Abstract
Tourette syndrome (TS) is a neurological disorder characterised by vocal and motor tics. It is associated with cortical–striatal–thalamic–cortical circuit [CSTC] dysfunction and hyper-excitability of cortical motor regions. TS follows a developmental time course, in which tics often become increasingly more controlled during adolescence. Importantly, however, a substantial minority of patients continue to have debilitating tics into adulthood. This indicates that there may be important differences between adult TS patients and children and adolescents with the disorder. We use TMS to examine cortical motor excitability in a sample of children, adolescents and young adults with TS. We demonstrate that, in contrast to studies of adult patients, resting motor threshold and the variability of MEP responses are increased in children with TS, while the gain of motor excitability in reduced. Importantly, we demonstrate that these differences normalise with age over adolescence. We conclude that these effects are likely due to a developmental delay in the maturation of key brain networks in TS, consistent with recent brain imaging studies of structural and functional brain connectivity. Importantly, these findings suggest that the alterations in brain network structure and function associated with TS may be quite different in children and adult patients with the condition.
Keywords: Tourette syndrome, Motor excitability, Transcranial magnetic stimulation (TMS), Motor threshold, Children and adolescents
1. Introduction
Tourette syndrome (TS) is a neurological disorder that lies at the extreme of the tic disorder spectrum and is characterised by the presence of chronic vocal and motor tics (Cohena et al., 2013). Tics are involuntary, repetitive, stereotyped behaviours that occur with a limited duration, often many times in a single day (Cohena et al., 2013). TS is highly heritable, is more often seen in males than females (∼4:1), and affects approximately 1% of individuals aged 5–18 years (Cohena et al., 2013).
Importantly, TS often follows a developmental time course in which tics become increasingly more controlled during adolescence in many individuals. TS first presents during early childhood (∼4–7 years) and the severity of tics follow a remitting pattern with increasing age. Tic severity is often maximal between 11 and 14 years with tics decreasing by early adulthood (Cohena et al., 2013). This suggests that the majority of individuals with TS appear to develop a means of controlling and effectively suppressing their tics by early adulthood, however a substantial minority (∼20–30%) continue to have debilitating tics into adulthood, with symptoms becoming more severe in some cases and resistant to treatment (Cohena et al., 2013).
While the neurobiological basis of TS remains unclear, it is generally acknowledged that cortical–striatal–thalamic–cortical circuits [CSTC] are dysfunctional in TS, with subsets of striatal projection neurons becoming active within inappropriate contexts, resulting in the disinhibition of thalamo-cortical projections (Albin and Mink, 2006) and hyper-excitability of cortical motor regions (Gilbert et al., 2004, Orth et al., 2008, Heise et al., 2010) that in turn lead to the occurrence of tics (Bohlhalter et al., 2006). In particular, TS has been associated with dysfunctional signalling of the neuromodulator dopamine (DA) (Buse et al., 2013), which is linked to mechanisms of reinforcement learning (Schultz, 1997), and the neurotransmitter GABA (Ramamoorthi and Lin, 2011, Clarke et al., 2012). Dysfunctional signalling of DA and GABA may each contribute to impairment in TS in the operation of the cortical–striatal–thalamic–cortical [CSTC] brain circuits that are implicated in motor learning, particularly habit formation, and the selection of actions according to behavioural context (Albin and Mink, 2006, Graybiel, 2008).
Alterations in cortical excitability and physiological inhibition have previously been studied using brain stimulation (e.g., transcranial magnetic stimulation [TMS]) techniques (for review see Orth, 2009). TMS can be used to stimulate the primary motor cortex and induce a measurable motor evoked potential [MEP] in a targeted muscle; it is therefore a useful tool to non-invasively measure corticospinal excitability [CSE], both at rest and during the execution of behaviour. Several different measurements can be obtained using TMS in order to quantify different aspects of CSE. Key studies in TS have examined resting and active motor threshold for each individual, TMS recruitment curves, and the peak-to-peak amplitude of the MEP at different time points during movement preparation.
Motor threshold is defined as the minimum intensity of stimulation required to reliably induces an MEP of a specific amplitude in a target muscle, either at rest (resting motor threshold [RMT]), or when the muscle is partly activated (active motor threshold [AMT]). A key theoretical construct is the ‘gain’ in CSE. This can be defined as the rate at which CSE increases. This construct can be operationsalised in several ways but is most often measured as the slope of the TMS recruitment curve or the rate at which MEPs increase in amplitude ahead of a volitional movement. TMS recruitment curves are assessed by using a stimulus-response TMS technique, where the intensity of TMS is systematically increased from RMT in order to measure the intrinsic capability of the motor cortex to ramp up global excitability in the resting muscle (which we will refer to as the ‘gain’ of motor excitability). Gain of motor excitability can also be measured during the preparation of a volitional movement in a resting muscle, where the TMS intensity is not altered, but the time in which the TMS pulse is given is altered. Typically, the closer to onset of the movement, the greater the MEP amplitude signalling that gains are made in motor excitability during movement preparation.
Two key findings are as follows: First, motor threshold values do not differ in individuals with TS relative to matched controls (Orth, 2009) (c.f. reference (Orth and Rothwell, 2009)). Importantly, it is suggested that equivalent RMTs in TS patients and controls indicates that neural populations recruited by TMS at threshold are in the same state in both samples (Orth, 2009).
Second, a number of studies have demonstrated that the gain of motor cortical excitability is reduced in individuals with TS. This is the case for both TMS-induced increases in motor excitability (i.e., TMS recruitment curves) (Orth et al., 2008, Draper et al., 2014) and gains in motor excitability during motor preparation, immediately preceding the execution of volitional movements (Heise et al., 2010, Draper et al., 2015). Importantly, the gain in cortical excitability is thought to depend upon the distribution of excitability within the population of corticospinal neurons (i.e., recruitment of neurons with different levels of excitability): thus it is concluded that a shallower gain function in TS reflects a reduction in the spread of excitability within this population (Orth, 2009).
Importantly, the relationship between individual TMS measurement values and tic severity scores in TS has been examined, however the evidence is rather mixed. Orth and colleagues reported, in a study of adults with TS, that the individual slope values for TMS recruitment curves were positively associated with some measures of complex, phonic, and finger tics (Heise et al., 2010). By contrast, they reported that clinical tic rating scales (i.e., the Yale Global Tic Severity Scale [YGTSS] (Leckman et al., 1989)) and other video measures (e.g., the Modified Rush Video Scale (Goetz et al., 1999)) were not associated with tic severity.
It is important to note that the majority of studies investigating cortical excitability and physiological inhibition in TS using TMS techniques have been conducted in adults with TS and must therefore be interpreted with some caution for the following reasons. First, TS is a disorder of childhood onset that typically follows a developmental time course in which in the majority of individuals, tics are absent or relatively mild by early adulthood. Adults with TS can be viewed therefore as unrepresentative of the more general TS population (i.e. children and adolescents with the disorder), but may nevertheless constitute an important group in which the clinical phenotype is stable and the compensatory plastic changes thought to bring about increased control over tic severity during adolescence (Jackson et al., 2011) have either failed to occur or have been ineffective. Second, brain imaging studies have consistently demonstrated that while there are widespread alterations in brain structure and function associated with TS (for review see Plessen et al., 2009), these effects differ quite markedly for adult and child samples, and have often been diametrically opposite (Plessen et al., 2009). Given the above, it is important to investigate whether the findings demonstrated in TMS studies investigating cortical excitability and physiological inhibition in adults with TS are replicated in children and adolescents with TS.
In this study we examine core measures, namely: resting motor threshold; TMS recruitment curves; and motor excitability during the preparation of volitional movements, in a sample of children, adolescents and young adults with TS compared to a sample of age- and gender matched typically developing individuals. We demonstrate that, consistent with previous studies of adult TS patients, children and adolescents with TS exhibit reduced gain in motor excitability when indexed by TMS recruitment (IO) curves and ahead of volitional movements. However, and in direct contrast to studies of adult patients, we show that: RMT is significantly different (higher) in children and adolescents with TS compared to age-matched controls; that differences in RMT vary with age and are most pronounced in the youngest individuals and absent in young adults (18 years or older); that TMS-induced MEP responses are more variable in children and adolescents with TS relative to controls; and, that individual measures of motor gain function are inversely related to motor tic severity scores, indicating that reduced gain values are associated with increased tic severity. The results are interpreted as consistent with the view that there may be a delay in the development of the structure and function of brain networks in TS that contributes to the occurrence of tics but which may normalise with age during adolescence in the majority of individuals with TS.
2. Method
2.1. Participants
17 adolescents and young adults with Tourette Syndrome (TS) were recruited to take part in two TMS studies (age range = 11.9–21.6 years, mean = 16.47 years ± 3.17, 3 females). The sum of motor and phonic tic scores ranged from 3 to 44, mean = 22.6 ± 11. 7. Participants in the TS Group suffered from additional co-morbidities besides TS. Three had an additional diagnosis of OCD, one had ADHD and three participants were diagnosed with ASD. See Table 1 for additional details of tic scores, co-morbidities and medication.
Table 1.
Clinical and biographical characteristics of the participants with TS that took part in the study including gender, age, tic severity, any comorbidities the participant has and medication.
| TS ID (n = 17) | Gender | Age (years) | YGSS | Motor tic score | Phonic tic score | Impairment score | Comorbidity | Medication |
|---|---|---|---|---|---|---|---|---|
| TS006 | M | 21.6 | 37 | 15 | 17 | 5 | None | Clonidine |
| TS013 | M | 18.3 | 13 | 13 | 0 | 0 | None | Clonidine (75 mg) |
| TS018 | M | 19.3 | 13 | 13 | 0 | 0 | None | None |
| TS028 | F | 19.0 | 51 | 13 | 13 | 25 | OCD | Fluoxetine (25 mg) |
| TS030 | M | 17.0 | 23 | 12 | 11 | 0 | None | None |
| TS031 | M | 18.5 | 47 | 16 | 11 | 20 | None | Fluoxetine |
| TS034 | M | 15.4 | 3 | 3 | 0 | 0 | None | None |
| TS048 | M | 16.0 | 19 | 8 | 6 | 5 | None | Clonidine |
| TS055 | M | 17.0 | 25 | 15 | 0 | 10 | ADHD | None |
| TS069 | M | 12.6 | 13 | 9 | 4 | 0 | None | None |
| TS071 | M | 13.6 | 67 | 19 | 18 | 30 | OCD | None |
| TS081 | F | 12.8 | 46 | 17 | 9 | 20 | OCD | Clonidine, Melatonin, Aripiprazole |
| TS082 | M | 11.9 | 17 | 12 | 0 | 5 | None | None |
| TS084 | F | 21.6 | 45 | 15 | 10 | 20 | Asperger's syndrome | Citalopram (40 mg) |
| TS088 | M | 12.4 | 58 | 18 | 20 | 20 | Asperger's syndrome | Clonidine |
| TS092 | M | 14.2 | 38 | 12 | 11 | 15 | ASD | None |
| TS103 | M | 18.9 | 64 | 22 | 22 | 20 | None | None |
For ethical reasons (all of the TS group were in full time education) we could not ask those children on medication to come off their medication for the purposes of this study. Accordingly, we conducted several stepwise regression analyses to determine whether medication status, having first accounted for age differences, predicted any of the core dependent measures (i.e., motor threshold, TMS recruitment curve slopes, or gain in motor excitability (slope)) prior to volitional movements. These analyses revealed that in all cases age was a significant predictor but medication status was not (resting motor threshold: effect of age t = 3.21, p < 0.006; effect of medication status = t < 1.0, p = 0.3. TMS recruitment curve slopes: effect of age t = −3.94, p = 0.001; effect of medication status = t < −1.0, p = 0.7. Gain in motor excitability (slope) prior to volitional movements: effect of age t = 4.94, p < 0.0005, effect of medication status = t < −1.0, p = 0.7).
17 gender and age-matched controls (CS) (3 females, age range = 11.9–21.8 years, mean = 16.59 ± 3.18) also took part in this study.
2.2. TMS protocol
An unpaired Magstim Bistim 2 machine and a 70 mm figure-of-eight coil were used to deliver single-pulse TMS (sp-TMS) to the motor hotspot of the first dorsal interosseous (FDI) muscle of the right hand. The coil was held at approximately a 45° angle in order to induce a posterior-anterior electric field for optimal stimulation of the motor cortex. First, the resting motor threshold (RMT) was found as the intensity that was required to reliably elicit an MEP of at least 150–200 μV in 5 out of 10 trials.
The location of this motor hotspot was continuously tracked throughout both experiments using the BrainSight 2 MRI based neuronavigation system. Two trackers were used: one was attached to the participants’ foreheads and the other to the TMS coil. Using a camera and software that aligns specific points on the subject's head to a virtual head on-screen, using automatic curvilinear reconstruction, allowed the experimenter to ensure the TMS coil was always placed directly over the target.
Once the motor hotspot was located, the TMS coil was stabilised using a Manfrotto arm device. The coil was continuously observed by the experimenter and adjusted whilst trials were delivered to ensure throughout that the coil was positioned over the target area. In order to record the muscle twitch from the FDI muscle, disposable electromyography (EMG) electrodes with a diameter of 5 mm were placed on the FDI muscle in a standard belly-tendon configuration. BrainVision Recorder software was used to record EMG responses to the TMS protocol and data were recorded at a sampling rate of 5000 Hz with a sampling interval of 200 μS.
2.2.1. Experiment 1: Measuring recruitment curves in TS
The participant rested their chin on a chin-rest whilst receiving 80 trials of sp-TMS at different percentages of each individual's RMT. There was an inter-trial interval (ITI) of 5 s. The TMS intensities delivered ranged from 95% to 130% of RMT in increments of 5% (producing 8 TMS intensities). Pulses were pseudo-randomised and organised into 8 blocks (i.e., each block contained eight trials that comprised of one trial at each TMS intensity). After each block the experimenter checked that the participant was tolerating the procedure well and would readjust the coil position if necessary.
2.2.2. Experiment 2: Single-pulse TMS delivered during movement preparation
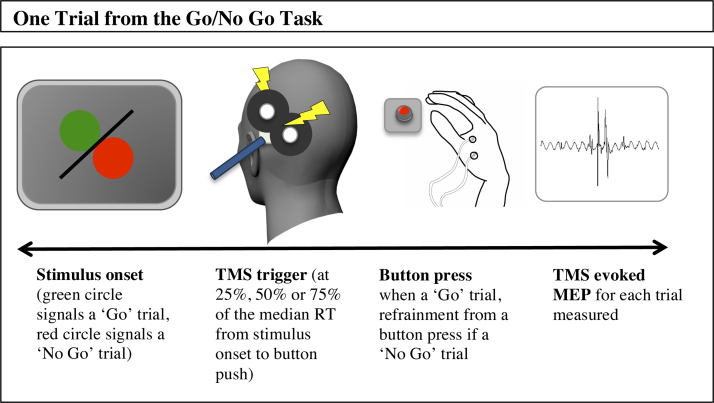
The participant sat with their chin placed on the chin rest, 50 cm away from a 17-inch monitor where the visual stimuli were displayed. The behavioural task was a simple Go/No Go decision task in which presentation of a green circle signalled a button press using their right hand and a red circle signalled that the participant should withhold their response. The stimuli were organised into 12 blocks of 9 trials. In each block there was a ratio of 1:8 NoGo to Go trials. The trial on which the No Go stimulus appeared was randomised within each block. 36 practice trials without TMS were used to calculate an initial median response time (RT). This was to estimate, for each participant, the initial time to trigger a TMS pulse during the sp-TMS task and also to familiarise the participants with the task. TMS pulses were triggered (in the main experiment) at 25%, 50% and 75% of each individual's estimated RT (as calculated by the median RT during the practice trials); this median RT estimate value was then constantly updated throughout the experiment after every 9 trials. Each trial was terminated by a button response or else was timed out after 2 s. A graphical representation of a single trial during the Go/No Go task can be seen in Fig. 1. TMS was triggered at 100% of RMT throughout the study.
Fig. 1.
A graphical representation of a single trial in the Go/No No task.
3. Results
EMG signals recorded during the experiment were analysed using EEGLAB in MATLAB. The peak-to-peak amplitude of the MEP was measured for each trial in both experiments. Data from each trial were visually inspected. If the trial was contaminated by any other activity (a tense muscle for example), the trial was excluded. All participants took part in both experiments.
3.1. Experiment 1: TMS induced input-output curves in TS
3.1.1. Motor threshold differences
RMTs for the TS group were higher than those for the CS group (mean = 48%, standard deviation (SD) = 10.45, mean = 37.6%, SD = 5.2 respectively). Thresholds for both groups were normally distributed but Levene's Test for equality of variances highlighted that the variances were not homogenous. An unpaired Student's t test (corrected for heterogeneous variance) confirmed that the difference between group means was statistically significant (t(23.45) = 3.677, p < 0.01). This finding conflicts with a number of previous studies in adults with TS that have demonstrated that RMTs do not differ between adults with TS and matched controls (Orth et al., 2008, Orth, 2009).
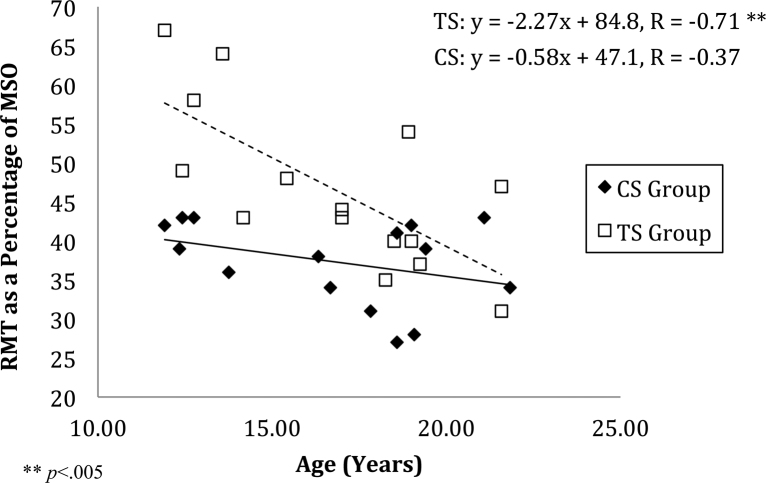
The differences in RMT that we observed appear more pronounced in younger participants. To investigate this further a stepwise regression was conducted to examine if age contributed to a linear model that included group as a predictor. Two variables were entered (Group and Age) and no variables were removed. The model was statistically significant, (F(2, 31) = 17.03, p < 0.0001) and explained approximately 50% of the variance (R2 = 0.524, adjusted R2 = 0.493). Group (Pearson's r = 0.55) and Age (r = −0.49) were similarly weighted with group being the primary predictor.
In summary, age is predictive of RMT, particularly in the TS Group, where RMT was substantially higher in younger children. Younger children in the TS Group require a higher intensity of stimulation to produce an MEP of a similar magnitude to older individuals with TS and to typically developing control children that are of the same age. Relevant data are presented in Fig. 2.
Fig. 2.
Resting motor threshold (RMT) calculated as percentage of maximum stimulator output (MSO) to elicit a motor evoked potential (MEP) of between 150 and 200 μV plotted against the age of participant. A stepwise regression was conducted and showed that both group and age was predictive of RMT (F(2, 31) = 17.03, p < .0001). These two factors explained roughly 50% of the variance (R2 = 0.524, Adjusted R2 = 0.493).
3.1.2. Group differences in global excitability indexed by TMS input-output curves
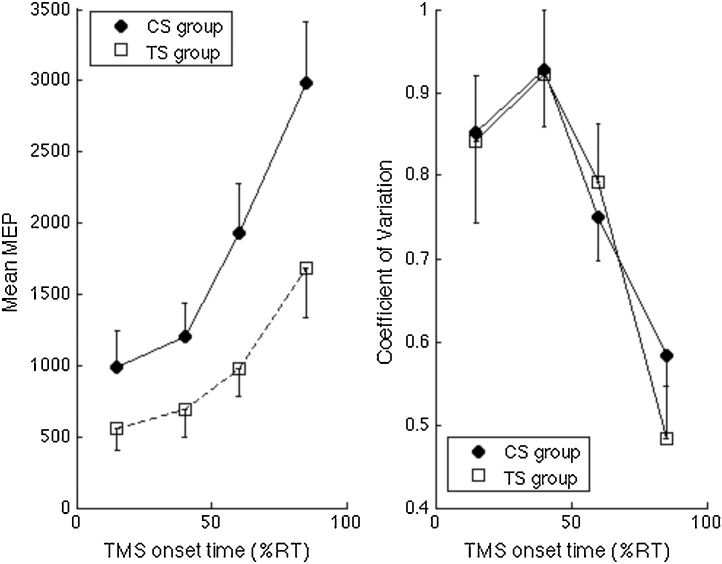
Raw MEPs were log-transformed to base 10 in order to conform values towards normality and to homogenise data variance. This allowed us to compare groups using parametric statistics without violating statistical assumptions. For completeness, however, raw MEP data and associated Coefficient of Variation data are presented in Fig. 3. Transformed MEP data were collapsed for each participant to produce mean MEP values for each TMS intensity.
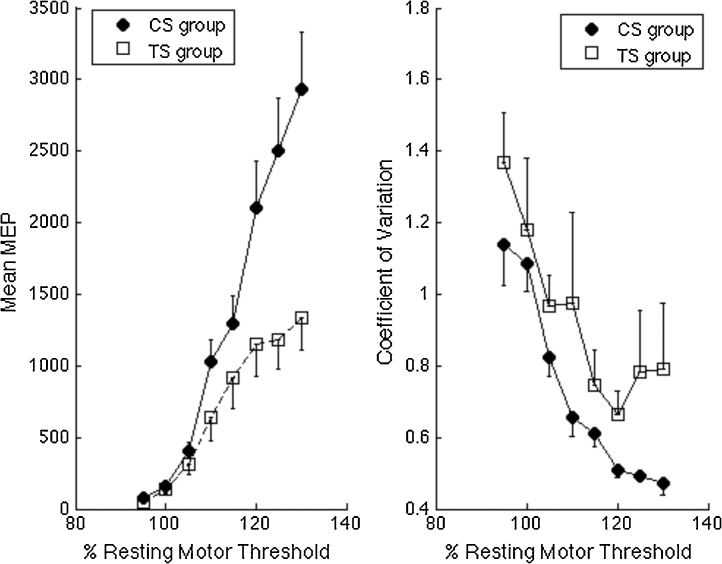
Fig. 3.
Left panel. Illustrates TMS recruitment curves (mean of individual median motor evoked potentials (MEP) values measured in microvolts) for each level of TMS stimulator output (defined relative to each individual's RMT). The graph shows the mean MEP for the control (CS) and Tourette syndrome (TS) groups. A two-way mixed ANOVA with age entered as a covariate demonstrated a within-subject main effect of intensity (F(3.1,95.7) = 2.924, p < .05) and a between-subject effect of group ((F1,31) = 6.606, p < .05). Right panel. Mean coefficient of variation (CoV) values for TMS recruitment curves. CoV values reduce with age and are significantly elevated in the TS group.
A two-way mixed ANOVA was used to analyse the log-transformed mean MEP data for all TMS intensities. The ANOVA consisted of a within-subject factor of TMS intensity (95–130% of RMT) and a between-subject factor of Group (TS vs. CS). Age was entered as a covariate. As expected there was a significant within-subject main effect of TMS intensity (F(3.1,95.7) = 2.924, p < 0.05 [Greenhouse–Geisser corrected]) and a significant between-subjects main effect of Group (F(1,31) = 6.606, p < 0.05). Age was a significant predictor of motor excitability and is examined in more detail below. There was no significant interaction between TMS intensity and Age (p = 0.45) and the TMS intensity × Group interaction also failed to reach statistical significance (p > 0.1).
It should be noted that Levene's test of equality of error variances indicated differences in variance between the groups. This can also be seen from an inspection of the error bars presented in Fig. 3 (left panel) that show mean TMS recruitment curves for each group at each TMS intensity. Inspection of this figure clearly indicates that MEP variability was larger in the TS group.
3.1.3. Coefficient of variation (CoV)
To investigate differences in variability between the groups, we computed the coefficient of variation for each individual and for each level of TMS intensity. Relevant data are presented in Fig. 3 (left panel). These data were entered into a mixed ANOVA with Group (TS vs. CS) entered as a between-subject factor, TMS intensity (95–130% of RMT) entered as a within-subject variable, and Age entered as a covariate. The ANOVA revealed a significant main effect of Group (F(1,31) = 4.18, p = 0.05) and a marginal of Age (F(1,31) = 3.27, p = 0.08). MEP variability was larger in the TS group and decreased with age in both groups. The main effect of TMS intensity was not significant (F(3.3,102.5) = 2.03, p = 0.108) and there was no Group × TMS intensity interaction effect (F(3.3,102.5) < 1.0, p = 0.425). By contrast, there was a significant TMS intensity × Age interaction (F(3.3,102.5) = 4.234, p < 0.01).
Overall, these findings confirm that the TS group exhibited more variability, as indexed by the CoV, in their MEP responses than matched typically developing controls and that for both groups, MEP variability decreases with age. Importantly, the rate of this decrease in variability is comparable across the groups.
3.1.4. Relationship between TMS recruitment curve slope values and tic severity
A previous study has demonstrated that the individual slope values that describe the TMS-induced recruitment (IO) curves in adults with TS were positively associated with some tic severity measures (Orth et al., 2008). By contrast, a recent study of children and adolescents with TS that measured the individual slope values that describe the rise in motor excitability ahead of volitional movements demonstrated that these slopes were inversely related to motor tic severity scores (Draper et al., 2015).
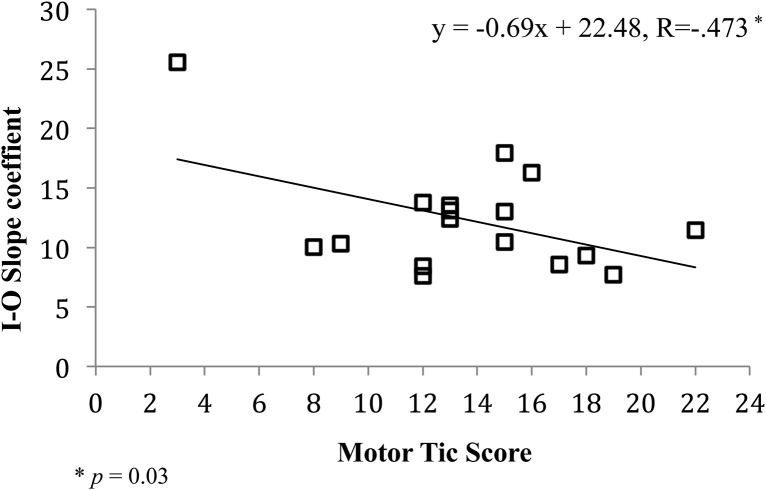
To investigate the relationship between IO slope and tic severity in the TS group, a stepwise regression was conducted with age and motor tic severity scores entered as predictors. This analysis revealed that both age and motor tic severity each significantly contribute to a model predicting the I–O slope (F(2,14) = 5.74, p < 0.05) and explained approximately 40% of the variance (R2 = 0.45, Adjusted R2 = 0.37). Motor tic scores were weighted highest as the primary predictor (r = −0.47) however age was positively associated with IO slopes (r = 0.48). Relevant data illustrating the relationship between I–O slope and motor tics are presented in Fig. 4.
Fig. 4.
Scatter plot illustrating the relationship between the slopes describing motor gain function and motor tic severity scores in the TS Group.
3.2. Experiment 2 – Single pulse TMS with the Go/No Go task
3.2.1. Behavioural data
Behavioural data (response times [RT] and accuracy) was first analysed to check for differences between groups. RT estimates for correct GO trials were based upon button press responses. However, since EMG data was also collected from the responding hand on all trials, including NOGO trials, reaction time effects can also be verified using the EMG defined onset of movement. This might allow for an earlier, and potentially more sensitive measurement of RT on GO trials, and the identification of self-corrected error responses on NOGO trials, which is not possible from analysis of button-press responses. A two-tailed independent groups t-test was conducted for RT for correct GO trials. This revealed a significant difference between groups with the TS group responding more slowly on average (t(32) = −2.319, p < 0.05). By contrast, there were no differences in accuracy measures. Specifically, both groups performed at 100% accuracy for GO Trials and there were no between group differences in errors made on NOGO Trials. This was the case when looking at correct button-press responses and also when we used the EMG recordings to identify movement-contaminated (i.e., self-corrected movement) trials (two-tailed t tests, p = 0.634, p = 0.371 respectively).
3.2.2. MEP data
The peak-to-peak amplitude of the MEP was measured for each trial. Each trial was individually inspected for contamination by any other muscle activity and spoiled trials were excluded. Each MEP was time-stamped as the percentage of the response time of that trial that the TMS was triggered. MEPs were then binned into four different time periods of movement preparation according to their time stamps: 0–29%, 30–49%, 50–69% and 70–100% of that individual's RT for that trial. Median MEPs (measured in microvolts) for each bin were calculated for each individual and then averaged for each group. Finally, MEPs were log-transformed to base 10.
A two-way mixed ANOVA was conducted on the log-transformed MEP data with TMS onset time (0–29%, 30–49%, 50–69%, 70–100% of RT) entered as a within-subject variable and Group (TS vs. CS) entered a between-subject variable. Age was also entered as a covariate. The ANOVA revealed that there was no main effect of TMS onset time (p = 0.24), no significant Group × TMS onset time interaction (p = 0.36), and no interaction between TMS onset time and Age (p = 0.91). By contrast, the ANOVA revealed that there were significant main effects of Age (F(1,31) = 11.36, p < 0.005) and Group (F(1,31) = 5.77, p < 0.05). Relevant data are presented in Fig. 5.
Fig. 5.
Left panel. Illustrates motor gain ahead of volitional movement (as measured by TMS induced motor evoked potentials (MEP) values measured in microvolts). The graph shows the mean MEP for the control (CS) and Tourette syndrome (TS) groups. Right panel. Mean coefficient of variation (CoV) values for TMS induced MEP.
A stepwise regression analysis was conducted to further examine the direction of these effects. A model that included Age and Group as factors was statistically significant (F(2,31) = 6.628, p < 0.005) and explained between 25 and 30% of the variance (R2 = .299, Adjusted R2 = .254). Age (r = 0.41) and Group (r = −0.38) were similarly weighted predictors, and Age was shown to be positively associated with cortical excitability.
These data confirm that the TS group exhibit reduced motor excitability ahead of volitional movements compared to an age-matched group of typically developing young adults. The effects of age are further explored below.
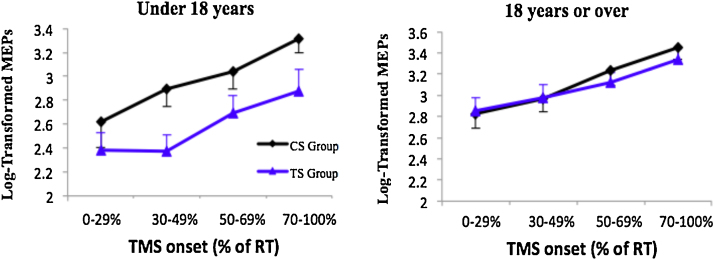
The between group differences in motor excitability preceding volitional movement appear to be driven primarily by the younger participants. This can be seen more clearly by separating participants into adolescent (i.e., under 18 years; N = 10) and young adult (aged 18 years or over; N = 7) sub-groups. Relevant data are presented in Fig. 6. Inspection of this figure clearly indicates that whereas the young adult group (18+ years) exhibit no between-group difference in their motor excitability during movement preparation, adolescents (under 18 years) with TS exhibit substantially reduced motor excitability preceding volitional movements compared to typically developing age-matched individuals.
Fig. 6.
Log-transformed MEP values (base 10) were binned into 4 stages of movement preparation and means were plotted. Error bars are standard error of the mean. For illustrative purposes only, data was split into two groups: those under 18 (n = 10) and those above 18 (n = 7).
3.2.3. Coefficient of variation (CoV)
To investigate differences in variability between the groups we computed the CoV for each individual at each TMS onset time using the log-transformed MEP data. These data were entered into a mixed ANOVA with Group (TS vs. CS) as a between-subject factor, TMS onset time (0–29%, 30–49%, 50–69%, 70–100% of RT) as a within-subject variable and age as a covariate. The ANOVA revealed a main effect of age (F(1,31) = 4.5, p < 0.05). By contrast, the main effect of TMS onset time was not significant (F(2.5,75.8) = 1.78, p = 1.7), and the main effect of Group, and the Group × TMS onset time interaction, were not statistically significant (maximum F = 1.02, p = 0.38).
These findings confirm that for both groups, TMS variability measured during movement preparation decreases with age. Furthermore, while the mean MEP variability was larger in the TS group compared to controls, this difference did not reach conventional levels of statistical significance.
3.2.4. Slope during movement preparation
As noted above, previous studies have demonstrated that the gain in motor excitability immediately preceding volitional movements is substantially reduced in individuals with TS (Heise et al., 2010, Draper et al., 2014) and slope values are inversely related to motor tic severity in adolescents with TS (Draper et al., 2015).
To investigate this we calculated the slope coefficient for each individual's gain in cortical–spinal excitability (CSE) within the motor preparation period and investigated whether the slope were different between groups. To maintain consistency with previous studies we modelled the gain in CSE as a linear function fitted to raw MEP for each individual. An independent groups t test (one-tailed) demonstrated that, as predicted, the CS group (mean = 33.06, SD = 30.5) exhibited a significantly steeper slope value than the TS group (mean = 16.71, SD = 13.76): t(22.25) = 2.015, p = 0.028 (equal variances not assumed). This result confirms the finding reported previously demonstrating that individuals with TS exhibit a reduced gain in motor excitability ahead of volitional movements (Heise et al., 2010, Draper et al., 2015).
3.2.5. Comparison of CSE gain functions across Experiments 1 and 2
We examined whether the individual CSE gain functions observed in Experiment 1 (i.e., TMS-induced IO curves – when calculated as a linear function) and Experiment 2 (i.e., increase in CSE preceding volitional movements) were linearly associated for each group. A Pearson correlation was conducted for each group. These analyses revealed that for both groups the individual CSE gain functions obtained in Experiments 1 and 2 were strongly positively correlated (CS Group: R = 0.79, N = 17, p < 0.0001, TS Group: R = 0.64, N = 17, p < 0.01). Furthermore, a Z-test was conducted to determine whether these two correlation coefficients were significantly different and demonstrated that the were not (Z = 0.83).
4. Discussion
We used TMS to investigate differences in several key measures of motor cortical excitability in a sample of children and adolescents with TS relative to an age- and gender-matched group of typically developing individuals, specifically: resting motor threshold (RMT); TMS recruitment (IO) curves; and the gain in motor excitability during the period immediately proceeding the execution of volitional movements. Importantly, a key aim of this study was to examine how these measurements might differ from similar measures that had previously been reported in adult TS patients. The main findings of this study are summarised below.
First, in contrast to previous studies in adult TS patients (Orth et al., 2008, Orth, 2009) that reported that motor thresholds in individuals with TS and neurologically normal controls were equivalent, we demonstrated that motor thresholds were significantly higher in individuals with TS compared to age-matched controls. Furthermore, we demonstrated that between-group differences in motor threshold were most apparent in younger individuals with TS, were absent in young adults with TS, and that threshold values were significantly predicted by age within the TS group.
Second, we demonstrated that gain in motor excitability was significantly decreased in individuals with TS compared to age- and gender-matched controls. Importantly, this finding was observed for both TMS-induced increases in motor excitability (i.e., TMS recruitment curves) and with respect to the increase in motor excitability that precedes the execution of volitional movements. Furthermore, in the case of TMS recruitment curve data, the gain in motor excitability was demonstrated to be inversely related to tic severity scores and positively associated with the age of the patient, indicating that motor cortical excitability may normalise with age. Similarly, with respect to the gain in motor excitability, our analyses confirmed that motor excitability was positively associated with age and that reductions in MEP amplitude, relative to age-match controls, were only observed in younger TS patients.
Third, our analyses of the variability of TMS response (operationalised as the coefficient of variation in TMS-induced MEPs) differed as a function of both age and group. Variability in MEP response decreased with increasing age. This was the case for the TMS recruitment curve data and for increases in motor excitability that precede the execution of volitional movements. Importantly, in both cases variability in MEP response was increased in the TS group relative to controls, and this difference was statistically significant for TMS recruitment curve data. These results are discussed below.
Motor threshold is thought to reflect the excitability of a population of corticospinal neurons that project to the targeted muscle, and to depend on the axonal membrane properties of neurons at the site of stimulation and the membrane properties of post-synaptic neurons (Orth, 2009, Ziemann, 2013). Previous findings, demonstrating that motor thresholds are equivalent in adults with TS and neurologically normal controls (Orth et al., 2008, Heise et al., 2010), have been interpreted as indicating that these properties are not different (i.e., they are in a similar state) in individuals with TS (Orth, 2009).
By contrast, the gain in motor excitability, for example following increases in suprathreshold TMS intensity, or during the preparation of volitional hand movements, is thought to depend upon the distribution of excitability within the population of corticospinal neurons, and the recruitment of neurons with different levels of excitability. More specifically, the amplitude of the recorded MEP values provides an estimate of the fraction of the population of neurons that are recruited by the TMS pulse. Previous studies have demonstrated that in neurologically healthy adults, corticospinal excitability (CSE) within the contralateral motor cortex increases progressively during the preparation of volitional hand movements (Rossini et al., 1988). Furthermore, increases in CSE during motor preparation are accompanied by limb-specific decreases in the variability of CSE that occur shortly before movement onset and are thought to track the state of preparation for movement of the limb (Klein-Flügge et al., 2013). Importantly, it has been suggested that decreases in the variability of CSE that are observed in typically developing individuals immediately preceding volitional movement, most likely reflect increasingly consistent firing patterns within the population of motor cortical neurons recruited during movement preparation (Churchland et al., 2006).
It should be noted that the amplitude of MEP measurements are the summation of a number of physiological signals that may reflect the modulatory effects of cortical inputs from secondary motor areas to motor cortex excitability, as well as the activity of local neural circuits within motor cortex (including GABA interneurons). Importantly, the coordination of firing patterns at the population level within motor cortex is thought to depend critically upon the operation of populations of GABAergic interneurons (Di Cristo, 2007) that may be dysfunctional in TS (Gilbert et al., 2004, Orth et al., 2008, Heise et al., 2010, Orth, 2009).
Previous studies have repeatedly demonstrated that the gain of cortical motor excitability is significantly reduced in TS (Orth et al., 2008, Heise et al., 2010, Draper et al., 2014, Draper et al., 2015), and that increases in motor excitability are not accompanied by a decrease in CSE variability, as is the case for typically developing individuals (Draper et al., 2015). One explanation for the decreased motor gain function observed in individuals ahead to volitional movements is that individuals with TS may gain control over their tics through an increase in tonic inhibition that may operate to alter the gain of motor excitability (Heise et al., 2010, Draper et al., 2014, Draper et al., 2015). Specifically, it has been argued that during the execution of volitional movements, individuals with TS require increased inhibitory control of motor cortical excitability in order to select an appropriate motor response and simultaneously to control for the occurrence of tics (Heise et al., 2010). Consistent with this proposal, a recent magnetic resonance spectroscopy (MRS) study of in vivo levels of the inhibitory neurotransmitter GABA has reported that individuals with TS exhibit significantly elevated levels of GABA, relative to matched controls, within the Supplementary Motor Area – a cortical region strongly linked to the genesis of motor tics (Bohlhalter et al., 2006) – and that GABA levels within the SMA are inversely associated with fMRI BOLD activation within the SMA, cortical excitability in primary motor cortex preceding volitional movements, and are predicted by motor tic severity scores (Goetz et al., 1999).
While the finding of a decreased gain function for motor excitability in TS patients is consistent with several previous studies of both adults and children with TS (Orth et al., 2008, Heise et al., 2010, Draper et al., 2014, Draper et al., 2015), the association between motor excitability measures and tic severity measurements has produced contradictory results in these studies. Orth and colleagues reported that, in a group of adults TS patients, the slope of MEP recruitment curves were positively associated with tic severity measurements (Orth et al., 2008). However, in a later study of adults with TS, it was reported that there was in fact an inverse relationship between tic severity and motor excitability (Heise et al., 2010). Specifically, these authors reported that individuals with the most severe tics exhibited the smallest MEP amplitudes during the period immediately preceding volitional movements (Heise et al., 2010). This latter finding was subsequently replicated in a study of children and adolescents with TS, where it was shown that tic severity was again inversely related to the slope value describing the gain in motor excitability preceding volitional movements (Draper et al., 2015). This latter finding has now been replicated in the current study, where we demonstrate that the slope values describing individual TMS recruitment curves are significantly negatively correlated with motor tic severity scores.
Perhaps the most important finding in the current study is our demonstration that, that our TMS measures of motor excitability are often predicted by the age of our TS patients, and that between-group differences between the TS group and age-matched typically developing controls appear to normalise by early adulthood. Specifically, we observed that motor threshold values were significantly higher than matched controls in the younger TS patients but this difference was absent in young adult (i.e., 18 years of age or older) TS patients. Similar, age was a significant predictor of the slope of the motor gain function for TMS recruitment curves and for the period preceding the execution of volitional movements. Importantly, in both instances motor excitability is substantially reduced in the TS group but is positively correlated with age, indicating that between-group differences in motor excitability may largely disappear with age during adolescence. Finally, variability in MEP responses was shown significantly decrease with increasing age.
One explanation for the range of findings observed in the current study is that there may be a developmental delay in the formation of the cortical–cortical and corticospinal motor networks in TS. As noted above, motor threshold is thought to depend upon the recruitment of a coherent population of corticospinal neurons that project to the targeted muscle, and increases in motor excitability and decreases in MEP variability, for example ahead of volitional movements, are thought to reflect increasingly consistent firing patterns within the population of motor cortical neurons recruited during movement preparation (Churchland et al., 2006). It is plausible therefore that a delay in the formation of relevant motor networks may lead to a reduced number of neurons being recruited by a TMS pulse, or the response to such a pulse being more variable. This would be expected to lead to higher motor thresholds, reduced MEP amplitudes, and increased variability of MEP response.
Evidence for the ‘immaturity’ of brain networks in children and adolescents with TS comes from recent functional and structural brain imaging studies. Church and colleagues (Church et al., 2009) examined functional connectivity in a group of 32 adolescents with TS using resting-state functional magnetic resonance imaging (rs-fMRI) and compared the results of their analyses to age-related connectivity values based upon a large group (210) of typically developing individuals. They reported that there were widespread differences in functional connectivity throughout the brain of adolescents with TS and that connections within the adolescent TS brain were significantly less mature that age-matched controls (Church et al., 2009). Other studies have investigated structural connectivity of white matter pathways using diffusion tensor imaging (DTI) and have reported widespread alterations in the microstructure of white matter (e.g., decreased fractional anisotropy and increased diffusivity) in adolescents with TS (Jackson et al., 2011), that are consistent with altered development of white matter pathways in child and adolescent TS patients. It is particularly important to note however that these findings do not extend to adults with TS, who exhibit quite the opposite pattern of results. Thus, Worbe and colleagues recently conducted investigations of both structural and functional connectivity in adult TS patients and found the opposite pattern of effects. Specifically, in a study investigating functional connectivity in a group of adult TS patients using rs-fMRI, these authors reported increased functional connectivity (i.e., increased number of interactions among brain regions) in adults with TS. Furthermore, they report that functional brain networks were highly disorganised in adults with TS and were characterised by shorter path lengths, stronger functional connectivity locally within brain regions, and by the absence of so-called network hubs that are a hallmark of efficient information transfer (Worbe et al., 2012). Importantly, Worbe and colleagues reported that these functional abnormalities in brain networks were positively associated with tic severity scores (Worbe et al., 2012).
Similarly, in a subsequent study Worbe and colleagues investigated the structural connectivity properties of the cortical–striatal–thalamic–cortical [CSTC] networks (known to be dysfunctional in TS) in a group of adult TS patients. They reported that there were widespread white matter abnormalities in the TS group, and in particular enhanced structural connectivity linking the striatum and thalamus with cortical sensorimotor areas that included: primary motor and sensory cortices and the SMA (Worbe et al., 2015). Furthermore, they again demonstrated that increased connectivity to the motor cortex was positively associated with tic severity scores, but was not influenced by age, medication status or gender (Worbe et al., 2015).
Taken together these results indicate that the functional and structural properties of brain networks that are implicated in the control of action may be ‘immature’ and characterised by under connectivity in children and adolescents with TS, but these networks may become normalised during adolescence. By contrast, individuals with TS who continue to experience debilitating tics into adulthood, and who often present with symptoms that become more severe and resistant to treatment, are characterised by functional and structural control networks that are characterised by over connectivity and disorganisation. These quite opposite patterns of results suggest that alterations in motor excitability observed in adolescents with TS and adults with TS may have quite different physiological explanations.
4.1. Summary
In summary, we propose that the our finding of increased motor threshold, increased MEP variability, and reduced gain of motor excitability in young TS patients, that normalises with age over adolescence, is likely due to a developmental delay in the maturation of key brain networks and consistent with recent brain imaging studies of structure and functional brain connectivity in children and adolescents with TS.
4.2. Limitations of the current study
The size of the sample reported in the current study is larger, or of a comparable size, to most previous published studies investigating this topic (e.g., Orth et al., 2008, Heise et al., 2010, Draper et al., 2015, Orth et al., 2005) nevertheless the sample size is relative modest and the results should be interpreted with this in mind. Several of our patient group presented with co-occurring conditions and a number were receiving medication either at the time of testing or had been medicated in the months preceding testing. The occurrence of co-morbid conditions is common in TS and our inclusion of individuals with co-occurring conditions is consistent with previously reported studies. In particular, previous studies have reported that TMS measures of motor threshold do not differ across TS subgroups presenting with co-occurring conditions relative to ‘pure’ TS patients (Orth and Rothwell, 2009).
In adult studies it has sometimes been possible to test patients who are taken off of their medication for the purpose. In the current study this was not possible. All of our patients were in full time education and could not be asked to come off of their medication. We did however explicitly test whether the medicated and unmedicated patients differed from one another on all dependent measures and we confirmed that there were no statistically significant effects of medication status. Nevertheless our findings should be interpreted with this in mind.
Finally, a key finding of the current study is that differences in resting motor threshold, motor excitability, and MEP variability that are observed for the TS group relative to age-matched controls are themselves modulated by the age of the patient and tend to normalise across adolescence. This is an important finding but it is important to note that this effect is based upon cross-sectional data. The influence of age on cortical excitability changes during adolescence would be best explored through a longitudinal study.
Acknowledgements
This work was funded by a grant from the James Tudor Foundation to SRJ. We are grateful to Jane Fowlie for her help with participant recruitment, to Tourettes Action, and to the patients who participated in this study and their families.
References
- Albin R.L., Mink J.W. Recent advances in Tourette syndrome research. Trends Neurosci. 2006;29:175–182. doi: 10.1016/j.tins.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Bohlhalter S. Neural correlates of tic generation in Tourette syndrome: an event-related functional MRI study. Brain. 2006;129:2029–2037. doi: 10.1093/brain/awl050. [DOI] [PubMed] [Google Scholar]
- Buse J., Schoenefeld K., Münchau A., Roessner V. Neuromodulation in Tourette syndrome: dopamine and beyond. Neurosci. Biobehav. Rev. 2013;37:1069–1084. doi: 10.1016/j.neubiorev.2012.10.004. [DOI] [PubMed] [Google Scholar]
- Church J.A., Fair D.A., Dosenbach N.U., Cohen A.L., Miezin F.M., Petersen S.E. Control networks in paediatric Tourette syndrome show immature and anomalous patterns of functional connectivity. Brain. 2009;132:225–238. doi: 10.1093/brain/awn223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchland M.M., Yu B.M., Ryu S.I., Santhanam G., Shenoy K.V. Neural variability in premotor cortex provides a signature of motor preparation. J. Neurosci. 2006;26:3697–3712. doi: 10.1523/JNEUROSCI.3762-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke R.A., Lee S., Eapen V. Pathogenetic model for Tourette syndrome delineates overlap with related neurodevelopmental disorders including Autism. Transl. Psychiatr. 2012;2:e158. doi: 10.1038/tp.2012.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohena S.C., Leckman J.F., Bloch M.H. Clinical assessment of Tourette syndrome and tic disorders. Neurosci. Biobehav. Rev. 2013;37:997–1007. doi: 10.1016/j.neubiorev.2012.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Cristo G. Development of cortical GABAergic circuits and its implications for neurodevelopmental disorders. Clin. Genet. 2007;72:1–8. doi: 10.1111/j.1399-0004.2007.00822.x. [DOI] [PubMed] [Google Scholar]
- Draper A., Stephenson M.C., Jackson G.M., Pepes S., Morgan P.S., Morris P.G., Jackson S.R. Increased GABA contributes to enhanced control over motor excitability in Tourette syndrome. Curr. Biol. 2014;24:2343–2347. doi: 10.1016/j.cub.2014.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draper A., Jude L., Jackson G.M., Jackson S.R. Motor excitability during movement preparation in Tourette syndrome. J. Neuropsychol. 2015;9:33–44. doi: 10.1111/jnp.12033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert D.L., Bansal A.S., Sethuraman G., Sallee F.R., Zhang J., Lipps T. Association of cortical disinhibition with tic, attention deficit hyperactivity disorder, and obsessive compulsive disorder severity in Tourette syndrome. Mov. Disord. 2004;19:416–425. doi: 10.1002/mds.20044. [DOI] [PubMed] [Google Scholar]
- Goetz C.G., Pappert E.J., Louis E.D., Raman R., Leurgans S. Advantages of a modified scoring method for the Rush Video-Based Tic Rating Scale. Mov. Disord. 1999;14:502–506. doi: 10.1002/1531-8257(199905)14:3<502::aid-mds1020>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Graybiel A.M. Habits, rituals, and the evaluative brain. Ann. Rev. Neurosci. 2008;31:359–387. doi: 10.1146/annurev.neuro.29.051605.112851. [DOI] [PubMed] [Google Scholar]
- Heise K.F., Steven B., Liuzzi G. Altered modulation of intracortical excitability during movement preparation in Gilles de la Tourette syndrome. Brain. 2010;133:580–590. doi: 10.1093/brain/awp299. [DOI] [PubMed] [Google Scholar]
- Jackson S.R., Parkinson A., Jung J., Ryan S.E., Morgan P.S., Hollis C. Compensatory neural reorganization in Tourette syndrome. Curr. Biol. 2011;21:580–585. doi: 10.1016/j.cub.2011.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein-Flügge M.C., Nobbs D., Pitcher J.B., Bestmann S. Variability of human corticospinal excitability tracks the state of action preparation. J. Neurosci. 2013;33:5564–5572. doi: 10.1523/JNEUROSCI.2448-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leckman J.F., Riddle M.A., Hardin M.T., Ort S.I., Swartz K.L., Stevenson J. The Yale Global Tic Severity Scale: initial testing of a clinician-rated scale of tic severity. J. Am. Acad. Child Adolesc. Psychiatry. 1989;28:566–573. doi: 10.1097/00004583-198907000-00015. [DOI] [PubMed] [Google Scholar]
- Orth M. Transcranial magnetic stimulation in Gilles de la Tourette syndrome. J. Psychosom. Res. 2009;67:591–598. doi: 10.1016/j.jpsychores.2009.07.014. [DOI] [PubMed] [Google Scholar]
- Orth M., Rothwell J.C. Motor cortex excitability and comorbidity in Gilles de la Tourette syndrome. J. Neurol. Neurosurg. Psychiatry. 2009;80:29–34. doi: 10.1136/jnnp.2008.149484. [DOI] [PubMed] [Google Scholar]
- Orth M., Amann B., Robertson M.M., Rothwell J.C. Excitability of motor cortex inhibitory circuits in Tourette syndrome before and after single dose nicotine. Brain. 2005;128:1292–1300. doi: 10.1093/brain/awh473. [DOI] [PubMed] [Google Scholar]
- Orth M., Munchau A., Rothwell J.C. Corticospinal system excitability at rest is associated with tic severity in Tourette syndrome. Biol. Psychiatry. 2008;64:248–251. doi: 10.1016/j.biopsych.2007.12.009. [DOI] [PubMed] [Google Scholar]
- Plessen K.J., Bansal R., Peterson B.S. Imaging evidence for anatomical disturbances and neuroplastic compensation in persons with Tourette syndrome. J. Psychosom. Res. 2009;67:559–573. doi: 10.1016/j.jpsychores.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramamoorthi K., Lin Y. The contribution of GABAergic dysfunction to neurodevelopmental disorders. Trends Mol. Med. 2011;17:452–462. doi: 10.1016/j.molmed.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossini P.M., Zarola F., Stalberg E., Caramia M. Pre-movement facilitation of motor-evoked potentials in man during transcranial stimulation of the central motor pathways. Brain Res. 1988;458:20–30. doi: 10.1016/0006-8993(88)90491-x. [DOI] [PubMed] [Google Scholar]
- Schultz W. Dopamine neurons and their role in reward mechanisms. Curr. Opin. Neurobiol. 1997;7:191–197. doi: 10.1016/s0959-4388(97)80007-4. [DOI] [PubMed] [Google Scholar]
- Worbe Y., Malherbe C., Hartmann A., Pelegrini-Issac M., Messe A., Vidailhet M. Functional immaturity of cortico-basal ganglia networks in Gilles de la Tourette syndrome. Brain. 2012;135:1937–1946. doi: 10.1093/brain/aws056. [DOI] [PubMed] [Google Scholar]
- Worbe Y., Marrakchi-Kacem L., Lecomte S., Valabregue R., Poupon F., Guevara P. Altered structural connectivity of cortico-striato-pallido-thalamic networks in Gilles de la Tourette syndrome. Brain. 2015;138:472–482. doi: 10.1093/brain/awu311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemann U. vol. 116. 2013. Pharmaco-transcranial magnetic stimulation studies of motor excitability; pp. 387–397. (Handbook of Clinical Neurology). [DOI] [PubMed] [Google Scholar]