 2005;43(10):1263–1279. doi: 10.1016/j.brat.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Vasey M., Crnic K., Carter W. Worry in childhood: a developmental perspective. Cognit. Ther. Res. 1994;18(6):529–549. [Google Scholar]
- Velanova K., Wheeler M.E., Luna B. Maturational changes in anterior cingulate and frontoparietal recruitment support the development of error processing and inhibitory control. Cereb. Cortex. 2008;18(11):2505–2522. doi: 10.1093/cercor/bhn012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt B.A., Finch D.M., Olson C.R. Functional heterogeneity in cingulate cortex: the anterior executive and posterior evaluative regions. Cereb. Cortex. 1992;2(6):435–443. doi: 10.1093/cercor/2.6.435-a. [DOI] [PubMed] [Google Scholar]
- Watson D., O’Hara M.W., Naragon-Gainey K., Koffel E., Chmielewski M., Kotov R., Ruggero C.J. Development and validation of new anxiety and bipolar symptom scales for an expanded version of the IDAS (the IDAS-II) Assessment. 2012;19(4):399–420. doi: 10.1177/1073191112449857. [DOI] [PubMed] [Google Scholar]
- Weinberg A., Olvet D.M., Hajcak G. Increased error-related brain activity in generalized anxiety disorder. Biol. Psychol. 2010;85(3):472–480. doi: 10.1016/j.biopsycho.2010.09.011. [DOI] [PubMed] [Google Scholar]
- Weinberg A., Klein D.N., Hajcak G. Increased error-related brain activity distinguishes Generalized Anxiety Disorder with and without comorbid Major Depressive Disorder. J. Abnorm. Psychol. 2012 doi: 10.1037/a0028270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg A., Riesel A., Hajcak G. Integrating multiple perspectives on error-related brain activity: the ERN as a neural indicator of trait defensive reactivity. Motiv. Emot. 2012:1–17. [Google Scholar]
- Weinberg A., Kotov R., Proudfit G.H. Neural indicators of error processing in generalized anxiety disorder, obsessive-compulsive disorder, and major depressive disorder. J. Abnorm. Psychol. 2015;124(1):172. doi: 10.1037/abn0000019. [DOI] [PubMed] [Google Scholar]
- Weinberg A., Meyer A., Hale-Rude E., Perlman G., Kotov R., Klein D.N., Hajcak G. Error-related negativity (ERN) and sustained threat: conceptual framework and empirical evaluation in an adolescent sample. Psychophysiology. 2016;53(3):372–385. doi: 10.1111/psyp.12538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittchen H.U., Lieb R., Pfister H., Schuster P. The waxing and waning of mental disorders: evaluating the stability of syndromes of mental disorders in the population. Compr. Psychiatry. 2000;41(2 Suppl. 1):122–132. doi: 10.1016/s0010-440x(00)80018-8. [DOI] [PubMed] [Google Scholar]
- Xiao Z., Wang J., Zhang M., Li H., Tang Y., Wang Y., Fromson J.A. Error-related negativity abnormalities in generalized anxiety disorder and obsessive-compulsive disorder. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2011;35(1):265–272. doi: 10.1016/j.pnpbp.2010.11.022. [DOI] [PubMed] [Google Scholar]
- Yancey J.R., Venables N.C., Patrick C.J. Psychoneurometric operationalization of threat sensitivity: relations with clinical symptom and physiological response criteria. Psychophysiology. 2016;53(3):393–405. doi: 10.1111/psyp.12512. [DOI] [PMC free article] [PubMed] [Google Scholar]