Abstract
Background
Stage IIIA non-small cell lung cancer (NSCLC) is a heterogeneous group of patients, often requiring variable and individualized approaches. The dilemma to operate or not frequently arises, since more than 75% of the cases of NSCLC are diagnosed in advanced stages (IIIA). The main objective of this study was to assess whether the benefits outweigh surgical risks for the T4N0–1M0 subgroup.
Methods
Data from 857 patients with locally advanced T4 NSCLC were retrospectively collected from two different institutions, between 2002 and 2017. Clinical data that were retrieved and analyzed, included demographics, comorbidities, surgical details, neoadjuvant or/and adjuvant therapy and postoperative complications.
Results
Twelve patients were in the cardiopulmonary bypass (CPB) group and thirty in the non-CPB. The most common types of lung cancer were squamous cell carcinoma (50.0%) and adenocarcinoma (35.7%). The most frequent invasion of the tumor was seen in main pulmonary artery and the superior vena cava. Significantly more patients of the CPB group underwent pneumonectomy as their primary lung resection (P=0.006). In all patients R0 resection was achieved according to histological reports. The overall 5-year survival was 60%, while the median overall survival was 22.5 months. Analysis revealed that patient age (P=0.027), preoperative chronic obstructive pulmonary disease (COPD) (P=0.001), tumor size (4.0 vs. 6.0 cm) (P=0.001), postoperative respiratory dysfunction (P=0.001) and postoperative atelectasis (P=0.036) are possible independent variables that are significantly correlated with patient outcome.
Conclusions
We suggest that in patients with stage IIIA/T4 NSCLC, complete resection of the T4 tumor, although challenging, can be performed in highly selected patients. Such an approach seems to result in improved long-term survival. More specific studies on this area of NSCLC probably will further enlighten this field, and may result in even better outcomes, as advanced systemic perioperative approaches such as modern chemotherapy, immunotherapy and improvements in radiation therapy have been incorporated in daily practice.
Keywords: Extended resection, carcinoma, non-small cell lung cancer (NSCLC), cardiopulmonary bypass (CPB)
Introduction
It is known that stage IIIA non-small cell lung cancer (NSCLC) is a heterogeneous group of patients, often requiring variable and individualized approaches. In particular, for T4 tumors, although a complete resection is really challenging even for experienced thoracic surgeons, it remains unclear if there is a true benefit for the patients. T4 lesions are defined by local invasion of structures, such as the heart, great vessels, esophagus, trachea, carina, recurrent laryngeal nerve, vertebral body, so that they are frequently considered unresectable. Surgical attempts of resecting such tumors require advanced techniques, often including the use of cardiopulmonary bypass (CPB).
The dilemma to operate or not frequently arises, since more than 75% of the cases of NSCLC are diagnosed in advanced stages (IIIA) (1). Patients with locally advanced T4 NSCLC have a poor prognosis with systemic treatments, such as chemotherapy with or without radiation; the achievement of en bloc R0 resection seems to be a hopeful strategy, as it may yield a 43% 5-year survival rate (2). However, few surgical series have been reported examining separately the outcome in T4/stage IIIA NSCLC, so that the question of the value of resection of such tumors is not clearly established, for T4N0–1 patients (3). Determination of resectability, surgical staging, and extent of pulmonary resection should be made by a multidisciplinary approach. Accordingly, highly selected patients may benefit from multimodal therapy, including surgery (4).
In this study, we retrospectively analyzed the characteristics and the outcomes of patients with clinical stage IIIA (T4N0–1M0) NSCLC, who underwent an intended curative resection at two different cardiothoracic surgical centers in Europe. The main objectives of this study were to assess whether the benefits outweigh the surgical risks and if CPB was associated with worse outcome.
The study was approved by institutional ethics board of European Interbalkan Medical Center (No. 1287), while all patients had pre-operatively been through an informed-consent process where future publication of data was pre-authorized.
Methods
Patients
Data from 857 patients with locally advanced T4 NSCLC were retrospectively collected from two different institutions between 2002 and 2017. At the Cardiothoracic Department of Interbalkan Medical Center of Thessaloniki (Greece), 29 patients underwent pulmonary resections for T4 NSCLC and by the Thoracic Department of St James University Hospital of Leeds (UK), 13 patients were treated for the same reason, for a total of 42 patients.
Clinical data that were retrieved and analyzed, included demographics (age, gender), comorbidities [chronic obstructive pulmonary disease (COPD), coronary artery disease (CAD), myocardial infarction (MI), diabetes, stroke, hypertension, renal dysfunction], tumor characteristics (histology, T status, pathological stage, topography), lymph node invasion (pathological N status), organ invasion (thoracic inlet, superior vena cava, inferior vena cava, trachea, carina, pericardium, intra-pericardial pulmonary artery, left atrium, right atrium, thoracic aorta, esophagus, diaphragm, vertebra), surgical approach, resection type, use of CPB, use of neoadjuvant therapy, use of adjuvant therapy and postoperative complications [atrial fibrillation (AF), MI, stroke, pneumonia, atelectasis, respiratory failure (as defined by prolonged need for non-invasive oxygenation due to desaturation), renal failure (as defined by exceeding the baseline serum creatinine level), reoperation for bleeding].
Preoperative workup
As per routine, all patients underwent a preoperative evaluation, including staging [chest computed tomography (CT) scan, brain CT, upper abdomen CT and bone scan or brain magnetic resonance imaging (MRI) and positron emission tomography with CT (PET-CT scan)], flexible fiberoptic bronchoscopy, respiratory mechanics tests (spirometry), lung parenchyma function tests [diffusion capacity for carbon monoxide (DLCO), arterial blood gas analysis], cardiopulmonary interaction tests (stair climbing, 6-minute walk, VO2max), transthoracic echocardiogram, routine biochemical profile and blood tests.
On clinical grounds, a few patients underwent additionally chest MRI, ventilation perfusion lung scintigraphy and vibration response imaging (VRIxp™). Mediastinoscopy or endobronchial ultrasound (EBUS) was performed only in patients with a mediastinal lymph node larger than 1 cm or PET-positive. We excluded in this study patients, who had only chest wall invasion. All selected cases were discussed in a multidisciplinary team meeting involving chest physicians, thoracic surgeons, chest radiologist, clinical and medical oncologists and histopathologists; such setting is available in both centers.
Operative methods
All patients underwent general anesthesia with double lumen tube, except of cases with central airway invasion, where special ventilation techniques, namely jet ventilation, time limited apneic diffusion oxygenation or use of CPB/extracorporeal membrane oxygenation (ECMO). We selected the most appropriate surgical approach according to tumor invasion: median sternotomy, posterolateral thoracotomy, clamshell or hemi clamshell, Dartevelle, Shaw-Paulson and cervical incision. The decision of using CPB was performed in selected patients intraoperatively, with peripheral cannulation sites and beating heart. In cases with invasion of great vessels or atrium, after resection, the affected structure was reconstructed by autologous or heterologous pericardial patch.
All patients were followed up initially at the tertiary center for 6 months. Subsequently, the follow-up protocol was organized by the medical oncologist; outpatient visits were planned every 3 months for the first 2 years and annually thereafter.
Statistical analysis
Statistical analysis was performed using the statistical package IMB SPSS Statistics v.20.0. All results are expressed as medians and ranges or as absolute numbers and percentages. Statistical significance was evaluated using the χ2 test with P value of less than 0.05. Survival curves were calculated using the Kaplan-Meier method. Univariate comparison was performed using a Cox proportional hazard model. Two post hoc groups, one with CPB and the non-CBP groups were analyzed separately and outcomes were compared.
Results
Patient characteristics
A summary of the demographic and clinical data recorded is listed in Table 1. There were 11 female patients (26.2%) among the 42 patients. Twelve patients were in the CPB group and 30 in the non-CPB. The median age was 65.0±12.8 (range, 28–75) years for the CPB group and 63.5±11.8 (range, 24–70) years for the non-CPB group. No statistically significant differences were found for medical comorbidities between the two groups. The median size of the tumor was 4.0±1.9 cm in the non-CPB group and 6.0±2.2 cm in the CPB group. The most common types of lung cancer were squamous cell carcinoma (SCC) (50.0%) and adenocarcinoma (35.7%). The most frequent invasion of the tumor was seen in main pulmonary artery and the superior vena cava.
Table 1. Patient demographics and comorbidities—tumor characteristics.
| Variables | Total | No CPB or standby | On CPB | P value | |||||
|---|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | ||||
| Demographic | |||||||||
| Patient number | 42 | 100.0 | 30 | 71.4 | 12 | 28.6 | – | ||
| Age, years (median) | 64.5±12.0 | 63.5±11.8 | 65.0±12.8 | 0.811 | |||||
| Sex | 0.464 | ||||||||
| Male | 31 | 73.8 | 21 | 50.0 | 10 | 23.8 | |||
| Female | 11 | 26.2 | 9 | 21.4 | 2 | 4.8 | |||
| Center | 0.007 | ||||||||
| GR | 29 | 69.0 | 17 | 40.5 | 12 | 28.6 | |||
| UK | 13 | 31.0 | 13 | 31.0 | 0 | 0.0 | |||
| Medical comorbidities | |||||||||
| COPD | 14 | 33.3 | 9 | 21.4 | 5 | 11.9 | 0.491 | ||
| Hypertension | 3 | 7.1 | 2 | 4.8 | 1 | 2.4 | 1.000 | ||
| CAD | 2 | 4.8 | 2 | 4.8 | 0 | 0.0 | 1.000 | ||
| MI | 2 | 4.8 | 2 | 4.8 | 0 | 0.0 | 1.000 | ||
| Diabetes | 3 | 7.1 | 3 | 7.1 | 0 | 0.0 | 0.541 | ||
| Stroke | 1 | 2.4 | 1 | 2.4 | 0 | 0.0 | 1.000 | ||
| Renal dysfunction | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | – | ||
| Smoking | 30 | 71.4 | 21 | 50.0 | 9 | 21.4 | 1.000 | ||
| Tumor characteristics | |||||||||
| Size, cm (median) | 4.5±2.0 | 4.0±1.9 | 6.0±2.2 | 0.530 | |||||
| Pathology | |||||||||
| Squamous | 21 | 50.0 | 18 | 42.8 | 3 | 7.1 | 0.040 | ||
| Adenocarcinoma | 15 | 35.7 | 8 | 19.0 | 7 | 16.7 | 0.053 | ||
| Large cell | 1 | 2.4 | 1 | 2.4 | 0 | 0.0 | 0.522 | ||
| Atypical carcinoid | 2 | 4.8 | 2 | 4.8 | 0 | 0.0 | 0.359 | ||
| Adenocystic | 2 | 4.8 | 1 | 2.4 | 1 | 2.4 | 0.492 | ||
| Glomus | 1 | 2.4 | 0 | 0.0 | 1 | 2.4 | 0.110 | ||
| Adjacent anatomic structure invasion | |||||||||
| Thoracic inlet | 4 | 9.5 | 3 | 7.1 | 1 | 2.4 | 0.931 | ||
| SVC | 8 | 19.0 | 6 | 14.2 | 2 | 4.8 | 0.804 | ||
| IVC | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | – | ||
| Trachea | 2 | 4.8 | 0 | 0.0 | 2 | 4.8 | 0.022 | ||
| Carina | 6 | 14.3 | 4 | 9.5 | 2 | 4.8 | 0.780 | ||
| Pericardium | 7 | 16.7 | 2 | 4.8 | 5 | 11.9 | 0.006 | ||
| Pulmonary artery | 9 | 21.4 | 8 | 19.0 | 1 | 2.4 | 0.191 | ||
| Intrapericardial PA | 4 | 9.5 | 1 | 2.4 | 3 | 7.1 | 0.031 | ||
| Left atrium | 3 | 7.1 | 1 | 2.4 | 2 | 4.8 | 0.130 | ||
| Right atrium | 1 | 2.4 | 0 | 0.0 | 1 | 2.4 | 0.110 | ||
| Thoracic aorta | 3 | 7.1 | 0 | 0.0 | 3 | 7.1 | 0.004 | ||
| Esophagus | 1 | 2.4 | 0 | 0.0 | 1 | 2.4 | 0.110 | ||
| Diaphragm | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | – | ||
| Vertebra | 2 | 4.8 | 2 | 4.8 | 0 | 0.0 | 0.359 | ||
| Surgical approach | |||||||||
| Thoracotomy | 33 | 78.6 | 26 | 61.9 | 7 | 16.7 | 0.086 | ||
| Sternotomy | 1 | 2.4 | 0 | 0.0 | 1 | 2.4 | 0.110 | ||
| Cervical | 2 | 4.8 | 0 | 0.0 | 2 | 4.8 | 0.022 | ||
| Clamshell | 2 | 4.8 | 0 | 0.0 | 2 | 4.8 | 0.022 | ||
| Hemi-Clamshell | 1 | 2.4 | 1 | 2.4 | 0 | 0.0 | 0.522 | ||
| Dartevelle | 1 | 2.4 | 1 | 2.4 | 0 | 0.0 | 0.522 | ||
| Shaw-Paulson | 2 | 4.8 | 2 | 4.8 | 0 | 0.0 | 0.359 | ||
| Pulmonary resection | |||||||||
| Lobectomy | 14 | 33.3 | 11 | 26.2 | 3 | 7.1 | 0.469 | ||
| Sleeve lobectomy | 8 | 19.0 | 7 | 16.7 | 1 | 2.4 | 0.263 | ||
| Double sleeve lobectomy | 6 | 14.3 | 6 | 14.3 | 0 | 0.0 | 0.094 | ||
| Pneumonectomy | 7 | 16.7 | 2 | 4.8 | 5 | 11.9 | 0.006 | ||
| Sleeve pneumonectomy | 4 | 9.5 | 3 | 7.1 | 1 | 2.4 | 0.868 | ||
| Resection of trachea | 3 | 7.1 | 1 | 2.4 | 2 | 4.8 | 0.130 | ||
| CBP details | |||||||||
| Cannulation site | – | – | – | – | Femo-femoral | – | |||
| Time (min) | – | – | – | – | 45.0±7.0 | – | |||
CPB, cardiopulmonary bypass; COPD, chronic obstructive pulmonary disease; CAD, coronary artery disease; MI, myocardial infraction; SVC, superior vena cava; IVC, inferior vena cava; PA, pulmonary artery.
Surgical details
The most preferable surgical approach was the posterolateral thoracotomy (61.9%). Significantly more patients of the CPB group underwent pneumonectomy as their primary lung resection (P=0.006). The surgical operation was completed in most cases without the usage of the CPB (30 vs. 12 patients). For the patients who required CBP, the preferable cannulation site was the right femo-femoral site, with mean duration 45.0±7.0 minutes. The use of the CPB was intraoperatively decided in 14 cases, but only used at the 12 cases (CPB standby in 2 cases). In all patients R0 resection was achieved according to histological reports. A small sample of surgical techniques is presented in Figures 1-3.
Figure 1.
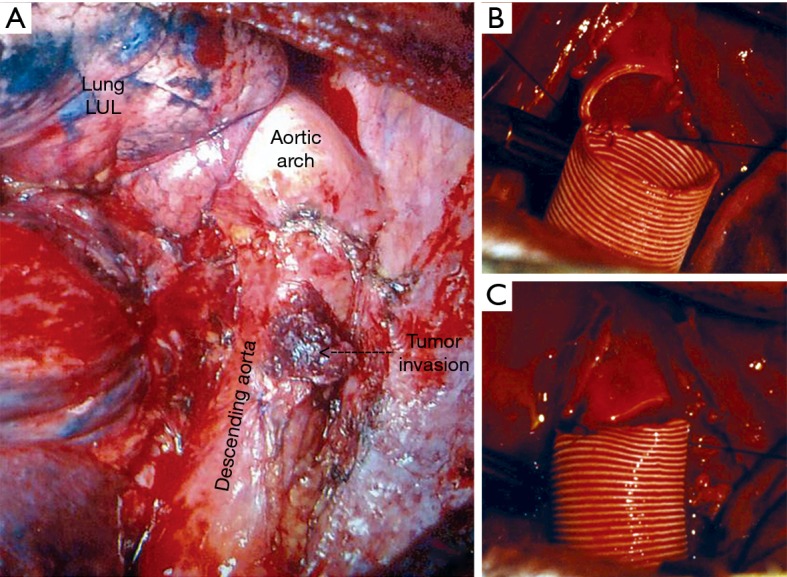
Descending aorta invasion resection and reconstruction with synthetic graft. (A) Tumor invading the wall of descending aorta; (B) the begging of end to end proximal aorta-graft anastomosis; (C) completing of proximal aorta-graft anastomosis. LUL, left upper lobe.
Figure 2.
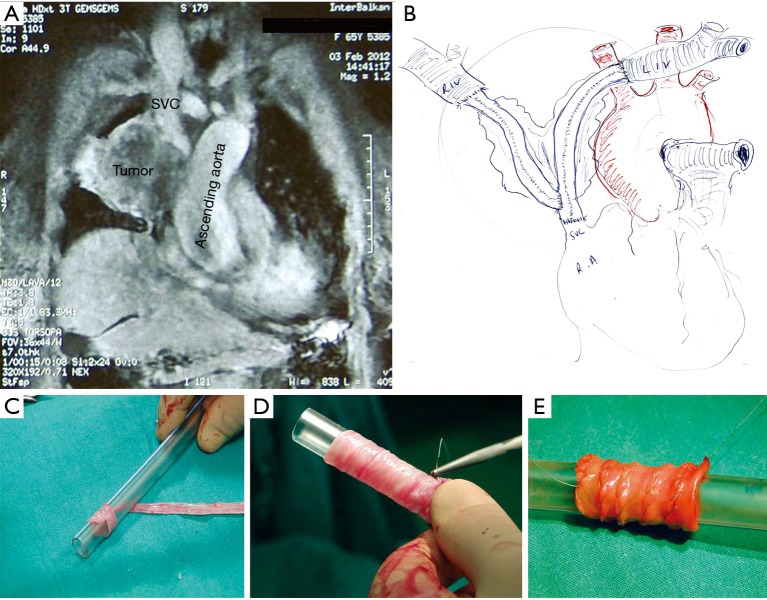
Superior vena cava (SVC) invasion resection and reconstruction with native venous-graft. (A) Tumor invading the SVC (chest MRI—coronal view); (B) handmade sketch of SVC and anonymous reconstruction with native graft constructed by saphenous vein; (C,D,E) stages of native venous-grafts construction by saphenous vein. MRI, magnetic resonance imaging.
Figure 3.
Surgical field after resection of tumor invasion of thoracic spine and spondylodesis.
Postoperative complications
The postoperative outcomes are presented at the Table 2. The median length of the in overall hospital stay was similar in the two groups (8.0±4.7 days). The rate of major postoperative complications was different in the two groups. The most frequent observed complications were AF (14.3%) and atelectasis (14.3%). Blood transfusion was significantly higher in the CBP group (P=0.010). Thirty-day mortality was zero in both groups. Most of the patient received adjuvant chemotherapy (76.2%), while fewer received additionally adjuvant radiotherapy (40.5%). Hematogenous tumor dissemination of patients undergoing CPB was not observed.
Table 2. Patient postoperative characteristics.
| Characteristics | Total | No CPB or standby | On CPB | P value | |||||
|---|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | ||||
| Adjuvant therapy | |||||||||
| Chemotherapy | 32 | 76.2 | 22 | 52.4 | 10 | 23.8 | 0.696 | ||
| Radiotherapy | 17 | 40.5 | 11 | 26.2 | 6 | 14.3 | 0.498 | ||
| In hospital stay (days) median | 8.0±4.7 | 8.0±5.5 | 8.0±1.5 | 0.590 | |||||
| Postoperative complications | |||||||||
| AF | 6 | 14.3 | 3 | 7.1 | 3 | 7.1 | 0.329 | ||
| MI | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | – | ||
| Pneumonia | 4 | 9.5 | 3 | 7.1 | 1 | 2.4 | 1.000 | ||
| Respiratory failure | 2 | 4.8 | 2 | 4.8 | 0 | 0.0 | 1.000 | ||
| Atelectasis | 6 | 14.3 | 5 | 11.9 | 1 | 2.4 | 0.655 | ||
| Reoperation | 1 | 2.4 | 1 | 2.4 | 0 | 0.0 | 1.000 | ||
| Pulmonary edema | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | – | ||
| Renal hemodialysis | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | – | ||
| Blood transfusion (units) | 1.3±1.1 | 0.8±1.0 | 2.4±0.6 | 0.010 | |||||
| 30-day mortality | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | – | ||
CPB, cardiopulmonary bypass; AF, atrial fibrillation; MI, myocardial infraction.
Overall survival (OS)
The overall 5-year survival was 60%, while the median OS was 22.5 months (Figure 4). Between the two groups there was no statistically significant difference in OS (P=0.353), as presented in Figure 5. We tried to identify a possible predictive factor for survival, using the Cox regression analysis by various characteristics and the results are shown on Table 3. Analysis revealed that patient age (P=0.027), preoperative COPD (P=0.001), tumor size (4.0 vs. 6.0 cm) (P=0.001), postoperative respiratory dysfunction (P=0.001) and postoperative atelectasis (P=0.036) are possible independent variables that are significant correlated with patient outcome (Figure 6).
Figure 4.
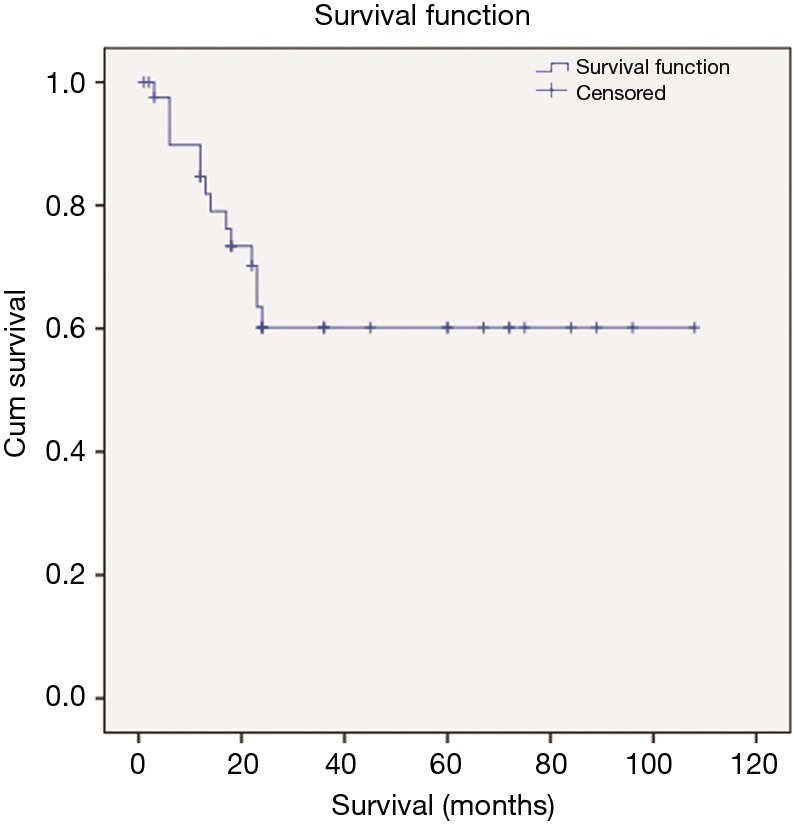
Kaplan-Meier plot for the full group of participants.
Figure 5.
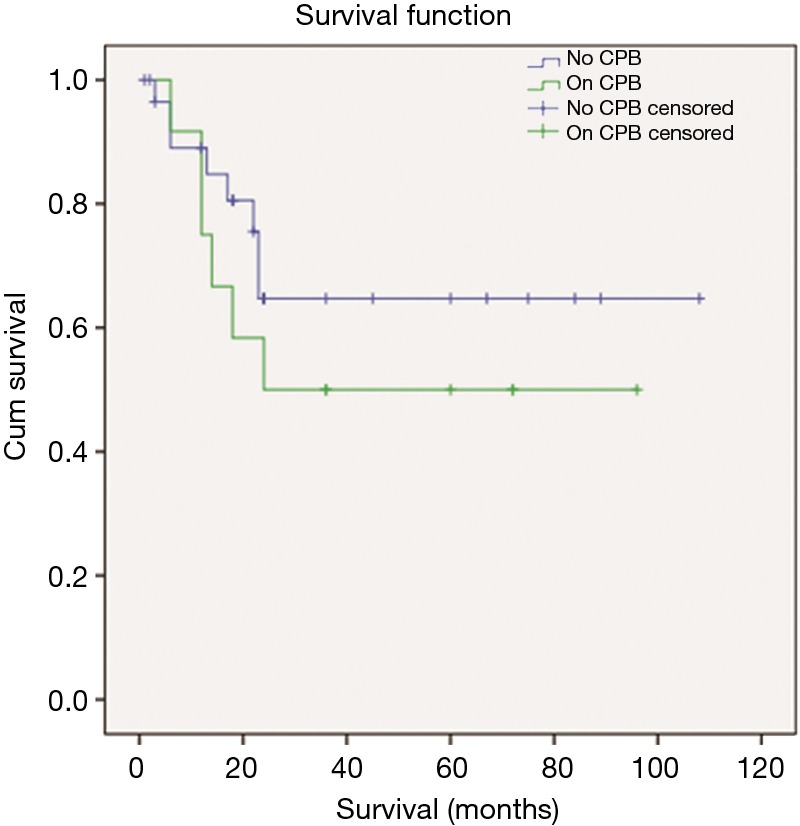
Kaplan-Meier plot for the groups A (no CPB) and B (on CPB). CPB, cardiopulmonary bypass.
Table 3. Cox regression in survival.
| Variable | P value |
|---|---|
| Demographic | |
| Age (years) | 0.027 |
| Medical comorbidities | |
| COPD | 0.001 |
| Hypertension | 0.999 |
| CAD | 0.390 |
| MI | 0.390 |
| Diabetes | 0.999 |
| Stroke | 0.257 |
| Renal dysfunction | – |
| Smoking | 0.244 |
| Tumor characteristics | |
| Size (cm) | 0.001 |
| Pathology | 0.288 |
| Adjacent anatomic structure invasion | 0.648 |
| Surgical | |
| Approach | 0.124 |
| CPB | 0.359 |
| Resection type | 0.345 |
| Postoperative | 0.017 |
| AF | 0.224 |
| Pneumonia | 0.085 |
| Respiratory dysfunction | 0.001 |
| Atelectasis | 0.036 |
| Adjuvant therapy | |
| Chemotherapy | 0.886 |
| Radiotherapy | 0.775 |
COPD, chronic obstructive pulmonary disease; CAD, coronary artery disease; MI, myocardial infraction; CPB, cardiopulmonary bypass; AF, atrial fibrillation.
Figure 6.
Cox proportional-hazards model (independent variables that are significant correlated with patient outcome). COPD, chronic obstructive pulmonary disease.
Discussion
This retrospective study was undertaken to quantify outcomes of surgical management of the primary pulmonary tumor in patients with clinical stage IIIA (T4N0–1M0), using data of two different centers. In order to assess the clinical usefulness of such resections, we attempted to identify patient and tumor factors associated with survival. Such an endeavor might identify parameters that could potentially improve future patient selection. Additionally, we intended to test the hypothesis, that surgical resection with CPB is associated with worst perioperative or long-term survival compared to surgical resection without CPB.
The limited number of patients with potentially operable stage IIIA disease is a main reason why current evidence for this topic is generally limited to small single-institution studies (Table 4). T4 tumors that invade the heart, great vessels, thoracic vertebrae, or esophagus comprise a heterogeneous group of locally invasive lung cancers. Current National Comprehensive Cancer Network guidelines do not recommend surgery for T4 extension with N2–3 disease (stage IIIB). However, biopsy-proven T4N0–1 (stage IIIA) may be operable (5). Localized tumors with invasion of the aorta, pulmonary artery, left atrium, thoracic vertebrae, or esophagus represent only small subset of T4 disease.
Table 4. Review of publication for extended lung resections.
| Publication year | Author | Country | No. patient | Period | |
|---|---|---|---|---|---|
| From | To | ||||
| 1971 | Charles P. Bailey | USA | 2 | – | – |
| 1991 | Takayuki Shirakusa | Japan | 12 | – | – |
| 1993 | Maeda | Japan | 42 | – | – |
| 1994 | Ryosuke Tsuchiya | Japan | 101 | 1962 | 1991 |
| 1994 | Nael Martini | USA | 44 | 1974 | 1984 |
| 1994 | Thomas | France | 15 | 1981 | 1991 |
| 1994 | Roviaro | – | 28 | 1983 | 1992 |
| 1995 | Dartevelle | France | 14 | – | – |
| 1995 | Jakob R. Izbicki | Germany | 94 | 1987 | 1990 |
| 1995 | Tatsuo Fukuse | Japan | 42 | 1976 | 1993 |
| 1996 | Dartevelle and Macchiarini | – | 60 | – | – |
| 1996 | Pitz | The Netherlands | 70 | 1977 | 1993 |
| 1997 | Fukuse | Japan | 42 | 1976 | 1993 |
| 1999 | Takao Takahashi | Japan | 49 | 1980 | 1996 |
| 1999 | Walter Klepetko | Austria | 7 | 1991 | 1996 |
| 1999 | Mitchell | USA | 135 | 1962 | 1999 |
| 2000 | Spaggiari | France | 25 | 1983 | 1996 |
| 2001 | Mitchell | USA | 60 | 1973 | 1998 |
| 2001 | Victor A. Tarasov | Russia | 50 | – | – |
| 2001 | Oda | Japan | 24 | 1981 | 1999 |
| 2001 | Bernard A | France | 77 | 1990 | 1998 |
| 2002 | Mezzetti | Italy | 27 | 1979 | 1999 |
| 2002 | Spaggiari | France | 93 | 1985 | 2000 |
| 2002 | Ara A. Vaporciyan | USA | 19 | – | – |
| 2002 | Porhanov | Russia | 151 | 1979 | 2002 |
| 2003 | Cordula C.M. Pitz | The Netherlands | 89 | – | – |
| 2003 | Seiki Hasegawa | Japan | 11 | – | – |
| 2003 | Pitz | The Netherlands | 89 | 1977 | 1993 |
| 2004 | Spaggiari | Italy | 15 | 1963 | 2000 |
| 2004 | John G. Byrne | USA | 14 | – | – |
| 2004 | Ratto | Italy | 19 | 1996 | 2004 |
| 2004 | Bobbio | Italy | 23 | 1982 | 2001 |
| 2005 | Regnard | France | 65 | 1983 | 2002 |
| 2005 | Shargall | Canada | 15 | 1988 | 2003 |
| 2005 | Lorenzo Spaggiari | Italy | 15 | – | – |
| 2005 | Mitsunori Ohta | Japan | 16 | – | – |
| 2006 | Macchiarini | Spain | 50 | 2000 | 2006 |
| 2006 | Roviaro | Italy | 53 | 1983 | 2004 |
| 2006 | De Perrot | France | 119 | 1981 | 2004 |
| 2008 | Rea | Italy | 49 | 1982 | 2005 |
| 2008 | Francesco Petrella | Italy | 21 | – | – |
| 2008 | Bedrettin Yýldýzeli | France | 271 | – | – |
| 2009 | Wu | China | 46 | 2000 | 2006 |
| 2010 | Wang | China | 48 | 1996 | 2008 |
| 2013 | Lorenzo Spaggiari | Italy | 125 | 1998 | 2010 |
| 2014 | Geraud Galvaing | – | 19 | 2004 | 2012 |
| 2016 | Waldemar Schreiner1 | Germany | 9 | – | – |
| 2016 | Langer | France | 373 | 1980 | 2013 |
Randomized data providing proof of a survival advantage in patients undergoing extended resections for these neoplasms are lacking. However, accumulated published experience seems to support the merits of a surgical approach on individualizing basis (5). We found, in agreement with literature (6), that the surgical approach was an appropriate therapeutic option in our selected patients, as the median OS was 22.5 months, and the overall 5-year survival was 60%.
The value of CPB has been reported for thoracic malignancies invading the heart or great vessels (7,8). CPB was used to resect tumor invading the aortic arch, the descending aorta, the pulmonary artery bifurcation, the left atrium, and the carina. However, few authors have reported their experience with CPB in lung cancer (9,10). In these studies authors confirmed the safety of CPB for NSCLC invading the great vessels and/or the left atrium in well-selected cases (7 patients in each of them), but they did not report long-term survival. Within the limitations of the small numbers of our subjects and no data related to the cause of deaths, we found no difference on (I) postoperative complications, (II) 30-day mortality and (III) the OS between the 12 patients underwent CBP and the 30 patients that weren’t. To our experience, CPB was not associated with cancer dissemination. Our working hypothesis is that the application of adjuvant chemotherapy played a role in suppressing possible recurrences.
Long-term outcome of patients with locally advanced lung cancer depends primarily on the completeness of resection (R0). Data reported a series of lung cancer invading the mediastinum, and observed that the 5-year survival rate was 30%, if the tumor was completely resected, whereas it was only 14%, if it was incompletely resected (11). Others made similar observations in a series of lung cancers invading the heart or great vessels, with a 5-year survival of 40%, if the tumor was completely resected and much lower if the tumor was incompletely resected (12). In another study, the completeness of surgical resection in NSCLC stage IV invading the pulmonary sulcus and spine was a statistically significant predictor for 5-year survival; patients with an R0 resection have a 69% survival compared with 0% when the resection was incomplete (13). We suggest that a detailed mapping of the lung tumor extension and a preoperational decision regarding the use of CBP, give the best results. Of the 14 patients scheduled to undergo CBP, we eventually canceled only two, as it was not necessary. We succeed in all patients complete resection of the tumor. Postoperative complications were reasonable and the 30-day mortality was zero. Additionally, in agreement with the literature (14,15), the use of CPB does not appear to increase the risk of cancer dissemination as none of our patients had evidence of intrathoracic disease 5 years after the procedure. If the tumor extent remains a problem, in a subgroup of patients re-implantation or auto-transplantation techniques can be considered for cases in which is technically feasible, as extensive pulmonary resection can thus be performed, while minimizing the loss of pulmonary reserve (16,17).
It is important to identify prognostic factors to help select optimal surgical candidates. In that regard, there have been few publications with inconclusive results. In one study patient gender, EGFR mutation, N factor, and M factor showed no statistically significant effect on survival. However, not having SCC and being in the M-better group were significantly associated with improved survival (6). Others, in univariate analysis reported that smaller tumor size, fewer pack-years of cigarettes smoked, female sex, lower T factor, N factor, and overall pathologic stage were associated with improved survival, but only female gender remained an independent predictor of survival in multivariate analysis (18). We were able to identify patient age, preoperative history of COPD, tumor size, postoperative respiratory dysfunction and postoperative atelectasis are possible independent variables correlating with patient outcome. Among them, preoperative COPD, tumor size and postoperative respiratory dysfunctions can strongly influence the OS.
There are several limitations of this study. First, because of the retrospective nature of this study, the results are based on a highly selected group of patients, so that inherent biases cannot be excluded. Second, as the incidence of this entity is low, it was difficult to make conclusions about certain subgroups, such as different histology or to distinguish outcomes achieved in patients with different sites of lung invasion. Despite these serious limitations, dome guidance helping patient selection for this kind of surgical procedure can be discerned.
In conclusion, we suggest that in patients with stage IIIA (T4N0–1M0) NSCLC, the complete resection of the T4 tumor, although challenging, can be performed in highly selected patients. Such an approach seems to result in improved long-term survival. More specific studies on this area of NSCLC probably will further enlighten this field, and may result in even better outcomes incorporating advanced systemic perioperative approaches such as modern chemotherapy, immunotherapy and improvements in radiation therapy.
Acknowledgments
None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by institutional ethics board of European Interbalkan Medical Center (No. 1287), while all patients had pre-operatively been through an informed-consent process where future publication of data was pre-authorized.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Varela G, Thomas PA. Surgical management of advanced non-small cell lung cancer. J Thorac Dis 2014;6 Suppl 2:S217-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Langer NB, Mercier O, Fabre D, et al. Outcomes After Resection of T4 Non-Small Cell Lung Cancer Using Cardiopulmonary Bypass. Ann Thorac Surg 2016;102:902-10. 10.1016/j.athoracsur.2016.03.044 [DOI] [PubMed] [Google Scholar]
- 3.Dartevelle PG, Mitilian D, Fadel E. Extended surgery for T4 lung cancer: a 30 years’ experience. Gen Thorac Cardiovasc Surg 2017;65:321-8. 10.1007/s11748-017-0752-6 [DOI] [PubMed] [Google Scholar]
- 4.Galvaing G, Chadeyras JB, Merle P, et al. Extended resection of non-small cell lung cancer invading the left atrium, is it worth the risk? Chin Clin Oncol 2015;4:43. [DOI] [PubMed] [Google Scholar]
- 5.Goldstraw P, Chansky K, Crowley J, et al. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J Thorac Oncol 2016;11:39-51. 10.1016/j.jtho.2015.09.009 [DOI] [PubMed] [Google Scholar]
- 6.Chikaishi Y, Shinohara S, Kuwata T, et al. Complete resection of the primary lesion improves survival of certain patients with stage IV non-small cell lung cancer. J Thorac Dis 2017;9:5278-87. 10.21037/jtd.2017.11.67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vaporciyan AA, Rice D, Correa AM, et al. Resection of advanced thoracic malignancies requiring cardiopulmonary bypass. Eur J Cardiothorac Surg 2002;22:47-52. 10.1016/S1010-7940(02)00204-X [DOI] [PubMed] [Google Scholar]
- 8.Byrne JG, Leacche M, Agnihotri AK, et al. The use of cardiopulmonary bypass during resection of locally advanced thoracic malignancies: a 10-year two-center experience. Chest 2004;125:1581-6. 10.1378/chest.125.4.1581 [DOI] [PubMed] [Google Scholar]
- 9.Tsuchiya R, Asamura H, Kondo H, et al. Extended resection of the left atrium, great vessels, or both for lung cancer. Ann Thorac Surg 1994;57:960-5. 10.1016/0003-4975(94)90214-3 [DOI] [PubMed] [Google Scholar]
- 10.de Perrot M, Fadel E, Mussot S, et al. Resection of locally advanced (T4) non-small cell lung cancer with cardiopulmonary bypass. Ann Thorac Surg 2005;79:1691-6; discussion 1697. [DOI] [PubMed]
- 11.Martini N, Yellin A, Ginsberg RJ, et al. Management of non-small cell lung cancer with direct mediastinal involvement. Ann Thorac Surg 1994;58:1447-51. 10.1016/0003-4975(94)91933-X [DOI] [PubMed] [Google Scholar]
- 12.Fukuse T, Wada H, Hitomi S. Extended operation for non-small cell lung cancer invading great vessels and left atrium. Eur J Cardiothorac Surg 1997;11:664-9. 10.1016/S1010-7940(96)01140-2 [DOI] [PubMed] [Google Scholar]
- 13.Collaud S, Waddell TK, Yasufuku K, et al. Long-term outcome after en bloc resection of non-small-cell lung cancer invading the pulmonary sulcus and spine. J Thorac Oncol 2013;8:1538-44. 10.1097/01.JTO.0000437419.31348.a4 [DOI] [PubMed] [Google Scholar]
- 14.Klepetko W, Wisser W, Bîrsan T, et al. T4 lung tumors with infiltration of the thoracic aorta: is an operation reasonable? Ann Thorac Surg 1999;67:340-4. 10.1016/S0003-4975(98)01244-2 [DOI] [PubMed] [Google Scholar]
- 15.Horita K, Higuchi S, Nakayama Y, et al. An updated report of a case of lung cancer resected using cardiopulmonary bypass. Thorac Cardiovasc Surg 1997;45:100-1. 10.1055/s-2007-1013698 [DOI] [PubMed] [Google Scholar]
- 16.Tryfon S, Zarogoulidis P, Tsavlis D, et al. Ex situ reimplantation technique, in central lung tumors. Ann Transl Med 2015;3:178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Emmanouilides C, Tryfon S, Baka S, et al. Operation for preservation of lung parenchyma in central lung cancer--in vivo and ex situ reimplantation techniques. Anticancer Res 2015;35:1675-81. [PubMed] [Google Scholar]
- 18.Finley DJ, Yoshizawa A, Travis W, et al. Predictors of outcomes after surgical treatment of synchronous primary lung cancers. J Thorac Oncol 2010;5:197-205. 10.1097/JTO.0b013e3181c814c5 [DOI] [PubMed] [Google Scholar]