Abstract
Mechanistic understanding of atrial fibrillation (AF) pathophysiology and the complex bidirectional relationship with thromboembolic risk remains limited. Oral anticoagulation is a mainstay of AF management. An emerging concept is that anticoagulants may themselves have potential pleiotropic disease-modifying effects. We here review the available evidence for hemostasis-independent actions of the oral anticoagulants on electrical and structural remodeling, and the inflammatory component of the vulnerable substrate.
Keywords: Atrial fibrillation, anticoagulants, stroke, protease-activated receptors, calcium, thrombin
1. Arrhythmia and coagulation: a two-way road
Atrial fibrillation (AF) is the most common arrhythmia, with an estimated lifetime risk of up to 26% [1, 2]. A major burden associated with AF is the increased risk of thromboembolism and ischemic stroke. Mechanistic understanding of AF pathophysiology, consisting of structural, electrical, calcium-handling and neuronal remodeling, and development of a procoagulant state, remains inadequate. Because of this limitation, mechanism-based treatment options to prevent or ideally reverse atrial cardiomyopathy and the associated thromboembolic propensity are an unmet need in modern cardiology. This concept and the hopeful perspective that greater mechanistic insight will also bring advances in AF therapy, have been comprehensively discussed previously [3–6].
Current AF treatment encompasses antiarrhythmic pharmacotherapy or ablation. Recent studies suggest that the two avenues provide comparable benefit, with ablation perhaps a nose ahead [7]. However, this invasive technique is completely impractical as an approach to treat millions of afflicted patients. In addition, catheter ablation does not cure AF at the molecular and cellular level. Broad-scale therapy therefore continues to rely on currently available antiarrhythmics, which have their own risks and limitations. New emerging means of rhythm control have been reviewed recently [7]. A further pillar of AF management is anticoagulation. AF is considered a pro-coagulant state [8], with embolic stroke representing the predominant cause of morbidity and mortality in afflicted patients, particularly those with coronary artery disease (CAD). The relationship between AF and thromboembolic risk, however, appears to be much more complex than can be explained with Virchow`s triad of impaired hemostasis, vessel wall dysfunction and hypercoagulation and the phenomenon of blood stasis, leading to local atrial thrombosis. There appears to be a temporal dissociation between atrial arrhythmias and thrombosis, as highlighted by the IMPACT study [9], with abnormal platelet activation not consistently observed in patients with AF. In addition, AF per se can impact on platelet procoagulant phenotype [10], while coagulation itself is associated with increased risk of arrhythmia: in a large prospective study with a follow-up time of more than 16 years, a strong and independent relation was shown between various coagulation markers and new-onset AF [11]. Finally, atrial cardiomyopathy may also cause stroke independently of AF [12], therefore strategies targeting the atrium itself are likely to be beneficial for stroke prevention. Overall, the emerging picture supports a concept of a bidirectional mechanistic highway between AF and stroke, underscoring the critical need for appropriate anticoagulant management in patients with AF.
2. Direct oral anticoagulants
The vitamin K-dependent coumarin-derivative warfarin, and in numerous countries the related compound phenprocoumon, were for a long time the pillar of oral anticoagulant therapy in AF. Their main mechanism of action is inhibition of hepatic synthesis of the coagulant factors FII (thrombin), FVII, FX and FIX. The relatively narrow therapeutic range, the strict requirement for monitoring and high susceptibility for pharmacokinetic interactions with numerous drugs and food are only some of the factors that drove the search for improved anticoagulant agents. The past decade or so has brought forth a new group of oral therapeutic agents which directly inhibit activated thrombin and FXa, and which are increasingly preferred over the coumarin-derivatives. Besides a more controlled anticoagulation, the newer agents also possess favorable effects on fibrin clot formation and fibrinolysis [13, 14]. Currently available DOAC comprise the so-called xabans (rivaroxaban, apixaban, edoxaban) which target FXa, and the gatrans (to date only dabigatran) which inhibit thrombin. In Europe, these agents are also gaining increasing relevance in reducing thromboembolic risk in patients undergoing electrical and pharmacological cardioversion [15, 16]. Excellent overviews of DOAC safety, efficacy and use in patients with cardiac arrhythmias have recently been provided [17–20].
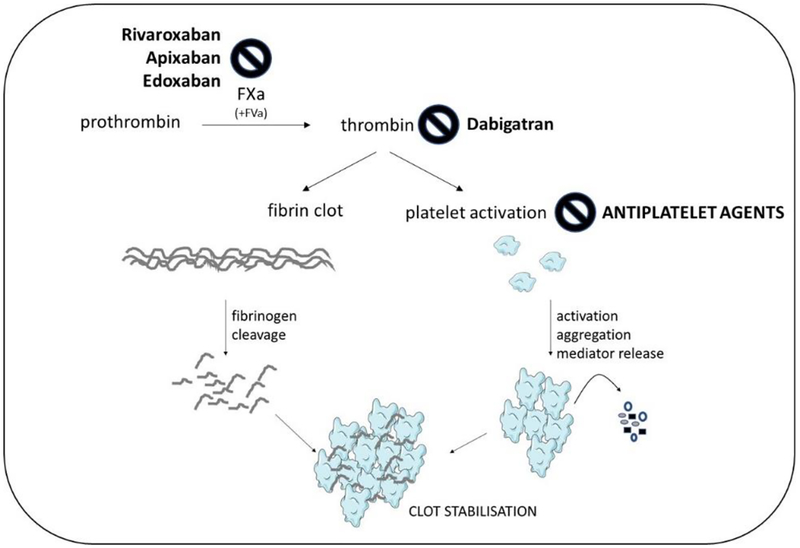
FXa and thrombin are central to the common pathway of coagulation. In a final step of the coagulation cascade (Figure 1), the prothrombinase complex comprising FXa and FVa mediates activation of prothrombin to thrombin. Thrombin is both a potent platelet activator and responsible for the cleavage of fibrinogen to fibrin, thereby contributing to both initial platelet plug formation, and fibrin clot stabilization.
Fig. 1: DOAC inhibition sites.
Coagulant pathways converge in a common step culminating in the FXa-mediated proteolysis of prothrombin to active thrombin. Thrombin potently activates platelets and cleaves fibrinogen to fibrin, leading to clot stabilization. Classic antiplatelets agents prevent secondary platelet activation. The DOAC either inhibit FXa enzymatic activity and hence thrombin activation, or directly inhibit thrombin. The vitamin K-dependent oral anticoagulants like warfarin by contrast suppress block coagulant activity indirectly by preventing synthesis of the precurser factors FII (thrombin), FVII, FIX and FX.
Given the prime position of thrombin and FXa in hemostasis and thrombosis, DOAC can be seen as the best available option for stroke prevention in patients with AF. Recently, the concept of a bidirectionality between coagulation and AF is gaining interest, with AF promoting a hypercoagulant state on the one hand, and an altered hemostatic balance on the other hand supporting AF development and progression. Increased thrombin levels may also be a culprit in ventricular arrhythmias, the prime cause of sudden cardiac death. Patients with myocardial ischemia (MI) also exhibiting ventricular fibrillation show elevated markers of thrombin generation during the acute phase of MI [21]. This review aims to give an overview of experimental and clinical evidence for the notion that DOAC may provide therapeutic benefits beyond thromboprophylaxis, by preventing cardiac arrhythmogenesis and the progression to persistent arrhythmia forms.
3. Pleiotropic cellular actions of thrombin and FXa
The idea that AF potentiates blood coagulation has been fixed for decades, but the molecular mechanisms of activated blood coagulation on atrial remodeling and the progression of AF are not fully understood. The causal role of a pro-coagulant state in AF development and the possible efficacy of anticoagulant drugs on the evolution of AF were elegantly demonstrated in a recent experimental study [22]. Transgenic mice with a pro-coagulant phenotype (TMpro/pro) exhibited augmented AF susceptibility and an increase in AF duration in response to pacing, while in goats with pacing-induced sustained AF, FXa inhibition abrogated AF substrate complexity, suggesting potential antiarrhythmic effects of anticoagulant drugs. It is important to note that the apparent pro-arrhythmic effects of enhanced coagulation, and conversely the anti-arrhythmic effects of FXa inhibition, were not attributable to hemostatic modulation, but rather to alterations in signaling responses elicited at the cellular level by the coagulant factors themselves.
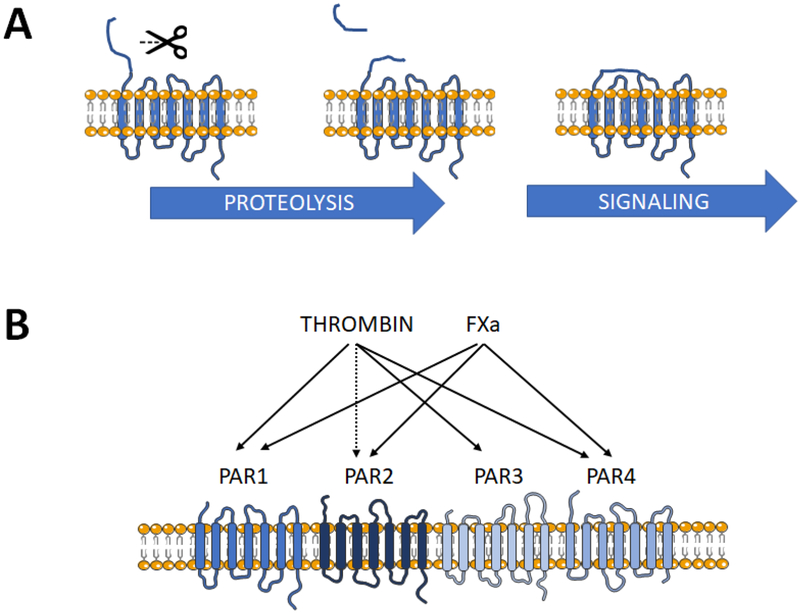
Thrombin and FXa are both serine proteases, and as such are able to proteolytically cleave a number of substrates beyond their classical hemostatic targets. One such group of substrates is the protease-activated receptor (PAR) family, of which four members PAR1–4 have been identified to date (Figure 2). PAR1 is the prototypic receptor, first described in human platelets, but since then shown to be almost ubiquitously expressed. PAR1 is responsible for thrombin- and FXa-driven cellular growth and differentiation, inflammatory signaling, migration and chemotaxis [23–26], as well as generation of reactive oxygen species [27, 28], themselves are critical factors in arrhythmogenesis [29]. Subsequently PAR2, PAR3 and PAR4 were discovered, exhibiting distinct distribution, function and regulation. All four PAR are G protein-coupled receptors, activated by proteolytic cleavage of the extracellular domain to generate a new N-terminus, which binds to and auto-activates the corresponding receptor. Short peptides mimicking the N-terminal tethered ligand motif can activate individual PAR independent of cleavage and with variable specificity. PAR1 represents the predominant receptor mediating the cellular actions of thrombin and FXa, while PAR2 is preferentially activated by FXa/FVII and tryptic enzymes, although activation by supraphysiological concentrations of thrombin has been reported [30]. PAR3 responds to thrombin and is generally seen as a co-factor for other PAR, not able to signal independently because of a shortened cytoplasmic domain. Expression of PAR4, which responds to thrombin, FXa and a range of inflammatory cell-derived proteases, is generally low to non-detectable in non-platelet cells under physiological conditions, but is rapidly and adaptively upregulated in settings of thromboinflammatory stress [31].
Fig. 2. Protease-activated receptors (PAR).
A) Simplified scheme depicting PAR activation. The N-terminal domain of the receptor is proteolytically cleaved at a specific recognition motif to generate a new N-terminus. The “tethered ligand” domain binds to and auto-activates the receptor, initiating G-protein-dependent signaling (possibly not in the case of PAR3). B) The relative selectivity of PAR for activation by thrombin and FXa. PAR1 is the predominant receptor for both coagulant proteases, PAR2 is potently activated by FXa and possibly by supraphysiological thrombin levels. PAR3 is cleaved by thrombin. PAR4 responds to thrombin (with 100-fold lower affinity than for PAR1) and modestly to FXa.
PAR pharmacology is quite complex, with individual PAR able to homo- and/or heterodimerize with a variety of other receptor types, displaying biased and non-canonical signaling properties, distinct activation and inactivation kinetics, and different reactivity towards a range of coagulant and inflammatory protease agonists [23, 24]. Thus, the net pharmacological effects of DOAC may differ at the cellular level, even if comparable degrees of anticoagulation are achieved in the blood.
4. Potential antiarrhythmic actions independent of hemostatic effects
The thrombin/FXa-receptor PAR1 is abundantly expressed in the heart, and mounting evidence supports its role in pro-arrhythmic electrical and structural remodeling. Mechanistic information on how thrombin and PAR1 may support cardiac arrhythmias was actually obtained 20 to 30 years ago. These early studies in isolated rat ventricular cardiomyocytes showed that thrombin elevates intracellular inositol trisphosphate (IP3) levels and raises diastolic and systolic Ca2+ levels, thereby promoting early afterdepolarizations (EADs) and increasing beating rate [32, 33].
Thrombin was also shown to increase the beating rate of depolarized canine Purkinje fibers, without affecting fibers held at normal maximal diastolic potential, and to prolong the action potential (AP) duration (APD) in fibers driven at a constant cycle length. This latter action was sensitive to inhibition with nisoldipine, implicating L-type calcium channels in the thrombin response [32]. APD of ventricular papillary muscle from guinea pigs was also prolonged with thrombin, although this effect was sensitive to tetrodotoxin, suggesting involvement of voltage-gated Na+ channels [34]. Follow-up studies with selective PAR-activating peptides demonstrated that the thrombin-induced changes in cardiomyocyte Ca2+ fluxes and spontaneous beating were predominantly mediated through PAR1 [35, 36]. Accordingly, the PAR1-activating peptide TRAP was found to stimulate phosphoinositide hydrolysis and to increase the beating rate of spontaneously contracting ventricular cardiomyocytes to a similar extent as thrombin [36]. TRAP was also reported to raise cytosolic Ca2+ in field-stimulated ventricular cardiomyocytes, but this effect appeared to be independent of IP3 accumulation, protein kinase C stimulation, L-type Ca2+ channel activation or Ca2+ influx. However, the TRAP-mediated increase of cytosolic Ca2+ was prevented by depletion of sarcoplasmic reticulum (SR) Ca2+ stores with caffeine, suggesting a potential involvement of type-2 ryanodine receptor channel activation by thrombin [37].
Subsequent studies addressed the role of thrombin and its receptor for the development of ventricular arrhythmias in isolated rat hearts subjected to acute myocardial infarction (AMI). In such systems, local application of thrombin or TRAP caused ventricular tachycardia and fibrillation, while the thrombin inhibitor hirudin attenuated these arrhythmias, implicating local thrombin formation in AMI-associated arrhythmias. Pretreatment of rats with glibenclamide abrogated the response to thrombin and TRAP increased a glibenclamide-sensitive K+ current (IK,ATP) in isolated rat ventricular cardiomyocytes [38, 39]. More recently, the PAR1-selective antagonist SCH79797 also reduced the number of premature contractions, as well as the prevalence and duration of ventricular tachycardia and fibrillation in this model, further validating the involvement of IK,ATP, in part via modulation of nitric oxide synthase activity [40].
Multiple pro-arrhythmogenic signaling mediators are modulated by thrombin and hence represent putative targets for coagulation-independent inhibition by DOAC. Lysophosphatidylcholine (LPC) is a pro-arrhythmic lipid mediator [41] generated by phospholipase A2 (PLA2). LPC accumulation is strongly implicated in arrhythmogenesis upon AMI, and in isolated ventricular cardiomyocytes, LPC formation is triggered by thrombin via PAR1 [42–44]. There is no report to date showing whether LPC formation is affected by anticoagulant application. The same holds for the plasmalemmal Na+-H+ exchanger NHE-1, the expression of which is significantly augmented in AF and severe HF [45]. Enhanced NHE-1 causes Ca2+ overload and contributes to arrhythmia susceptibility after MI, and NHE-1 inhibition prevents ventricular fibrillation in this context [46, 47]. Thrombin stimulates NHE-1 activity in isolated cardiomyocytes [48], but if this increase is prevented by DOAC treatment remains to be examined.
The majority of work examining the pro-arrhythmogenic effects of thrombin and PAR was performed in ventricular cardiomyocytes or whole hearts. However, expression of pro-thrombin, thrombin and PAR-1 is higher in human atrium than in human ventricle, and their levels are upregulated in atria from patients with AF [49]. This makes it likely that the local thrombin system is not just a bystander in AF pathophysiology. In support of this, an elegant study in freshly isolated human atrial cardiomyocytes subjected to whole-cell patch-clamp, showed an augmented late (persistent) Na+ current in response to thrombin and PAR1 activation [34].
Such observations raise the possibility that DOAC, by inhibiting thrombin- and/or FXa-triggered PAR activation and hence independently of their anticoagulant actions, may directly modify atrial electrophysiological properties. This capacity may set the DOAC apart from warfarin, which does not inhibit the coagulant factors themselves, but rather their biosynthesis. Rivaroxaban directly abbreviates left atrial APD and increases both L-type Ca2+-current and ultra-rapid delayed rectifier K+ current in isolated left atrial cardiomyocytes, without affecting transient outward K+ current [50]. Again, the electrophysiological response to rivaroxaban in the absence of exogenously added coagulant strongly supports the idea of a local DOAC-sensitive system able to generate active FXa and/or thrombin. Alternatively, rivaroxaban and perhaps other DOAC may exert direct pleiotropic effects fully independent of thrombin and FXa inhibition. Such observations have not been reported to date, but would of course be highly relevant in the context of tailored therapy.
What is the significance of warfarin in this context? Meta-analysis of the specific causes of death in anticoagulated AF patients across the four landmark trials RE-LY, ROCKET AF ARISTOTLE and ENGAGE AF-TIMI 48, highlighted cardiac-related deaths as the major contributor (46%), while only approximately 10% of deaths were attributable to stroke and bleeding combined [51]. Expectedly, bleeding-related mortality was higher in patients on warfarin, but deaths due to sudden death, dysrhythmia, MI or heart failure (HF) was actually comparable between DOAC and warfarin groups. A coagulation-independent influence on cardiac function may therefore be common to all agents that somehow target PAR-activating proteases, either directly or indirectly.
One recently published study provided a direct comparison of the acute electrophysiological properties of apixaban and the vitamin K-dependent anticoagulants warfarin and fluindione, in rabbit pulmonary veins (PV) [52]. Focal ectopic (triggered) activity originating from the PV sleeves was considered as an important source of triggers and perpetuators for the development of AF. In this model, all anticoagulants exerted significant electrophysiological effects. Notably apixaban prolonged APD and decreased the occurrence of both late-phase 3 EADs and delayed afterdepolarizations (DADs). These observations are consistent with a net anti-arrhythmic profile, attributable largely to PAR1-mediated changes in atrial electrical properties and Ca2+ handling. By contrast, warfarin abbreviated the effective atrial refractive period and increased the frequency of late phase 3 EADs and DADs, unmasking a pro-arrhythmic potential of warfarin [52]. The apixaban-stimulated APD prolongation is at odds with the APD-abbreviating effects of rivaroxaban in isolated human atrial cardiomyocytes [50], pointing to the possibility that the effects of DOACs might differ in the remodeled and non-remodeled atrium or alternatively the different anticoagulants may distinctly affect cardiac electrophysiology. Another study in rabbit PVs for example showed a slowing in spontaneous PV firing from dabigatran-treated rabbits, while thrombin and blood clot solution from untreated rabbits reduced spontaneous beating rates, induced DADs and increased ectopic firing [53]. Clearly further systematic investigation is required to fully clarify if DOAC are truly “anti-arrhythmic,” but the stage for such coagulation-independent benefits is certainly set.
At this point it must be mentioned that heparins are likely to modify cardiac electrophysiology through distinct mechanisms. Heparin, and heparin-derived oligosaccharides such as enoxaparin in particular, were recently hailed as a potential new class of antiarrhythmic agents, preventing arrhythmia development in isolated atria ex vivo and in vivo [54, 55]. These actions are presumably independent of coagulation factor inhibition. Heparin is a known inhibitor of IP3, which triggers Ca2+-dependent positive inotropic and arrhythmogenic effects. Accordingly, heparin opposes the ability of IP3 to increase Ca2+ spark frequency, decreases spark amplitude, and lower Ca2+ load of the sarcoplasmic reticulum (SR) in permeabilized atrial and ventricular myocytes [56–58]. Heparin also interacts directly with dihydropyridine-sensitive L-type Ca2+ channels to decrease cardiomyocyte Ca2+ transient amplitude and cellular contractility [59, 60], and stimulates NCX thereby reducing Na+ loading of ventricular cardiomyocytes [61]. A physical interaction with phospholamban also allows heparin to directly stimulate SERCA activity [62]. The antiarrhythmic effect of heparin is already evident in the developing heart. In isolated perfused embryonic mouse hearts, heparin increased AP amplitude and maximal rate of increase, reduced frequency and duration of spontaneous beats, and increased electrical excitability and conduction velocity of atria and ventricles. Atrio-ventricular latency was attenuated, while the magnitude of atrial and ventricular contractions was improved [63].
5. Inhibition of the fibro-inflammatory substrate supporting AF maintenance
Fibrosis and inflammation are integral components of atrial structural remodeling and AF pathophysiology (reviewed in [64] and [65]). Matrix metalloproteinases (MMP) play an essential role in this regard. Activated MMP-9 in particularly is thought to be both cause and biomarker of fibrotic remodeling predisposing to arrhythmias [66–69]. Thrombin and FXa both regulate MMP-9 expression and activity [70–75] while DOAC treatment reduces MMP-9 levels [76–78], which may be one means by which DOAC could limit the structural changes that support AF maintenance. Fibrotically remodeled atrial tissue is characterized by excess collagen that compromises impulse propagation that supports arrhythmia development [79–81]. In atria obtained at autopsy, thrombin and PAR1 are found in close association with fibrotic and inflammatory markers, particularly in tissue from patients with AF [49]. Mounting evidence indicates that DOAC treatment may have a beneficial impact on structural remodeling, independent of anticoagulatory effects. Thrombin, via PAR1 cleavage, induces effector kinase phosphorylation and myofibroblast differentiation, proliferation, collagen synthesis and secretion of monocyte chemotactic substances in primary atrial fibroblasts. This pro-fibrotic and pro-inflammatory spectrum is blunted in vitro by direct addition of dabigatran [82], consistent with a direct cellular site of action of DOAC independently of hemostatic processes. Similar findings were reported in adult rat atrial fibroblasts [22]. Presumably the same will hold true in vivo, since inhibition of FXa-mediated thrombin generation with the low-molecular weight heparin nadroparin reduced atrial cell–cell distances and the total number of αSMA-positive myofibroblasts in goats with AF [22].
Evidently counteracting thrombin activity in vivo results in anti-fibrotic actions at the cellular level in the heart, raising the question of how warfarin fares in this regard. Warfarin prevents the synthesis of not only thrombin and FX, but also FVII and FIX. Part of the benefit might therefore stem from inhibiting FIX, which has been associated with focal ventricular fibrosis. Accordingly, transgenic mice overexpressing human factor IX die at much younger ages [83]. Conversely, low levels of FVII leads to progressive fibrotic and inflammatory cardiomyopathy in transgenic mice. This is seen from an early age, together with compromised ventricular diastolic and systolic function [84]. The authors suggested intracardiac bleeding as a likely triggering event. How much of the subsequent fibro-inflammatory dysfunction is attributable to the tissue damage and how much to local PAR activation has not been dissected.
There is some evidence that warfarin reduces systemic inflammatory burden, with significant reductions in C reactive protein and interleukin (IL)-6 tightly coupled to reaching target INR [85]. A cellular anti-inflammatory action was also reported for low concentrations of warfarin applied directly to in mouse macrophages stimulated with tumor necrosis factor (TNF)-α, leading to reduced IL-6 secretion. Apparently, warfarin could specifically suppress IKB phosphorylation, phosphorylation of other TNF-α targets was not affected. Higher levels of warfarin however led to a direct cytotoxic action with a net pro-inflammatory action [86]. An influence on cardiac fibrotic remodeling has not been described for warfarin. In a model of fibrotic lung injury, however, warfarin failed to protect against fibrosis, while dabigatran showed significant protection. Mechanistically, this was attributed to reduced activation of PAR1, integrins and TGF-β in dabigatran-treated animals, while warfarin treatment did not downregulate the PAR1/αvβ6/TGF-β axis [87]. Heparin and fractionated derivatives including fondaparinux also possess cardioprotective and anti-inflammatory properties in vivo, distinct from their anticoagulant properties [88–90], even though heparin released from infiltrated mast cells instead promotes perimyocytic and interstitial fibrosis [91]. With exogenous application, inflammatory downregulation is seen both systemically and at the cardiac level [92, 93], as well as in isolated hearts [94]. Similarly, an antifibrotic action can be reproduced in isolated human cardiac myofibroblasts [95], confirming that counteraction of fibro-inflammation by heparins is largely independent of hemostatic actions. Most of these studies investigated heparin under settings of MI. Whether heparins counter chronic low-level inflammation and fibrotic signaling in the absence of ischemic priming as effectively requires further study, as does the contribution of inhibited PAR signaling in this context.
Assuming DOAC limit cardiac myofibroblast differentiation in the same way in patients, this anticoagulant approach could conceivably help to prevent heterogeneous conduction slowing, a consequence of heterocellular gap junctional coupling of myofibroblasts and cardiomyocytes. This phenomenon is elegantly demonstrated by single-cell patch clamp electrophysiology and optical mapping of impulse conduction in neonatal rat ventricular cells [96], and is considered to contribute to arrhythmogenesis in fibrotically remodeled hearts, although direct demonstration in intact hearts in vivo is still lacking. In a rat model of HF with left atrial dilation, dabigatran was indeed found to reduce atrial fibrotic remodeling and concomitantly suppress the duration of AF episodes induced by burst pacing [97], supporting the idea that DOAC could help to slow the progression of the underlying arrhythmogenic substrate promoting AF maintenance. In the latter study, the authors explored the concentration-dependence of the dual impact of dabigatran on hemostasis and on the arrhythmogenic substrate. At least in this rat model, inhibition of circulating thrombin activity was directly related to the plasma concentration of dabigatran, while its anti-remodeling effect was observed within a window of 35 to 100 nmol/l, but was lost at higher concentrations [97]. Since the concentrations of DOAC within the atrium are usually unknown it is very difficult to predict whether DOAC exerts anti-arrhythmic effects on individual patient basis. Extensive clinical validation would be needed to delineate the potential anti-arrhythmic effects of DOAC in the clinical setting.
The available mechanistic data suggest that the anti-remodeling and anti-proliferative potential of DOAC is likely to be mediated through inhibition of PAR1 signaling in response to both thrombin and FXa [98–101]. PAR1 is the predominantly expressed PAR in both ventricular and atrial fibroblasts in heart [22, 82, 102]; other PAR isoforms are expressed at much lower levels. However, a contribution by FXa-activated PAR2 to atrial remodeling processes has been documented [103, 104]. Notably, a direct inhibitory effect of rivaroxaban on FXa/PAR2-mediated fibro-inflammatory processes has been shown in human atrial tissue slices [103], with rivaroxaban halting the feed-forward upregulation of PAR2 [103], much as we have seen in other cell types [105]. Additional rapid pacing (4-Hz) of human atrial slices led to upregulation of PAR1 as well, and to a further augmented pro-inflammatory gene expression [103]. So, within atria, FXa, and presumably thrombin, act synergistically with the high atrial rate to drive expression of PAR and of inflammatory mediators. In mouse ventricular fibroblasts FXa may even be the more potent trigger of remodeling, causing a stronger migration and proliferation, and augmented generation of H2O2, TGF-β, and the transcription factors AP-1 and NF-KB, than elicited by equimolar thrombin [106]. Whether a xaban is a superior inhibitor or fibro-inflammatory remodeling than a gatran is unclear and will need specific assessment in systematic head-to-head comparative studies.
One recent player in AF initiation and progression is the NLRP3 inflammasome, a multimeric signaling structure comprising auto-activated caspase-1 responsible for maturation of IL-1β and IL-18. The NLRP3 inflammasome was recently shown to mediate sterile inflammation in atrial cardiomyocytes, contributing causally to AF pathophysiology [107–109]. Ventricular arrhythmias in a diabetic mouse model were also effectively prevented by inhibiting either the IL-1 receptor or the NLRP3 inflammasome [110]. Thrombin can activate NLRP3 signaling in numerous cell types [111], and inhibition of this might contribute to the ability of DOAC to reduce circulating IL-1β levels in vivo [112–114]. In mice this anti-inflammatory action is associated with a concomitant reduction of atrial remodeling and AF episode duration [114].
6. Anticoagulation for all, regardless of rhythm?
Taken together, anticoagulation seems a feasible approach to limit and prevent the adverse inflammatory, structural and electrical effects of thrombin and FXa even in patients with no indication for anticoagulation. Particularly patients with HF, who present with an increased risk of arrhythmias, might be thought to benefit. The WARCEF trial [115] was designed to compare warfarin and aspirin in patients in sinus rhythm with reduced left ventricular ejection fraction. Over 2300 patients were followed for up to 6 years. The study did not specifically assess arrhythmia-associated events or deaths, primary outcome was the time to the first event in a composite end point of ischemic stroke, intracerebral hemorrhage, or death from any cause. With respect to this combined end-point, there was no significant overall difference between the two treatments, a modestly reduced risk of ischemic stroke with warfarin was offset by an increased risk of major bleeding. The more recent COMPASS trial compared rivaroxaban with aspirin, both given alone or in combination. Adding rivaroxaban to aspirin reduced the relative risk of major adverse cardiovascular events by 32% in patients with HF. Net clinical benefit was improved by 31%, particularly since the increase in bleeding caused by the dual approach was smaller in patients with HF, compared to those without HF. The benefit of rivaroxaban alone versus aspirin was much weaker. The subsequent COMMANDER HF trial specifically investigated rivaroxaban in HF patients with sinus rhythm. Nearly all (93%) were on background aspirin [116]. Numerically, the group treated with low-dose rivaroxaban showed lower all-cause mortality, MI and stroke, but overall the primary endpoints were comparable in those with and without the DOAC. It is presumed that higher doses would merely elevate bleeding risk without the hoped-for benefit, and that the same would likely be true for other anticoagulants. It seems unlikely that this question will be addressed in a similar large-scale study. Unfortunately, these studies do not provide specific information on electrical and fibro-inflammatory impacts of the treatments, but the weak net survival benefit does not warrant anticoagulant use to limit these detrimental remodeling effects in the absence of a clear indication for anticoagulation.
7. Conclusion
DOAC at clinically relevant concentrations appear able to modify ionic currents and relevant arrhythmogenic processes. Whether this translates into a clinical efficacy potentially suppressing AF progression or recurrence, particularly with long-term use, is not evident from the major trials performed to date. Unfortunately, all clinical trials with the DOACs did not include ECGs monitoring of incident AF, so it is not possible to directly judge their disease-modifying effects on AF per se. Such data would be invaluable when considering the apparent temporal disconnection between AF and stroke, and how anticoagulants might impact directly on these two distinct but intertwined entities. The emerging picture however is clearly of a multidirectional interplay between high atrial rate, pro-coagulant changes and fibro-inflammatory processes, all contributing to atrial arrhythmogenesis and AF progression. Future prospective clinical studies will be needed to prove the anti-arrhythmic potential of DOACs in AF and perhaps other arrhythmia forms.
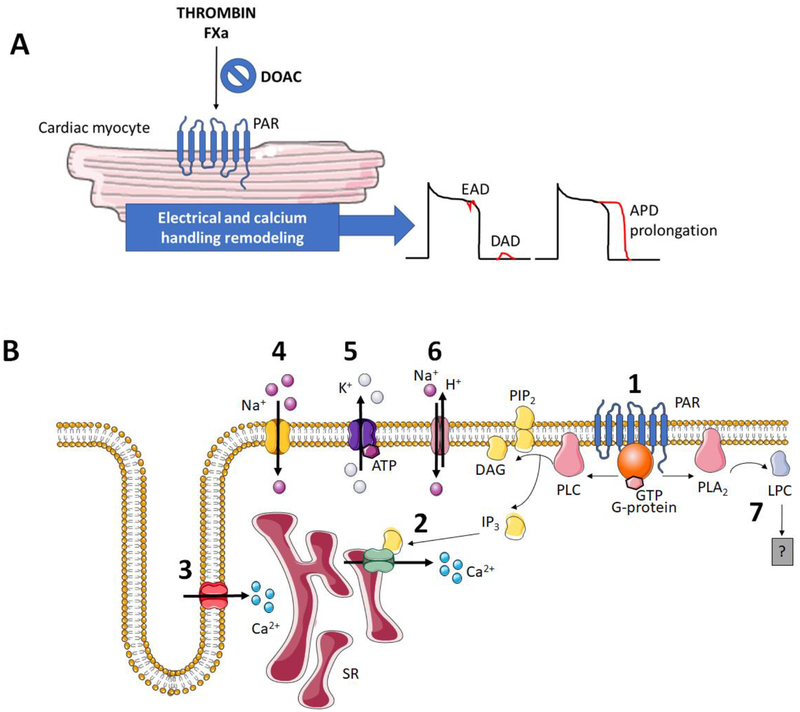
Fig. 3. Candidate anti-arrhythmic mechanisms of anticoagulants.
A) Cellular PAR activation by FXa and/or thrombin leads to alterations in cardiomyocyte ion channels, Ca2+ handling, leading to prolonged action potential duration (APD) with enhanced susceptibility to early and delayed afterdepolarizations (EADs and DADs, respectively) and B) Summary of cellular effectors and ion channels reported to be modified downstream of PAR1-activation in cardiomyocytes, which potentially contribute to pro-arrhythmic responses to coagulant proteases: (1) PAR1 is coupled to activation of phospholipase C (PLC), generating the signaling mediators diacylglycerol (DAG) and inositol trisphosphate (IP3). (2) IP3 activates ligand-gated Ca2+ channels of the sarcoplasmic reticulum (SR) to raise intracellular Ca2+. PAR1 activation has been reported to modulate voltage-gated ion channels, including (3) L-type Ca2+ channels and (4) Na+ channels, and (5) and K+ channels such as ATP-sensitive K+ channels. Additional candidate mediators of PAR1-triggered arrhythmogenesis include (6) the Na+-H+ exchanger (NHE-1) and (7) phospholipase A2-derived lysophosphatidylcholine (LPC). The precise mechanism how LPC supports rhythm disorders requires further elucidation, although the generation of reactive oxygen species is a candidate mechanism.
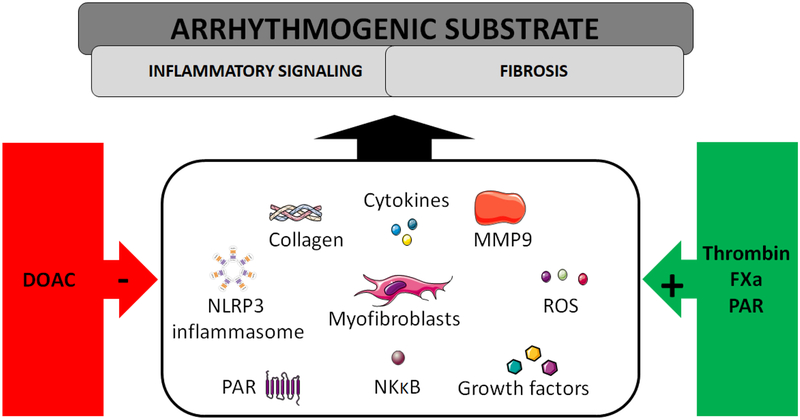
Fig. 4. Candidate mechanisms how anticoagulants may target the pro-arrhythmogenic substrate.
Inflammatory and fibrotic processes contribute to the vulnerable substrate. Some of them have been shown to be directly amplified by coagulant proteases and PAR activation, including myofibroblast differentiation with increased collagen deposition, cytokine and growth factor secretion, activation of NFKB-dependent inflammatory gene expression, production of reactive oxygen species (ROS), matrix metalloproteinase (especially MMP9) expression and activation, NLRP3 inflammasome priming and triggering, and feed-forward regulation of PAR activation. Application of DOACs suppresses these fibro-inflammatory mediators and may thus limit the progression to persistent arrhythmia forms.
Sources of funding
The authors’ work was supported by grants from the National Institutes of Health (R01-HL131517 and R01-HL136389 to DD) and the German Research Foundation (DFG, Do 769/4-1 to DD).
Footnotes
Declarations of interest: none (all authors)
References
- [1].Andrade J, Khairy P, Dobrev D Nattel S, The clinical profile and pathophysiology of atrial fibrillation: relationships among clinical features, epidemiology, and mechanisms, Circ Res, 2014;114:1453–1468, 10.1161/CIRCRESAHA.114.303211 [DOI] [PubMed] [Google Scholar]
- [2].Kirchhof P, Breithardt G, Bax J, Benninger G, Blomstrom-Lundqvist C, Boriani G, Brandes A, Brown H, Brueckmann M, Calkins H, Calvert M, Christoffels V, Crijns H, Dobrev D, Ellinor P, Fabritz L, Fetsch T, Freedman SB, Gerth A, Goette A, Guasch E, Hack G, Haegeli L, Hatem S, Haeusler KG, Heidbuchel H, Heinrich-Nols J, Hidden-Lucet F, Hindricks G, Juul-Moller S, Kaab S, Kappenberger L, Kespohl S, Kotecha D, Lane DA, Leute A, Lewalter T, Meyer R, Mont L, Munzel F, Nabauer M, Nielsen JC, Oeff M, Oldgren J, Oto A, Piccini JP, Pilmeyer A, Potpara T, Ravens U, Reinecke H, Rostock T, Rustige J, Savelieva I, Schnabel R, Schotten U, Schwichtenberg L, Sinner MF, Steinbeck G, Stoll M, Tavazzi L, Themistoclakis S, Tse HF, Van Gelder IC, Vardas PE, Varpula T, Vincent A, Werring D, Willems S, Ziegler A, Lip GY Camm AJ, A roadmap to improve the quality of atrial fibrillation management: proceedings from the fifth Atrial Fibrillation Network/European Heart Rhythm Association consensus conference, Europace, 2016;18:37–50, 10.1093/europace/euv304 [DOI] [PubMed] [Google Scholar]
- [3].Dobrev D, Aguilar M, Heijman J, Guichard J-B Nattel S, Postoperative atrial fibrillation: mechanisms, manifestations and management, Nature Reviews Cardiology, 2019, 10.1038/s41569-019-0166-5 [DOI] [PubMed] [Google Scholar]
- [4].Heijman J Dobrev D, Challenges to the translation of basic science findings to atrial fibrillation therapies, Future Cardiol, 2016;12:251–254, 10.2217/fca-2016-0007 [DOI] [PubMed] [Google Scholar]
- [5].Heijman J, Voigt N, Nattel S Dobrev D, Cellular and molecular electrophysiology of atrial fibrillation initiation, maintenance, and progression, Circ Res, 2014;114:1483–1499, 10.1161/CIRCRESAHA.114.302226 [DOI] [PubMed] [Google Scholar]
- [6].Nattel S Dobrev D, Electrophysiological and molecular mechanisms of paroxysmal atrial fibrillation, Nat Rev Cardiol, 2016;13:575–590, 10.1038/nrcardio.2016.118 [DOI] [PubMed] [Google Scholar]
- [7].Dan GA Dobrev D, Antiarrhythmic drugs for atrial fibrillation: Imminent impulses are emerging, Int J Cardiol Heart Vasc, 2018;21:11–15, 10.1016/j.ijcha.2018.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Badimon L Cubedo J, Hypercoagulability and atrial fibrillation: a two-way road?, Eur Heart J, 2017;38:51–52, 10.1093/eurheartj/ehw108 [DOI] [PubMed] [Google Scholar]
- [9].Martin DT, Bersohn MM, Waldo AL, Wathen MS, Choucair WK, Lip GY, Ip J, Holcomb R, Akar JG, Halperin JL Investigators I, Randomized trial of atrial arrhythmia monitoring to guide anticoagulation in patients with implanted defibrillator and cardiac resynchronization devices, Eur Heart J, 2015;36:1660–1668, 10.1093/eurheartj/ehv115 [DOI] [PubMed] [Google Scholar]
- [10].Wysokinski WE, Tafur A, Ammash N, Asirvatham SJ, Wu Y, Gosk-Bierska I, Grill DE, Slusser JP, Mruk J McBane RD, Impact of atrial fibrillation on platelet gene expression, Eur J Haematol, 2017;98:615–621, 10.1111/ejh.12879 [DOI] [PubMed] [Google Scholar]
- [11].Alonso A, Tang W, Agarwal SK, Soliman EZ, Chamberlain AM Folsom AR, Hemostatic markers are associated with the risk and prognosis of atrial fibrillation: the ARIC study, Int J Cardiol, 2012;155:217–222, 10.1016/j.ijcard.2010.09.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Goette A, Kalman JM, Aguinaga L, Akar J, Cabrera JA, Chen SA, Chugh SS, Corradi D, D’Avila A, Dobrev D, Fenelon G, Gonzalez M, Hatem SN, Helm R, Hindricks G, Ho SY, Hoit B, Jalife J, Kim YH, Lip GY, Ma CS, Marcus GM, Murray K, Nogami A, Sanders P, Uribe W, Van Wagoner DR, Nattel S Document R, EHRA/HRS/APHRS/SOLAECE expert consensus on atrial cardiomyopathies: definition, characterization, and clinical implication, Europace, 2016;18:1455–1490, 10.1093/europace/euw161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Konigsbrugge O, Weigel G, Quehenberger P, Pabinger I Ay C, Plasma clot formation and clot lysis to compare effects of different anticoagulation treatments on hemostasis in patients with atrial fibrillation, Clin Exp Med, 2018;18:325–336, 10.1007/s10238-018-0490-9 [DOI] [PubMed] [Google Scholar]
- [14].Lau YC, Xiong Q, Shantsila E, Lip GY Blann AD, Effects of non-vitamin K antagonist oral anticoagulants on fibrin clot and whole blood clot formation, integrity and thrombolysis in patients with atrial fibrillation, J Thromb Thrombolysis, 2016;42:535–544, 10.1007/s11239-016-1399-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Papp J, Zima E, Bover R, Karaliute R, Rossi A, Szymanski C, Troccoli R, Schneider J, Fagerland MW, Camm AJ Atar D, Changes in oral anticoagulation for elective cardioversion: results from a European cardioversion registry, Eur Heart J Cardiovasc Pharmacother, 2017;3:147–150, 10.1093/ehjcvp/pvx003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Sulzgruber P Niessner A, Changing paradigms in oral anticoagulation during cardioversion in Europe, Eur Heart J Cardiovasc Pharmacother, 2018;4:2–3, 10.1093/ehjcvp/pvx025 [DOI] [PubMed] [Google Scholar]
- [17].Dimitropoulos G, Rahim SMZ, Moss AS Lip GYH, New anticoagulants for venous thromboembolism and atrial fibrillation: what the future holds, Expert Opin Investig Drugs, 2018;27:71–86, 10.1080/13543784.2018.1416090 [DOI] [PubMed] [Google Scholar]
- [18].Hochtl T Huber K, New anticoagulants for the prevention of stroke in atrial fibrillation, Fundam Clin Pharmacol, 2012;26:47–53, 10.1111/j.1472-8206.2011.00982.x [DOI] [PubMed] [Google Scholar]
- [19].Masarone D, Limongelli G, Rubino M, Valente F, Vastarella R, Ammendola E, Gravino R, Verrengia M, Salerno G Pacileo G, Management of Arrhythmias in Heart Failure, J Cardiovasc Dev Dis, 2017;4, 10.3390/jcdd4010003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Pabinger I, Lang W, Roithinger FX, Weidinger F, Eichinger-Hasenauer S, Glehr R, Halbmayer WM, Haring HP, Jilma B, Korninger HC, Kozek-Langenecker S, Kyrle P, Watzke H, Weltermann A, Willeit J, Huber K, Osterreichische Kardiologische G, Osterreichische Gesellschaft fur H, Medizinische O, Osterreichische S-G, Osterreichische Gesellschaft fur Innere M, Osterreichische Gesellschaft fur Anasthesiologie RuI Osterreichische Gesellschaft fur Allgemein- und F, [Consensus statement: Stroke prevention in nonvalvular atrial fibrillation in special consideration of the new direct oral anticoagulants], Wien Klin Wochenschr, 2014;126:792–808, 10.1007/s00508-014-0586-5 [DOI] [PubMed] [Google Scholar]
- [21].Elmas E, Kaelsch T, Wolpert C, Sueselbeck T, Bertsch T, Dempfle CE Borggrefe M, Assessment of markers of thrombin generation in patients with acute myocardial infarction complicated by ventricular fibrillation, Clin Cardiol, 2006;29:165–169, [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Spronk HM, De Jong AM, Verheule S, De Boer HC, Maass AH, Lau DH, Rienstra M, van Hunnik A, Kuiper M, Lumeij S, Zeemering S, Linz D, Kamphuisen PW, Ten Cate H, Crijns HJ, Van Gelder IC, van Zonneveld AJ Schotten U, Hypercoagulability causes atrial fibrosis and promotes atrial fibrillation, Eur Heart J, 2017;38:38–50, 10.1093/eurheartj/ehw119 [DOI] [PubMed] [Google Scholar]
- [23].Gieseler F, Ungefroren H, Settmacher U, Hollenberg MD Kaufmann R, Proteinase-activated receptors (PARs) – focus on receptor-receptor-interactions and their physiological and pathophysiological impact, Cell Comm Signal, 2013;11:86, [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Coughlin SR, Thrombin signalling and protease-activated receptors, Nature, 2000;407:258–264, 10.1038/35025229 [DOI] [PubMed] [Google Scholar]
- [25].Borensztajn K Spek CA, Blood coagulation factor Xa as an emerging drug target, Expert Opin Ther Targets, 2011;15:341–349, 10.1517/14728222.2011.553608 [DOI] [PubMed] [Google Scholar]
- [26].Spronk HM, de Jong AM, Crijns HJ, Schotten U, Van Gelder IC Ten Cate H, Pleiotropic effects of factor Xa and thrombin: what to expect from novel anticoagulants, Cardiovasc Res, 2014;101:344–351, 10.1093/cvr/cvt343 [DOI] [PubMed] [Google Scholar]
- [27].Antoniak S, Sparkenbaugh E Pawlinski R, Tissue factor, protease activated receptors and pathologic heart remodelling, Thromb Haemost, 2014;112:893–900, 10.1160/TH14-03-0243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Antoniak S, Pawlinski R Mackman N, Protease-activated receptors and myocardial infarction, IUBMB Life, 2011;63:383–389, 10.1002/iub.441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Tse G, Yan BP, Chan YW, Tian XY Huang Y, Reactive Oxygen Species, Endoplasmic Reticulum Stress and Mitochondrial Dysfunction: The Link with Cardiac Arrhythmogenesis, Front Physiol, 2016;7:313, 10.3389/fphys.2016.00313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Mihara K, Ramachandran R, Saifeddine M, Hansen KK, Renaux B, Polley D, Gibson S, Vanderboor C Hollenberg MD, Thrombin-Mediated Direct Activation of Proteinase-Activated Receptor-2: Another Target for Thrombin Signaling, Mol Pharmacol, 2016;89:606–614, 10.1124/mol.115.102723 [DOI] [PubMed] [Google Scholar]
- [31].Fender AC, Rauch BH, Geisler T Schror K, Protease-Activated Receptor PAR-4: An Inducible Switch between Thrombosis and Vascular Inflammation?, Thromb Haemost, 2017;117:2013–2025, 10.1160/TH17-03-0219 [DOI] [PubMed] [Google Scholar]
- [32].Steinberg SF, Robinson RB, Lieberman HB, Stern DM Rosen MR, Thrombin modulates phosphoinositide metabolism, cytosolic calcium, and impulse initiation in the heart, Circ Res, 1991;68:1216–1229, [DOI] [PubMed] [Google Scholar]
- [33].Albitz R, Droogmans G, Nilius B Casteels R, Thrombin stimulates L-type calcium channels of guinea pig cardiomyocytes in cell-attached patches but not after intracellular dialysis, Cell Calcium, 1992;13:203–210, [DOI] [PubMed] [Google Scholar]
- [34].Pinet C, Algalarrondo V, Sablayrolles S, Le Grand B, Pignier C, Cussac D, Perez M, Hatem SN Coulombe A, Protease-activated receptor-1 mediates thrombin-induced persistent sodium current in human cardiomyocytes, Mol Pharmacol, 2008;73:1622–1631, 10.1124/mol.107.043182 [DOI] [PubMed] [Google Scholar]
- [35].Sabri A, Muske G, Zhang H, Pak E, Darrow A, Andrade-Gordon P Steinberg SF, Signaling properties and functions of two distinct cardiomyocyte protease-activated receptors, Circ Res, 2000;86:1054–1061, [DOI] [PubMed] [Google Scholar]
- [36].Jiang T, Danilo P Jr. Steinberg SF, The thrombin receptor elevates intracellular calcium in adult rat ventricular myocytes, J Mol Cell Cardiol, 1998;30:2193–2199, [DOI] [PubMed] [Google Scholar]
- [37].Jiang T, Kuznetsov V, Pak E, Zhang H, Robinson RB Steinberg SF, Thrombin receptor actions in neonatal rat ventricular myocytes, Circ Res, 1996;78:553–563, [DOI] [PubMed] [Google Scholar]
- [38].Jacobsen AN, Du XJ, Lambert KA, Dart AM Woodcock EA, Arrhythmogenic action of thrombin during myocardial reperfusion via release of inositol 1,4,5-triphosphate, Circulation, 1996;93:23–26, [DOI] [PubMed] [Google Scholar]
- [39].Tang L, Deng C, Long M, Tang A, Wu S, Dong Y, Saravolatz LD Gardin JM, Thrombin receptor and ventricular arrhythmias after acute myocardial infarction, Mol Med, 2008;14:131–140, 10.2119/2007-00097.Tang [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Mohamed OY, Al-Masri AA, El Eter EA Lateef R, SCH 79797, a selective PAR1 antagonist, protects against ischemia/reperfusion-induced arrhythmias in the rat hearts, Eur Rev Med Pharmacol Sci, 2016;20:4796–4800, [PubMed] [Google Scholar]
- [41].Man RY Choy PC, Lysophosphatidylcholine causes cardiac arrhythmia, J Mol Cell Cardiol, 1982;14:173–175, [DOI] [PubMed] [Google Scholar]
- [42].McHowat J Creer MH, Thrombin activates a membrane-associated calcium-independent PLA2 in ventricular myocytes, Am J Physiol, 1998;274:C447–454, [DOI] [PubMed] [Google Scholar]
- [43].McHowat J Creer MH, Lysophosphatidylcholine accumulation in cardiomyocytes requires thrombin activation of Ca2+-independent PLA2, Am J Physiol, 1997;272:H1972–1980, 10.1152/ajpheart.1997.272.4.H1972 [DOI] [PubMed] [Google Scholar]
- [44].Park TH, McHowat J, Wolf RA Corr PB, Increased lysophosphatidylcholine content induced by thrombin receptor stimulation in adult rabbit cardiac ventricular myocytes, Cardiovasc Res, 1994;28:1263–1268, [DOI] [PubMed] [Google Scholar]
- [45].Hui Y, Junzhu C Jianhua Z, Gap junction and Na+-H+ exchanger alternations in fibrillating and failing atrium, Int J Cardiol, 2008;128:147–149, 10.1016/j.ijcard.2007.06.070 [DOI] [PubMed] [Google Scholar]
- [46].Gumina RJ, Daemmgen J Gross GJ, Inhibition of the Na(+)/H(+) exchanger attenuates phase 1b ischemic arrhythmias and reperfusion-induced ventricular fibrillation, Eur J Pharmacol, 2000;396:119–124, [DOI] [PubMed] [Google Scholar]
- [47].Ayoub IM, Kolarova J, Yi Z, Trevedi A, Deshmukh H, Lubell DL, Franz MR, Maldonado FA Gazmuri RJ, Sodium-hydrogen exchange inhibition during ventricular fibrillation: Beneficial effects on ischemic contracture, action potential duration, reperfusion arrhythmias, myocardial function, and resuscitability, Circulation, 2003;107:1804–1809, 10.1161/01.Cir.0000058704.45646.0d [DOI] [PubMed] [Google Scholar]
- [48].Avkiran M Haworth RS, Regulatory effects of G protein-coupled receptors on cardiac sarcolemmal Na+/H+ exchanger activity: signalling and significance, Cardiovasc Res, 2003;57:942–952, [DOI] [PubMed] [Google Scholar]
- [49].Ito K, Date T, Ikegami M, Hongo K, Fujisaki M, Katoh D, Yoshino T, Anzawa R, Nagoshi T, Yamashita S, Inada K, Matsuo S, Yamane T Yoshimura M, An immunohistochemical analysis of tissue thrombin expression in the human atria, PLoS One, 2013;8:e65817, 10.1371/journal.pone.0065817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Chang CJ, Chen YC, Lin YK, Huang JH, Chen SA Chen YJ, Rivaroxaban modulates electrical and mechanical characteristics of left atrium, J Biomed Sci, 2013;20:17, 10.1186/1423-0127-20-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Gomez-Outes A, Lagunar-Ruiz J, Terleira-Fernandez AI, Calvo-Rojas G, Suarez-Gea ML Vargas-Castrillon E, Causes of Death in Anticoagulated Patients With Atrial Fibrillation, J Am Coll Cardiol, 2016;68:2508–2521, 10.1016/j.jacc.2016.09.944 [DOI] [PubMed] [Google Scholar]
- [52].Font J, Simeon M, Simard C, Allouche S, Plane AF, Ferchaud V, Brionne M, Rouet R, Nowoczyn M, Manrique A, Puddu PE, Milliez P Alexandre J, PAR1 contribution in acute electrophysiological properties of oral anticoagulants in rabbit pulmonary vein sleeve preparations, Fundam Clin Pharmacol, 2018;32:378–391, 10.1111/fcp.12365 [DOI] [PubMed] [Google Scholar]
- [53].Chang CJ, Chen YC, Kao YH, Lin YK, Chen SA Chen YJ, Dabigatran and thrombin modulate electrophysiological characteristics of pulmonary vein and left atrium, Circ Arrhythm Electrophysiol, 2012;5:1176–1183, 10.1161/circep.112.971556 [DOI] [PubMed] [Google Scholar]
- [54].de Godoy CMG, Vasques ER, Caricati-Neto A, Tavares JGP, Alves BJ, Duarte J, Miranda-Ferreira R, Lima MA, Nader HB Tersariol I, Heparin Oligosaccharides Have Antiarrhythmic Effect by Accelerating the Sodium-Calcium Exchanger, Front Cardiovasc Med, 2018;5:67, 10.3389/fcvm.2018.00067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Kucukhuseyin O, Khalid S, Sabitaliyevich UY Kucukhuseyin C, The role of PLC-IP3 cascade on 4-aminopyridine (4-AP) contracture in electrically-driven rat atrial and diaphragmatic strips: new evidence by neomycin and heparin, Cell Mol Biol (Noisy-le-grand), 2018;64:26–32, [PubMed] [Google Scholar]
- [56].Domeier TL, Zima AV, Maxwell JT, Huke S, Mignery GA Blatter LA, IP3 receptor-dependent Ca2+ release modulates excitation-contraction coupling in rabbit ventricular myocytes, Am J Physiol Heart Circ Physiol, 2008;294:H596–604, 10.1152/ajpheart.01155.2007 [DOI] [PubMed] [Google Scholar]
- [57].Zima AV, Bare DJ, Mignery GA Blatter LA, IP3-dependent nuclear Ca2+ signalling in the mammalian heart, J Physiol, 2007;584:601–611, 10.1113/jphysiol.2007.140731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Felzen B, Berke G, Gardner P Binah O, Involvement of the IP3 cascade in the damage to guinea-pig ventricular myocytes induced by cytotoxic T lymphocytes, Pflugers Arch, 1997;433:721–726, [DOI] [PubMed] [Google Scholar]
- [59].Garcia MC, Sanchez JA, Sharma VK Sheu SS, Extracellular heparin inhibits Ca2+ transients and contraction in mammalian cardiac myocytes, Pflugers Arch, 1995;431:84–90, [DOI] [PubMed] [Google Scholar]
- [60].Knaus HG, Scheffauer F, Romanin C, Schindler HG Glossmann H, Heparin binds with high affinity to voltage-dependent L-type Ca2+ channels. Evidence for an agonistic action, J Biol Chem, 1990;265:11156–11166, [PubMed] [Google Scholar]
- [61].Barry WH, Zhang XQ, Halkos ME, Vinten-Johansen J, Saegusa N, Spitzer KW, Matsuoka N, Sheets M, Rao NV Kennedy TP, Nonanticoagulant heparin reduces myocyte Na+ and Ca2+ loading during simulated ischemia and decreases reperfusion injury, Am J Physiol Heart Circ Physiol, 2010;298:H102–111, 10.1152/ajpheart.00316.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Hughes E, Edwards R Middleton DA, Heparin-derived oligosaccharides interact with the phospholamban cytoplasmic domain and stimulate SERCA function, Biochem Biophys Res Commun, 2010;401:370–375, 10.1016/j.bbrc.2010.09.056 [DOI] [PubMed] [Google Scholar]
- [63].Alanis J, Arguello C Polo L, Effects of heparin on the electrophysiological and mechanical properties of early embryonic chick hearts, J Mol Cell Cardiol, 1997;29:2503–2511, [DOI] [PubMed] [Google Scholar]
- [64].Heijman J Dobrev D, Rat engineered heart tissue: a novel tool in the safety pharmacology toolkit?, Basic Res Cardiol, 2014;109:437, 10.1007/s00395-014-0437-6 [DOI] [PubMed] [Google Scholar]
- [65].Molina CE, Abu-Taha IH, Wang Q, Rosello-Diez E, Kamler M, Nattel S, Ravens U, Wehrens XHT, Hove-Madsen L, Heijman J Dobrev D, Profibrotic, Electrical, and Calcium-Handling Remodeling of the Atria in Heart Failure Patients With and Without Atrial Fibrillation, Front Physiol, 2018;9:1383, 10.3389/fphys.2018.01383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Nakano Y, Niida S, Dote K, Takenaka S, Hirao H, Miura F, Ishida M, Shingu T, Sueda T, Yoshizumi M Chayama K, Matrix metalloproteinase-9 contributes to human atrial remodeling during atrial fibrillation, J Am Coll Cardiol, 2004;43:818–825, 10.1016/j.jacc.2003.08.060 [DOI] [PubMed] [Google Scholar]
- [67].Stanciu AE, Vatasescu RG, Stanciu MM, Serdarevic N Dorobantu M, The role of pro-fibrotic biomarkers in paroxysmal and persistent atrial fibrillation, Cytokine, 2018;103:63–68, 10.1016/j.cyto.2017.12.026 [DOI] [PubMed] [Google Scholar]
- [68].Weng CH, Chung FP, Chen YC, Lin SF, Huang PH, Kuo TB, Hsu WH, Su WC, Sung YL, Lin YJ, Chang SL, Lo LW, Yeh HI, Chen YJ, Hong YR, Chen SA Hu YF, Pleiotropic Effects of Myocardial MMP-9 Inhibition to Prevent Ventricular Arrhythmia, Sci Rep, 2016;6:38894, 10.1038/srep38894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Xu J, Cui G, Esmailian F, Plunkett M, Marelli D, Ardehali A, Odim J, Laks H Sen L, Atrial extracellular matrix remodeling and the maintenance of atrial fibrillation, Circulation, 2004;109:363–368, 10.1161/01.cir.0000109495.02213.52 [DOI] [PubMed] [Google Scholar]
- [70].Fang Q, Liu X, Al-Mugotir M, Kobayashi T, Abe S, Kohyama T Rennard SI, Thrombin and TNF-alpha/IL-1beta synergistically induce fibroblast-mediated collagen gel degradation, Am J Respir Cell Mol Biol, 2006;35:714–721, 10.1165/rcmb.2005-0026OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Wang L, Luo J He S, Induction of MMP-9 release from human dermal fibroblasts by thrombin: involvement of JAK/STAT3 signaling pathway in MMP-9 release, BMC Cell Biol, 2007;8:14, 10.1186/1471-2121-8-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Chang CJ, Hsu LA, Ko YH, Chen PL, Chuang YT, Lin CY, Liao CH Pang JH, Thrombin regulates matrix metalloproteinase-9 expression in human monocytes, Biochem Biophys Res Commun, 2009;385:241–246, 10.1016/j.bbrc.2009.05.049 [DOI] [PubMed] [Google Scholar]
- [73].Abdulkhalek S, Guo M, Amith SR, Jayanth P Szewczuk MR, G-protein coupled receptor agonists mediate Neu1 sialidase and matrix metalloproteinase-9 cross-talk to induce transactivation of TOLL-like receptors and cellular signaling, Cell Signal, 2012;24:2035–2042, 10.1016/j.cellsig.2012.06.016 [DOI] [PubMed] [Google Scholar]
- [74].Kadoglou NP, Moustardas P, Katsimpoulas M, Kapelouzou A, Kostomitsopoulos N, Schafer K, Kostakis A Liapis CD, The beneficial effects of a direct thrombin inhibitor, dabigatran etexilate, on the development and stability of atherosclerotic lesions in apolipoprotein E-deficient mice : dabigatran etexilate and atherosclerosis, Cardiovasc Drugs Ther, 2012;26:367–374, 10.1007/s10557-012-6411-3 [DOI] [PubMed] [Google Scholar]
- [75].Zuo P, Zhou Q, Zuo Z, Wang X, Chen L Ma G, Effects of the factor Xa inhibitor, fondaparinux, on the stability of atherosclerotic lesions in apolipoprotein E-deficient mice, Circ J, 2015;79:2499–2508, 10.1253/circj.CJ-15-0285 [DOI] [PubMed] [Google Scholar]
- [76].Dong A, Mueller P, Yang F, Yang L, Morris A Smyth SS, Direct thrombin inhibition with dabigatran attenuates pressure overload-induced cardiac fibrosis and dysfunction in mice, Thromb Res, 2017;159:58–64, 10.1016/j.thromres.2017.09.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Monux G, Zamorano-Leon JJ, Marques P, Sopena B, Garcia-Garcia JM, Laich de Koller G, Calvo-Rico B, Garcia-Fernandez MA, Serrano J Lopez-Farre A, FXa inhibition by rivaroxaban modifies mechanisms associated with the pathogenesis of human abdominal aortic aneurysms, Br J Clin Pharmacol, 2017;83:2661–2670, 10.1111/bcp.13383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Chan MY, Lin M, Lucas J, Moseley A, Thompson JW, Cyr D, Ueda H, Kajikawa M, Ortel TL Becker RC, Plasma proteomics of patients with non-valvular atrial fibrillation on chronic anti-coagulation with warfarin or a direct factor Xa inhibitor, Thromb Haemost, 2012;108:1180–1191, 10.1160/th12-05-0310 [DOI] [PubMed] [Google Scholar]
- [79].Rohr S, Arrhythmogenic implications of fibroblast-myocyte interactions, Circ Arrhythm Electrophysiol, 2012;5:442–452, 10.1161/CIRCEP.110.957647 [DOI] [PubMed] [Google Scholar]
- [80].de Bakker JM, van Capelle FJ, Janse MJ, Tasseron S, Vermeulen JT, de Jonge N Lahpor JR, Slow conduction in the infarcted human heart. ‘Zigzag’ course of activation, Circulation, 1993;88:915–926, [DOI] [PubMed] [Google Scholar]
- [81].Rohr S, Myofibroblasts in diseased hearts: new players in cardiac arrhythmias?, Heart Rhythm, 2009;6:848–856, 10.1016/j.hrthm.2009.02.038 [DOI] [PubMed] [Google Scholar]
- [82].Altieri P, Bertolotto M, Fabbi P, Sportelli E, Balbi M, Santini F, Brunelli C, Canepa M, Montecucco F Ameri P, Thrombin induces protease-activated receptor 1 signaling and activation of human atrial fibroblasts and dabigatran prevents these effects, Int J Cardiol, 2018;271:219–227, 10.1016/j.ijcard.2018.05.033 [DOI] [PubMed] [Google Scholar]
- [83].Ameri A, Kurachi S, Sueishi K, Kuwahara M Kurachi K, Myocardial fibrosis in mice with overexpression of human blood coagulation factor IX, Blood, 2003;101:1871–1873, 10.1182/blood-2002-05-1581 [DOI] [PubMed] [Google Scholar]
- [84].Xu H, Noria F, Sandoval-Cooper MJ, Menchen H, Donahue DL, Ploplis VA Castellino FJ, Severe deficiency of coagulation Factor VII results in spontaneous cardiac fibrosis in mice, J Pathol, 2009;217:362–371, 10.1002/path.2454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Maclean PS, Tait RC, Rumley A, McMahon AD Lowe GD, Anticoagulation with warfarin downregulates inflammation, J Thromb Haemost, 2003;1:1838–1839, [DOI] [PubMed] [Google Scholar]
- [86].Kater AP, Peppelenbosch MP, Brandjes DP Lumbantobing M, Dichotomal effect of the coumadin derivative warfarin on inflammatory signal transduction, Clin Diagn Lab Immunol, 2002;9:1396–1397, [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Shea BS, Probst CK, Brazee PL, Rotile NJ, Blasi F, Weinreb PH, Black KE, Sosnovik DE, Van Cott EM, Violette SM, Caravan P Tager AM, Uncoupling of the profibrotic and hemostatic effects of thrombin in lung fibrosis, JCI Insight, 2017;2, 10.1172/jci.insight.86608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Downing LJ, Strieter RM, Kadell AM, Wilke CA, Greenfield LJ Wakefield TW, Low-dose low-molecular-weight heparin is anti-inflammatory during venous thrombosis, J Vasc Surg, 1998;28:848–854, [DOI] [PubMed] [Google Scholar]
- [89].Shaker RA, Abboud SH, Assad HC Hadi N, Enoxaparin attenuates doxorubicin induced cardiotoxicity in rats via interfering with oxidative stress, inflammation and apoptosis, BMC Pharmacol Toxicol, 2018;19:3, 10.1186/s40360-017-0184-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Montaigne D, Marechal X, Lancel S, Decoster B, Asseman P Neviere R, The synthetic pentasaccharide fondaparinux prevents coronary microvascular injury and myocardial dysfunction in the ischemic heart, Thromb Haemost, 2008;100:912–919, [PubMed] [Google Scholar]
- [91].Li QY, Raza-Ahmad A, MacAulay MA, Lalonde LD, Rowden G, Trethewey E Dean S, The relationship of mast cells and their secreted products to the volume of fibrosis in posttransplant hearts, Transplantation, 1992;53:1047–1051, [DOI] [PubMed] [Google Scholar]
- [92].Thourani VH, Brar SS, Kennedy TP, Thornton LR, Watts JA, Ronson RS, Zhao ZQ, Sturrock AL, Hoidal JR Vinten-Johansen J, Nonanticoagulant heparin inhibits NF-kappaB activation and attenuates myocardial reperfusion injury, Am J Physiol Heart Circ Physiol, 2000;278:H2084–2093, 10.1152/ajpheart.2000.278.6.H2084 [DOI] [PubMed] [Google Scholar]
- [93].Deepa PR Varalakshmi P, Favourable modulation of the inflammatory changes in hypercholesterolemic atherogenesis by a low-molecular-weight heparin derivative, Int J Cardiol, 2006;106:338–347, 10.1016/j.ijcard.2005.02.012 [DOI] [PubMed] [Google Scholar]
- [94].Pevni D, Frolkis I, Shapira I, Schwartz D, Yuhas Y, Schwartz IF, Chernichovski T Uretzky G, Heparin added to cardioplegic solution inhibits tumor necrosis factor-alpha production and attenuates myocardial ischemic-reperfusion injury, Chest, 2005;128:1805–1811, 10.1378/chest.128.3.1805 [DOI] [PubMed] [Google Scholar]
- [95].Park DSJ, Mewhort HEM, Teng G, Belke D, Turnbull J, Svystonyuk D, Guzzardi D, Kang S Fedak PWM, Heparin Augmentation Enhances Bioactive Properties of Acellular Extracellular Matrix Scaffold, Tissue Eng Part A, 2018;24:128–134, 10.1089/ten.TEA.2017.0004 [DOI] [PubMed] [Google Scholar]
- [96].Grand T, Salvarani N, Jousset F Rohr S, Aggravation of cardiac myofibroblast arrhythmogeneicity by mechanical stress, Cardiovasc Res, 2014;104:489–500, 10.1093/cvr/cvu227 [DOI] [PubMed] [Google Scholar]
- [97].Jumeau C, Rupin A, Chieng-Yane P, Mougenot N, Zahr N, David-Dufilho M Hatem SN, Direct Thrombin Inhibitors Prevent Left Atrial Remodeling Associated With Heart Failure in Rats, JACC Basic Transl Sci, 2016;1:328–339, 10.1016/j.jacbts.2016.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Pawlinski R, Tencati M, Hampton CR, Shishido T, Bullard TA, Casey LM, Andrade-Gordon P, Kotzsch M, Spring D, Luther T, Abe J, Pohlman TH, Verrier ED, Blaxall BC Mackman N, Protease-activated receptor-1 contributes to cardiac remodeling and hypertrophy, Circulation, 2007;116:2298–2306, 10.1161/CIRCULATIONAHA.107.692764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Shi G, Yang X, Pan M, Sun J, Ke H, Zhang C Geng H, Apixaban attenuates ischemia-induced myocardial fibrosis by inhibition of Gq/PKC signaling, Biochem Biophys Res Commun, 2018;500:550–556, 10.1016/j.bbrc.2018.04.071 [DOI] [PubMed] [Google Scholar]
- [100].Bulani Y, Srinivasan K Sharma SS, Attenuation of type-1 diabetes-induced cardiovascular dysfunctions by direct thrombin inhibitor in rats: a mechanistic study, Mol Cell Biochem, 2018, 10.1007/s11010-018-3394-9 [DOI] [PubMed] [Google Scholar]
- [101].Bulani Y Sharma SS, Argatroban Attenuates Diabetic Cardiomyopathy in Rats by Reducing Fibrosis, Inflammation, Apoptosis, and Protease-Activated Receptor Expression, Cardiovasc Drugs Ther, 2017;31:255–267, 10.1007/s10557-017-6732-3 [DOI] [PubMed] [Google Scholar]
- [102].Snead AN Insel PA, Defining the cellular repertoire of GPCRs identifies a profibrotic role for the most highly expressed receptor, protease-activated receptor 1, in cardiac fibroblasts, FASEB J, 2012;26:4540–4547, 10.1096/fj.12-213496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Bukowska A, Zacharias I, Weinert S, Skopp K, Hartmann C, Huth C Goette A, Coagulation factor Xa induces an inflammatory signalling by activation of protease-activated receptors in human atrial tissue, Eur J Pharmacol, 2013;718:114–123, 10.1016/j.ejphar.2013.09.006 [DOI] [PubMed] [Google Scholar]
- [104].Chung CC, Lin YK, Chen YC, Kao YH, Yeh YH Chen YJ, Factor Xa inhibition by rivaroxaban regulates fibrogenesis in human atrial fibroblasts with modulation of nitric oxide synthesis and calcium homeostasis, J Mol Cell Cardiol, 2018;123:128–138, 10.1016/j.yjmcc.2018.09.003 [DOI] [PubMed] [Google Scholar]
- [105].Jobi K, Rauch BH, Dangwal S, Freidel K, Doller A, Eberhardt W, Fischer JW, Schror K Rosenkranz AC, Redox regulation of human protease-activated receptor-2 by activated factor X, Free Radic Biol Med, 2011;51:1758–1764, 10.1016/j.freeradbiomed.2011.08.003 [DOI] [PubMed] [Google Scholar]
- [106].Kitasato L, Yamaoka-Tojo M, Hashikata T, Ishii S, Kameda R, Shimohama T, Tojo T Ako J, Factor Xa in mouse fibroblasts may induce fibrosis more than thrombin, Int Heart J, 2014;55:357–361, [DOI] [PubMed] [Google Scholar]
- [107].Chen G, Chelu MG, Dobrev D Li N, Cardiomyocyte Inflammasome Signaling in Cardiomyopathies and Atrial Fibrillation: Mechanisms and Potential Therapeutic Implications, Front Physiol, 2018;9:1115, 10.3389/fphys.2018.01115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Yao C, Veleva T, Scott L Jr., Cao S, Li L, Chen G, Jeyabal P, Pan X, Alsina KM, Abu-Taha I, Ghezelbash S, Reynolds CL, Shen YH, LeMaire SA, Schmitz W, Muller FU, El-Armouche A, Eissa NT, Beeton C, Nattel S, Wehrens XHT, Dobrev D Li N, Enhanced Cardiomyocyte NLRP3 Inflammasome Signaling Promotes Atrial Fibrillation, Circulation, 2018, 10.1161/CIRCULATIONAHA.118.035202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Scott L Jr., Li N Dobrev D, Role of inflammatory signaling in atrial fibrillation, Int J Cardiol, 2018, 10.1016/j.ijcard.2018.10.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Monnerat G, Alarcon ML, Vasconcellos LR, Hochman-Mendez C, Brasil G, Bassani RA, Casis O, Malan D, Travassos LH, Sepulveda M, Burgos JI, Vila-Petroff M, Dutra FF, Bozza MT, Paiva CN, Carvalho AB, Bonomo A, Fleischmann BK, de Carvalho AC Medei E, Macrophage-dependent IL-1beta production induces cardiac arrhythmias in diabetic mice, Nat Commun, 2016;7:13344, 10.1038/ncomms13344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Ye X, Zuo D, Yu L, Zhang L, Tang J, Cui C, Bao L, Zan K, Zhang Z, Yang X, Chen H, Tang H, Zu J, Shi H Cui G, ROS/TXNIP pathway contributes to thrombin induced NLRP3 inflammasome activation and cell apoptosis in microglia, Biochem Biophys Res Commun, 2017;485:499–505, 10.1016/j.bbrc.2017.02.019 [DOI] [PubMed] [Google Scholar]
- [112].Hara T, Fukuda D, Tanaka K, Higashikuni Y, Hirata Y, Yagi S, Soeki T, Shimabukuro M Sata M, Inhibition of activated factor X by rivaroxaban attenuates neointima formation after wire-mediated vascular injury, Eur J Pharmacol, 2018;820:222–228, 10.1016/j.ejphar.2017.12.037 [DOI] [PubMed] [Google Scholar]
- [113].Song K, Wang Y, Sheng J, Ma C Li H, Effects of dabigatran regulates noreflow phenomenon in acute myocardial infarction mice through antiinflammatory and antioxidative activities and connective tissue growth factor expression, Mol Med Rep, 2018;17:580–585, 10.3892/mmr.2017.7861 [DOI] [PubMed] [Google Scholar]
- [114].Kondo H, Abe I, Fukui A, Saito S, Miyoshi M, Aoki K, Shinohara T, Teshima Y, Yufu K Takahashi N, Possible role of rivaroxaban in attenuating pressure-overload-induced atrial fibrosis and fibrillation, J Cardiol, 2018;71:310–319, 10.1016/j.jjcc.2017.08.007 [DOI] [PubMed] [Google Scholar]
- [115].Homma S, Thompson JL, Pullicino PM, Levin B, Freudenberger RS, Teerlink JR, Ammon SE, Graham S, Sacco RL, Mann DL, Mohr JP, Massie BM, Labovitz AJ, Anker SD, Lok DJ, Ponikowski P, Estol CJ, Lip GY, Di Tullio MR, Sanford AR, Mejia V, Gabriel AP, del Valle ML, Buchsbaum R Investigators W, Warfarin and aspirin in patients with heart failure and sinus rhythm, N Engl J Med, 2012;366:1859–1869, 10.1056/NEJMoa1202299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Zannad F, Anker SD, Byra WM, Cleland JGF, Fu M, Gheorghiade M, Lam CSP, Mehra MR, Neaton JD, Nessel CC, Spiro TE, van Veldhuisen DJ, Greenberg B Investigators CH, Rivaroxaban in Patients with Heart Failure, Sinus Rhythm, and Coronary Disease, N Engl J Med, 2018;379:1332–1342, 10.1056/NEJMoa1808848 [DOI] [PubMed] [Google Scholar]