Highlights
-
•
Grasp height reveals how grasps are planned.
-
•
ASD children without intellectual disability show the grasp height effect.
-
•
Modulation of grasp height is similar in ASD and TD children.
-
•
Some aspects of prospective sensorimotor control are intact in autism.
Keywords: Autism, Prospective control, Grasp height effect, Sensorimotor, Kinematics, Joint action
Abstract
Where grasps are made reveals how grasps are planned. The grasp height effect predicts that, when people take hold of an object to move it to a new position, the grasp height on the object is inversely related to the height of the target position. In the present study, we used this effect as a window into the prospective sensorimotor control of children with autism spectrum disorders without accompanying intellectual impairment. Participants were instructed to grasp a vertical cylinder and move it from a table (home position) to a shelf of varying height (target position). Depending on the conditions, they performed the task using only one hand (unimanual), two hands (bimanual), or with the help of a co-actor (joint). Comparison between the performance of typically developing children and children with autism revealed no group difference across tasks. We found, however, a significant influence of IQ on grasp height modulation in both groups. These results provide clear evidence against a general prospective sensorimotor planning deficit and suggest that at least some form of higher order planning is present in autism without accompanying intellectual impairment.
1. Introduction
The ability to accurately anticipate and predict forthcoming actions and their effects is essential to solve daily sequential tasks, such as using a knife to spread jam on bread or grasping a bottle to pour a liquid without spilling it. A useful way to study this ability is to observe adaptations in one’s behaviour as a function of the behaviour that follows. If an action differs depending on the subsequent action, then the anticipatory effect can be said to reflect prospective sensorimotor control (Ansuini et al., 2015, Rosenbaum et al., 2012).
Anticipatory changes of this sort have been studied extensively in object manipulation (Ansuini et al., 2008, Ansuini et al., 2006, Armbrüster and Spijkers, 2006, Becchio et al., 2012, Becchio et al., 2008, Cohen and Rosenbaum, 2004, Crajé et al., 2011, Johnson-Frey et al., 2003, Marteniuk et al., 1987, Rosenbaum et al., 1990, Rosenbaum et al., 1993, Sartori et al., 2009, Schuboe et al., 2008). For example, it is already well known that individuals tend to grasp objects differently depending on what they plan to do with the objects (Ansuini et al., 2015). A clear demonstration of prospective sensorimotor control for object manipulation is provided by the grasp height effect, i.e., the tendency to take hold of objects at a height that is inversely related to the height of the target location that they are attempting to reach (for review, see Rosenbaum et al., 2012). For example, when placing a book on a shelf, the higher the shelf, the lower individuals tend to grasp the book. Doing so has been shown to promote not just comfort of the end posture (i.e., end-state comfort) but also better control at the time of task completion (Rosenbaum et al., 2006). Thus, the initial grip of the book reflects an anticipation of the posture the body will be in once the target location of the action is reached.
Behaviours that reflect this effect have been reported when adult participants manipulate objects with only one hand (unimanual object manipulation; Cohen and Rosenbaum, 2004, Rosenbaum and Jorgensen, 1992, Weigelt et al., 2007) as well as when they use two (bimanual object manipulation; Haggard, 1998, Meyer et al., 2013, Rosenbaum et al., 1990). Moreover, there is evidence of grasp height effect in typically developing children from 7 to 12 years of age, with an increase of the effect as their age develops within this range (Janssen and Steenbergen, 2011).
A far less studied aspect of prospective sensorimotor control is the planning of cooperative actions with others. Acting jointly with another person requires one to consider and integrate not only one’s own but also their partner’s next action (Sebanz et al., 2006). Consider, for example, one person handing books to another when filling a bookshelf together. Formalizing this example, Meyer et al. (2013) found that adult participants modulated the choice of the grasp height to accommodate not only their own end-state but also their action partner’s end-state. This result has been taken to signify similarity in mechanisms underlying prospective control of individual and joint action sequences. However, the exact mechanism supporting joint action planning remains unclear. Do individuals represent their action partner’s discomfort and therefore adjust their own actions accordingly? If so, does joint action planning depend on the ability to represent others’ internal states? More broadly, does it relate to social functioning?
Abnormalities in social functions are a striking feature of autism, a neurodevelopmental disorder defined by characteristic deficits in social interaction and communication − so-called social symptoms. Even individuals with autism spectrum disorders exhibit deficits in coordinating gaze and action with others and understanding the mental states and social intentions of other people (Happé and Frith, 2014). Yet, this condition is also defined by a less well-researched range of non-social motor symptoms (Cook, 2016; for meta-analysis, see Fournier et al., 2010), including impairments in basic motor control (Adrien et al., 1993, Jansiewicz et al., 2006, Teitelbaum et al., 1998), difficulties performing skilled motor gestures (Mostofsky et al., 2006), abnormal patterns of motor learning (Haswell et al., 2009), and disturbances in the reach-to-grasp movement (Mari et al., 2003, Noterdaeme et al., 2002). Comparison between the performance of typically developing children and children with autism spectrum disorders may thus inform us about the link between prospective sensorimotor control, motor skills, and more complex socio-cognitive skills.
With this in mind, in the present study, we examined prospective planning for self and other people’s actions in typically developing children and children with autism spectrum disorders without accompanying intellectual impairment. To study whether participants altered their initial grasp in anticipation of what they or their action partner planned to do with the object, we implemented a simple object manipulation task in which a cylinder had to be transported from a table (i.e., home position) to a shelf of varying height (i.e., target position). The number of moves varied depending on the task: unimanual, bimanual, joint. In the unimanual task, participants picked up the cylinder with their right hand and then moved it to the target position. In the bimanual task, they picked it up with their right hand and passed it to their left to move it to the target position. The joint task was similar, except that they picked up the cylinder with their right hand and passed it to a co-actor to move it to the target position. We used the height at which the cylinder was grasped (i.e., grasp height) as a continuous measure for prospective sensorimotor control across tasks. Grasp heights were analysed in a mixed factorial ANCOVA with task (unimanual, bimanual, joint) and target position height (low, middle, high) as within-subject factors, group (ASD, TD) as between-subject factor, and age, stature, and Full Scale IQ as covariates. In addition, to investigate whether prospective control was linked to motor, executive, and language function, in each group we correlated grasp height measures with standardized measures of motor skills, executive planning, and receptive vocabulary. Finally, we also correlated the grasp height measures with the severity of autism symptoms as measured by the Autism Diagnostic Observation Schedule.
2. Materials and methods
2.1. Participants
Seventeen children with Autism Spectrum Disorder without accompanying intellectual impairment (ASD group: 15 males) and 20 age-matched typically developing children (TD group: 16 males) were recruited from the Child Neuropsychiatry Unit of the ‘Giannina Gaslini’ Hospital and schools in Genova. All participants had normal or corrected-to-normal vision and were screened for exclusion criteria (dyslexia, epilepsy, and any other neurological or psychiatric conditions). Participants in the ASD group were diagnosed according to DSM-5 (American Psychiatric Association, 2013) criteria. The Autism Diagnostic Observation Scale (ADOS-2; Lord et al., 2012) and the Autism Diagnostic Interview-Revised (ADI-R; Rutter et al., 2003) were administered by two skilled professionals (a child neuropsychiatrist and a neuropsychologist). All participants met the cut-off criteria for ASD with respect to the total ADOS score and the communication and reciprocal social interaction subscales (see Table 1).
Table 1.
ADOS-2 and ADI-R scores for participants in the ASD group.
| Participant | ADOS-2 |
ADI-R |
||||||
|---|---|---|---|---|---|---|---|---|
| Total Score | SA | RRB | Total Score | A) | B) | C) | D) | |
| 1 | 8 | 6 | 2 | 30 | 12 | 9 | 7 | 2 |
| 2 | 8 | 6 | 2 | 28 | 10 | 11 | 5 | 2 |
| 3 | 9 | 8 | 1 | 25 | 10 | 8 | 5 | 2 |
| 4 | 8 | 6 | 2 | 28 | 11 | 9 | 4 | 4 |
| 5 | 8 | 7 | 1 | 31 | 8 | 17 | 5 | 1 |
| 6 | 8 | 7 | 1 | 49 | 20 | 15 | 10 | 4 |
| 7 | 13 | 11 | 2 | 21 | 9 | 8 | 3 | 1 |
| 8 | 9 | 8 | 1 | 41 | 18 | 19 | 3 | 1 |
| 9 | 8 | 7 | 1 | 30 | 11 | 11 | 5 | 3 |
| 10 | 8 | 7 | 1 | 24 | 10 | 8 | 5 | 1 |
| 11 | 10 | 8 | 2 | 29 | 11 | 8 | 5 | 5 |
| 12 | 9 | 8 | 1 | 32 | 12 | 11 | 6 | 3 |
| 13 | 8 | 7 | 1 | 24 | 10 | 9 | 4 | 1 |
| 14 | 9 | 8 | 1 | 24 | 10 | 7 | 6 | 2 |
| 15 | 8 | 7 | 1 | 25 | 11 | 9 | 3 | 2 |
| 16 | 8 | 6 | 2 | 24 | 10 | 5 | 7 | 2 |
| 17 | 7 | 6 | 1 | 27 | 14 | 3 | 6 | 4 |
Note: ADOS-2 (Autism Diagnostic Observation Scale 2) subtests: SA (Social Affect); RRB (Restricted and Repetitive Behaviors). Cut-off score for ADOS-2 Total Score (SA + RRB): (autism = 9; autism spectrum = 7). ADOS-2 Total score range (0–28). ADI-R (Autism Diagnostic Interview-Revised) subtests: A) Qualitative Abnormalities in Reciprocal Social Interaction (cut-off score = 10); B) Qualitative Abnormalities in Communication (cut-off score = 8); C) Restricted, Repetitive, and Stereotyped Patterns of Behavior (cut-off score = 3); D) Abnormality of Development Evident at or Before 36 Months (cut-off score = 1). ADI-R Total score range (0–78). The scores in Italics meet cut-off criteria.
Groups were matched for age (ASD M ± SD = 9.9 ± 1.6 years.months; TD M ± SD = 9.5 ± 1.5 years.months; t(35) = 0.804, p > 0.05), gender (ASD M:F = 15:17; TD M:F = 16:20), stature (ASD M ± SD = 141.2 ± 8.7; TD M ± SD = 138 ± 9.1 cm; t(35) = 1.177, p > 0.05), and Full Scale IQ (FS IQ) as measured by the Wechsler Scale of Intelligence (WISC IV; Wechsler, 2003) (ASD M ± SD = 96.3 ± 10.2; TD M ± SD = 102.8 ± 9.4; t(35) = −2.020, p > 0.05; see Table 2). All but two of the participants (one in the ASD group and one in the TD group) were right-handed according to the Edinburgh Handedness Inventory (Oldfield, 1971). For all children, parental written informed consent was obtained. The study was approved by the local ethics committee (ASL3 Genovese) and performed in accordance with the principles of the revised Helsinki Declaration (World Medical Association General Assembly, 2008).
Table 2.
Summary of ASD and TD group characteristics and Full Scale IQ.
| Group | M | SD | Min | Max | |
|---|---|---|---|---|---|
| Age (years.months) | ASD | 9.9 | 1.6 | 7.1 | 12.9 |
| TD | 9.5 | 1.5 | 7.1 | 12.5 | |
| Stature (centimetre) | ASD | 141.2 | 8.7 | 126 | 156 |
| TD | 138 | 9.1 | 122 | 160 | |
| Full Scale IQ | ASD | 96.3 | 10.2 | 81 | 113 |
| TD | 102.8 | 9.4 | 83 | 115 | |
Note: ASD = Autism Spectrum Disorder; TD = Typically Developing; Total standard scores reported for Full Scale IQ; M = Mean; SD = Standard Deviation; Min = Minimum; Max = Maximum.
2.2. Motor and cognitive assessment
Every child was tested on the following motor and cognitive tests: the Movement Assessment Battery for Children (MABC-2), the Tower of London (TOL), and the Peabody Picture Vocabulary Test-Revised (PPVT-R).
2.2.1. Movement Assessment Battery for Children (MABC-2)
The MABC-2 (Henderson et al., 2007) is a validated measure of movement skill in children and was used to test participants’ overall motor performance. This measure comprises three subscales: manual dexterity, ball skills, and balance. Percentile scores can be used as an indicator of motor difficulties, with scores below the 5th percentile suggesting a significant motor difficulty (red zone), between the 6th and 15th percentiles signifying a borderline motor difficulty (amber zone), and above the 15th percentile indicating no motor difficulty (green zone). While the majority of TD children (75%) were classified in the green zone of MABC-2 assessment, 65% of children with ASD were classified in the red and in the amber zone, indicating that they experienced or were at risk of motor difficulties or delays. In line with this, total MABC-2 score was significantly lower in the ASD group compared to the TD group (ASD M = 15.35 vs. TD = M 31.85; t(35) = −2.455, p < 0.05; see Table 3).
Table 3.
Motor and cognitive details for participants in ASD and TD group.
| Participant | Group | MABC-2 |
PPVT-R | TOL | |||
|---|---|---|---|---|---|---|---|
| Total Score | Manual Dexterity | Ball Skills | Balance | ||||
| 1 | ASD | 4 | <15 | >15 | >15 | 110 | 30 |
| 2 | ASD | 70 | >15 | >15 | >15 | 106 | 90 |
| 3 | ASD | 36 | <15 | >15 | >15 | 103 | 85 |
| 4 | ASD | 1 | <15 | <15 | <15 | 82 | 65 |
| 5 | ASD | 1 | <15 | >15 | <15 | 103 | 65 |
| 6 | ASD | 1 | <15 | >15 | <15 | 110 | 30 |
| 7 | ASD | 6 | <15 | >15 | >15 | 106 | 95 |
| 8 | ASD | 2 | <15 | <15 | >15 | 103 | 40 |
| 9 | ASD | 29 | <15 | >15 | >15 | 106 | 90 |
| 10 | ASD | 13 | >15 | <15 | >15 | 100 | 35 |
| 11 | ASD | 1 | <15 | <15 | <15 | 84 | 75 |
| 12 | ASD | 36 | >15 | >15 | >15 | 106 | 15 |
| 13 | ASD | 18 | <15 | >15 | >15 | 72 | 40 |
| 14 | ASD | 40 | <15 | >15 | >15 | 100 | 65 |
| 15 | ASD | 1 | <15 | <15 | <15 | 84 | 90 |
| 16 | ASD | 1 | <15 | <15 | >15 | 100 | 80 |
| 17 | ASD | 1 | <15 | <15 | <15 | 77 | 90 |
| 1 | TD | 65 | >15 | >15 | >15 | 98 | 90 |
| 2 | TD | 13 | >15 | <15 | >15 | 104 | 65 |
| 3 | TD | 18 | <15 | >15 | >15 | 107 | 95 |
| 4 | TD | 3 | <15 | >15 | >15 | 124 | 95 |
| 5 | TD | 18 | <15 | >15 | >15 | 115 | 95 |
| 6 | TD | 29 | <15 | >15 | >15 | 99 | 60 |
| 7 | TD | 1 | <15 | <15 | <15 | 107 | 15 |
| 8 | TD | 5 | <15 | >15 | >15 | 123 | 95 |
| 9 | TD | 45 | <15 | >15 | >15 | 100 | 25 |
| 10 | TD | 45 | <15 | >15 | >15 | 125 | 60 |
| 11 | TD | 36 | <15 | >15 | >15 | 118 | 70 |
| 12 | TD | 11 | <15 | >15 | >15 | 110 | 85 |
| 13 | TD | 40 | <15 | >15 | >15 | 109 | 85 |
| 14 | TD | 65 | >15 | >15 | >15 | 108 | 90 |
| 15 | TD | 36 | >15 | >15 | >15 | 122 | 60 |
| 16 | TD | 45 | <15 | >15 | >15 | 122 | 75 |
| 17 | TD | 65 | >15 | >15 | >15 | 75 | 55 |
| 18 | TD | 16 | <15 | >15 | >15 | 107 | 30 |
| 19 | TD | 45 | <15 | >15 | >15 | 84 | 60 |
| 20 | TD | 36 | <15 | >15 | >15 | 93 | 5 |
Note: ASD = Autism Spectrum Disorder; TD = Typically Developing; Percentile intervals reported for MABC-2 (Movement Assessment Battery for Children-2; cut-off at the 15th percentile); Total standard scores reported for PPVT-R (Peabody Picture Vocabulary Test-Revised; cut-off at the standard score of 85 or lower, i.e., 1 or more SDs below age-based corrected normative data); Percentile values reported for TOL (Tower of London; cut-off at the 15th percentile). Scores meeting cut-off criteria are reported in Italics.
2.2.2. Tower of London (TOL)
The TOL (Anderson et al., 1996) is a widely used neuropsychological test of planning and problem solving in which one must move coloured discs from an initial state to their desired goal state. To achieve this in as few moves as possible, which is the aim of the task, requires executive planning. When considering the performance at this test, no significant differences were found between ASD and TD children (ASD M = 63.53 vs. TD M = 65.5; t(35) = −0. 219, p > 0.05; see Table 3).
2.2.3. Peabody Picture Vocabulary Test-Revised (PPVT-R)
For the assessment of receptive vocabulary, children were administered the Italian version of the Peabody Picture Vocabulary Test PPVT-R (Dunn and Dunn, 1997; for Italian standardization, Stella et al., 2000). The PPVT-R is a standardized measure of receptive vocabulary, with good test–retest, split half, and alternate form reliability, and strong criterion-related validity. PPVT-R results can be reported as age-based scores (with a mean of 100 and a standard deviation of 15) that range from 20 to 160. While both groups scored at the level expected for their age, ASD children showed lower verbal abilities than TD children (ASD M = 97.18 vs. TD M = 107.5; t(35) = −2.421, p < 0.05; see Table 3).
2.3. Procedure
Fig. 1 shows the experimental set up. The participant was asked to stand on a floor marking tape parallel to the lateral edge of a table (at about 22 cm from the table). At the start of each trial, a white plastic cylinder (height = 30 cm; diameter = 1.6 cm; weight = 135 g) with a thin plastic base (height = 0.5 cm; diameter = 10 cm) was placed on the table at a distance of 25 cm in front of the participant (home position). A wired grid stand with a grid shelf (15 × 30 cm) attached to it stood parallel to the short side of the table, to the left of the participant (see Fig. 1). The grid shelf was designated as the target position.
Fig. 1.
A schematic representation of materials and experimental set-up that were used to test grasp height effect. The position of the markers on the participant’s right hand and the cylinder were used to measure the grasp height effect during the unimanual, bimanual, and joint tasks.
Both the height of the home position and the height of the target position were adjusted to the participant’s height. The initial height of the table was levelled with the elbow height of the participant when standing (please refer to Supplementary Table 1 for details). The grid shelf (target position) could be positioned at one of three heights: at the same height of the home position (middle), 20 cm higher, (high) or 20 cm lower (low) than the home position. This allowed for comfortable initial and final postures (e.g., no need for bending or arm stretching).
Throughout all experimental sessions, the same female experimenter, kneeling at the opposite side of the table, interacted with the participants. The grasp height effect was tested in three tasks:
Unimanual task: The participant reached towards, grasped the cylinder with the right hand and then moved it from the home position to the target position;
Bimanual task: The participant grasped the cylinder with their right hand, passed it on to their left hand and then moved it to the target position;
Joint task: The participant grasped the cylinder with their right hand, and then passed it on to the experimenter, who took hold of it with their right hand and moved it to the target position. The experimenter was at a distance of about 1 m from the participant.
Participants performed a series of three consecutive movements for each of the three target position heights (low, middle, high) for each of the three tasks (unimanual, bimanual, joint), making a total of 27 movements. The order of tasks and target position heights were balanced across participants.
At the start of the first trial, the child was instructed to stand on the floor marking tape. Once the child stood on the mark, the experimenter positioned the grid shelf at one of the three heights (low, middle, or high) and instructed the child on how to perform. Participants were asked to keep their left hands by their sides at all times and to keep their right hands by their sides between trials. They were asked to take hold of the cylinder with their right hand and, after completion of the task, to return that hand to the side of their body (i.e., let it hang down). At the end of the trial, the experimenter returned the cylinder to the home position. This procedure was repeated three times. When three trials were completed, the experimenter removed the grid shelf and, consulting a previously prepared design sheet, positioned the shelf to another height, whereupon the sequence of the three movements was repeated.
Children were asked to perform in a relaxed manner, moving at a comfortable speed. Throughout the experiment, the experimenter carefully monitored the children’s performance and reminded them of the instructions, if necessary.
In order to become familiarized with the procedure, the children performed two practice trials before each experimental task. There was a short pause of approximately 20 s between each trial and a longer pause of about 2 min between tasks. The entire experiment lasted around 20 min.
2.4. Data acquisition
To track and record the children’s grasp height, we used a near-infrared camera motion capture system (frame rate = 100 Hz; Bonita Vicon Motion Systems Ltd, Oxford, UK). Six cameras were located in a semicircle at a distance of 1.5–2 m from the table on which the plastic cylinder was placed (see Fig. 1). Each child was outfitted with three lightweight retro-reflective hemispheric markers (10 mm in diameter) placed on the metacarpal-phalangeal joint of the index and little fingers as well as on the radial aspect of the wrist of the right hand (see Fig. 1). A retro-reflective hemispheric marker was also placed on the base of the cylinder (see Fig. 1). After data collection, each trial was individually inspected for correct marker identification and then run through a low-pass Butterworth filter with a 6 Hz cut-off. For data processing and analyses, a custom software (Matlab; MathWorks, Natick, MA) was used to compute the grasp height, defined as the distance (mm) between the marker placed on the index metacarpal-phalangeal joint of the hand and the marker placed on the cylinder at lift onset (i.e., the first frame in which the vertical displacement of the marker on the cylinder exceeded 0.5 mm).
2.5. Statistical analyses
We performed two complementary analyses. In our first analysis, to compare grasp height across tasks and groups, we performed a mixed factorial Analysis of Covariance (ANCOVA) with task (three levels: unimanual, bimanual, joint) and target position height (three levels: low, middle, high) as within-subjects factors and group (two levels: ASD, TD) as between-subjects factors. Chronological age, stature, and FS IQ were closely matched for ASD and TD children (see ‘Participants’ section). Despite this careful matching, with nearly identical age, stature, and FS IQ averages between groups, there is always some remaining variation across participants in these measures. To control for this, we entered the children’s age, stature, and FS IQ as covariates (for a description of a similar rationale, please refer to Pallett et al., 2014). Analysis of covariance allowed us to reduce within-group error variance while testing the between-group differences adjusted for the covariates (see Field, 2013). We did not include MABC-2, TOL, and PPVT-R as covariates because these variables measure attributes that are intrinsic to the disorder and hence their inclusion would lead to erosion of the effect of group (Adams et al., 1985, Evans and Anastasio, 1968, Lord, 1967, Lord, 1969, Miller and Chapman, 2001, Tupper and Rosenblood, 1984). Post-hoc tests (Bonferroni’s correction; p < 0.05) were applied to explore significant effects and interactions.
In a second analysis, to capture the relationship between motor and cognitive functioning and prospective control, we correlated (by means of Pearson’s correlation) the difference in grasp height with movement skills (as measured by MABC-2, total score), executive planning skills (as measured by the TOL), and receptive vocabulary (as measured by the PPVT-R). In the ASD group, the difference in grasp height was further correlated with the degree of autistic severity (as measured by ADOS-2 and ADI-R tests). The difference in grasp height was calculated by the difference between the average grasp height when placing the cylinder on the lower target position and the average grasp height when placing the cylinder on the higher target position. The calculation was made for each participant in the TD and ASD groups, and for the unimanual, bimanual, and joint tasks separately.
3. Results
Missing values accounted for <1% of the data (3 over 459 trials in ASD group and 1 over 540 in the TD group). No values were removed as outliers (defined as grasp heights deviating 2.5 SD from their respective averages).
3.1. Distribution of grasp height as a function of the target position in TD and ASD individuals
The distribution of grasp height for each task in TD and ASD individuals is illustrated in Fig. 2. To favour comparison, data for each TD and ASD participant are reported within the same graph. For the unimanual task (Fig. 2a), the distribution of grasp heights tended to cluster as a function of the target position, with participants being more likely to grasp the cylinder lower when the target position was high (red dots) than when it was low (blue dots). This pattern was apparent in both TD and ASD children.
Fig. 2.
Grasp height as a function of the target position. Photos, dot plots and bar graphs illustrating performance in the unimanual (a), bimanual (b), and joint tasks (c). Dot plots illustrate grasp height by individual participants (in the order in which they were recruited) in the TD (Typically Developing) and the ASD (Autism Spectrum Disorder) group. Each dot represents a trial and is colour-coded by target position. Bar graphs represent mean grasp height in the TD and the ASD group. Error bars represent standard error of the mean. (For interpretation of the references to colour in this figure caption, the reader is referred to the web version of this article).
As illustrated in Fig. 2b, in the bimanual task, in both groups, grasp heights for high and low target positions showed a larger degree of overlap, with just a few children grasping the cylinder higher when the target position was high and lower when it was low, as predicted by the grasp height effect for bimanual actions. Interestingly, a larger proportion of children in both groups grasped the object in the same way as in the unimanual task (i.e., lower when the target position was high, higher when target position was low), thus violating the grasp height effect.
As for the joint task, inspection of grasp heights in Fig. 2c suggests that, aside from a small number of children in the TD group (i.e., five children who grasped the cylinder higher when the target position was high, lower when the target position was low), the majority of children in both groups did not show a clear grasp height modulation to the partner’s end posture. Qualitatively, it thus appears that prospective sensorimotor control for joint actions was not yet fully developed in the tested age range.
3.2. Grasp height across tasks in TD and ASD group
The ANCOVA with age, stature, and FS IQ as covariates revealed no main effect of group (F(1, 32) = 3.012; p = 0.092; ηp2 = 0.086), but it did show a significant group by task interaction (quadratic effect: F(1, 32) = 5.069; p = 0.031; ηp2 = 0.137). Post-hoc contrasts indicated that children in the ASD group grasped the cylinder lower in the joint task than in the unimanual task (p = 0.001) and in the bimanual task (p = 0.035). No similar differences were found for the TD group (ps > 0.8341). The interaction task by target position height was also significant (quadratic effect: [F(1, 32) = 8.828; p = 0.006; ηp2 = 0.216]), resulting from a significant grasp height effect in the unimanual task (ps < 0.005) but not in the joint task (ps > 0.529). As for the bimanual task, post-hoc contrast revealed that children grasped the object lower when the target position was high compared to low (p = 0.016). This pattern violates the grasp height effect, thus confirming the impression gleaned from Fig. 2b. No other contrast was significant (ps > 0.148).
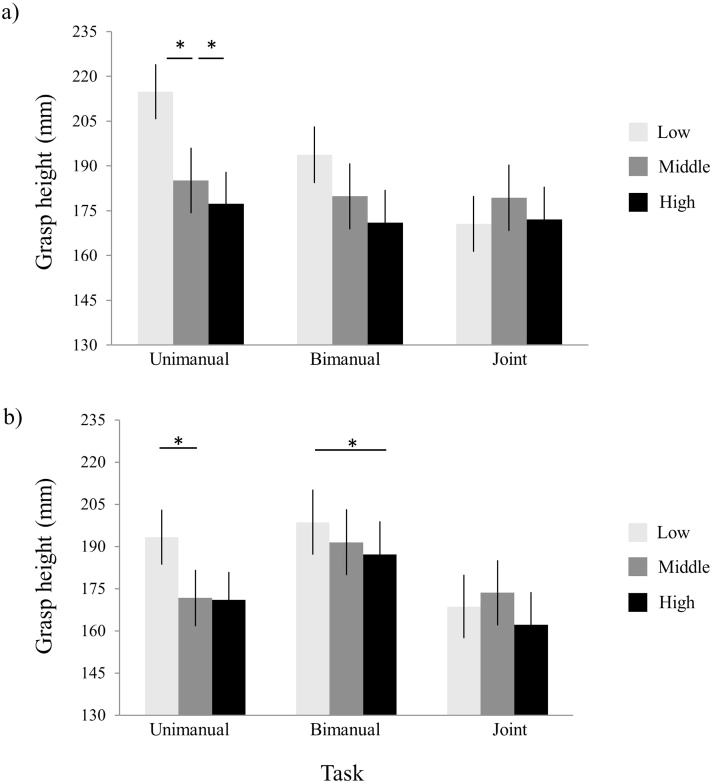
The interaction task by target position height was further qualified by a three-way task by target position height by FS IQ interaction [linear effect; F(1, 32) = 4.877; p = 0.034; ηp2 = 0.132], indicating that the children’s IQ influenced the modulation of the effect of shelf height across tasks. To explore this effect, we examined the task by target position height interaction at different levels of FS IQ: lower IQ (FS IQ < 100; N = 17) and higher IQ (FS IQ ≥ 100; N = 20). In the unimanual task, children with a higher IQ adjusted their initial grasp height such that a comfortable end-state was achieved at all three target positions (ps < 0.039; see Fig. 3a). Children with a lower IQ, in contrast, only showed a significant modulation of grasp height for low compared to middle target positions (p = 0.048; see Fig. 3b). In the bimanual task, whereas children with higher IQ showed no modulation of grasp height (ps > 0.448), children with a lower IQ tended to grasp the cylinder higher when the target position was low than when it was high (p = 0.028), thus showing a pattern opposite to that predicted by the grasp height effect (Fig. 3). No modulation of grasp height to target position was found for children with either a higher or lower IQ in the joint task (all ps > 0.198).
Fig. 3.
Graphical representation of the ‘Task’ by ‘Target Position’ interaction at different levels of FS IQ. Grasp heights for low (light grey bars), middle (dark grey bars), and high (black bars) target positions in the unimanual, bimanual and joint tasks for higher IQ (a) and lower IQ children (b). Asterisks indicate significant differences (p < 0.05). Vertical lines represent standard errors.
Finally, the ANCOVA revealed a significant task by stature interaction (linear effects: F(1, 32) = 5.619; p = 0.024; ηp2 = 0.149), resulting from children with lower stature (<139 cm; N = 18) grasping the cylinder lower in the joint task than in the unimanual task (p = 0.002). Children with a higher stature (≥139 cm; N = 19), in contrast, did not show differences in grasp height as a function of task (p = 1). In the joint task, children were requested to grasp the target object and hand it to experimenter, the distance between the child and the experimenter being of about 1 m. One possible explanation is thus that children with lower stature (and possibly shorter arms) adopted a lower grasp so to minimize the awkwardness of the hand posture when handing the object over to the partner.
3.3. Relationship between motor and cognitive functioning and prospective control
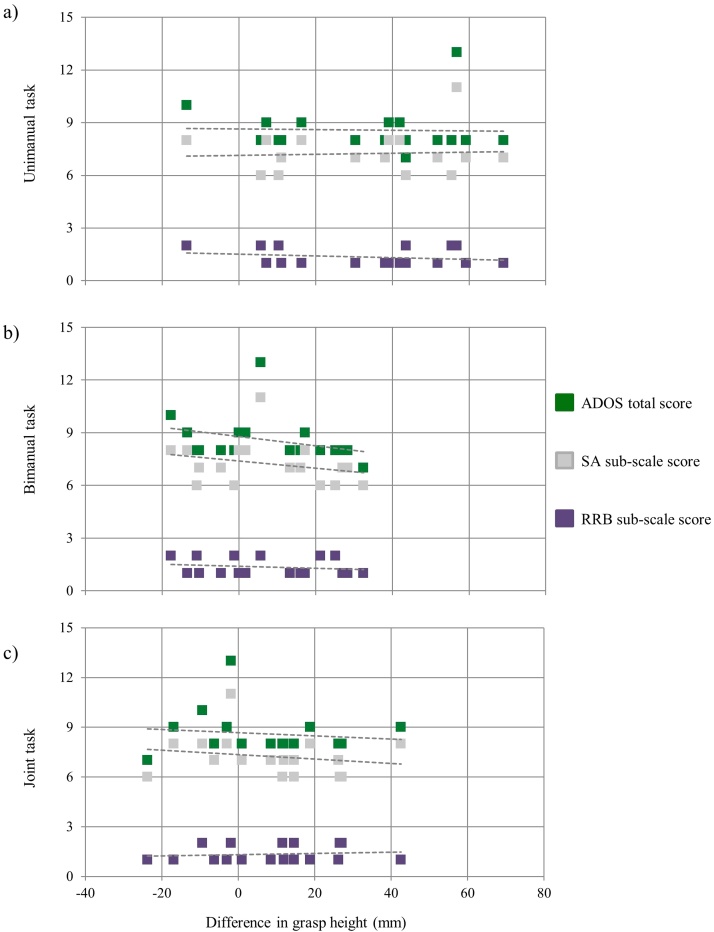
The correlation analysis revealed no significant association between motor, linguistic, and executive functions (as measured by MABC-2, PPVT-R, and TOL) nor differences in grasp height in either the TD group (ps > 0.199) or the ASD group (ps > 0.53). The association between the degree of autistic severity and differences in grasp height in ASD children was also not significant (ADOS Total Score: ps > 0.209; ADOS Social Affect: ps > 0.300; ADOS Restricted and Repetitive Behaviors: ps > 0.383; ADI-R: ps > 0.083; see Fig. 4).
Fig. 4.
Mean difference in grasp height (mm) as a predictor of social, communicative, and stereotyped behaviour used for the diagnosis of autism. Scores at the level of global performance (ADOS Total Score; green), Social Affect (SA) and Restrictive and Repetitive Behaviors (RRB) ADOS subscales (grey and purple). The x-axis represents the difference in grasp height in the unimanual (a), bimanual, (b) and joint tasks (c). (For interpretation of the references to colour in this figure caption, the reader is referred to the web version of this article).
4. Discussion
Altered motor behaviour in ASD is commonly reported using clinical and standardized measures of motor performance, such as the MABC-2–a minimal set of tasks grouped in three categories: manual dexterity, ball skills and balance. While these categories may be helpful in considering how an individual needs support in everyday life, however, they provide little information about the underlying motor processes (Gowen and Hamilton, 2013). Deficits in manual dexterity and ball skills as measured by the MABC-2, for example, could be related to sensory, planning or execution aspects of motor control. The aim of current work was to isolate the prospective sensorimotor control component in autistic motor ability.
To this end, we tested the grasp height effect in 17 children with an independent clinical diagnosis of ASD and compared their performance to that of 20 TD children matched for age, gender, stature, handedness, and IQ. ASD children were significantly impaired in motor skills, as evaluated by MABC-2. Nevertheless, in the three object manipulation tasks, which assessed unimanual, bimanual, and joint prospective control, they performed as well as TD children. In both groups, we found a significant grasp height effect in the unimanual task, but not in the bimanual and joint tasks. These findings challenge the hypothesis of a general prospective sensorimotor planning deficit in autism and suggest that not all motor processes are impaired in individuals with autism spectrum disorder.
In what follows, we first consider the relationship between our results and other studies examining prospective control in autism. Next, we discuss some of the factors that may account for modulation of grasp height across the three tasks.
4.1. Do children plan ahead? TD versus ASD children
Previous studies that have sought to examine prospective sensorimotor planning in children with ASD have yielded conflicting results. Some studies indicate that prospective control is impaired in children with ASD (Hughes, 1996, Scharoun and Bryden, 2016). For example, Hughes (1996) found that 12- and 13-year-old children transported a dowel using an underhand grip as opposed to the overhand grip used by younger (4-year-old), typically developing children. The underhand grip resulted in an uncomfortable end-state posture, indicating a lack of prospective planning. Other studies, however, revealed no significant group differences (Gonzalez et al., 2011, Hamilton et al., 2007, van Swieten et al., 2010. Using an orientation matching task, van Swieten et al. (2010), for example, report that 9- to 14-year-old children with ASD chose postures that led to end-state comfort about 50% of the time, which was similar to the age-matched controls. Hamilton et al. (2007) also tested a group of twenty-five autistic children on the grip selection task and found no group differences.
There are several possible causes for these inconsistencies, including differences between the task and procedures, the sample size and age of participants, as well as their cognitive and motor development. Our study rectifies these limitations in three ways. Firstly, we performed a comprehensive set of measurements spanning higher order planning for both individual and joint object manipulations. Secondly, unlike in other studies, participants were matched for age, gender, stature, handedness, and FS IQ. Finally, whereas all previous studies employed video-analysis of dichotomous outcome measures (i.e., grip selection), we used kinematic recordings of a more sensitive continuous measure, namely the height at which the object was initially grasped to later be moved to the target position. This is important as dichotomous measures may potentially cloud differences in motor patterning (Janssen and Steenbergen, 2011).
Kinematic analysis revealed a significant grasp height effect in the unimanual task, yet the measured effect was similar in the TD and ASD groups. Similarly, we found no differences in grasp height between groups in the bimanual and joint tasks. We emphasize that it is not that we failed to measure any effect in either the TD or ASD group; to the contrary, we reliably measured a grasp height effect in the unimanual task and a significant inversion of this effect in the bimanual task. This provides strong evidence that the lack of measurable differences between the TD and the ASD populations is not a consequence of poor resolving power associated with our paradigm.
4.2. How far do children plan ahead?
Children in both groups showed the grasp height effect in the unimanual task but not in the bimanual and joint task. What factors may account for these task modulations?
4.2.1. Orders of planning and planning span
One factor that could account for the observed modulations is related to the orders of planning, i.e., what needs to be done one (second-order), two (third-order), or several actions later. The unimanual task looked for second-order effects, reflecting the influence of what the subject intends to do next with the object (i.e., move the object to the target position). The bimanual and the joint tasks looked for third-order effects, reflecting the influence of what is to be done after that (i.e., pass the object to the other hand or to the co-actor to move it to the target position; see Rosenbaum et al., 2013). It is thus possible that the task-dependent modulations reflect differences in the planning span (Rosenbaum et al., 2013), with third-order planning exceeding the action planning capabilities of 7- to 11-year-old TD and ASD children. Contrary to this, however, recent work has shown that 7-year-old children, but not 3- and 5-year old children, demonstrate evidence for third-order joint action planning (Paulus, 2016). Moreover, an explanation in terms of planning span cannot account for differences in the bimanual and joint task. While the number of action steps may contribute to the observed patterns, it seems thus unlikely that the planning span is the only critical factor.
4.2.2. Unimanual bias in bimanual prospective control
Although results did not reveal a significant grasp height effect in the bimanual task, it would be incorrect to say that children totally failed to consider the more distal action goal in this task. Both qualitative as well as quantitative evidence indicate that in the bimanual task a good proportion of participants regularly grasped the cylinder higher (with their right hand) when the target position was low compared to when it was high. This shows that children did not ignore the height of the target position; the error rather points to a specific problem in planning the appropriate sequence of moves. At first glance, this might appear as an executive planning deficit, reflecting the inability to represent the sequence of intermediate choices or moves that must be arranged in order to achieve a desired end-state. However, if this were the case, we would expect an association with executive function performance. Correlation analysis showed that this was not case (see also van Swieten et al., 2010, Wunsch et al., 2016).
What the grasping patterning in the bimanual task suggests is rather that children engaged in action planning, but they did so in a ‘unimanual’ way. In other words, they grasped the object with their right hand at the height that would have been appropriate as if they intended to move it with their right hand to the target position. This behaviour may reflect the potential conflict between unimanual and bimanual planning constraints. Conflict between grasping strategies has been shown to increase the overall cognitive demands of a task and interfere with the ability to integrate proximal and distal action segments into a single action plans (Stockel and Hughes, 2015). As a result, children who do not possess the cognitive resources to resolve the conflict may be biased to select a non-compliant grasp posture (Paulus, 2016, Stockel et al., 2012). In our task, grasping the object with one’s right hand in the bimanual task may have triggered the planning that would have been appropriate to complete the task with one hand. This interpretation is further strengthened by the fact that this ‘unimanual bias’ was most pronounced in children with lower IQ, i.e., children who arguably did not possess the cognitive resources to deal with the conflict.
All of these considerations raise questions about the grasping pattern displayed by higher IQ participants. Could this apparently random pattern reflect the not yet fully developed ability to select an appropriate grasp when unimanual and bimanual action planning are in conflict? Would removing the conflict facilitate compliance with the bimanual grasp height effect? This could be tested by manipulating task constraints (e.g., by asking participants to initially grasp with their left hand in the bimanual task so to avoid conflict with the unimanual strategy; see Stockel and Hughes, 2015, for a similar approach). If removing conflict decreases cognitive costs, we should see an improvement in grasp height performance.
4.2.3. Effect of social context
Finally, it is also worth considering the differences between third-order bimanual and joint action planning. Bimanual and joint object manipulation differ in a trivial sense because, in a joint task, each actor is responsible for only half of the task, i.e., for one hand, so to say. However, accumulating evidence indicates that when two adult co-actors perform a task together, each actor integrates the co-actor’s action in his or her action planning (Sebanz et al., 2006). Co-representation effects of this sort have also been reported in children aged 5 years and up (Milward et al., 2014; see also Meyer et al., 2016). However, it remains unknown whether and to what extent young children spontaneously represent the co-actor’s end-state in a joint task. Paulus (2016), for example, found that 7-year-old children, but not 3- and 5-year-old children, adjusted their own motor planning to accommodate the end-state of another person. In the study by Paulus (2016), however, children received some critical feedback when not performing adequately – the partner frowned, uttered a sceptical “mhmm” and waited for 3 s, demonstrating their difficulty in dealing with the problem.
In the present study, in contrast, children received no feedback whatsoever. Moreover, because of the nature of the task, it is implausible that they perceived the partner’s discomfort when not performing adequately. It is thus possible that they did not represent the partner’s end-state. Again, however, it would be incorrect to say that children totally failed to consider joint task constraints. In the joint task, we found no evidence of unimanual bias. This is at odds with the bimanual task in which a good proportion of children grasped the object in a ‘unimanual way’. Why might this be? While the task design does not allow us to draw conclusions regarding the underlying computational mechanisms, it suggests that grasp performance was influenced by the social context of the task, i.e., the presence of someone else in the action scene. This is further supported by the finding that, regardless of the height of the target position, ASD children grasped the object lower in the joint task. Although this effect does not reflect co-representation of the partner’s end state, it suggests that ASD children adjusted their behaviour to accommodate the other’s action. Future studies will be necessary to understand the exact computational characteristics of this phenomenon. A speculative possibility is that having difficulties in anticipating the partner’s movements, children in the ASD group used a lower grasp to allow more space for the partner’s hand.
4.3. Limitations and future directions
Our results suggest that grasp height is sensitive to forthcoming task demands to a similar degree in autistic and typically developing children. In the present study, however, we only examined where the object was grasped, i.e., the spatial aspect of the tasks. It would be thus premature to assume, based on our data, the integrity of prospective sensorimotor planning in ASD children. An important aspect of prospective planning concerns the organization of the temporal aspect of the action sequence (Gowen and Hamilton, 2013). It is possible that, in the unimanual task, children with autism were able to plan grasp height of an action as a function of the action that followed but had more difficulty organizing the temporal chaining of successive movements into an overall action sequence. In line with this, using a grasp-to-eat task, Cattaneo et al. (2007) found that electromyography (EMG) activity related to mouth opening was delayed in children with ASD compared to typical controls. Specifically, whereas EMG activity in typically developing children started before the hand even grasped the object, EMG activity in the autistic children started much later, when their hand was bringing the food to their mouth (although Pascolo and Cattarinussi, 2012 have recently failed to replicate this finding). The present study was not designed to test temporal aspects; therefore, we cannot rule out that the temporal details of the action planning across tasks differ across groups. Future studies examining when the hand starts to adjust to future task demands may help to clarify this issue.
A further limitation of the current study is that we cannot exclude that ASD children used different processes to anticipate future task demands. Cognitive neuroscientists have identified several brain regions thought to be important for prospective sensorimotor control of grasping movements. These include the inferior frontal gyrus, the supramargynal gyrus, and the intraparietal sulcus (Tunik et al., 2008, Króliczak et al., 2008). As structural and functional alterations within these areas have been reported in ASD (see Patriquin et al., 2016 for a meta-analysis), it will be important for future studies to investigate continuity and differences in these areas in TD and ASD individuals during prospective planning of individual and joint actions.
Finally, our results do not allow us to rule out the emergence of group differences with age. This applies in particular to bimanual and joint action planning as our results suggest that these abilities are not yet fully developed at 7–11 years of age. While we do not observe differences between groups in this age range, it is thus possible that developmental differences manifest in older children. Longitudinal studies extending the age-range are needed to address this issue.
5. Conclusions
It has been proposed that children with ASD may not take the final goal into account when planning their actions (e.g., Fabbri-Destro et al., 2009). Our results argue against this view and suggest that at least some aspects of prospective sensorimotor control are intact in ASD children who have clear deficits in movement execution. This indicates that prospective sensorimotor control cannot be considered as a single factor explanation of impaired action and interaction in autism.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding
This work received funding from the European Research Council under the European Union’s Seventh Framework Programme (FP7/2007-2013)/ERC grant agreement n. 312919.
Acknowledgements
We would like to thank the families and the children for their participation to this research. We further thank Marco Jacono for his help with kinematics measuring, Laura Taverna for her help with figure preparation, and Tullio Ferracciolo for his contribution to data acquisition. Finally, we thank Martina Semino, Eugenia Dufour, Alessandra Gamucci, and Maria Pintaudi for their help with the administration of the motor and neuropsychological tests.
Footnotes
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.dcn.2017.02.009.
Appendix A. Supplementary data
The following are Supplementary data to this article:
References
- Adams K.M., Brown G.G., Grant I. Analysis of covariance as a remedy for demographic mismatch of research subject groups: some sobering simulations. J. Clin. Exp. Neuropsychol. 1985;7(4):445–462. doi: 10.1080/01688638508401276. [DOI] [PubMed] [Google Scholar]
- Adrien J.L., Lenoir P., Martineau J., Perrot A., Hameury L., Larmande C., Sauvage D. Blind ratings of early symptoms of autism based upon family home movies. J. Am. Acad. Child Adolesc. Psychiatry. 1993;32(3):617–626. doi: 10.1097/00004583-199305000-00019. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association . 5th ed. American Psychiatric Association Press; Washington: 2013. Diagnostic and Statistical Manual of Mental Disorders. [Google Scholar]
- Anderson P., Anderson V., Lajoie G. The tower of London test: validation and standardization for pediatric populations. Clin. Neuropsychol. 1996;10(1):54–65. [Google Scholar]
- Ansuini C., Santello M., Massaccesi S., Castiello U. Effects of end-goal on hand shaping. J. Neurophysiol. 2006;95(4):2456–2465. doi: 10.1152/jn.01107.2005. [DOI] [PubMed] [Google Scholar]
- Ansuini C., Giosa L., Turella L., Altoè G., Castiello U. An object for an action, the same object for other actions: effects on hand shaping. Exp. Brain Res. 2008;185(1):111–119. doi: 10.1007/s00221-007-1136-4. [DOI] [PubMed] [Google Scholar]
- Ansuini C., Cavallo A., Bertone C., Becchio C. Intentions in the brain: the unveiling of Mister Hyde. Neuroscientist. 2015;21(2):126–135. doi: 10.1177/1073858414533827. [DOI] [PubMed] [Google Scholar]
- Armbrüster C., Spijkers W. Movement planning in prehension: do intended actions influence the initial reach and grasp movement? Motor Control. 2006;10(4):311–329. doi: 10.1123/mcj.10.4.311. [DOI] [PubMed] [Google Scholar]
- Becchio C., Sartori L., Bulgheroni M., Castiello U. The case of Dr. Jekyll and Mr. Hyde: a kinematic study on social intention. Conscious. Cogn. 2008;17(3):557–564. doi: 10.1016/j.concog.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Becchio C., Manera V., Sartori L., Cavallo A., Castiello U. Grasping intentions: from thought experiments to empirical evidence. Front. Hum. Neurosci. 2012;6:117. doi: 10.3389/fnhum.2012.00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattaneo L., Fabbri-Destro M., Boria S., Pieraccini C., Monti A., Cossu G., Rizzolatti G. Impairment of actions chains in autism and its possible role in intention understanding. Proc. Natl. Acad. Sci. 2007;104(45):17825–17830. doi: 10.1073/pnas.0706273104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen R., Rosenbaum D. Where grasps are made reveals how grasps are planned: generation and recall of motor plans. Exp. Brain Res. 2004;157(4):486–495. doi: 10.1007/s00221-004-1862-9. [DOI] [PubMed] [Google Scholar]
- Cook J. From movement kinematics to social cognition: the case of autism. Phil. Trans. R. Soc. B. 2016;371(1693):20150372. doi: 10.1098/rstb.2015.0372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crajé C., Lukos J.R., Ansuini C., Gordon A.M., Santello M. The effects of task and content on digit placement on a bottle. Exp. Brain Res. 2011;212(1):119–124. doi: 10.1007/s00221-011-2704-1. [DOI] [PubMed] [Google Scholar]
- Dunn L.M., Dunn L.M. American Guidance Service; Circle Pines: 1997. PPVT-III: Peabody Picture Vocabulary Test, ed. [Google Scholar]
- Evans S.H., Anastasio E.J. Misuse of analysis of covariance when treatment effect and covariate are confounded. Psychol. Bull. 1968;69(4):225–234. doi: 10.1037/h0025666. [DOI] [PubMed] [Google Scholar]
- Fabbri-Destro M., Cattaneo L., Boria S., Rizzolatti G. Planning actions in autism. Exp. Brain Res. 2009;192(3):521–525. doi: 10.1007/s00221-008-1578-3. [DOI] [PubMed] [Google Scholar]
- Field A.P. 4th ed. Sage; London: 2013. Discovering Statistics Using IBM SPSS Statistics. [Google Scholar]
- Fournier K.A., Hass C.J., Naik S.K., Lodha N., Cauraugh J.H. Motor coordination in autism spectrum disorders: a synthesis and meta-analysis. J. Autism Dev. Disord. 2010;40(10):1227–1240. doi: 10.1007/s10803-010-0981-3. [DOI] [PubMed] [Google Scholar]
- Gonzalez D.A., Studenka B.E., Glazebrook C.M., Lyons J.L. Extending end-state comfort effect: do we consider the beginning state comfort of another? Acta Psychol. 2011;136(3):347–353. doi: 10.1016/j.actpsy.2010.12.009. [DOI] [PubMed] [Google Scholar]
- Gowen E., Hamilton A. Motor abilities in autism: a review using a computational context. J. Autism Dev. Disord. 2013;43(2):323–344. doi: 10.1007/s10803-012-1574-0. [DOI] [PubMed] [Google Scholar]
- Haggard P. Planning of action sequences. Acta Psychol. 1998;99(2):201–215. [Google Scholar]
- Hamilton A.F.D.C., Brindley R.M., Frith U. Imitation and action understanding in autistic spectrum disorders: how valid is the hypothesis of a deficit in the mirror neuron system? Neuropsychologia. 2007;45(8):1859–1868. doi: 10.1016/j.neuropsychologia.2006.11.022. [DOI] [PubMed] [Google Scholar]
- Happé F., Frith U. Annual research review: towards a developmental neuroscience of atypical social cognition. J. Child Psychol. Psychiatry. 2014;55(6):553–577. doi: 10.1111/jcpp.12162. [DOI] [PubMed] [Google Scholar]
- Haswell C.C., Izawa J., Dowell L.R., Mostofsky S.H., Shadmehr R. Representations of internal models of action in the autistic brain. Nat. Neurosci. 2009;12(8):970–972. doi: 10.1038/nn.2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson S.E., Sugden D.A., Barnett A.L. 2nd ed. Harcourt Assessment; London: 2007. Movement Assessment Battery for Children. [Google Scholar]
- Hughes C. Brief report: planning problems in autism at the level of motor control. J. Autism Dev. Disord. 1996;26(1):99–107. doi: 10.1007/BF02276237. [DOI] [PubMed] [Google Scholar]
- Jansiewicz E.M., Goldberg M.C., Newschaffer C.J., Denckla M.B., Landa R., Mostofsky S.H. Motor signs distinguish children with high functioning autism and Asperger’s syndrome from controls. J. Autism Dev. Disord. 2006;36(5):613–621. doi: 10.1007/s10803-006-0109-y. [DOI] [PubMed] [Google Scholar]
- Janssen L., Steenbergen B. Typical and atypical (cerebral palsy) development of unimanual and bimanual grasp planning. Res. Dev. Disabil. 2011;32(3):963–971. doi: 10.1016/j.ridd.2011.02.002. [DOI] [PubMed] [Google Scholar]
- Johnson-Frey S.H., Maloof F.R., Newman-Norlund R., Farrer C., Inati S., Grafton S.T. Actions or hand-object interactions? Human inferior frontal cortex and action observation. Neuron. 2003;39(6):1053–1058. doi: 10.1016/s0896-6273(03)00524-5. [DOI] [PubMed] [Google Scholar]
- Kroliczak G., McAdam T.D., Quinlan D.J., Culham J.C. The human dorsal stream adapts to real actions and 3D shape processing: a functional magnetic resonance imaging study. J. Neurophysiol. 2008;100(5):2627–2639. doi: 10.1152/jn.01376.2007. [DOI] [PubMed] [Google Scholar]
- Lord C., Rutter M., DiLavore P., Risi S., Gotham K., Bishop S. 2nd ed. Western Psychological Corporation; Los Angeles: 2012. Autism Diagnostic Observation Schedule. [Google Scholar]
- Lord F.M. A paradox in the interpretation of group comparisons. Psychol. Bull. 1967;68(5):304–305. doi: 10.1037/h0025105. [DOI] [PubMed] [Google Scholar]
- Lord F.M. Statistical adjustments when comparing preexisting groups. Psychol. Bull. 1969;72(5):336–337. [Google Scholar]
- Mari M., Castiello U., Marks D., Marraffa C., Prior M. The reach-to-grasp movement in children with autism spectrum disorder. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2003;358(1430):393–403. doi: 10.1098/rstb.2002.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marteniuk R.G., MacKenzie C.L., Jeannerod M., Athenes S., Dugas C. Constraints on human arm movement trajectories. Can. J. Psychol. 1987;41(3):365. doi: 10.1037/h0084157. [DOI] [PubMed] [Google Scholar]
- Meyer M., van der Wel R.P.R.D., Hunnius S. Higher-order action planning for individual and joint object manipulations. Exp. Brain Res. 2013;225(4):579–588. doi: 10.1007/s00221-012-3398-8. [DOI] [PubMed] [Google Scholar]
- Meyer M., van der Wel R.P., Hunnius S. Planning my actions to accommodate yours: joint action development during early childhood. Phil. Trans. R. Soc. B. 2016;371(1693):20150371. doi: 10.1098/rstb.2015.0371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G.A., Chapman J.R. Misunderstanding analysis of covariance. J. Abnorm. Psychol. 2001;110(1):40–48. doi: 10.1037//0021-843x.110.1.40. [DOI] [PubMed] [Google Scholar]
- Milward S.J., Kita S., Apperly I.A. The development of co-representation effects in a joint task: do children represent a co-actor? Cognition. 2014;132(3):269–279. doi: 10.1016/j.cognition.2014.04.008. [DOI] [PubMed] [Google Scholar]
- Mostofsky S.H., Dubey P., Jerath V.K., Jansiewicz E.M., Goldberg M.C., Denckla M.B. Developmental dyspraxia is not limited to imitation in children with autism spectrum disorders. J. Int. Neuropsychol. Soc. 2006;12(3):314–326. doi: 10.1017/s1355617706060437. [DOI] [PubMed] [Google Scholar]
- Noterdaeme M., Mildenberger K., Minow F., Amorosa H. Evaluation of neuromotor deficits in children with autism and children with a specific speech and language disorder. Eur. Child Adolesc. Psychiatry. 2002;11(5):219–225. doi: 10.1007/s00787-002-0285-z. [DOI] [PubMed] [Google Scholar]
- Oldfield R.C. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9(1):97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Pallett P.M., Cohen S.J., Dobkins K.R. Face and object discrimination in autism, and relationship to IQ and age. J. Autism Dev. Disord. 2014;44(5):1039–1054. doi: 10.1007/s10803-013-1955-z. [DOI] [PubMed] [Google Scholar]
- Pascolo P.B., Cattarinussi A. On the relationship between mouth opening and broken mirror neurons in autistic individuals. J. Electromyogr. Kinesiol. 2012;22(1):98–102. doi: 10.1016/j.jelekin.2011.07.009. [DOI] [PubMed] [Google Scholar]
- Patriquin M.A., DeRamus T., Libero L.E., Laird A., Kana R.K. Neuroanatomical and neurofunctional markers of social cognition in autism spectrum disorder. Hum. Brain Mapp. 2016;37(11):3957–3978. doi: 10.1002/hbm.23288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus M. The development of action planning in a joint action context. Dev. Psychol. 2016;52(7):1052. doi: 10.1037/dev0000139. [DOI] [PubMed] [Google Scholar]
- Rosenbaum D.A., Jorgensen M.J. Planning macroscopic aspects of manual control. Hum. Mov. Sci. 1992;11(1):61–69. [Google Scholar]
- Rosenbaum D.A., Marchak F., Barnes H.J., Vaughan J., Slotta J., Jorgensen M. Constraints for action selection: overhand versus underhand grips. In: Jeannerod M., editor. Attention and Performance XIII: Motor Representation and Control. Lawrence Erlbaum Associates; Hillsdale: 1990. pp. 321–342. [Google Scholar]
- Rosenbaum D.A., Engelbrecht S.E., Bushe M.M., Loukopoulos L.D. Knowledge model for selecting and producing reaching movements. J. Mot. Behav. 1993;25(3):217–227. doi: 10.1080/00222895.1993.9942051. [DOI] [PubMed] [Google Scholar]
- Rosenbaum D.A., Halloran E.S., Cohen R.G. Grasping movement plans. Psychon. B. Rev. 2006;13(5):918–922. doi: 10.3758/bf03194019. [DOI] [PubMed] [Google Scholar]
- Rosenbaum D.A., Chapman K.M., Weigelt M., Weiss D.J., van der Wel R. Cognition, action, and object manipulation. Psychol. Bull. 2012;138(5):924. doi: 10.1037/a0027839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum D.A., Chapman K.M., Coelho C.J., Gong L., Studenka B.E. Choosing actions. Front. Psychol. 2013;4:273. doi: 10.3389/fpsyg.2013.00273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutter M., Le Coteur A., Lord C. Western Psychological Services; Los Angeles: 2003. Autism Diagnostic Interview-revised, ed. [Google Scholar]
- Sartori L., Becchio C., Bara B.G., Castiello U. Does the intention to communicate affect action kinematics? Conscious. Cogn. 2009;18(3):766–772. doi: 10.1016/j.concog.2009.06.004. [DOI] [PubMed] [Google Scholar]
- Scharoun S.M., Bryden P.J. Anticipatory planning in children with Autism Spectrum Disorder: an assessment of independent and joint action tasks. Front. Integr. Neurosci. 2016;10:29. doi: 10.3389/fnint.2016.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuboe A., Maldonado A., Stork S., Beetz M. Subsequent actions influence motor control parameters of a current grasping action. The 17th IEEE International Symposium Robot and Human Interactive Communication, RO-MAN 2008; Munich; 2008. pp. 389–394. [Google Scholar]
- Sebanz N., Bekkering H., Knoblich G. Joint action: bodies and minds moving together. Trends Cogn. Sci. 2006;10(2):70–76. doi: 10.1016/j.tics.2005.12.009. [DOI] [PubMed] [Google Scholar]
- Stella G., Pizzoli C.E., Tressoldi P.E. Torino; Omega Edizioni: 2000. Peabody test di vocabolario recettivo, ed. [Google Scholar]
- Stöckel T., Hughes C.M. Effects of multiple planning constraints on the development of grasp posture planning in 6- to 10-year-old children. Dev. Psychol. 2015;51(9):1254–1261. doi: 10.1037/a0039506. [DOI] [PubMed] [Google Scholar]
- Stöckel T., Hughes C.M., Schack T. Representation of grasp postures and anticipatory motor planning in children. Psychol. Res. 2012;76(6):768–776. doi: 10.1007/s00426-011-0387-7. [DOI] [PubMed] [Google Scholar]
- Teitelbaum P., Teitelbaum O., Nye J., Fryman J., Maurer R.G. Movement analysis in infancy may be useful for early diagnosis of autism. Proc. Natl. Acad. Sci. U. S. A. 1998;95(23):13982–13987. doi: 10.1073/pnas.95.23.13982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunik E., Lo O.Y., Adamovich S.V. Transcranial magnetic stimulation to the frontal operculum and supramarginal gyrus disrupts planning of outcome-based hand–object interactions. J. Neurosci. 2008;28(53):14422–14427. doi: 10.1523/JNEUROSCI.4734-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tupper D.E., Rosenblood L.K. Methodological considerations in the use of attribute variables in neuropsychological research. J. Clin. Exp. Neuropsychol. 1984;6(4):441–453. doi: 10.1080/01688638408401234. [DOI] [PubMed] [Google Scholar]
- Wechsler D. 4th ed. Harcourt Assessment; San Antonio: 2003. Wechsler Intelligence Scale for Children. [Google Scholar]
- Weigelt M., Cohen R., Rosenbaum D.A. Returning home: location memory versus posture memory in object manipulation. Exp. Brain Res. 2007;179(2):191–198. doi: 10.1007/s00221-006-0780-4. [DOI] [PubMed] [Google Scholar]
- World Medical Association General Assembly Declaration of Helsinki. Ethical principles for medical research involving human subjects. World Med. J. 2008;54:122–125. [Google Scholar]
- Wunsch K., Pfister R., Henning A., Aschersleben G., Weigelt M. No interrelation of motor planning and executive functions across young ages. Front. Psychol. 2016;7:1031. doi: 10.3389/fpsyg.2016.01031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Swieten L.M., van Bergen E., Williams J.H.G., Wilson A.D., Plumb M.S., Kent S.W., Mon-Williams M.A. A test of motor (not executive) planning in developmental coordination disorder and autism. J. Exp. Psychol. 2010;36(2):493–499. doi: 10.1037/a0017177. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.