Highlights
-
•
Brain responsiveness to social and monetary reward were studied in ADHD versus ASD.
-
•
Clinical groups versus TDC had different ventral striatum activation to both rewards.
-
•
Medial prefrontal overactivation in response to social reward was ADHD specific.
-
•
Ventral striatal underactivation in response to monetary reward was ASD specific.
-
•
Fronto-striato-parietal underactivation for money was shared by both clinical groups.
Keywords: Autism spectrum disorder, ADHD, Social reward, Monetary reward, Ventral striatum, Medial prefrontal cortex
Abstract
Although attention deficit hyperactivity disorders (ADHD) and autism spectrum disorders (ASD) share certain neurocognitive characteristics, it has been hypothesized to differentiate the two disorders based on their brain's reward responsiveness to either social or monetary reward. Thus, the present fMRI study investigated neural activation in response to both reward types in age and IQ-matched boys with ADHD versus ASD relative to typically controls (TDC). A significant group by reward type interaction effect emerged in the ventral striatum with greater activation to monetary versus social reward only in TDC, whereas subjects with ADHD responded equally strong to both reward types, and subjects with ASD showed low striatal reactivity across both reward conditions. Moreover, disorder-specific neural abnormalities were revealed, including medial prefrontal hyperactivation in response to social reward in ADHD versus ventral striatal hypoactivation in response to monetary reward in ASD. Shared dysfunction was characterized by fronto-striato-parietal hypoactivation in both clinical groups when money was at stake. Interestingly, lower neural activation within parietal circuitry was associated with higher autistic traits across the entire study sample. In sum, the present findings concur with the assumption that both ASD and ADHD display distinct and shared neural dysfunction in response to reward.
1. Introduction
There is growing evidence to suggest that motivation deficits contribute to the core clinical symptomatology of both autism spectrum disorders (ASD) and attention deficit hyperactivity disorders (ADHD) (Taurines et al., 2012). However, the neurobiological mechanisms causing and maintaining these deficits are currently not well understood but they may be divergent among the two disorders. While ASD and ADHD share certain behavioral (e.g., attention deficits), cognitive (e.g., executive dysfunction) and neurobiological characteristics (e.g., fronto-striatal system dysfunction) and exhibit a high rate of co-occurrence (Rommelse et al., 2011), it has been hypothesized to differentiate both disorders at the brain level based on their reward circuitry reactivity to either social or monetary reward, with greatest differences to be expected for social rewards (Kohls et al., 2009a, Kohls et al., 2009b). This, however, has not been studied to date.
In the case of ADHD, prevailing theories propose that the characteristic symptoms such as inattention, impulsivity and hyperactivity may result from dysregulated reward-seeking tendencies, including oversensitivity to reward contingencies as well as an abnormal preference for immediate over delayed rewards (“delay aversion”). This is most likely caused by disruptions of the dopaminergic reward pathway, including striatum and orbitofrontal cortex (Luman et al., 2005, Luman et al., 2010, Sonuga-Barke, 2011, Volkow et al., 2011). In a previous behavioral study, we could demonstrate that boys with ADHD were particularly hyperresponsive to social rewards (i.e., smiles and friendly faces), indicating that the motivational context as defined by the type of attainable reward plays a significant role in influencing goal-directed behavior in children and adolescents with ADHD versus typically developing controls (Geurts et al., 2008, Kohls et al., 2009b, Konrad et al., 2000, Krauel et al., 2007). This was recently also demonstrated for temporal discounting tasks (Demurie et al., 2013).
Considering brain function, deficits within the fronto-striatal reward circuitry have been reported in ADHD for monetary rewards using functional magnetic resonance imaging (fMRI), although the direction of the deviations is inconsistent. While there is evidence for diminished brain activation in ventral striatum during reward anticipation (Plichta and Scheres, 2014, Scheres et al., 2007), other studies reported enhanced striatal and medial prefrontal/orbitofrontal reactivity during reward outcome processing in children and adolescents with ADHD relative to TDC (Bjork et al., 2010, Gatzke-Kopp et al., 2009, Paloyelis et al., 2012, Rubia et al., 2009a). Given that all fMRI studies to date exclusively employed monetary rewards, it remains unclear to what extent day-to-day social incentives may affect neural reward responsiveness in youth with ADHD.
In the field of ASD, it has been suggested that the core social-communicative symptoms in affected individuals are closely linked to an under-responsiveness to social incentives, apparent as a lack of motivation to attend to social stimuli, and to seek and enjoy reciprocal social interactions and relationships (Chevallier et al., 2012, Dawson et al., 2005, Kohls et al., 2012, Mundy, 1995, Schultz, 2005). In fact, behavioral treatment curricula have shown that youth with ASD benefit less from the use of social rewards than from nonsocial rewards (Koegel et al., 2001, Margolies, 1977, Matson et al., 1996), and experimental studies have confirmed that the performance of children with ASD is only minimally affected by positive social reinforcement (Demurie et al., 2011, Freitag, 1970, Garretson et al., 1990, Geurts et al., 2008). However, the specificity of reward dysfunction for social rewards has also been questioned given behavioral and neural evidences in support of a more generalized reward processing deficit comprising social as well as nonsocial types of reward (Kohls et al., 2012). For instance, recent fMRI investigations in ASD revealed abnormal activation patterns of reward structures, including striatum, ventromedial prefrontal/orbitofrontal cortex, anterior cingulate and amygdala, not only in response to social rewards, but also for monetary reward, food items, and typical autism-specific objects of interest (Cascio et al., 2012, Delmonte et al., 2012, Dichter et al., 2012c, Dichter et al., 2012b, Kohls et al., 2013b, Richey et al., 2012, Schmitz et al., 2008, Scott-Van Zeeland et al., 2010).
Taken together, the independent fMRI findings in ADHD and ASD provide a mixed and incomplete picture of aberrant neural reward responsiveness, precluding any firm conclusions. However, the existing data do suggest a dysfunction of the fronto-striatal reward pathway in both clinical groups. Still, no study to date has directly compared brain correlates of reward processing in individuals with ADHD versus ASD, leaving the question unanswered about potential commonalities along with disorder-specific reward deficits, particularly with regard to social reward. However, closely related to this question, two recent fMRI investigations compared neural responses between children with ADHD and ASD using a sustained attention task (Christakou et al., 2013) and a resting-state paradigm (Di Martino et al., 2013), but without reward contingencies. While shared functional abnormalities spanning both disorders were observed in fronto-striatal as well as superior parietal areas including precuneus, atypical neural activation in other brain regions were disorder-specific and included ADHD-related deficits in dorsolateral prefrontal cortex and striatal pallidum, in contrast to ASD-related dysfunction in temporo-limbic and cerebellar nodes.
One paradigm that was adapted from animal research (Schultz et al., 1997) in order to assess reward responsiveness in children with and without ASD or ADHD is the incentive go/no-go task (Kohls et al., 2009a, Kohls et al., 2009b, Pankert et al., 2014). Unlike the “classic” incentive delay task (Knutson et al., 2005), the incentive go/no-go task allows examining the extent to which specific subcomponents of goal-directed behavior – i.e., response initiation versus response inhibition – and their underlying neural mechanisms can be differentially influenced by reward. Previously, we used the incentive go/no-go task with monetary and social reward contingencies and found diminished reward circuitry activation (e.g., ventral striatum) in children with ASD relative to healthy controls under both social and monetary reward conditions that required an active response to gain a reward (Kohls et al., 2013b).
Thus, the goal of the present fMRI study was to further differentiate ASD from ADHD on the basis of their brain's reward system responsivity to social versus monetary reward. To do so, we recruited an age- and IQ-matched group of subjects with ADHD to add to our previously reported sample of boys with ASD and typically developing male controls (TDC) between the ages of 9–18 years. Based on our prior report, we predicted that under-reactivity of the reward system (e.g., ventral striatum (VS)) in response to social as well monetary reward would be ASD-specific, whereas over-reactivity particularly under social reward conditions would be ADHD-specific (Kohls et al., 2009a), with both disorder groups showing shared functional abnormalities within a fronto-striato-parietal network relative to TDC (Christakou et al., 2013, Di Martino et al., 2013).
2. Method
2.1. Participants
A total of 55 right-handed male children and adolescents between the ages of 9 and 18 years participated in this the study, including 19 individuals with ADHD, 18 individuals with ASD and 18 matched TDC. Subsequently, three ADHD and three ASD participants were excluded because of excessive head movements, i.e., more than 3 mm of translational motion and/or more than one degree of rotation in any direction, during the scan. One TDC participant was excluded due to scores exceeding the cut-offs on screening measures for ASD (Social Communication Questionnaire: SCQ (Rutter et al., 2003), Social Responsiveness Scale: SRS (Constantino and Gruber, 2005)) and for overall psychopathology (Child Behavior Checklist: CBCL 4–18 (Achenbach, 1991)). All included participants (ADHD group n = 16: 13 combined subtype and 3 inattentive subtype; ASD group n = 15; TDC group n = 17) had a full-scale IQ ≥ 80 (estimated based on a short version of the WISC-III). The groups did not differ with respect to age and estimated IQ (all p-values > 0.1; Table 1). All participants had normal or corrected-to-normal vision.
Table 1.
Summary and comparisons of demographic and clinical characteristics for the study sample.
| Measure | ASD (n = 15) | ADHD (n = 16) | TDC (n = 17) | p-Valuesa | Pairwise group comparisonsb |
|---|---|---|---|---|---|
| M (SD) | M (SD) | M (SD) | |||
| Age (years) | 14.6 (3.3) | 14.5 (2.6) | 13.9 (3.0) | ns | |
| IQ (WISC-III) | 109.8 (12.1) | 104.6 (13.0) | 112.9 (12.6) | ns | |
| ADOS-G social | 8.3 (2.6) | NA | NA | ||
| ADOS-G communication | 4.1 (1.7) | NA | NA | ||
| ADOS-G stereotypy | 1.5 (1.0) | NA | NA | ||
| ADI-R social | 16.2 (4.4) | NA | NA | ||
| ADI-R communication | 16.8 (4.6) | NA | NA | ||
| ADI-R stereotypy | 5.5 (2.6) | NA | NA | ||
| FBB-HKS (total) | 22.5 (14.3) | 22.2 (8.3) | 5.6 (3.9) | <0.001 | TDC < ADHD = ASD |
| AQ (short; total) | 14.5 (7.8) | 10.1 (3.7) | 6.2 (3.1) | <0.001 | TDC = ADHD < ASD |
| GEM (total) | −29.1 (18.8) | 19.4 (27.7) | 29.4 (26.0) | <0.001 | TDC = ADHD < ASD |
| ATS (total) | −35.5 (16.5) | 14.4 (19.2) | 38.8 (20.1) | <0.001 | TDC > ADHD > ASD |
| ICS (total) | 4.2 (0.8) | 4.8 (0.6) | 5.6 (0.5) | <0.001 | TDC > ADHD > ASD |
Note: ASD = autism spectrum disorders, ADHD = attention deficit hyperactivity disorder, TDC = typically developing children, WISC-III = Wechsler Intelligence Scale for Children, ADOS-G = Autism Diagnostic Observation Schedule-Generic, ADI-R = Autism Diagnostic Interview-Revised, FBB-HKS = German Parental.
Report on ADHD symptoms according to ICD-10 and DSM-IV, AQ (short; total) = Autism Spectrum Quotient (33 items short version) total score, GEM = Griffith Empathy Measure total score, ATS = Affiliative Tendency Scale total score, ICS = Interpersonal Competence Scale total score.
p-values based on F-tests.
Tukey HSD tests (p-values ≤ 0.05).
Note that data from 15 subjects with ASD and 17 TDC had been previously reported (Kohls et al., 2013b). However, an age- and IQ matched group of subjects with ADHD was added in the present study in order to investigate the specificity of our previous findings. Any fMRI analyses and resulting findings presented in this article are novel and have not been published before.
Participants with ADHD and ASD were recruited through the Department of Child and Adolescent Psychiatry and Psychotherapy of the University Hospital Aachen, Germany. Diagnoses were given by an expert child and adolescent psychiatrist according to standard ICD-10 and DSM-IV criteria using the K-SADS-PL (Kaufman et al., 1997). In the ASD group, diagnoses were confirmed using the Autism Diagnostic Observation Schedule-Generic (ADOS-G; Lord et al., 2000) and the Autism Diagnostic Interview-Revised (ADI-R; Lord et al., 1994) conducted by experienced clinicians. Additionally, parents were asked to complete the SCQ and the SRS. None of the included ASD participants had a confirmed comorbid ADHD diagnosis or was taking any psychotropic medication at time of testing, and ADHD participants did not fulfill criteria for ASD. In the ADHD group, psychiatric classification was based on the diagnostic interview (K-SADS-PL) and a parental report on ADHD symptoms according to ICD-10 and DSM-IV (FBB-HKS; Döpfner and Lehmkuhl, 1998). None of the ADHD participants used any psychotropic medications other than short- or long acting stimulants (n = 9; medicated for several years), which were discontinued at least 48 h prior to scanning. The TDC group was recruited from local schools and underwent an extensive psychiatric examination (K-SADS-PL). In addition, parents evaluated the behavior of their children with regard to psychopathology using the CBCL. None of the TDC had a history or the presence of psychiatric or neurological disorders or was taking psychotropic medications.
Additionally, all parents were asked to complete the Griffith Empathy Measure (GEM) (Dadds et al., 2008), an abbreviated German version of the Autism-Spectrum Quotient-adolescent version (AQ) (Baron-Cohen et al., 2001), the Affiliative Tendency Scale (ATS) (Mehrabian, 1997) and the Interpersonal Competence Scale (ICS) (Cairns et al., 1995) in order to assess dimensionally different aspects of empathy, social motivation and social functions across all study participants (see for details Table 1).
Participants were compensated for their participation in the study. Informed consent was obtained from all participants and their parents. The study was approved by the local Ethics Committee.
2.2. FMRI task
We used an incentive go/no-go task with monetary and social reward contingencies in a blocked-design (Kohls et al., 2011, Kohls et al., 2013b) that was adapted from animal research (Schultz et al., 1997) and has previously shown robust reward circuit activation in adult samples (Goldstein et al., 2007, Thut et al., 1997). Altogether, 18 go blocks and 18 no-go blocks were presented pseudorandomly (counterbalanced across participants), including three different incentive conditions: non-reward (NR), social reward (SR), and monetary reward (MR). Each reward condition comprised six go and six no-go blocks. Every block consisted of five trials, which were either go or no-go trails. In go blocks, all five trials were go trials (=30 go trials per reward condition). In no-go blocks, on average, 65% were go trials and 35% were no-go trials (=19 go and 11 no-go trials per reward condition). Blocks started with an individual block cue (for 2950 ms) signaling the reward type that could be obtained in the ongoing block for correct performance. Each trial started with an instruction cue (for 250 ms), indicating a go trial (downward arrow) or a no-go trial (upward arrow). One second after the cue, the target stimulus (black square) was presented for 500 ms. The pre-target period, showing a fixation cross, served as an anticipation phase. Participants were instructed to respond with their index finger of the right hand on a MR-compatible response console as quickly and accurately as possible upon seeing the target after the go cue and to refrain from responding upon seeing the target after the no-go cue. Feedback was presented for 1500 ms immediately after the target disappearance, followed by an intertrial interval of 1000 ms. Altogether, each trial had a length of 4250 ms, and the block length was 24.2 s.
Depending on the reward condition, participants were rewarded for successful task performance (i.e., an accurate button press in go trials within a response time window of 500 ms and a correct inhibitory response in no-go trials) with a probability of 80% in order to strongly drive neural reward circuitry. In the SR condition, positive facial expressions served as rewards and neutral faces were shown after errors. Correct task performance in the MR condition was rewarded with money, symbolized by different wallets, each filled with a 50 Eurocent coin. Empty wallets were shown after errors. All participants were told that better performance would result in a larger amount of money paid after the experimental session. In the NR condition, meaningless feedback (represented by mosaic pictures) was given for both successful and failed task performance. Mosaic pictures were produced to resemble the social and monetary feedback pictures in complexity, size, and luminance. Visual stimulation was displayed on a rear projection LCD screen and viewed by the participant through a mirror attached to the head coil. Behavioral data collection and stimulus presentation were controlled by the Presentation 12.0 software (Neurobehavioral Systems, Albany, CA, USA).
To ensure that all participants understood the task instructions, the experimental procedure was preceded by 10 practice trials in each reward condition outside the magnet. After the experimental procedure, participants were asked to complete a rating questionnaire to assess their insight into aspects of task manipulations.
2.3. Subjective rating questionnaire
Following the experimental procedure, participants were asked separately for the three reward conditions (a) how motivating they found the condition, and (b) how rewarding they found the feedback stimuli. The participants were also asked how much they were motivated with regard to doing the task prior to the scan. Participants indicated their answers by marking a 10-cm, horizontal visual-analog scale min = 0, max = 100.
2.4. Behavioral data analysis
The two scales of the subjective rating questionnaire were analyzed within a MANOVA, with incentive type as a within-subjects repeated factor (NR, SR, MR) and group (ASD, ADHD, TDC) as the between-subjects factor, followed by univariate ANOVAs. Reaction times (RT) for go hits (in ms) as well as performance accuracy (go hit rate in %; no-go rejection rate in %) on the reward task were analyzed using 3 × 3 (reward × group) repeated measures ANOVA models, followed by planned contrasts. Since omission errors were very infrequent (below 2%), they were not included in the analysis. As age and IQ did not correlate with the dependent measures, these variables were not included as covariates in the data analyses. The alpha level was set at 0.05. Effect sizes were calculated using partial eta square (ηp2).
2.5. Image acquisition
T2* weighted BOLD images were obtained with echoplanar imaging using a Siemens Trio 3.0 T scanner (Erlangen, Germany) and a multichannel head coil. Whole brain volumes of 36, 3-mm thick axial images (TR/TE = 2200/30 ms, gap = 0.6 mm, flip angle = 90°, 64 × 64 matrix, voxel size = 3.1 × 3.1 × 3 mm3, FOV = 200 mm × 200 mm) were obtained continuously through one 15 min functional run. Altogether, 403 volumes (i.e., 66 volumes per condition) were acquired per participant preceded by four ‘dummy’ scans allowing for T1 magnetic saturation. For each participant, 176 high-resolution T1-weighted MPRAGE sagittal images of the entire brain were obtained following the functional run (TR/TE = 2250/3.93 ms, 256 × 256 matrix, voxel size = 1 × 1 × 1 mm3, FOV = 256 mm × 256 mm).
2.6. Image analysis
Image processing and statistical analyses were carried out using FEAT v5.98, part of the FSL analysis package v4.1.4. Prior to image analysis, the first four images of the functional data set were discarded because of the non-equilibrium state of magnetization. For pre-processing, functional volumes for each participant were skull-stripped, motion-corrected, temporally high-pass filtered, and spatially smoothed using a Gaussian kernel (FWHM = 5 mm).
Regression analysis was carried out on the pre-processed functional time series of each participant using FMRIB's Improved Linear Model with autocorrelation correction. Eight ‘block’ regressors (i.e., Go blocks: NR, SR, MR, and R = social and monetary reward combined; No-go blocks: NR, SR, MR, and R) were included in the general linear regression model and convolved with a double-gamma hemodynamic response function, along with its temporal derivative. Error trials and motion parameters were entered as nuisance regressors. The groups did not differ with respect to the amount of head movement during the scan (absolute displacement: ASD = 0.37 mm ± 0.18 mm; ADHD = 0.38 mm ± 0.16; TDC = 0.36 mm ± 0.19 mm; p = 0.90). Each regressor resulted in a voxelwise effect-size parameter estimate (β values) image reflecting the magnitude of brain activation associated with that regressor. In order to create comparisons of interest, β value images were contrasted. Functional data were registered to MNI space using affine transformations.
Group inferential statistical analyses were carried out using FMRIB's Linear Analysis of Mixed Effects (FLAME 1 + 2). Within-group mixed effects models were run for each contrast of interest. All Z statistical maps were cluster-corrected with a mean cluster threshold of Z > 2.3 and a whole-brain corrected cluster significance threshold of p ≤ 0.05 using Gaussian random field theory (Worsley et al., 1992). Next, a region-of-interest (ROI) analysis was performed on individual β values extracted from the VS, including ventromedial caudate and Nacc as structurally outlined in the Harvard-Oxford structural probabilistic atlas, for the two high-level contrasts of interest, i.e., SR > NR and MR > NR, in order to run a 2 × 3 (reward × group) repeated measures ANOVA followed by planned contrasts. Moreover, conjunction analyses with strict minimum statistics (Nichols et al., 2005) were run on contrasts of interest (e.g., SR > NR and MR > NR) to find reward areas that were abnormally activated in both clinical groups compared to healthy controls (ASDvsTDC ∩ ADHDvsTDC) or that were specifically atypical in one clinical group (ASDvsTDC ∩ ASDvsADHD; ADHDvsTDC ∩ ADHDvsASD). All activation maps were cluster-corrected with a mean cluster threshold of Z > 2.3 and a whole-brain corrected cluster significance threshold of p ≤ 0.05 using Gaussian random field theory.
3. Results
3.1. Subjective pre- and post-test ratings
The three groups started the experimental procedure equally motivated according to self-ratings (see Table 2). The post-test questions revealed a significant main effect of reward on the subjective rating scales (F(4, 42) = 36.32, p < 0.001, ηp2 = 0.78), which was related to both motivation (p < 0.001, ηp2 = 0.61) and reward value ratings (p < 0.001, ηp2 = 0.66), with the highest ratings for the MR condition, the lowest ratings for the NR condition and with the SR condition intermediate (all ps ≤ 0.001). These data demonstrate that reward manipulation within the experimental paradigm was successful. The group by reward interaction effect (F(8, 86) = 1.92, ns, ηp2 = 0.15) and the group effect (F(4, 90) = 0.77, ns, ηp2 = 0.03) were found to be non-significant, suggesting that the different reward conditions similarly affected subjective experiences associated with task performance in all groups.
Table 2.
Main performance variables of the incentive go/no-go task and subjective motivation ratings by group and incentive condition.
| Measures | ASD (n = 15) | ADHD (n = 16) | TDC (n = 17) | p-Valuesa |
|---|---|---|---|---|
| M (SD) | M (SD) | M (SD) | ||
| Motivation rating (max. 100) | ||||
| Start | 67.0 (24.7) | 66.3 (25.5) | 75.9 (11.8) | ns |
| Non-reward | 41.3 (28.9) | 48.8 (23.6) | 45.8 (30.2) | ns |
| Social reward | 82.0 (19.9) | 72.5 (11.8) | 80.6 (15.6) | ns |
| Monetary reward | 86.7 (13.9) | 87.5 (12.4) | 94.1 (7.9) | ns |
| RT for go hits (in ms) | ||||
| Non-reward | 244.8 (26.5) | 257.2 (32.2) | 236.6 (28.7) | ns |
| Social reward | 240.9 (23.7) | 255.1 (40.6) | 234.4 (33.9) | ns |
| Monetary reward | 243.4 (28.7) | 260.9 (37.2) | 231.4 (29.2) | ns |
| Go hit rate (accuracy in %) | ||||
| Non-reward | 87.2 (10.3) | 83.0 (12.3) | 91.3 (8.0) | ns |
| Social reward | 89.9 (7.7) | 87.7 (8.9) | 91.1 (7.9) | ns |
| Monetary reward | 88.7 (8.7) | 88.5 (7.3) | 92.5 (6.1) | ns |
| No-go rejection rate (accuracy in %) | ||||
| Non-reward | 96.2 (4.1) | 96.4 (6.1) | 95.5 (7.2) | ns |
| Social reward | 98.4 (2.9) | 97.0 (5.9) | 97.5 (3.4) | ns |
| Monetary reward | 98.4 (2.3) | 97.0 (4.6) | 97.8 (3.8) | ns |
Note: ASD = autism spectrum disorders, ADHD = attention deficit hyperactivity disorder, TDC = typically developing children.
p-values based on F-tests.
3.2. Task performance
The analysis of reaction time for go hits (in ms) revealed neither a main effect of reward (F(1, 45) = 0.18, ns, ηp2 = 0.004), nor a main effect of group (F(1, 45) = 2.62, ns, ηp2 = 0.10) or a significant group by reward interaction effect (F(2, 45) = 0.38, ns, ηp2 = 0.04), indicating that reward contingencies did not influence response speed substantially within this study sample. The analysis of go hit rates (i.e., accuracy in %) revealed a significant main effect of reward (F(1, 45) = 8.60, p = 0.005, ηp2 = 0.16), with particularly better performance accuracy under MR versus NR conditions (p = 0.005, ηp2 = 0.16; SR versus NR: p = 0.068, ηp2 = 0.07; SR versus MR: p = 0.48, ηp2 = 0.01). The group by reward interaction effect (F(2, 45) = 1.64, ns, ηp2 = 0.07) and the group effect (F(2, 45) = 1.87, ns, ηp2 = 0.08) were found to be non-significant, indicating that all three groups performed equally well under the different reward conditions. The analysis of no-go rejection rates (i.e., accuracy in %) did not reveal any significant main or interaction effects (all ps > 0.05; see Table 2).
3.3. BOLD activation during go blocks
3.3.1. Reward circuit reactivity across the three study groups
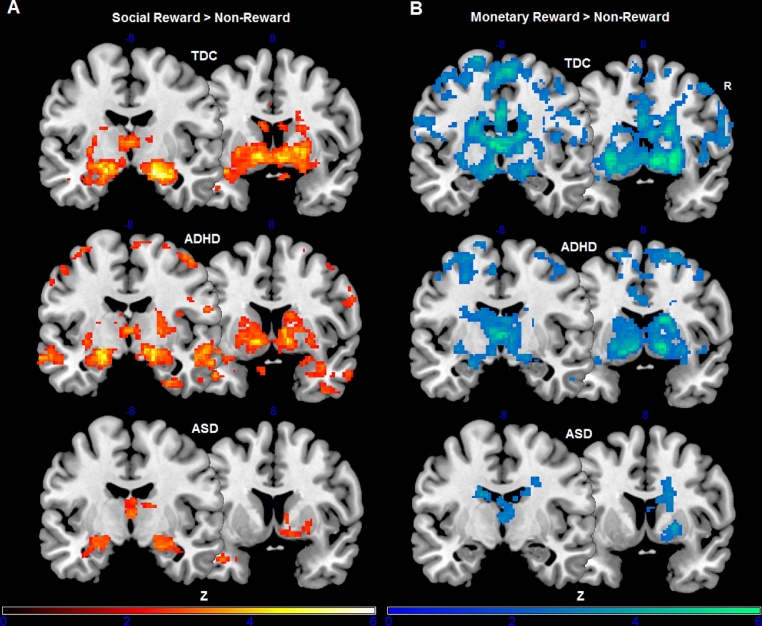
Reward circuitry activations separately for each group are listed in Table 3. The reward versus non-reward contrast (R > NR) revealed robust brain activation in ventral striatum (with nucleus accumbens), dorsal striatum (with caudate and putamen), thalamus, insula, amygdala and OFC across all three groups. Reward system reactivity for the two high-level contrasts, i.e., social reward versus non-reward (SR > NR) and monetary reward versus non-reward (MR > NR), is depicted in Fig. 1.
Table 3.
Reward circuitry and social brain activations across all three study groups.
| Anatomical region | Side | x | y | z | Maximum Z score |
|---|---|---|---|---|---|
| R > NR | |||||
| TDC | |||||
| Amygdala | r | 20 | −6 | −12 | 4.81 |
| Caudate | r | 18 | 6 | 18 | 4.70 |
| l | −18 | 0 | 20 | 4.06 | |
| Insula | r | 28 | 16 | −10 | 4.05 |
| Orbitofrontal cortex | r | 32 | 20 | −16 | 3.25 |
| Putamen | r | 16 | 8 | −4 | 5.64 |
| Ventral striatum/Nacc | r | 8 | 8 | −6 | 6.00 |
| l | −10 | 10 | −4 | 5.72 | |
| Thalamus | r | 4 | −16 | 12 | 6.53 |
| l | −2 | −18 | 10 | 5.72 | |
| ASD | |||||
| Amygdala | r | 22 | −2 | −14 | 3.16 |
| Caudate | r | 18 | 16 | 12 | 3.82 |
| l | −18 | 0 | 20 | 2.92 | |
| Insula | r | 30 | 16 | −10 | 3.13 |
| Orbitofrontal cortex | r | 30 | 20 | −14 | 3.46 |
| Putamen | r | 20 | 12 | 0 | 3.87 |
| Ventral striatum/Nacc | r | 12 | 8 | −6 | 3.01 |
| l | −10 | 10 | −8 | 3.09 | |
| Thalamus | r | 2 | −16 | 10 | 4.53 |
| l | 0 | −12 | 8 | 4.54 | |
| ADHD | |||||
| Amygdala | r | 22 | −2 | −16 | 4.60 |
| Caudate | r | 18 | 10 | 16 | 4.44 |
| l | −18 | 4 | 18 | 3.00 | |
| Orbitofrontal cortex | r | 28 | 18 | −14 | 4.01 |
| Putamen | r | 16 | 8 | −4 | 5.92 |
| Ventral striatum/Nacc | r | 12 | 8 | −4 | 6.33 |
| l | −12 | 6 | −8 | 5.35 | |
| Thalamus | r | 4 | −14 | 6 | 5.34 |
| l | 0 | −18 | 4 | 4.80 | |
| SR > MR | |||||
| TDC | |||||
| Amygdala | r | 20 | −8 | −18 | 4.56 |
| l | −20 | −8 | −18 | 3.07 | |
| Temporal pole | r | 26 | 10 | −34 | 2.93 |
| l | −40 | 20 | −32 | 4.25 | |
| Medial prefrontal cortex | r | 4 | 52 | −18 | 5.52 |
| ASD | |||||
| Amygdala | r | 24 | −4 | −20 | 4.04 |
| l | −28 | −6 | −18 | 3.73 | |
| Temporal pole | r | 28 | 12 | −28 | 3.02 |
| l | −40 | 12 | −30 | 3.35 | |
| Medial prefrontal cortex | l | −6 | 42 | −18 | 3.78 |
| ADHD | |||||
| Amygdala | r | 30 | −2 | −24 | 3.75 |
| l | −28 | −2 | −20 | 3.45 | |
| Temporal pole | r | 28 | 10 | −28 | 2.91 |
| l | −40 | 10 | −30 | 3.43 | |
| Medial prefrontal cortex | l | −2 | 44 | −20 | 3.94 |
| MR > SR | |||||
| TDC | |||||
| Anterior cingulate cortex | r | 8 | 36 | 18 | 4.26 |
| Caudate | r | 18 | −4 | 26 | 5.03 |
| l | −18 | 4 | 22 | 4.76 | |
| Ventral striatum/Nacc | r | 14 | 16 | −2 | 2.82 |
| Thalamus | r | 6 | −16 | 14 | 4.87 |
| ASD | |||||
| No significant activation differences | |||||
| ADHD | |||||
| Anterior cingulate cortex | r | 8 | 36 | 24 | 4.10 |
Note: x, y, z refers to axis in MNI (Montreal Neurological Institute) space; l = left hemisphere; r = right hemisphere; MR = monetary reward; SR = social reward; NR = non-reward; R = reward (i.e., social and monetary reward combined). Z scores are based on cluster-level correction for multiple comparisons across the whole brain with a mean cluster threshold of Z > 2.3 and a cluster-corrected significance threshold of p ≤ 0.05. Please note that results are shown for go conditions only. Activation differences between the high-level reward versus non-reward contrasts (i.e., R > NR, SR > NR, and MR > NR) across the three study groups as well as group comparisons were found to be non-significant during no-go conditions.
Fig. 1.
Z-statistic activation maps depicting reward circuitry activation in the three groups separately for the two high-level contrasts social reward versus non-reward (A) and monetary reward versus non-reward (B). Z scores are based on cluster-level correction for multiple comparisons across the whole brain with a mean cluster threshold of Z > 2.3 and a cluster-corrected significance threshold of p ≤ 0.05 using Gaussian random field theory. Color bars indicate Z-statistics. Results shown are for go blocks only.
3.3.2. Region-of-interest analysis of ventral striatum activation
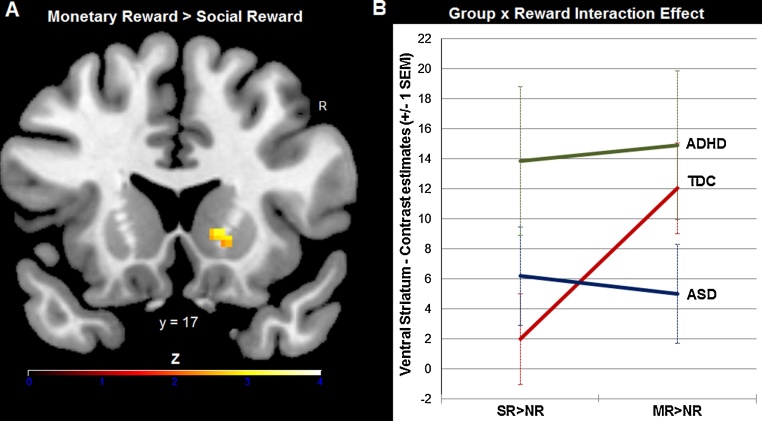
Following up our a priori hypothesis on differential striatal reactivity among the three groups dependent on the reward type at stake, we next performed a ROI analysis on individual β values from the ventral striatum for SR > NR and MR > NR. We found a significant reward by group interaction effect (F(2, 45) = 4.18, p = 0.02, ηp2 = 0.16), related to stronger striatal activation on the right hemisphere for monetary versus social reward in TDC (p = 0.001, Cohen's d = −0.94), which was absent in both clinical groups (ASD: p = 0.71, ADHD: p = 0.74). Follow-up analyses showed that the ASD group had relatively low ventral striatum reactivity for both reward types (ps ≥ 0.1 based on one-sample t tests against zero within the ASD group for SR > NR and MR > NR), while subjects with ADHD responded equally strong to social and monetary reward (ps ≤ 0.019 based on one-sample t tests against zero within the ADHD group for SR > NR and MR > NR). Note, though, that the peak of this interaction effect was located in the caudate head, as part of the ventral striatum (Mawlawi et al., 2001), but not in the nucleus accumbens proper (see Fig. 2).
Fig. 2.
A significant group by reward type interaction effect emerged in the ventral striatum. While healthy controls showed stronger striatal activation to monetary reward than social reward (A), this effect was absent in both clinical groups: the ASD group had low striatum reactivity across both social reward (SR > NR) and monetary reward (MR > NR) conditions, subjects with ADHD responded equally strong to both reward types (B). Z scores in panel A are based on cluster-level correction for multiple comparisons across the whole brain with a mean cluster threshold of Z > 2.3 and a cluster-corrected significance threshold of p ≤ 0.05 using Gaussian random field theory. Color bars indicate Z-statistics. The line graph in panel B depicts mean contrast estimates extracted by group from the ventral striatum for the two high-level contrasts SR > NR and MR > NR. Results shown are for go blocks only.
3.3.3. Shared and disorder-specific abnormalities in neural activation for reward
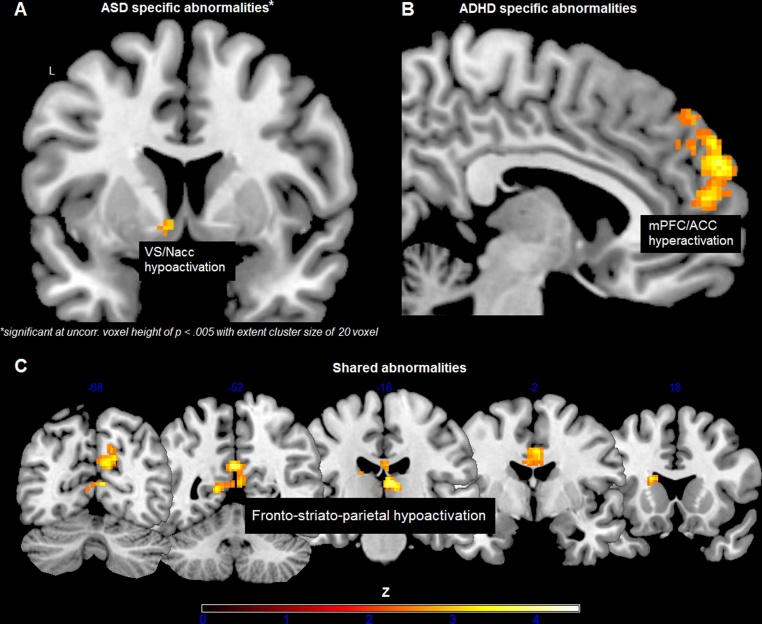
Relative to TDC and ASD, the ADHD group showed enhanced activation in a medial prefrontal cluster during social reward processing, including dorsal anterior cingulate cortex (right: peak MNI = −2, 50, 16; Z = 3.26) and superior frontal gyrus (right: peak MNI = 8, 58, 26; Z = 3.74). For the ASD group, no disorder-specific abnormalities were revealed using random field theory, i.e., FWE, corrections. However, lowering the threshold to an uncorrected voxel height of p < 0.005 with an extent cluster size of at least 20 voxels (according to Scott-Van Zeeland et al., 2010), which still produces desirable balance between Types I and II errors comparable to false discovery rate (Lieberman and Cunningham, 2009), the ASD group, compared to TDC and ADHD, showed diminished activation in ventral striatum in response to monetary reward (left: peak MNI = −8, 6, −6; Z = 3.07). Both clinical groups versus TDC showed hypoactivation in dorsal striatum (left caudate: peak MNI = −18, 18, 16; Z = 3.25), thalamus (right: peak MNI = 8, −20, 10; Z = 3.74), dorsal cingulate (right: peak MNI = 4, 2, 36; Z = 3.86), posterior cingulate (left: peak MNI = −2, −52, 26; Z = 3.86), and precuneus (right: peak MNI = 8, −66, 28; Z = 3.49) when money was at stake (see Fig. 3).
Fig. 3.
Conjunction analyses with strict minimum statistics revealed disorder-specific and shared neural abnormalities during reward processing, including (A) ventral striatum hypoactivation in response to monetary reward in ASD versus TDC and ADHD, (B) medial prefrontal hyperactivation in response to social reward in ADHD versus TDC and ASD, and (C) fronto-striato-parietal hypoactivation in both clinical groups versus TDC when money was at stake, comprising precuneus, posterior cingulate, thalamus, dorsal anterior cingulate and dorsal striatum (i.e., caudate) (from left to right). Z scores in panels B and C are based on cluster-level correction for multiple comparisons across the whole brain with a mean cluster threshold of Z > 2.3 and a cluster-corrected significance threshold of p ≤ 0.05 using Gaussian random field theory. Color bars indicate Z-statistics. Results shown are for go blocks only.
3.3.4. Brain-behavior correlational analyses
In order to investigate the extent to which abnormally activated brain regions in both clinical groups (versus TDC) were associated with clinical symptoms and phenotypic variations, we extracted individual mean contrast estimates from the respective activation clusters (i.e., conjunction analyses) and calculated Pearson correlations between activation magnitudes and the dependent measures, including ADOS-G and ADI-R for ASD only, FBB-HKS for ADHD only, and AQ for the entire sample. Bonferroni corrections were applied to adjust the alpha level for multiple comparisons.
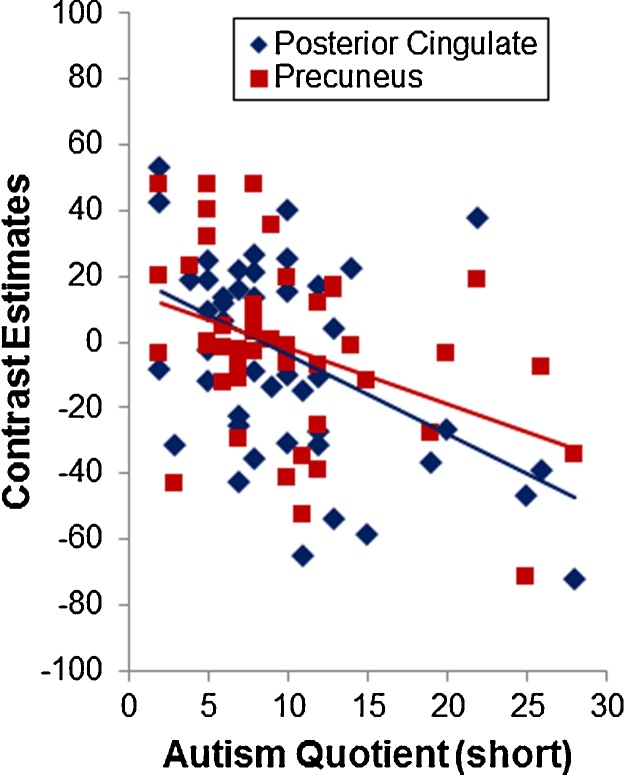
No significant correlations were detected for ASD only or ADHD only. However, considering the whole sample significant negative correlations were observed between the AQ total score and neural responses within posterior cingulate (r = −0.45, p = 0.002) and precuneus (r = −0.40, p = 0.006), suggesting that weaker brain activation within parietal areas was associated with higher autistic traits beyond diagnostic categories (Fig. 4). The correlations remained significant when age, IQ and ADHD severity were controlled for.
Fig. 4.
Brain responses within parietal areas of the reward and salience network was significantly negatively correlated with the Autism-Spectrum Quotient (AQ) total score, suggesting that weaker reactivity of this neural circuit was associated with higher autistic traits across the entire study sample beyond diagnostic boundaries.
3.4. BOLD activation during no-go blocks
Activation differences between the high-level reward versus non-reward contrasts (i.e., R > NR, SR > NR, and MR > NR) across the three study groups as well as group comparisons were found to be non-significant using whole-brain cluster corrections and ROI analyses (e.g., VS).
4. Discussion
In the present fMRI study we compared reward circuitry responses in children with ASD versus children with ADHD relative to healthy controls using a go/no-go incentive delay task with social and monetary reward contingencies. While the two disorder groups showed equal subjective motivation ratings as well as behavioral task performances as controls, our fMRI analyses revealed the expected differential patterns of aberrant reward system reactivity in both clinical groups versus TDC, particularly in ventral striatum. In addition, disorder-specific and shared functional abnormalities during reward processing were found, including (a) medial prefrontal hyperactivation in response to social reward in ADHD relative to TDC and ASD, (b) ventral striatal hypoactivation in response to monetary reward in ASD relative to TDC and ADHD (but only when a less stringent whole-brain correction threshold was considered, and thus needs to be interpreted with caution), and (c) fronto-striato-parietal hypoactivation in both clinical groups relative to TDC when money was at stake, comprising dorsal cingulate, caudate, thalamus, posterior cingulate and precuneus. Interestingly, lower neural reactivity within the parietal circuitry was associated with higher autistic traits across the entire study sample.
The current imaging finding of differential ventral striatum responsivity to monetary versus social reward among the three study groups is consistent with our earlier work on reward responsiveness in youth with and without ASD or ADHD (Kohls et al., 2009a, Kohls et al., 2009b, Kohls et al., 2011, Kohls et al., 2013b, Vloet et al., 2011). For instance, we previously found in typically developing children that monetary reward had a substantially larger beneficial effect on behavioral task performance in a rewarded go/no-go paradigm than social reward (i.e., smiles and friendly faces), suggesting that specific types of social incentives, like those used in the present study, do not have an equally strong reward value as compared to monetary incentives in TDC (Kohls et al., 2009b). This is most likely reflected in greater ventral striatum activity for more salient and preferred rewards (e.g., smile versus €0.20), which has been reported for healthy adults (Spreckelmeyer et al., 2009), but also holds true for healthy children as demonstrated here.
However, in both youth with ASD and ADHD this differential striatum reactivity to monetary versus social reward was absent, but the deviant activation pattern was opposite in the two groups. While individuals with ADHD had equally high striatal activation to monetary and social reward, subjects with ASD displayed low striatum responses to both reward types. Low ventral striatum activation in ASD further emphasizes recent imaging findings of reward circuitry dysfunction in ASD not only in response to social rewards (Delmonte et al., 2012, Scott-Van Zeeland et al., 2010), but also for tangible rewards like money (Dichter et al., 2012c, Dichter et al., 2012b, Kohls et al., 2011, Kohls et al., 2013b, Richey et al., 2012, Schmitz et al., 2008). Taken together, there is evidence for a general reward processing dysfunction in ASD. However, money has strong social connotations and, thus, exerts substantial impact on pro-social behavior (Vohs et al., 2006). In this regard, aberrant ventral striatum reactivity for monetary reward is not necessarily at odds with the core ASD social phenotype (Kohls et al., 2012).
It should be pointed out that we did not find greater malfunctions in the reward circuitry in response to social reward compared to monetary reward in children with ASD (Delmonte et al., 2012, Scott-Van Zeeland et al., 2010). It is beyond the scope of this discussion to speculate upon the diverse subject- and method-related factors that might have contributed to this inconsistency (for a thorough discussion, see Kohls et al., 2012). However, it seems evident that more ecologically valid social reward stimulus sets and paradigms are necessary that are closer to real-life social encounters in order to provide a clearer picture about reward circuitry dysfunction in ASD which would be most consistent with the core clinical phenotype (Gossen et al., 2014, Kohls et al., 2013a).
In stark contrast to ASD, children with ADHD had equally strong ventral striatum activation to both social and monetary reward, which was accompanied by ADHD-specific medial prefrontal overactivation under social reward conditions. This is consistent with earlier findings of greater behavioral sensitivity to social incentives, including social rewards, in children with ADHD (Geurts et al., 2008, Kohls et al., 2009a, Krauel et al., 2007, Matthys et al., 1998). As mentioned above, to the best of our knowledge the current study is the first to investigate reward circuit responsivity to social reward in ADHD. Prior imaging work has almost exclusively applied monetary incentives (but see Wilbertz et al. (2012) who used monetary reward versus performance feedback) with somewhat mixed findings, primarily with regard to the direction of potential striatum dysfunction. Using event-related task designs a number of studies reported diminished striatal responses during monetary reward anticipation (Carmona et al., 2012, Hoogman et al., 2011, Plichta and Scheres, 2014, Scheres et al., 2007, Ströhle et al., 2008), whereas others found enhanced striatum reactivity in response to monetary reward delivery in individuals with ADHD (Paloyelis et al., 2012, Ströhle et al., 2008). While the present study applied a blocked design, which does not allow the reliable disentanglement between brain responses for reward anticipation and consumption and, thus, hampers comparisons with prior studies, the current results corroborate and extent the earlier findings by revealing an atypical pattern of ventral striatum activation when social versus monetary reward were at stake.
The heightened ventral striatum reactivity was complemented by ADHD-specific medial prefrontal overactivation under social reward conditions. In ADHD, stronger medial prefrontal activation, including anterior cingulate and orbitofrontal cortex, in response to reward (i.e., money) has been quite consistently reported before (Bjork et al., 2010, Gatzke-Kopp et al., 2009, Ströhle et al., 2008, Wilbertz et al., 2012) and has been shown to be normalized through MPH administration (Rubia et al., 2009a). Our finding of medial prefrontal overreactivity in response to social reward can be seen in line with Wilbertz and colleagues who reported stronger orbitofrontal activity for non-monetary reward (i.e., checkmark for accurate task performance) in adults with ADHD (Wilbertz et al., 2012).
Both ventral striatum and medial prefrontal cortex have critical roles in reward responsiveness (Haber and Knutson, 2010). While the ventral striatum signals the anticipatory drive for a motivational incentive, the medial prefrontal cortex encodes and acts upon reward values (Berridge et al., 2009). Applied to the ADHD data at hand, the neural overreactivity for social reward may, at least partly, underlie atypical reward responsiveness, particularly in social contexts (Kohls et al., 2009a). On an everyday basis, these strongly valuated and sought social incentives are most likely to disrupt goal-directed, adapted social behavior through impulsive acts and may have serious consequences on establishing and maintaining interpersonal relationships (Nigg and Casey, 2005). It is plausible to assume that such urge for social reward-driven behavioral tendencies may be triggered as a compensatory reaction to frequent personal rejections or/and a lack in self-confidence among youth with ADHD (Becker et al., 2012). However, future research is needed to address this assumption.
Both neurodevelopmental disorders shared functional abnormalities within a fronto-striato-parietal network during monetary reward processing, comprising diminished activation in dorsal cingulate, caudate, thalamus, posterior cingulate and precuneus. This is in line with findings from Christakou et al. who revealed aberrant brain activation in the fronto-striato-parietal circuitry in ASD and ADHD versus TDC for to-be-attended stimuli during a vigilance task (Christakou et al., 2013, Rubia et al., 2009b). Additionally, Di Martino et al. reported overlapping functional connectivity abnormalities in the parietal lobule including precuneus (Di Martino et al., 2013). Given the involvement of the fronto-striato-parietal circuitry in voluntary (i.e., motivational) attention control processes, it appears that both clinical groups are characterized by deficient recruitment of a neural network that most likely mediates the reactivity to salient stimuli, like money, which then enables an individual to act upon such stimuli in a goal-directed way, e.g., approach or avoidance (Seeley et al., 2007). In sum, the current findings further highlight that fronto-striato-parietal aberrations may underlie the shared ASD-ADHD clinical phenotype, including executive and motivation dysfunction; both domains constitute the most consistent cognitive deficits among the two disorders (Rommelse et al., 2011). Interestingly, the degree of neural activation within parietal circuitry was associated with Autism-Spectrum Quotient scores across the entire study sample, suggesting that this circuitry may also be closely related to the manifestation of autistic traits (e.g., social attention etc.) spanning both clinical groups and the normal population.
Noteworthy, the three groups did not differ in any task performance measures and were similarly affected by the different reward contingencies. This indicates that the motivational manipulations were effective and that the detected group differences in brain activation were not confounded by task performance differences (Murphy and Garavan, 2004). The absence of this potential confound underscores the uniqueness of neuroimaging data in unraveling aberrant reward mechanisms in patients with mental disorders. However, this raises the question as to how group differences in reward network activation are meaningful when behavioral differences are nonexistent. Although one can argue that behavioral measures, such as accuracy and reaction time, are non-optimal surrogates of performance that do not properly capture reward processing, it is also plausible to assume that comparable behavior in the presence of different brain activation could reflect the implementation of an alternate, though effective, performance strategy while the underlying neural mechanisms are yet divergent (“behavioral phenocopy”, Church et al., 2010).
Another issue of clinical imaging is that neural activation differences observed between individuals with ASD and TDC or ADHD could be due to differences in neurovascular coupling and/or oxygen consumption that underlie the BOLD signal in fMRI, and thus are artifacts (Reynell and Harris, 2013). Studies have just begun to explore neurophysiological aberrations in ASD that may potentially alter the relationship between neuronal activity and the BOLD response. Thus, it will be important for future research to implement study designs that are able to disentangle neurovascular abnormalities, if existent, from “true” task-related BOLD hyper- or hypoactivation in individuals with ASD relative to comparison groups (Feczko et al., 2012).
The following study limitations should be considered. Given phenotypic and neural heterogeneity within each of the disorder groups, it is likely that reward circuit dysfunction contributes with variable degree to the clinical manifestation of individuals with ASD and ADHD. Overall the sample size was relatively small, which may limit reproducibility of results (Button et al., 2013), but was comparable to other relevant fMRI studies (Christakou et al., 2013, Richey et al., 2012, Rubia et al., 2009b). Thus, any firm conclusions are premature and require larger and more diverse study samples that also include females. The sample composition could also explain the absence of brain-behavior correlations between clinical symptoms and neural reward responsiveness. The ability to reveal robust associations in both disorder groups was likely weakened by the limited range in severity scores on the disorder-specific clinical measures. One further shortcoming relates to the use of MPH medications particularly in ADHD versus ASD. Although medications were discontinued at least 48 h prior to testing, research has shown that chronic MPH application is able to upregulate striatal dopamine turnover (Wang et al., 2013). Therefore, MPH treatment history could have biased the current findings of differential striatum reactivity in ADHD versus the two other study groups. Concerning ASD, several patients scored high on the ADHD-specific symptom measure (i.e., FBB-HKS (Döpfner and Lehmkuhl, 1998)), although none did meet diagnostic criteria for ADHD. This might challenge the ascription of findings specifically to ASD. However, ADHD symptoms are very common among youth with ASD (Rommelse et al., 2011), making our ASD sample representative for this clinical disorder.
Despite these limitations, the present study on reward circuit responsivity in ASD versus ADHD demonstrates unique (i.e., striatal hypoactivation for monetary reward in ASD, and mPFC hyperactivation for social reward in ADHD) as well as shared neural dysfunction (i.e., fronto-striato-parietal hypoactivation for monetary reward in both clinical groups). Although no direct link between brain dysfunction and clinical manifestation could be revealed, based on the available literature and current neurobiological models (Rommelse et al., 2011, Taurines et al., 2012), it is plausible to assume that reward-based motivation deficits may lie at the core of both ASD and ADHD (Dichter et al., 2012a). While disorder-specific dysfunctions likely give rise to the distinct clinical picture of each of the syndromes, shared aberrations may underlie the phenotypic overlap and co-occurrence of both disorders, possibly mediated by pleiotropic genes. The evidence for overlapping neural dysfunction in response to reward emphasizes the need for more systematic research (for instance, as outlined in Rommelse et al., 2011) or in the NIMH Research Domain Criteria (RDoC) Project (Insel et al., 2010) on other common patterns of dysregulated brain function in relation to clinical phenotypes with the ultimate goal to better inform targeted interventions.
Acknowledgments
This study was supported by the German Research Foundation (Deutsche Forschungsgemeinschaft, IRTG 1328). We would like to thank all young volunteers and their families who participated in this study. We are also grateful to two anonymous reviewers for their helpful comments on an earlier version of the manuscript.
Footnotes
Available online 17 August 2014
References
- Achenbach T.M. University of Vermont, Department of Psychiatry; Burlington, VT: 1991. Manual for the Child Behavior Checklist/4-18 and 1991 Profile. [Google Scholar]
- Baron-Cohen S., Wheelwright S., Skinner R., Martin J., Clubley E. The autism-spectrum quotient (AQ): evidence from Asperger syndrome/high-functioning autism, males and females, scientists and mathematicians. J. Autism Dev. Disord. 2001;31(1):5–17. doi: 10.1023/a:1005653411471. [DOI] [PubMed] [Google Scholar]
- Becker S.P., Luebbe A.M., Langberg J.M. Co-occurring mental health problems and peer functioning among youth with attention-deficit/hyperactivity disorder: a review and recommendations for future research. Clin. Child Fam. Psychol. Rev. 2012;15(4):279–302. doi: 10.1007/s10567-012-0122-y. [DOI] [PubMed] [Google Scholar]
- Berridge K.C., Robinson T.E., Aldridge J.W. Dissecting components of reward: “liking”, “wanting”, and learning. Curr. Opin. Pharmacol. 2009;9(1):65–73. doi: 10.1016/j.coph.2008.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjork J.M., Chen G., Smith A.R., Hommer D.W. Incentive-elicited mesolimbic activation and externalizing symptomatology in adolescents. J. Child Psychol. Psychiatry. 2010;51(7):827–837. doi: 10.1111/j.1469-7610.2009.02201.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Button K.S., Ioannidis J.P.A., Mokrysz C., Nosek B.A., Flint J., Robinson E.S.J., Munafò M.R. Power failure: why small sample size undermines the reliability of neuroscience. Nat. Rev. Neurosci. 2013;14(5):365–376. doi: 10.1038/nrn3475. [DOI] [PubMed] [Google Scholar]
- Cairns R.B., Leung M.-C., Gest S.D., Cairns B.D. A brief method for assessing social development: structure, reliability, stability, and developmental validity of the Interpersonal Competence Scale. Behav. Res. Ther. 1995;33(6):725–736. doi: 10.1016/0005-7967(95)00004-h. [DOI] [PubMed] [Google Scholar]
- Carmona S., Hoekzema E., Ramos-Quiroga J.A., Richarte V., Canals C., Bosch R., Vilarroya O. Response inhibition and reward anticipation in medication-naïve adults with attention-deficit/hyperactivity disorder: a within-subject case-control neuroimaging study. Hum. Brain Mapp. 2012;33(10):2350–2361. doi: 10.1002/hbm.21368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cascio C.J., Foss-Feig J.H., Heacock J.L., Newsom C.R., Cowan R.L., Benningfield M.M., Cao A. Response of neural reward regions to food cues in autism spectrum disorders. J. Neurodev. Disord. 2012;4(1):9. doi: 10.1186/1866-1955-4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevallier C., Kohls G., Troiani V., Brodkin E.S., Schultz R.T. The social motivation theory of autism. Trends Cogn. Sci. 2012;16(4):231–239. doi: 10.1016/j.tics.2012.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christakou A., Murphy C.M., Chantiluke K., Cubillo A.I., Smith A.B., Giampietro V., Rubia K. Disorder-specific functional abnormalities during sustained attention in youth with Attention Deficit Hyperactivity Disorder (ADHD) and with autism. Mol. Psychiatry. 2013;18(2):236–244. doi: 10.1038/mp.2011.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church J.A., Petersen S.E., Schlaggar B.L. The “Task B problem” and other considerations in developmental functional neuroimaging. Hum. Brain Mapp. 2010;31(6):852–862. doi: 10.1002/hbm.21036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantino J., Gruber C. Western Psychological Services; Los Angeles, CA: 2005. The Social Responsiveness Scale (SRS) Manual. [Google Scholar]
- Dadds M.R., Hunter K., Hawes D.J., Frost A.D.J., Vassallo S., Bunn P., Masry Y.E. A measure of cognitive and affective empathy in children using parent ratings. Child Psychiatry Hum. Dev. 2008;39(2):111–122. doi: 10.1007/s10578-007-0075-4. [DOI] [PubMed] [Google Scholar]
- Dawson G., Webb S.J., McPartland J. Understanding the nature of face processing impairment in autism: insights from behavioral and electrophysiological studies. Dev. Neuropsychol. 2005;27(3):403–424. doi: 10.1207/s15326942dn2703_6. [DOI] [PubMed] [Google Scholar]
- Delmonte S., Balsters J.H., McGrath J., Fitzgerald J., Brennan S., Fagan A.J., Gallagher L. Social and monetary reward processing in autism spectrum disorders. Mol. Autism. 2012;3(1):7. doi: 10.1186/2040-2392-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demurie E., Roeyers H., Baeyens D., Sonuga-Barke E. Common alterations in sensitivity to type but not amount of reward in ADHD and autism spectrum disorders. J. Child Psychol. Psychiatry. 2011;52(11):1164–1173. doi: 10.1111/j.1469-7610.2010.02374.x. [DOI] [PubMed] [Google Scholar]
- Demurie E., Roeyers H., Baeyens D., Sonuga-Barke E. Domain-general and domain-specific aspects of temporal discounting in children with ADHD and autism spectrum disorders (ASD): a proof of concept study. Res. Dev. Disabil. 2013;34(6):1870–1880. doi: 10.1016/j.ridd.2013.03.011. [DOI] [PubMed] [Google Scholar]
- Di Martino A., Zuo X.-N., Kelly C., Grzadzinski R., Mennes M., Schvarcz A., Milham M.P. Shared and distinct intrinsic functional network centrality in autism and attention-deficit/hyperactivity disorder. Biol. Psychiatry. 2013 doi: 10.1016/j.biopsych.2013.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dichter G.S., Damiano C.A., Allen J.A. Reward circuitry dysfunction in psychiatric and neurodevelopmental disorders and genetic syndromes: animal models and clinical findings. J. Neurodev. Disord. 2012;4(1):19. doi: 10.1186/1866-1955-4-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dichter G.S., Felder J.N., Green S.R., Rittenberg A.M., Sasson N.J., Bodfish J.W. Reward circuitry function in autism spectrum disorders. Soc. Cogn. Affect. Neurosci. 2012;7(2):160–172. doi: 10.1093/scan/nsq095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dichter G.S., Richey J.A., Rittenberg A.M., Sabatino A., Bodfish J.W. Reward circuitry function in autism during face anticipation and outcomes. J. Autism Dev. Disord. 2012;42(2):147–160. doi: 10.1007/s10803-011-1221-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Döpfner M., Lehmkuhl G. Huber Verlag; Bern: 1998. Diagnostik-System für psychische Störungen im Kindes- und Jugendalter nach ICD-10 und DSM-IV. [Google Scholar]
- Feczko E., Miezin F.M., Constantino J.N., Schlaggar B.L., Petersen S.E., Pruett J.R. The hemodynamic response in children with Simplex Autism. Dev. Cogn. Neurosci. 2012;2(4):396–408. doi: 10.1016/j.dcn.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freitag G. An experimental study of the social responsiveness of children with autistic behaviors. J. Exp. Child Psychol. 1970;9(3):436–453. doi: 10.1016/0022-0965(70)90030-5. [DOI] [PubMed] [Google Scholar]
- Garretson H.B., Fein D., Waterhouse L. Sustained attention in children with autism. J. Autism Dev. Disord. 1990;20(1):101–114. doi: 10.1007/BF02206860. [DOI] [PubMed] [Google Scholar]
- Gatzke-Kopp L.M., Beauchaine T.P., Shannon K.E., Chipman J., Fleming A.P., Crowell S.E., Aylward E. Neurological correlates of reward responding in adolescents with and without externalizing behavior disorders. J. Abnorm. Psychol. 2009;118(1):203–213. doi: 10.1037/a0014378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geurts H.M., Luman M., van Meel C.S. What's in a game: the effect of social motivation on interference control in boys with ADHD and autism spectrum disorders. J. Child Psychol. Psychiatry. 2008;49(8):848–857. doi: 10.1111/j.1469-7610.2008.01916.x. [DOI] [PubMed] [Google Scholar]
- Goldstein R.Z., Alia-Klein N., Tomasi D., Zhang L., Cottone L.A., Maloney T., Volkow N.D. Is decreased prefrontal cortical sensitivity to monetary reward associated with impaired motivation and self-control in cocaine addiction? Am. J. Psychiatry. 2007;164(1):43–51. doi: 10.1176/appi.ajp.164.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gossen A., Groppe S.E., Winkler L., Kohls G., Herrington J., Schultz R.T., Spreckelmeyer K.N. Neural evidence for an association between social proficiency and sensitivity to social reward. Soc. Cogn. Affect. Neurosci. 2014;9(5):661–670. doi: 10.1093/scan/nst033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber S.N., Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology. 2010;35(1):4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoogman M., Aarts E., Zwiers M., Slaats-Willemse D., Naber M., Onnink M., Franke B. Nitric oxide synthase genotype modulation of impulsivity and ventral striatal activity in adult ADHD patients and healthy comparison subjects. Am. J. Psychiatry. 2011;168(10):1099–1106. doi: 10.1176/appi.ajp.2011.10101446. [DOI] [PubMed] [Google Scholar]
- Insel T., Cuthbert B., Garvey M., Heinssen R., Pine D.S., Quinn K., Wang P. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Am. J. Psychiatry. 2010;167(7):748–751. doi: 10.1176/appi.ajp.2010.09091379. [DOI] [PubMed] [Google Scholar]
- Kaufman J., Birmaher B., Brent D., Rao U., Flynn C., Moreci P., Ryan N. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J. Am. Acad. Child Adolesc. Psychiatry. 1997;36(7):980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Knutson B., Taylor J., Kaufman M., Peterson R., Glover G. Distributed neural representation of expected value. J. Neurosci. 2005;25(19):4806–4812. doi: 10.1523/JNEUROSCI.0642-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koegel R.L., Koegel L.K., McNerney E.K. Pivotal areas in intervention for autism. J. Clin. Child Psychol. 2001;30(1):19–32. doi: 10.1207/S15374424JCCP3001_4. [DOI] [PubMed] [Google Scholar]
- Kohls G., Chevallier C., Troiani V., Schultz R.T. Social “wanting” dysfunction in autism: neurobiological underpinnings and treatment implications. J. Neurodev. Disord. 2012;4:10. doi: 10.1186/1866-1955-4-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohls G., Herpertz-Dahlmann B., Konrad K. Hyperresponsiveness to social rewards in children and adolescents with attention-deficit/hyperactivity disorder (ADHD) Behav. Brain Funct. 2009;5:20. doi: 10.1186/1744-9081-5-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohls G., Peltzer J., Herpertz-Dahlmann B., Konrad K. Differential effects of social and non-social reward on response inhibition in children and adolescents. Dev. Sci. 2009;12(4):614–625. doi: 10.1111/j.1467-7687.2009.00816.x. [DOI] [PubMed] [Google Scholar]
- Kohls G., Peltzer J., Schulte-Rüther M., Kamp-Becker I., Remschmidt H., Herpertz-Dahlmann B., Konrad K. Atypical brain responses to reward cues in autism as revealed by event-related potentials. J. Autism Dev. Disord. 2011;41(11):1523–1533. doi: 10.1007/s10803-011-1177-1. [DOI] [PubMed] [Google Scholar]
- Kohls G., Perino M.T., Taylor J.M., Madva E.N., Cayless S.J., Troiani V., Schultz R.T. The nucleus accumbens is involved in both the pursuit of social reward and the avoidance of social punishment. Neuropsychologia. 2013;51(11):2062–2069. doi: 10.1016/j.neuropsychologia.2013.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohls G., Schulte-Rüther M., Nehrkorn B., Müller K., Fink G.R., Kamp-Becker I., Konrad K. Reward system dysfunction in autism spectrum disorders. Soc. Cogn. Affect. Neurosci. 2013;8(5):565–572. doi: 10.1093/scan/nss033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konrad K., Gauggel S., Manz A., Schöll M. Lack of inhibition: a motivational deficit in children with attention deficit/hyperactivity disorder and children with traumatic brain injury. Child Neuropsychol. 2000;6(4):286–296. doi: 10.1076/chin.6.4.286.3145. [DOI] [PubMed] [Google Scholar]
- Krauel K., Duzel E., Hinrichs H., Santel S., Rellum T., Baving L. Impact of emotional salience on episodic memory in attention-deficit/hyperactivity disorder: a functional magnetic resonance imaging study. Biol. Psychiatry. 2007;61(12):1370–1379. doi: 10.1016/j.biopsych.2006.08.051. [DOI] [PubMed] [Google Scholar]
- Lieberman M.D., Cunningham W.A. Type I and Type II error concerns in fMRI research: re-balancing the scale. Soc. Cogn. Affect. Neurosci. 2009;4(4):423–428. doi: 10.1093/scan/nsp052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C., Risi S., Lambrecht L., Cook E.H., Jr., Leventhal B.L., DiLavore P.C., Rutter M. The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. J. Autism Dev. Disord. 2000;30(3):205–223. [PubMed] [Google Scholar]
- Lord C., Rutter M., Le Couteur A. Autism Diagnostic Interview-Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J. Autism Dev. Disord. 1994;24(5):659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Luman M., Oosterlaan J., Sergeant J.A. The impact of reinforcement contingencies on AD/HD: a review and theoretical appraisal. Clin. Psychol. Rev. 2005;25(2):183–213. doi: 10.1016/j.cpr.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Luman M., Tripp G., Scheres A. Identifying the neurobiology of altered reinforcement sensitivity in ADHD: a review and research agenda. Neurosci. Biobehav. Rev. 2010;34(5):744–754. doi: 10.1016/j.neubiorev.2009.11.021. [DOI] [PubMed] [Google Scholar]
- Margolies P.J. Behavioral approaches to the treatment of early infantile autism: a review. Psychol. Bull. 1977;84(2):249–264. [PubMed] [Google Scholar]
- Matson J.L., Benavidez D.A., Compton L.S., Paclawskyj T., Baglio C. Behavioral treatment of autistic persons: a review of research from 1980 to the present. Res. Dev. Disabil. 1996;17(6):433–465. doi: 10.1016/s0891-4222(96)00030-3. [DOI] [PubMed] [Google Scholar]
- Matthys W., van Goozen S.H., de Vries H., Cohen-Kettenis P.T., van Engeland H. The dominance of behavioural activation over behavioural inhibition in conduct disordered boys with or without attention deficit hyperactivity disorder. J. Child Psychol. Psychiatry. 1998;39(5):643–651. [PubMed] [Google Scholar]
- Mawlawi O., Martinez D., Slifstein M., Broft A., Chatterjee R., Hwang D.-R., Laruelle M. Imaging human mesolimbic dopamine transmission with positron emission tomography: I. Accuracy and precision of D2 receptor parameter measurements in ventral striatum. J. Cereb. Blood Flow Metab. 2001;21(9):1034–1057. doi: 10.1097/00004647-200109000-00002. [DOI] [PubMed] [Google Scholar]
- Mehrabian A. Analysis of affiliation-related traits in terms of the PAD Temperament Model. J. Psychol. 1997;131(1):101–117. doi: 10.1080/00223989709603508. [DOI] [PubMed] [Google Scholar]
- Mundy P. Joint attention and social-emotional approach behavior in children with autism. Dev. Psychopathol. 1995;7(01):63–82. [Google Scholar]
- Murphy K., Garavan H. Artifactual fMRI group and condition differences driven by performance confounds. Neuroimage. 2004;21(1):219–228. doi: 10.1016/j.neuroimage.2003.09.016. [DOI] [PubMed] [Google Scholar]
- Nichols T., Brett M., Andersson J., Wager T., Poline J.-B. Valid conjunction inference with the minimum statistic. Neuroimage. 2005;25(3):653–660. doi: 10.1016/j.neuroimage.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Nigg J.T., Casey B.J. An integrative theory of attention-deficit/hyperactivity disorder based on the cognitive and affective neurosciences. Dev. Psychopathol. 2005;17(3):785–806. doi: 10.1017/S0954579405050376. [DOI] [PubMed] [Google Scholar]
- Paloyelis Y., Mehta M.A., Faraone S.V., Asherson P., Kuntsi J. Striatal sensitivity during reward processing in attention-deficit/hyperactivity disorder. J. Am. Acad. Child Adolesc. Psychiatry. 2012;51(7) doi: 10.1016/j.jaac.2012.05.006. 722–732.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pankert A., Pankert K., Herpertz-Dahlmann B., Konrad K., Kohls G. Responsivity to familiar versus unfamiliar social reward in children with autism. J. Neural Transm. (Vienna, Aust.: 1996) 2014 doi: 10.1007/s00702-014-1210-6. [DOI] [PubMed] [Google Scholar]
- Plichta M.M., Scheres A. Ventral–striatal responsiveness during reward anticipation in ADHD and its relation to trait impulsivity in the healthy population: a meta-analytic review of the fMRI literature. Neurosci. Biobehav. Rev. 2014;38:125–134. doi: 10.1016/j.neubiorev.2013.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynell C., Harris J.J. The BOLD signal and neurovascular coupling in autism. Dev. Cogn. Neurosci. 2013;6:72–79. doi: 10.1016/j.dcn.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richey J.A., Rittenberg A., Hughes L., Damiano C.A., Sabatino A., Miller S., Dichter G.S. Common and distinct neural features of social and nonsocial reward processing in autism and social anxiety disorder. Soc. Cogn. Affect. Neurosci. 2012 doi: 10.1093/scan/nss146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rommelse N.N.J., Geurts H.M., Franke B., Buitelaar J.K., Hartman C.A. A review on cognitive and brain endophenotypes that may be common in autism spectrum disorder and attention-deficit/hyperactivity disorder and facilitate the search for pleiotropic genes. Neurosci. Biobehav. Rev. 2011;35(6):1363–1396. doi: 10.1016/j.neubiorev.2011.02.015. [DOI] [PubMed] [Google Scholar]
- Rubia K., Halari R., Cubillo A., Mohammad A.-M., Brammer M., Taylor E. Methylphenidate normalises activation and functional connectivity deficits in attention and motivation networks in medication-naïve children with ADHD during a rewarded continuous performance task. Neuropharmacology. 2009;57(7–8):640–652. doi: 10.1016/j.neuropharm.2009.08.013. [DOI] [PubMed] [Google Scholar]
- Rubia K., Smith A.B., Halari R., Matsukura F., Mohammad M., Taylor E., Brammer M.J. Disorder-specific dissociation of orbitofrontal dysfunction in boys with pure conduct disorder during reward and ventrolateral prefrontal dysfunction in boys with pure ADHD during sustained attention. Am. J. Psychiatry. 2009;166(1):83–94. doi: 10.1176/appi.ajp.2008.08020212. [DOI] [PubMed] [Google Scholar]
- Rutter M., Bailey A., Lord C. Western Psychological Services; Los Angeles, CA: 2003. The Social Communication Questionnaire. [Google Scholar]
- Scheres A., Milham M.P., Knutson B., Castellanos F.X. Ventral striatal hyporesponsiveness during reward anticipation in attention-deficit/hyperactivity disorder. Biol. Psychiatry. 2007;61(5):720–724. doi: 10.1016/j.biopsych.2006.04.042. [DOI] [PubMed] [Google Scholar]
- Schmitz N., Rubia K., van Amelsvoort T., Daly E., Smith A., Murphy D.G.M. Neural correlates of reward in autism. Br. J. Psychiatry. 2008;192(1):19–24. doi: 10.1192/bjp.bp.107.036921. [DOI] [PubMed] [Google Scholar]
- Schultz R.T. Developmental deficits in social perception in autism: the role of the amygdala and fusiform face area. Int. J. Dev. Neurosci. 2005;23(2–3):125–141. doi: 10.1016/j.ijdevneu.2004.12.012. [DOI] [PubMed] [Google Scholar]
- Schultz W., Dayan P., Montague P.R. A neural substrate of prediction and reward. Science. 1997;275(5306):1593–1599. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- Scott-Van Zeeland A.A., Dapretto M., Ghahremani D.G., Poldrack R.A., Bookheimer S.Y. Reward processing in autism. Autism Res. 2010;3(2):53–67. doi: 10.1002/aur.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley W.W., Menon V., Schatzberg A.F., Keller J., Glover G.H., Kenna H., Greicius M.D. Dissociable intrinsic connectivity networks for salience processing and executive control. J. Neurosci. 2007;27(9):2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonuga-Barke E.J.S. Editorial: ADHD as a reinforcement disorder – moving from general effects to identifying (six) specific models to test. J. Child Psychol. Psychiatry. 2011;52(9):917–918. doi: 10.1111/j.1469-7610.2011.02444.x. [DOI] [PubMed] [Google Scholar]
- Spreckelmeyer K.N., Krach S., Kohls G., Rademacher L., Irmak A., Konrad K., Gründer G. Anticipation of monetary and social reward differently activates mesolimbic brain structures in men and women. Soc. Cogn. Affect. Neurosci. 2009;4(2):158–165. doi: 10.1093/scan/nsn051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ströhle A., Stoy M., Wrase J., Schwarzer S., Schlagenhauf F., Huss M., Heinz A. Reward anticipation and outcomes in adult males with attention-deficit/hyperactivity disorder. Neuroimage. 2008;39(3):966–972. doi: 10.1016/j.neuroimage.2007.09.044. [DOI] [PubMed] [Google Scholar]
- Taurines R., Schwenck C., Westerwald E., Sachse M., Siniatchkin M., Freitag C. ADHD and autism: differential diagnosis or overlapping traits? A selective review. Atten. Deficit Hyperact. Disord. 2012;4(3):115–139. doi: 10.1007/s12402-012-0086-2. [DOI] [PubMed] [Google Scholar]
- Thut G., Schultz W., Roelcke U., Nienhusmeier M., Missimer J., Maguire R.P., Leenders K.L. Activation of the human brain by monetary reward. Neuroreport. 1997;8(5):1225–1228. doi: 10.1097/00001756-199703240-00033. [DOI] [PubMed] [Google Scholar]
- Vloet T.D., Konrad K., Herpertz-Dahlmann B., Kohls G. The effect of social and monetary reward on inhibitory control in boys with hyperkinetic conduct disorder. Z. Kinder Jugendpsychiatr. Psychother. 2011;39(5):341–349. doi: 10.1024/1422-4917/a000127. [DOI] [PubMed] [Google Scholar]
- Vohs K.D., Mead N.L., Goode M.R. The psychological consequences of money. Science. 2006;314(5802):1154–1156. doi: 10.1126/science.1132491. [DOI] [PubMed] [Google Scholar]
- Volkow N.D., Wang G.-J., Newcorn J.H., Kollins S.H., Wigal T.L., Telang F., Swanson J.M. Motivation deficit in ADHD is associated with dysfunction of the dopamine reward pathway. Mol. Psychiatry. 2011;16(11):1147–1154. doi: 10.1038/mp.2010.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G.-J., Volkow N.D., Wigal T., Kollins S.H., Newcorn J.H., Telang F., Swanson J.M. Long-term stimulant treatment affects brain dopamine transporter level in patients with attention deficit hyperactive disorder. PLoS ONE. 2013;8(5):e63023. doi: 10.1371/journal.pone.0063023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilbertz G., Tebartz van Elst L., Delgado M.R., Maier S., Feige B., Philipsen A., Blechert J. Orbitofrontal reward sensitivity and impulsivity in adult attention deficit hyperactivity disorder. Neuroimage. 2012;60(1):353–361. doi: 10.1016/j.neuroimage.2011.12.011. [DOI] [PubMed] [Google Scholar]
- Worsley K.J., Evans A.C., Marrett S., Neelin P. A three-dimensional statistical analysis for CBF activation studies in human brain. J. Cereb. Blood Flow Metab. 1992;12(6):900–918. doi: 10.1038/jcbfm.1992.127. [DOI] [PubMed] [Google Scholar]