Abstract
Background
Malnutrition is one of the most important comorbidities in patients with heart failure with preserved ejection fraction. We recently reported the prognostic significance of serum cholinesterase level and superior predictive power of cholinesterase level to other objective nutritional indices such as the controlling nutritional status score, prognostic nutritional index, and geriatric nutritional risk index in patients with acute decompensated heart failure. The aim of this study was to clarify the prognostic role of cholinesterase in patients with heart failure with preserved ejection fraction/acute decompensated heart failure and investigate incremental cholinesterase value.
Methods and Results
We prospectively studied 274 consecutive patients from the PURSUIT‐HFpEF (Prospective Multicenter Observational Study of Patients with Heart Failure With Preserved Ejection Fraction) study. During a follow‐up period of 1.2±0.6 years, 56 patients reached the composite end points (cardiovascular death and readmission for worsening heart failure). In the multivariable Cox analysis, cholinesterase level was significantly associated with the composite end points after adjustment for major confounders. A Kaplan–Meier analysis revealed that patients with low cholinesterase levels (stratified by tertile) had significantly greater risk of reaching the composite end points than those with middle or high cholinesterase levels (P=0.0025). Cholinesterase level showed the best C‐statistics (0.703) for prediction of the composite end points among the objective nutritional indices. C‐statistics of the Meta‐Analysis Global Group in Chronic Heart Failure (MAGGIC) risk score for prediction of the composite end points were improved when cholinesterase level was added (C‐statistics, from 0.601 to 0.705; P=0.0408).
Conclusions
Cholinesterase was a useful prognostic marker for prediction of adverse outcome in patients with heart failure with preserved ejection fraction/acute decompensated heart failure.
Keywords: cholinesterase, heart failure, malnutrition, nutritional indices, risk stratification
Subject Categories: Heart Failure
Clinical Perspective
What Is New?
In acute heart failure patients with preserved left ventricular ejection fraction, serum cholinesterase level is a simple and strong prognostic factor for prediction of poor outcome and tends to provide more‐powerful prognostic information than other objective nutritional indices (eg, the Controlling Nutritional Status score, Prognostic Nutritional Index, and Geriatric Nutritional Risk Index).
Serum cholinesterase level provides incremental value over the Meta‐Analysis Global Group in Chronic Heart Failure (MAGGIC) risk score in acute heart failure patients with preserved left ventricular ejection fraction.
What Are the Clinical Implications?
Cholinesterase provides a more‐accurate nutritional risk evaluation of patients than other objective nutritional indices.
This may allow early implementation of appropriate nutritional intervention in daily practice and lead to better outcomes in acute heart failure patients with preserved left ventricular ejection fraction.
Introduction
Although treatment of heart failure (HF) has remarkably progressed over the past several decades, HF is still a serious disease characterized by high risks of mortality and repetitive hospitalization.1 Given that the number of patients with HF is increasing, HF is becoming a major health problem.2 Recently, approximately half of patients with HF have been shown to have HF with preserved left ventricular ejection fraction (HFpEF), and patients with HFpEF had a higher comorbidity burden than those with HF with reduced ejection fraction.3 Malnutrition, which is one of the most important comorbidities of HF, has been reported as a powerful prognostic factor in patients with HFpEF.4, 5, 6, 7, 8 Some recent studies have shown the potential of dietary intervention to improve HF symptoms or cardiorespiratory fitness.9, 10, 11 Therefore, accurate nutritional evaluation is indispensable to improve the prognosis of patients with HF.12
Serum cholinesterase level has been used for the evaluation of nutritional status in daily practice,13 and its prognostic value was reported in patients with chronic HF (CHF).14 In addition, we have recently reported the prognostic significance of cholinesterase level and shown that cholinesterase level has superior predictive power to other objective nutritional indices, such as the Controlling Nutritional Status (CONUT) score, Prognostic Nutritional Index (PNI), and Geriatric Nutritional Risk Index (GNRI), in patients with acute decompensated HF (ADHF).15 However, the prognostic value of cholinesterase remains to be fully evaluated in ADHF patients with preserved ejection fraction.
Risk stratification of patients with HFpEF is clinically relevant because of the lack of therapies with proven efficacies. As one of the several HF risk models, the Meta‐Analysis Global Group in Chronic Heart Failure (MAGGIC) risk score was originally developed from a large international database of patients with CHF, and its prognostic usefulness was externally validated in CHF patients with preserved ejection fraction16 and patients with ADHF.17 Therefore, the present study was conducted to achieve the following objectives using the multicenter HFpEF registry: (1) investigation of the prognostic role of cholinesterase level and comparison with other nutritional indexes in HFpEF‐ADHF and (2) investigation of the prognostic usefulness of the MAGGIC risk score in patients with ADHF with preserved ejection fraction and the incremental prognostic value of cholinesterase level over the MAGGIC risk score.
Methods
Our study data will not be made available to other researchers for purposes of reproducing the results because of institutional review board restrictions. However, the study materials that support the findings of this study and the methods used in the analyses will be provided by the corresponding author upon reasonable request.
Subjects
Patient data were obtained from the PURSUIT‐HFpEF (Prospective Multicenter Observational Study of Patients with Heart Failure With Preserved Ejection Fraction) study. The PURSUIT‐HFpEF study is a prospective, multicenter, observational study in which collaborating hospitals in Osaka record clinical, echocardiographic, and outcome data of patients with ADHF and preserved left ventricular ejection fraction (LVEF; ≥50%; UMIN‐CTR ID: UMIN000021831). Consecutive patients with ADHF and preserved ejection fraction were prospectively registered and agreed to be followed up for collection of outcome data. ADHF was diagnosed on the basis of the following criteria: (1) clinical symptoms and signs according to the Framingham Heart Study criteria and (2) serum NT‐proBNP (N‐terminal pro‐B‐type natriuretic peptide) level of ≥400 pg/mL or brain natriuretic peptide level of ≥100 pg/mL. Between June 2016 and December 2017, 345 patients were enrolled. We excluded patients with in‐hospital death (n=8), missing cholinesterase data (n=45), or missing follow‐up data (n=18). Ultimately, 274 patients with ADHF were analyzed in this study. All patients provided written informed consent for participation in this study, which was approved by the ethics committee of each participating hospital. This study conformed to the ethical guidelines outlined in the Declaration of Helsinki.
Data Collection
All patients underwent echocardiography and venous blood sampling at discharge. On echocardiography, left ventricular end‐diastolic dimension and end‐systolic dimensions and left atrial dimension were measured using the standard technique. LVEF was measured using the modified Simpson method. Diastolic left ventricular function was measured using the ratio of early transmitral filling velocity, which was measured with pulse‐wave Doppler ultrasonography, to early diastolic mitral annular velocity, which was determined using spectral tissue Doppler imaging (E/e′). Systolic right ventricular function was measured using tricuspid annular plane systolic excursion. Tricuspid regurgitation pressure gradient was measured using continuous‐wave Doppler imaging. Blood samples were obtained at discharge for assessment of complete blood count and serum levels of sodium, chloride, potassium, creatinine, blood urea nitrogen, albumin, uric acid, aspartate aminotransaminase, alanine aminotransferase, total bilirubin, gamma‐glutamyl transpeptidase, alkaline phosphatase, C‐reactive protein, cholinesterase, and NT‐proBNP levels.
The CONUT score was calculated from 3 variables, namely serum albumin level, total cholesterol level, and lymphocyte count (range, 0–12, with higher values indicating worse nutritional status), as previously reported.18 We also calculated PNI (10×serum albumin level [g/dL]+0.005×total lymphocyte count [/mm3]) and GNRI (14.89×serum albumin level+41.7×body mass index [BMI]/22).19, 20
The MAGGIC risk score was calculated from 13 variables as follows: age, sex, BMI, systolic blood pressure, LVEF, creatinine level, current smoking, diabetes mellitus, chronic obstructive pulmonary disease, New York Heart Association class, HF duration of >18 months, β‐blocker use, and angiotensin‐converting enzyme inhibitor use, as previously reported.21
Clinical Outcomes
After discharge, all patients were followed up in each hospital. Survival data were obtained by dedicated coordinators and investigators by direct contact with patients and their physicians at the hospital or in an outpatient setting or by a telephone interview with their families or by mail. The primary end points of this study were the composites of cardiovascular death and hospitalization for worsening HF (composite end points). The secondary end points were all‐cause mortality and cardiovascular death.
Statistical Analyses
All continuous variables are expressed as mean (SD) or median (25th–75th percentile), as appropriate, and categorical variables are expressed as percentage. Patients were stratified according to the tertile of serum cholinesterase value. The Student t test or 1‐factor ANOVA was used to compare differences in normally distributed continuous variables. The Kruskal–Wallis rank‐sum test was used to compare differences in nonnormally distributed data. Fisher's exact test and chi‐square test were used to compare between‐group differences in categorical variables. Correlations of serum cholinesterase level to serum levels of albumin, aspartate aminotransaminase, alanine aminotransferase, alkaline phosphatase, total bilirubin, and C‐reactive protein and BMI, CONUT score, GNRI, and PNI were assessed using Spearman correlation coefficients. The Kaplan–Meier method and log‐rank test were used to estimate cardiac event‐free rates. Cox regression analyses were conducted to investigate the impact of low and middle level of cholinesterase on study outcomes, while taking hazard ratio (HR) of high cholinesterase group as a reference. Cox proportional hazards regression models were used to identify patients at risk of the primary and secondary end points to calculate multivariable‐adjusted HRs and 95% CIs. Two multivariable models (clinical and biomarker models) were constructed. Covariates considered to be clinically relevant were selected a priori and forced into the models. The clinical model included age, sex, BMI, and history of diabetes mellitus, which were selected because they were basic clinical parameters and known as factors that could influence cholinesterase value.13 The biomarker model included hemoglobin level, estimated glomerular filtration rate, albumin level, and log‐transformed NT‐proBNP level. These variables were selected because comorbidities, such as anemia, renal failure, malnutrition, and NT‐proBNP level, were well‐established risk predictors in patients with HFpEF.16, 22, 23 To avoid overfitting, especially in the prediction of cardiovascular death, the covariates of the clinical model were restricted to age and sex and those of the biomarker model to hemoglobin and estimated glomerular filtration rate. To evaluate for potential collinearity among the covariates, we calculated the variance inflation factor for each adjusted model after the models were constructed. Multicollinearity was defined such that the maximum variance inflation factor value exceeded a recommended threshold of 10.24 The areas under the curve of cholinesterase level and other objective nutritional indices were compared using a receiver‐operating characteristic curve analysis for prediction of the composite end point within 1 year. The significance criterion was adjusted to P<0.017 by using Bonferroni's correction to control the multitesting issue. Furthermore, to clarify improvement of risk prediction by cholinesterase level over the MAGGIC risk score, a receiver‐operating characteristic curve analysis was performed for the logistic regression models of MAGGIC only and MAGGIC plus cholinesterase, for prediction of the composite end point within 1 year. Areas under the curve were compared according to the method of DeLong et al.25 Net reclassification improvement and integrated discrimination improvement attained by the addition of cholinesterase to the MAGGIC risk score were also calculated. MedCalc (version 17.11.564 bit; MedCalc Software bvba, Ostend, Belgium) and EZR software (version 1.03; Saitama Medical Center, Jichi Medical University, Saitama, Japan) were used to perform all statistical analyses. P<0.05 was considered statistically significant.
Results
Baseline Patient Characteristics
Distribution of cholinesterase levels is shown in Figure 1. Median value (interquartile range) was 208 U/L (166–255 U/L). The study population (n=274) was categorized by cholinesterase level tertiles as follows: low cholinesterase level ≤180 U/L (n=93); 180 U/L <cholinesterase level ≤236 U/L (n=90); and cholinesterase level >236 U/L (n=91).
Figure 1.
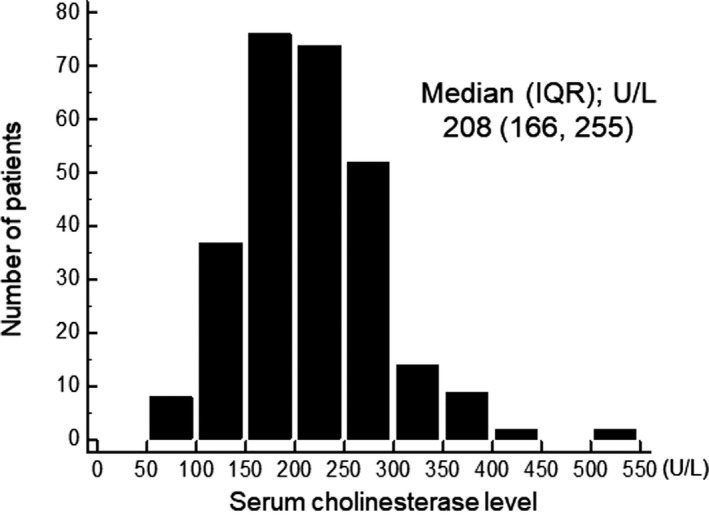
Distribution of serum cholinesterase level. IQR indicates interquartile range.
In all study patients, mean age was 80 years, the proportion of male patients was 46%, and the LVEF was 61%±7%. Patients’ baseline characteristics stratified according to cholinesterase level tertiles are shown in Table 1. Compared with patients with high cholinesterase levels, those with low cholinesterase levels had significantly higher age, higher proportion of males, lower BMI, lower incidence rate of dyslipidemia, smaller left ventricular end‐diastolic dimension and tricuspid annular plane systolic excursion, lower hemoglobin level, platelet count, and sodium and albumin levels, and higher NT‐proBNP and gamma‐glutamyl transpeptidase levels. As for the nutritional indices, patients with lower cholinesterase had a significantly higher CONUT score and lower PNI and GNRI.
Table 1.
Baseline Characteristics of the Patients With Acute Decompensated Heart Failure Stratified by Serum Cholinesterase Level Tertile
| Overall (n=274) | Lowest Tertile Cholinesterase Level ≤180 (n=93) | Middle Tertile180 <Cholinesterase Level ≤236 (n=90) | Highest Tertile236 <Cholinesterase Level (n=91) | P Value | |
|---|---|---|---|---|---|
| Clinical data | |||||
| Age, y | 80±10 | 82±7 | 81±9 | 76±11 | <0.001 |
| Sex (male, %) | 46 | 52 | 51 | 34 | 0.0255 |
| BMI | 21.8±4.8 | 20.1±3.6 | 21.6±4.3 | 23.5±4.6 | <0.001 |
| NYHA class I/II/III, % | 42/52/4 | 35/55/10 | 40/55/5 | 51/46/3 | 0.096 |
| SBP, mm Hg | 119±17 | 118±18 | 119±19 | 121±16 | 0.528 |
| Heart rate, bpm | 71±12 | 71±12 | 71±12 | 70±12 | 0.588 |
| Atrial fibrillation, % | 36 | 31 | 44 | 32 | 0.111 |
| Hypertension, % | 87 | 89 | 82 | 88 | 0.339 |
| Diabetes mellitus, % | 35 | 30 | 30 | 44 | 0.086 |
| Dyslipidemia, % | 47 | 40 | 44 | 61 | 0.019 |
| Previous HF hospitalization, % | 29 | 34 | 30 | 22 | 0.172 |
| Medications | |||||
| ACEI or ARB, % | 52 | 42 | 54 | 59 | 0.051 |
| β‐blocker, % | 57 | 52 | 57 | 63 | 0.319 |
| Loop diuretics, % | 97 | 97 | 97 | 99 | 0.748 |
| Aldosterone blocker, % | 37 | 37 | 33 | 40 | 0.685 |
| Tolvaptan, % | 17 | 21 | 13 | 15 | 0.446 |
| Statin, % | 33 | 25 | 37 | 37 | 0.122 |
| Echocardiography | |||||
| LVEF, % | 61±7 | 62±8 | 62±7 | 61±7 | 0.559 |
| LVDd, mm | 46±7 | 44±8 | 45±6 | 47±6 | 0.013 |
| LAD, mm | 44±8 | 43±9 | 44±9 | 45±7 | 0.475 |
| E/e′ | 13 (10–17) | 13 (10–18) | 13 (10–17) | 13 (10–18) | 0.912 |
| TAPSE, mm | 18±5 | 16±4 | 18±5 | 18±5 | 0.011 |
| TRPG, mm Hg | 26 (21–31) | 28 (22–34) | 25 (22–30) | 26 (21–31) | 0.137 |
| IVC diameter, mm | 14 (10–16) | 14 (9–18) | 14 (11–16) | 13 (10–16) | 0.827 |
| Laboratory data | |||||
| Hemoglobin, g/dL | 11.1 (9.8–12.6) | 10.4 (8.9–11.6) | 11.5 (10.2–13.1) | 11.7 (10.7–13.1) | <0.001 |
| Platelet count, 104/mL | 21 (17–27) | 19 (15–24) | 22 (18–25) | 22 (18–29) | 0.049 |
| Lymphocyte count, count/mL | 1391 (1015–1815) | 1173 (918–1474) | 1363 (1034–1779) | 1645 (1260–1952) | <0.001 |
| Sodium, mEq/L | 138±4 | 137±4 | 139±3 | 139±4 | <0.001 |
| Chloride, mEq/L | 102±4 | 102±4 | 103±4 | 103±4 | 0.738 |
| Potassium, mEq/L | 4.2±0.6 | 4.1±0.6 | 4.3±0.6 | 4.3±0.5 | 0.018 |
| Creatinine, mg/dL | 1.10 (0.90–1.50) | 1.10 (0.90–1.60) | 1.20 (0.90–1.60) | 1.00 (0.80–1.50) | 0.110 |
| BUN, mg/dL | 24 (18–35) | 24 (17–35) | 26 (20–38) | 23 (18–34) | 0.218 |
| eGFR | 41 (30–55) | 40 (26–54) | 41 (28–55) | 44 (33–57) | 0.357 |
| Uric acid, mg/dL | 6.7±2.0 | 6.3±2.0 | 7.1±2.0 | 6.8±2.1 | 0.034 |
| Albumin, g/dL | 3.4±0.5 | 3.1±0.5 | 3.5±0.4 | 3.7±0.4 | <0.001 |
| Total cholesterol, mg/dL | 161±35 | 147±34 | 164±31 | 171±37 | <0.001 |
| C‐reactive protein, mg/dL | 0.25 (0.11–0.81) | 0.32 (0.12–1.23) | 0.24 (0.11–0.89) | 0.20 (0.10–0.44) | 0.053 |
| Log NT‐proBNP | 7.0±1.3 | 7.6±1.3 | 7.0±1.1 | 6.5±1.1 | <0.001 |
| AST, U/L | 23 (17–30) | 23 (18–31) | 23 (17–32) | 21 (17–27) | 0.256 |
| ALT, U/L | 15 (10–24) | 15 (10–23) | 17 (11–26) | 14 (10–24) | 0.666 |
| GGT, U/L | 34 (20–62) | 43 (22–78) | 32 (20–69) | 27 (19–46) | 0.024 |
| ALP, U/L | 257 (202– 322) | 261 (209–363) | 245 (196–294) | 257 (195–3203) | 0.087 |
| Total bilirubin, mg/dL | 0.60 (0.40–0.80) | 0.60 (0.43–0.80) | 0.60 (0.40–0.73) | 0.60 (0.50–0.80) | 0.760 |
| Cholinesterase, U/L | 208 (166–255) | 151 (126–167) | 209 (198–219) | 274 (255–311) | <0.001 |
| Nutritional indices | |||||
| CONUT score | 3 (2–5) | 5 (3–6) | 3 (2–5) | 2 (1–3) | <0.001 |
| PNI | 42±6 | 38±6 | 42±5 | 45±6 | <0.001 |
| GNRI | 92±12 | 85±11 | 92±11 | 99±11 | <0.001 |
| Heart failure model | |||||
| MAGGIC risk score | 24±5 | 26±4 | 25±5 | 21±5 | <0.001 |
ACEI indicates angiotensin‐converting enzyme inhibitor; ALP, alkaline phosphatase; ALT, alanine aminotransferase; ARB, angiotensin receptor blocker; AST, aspartate aminotransaminase; BMI, body mass index; BNP, brain natriuretic peptide; BUN, blood urea nitrogen; CONUT, the Controlling Nutritional Status score; GGT, gamma‐glutamyl transpeptidase; GNRI, Geriatric Nutritional Risk Index; HF, heart failure; IVC, inferior vena cava; LAD, left atrial dimension; LVDd, left ventricular end‐diastolic dimension; LVDs, left ventricular end‐systolic dimension; LVEF, left ventricular ejection fraction; MAGGIC risk score, the Meta‐Analysis Global Group in Chronic Heart Failure risk score; NYHA, New York Heart Association; PNI, Prognostic Nutritional Index; SBP, systolic blood pressure; TAPSE, tricuspid annular plane systolic excursion; TRPG, tricuspid regurgitant pressure gradient.
Serum cholinesterase level had moderate correlations with albumin level (r=0.448; P<0.0001), BMI (r=0.327; P<0.0001), CONUT score (r=−0.528; P<0.0001), PNI (r=0.533; P<0.0001), and GNRI (r=0.499; P<0.0001). Cholinesterase level had weak correlations with C‐reactive protein level (r=−0.152; P=0.0122). Cholinesterase level had no significant correlation with aspartate aminotransaminase (r=−0.084; P=0.1675), alkaline phosphatase (r=−0.106; P=0.0800), total bilirubin (r=−0.047; P=0.4434), or alanine aminotransferase level (r=0.047; P=0.4396).
Clinical Outcomes and Prognostic Analysis
During a mean follow‐up period of 1.3±0.5 years, 56 patients reached the composite end points, 38 patients died, and 15 patients had a cardiovascular death.
The Kaplan–Meier analysis revealed that patients with low cholinesterase levels (≤80 U/L defined by tertile) had a significantly greater risk of reaching the composite end points than those with middle or high cholinesterase levels (31% versus 16% versus 14%; P=0.0025; adjusted HR, 2.56 [95% CI, 1.33–4.93]; adjusted HR, 1.27 [95% CI, 0.59–2.76]; Figure 2). Patients with low cholinesterase levels had greater risks of all‐cause mortality and cardiovascular death than those with middle or high cholinesterase levels (Figure 2).
Figure 2.
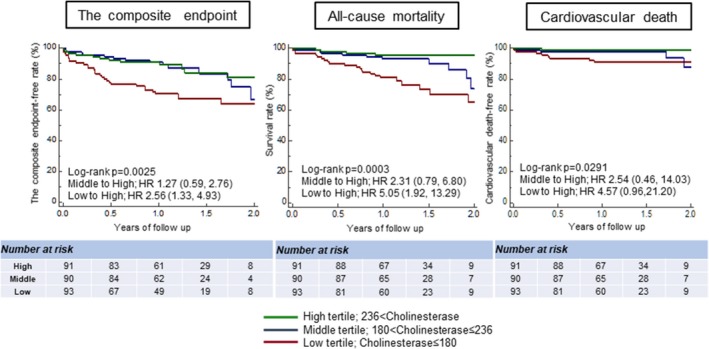
Kaplan–Meier estimates of the composite end‐point–free survival rate and cardiovascular‐death–free curves for patients stratified by serum cholinesterase level tertile. HR indicates hazard ratio.
Results of the multivariable Cox proportional hazards analysis for prediction of the composite end points, all‐cause mortality, and cardiovascular death are shown in Table 2. Cholinesterase level was significantly associated with the composite end points, all‐cause mortality, and cardiovascular mortality in both the clinical and biomarker models. No collinearity was identified among the covariates used in the analyses because all the variance inflation factor values were <10.
Table 2.
Cox Multivariable Proportional Hazard Models of Cholinesterase for the Prediction of Composite End Points, All‐Cause Mortality, and Cardiovascular Death
| Clinical Model | Biomarker Model | |||
|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | |
| Composite end point | 0.90 (0.86–0.95)a | 0.0001 | 0.93 (0.88–0.99)b | 0.0166 |
| All‐cause mortality | 0.85 (0.80–0.92)a | <0.0001 | 0.88 (0.82–0.95)b | 0.0007 |
| Cardiovascular death | 0.86 (0.77–0.96)c | 0.0059 | 0.88 (0.79–0.98)d | 0.0164 |
BMI indicates body mass index; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate; HR, hazard ratio; log NT‐pro BNP, log‐transformed n‐terminal pro‐brain natriuretic peptide.
Clinical model: Cholinesterase level (10 U/L) was adjusted for age, sex, BMI, and DM.
Biomarker model: Cholinesterase level (10 U/L) was adjusted for eGFR, hemoglobin level, log NT‐proBNP level, and albumin level.
Clinical model: Cholinesterase level (10 U/L) was adjusted for age and sex.
Biomarker model: Cholinesterase level (10 U/L) was adjusted for eGFR and hemoglobin level.
Comparison With Other Nutritional Indices
The receiver‐operating characteristic analysis for identification of the composite end point within 1 year is shown in Figure 3. Cholinesterase level had the greatest areas under the curve among all the objective nutritional indices for identification of the composite end points (cholinesterase level, 0.703 [95% CI, 0.642–0.758]; CONUT score, 0.672 [95% CI, 0.610–0.729]; PNI, 0.676 [95% CI, 0.615–0.733]; GNRI, 0.619 [95% CI, 0.556–0.679]; Figure 3). Differences between cholinesterase level and the other nutritional indices were not statistically significant (cholinesterase level versus CONUT score, P=0.5631; cholinesterase level versus PNI, P=0.6418; cholinesterase level versus GNRI, P=0.1724).
Figure 3.
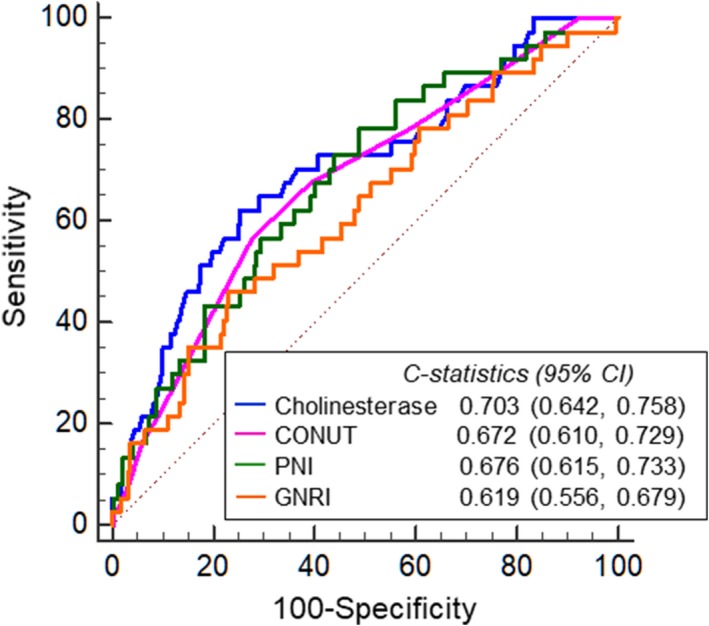
Receiver‐operating characteristic curve analysis of cholinesterase level, Controlling Nutritional Status score, Prognostic Nutritional Index, and Geriatric Nutritional Risk Index for the prediction of the composite endpoint within 1 year. CHE indicates Cholinesterase; CONUT, the Controlling Nutritional Status score; PNI, Prognostic Nutritional Index; GNRI, Geriatric Nutritional Risk Index.
Prognostic Value of HF Risk Scores and the Incremental Prognostic Value of Cholinesterase Level Over the HF Risk Scores
The MAGGIC risk score tended to be greater in patients with than in those without the composite end points (25±6 versus 24±5; P=0.0877). The MAGGIC risk score was significantly associated with the composite end point (HR, 1.05 [95% Cl, 1.00–1.11]; P=0.0455), all‐cause mortality (HR, 1.15 [95% CI, 1.08–1.23]; P<0.0001), and cardiovascular death (HR, 1.13 [95% CI, 1.02–1.26]; P=0.0260).
C‐statistics of the MAGGIC risk score for prediction of the composite end points within 1 year was significantly improved when cholinesterase level was added to the MAGGIC risk score (from 0.601 to 0.705, P=0.0408; Figure 4). Net reclassification improvement and integrated discrimination improvement attained by the addition of cholinesterase level to the MAGGIC risk score were also statistically significant for prediction of the composite end points within 1 year (net reclassification improvement: 0.6313 [95% CI, 0.3149–0.9477], P<0.0001; integrated discrimination improvement: 0.0687 [95% CI, 0.0033–0.1043], P=0.0002).
Figure 4.
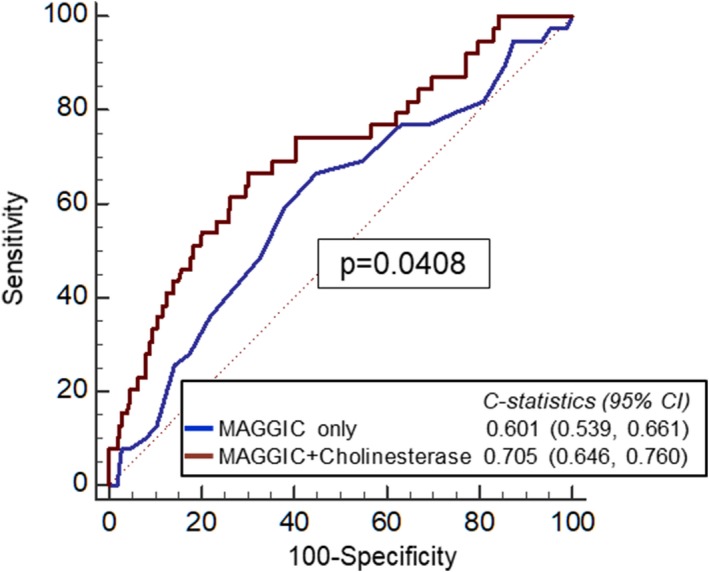
Receiver‐operating characteristic curve analysis of the MAGGIC (Meta‐Analysis Global Group in Chronic Heart Failure) risk score and MAGGIC plus cholinesterase level for the prediction of the composite end point within 1 year. CHE indicates Cholinesterase.
Discussion
The present study examined patients admitted for ADHF with preserved LVEF and demonstrated that low cholinesterase level was independently associated with poor outcome. In addition, predictive power of cholinesterase level tended to be greater than those of other objective indices. Furthermore, cholinesterase level provided an incremental value over the MAGGIC risk score in ADHF with preserved LVEF.
Previous Studies
Evaluating comorbidities is clinically important, especially in patients with HFpEF because the prognosis of HFpEF is strongly influenced by comorbidities.26 As for malnutrition, Kinugasa et al used GNRI and showed that malnutrition risk was associated with functional decline and poor prognosis in patients with HFpEF.5 They found that GNRI had better predictive power than BMI or serum albumin level alone by their complementary effects. Cheng et al showed the long‐term prognostic role of PNI in patients with HFpEF.6 As for the evidence to support the usefulness of cholinesterase level, Sato et al showed the prognostic role of cholinesterase level in patients with CHF.14 In addition, we previously showed the prognostic value of cholinesterase level in patients with ADHF. In our previous study, we demonstrated its prognostic role regardless of LVEF by conducting a subgroup analysis using heart failure with reduced ejection fraction (LVEF<40%), HF with mid‐range ejection fraction (40%; ≤LVEF<50%), and HFpEF (LVEF≥50%).15 However, because the data were obtained from a single‐center registry study, the number of study subjects with HFpEF was relatively limited. To overcome the limitation of our previous study, this study was conducted by using data from a multicenter HFpEF registry (PURSUIT‐HFpEF registry). The present study demonstrated that low cholinesterase levels provided prognostic information in ADHF patients with preserved LVEF.
Possible Mechanism
Decreased serum cholinesterase level is observed in many clinical conditions such as malnutrition, inflammation, and liver damage. In the present study, patients with low cholinesterase levels showed significantly lower BMI, lower albumin level, higher CONUT score, and lower PNI and GNRI. Furthermore, cholinesterase level also had a moderate correlation with albumin level, BMI, and the other nutritional indices and a weak or no correlation with liver function test results and C‐reactive protein level. These results support our view that cholinesterase level was mainly driven by malnutrition, rather than inflammation or hepatic function, in our study patients.
Considering the phenotypes of malnutrition, such as cachexia, sarcopenia, and sarcopenic obesity, is clinically important because they affect patients’ cardiorespiratory fitness and quality of life, and they have different prognoses.27, 28 Cholinesterase level has been reported to be a good biomarker for identifying elderly subjects at risk of sarcopenia.29 Furthermore, cholinesterase plays an important role in lipid metabolism, whether directly or through a synergistic action with cholesterol esterase.13 Thus, theoretically, cholinesterase level can be a comprehensive malnutritional biomarker of both sarcopenia and cachexia.
The mechanism of malnutrition in patients with HF has been explained in a complicated pathway. Advanced HF causes peripheral hypoperfusion, which leads to enhanced neurohormonal activation, oxidative stress, and systemic inflammatory activity.30, 31 These conditions would lead to insulin resistance, impaired protein and lipid metabolisms, anabolic deficiency, and anabolic‐catabolic imbalance. Thus, pleiotropic nutritional indices, such as the CONUT score, PNI, and GNRI, would be more‐appropriate screening tools for malnutrition in HF than albumin level or BMI alone. In that context, serum cholinesterase level is a better pleiotropic biomarker because it reflects various factors such as malnutrition, systemic inflammation, and hepatocellular impairment.13
Cholinesterase is a family of enzymes that hydrolyze acetylcholine and other choline esters.13 It is of 2 main types: acetylcholinesterase and butyrylcholinesterase. We usually measure only butyrylcholinesterase in daily blood chemistry and call it cholinesterase. Although butyrylcholinesterase has been known for decades as a marker of nutrition and hepatic protein synthesis, its specific physiological role had not been clarified until recently. Chen et al reported that butyrylcholinesterase has a specific physiological role in hydrolyzing the appetite‐promoting hormone, ghrelin.32, 33 Their group also reported that butyrylcholinesterase was not just a hydrolase of ghrelin, but also an important regulator of the butyrylcholinesterase‐ghrelin axis.34
On the other hand, one of the mechanisms of malnutrition in patients with HF has been considered to be catabolic‐anabolic imbalance and appetite loss, which is partly caused by ghrelin resistance.35 Elevated ghrelin concentration has also been reported to be a compensatory mechanism in patients with malnutrition and a powerful prognostic factor in HF patients.36, 37, 38 Taking into account these findings altogether, butyrylcholinesterase level might be decreased to elevate ghrelin level to maintain homeostasis as a compensatory mechanism in patients with ghrelin resistance and serves as a severity marker of catabolic‐anabolic imbalance. Thus, the predictive power of cholinesterase level could be explained not only by its pleiotropic characteristics, but also by identifying patients at high risk of malnutrition by the butyrylcholinesterase‐ghrelin axis, which is one of the leading mechanisms of malnutrition in HF.
Butyrylcholinesterase as a Therapeutic Target
In addition, butyrylcholinesterase inhibition has the potential to treat malnutrition in HF by elevating the activated ghrelin concentration. In fact, the appetite‐improving effect of rivastigmine, which is an acetylcholinesterase and butyrylcholinesterase inhibitor used for patients with dementia in daily practice, has already been observed in patients with dementia treated with rivastigmine by increased active‐to‐inactive ghrelin ratio.39 However, considering that butyrylcholinesterase level is already decreased as a compensatory mechanism in malnourished patients with HF, whether further butyrylcholinesterase inhibition is effective remains to be clarified.
Incremental Value of Cholinesterase Level Over the HF Risk Model
Although several HF risk models have been developed, the number of validated risk models for patients with HFpEF is relatively limited. The MAGGIC risk score is a well‐established comprehensive risk model and was externally validated for CHF patients with preserved LVEF16 and patients with ADHF.17 In the present study, we first demonstrated its prognostic value in ADHF patients with preserved LVEF. Furthermore, we showed the incremental prognostic role of cholinesterase level over the MAGGIC risk score, which is mainly composed of daily applicable measurements. The MAGGIC risk score also includes BMI, which is a component of GNRI. However, cholinesterase level was associated with poor outcome even after adjustment for BMI in the present study, which supports the concept that cholinesterase level has an incremental prognostic value over the MAGGIC risk score.
Clinical Implications
As a clinical prognostic factor, simplicity is indispensable for daily use. Cholinesterase level is a more easily available biomarker than other nutritional indices in daily practice, although the statistical superiority of cholinesterase level to the other nutritional indices was not shown in this study. This biomarker possibly provides a more‐accurate nutritional risk evaluation of patients with HFpEF than other objective nutritional indices, possibly allowing early implementation of appropriate nutritional intervention in daily practice, which leads to better outcomes in patients with HF.
Study Limitations
Several limitations of this study should be acknowledged. First, the relatively small and empirically chosen population sample size was a major limitation, and the follow‐up period was relatively short. Second, the patients in our study population were significantly older and had lower BMIs than those in a previous large‐scale HFpEF study.40 Furthermore, ethnic differences should be taken into account when generalizing our results to non‐Japanese populations. Third, although, ideally, individual body composition must be considered in defining sarcopenia, cachexia, or sarcopenic obesity when discussing malnutrition in patients with HF,27 body composition data obtained using dual‐energy x‐ray absorptiometry or bioelectric impedance analysis or body‐weight data were not available. Fourth, serum cholinesterase level would be strongly influenced by the patient's dietary intake, but precise dietary information or nutritional history taken before HF admission and after discharge was not available in the present study. Finally, we could not obtain data on serum prealbumin level, which has also been established as a marker of cardiac cachexia.41 However, in our previous study,15 cholinesterase level remained a significant predictor of all‐cause mortality that was independent of prealbumin level.
Conclusions
Serum cholinesterase level was a simple prognostic indicator for prediction of adverse outcome and tended to provide more‐powerful prognostic information than other objective nutritional indices in ADHF patients with preserved LVEF. Furthermore, cholinesterase level provided an incremental value over the MAGGIC risk score in ADHF with preserved LVEF. These findings suggest that serum cholinesterase level is a clinically significant risk‐stratification biomarker in patients with HFpEF.
Sources of Funding
This work was funded by Roche Diagnostics K.K. and Fuji Film Toyama Chemical Co. Ltd.
Disclosures
Shungo Hikoso has received personal fees from Daiichi Sankyo Company (modest), Bayer (modest), Astellas Pharma (modest), Pfizer Pharmaceuticals (modest), and Boehringer Ingelheim Japan (modest) and received grants from Roche Diagnostics (significant), FUJIFILM Toyama Chemical (significant), and Actelion Pharmaceuticals (significant). Daisaku Nakatani received a personal fee from the Daiichi–Sankyo Company (modest). Yasushi Sakata received personal fees from Otsuka Pharmaceutical (significant), Ono Pharmaceutical (modest), Daiichi Sankyo Company (significant), Mitsubishi Tanabe Pharma Corporation (modest), and Actelion Pharmaceuticals (modest) and received significant grants form Roche Diagnostic, FUJIFILM Toyama Chemical, ABbott Medical Japan, Otsuka Pharmaceutical, Daiichi Sankyo Company, Mitsubishi Tanabe Pharma Corporation, and Biotronik. The remaining authors have no disclosures to report.
Supporting information
Data S1. The OCVC‐Heart Failure Investigators.
Acknowledgments
A complete list of the OCVC‐Heart Failure Investigators can be found in Data S1.
Author Contributions: Takahisa Yamada, Shunsuke Tamaki, Shungo Hikoso, and Yasushi Sakata contributed to the data analysis and assisted in writing the manuscript. Hiroyuki Kurakami provided expert statistical advice. All the other authors contributed to the data collection and interpretation and critically reviewed the manuscript.
(J Am Heart Assoc. 2020;9:e014100 . DOI: 10.1161/JAHA.119.014100.)
References
- 1. Levy D, Kenchaiah S, Larson MG, Benjamin EJ, Kupka MJ, Ho KK, Murabito JM, Vasan RS. Long‐term trends in the incidence of and survival with heart failure. N Engl J Med. 2002;347:1397–1402. [DOI] [PubMed] [Google Scholar]
- 2. Dunlay SM, Redfield MM, Weston SA, Therneau TM, Hall Long K, Shah ND, Roger VL. Hospitalizations after heart failure diagnosis a community perspective. J Am Coll Cardiol. 2009;54:1695–1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ather S, Chan W, Bozkurt B, Aguilar D, Ramasubbu K, Zachariah AA, Wehrens XH, Deswal A. Impact of noncardiac comorbidities on morbidity and mortality in a predominantly male population with heart failure and preserved versus reduced ejection fraction. J Am Coll Cardiol. 2012;59:998–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Liu M, Chan CP, Yan BP, Zhang Q, Lam YY, Li RJ, Sanderson JE, Coats AJ, Sun JP, Yip GW, Yu CM. Albumin levels predict survival in patients with heart failure and preserved ejection fraction. Eur J Heart Fail. 2012;14:39–44. [DOI] [PubMed] [Google Scholar]
- 5. Kinugasa Y, Kato M, Sugihara S, Hirai M, Yamada K, Yanagihara K, Yamamoto K. Geriatric nutritional risk index predicts functional dependency and mortality in patients with heart failure with preserved ejection fraction. Circ J. 2013;77:705–711. [DOI] [PubMed] [Google Scholar]
- 6. Cheng YL, Sung SH, Cheng HM, Hsu PF, Guo CY, Yu WC, Chen CH. Prognostic nutritional index and the risk of mortality in patients with acute heart failure. J Am Heart Assoc. 2017;6:e004876 DOI: 10.1161/JAHA.116.004876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Minamisawa M, Seidelmann SB, Claggett B, Hegde SM, Shah AM, Desai AS, Lewis EF, Shah SJ, Sweitzer NK, Fang JC, Anand IS, O'Meara E, Rouleau JL, Pitt B, Solomon SD. Impact of malnutrition using geriatric nutritional risk index in heart failure with preserved ejection fraction. JACC Heart Fail. 2019;7:664–675. [DOI] [PubMed] [Google Scholar]
- 8. Nishi I, Seo Y, Hamada‐Harimura Y, Yamamoto M, Ishizu T, Sugano A, Sato K, Sai S, Obara K, Suzuki S, Koike A, Aonuma K, Ieda M. Geriatric nutritional risk index predicts all‐cause deaths in heart failure with preserved ejection fraction. ESC Heart Fail. 2019;6:396–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Carbone S, Canada JM, Buckley LF, Trankle CR, Billingsley HE, Dixon DL, Mauro AG, Dessie S, Kadariya D, Mezzaroma E, Buzzetti R, Arena R, Van Tassell BW, Toldo S, Abbate A. Dietary fat, sugar consumption, and cardiorespiratory fitness in patients with heart failure with preserved ejection fraction. JACC Basic Transl Sci. 2017;2:513–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Carbone S, Billingsley HE, Canada JM, Kadariya D, Medina de Chazal H, Rotelli B, Potere N, Paudel B, Markley R, Dixon DL, Trankle CR, Van Tassell BW, Celi FS, Abbate A. Unsaturated fatty acids to improve cardiorespiratory fitness in patients with obesity and HFpEF: the UFA‐Preserved Pilot Study. JACC Basic Transl Sci. 2019;4:563–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hummel SL, Karmally W, Gillespie BW, Helmke S, Teruya S, Wells J, Trumble E, Jimenez O, Marolt C, Wessler JD, Cornellier ML, Maurer MS. Home‐delivered meals postdischarge from heart failure hospitalization. Circ Heart Fail. 2018;11:e004886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, Gonzalez‐Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GMC, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37:2129–2200. [DOI] [PubMed] [Google Scholar]
- 13. Santarpia L, Grandone I, Contaldo F, Pasanisi F. Butyrylcholinesterase as a prognostic marker: a review of the literature. J Cachexia Sarcopenia Muscle. 2013;4:31–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sato T, Yamauchi H, Suzuki S, Yoshihisa A, Yamaki T, Sugimoto K, Kunii H, Nakazato K, Suzuki H, Saitoh S, Takeishi Y. Serum cholinesterase is an important prognostic factor in chronic heart failure. Heart Vessels. 2015;30:204–210. [DOI] [PubMed] [Google Scholar]
- 15. Seo M, Yamada T, Tamaki S, Morita T, Furukawa Y, Iwasaki Y, Kawasaki M, Kikuchi A, Kawai T, Abe M, Nakamura J, Yamamoto K, Kayama K, Kawahira M, Tanabe K, Kimura T, Ueda K, Sakamoto D, Sakata Y, Fukunami M. Prognostic significance of serum cholinesterase in patients with acute decompensated heart failure: a prospective comparative study with other nutritional indices. Am J Clin Nutr. 2019;110:330–339. [DOI] [PubMed] [Google Scholar]
- 16. Rich JD, Burns J, Freed BH, Maurer MS, Burkhoff D, Shah SJ. Meta‐analysis global group in chronic (MAGGIC) heart failure risk score: validation of a simple tool for the prediction of morbidity and mortality in heart failure with preserved ejection fraction. J Am Heart Assoc. 2018;7:e009594 DOI: 10.1161/JAHA.118.009594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sawano M, Shiraishi Y, Kohsaka S, Nagai T, Goda A, Mizuno A, Sujino Y, Nagatomo Y, Kohno T, Anzai T, Fukuda K, Yoshikawa T. Performance of the MAGGIC heart failure risk score and its modification with the addition of discharge natriuretic peptides. ESC Heart Fail. 2018;5:610–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ignacio de Ulibarri J, Gonzalez‐Madrono A, de Villar NG, Gonzalez P, Gonzalez B, Mancha A, Rodriguez F, Fernandez G. CONUT: a tool for controlling nutritional status. First validation in a hospital population. Nutr Hosp. 2005;20:38–45. [PubMed] [Google Scholar]
- 19. Alvares‐da‐Silva MR, Reverbel da Silveira T. Comparison between handgrip strength, subjective global assessment, and prognostic nutritional index in assessing malnutrition and predicting clinical outcome in cirrhotic outpatients. Nutrition. 2005;21:113–117. [DOI] [PubMed] [Google Scholar]
- 20. Bouillanne O, Morineau G, Dupont C, Coulombel I, Vincent JP, Nicolis I, Benazeth S, Cynober L, Aussel C. Geriatric nutritional risk index: a new index for evaluating at‐risk elderly medical patients. Am J Clin Nutr. 2005;82:777–783. [DOI] [PubMed] [Google Scholar]
- 21. Pocock SJ, Ariti CA, McMurray JJ, Maggioni A, Kober L, Squire IB, Swedberg K, Dobson J, Poppe KK, Whalley GA, Doughty RN. Predicting survival in heart failure: a risk score based on 39 372 patients from 30 studies. Eur Heart J. 2013;34:1404–1413. [DOI] [PubMed] [Google Scholar]
- 22. Kasahara S, Sakata Y, Nochioka K, Tay WT, Claggett BL, Abe R, Oikawa T, Sato M, Aoyanagi H, Miura M, Shiroto T, Takahashi J, Sugimura K, Teng TK, Miyata S, Shimokawa H. The 3a3b score: the simple risk score for heart failure with preserved ejection fraction—a report from the CHART‐2 Study. Int J Cardiol. 2019;284:42–49. [DOI] [PubMed] [Google Scholar]
- 23. Chen YJ, Sung SH, Cheng HM, Huang WM, Wu CL, Huang CJ, Hsu PF, Yeh JS, Guo CY, Yu WC, Chen CH. Performance of AHEAD score in an Asian cohort of acute heart failure with either preserved or reduced left ventricular systolic function. J Am Heart Assoc. 2017;6:e004297 DOI: 10.1161/JAHA.116.004297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kutner MH, Nachtsheim CJ, Neter J. Applied Linear Regression Models, 4th ed. New York, NY: McGraw Hill/Irwin; 2004. [Google Scholar]
- 25. DeLong ER, DeLong DM, Clarke‐Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–845. [PubMed] [Google Scholar]
- 26. Marechaux S, Six‐Carpentier MM, Bouabdallaoui N, Montaigne D, Bauchart JJ, Mouquet F, Auffray JL, Le Tourneau T, Asseman P, LeJemtel TH, Ennezat PV. Prognostic importance of comorbidities in heart failure with preserved left ventricular ejection fraction. Heart Vessels. 2011;26:313–320. [DOI] [PubMed] [Google Scholar]
- 27. Carbone S, Billingsley HE, Rodriguez‐Miguelez P, Kirkman DL, Garten R, Franco RL, Lee DC, Lavie CJ. Lean mass abnormalities in heart failure: the role of sarcopenia, sarcopenic obesity, and cachexia. Curr Probl Cardiol. 2019. Available at: https://www.sciencedirect.com/science/article/abs/pii/S014628061930057X?via%3Dihub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Emami A, Saitoh M, Valentova M, Sandek A, Evertz R, Ebner N, Loncar G, Springer J, Doehner W, Lainscak M, Hasenfuss G, Anker SD, von Haehling S. Comparison of sarcopenia and cachexia in men with chronic heart failure: results from the Studies Investigating Co‐morbidities Aggravating Heart Failure (SICA‐HF). Eur J Heart Fail. 2018;20:1580–1587. [DOI] [PubMed] [Google Scholar]
- 29. Cacciatore F, Della‐Morte D, Basile C, Curcio F, Liguori I, Roselli M, Gargiulo G, Galizia G, Bonaduce D, Abete P. Butyryl‐cholinesterase is related to muscle mass and strength. A new biomarker to identify elderly subjects at risk of sarcopenia. Biomark Med. 2015;9:669–678. [DOI] [PubMed] [Google Scholar]
- 30. Levine B, Kalman J, Mayer L, Fillit HM, Packer M. Elevated circulating levels of tumor necrosis factor in severe chronic heart failure. N Engl J Med. 1990;323:236–241. [DOI] [PubMed] [Google Scholar]
- 31. Rossignol P, Masson S, Barlera S, Girerd N, Castelnovo A, Zannad F, Clemenza F, Tognoni G, Anand IS, Cohn JN, Anker SD, Tavazzi L, Latini R. Loss in body weight is an independent prognostic factor for mortality in chronic heart failure: insights from the GISSI‐HF and Val‐HeFT trials. Eur J Heart Fail. 2015;17:424–433. [DOI] [PubMed] [Google Scholar]
- 32. Chen VP, Gao Y, Geng L, Parks RJ, Pang YP, Brimijoin S. Plasma butyrylcholinesterase regulates ghrelin to control aggression. Proc Natl Acad Sci USA. 2015;112:2251–2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chen VP, Gao Y, Geng L, Brimijoin S. Butyrylcholinesterase regulates central ghrelin signaling and has an impact on food intake and glucose homeostasis. Int J Obes (Lond). 2017;41:1413–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Brimijoin S, Chen VP, Pang YP, Geng L, Gao Y. Physiological roles for butyrylcholinesterase: a BChE‐ghrelin axis. Chem Biol Interact. 2016;259:271–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Attanasio P, Anker SD, Doehner W, von Haehling S. Hormonal consequences and prognosis of chronic heart failure. Curr Opin Endocrinol Diabetes Obes. 2011;18:224–230. [DOI] [PubMed] [Google Scholar]
- 36. Chen Y, Ji XW, Zhang AY, Lv JC, Zhang JG, Zhao CH. Prognostic value of plasma ghrelin in predicting the outcome of patients with chronic heart failure. Arch Med Res. 2014;45:263–269. [DOI] [PubMed] [Google Scholar]
- 37. Nagaya N, Uematsu M, Kojima M, Date Y, Nakazato M, Okumura H, Hosoda H, Shimizu W, Yamagishi M, Oya H, Koh H, Yutani C, Kangawa K. Elevated circulating level of ghrelin in cachexia associated with chronic heart failure: relationships between ghrelin and anabolic/catabolic factors. Circulation. 2001;104:2034–2038. [DOI] [PubMed] [Google Scholar]
- 38. Nagaya N, Uematsu M, Kojima M, Ikeda Y, Yoshihara F, Shimizu W, Hosoda H, Hirota Y, Ishida H, Mori H, Kangawa K. Chronic administration of ghrelin improves left ventricular dysfunction and attenuates development of cardiac cachexia in rats with heart failure. Circulation. 2001;104:1430–1435. [DOI] [PubMed] [Google Scholar]
- 39. Furiya Y, Tomiyama T, Izumi T, Ohba N, Ueno S. Rivastigmine improves appetite by increasing the plasma Acyl/Des‐Acyl ghrelin ratio and cortisol in Alzheimer disease. Dement Geriatr Cogn Dis Extra. 2018;8:77–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Haass M, Kitzman DW, Anand IS, Miller A, Zile MR, Massie BM, Carson PE. Body mass index and adverse cardiovascular outcomes in heart failure patients with preserved ejection fraction: results from the irbesartan in heart failure with preserved ejection fraction (I‐PRESERVE) trial. Circ Heart Fail. 2011;4:324–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Araujo JP, Lourenco P, Rocha‐Goncalves F, Ferreira A, Bettencourt P. Nutritional markers and prognosis in cardiac cachexia. Int J Cardiol. 2011;146:359–363. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. The OCVC‐Heart Failure Investigators.


