Abstract
Background
QFT-Plus is a recently developed next-generation QuantiFERON-TB Gold In-Tube (QFT-GIT) test. Unlike the QFT-GIT test, it includes a TB2 antigen tube with peptides that may stimulate CD8+ T cells. This study evaluated the diagnostic performance of QFT-Plus and compared it with that of QFT-GIT.
Methods
QFT-Plus and QFT-GIT tests were performed in 33 patients with active tuberculosis (TB) and 57 healthy controls including subjects with latent TB infection (LTBI). Positivity and negativity of IFN-γ responses were compared between tests, and total concordance of the outcome was analyzed.
Results
Positive and negative outcomes of QFT-Plus and QFT-GIT tests showed substantial agreement (91.1%, kappa=0.8). The sensitivity and the specificity of QFT-Plus (93.9% sensitivity, 92.6% specificity) were similar with those of QFT-GIT (93.9% sensitivity, 100% specificity). Of eight discordant results, five (5.56%) and three (3.3%) were positive in QFT-GIT alone and QFT-plus alone, respectively. Reactivity in the TB2 tube contributes to the difference between QFT-GIT and QFT-Plus. Median IFN-γ production in TB2 (10.0 IU/mL in TB, 3.850 IU/mL in LTBI, P=0.001) is significantly higher in the TB group than the LTBI group. The QFT-Plus did not clearly discriminate between active TB and latent TB, although it showed significantly lower IFN-γ concentrations compared with the QFT-GIT in individuals with LTBI (3.850 vs. 7.205 IU/mL).
Conclusions
Similar accuracy of detecting Mycobacterium tuberculosis infection was observed for QFT-Plus and QFT-GIT tests in immunocompetent patients and healthy controls, including those with LTBI. Improved efficacy for identifying M. tuberculosis infection was not found with the QFT-Plus, but further studies in a larger population may confirm the clinical significance of positive response in the TB2 tube of QFT-Plus.
Keywords: Diagnosis, latent tuberculosis infection (LTBI), Mycobacterium tuberculosis (M. tb), QuantiFERON-TB Gold In-Tube (QFT-GIT), QFT-Plus, tuberculosis (TB)
Introduction
Tuberculosis (TB) is a global disease caused by infection with Mycobacterium tuberculosis (M. tb) (1). There were an estimated 10 million cases of TB and 1.3 million deaths worldwide in 2017. While latent TB infection (LTBI) has no risk of transmission, it is a reservoir of TB disease that may progress to active TB in 5–15% of infected subjects during their lifetime (2). Thus, early diagnosis and treatment of LTBI is important for ultimately controlling TB (3). In South Korea, TB incidence and death rate are still the highest among Organization for Economic Co-operation and Development (OECD) countries (incidence: 70/100,000, death: 5/100,000), although they decline every year (1,4). Based on the seventh Korean National Health and Nutrition Examination Survey, estimated LTBI prevalence in South Korea is 33.2% (5). The prevalence of LTBI using the interferon-γ release assay (IGRA) test ranged from 2.1% to 34% (total 11.6%) among congregated settings (6). The Korean Centers for Disease Control and prevention established a TB prevention project by providing employees working at medical institutions or daycare centers with LTBI checkups and treatment (7).
Diagnosis of LTBI has been performed by tuberculin skin test (TST) and T-cell IGRA. Compared with the TST, IGRAs only require one patient visit and do not yield false-positive results in Bacillus-Calmette-Guerin vaccinated subjects (8). However, IGRA has poor sensitivity in immunocompromised patients and children (9,10). It cannot distinguish between active TB and LTBI (8) and poorly correlates with developing active disease (11). To improve the efficacy of IGRA, QuantiFERON-TB Gold Plus (QFT-Plus) has been recently developed as a next-generation QuantiFERON-TB Gold In-Tube (QFT-GIT) test. QFT-Plus contains two TB-specific antigen coated tubes, TB1 and TB2.The antigen tubes contain 6-kDa early secretory antigenic target (ESAT-6) and 10-kDa culture filtrate protein (CFP-10), but not TB7.7. The long synthetic peptides in TB1 tubes are designed to stimulate CD4-T cells, as does QFT-GIT. However, the QFT-Plus includes an additional TB2 antigen tube which contains six short peptides that may also induce CD8 T-cell specific immune responses (12). Test performance of the QFT-Plus has mostly been reported in low incidence settings (13-16) or in immuncompromised patients (17). The diagnostic sensitivity of QFT-Plus was similar with QFT-GIT in a country where TB prevalence is low (13,14), but the data on the performance of two assays are controversial; Tsuyuzaki et al. reported that QFT-Plus revealed higher positivity and increased IFN-γ production compared with QFT-GIT in TB contact investigation (18). However, lower IFN-γ concentration was observed by QFT-Plus compared with QFT-GIT based on the report by Yi et al. (15). In addition, evaluation of QFT-Plus is still lacking in countries with intermediate/high TB burden. Therefore, the new version of IGRA, QFT-Plus needs to be investigated in diverse population including endemic settings.
In this study, the performance of QFT-Plus was tested in immunocompetent subjects including patients with active TB, individuals with LTBI, and non-infected healthy controls. IFN-γ responses to TB1 and TB2 antigens were analyzed alone and combined. The efficacy of the QFT-Plus for identifying M. tb infection was compared with the performance of QFT-GIT, a conventional IGRA.
Methods
Enrollment of study subjects
This study was approved by the ethical committee of Chuncheon Sacred Heart Hospital (Approval number: 2017-27). All subjects gave written informed consent to participate in this study. Enrolled patients with active TB were classified as “confirmed TB” and “clinical TB”. “Confirmed TB” was diagnosed by a positive culture for M. tb from sputum or pleural effusion. “Clinical TB” was identified based on clinical and radiologic criteria, which included typical cavities and branching centrilobular nodules evident on high-resolution computed tomography, and appropriate responses to anti-TB treatment (19). Extent of lung lesion was divided into three; mild (one-third of lung field), moderate (one-half of lung field) and severe (more than half of lung field). Individuals with diabetes mellitus, malignancy, end-stage renal disease, or those who received immunosuppressive therapy such as anti-cancer chemotherapy or steroids were excluded.
LTBI was characterized by a positive QFT-GIT (Qiagen, Hilden, Germany) in the absence of radiologic findings or clinical symptoms of active TB. Healthy controls consisted of healthy adults with a negative QFT-GIT who did not have any contact with active pulmonary TB patients and had no respiratory symptoms. Finally, we prospectively enrolled 33 patients with active TB, 30 LTBI subjects, and 27 healthy controls. Table 1 shows the demographic information of the study population.
Table 1. Basic characteristics of study populations.
| Characteristics | Active TB (n=33) | LTBI (n=30) | HC (n=27) |
|---|---|---|---|
| Sex, M/F | 29/4 | 4/26 | 5/22 |
| Age (IQR) | 17 [17–24] | 41 [36–46] | 42 [35–46] |
| Extrapulmonary TB, n (%) | 2 (6.1) | ||
| AFB smear (+), n (%) | 3 (9.1) | ||
| AFB culture (+), n (%) | 15 (45.5) | ||
| Cavity, n (%) | 3 (9.1) | ||
| Lung lesion, n (%) | |||
| Mild | 29 (87.9) | ||
| Moderate/severe | 4 (12.1) |
The numbers in brackets represent percentages in each category. AFB, acid fast bacilli; LTBI, latent tuberculosis infection; HC, healthy control; M/F, male/female; IQR, interquartile range; n, number.
QuantiFERON-TB Gold In Tube (QFT-GIT) and QuantiFERON-TB Gold Plus (QFT-Plus)
The QFT-GIT and QFT-Plus tests were performed in accordance with the manufacturer’s instructions. Briefly, whole blood (8 mL in total) was drawn into two blood-collecting vessels containing lithium-heparin. 1 mL of blood samples were collected in each tube and incubated at 37 °C for 16–24 hours. Conventional QFT-GIT consisted of three tubes: nil (no antigen, negative control), TB, and mitogen (positive control). QFT-Plus consisted of four tubes: nil, TB1, TB2, and mitogen. After incubation, the tubes were centrifuged at 2,000 ×g for 10 minutes. A total of 300 µL of plasma supernatant was harvested and stored at −80 °C until enzyme-linked immunosorbent assay (ELISA) was performed. The frozen plasma samples were thawed at room temperature and re-centrifuged at 1,750 ×g for 10 minutes. Every sample was analyzed in duplicate and samples from 8 subjects were on a single plate in each run of ELISA. The optimal density of each well was measured on a plate reader (Multiskan JX, Thermo Scientific) and the concentration of IFN-γ was determined using a QFT-GIT analysis software provided by the manufacturer. The concentration of released IFN-γ in each tube was calculated by subtracting the value of the nil tube. The results were interpreted as positive when the IFN-γ concentration was ≥0.35 IU/mL and ≥25% of the nil value.
Statistical analysis
For the purpose of the study, we computed a sample size of 81 patients, given an α error of 5% (one-sided) and a β error of 80%, with a standard deviation for the sensitivity equal to 93% and a non-inferiority limit of 10%. After considering potential drop-outs (5%), required minimum sample size of 85 was estimated. Data were analyzed using SPSS software (V 21.0; SPSS, Chicago, IL, USA) and GraphPad Prism 6 (GraphPad, San Diego, CA, USA). Data are presented as median and interquartile range (IQR). Categorical variables were analyzed using the Chi-Square test and Kruskal-Wallis test with Dunn’s multiple comparison. Agreement between the QFT-GIT and QFT-Plus tests (test concordance) was assessed by kappa statistics (κ coefficients); κ≤0.20 was considered “slight”; 0.20<κ≤0.40 was considered “fair”, 0.40<κ≤0.60 was considered “moderate”, 0.60<κ≤0.80 was considered “substantial”, and 0.80<κ≤1.00 was considered “optimal”.
Results
Characteristics of study participants
Among 33 patients with active TB, M. tb was identified by acid fast bacilli (AFB) staining and culture (one patient had an extrapulmonary form) in 15 patients (Table 1). The other 18 patients were clinically diagnosed with TB. All patients were BCG-vaccinated. The extent of lung lesions was divided into thirds (mild, moderate, and severe), and 29 patients (87.9%) had mild pulmonary lesions constituting less than one-thirds of the lung field. Age and gender distributions were not matched among the three groups; patients with active TB were younger than LTBI subjects and healthy controls (median age: 17 in active TB, 41 in LTBI, 42 in healthy control). The active TB group included more men compared with the LTBI and healthy control groups (active TB: 87.9%, LTBI: 13.3%, healthy control: 18.5%).
Results of QFT-GIT and QFT-Plus tests in study subjects
Among a total of 90 subjects, 61 (61/90, 67.8%) and 59 (59/90, 65.6%) subjects showed positive IFN-γ responses (cut-off value 0.35 IU/mL) with the conventional QFT-GIT and QFT-Plus, respectively. Both tests had positive outcomes in 31 patients with active TB, but different outcomes were observed in LTBI and control groups. QFT-GIT gave 30 positive results with LTBI, whereas QFT-Plus gave 26 and 2 positive results in LTBI and control groups, respectively (Table 2).
Table 2. QFT-Plus response among different groups.
| Variable | Active TB (n=33) | LTBI (n=30) | HC (n=27) | ||
|---|---|---|---|---|---|
| Total | AFB Cx(+) (n=16) | AFB Cx(−) (n=17) | |||
| QFT-GIT | |||||
| TB+ | 31 (93.9) | 15 (93.8) | 16 (94.1) | 30 (100.0) | 0 (0.0) |
| QFT-Plus | |||||
| TB1+ or TB2+ | 31 (93.9) | 15 (93.8) | 16 (94.1) | 26 (86.7) | 2 (7.4) |
| TB1+ | 29 (87.9) | 13 (81.3) | 16 (94.1) | 26 (86.7) | 0 (0.0) |
| TB2+ | 31 (93.9) | 15 (93.8) | 16 (94.1) | 24 (80.0) | 2 (7.4) |
| Only TB1+ | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (6.7) | 0 (0.0) |
| Only TB2+ | 2 (6.1) | 2 (12.5) | 0 (0.0) | 0 (0.0) | 2 (7.4) |
| TB1 and TB2 | 29 (87.9) | 13 (81.3) | 16 (94.1) | 24 (80.0) | 0 (0.0) |
The numbers in brackets represent percentages in each category. AFB, acid fast bacilli; Cx, culture; n, number; LTBI, latent tuberculosis infection; HC, healthy control; QFT-GIT, QuantiFERON-TB Gold In-Tube; QFT Plus, QuantiFERON-TB Gold Plus.
The sensitivity of QFT-Plus in active TB cases, based on the response to either TB1 or TB2, was 93.9%. The specificity calculated on healthy controls was 92.6%. These values are similar to those of the QFT-GIT test (sensitivity 93.9%, specificity 100%). Total concordance between the two tests was substantial (κ=0.8) (Table 3). Each of the QFT-Plus TB antigens showed similar concordance with QFT-GIT TB antigen in all subjects (TB1: κ=0.855, TB2: κ=0.755) (Table 3). In active TB, substantial or moderate agreement was achieved based on TB antigen (TB1: κ=0.637, TB: κ=0.468).
Table 3. Concordance between QFT-GIT and QFT-Plus.
| QFT-GIT | QTF Plus | TB1 | TB2 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| P | N | κ | P | N | κ | P | N | κ | |||
| P | 56/90 | 5/90 | 0.8 | 55/90 | 6/90 | 0.855 | 54/90 | 7/90 | 0.755 | ||
| N | 3/90 | 26/90 | 0/90 | 29/90 | 3/90 | 26/90 | |||||
P, positive, N, negative; QFT-GIT, QuantiFERON-TB Gold In-Tube; QFT Plus, QuantiFERON-TB Gold Plus.
Based on the responses to TB1 and TB2 antigens, IFN-γ responses were analyzed for the different groups; positive responses to both TB1 and TB2 antigens were found in 29 patients with active TB (87.9%) and 24 subjects with LTBI (81.3%) (Table 2). The proportion of responders to TB1 antigen were similar for active TB and LTBI (87.9% vs. 86.7%), whereas the proportion of responders to TB2 antigen was higher with active TB compared with LTBI (93.9% vs. 80.0%). Interestingly, two exclusive responders to TB2 alone were identified with active TB (2/33, 6.1%). A higher proportion of patients responded to TB2 antigen compared withTB1 (TB1 vs. TB2; 87.9% vs. 93.9%) in active TB, whereas fewer TB2 responders were observed in LTBI (TB1 vs. TB2; 86.7% vs. 80.0%) (Table 2).
Individual discrepancies of results between QFT-GIT and QFT-Plus
The alternative IGRA tests yielded discrepant results in eight study subjects. Five (5/8) subjects, including one patient with active TB and four individuals with LTBI, were IFN-γ positive by QFT-GIT test, whereas they did not respond to antigens of the QFT-Plus. Five subjects with positive QFT-GIT and negative QFT-Plus showed weak positivity by QFT-GIT test; the detection levels of IFN-γ were around 1 IU/mL or below. Two healthy controls and one patient with active TB (3/8) showed positive IFN-γ responses by the QFT-Plus test while negative results were observed in the QFT-GIT test (Table 4). All study participants who had QFT-Plus positive and QFT-GIT negative (G−P+) results responded to TB2 antigen alone in the QFT-Plus test (Table 4).
Table 4. Cases with discrepancies between QFT-GIT and QFT-Plus (indicated values: interferon-γ in IU/mL).
| Variable | QFT-GIT | QFT-Plus | |
|---|---|---|---|
| TB1 | TB2 | ||
| G+P− | |||
| LTBI | 0.42 | 0.17 | 0.15 |
| 0.57 | −0.29 | 0.21 | |
| 0.95 | −0.79 | −1.85 | |
| 1.03 | −0.14 | −0.07 | |
| Active TB | 0.43 | 0.14 | 0.17 |
| G−P+ | |||
| HC | −0.34 | −0.09 | 0.63 |
| 0.07 | −0.32 | 0.87 | |
| Active TB | −0.01 | −0.03 | 2.02 |
LTBI, latent tuberculosis infection; HC, healthy control; QFT-GIT, QuantiFERON-TB Gold In-Tube; QFT Plus, QuantiFERON-TB Gold Plus; G+P−, QFT-GIT positive and QFT-Plus negative group; G−P+, QFT-GIT negative and QFT-Plus positive group.
Comparison of quantitative IFN-γ responses between QFT-GIT and QFT-Plus tests
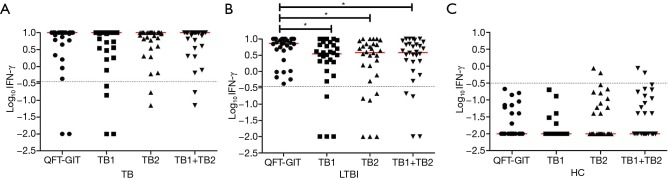
The median value of IFN-γ discriminated between QFT-GIT and QFT-Plus antigens in subjects with LTBI (Figure 1A); QFT-GIT antigen-induced IFN-γ responses (7.205 IU/mL, IQR: 3.858–10.00) were significantly higher than those induced by TB1 (3.485 IU/mL, IQR: 1.728–6.945, P<0.05) and TB2 (3.850 IU/mL, IQR: 0.705–7.023 IU/mL, P<0.05) in QFT-Plus. However, no significant differences were found in patients with active TB and healthy controls (Figure 1B,C).
Figure 1.
Quantitative IFN-γ response of patients with active TB (B), subjects with LTBI (A), and healthy controls (C). Horizontal lines in red indicate median production and dotted lines indicate cut-off (Log10 0.35=−0.456). *, P<0.05. IFN, interferon; IU, international unit; LTBI, latent tuberculosis infection; HC, healthy control; QFT-GIT, QuantiFERON-TB Gold In-Tube.
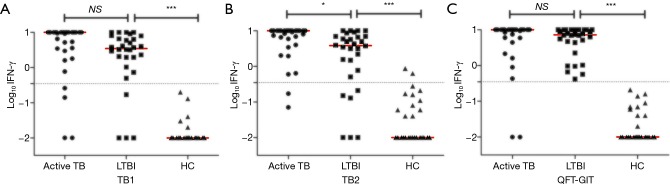
Comparative analyses based on antigen tubes showed that all TB antigens of QFT-Plus and QFT-GIT induced significantly higher IFN-γ responses in active TB and individuals with LTBI compared with controls (Figure 2). In response to TB2 in QFT-Plus, median IFN-γ responses were significantly higher in patients with active TB than in subjects with LTBI (10.00 IU/mL, IQR: 4.97–10.00 vs. 3.85 IU/mL, IQR: 0.70–7.023, P<0.05 Figure 2B). IFN-γ responses to TB1were not different between patients with active TB and subjects with LTBI (10.00 IU/mL, IQR: 3.225–10.00 vs. 3.485 IU/mL, IQR: 1.728–6.945, P>0.05, Figure 2A). Likewise, median IFN-γ responses did not vary between active TB and LTBI by QFT-GIT tests (P>0.05, Figure 2C).
Figure 2.
Quantitative IFN-γ responses to QFT-Plus antigen TB1 (A), TB2 (B), and QFT-GIT antigen (C). Horizontal lines in red indicate median production and dotted lines indicate cut-off (Log10 0.35=−0.456). *, P<0.05; ***, P<0.001. IFN, interferon; IU, international unit; LTBI, latent tuberculosis infection; HC, healthy control; QFT-GIT, QuantiFERON-TB Gold In-Tube; NS, not significant.
Discussion
We examined the efficacy of the recently upgraded IGRA, QFT-Plus, and compared it with QFT-GIT in a setting with intermediate TB burden. Although the CD8-T cell specific immune responses might be induced by additional TB2 peptides in QFT-Plus, the efficacy of QFT-Plus did not seem superior to that of conventional QFT-GIT. Substantial agreement with the same sensitivity and similar specificity was observed between the two assays.
Several studies have reported variable diagnostic outcomes in a borderline range (0.20–0.99 IU/mL) by QFT-GIT tests, and retesting with a raised cut-off for positivity has been recommended to reduce false positives (20-24). Conversions and reversions were detected in all high, intermediate, and low-incidence settings (22-24). In our study, all discordant results between QFT-GIT and QFT-Plus were observed at 0.4–1.0 IU/mL, except for one patient with active TB whose IFN-γ response to TB2 was 2.0 IU/mL. This suggests that total concordance between the two tests may be increased by a raised cut-off (>1.0 IU/mL). Meanwhile, minus values of the IFN-γ (TB-Nil) were observed in some patients, and it was due to higher IFN-γ levels of Nil tubes compared with TB antigen tubes. The result with over 8 IU/mL of Nil IFN-γ should be interpreted as indeterminate (25). In this study, all the Nil IFN-γ values were less than 8 IU/mL, and the minus values of IFN-γ (TB-Nil) were regarded as negative results.
The IFN-γ responses to TB2 alone were associated with active TB, whereas positive results by QFT-Plus in the LTBI group were mainly due to responses to TB1.A previous study performed in Italy, a low TB endemic country (13), showed similar data as ours; the majority of subjects with LTBI responded to TB1 antigen stimulation (98%) and no subjects with LTBI showed exclusive response to TB2 antigen. Only patients with active TB responded to TB2 antigen alone, suggesting that CD8 T cells may play a role in recognition of replicating M. tb. Several studies reported that CD8 T cell response correlates with mycobacterial load and declines after TB therapy (11,26,27). Day et al. found that M. tb-specific CD8 T cells increased in patients with active TB, and specific polyfunctional IFN-γ+IL-2+TNF-α+CD8 T cells were impaired in patients with smear positive TB (11,26). In addition, successful treatment led to increased polyfunctional CD8 T cells and reduction of single IFN-γ producing cells (26,27). Theoretically, mycobacteria specific-CD8 T cells can secrete IFN-γ aside from CD4 T cells. Barcellini et al. showed that IFN-γ can be amplified by CD8 T cell-stimulating antigens (28). In this study, there was no significant difference in IFN-γ responses between TB1 and TB2 antigen stimulation, in accordance with a study in Japan (15). Differences in the proportions of smear-positive patients may explain the discrepancy among studies.
The QFT-Plus does not contain TB 7.7, which is included in the QFT-GIT. The absence of TB7.7 in QFT-Plus antigen tubes might cause different IFN-γ responses in LTBI groups, although the active TB and control groups were not affected by the antigen difference. Patients with active TB had higher levels of IFN-γ by QFT-GIT than by QFT-Plus in Japan and Germany (14,15), whereas similar responses were found between the alternative assays in Italy (13).
When comparing quantitative levels of IFN-γ, patients with active TB showed statistically higher IFN-γ levels to TB2 stimulation compared to individuals with LTBI. A similar study was reported in the Netherlands (29); median IFN-γ production byTB2 was significantly higher with active TB than with LTBI without prophylactic therapy. However, active TB and LTBI were not clearly differentiated by the QFT-Plus test due to overlapping responses to the TB1 antigen. Increased IP-10 responses were found in patients with active TB and high bacterial loads or radiologic severity compared to patients with a clinical diagnosis (30). However, IP-10 responses by QFT-Plus did not distinguish between active TB and LTBI (30).
More data are needed to demonstrate the utility of QFT-Plus distinct from QFT-GIT in a larger sample size, and the variability of QFT-Plus should be evaluated. In this study, the number of subjects was limited as the enrollment of subjects had been conducted in a single institution within a restricted period. More patients and controls who match general backgrounds including age distribution should be participated for validation of the study results.
In conclusion, we confirmed that QFT-Plus and QFT-GIT assays have excellent agreement and similar accuracy for identifying M. tb infection in immunocompetent subjects in South Korea, which has intermediate TB prevalence. IFN-γ responses exclusive to TB1 or TB2 antigens were observed in LTBI or active TB, respectively. Further surveys are needed to evaluate the role of QFT-Plus in determining patients with different stages of M. tb infection or to monitor treatment effect.
Acknowledgments
Funding: This study was supported by the National Research Foundation of Korea grant (NRF-2018R1D1A1A02049260) funded by the Ministry of Education, and by the Bio & Medical Technology Development Program (NRF-2017M3A9E8033225) in South Korea.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by the Ethics committee of Chuncheon Sacred Heart Hospital (No-2017-27).
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.World Health Organizaiton, Global tuberculosis report 2018, Available online: https://apps.who.int/iris/handle/10665/274453
- 2.Marks GB, Bai J, Simpson SE, et al. Incidence of tuberculosis among a cohort of tuberculin-positive refugees in Australia: reappraising the estimates of risk. Am J Respir Crit Care Med 2000;162:1851-4. 10.1164/ajrccm.162.5.2004154 [DOI] [PubMed] [Google Scholar]
- 3.Lonnroth K, Migliori GB, Abubakar I, et al. Towards tuberculosis elimination: an action framework for low-incidence countries. Eur Respir J 2015;45:928-52. 10.1183/09031936.00214014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Available online: http://www.mohw.go.kr/react/al/sal0301vw.jsp?PAR_MENU_ID=04&MENU_ID=0403&page=1&CONT_SEQ=348699. Ministry of Health and welfare, Korea.
- 5.Lee SH. Diagnosis and treatment of latent tuberculosis infection. Tuberc Respir Dis (Seoul) 2015;78:56-63. 10.4046/trd.2015.78.2.56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yedla S. Replication of urban innovations - prioritization of strategies for the replication of Dhaka's community-based decentralized composting model. Waste Manag Res 2012;30:20-31. 10.1177/0734242X10380116 [DOI] [PubMed] [Google Scholar]
- 7.Plumridge N. Information systems. Capturing the costs of community services. Health Serv J 2010;120:20-1. [PubMed] [Google Scholar]
- 8.Goletti D, Sanduzzi A, Delogu G. Performance of the tuberculin skin test and interferon-gamma release assays: an update on the accuracy, cutoff stratification, and new potential immune-based approaches. J Rheumatol Suppl 2014;91:24-31. 10.3899/jrheum.140099 [DOI] [PubMed] [Google Scholar]
- 9.Vincenti D, Carrara S, Butera O, et al. Response to region of difference 1 (RD1) epitopes in human immunodeficiency virus (HIV)-infected individuals enrolled with suspected active tuberculosis: a pilot study. Clin Exp Immunol 2007;150:91-8. 10.1111/j.1365-2249.2007.03462.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Getahun H, Matteelli A, Abubakar I, et al. Management of latent Mycobacterium tuberculosis infection: WHO guidelines for low tuberculosis burden countries. Eur Respir J 2015;46:1563-76. 10.1183/13993003.01245-2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Day CL, Abrahams DA, Lerumo L, et al. Functional capacity of Mycobacterium tuberculosis-specific T cell responses in humans is associated with mycobacterial load. J Immunol 2011;187:2222-32. 10.4049/jimmunol.1101122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.QUIAGEN. QuantiFERON®-TB Gold Plus, ELISA Package Insert. Available online: http://www.quantiferon.com/irm/content/PI/QFT/PLUS/2PK-Elisa/UK.pdf.
- 13.Petruccioli E, Vanini V, Chiacchio T, et al. Analytical evaluation of QuantiFERON- Plus and QuantiFERON- Gold In-tube assays in subjects with or without tuberculosis. Tuberculosis (Edinb) 2017;106:38-43. 10.1016/j.tube.2017.06.002 [DOI] [PubMed] [Google Scholar]
- 14.Hoffmann H, Avsar K, Gores R, et al. Equal sensitivity of the new generation QuantiFERON-TB Gold plus in direct comparison with the previous test version QuantiFERON-TB Gold IT. Clin Microbiol Infect 2016;22:701-3. 10.1016/j.cmi.2016.05.006 [DOI] [PubMed] [Google Scholar]
- 15.Yi L, Sasaki Y, Nagai H, et al. Evaluation of QuantiFERON-TB Gold Plus for Detection of Mycobacterium tuberculosis infection in Japan. Sci Rep 2016;6:30617. 10.1038/srep30617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moon HW, Gaur RL, Tien SS, et al. Evaluation of QuantiFERON-TB Gold-Plus in Health Care Workers in a Low-Incidence Setting. J Clin Microbiol 2017;55:1650-7. 10.1128/JCM.02498-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ryu MR, Park MS, Cho EH, et al. Comparative Evaluation of QuantiFERON-TB Gold In-Tube and QuantiFERON-TB Gold Plus in Diagnosis of Latent Tuberculosis Infection in Immunocompromised Patients. J Clin Microbiol 2018;56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tsuyuzaki M, Igari H, Okada N, et al. Variation in interferon-gamma production between QFT-Plus and QFT-GIT assays in TB contact investigation. Respir Investig 2019. 10.1016/j.resinv.2019.07.002 [DOI] [PubMed] [Google Scholar]
- 19.Treatment of Tuberculosis: Guidelines for National Programmes. Geneva, Switzerland: World Health Organization, 2003. [Google Scholar]
- 20.Metcalfe JZ, Cattamanchi A, McCulloch CE, et al. Test variability of the QuantiFERON-TB gold in-tube assay in clinical practice. Am J Respir Crit Care Med 2013;187:206-11. 10.1164/rccm.201203-0430OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jonsson J, Westman A, Bruchfeld J, et al. A borderline range for Quantiferon Gold In-Tube results. PLoS One 2017;12:e0187313. 10.1371/journal.pone.0187313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nemes E, Rozot V, Geldenhuys H, et al. Optimization and Interpretation of Serial QuantiFERON Testing to Measure Acquisition of Mycobacterium tuberculosis Infection. Am J Respir Crit Care Med 2017;196:638-48. 10.1164/rccm.201704-0817OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hur YG, Hong JY, Choi DH, et al. A Feasibility Study for Diagnosis of Latent Tuberculosis Infection Using an IGRA Point-of-Care Platform in South Korea. Yonsei Med J 2019;60:375-80. 10.3349/ymj.2019.60.4.375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moses MW, Zwerling A, Cattamanchi A, et al. Serial testing for latent tuberculosis using QuantiFERON-TB Gold In-Tube: A Markov model. Sci Rep 2016;6:30781. 10.1038/srep30781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Powell RD, 3rd, Whitworth WC, Bernardo J, et al. Unusual interferon gamma measurements with QuantiFERON-TB Gold and QuantiFERON-TB Gold In-Tube tests. PLoS One 2011;6:e20061. 10.1371/journal.pone.0020061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Prezzemolo T, Guggino G, La Manna MP, et al. Functional Signatures of Human CD4 and CD8 T Cell Responses to Mycobacterium tuberculosis. Front Immunol 2014;5:180. 10.3389/fimmu.2014.00180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nyendak MR, Park B, Null MD, et al. Mycobacterium tuberculosis specific CD8(+) T cells rapidly decline with antituberculosis treatment. PLoS One 2013;8:e81564. 10.1371/journal.pone.0081564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barcellini L, Borroni E, Brown J, et al. First independent evaluation of QuantiFERON-TB Plus performance. Eur Respir J 2016;47:1587-90. 10.1183/13993003.02033-2015 [DOI] [PubMed] [Google Scholar]
- 29.Hofland RW, Bossink AWJ, Nierkens S, et al. QuantiFERON-plus does not discriminate between active and latent tuberculosis. Infect Dis (Lond) 2018;50:479-82. 10.1080/23744235.2018.1425550 [DOI] [PubMed] [Google Scholar]
- 30.Petrone L, Vanini V, Chiacchio T, et al. Evaluation of IP-10 in Quantiferon-Plus as biomarker for the diagnosis of latent tuberculosis infection. Tuberculosis (Edinb) 2018;111:147-53. 10.1016/j.tube.2018.06.005 [DOI] [PubMed] [Google Scholar]