Abstract
Background
In this real-world multicenter study we addressed the activity of post-progression anticancer treatments after first-line pembrolizumab in advanced non-small cell lung cancer (NSCLC) patients with PD-L1 ≥50%.
Methods
Clinico-pathological data of PD-L1 ≥50% advanced NSCLCs who failed first-line pembrolizumab were collected in 14 Oncologic Centers from different European countries. Types of subsequent anticancer treatment and outcomes on salvage chemotherapy or pembrolizumab beyond progression with or without the addition of local ablative therapies were reported.
Results
Out of 173 patients, 100 had progressed on pembrolizumab, of which 60 patients (60%) met eligibility criteria and were treated with either salvage chemotherapy (42/60, 70%) or pembrolizumab beyond progression (18/60, 30%). Overall, median age was 66 years, 63.3% were male, 60.0% had a performance status of 0–1, 88.3% were smokers and 61.7% had adenocarcinoma histology. In patients evaluable for response, objective response rate to salvage chemotherapy was 41.9%, with no significant difference according to the type of regimen (42.9% for platinum-based and 40.0% for single-agent chemotherapy). Median progression-free survival (PFS) to salvage chemotherapy was 4.5 months. Among patients treated with pembrolizumab beyond progression, 13 out of 18 patients (72.2%) had progressive disease in ≤2 organ sites, of whom 9 (69.2%) were managed with the addition of local ablative therapies consisting of radiation at progressive lesion(s). No significant difference was noted in terms of post-progression survival between the salvage chemotherapy and the pembrolizumab beyond progression groups of patients (6.9 versus 8.1 months, respectively, P=0.08).
Conclusions
In PD-L1 ≥50% advanced NSCLCs who progress on first-line pembrolizumab, salvage chemotherapy is associated with a remarkable anticancer activity, while select patients may benefit from continuation of pembrolizumab beyond progression, with the possible addition of local ablative radiotherapy in oligoprogressive cases.
Keywords: Local ablative therapy, non-small cell lung cancer (NSCLC), PD-L1 ≥50%, pembrolizumab beyond progression, salvage chemotherapy
Introduction
Ever since its introduction for clinical use, immunotherapy with anti-programmed cell death 1 (PD-1) or anti-programmed cell death ligand 1 (PD-L1) has revolutionized the treatment of patients with advanced non-small cell lung cancer (NSCLC) with no actionable mutations in the epidermal growth factor receptor (EGFR) or anaplastic lymphoma kinase (ALK) genes (1). In fact, while early trials demonstrated the superiority of anti-PD-1/PD-L1 agents over second-line chemotherapy in platinum-pretreated patients (2-5), more recent studies have shown that inhibition of PD-1 axis is also effective as first-line treatment (6-8).
PD-L1 expression levels on tumor cells have emerged as a biologic predictor of response to PD-1 axis inhibitor therapy, with a recent phase 3 trial showing improved survival with the anti-PD-1 agent pembrolizumab over platinum-based chemotherapy for treatment-naïve patients with a PD-L1 expression ≥50% (6,7). These results led to a paradigm change in the treatment algorithm of advanced NSCLCs, which led to the use of pembrolizumab instead of chemotherapy as first-line treatment of patients with PD-L1 expression ≥50%. In this context, interest has partly shifted to subsequent anticancer treatment to be offered after progression on pembrolizumab. In fact, it has not yet been fully elucidated to what extent PD-L1 ≥50% patients would benefit from salvage chemotherapy administered after pembrolizumab.
Characterizing the activity of salvage chemotherapy administered sequentially after pembrolizumab is of particular relevance in view of the great efficacy recently shown by pembrolizumab in combination with chemotherapy as first-line treatment irrespective of PD-L1 expression levels (8). For the same reason, as many patients will be exposed to the combination of pembrolizumab with chemotherapy in the first-line setting, treatment with pembrolizumab beyond progression has become an area of interest. However, data on the potential benefits of PD-1 axis inhibitors given beyond progression in NSCLC have been limited so far and mostly confined to the setting of patients pretreated with platinum-based chemotherapy (9-11). Therefore, at the present time, data on the use of pembrolizumab beyond progression in patients with PD-L1 ≥50% are scant, and the clinical benefit of such an approach is unknown. In addition, although local ablative therapies represent a consolidated approach in oligoprogressive patients with oncogene-addicted NSCLC treated with a tyrosine kinase inhibitor (12), the impact of local treatments in patients who continue treatment with PD-1 axis inhibitors beyond progression has been poorly addressed.
On this basis, we ran a real-world multicenter study in advanced NSCLC patients with PD-L1 ≥50% in which we described the types of post-progression anticancer treatment and the clinical outcome after first-line pembrolizumab, focusing on strategies such as salvage chemotherapy and pembrolizumab beyond progression. In addition, in the latter group of patients, we focused on those individuals who received local ablative therapies in order to address the relevance of such a strategy in the pembrolizumab beyond progression group.
Methods
Study population
This retrospective study aimed at describing the types and outcomes of subsequent anticancer treatments received after progression on first-line pembrolizumab in non-oncogene addicted advanced NSCLC patients with PD-L1 ≥50%. The medical charts were reviewed and data were extracted from 14 Oncologic Centers operating in four different countries (Greece n=7; Italy n=5; Switzerland n=1; Spain n=1). As this was a real-life study, there were no clinical or pathological restrictions for patient enrollment, provided that eligible patients had been treated with first-line pembrolizumab, and had an EGFR wild type, ALK negative, and PD-L1 ≥50% biological profile of the tumor (as per local assessment). Disease progression on pembrolizumab was defined as progressive disease (PD) as per RECIST 1.1 that occurred during treatment or within 6 months of the last dose of pembrolizumab.
The following data were collected: clinico-pathological characteristics, types of post-progression anticancer treatment, response to first-line pembrolizumab and to salvage chemotherapy as per RECIST 1.1 [complete response (CR), partial response (PR), stable disease (SD) and PD, with overall response rate (ORR) being the sum of CR and PR, and disease control rate being the sum of CR, PR and SD], progression-free survival (PFS) of salvage chemotherapy (calculated from the time of initiation of treatment to the radiographic evidence of disease progression or death of the patient in the absence of disease progression, with alive patients without documented radiographic progression being censored at the time of last follow-up). In addition, post-progression survival (PPS) was evaluated for either the salvage chemotherapy and the pembrolizumab beyond progression groups of patients (calculated from the date of progression on pembrolizumab to death of the patient from any cause, with alive patients being censored at the time of the last follow-up). The collection of data on local ablative therapies was limited to patients who were treated with pembrolizumab beyond progression as this was among the outcomes of the present analysis. Since this was a real-world study, the decision on whether patients progressing on first-line pembrolizumab were treated with salvage chemotherapy or pembrolizumab beyond progression was at physicians’ discretion.
Statistical analysis
Descriptive statistics were performed using frequencies, percentages, frequency tables for categorical variables, median and range for quantitative variables. Non-parametric Mann-Whitney test was performed to compare continuous variable with no normal distribution. Categorical variables were evaluated by chi-square analysis or Fisher’s exact test where appropriate.
PFS and PPS were analyzed according to Kaplan-Meier method and survival curves were compared using the log-rank test. Cox model was used to estimate hazard ratio and related 95% confidence interval (CI). Given the retrospective nature of the study, statistical significance should be used in an exploratory view and median time estimation with their 95% CI: reported to better interpret the data. All estimates were achieved using STATA 14.2 (Stata Corp Ltd., College Station, TX, USA).
Results
Patients
Figure 1 shows study flow-chart. Out of 173 patients treated with first-line pembrolizumab from January 2017 to March 2019, 100 patients experienced PD. Of them, 40 patients did not receive any subsequent anticancer treatments because of rapid deterioration of clinical conditions, while 60 patients received a post-progression treatment. Table 1 lists the types of subsequent anticancer treatments, consisting of salvage chemotherapy in 42 patients and pembrolizumab beyond progression in 18 cases. Platinum-based chemotherapy was delivered in approximately three quarters of patients who received salvage chemotherapy, with platinum/pemetrexed being the most commonly administered chemotherapeutic regimen. Table 2 shows the clinical characteristics of the 60 patients according to whether they received salvage chemotherapy or pembrolizumab beyond progression. No significant differences were seen between the two groups, except: (I) higher median age reported in the pembrolizumab beyond progression group (71 years versus 64 years, borderline significance); (II) more patients in the salvage chemotherapy group had adenocarcinoma histology (69.0% versus 44.5%, borderline significance); (III) more patients in the pembrolizumab beyond progression group experienced a PR or SD as best response to prior pembrolizumab (77.8% versus 47.6%); (IV) the same group also had longer median duration of prior pembrolizumab (5.9 versus 3.6 months).
Figure 1.
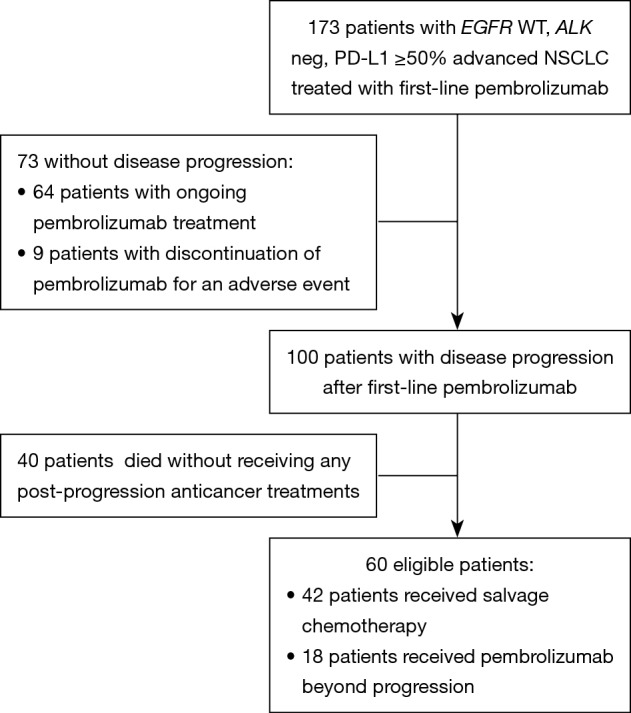
Study flowchart. WT, wild type; NSCLC, non-small cell lung cancer.
Table 1. Post-progression anticancer treatments.
| Types of post-progression anticancer treatment | Total patients (n=60) (%) |
|---|---|
| Salvage chemotherapy | 42 (70.0) |
| Platinum-based chemotherapy* | 31 out of 42 (73.8) |
| Mono-chemotherapy** | 11 out of 42 (26.2) |
| Pembrolizumab beyond progression | 18 (30.0) |
*, n=12 carboplatin/pemetrexed; n=5 carboplatin/gemcitabine; n=4 cisplatin/pemetrexed; n=3 carboplatin/paclitaxel; n=3 carboplatin/nab-paclitaxel; n=2 carboplatin/paclitaxel/bevacizumab; n=1 cisplatin/gemcitabine; n=1 carboplatin. **, n=7 gemcitabine; N=4 docetaxel.
Table 2. Characteristics of patients.
| Characteristic | Total patients (n=60) (%) | Salvage chemotherapy (n=42) (%) | Pembrolizumab beyond progression (n=18) (%) | P value |
|---|---|---|---|---|
| Median age, years [range] | 66 [19–86] | 64 [19–84] | 71 [49–86] | 0.09 |
| Gender | 0.81 | |||
| Male | 38 (63.3) | 27 (64.3) | 11 (61.1) | |
| Female | 22 (36.7) | 15 (35.7) | 7 (38.9) | |
| Performance status | 0.47 | |||
| 0–1 | 36 (60.0) | 24 (57.1) | 12 (66.7) | |
| 2 | 24 (40.0) | 18 (42.9) | 6 (33.3) | |
| Smoking history | 0.57 | |||
| Never smokers* | 7 (11.7) | 4 (9.5) | 3 (16.7) | |
| Ever smokers | 53 (88.3) | 38 (90.5) | 15 (8.3) | |
| Histology | 0.07 | |||
| Adenocarcinoma | 37 (61.7) | 29 (69.0) | 8 (44.5) | |
| Squamous | 14 (23.3) | 8 (19.0) | 6 (33.3) | |
| Other | 9 (15.0) | 5 (11.9)** | 4 (22.2)*** | |
| Brain metastases | 0.16 | |||
| Yes | 16 (26.7) | 9 (21.4) | 7 (38.9) | |
| No | 44 (73.3)∞ | 33 (78.6) | 11 (61.1) | |
| PD-L1 TPS§ | 0.43 | |||
| ≥50–74% | 41 (68.3) | 30 (71.4) | 11 (61.1) | |
| ≥75–100% | 19 (31.7) | 12 (28.6) | 7 (38.9) | |
| Response to prior pembrolizumab | <0.05 | |||
| PR or SD | 34 (56.7) | 20 (47.6) | 14 (77.8) | |
| PD | 26 (43.3) | 22 (52.4) | 4 (22.2) | |
| Median duration of prior pembrolizumab, months (range) | 3.7 (0.4–18.2) | 3.6 (0.7–14.6) | 5.9 (0.4–18.1) | <0.05 |
*, less than 100 cigarettes in the lifetime; **, 3 non-small cell lung cancers not otherwise specified, 1 large cell carcinoma, 1 adenosquamous; ***, 2 non-small cell lung cancers not otherwise specified, 2 sarcomatoid carcinomas; ∞, includes 6 patients not staged for brain metastases, 3 in each group; §, as assessed by immunohistochemistry (Dako 22C3 PharmDx). PD, progressive disease; PR, partial response; SD, stable disease; TPS, tumor proportion score.
At the time of this analysis (June 2019), median follow-up time calculated from the date of progression to first-line pembrolizumab (based on the objectives of this analysis) was 5.7 months (range, 0.7–16.0 months) in the salvage chemotherapy group and 5.5 months (range, 1.2–20.9 months) in the pembrolizumab beyond progression group.
Efficacy of salvage chemotherapy
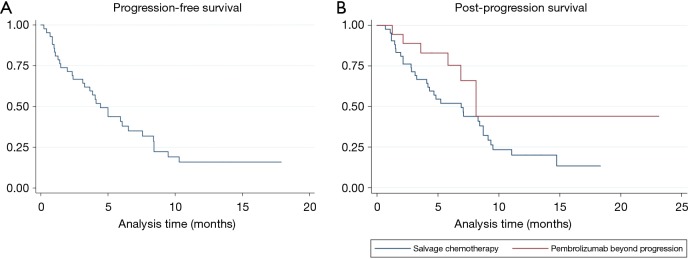
Among the 42 patients who received salvage chemotherapy, 31 had at least one follow-up radiographic assessment, and were therefore evaluable for activity. Table 3 lists the activity of salvage chemotherapy. Overall, an ORR was observed in 41.9% of patients, with a disease control rate of 54.8%. Thirty-three patients had died or had disease progression, and the median PFS was 4.5 months (95% CI: 3.1–6.5 months) (Figure 2A).
Table 3. Activity of salvage chemotherapy.
| Variable | All evaluable patients (n=31) (%) | Platinum-based chemotherapy (n=21) (%) | Single-agent chemotherapy (n=10) (%) |
|---|---|---|---|
| Response | 0 | ||
| CR | 1 (3.2) | 1 (4.8) | 4 (40.0) |
| PR | 12 (38.7) | 8 (38.1) | 2 (20.0) |
| SD | 4 (12.9) | 2 (9.5) | 4 (40.0) |
| PD | 14 (45.2) | 10 (47.6) | |
| Objective response | |||
| No. of patients | 13 (41.9) | 9 (42.9) | 4 (40.0) |
| 95% CI | 25.6–60.3 | 25.5–67.0 | 8.9–71.9 |
| Disease control | |||
| No. of patients | 17 (54.8) | 11 (52.4) | 6 (60.0) |
CR, complete response; PD, progressive disease; PR, partial response; SD, stable disease; CI, confidence interval.
Figure 2.
Survival curves. (A) Kaplan-Meier estimate of progression-free survival for patients treated with salvage chemotherapy; (B) Kaplan-Meier estimates of post-progression survival for patients treated with salvage chemotherapy (blue line) and for patients treated with pembrolizumab beyond progression (red line).
No significant difference was seen in terms of ORR between the 23 patients who were treated with platinum-based chemotherapy versus the ten patients who received single-agent chemotherapy, the ORR being 42.9% and 40.0%, respectively (Table 3).
We performed an exploratory analysis to determine whether any clinical or pathologic features were associated with PFS. However, at the univariate analysis none of the variables considered (age; male versus female; PS 0–1 versus 2; never smokers versus ever smokers; adenocarcinoma versus other histologies; brain metastases versus no brain metastases; PD-L1 ≥50–74% versus ≥75–100%; PR or SD versus PD on prior pembrolizumab) were significantly associated with clinical outcome; these evidences were confirmed in the multivariate approach.
Pembrolizumab beyond progression
Eighteen patients were treated with pembrolizumab beyond progression. Table 4 lists the patients for whom it was deemed clinically appropriate by the investigator to continue on pembrolizumab beyond progression with or without the addition of local ablative therapies. Overall, local treatment was delivered in 11 patients, and consisted of pericardial drainage in two patients who had developed symptomatic pericardial effusions and local ablative radiotherapy in the remainder 9 cases.
Table 4. Patients who were treated with pembrolizumab beyond disease progression.
| Study case No. | Age, years | Gender (M/F) | Histology | PD-L1 TPS | Best response on pembrolizumab | Sites of disease progression on pembrolizumab | Type of local ablative treatment | PPS (months) | PPS rates at 6 and 12 months |
|---|---|---|---|---|---|---|---|---|---|
| 01& | 62 | M | Sarcomatoid carcinoma | 75% | PR | Brain (new) | WBRT | 6.9 | 88.9% and 71.1% |
| 02& | 48 | M | Squamous | 60% | PR | Single lymph-nodal station | Lymph-nodal RT | 7.4+ | |
| 03& | 72 | M | Squamous | 100% | PR | Single lung lesion | Lung stereotactic RT | 14.7+ | |
| 04& | 62 | F | Adenoca. | 80% | PR | Single renal lesion | Renal RT | 24.6+ | |
| 05& | 78 | F | Squamous | 70% | PR | Single lung and lymph-nodal station | Lung and lymph-nodal stereotactic RT | 4.6+ | |
| 06& | 69 | F | NSCLC NOS | 70% | SD | Brain (new) | WBRT | 7.1+ | |
| 07& | 87 | M | Adenoca. | 60% | PR | Single lung lesion | Lung RT | 4.2+ | |
| 08& | 60 | M | Adenoca. | 70% | PR | Brain (pre-existing) | WBRT | 4.0+ | |
| 09& | 51 | M | Adenoca. | 60% | PD | Brain (new) | WBRT | 4.6+ | |
| 10# | 69 | F | Adenoca. | 60% | PD | Pericardial effusion* | – | 2.1 | 63.3% and 0% |
| 11# | 64 | F | Adenoca. | 80% | PR | Pericardial effusion* | – | 6.9+ | |
| 12# | 79 | M | Adenoca. | 75% | SD | Lung and pleura | – | 3.5 | |
| 13# | 72 | M | NSCLC NOS | 95% | PR | Lung | – | 7.1+ | |
| 14 | 58 | F | Squamous | 80% | PD | Lung, liver, and bones** | – | 1.2 | |
| 15 | 71 | M | Squamous | 95% | PD | Lung, pleura, and soft tissues | – | 5.8 | |
| 16 | 72 | M | Squamous | 90% | SD | Multiple lymph-nodal stations | – | 8.0 | |
| 17 | 74 | M | Sarcomatoid carcinoma | 60% | PR | Multiple lung lesions | – | 5.9+ | |
| 18 | 72 | F | Adenoca. | 60% | SD | Lung, liver, and extra-thoracic lymph-nodal stations | – | 3.4+ |
&, patients with progressive disease at ≤2 organ sites treated with local ablative radiotherapy; #, patients with progressive disease at ≤2 organ sites; *, treated by pericardial drainage only; **, palliative bone radiotherapy administered; +, sign indicates alive patients at the time of the last contact. Adenoca., adenocarcinoma; No., number; NSCLC, non-small cell lung cancer; NOS, not otherwise specified; PD, progressive disease; PR, partial response; PPS, post-progression survival; RT, radiotherapy; SD, stable disease; TPS, tumor proportion score; WBRT, whole brain radiotherapy.
Among the 13 patients with PD in ≤2 organ sites, local ablative radiotherapy at progressive organ sites/lesion(s) was administered in 9 cases (69.2%). It consisted of whole brain radiotherapy in four cases of isolated central nervous system progression with multiple (>3) brain metastasis (n=3 patients with new brain metastases, n=1 patient with pre-existing brain metastases) and stereotactic radiation to extra-cranial progressive lesion(s) in 5 other cases (n=4 patients at a single progressive lesion, n=1 patient at two progressive lesions).
Post-progression survival
Thirty-two patients in the salvage chemotherapy group and six patients in the pembrolizumab beyond progression group had died, respectively. Overall, median PPS was 8.2 months (95% CI: 4.8–9.1 months): median PPS was 6.9 months (95% CI: 4.0–8.7 months) in the salvage chemotherapy and 8.1 months (95% CI: 5.8–NR) in the pembrolizumab beyond progression groups of patients, P=0.08 (Figure 2B). Among the nine patients with PD in ≤2 organ sites who were treated with pembrolizumab beyond progression with the addition of local ablative radiotherapy the PPS rates at 6 and 12 months were 88.9% (95% CI: 43.3–98.4%) and 71.1% (95% CI: 23.3–92.3%), respectively, while the same values for the remainder nine patients were 63.5% (95% CI: 23.8–86.6%) and 0%, respectively (Table 4).
Discussion
In this analysis, we focused on the types of post-progression anticancer treatments and on the clinical outcome of subsequent anticancer therapies after first-line pembrolizumab in PD-L1 ≥50% advanced NSCLC patients. This clinical question is highly relevant in view of the existence of two competing first-line standards of care for this group of patients, either pembrolizumab as single-agent or pembrolizumab in combination with chemotherapy (6-8).
An interesting issue is whether prior exposure to PD-1 axis inhibitor therapy may affect response to salvage chemotherapy (13). Some authors have reported a high anticancer activity for chemotherapy administered immediately after anti-PD-1/PD-L1 agents across multiple cancers (14-17). With regard to NSCLC, Park et al. found an outstanding response rate of 66.7% for platinum-based chemotherapy administered as salvage treatment after PD-1 axis inhibitors as opposed to 39.5% for platinum-based chemotherapy administered prior to anti-PD-1/PD-L1 agents (16). Of note, the same authors reported an outstanding ORR of 53% even for single-agent chemotherapy when administered after PD-1 axis inhibitors. Similarly, another study reported an ORR of 39% with single-agent docetaxel administered sequentially after anti-PD-1/PD-L1 agents (17). Likewise, our data showed an excellent activity for salvage chemotherapy, as we reported an ORR of 42.9% for platinum-based chemotherapy and an ORR of 40% for single-agent chemotherapy (Table 3). However, while prior studies mainly focused on heavily pretreated NSCLC patients irrespective of PD-L1 expression, the present analysis suggests that salvage chemotherapy could also be highly effective when administered after first-line pembrolizumab in PD-L1 ≥50% advanced NSCLC patients, with an ORR of 41.9%, a disease control rate of 54.8% and a median PFS of 4.5 months (Table 3, Figure 2A).
Of note, responses have been reported by some authors in patients who were re-challenged with PD-1 axis inhibitors after a treatment break (9,18). In addition, two retrospective series showed survival benefit in patients who were treated with PD-1 axis inhibitors beyond progression compared to those whose treatment was stopped at the time of progression or those who switched to other anticancer treatments (10,11). In this study we found that pembrolizumab beyond progression could be beneficial in select patients, as no significant differences were noted in terms of PPS between patients treated with salvage chemotherapy and those who received pembrolizumab beyond progression (6.9 versus 8.1 months, respectively, P=0.08) (Figure 2B). As expected, the majority of patients who were treated with pembrolizumab beyond progression had experienced either PR or SD (14 out of 18 patients, 77.8%) as best response to pembrolizumab and had undergone PD in ≤2 organ sites (13 out of 18 patients, 72.2%) (Table 4). Importantly, in 9 of the 13 patients (69.2%) with oligoprogressive disease in ≤2 organ sites the benefits from pembrolizumab beyond progression were maximized through the addition of local ablative radiotherapy, with a reported 6- and 12-month PPS of 88.9% and 71.1%, respectively. Consistently, a small retrospective study involving 26 patients with acquired resistance to anti-PD-1/PD-L1 agents found an outstanding 2-year survival rate of 92% for the 15 patients who were managed with the addition of local ablative therapies (mainly radiotherapy) for oligoprogressive disease with or without treatment with PD-1 axis inhibition beyond progression (9). Whether local ablative radiotherapy may have contributed to the control of occult disease through additional abscopal effects in the nine patients from our series who achieved a long-term survival beyond progression is a fascinating hypothesis, and ongoing clinical studies are currently evaluating the abscopal potential of local tumor radiation with PD-1 axis inhibitor therapy (19).
A few limitations should be acknowledged in the present study. First of all, this is a retrospective study with potential biases affecting the results. Second, the number of patients included is relatively small. Third, patients treated with salvage chemotherapy and pembrolizumab beyond progression are not formally comparable because of lack of randomization, as those who continued pembrolizumab beyond progression could have had a better clinical status than patients who were switched to salvage chemotherapy. However, the fact that patients in the salvage chemotherapy group were clinically fit in order to receive salvage chemotherapy suggests that they were not expected to undergo rapid clinical deterioration at the time of treatment initiation. Lastly, it may be argued that the benefits of pembrolizumab beyond progression could be ascribed to the existence of an atypical response pattern termed pseudoprogression, which consists of an initial radiological assessment meeting conventional response criterion for PD, followed by a decrease in tumor burden at subsequent radiological assessments (20). Nevertheless, while pseudoprogression seems to be relatively common in melanoma, it is much less frequent in NSCLC, occurring in approximately 2% of patients (21). In addition, since most of patients treated with pembrolizumab beyond progression had experienced a PR or SD as best response to prior pembrolizumab, it seems likely that our patients were experiencing acquired resistance rather than pseudoprogression. Despite these limitations, this study has the strength of describing in a real-life scenario how patients with PD-L1 ≥50% advanced NSCLC perform on subsequent anticancer therapies at the time of failure on first-line pembrolizumab. This remains a relevant question as not all of these patients will be treated upfront with the combination of PD-1 axis inhibitor therapy and chemotherapy.
In conclusion, we showed that chemotherapy is a highly effective rescue treatment for PD-L1 ≥50% advanced NSCLC patients who failed first-line pembrolizumab. On the other hand, continuation of pembrolizumab beyond progression should be considered in select cases depending on the benefit on prior pembrolizumab and/or number of progressive organ sites and lesions, along with local ablative radiotherapy which may add to the overall clinical benefit of such a strategy in oligoprogressive cases.
Acknowledgments
None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Patients provided written informed consent for the collection of clinical outcomes information. Patients provided written informed consent for the collection of clinical outcomes information. All procedures were performed in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments.
Footnotes
Conflict of Interest: This work was presented in abstract form at World Conference on Lung Cancer 2019. Giulio Metro has had consulting/advisory relationship with Boehringher-Ingelheim; Giuseppe Banna has had consulting/advisory relationships with Boehringher-Ingelheim, Janssen-Cilag, Roche; Athina Christopoulou has had consulting/advisory relationships with Bristol Myers Squibb, Merck, Novartis, Pfizer, Roche, Sanofi; Antonio Calles received honorary/consulting fees from AstraZeneca, Boehringher-Ingelheim, Bristol Myers Squibb, Eli Lilly, Merck, Novartis, Pfizer, Roche; Helena Linardou has had consulting/advisory relationships with AstraZeneca, Boehringher-Ingelheim, Bristol Myers Squibb, Merck, Novartis, Roche; Paris Kosmidis has had consulting/advisory relationships with Bristol Myers Squibb, Merck, Novartis, Pfizer. The other authors have no conflicts of interest to declare.
References
- 1.Toschi L, Rossi S, Finocchiaro G, et al. Non-small cell lung cancer treatment (r)evolution: ten years of advances and more to come. Ecancermedicalscience 2017;11:787. 10.3332/ecancer.2017.787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced non-squamous non-small cell lung cancer. N Engl J Med 2015;373:1627-39. 10.1056/NEJMoa1507643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med 2015;373:123-35. 10.1056/NEJMoa1504627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 2016;387:1540-50. 10.1016/S0140-6736(15)01281-7 [DOI] [PubMed] [Google Scholar]
- 5.Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet 2017;389:255-65. 10.1016/S0140-6736(16)32517-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reck M, Rodríguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med 2016;375:1823-33. 10.1056/NEJMoa1606774 [DOI] [PubMed] [Google Scholar]
- 7.Reck M, Rodríguez-Abreu D, Robinson AG, et al. Updated analysis of KEYNOTE-024: pembrolizumab versus platinum-based chemotherapy for advanced non-small-cell lung cancer with PD-L1 tumor proportion score of 50% or greater. J Clin Oncol 2019;37:537-46. 10.1200/JCO.18.00149 [DOI] [PubMed] [Google Scholar]
- 8.Addeo A, Banna GL, Metro G, Di Maio M. Chemotherapy in combination with immune checkpoint inhibitors for the first-line treatment of patients with advanced non-small cell lung cancer: a systematic review and literature-based meta-analysis. Front Oncol 2019;9:264. 10.3389/fonc.2019.00264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gettinger SN, Wurtz A, Goldberg SB, et al. Clinical features and management of acquired resistance to PD-1 axis inhibitors in 26 patients with advanced non-small cell lung cancer. J Thorac Oncol 2018;13:831-9. 10.1016/j.jtho.2018.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gandara DR, von Pawel J, Mazieres J, et al. Atezolizumab treatment beyond progression in advanced NSCLC: results from the randomized, phase III OAK Study. J Thorac Oncol 2018;13:1906-18. 10.1016/j.jtho.2018.08.2027 [DOI] [PubMed] [Google Scholar]
- 11.Ricciuti B, Genova C, Bassanelli M, et al. Safety and efficacy of nivolumab in patients with advanced non-small-cell lung cancer treated beyond progression. Clin Lung Cancer 2019;20:178-185.e2. 10.1016/j.cllc.2019.02.001 [DOI] [PubMed] [Google Scholar]
- 12.McDonald F, Hanna GG. Oligoprogressive Oncogene-addicted Lung Tumours: Does Stereotactic Body Radiotherapy Have a Role? Introducing the HALT Trial. Clin Oncol (R Coll Radiol) 2018;30:1-4. 10.1016/j.clon.2017.10.013 [DOI] [PubMed] [Google Scholar]
- 13.Saleh K, Khalifeh-Saleh N, Kourie HR, et al. Do immune checkpoint inhibitors increase sensitivity to salvage chemotherapy? Immunotherapy 2018;10:163-5. 10.2217/imt-2017-0153 [DOI] [PubMed] [Google Scholar]
- 14.Dwary AD, Master S, Patel A, et al. Excellent response to chemotherapy post immunotherapy. Oncotarget 2017;8:91795-802. 10.18632/oncotarget.20030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Szabados B, van Dijk N, Tang YZ, et al. Response rate to chemotherapy after immune checkpoint inhibition in metastatic urothelial cancer. Eur Urol 2018;73:149-52. 10.1016/j.eururo.2017.08.022 [DOI] [PubMed] [Google Scholar]
- 16.Park SE, Lee SH, Ahn JS, et al. Increased response rates to salvage chemotherapy administered after PD-1/PD-L1 inhibitors in patients with non-small cell lung cancer. J Thorac Oncol 2018;13:106-11. 10.1016/j.jtho.2017.10.011 [DOI] [PubMed] [Google Scholar]
- 17.Schvartsman G, Peng SA, Bis G, et al. Response rates to single-agent chemotherapy after exposure to immune checkpoint inhibitors in advanced non-small cell lung cancer. Lung Cancer 2017;112:90-5. 10.1016/j.lungcan.2017.07.034 [DOI] [PubMed] [Google Scholar]
- 18.Garon EB, Hellmann MD, Rizvi NA, et al. Five-year overall survival for patients with advanced non-small-cell lung cancer treated with pembrolizumab: results from the phase I KEYNOTE-001 study. J Clin Oncol 2019;37:2518-27. 10.1200/JCO.19.00934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Walker J, Loo BW., Jr radiotherapy and immunotherapy-shining further together. JAMA Oncol 2019. [Epub ahead of print]. 10.1001/jamaoncol.2019.1448 [DOI] [PubMed] [Google Scholar]
- 20.Hodi FS, Hwu WJ, Kefford R, et al. Evaluation of immune-related response criteria and RECIST v1.1 in patients with advanced melanoma treated with pembrolizumab. J Clin Oncol 2016;34:1510-7. 10.1200/JCO.2015.64.0391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Katz SI, Hammer M, Bagley SJ, et al. Radiologic pseudoprogression during anti-PD-1 therapy for advanced non-small cell lung cancer. J Thorac Oncol 2018;13:978-86. 10.1016/j.jtho.2018.04.010 [DOI] [PubMed] [Google Scholar]