Abstract
Background
Although abnormal head and neck postures are defining features of cervical dystonia (CD), head tremor (HT) is also common. However, little is known about the relationship between abnormal postures and HT in CD.
Methods
We analyzed clinical data and video recordings from 185 patients enrolled by the Dystonia Coalition. We calculated the likelihood of their HT and HT type (“regular” vs. “jerky”) given directionality of abnormal head postures, disease duration, sex, and age.
Results
Patients with retrocollis were more likely to have HT than patients with anterocollis (X2 (1, N = 121) = 7.98, p = 0.005). There was no difference in HT likelihood given left or right turning in laterocollis and rotation. Patients with HT had longer disease duration (t(183) = 2.27, p = 0.024). There was no difference in age between patients with and without HT. In a logistic regression model, anterocollis/retrocollis direction (X2 (1, N = 121) = 6.04, p = 0.014), disease duration (X2 (1, N = 121) = 7.28, p = 0.007), and the interaction term between age and disease duration (X2 (1, N = 121) = 7.77, p = 0.005) collectively contributed to HT likelihood. None of the postural directionality or demographic variables were associated with differential likelihood of having regular versus jerky HT.
Discussion
We found that HT is more likely for CD patients with a specific directionality in their predominant posture. Our finding that CD patients with longer disease duration have a higher likelihood of HT also raises the question of whether HT becomes more likely over time in individual patients.
Keywords: Cervical dystonia, head tremor, posture, disease duration, tremor type
Introduction
Although abnormal head posture is a defining feature of cervical dystonia (CD), head tremor (HT) is also common,1 affecting more than half of CD patients.2,3 Both abnormal posture and HT lead to substantially reduced quality of life. The type of HT can be characterized as “jerky” or “regular.” Dystonic tremor often appears asymmetrical and jerky with a higher frequency, whereas a tremor that appears symmetrical with a regular typically lower frequency resembles essential tremor.4–6 Although tremor commonly occurs in CD, and abnormal posture and tremor usually occur in the same body region, the relationship between head posture and HT remains unclear. A in-depth understanding of how posture and tremor relate in CD may also shed light on their pathophysiological mechanisms. Furthermore, delineating this relationship may inform clinical management because chemodenervation strategies for dystonic posture may differ from treatment for tremor. In this study, we tested the hypotheses that the presence of HT and its type depend on a patient’s predominant posture. We defined predominant posture as the head position in its spontaneous natural dystonic state. Given the conventional view that the isolated focal dystonias, including CD, are usually nondegenerative disorders, we further hypothesized that the presence of HT and its type do not depend on disease duration or age.
Methods
We analyzed data collected from 208 patients with a clinical diagnosis of isolated CD enrolled across 10 sites in a previous rating scale validation study under the auspices of the Dystonia Coalition (https://clinicaltrials.gov/ct2/show/NCT01373424). All patients provided informed consent prior to their participation in the study. The protocols for original data collection and subsequent analyses were approved by the Human Research Protection Offices at the Washington University School of Medicine (WUSM), Rush University Medical Center (RUMC), and the University of California, San Diego (UCSD; protocol 111255X). All patients were assessed three or more months after their last BoNT injections. They were video-recorded during a standard examination protocol between March 2011 and January 2013. We calculated the duration patients had CD by subtracting their reported age of onset from their age at the time the protocol was administered. Movement disorders neurologists evaluated each patient using the revised Toronto Western Spasmodic Torticollis Rating Scale (TWSTRS-2) and further assessed the presence or absence of HT. If tremor was present, the investigator indicated whether the HT type was “regular” or “jerky.” The TWSTRS-2 Motor Severity scale included, among others, items that characterize deviation from a neutral head position in each of the three axes of rotation: pitch (“anterocollis” and “retrocollis”), roll (“laterocollis”), and yaw (“rotation”). Each of the four items was scored as none (0), slight (1), mild (2), moderate (3), or severe (4) deviation of head. All video recordings were also reviewed by Qiyu Chen (QC) to generate predominant directionality data not recorded in the TWSTRS Rotation and Laterocollis items. Predominant posture directionality in the roll and yaw axes was assessed by observing their head orientation during a step in the video protocol in which patients were seated in a chair without head support, feet resting on the floor, and instructed to let their head drift to its most comfortable (dystonic) position with eyes closed. In each of these axes, if the patient had a TWSTRS severity score of 0, their direction was considered neutral; otherwise the observed patient posture was identified as either left or right. Of the 208 patients, 20 patients were excluded due to missing data, 1 patient was excluded due to an ambiguous HT presence evaluation, and 2 patients were excluded because they had nonzero scores for both anterocollis and retrocollis.
We used chi-squared tests to examine the likelihood of having HT or type of HT given predominant postural direction. We used t-tests to evaluate differences in age, age of onset, and disease duration between patients with HT and without HT, and between patients with regular HT and jerky HT. We used nominal logistic regression to characterize the contribution of directionality in each of the three axes of rotation, along with age, disease duration, and the interaction term between age and disease duration to predict the presence of HT and the likelihood of having either regular or jerky HT. All statistical analysis was performed with John’s Macintosh Project (JMP)[JMP.13.0. ed.]. We used an alpha level of 0.05 to determine significance.
Results
In this study, we investigated a total of 185 patients, of which 117 had HT and 68 had no HT. Among the 117 patients with HT, all had postural abnormalities, 33 had regular HT, and 84 had jerky HT. There were no differences in age or proportion of female patients for those who had HT versus those who did not have HT (Table 1). Likewise, there was no difference in age or proportion of female patients for those who had a jerky HT versus who had a regular type of HT.
Table 1.
CD and Tremor: Demographics
| Head Tremor? | Head Tremor Type | |||||
|---|---|---|---|---|---|---|
| NO | Yes | Jerky | Regular | |||
| N | 68 | 117 | 84 | 33 | ||
| Age, in yrs | 58.1 +/- 10.7 | 60.6 +/- 10.1 | t =1.57 (.118) | 60.2 +/- 10.7 | 61.7 +/- 8.3 | t = 0.72 (.474) |
| Sex, in %F | 72% | 77% | X2 = 0.54 (.463) | 76% | 79% | X2 = 0.09 (.763) |
| (p values in parentheses) | ||||||
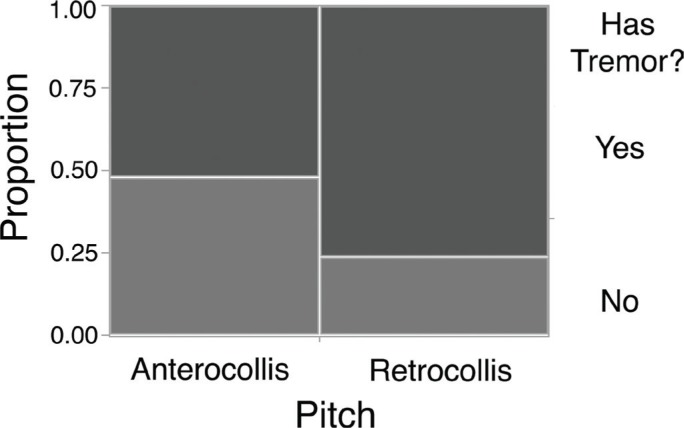
We investigated the relationship between HT and the predominant posture in each axis of head movement. In the pitch axis, we compared patients with predominantly anterocollis to patients with predominantly retrocollis (Figure 1). Patients with retrocollis were more likely to have HT than patients with anterocollis (X2 (1, N = 121) = 7.98, p = 0.005) (Figure 1). There was no difference in the likelihood of having a specific type of HT given pitch direction (X2 (1, N = 78) = 2.17, p = 0.141). In terms of laterocollis, we compared patients with predominantly left tilt to patients with predominantly right tilt. There was no difference in the likelihood of having HT (X2 (1, N = 155) = 3.05, p = 0.081) and the specific type of HT (X2 (1, N = 100) = 0.01, p = 0.918). In terms of rotation, we compared patients with predominantly left turn to patients with predominantly right turn. There was no difference in the likelihood of having HT (X2 (1, N = 169) = 0.22, p = 0.637) and the specific type of HT (X2 (1, N = 111) = 0.47, p = 0.495).
Figure 1.
Mosaic Plot Depicting Likelihood of Head Tremor in Regard to Pitch. The area of each rectangle is proportional to the number of patients.
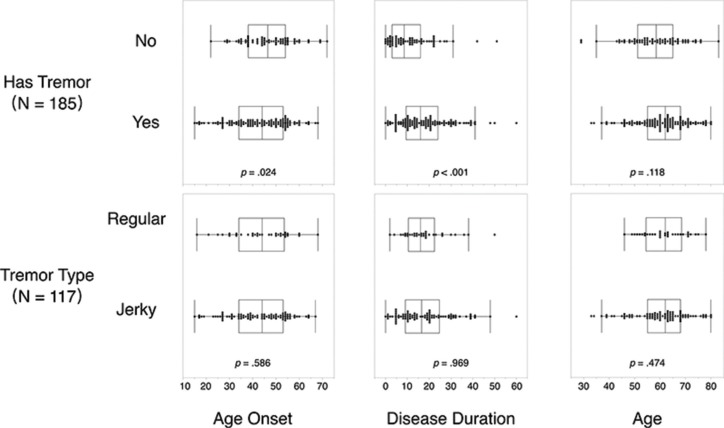
We also investigated the relationship between HT and patients’ age of onset and disease duration (Figure 2). Patients with HT (Mean [M] = 42.7, standard deviation [SD] = 12.4) had earlier age of onset compared to patients without HT (M = 46.8, SD = 10.6), t(183) = 2.27, p = 0.024. Age of onset of patients with regular HT (M = 43.7, SD =12.2) did not differ from patients with jerky HT (M=42.3, SD = 12.5), t(115) = 0.55, p = 0.586. Patients with HT (M = 17.9, SD = 11.6) had longer disease duration compared to patients without HT (M = 11.3, SD = 10.1), t(183) = -3.87, p < 0.001. Disease duration did not differ between patients with regular HT (M = 17.9, SD = 10.3) and those with jerky HT (M = 17.9, SD = 12.1), t(115) = 0.04, p = 0.969.
Figure 2.
Distribution of Age of Onset, Disease Duration, and Age for Patients without versus with Head Tremor (Upper), and for Head Tremor Patients Whose Head Tremor Type Was Regular versus Jerky. Each data point represents one patient.
We combined disease duration, age, and predominant posture direction as predictor variables in nominal logistic regressions to predict the presence and type (regular vs. jerky) of HT. We used separate regression models for each of the three axes of rotation: pitch (anterocollis and retrocollis), roll (laterocollis), and yaw (rotation). Pitch (X2 (1, N = 121) = 6.04, p = 0.014), disease duration (X2 (1, N = 121) = 7.28, p = 0.007), and the interaction term between disease duration and age (X2 (1, N = 121) = 7.77, p = 0.005) were significant predictors of the presence of HT. The specific type of HT was not associated with pitch, age, disease duration, or the interaction between age and disease duration. Laterocollis, age, and the interaction between age and disease duration were not predictive of the likelihood of having HT, but disease duration alone was (X2 (1, N = 155) = 10.65, p = 0.001). The specific type of HT was not associated with the presence of laterocollis, age, disease duration, or the interaction term between age and disease duration. Rotation, age, and the interaction term between age and disease duration were not predictive of the likelihood of having HT, but disease duration was (X2 (1, N = 169) = 14.93, p < 0.001). The likelihood of having a specific type of HT was not associated with rotation, age, disease duration, or the interaction term between age and disease duration.
Discussion
Posture
In this study we tested the hypothesis that the presence of HT and its type depend on a CD patient’s predominant posture. The proportion of our CD cohort that had HT (63%) was consistent with previous reports (57–68%).2, 3 We found that patients with retrocollis are more likely to have HT than patients with anterocollis. The reasons for this are unclear. There are anecdotal reports that pure anterocollis may represent an entity other than CD, and that those patients almost never have any HT. If that was the case, it could be influencing our finding that HT is more likely with retrocollis than with antercollis. However, two factors make this unlikely. First, one of our cohort inclusion criteria was isolated dystonia, making the inclusion of patients without isolated primary CD in our CD cohort extremely unlikely. Second, in a posthoc analysis, when we limited our anterocollis patients to only “pure” anterocollis (operationally defined as having severity ratings of less than 2 in both laterocollis and rotation), the majority (five out of seven) had HT. Therefore, the findings in our cohort do not support the concept that pure anterocollis is not associated with HT. Given the bilateral neck muscle involvement in anterocollis and retrocollis, HT in the context of those postures must involve asynchronous activity in left- and right-side muscles. It is curious that the likelihood of HT is higher for retrocollis than for anterocollis. It may be because of a greater number of opposing force vectors produced by a greater number of overactive muscle groups. Retrocollis involves a slightly higher number of muscle groups than anterocollis. Retrocollis involves bilateral dystonic contraction of semispinalis, upper trapezius, splenius capitis, longissimus capitis, and obliquus capitis. Antercollis involves bilateral longus colli, sternocleidomastoid, scalene complex, and digastric. It is also possible that the anterocollis versus retrocollis muscle groups are controlled by subtly different central motor networks. Some have proposed that HT associated with CD describes a subtype of CD with specific activation of different brain regions as reflected in animal models.7 Thus, we hypothesize that the subtly different central motor networks that control the muscles involved in retrocollis versus anterocollis are differentially susceptible to pathological oscillations.
Disease duration
Given the conventional view that the isolated focal dystonias, including CD, are usually nondegenerative disorders, we initially hypothesized that the presence of HT and its type do not depend on disease duration or age. However, our results indicate that HT is more likely for CD patients who have had longer disease duration. This is consistent with results from two other studies. In a study using the Dystonia Coalition database and including 1,068 CD patients,8 the 291 tremor-dominant patients had longer disease duration than nontremor-dominant CD (p < 0.001). In another smaller cohort of 25 CD patients, Antelmi et al.9 found that CD patients with tremor had longer average disease duration (17 years) than those without (14 years), although the difference was not statistically significant. Notably, 5 of the 13 CD patients with tremor in Antelmi et al.’s cohort exhibited arm tremor as well as HT. Collectively these cross-sectional studies raise the possibility that the chance of tremor occurring with CD increases with disease duration. This could represent progression of CD which would counter the commonly held belief that CD is not a progressive disorder. On the other hand, a longer disease duration associated with HT in CD could also result from earlier onset.
Age of onset
In our cohort, patients with HT had earlier age of onset compared to patients without HT. In contrast, previous studies in smaller cohorts found that tremor is more common in patients with later onset dystonia,10 even in studies with a cohort limited to adult onset dystonias.11 Tremor is less common in studies focused on young onset dystonia12 compared to studies focused on adult onset dystonia.11 In a study that did not report whether its patient cohort consisted of only adult onset dystonia patients, no difference was found between mean ages of onset of CD between patients with HT and patients without HT.3 The discrepancy in results among these studies may be related to differences in the distribution of onset age, dystonia subtypes included, or forms of tremor assessed in their patient cohorts. Our cohort consisted of a large number of patients with CD and demonstrated that HT preferentially affects CD patients with younger age of onset. In studies suggesting a higher prevalence of tremor in patients with later onset age, the cohorts may have included a broader spectrum of dominant body regions affected, and HT likelihood was not evaluated separately for the different phenotypes. In studies suggesting no difference in prevalence of HT in patients regarding age of onset, only regular (but not jerky) head oscillations were considered HT.3 Information about the relative timing of CD and HT onset are equivocal: other studies suggest that tremor onset can precede,4,5,11 coincide with,4,11 or follow dystonia onset.4,11 As with most studies of this nature, the age of CD onset was based on patient reports and is therefore susceptible to recall bias. As but one example, because HT may be more visible than mild postural abnormalities, CD patients may be more likely to report age of onset earlier if HT is their initially presenting symptom.
Age
In the logistic regression model, age did not play a significant role in determining HT likelihood. However, the interaction of age and disease duration played a significant role, and there was a trend toward patients with HT being older than those without HT in our cohort when age was assessed in isolation. Collectively these results are consistent with the findings of Merola et al.8
Sex
The proportion of patients with HT who were female was higher than for patients without HT, but this difference did not reach statistical significance. Men and women had similar representation in those who did not have HT. Merola et al.8 and Pal et al.3 found the same trend with statistical significance in their cohorts that may reflect their larger patient cohort. In Pal et al.’s study, because patients with only jerky HT were classified as having no HT, their reported relationship between gender and HT prevalence may be sensitive to HT type.
HT type
There was no difference in the likelihood of having regular versus jerky HT given a patient’s predominant posture, age of onset, disease duration, age and sex. It may be that – when comparing regular and jerky HT in CD – the distributions of predominant posture and these demographic variables are all overlapped because the phenomena coexist to varying degrees within patients, as recently illustrated with magnetic coil measurements of HT in a cohort of 14 CD patients.13 On the other hand, dystonic and essential tremor are associated with different network activity, at least based on functional connectivity measures from functional magnetic resonance imaging (MRI) recorded during grip-force task modulated by visual feedback.14 We hypothesize that objective quantification of HT in larger cohorts of CD patients with a broader array of HT regularity metrics may reveal distinct subgroups of HT in CD that are otherwise difficult to detect in small cohorts with low-dimensional measures. This would also enable future studies of HT in which the motor features are characterized at a level of precision commensurate with the brain network assays.
Limitations
One of the limitations of this study is that we do not have any data on the relative timing of onset of the patients’ CD versus HT because our patients were not asked about age at onset of HT. So we do not know the proportion of patients for whom HT was the initially presenting symptom or how long after CD onset their HT developed. This information should be acquired in future longitudinal studies. Another limitation is regarding the assessment of HT type. It can be difficult to distinguish regular versus jerky HT clinically; therefore, this dichotomy may have limited accuracy.
Conclusion
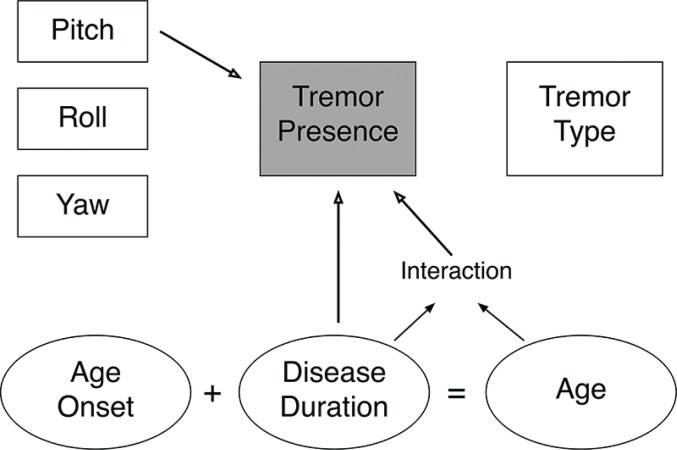
In summary, we found that the pitch axis of postural abnormality in CD and disease duration and age relate to HT in CD (Figure 3). None of these variables were differentially associated with the qualitative type of HT. We found HT to be more common in CD patients with retrocollis than anterocollis, which raises the question of whether other aspects of HT not captured in our data also depend on postural directionality. For instance, does the directionality of the HT relate to the directionality of a patient’s predominant posture? If directional elements of the posture respond differentially to the sensory trick or treatment, do the directional aspects of the HT respond in the same way? Relatedly, does the predominant axis of rotation, rather than the direction within each individual axis, also influence the likelihood of HT? The field can begin to be able to address these questions with the objective nature and temporal precision of emerging methods to capture these phenomena with inertial measurement units and computer vision-based analyses of video recordings. Collectively such investigations should help optimize CD treatment and help inform theories of the network pathophysiology that cause abnormal posture and HT in CD.
Figure 3.
Summary Schematic of the Interaction between Pitch, Head Tremor, and Time. Arrows indicate significant associations.
Acknowledgments
We gratefully acknowledge Laura Wright and Matt Hicks (WUSM) for assistance with providing data access.
Footnotes
Citation: Chen Q, Vu JP, Cisneros E, Benadof CN, Zhang Z, Barbano RL, et al. Postural directionality and head tremor in cervical dystonia. Tremor Other Hyperkinet Mov. 2020: 10. doi: 10.7916/tohm.v0.745
Editor: Elan D. Louis, Yale University, USA
Funding: This research was conducted by the Dystonia Coalition, which is part of the Rare Diseases Clinical Research Network, an initiative funded by the Office of Rare Diseases Research at the National Center for Advancing Translational Sciences (U54 TR001456) in collaboration with the National Institute of Neurological Disorders and Stroke (U54 NS065701) at the National Institutes of Health (NIH). This work was supported by the Office of the Assistant Secretary of Defense for Health Affairs, through the Peer-Reviewed Medical Research Program under Award No. W81XWH-17-1-0393. Opinions, interpretations, conclusions, and recommendations made in this article are those of the author and are not necessarily endorsed by the Department of Defense.
Financial Disclosures: None.
Conflicts of Interest: The authors report no conflicts of interest.
Ethics Statement: This study was performed in accordance with the ethical standards detailed in the Declaration of Helsinki. The authors’ institutional ethics committee has approved this study and all patients have provided written informed consent.
Authors’ contribution
(1) Research project: A. Conception, B. Organization, C. Execution; (2) Statistical Analysis: A. Design, B. Execution, C. Review and Critique; (3) Manuscript Preparation: A. Writing of the first draft, B. Review and Critique.
Q.C. was involved in the conception, organization, and execution of the research; review and critique of the article; and writing of the first draft of the article. J.P.V, E.C., C.N.B., Z.Z., R.L.B., C.G.G., J.J., H.A.J., and J.S.P. were responsible for the execution of the research and review and critique of the article. M.I.A. was responsible for the design of the study and review and critique of the article. G.T.S. was responsible for the organization, execution and design of the study and review and critique of the article. C.L.C. was responsible for the organization and execution of the study and review and critique of the article. D.A.P. was responsible for the conception, organization, execution and design of the study; writing of the first draft; and review and critique of the article.
References
- 1.Gövert F, Deuschl G. Tremor entities and their classification: an update. Curr Opin Neurol 2015;28(4):393–399. doi: 10.1097/WCO.0000000000000211 [DOI] [PubMed] [Google Scholar]
- 2.Hvizdosova L, Nevrly M, Otruba P, Kanovsky P. The prevalence of dystonic tremor and tremor associated with dystonia (TAWD) in patients with cervical dystonia. Mov Disord 2018;33:S322. doi: 10.1002/mds.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pal PK, Samii A, Schulzer M, Mak E, Tsui JK. Head tremor in cervical dystonia. Can J Neurol Sci 2000;27(2):137–142. doi: 10.1017/s0317167100052240 [DOI] [PubMed] [Google Scholar]
- 4.Pandey S, Sarma N. Tremor in dystonia. Parkinsonism Relat Disord 2016;29:3–9. doi: 10.1016/j.parkreldis.2016.03.024 [DOI] [PubMed] [Google Scholar]
- 5.Albanese A, Sorbo FD. Dystonia and tremor: the clinical syndromes with isolated tremor. Tremor Other Hyperkinet Mov 2016;6:319. doi: 10.7916/D8X34XBM [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shaikh AG, Zee DS, Jinnah HA. Oscillatory head movements in cervical dystonia: dystonia, tremor, or both? Mov Disord 2015;30(6):834–842. doi: 10.1002/mds.26231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jinnah HA, Neychev V, Hess EJ. The anatomical basis for dystonia: the motor network model. Tremor Other Hyperkinet Mov 2017;7:506. doi: 10.7916/D8V69X3S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Merola A, Dwivedi AK, Shaikh AG, Tareen TK, Da Prat GA, Kauffman MA, et al. Head tremor at disease onset: an ataxic phenotype of cervical dystonia. J Neurol 2019;266(8):1844–1851. doi: 10.1007/s00415-019-09341-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Antelmi E, Di Stasio F, Rocchi L, Erro R, Liguori R, Ganos C, et al. Impaired eye blink classical conditioning distinguishes dystonic patients with and without tremor. Parkinsonism Relat Disord 2016;31:23–27. doi: 10.1016/j.parkreldis.2016.06.011 [DOI] [PubMed] [Google Scholar]
- 10.Defazio G, Conte A, Gigante AF, Fabbrini G, Berardelli A. Is tremor in dystonia a phenotypic feature of dystonia? Neurology 2015;84(10):1053–1059. doi: 10.1212/WNL.0000000000001341 [DOI] [PubMed] [Google Scholar]
- 11.Defazio G, Gigante AF, Abbruzzese G, Bentivoglio AR, Colosimo C, Esposito M, et al. Tremor in primary adult-onset dystonia: prevalence and associated clinical features. J Neurol Neurosurg Psychiatry 2013;84(4):404–408. doi: 10.1136/jnnp-2012-303782 [DOI] [PubMed] [Google Scholar]
- 12.Koukouni V, Martino D, Arabia G, Quinn NP, Bhatia KP. The entity of young onset primary cervical dystonia. Mov Disord 2007;22(6):843–847. doi: 10.1002/mds.21421 [DOI] [PubMed] [Google Scholar]
- 13.Beylergil SB, Singh AP, Zee DS, Jinnah HA, Shaikh AG. Relationship between jerky and sinusoidal oscillations in cervical dystonia. Parkinsonism Relat Disord 2019;66:130–137. doi: 10.1016/j.parkreldis.2019.07.024 [DOI] [PubMed] [Google Scholar]
- 14.DeSimone JC, Archer DB, Vaillancourt DE, Wagle Shukla A. Network-level connectivity is a critical feature distinguishing dystonic tremor and essential tremor. Brain 2019;142(6):1644–1659. doi: 10.1093/brain/awz085 [DOI] [PMC free article] [PubMed] [Google Scholar]