Introduction
In the United States, >1 million people are living with HIV, and ≈50% are >50 years old.1 Potent combination antiretroviral therapy (ART) has significantly reduced the morbidity and mortality of people living with HIV (PLWH).1, 2 However, as AIDS‐related mortality has declined, death due to cardiovascular disease (CVD) and non–AIDS‐associated malignancies has increased.3, 4 This changing pattern of mortality can be partly attributed to the fact that PLWH are living longer on effective ART and thus are now at risk for age‐related diseases, but PLWH are dying from these diseases at higher rates and at younger ages than the general population.1, 5, 6
Epidemiologic research has demonstrated higher rates of heart failure (HF),7, 8, 9, 10 coronary artery disease (CAD),11, 12 pulmonary hypertension,13 sudden cardiac death,14, 15 and stroke8 in PLWH on ART compared with those without HIV, even when controlling for traditional cardiovascular risk factors. PLWH on ART have also been shown to have higher rates of subclinical CVD. including myocardial fibrosis and steatosis,16, 17, 18 as well as impaired systolic and diastolic function19, 20 compared with controls without HIV. For these reasons, clinical cardiovascular specialists are likely to encounter PLWH who are at risk for or who have overt CVD, and HIV‐related CVD is poised to become a major public health problem in the coming decades.
In the ART era, CVD research has focused largely on mechanisms and prevention of CAD in PLWH, motivated in part by the fact that treatment with ART (particularly older, protease inhibitor–containing regimens) can directly and quickly lead to the development of an abnormal lipid profile that is amplified by the chronic inflammation associated with HIV.21, 22, 23, 24, 25 Although the link between inflammation and atherosclerosis is well established,26 chronic inflammation is increasingly being understood as a key mediator of other types of CVD, including HF, and may be a prominent mediator of the increased risk of CVD in PLWH.27, 28, 29, 30, 31
Coronary microvascular dysfunction (CMD) is an important pathophysiologic link between cardiovascular risk factors, chronic inflammation, endothelial activation, and the development of clinical CVD.17, 32, 33 CMD is also a risk marker and can be used to risk stratify and to study the effects of particular interventions on coronary microvascular function. Given the elevated rates of both subclinical and clinical CVD among PLWH, along with the lack of evidence‐based therapeutics that can be used to specifically target the increased risk of CVD in treated HIV, we believe it is critical to investigate specific mechanisms, such as CMD, that might provide insight into the mechanisms underlying CVD in PLWH. In addition, CMD in PLWH may also provide a unique model to better understand the relationship among immune activation, inflammation, and CVD in people without HIV. In this review, we describe (1) the relationship between inflammation and endothelial dysfunction, as well as methods for the assessment of peripheral endothelial function; (2) the pathophysiology of CMD and its possible contribution to CVD in PLWH; (3) strengths and limitations of prior studies that have examined CMD in PLWH; and (4) unmet needs and future directions for the study of CMD in PLWH.
Inflammation, Endothelial Activation, and Peripheral Endothelial Dysfunction
Endothelial cells, like circulating cells of the innate immune system, express immune receptors, and binding of damage‐associated molecular patterns, pathogen‐associated molecular patterns, and inflammatory cytokines lead to upregulation and release of proinflammatory cytokines such as interleukin‐6 (IL‐6), interleukin‐1 alpha (IL‐1α), and interleukin‐1 beta (IL‐1β); increased expression of adhesion molecules such as vascular cell adhesion molecule‐1 (VCAM‐1) and intercellular adhesion molecule‐1 (ICAM‐1); and detachment of endothelial cells from their underlying supporting matrix.34, 35
Endothelial activation can lead to endothelial dysfunction via the inhibition of endothelial nitric oxide synthase by reactive oxygen species, thereby decreasing nitric oxide production.36 Nitric oxide–dependent pathways are able to prevent endothelial activation by decreasing expression of cell adhesion markers and by inhibiting platelet activation by promoting cGMP signaling.37 Platelets themselves, once thought of nearly exclusively in the context of thrombosis and hemostasis, are now understood as important mediators and effectors of inflammation. Platelets express toll‐like receptors, which, when bound by ligands such as LPS (lipopolysaccharide), release proinflammatory cytokines such as IL‐1β.38 Activated platelets also can induce the transcription of inflammatory proteins in leukocytes via the interaction of P‐selectin, expressed on the surface of activated platelets, and PSGL‐1 (P‐selectin glycoprotein ligand 1), a receptor constitutively expressed by leukocytes.39 In addition, the interaction of P‐selectin and PSGL‐1 can lead to leukocyte expression of tissue factor and localization of leukocytes to the vascular endothelium, promoting inflammation within the microvasculature.38, 39, 40
Endothelial dysfunction, a consequence of impaired nitric oxide signaling, is an early event in the development of diseases such as CAD41, 42 and HF (specifically, HF with preserved ejection fraction)43 and of extracardiac diseases such as chronic kidney disease.44 The endothelial dysfunction seen in these diseases is also seen, as one might expect, in the risk factors associated with these conditions, such as obesity, hypertension, and diabetes mellitus.45 Endothelial dysfunction is also present in chronic inflammatory states such as systemic lupus erythematosus, rheumatoid arthritis, and psoriasis and correlates with the increased cardiac risk seen in these populations.43, 46, 47 Systemic endothelial function can be assessed through serum and urine analyses, and peripheral endothelial function can be assessed with both invasive and noninvasive functional testing.35, 48, 49, 50, 51, 52, 53
The most frequently used functional tests of peripheral endothelial function are brachial flow‐mediated dilation (FMD) and peripheral arterial tonometry. These tests noninvasively measure endothelial function by assessing the endothelium‐dependent response to ischemia induced by occlusion of peripheral arteries in the forearm (ie, the brachial artery or its distal branches). The health of the endothelium, as measured by brachial FMD and peripheral arterial tonometry, has been shown to correlate with cardiovascular outcomes, although results from these 2 tests do not consistently correlate with one another.41, 54
PLWH have evidence of chronic immune activation and systemic and peripheral endothelial dysfunction. PLWH have increased levels of inflammation markers such as high‐sensitivity C‐reactive protein and IL‐6, that decrease but do not normalize with ART and that are associated with increased morbidity and mortality.55, 56 Increased inflammatory markers have been observed before and after ART initiation (in those treated during acute or chronic stages of infection) and in PLWH who maintain an undetectable viral load without ART (ie, elite HIV controllers).57, 58 Importantly, the pivotal Strategies for Management of Antiretroviral Therapies (SMART) trial demonstrated decreased levels of inflammation and improved cardiovascular outcomes in those treated continuously with ART, in contrast to those whose exposure to ART was minimized, although there may be differences in the reduction of inflammation by ART class.59, 60 Notably, contemporary ART regimens, which frequently include an integrase inhibitor such as dolutegravir, are associated with weight gain more than the older ART regimens, and the long‐term effects of these agents on vascular inflammation and CVD are incompletely understood and should be investigated by future studies.61 The increased inflammation and immune activation seen in PLWH is hypothesized to be multifactorial: gut microbial translocation, immune dysregulation, low‐level viremia, and viral coinfections (eg, cytomegalovirus).62, 63, 64 There is also a higher prevalence of traditional cardiovascular risk factors such as smoking and hypertension that may also contribute to the chronic inflammatory state seen in PLWH on ART.65, 66
There is evidence of endothelial dysfunction in PLWH, as measured by elevations in circulating markers, such as ICAM‐1 and VCAM‐1, and by FMD.62, 67, 68, 69, 70, 71, 72, 73 Although in vitro studies have shown that ART can lead directly to endothelial activation, most in vivo studies have demonstrated decreased levels of endothelial activation and improved endothelial function with ART initiation, although the degree of improvement may vary by drug class and typically does not normalize to levels seen in people without HIV.71, 74 Although there is evidence of endothelial dysfunction in PLWH, studies that have found increased endothelial dysfunction (by FMD) in PLWH compared with controls have frequently been complicated by small sample sizes and inclusion of PLWH not on ART and with detectable HIV RNA levels (Table 1).42, 54, 69, 75, 76, 77, 78, 79, 80, 81
Table 1.
Brachial FMD in PLWH and Controls
| Reference | Year Published | Participants (HIV/Control) | HIV Treated | Cardiovascular Risk Factors | FMD Results | Limitations |
|---|---|---|---|---|---|---|
| Nolan et al75 | 2003 | 24/24 (adult) | On PI for >9 mo | Excluded: ACEI, lipid‐lowering drug use, DM renal failure | No significant difference in FMD | Small sample size, specific to PI treatment |
| Bonnet et al76 | 2004 | 49/24 (pediatric) | 15 treatment naïve | Excluded: DM, HTN, renal failure | Significantly lower FMD in HIV+ compared with controls | Participants not on ART |
| Charakida et al77 | 2005 | 83/59 (pediatric) | 27 treatment naïve | Excluded: DM, HTN, renal failure | Significantly lower FMD in HIV+ compared with controls | Participants not on ART |
| Solages et al42 | 2006 | 76/227 (adult) | 12 off ART | Excluded: CVD, DM, HTN | Significantly lower FMD in HIV+ compared with controls | Participants not on ART |
| van Wijk et al78 | 2006 | 37/27 (13 with DM, 14 without DM) (adult) | 100% ART | Excluded: antihypertensive use, DM (except control DM arm), lipid‐lowering drug use, renal disease | Significantly lower FMD in HIV+ compared with controls, HIV without MetS comparable to HIV− with DM | Small sample size, treatment options have changed markedly since 2006 |
| Rose et al69 | 2013 | 36/50 (published reference group; adult) | 100% treatment naïve | Excluded: BMI >27, lipid‐lowering drug use | Significantly lower FMD in HIV+ compared with controls | Participants not on ART |
| Gleason et al79 | 2015 | 281/36 (adult) | 51 treatment naïve/230 treated | Excluded: DM | Worse FMD if treatment with efavirenz or ritonavir boosted lopinavir compared with those on nevirapine or treatment naive | Inclusion without analysis of HIV vs controls, participants not on ART |
| Koethe et al80 | 2016 | 70 (35 nonobese, 35 obese)/30 obese (adult) | 100% on ART | Excluded: CVD, DM, statin use | No significant differences in FMD between obese HIV+ and obese HIV− | Small sample size |
| Dysangco et al54 | 2017 | 72/39 (adult) | 44 off ART, 28 on ART | Excluded: CVD, DM, statin use, uncontrolled HTN | No significant differences in FMD among 3 groups (treated, untreated, control) | Participants not on ART |
| Sharma et al81 | 2018 | 43/25 (adult) | Treatment naïve | Excluded: BMI >25, CVD, DM, HTN, renal disease | Significantly lower FMD in HIV+ compared with controls | Participants not on ART |
Types of ART: non‐nucleoside reverse transcriptase inhibitors: efavirenz, nevirapine; PIs: ritonavir, boosted lopinavir. ACEI indicates angiotensin‐converting enzyme inhibitor; ART, antiretroviral therapy; BMI, body mass index; CVD, cardiovascular disease; DM, diabetes mellitus; FMD, flow‐mediated dilation; HTN, hypertension; MetS, metabolic syndrome; PI, protease inhibitor; PLWH, people living with HIV.
In addition, evidence shows that platelets in PLWH show signs of increased activation, increased spontaneous and induced aggregation, decreased responsiveness to antiplatelet agents, and increased platelet‐mediated endothelial and leukocyte activation.82, 83, 84 Notably, the directionality of platelet dysfunction has not been well established; it is not known whether platelet activation is a consequence of impaired endothelial function and decreased cGMP signaling or whether abnormalities in platelet function lead to or amplify endothelial activation and dysfunction.
Normal and Abnormal Coronary Microvascular Function
Although peripheral tests of endothelial function have been shown to correlate with cardiovascular outcomes, studying the vascular bed of interest—the coronary circulation—provides more targeted information. CMD describes abnormalities in cardiac blood flow that are not the result of stenosis in major epicardial arteries.85 The coronary microvasculature includes both coronary prearterioles and arterioles; extramyocardial prearterioles dilate and constrict largely due to changes in pressure and flow, whereas the arterioles, embedded within the myocardium, respond primarily to changes in myocardial demand via metabolic signaling across the vascular endothelium.86 Both segments, however, rely on endothelium‐dependent nitric oxide signaling for dilation.86 Abnormal coronary microvascular function may be the result of endothelial cell dysfunction, vascular smooth muscle cell dysfunction, or abnormal vascular remodeling.87 Endothelial dysfunction in the coronary microvasculature can lead to myocardial ischemia, with or without anginal symptoms; increased platelet aggregation and activation; and leukocyte adhesion to and transmigration across the vascular endothelium—processes key to the development of coronary heart disease and HF via atherosclerosis and myocardial fibrosis, respectively.88
Although the coronary microvascular circulation cannot be visualized directly, invasive and noninvasive techniques can assess coronary microvascular function. Coronary microvascular function is assessed by comparing resting and hyperemic myocardial or coronary blood flow, reported as the myocardial or coronary flow reserve. Hyperemia can be induced either through the infusion of vasoactive substances, such as adenosine, acetylcholine, or dipyridamole, or through a maneuver such as cold‐pressor testing.87 Myocardial flow reserve can be assessed invasively using a blush score or noninvasively using myocardial contrast echocardiography, cardiac magnetic resonance, or positron emission tomography/computed tomography (PET/CT) imaging.89, 90 Coronary flow reserve can be measured invasively, using coronary Doppler wires or thermodilution, or noninvasively, using Doppler echocardiography.91 Coronary endothelial function does not specifically assess the microvasculature but is an important potential contributor to CMD in those for whom CMD is hypothesized to be secondary to inflammation and subsequent endothelial dysfunction (eg, PLWH). Coronary endothelial function is typically assessed invasively with coronary angiography or ultrasound using either medications (eg, acetylcholine, salbutamol), pacing, or exercise to induce endothelium‐dependent changes in epicardial blood flow.89, 92 Detailed reviews of the methods of assessing CMD can be found elsewhere.93, 94
Importantly, both intrinsic and extrinsic factors can result in a diagnosis of CMD because of the indirect nature of each of the aforementioned diagnostic techniques for measuring myocardial or coronary blood flow. Besides intrinsic factors (eg, primary endothelial or vascular smooth muscle dysfunction), extrinsic factors such as elevated diastolic intracardiac pressures, interstitial myocardial fibrosis, and cardiomyocyte hypertrophy with insufficient coronary microvascular blood supply can each cause a reduction in myocardial or coronary blood flow. In addition, if endothelial dysfunction is severe enough, the endothelial cells can undergo apoptosis, leading to coronary microvascular (capillary) rarefaction. Understanding which of these factors (alone or in combination) results in reduced coronary or microvascular blood flow has important implications for determining the optimal course of treatment for patients with CMD.
Abnormal coronary microvascular function is independently associated with an increased risk of cardiovascular events and increased all‐cause mortality in specific populations. For example, CMD is associated with increased all‐cause mortality in patients with microvascular angina (ie, ischemia and no obstructive CAD),95, 96 increased cardiovascular events in chronic kidney disease,97 and increased cardiovascular mortality in those with suspected or known CAD, hypertrophic cardiomyopathy, or dilated cardiomyopathy.86, 98, 99 CMD is also seen in those with cardiovascular risk factors such as diabetes mellitus, hyperlipidemia, and hypertension and has been shown to improve when these risk factors are treated appropriately.86, 91 In addition, those with autoimmune and inflammatory diseases, without traditional cardiac risk factors, may have CMD, and treatment of the rheumatologic disease improves their coronary microvascular function.100, 101
More than a decade ago, Camici and Crea proposed a framework that divided CMD into 4 categories: (1) CMD without obstructive CAD or myocardial disease, (2) CMD without obstructive CAD but with myocardial disease, (3) CMD with obstructive CAD, and (4) iatrogenic CMD.86 Although PLWH may have CMD that falls into any of these categories, given elevated rates of atherosclerotic diseases, myocardial fibrosis, and greater prevalence of cardiovascular risk factors, the degree to which CMD exists in PLWH as a consequence of upregulated inflammatory pathways is of particular interest because it may provide insight into the unique mechanisms of cardiovascular risk seen in this population (Figures 1 and 2). Furthermore, understanding if and how proinflammatory conditions like HIV are related to CMD may provide insight into other etiologies of CMD in people without HIV.
Figure 1.
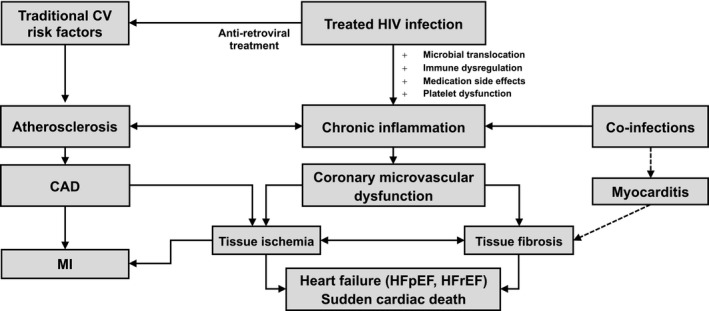
Proposed mechanisms of coronary microvascular dysfunction in treated HIV. People living with HIV (PLWH) have elevated markers of inflammation secondary to both traditional cardiovascular risk factors (eg, smoking, hypertension) and risk factors associated with HIV infection, its treatment, and coinfections. Chronic inflammation can lead to endothelial dysfunction through suppression of endothelial nitric oxide synthase in both systemic and coronary circulation, leading to coronary microvascular dysfunction (CMD), tissue ischemia, and fibrosis. Ultimately, CMD, along with atherosclerosis and its sequelae, may lead to the cardiovascular conditions overrepresented in PLWH including heart failure and sudden cardiac death. CAD indicates coronary artery disease; CV, cardiovascular; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; MI, myocardial infarction.
Figure 2.
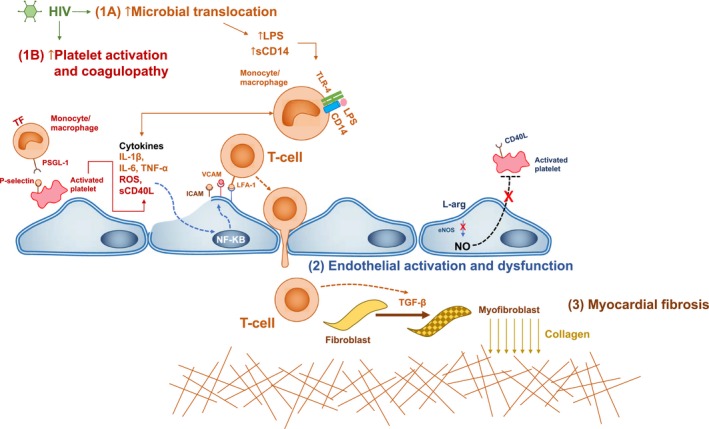
Proposed molecular mechanisms underlying inflammation, coronary microvascular dysfunction, and myocardial fibrosis in treated HIV. People living with HIV (PLWH) have increased levels of LPS (lipopolysaccharide), soluble CD14 (cluster of differentiation 14; sCD14), IL‐6 (interleukin 6), tissue factor (TF)–expressing monocytes/macrophages, signs of platelet activation, and increased levels of ICAM‐1 (intercellular adhesion molecule 1) and VCAM‐1 (vascular cell adhesion molecule 1). We propose the interrelated pathways that may lead to coronary microvascular dysfunction in HIV. 1A, LPS and CD14 interaction, secondary to microbial translocation, lead to macrophage activation, TF expression and release of proinflammatory cytokines including IL‐1, IL‐6, and TNF‐α (tumor necrosis factor α). This leads upregulation of inflammatory pathways in endothelial cells mediated by NF‐κB (nuclear factor κB), leading to increased ICAM‐1 and VCAM‐1 expression and downregulation of endothelial nitric oxide (NO) synthase (eNOS) leads to decreased conversion of the amino acid L‐arginine (L‐arg) to NO. 1B, Platelet activation leads to the release of cytokines and procoagulopathic molecules as well as cell‐surface expression of CD40L (cluster of differentiation 40 ligand) and P‐selectin. Platelets interact with lymphocytes via P‐selectin and PSGL‐1 (P‐selectin glycoprotein ligand 1) facilitating lymphocyte rolling and interactions with endothelial cells via adhesion molecules (e.g., ICAM‐1 and VCAM‐1) and integrins (e.g., lymphocyte function associated antigen‐1 [LFA‐1]). 2, Endothelial cell activation and nitric oxide downregulation leads to increased platelet activation, increased inflammatory and endothelial interaction, and lymphocyte transmigration across the vessel wall. 3, Lymphocyte transmigration leads to increased TGF‐β (transforming growth factor β) expression in the subendothelial space, leading to the transformation of fibroblasts into myofibroblasts with subsequent collagen deposition and fibrosis. ROS indicates reactive oxygen species.
CMD in HIV
To our knowledge, 2 studies have investigated the frequency and associations of CMD through assessment of myocardial flow reserve, and 3 studies have investigated coronary endothelial dysfunction by measuring changes in coronary artery blood flow in PLWH compared with controls. Two other studies have quantified CMD in those with HIV but without the inclusion of controls (Table 2).102, 103, 104, 105, 106, 107, 108
Table 2.
Coronary Microvascular and Endothelial Function in PLWH
| Reference | Publication Year | Imaging Modality (Stressor) | No. HIV+ Participants (Subgroups)/No. Controls | Cardiovascular RF (Exclusion Criteria, Baseline Characteristics of PLWH vs Controls) | HIV Characteristics | Results | MFR/CEF | Limitations |
|---|---|---|---|---|---|---|---|---|
| Lebech et al102 | 2008 | PET/CT (dipyridamole) | 25 (13 with TC ≤215 mg/dL; 12 with TC ≥254 mg/dL)/14 |
Excluded: antihypertensive therapy, CVD, DM, (current) smoking, statin use; in TC <215 mg/dL, lower HDL, higher TC vs controls |
100% ART, 60% on PI; mean CD4, 653±273 cells/mm3; VL suppressed in 80% | No significant difference in MFR, FMD, and NMD | MFR in PLWH: 3.2±0.3 (normal TC), 3.2±0.3 (elevated TC); MFR in controls: 3.0±0.3 | Small sample size, restrictive inclusion criteria |
| Kristoffersen et al103 | 2010 | PET/CT (cold‐pressor testing, dipyridamole) | 12/0 | Excluded: DM, HTN, ischemic heart disease, lipid‐lowering treatment |
Baseline: 0% on ART; mean CD4, 251±68 cells/mm3; VL unsuppressed; after ART: not reported |
FMD, MFR significantly lower after ART initiation; not significant in cold‐pressor reserve | MFR at baseline: 3.11±0.32; after treatment: 2.48±0.25 | No control group, small sample size |
| Knudsen et al104, a | 2015 | PET/CT (adenosine) | 56/25 | No cardiovascular RF exclusion criteria; increased TC and HDL in PLWH | 100% on ART (34% on PI); median CD4, 675 cells/mm3 (range: 285–1390); 100% VL <40 copies/mL | No significant differences in MFR | MFR in PLWH: 2.97±0.11; MFR in controls: 3.13±0.17 | Danish cohort, generalizable to US population? |
| Iantorno et al105, b | 2017 | cMRI (IHE) | 35 (18 CAD−, 17 CAD+)/77 (36 CAD−, 41 CAD+) |
HIV−/CAD−: ≤50 y excluded CAD, DM, >1 cardiovascular RF; >50 y (HIV+/HIV−): CACS=0, CAD+; >50 y (HIV−/HIV+): 30–70% luminal stenosis, HIV+ significantly higher HCV positivity |
CAD−: 94% on ART (60% on PI); mean CD4, 614±93 cells/mm3; VL suppressed in 83%; CAD+: 100% on ART (58% on PI); mean CD4, 629±63 cells/mm3; VL suppressed in 95% |
HIV+/CAD− significantly impaired CEF compared with HIV−/CAD−; IL‐6 inversely correlated with CEF in HIV+ |
HIV−/CAD− CSA change: +10.2±0.9%; CBF change: +41.2±5.1%; HIV+/CAD− CSA change: +3.22±1.2% CBF change: (+6.2±3.0%) |
Use of new method to assess CEF, small subgroups, frequent PI use, variability in VL suppression |
| Knudsen et al106, a | 2018 | PET/CT (adenosine) | 94 (50 men, 44 women)/0 |
No exclusion based on cardiovascular RF; women younger, less likely on antihypertensives and statins, higher DBP and HDL, lower creatinine and FRS |
Men: 100% on ART (52% on PI); median CD4, 645 cells/mm3 (range: 285–1390); 94% with suppressed VL; women: 100% on ART (27% PI); median CD4, 644 cells/mm3 (range: 222–1780); 93% with suppressed VL |
Women significantly lower MFR than men; significant (negative) association between CMV IgG and MFR in women |
Women with HIV: 2.13±0.10; men with HIV: 2.57±0.11 | No control group, significant differences in baseline cardiovascular RF |
| Iantorno et al107, b | 2018 | cMRI (IHE) | 51 (36 CAD−, 15 CAD+)/14 CAD− |
HIV−: excluded CAD or any CAD RF; if >50 y, excluded CACS >0; HIV+/CAD−: excluded CACS >0; in HIV+/CAD+, 30% to 70% luminal stenosis |
CAD−: 94% on ART (16% on PI); mean CD4, 611±480 cells/mm3; 89% with suppressed VL; CAD+: 73% on ART (40% on PIs); mean CD4, 619±254 cells/mm3; 80% with suppressed VL |
CEF significantly impaired in HIV+; epicardial adipose tissue correlated with CSA change in HIV+ |
HIV−/CAD− CSA change, +10.1±5.5%; CBF change, +33.2±15.2%; HIV+/CAD− CSA change, +0.2±12.3%; CBF change, +0.2±22.5%; HIV+/CAD+ CSA change, −1.1±3.8%; CBF change, +2.3±17.5% |
Participants not on ART, use of new method to assess CEF |
| Leucker et al108 | 2018 | cMRI (IHE) | 48/15 |
HIV−: excluded CVD if >1 cardiovascular RF; if ≥40 y, CACS >0; HIV+: excluded CACS >0, CVD, significantly lower HDL, higher statin use; higher % men in HIV+ |
100% on ART (0% on PI); 100% with suppressed VL; median CD4, 665 (range: 456–933) |
CEF significantly impaired in HIV+ Inverse relationship between CSA change and PCSK9 level |
HIV−/CAD− CSA change: +11.1±‐3.7% CBF change: +37.5±10.0% HIV+/CAD− CSA change: +2.9±9.6% CBF change: +6.2±19.2% |
Use of new method to assess CEF, differences in cardiovascular RF between groups |
ART indicates antiretroviral therapy; CACS, coronary artery calcium score; CAD, coronary artery disease; CBF, coronary blood flow; CEF, coronary endothelial function; cMRI, cardiac magnetic resonance imaging; CMV, cytomegalovirus; CSA, coronary surface area; CVD, cardiovascular disease; DBP, diastolic blood pressure; DM, diabetes mellitus; FMD, flow‐mediated dilation; FRS, Framingham risk score; HCV, hepatitis C virus; HDL, high‐density lipoprotein; HTN, hypertension; IHE, isometric handgrip exercise; IL, interleukin; MFR, myocardial flow reserve; NMD, nitroglycerine‐mediated dilation; PET/CT, positron emission tomography/computed tomography; PI, protease inhibitor; PLWH, people living with HIV; RF, risk factor; TC, total cholesterol; TG, triglycerides; VL, viral load.
Some participants were included in both studies.
VL suppressed: <20 copies/mL.
Lebech et al measured coronary microvascular function using PET/CT in PLWH on ART who had minimal traditional cardiovascular risk factors, apart from a subgroup with elevated total cholesterol (≥254 mg/dL).102 They paired their investigation with an assessment of brachial FMD and nitroglycerin‐mediated dilation. Global myocardial blood flow was assessed at rest and after dipyridamole infusion. Myocardial flow reserve was calculated as a ratio of global myocardial blood flow at stress to global myocardial blood flow at rest. No differences were found in myocardial flow reserve, FMD, or nitroglycerin‐mediated dilation among the 3 groups.
Knudsen et al also measured myocardial flow reserve using PET/CT in PLWH on ART compared with controls without HIV.104 Left ventricular ejection fraction and coronary artery calcium score were simultaneously assessed. Baseline characteristics were similar in the 2 groups, apart from higher systolic blood pressure, LDL (low‐density lipoprotein), and HDL (high‐density lipoprotein) in those with HIV compared with controls. The mean Framingham risk score was <10% in both groups, and the median coronary artery calcium score in both groups was zero. Myocardial blood flow was assessed at rest and after adenosine infusion. Myocardial flow reserve, calculated as described earlier, was similar in PLWH and controls. Notably, myocardial blood flow at rest and at stress was significantly lower in PLWH compared with controls, as was mean left ventricular ejection fraction. No correlations were found among myocardial flow reserve, current CD4 count, nadir CD4 count, use of a protease inhibitor, or duration of HIV.
Three additional studies measured coronary endothelial function using cardiac magnetic resonance imaging and isometric handgrip exercise. In all studies, coronary endothelial function was assessed by cross‐sectional images of a native coronary artery with <30% luminal stenosis, and the changes in coronary cross‐sectional area and coronary flow velocity during handgrip exercise were assessed. Prior research has shown that handgrip exercise–mediated changes in coronary blood flow and surface area are mediated by nitric oxide, and coronary endothelial function measurements have shown good reproducibility in a small cohort of healthy and CAD patients.109 Although all 3 studies used the same methodology, the groups enrolled and the variables measured varied among the groups and will be described individually.
Iantorno et al enrolled PLWH on ART and HIV‐negative controls with and without a history of CAD, leading to the creation of 4 groups.105 PLWH without a history of CAD (HIV+/CAD−) were found to have significantly impaired coronary endothelial function compared with controls, as measured by change in mean coronary flow velocity and coronary artery surface area. Coronary endothelial function did not significantly differ between the 2 groups with CAD (HIV+/CAD+ and HIV−/CAD+). Notably, coronary endothelial function in PLWH without CAD was not significantly different from both groups with CAD. Coronary endothelial function was found to be significantly inversely correlated with IL‐6 levels among those with HIV, and no associations between coronary endothelial function and HIV‐specific parameters (CD4 count at time of testing, viral load, ART regimen) were reported.
Iantorno et al conducted an additional study investigating the relationship between coronary endothelial function and local epicardial fat in PLWH and HIV‐negative controls.107 Three groups were included: PLWH with and without CAD and controls without CAD (HIV+/CAD−, HIV+/CAD+, HIV−/CAD−). Data from their prior study were included in this study. Coronary endothelial function was calculated for all groups. Changes in coronary artery surface area and coronary blood flow were significantly lower in those with HIV, with and without CAD, compared with HIV‐negative controls. Local epicardial adipose tissue was also inversely correlated with the change in coronary artery surface area (but not coronary artery flow velocity) among those with HIV.
The third study, from Leuker et al, measured coronary endothelial function in PLWH and HIV‐negative controls without CAD (HIV+/CAD−, HIV−/CAD−). This study again demonstrated impaired coronary endothelial function in those with HIV compared with controls.108 The authors also found a significant inverse relationship between proprotein convertase subtilisin/kexin type 9 (PCSK9) levels and coronary artery surface area dilation among those with HIV but not among controls without HIV.
Although the following 2 studies did not enroll controls without HIV, they assessed CMD in PLWH on ART using PET/CT. Kristoffersen et al enrolled 12 PLWH before ART initiation and measured myocardial flow reserve and FMD before treatment and then again after therapy was initiated, 24 to 67 days later.103 Myocardial flow reserve, assessed by PET/CT, and dipyridamole infusion as well as cold‐pressor testing were used to induce hyperemia. The authors were able to obtain a full set of images from only 9 participants but found a significant decrease in myocardial flow reserve (20%), driven by a decrease in the maximal blood flow after dipyridamole infusion, and a significant decrease in FMD after ART initiation.
Knudsen et al, building on their earlier study, compared myocardial flow reserve in men and women living with HIV.106 They used PET/CT and adenosine infusion to induce hyperemia. Male participants from their previous study were included, and women were recruited from the Study of HIV, Cervical Abnormalities, and Infections in Women from Denmark (SHADE) cohort, which follows women with HIV in Denmark. They found that women with HIV had significantly lower myocardial flow reserve than men, and myocardial flow reserve in women was found to inversely correlate with cytomegalovirus IgG, whereas no such association was found for men.
A number of possible explanations exist for the inconsistent results of studies comparing coronary microvascular or endothelial function in PLWH and controls without HIV. First, although all studies aimed to enroll participants without a high cardiovascular risk burden (apart from the studies that specifically enrolled patients with CAD), they varied significantly in their enrollment criteria; some excluded participants with any cardiovascular risk factors, whereas others allowed patients with diabetes mellitus, hypertension, and CAD to enroll. Most studies reported no significant differences in risk factor burden between PLWH and controls, but study groups were small, with ranges of 13 to 56 participants with HIV and 14 to 44 participants without HIV included in the studies.
In addition, although HIV‐specific parameters were similar in all studies, viral suppression (viral load <20 copies/mL) and ART use varied between studies, with 77% to 100% suppressed and 73% to 100% on ART. Mean or median current CD4+ T‐cell count was measured in all studies, and counts were consistently between 600 and 700 cells/μL. Conversely, nadir CD4 count was reported in only 2 studies (although tests of association were done in 3 studies).102, 104, 106 Notably, no study reported any associations between HIV‐related parameters (including use of protease inhibitors, nadir CD4 count, current CD4 count, HIV RNA levels, or hepatitis C virus coinfection) and myocardial flow reserve or coronary endothelial function.
All studies were done by researchers from just 2 academic hospitals, one in the United States and one in Denmark. The 2 Danish studies found no differences in myocardial blood flow in PLWH compared with controls, whereas all 3 studies from the United States found significant differences between the 2 groups. The geographic differences also corresponded to differences in imaging modality and stressor. Although both adenosine and dipyridamole are commonly used to assess coronary microvascular function, they have been shown to act in both an endothelium‐dependent and endothelium‐independent manner to promote vasodilation.110 In contrast, isometric handgrip exercise has been shown to act primarily in an endothelium‐dependent manner via nitric oxide signaling. Both adenosine and dipyridamole have been used in studies that found abnormal myocardial flow reserve or coronary flow reserve in patients with chronic inflammatory diseases compared with controls; however, CMD in PLWH may be more specifically related to abnormalities at the level of the endothelium, and thus fewer abnormalities may be noted with adenosine or dipyridamole.
The localization of abnormalities of coronary blood flow to the endothelium is consistent with the evidence of immune activation and peripheral endothelial dysfunction seen in PLWH. In addition, 2 of the above studies showed an association between abnormalities in coronary function and markers of inflammation—specifically, between IL‐6 levels and coronary endothelial function105 and between cytomegalovirus IgG levels and myocardial flow reserve.106
Future Directions and Unmet Needs
Given the conflicting results, the relatively small numbers of patients examined, and the cross‐sectional nature of the aforementioned prior studies, further studies assessing coronary microvascular function in PLWH are required to better understand the epidemiology, time course, and mechanisms of CMD in PLWH on ART. All studies thus far, which have assessed CMD in PLWH compared with controls, have examined CMD at one time point and so have not been able to assess for possible changes in coronary endothelial function that may occur over the course of treated HIV infection. In addition, by imposing strict inclusion and exclusion criteria with regard to traditional cardiovascular risk factors, studies may prevent an appreciation of the additive or multiplicative effect that HIV infection may have with traditional cardiovascular risk factors such as smoking, diabetes mellitus, and hypertension and limit their generalizability, given the high rates of comorbid conditions in PLWH.
Future studies should prioritize simultaneous investigation of CMD and cardiac structure (ie, presence of fibrosis or steatosis) and function (ie, systolic and diastolic function) to examine the degree to which CMD relates to myocardial abnormalities observed in PLWH. Given the multiple etiologies of CMD, careful matching of participants and inclusion of people with well‐controlled HIV (eg, suppressed viral load, consistent ART use) will be crucial to minimize confounding. Animal models of HIV infection, for example, SIV‐infected rhesus macaques and HIV‐1 transgenic rats,111 have helped identify cellular mechanisms that may drive vascular dysfunction and increased myocardial fibrosis associated with HIV infection, but studies assessing CMD in animal models of HIV have not been performed.112, 113, 114 Such studies would help isolate specific mechanisms underlying CMD in HIV infection and disentangle the confounding or additive effects that traditional cardiovascular risk factors (eg, hypertension, smoking, diabetes mellitus), ART, and coinfections may have on CMD. Noninvasive assessments of CMD have been performed successfully in a variety of animal models to study the pathophysiology of CMD; these methodologies could be applied to animal models of HIV.91
CMD may be an important target for therapeutic interventions and a useful parameter for assessing and following CVD risk among HIV‐infected populations. Randomized control trials and longitudinal studies will help to determine the efficacy of specific interventions (eg, anti‐inflammatory agents) on CMD in PLWH and clarify the risk factors, causal pathways, and prognostic value of myocardial flow reserve, coronary flow reserve, and coronary endothelial function in PLWH. Finally, novel therapeutics in development for CMD for other conditions (eg, myeloperoxidase inhibition for CMD in HF with preserved ejection fraction [NCT03756285]) may have utility in PLWH and could be tested in the future in these patients if a firmer link between HIV and CMD were ultimately established. Table 3 lists future directions and unmet needs in the field of CMD in HIV.
Table 3.
CMD in PLWH: Future Directions and Unmet Needs
| Question | Significance | Future Directions |
|---|---|---|
| What is the prevalence of CMD in PLWH on ART with well‐controlled cardiovascular RFs? | Important to understand whether CMD reflects traditional cardiovascular RFs (HTN, HLD, DM, smoking), HIV infection, or a combination/interaction of HIV and cardiovascular RFs | Cross‐sectional studies of PLWH on ART and controls, comparing those with well‐controlled cardiovascular RF and no cardiovascular RF |
| If there are increased rates of CMD in PLWH, what is the mechanism? | CMD may be secondary to myocardial disease, vascular rarefication, atherosclerosis, or RFs including chronic inflammation; mechanism is important for determining therapeutic interventions | Studies in SIV/HIV+ animal models; studies that incorporate simultaneous assessment of atherosclerosis (eg, coronary CT calcium scan), myocardial structure/function, and inflammatory milieu |
| Are there differences in CMD in men and women with HIV? | Although women frequently were not included in studies of PLWH, one study showed impaired CMD in women with HIV compared with men; different pathophysiologic pathways may be responsible | Prioritize research that focuses on CMD in women living with HIV |
| What therapeutic interventions may improve CMD in PLWH? | Drug studies that have targeted inflammatory pathways in PLWH have been largely negative using brachial FMD as a surrogate end point; a more specific surrogate may be useful | Randomized trials of antifibrotic and anti‐inflammatory medications and intensive RF control using a change in CFR/MRF/CEF as a surrogate end point |
| Can brachial FMD or EndoPAT (Itamar Medical) be used as screening tools to identify CMD in PLWH? | CFR/MFR/CEF may be impractical for many patients; noninvasive peripheral testing could help identify those in need of further testing | Prospective studies using concurrent brachial FMD or EndoPAT and CFR/MFR/CEF assessment |
| What is the prognostic significance of CMD in PLWH? | Whether CMD correlates with adverse cardiovascular outcomes in PLWH has significant implications for its value as a screening or risk‐stratification tool | Additional testing in PLWH using modalities with proven prognostic value (PET/CT, cMR, or TTE) and prospective studies of PLWH on ART with long‐term follow‐up |
ART indicates antiretroviral therapy; CEF, coronary endothelial function; CFR, coronary flow reserve; CMD, coronary microvascular dysfunction; cMR, cardiac magnetic resonance; CT, computed tomography; DM, diabetes mellitus; FMD, flow‐mediated dilation; HLD, hyperlipidemia; HTN, hypertension; MFR, myocardial flow reserve; PET/CT, positron emission tomography/computed tomography; PLWH, people living with HIV; RF, risk factor; TTE, transthoracic echocardiography.
Conclusions
CMD may be more common in PLWH on ART than in controls without HIV and with similar (traditional) cardiovascular risk profiles; however, further research is needed. Research focusing on CMD in PLWH should be prioritized because it may provide a novel pathophysiologic link among chronic inflammation, immune activation, and adverse cardiovascular outcomes observed in PLWH and in non‐HIV disease states. In addition, because CMD can be reversed with proper therapeutic intervention or lead to cardiovascular remodeling if not addressed, it may be an important metric by which PLWH can be risk stratified, enrolled into trials of anti‐inflammatory or antifibrotic agents that improve coronary microvascular function directly, or referred for aggressive cardiovascular risk management. As PLWH age on ART and increasingly present for CVD management, it is imperative that the mechanisms responsible for their increased risk of CVD be elucidated so that the tremendous success of ART is not lessened by premature death or disability from CVD.
Sources of Funding
Rethy was supported with funding from the Sarnoff Cardiovascular Research Foundation. Shah is supported by the US National Institutes of Health (grants R01 HL107577, R01HL127028, R01 HL140731, and R01 HL149423) and the American Heart Association (grants 16SFRN28780016 and 15CVGPSD27260148).
Disclosures
Shah has received research grants from Actelion, AstraZeneca, Corvia, and Novartis and consulting fees from Actelion, Amgen, AstraZeneca, Bayer, Boehringer‐Ingelheim, Cardiora, Eisai, Ironwood, Merck, MyoKardia, Novartis, Sanofi, and United Therapeutics. The remaining authors have no disclosures to report.
J Am Heart Assoc. 2020;9:e014018 DOI: 10.1161/JAHA.119.014018.
References
- 1. Division of HIV/AIDS Prevention NCfHA, Viral Hepatitis, STD, and TB Prevention, Centers for Disease Control and Prevention . HIV among people aged 50 and older. July 12, 2019.
- 2. Centers for Disease Control and Prevention . HIV Surveillance Report, 2017. Published on November 2018. Available at: http://www.cdc.gov/hiv/library/reports/hiv-surveillance.html. Accessed June 1, 2019.
- 3. Morlat P, Roussillon C, Henard S, Salmon D, Bonnet F, Cacoub P, Georget A, Aouba A, Rosenthal E, May T, Chauveau M, Diallo B, Costagliola D, Chene G. Causes of death among HIV‐infected patients in France in 2010 (national survey): trends since 2000. AIDS. 2014;28:1181–1191. [DOI] [PubMed] [Google Scholar]
- 4. Feinstein MJ, Bahiru E, Achenbach C, Longenecker CT, Hsue P, So‐Armah K, Freiberg MS, Lloyd‐Jones DM. Patterns of cardiovascular mortality for HIV‐infected adults in the United States: 1999 to 2013. Am J Cardiol. 2016;117:214–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bhaskaran K, Hamouda O, Sannes M, Boufassa F, Johnson AM, Lambert PC, Porter K. Changes in the risk of death after HIV seroconversion compared with mortality in the general population. JAMA. 2008;300:51–59. [DOI] [PubMed] [Google Scholar]
- 6. Mahy M, Autenrieth CS, Stanecki K, Wynd S. Increasing trends in HIV prevalence among people aged 50 years and older: evidence from estimates and survey data. AIDS. 2014;28(suppl 4):S453–S459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Freiberg MS, Chang CH, Skanderson M, Patterson OV, DuVall SL, Brandt CA, So‐Armah KA, Vasan RS, Oursler KA, Gottdiener J, Gottlieb S, Leaf D, Rodriguez‐Barradas M, Tracy RP, Gibert CL, Rimland D, Bedimo RJ, Brown ST, Goetz MB, Warner A, Crothers K, Tindle HA, Alcorn C, Bachmann JM, Justice AC, Butt AA. Association between HIV infection and the risk of heart failure with reduced ejection fraction and preserved ejection fraction in the antiretroviral therapy era: results from the Veterans Aging Cohort Study. JAMA Cardiol. 2017;2:536–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Barnes RP, Lacson JC, Bahrami H. HIV infection and risk of cardiovascular diseases beyond coronary artery disease. Curr Atheroscler Rep. 2017;19:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Erqou S, Lodebo BT, Masri A, Altibi AM, Echouffo‐Tcheugui JB, Dzudie A, Ataklte F, Choudhary G, Bloomfield GS, Wu WC, Kengne AP. Cardiac dysfunction among people living with HIV: a systematic review and meta‐analysis. JACC Heart Fail. 2019;7:98–108. [DOI] [PubMed] [Google Scholar]
- 10. Feinstein MJ, Steverson AB, Ning H, Pawlowski AE, Schneider D, Ahmad FS, Sanders JM, Sinha A, Nance RM, Achenbach CJ, Christopher Delaney JA, Heckbert SR, Shah SJ, Hanna DB, Hsue PY, Bloomfield GS, Longenecker CT, Crane HM, Lloyd‐Jones DM. Adjudicated heart failure in HIV‐infected and uninfected men and women. J Am Heart Assoc. 2018;7:e009985 DOI: 10.1161/JAHA.118.009985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Silverberg MJ, Leyden WA, Xu L, Horberg MA, Chao CR, Towner WJ, Hurley LB, Quesenberry CP Jr, Klein DB. Immunodeficiency and risk of myocardial infarction among HIV‐positive individuals with access to care. J Acquir Immune Defic Syndr. 2014;65:160–166. [DOI] [PubMed] [Google Scholar]
- 12. Klein DB, Leyden WA, Xu L, Chao CR, Horberg MA, Towner WJ, Hurley LB, Marcus JL, Quesenberry CP Jr, Silverberg MJ. Declining relative risk for myocardial infarction among HIV‐positive compared with HIV‐negative individuals with access to care. Clin Infect Dis. 2015;60:1278–1280. [DOI] [PubMed] [Google Scholar]
- 13. Barnett CF, Hsue PY. Human immunodeficiency virus‐associated pulmonary arterial hypertension. Clin Chest Med. 2013;34:283–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tseng ZH, Secemsky EA, Dowdy D, Vittinghoff E, Moyers B, Wong JK, Havlir DV, Hsue PY. Sudden cardiac death in patients with human immunodeficiency virus infection. J Am Coll Cardiol. 2012;59:1891–1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Alvi RM, Neilan AM, Tariq N, Hassan MO, Awadalla M, Zhang L, Afshar M, Rokicki A, Mulligan CP, Triant VA, Zanni MV, Neilan TG. The risk for sudden cardiac death among patients living with heart failure and human immunodeficiency virus. JACC Heart Fail. 2019;7:759–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ntusi N, O'Dwyer E, Dorrell L, Wainwright E, Piechnik S, Clutton G, Hancock G, Ferreira V, Cox P, Badri M, Karamitsos T, Emmanuel S, Clarke K, Neubauer S, Holloway C. HIV‐1‐related cardiovascular disease is associated with chronic inflammation, frequent pericardial effusions, and probable myocardial edema. Circ Cardiovasc Imaging. 2016;9:e004430. [DOI] [PubMed] [Google Scholar]
- 17. Holloway CJ, Ntusi N, Suttie J, Mahmod M, Wainwright E, Clutton G, Hancock G, Beak P, Tajar A, Piechnik SK, Schneider JE, Angus B, Clarke K, Dorrell L, Neubauer S. Comprehensive cardiac magnetic resonance imaging and spectroscopy reveal a high burden of myocardial disease in HIV patients. Circulation. 2013;128:814–822. [DOI] [PubMed] [Google Scholar]
- 18. Luetkens JA, Doerner J, Schwarze‐Zander C, Wasmuth JC, Boesecke C, Sprinkart AM, Schmeel FC, Homsi R, Gieseke J, Schild HH, Rockstroh JK, Naehle CP. Cardiac magnetic resonance reveals signs of subclinical myocardial inflammation in asymptomatic HIV‐infected patients. Circ Cardiovasc Imaging. 2016;9:e004091. [DOI] [PubMed] [Google Scholar]
- 19. Hsue PY, Hunt PW, Ho JE, Farah HH, Schnell A, Hoh R, Martin JN, Deeks SG, Bolger AF. Impact of HIV infection on diastolic function and left ventricular mass. Circ Heart Fail. 2010;3:132–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Moyers BS, Secemsky EA, Vittinghoff E, Wong JK, Havlir DV, Hsue PY, Tseng ZH. Effect of left ventricular dysfunction and viral load on risk of sudden cardiac death in patients with human immunodeficiency virus. Am J Cardiol. 2014;113:1260–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. D'Ascenzo F, Cerrato E, Biondi‐Zoccai G, Moretti C, Omede P, Sciuto F, Bollati M, Modena MG, Gaita F, Sheiban I. Acute coronary syndromes in human immunodeficiency virus patients: a meta‐analysis investigating adverse event rates and the role of antiretroviral therapy. Eur Heart J. 2012;33:875–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lacson JC, Barnes RP, Bahrami H. Coronary artery disease in HIV‐infected patients: downside of living longer. Curr Atheroscler Rep. 2017;19:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Freiberg MS, Chang CC, Kuller LH, Skanderson M, Lowy E, Kraemer KL, Butt AA, Bidwell Goetz M, Leaf D, Oursler KA, Rimland D, Rodriguez Barradas M, Brown S, Gibert C, McGinnis K, Crothers K, Sico J, Crane H, Warner A, Gottlieb S, Gottdiener J, Tracy RP, Budoff M, Watson C, Armah KA, Doebler D, Bryant K, Justice AC. HIV infection and the risk of acute myocardial infarction. JAMA Intern Med. 2013;173:614–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Waters DD, Hsue PY. Lipid abnormalities in persons living with HIV infection. Can J Cardiol. 2019;35:249–259. [DOI] [PubMed] [Google Scholar]
- 25. Feinstein MJ, Hsue PY, Benjamin LA, Bloomfield GS, Currier JS, Freiberg MS, Grinspoon SK, Levin J, Longenecker CT, Post WS. Characteristics, prevention, and management of cardiovascular disease in people living with HIV: a scientific statement from the American Heart Association. Circulation. 2019;140:e98–e124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Libby P, Loscalzo J, Ridker PM, Farkouh ME, Hsue PY, Fuster V, Hasan AA, Amar S. Inflammation, immunity, and infection in atherothrombosis: JACC review topic of the week. J Am Coll Cardiol. 2018;72:2071–2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Paulus WJ, Tschope C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol. 2013;62:263–271. [DOI] [PubMed] [Google Scholar]
- 28. Deswal A, Petersen NJ, Feldman AM, Young JB, White BG, Mann DL. Cytokines and cytokine receptors in advanced heart failure: an analysis of the cytokine database from the Vesnarinone trial (VEST). Circulation. 2001;103:2055–2059. [DOI] [PubMed] [Google Scholar]
- 29. Cesari M, Penninx BW, Newman AB, Kritchevsky SB, Nicklas BJ, Sutton‐Tyrrell K, Rubin SM, Ding J, Simonsick EM, Harris TB, Pahor M. Inflammatory markers and onset of cardiovascular events: results from the Health ABC study. Circulation. 2003;108:2317–2322. [DOI] [PubMed] [Google Scholar]
- 30. Khalid U, Ahlehoff O, Gislason GH, Kristensen SL, Skov L, Torp‐Pedersen C, Hansen PR. Psoriasis and risk of heart failure: a nationwide cohort study. Eur J Heart Fail. 2014;16:743–748. [DOI] [PubMed] [Google Scholar]
- 31. Giles JT, Fernandes V, Lima JA, Bathon JM. Myocardial dysfunction in rheumatoid arthritis: epidemiology and pathogenesis. Arthritis Res Ther. 2005;7:195–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Thiara DK, Liu CY, Raman F, Mangat S, Purdy JB, Duarte HA, Schmidt N, Hur J, Sibley CT, Bluemke DA, Hadigan C. Abnormal myocardial function is related to myocardial steatosis and diffuse myocardial fibrosis in HIV‐infected adults. J Infect Dis. 2015;212:1544–1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Feinstein MJ, Mitter SS, Yadlapati A, Achenbach CJ, Palella FJ Jr, Gonzalez PE, Meyers S, Collins JD, Shah SJ, Lloyd‐Jones DM. HIV‐related myocardial vulnerability to infarction and coronary artery disease. J Am Coll Cardiol. 2016;68:2026–2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Khakpour S, Wilhelmsen K, Hellman J. Vascular endothelial cell Toll‐like receptor pathways in sepsis. Innate Immun. 2015;21:827–846. [DOI] [PubMed] [Google Scholar]
- 35. Gimbrone MA Jr, Garcia‐Cardena G. Endothelial cell dysfunction and the pathobiology of atherosclerosis. Circ Res. 2016;118:620–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Liao JK. Linking endothelial dysfunction with endothelial cell activation. J Clin Invest. 2013;123:540–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wang GR, Zhu Y, Halushka PV, Lincoln TM, Mendelsohn ME. Mechanism of platelet inhibition by nitric oxide: in vivo phosphorylation of thromboxane receptor by cyclic GMP‐dependent protein kinase. Proc Natl Acad Sci USA. 1998;95:4888–4893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Morrell CN, Aggrey AA, Chapman LM, Modjeski KL. Emerging roles for platelets as immune and inflammatory cells. Blood. 2014;123:2759–2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rondina MT, Weyrich AS, Zimmerman GA. Platelets as cellular effectors of inflammation in vascular diseases. Circ Res. 2013;112:1506–1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Polgar J, Matuskova J, Wagner DD. The P‐selectin, tissue factor, coagulation triad. J Thromb Haemost. 2005;3:1590–1596. [DOI] [PubMed] [Google Scholar]
- 41. Faccini A, Kaski JC, Camici PG. Coronary microvascular dysfunction in chronic inflammatory rheumatoid diseases. Eur Heart J. 2016;37:1799–1806. [DOI] [PubMed] [Google Scholar]
- 42. Solages A, Vita JA, Thornton DJ, Murray J, Heeren T, Craven DE, Horsburgh CR Jr. Endothelial function in HIV‐infected persons. Clin Infect Dis. 2006;42:1325–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ter Maaten JM, Damman K, Verhaar MC, Paulus WJ, Duncker DJ, Cheng C, van Heerebeek L, Hillege HL, Lam CS, Navis G, Voors AA. Connecting heart failure with preserved ejection fraction and renal dysfunction: the role of endothelial dysfunction and inflammation. Eur J Heart Fail. 2016;18:588–598. [DOI] [PubMed] [Google Scholar]
- 44. Zungsontiporn N, Ndhlovu LC, Mitchell BI, Stein JH, Kallianpur KJ, Nakamoto B, Keating SM, Norris PJ, Souza SA, Shikuma CM, Chow DC. Serum amyloid P (SAP) is associated with impaired brachial artery flow‐mediated dilation in chronically HIV‐1 infected adults on stable antiretroviral therapy. HIV Clin Trials. 2015;16:228–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Tschope C, Van Linthout S. New insights in (inter)cellular mechanisms by heart failure with preserved ejection fraction. Curr Heart Fail Rep. 2014;11:436–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ganz P, Hsue PY. Endothelial dysfunction in coronary heart disease is more than a systemic process. Eur Heart J. 2013;34:2025–2027. [DOI] [PubMed] [Google Scholar]
- 47. Ikonomidis I, Makavos G, Papadavid E, Varoudi M, Andreadou I, Gravanis K, Theodoropoulos K, Pavlidis G, Triantafyllidi H, Parissis J, Paraskevaidis I, Rigopoulos D, Lekakis J. Similarities in coronary function and myocardial deformation between psoriasis and coronary artery disease: the role of oxidative stress and inflammation. Can J Cardiol. 2015;31:287–295. [DOI] [PubMed] [Google Scholar]
- 48. Hamburg NM, Benjamin EJ. Assessment of endothelial function using digital pulse amplitude tonometry. Trends Cardiovasc Med. 2009;19:6–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kuvin JT, Patel AR, Sliney KA, Pandian NG, Sheffy J, Schnall RP, Karas RH, Udelson JE. Assessment of peripheral vascular endothelial function with finger arterial pulse wave amplitude. Am Heart J. 2003;146:168–174. [DOI] [PubMed] [Google Scholar]
- 50. Green DJ, Dawson EA, Groenewoud HM, Jones H, Thijssen DH. Is flow‐mediated dilation nitric oxide mediated? A meta‐analysis. Hypertension. 2014;63:376–382. [DOI] [PubMed] [Google Scholar]
- 51. Celermajer DS, Sorensen KE, Gooch VM, Spiegelhalter DJ, Miller OI, Sullivan ID, Lloyd JK, Deanfield JE. Non‐invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet. 1992;340:1111–1115. [DOI] [PubMed] [Google Scholar]
- 52. Feldt‐Rasmussen B. Microalbuminuria, endothelial dysfunction and cardiovascular risk. Diabetes Metab. 2000;26(suppl 4):64–66. [PubMed] [Google Scholar]
- 53. Malik AR, Sultan S, Turner ST, Kullo IJ. Urinary albumin excretion is associated with impaired flow‐ and nitroglycerin‐mediated brachial artery dilatation in hypertensive adults. J Hum Hypertens. 2007;21:231–238. [DOI] [PubMed] [Google Scholar]
- 54. Dysangco A, Liu Z, Stein JH, Dube MP, Gupta SK. HIV infection, antiretroviral therapy, and measures of endothelial function, inflammation, metabolism, and oxidative stress. PLoS One. 2017;12:e0183511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Tien PC, Choi AI, Zolopa AR, Benson C, Tracy R, Scherzer R, Bacchetti P, Shlipak M, Grunfeld C. Inflammation and mortality in HIV‐infected adults: analysis of the FRAM study cohort. J Acquir Immune Defic Syndr. 2010;55:316–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Hsu DC, Ma YF, Hur S, Li D, Rupert A, Scherzer R, Kalapus SC, Deeks S, Sereti I, Hsue PY. Plasma IL‐6 levels are independently associated with atherosclerosis and mortality in HIV‐infected individuals on suppressive antiretroviral therapy. AIDS. 2016;30:2065–2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Krishnan S, Wilson EM, Sheikh V, Rupert A, Mendoza D, Yang J, Lempicki R, Migueles SA, Sereti I. Evidence for innate immune system activation in HIV type 1‐infected elite controllers. J Infect Dis. 2014;209:931–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Deeks SG, Tracy R, Douek DC. Systemic effects of inflammation on health during chronic HIV infection. Immunity. 2013;39:633–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Hileman CO, Funderburg NT. Inflammation, immune activation, and antiretroviral therapy in HIV. Curr HIV/AIDS Rep. 2017;14:93–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. El‐Sadr WM, Lundgren J, Neaton JD, Gordin F, Abrams D, Arduino RC, Babiker A, Burman W, Clumeck N, Cohen CJ, Cohn D, Cooper D, Darbyshire J, Emery S, Fatkenheuer G, Gazzard B, Grund B, Hoy J, Klingman K, Losso M, Markowitz N, Neuhaus J, Phillips A, Rappoport C. CD4+ count‐guided interruption of antiretroviral treatment. N Engl J Med. 2006;355:2283–2296. [DOI] [PubMed] [Google Scholar]
- 61. Sax PE, Erlandson KM, Lake JE, McComsey GA, Orkin C, Esser S, Brown TT, Rockstroh JK, Wei X, Carter CC, Zhong L, Brainard DM, Melbourne K, Das M, Stellbrink HJ, Post FA, Waters L, Koethe JR. Weight gain following initiation of antiretroviral therapy: risk factors in randomized comparative clinical trials. Clin Infect Dis. 2019;ciz999 Available at: https://academic.oup.com/cid/advance-article/doi/10.1093/cid/ciz999/5586728. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Sinha A, Ma Y, Scherzer R, Hur S, Li D, Ganz P, Deeks SG, Hsue PY. Role of T‐cell dysfunction, inflammation, and coagulation in microvascular disease in HIV. J Am Heart Assoc. 2016;5:e004243 DOI: 10.1161/JAHA.116.004243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Nou E, Lo J, Grinspoon SK. Inflammation, immune activation, and cardiovascular disease in HIV. AIDS. 2016;30:1495–1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Blodget E, Shen C, Aldrovandi G, Rollie A, Gupta SK, Stein JH, Dube MP. Relationship between microbial translocation and endothelial function in HIV infected patients. PLoS One. 2012;7:e42624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Fahme SA, Bloomfield GS, Peck R. Hypertension in HIV‐infected adults: novel pathophysiologic mechanisms. Hypertension. 2018;72:44–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Mdodo R, Frazier EL, Dube SR, Mattson CL, Sutton MY, Brooks JT, Skarbinski J. Cigarette smoking prevalence among adults with HIV compared with the general adult population in the United States: cross‐sectional surveys. Ann Intern Med. 2015;162:335–344. [DOI] [PubMed] [Google Scholar]
- 67. Grome HN, Barnett L, Hagar CC, Harrison DG, Kalams SA, Koethe JR. Association of T cell and macrophage activation with arterial vascular health in HIV. AIDS Res Hum Retroviruses. 2017;33:181–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Wohl DA, Arnoczy G, Fichtenbaum CJ, Campbell T, Taiwo B, Hicks C, McComsey GA, Koletar S, Sax P, Tebas P, Ha B, Massengale K, Walsh K, Stein JH. Comparison of cardiovascular disease risk markers in HIV‐infected patients receiving abacavir and tenofovir: the nucleoside inflammation, coagulation and endothelial function (NICE) study. Antivir Ther. 2014;19:141–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Rose H, Low H, Dewar E, Bukrinsky M, Hoy J, Dart A, Sviridov D. The effect of HIV infection on atherosclerosis and lipoprotein metabolism: a one year prospective study. Atherosclerosis. 2013;229:206–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Blanco JJ, Garcia IS, Cerezo JG, de Rivera JM, Anaya PM, Raya PG, Garcia JG, Lopez JR, Hernandez FJ, Rodriguez JJ. Endothelial function in HIV‐infected patients with low or mild cardiovascular risk. J Antimicrob Chemother. 2006;58:133–139. [DOI] [PubMed] [Google Scholar]
- 71. Torriani FJ, Komarow L, Parker RA, Cotter BR, Currier JS, Dube MP, Fichtenbaum CJ, Gerschenson M, Mitchell CK, Murphy RL, Squires K, Stein JH. Endothelial function in human immunodeficiency virus‐infected antiretroviral‐naive subjects before and after starting potent antiretroviral therapy: the ACTG (AIDS Clinical Trials Group) Study 5152s. J Am Coll Cardiol. 2008;52:569–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Arildsen H, Sorensen KE, Ingerslev JM, Ostergaard LJ, Laursen AL. Endothelial dysfunction, increased inflammation, and activated coagulation in HIV‐infected patients improve after initiation of highly active antiretroviral therapy. HIV Med. 2013;14:1–9. [DOI] [PubMed] [Google Scholar]
- 73. Mosepele M, Mohammed T, Mupfumi L, Moyo S, Bennett K, Lockman S, Hemphill LC, Triant VA. HIV disease is associated with increased biomarkers of endothelial dysfunction despite viral suppression on long‐term antiretroviral therapy in Botswana. Cardiovasc J Afr. 2018;29:155–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Zanni MV, Schouten J, Grinspoon SK, Reiss P. Risk of coronary heart disease in patients with HIV infection. Nat Rev Cardiol. 2014;11:728–741. [DOI] [PubMed] [Google Scholar]
- 75. Nolan D, Watts GF, Herrmann SE, French MA, John M, Mallal S. Endothelial function in HIV‐infected patients receiving protease inhibitor therapy: does immune competence affect cardiovascular risk? QJM. 2003;96:825–832. [DOI] [PubMed] [Google Scholar]
- 76. Bonnet D, Aggoun Y, Szezepanski I, Bellal N, Blanche S. Arterial stiffness and endothelial dysfunction in HIV‐infected children. AIDS. 2004;18:1037–1041. [DOI] [PubMed] [Google Scholar]
- 77. Charakida M, Donald AE, Green H, Storry C, Clapson M, Caslake M, Dunn DT, Halcox JP, Gibb DM, Klein NJ, Deanfield JE. Early structural and functional changes of the vasculature in HIV‐infected children: impact of disease and antiretroviral therapy. Circulation. 2005;112:103–109. [DOI] [PubMed] [Google Scholar]
- 78. van Wijk JP, de Koning EJ, Cabezas MC, Joven J, op't Roodt J, Rabelink TJ, Hoepelman AM. Functional and structural markers of atherosclerosis in human immunodeficiency virus‐infected patients. J Am Coll Cardiol. 2006;47:1117–1123. [DOI] [PubMed] [Google Scholar]
- 79. Gleason RL Jr, Caulk AW, Seifu D, Parker I, Vidakovic B, Getenet H, Assefa G, Amogne W. Current Efavirenz (EFV) or ritonavir‐boosted lopinavir (LPV/r) use correlates with elevate markers of atherosclerosis in HIV‐infected subjects in Addis Ababa, Ethiopia. PLoS One. 2015;10:e0117125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Koethe JR, Grome H, Jenkins CA, Kalams SA, Sterling TR. The metabolic and cardiovascular consequences of obesity in persons with HIV on long‐term antiretroviral therapy. AIDS. 2016;30:83–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Sharma A, Gupta N, Srivastava D. Carotid intima‐media thickness, flow‐mediated dilatation and proteinuria in patients of human immunodeficiency virus‐positive patients: a case‐control study. J Family Med Prim Care. 2018;7:362–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Hauguel‐Moreau M, Boccara F, Boyd A, Salem JE, Brugier D, Curjol A, Hulot JS, Kerneis M, Galier S, Cohen A, Montalescot G, Collet JP, Silvain J. Platelet reactivity in human immunodeficiency virus infected patients on dual antiplatelet therapy for an acute coronary syndrome: the EVERE2ST‐HIV study. Eur Heart J. 2017;38:1676–1686. [DOI] [PubMed] [Google Scholar]
- 83. Marcantoni E, Allen N, Cambria MR, Dann R, Cammer M, Lhakhang T, O'Brien MP, Kim B, Worgall T, Heguy A, Tsirigos A, Berger JS. Platelet transcriptome profiling in HIV and ATP‐binding cassette subfamily C member 4 (ABCC4) as a mediator of platelet activity. JACC Basic Transl Sci. 2018;3:9–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. O'Brien MP, Hunt PW, Kitch DW, Klingman K, Stein JH, Funderburg NT, Berger JS, Tebas P, Clagett B, Moisi D, Utay NS, Aweeka F, Aberg JA. A randomized placebo controlled trial of aspirin effects on immune activation in chronically human immunodeficiency virus‐infected adults on virologically suppressive antiretroviral therapy. Open Forum Infect Dis. 2017;4:ofw278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Recio‐Mayoral A, Mason JC, Kaski JC, Rubens MB, Harari OA, Camici PG. Chronic inflammation and coronary microvascular dysfunction in patients without risk factors for coronary artery disease. Eur Heart J. 2009;30:1837–1843. [DOI] [PubMed] [Google Scholar]
- 86. Camici PG, Crea F. Coronary microvascular dysfunction. N Engl J Med. 2007;356:830–840. [DOI] [PubMed] [Google Scholar]
- 87. Crea F, Camici PG, Bairey Merz CN. Coronary microvascular dysfunction: an update. Eur Heart J. 2014;35:1101–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Forstermann U, Sessa WC. Nitric oxide synthases: regulation and function. Eur Heart J. 2012;33:829–837, 837a–837d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Flammer AJ, Anderson T, Celermajer DS, Creager MA, Deanfield J, Ganz P, Hamburg NM, Luscher TF, Shechter M, Taddei S, Vita JA, Lerman A. The assessment of endothelial function: from research into clinical practice. Circulation. 2012;126:753–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Feher A, Sinusas AJ. Quantitative assessment of coronary microvascular function: dynamic single‐photon emission computed tomography, positron emission tomography, ultrasound, computed tomography, and magnetic resonance imaging. Circ Cardiovasc Imaging. 2017;10:e006427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Gan LM, Wikstrom J, Fritsche‐Danielson R. Coronary flow reserve from mouse to man–from mechanistic understanding to future interventions. J Cardiovasc Transl Res. 2013;6:715–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Halcox JP, Schenke WH, Zalos G, Mincemoyer R, Prasad A, Waclawiw MA, Nour KR, Quyyumi AA. Prognostic value of coronary vascular endothelial dysfunction. Circulation. 2002;106:653–658. [DOI] [PubMed] [Google Scholar]
- 93. Camici PG, d'Amati G, Rimoldi O. Coronary microvascular dysfunction: mechanisms and functional assessment. Nat Rev Cardiol. 2015;12:48–62. [DOI] [PubMed] [Google Scholar]
- 94. Taqueti VR, Di Carli MF. Coronary microvascular disease pathogenic mechanisms and therapeutic options: JACC state‐of‐the‐art review. J Am Coll Cardiol. 2018;72:2625–2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Sicari R, Rigo F, Cortigiani L, Gherardi S, Galderisi M, Picano E. Additive prognostic value of coronary flow reserve in patients with chest pain syndrome and normal or near‐normal coronary arteries. Am J Cardiol. 2009;103:626–631. [DOI] [PubMed] [Google Scholar]
- 96. Bairey Merz CN, Pepine CJ, Walsh MN, Fleg JL. Ischemia and no obstructive coronary artery disease (INOCA): developing evidence‐based therapies and research agenda for the next decade. Circulation. 2017;135:1075–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Nakanishi K, Fukuda S, Shimada K, Miyazaki C, Otsuka K, Kawarabayashi T, Watanabe H, Yoshikawa J, Yoshiyama M. Prognostic value of coronary flow reserve on long‐term cardiovascular outcomes in patients with chronic kidney disease. Am J Cardiol. 2013;112:928–932. [DOI] [PubMed] [Google Scholar]
- 98. Indorkar R, Kwong RY, Romano S, White BE, Chia RC, Trybula M, Evans K, Shenoy C, Farzaneh‐Far A. Global coronary flow reserve measured during stress cardiac magnetic resonance imaging is an independent predictor of adverse cardiovascular events. JACC Cardiovasc Imaging. 2019;12:1686–1695. [DOI] [PubMed] [Google Scholar]
- 99. Murthy VL, Naya M, Foster CR, Hainer J, Gaber M, Di Carli G, Blankstein R, Dorbala S, Sitek A, Pencina MJ, Di Carli MF. Improved cardiac risk assessment with noninvasive measures of coronary flow reserve. Circulation. 2011;124:2215–2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Amigues I, Russo C, Giles JT, Tugcu A, Weinberg R, Bokhari S, Bathon JM. Myocardial microvascular dysfunction in rheumatoid arthritis: quantitation by (13)N‐ammonia positron emission tomography/computed tomography. Circ Cardiovasc Imaging. 2019;12:e007495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Erre GL, Buscetta G, Paliogiannis P, Mangoni AA, Carru C, Passiu G, Zinellu A. Coronary flow reserve in systemic rheumatic diseases: a systematic review and meta‐analysis. Rheumatol Int. 2018;38:1179–1190. [DOI] [PubMed] [Google Scholar]
- 102. Lebech AM, Kristoffersen US, Wiinberg N, Kofoed K, Andersen O, Hesse B, Petersen CL, Gerstoft J, Kjaer A. Coronary and peripheral endothelial function in HIV patients studied with positron emission tomography and flow‐mediated dilation: relation to hypercholesterolemia. Eur J Nucl Med Mol Imaging. 2008;35:2049–2058. [DOI] [PubMed] [Google Scholar]
- 103. Kristoffersen US, Wiinberg N, Petersen CL, Gerstoft J, Gutte H, Lebech AM, Kjaer A. Reduction in coronary and peripheral vasomotor function in patients with HIV after initiation of antiretroviral therapy: a longitudinal study with positron emission tomography and flow‐mediated dilation. Nucl Med Commun. 2010;31:874–880. [DOI] [PubMed] [Google Scholar]
- 104. Knudsen A, Christensen TE, Ghotbi AA, Hasbak P, Lebech AM, Kjaer A, Ripa RS. Normal myocardial flow reserve in HIV‐infected patients on stable antiretroviral therapy: a cross‐sectional study using rubidium‐82 PET/CT. Medicine (Baltimore). 2015;94:e1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Iantorno M, Schar M, Soleimanifard S, Brown TT, Moore R, Barditch‐Crovo P, Stuber M, Lai S, Gerstenblith G, Weiss RG, Hays AG. Coronary artery endothelial dysfunction is present in HIV‐positive individuals without significant coronary artery disease. AIDS. 2017;31:1281–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Knudsen A, Thorsteinsson K, Christensen TE, Hasbak P, Ripa RS, Panum I, Lebech AM, Kjaer A. Cardiac microvascular dysfunction in women living with HIV is associated with cytomegalovirus immunoglobulin G. Open Forum Infect Dis. 2018;5:ofy205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Iantorno M, Soleimanifard S, Schar M, Brown TT, Bonanno G, Barditch‐Crovo P, Mathews L, Lai S, Gerstenblith G, Weiss RG, Hays AG. Regional coronary endothelial dysfunction is related to the degree of local epicardial fat in people with HIV. Atherosclerosis. 2018;278:7–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Leucker TM, Weiss RG, Schar M, Bonanno G, Mathews L, Jones SR, Brown TT, Moore R, Afework Y, Gerstenblith G, Hays AG. Coronary endothelial dysfunction is associated with elevated serum PCSK9 Levels in people with HIV independent of low‐density lipoprotein cholesterol. J Am Heart Assoc. 2018;7:e009996 DOI: 10.1161/JAHA.118.009996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Hays AG, Iantorno M, Soleimanifard S, Steinberg A, Schar M, Gerstenblith G, Stuber M, Weiss RG. Coronary vasomotor responses to isometric handgrip exercise are primarily mediated by nitric oxide: a noninvasive MRI test of coronary endothelial function. Am J Physiol Heart Circ Physiol. 2015;308:H1343–H1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Smits P, Williams SB, Lipson DE, Banitt P, Rongen GA, Creager MA. Endothelial release of nitric oxide contributes to the vasodilator effect of adenosine in humans. Circulation. 1995;92:2135–2141. [DOI] [PubMed] [Google Scholar]
- 111. Hatziioannou T, Evans DT. Animal models for HIV/AIDS research. Nat Rev Microbiol. 2012;10:852–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Shannon RP. SIV cardiomyopathy in non‐human primates. Trends Cardiovasc Med. 2001;11:242–246. [DOI] [PubMed] [Google Scholar]
- 113. Walker JA, Beck GA, Campbell JH, Miller AD, Burdo TH, Williams KC. Anti‐alpha4 integrin antibody blocks monocyte/macrophage traffic to the heart and decreases cardiac pathology in a SIV infection model of AIDS. J Am Heart Assoc. 2015;4:e001932 DOI: 10.1161/JAHA.115.001932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Kelly KM, Tocchetti CG, Lyashkov A, Tarwater PM, Bedja D, Graham DR, Beck SE, Metcalf Pate KA, Queen SE, Adams RJ, Paolocci N, Mankowski JL. CCR5 inhibition prevents cardiac dysfunction in the SIV/macaque model of HIV. J Am Heart Assoc. 2014;3:e000874 DOI: 10.1161/JAHA.114.000874. [DOI] [PMC free article] [PubMed] [Google Scholar]


