Highlights
-
•
Changes in ERPs and oscillatory dynamic occur during auditory sentence processing.
-
•
Adults are significantly better at identifying syntactic errors compared to children.
-
•
Adults display a significant P600 effect and theta/beta power decrease.
-
•
Children display a significant N400 effect and smaller decrease in theta/beta power.
-
•
These findings suggest syntactic processing skills are still developing by age 12.
Keywords: Time-frequency analysis, Language, ERP, Development
Abstract
Although very young children process ongoing language quickly and effortlessly, research indicates that they continue to improve and mature in their language skills through adolescence. This prolonged development may be related to differing engagement of semantic and syntactic processes. This study used event related potentials and time frequency analysis of EEG to identify developmental differences in neural engagement as children (ages 10–12) and adults performed an auditory verb agreement grammaticality judgment task. Adults and children revealed very few differences in comprehending grammatically correct sentences. When identifying grammatical errors, however, adults displayed widely distributed beta and theta power decreases that were significantly less pronounced in children. Adults also demonstrated a significant P600 effect, while children exhibited an apparent N400 effect. Thus, when identifying subtle grammatical errors in real time, adults display greater neural activation that is traditionally associated with syntactic processing whereas children exhibit greater activity more commonly associated with semantic processing. These findings support previous claims that the cognitive and neural underpinnings of syntactic processing are still developing in adolescence, and add to them by more clearly identifying developmental changes in the neural oscillations underlying grammatical processing.
1. Introduction
Real-time language comprehension is a fast-paced, complex task that includes retrieving and integrating phonological, semantic, syntactic, and pragmatic information with millisecond-level precision. Behavioral and neuroimaging research indicate that the development of adult-like language abilities and the neural structures underlying those abilities is prolonged, continuing through age 12 or later (Atchley et al., 2006, Friedrich and Friederici, 2004, Friederici and Hahne, 2001, Silva-Pereya et al., 2005, Nuñez et al., 2011). Performing well during natural, everyday language tasks but exhibiting subtle processing differences when language capabilities are taxed indicates that children may engage somewhat different skills or strategies than adults during language comprehension (Holland et al., 2007). To better understand the nature of these differences we used event-related potentials (ERPs) and time frequency analysis of EEG to examine the neural oscillations underlying naturally paced sentence comprehension in children and adults.
Many theories have noted that the development of effective semantic integration and syntactic unification may contribute to the prolonged development of language skills (e.g., Brauer and Friederici, 2007, Chou et al., 2006). One must quickly retrieve semantic representations related to each incoming word and then, as each new word in the sentence is encountered, integrate it to form a coherent semantic representation. For example, when hearing the phrase the hairy, it is easier to integrate the word dog with that phrase than table, because a hairy dog refers to a logical semantic representation in a way that a hairy table does not. Syntactic unification is also necessary for successful language comprehension. Continuing our example, in English, adjectives are often followed by nouns; thus, one can integrate the syntactic information in the hairy dog to form a meaningful representation but not the hairy eat.
Research using ERPs consistently reports that semantic and syntactic abilities develop through early adolescence to support language comprehension (e.g., Atchley et al., 2006, Friederici and Hahne, 2001). Participants in these studies read or hear sentences containing a semantic error (She buttered her toast with a dress) or a grammatical error (The goose was in the fed). Compared to correct sentences, children and adults exhibit a larger N400 to semantic errors and a larger P600 to grammatical errors. Although study specifics vary, children generally display an N400 that is later, larger and more broadly distributed and a P600 that is larger and later compared to adults (Benau et al., 2011, Friederici and Männel, 2013, Hahne et al., 2004, Friedrich and Friederici, 2004, Friederici, 2006). These developmental differences are thought to reflect higher cognitive demands when children perform the same language task as adults. These findings are informative about the development of early language skills but, due to the process of averaging the EEG signal to produce an ERP, non-phase locked dynamics, providing important information related to semantics and syntax, can be lost. Recent computational advances, such as time frequency analysis, provide different means of analyzing EEG data by decomposing the signal to identify changes in the amplitude, or power, of the response within frequency bands of interest (Davidson and Indefrey, 2007, Cohen, 2014). Given this advantage, time frequency analysis may identify differences in processing that are lost due to the averaging process used in traditional ERP analysis.
Changes in the beta frequency band (12–30 Hz) have been related to syntactic unification (e.g., Bastiaansen et al., 2010, Davidson and Indefrey, 2007). According to theories of syntactic unification, each incoming word in a sentence activates multiple syntactic possibilities, called lexical frames (Vosse and Kempen, 2000). These lexical frames specify the potential structural environment for each incoming word, and are combined based on various features and constraints to create one stable syntactic structure by which the meaning of the sentence can be decoded. Related to time frequency analysis, beta increases with each word in a visually presented grammatically correct sentence, but decreases at the point of a syntactic error in a sentence, when syntactic unification fails (Bastiaansen et al., 2010, Davidson and Indefrey, 2007). Further, when the words of a sentence are presented in a random order, no increase in beta occurs, presumably due to the lack of syntactic information (Bastiaansen et al., 2010). Although beta responds differently to a syntactic violation than the P600 ERP component, both appear to play an important role in identifying changes in syntactic processing.
Similar to beta, theta power also increases with each word during sentence reading (Bastiaansen et al., 2010). However, at the point of a semantically incongruent word in the sentence, theta power is greater than when the words are semantically congruent. Similar to the N400, the amount of theta increase may be linked to how difficult it is to semantically integrate the current information with the preceding context (Davidson and Indefrey, 2007, Hald et al., 2006). However, to date, no research that we know of has studied the neural oscillations underlying the semantic aspects of sentence processing in children compared to adults.
While theta and beta increase during reading of sentences, they diverge in how they respond to an error in the sentence - theta increases to a semantic error whereas beta decreases to a syntactic error (Bastiaansen et al., 2002, Bastiaansen et al., 2010). On the surface, it seems that theta and beta are similar to the N400 and P600 ERP responses, indexing semantic integration and syntactic unification, respectively; however, further research is needed to identify the relationship between language processes and underlying neural activity. The current study uses ERP (e.g., P600, N400) and time frequency (e.g., theta, beta) analyses to investigate neural processing in children 10–12 years old and adults during a grammaticality judgment task in which they listen to sentences containing either no grammatical error or a verb agreement error (e.g., she walk). For both groups, we predicted theta and beta increases for grammatically correct sentences and a beta decrease/P600 effect following the agreement error. We examined the possibility of a theta increase/N400 because children seem to engage different strategies than adults during language processing. Further, we performed analyses to better identify the relationships between ERPs, changes in power, and behavioral measures.
2. Methods
2.1. Participants
Eighteen right-handed, monolingual English-speaking adults ages 18–31 (9 male, 9 female; M = 24.41, SD = 4.37) and eighteen right-handed, monolingual English-speaking children ages 10–12 years (9 male, 9 female; M = 10.94, SD = 0.94) participated in the study. All participants had no history of significant neurological issues (traumatic brain injury, CVA, seizure disorders, history of high fevers, tumors, or learning disabilities), based on adult self-report and parental report for child participants. Exclusion criteria included left-handedness, use of alcohol or controlled substances within 24 h of testing, and medications other than over-the-counter analgesics and contraceptives.
2.2. Stimuli
Participants completed a grammaticality judgment task in which they heard a sentence and indicated via button press whether the sentence was grammatical or ungrammatical. Each sentence began with a prepositional phrase followed by either a plural (we/they) or singular (he/she) pronoun subject followed by an action verb (e.g., jump; jumps) with all words in the sentence found in children's early vocabularies (Fenson et al., 1994). In ungrammatical sentences, the grammatical violation was a noun–verb agreement error occurring at the verb (e.g., he walk, they walks). Importantly, the current study design utilizes verb forms with and without the morphological ending –s. Both conditions were equally likely to occur in both the grammatical and ungrammatical conditions, therefore eliminating differences in processing the acoustical properties of the word as a confounding variable. Sentences were either simple (one critical noun–verb pairing) or compound (two critical noun–verb pairings). To ensure that participants were fully engaged in the process of sentence parsing before the onset of the critical verb, and to avoid interference of wrap-up effects at the sentence-final position, there were at least three words preceding the pronoun and critical verb, and at least two words following the critical verb (Hagoort et al., 1993). Ungrammatical compound sentences contained only one ungrammatical phrase; two grammatical violations never occurred in the same sentence. Example sentences can be found in Table 1. To create the auditory stimuli, grammatically correct sentences were recorded by a female native English speaker using typical intonation. A splicing technique, using Cool Edit Pro 2.1 (Adobe Systems Inc.), was applied to create all ungrammatical sentences from the recorded grammatical sentences in order to control for changes in intonation early in the sentence that may imply the occurrence of an error.
Table 1.
Examples of grammatical sentences. All sentences began with a prepositional phrase and were followed by the critical noun–verb pairing, which are underlined in the above examples. Simple sentences contained one critical noun–verb pairing while compound sentences contained two.
| Singular | Plural | |
|---|---|---|
| Simple | In the gym he jumps higher than me | In the gym they jump higher than me |
| Compound | In the gym he jumps high but they jump higher. | In the gym we jump high but he jumps higher. |
During the grammaticality judgment task, each subject heard 160 sentences: 80 simple sentences (40 grammatical/40 ungrammatical) and 80 compound sentences (40 grammatical/20 early error ungrammatical/20 later error ungrammatical). Compound sentences were included to ensure the participant attended to the whole sentence, rather than just the first verb. To encourage participants to attend to the complete sentence they were asked to withhold their response until after the sentence was complete. This study focused on identifying the processes underlying processing of correct grammaticality judgments; thus, EEG responses corresponding to incorrectly responded to trials were removed from all analyses. Only correct response trials were included to ensure processing differences were not related to performance differences.
2.3. Procedure
Participants sat in a chair one meter below a centralized speaker while wearing a Neuroscan high-density 64-electrode Quickcap. EEG data were acquired with a Synamps2 amplifier (Compumedics, Inc.) and Neuroscan 4.3.2 software, sampled at 1 kHz, hardware filtered at 200 Hz and high pass filtered at 0.15 Hz. Electrode impedances were typically below 5 kΩ.
Participants were told they would hear a sentence that may or may not contain a grammatical error. They were instructed that, following the completion of the sentence, they should press one button if the sentence was grammatical and a different button if the sentence was ungrammatical. The handedness of the button responses was counterbalanced across participants to remove motor related laterality differences. Training on how to engage in a grammaticality judgment task took place prior to the experimental stimuli being presented. During training, participants performed a shortened version of the task, including eight sentences that were both grammatical and ungrammatical. Feedback was provided during training but not during the test session. A break, occurring halfway into the test session, was provided to allow participants to move around without interfering with data collection. The total duration of recording was approximately 22 min for adults and 26 min for children. Participants were told to respond following the completion of the sentence; however, there was no time constraint on how quickly the participant had to respond, making the task self-paced. This discrepancy in participants’ break time between sentences is directly related the total average length of the task for each group.
2.4. EEG processing
Using Neuroscan's Edit program each continuous EEG was high-pass filtered at 0.15 Hz. Areas of muscle activity, electrode drift, and eye-blink artifacts were removed after visual inspection by deleting bad blocks and using a spatial filter in the Neuroscan Edit program. Data were referenced online to an electrode located near the vertex and re-referenced offline to average over the entire head. A spline-based estimate of the average scalp potential (Ferree, 2006) was computed using spherical splines (Perrin et al., 1989) from a standardized set of electrode positions to interpolate bad electrodes, yielding 62 data channels in each subject. The continuous data were epoched with each epoch spanning 500 msec prior to the onset of the critical verb to 1500 msec after the onset of the critical verb.
2.5. Event related potentials
Using the EEGlab toolbox in Matlab the average referenced data were low-pass filtered at 30 Hz. For each trial and electrode, the mean amplitude of the pre-stimulus interval (−100 to 0 msec) was subtracted from each time point in the post-stimulus interval to correct for baseline differences. Single trials were averaged together to obtain a stable waveform ERP for each condition and each electrode for every subject.
2.6. Time frequency analysis
Time frequency analysis was used to quantify event-related spectral perturbations (ERSP). Fourier power spectra were computed using a slight change of the pwelch function, executed in Matlab (Mathworks, Inc.) and applied to 0.5 s windows. In each epoch and time window, the time series was linearly detrended and mean subtracted to reduce spectral leakage from the zero-frequency bin, cosine tapered to reduce spectral leakage, and zero-padded for a 1 s duration to achieve 1 Hz frequency resolution. To obtain the power spectral density (PSD) in units of μV2/Hz, each window was Fourier transformed, magnitude squared, and suitably normalized. The results were averaged across trials to obtain the best statistical estimate of the PSD in each window. By keeping the raw power values, rather than the log power values minus the baseline, our use of the term ERSP differs slightly from that of Delorme and Makeig (2004).
Throughout the peri-stimulus interval, the time-dependent PSD was estimated in 0.5 second sliding windows, moving in 0.05 second steps. The time of each window is defined as the center of the nonzero data in that window. To calculate the baseline spectrum from each condition, the 1 second baseline interval was divided into three 0.5 s windows with 50% overlap (Welch, 1967). This interval was averaged across all trials, within each condition, and the mean baseline power at each electrode and frequency was subtracted so that power differences revealed are relative to baseline (Delorme and Makeig, 2004).
3. Results
3.1. Behavioral results
Following the presentation of each sentence, participants made a grammaticality judgment via button response. In comparing error rates, a 2 sentence type (grammatical, ungrammatical) × 2 age group (child, adult) repeated measures two-way ANOVA revealed a main effect of age (F(1,35) = 9.67, p < 0.005), with no effect of sentence type (F(1,35) = 4.01, p = .053), nor an interaction (F(1,35) = 1.67, p = .206). Overall, children made significantly more errors compared to adults: 17.17% and 6.13%, respectively (F(1,35) = 12.06, p < 0.001; see Table 2). Additionally, a 2 age (children, adults) × 2 condition (singular, plural) ANOVA revealed a main effect of age (F(1,34) = 9.79, p < 0.01), but no main effect of condition (F(1,34) = 0.00006, p = .894), nor an interaction of age × condition (F(1,34) = 2.73, p = .107). These findings suggest that plural and singular conditions had no effect on processing of sentences; therefore, both conditions were combined to improve power. The behavioral data support previous reports that children continue to struggle with agreement error identification through age 12 (Clark, 2003).
Table 2.
Types of errors and percentages misidentified. Numbers shown represent the percent missed by children or adults for each sentence type.
| Singular |
Plural |
|||
|---|---|---|---|---|
| Grammatical | Ungrammatical | Grammatical | Ungrammatical | |
| Every morning he picks out what he is going to wear. | Every morning he pick out what he is going to wear. | Every morning they pick out what they are going to wear. | Every morning they picks out what they are going to wear. | |
| Children | 15.15% | 21.18% | 13.09% | 19.27% |
| Adults | 5.13% | 5.25% | 6.25% | 7.88% |
3.2. EEG results
3.2.1. Event related potentials
ERPs were only calculated for items that were responded to correctly and are epoched at the onset of the verb in the sentence (−100 to 1200 msec). This resulted in fewer trials per condition for children (Grammatically correct, M = 34.556; SD = 19.77; Grammatical errors, M = 21.83; SD = 16.24) than adults (Grammatically correct, M = 64.78; SD = 24.88; Grammatical errors, M = 39.44; SD = 16.61; F(1,34) = 15.752, p = .001, MSE = 45.435) and more grammatical trials than ungrammatical in both age groups. To improve statistical power, grammatical and ungrammatical sentences were created from both simple and compound sentences. Simple and compound sentences produced no significant differences related to the grammaticality ERP effects (F(1,35) = 2.071, p = NS). Additionally, there was no effect of condition for singular and plural conditions, nor was there an interaction with age; therefore, plural and singular conditions were combined to improve the signal-to-noise ratio.
To calculate the P600 amplitude we computed the average amplitude for central and parietal sites (‘cp3’,‘p1’,‘cpz’,‘pz’,‘p2’,‘cp4’) at three separate time windows (500–700, 700–900, and 900–1100 msec) similar to previous studies (Kaan and Swaab, 2002). The mean amplitude was then computed across selected electrodes within the designated time windows. Each time point, across each electrode, was averaged separately for grammatical conditions and ungrammatical conditions. The mean amplitudes for each condition were calculated for adults and children and input into a 2 (age) × 2 (grammaticality) repeated measures ANOVA. To calculate the N400 amplitude, we compared the average amplitude for midline, frontal, and parietal sites (‘cz’, ‘fz’, ‘fcz’,‘cpz’,‘pz’, ‘cp3’, ‘cp4’) at the time window of 350–450 msec, similar to past research (Benau et al., 2011, Kutas and Federmeier, 2011, Van Petten and Luka, 2006). The mean amplitude was calculated similar to that of the P600, however, the electrodes included in the N400 analysis differed and were averaged only for one time window, across time points between 350 and 450 msec. To determine significance, a 2 (age) × 2 (grammaticality) ANOVA was used.
To account for any potential influence of differences in the number of trials between children and adults, we repeated all ANOVAs described above with equal numbers of trials between groups. To do so, we randomly selected and removed trials from each adult's data, within each condition, resulting in equivalent average numbers of trials between adults and children. This corrected analysis revealed the same pattern of results as those presented below.
P600. In the earliest time window (500–700 msec post-critical verb onset), a 2 (age) × 2 (grammaticality) ANOVA on P600 amplitude revealed a significant main effect of age (F(1,35) = 5.149, p < 0.04) and a significant interaction between age and grammaticality (F(1,35) = 7.159, p < 0.02) at widespread posterior electrodes. For children, a paired-samples t-test comparing grammatical and ungrammatical items revealed a significantly more positive amplitude for grammatical items versus ungrammatical items (t(17) = 2.539, p < 0.05). No significant differences between grammatical and ungrammatical items were present for adults in this time window.
In the middle time window (700–900 msec post-critical verb), a 2 (age) × 2 (grammaticality) ANOVA on P600 amplitude revealed a significant main effect of age (F(1,35) = 9.624, p < 0.01) and a significant interaction between age and grammaticality (F(1,35) = 7.390, p < 0.02). There were no significant differences between grammatical and ungrammatical items for children, although the mean amplitude was more positive for grammatical versus ungrammatical items. For adults, a paired-samples t-test revealed a significantly more positive amplitude for ungrammatical versus grammatical items (t(17) = −3.137, p < 0.01).
In the last time window (900–1100 msec post-critical verb), a 2 (age) × 2 (grammaticality) ANOVA on the P600 amplitude revealed a significant main effect of grammaticality (F(1,35) = 8.175, p < 0.01) at widespread posterior electrodes. Like the middle time window, no significant differences between grammatical and ungrammatical items were present for children although, the mean amplitude was more positive for ungrammatical versus grammatical items. For adults, a paired-samples t-test revealed a significantly more positive amplitude for ungrammatical versus grammatical items (t(17) = −4.547, p < .001). Fig. 1 presents the ERP results from this analysis.
Fig. 1.
ERPs for both adults and children at widespread electrode sites. Adults (top headmap) demonstrated a significantly larger P600 for ungrammatical (blue) than grammatical (red sentences). Children (bottom headmap) demonstrated a larger N400 for ungrammatical (blue) than grammatical (red) sentences, and a later P600 compared to adults. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
N400. A 2 (age) × 2 (grammaticality) ANOVA on N400 amplitude revealed a significant main effect of grammaticality (F(1,35) = 4.666, p < 0.05) and a significant interaction between age and grammaticality (F(1,35) = 7.403, p < 0.02) at widespread frontal, midline, and parietal electrodes 350–450 msec after the onset of the critical verb. For children, a paired-samples t-test comparing grammatical and ungrammatical items revealed a significant difference (t(17) = 2.70, p < 0.02) in which the N400 amplitude was more negative for the ungrammatical items. There were no significant differences between grammatical and ungrammatical items for adults. Fig. 1 presents the ERP results from this analysis.
3.2.2. Time frequency analysis
To examine developmental differences in neural engagement during grammaticality judgments, theta and beta power changes, post-critical verb onset for grammatical/ungrammatical conditions, were compared between children and adults. Similar to methods proposed by Maris and Oostenveld (2007) and Bastiaansen et al. (2010), permutation statistics were conducted to control for multiple comparisons using the EEGLab toolbox, an open-source, interactive Matlab toolbox for processing continuous and event-related EEG (Delorme and Makeig, 2004). The permutation involves: 1. Collecting EEG data for each of the experimental conditions, 2. Drawing as many trials from each combined data set as there are conditions and placing these additional trials within separate subsets (referred to as random partitioning) and 3. Calculating the test statistic based on this random partition. Steps 2 and 3 are repeated a large number of times, based on data size and number of variables, then random test statistics are compared to the observed test statistic. The permutation p-value is the proportion of partitions where the observed test statistic is larger than the value drawn from the permutation test statistic. Permutation accuracy can be quantified by means of the well-known confidence interval of its binomial distribution (Ernst, 2004, Maris and Oostenveld, 2007). Fortunately, this statistical measure is well-designed within the EEGlab toolbox of Matlab and, therefore, has been calculated specifically to deal with the type of multidimensional data found in EEG studies. It is important to note that these analyses focus on how each frequency band changes during sentence processing compared to each individual's pre-stimulus baseline. Thus, group differences are related to changes compared to each individual's own baseline and should not be influenced by slight developmental changes in resting state EEG between adolescence and adulthood.
Using the permutation analysis, we conducted a 2 (age) × 2 (grammaticality) repeated measures ANOVA across all electrodes for both beta (12–30 Hz) and theta (4–8 Hz). Related to beta, based on previous research our analysis includes the time windows 500–700, 700–900, and 900–1100 msec after critical word onset, which is similar to the P600 (Kaan and Swaab, 2002, Davidson and Indefrey, 2007). For theta, we focused on an earlier time window (350–450 msec), which is linked to both the N400 and to previous findings relating theta to changes in semantic processing (Davidson and Indefrey, 2007). To limit the potential of type 1 error, we only focused on clusters of three or more significant electrodes (p < 0.05). From one of these statistically significant electrodes, we included a more detailed ERSP representation for each frequency band at the point of the critical verb onset (−500–1500 msec), which provide more specific information about the timing and frequency of the effects. The information included in the ERSP figures are based on the statistical output from the cluster analysis, and only analyses for the time periods of interest for theta, early beta, middle beta, and late beta (350–450, 500–700, 700–900, and 900–1100 msec, respectively) are included. Fig. 2, Fig. 3, Fig. 4, Fig. 5, Fig. 6, Fig. 7, Fig. 8, Fig. 9, Fig. 10 present this analysis.
Fig. 2.
Beta changes in the 500–700 msec time window highlighting electrodes that exhibit significant differences between grammatical and ungrammatical conditions. This figure illustrates increases (yellow/red) and decreases (blue) in beta power (12–30 Hz) across all electrodes plotted on the headmap. Red electrodes on the far right headmap demonstrate the significant main effect of grammaticality. A main effect of age did not exist. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Fig. 3.
ERSP changes in beta at P4 highlighting 500–700 msec differences. This figure illustrates the increases/decreases in beta power between grammatical and ungrammatical conditions from −100 to 1200 msec. The yellow in the first box demonstrates power increases for grammatical sentences, while the blue in the second box demonstrates power decreases for ungrammatical sentences. Masking was used to highlight the significant interaction of age and grammaticality from 500 to 700 msec pulled out in the first analysis. The red in the far right box highlights the significant main effect of grammaticality between 12 and 30 Hz (p < 0.05). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Fig. 4.
Beta changes in the 700–900 msec time window highlighting electrodes that exhibit significant differences between age and grammaticality. This figure illustrates increases (yellow/red) and decreases (blue) in beta power (12–30 Hz) across all electrodes plotted on the headmap. Red electrodes on the bottom, right headmap highlight the significant interaction of age and grammaticality (p < 0.05) that is driven by a power decrease (blue) in the adult's ungrammatical condition, that is lacking in children. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Fig. 5.
ERSP changes in beta at CPz highlighting 700–900 msec differences. This figure illustrates the increases/decreases in beta power between conditions from −100 to 1200 msec. Masking was used to highlight the significant interaction of age and grammaticality from 700 to 900 msec pulled out in the first analysis. The blue in the top box highlights beta power decreases for ungrammatical sentences in adults, that was lacking in children. The red in the bottom, right box highlights the significant interaction of age and grammaticality between 12 and 30 Hz (p < 0.05). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Fig. 6.
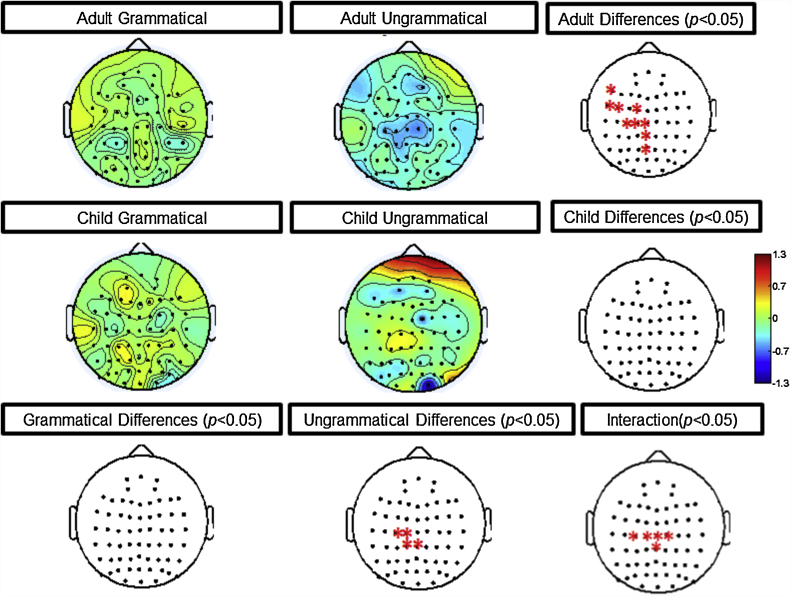
Beta changes in the 900–1100 msec time window highlighting electrodes that exhibit significant differences between age and grammaticality. This figure illustrates increases (yellow/red) and decreases (blue) in beta power (12–30 Hz) across all electrodes plotted on the headmap. Red electrodes on the bottom, right headmap highlight the significant interaction of age and grammaticality (p < 0.05) that is driven by decreases (blue) in the adult's ungrammatical condition that is lacking in children. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Fig. 7.
ERSP changes in beta at C1 highlighting 900–1100 msec differences. This figure illustrates the increases/decreases in beta power between conditions from −100 to 1200 msec. Masking was used to highlight the significant interaction of age and grammaticality from 900 to 1100 msec pulled out in the first analysis. The blue in the top, central box highlights decreases in beta power for ungrammatical sentences in adults that was lacking in children. The red in the bottom, right box highlights the significant interaction of age and grammaticality between 12 and 30 Hz (p < 0.05). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Fig. 8.
Theta changes from 350 to 450 msec highlighting electrodes that exhibit significant differences between age and grammaticality. This figure illustrates increases (yellow/red) and decreases (blue) in theta power (4–8 Hz) across all electrodes plotted on the headmap. Red electrodes on the bottom, right headmap highlight the significant interaction of age and grammaticality (p < 0.05) that is driven by decreases (blue) in the adult's ungrammatical condition, that was not evident in children's processing of ungrammatical sentences. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Fig. 9.
ERSP changes in theta at F1 highlighting 350–450 msec differences. This figure illustrates the increases/decreases in theta power between conditions from −100 to 1200 msec. Masking was used to highlight the significant interaction of age and grammaticality from 350 to 450 msec pulled out in first analysis. The blue in the top box highlights adult theta power decreases for ungrammatical sentences, which were not evident in children's processing of ungrammatical sentences. The red in the bottom, right box highlights the significant interaction of age and grammaticality between 4 and 8 Hz (p < 0.05). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Fig. 10.
ERSP changes in theta at POz highlighting 350–450 msec differences. This figure illustrates the increases/decreases between conditions from −100 to 1200 msec. Masking was used to highlight the significant interaction of age and grammaticality from 350 to 450 msec pulled out in first analysis. The blue in the top box highlights adult decreases in theta for ungrammatical sentences, that was not present in children's processing of ungrammatical sentences. The red in the bottom, right box highlights the significant interaction of age and grammaticality between 4 and 8 Hz (p < 0.05). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Beta. The repeated measures two-way ANOVA for early beta (500–700 msec) revealed no significant interactions, but did reveal a significant effect of grammaticality for adults. Specifically, as shown in Fig. 2, ungrammatical sentences elicited a significant beta power decrease at right parietal electrodes compared to grammatical sentences (p < 0.05). Fig. 3 shows the ERSP changes for each condition (−100 to 1200 msec) at one statistically significant electrode (CP4) with plotting analyses only corresponding to the time period of interest (500–700 msec).
The repeated measures two-way ANOVA for middle beta (700–900 msec) revealed a significant age x grammaticality interaction (p < 0.05; see Fig. 4). This interaction was driven by a significant decrease in beta for ungrammatical sentences at widespread electrodes in adults, whereas children did not demonstrate this pattern of beta activity. Fig. 5 shows the corresponding ERSP changes for each condition (−100 to 1200 msec) at one statistically significant electrode (CPz), plotting analyses only corresponding to the time period of interest (700–900 msec).
The repeated measures two-way ANOVA for late beta (900–1100 msec) revealed a significant age × grammaticality interaction (p < 0.05; see Fig. 6). This interaction was driven by a significant decrease in beta for ungrammatical sentences at widespread left hemisphere electrodes for adults, whereas children did not demonstrate this pattern of beta activity. Fig. 7 shows the corresponding ERSP changes for each condition (−100 to 1200 msec) at one statistically significant electrode (C1), plotting analyses only corresponding to the time period of interest (900–1100 msec).
Theta. The repeated measures two-way ANOVA for theta (4–8 Hz) revealed a significant interaction of age and grammaticality from 350 to 450 msec (p < 0.05). This interaction was driven by frontal and parietal theta power decreases for ungrammatical sentences in adults that was not present in children (see Fig. 8). Fig. 9 shows the frontal ERSP changes and Fig. 10 shows the parietal ERSP changes for each condition (−100 to 1200 msec) at a statistically significant electrode (F1/POz), plotting analyses only corresponding to the time period of interest (350–450 msec).
3.2.3. Correlation analysis of ERPs, time frequency power changes, and behaviors
To clarify the relationship between theta changes and the N400 and beta changes and the P600, as well as how these changes relate to behavioral performance, we performed two sets of correlations (see Table 3, Table 4, Table 5, Table 6). Correlations were performed by computing the average amplitude for ERPs and ERSPs separately, across the time window of interest (i.e. for the N400, the average amplitude was between 350 and 450 msec). A Pearson's R correlation was then conducted to determine if a significant relationship between each ERP and ERSP average existed. It should be noted that there were few significant correlations. Of note, there was a significant correlation between beta and the P600 in the middle time period (700–900 msec) and between the P600 in the late time period (900–1100 msec) and error rates on grammatically incorrect items.
Table 3.
Correlation analysis of average amplitudes for early beta power changes to the early P600 ERP effect given in both children and adults (500–700 msec).
| Early P600 | Early beta effect | Percent correct grammatical | ||
|---|---|---|---|---|
| Early beta effect | Pearson correlation | −0.057 | ||
| Sig. (1-tailed) | 0.371 | |||
| Percent correct grammatical | Pearson correlation | −0.005 | −0.008 | |
| Sig. (1-tailed) | 0.488 | 0.483 | ||
| Percent correct ungrammatical | Pearson correlation | −0.025 | 0.18 | 0.609*** |
| Sig. (1-tailed) | 0.443 | 0.146 | 0.000 | |
* Correlation is significant at the 0.05 level; ** Correlation is significant at the 0.01 level.
Correlation is significant at the 0.001 level.
Table 4.
Correlation analysis of average amplitudes for middle beta power changes to the middle P600 ERP effect given in both children and adults (700–900 msec).
| Middle P600 | Middle beta effect | Percent correct grammatical | ||
|---|---|---|---|---|
| Middle beta effect | Pearson correlation | −0.371* | ||
| Sig. (1-tailed) | 0.013 | |||
| Percent correct grammatical | Pearson correlation | 0.072 | −0.014 | |
| Sig. (1-tailed) | 0.339 | 0.468 | ||
| Percent correct ungrammatical | Pearson correlation | 0.011 | −0.045 | 0.609*** |
| Sig. (1-tailed) | 0.475 | 0.396 | 0.000 | |
Correlation is significant at the 0.05 level.
** Correlation is significant at the 0.01 level.
Correlation is significant at the 0.001 level.
Table 5.
Correlation analysis of average amplitudes for late beta power changes to the late P600 ERP effect given in both children and adults (900–1100 msec).
| Late P600 | Late beta effect | Percent correct grammatical | ||
|---|---|---|---|---|
| Late beta effect | Pearson correlation | −0.256 | ||
| Sig. (1-tailed) | 0.066 | |||
| Percent correct grammatical | Pearson correlation | −0.021 | 0.005 | |
| Sig. (1-tailed) | 0.452 | 0.488 | ||
| Percent correct ungrammatical | Pearson correlation | −0.318* | 0.264 | 0.609*** |
| Sig. (1-tailed) | 0.029 | 0.06 | 0.000 | |
Correlation is significant at the 0.05 level.
** Correlation is significant at the 0.01 level.
Correlation is significant at the 0.001 level.
Table 6.
Correlation analysis of average amplitudes for theta power changes to the N400 ERP effect given in both children and adults (350–450 msec).
| N400 | Theta effect | Percent correct grammatical | ||
|---|---|---|---|---|
| Theta effect | Pearson correlation | −0.029 | ||
| Sig. (1-tailed) | 0.433 | |||
| Percent correct grammatical | Pearson correlation | 0.121 | −0.021 | |
| Sig. (1-tailed) | 0.242 | 0.066 | ||
| Percent correct ungrammatical | Pearson correlation | 0.378* | −0.034 | 0.609*** |
| Sig. (1-tailed) | 0.012 | 0.421 | 0.000 | |
Correlation is significant at the 0.05 level.
** Correlation is significant at the 0.01 level.
Correlation is significant at the 0.001 level.
4. Discussion
This paper is the first to investigate developmental changes in neural oscillations underlying auditory sentence processing. As expected, adults performed better than the 10–12 year olds on a grammaticality judgment task related to verb agreement errors. To investigate whether a developmental difference in the ability to identify errors might be associated with differences in the neural processes underlying common, day-to-day language comprehension, we analyzed the ERPs and neural oscillations underlying grammatically correct and incorrect sentences. In adult participants, grammatical violations were associated with a P600 ERP effect and beta power decrease- both of which have been related to effective syntactic processing in past literature (Hahne et al., 2004, Bastiaansen et al., 2010, Davidson and Indefrey, 2007). Children demonstrated a different pattern of engagement, specifically an apparent N400 effect, and lack of beta or theta decrease for grammatical violations compared to adults. Thus, developmental differences existed in our ERP findings, and these findings were further supported by differences in the underlying neural oscillations.
We interpret the P600 effect and beta decrease to verb agreement errors in adults as indicating effective syntactic unification. Recall that studies using time frequency analysis for adults reported beta power increases related to syntactic unification and beta decreases when a violation disrupts the syntactic unification process (Bastiaansen et al., 2010, Davidson and Indefrey, 2007, Weiss et al., 2005, Weiss and Mueller, 2012). Building on past research, our findings indicate that adults effectively use syntactic information to identify a verb agreement error. The lack of an N400 effect in adults is further in line with past research, given that the N400 is commonly associated with semantic errors (Atchley et al., 2006, Friederici and Hahne, 2001, Friedrich and Friederici, 2004, Hahne and Friederici, 1999, Juottonen et al., 1996, Silva-Pereya et al., 2005). Based on previous work (Bastiaansen et al., 2002, Bastiaansen et al., 2010), we expected to see a theta increase as adults integrated the semantic information related to the presentation of each new word in the sentence. Indeed, there was a slight increase in theta for the grammatically correct sentences and an unexpected significant decrease in theta after the critical verb for ungrammatical sentences. Theta increases are generally related to semantic integration (Davidson and Indefrey, 2007, Hagoort et al., 2004, Hald et al., 2006, Klimesch et al., 1994, Maguire et al., 2010, Wang et al., 2012, Bastiaansen et al., 2002, Bastiaansen et al., 2005, Bastiaansen et al., 2010); therefore, we speculate that our identified theta decrease in adults’ processing of grammatical violations occurs because adults have enough syntactic information to make the appropriate grammaticality judgment and no longer need to integrate semantic information.
Unlike adults, children's ERPs exhibited an ongoing negativity from 200 to 900 msec for agreement errors. During this time period the ungrammatical condition was more negative in amplitude than the grammatical condition, similar to a sustained N400 effect; however, after 900 msec, the ungrammatical condition begin to demonstrate a more positive amplitude compared to the grammatical condition in children, similar to a delayed P600 effect. Evidence from the time frequency analysis is consistent with our ERP findings such that the predicted beta differences between grammatical and ungrammatical sentences were not present in children. In the earlier time window (500–700 msec), children demonstrated a decrease in beta power; however this decrease was not as robust as in adults. In the later time windows (700–900 msec and 900–1100 msec) children exhibited less of a decrease in beta power than adults for the ungrammatical condition. The ERP and time frequency evidence, taken together, indicate that 10–12 year old children do not engage syntactic unification skills similar to adults. This suggests that while the P600 and beta may be related, future studies are needed to better clarify this connection, or determine beta's role in sentence processing. Additionally, we found that children demonstrated a significant N400, but lacked the decrease in theta present in adults for ungrammatical sentences. These findings suggest that children integrate semantic information while making a grammaticality judgment differently than adults.
Although children did not demonstrate the expected P600 effect or beta power decrease during processing of the agreement error, very few differences were observed between children and adults in beta or theta for grammatical sentences. Therefore, it appears that developmental differences appear primarily during the error detection process and could be due to the speed and subtlety of the task. Specifically, this task differed from others in that it involved auditory, real-time sentence processing. In visual tasks, semantic and morphosyntactic information is available at the same time, whereas in auditory tasks, semantic and syntactic information can become available at different times, adding to the level of difficulty (Molinaro et al., 2011). Further, the error was an agreement error (i.e. he go, they goes), which is a very subtle error that may not be as obvious as other errors used in past literature, such as word order or gender violations. Although participants were instructed to respond after the completion of the sentence to avoid measuring motor responses during the sentence and ensure they attend to the entire sentence, ultimately making reaction time measures unnecessary, past studies have identified differences in how error types are processed using reaction times. A reaction time study revealed that children ages 7–9 years old were better at detecting word-order violations than morphological errors, which the authors contributed to the fact that the English language is a strong word order language with a weak inflectional morphology system (Wulfeck, 1993). Therefore, we speculate that the modality and error type could have influenced children's performance and underlying neural processes during this task. Future studies should aim to better identify these influences by measuring reaction times immediately following the error.
The lack of a P600 effect between 200 and 900 msec was unexpected given that previous research reported a P600-like positivity as early as 3- to 4-years old (Silva-Pereyra et al., 2005a; for review, see Friederici, 2006); however, the finding of a negativity in children's ERPs related to a grammatical error is not unique to this study. Hahne et al. (2004) found that, during a grammaticality judgment task, children 7–10 years of age demonstrated a sustained anterior negativity in response to errors, and did not exhibit a P600 effect similar to adults until 13 years of age. The sustained negativity, similar to what we interpret as a prolonged N400 effect in the current study, could be a developmental precursor necessary for future syntactic processing. Behavioral studies have found similar results where function words, or words that are grammatical operators in a language, are not processed quickly and independently from semantic variables until at least 10 years of age. Alternatively, content words, words that bear the semantic bulk of a language, showed adult-like use by 5 years of age (Friederici, 1983). Therefore, words that are semantically robust develop earlier and assist in semantic processing, while words that are the grammatical operators in a language continue to develop, and could contribute to the prolonged development of syntactic processing skills. Cellular research has also found that adults show symmetry in BA 45, engaged in semantic processing, and BA 44, engaged in syntactic processing, while children do not show this symmetry for BA 44 (syntax) until at least eleven years of age, but BA 45 (semantics) develops by age five (Amunts et al., 2003 as cited by Hahne et al., 2004).
Findings from the present study lend support to evidence from behavioral, cellular, and ERP research that when syntactic unification skills are not yet at the level necessary to effectively perform a grammaticality judgment task (as demonstrated by the lack of a P600 effect and beta power decreases) children may engage other cognitive processes. Although the N400 has been related to semantic integration, the lack of correlation to changes in theta suggests children may be using another compensatory strategy to identify grammatical errors. In fact, theta power changes have been related to lexical retrieval (Bastiaansen et al., 2005, Bastiaansen et al., 2008) and working memory (Bastiaansen et al., 2002). Future research is needed to determine which compensatory strategy(s) children engage as an important precursor for the successful development of syntactic processing. Although children may have had a lower signal-to-noise ratio due to having fewer trials than adults, it seems unlikely to have influenced our overall findings based on the replication of our results when the number of trials were evenly matched and the presence of a significant N400 in children. Rather, the N400 effect and lack of beta power decreases at such a late age in this study is likely due to the difficulty associated with identifying a subtle morphosyntatic error when presented in naturally paced auditory sentences.
The present study adds to past literature by investigating the role of neural oscillations in language development during real-time sentence processing. Although the ERP findings in this study identified differences in the processing of syntactic errors, time frequency analysis allowed us to further decompose the multidimensional EEG data, which contains frequency as one of its dimensions, allotting us greater opportunities to link raw EEG data to neurophysiological processes (Cohen, 2014). This is contrary to ERP data which only represent a fraction of the entire EEG; therefore, there are many task-related dynamics related to sentence processing within EEG that are only retrievable by uncovering the underlying neural oscillations. As a result, there has been a large increase in interest in neural oscillations during language processing in adults, though little work has focused on how these processes develop. We feel the addition of the neural oscillation data uncovered important findings related to language development. Specifically, the present study found no correlation between the N400 and theta, indicating that theta may be identifying the engagement of an additional cognitive process, which is unidentified within the ERP. This finding could potentially add a new dimension to our previous understanding of sentence processing, by implying working memory, or another cognitive process, is necessary. Further research within the field is necessary to better clarify this link; however, this is the first study to investigate the neural oscillations engaged during auditory language processing in children. These preliminary findings support previous claims that the cognitive and neural underpinnings of syntactic processing are still developing in adolescence, and add to them by more clearly identifying developmental changes in the neural oscillations underlying grammatical processing.
References
- Amunts K., Schleicher A., Ditterich A., Zilles K. Broca's region: cytoarchitectonic asymmetry and developmental changes. J. Comp. Neurol. 2003;465:72–89. doi: 10.1002/cne.10829. [DOI] [PubMed] [Google Scholar]
- Atchley R.A., Rice M.L., Betz S.K., Kwasney K.M., Sereno J.A., Jongman A. A comparison of semantic and syntactic event related potentials generated by children and adults. Brain Lang. 2006;99:236–246. doi: 10.1016/j.bandl.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Bastiaansen M.C.M., Magyari L., Hagoort P. Syntactic unification operations are reflected in oscillatory dynamics during on-line sentence comprehension. J. Cogn. Neurosci. 2010;22:1333–1347. doi: 10.1162/jocn.2009.21283. [DOI] [PubMed] [Google Scholar]
- Bastiaansen M.C.M., Van Berkum J.J.A., Hagoort P. Event-related theta power increases in the human EEG during online sentence processing. Neurosci. Lett. 2002;323(1):13–16. doi: 10.1016/s0304-3940(01)02535-6. [DOI] [PubMed] [Google Scholar]
- Bastiaansen M.C.M., Oostenveld R., Jensen O., Hagoort P. I see what you mean: theta power increases are involved in the retrieval of lexical semantic information. Brain Lang. 2008;106:15–28. doi: 10.1016/j.bandl.2007.10.006. [DOI] [PubMed] [Google Scholar]
- Bastiaansen M.C.M., Van der Linden M., Ter Keurs M., Dijkstra T., Hagoort P. Theta responses are involved in lexico-semantic retrieval during language processing. J. Cogn. Neurosci. 2005;17:530–541. doi: 10.1162/0898929053279469. [DOI] [PubMed] [Google Scholar]
- Benau E.M., Morris J., Couperus J.W. Semantic processing in children and adults: incongruity and the N400. J. Psycholinguist. Res. 2011;40(3):225–239. doi: 10.1007/s10936-011-9167-1. [DOI] [PubMed] [Google Scholar]
- Brauer J., Friederici A.D. Functional neural networks of semantic and syntactic processes in the developing brain. J. Cogn. Neurosci. 2007;19:1609–1623. doi: 10.1162/jocn.2007.19.10.1609. [DOI] [PubMed] [Google Scholar]
- Chou T.-L., Booth J.R., Bitan T., Burman D.D., Bigio J.D., Cone N.E., Lu D., Cao F. Developmental and skill effects on the neural correlates of semantic processing to visually presented words. Hum. Brain Mapp. 2006;27:915–924. doi: 10.1002/hbm.20231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark E.V. Cambridge University Press; Cambridge: 2003. First Language Acquisition. [Google Scholar]
- Cohen M.X. The MIT Press; Cambridge: 2014. Analyzing Neural Time Series Data: Theory and Practice. [Google Scholar]
- Davidson D.J., Indefrey P. Inverse relation between event-related and time-frequency violation responses in sentence processing. Brain Res. 2007;1158:81–92. doi: 10.1016/j.brainres.2007.04.082. [DOI] [PubMed] [Google Scholar]
- Delorme A., Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J. Neurosci. Methods. 2004;134:9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Ernst M.D. Permutation methods: a basis for exact inference. Stat. Sci. 2004;19:676–685. [Google Scholar]
- Fenson L., Dale P.S., Reznick J.S., Bates E., Thal D.J., Pethick S.J. Variability in early communicative development. Monogr. Soc. Res. Child Dev. 1994 [PubMed] [Google Scholar]
- Ferree T.C. Spherical splines and average referencing in scalp electroencephalography. Brain Topogr. 2006;19:43–52. doi: 10.1007/s10548-006-0011-0. [DOI] [PubMed] [Google Scholar]
- Friedrich M., Friederici A.D. N400-like semantic incongruity effects in 19-month-olds: processing known words in picture contexts. J. Cogn. Neurosci. 2004;16(8):1465–1477. doi: 10.1162/0898929042304705. [DOI] [PubMed] [Google Scholar]
- Friederici A.D. Aphasics’ perception of words in sentential context: some real-time processing evidence. Neuropsychologia. 1983;21:351–358. doi: 10.1016/0028-3932(83)90021-0. [DOI] [PubMed] [Google Scholar]
- Friederici A.D. The neural basis of language development and its impairment. Neuron. 2006;52(6):941–952. doi: 10.1016/j.neuron.2006.12.002. [DOI] [PubMed] [Google Scholar]
- Friederici A.D., Hahne A. Development patterns of brain activity reflecting semantic and syntactic processes. In: Weissenborn J., Joule B., editors. Approaches to Bootstrapping: Phonological, Lexical, Syntactic and Neurophysiological Aspects of Early Language Acquisition. John Benjamins Publishing; Amsterdam: 2001. pp. 231–246. [Google Scholar]
- Friederici A.D., Männel C. Neural correlates of the development of speech perception and comprehension. In: Ochsnet K., Kosslyn S.M., editors. vol. 1. OUP; USA: 2013. pp. 171–192. (The Oxford Handbook of Cognitive Neuroscience: Core Topics). [Google Scholar]
- Hagoort P., Brown C., Groothusen J. The syntactic positive shift (SPS) as an ERP measure of syntactic processing. Lang. Cogn Process. 1993;8(4):439–483. [Google Scholar]
- Hagoort P., Hald L., Bastiaansen M., Petersson K.M. Integration of word meaning and world knowledge in language comprehension. Science. 2004;304(5669):438–441. doi: 10.1126/science.1095455. [DOI] [PubMed] [Google Scholar]
- Hahne A., Eckstein K., Friederici A.D. Brain signatures of syntactic and semantic processes during children's language development. J. Cogn. Neurosci. 2004;16(7):1302–1318. doi: 10.1162/0898929041920504. [DOI] [PubMed] [Google Scholar]
- Hahne A., Friederici A.D. Rule application during language comprehension in the adult and the child. In: Friederici A.D., Menzel R., editors. Learning: Rule Extraction and Representation. Walter de Gruyeter; Berlin: 1999. pp. 71–88. [Google Scholar]
- Hald L.A., Bastiaansen M.C.M., Hagoort P. EEG theta and gamma responses to semantic violations in online sentence processing. Brain Lang. 2006;96(1):90–105. doi: 10.1016/j.bandl.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Holland S.K., Vannest J., Mecoli M., Jacola L.M., Tillema J.-M., Karunanayaka P.R., Schmithorst V.J., Yuan W., Plante E., Byars A.W. Functional MRI of language lateralization during development in children. Int. J. Audiol. 2007;46:533–551. doi: 10.1080/14992020701448994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juottonen K., Revonsuo A., Lang H. Dissimilar age influences on two ERP waveforms (LPC and N400) reflecting semantic context effect. Cogn. Brian Res. 1996;4:99–107. [PubMed] [Google Scholar]
- Kaan E., Swaab T.Y. The brain circuitry of syntactic comprehension. Trends Cogn. Sci. 2002;6(8):350. doi: 10.1016/s1364-6613(02)01947-2. [DOI] [PubMed] [Google Scholar]
- Klimesch W., Schimke H., Schwaiger J. Episodic and semantic memory: an analysis in the EEG theta and alpha band. Electroencephalogr. Clin. Neurophysiol. 1994;91(6):428–441. doi: 10.1016/0013-4694(94)90164-3. [DOI] [PubMed] [Google Scholar]
- Kutas M., Federmeier K.D. Thirty years and counting: finding meaning in the N400 component of the event-related brain potential (ERP) Annu. Rev. Psychol. 2011;67 doi: 10.1146/annurev.psych.093008.131123. 14.1–14.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire M.J., Hirsh-Pasek K., Golinkoff R.M., Imai M., Haryu E., Vanegas S., Okada H., Pulverman R., Sanchez-Davis B. A developmental shift from similar to language-specific strategies in verb acquisition: a comparison of English, Spanish, and Japanese. Cognition. 2010;114:299–319. doi: 10.1016/j.cognition.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maris E., Oostenveld R. Nonparametric statistical testing of EEG- and MEG-data. J. Neurosci. Methods. 2007;164(1):177–190. doi: 10.1016/j.jneumeth.2007.03.024. [DOI] [PubMed] [Google Scholar]
- Molinaro N., Barber H.A., Carreiras M. Grammatical agreement processing in reading: ERP findings and future directions. Cortex: J. Devoted Stud. Nerv. Syst. Behav. 2011;47(8):908–930. doi: 10.1016/j.cortex.2011.02.019. [DOI] [PubMed] [Google Scholar]
- Nuñez S.C., Dapretto M., Katzir T., Starr A., Bramen J. fMRI of syntactic processing in typically developing children: neural correlates in the inferior frontal gyrus. Dev. Cogn. Neurosci. 2011;1:313–323. doi: 10.1016/j.dcn.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrin F., Pernier J., Bertrand O., Echallier J.F. Spherical splines for scalp potential and current density mapping. Electroencephalogr. Clin. Neurophysiol. 1989;72(2):184–187. doi: 10.1016/0013-4694(89)90180-6. [DOI] [PubMed] [Google Scholar]
- Silva-Pereyra J., Klarman L., Lin L.J., Kuhl P.K. Sentence processing in 30-month-old children: an event-related potential study. Neuroreport. 2005;16:645–648. doi: 10.1097/00001756-200504250-00026. [DOI] [PubMed] [Google Scholar]
- Silva-Pereya J., Rivera-Gaxiola M., Kuhl P. An event-related brain potential study of sentence comprehension in preschoolers: semantic and morphosyntactic processing. Cogn. Brain Res. 2005;23:247–258. doi: 10.1016/j.cogbrainres.2004.10.015. [DOI] [PubMed] [Google Scholar]
- Van Petten C., Luka B.J. Neural localization of semantic context effects in electromagnetic and hemodynamic studies. Brain Lang. 2006;97(3):279–293. doi: 10.1016/j.bandl.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Vosse T., Kempen G. Syntactic structure assembly in human parsing: a computational model based on competitive inhibition and a lexicalist grammar. Cognition. 2000;75:105–143. doi: 10.1016/s0010-0277(00)00063-9. [DOI] [PubMed] [Google Scholar]
- Wang L., Zhu Z., Bastiaansen M. Integration or predictability? A further specification of the functional role of gamma oscillations in language comprehension. Front. Psychol. 2012;3(187):1–12. doi: 10.3389/fpsyg.2012.00187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss S., Mueller H.M. Too many betas do not spoil the broth: the role of beta brain oscillations in language processing. Front. Psychol. 2012:3. doi: 10.3389/fpsyg.2012.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss S., Mueller H.M., Schack B., King J.W., Kutas M., Rappelsberger P. Increased neuronal communication accompanying sentence comprehension. Int. J. Psychophysiol. 2005;57:129–141. doi: 10.1016/j.ijpsycho.2005.03.013. [DOI] [PubMed] [Google Scholar]
- Welch P.D. The use of fast Fourier transform for the estimation of power spectra: a method based on time averaging over short, modified periodograms. IEEE Trans. Audio Electroacoust. 1967;15(2):70–73. [Google Scholar]
- Wulfeck B.B. A reaction time study of grammaticality judgments in children. J. Speech Hear. Res. 1993;36(6):1208–1215. doi: 10.1044/jshr.3606.1208. [DOI] [PubMed] [Google Scholar]