Highlights
-
•
Defining vulnerabilities in the life-cycle of Babesia parasites may lead to designing new control measures for babesiosis.
-
•
Poorly understood interactions between Babesia parasites and their hosts can be effectively addressed with new technologies.
-
•
Effective control of babesiosis will require additional extensive integrative research strategies.
Keywords: Babesia, Babesiosis, Tick fever, Parasite vaccines, Pathogenesis
Abstract
The global impact of bovine babesiosis caused by the tick-borne apicomplexan parasites Babesia bovis, Babesia bigemina and Babesia divergens is vastly underappreciated. These parasites invade and multiply asexually in bovine red blood cells (RBCs), undergo sexual reproduction in their tick vectors (Rhipicephalus spp. for B. bovis and B. bigemina, and Ixodes ricinus for B. divergens) and have a trans-ovarial mode of transmission. Babesia parasites can cause acute and persistent infections to adult naïve cattle that can occur without evident clinical signs, but infections caused by B. bovis are associated with more severe disease and increased mortality, and are considered to be the most virulent agent of bovine babesiosis. In addition, babesiosis caused by B. divergens has an important zoonotic potential. The disease caused by B. bovis and B. bigemina can be controlled, at least in part, using therapeutic agents or vaccines comprising live-attenuated parasites, but these methods are limited in terms of their safety, ease of deployability and long-term efficacy, and improved control measures are urgently needed. In addition, expansion of tick habitats due to climate change and other rapidly changing environmental factors complicate efficient control of these parasites. While the ability to cause persistent infections facilitates transmission and persistence of the parasite in endemic regions, it also highlights their capacity to evade the host immune responses. Currently, the mechanisms of immune responses used by infected bovines to survive acute and chronic infections remain poorly understood, warranting further research. Similarly, molecular details on the processes leading to sexual reproduction and the development of tick-stage parasites are lacking, and such tick-specific molecules can be targets for control using alternative transmission blocking vaccines. In this review, we identify and examine key phases in the life-cycle of Babesia parasites, including dependence on a tick vector for transmission, sexual reproduction of the parasite in the midgut of the tick, parasite-dependent invasion and egression of bovine RBCs, the role of the spleen in the clearance of infected RBCs (IRBCs), and age-related disease resistance in cattle, as opportunities for developing improved control measures. The availability of integrated novel research approaches including “omics” (such as genomics, transcriptomics, and proteomics), gene modification, cytoadhesion assays, RBC invasion assays and methods for in vitro induction of sexual-stage parasites will accelerate our understanding of parasite vulnerabilities. Further, producing new knowledge on these vulnerabilities, as well as taking full advantage of existing knowledge, by filling important research gaps should result in the development of next-generation vaccines to control acute disease and parasite transmission. Creative and effective use of current and future technical and computational resources are needed, in the face of the numerous challenges imposed by these highly evolved parasites, for improving the control of this disease. Overall, bovine babesiosis is recognised as a global disease that imposes a serious burden on livestock production and human livelihood, but it largely remains a poorly controlled disease in many areas of the world. Recently, important progress has been made in our understanding of the basic biology and host-parasite interactions of Babesia parasites, yet a good deal of basic and translational research is still needed to achieve effective control of this important disease and to improve animal and human health.
1. Introduction
Apicomplexans are obligate intracellular parasites defined by the presence of a cell invasion apparatus consisting of rhoptries, micronemes, and conoid organelles, known as the apical complex, which facilitates invasion of these parasites into their host red blood cells (RBCs). Members of Babesia, of which more than 100 species have been described (Chauvin et al., 2009), reside within the order of Piroplasmida and can infect domestic and wild vertebrates as well as becoming an emerging pathogen of humans, of particular importance in transfusion medicine (Lobo et al., 2013). These tick-borne apicomplexan parasites are trans-ovarially transmitted by Ixodidae ticks and similar to other related Alveolata, including dinoflagellates, they contain a remnant membrane-bound, DNA-containing plastid organelle, derived from a vestigial endosymbiotic photosynthetic organism, most likely a red alga (Arisue and Hashimoto, 2015). Babesia parasites contain most apicomplexan invading organelles, except conoids. The evolutionary origin of Babesia parasites, similar to other apicomplexans, is most probably related to a photosynthetic colpodellid-like organism (Kuvardina et al., 2002, Leander et al., 2003, Mathur et al., 2018). How apicomplexans switched from an autotrophic to a parasitic life-style is currently an active field of research that may provide clues towards our understanding of the mechanisms involved in the evolution of virulence in these organisms of both veterinary and medical importance.
Babesiosis is a globally important tick-borne disease caused by apicomplexan parasites of the genus Babesia. Babesia spp. are highly successful intracellular parasites, which acquired highly sophisticated mechanisms for survival during long term co-evolution with their hosts. As a result, these parasites have developed a complex life-cycle involving hard ticks as definitive hosts and vertebrates as intermediate hosts. Thus, Babesia spp. invade and multiply asexually by binary fission inside RBCs of their infected vertebrate hosts and produce sexual forms in the midgut of the ixodid tick vectors, their definitive hosts, where they undergo sexual multiplication (Mehlhorn and Shein, 1984).
Bovine babesiosis is mainly caused by Babesia bovis and Babesia bigemina in tropical and sub-tropical regions worldwide, and these parasites remain by far the most studied agents of tick fever. In addition, Babesia divergens is responsible for bovine babesiosis in Europe and an important zoonotic pathogen that may also infect immunocompromised humans and can cause a potentially life-threatening disease (Beugnet and Moreau, 2015, Rozej-Bielicka et al., 2015). Bovine babesiosis has an enormous economic and social impact on beef and dairy industries worldwide (Suarez and Noh, 2011). Despite the availability and use of live vaccines comprising attenuated parasites (De Vos and Bock, 2000, Florin-Christensen et al., 2014) in a few countries, bovine babesiosis remains poorly controlled, confirming the urgent need for novel vaccines to prevent the development of acute disease and expansion of the parasites into non-endemic areas. Currently, the control of bovine babesiosis is increasingly under threat due to climatic change that favors vector development and expansion, and other environmental factors (Dantas-Torres, 2015, Sonenshine, 2018). Recent investigations in the biology of the tick-borne apicomplexan Babesia parasites revealed an impressive repertoire of resources available to these parasites, allowing them to survive the constant changes and pressures occurring in their environments, including overcoming the hostile activity of the host’s immune system. While Babesia parasites are able to use a seemingly unlimited and diverse repertoire of resources to survive and develop in their tick vector and vertebrate hosts, this also suggests increasing challenges in our search for new resources and strategies toward improved control, treatment and preventative measures. However, as our research toolbox expands due to emergence of novel technologies, our knowledge of the complexities in parasite biology and host-parasite interactions also increases at a faster rate.
Improved control of babesiosis relies on practical, sensitive and specific methods for serological and molecular diagnosis of the disease. Diagnostic and genotyping methods are useful not only to demonstrate the presence and impact of the disease in the field, but also to inform on the complexity of the strain variation among and between Babesia populations, their relative virulence and the presence of co-infections. In addition, the variable results usually derived from the application of serological diagnostic methods based on apparently widely conserved antigens can provide important clues on the antigenicity of key molecules that can be required to elicit protective immunity. Serological diagnosis can also inform on the evolution of the immune responses in endemic areas, since diagnostic data can provide valuable evidence that may suggest hypotheses on the mechanisms involved in the variations of the antigens involved. Therefore, diagnostic tools are also highly relevant for vaccine development, and for controlling the disease. Control of babesiosis can also be achieved by the elimination of the parasites’ tick vectors using acaricides. So far, none of the strategies applied to control babesiosis have succeeded in complete elimination of the disease in endemic areas, with the sole exception of a long-term campaign (the Cattle Fever Tick Eradication Program) that was developed in the United States (USA) from ∼1906–1943 and beyond, based on the eradication of Rhipicephalus ticks (https://www.aphis.usda.gov/animal_health/animal_diseases/tick/downloads/draft_eis_document.pdf). This campaign succeeded under very special circumstances (Bowman, 2006) and could not be reproduced elsewhere, despite important efforts using a similar approach developed in several countries including, for instance, major beef producers such as Australia and Argentina (Pegram et al., 2000, Bowman, 2006). In addition to the development of acaricide resistance in ticks, the impact that would result as a consequence of the application of the massive amounts of acaricides required for a large-scale tick eradication campaign would be unacceptable nowadays under current environmental and public health standards.
In this review, we focus on recounting what is currently known about some of the key mechanisms in the life cycle of Babesia parasites and the potential application of novel research strategies to better understand these mechanisms, with the expectation that well-aimed and focused, basic and translational research, will succeed in finding “Achilles heels” that can be exploited to our advantage in the fight against these highly evolved and globally important parasites.
2. Current methods of control
Currently, control of bovine babesiosis is mainly based on the use of drugs, live vaccines and tick vector control strategies. In endemic areas, drugs are of limited use since they cannot be used to prevent infections, are expensive, and may leave residual metabolites in milk and/or meat. In addition, successful drug treatment is dependent on early diagnosis, correct administration, and proper storage of the drugs. For bovine babesiosis, and babesiosis in other animals (such as infections caused by Babesia caballi for example), imidocarb dipropionate and diminazene aceturate are the usual treatment of choice (Vial and Gorenflot, 2006, Mosqueda et al., 2012). However, continued or inappropriate use of drugs can lead to drug resistance (Mosqueda et al., 2012) and they have the risk of generating chemical residues in meat and milk with potential toxic effects (Coldham et al., 1995), so researchers urgently need to find or develop new alternative effective and affordable drugs with low toxicity. Recent work resulted in the identification of several new drug candidates (Table 1). Studies have shown that strategies based on the combination of chemotherapeutics are significantly more effective for the elimination of parasites, and importantly, result in reduced risk of development of drug-resistance than the application of a single drug therapy (Pritchard et al., 2013). Advantages of a combination of chemotherapeutics include high effectiveness, reduced dosage (which may lead to reduced toxic side effects), lower induction of drug resistance, and recrudescence. To this end, it is also preferable to define drugs that are able to inhibit distinct pathways, leading to synergistic effects, an approach that can also help in preventing development of anti-drug resistance (Lawres et al., 2016). In most apicomplexan parasites a mitochondrial and apicoplast organelle are commonly found. The apicoplast is a plastid like organelle that is described as essential for long-term parasite viability and is therefore considered a good target for specific drugs (Fichera and Roos, 1997, Tuvshintulga et al., 2017). The mitochondria were likely obtained through an endosymbiotic process by incorporation of an α-proteobacterium (Gray et al., 1999, van der Giezen, 2011). Apicomplexan mitochondria present as an interesting target due to distinct differences compared with the mammalian host which has led to the development of anti-parasitic therapeutics, as reviewed in Monzote and Gille (2010). In addition, although chemotherapeutics can be effective in preventing the death of infected animals, they may interfere with the process of developing protective immunity in the herd, and thus are not considered an ideal method of control. The use of live vaccines based on attenuated parasites is the cornerstone to control bovine babesiosis in several countries such as Australia, Israel, and Argentina. However, such live vaccines are also cumbersome and expensive to produce, and have many limitations such as the need of a cold chain, contamination with other infectious agents, a high risk of reversion to virulence due to several possible mechanisms, can only be applied to young calves, etc. Yet, while safer sub-unit vaccines are not available, they are, arguably, the most sustainable and desirable method for control of bovine babesiosis (Brown et al., 2006, Florin-Christensen et al., 2014). Babesia strains consist of several subpopulations of parasites that may have variable degrees of virulence. It has been theorised that the occurrence of a virulent or attenuated virulence phenotype of a strain depends of the sum of the contributions of each subpopulation, and hence, virulence was characterised as a “fluid” phenotype (Lau et al., 2011). Live vaccines are usually based on attenuated parasites that are derived from virulent strains by “quick passage” (B. bovis), or “slow passage” (B. bigemina) in splenectomised (for B. bovis) or spleen-intact calves (for B. bigemina). The resulting production of pairs of virulent and derived-attenuated strains, generated by this process, suggested the possibility of comparative genomic and transcriptomic studies towards identifying virulence and/or attenuation factors (Lau et al., 2011, Pedroni et al., 2013, Gallego-Lopez et al., 2018).
Table 1.
Drugs used to inhibit the growth of bovine Babesia parasites.
| Drug | Molecular target | In field use | Babesia spp. | Stage |
IC50 |
LD100 | References | |
|---|---|---|---|---|---|---|---|---|
| In vivo | In vitro | (μM) | (μM) | |||||
| Diminazene aceturate (DA) | Inhibits the mitochondrial topoisomerase IIa | Yes |
B. bovis B. bigemina B. divergens |
Yes (3–5 mg/kg)b Yes (3–5 mg/kg)b Yes (3–5 mg/kg)b |
Yes Yes Yes |
0.19 ± 0.04c 0.48 ± 0.09d 1.85 ± 0.1c 0.21 ± 0.06d 0.38 ± 0.04c 0.14 ± 0.03d |
bKuttler (1981). aPeregrine and Mamman (1993), dRizk et al. (2017), cGuswanto et al. (2018) | |
| Imidocarb dipropionate | Interference with the production/use of polyaminese, or with the entry of inositol into the parasitised erythrocytef | Yes |
B. bovis B. bigemina |
Yes (1–3 mg/kg)b Yes (1–3 mg/kg)b |
Yes Yes |
8.6 nMg 0.08 nMh |
eBacchi et al. (1981), bKuttler (1981), fMcHardy et al. (1986), gNott et al. (1990), hSilva et al. (2018b) | |
| Draxxin® (Tulathromycin) | Interference with protein synthesis. 23S prokaryotic rRNA | Yes, but not for babesiosis |
B. bovis B. bigemina |
No No |
Yes Yes |
0.02 ± 0.0006 0.006 ± 0.0002 |
0.04 0.01 |
Silva et al. (2018b) |
| N-acetyl-l-cysteine | No |
B. bovis B. bigemina B. divergens |
No No No |
Yes Yes Yes |
332.1 ± 33.1 229.2 ± 37.5 117.2 ± 8.0 |
Rizk et al., (2017) | ||
| Clofazimine | Associated with enhanced activity of phospholipase A2 | No |
B. bovis B. bigemina |
Yes Yes |
4.5 ± 0.30 3.0 ± 0.20 |
Tuvshintulga et al. (2016) | ||
| Nitidine chloride | Topoisomerases | No |
B. bovis B. bigemina |
No No |
Yes Yes |
1.01 ± 0.2 5.34 ± 1.0 |
4 10 |
Tayebwa et al. (2018) |
| Camptothecin | Topoisomerases | No |
B. bovis B. bigemina |
No No |
Yes Yes |
11.67 ± 1.6 4.00 ± 1.0 |
48 8 |
Tayebwa et al. (2018) |
| 17-dimethylaminoethylamino-17-demethoxygeldanamycin (17-DMAG) | Heat shock protein 90 | No |
B. bovis B. bigemina B. divergens |
No No No |
Yes Yes Yes |
0.08 ± 0.0029 0.06 ± 0.002 0.18 ± 0.003 |
Guswanto et al. (2018) | |
| Atovaquone (AV) | Inhibits the rate of oxygen consumptioni | Yes, but not for babesiosis |
B. bovis B. bigemina B. divergens |
No No Yes (1 mg/kg)j |
Yes Yes Yes |
0.03 ± 0.002c 0.71 ± 0.04c 0.01 ± 0.001c |
jPudney and Gray (1997), iMurphy and Lang-Unnasch (1999), cGuswanto et al. (2018) | |
| DA + AV | No |
B. bovis B. bigemina B. divergens |
No No No |
Yes Yes Yes |
0.75 1.06 1.21 |
Guswanto et al. (2018) | ||
| 17-DMAG + AV | No |
B. bovis B. bigemina B. divergens |
No No No |
Yes Yes Yes |
1.26 1.18 2.07 |
Guswanto et al. (2018) | ||
| 17-DMAG + DA | No |
B. bovis B. bigemina B. divergens |
No No No |
Yes Yes Yes |
0.87 1.35 1.85 |
Guswanto et al. (2018) | ||
| Trifluralin analogues | Disrupt microtubules | No |
B. bovis B. bigemina |
No No |
Yes Yes |
18.7–8.5k 1.01–8.2l |
kSilva et al. (2013, l2016) | |
Note: In vivo dosage is per kg of body weight.
IC50, half maximal inhibitory concentration; LD100, letal dose, 100%.
3. Parasite vulnerabilities
Despite their similarities, B. bovis and B. bigemina also have fundamental differences. Both parasites use similar tick vectors including Rhipicephalus microplus and Rhipicephalus annulatus, although B. bigemina has an extended range of potential tick vectors including Rhipicephalus decoloratus. In addition, although both parasites are able to transmit transovarially, the larvae are the infective stage for B. bovis, whereas nymphs are responsible for transmitting B. bigemina during tick feeding, which can be considered as a vulnerability (V#1; Table 2, Fig. 1) that can be used for controlling the parasite). Babesia bovis is usually considered more virulent than B. bigemina, and tends to cause more dramatic clinical signs and symptoms, such as vascular sequestration and vaso-occlusion and, overall, increased mortality in the vertebrate host. Babesia divergens and Babesia major are the causative agents of bovine babesiosis in Europe, with B. divergens being the more common cause of infection (Zintl et al., 2003, Michelet et al., 2014; Wilson, S.G. (1966). The incidence of Babesia major in the Netherlands. In Corradetti, A., (Ed.) Proceedings of the First International Congress of Parasitology, Pergamon, pp. 276–277). Babesia ovata and Babesia orientalis are predominantly found in Asia with B. orientalis being the major causative agent of babesiosis in buffalo (Sivakumar et al., 2016, He et al., 2017). The tick vectors for these Babesia parasites have been reviewed elsewhere (Uilenberg, 2006).
Table 2.
Parasite vulnerabilities and intervention strategies to protect against bovine babesiosis.
| Vulnerability # | Justification | Targets – strategies |
|---|---|---|
| 1: Ticks needed for transmission | Tick control impedes the expansion of the parasite | Tick vaccines, new acaricides, management strategies |
| 2: Sexual reproduction in midgut, invasion of tick tissues | Antibodies against tick-specific stages may interfere with sexual cell fusion in the midgut and other tick stages | Sexual stage-specific Babesia antigens: HAP2, 6-Cys, CCp, CPW-WPC |
| 3: RBC invasion | Discovering key molecules involved in the process of parasite attachment and invasion can lead to the development of invasion-interfering strategies | Babesia surface exposed antigens: MSAs, RAP-1, CLAMP, MIC-1, TRAP, AMA-1 |
| 4: IRBC egression | Interfering with mechanisms for egression can be targeted by drugs | Drugs that inhibit egression such as bumped kinases |
| 5: Trapping of IRBC by the spleen | Babesia bovis avoids trapping using sequestration, a mechanism that can be a target for interference | Antigens exposed in the erythrocyte surface, Ves1 and Ves2 antigens, SmORFs? Spherical body proteins? |
| 6: Young calves have increased resistance compared to older | Discovering the bases for increased resistance to bovine babesiosis in calves may guide vaccine design | Immuno-stimulants, interleukins, vaccine adjuvants able to bias the immune responses |
RBC, red blood cell; IRBC, infected RBC; HAP2, HAPLESS2/GCS1; MSAs, Merzoite Surface Antigens; Rap-1, Rhoptry Associated Protein-1; MIC-1, Microneme-1; TRAP, thrombospondin-related anonymous protein-1; AMA-1, Apical membrane antigen-1; Ves, Variable erythrocyte surface; SmORF, Small Open Reading Frame.
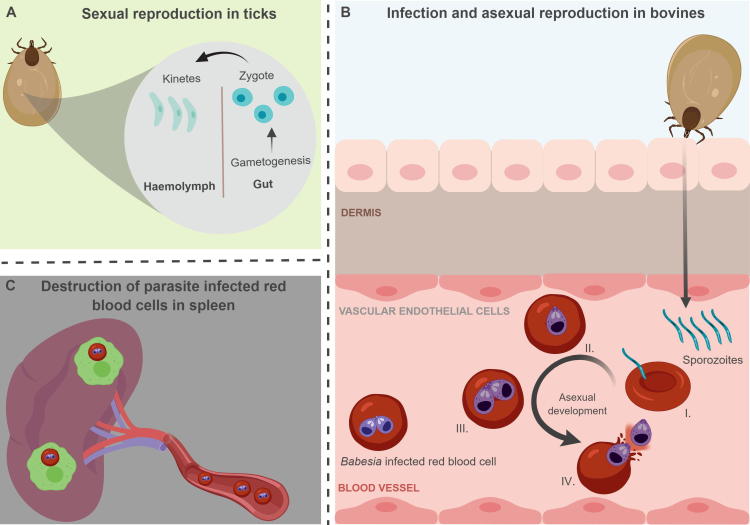
Fig. 1.
Schematic representation of a simplified and partial life cycle of Babesia parasites. (A) Representation of the life-cycle of Babesia parasites in an adult, female tick (e.g. Rhipicephalus microplus) after taking a blood meal from an infected animal. Upon ingestion, the parasite develops sexual forms that fuse to form zygotes. Zygotes mature into kinetes upon invasion of the midgut epithelial cells of the tick which invade the tick hemolymph, where they can invade the ovaries and ultimately infect the larvae of the next generation of ticks. (B) Representation of Babesia infection and asexual reproduction in the bovine host. Sporozoites are introduced from tick saliva into the blood of their bovine host during blood feeding. (I) Sporozoites invade red blood cells (RBCs) and undergo asexual development. (II) Sporozoites mature into trophozoites inside the infected RBC. (III) Trophozoites divide asexually into two daughter merozoites inside infected RBCs. (IV) Merozoites are released into the blood following RBC lysis and then rapidly invade new RBCs. (C) Representation of splenic macrophage-mediated destruction of Babesia-infected RBCs in the blood circulation. Figure generated using BioRender.
Babesia parasites are able to cause both acute and persistent infections in their vertebrate hosts, where they solely invade RBCs, and depend on female ticks feeding on an infected host for subsequent transovarial transmission (V#2, Table 2, Fig. 1). Babesia parasites have a complex life-cycle in their tick definitive host, as briefly described here, and expanded later in the review (Fig. 1). When a tick acquires parasites during blood feeding on a Babesia-infected animal, the parasites undergo sexual reproduction in the tick midgut, leading to the production of kinetes that invade the ovaries resulting in eggs in the next generation of ticks being infected (transovarial transmission). Further, sporozoites that reside and reproduce asexually in the salivary glands of the larvae of the next generation of ticks (Mehlhorn and Shein, 1984) are transmitted to a new bovine host during the blood feeding activity of larval (for B. bovis) or nymphal (for B. bigemina) stages, thereby completing the lifecycle. The impact of Babesia spp. infections on the tick has not been thoroughly characterised, but some evidence suggests that infection may result in an overall decrease in tick fitness (de Vos et al., 1989, Cen-Aguilar et al., 1998, Florin-Christensen and Schnittger, 2009).
3.1. Transmission of Babesia parasites
Control at the level of transmission of Babesia parasites may be achieved using a combination of strategies including measures targeting the tick vectors and sexual stages of the parasites. Novel approaches include the development of anti-tick vaccines as an alternative to acaricides, which can generate resistance and may have negative impacts on the environment. The development of tick vaccines was recently reviewed by Valle and Guerrero (2018).
Understanding the mechanisms involved in sexual reproduction of the parasites and their transmission by ticks is also essential for development of novel control strategies aimed at impairing the expansion of the parasite in endemic areas. This can be achieved, at least in theory, by the development of effective transmission-blocking vaccines (TBVs) which is an important goal for controlling arthropod-transmitted parasites in general. It is currently accepted that gametogenesis in Babesia spp. occurs mainly in the gut of the tick. As a result of fertilisation of macrogametes by the microgametes, oocytes emerge which then invade epithelial cells of the tick midgut. Kinetes then emerge from infected gut cells into the circulation in the tick haemolymph. These forms are able to migrate to the ovary of the ticks, as well as other tick tissues, where they penetrate into the eggs of the tick progeny, resulting in transovarial transmission. The process culminates with the invasion of the cells in the salivary gland of tick larvae, where sporozoites are formed. The sporozoites are the infective stage which is injected into the blood and s.c. tissue of the next bovine host upon feeding by larval (B. bovis) or nymphal (B. bigemina) tick stages (Suarez and Noh, 2011). The development of TBVs relies heavily on the discovery of parasite antigens that are differentially expressed in sexual stages of Babesia parasites, and testing of the effect of antibodies directed against such key antigens while parasites reside in the midgut of tick vector. However, other approaches targeting other tick stages of the parasites such as kinetes are also at least theoretically possible.
Recent work has identified several genes and gene families of B. bovis that are differentially expressed in tick stages. The first of such studies focused on the members of the CCp and 6-cys gene families. A highly conserved family of six multi-domain adhesion proteins, termed the CCp family, was initially identified in Plasmodium parasites. Importantly, it was demonstrated that the CCp proteins in Plasmodium are expressed on the surface of gametocytes, and that knock-out (KO) of the Plasmodium CCp genes blocks sporozoite formation, thus preventing the development of the parasite in the mosquito vectors (Pradel et al., 2004, Lavazec et al., 2009). A feature of the CCp proteins is the presence of at least one signature domain, Limulus coagulation factor C (LCCL), and additional adhesion domains, suggesting that these proteins may mediate cell contact of gametocytes (Delrieu et al., 2002, Pradel et al., 2004, Simon et al., 2009). These data suggest that CCp proteins are potential targets for the development of TBVs to control the expansion of Plasmodium parasites (Pradel et al., 2004). Furthermore, at least three CCp genes (ccp1, ccp2 and ccp3) were also identified in B. bovis and B. bigemina. Experiments performed in an in vitro system for induction of sexual stage forms of B. bigemina demonstrated significant upregulation of the expression of all three CCp genes upon induction. Increased production of all three CCp proteins was also found in induced sexual forms of B. bigemina using immunoblotting and immunofluorescence (Bastos et al., 2013). In contrast, no expression of the CCp genes could be detected in samples collected from ticks infected with B. bovis, and further work will be required in order to clarify the pattern of expression of the CCp proteins in this parasite (Bastos et al., 2013).
Work in Plasmodium has also resulted in the initial identification of the 6-Cys gene family. Three of the Plasmodium 6-Cys genes (Pfs230, Pf48/45 and Pfs47) are expressed in gametes and are prime candidates for the development of TBVs against Plasmodium (Williamson, 2003, Eksi et al., 2006). Main features of 6-Cys proteins include the presence of a domain defined by a conserved arrangement of 6-cysteine residues, and other alternative arrangements (Silva et al., 2011, Alzan et al., 2016b). In silico comparative genomic analysis revealed the presence of at least 10 6-Cys domain-containing protein-coding orthologous genes in B. bovis (Silva et al., 2011, Alzan et al., 2016b). Transcription and immunoblot analyses demonstrated that at least two members of the B. bovis 6-Cys gene family, denominated 6-Cys A and B, can be considered as markers for sexual stages, since they are differentially expressed in the tick stages of the parasites, and not during its asexual blood stages (Alzan et al., 2016b). An important limitation for the study of the biology of sexual stages in Babesia parasites is their development in the midgut of the tick. The haemoglobin-rich milieu of the tick midgut is extremely complex due to the abundance of tick and bovine cells, which makes the identification of sexual forms of the parasite particularly difficult. A significant advance was the development of an in vitro system for the induction of sexual forms of B. bovis using blood cultures (Hussein et al., 2017) with similar systems developed for B. bigemina (Mosqueda et al., 2004, Bastos et al., 2013, Bohaliga et al., 2018). Use of this novel experimental tool will facilitate further clarification of the biology of the parasite, especially aspects related to its transition into sexual forms. In fact, this system already allowed confirmation of the expression of the 6-Cys A protein in the surface of the sexual stage forms using live immunofluorescence analysis (Hussein et al., 2017). Furthermore, the system was also essential for the characterisation of HAP2, a protein encoded by a highly conserved gene family also identified previously in Plasmodium (Angrisano et al., 2017). Malaria parasites, as well as other related apicomplexans, express a protein known as HAPLESS2/GCS1 (HAP2) on the surface of microgametes that occur in the mosquito gut lumen, which is also a candidate for TBV against Plasmodium. The B. bovis HAP2 was also found exclusively expressed in the surface of in vitro induced sexual forms, but not in in vitro cultured asexual stages of the parasite. Parasites with a KO in the HAP2 gene are able to grow in in vitro cultures, suggesting that the expression of this gene is irrelevant for its development in RBCs (Hussein et al., 2017). However, HAP2 KO B. bovis parasites cannot be induced into sexual stages using the in vitro induction system, suggesting that expression of HAP2 is required for the formation of sexual forms in B. bovis (Hussein et al., 2017). Altogether, this research suggested HAP2 protein as an additional prime candidate for the development of TBVs against B. bovis.
Additional novel proteomic tools were also recently applied to explore the proteome of kinete stages of B. bovis; a stage of the parasite that exclusively occurs during its development in ticks (Johnson et al., 2017). The study identified 10 proteins that are differentially expressed in kinetes that may also represent vulnerable targets for intervention against parasite transmission.
Finally, identification of proteins that are essential for the completion of the parasite life-cycle in the tick can also be used for the development of subunit vaccines that are not transmissible by ticks. Importantly, genes required in tick stages are usually irrelevant for the development of the parasites in asexual blood stages, greatly facilitating the process of KO parasites, since gene editing and transfection methods are usually applied in blood stage parasites and mutations affecting such non-essential genes generally result in viable blood stage parasites (Suarez et al., 2017). This can now be achieved by generating gene KO parasites, based on the use of recently developed B. bovis and B. bigemina gene manipulation methods (Silva et al., 2018a).
Overall, important progress has been made recently, leading to the identification of novel targets that can be exploited towards controlling bovine babesiosis. These advances occurred mainly by the application of novel KO techniques, in vitro sexual induction systems and proteomic techniques. However, it remains unknown whether TBVs based on the elicitation of antibodies that need to act in the milieu of the tick midgut, which has a distinct pH and is rich in proteases and other enzymes that can modify and inactivate the antibodies, is a workable approach. Future critical research is still required on testing new subunit vaccines based on tick stage antigens, with novel vaccine formulation and delivery systems that are able to elicit the high titers of antibodies likely required for the success of this novel vaccination approach. In addition, such subunit vaccine development requires considerable time to reach the end user, thus it would be feasible and practical to use attenuated parasite vaccines that cannot be transmissible in ticks. This can be possible, at least in theory, using KO approaches targeting genes required for the development of sexual stages of Babesia parasites.
3.2. Red blood cell invasion
Babesia sporozoites injected into the bovine host during tick feeding reach the circulation and invade RBCs (V#3, Table 2). RBCs are terminally-differentiated cells which lack any of their own genetic information and the ability to synthesise proteins, lipids and carbohydrates that, in addition to their low metabolic activity, makes for a relatively safe environment for pathogens to reside in. However, Babesia-infected RBCs are equipped with a source of introduced genetic information, which is now provided and operated by the parasite, and as a result, the parasite is relatively free to modify and control the red blood cell in order to fulfill its needs. Therefore, and similar to Plasmodium parasites, B. bovis dramatically modifies the composition and architecture of the infected RBCs (IRBCs). These changes include the protein and lipid composition of the RBC membrane (Florin-Christensen et al., 2000, Allred and Al-Khedery, 2006) and hence its relative permeability and deformability. Indeed, RBCs appear as ideal host cells for these parasite since they freely circulate through every organ of the host providing easy access to a variety of tissues that may be sites for capillary sequestration (in the case of B. bovis-IRBCs), they lack lysosomes and, importantly, are unable to present antigens to the immune system, thus facilitating escape of the parasite from immune effectors. RBCs are also unable to phagocytose and internalise other cells or nutrients by endocytosis, implying that the parasite must be the active force required for invasion to occur (V#3, Table 2). Merozoites reproduce asexually and mature inside RBCs until they egress and then invade new RBCs (V#4, Table 2). However, RBCs also transit through the spleen, an organ responsible for the clearance of IRBCs (V#5, Table 2).
Babesia parasites are equipped with an apical complex, a complement of secretory organelles that are required for RBC and target cell invasion. The apical complex organelle includes the pear shaped rhoptries, the microneme vesicles, spherical bodies (homologous to dense granules in Plasmodium and Toxoplasma) and other structures. The components of each of these organelles represent a knowledge gap in Babesia, but they are better defined in other related and more studied apicomplexans such as Toxoplasma and Plasmodium (Gubbels and Duraisingh, 2012). However, some apical complex molecules are species-specific and not widely conserved among apicomplexans, perhaps as a result of immune selective pressure during many years of co-evolution with distinct vertebrate hosts, and are poorly defined in Babesia parasites. However, Babesia parasites are equipped with a core of typical and highly conserved apicomplexan molecules with a known role in invasion such as AMA-1, TRAP, MIC-1, (Gaffar et al., 2004a, Gaffar et al., 2004b, Cerede et al., 2005) CLAMP (Suarez et al., 2017), and rhoptry neck proteins (Kemp et al., 2013), which are conserved among most apicomplexans.
In contrast to the related malaria parasites, babesia sporozoites solely infect RBCs, which is the only cell targeted for invasion in the bovine host (Mehlhorn and Shein, 1984, Chauvin et al., 2009). The process of RBC invasion by Babesia sporozoites and merozoites remains an important knowledge gap. Unraveling this process might lead to the design of novel methods for the control of bovine babesiosis. The mechanics of RBC invasion were revealed using transgenic parasites (Asada et al., 2012), but the molecular events involved remain obscure. Possibly, the initial event occurring in invasion is the recognition of a target cell by a gliding parasite apparently by random interactions, likely using glycosylphosphatidylinositol (GPI)-anchored surface exposed molecules such as MSA-1 and MSA-2 in B. bovis (Hines et al., 1992, Suarez et al., 2000, Florin-Christensen et al., 2002) and GP-45 B. bigemina proteins (Fisher et al., 2001), but it is also possible that it can be, at least in part, driven by recently discovered differences in the electric potential in the RBC and parasite surfaces (Scudiero et al., 2018). An important element involved in invasion is the high level of motility of the babesial invading sporozoites and merozoites, which is achieved by a gliding motion, provided by the activity of a highly efficient actin motor (Asada et al., 2012). Once the parasite is able to establish an interaction with a suitable RBC, it adheres then re-orients in order to have its apical end in direct contact with the surface of the RBC, resulting in high affinity docking involving membrane fusions, mediated by parasite microneme, and perhaps rhoptry, proteins. All these mechanisms, initial recognition, gliding, and re-orientation, can be potentially targeted by new control methods, either by drugs or vaccines. Intriguingly, similar to other apicomplexans, Babesia parasites form a parasitophorous vacuolar membrane (PVM) and reside within a vacuole immediately following invasion of the RBC but, in contrast to other related apicomplexans, the PVM is rapidly lost, leaving the parasite in direct contact with the cytoplasmic content of the RBC, as is consistently observed for B. bovis, B. bigemina, and B. divergens (Rudzinska et al., 1976, Potgieter and Els, 1976, Gohil et al., 2010). Babesia parasites contain genes encoding other widely conserved proteins such as serine rhomboid proteases which might be involved in releasing the parasite into the PV (Li et al., 2012), and so may also play important roles in the process of RBC invasion. The PVM is formed with the contribution of molecules secreted by the apical complex of the parasites, likely including the spherical bodies, which are homologous with the dense granules in Plasmodium and other apicomplexans in addition to the incorporation of host RBC proteins (Soldati et al., 2004, Repnik et al., 2015). Recent work has identified agents that are able to inhibit the egress of Babesia parasites (Pedroni et al., 2016), so future exploitation of these may not only directly facilitate the control of babesiosis, but importantly, also enhance our understanding of the mechanisms involved in the egress of the parasite from IRBCs.
3.3. Sequestration and persistence in infected animals
The growth of Babesia parasites in RBCs results in dramatic changes to the architecture of the IRBCs which display features that are highly atypical for normal RBCs (Gohil et al., 2010). These changes include modifications to the RBC that likely favor escape of the effector arms of the immune system by the parasite, such as increased cellular adhesiveness (in the case of B. bovis) and high antigenic variability. While Babesia parasites have a relatively small genome (approximately 7.0–16 Mbp) (Jackson et al., 2014), compared with the larger genome (∼23 Mbp) of related Plasmodium parasites (Gardner et al., 2002), it is efficiently used by the parasites to create their own defense mechanisms and modulate the host responses for long-term survival. Importantly, comparative genomic analysis of Babesia parasites demonstrated a large expansion of genes involved in antigenic variation, a mechanism used by the parasites for escaping the immune pressure of the vertebrate hosts. This is exemplified by the presence of the large ves (variable erythrocyte surface antigen) gene family comprising more than 150 genes in B. bovis. Interestingly, members of the ves family have also been identified in the genomes of both B. bigemina and B. divergens (Jackson et al., 2014). It is known that, at least for B. bovis, that the ves family of genes encodes proteins responsible for generating rapid antigenic variation, which allows the parasite to escape immune responses of the bovine host. Therefore, the presence of such large gene families suggests that periodic switching of variant antigens expressed on the surface of IRBCs is likely concomitant with the acquisition of new antibodies created by the vertebrate hosts, a phenomenon also observed in P. falciparum with the variant antigen determinants (var) gene family (Biggs et al., 1991, Allred et al., 1993). The consistent investment of Babesia parasites in developing a mechanism based on gene family expansion aimed at creating antigenic diversity is a strong indication of the importance for the need of such avoidance mechanisms in Babesia spp. At least for B. bovis, the mechanisms involved include the expression of variant genes in a “locus of active transcription” (LAT), but antigenic variation can also be potentiated, likely by the activity of segmental gene conversion mechanisms, that can provide increased sequence changes in these genes (Al-Khedery and Allred, 2006). In addition, RBCs infected with B. bovis, but not B. divergens or B. bigemina, can adhere to host vascular endothelial cells and accumulate in the microvasculature to avoid destruction in the spleen. In contrast, B. bigemina parasites can express IgM receptors on their surface (Echaide et al., 1998), although the mechanisms and consequences derived from the expression of such molecules in B. bigemina remain unexplained. Interestingly, similar IgM masking was also described to occur in malaria parasites as a mechanism to escape protective immunity (Barfod et al., 2011). These very intriguing findings are suggestive of the existence of an array of diverse evasion maneuvers that are employed by these parasites. Therefore, cytoadhesion and vascular sequestration in B. bovis appears to be mediated by VESA1 antigens, which are aptly expressed on the surface of the IRBCs. Similar to in the situation with malaria parasites, B. bovis affects changes to the surface architecture of IRBCs with the formation of ridge-like structures (Gohil et al., 2010) (Fig. 2). The molecular composition of these ridges has still not been identified but they likely contain the VESA1 antigens and are involved in the interactions of the IRBCs with vascular endothelial cells, resulting in cytoadhesion and their accumulation in the microvasculature. However, B. bigemina and B. divergens, despite expressing genes that are homologous to ves genes (Jackson et al., 2014), neither express ridges on the surface of IRBCs nor do IRBCs sequester in the microvasculature (Hutchings et al., 2007, Gohil et al., 2010, Scudiero et al., 2018). It is possible that such ridges on B. bovis-IRBCs act as a platform to anchor and cluster the cytoadhesion ligand on the IRBC (similar to knobs in P. falciparum) (Crabb et al., 1997, Gohil et al., 2010). The lack of such ridges on the surface of RBCs infected with either B bigemina or B. divergens may imply that the VES proteins are not correctly presented or anchored to be functional in vivo in these two non-sequestering parasites. However, the lack of cytoadhesion of B. bigemina-IRBCs may have a structural basis, since the VES proteins in B. divergens and B. bigemina lack cysteine-rich CKRD and VDCS domains (Jackson et al., 2014). In addition, B. bigemina and B. divergens ves genes seem not to be organised into a locus of active transcription (LAT) as described in B. bovis (Jackson et al., 2014). In addition, the Small Open Reading Frame (SmORF) family in B. bovis could assist in capillary sequestration together with VESA1 proteins in a manner similar to STEVOR proteins in Plasmodium falciparum. Babesia bovis SmORF genes are found within 4 kb of members of the ves1 family; an arrangement that is similar to the location of var, rifin and stevor genes in P. falciparum (McRobert et al., 2004, Sanyal et al., 2012, Tibúrcio et al., 2012). Furthermore, the SmORF gene family is present only in B. bovis and not in B. bigemina or B. divergens, suggesting a potential role of the SmORF family in cytoadhesion. To fully understand the virulence mechanisms in bovine babesiosis caused by B. bovis, we also need to identify the bovine receptor for IRBCs expressed on the surface of microvascular endothelial cells. Based on studies of P. falciparum, CD36, also known to be expressed on the surface of bovine endothelial cells, is a possible candidate as a receptor for B. bovis IRBCs, but this still remains to be demonstrated. New experimental tools including transfection combined with cytoadhesion assays will be helpful to define the molecular mechanisms mediating the process of cytoadhesion and vascular sequestration of IRBCs. This could include testing mutated ves genes lacking the putative receptor region containing the cystein-rich domain, or the expression of B. bovis wild type and mutated VES proteins, or other functionally relevant RBC surface proteins in normally non-adhesive B. bigemina parasites. These experiments are now made possible using the recently developed stable transfection system for B. bigemina (Silva et al., 2018a).
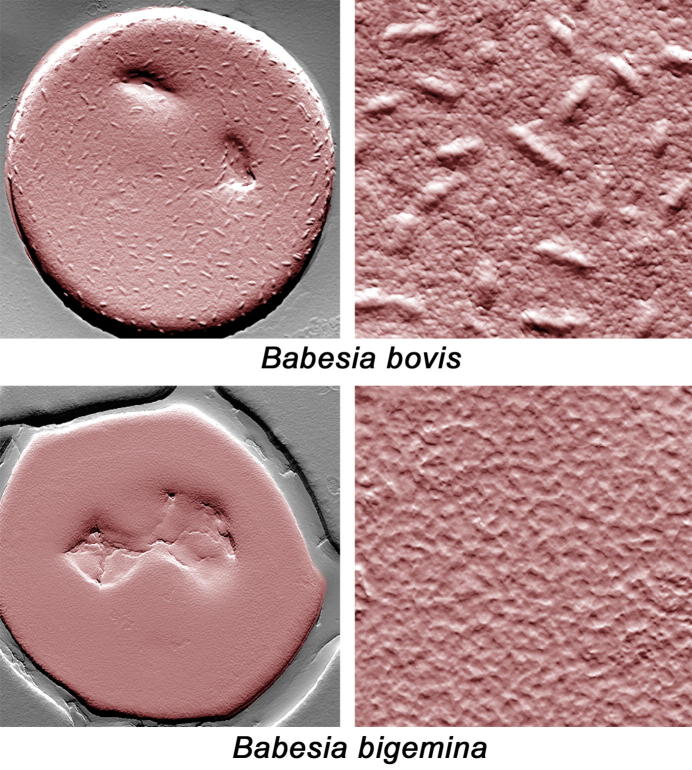
Fig. 2.
Pseudo-coloured atomic force microscopy images of the surface of bovine red blood cells (RBCs) infected with late stages of either Babesia bovis or Babesia bigemina. The unique ridge-like features present on the surface of B. bovis-infected RBCs are notably absent from RBCs infected with B. bigemina. The right-hand panels represent a higher magnification view of the surface of the infected RBCs shown in the left-hand panels. The atomic force microscopy appearance of the surface of normal, uninfected bovine RBCs is similar to RBCs infected with B. bigemina (not shown).
Until now, members of the VES family are the only B. bovis RBC surface-exposed proteins that have been characterised in any detail. However, the parasite is able to export additional, previously uncharacterised, proteins to the IRBC membrane and expose them on the cell surface to play important roles in host-parasite interactions (Gohil et al., 2013). This includes members of the poorly characterised SmORF gene family (Brayton et al., 2007, Ferreri et al., 2012). In fact, and similar to Plasmodium parasites, B. bovis has the ability to completely transform the architecture of the red blood cell in which it matures and divides. However, the proteins identified in Plasmodium parasites as responsible for similar changes seen in the architecture of the IRBCs are conspicuously absent in Babesia parasites, suggesting the operation of convergent evolutionary mechanisms resulting in the export of the required parasite proteins and in similar cytoadhesive phenotypes. A typical B. bovis-IRBC displaying surface modifications, as visualised by atomic force microscopy (AFM) is shown in Fig. 2. RBC modifications include the formation of RBC surface ridges that effectively increase the total surface area of the IRBC and appear to be essential to facilitate cytoadhesion of IRBCs in the host microvasculature, either by facilitating adhesion to endothelial cells or/and platelets (Hutchings et al., 2007). It is also possible that these modifications might also help the parasite avoid trapping by macrophages or interfere with the interactions with hosts’ antibodies, although all of these remain hypothetical (Scudiero et al., 2018). Consistently, no such RBC surface modifications are evident on the surface of non-cytoadhesive B. bigemina-IRBCs (Fig. 3) (Hutchings et al., 2007, Scudiero et al., 2018). It is likely that the changes in the IRBCs are mediated by proteins exported by the parasite, given that RBCs are terminally differentiated cells which lack any protein synthesis activity whatsoever, or alternatively, the result of the modification of existing RBC surface molecules by the parasite. Importantly, a Plasmodium export element (PEXEL)-like signal was identified in exported proteins in B. bovis such as Spherical Body Protein (SBP) 2. The bipartite functional domain ‘PEXEL’ appears to be an essential component of the molecular mechanisms involved in the export of Plasmodium proteins into the host cell. This domain consists of an N‐terminal eukaryotic signal sequence followed by a pentameric conserved motif with the consensus sequence, RxLxE/Q/D (Hiller et al., 2004, Marti et al., 2004). Exported proteins containing the PEXEL motif are directed to the endoplasmic reticulum, where they are subsequently cleaved by a protease (Boddey et al., 2010, Russo et al., 2010).
Fig. 3.
Schematic representation of protective immune responses in bovines infected with Babesia parasites. (A) Representation of innate immunity in young calves. The innate immunity in young calves is characterised by rapid activation of macrophages, abundant release of Interferon-γ (IFN-γ) and nitric oxide (NO). Young, naïve calves are naturally more resistant to infection and usually survive the challenge upon exposure to Babesia-infected ticks in endemic areas (a process also known as pre-munisation). In contrast, adult animals are more susceptible to Babesia infection and usually develop acute, often fatal, babesiosis. Animals which survive acute infections can develop chronic babesiosis and produce life-long protective immune responses. Further, innate immune responses appear to be more pronounced in young, rather than adult, animals. (B) Representation of adaptive immunity in persistently infected or vaccinated animals. Macrophages and protective neutralising antibodies appear to be essential for control of parasitemia in vaccinated and persistently infected animals. Figure generated using BioRender.
In addition, another form of antigenic variation in Babesia spp., based on allelic diversity in GPI-anchored variable merozoite surface antigens (VMSA) (Carcy et al., 2006), has also been identified at a population level (Suarez et al., 2000, Leroith et al., 2005, Berens et al., 2005, Lau et al., 2010). However, it remains premature to conclude that this mechanism could allow heterologous parasites to escape protective immunity as this could be due to variation in several genes and gene products that have not yet been explored. It is interesting that similar mechanisms for antigenic diversity and cytoadhesion co-evolved independently in distinct protozoa such as Plasmodium and Babesia. Antigenic variation using similar mechanisms have also been identified in other protozoans such as trypanosomes, and even prokaryotic organisms (Barbet et al., 2000) that can cause persistent disease. The presence of similar antigenic variation mechanisms that are useful to these otherwise distinct parasitic organisms which arose as a response to relatively similar and invariable immune systems of vertebrates is another interesting example of convergent evolution.
The concerted operation of these mechanisms results in parasites that are able to establish persistent infection of the bovine host, thus maximising their chances of transmission by tick vectors. Persistently infected bovines are apparently healthy and do not show clinical signs of Babesia spp. infection, unless the animal is exposed to stress, co-infections with other pathogens, or if it is immunocompromised, for example by splenectomy, which may result in the breakdown of its immunity to Babesia spp., and the resurgence of acute disease. It is not known exactly what factors are required for the bovine to remain apparently healthy during persistent infection. It is likely that by that point the immune response maturated enough to form high affinity antibodies and a robust cellular immunity based on regulatory CD4+ and regulatory CD8+ lymphocytes that secrete adequate classes and levels of cytokines which are able to keep in check the ability of the parasites to expand in the persistently infected animals (see below). Thus, it is possible that such a balance between a relatively healthy status and acute infection is provoked by constant immune stimulation by the parasite, an example of the establishment of a symbiotic relationship between the host and the parasite upon the development of persistent infection.
3.4. Immune responses to Babesia infection
The precise nature of protective immune responses involved in the control of acute babesiosis in bovines also remains an important research gap, largely due to practical, economic, and ethical restrictions associated with performing experiments in bovines and the lack of reliable small animal models for B. bovis and B. bigemina. Therefore, the lack of practical and reliable experimental systems for defining in detail the mechanisms involved in the development of protection critically affects our ability to design effective vaccines. Considerable progress was made in understanding the immune responses to B. bovis infections; however, the knowledge of immune responses to B. bigemina infections remains essentially unknown. Briefly, immunity to Babesia parasites in young animals and adult animals requires the strong induction of innate immune responses and development of effective adaptive immune responses, respectively (represented schematically in Fig. 3). It has also been suggested that successfully immunised animals and persistently infected animals that survive acute stage infections and are able to control parasitemia rely on antigen-specific CD4+ T cells that produce IFN-γ. This cytokine plays a central role since it can activate macrophages, is required for the clearance of parasites, and enhances production of the neutralising IgG2 antibody (Brown and Palmer, 1999, Homer et al., 2000, Brown, 2001, Estes and Brown, 2002). This antibody isotype, in combination with IgG1, was shown to passively protect cattle against homologous strain challenge (Mahoney, 1986). The identities of the antigens that are targeted by these protective neutralising antibodies, however, remain unknown. The mechanism of T cell activation and the role of the distinct T cell population (such as γδ-T cells) during a successful adaptive immune response in vaccinated/persistently infected adult cattle also remain to be explored. In summary, robust, innate immune responses are needed to survive the acute stages of infection, followed by efficient stimulation of immune mechanisms leading to the production of antibodies, which are critical effectors to control infection in vaccinated and persistently infected animals.
3.4.1. Age-related immune responses to Babesia infections in cattle
Several reviews have described models of protective immune mechanisms for B. bovis (Goff et al., 1998, Brown and Palmer, 1999, Brown, 2001). Experimental evidence indicates that the resolution of acute B. bovis infection in immunologically-naïve young animals depends on a relatively strong innate immune response concomitant with infiltration of large leukocytes (monocytes/macrophages, Natural Killer (NK) cells and immature dendritic cells (DCs), macrophage activation by IFN-γ or parasite-derived products and priming of immune cells to release toxic macrophage metabolites including Nitric Oxide (NO) (Goff et al., 2010, Goff et al., 2003, Goff et al., 2001). A closer look at the immune responses in the spleen of young animals revealed the occurrence of cross-talk (mediated by IL-15 secreted by immature DCs) between the NK cells and immature DCs, resulting in IFN-γ secretion by NK cells and subsequent activation of DCs (Schneider et al., 2011). Further, efforts to understand the immunological basis for age-related resistance in the spleen of young (6-month old) and adult animals infected with B. bovis showed interesting differences in the cytokine response and NO release (Goff et al., 2001). Young animals showed increased expression of IL-12 and IFN-γ in spleen (3–6 days p.i.) 3 days earlier than adult animals and plasma levels of IFN-γ were also detected earlier in young animals than adult animals. Further, the sign of inducible nitric oxide synthase (iNOS) message was detected in young animals around day 7–8p.i., concomitant with parasite clearance. Many in vitro and ex vivo studies showed that IFN-γ secretion and NO release from immune cells were critical for parasite clearance, suggesting that the increased expression of IFN-γ and potential NO release are associated with increased resistance in young animals. As opposed to these observations, a few reports have shown that NO produced by macrophages during B. bovis infection was only partially growth inhibitory in in vitro experiments and in vivo experiments showed the limited role of NO in parasite clearance (Gale et al., 1998, Shoda et al., 2000). Interestingly, an earlier study showed that a soluble factor under 14 kDa isolated from calf serum and not from adult serum was strongly growth inhibitory to B. bovis replication, suggesting the presence of an alternative parasite growth inhibitory product in addition to NO in young animals (Levy et al., 1982). The soluble factor in the serum from young animals shown to have babesiacidal activity remains unknown to date. Therefore, the innate branch of the immune system plays a crucial role, mainly in the control of the expansion of the parasite in the early stages of infection, especially in naïve young animals. The efficient induction of these innate immune mechanisms in the young animals can lead to the development of a protective adaptive immune response in the adult animals and prevent the establishment of persistent disease, or the death of the infected animal due to the devastating effects of uncontrolled acute infection. Enhanced understanding of the mechanisms of resistance in young naïve animals to acute B. bovis infection (innate immunity) and those required for controlling parasitemia to persistent levels in adult cattle that survive infection (adaptive immunity), is also critically important for developing strategies to induce a protective immune response by vaccination, and more research is required in order to close this important research gap.
3.4.2. Immunopathological effects on the host
The virulence of Babesia parasites is characterised by both parasite-mediated exploitation of the host and infection-mediated immunopathology. During an acute infection, especially in immunologically-naïve adult animals, Babesia parasites exploit the host and reproduce mostly unchecked, resulting in important destruction of host RBCs and a dramatic drop in haematocrit (Brown and Palmer, 1999). As a result, the infected host experiences decreased levels of oxygen in tissues and vital organs, causing apnea and respiratory distress, and responds to the infection with an increase in body temperature, with rectal fever that can go above 39 °C for several days. Hemolysis seems to be more dramatic in infections caused by B. bigemina, and the resulting hemolytic disease can be detected by the red color of the urine resulting from the secretion of molecules derived from the mechanisms of haemoglobin degradation in the liver. The high level of RBC destruction also causes jaundice and kidney damage, and the over-activity of the spleen resulting in part from IRBC trapping results in marked splenomegaly. It is possible that increased parasitemias usually found in B. bigemina infections, when compared with cytoadhesive and sequestering B. bovis parasites, also contribute to increased parasite trapping by the spleen concomitant with increased concentrations of haemoglobin and degradation products in the blood, resulting in increased kidney damage and haematuria in babesiosis caused by B. bigemina. In contrast, a hallmark of B. bovis infections is the presence of clinical neurological signs, concomitant with sequestration of parasite-IRBCs in the microvasculature of the brain. These two are the main and key signature pathological manifestations of acute bovine babesiosis in each of these parasites. Immunopathological effects on a naïve host are likely to be stronger due to the lack of immune system priming against Babesia infections and non-specific parasite clearance mechanisms (i.e. increased expression of inflammatory cytokines). The severe pathogenesis is thought to be partially immune-mediated, and over-production of soluble mediators including IFN-γ, TNF-α, and NO (Wright et al., 1988, Clark and Jacobson, 1998). Early induction (day 3–6p.i.) of TNF-α, IFN-γ, IL-12 and IL-18 together with late induction (≥day 10 6p.i.) of IL-10 by the immunocompetent cells of the host responding to the infection may result in increased inflammatory responses and damage in tissues containing sequestered IRBCs in B. bovis infections (Goff et al., 2002a, Goff et al., 2001, Goff et al., 2002b).
4. Current molecular toolbox and future perspectives
Despite numerous research gaps and biological challenges that must be overcome before we can generate more effective control methods to prevent or better control babesiosis, the constant influx of new discoveries and technologies in the field of both babesiosis and closely related apicomplexans, supports the successful development of new methods aimed at a more effective control of bovine babesiosis and, eventually, human babesiosis. Important advances in our understanding of the biology of the parasites can be directly and indirectly associated with the sequencing of relevant Babesia spp. genomes, which began with the publication of the first complete B. bovis genome in 2007 (Brayton et al., 2007). This was followed by the development of transfection systems for gene modification and functional analysis to accelerate vaccine candidate discovery (Suarez et al., 2017). Advancements in our understanding of the genomics of Babesia parasites has allowed, for instance, the complete characterisation of critical genes/gene families such as the large, antigenically-variable ves1 gene family involved in host immune evasion, genes involved in the development of sexual stages (6-Cys, CCp, CPW-WPC, HAP2, etc.) (Bastos et al., 2013, Alzan et al., 2016b, Hussein et al., 2017) and conserved master regulatory genes such as AP2 (Alzan et al., 2016a). The AP2 and associated genes are responsible for transcriptional control of the genes involved in parasite stage transitions, as it was previously discovered in Plasmodium and Theileria parasites (Painter et al., 2011, Pieszko et al., 2015, Alzan et al., 2016a).
The subsequent application of other “omic” methods such as transcriptomics for example, permitted the comparison of virulent and attenuated strains of Babesia spp. in order to address the definition of virulence factors and attenuation markers, one of the most important remaining fundamental research gaps limiting rational vaccine design. The conclusion of genomic comparisons among virulent and attenuated paired strains was the finding of decreased population complexity in the attenuated strains compared with their virulent pairs, but the studies failed in identifying candidate genes or sets of genes responsible for increased or decreased virulence (Lau et al., 2011). Recent studies comparing transcriptomic profiles of geographically distinct virulent B. bovis isolates (Mexican, Australian and Argentinian strains) with their attenuated derivatives resulted in the identification of a limited number of differences such as the recent identification of the SBP2t11 gene as a marker for attenuation of B. bovis parasites (Pedroni et al., 2013, Gallego-Lopez et al., 2018). Other pioneering studies used transcriptomic and proteomic approaches to compare gene expression profiles of blood and kinete stages of Babesia spp. (Johnson et al., 2017) and showed that different sets of genes are activated and repressed in order to support the development of the parasite in the completely distinct environments of the host and the tick. While the functions of the genes identified in this work remain unknown, this can also be addressed in the future using transfection, KO and gene-editing approaches.
Critical to future research is a complete elucidation of the molecular mechanisms involved in the establishment of persistent disease by these parasites. This includes the dissection of the mechanisms of persistence at a molecular level. We must also improve our mechanistic understanding of the ability of B. bovis-IRBCs to cytoadhere and sequester, include the identification of the changes in the architecture of the surface of the IRBCs and the mechanisms involved in the expression of ves genes in this parasite. Additionally, nothing is known about the role of these VES proteins in B. bigemina and the mechanisms used by this parasite for persistence. Discovering these mechanisms will guide new strategies and identify potential targets toward rational subunit vaccine design. More widespread use of currently available tools such as in vitro static and dynamic (flow-based) cytoadhesion assays (Hutchings et al., 2007, Gohil et al., 2010), that can model parasite sequestration in vivo, would provide better insight into these mechanisms and help with the identification of new therapeutic agents that inhibit cytoadhesion. Increased understanding of the factors involved in induction of strong innate immunity in young animals will provide educated ideas/strategies for adjuvant selection and vaccine design. Understanding the mechanism of strong innate immunity in young animals will inform the type of vaccine-induced response needed to control parasitemia. In addition, careful consideration must be given to adjuvant selection and vaccine design as over-production of soluble mediators of cell-mediated immune responses might lead to increased pathology in vaccinated animals. A close evaluation of protective immune mechanisms involved in the efficient clearance of IRBCs by the spleen of young animals and understanding the balance of protective type 1 immune responses is important to design an effective subunit vaccine with limited vaccine-associated pathology in animals.
Recent work has focused on development of in vitro sexual-stage induction systems, due in part to the practical difficulties associated with the study of tick stages in the tick midgut milieu. Application of this system to B. bovis has identified the requirement of the HAP2 gene for the development of sexual-stage parasites and its link with the expression of the sexual-stage marker genes 6-Cys A and B (Hussein et al., 2017). The recent development of this in vitro method for the induction of Babesia sexual stages from cultured parasites will facilitate the study of the antigenic and morphological changes that the parasites undergo, the definition of possible cell surface-exposed immunological targets, and the testing of the effects of specific antibodies against key antigens (Camacho-Nuez et al., 2017, Hussein et al., 2017, Bohaliga et al., 2018). These developments can potentially accelerate the rate of discovery towards the development of transmission blocking vaccines.
Taken together, these new data and newly developed genetic manipulation systems are fundamental for the development of novel, next-generation vaccines that can target multiple stages of the parasites’ life-cycle, which may be essential for the eventual control of this disease.
5. Conclusions
Advancement of research in the biology of Babesia parasites is heavily influenced by the constant advances in molecular and cellular biology, immunology, computational sciences and vaccinology. In this review, we have attempted to link the need for a more complete understanding of the biology of Babesia parasites, including a better and more detailed characterisation of different phases in the parasite life-cycle and the distinct phases of the interactions of Babesia parasites with their host. Overall, the road ahead is still long and winding. However, we have also presented evidence supporting that we are acquiring an appropriately-equipped toolbox for guiding us on a successful journey towards babesiosis control. In practical terms, research will require improved and reliable experimental systems, especially when the natural systems present difficult practical obstacles and ethical concerns.
Currently, robust transfection methods are available for genetic modification in B. bovis and B. bigemina. In addition, our ability to efficiently manipulate the Babesia genomes will be augmented by the development of CRISPR/Cas9 gene editing methods. These gene manipulation methods combined with in vitro static and haemodynamic assays for the analysis of the interactions between IRBCs and host vascular endothelial cells, as well as studies using transgenic parasites in vivo, will allow a more detailed dissection of cytoadhesive mechanisms leading to the creation of new vaccines that might interfere with parasite adhesion and sequestration. The future development of efficient sub-unit vaccines against bovine babesiosis remains a challenge. Importantly, more research is needed to better understand the nature of protective immune responses in young animals and successfully vaccinated animals, and inform effective strategies for vaccine design. Additionally, other current and future methods of analysis, such as in vitro tick artificial feeding systems (Trentelman et al., 2017), will also facilitate the study of the interactions of the parasites with their tick hosts. These techniques, added to the in vitro sexual-stage induction methods, will provide a detailed analysis of the parasite-tick interface, which will allow in turn the identification and simplified method of testing of vaccine candidates with the potential to block the transmission of the parasite. Evaluation of the most promising antigens, those with the potential to limit blood stage infection and tick transmission, as vaccines in cattle in proof of concept trials will aid in the selection of handful of antigens with effective protection efficacy. Ideally, a multivalent subunit vaccine containing the selected antigens would be designed with the purpose of blocking both acute infection and transmission of the parasites.
Finally, although we may gain understanding of the function of individual genes and their biological relevance in different life-cycle stages of the parasite, a better understanding of the ‘big picture’ of this disease is still needed. Clearly, controlling the parasite presents an immense, but important, intellectual challenge, but creative and effective use of the current and future technical resources suggest that the ‘sky is our limit’ in our search for improved control measures for babesiosis that impose an important burden for humans and for food production, with an ultimate impediment to the progress of humanity in the future.
Acknowledgements
We would like to acknowledge generous financial support from the International Development Research Center (IDRC), funded by the Canadian Government and the Bill and Melinda Gates Foundation, the US Department of Agriculture and the Australian Research Council towards the improvement of our basic knowledge of Babesia parasites and the pathology of bovine babesiosis, as well as the development of novel methods for the future control of bovine babesiosis. We also acknowledge the contributions of Jacob Laughery and Paul Lacy for their valuable input into this review.
Contributor Information
Carlos E. Suarez, Email: ces@vetmed.wsu.edu.
Brian M. Cooke, Email: brian.cooke@monash.edu.
References
- Al-Khedery B., Allred D.R. Antigenic variation in Babesia bovis occurs through segmental gene conversion of the ves multigene family, within a bidirectional locus of active transcription. Mol. Microbiol. 2006;59:402–414. doi: 10.1111/j.1365-2958.2005.04993.x. [DOI] [PubMed] [Google Scholar]
- Allred D.R., Al-Khedery B. Antigenic variation as an exploitable weakness of babesial parasites. Vet. Parasitol. 2006;138:50–60. doi: 10.1016/j.vetpar.2006.01.039. [DOI] [PubMed] [Google Scholar]
- Allred D.R., Hines S.A., Ahrens K.P. Isolate-specific parasite antigens of the Babesia bovis-infected erythrocyte surface. Mol. Biochem. Parasitol. 1993;60:121–132. doi: 10.1016/0166-6851(93)90035-v. [DOI] [PubMed] [Google Scholar]
- Alzan H.F., Knowles D.P., Suarez C.E. Comparative bioinformatics analysis of transcription factor genes indicates conservation of key regulatory domains among Babesia bovis, Babesia microti, and Theileria equi. PLoS Negl. Trop. Dis. 2016;10 doi: 10.1371/journal.pntd.0004983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alzan H.F., Lau A.O., Knowles D.P., Herndon D.R., Ueti M.W., Scoles G.A., Kappmeyer L.S., Suarez C.E. Expression of 6-Cys gene superfamily defines Babesia bovis sexual stage development within Rhipicephalus microplus. PLoS ONE. 2016;11 doi: 10.1371/journal.pone.0163791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angrisano F., Sala K.A., Da D.F., Liu Y., Pei J., Grishin N.V., Snell W.J., Blagborough A.M. Targeting the conserved fusion loop of HAP2 inhibits the transmission of Plasmodium berghei and falciparum. Cell Rep. 2017;21:2868–2878. doi: 10.1016/j.celrep.2017.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arisue N., Hashimoto T. Phylogeny and evolution of apicoplasts and apicomplexan parasites. Parasitol. Int. 2015;64:254–259. doi: 10.1016/j.parint.2014.10.005. [DOI] [PubMed] [Google Scholar]
- Asada M., Goto Y., Yahata K., Yokoyama N., Kawai S., Inoue N., Kaneko O., Kawazu S. Gliding motility of Babesia bovis merozoites visualized by time-lapse video microscopy. PLoS ONE. 2012;7 doi: 10.1371/journal.pone.0035227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacchi C.J., Nathan H.C., Hutner S.H., Duch D.S., Nichol C.A. Prevention by polyamines of the curative effect of amicarbalide and imidocarb for Trypanosoma brucei infections in mice. Biochem. Pharmacol. 1981;30:883–886. doi: 10.1016/s0006-2952(81)80011-1. [DOI] [PubMed] [Google Scholar]
- Barbet A.F., Lundgren A., Yi J., Rurangirwa F.R., Palmer G.H. Antigenic variation of Anaplasma marginale by expression of MSP2 mosaics. Infect. Immun. 2000;68:6133–6138. doi: 10.1128/iai.68.11.6133-6138.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barfod L., Dalgaard M.B., Pleman S.T., Ofori M.F., Pleass R.J., Hviid L. Evasion of immunity to Plasmodium falciparum malaria by IgM masking of protective IgG epitopes in infected erythrocyte surface-exposed PfEMP1. Proc. Natl. Acad. Sci. USA. 2011;108:12485–12490. doi: 10.1073/pnas.1103708108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastos R.G., Suarez C.E., Laughery J.M., Johnson W.C., Ueti M.W., Knowles D.P. Differential expression of three members of the multidomain adhesion CCp family in Babesia bigemina, Babesia bovis and Theileria equi. PLoS ONE. 2013;8 doi: 10.1371/journal.pone.0067765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berens S.J., Brayton K.A., Molloy J.B., Bock R.E., Lew A.E., McElwain T.F. Merozoite surface antigen 2 proteins of Babesia bovis vaccine breakthrough isolates contain a unique hypervariable region composed of degenerate repeats. Infect. Immun. 2005;73:7180–7189. doi: 10.1128/IAI.73.11.7180-7189.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beugnet F., Moreau Y. Babesiosis. Rev. Sci. Tech. (International Office of Epizootics) 2015;34:627–639. doi: 10.20506/rst.34.2.2385. [DOI] [PubMed] [Google Scholar]
- Biggs B.A., Goozé L., Wycherley K., Wollish W., Southwell B., Leech J.H., Brown G.V. Antigenic variation in Plasmodium falciparum. Proc. Natl. Acad. Sci. USA. 1991;88:9171–9174. doi: 10.1073/pnas.88.20.9171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boddey J.A., Hodder A.N., Günther S., Gilson P.R., Patsiouras H., Kapp E.A., Pearce J.A., de Koning-Ward T.F., Simpson R.J., Crabb B.S., Cowman A.F. An aspartyl protease directs malaria effector proteins to the host cell. Nature. 2010;463:627. doi: 10.1038/nature08728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohaliga G.A.R., Johnson W.C., Taus N.S., Hussein H.E., Bastos R.G., Suarez C.E., O'Connor R., Ueti M.W. Identification of a putative methyltransferase gene of Babesia bigemina as a novel molecular biomarker uniquely expressed in parasite tick stages. Parasit. Vectors. 2018;11:480. doi: 10.1186/s13071-018-3052-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman D.D. Successful and currently ongoing parasite eradication programs. Vet. Parasitol. 2006;139:293–307. doi: 10.1016/j.vetpar.2006.04.020. [DOI] [PubMed] [Google Scholar]
- Brayton K.A., Lau A.O., Herndon D.R., Hannick L., Kappmeyer L.S., Berens S.J., Bidwell S.L., Brown W.C., Crabtree J., Fadrosh D., Feldblum T., Forberger H.A., Haas B.J., Howell J.M., Khouri H., Koo H., Mann D.J., Norimine J., Paulsen I.T., Radune D., Ren Q., Smith R.K., Jr., Suarez C.E., White O., Wortman J.R., Knowles D.P., Jr., McElwain T.F., Nene V.M. Genome sequence of Babesia bovis and comparative analysis of apicomplexan hemoprotozoa. PLoS Path. 2007;3:1401–1413. doi: 10.1371/journal.ppat.0030148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown W.C. Molecular approaches to elucidating innate and acquired immune responses to Babesia bovis, a protozoan parasite that causes persistent infection. Vet. Parasitol. 2001;101:233–248. doi: 10.1016/s0304-4017(01)00569-6. [DOI] [PubMed] [Google Scholar]
- Brown W.C., Norimine J., Knowles D.P., Goff W.L. Immune control of Babesia bovis infection. Vet. Parasitol. 2006;138:75–87. doi: 10.1016/j.vetpar.2006.01.041. [DOI] [PubMed] [Google Scholar]
- Brown W.C., Palmer G.H. Designing blood-stage vaccines against Babesia bovis and B. bigemina. Parasitol. Today (Personal ed) 1999;15:275–281. doi: 10.1016/s0169-4758(99)01471-4. [DOI] [PubMed] [Google Scholar]
- Camacho-Nuez M., Hernández-Silva D.J., Castañeda-Ortiz E.J., Paredes-Martínez M.E., Rocha-Martínez M.K., Alvarez-Sánchez M.E., Mercado-Curiel R.F., Aguilar-Tipacamu G., Mosqueda J. Hap2, a novel gene in Babesia bigemina is expressed in tick stages, and specific antibodies block zygote formation. Parasit. Vectors. 2017;10:568. doi: 10.1186/s13071-017-2510-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carcy B., Precigout E., Schetters T., Gorenflot A. Genetic basis for GPI-anchor merozoite surface antigen polymorphism of Babesia and resulting antigenic diversity. Vet. Parasitol. 2006;138:33–49. doi: 10.1016/j.vetpar.2006.01.038. [DOI] [PubMed] [Google Scholar]
- Cen-Aguilar J.F., Rodriguez-Vivas R.I., Dominguez-Alpizar J.L., Wagner G.G. Studies on the effect of infection by Babesia sp. on oviposition of Boophilus microplus engorged females naturally infected in the Mexican tropics. Vet. Parasitol. 1998;78:253–257. doi: 10.1016/s0304-4017(98)00148-4. [DOI] [PubMed] [Google Scholar]
- Cerede O., Dubremetz J.F., Soete M., Deslee D., Vial H., Bout D., Lebrun M. Synergistic role of micronemal proteins in Toxoplasma gondii virulence. J. Exp. Med. 2005;201:453–463. doi: 10.1084/jem.20041672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauvin A., Moreau E., Bonnet S., Plantard O., Malandrin L. Babesia and its hosts: adaptation to long-lasting interactions as a way to achieve efficient transmission. Vet. Res. 2009;40:37. doi: 10.1051/vetres/2009020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark I.A., Jacobson L.S. Do babesiosis and malaria share a common disease process? Ann. Trop. Med. Parasitol. 1998;92:483–488. doi: 10.1080/00034989859456. [DOI] [PubMed] [Google Scholar]
- Coldham N.G., Moore A.S., Dave M., Graham P.J., Sivapathasundaram S., Lake B.G., Sauer M.J. Imidocarb residues in edible bovine tissues and in vitro assessment of imidocarb metabolism and cytotoxicity. Drug Metab. Dispos. 1995;23:501–505. [PubMed] [Google Scholar]
- Crabb B.S., Cooke B.M., Reeder J.C., Waller R.F., Caruana S.R., Davern K.M., Wickham M.E., Brown G.V., Coppel R.L., Cowman A.F. Targeted gene disruption shows that knobs enable malaria-infected red cells to cytoadhere under physiological shear stress. Cell. 1997;89:287–296. doi: 10.1016/s0092-8674(00)80207-x. [DOI] [PubMed] [Google Scholar]
- Dantas-Torres F. Climate change, biodiversity, ticks and tick-borne diseases: the butterfly effect. Int. J. Parasitol. Parasites Wildl. 2015;4:452–461. doi: 10.1016/j.ijppaw.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vos A.J., Bock R.E. Vaccination against bovine babesiosis. Ann. N. Y. Acad. Sci. 2000;916:540–545. doi: 10.1111/j.1749-6632.2000.tb05333.x. [DOI] [PubMed] [Google Scholar]
- de Vos A.J., Stewart N.P., Dalgliesh R.J. Effect of different methods of maintenance on the pathogenicity and infectivity of Babesia bigemina for the vector Boophilus microplus. Res. Vet. Sci. 1989;46:139–142. [PubMed] [Google Scholar]
- Delrieu I., Waller C.C., Mota M.M., Grainger M., Langhorne J., Holder A.A. PSLAP, a protein with multiple adhesive motifs, is expressed in Plasmodium falciparum gametocytes. Mol. Biochem. Parasitol. 2002;121:11–20. doi: 10.1016/s0166-6851(02)00016-6. [DOI] [PubMed] [Google Scholar]
- Echaide I.E., Hines S.A., McElwain T.F., Suarez C.E., McGuire T.C., Palmer G.H. In vivo binding of immunoglobulin M to the surfaces of Babesia bigemina-infected erythrocytes. Infect. Immun. 1998;66:2922–2927. doi: 10.1128/iai.66.6.2922-2927.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eksi S., Czesny B., van Gemert G.J., Sauerwein R.W., Eling W., Williamson K.C. Malaria transmission-blocking antigen, Pfs230, mediates human red blood cell binding to exflagellating male parasites and oocyst production. Mol. Microbiol. 2006;61:991–998. doi: 10.1111/j.1365-2958.2006.05284.x. [DOI] [PubMed] [Google Scholar]
- Estes D.M., Brown W.C. The type 1/type 2 paradigm and regulation of humoral immune responses in cattle. Vet. Immunol. Immunopathol. 2002;90:10. doi: 10.1016/s0165-2427(02)00201-5. [DOI] [PubMed] [Google Scholar]
- Ferreri L.M., Brayton K.A., Sondgeroth K.S., Lau A.O.T., Suarez C.E., McElwain T.F. Expression and strain variation of the novel “small open reading frame” (smorf) multigene family in Babesia bovis. Int. J. Parasitol. 2012;42:131–138. doi: 10.1016/j.ijpara.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fichera M.E., Roos D.S. A plastid organelle as a drug target in apicomplexan parasites. Nature. 1997;390:407–409. doi: 10.1038/37132. [DOI] [PubMed] [Google Scholar]
- Fisher T.G., McElwain T.F., Palmer G.H. Molecular basis for variable expression of merozoite surface antigen gp45 among American isolates of Babesia bigemina. Infect. Immun. 2001;69:3782–3790. doi: 10.1128/IAI.69.6.3782-3790.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florin-Christensen J., Suarez C.E., Florin-Christensen M., Hines S.A., McElwain T.F., Palmer G.H. Phosphatidylcholine formation is the predominant lipid biosynthetic event in the hemoparasite Babesia bovis. Mol. Biochem. Parasitol. 2000;106:147–156. doi: 10.1016/s0166-6851(99)00209-1. [DOI] [PubMed] [Google Scholar]
- Florin-Christensen M., Schnittger L. Piroplasmids and ticks: a long-lasting intimate relationship. Front. Biosci. (Landmark Ed.) 2009;1(14):3064–3073. doi: 10.2741/3435. [DOI] [PubMed] [Google Scholar]
- Florin-Christensen M., Suarez C.E., Hines S.A., Palmer G.H., Brown W.C., McElwain T.F. The Babesia bovis merozoite surface antigen 2 locus contains four tandemly arranged and expressed genes encoding immunologically distinct proteins. Infect. Immun. 2002;70:3566–3575. doi: 10.1128/IAI.70.7.3566-3575.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florin-Christensen M., Suarez C.E., Rodriguez A.E., Flores D.A., Schnittger L. Vaccines against bovine babesiosis: where we are now and possible roads ahead. Parasitology. 2014;141:1563–1592. doi: 10.1017/S0031182014000961. [DOI] [PubMed] [Google Scholar]
- Gaffar F.R., Yatsuda A.P., Franssen F.F., de Vries E. A Babesia bovis merozoite protein with a domain architecture highly similar to the thrombospondin-related anonymous protein (TRAP) present in Plasmodium sporozoites. Mol. Biochem. Parasitol. 2004;136:25–34. doi: 10.1016/j.molbiopara.2004.02.006. [DOI] [PubMed] [Google Scholar]
- Gaffar F.R., Yatsuda A.P., Franssen F.F., de Vries E. Erythrocyte invasion by Babesia bovis merozoites is inhibited by polyclonal antisera directed against peptides derived from a homologue of Plasmodium falciparum apical membrane antigen 1. Infect. Immun. 2004;72:2947–2955. doi: 10.1128/IAI.72.5.2947-2955.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale K.R., Waltisbuhl D.J., Bowden J.M., Jorgensen W.K., Matheson J., East I.J., Zakrzewski H., Leatch G. Amelioration of virulent Babesia bovis infection in calves by administration of the nitric oxide synthase inhibitor aminoguanidine. Parasite Immunol. 1998;20:441–445. doi: 10.1046/j.1365-3024.1998.00169.x. [DOI] [PubMed] [Google Scholar]
- Gallego-Lopez G.M., Lau A.O.T., Brown W.C., Johnson W.C., Ueti M.W., Suarez C.E. Spherical body protein 2 truncated copy 11 as a specific Babesia bovis attenuation marker. Parasit. Vectors. 2018;11:169. doi: 10.1186/s13071-018-2782-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner M.J., Hall N., Fung E., White O., Berriman M., Hyman R.W., Carlton J.M., Pain A., Nelson K.E., Bowman S., Paulsen I.T., James K., Eisen J.A., Rutherford K., Salzberg S.L., Craig A., Kyes S., Chan M.S., Nene V., Shallom S.J., Suh B., Peterson J., Angiuoli S., Pertea M., Allen J., Selengut J., Haft D., Mather M.W., Vaidya A.B., Martin D.M., Fairlamb A.H., Fraunholz M.J., Roos D.S., Ralph S.A., McFadden G.I., Cummings L.M., Subramanian G.M., Mungall C., Venter J.C., Carucci D.J., Hoffman S.L., Newbold C., Davis R.W., Fraser C.M., Barrell B. Genome sequence of the human malaria parasite Plasmodium falciparum. Nature. 2002;419:498–511. doi: 10.1038/nature01097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goff W.L., Bastos R.G., Brown W.C., Johnson W.C., Schneider D.A. The bovine spleen: interactions among splenic cell populations in the innate immunologic control of hemoparasitic infections. Vet. Immunol. Immunopathol. 2010;138:1–14. doi: 10.1016/j.vetimm.2010.07.006. [DOI] [PubMed] [Google Scholar]
- Goff W.L., Johnson W.C., Cluff C.W. Babesia bovis immunity. In vitro and in vivo evidence for IL-10 regulation of IFN-gamma and iNOS. Ann. N. Y. Acad. Sci. 1998;849:161–180. doi: 10.1111/j.1749-6632.1998.tb11046.x. [DOI] [PubMed] [Google Scholar]
- Goff W.L., Johnson W.C., Horn R.H., Barrington G.M., Knowles D.P. The innate immune response in calves to Boophilus microplus tick transmitted Babesia bovis involves type-1 cytokine induction and NK-like cells in the spleen. Parasite Immunol. 2003;25:185–188. doi: 10.1046/j.1365-3024.2003.00625.x. [DOI] [PubMed] [Google Scholar]
- Goff W.L., Johnson W.C., Parish S.M., Barrington G.M., Elsasser T.H., Davis W.C., Valdez R.A. IL-4 and IL-10 inhibition of IFN-gamma- and TNF-alpha-dependent nitric oxide production from bovine mononuclear phagocytes exposed to Babesia bovis merozoites. Vet. Immunol. Immunopathol. 2002;84:237–251. doi: 10.1016/s0165-2427(01)00413-5. [DOI] [PubMed] [Google Scholar]
- Goff W.L., Johnson W.C., Parish S.M., Barrington G.M., Tuo W., Valdez R.A. The age-related immunity in cattle to Babesia bovis infection involves the rapid induction of interleukin-12, interferon-gamma and inducible nitric oxide synthase mRNA expression in the spleen. Parasite Immunol. 2001;23:463–471. doi: 10.1046/j.1365-3024.2001.00402.x. [DOI] [PubMed] [Google Scholar]
- Goff W.L., Johnson W.C., Tuo W., Valdez R.A., Parish S.M., Barrington G.M., Davis W.C. Age-related innate immune response in calves to Babesia bovis involves IL-12 induction and IL-10 modulation. Ann. N. Y. Acad. Sci. 2002;969:164–168. doi: 10.1111/j.1749-6632.2002.tb04371.x. [DOI] [PubMed] [Google Scholar]
- Gohil S., Herrmann S., Gunther S., Cooke B.M. Bovine babesiosis in the 21st century: advances in biology and functional genomics. Int. J. Parasitol. 2013;43:125–132. doi: 10.1016/j.ijpara.2012.09.008. [DOI] [PubMed] [Google Scholar]
- Gohil S., Kats L.M., Sturm A., Cooke B.M. Recent insights into alteration of red blood cells by Babesia bovis: moovin' forward. Trends Parasitol. 2010;26:591–599. doi: 10.1016/j.pt.2010.06.012. [DOI] [PubMed] [Google Scholar]
- Gray M.W., Burger G., Lang B.F. Mitochondrial evolution. Science. 1999;283:1476–1481. doi: 10.1126/science.283.5407.1476. [DOI] [PubMed] [Google Scholar]
- Gubbels M.-J., Duraisingh M.T. Evolution of apicomplexan secretory organelles. Int. J. Parasitol. 2012;42:1071–1081. doi: 10.1016/j.ijpara.2012.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guswanto A., Nugraha A.B., Tuvshintulga B., Tayebwa D.S., Rizk M.A., Batiha G.E., Gantuya S., Sivakumar T., Yokoyama N., Igarashi I. 17-DMAG inhibits the multiplication of several Babesia species and Theileria equi on in vitro cultures, and Babesia microti in mice. Int. J. Parasitol. Drugs Drug Resist. 2018;8:104–111. doi: 10.1016/j.ijpddr.2018.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He L., Liu Q., Yao B., Zhou Y., Hu M., Fang R., Zhao J. A historical overview of research on Babesia orientalis, a protozoan parasite infecting water buffalo. Front. Microbiol. 2017;8:1323. doi: 10.3389/fmicb.2017.01323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiller N.L., Bhattacharjee S., van Ooij C., Liolios K., Harrison T., Lopez-Estrano C., Haldar K. A host-targeting signal in virulence proteins reveals a secretome in malarial infection. Science. 2004;306:1934–1937. doi: 10.1126/science.1102737. [DOI] [PubMed] [Google Scholar]
- Hines S.A., Palmer G.H., Jasmer D.P., McGuire T.C., McElwain T.F. Neutralization-sensitive merozoite surface antigens of Babesia bovis encoded by members of a polymorphic gene family. Mol. Biochem. Parasitol. 1992;55:85–94. doi: 10.1016/0166-6851(92)90129-8. [DOI] [PubMed] [Google Scholar]
- Homer M.J., Aguilar-Delfin I., Telford S.R., 3rd, Krause P.J., Persing D.H. Babesiosis. Clin. Microbiol. Rev. 2000;13:451–469. doi: 10.1128/cmr.13.3.451-469.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussein H.E., Bastos R.G., Schneider D.A., Johnson W.C., Adham F.K., Davis W.C., Laughery J.M., Herndon D.R., Alzan H.F., Ueti M.W. The Babesia bovis hap2 gene is not required for blood stage replication, but expressed upon in vitro sexual stage induction. PLoS Negl. Trop. Dis. 2017;11 doi: 10.1371/journal.pntd.0005965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchings C.L., Li A., Fernandez K.M., Fletcher T., Jackson L.A., Molloy J.B., Jorgensen W.K., Lim C.T., Cooke B.M. New insights into the altered adhesive and mechanical properties of red blood cells parasitized by Babesia bovis. Mol. Microbiol. 2007;65:1092–1105. doi: 10.1111/j.1365-2958.2007.05850.x. [DOI] [PubMed] [Google Scholar]
- Jackson A.P., Otto T.D., Darby A., Ramaprasad A., Xia D., Echaide I.E., Farber M., Gahlot S., Gamble J., Gupta D., Jackson L., Malandrin L., Malas T.B., Moussa E., Nair M., Reid A.J., Sanders M., Sharma J., Tracey A., Quail M.A., Weir W., Wastling J.M., Hall N., Willadsen P., Lingelbach K., Shiels B., Tait A., Berriman M., Allred D.R., Pain A. The evolutionary dynamics of variant antigen genes in Babesia reveal a history of genomic innovation underlying host-parasite interaction. Nucleic Acids Res. 2014;42:7113–7131. doi: 10.1093/nar/gku322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson W.C., Taus N.S., Reif K.E., Bohaliga G.A., Kappmeyer L.S., Ueti M.W. Analysis of stage-specific protein expression during Babesia bovis development within female Rhipicephalus microplus. J. Proteome Res. 2017;16:1327–1338. doi: 10.1021/acs.jproteome.6b00947. [DOI] [PubMed] [Google Scholar]
- Kemp L.E., Yamamoto M., Soldati-Favre D. Subversion of host cellular functions by the apicomplexan parasites. FEMS Microbiol. Rev. 2013;37:607–631. doi: 10.1111/1574-6976.12013. [DOI] [PubMed] [Google Scholar]
- Kuttler K.L. Chemotherapy of babesiosis: a review. In: Ristic M., Kreier J.P., editors. Babesiosis. Academic press; New York: 1981. pp. 65–85. [Google Scholar]
- Kuvardina O.N., Leander B.S., Aleshin V.V., Myl'nikov A.P., Keeling P.J., Simdyanov T.G. The phylogeny of colpodellids (Alveolata) using small subunit rRNA gene sequences suggests they are the free-living sister group to apicomplexans. J. Eukaryot. Microbiol. 2002;49:498–504. doi: 10.1111/j.1550-7408.2002.tb00235.x. [DOI] [PubMed] [Google Scholar]
- Lau A.O., Cereceres K., Palmer G.H., Fretwell D.L., Pedroni M.J., Mosqueda J., McElwain T.F. Genotypic diversity of merozoite surface antigen 1 of Babesia bovis within an endemic population. Mol. Biochem. Parasitol. 2010;172:107–112. doi: 10.1016/j.molbiopara.2010.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau A.O., Kalyanaraman A., Echaide I., Palmer G.H., Bock R., Pedroni M.J., Rameshkumar M., Ferreira M.B., Fletcher T.I., McElwain T.F. Attenuation of virulence in an apicomplexan hemoparasite results in reduced genome diversity at the population level. BMC Genomics. 2011;12:410. doi: 10.1186/1471-2164-12-410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavazec C., Moreira C.K., Mair G.R., Waters A.P., Janse C.J., Templeton T.J. Analysis of mutant Plasmodium berghei parasites lacking expression of multiple PbCCp genes. Mol. Biochem. Parasitol. 2009;163:1–7. doi: 10.1016/j.molbiopara.2008.09.002. [DOI] [PubMed] [Google Scholar]
- Lawres L.A., Garg A., Kumar V., Bruzual I., Forquer I.P., Renard I., Virji A.Z., Boulard P., Rodriguez E.X., Allen A.J., Pou S., Wegmann K.W., Winter R.W., Nilsen A., Mao J., Preston D.A., Belperron A.A., Bockenstedt L.K., Hinrichs D.J., Riscoe M.K., Doggett J.S., Ben Mamoun C. Radical cure of experimental babesiosis in immunodeficient mice using a combination of an endochin-like quinolone and atovaquone. J. Exp. Med. 2016;213:1307–1318. doi: 10.1084/jem.20151519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leander B.S., Kuvardina O.N., Aleshin V.V., Mylnikov A.P., Keeling P.J. Molecular phylogeny and surface morphology of Colpodella edax (Alveolata): insights into the phagotrophic ancestry of apicomplexans. J. Eukaryot. Microbiol. 2003;50:334–340. doi: 10.1111/j.1550-7408.2003.tb00145.x. [DOI] [PubMed] [Google Scholar]
- Leroith T., Brayton K.A., Molloy J.B., Bock R.E., Hines S.A., Lew A.E., McElwain T.F. Sequence variation and immunologic cross-reactivity among Babesia bovis merozoite surface antigen 1 proteins from vaccine strains and vaccine breakthrough isolates. Infect. Immun. 2005;73:5388–5394. doi: 10.1128/IAI.73.9.5388-5394.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy M.G., Clabaugh G., Ristic M. Age resistance in bovine babesiosis: role of blood factors in resistance to Babesia bovis. Infect. Immun. 1982;37:1127–1131. doi: 10.1128/iai.37.3.1127-1131.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Child M.A., Bogyo M. Proteases as regulators of pathogenesis: examples from the Apicomplexa. Biochim. Biophys. Acta. 2012;1824:177–185. doi: 10.1016/j.bbapap.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobo C.A., Cursino-Santos J.R., Alhassan A., Rodrigues M. Babesia: an emerging infectious threat in transfusion medicine. PLoS Path. 2013;9 doi: 10.1371/journal.ppat.1003387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahoney D.F. In: The Ruminant Immune System in Health and Disease. Morrison W.I., editor. Cambridge University Press; Cambridge: 1986. pp. 539–545. [Google Scholar]
- Marti M., Good R.T., Rug M., Knuepfer E., Cowman A.F. Targeting malaria virulence and remodeling proteins to the host erythrocyte. Science. 2004;306:1930–1933. doi: 10.1126/science.1102452. [DOI] [PubMed] [Google Scholar]
- Mathur V., Del Campo J., Kolisko M., Keeling P.J. Global diversity and distribution of close relatives of apicomplexan parasites. Environ. Microbiol. 2018;20:2824–2833. doi: 10.1111/1462-2920.14134. [DOI] [PubMed] [Google Scholar]
- McHardy N., Woollon R.M., Clampitt R.B., James J.A., Crawley R.J. Efficacy, toxicity and metabolism of imidocarb dipropionate in the treatment of Babesia ovis infection in sheep. Res. Vet. Sci. 1986;41:14–20. [PubMed] [Google Scholar]
- McRobert L., Preiser P., Sharp S., Jarra W., Kaviratne M., Taylor M.C., Renia L., Sutherland C.J. Distinct trafficking and localization of STEVOR proteins in three stages of the Plasmodium falciparum life cycle. Infect. Immun. 2004;72:6597–6602. doi: 10.1128/IAI.72.11.6597-6602.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehlhorn H., Shein E. The piroplasms: life cycle and sexual stages. Adv. Parasitol. 1984;23:37–103. doi: 10.1016/s0065-308x(08)60285-7. [DOI] [PubMed] [Google Scholar]
- Michelet L., Delannoy S., Devillers E., Umhang G., Aspan A., Juremalm M., Chirico J., van der Wal F.J., Sprong H., Boye Pihl T.P., Klitgaard K., Bødker R., Fach P., Moutailler S. High-throughput screening of tick-borne pathogens in Europe. Front. Cell. Infect. Microbiol. 2014;4:103. doi: 10.3389/fcimb.2014.00103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monzote L., Gille L. Mitochondria as a promising antiparasitic target. Curr Clin Pharmacol. 2010;5:55–60. doi: 10.2174/157488410790410605. [DOI] [PubMed] [Google Scholar]
- Mosqueda J., Falcon A., Antonio Alvarez J., Alberto Ramos J., Oropeza-Hernandez L.F., Figueroa J.V. Babesia bigemina sexual stages are induced in vitro and are specifically recognized by antibodies in the midgut of infected Boophilus microplus ticks. Int. J. Parasitol. 2004;34:1229–1236. doi: 10.1016/j.ijpara.2004.07.003. [DOI] [PubMed] [Google Scholar]
- Mosqueda J., Olvera-Ramírez A., Aguilar-Tipacamú G., Cantó G.J. Current advances in detection and treatment of babesiosis. Curr. Med. Chem. 2012;19:1504–1518. doi: 10.2174/092986712799828355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy A.D., Lang-Unnasch N. Alternative oxidase inhibitors potentiate the activity of atovaquone against Plasmodium falciparum. Antimicrob. Agents Chemother. 1999;43:651–654. doi: 10.1128/aac.43.3.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nott S.E., O'Sullivan W.J., Gero A.M., Bagnara A.S. Routine screening for potential babesicides using cultures of Babesia bovis. Int. J. Parasitol. 1990;20:797–802. doi: 10.1016/0020-7519(90)90014-e. [DOI] [PubMed] [Google Scholar]
- Painter H.J., Campbell T.L., Llinas M. The apicomplexan AP2 family: integral factors regulating Plasmodium development. Mol. Biochem. Parasitol. 2011;176:1–7. doi: 10.1016/j.molbiopara.2010.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedroni M.J., Sondgeroth K.S., Gallego-Lopez G.M., Echaide I., Lau A.O. Comparative transcriptome analysis of geographically distinct virulent and attenuated Babesia bovis strains reveals similar gene expression changes through attenuation. BMC Genomics. 2013;14:763. doi: 10.1186/1471-2164-14-763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedroni M.J., Vidadala R.S., Choi R., Keyloun K.R., Reid M.C., Murphy R.C., Barrett L.K., Van Voorhis W.C., Maly D.J., Ojo K.K., Lau A.O.T. Bumped kinase inhibitor prohibits egression in Babesia bovis. Vet. Parasitol. 2016;215:22–28. doi: 10.1016/j.vetpar.2015.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegram R.G., Wilson D.D., Hansen J.W. Past and present national tick control programs. Why they succeed or fail. Ann. N. Y. Acad. Sci. 2000;916:546–554. doi: 10.1111/j.1749-6632.2000.tb05334.x. [DOI] [PubMed] [Google Scholar]
- Peregrine A.S., Mamman M. Pharmacology of diminazene: a review. Acta Trop. 1993;54:185–203. doi: 10.1016/0001-706x(93)90092-p. [DOI] [PubMed] [Google Scholar]
- Pieszko M., Weir W., Goodhead I., Kinnaird J., Shiels B. ApiAP2 factors as candidate regulators of stochastic commitment to merozoite production in Theileria annulata. PLoS Negl. Trop. Dis. 2015;9 doi: 10.1371/journal.pntd.0003933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potgieter F.T., Els H.J. Light and electron microscopic observations on the development of small merozoites of Babesia bovis in Boophilus microplus larvae. Onderstepoort J. Vet. Res. 1976;43:123–128. [PubMed] [Google Scholar]
- Pradel G., Hayton K., Aravind L., Iyer L.M., Abrahamsen M.S., Bonawitz A., Mejia C., Templeton T.J. A multidomain adhesion protein family expressed in Plasmodium falciparum is essential for transmission to the mosquito. J. Exp. Med. 2004;199:1533–1544. doi: 10.1084/jem.20031274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchard J.R., Bruno P.M., Gilbert L.A., Capron K.L., Lauffenburger D.A., Hemann M.T. Defining principles of combination drug mechanisms of action. Proc. Natl. Acad. Sci. USA. 2013;110:E170–E179. doi: 10.1073/pnas.1210419110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pudney M., Gray J.S. Therapeutic efficacy of atovaquone against the bovine intraerythrocytic parasite, Babesia divergens. J. Parasitol. 1997;83:307–310. [PubMed] [Google Scholar]
- Repnik U., Gangopadhyay P., Bietz S., Przyborski J.M., Griffiths G., Lingelbach K. The apicomplexan parasite Babesia divergens internalizes band 3, glycophorin A and spectrin during invasion of human red blood cells. Cell. Microbiol. 2015;17:1052–1068. doi: 10.1111/cmi.12422. [DOI] [PubMed] [Google Scholar]
- Rizk M.A., El-Sayed S.A.E., AbouLaila M., Yokoyama N., Igarashi I. Evaluation of the inhibitory effect of N-acetyl-l-cysteine on Babesia and Theileria parasites. Exp. Parasitol. 2017;179:43–48. doi: 10.1016/j.exppara.2017.06.003. [DOI] [PubMed] [Google Scholar]
- Rozej-Bielicka W., Stypulkowska-Misiurewicz H., Golab E. Human babesiosis. Przegl. Epidemiol. 2015;69:605–608. [PubMed] [Google Scholar]
- Rudzinska M.A., Trager W., Lewengrub S.J., Gubert E. An electron microscopic study of Babesia microti invading erythrocytes. Cell Tissue Res. 1976;169:323–334. doi: 10.1007/BF00219605. [DOI] [PubMed] [Google Scholar]
- Russo I., Babbitt S., Muralidharan V., Butler T., Oksman A., Goldberg D.E. Plasmepsin V licenses Plasmodium proteins for export into the host erythrocyte. Nature. 2010;463:632. doi: 10.1038/nature08726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanyal S., Egée S., Bouyer G., Perrot S., Safeukui I., Bischoff E., Buffet P., Deitsch K.W., Mercereau-Puijalon O., David P.H., Templeton T.J., Lavazec C. Plasmodium falciparum STEVOR proteins impact erythrocyte mechanical properties. Blood. 2012;119:e1–e8. doi: 10.1182/blood-2011-08-370734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider D.A., Yan H., Bastos R.G., Johnson W.C., Gavin P.R., Allen A.J., Barrington G.M., Herrmann-Hoesing L.M., Knowles D.P., Goff W.L. Dynamics of bovine spleen cell populations during the acute response to Babesia bovis infection: an immunohistological study. Parasite Immunol. 2011;33:34–44. doi: 10.1111/j.1365-3024.2010.01249.x. [DOI] [PubMed] [Google Scholar]
- Scudiero L., Mercado-Rojano W.J., Rudolph A., Wang J., Laughery J.M., Suarez C.E. Comparisons of the topographic characteristics and electrical charge distributions among Babesia-infected erythrocytes and extraerythrocytic merozoites using AFM. J. Microsc. 2018;271:84–97. doi: 10.1111/jmi.12697. [DOI] [PubMed] [Google Scholar]
- Shoda L.K., Palmer G.H., Florin-Christensen J., Florin-Christensen M., Godson D.L., Brown W.C. Babesia bovis-stimulated macrophages express interleukin-1beta, interleukin-12, tumor necrosis factor alpha, and nitric oxide and inhibit parasite replication in vitro. Infect. Immun. 2000;68:5139–5145. doi: 10.1128/iai.68.9.5139-5145.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva M.G., Domingos A., Esteves M.A., Cruz M.E., Suarez C.E. Evaluation of the growth-inhibitory effect of trifluralin analogues on in vitro cultured Babesia bovis parasites. Int. J. Parasitol. Drugs Drug Resist. 2013;3:59–68. doi: 10.1016/j.ijpddr.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva M.G., Knowles D.P., Mazuz M.L., Cooke B.M., Suarez C.E. Stable transformation of Babesia bigemina and Babesia bovis using a single transfection plasmid. Sci. Rep. 2018;8:6096. doi: 10.1038/s41598-018-23010-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva M.G., Knowles D.P., Suarez C.E. Identification of interchangeable cross-species function of elongation factor-1 alpha promoters in Babesia bigemina and Babesia bovis. Parasit. Vectors. 2016;9:576. doi: 10.1186/s13071-016-1859-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva M.G., Ueti M.W., Norimine J., Florin-Christensen M., Bastos R.G., Goff W.L., Brown W.C., Oliva A., Suarez C.E. Babesia bovis expresses Bbo-6cys-E, a member of a novel gene family that is homologous to the 6-cys family of Plasmodium. Parasitol. Int. 2011;60:13–18. doi: 10.1016/j.parint.2010.09.004. [DOI] [PubMed] [Google Scholar]
- Silva M.G., Villarino N.F., Knowles D.P., Suarez C.E. Assessment of Draxxin((R)) (tulathromycin) as an inhibitor of in vitro growth of Babesia bovis, Babesia bigemina and Theileria equi. Int J Parasitol Drugs Drug Resist. 2018;8:265–270. doi: 10.1016/j.ijpddr.2018.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon N., Scholz S.M., Moreira C.K., Templeton T.J., Kuehn A., Dude M.A., Pradel G. Sexual stage adhesion proteins form multi-protein complexes in the malaria parasite Plasmodium falciparum. J. Biol. Chem. 2009;284:14537–14546. doi: 10.1074/jbc.M808472200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivakumar T., Igarashi I., Yokoyama N. Babesia ovata: Taxonomy, phylogeny and epidemiology. Vet. Parasitol. 2016;229:99–106. doi: 10.1016/j.vetpar.2016.10.006. [DOI] [PubMed] [Google Scholar]
- Sonenshine D.E. Range expansion of tick disease vectors in North America: implications for spread of tick-borne disease. Int. J. Environ. Res. Public Health. 2018;15(3):478. doi: 10.3390/ijerph15030478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soldati D., Foth B.J., Cowman A.F. Molecular and functional aspects of parasite invasion. Trends Parasitol. 2004;20:567–574. doi: 10.1016/j.pt.2004.09.009. [DOI] [PubMed] [Google Scholar]
- Suarez C.E., Bishop R.P., Alzan H.F., Poole W.A., Cooke B.M. Advances in the application of genetic manipulation methods to apicomplexan parasites. Int. J. Parasitol. 2017;47:701–710. doi: 10.1016/j.ijpara.2017.08.002. [DOI] [PubMed] [Google Scholar]
- Suarez C.E., Florin-Christensen M., Hines S.A., Palmer G.H., Brown W.C., McElwain T.F. Characterization of allelic variation in the Babesia bovis merozoite surface antigen 1 (MSA-1) locus and identification of a cross-reactive inhibition-sensitive MSA-1 epitope. Infect. Immun. 2000;68:6865–6870. doi: 10.1128/iai.68.12.6865-6870.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez C.E., Noh S. Emerging perspectives in the research of bovine babesiosis and anaplasmosis. Vet. Parasitol. 2011;180:109–125. doi: 10.1016/j.vetpar.2011.05.032. [DOI] [PubMed] [Google Scholar]
- Tayebwa D.S., Tuvshintulga B., Guswanto A., Nugraha A.B., Batiha G.E., Gantuya S., Rizk M.A., Vudriko P., Sivakumar T., Yokoyama N., Igarashi I. The effects of nitidine chloride and camptothecin on the growth of Babesia and Theileria parasites. Ticks Tick Borne Dis. 2018;9:1192–1201. doi: 10.1016/j.ttbdis.2018.04.019. [DOI] [PubMed] [Google Scholar]
- Tibúrcio M., Niang M., Deplaine G., Perrot S., Bischoff E., Ndour P.A., Silvestrini F., Khattab A., Milon G., David P.H., Hardeman M., Vernick K.D., Sauerwein R.W., Preiser P.R., Mercereau-Puijalon O., Buffet P., Alano P., Lavazec C. A switch in infected erythrocyte deformability at the maturation and blood circulation of Plasmodium falciparum transmission stages. Blood. 2012;119:e172–e180. doi: 10.1182/blood-2012-03-414557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trentelman J.J., Kleuskens J.A., van de Crommert J., Schetters T.P. A new method for in vitro feeding of Rhipicephalus australis (formerly Rhipicephalus microplus) larvae: a valuable tool for tick vaccine development. Parasit. Vectors. 2017;10:153. doi: 10.1186/s13071-017-2081-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuvshintulga B., AbouLaila M., Davaasuren B., Ishiyama A., Sivakumar T., Yokoyama N., Iwatsuki M., Otoguro K., Omura S., Igarashi I. Clofazimine inhibits the growth of babesia and theileria parasites in vitro and in vivo. Antimicrob. Agents Chemother. 2016;60:2739–2746. doi: 10.1128/AAC.01614-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuvshintulga B., AbouLaila M., Sivakumar T., Tayebwa D.S., Gantuya S., Naranbaatar K., Ishiyama A., Iwatsuki M., Otoguro K., Omura S., Terkawi M.A., Guswanto A., Rizk M.A., Yokoyama N., Igarashi I. Chemotherapeutic efficacies of a clofazimine and diminazene aceturate combination against piroplasm parasites and their AT-rich DNA-binding activity on Babesia bovis. Sci. Rep. 2017;7:13888. doi: 10.1038/s41598-017-14304-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uilenberg G. Babesia–a historical overview. Vet. Parasitol. 2006;138:3–10. doi: 10.1016/j.vetpar.2006.01.035. [DOI] [PubMed] [Google Scholar]
- Valle M.R., Guerrero F.D. Anti-tick vaccines in the omics era. Front. Biosci. (Elite Ed). 2018;10:122–136. doi: 10.2741/e812. [DOI] [PubMed] [Google Scholar]
- van der Giezen M. Mitochondria and the rise of eukaryotes. Bioscience. 2011;61:594–601. [Google Scholar]
- Vial H.J., Gorenflot A. Chemotherapy against babesiosis. Vet. Parasitol. 2006;138:147–160. doi: 10.1016/j.vetpar.2006.01.048. [DOI] [PubMed] [Google Scholar]
- Williamson K.C. Pfs230: from malaria transmission-blocking vaccine candidate toward function. Parasite Immunol. 2003;25:351–359. doi: 10.1046/j.1365-3024.2003.00643.x. [DOI] [PubMed] [Google Scholar]
- Wright I.G., Goodger B.V., Clark I.A. Immunopathophysiology of Babesia bovis and Plasmodium falciparum infections. Parasitol. Today (Personal ed) 1988;4:214–218. doi: 10.1016/0169-4758(88)90161-5. [DOI] [PubMed] [Google Scholar]
- Zintl A., Mulcahy G., Skerrett H.E., Taylor S.M., Gray J.S. Babesia divergens, a bovine blood parasite of veterinary and zoonotic importance. Clin. Microbiol. Rev. 2003;16:622–636. doi: 10.1128/CMR.16.4.622-636.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]