Abstract
Background:
Bisphenol A (BPA) and phthalates metabolites are linked to a variety of adverse health consequences, but studies have not explored their impact on growth trajectories.
Objective:
Explore body mass index (BMI) trajectories for tertile exposures to BPA and phthalates metabolites.
Methods:
We constructed BMI (kg/m2) trajectories from birth to 14 years in a birth cohort of 249 children from Mexico City using tertiles of third trimester maternal urinary concentrations of BPA and phthalates metabolites. Fractional age polynomials and mixed effects models were fit separately by sex. Predicted models were plotted for each metabolite tertile with the covariates mother’s education and BMI centered at average values.
Results:
Highest predicted BMI trajectories for females were observed for third tertile exposure to the phthalate metabolite mono(2-ethyl-5-carboxypentyl) phthalate. In males, first tertile exposure to mono-isobutyl phthalate and monobenzyl phthalate and second tertile exposure to mono(2-ethylhexyl) phthalate and mono(2-ethyl-5-hydroxyhexyl) phthalate predicted the highest BMI trajectory in males by adolescence. There was no relationshsip between BPA and child growth trajectory.
Conclusions:
These results suggest sex-specific differences in BMI trajectories by levels of metabolite exposure. Additional studies are needed to consider growth through adolescence in assessing the impact of pregnancy exposures on child’s BMI.
Keywords: BMI, Growth trajectory, BPA, Phthalates
INTRODUCTION
Childhood obesity is a globally persistent disease with multifactorial causes and a wide range of complications that track into adulthood 1. Increasingly, evidence suggests that exposures to environmental endocrine-disrupting compounds such as Bisphenol A (BPA) and phthalates may influence body weight and physiology, such as insulin resistance 2-4. These multi-functional compounds are used in everyday products, leading to ubiquitous exposure 5,6. BPA is used in the production of polycarbonate plastics and epoxy resins and is commonly found in food and beverage containers, medical equipment, toys, the linings of canned foods, thermal receipt paper, and dental sealants 7. Phthalates are used similarly as plasticizers in many consumer and industrialized products and as a stabilizing and solubilizing material 8.
Associations between exposures to these compounds and increased body mass index (BMI) and adiposity differ by sex, population, and age of exposure, but many studies suggest positive associations 9-12. However, no longitudinal studies have investigated the role that in utero exposures to these compounds may have on growth trajectories. By assessing whether growth differs by levels of exposure, this could allow estimation of milestones, such as infancy peak or the adiposity rebound, which could be used to link childhood growth with adult diseases like obesity 13.
In this study, we add to our previously reported associations between in utero and childhood urinary concentrations of BPA and phthalates metabolites on childhood BMI and adiposity. We examine the BMI growth trajectories of children exposed to these compounds in utero to assess how different levels of exposures may influence growth. We applied an exploratory method of growth modeling to fit and assess the trajectories of BMI for males and females by tertiles of third trimester BPA and phthalates metabolites.
METHODS
Study population
The Early Life in Mexico to Environmental Toxicants (ELEMENT; n=249) is a 22- year long research collaboration with Mexico’s Instituto Nacional de Salud Pública (INSP) that consists of 3 sequentially-enrolled birth cohorts from Mexico City serving low-to-moderate income populations, recruited from 1997-2005, and described elsewhere 14. Briefly, women were recruited to ELEMENT to examine the influence of in utero exposures to environmental toxicants on child development and health. Similar exclusion were applied to all 3 cohorts including living outside of Mexico City, gestational diabetes, preeclampsia, or pregnancy-related hypertensive disorder. Across each of the 3 cohorts, women were recruited during the first trimester of pregnancy or at delivery. Follow-up of the cohorts were continuously maintained, with child measurements collected at each study visit when the children were 3, 6, and 12 months of age and then every 6 months until 60 months; these children were then maintained in follow-up with 1-3 additional observations when children ranged in age from 8-14 years, between 2006-2012, conducted by trained personnel. Maternal education was obtained from the interviewer-administered baseline questionnaire.. The research protocols were approved by the Ethics and Research Committees of INSP in Mexico, and the Institutional Review Boards at Harvard University and University of Michigan Schools of Public Health and informed consent and assent of minors were provided by all participants prior to enrollment.
Anthropometric measures
Children’s weights and heights were taken by study personnel using established research protocol, in hospital gowns and without shoes15. Weight (BAME Mod 420; Catálogo Médico) was measured to the nearest 0.1 kg and height (BAME Mod 420; Catálogo Médico) was measured to the nearest 0.1 cm. Duplicate measures were taken of heights and weights and, if intra-personal variability exceeded the measurement tolerance of 0.5 cm, an additional measurement was taken. The observed values were averaged. BMI was calculated as weight divided by height squared (kg/m2).
Urinary BPA and phthalates metabolites
A spot (second morning void) urine sample was collected in sterile cups and was aliquoted an hour after collection and frozen at −20°C, from women during their third-trimester visit (average gestational age of 34.06 weeks). These samples were then transferred to the project's research center and frozen at −80 °C and shipped to the University of Michigan for storage. Samples were analyzed for total (free + glucuronidated) BPA and 9 phthalate metabolites by NSF International (Ann Arbor, MI, USA) using validated modification of the Centers for Disease Control and Prevention (CDC) methods described elsewhere 16. The 9 phthalate metabolites included: monoethyl phthalate (MEP), metabolite of diethyl phthalate (DEP mono-n-butyl phthalate (MBP), metabolite of di-n-butyl phthalate (DBP mono-isobutyl phthalate (MiBP), metabolite of di-isobutyl-phthalate (DiBP mono(3-carboxypropyl) phthalate (MCPP), metabolite of DBP and di-n-octyl phthalate (DOP monobenzyl phthalate (MBzP), metabolite of butylbenzyl phthalate (BBzP and mono(2-ethylhexyl) phthalate (MEHP), mono(2-ethyl-5-hydroxyhexyl) phthalate (MEHHP), mono(2-ethyl-5-oxohexyl) phthalate (MEOHP), and mono(2-ethyl-5-carboxypentyl) phthalate (MECPP), metabolites of di(2-ethylhexyl) phthalate (DEHP). Specific gravity (SG) of urine samples were measured using a handheld digital refractometer (ATAGO Company Ltd., Tokyo, Japan) and metabolite concentrations below the limit of quantitation (LOQ) were assigned a value of LOQ/sqrt(2).
Individual metabolites were then corrected for SG, which takes urinary dilution into account, using: Pc=P[SGp-1)/(SGi-1)], where Pc is the SG-corrected BPA or phthalate metabolite concentration (ng/mL), P is the measured urinary BPA or phthalate metabolite concentration, SGi is the urinary specific gravity for the individual, and SGp is the median of the urinary specific gravities for the sample (SGp for mothers = 1.013).17
Covariates
At 1 month postpartum, maternal weight and height was measured to the nearest 0.1 kg and 0.1 cm, respectively (BAME Mod 420; Catálogo Médico). Sociodemographic characteristics of the mother were collected at the third trimester through questionnaires administered by study personnel. Socioeconomic status was represented by the years of completed schooling by the mother at enrollment.
Statistical analysis
Data analysis was completed using SAS (version 9.3; SAS Institute, Cary, NC, USA). SG-corrected metabolites were natural log-transformed to normality prior to analyses. Each child had 5 to 12 observations for BMI; values were checked for biological implausibility by visually assessing their growth trajectory; values such as decreases in height were removed (n=6 observations).
We fit sex-stratified models to assess the differences in predicted BMI trajectories associated with exposure to tertiles of BPA and phthalate metabolites measured during the third trimester of pregnancy. Following an approach similar to published methods used to characterize children’s BMI trajectories18, we fit mixed effects models with fixed effects using a fractional polynomial age approach 19. First, the optimal fractional polynomial to model the expected valued of BMI was selected from
where m is the model degree and the exponential pj is selected from a set of 8 chosen values, which included −2, −1, −0.5, log, 0.5, 1, 2, and 3. The fewest number of terms in the model degree was set at m = 3 and the maximum degree was set to m = 8. Therefore, 219 candidate models were considered, with 1 model of the 8th degree, 8 models of the 7th degree, etc., and 56 models of the 3rd degree. The optimal number of age terms were selected separately for males and females based on smallest Akaike information criterion (AIC) and Bayesian information criterion (BIC). All models contained random intercept and linear age to account for within-child correlation in the measures. Models for each sex are shown in Supplementary Table 1.
Second, for BPA and the 9 phthalates metabolite, tertiles of the metabolites and their interaction terms with the selected age variables were entered as fixed effects. Likelihood ratio tests were conducted with the null model containing the age polynomials and the a priori identified covariates mother’s years of schooling and BMI at 1 month postpartum centered at their means, against the fully-adjusted model additionally containing the SG-corrected metabolites and their interaction terms with the age polynomials. Estimated model coefficients from fully-adjusted models were used to obtain predicted BMI trajectories for ages 0.25 to 13.8 years for each tertile of exposure when P<0.05 from the likelihood ratio test. These plots demonstrate the estimated BMI trajectories for each tertile of the phthalate metabolite or BPA exposure for an individual with otherwise average characteristics.
RESULTS
Among 249 children, 47% were male (Table 1). The average follow-up for children was 10.3 years. Among 2,162 total visits, 78% occurred at or before 5 years of age. Distributions of BPA and phthalate metabolites in maternal third trimester urine show detectable concentrations in most pregnancy samples, with no differences by sex (Supplementary Table 2).
Table 1.
Characteristics of mothers and children.
| Characteristics | Males | Females | ||
|---|---|---|---|---|
| N | Mean (SD)/% | N | Mean (SD)/% | |
| Child's age at last visit (yr) | 117 | 10.3 (1.5) | 132 | 10.3 (1.7) |
| Child's BMI at last visit (kg/m2) | 117 | 19.1 (3.08) | 132 | 19.8 (3.98) |
| Total number of visits | 1033 | 1129 | ||
| where age ≤1 years old | 243 | 25 | 263 | 24 |
| where age>1 and ≤3 years old | 381 | 36 | 424 | 37 |
| where age>3 and ≤5 years old | 182 | 17 | 201 | 18 |
| where age >5 years old | 227 | 22 | 241 | 21 |
| Mother's BMI | 114 | 26.7(4.2) | 126 | 27.2 (3.6) |
| Mother's years of education | 115 | 11.3 (2.8) | 130 | 10.8 (2.8) |
Abbreviations: SD (standard deviation)
We constructed BMI trajectories for models that showed a better fit with the inclusion of the BPA or phthalates metabolite (Supplementary Table 3). In boys, the likelihood ratio tests showed a better fit for models including the phthalates metabolites MiBP, MBzP, MEHP, and MEHHP. In girls, model fit was best with the inclusion of MECPP. We did not find a relationship between BPA exposure and BMI trajectories.
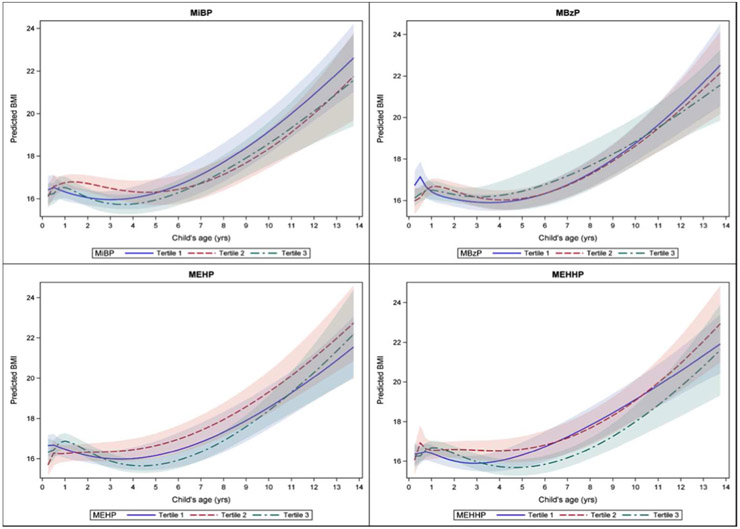
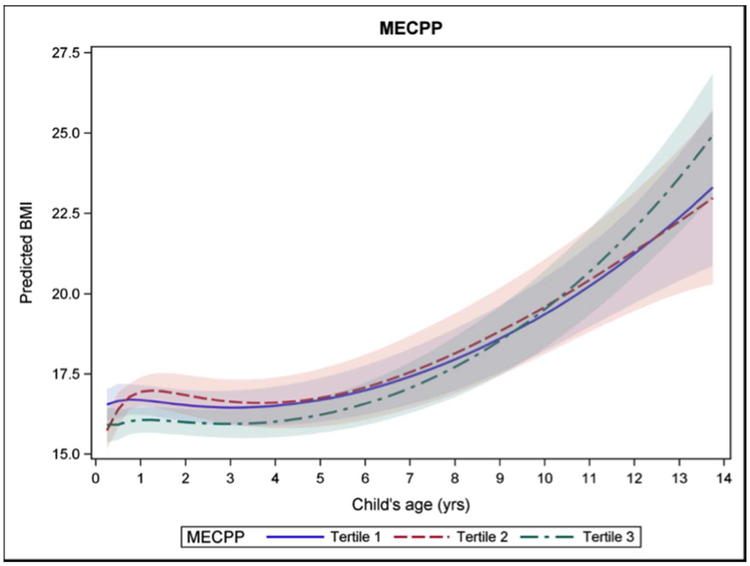
Trajectories for each tertile of metabolite exposure crossed at various times during the modeled ages, particularly in the first few years. Some BMI trajectories which appeared to be stable at the highest trajectory, such as the second tertile of exposure to MiBP in boys, did not remain so after the age of 5 years. Conversely, while exposure to the highest tertile of MEHP in infancy predicted the highest BMI trajectory in boys, after age 2 the highest trajectory was predicted by exposure to the second tertile of MEHP exposure. Confidence intervals widened as ages increased due to fewer observations. For modeled trajectories in boys, by age 14 the first and second tertiles of exposure had the highest predicted BMI trajectory (Figure 1). Exposure to the first tertile of MiBP and MBzP predicted the lowest BMI trajectory in infancy and early childhood, but crossed over to predict the highest BMI by age 14. With the phthalates metabolites MEHP and MEHHP, the second tertile of exposure was consistently predictive of the highest BMI trajectory from early childhood on. In girls, modeling showed that, by age 14, the third tertile of MECPP predicted the highest BMI trajectory for girls, though prior to age 9, the highest level of exposure predicted the lowest BMI trajectory (Figure 2).
Figure 1.
Male BMI trajectory for teritles of pregnancy MiBP, MBzP, MEHP, MEHHP exposures.
Figure 2.
Female BMI trajectory for teritles of pregnancy MECPP exposure.
DISCUSSION
In this prospective study modeling BMI trajectories, we observed that in utero exposure to phthalates metabolites differentially influenced the BMI trajectories of boys and girls. While tertile trajectories crossed frequently in early childhood, by age 14, we found that different tertiles of exposure predicted the highest attained BMI. This was observed with the highest tertile of exposure in girls, but the first or second tertile of exposure in boys. We did not detect any significant association with BPA.
Previous studies have found some positive associations between BPA and phthalates metabolites with child BMI; most longitudinal studies have outcomes in early childhood with few occurring in late childhood and early adolescence 11,12,20-23. Results from these longitudinal studies have been mixed, with differences reported by metabolites, sex, and age. The mixed findings from prenatal exposures and child BMI outcomes may be due to the different ages that child outcomes are measured, which may be influenced by the timing of puberty or adiposity rebound. Therefore, we explored the use of growth trajectories as an alternate method of assessing the potential impact of early life exposures. The fluctuating trajectories indicated that the age outcomes are assessed at can influence the interpretation of the relationship between levels of exposures with child outcome. This is likely a contributing reason why some metabolites are positively associated with child BMI at one age but not another.
This is illustrated by contrasting the results we obtained here, with previous work and studies by others. Deierlein et al. (2016) observed higher BMI for girls aged 7-13 years when they were exposed at 6-8 to medium vs. low concentrations of MBzP while, in previous work, we found an negative relationship between prenatal MBzP exposure and child’s BMI at ages 8-14 years with no modification by sex 10,12. However, we only observed a relationship between MBzP among boys in this study. Similarly, a pooled analysis of 3 prospective cohorts in the US showed sexually dimorphic associations with high molecular weight phthalates metabolites (MEHP, MEHHP, MEOHP, MECPP) negatively associated with BMI in girls, but no associations with boys 24. In previous work, we found urinary concentrations of MEHP in boys were negatively associated with child adiposity but did not find a relationship among girls 12. This was inconsistent with other studies which observed MEHP to be inversely associated with BMI in girls both cross-sectionally and longitudinally 9,10. Using BMI trajectory modeling to re-assess our findings of MEHP exposure in utero, we found a relationship among boys, but not girls. These differences in results may potentially be due to the close trajectory paths and overlapping confidence intervals. However, with further refinement, growth trajectory modeling could be a useful tool to assess how exposures may alter trajectories of growth in children and may aid in interpreting how different levels of exposures may result in different associations.
The observed sex differences are thought to be due to the endocrine-disrupting mechanisms of phthalates metabolites. Phthalates are anti-androgenic and are able to influence energy homeostasis through increasing the number of adipocytes, as well as increasing lipid accumulation; their action is not through direct interaction with the androgen receptors, but through stimulation of the peroxisome proliferator-activated receptors family of nuclear receptors (PPARs) 8,25. PPARα in particular has been found to have sex-differential effects, with exposure to MEHP significantly increasing body weight and fat pads of male, but not female mice 26.
Limitations of this study include the relatively small sample size and fewer number of observations of BMI measures after the age of 5, as follow-up was not systematic after that point in time. Children had 1-3 measurements after that age and few (n=21) had only 1 measurement after the age of 5. This modeling is exploratory in examining whether growth trajectories could be constructed using available data. Restricting growth modeling to observations before the age of 5 would have altered reported results and further work will need to ensure greater data support for more precise modeling. Our interpretations should therefore be viewed with caution until further studies are conducted with more observations. We also chose to use fractional age polynomials rather than splines to model growth trajectories; spline models are more flexible but require pre-defined decisions on the number and placement of age knots as well as requiring continuous data support, whereas we wanted to explore how growth curves would form based on the data, and much of our data on BMI was collected before the age of 5.
Another limitation was the use of a single spot urine in pregnancy for analyses of metabolites. Concentrations of metabolites are known to have short metabolic half-lives, with <24 hours for phthalates metabolites and <5-6 hours for BPA, and exhibit temporal variability; a single spot measure may therefore not be capable of accurately assessing individuals 27,28. However, individuals tend to exhibit consistent behaviors that would result in more consistent exposures, which has been shown by other studies 29. Our geometric mean urinary concentrations of metabolites were comparable or lower compared with those reported by others in the pregnancy period. In a Californian population, Harley et al. (2013) reported a GM of 1.2 ng/mL for BPA averaged across 2 pregnancy time points, which was higher than our 0.7 ng/mL and 0.9 ng/mL for boys and girls, respectively. Similarly, higher values were reported by Vafeiadi et al. (2016) among a Greek population during the first trimester of pregnancy (1.2 ng/mL). For phthalates metabolites, higher GM values were reported by Buckley et al. (2016) among 3 pooled cohorts from the US (μg/L; MEP: 154.1; MiBP: 6.45; MCPP: 2.08; MBzP: 11.6; MECPP: 35.7; MEHHP: 21.4; MEHP: 4.92; MEOHP: 18.0) and by Bellavia et al. (2017) among women in their first trimester of pregnancy in Boston (μg/L; MEP: 137.8; MEP: 0.9; MCPP: 2.1). While prenatal exposure to BPA was positively associated with BMI z-score in boys, but negatively in girls, at 4 years of age in the Rhea cohort 21,we did not find any differences in BMI trajectories by levels of BPA exposure, which may be due to the high proportion of values measured <LOQ. It is also possible that trimester-specific associations may reveal vulnerable windows for in utero exposures, but there are too few studies with trimester-specific exposures to draw any conclusions. Additionally, we also chose to correct for specific gravity when controlling for urinary dilution, rather than creatinine, as creatinine is influenced by factors such as age, sex, and muscle mass 30.
Exposures to BPA and phthalates metabolites are influenced by a variety of factors such as diet, physical activity, and use of personal care products. We did not include dietary intakes, consumer product use, or physical activity as potential confounders, which will impact the amount of residual confounding. We also relied on maternal BMI collected at 1 month postpartum rather than prepregnancy BMI due to limited availability of self-reported heights and weights of the mothers at recruitment into the original birth cohorts.
However, strengths of our study include the extensive follow-up within this prospective study design, allowing the construction of growth trajectories. Unlike cross-sectional studies, where there is possibility of reverse causality from higher urinary metabolites in heavier individuals due to increased dietary intake or volume of consumer product use, longitudinal studies provide a temporal sequence of events and we were able to measure exposures in utero, during a critical time of development. Additionally, the construction of BMI trajectories based on exposure in pregnancy is a novel method of assessing the potential impacts that these metabolites have on child growth on a continuous scale, rather than exploring these associations at various ages.
CONCLUSION
We found different levels of exposure to phthalates metabolites were associated with child growth by sex. In girls, the highest level of exposure to MECPP was associated with the highest predicted BMI by age 14, while lower levels of exposure to MiBP, MBzP, MEHP, and MEHHP were associated with the highest predicted BMI in boys. Our findings suggest that timing of outcome measurement may influence associations observed. Further follow-up in this study will provide us with anthropometric measures to capture trajectories through adolescence.Other studies are needed and should consider more evenly distributed measurements and confounding variables for growth modeling. Trajectory modeling is a potential method to better understand how exposures to endocrine-disrupting compounds may influence growth.
Supplementary Material
What is already known on this subject:
Exposures to endocrine-disrupting compounds like Bisphenol A and phthalates metabolites are widespread.
Studies have observed associations between exposures to these compounds and child BMI and adiposity, but results have been mixed.
Associations between exposures and child outcomes differ by sex.
What this study adds:
Novel growth trajectory modelling allowed exploration of how exposures during pregnancy may influence child growth.
Boys and girls have different BMI trajectories depending on level of exposure in utero.
Highest predicted BMI in adolescence for girls occurred with the highest exposure, while the opposite was observed in boys.
ACKNOWLEDGEMENTS
Author Affiliations: Department of Health Sciences, University of York, Heslington, York, United Kingdom (Tiffany C Yang Department of Environmental Health Sciences, University of Michigan, Ann Arbor, Michigan (Karen E Peterson, John D Meeker Department of Nutrition, Harvard School of Public Health, Boston, Massachusetts (Karen E Peterson Department of Biostatistics, University of Michigan, Ann Arbor, Michigan (Brisa N Sanchez, Zhenzhen Zhang Centro de Investigaciones en Salud Poblacional, Instituto Nacional de Salud Pública, Cuernavaca, Morelos, Mexico (Alejandra Cantoral, Maritsa Solano, Martha M Tellez-Rojo).
This study was supported by the University of Michigan School of Public Health Formative Children’s Environmental Health and Disease Prevention Research Center (Grant P20 ES018171/RD834800PI to K.E.P.); Lifecourse Exposures and Diet: Epigenetics, Maturation, and Metabolic Syndrome (Grant 1P01ES022844-01/RD-83543601 and Lifestage Exposures and Adult Disease (Grant P30 ES017885).
ABBREVIATIONS:
- BPA
bisphenol A
- BMI
body mass index
- INSP
Instituto Nacional de Salud Pública
- CDC
Centers for Disease Control and Prevention
- MEP
monoethyl phthalate
- DEP
diethyl phthalate
- MBP
mono-n-butyl phthalate
- DBP
di-n-butyl phthalate
- DiBP
di-isobutyl-phthalate
- MiBP
mono-isobutyl phthalate
- MCPP
mono(3-carboxypropyl) phthalate
- DOP
di-n-octyl phthalate
- MBzP
monobenzyl phthalate
- BBzP
butylbenzyl phthalate
- MEHP
mono(2-ethylhexyl) phthalate
- MEHHP
mono(2-ethyl-5-hydroxyhexyl) phthalate
- MEOHP
mono(2-ethyl-5-oxohexyl) phthalate
- MECPP
mono(2-ethyl-5-carboxypentyl) phthalate
- DEHP
di(2-ethylhexyl) phthalate
- SG
specific gravity
- LOQ
limit of quantitation
- AIC
Akaike information criterion
- BIC
Bayesian information criterion
REFERENCES
- 1.WHO. Report of the Commission on Ending Childhood Obesity. Who; 2016:30. doi:ISBN 978 92 4 151006 6. [Google Scholar]
- 2.Liu Y, Peterson KE. Maternal Exposure to Synthetic Chemicals and Obesity in the Offspring: Recent Findings. Curr Environ Heal Reports. 2015;2(4):339–347. doi: 10.1007/s40572-015-0068-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Menale C, Grandone A, Nicolucci C, et al. Bisphenol A is associated with insulin resistance and modulates adiponectin and resistin gene expression in obese children. Pediatr Obes. 2017;12(5):380–387. doi: 10.1111/ijpo.12154. [DOI] [PubMed] [Google Scholar]
- 4.Trasande L, Spanier AJ, Sathyanarayana S, Attina TM, Blustein J. Urinary phthalates and increased insulin resistance in adolescents. Pediatrics. 2013;132(3):e646–55. doi: 10.1542/peds.2012-4022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Silva MJ, Barr DB, Reidy JA, et al. Urinary levels of seven phthalate metabolites in the U.S. population from the National Health and Nutrition Examination Survey (NHANES) 1999-2000. Environ Health Perspect. 2004;112(3):331–338. doi: 10.1289/ehp.6723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Callan AC, Hinwood AL, Heffernan A, Eaglesham G, Mueller J, Odland JØ. Urinary bisphenol A concentrations in pregnant women. Int J Hyg Environ Health. November 2012:2–5. doi: 10.1016/j.ijheh.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 7.Vandenberg LN, Hauser R, Marcus M, Olea N, Welshons W V. Human exposure to bisphenol A (BPA). Reprod Toxicol. 2007;24(2):139–177. doi: 10.1016/j.reprotox.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 8.Kim SH, Park MJ. Phthalate exposure and childhood obesity. Ann Pediatr Endocrinol Metab. 2014;19(2):69–75. doi: 10.6065/apem.2014.19.2.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hatch EE, Nelson JW, Qureshi MM, et al. Association of urinary phthalate metabolite concentrations with body mass index and waist circumference: a cross-sectional study of NHANES data, 1999-2002. Environ Health. 2008;7:27. doi: 10.1186/1476-069X-7-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deierlein AL, Wolff MS, Pajak A, et al. Longitudinal Associations of Phthalate Exposures During Childhood and Body Size Measurements in Young Girls. Epidemiology. 2016;27(4):492–499. doi: 10.1097/EDE.0000000000000489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harley KG, Aguilar Schall R, Chevrier J, et al. Prenatal and postnatal bisphenol A exposure and body mass index in childhood in the CHAMACOS cohort. Environ Health Perspect. 2013;121(4):514–520, 520–6. doi: 10.1289/ehp.1205548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang TC, Peterson KE, Meeker JD, et al. Bisphenol A and phthalates in utero and in childhood: association with child BMI z-score and adiposity. Environ Res. 2017;156(March):326–333. doi: 10.1016/j.envres.2017.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dietz WH. Periods of risk in childhood for the development of adult obesity--what do we need to learn? J Nutr. 1997;127(9):1884S–1886S. [DOI] [PubMed] [Google Scholar]
- 14.Gonzalez-Cossio T, Peterson KE, Sanin L-H, et al. Decrease in Birth Weight in Relation to Maternal Bone-Lead Burden. Pediatrics. 1997;100(5):856–862. doi: 10.1542/peds.100.5.856. [DOI] [PubMed] [Google Scholar]
- 15.Lohman TG, Roche AF, Martorell R. Anthropometric Standardization Reference Manual. Champaign, IL: Human Kinetics Books; 1988. [Google Scholar]
- 16.Lewis RC, Meeker JD, Peterson KE, et al. Predictors of urinary bisphenol A and phthalate metabolite concentrations in Mexican children. Chemosphere. September 2013. doi: 10.1016/j.chemosphere.2013.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mahalingaiah S, Meeker JD, Pearson KR, et al. Temporal variability and predictors of urinary bisphenol A concentrations in men and women. Environ Health Perspect. 2008;116(2):173–178. doi: 10.1289/ehp.10605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wen X, Kleinman K, Gillman MW, Rifas-Shiman SL, Taveras EM. Childhood body mass index trajectories: modeling, characterizing, pairwise correlations and socio-demographic predictors of trajectory characteristics. BMC Med Res Methodol. 2012; 12(1):38. doi: 10.1186/1471-2288-12-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Royston P, Altman DG. Regression using Fractional Polynomials of Continuous Covariates: Parsimonious Parametric Modelling. Appl Stat. 1994;43(3):429–467. [Google Scholar]
- 20.Braun JM, Lanphear BP, Calafat AM, et al. Early-life bisphenol a exposure and child body mass index: a prospective cohort study. Environ Health Perspect. 2014;122(11): 1239–1245. doi: 10.1289/ehp.1408258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vafeiadi M, Roumeliotaki T, Myridakis A, et al. Association of early life exposure to bisphenol A with obesity and cardiometabolic traits in childhood. Environ Res. 2016;146:379–387. doi: 10.1016/j.envres.2016.01.017. [DOI] [PubMed] [Google Scholar]
- 22.Maresca MM, Hoepner LA, Hassoun A, et al. Prenatal exposure to phthalates and childhood body size in an urban cohort. Environ Health Perspect. 2016;124(4):514–520. doi: 10.1289/ehp.1408750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shoaff J, Papandonatos GD, Calafat AM, et al. Early-Life Phthalate Exposure and Adiposity at 8 Years of Age. Environ Health Perspect. 2017;125(9):97008. doi: 10.1289/EHP1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Buckley JP, Engel SM, Braun JM, et al. Prenatal Phthalate Exposures and Body Mass Index Among 4- to 7-Year-old Children: A Pooled Analysis. Epidemiology. 2016;27(3):449–458. doi: 10.1097/EDE.0000000000000436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taxvig C, Dreisig K, Boberg J, et al. Differential effects of environmental chemicals and food contaminants on adipogenesis, biomarker release and PPARγ activation. Mol Cell Endocrinol. 2012;361(1-2):106–115. [DOI] [PubMed] [Google Scholar]
- 26.Hao C, Cheng X, Xia H, Ma X. The endocrine disruptor mono-(2-ethylhexyl) phthalate promotes adipocyte differentiation and induces obesity in mice. Biosci Rep. 2012;32(6):619–629. doi: 10.1042/BSR20120042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koch HM, Bolt HM, Preuss R, Angerer J. New metabolites of di(2-ethylhexyl)phthalate (DEHP) in human urine and serum after single oral doses of deuterium-labelled DEHP. Arch Toxicol. 2005;79(7):367–376. doi: 10.1007/s00204-004-0642-4. [DOI] [PubMed] [Google Scholar]
- 28.Völkel W, Colnot T, Csanády GA, Filser JG, Dekant W. Metabolism and kinetics of bisphenol a in humans at low doses following oral administration. Chem Res Toxicol. 2002;15(10):1281–1287. [DOI] [PubMed] [Google Scholar]
- 29.Teitelbaum SL, Britton JA, Calafat a M, et al. Temporal variability in urinary concentrations of phthalate metabolites, phytoestrogens and phenols among minority children in the United States. Environ Res. 2008;106(2):257–269. doi: 10.1016/j.envres.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 30.Pearson MA, Lu C, Schmotzer BJ, Waller LA, Riederer AM. Evaluation of physiological measures for correcting variation in urinary output: Implications for assessing environmental chemical exposure in children. J Expo Sci Environ Epidemiol. 2009;19(3):336–342. doi: 10.1038/jes.2008.48. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.