Abstract
Background
Information is evolving on liver disease in pediatric patients with Fontan physiology. The purpose of this investigation is to evaluate the spectrum of liver disease in a pediatric population of patients with Fontan physiology and evaluate transient elastography (TE) as a noninvasive marker of liver disease.
Methods and Results
We prospectively enrolled all children with Fontan physiology. All patients underwent comprehensive liver evaluation including liver enzymes (alanine aminotransferase, aspartate aminotransferase, gamma‐glutamyl transferase, alkaline phosphatase), aspartate transaminase to platelet ratio index, albumin, bilirubin, international normalized ratio, complete blood cell count, abdominal ultrasound, and TE. Transjugular liver biopsies and hemodynamic measurements were performed in a subset of patients. A total of 76 children (median, 11.7; interquartile range, 8.4–14.8 [56% male]) were evaluated, with 17 having a transjugular liver biopsy (median 14.8 years; interquartile range, 14.3–17.4). All biopsies showed pathological changes. The severity of liver pathology did not correlate with TE. There was a positive correlation between TE and time since Fontan (R=0.42, P<0.01), aspartate transaminase to platelet ratio index (R=0.29, P=0.02), aspartate transaminase (R=−0.42, P<0.01), and platelets (R=−0.42, P<0.01). Splenomegaly on abdominal ultrasound was correlated with TE (z=−2.2, P=0.03), low platelet count (z=1.9, P=0.05), low aspartate transaminase (z=1.9, P=0.02), and low alkaline phosphatase (z=2.4, P=0.02).
Conclusions
Liver disease was ubiquitous in our cohort of pediatric patients with Fontan Physiology. Given the correlation between TE and time from Fontan, TE shows potential as a prospective marker of liver pathology. However, individual measurements with TE do not correlate with the severity of pathology. Given the prevalence of liver disease in this population, protective measures of liver health as well as routine liver health surveillance should be implemented with consideration for hepatology consultation and biopsy in the event of abnormal liver biochemical markers or imaging.
Keywords: congenital heart disease, Fontan procedure, liver
Subject Categories: Congenital Heart Disease
Clinical Perspective
What Is New?
The onset of liver disease in patients with Fontan physiology begins in childhood.
Single noninvasive assessment of the liver using transient elastography and aspartate transaminase to platelet ratio index does not correlate with severity of liver pathology based on biopsy.
What Are the Clinical Implications?
Surveillance of liver disease should be a routine part of clinical care of children and adolescents with Fontan physiology.
Invasive assessment with liver biopsy remains the gold standard to define severity of liver pathology in patients with Fontan physiology.
Introduction
The Fontan procedure is the final stage in the univentricular pathway that can be applied to a wide range of complex congenital heart conditions. Patients with Fontan physiology have improved survival in the current era, with many patients living well into adulthood.1 Despite this, the hemodynamic alteration following the Fontan procedure is associated with complications including thrombosis, protein losing enteropathy, and plastic bronchitis.1, 2, 3 Furthermore, there is growing awareness of clinically significant liver disease in the adult population of patients with Fontan physiology, making early recognition of liver disease in the pediatric cohort an area of growing concern.4, 5, 6, 7, 8, 9 Altered hemodynamics introduced after the Fontan procedure result in elevated central venous pressure, which is believed to trigger the cascade of sinusoidal dilation leading to sinusoidal fibrosis and cardiac cirrhosis.4, 6, 10, 11, 12, 13, 14, 15, 16 Fontan‐related liver disease also includes a component of portal injury leading to portal‐based fibrosis, the mechanism of which is unknown17, 18 but it has been proposed that it begins before Fontan palliation.17 The progressive nature of liver disease in patients with Fontan physiology is supported by a retrospective cohort of adult patients with Fontan physiology which showed that only 57% of patients 30 years post‐Fontan physiology were free of cirrhosis.7, 8 Subclinical liver changes have been observed in pediatric patients with Fontan physiology in recent years. These include elevated liver enzymes, impaired synthetic liver function, structural abnormalities on abdominal ultrasound, increased liver stiffness, and pathologic changes in liver biopsy specimens.5, 10, 11, 12, 15, 19, 20, 21 Despite growing awareness, the scope and natural history of liver disease in patients with Fontan physiology remains unclear. This is especially the case with respect to the timing, onset, and rate of progression within the pediatric population.1, 4, 5 To date, there is no consensus on a reliable noninvasive measure to identify or monitor liver disease in patients with Fontan physiology.
Minimally invasive measures of liver health including laboratory indices, imaging, and elastography are abnormal in patients with Fontan physiology, but the utility of these measurements in identifying pathologic liver changes remains unclear.4, 15, 22 In particular, TE measurements of liver stiffness are well documented to significantly increase immediately after the Fontan operation,14, 23 but the change in liver stiffness over time is not well defined. Liver biopsy remains the only method to definitively identify liver disease in this population and a noninvasive correlate to biopsy remains elusive. TE and the aspartate transaminase (AST) to platelet ratio index (APRI) are two validated noninvasive measures of fibrosis in pediatric patients with primary liver disease.14, 24 TE is an ultrasound‐based technology that measures liver stiffness. The APRI can be calculated from serum AST and platelet values. The ease with which these investigations can be performed in a serial manner in pediatric patients would make them ideal for prospectively monitoring liver health in patients with Fontan physiology.
The purpose of this investigation was to apply a standardized multimodal clinical pathway utilizing laboratory indices of liver health, APRI, liver stiffness measurement using TE, abdominal ultrasound, hemodynamic measurements, and liver biopsy to define the spectrum of liver pathology in pediatric patients with Fontan physiology and evaluate the utility of noninvasive modalities to identify liver pathology.
Methods
Patients with Fontan physiology were enrolled prospectively to undergo annual assessment of liver health. All patients with Fontan physiology at BC Children's Hospital were invited to participate. Written informed consent and assent was obtained from parents and patients, respectively. This study was approved by The University of British Columbia Children's and Women's Health Centre's clinical research ethics board.
A liver health pathway (Table S1) was jointly developed by pediatric gastroenterologists and cardiologists and adopted for clinical care of all patients with Fontan physiology at BC Children's Hospital.25 Each patient was evaluated with physical examination, laboratory studies, echocardiogram, abdominal ultrasound, and TE. The APRI was calculated using the measured AST level, platelet count, and the upper value of normal for AST at the BC Children's Hospital Biochemistry laboratory. TE measurements were obtained using a FibroScan device (Echosens), which was performed by trained personnel. Ten valid measurements were taken from the right lobe of the liver between the 7th and 8th ribs in the midaxillary line. The median value was recorded to represent the liver stiffness score in kilopascals. Values were accepted if the interquartile range was within 30% of the liver stiffness measurement. Laboratory measurements performed within 30 days of TE were used for correlation analysis.
As a part of routine management of patients with Fontan physiology at BC Children's Hospital, patients underwent catheterization for hemodynamic evaluation and angiography ≈10 years post‐Fontan, or earlier if clinically indicated. Hemodynamic measurements including pressure measurements and oxygen saturation were obtained during catheterization and pulmonary vascular resistance (PVR) was calculated. For these calculations, indexed oxygen consumption was estimated from reference tables.26 Transjugular hepatic biopsies were routinely performed at the same time as cardiac catheterization. A single pathologist blinded to laboratory results and TE scores assessed all liver biopsy specimens. Each liver biopsy was assessed for sinusoidal fibrosis (0–4), sinusoidal dilatation (0–3), portal inflammation (0–3), and portal fibrosis (0–4), as per the method described by Schwartz et al,18 which is partly based on the Scheuer system. The criteria for assessing severity of pathologic changes from Schwartz et al18 are reproduced in Table S2. For statistical analyses, the histologic parameters were categorized into mild (score 0–1/3 or 0–1/4), moderate (score 2/3 or 2/4), and severe (score 3/3, 3/4, or 4/4).
Categorical data are displayed as number (percentage) and continuous variables are displayed as median (interquartile range). Continuous variables were assessed using Spearman's rank order correlation, paired data were compared using the Wilcoxon rank‐sum test, and nonparametric data were compared using the Kruskall–Wallis test. Analyses were performed with STATA statistical software (version 14.2, StataCorp).
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Results
In total, 76 patients underwent comprehensive liver evaluation (Table 1). A total of 134 measurements of liver stiffness using TE were performed in 76 patients. The demographics and descriptive results for these patients are displayed in Table 1 and the demographics for the subset of patients who underwent liver biopsy are displayed in Table 2. There was no significant difference in the median liver stiffness based on the type of systemic ventricle (P=0.3) (Table S3).
Table 1.
Demographics and Median Results From Laboratory Investigations, Liver Stiffness, and Abdominal Ultrasound for All Patients Who Underwent Transient Elastography
| Parameter | Number (%) or median (IQR) |
|---|---|
| Patients, No. (% males) | 76 (50) |
| Age, median (IQR), y | 11.7 (8.4–14.8) |
| Duration since Fontan, median (IQR), y | 8.4 (4.6–11.4) |
| Age at Fontan, median (IQR), mo | 41 (36–48) |
| Dominant systemic ventricle, No. (%) | |
| Left | 33 (44) |
| Right | 35 (47) |
| Indeterminate | 8 (11) |
| Diagnosis, No. (%) | |
| HLHS | 21 (28) |
| DILV | 9 (12) |
| TA | 12 (16) |
| DORV | 8 (9) |
| AVSD | 8 (11) |
| PA | 4 (5) |
| ccTGA | 5 (7) |
| Ebsteins | 1 (1) |
| Univentricular | 8 (11) |
| Fontan type, No. (%) | |
| Extracardiac Fontan | 72 (96) |
| Lateral tunnel | 3 (4) |
| Laboratory median (IQR) | |
| APRI (reference range <0.7) | 0.4 (0.4–0.6) |
| ALT (reference range 10–45) | 37.0 (30.0–43.0) |
| AST (reference range 15–40) | 36.0 (30.0–43.0) |
| GGT (reference range 12–33) | 47.0 (32.0–64.0) |
| Platelets (reference range 180–440) | 211.0 (154.0–248.0) |
| Albumin (reference range 37–56) | 45.0 (41.0–47.0) |
| Bilirubin (reference range 2–17) | 9.0 (5.0–12.0) |
| Alkaline phosphatase (reference range 130–525) | 186.0 (140.0–240.0) |
| Transient elastography, median (IQR) | |
| Liver stiffness, kPa | 16.8 (13.3–21.1) |
| Abdominal ultrasound, No. (%) | |
| Hepatomegaly | 16 (12) |
| Splenomegaly | 15 (11) |
| Ascites | 6 (4) |
| Abnormal hepatic echotexture | 28 (21) |
| Nodules | 8 (6) |
ALT indicates alanine aminotransferase; APRI, aspartate transaminase to platelet ratio index; AST, aspartate aminotransferase; AVSD, atrioventricular septal defect; ccTGA, congenitally corrected transposition of the great arteries; DILV, double inlet left ventricle; DORV, double outlet right ventricle; GGT, gamma‐glutamyl transferase; HLHS, hypoplastic left heart syndrome; IQR, interquartile range; PA, pulmonary atresia; TA, tricuspid atresia.
Table 2.
Demographics, Median Liver Stiffness, and Hemodynamic Data for the Subset of Patients Who Underwent Transjugular Liver Biopsy
| Parameter | Number (%), median (IQR) |
|---|---|
| Patients, No. (% male) | 17 (65) |
| Age, median (IQR) | 14.8 (14.3–17.4) |
| Duration since Fontan, median (IQR), y | 11.9 (10.6–12.9) |
| Age at Fontan, median (IQR), y | 3.7 (3.0–4.3) |
| Dominant systemic ventricle, No. (%) | |
| Left | 8 (47) |
| Right | 8 (47) |
| Indeterminate | 1 (6) |
| Diagnosis, No. (%) | |
| HLHS | 3 (18) |
| DILV | 2 (12) |
| TA | 4 (24) |
| DORV | 2 (12) |
| AVSD | 3 (18) |
| PA | 1 (6) |
| ccTGA | 1 (6) |
| Ebsteins | 0 (0) |
| Univentricular | 1 (6) |
| Transient elastography, median (IQR) | |
| Liver stiffness, kPa | 21.3 (17.5–35.3) |
| Hemodynamics, mean (IQR) | |
| Fontan pressure, mm Hg | 13.0 (11–14) |
| PVR, mm Hg·min/L | 1.4 (0.9–1.8) |
AVSD indicates atrioventricular septal defect; ccTGA, congenitally corrected transposition of the great arteries; DILV, double inlet left ventricle; DORV, double outlet right ventricle; HLHS, hypoplastic left heart syndrome; IQR, interquartile range; PA, pulmonary atresia; PVR, pulmonary vascular resistance; TA, tricuspid atresia.
There was a significant correlation between the time since Fontan operation and liver stiffness measured by TE (ρ=0.41, P<0.001) (Figure). TE liver stiffness was correlated with APRI (ρ=0.4, P=0.01), AST (ρ=−0.4, P<0.01), and platelet count (ρ=−0.38, P<0.01). Neither Fontan pressure nor PVR were significantly associated with TE over time (Table 3). Splenomegaly identified on abdominal ultrasound was significantly associated with liver stiffness (z=−2.22, P=0.03) (Table 4). Furthermore, patients with splenomegaly were also found to have significantly low platelets, AST, and alkaline phosphatase (Table 5). Other findings on ultrasound were not found to be significant, which included hepatomegaly, development of ascites, abnormal hepatic echotexture, and hepatic nodules.
Figure 1.
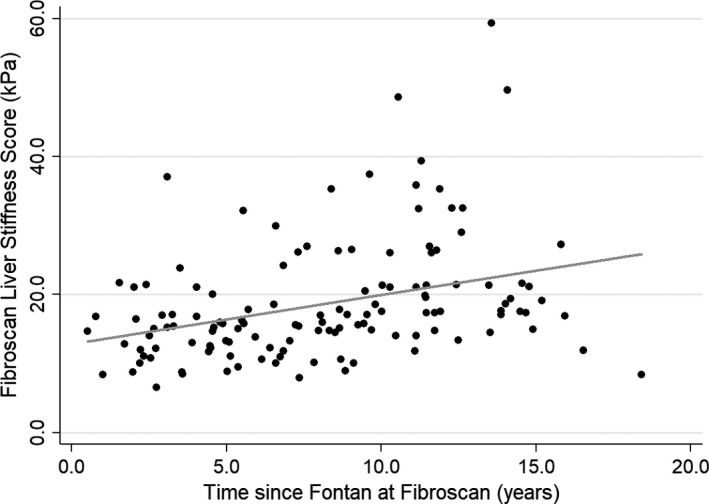
Scatter plot with regression line demonstrating the relationship between liver stiffness and time from Fontan. (R=0.41, P=<0.01).
Table 3.
Correlation Analysis of Both Liver Stiffness by Transient Elastography and the Time From the Fontan Procedure With Laboratory and Hemodynamic Parameters
| Investigation (Reference) | Median (IQR) | Liver Stiffness | Time From Fontan | ||
|---|---|---|---|---|---|
| ρ | P Value | ρ | P Value | ||
| APRI (<0.7) | 0.5 (0.4–0.6) | 0.2565 | 0.04* | 0.000 | 1.0 |
| ALT (10–45) | 37.0 (30.0–43.0) | −0.1952 | 0.09 | −0.2178 | 0.06 |
| AST (15–40) | 36.0 (30.0–43.0) | −0.4492 | <0.01* | −0.5489 | <0.01 |
| GGT (12–33) | 47.0 (32.0–64.0) | 0.0416 | 0.7 | 0.0895 | 0.4 |
| Bilirubin (2–17) | 11.0 (8.5–13.0) | 0.0762 | 0.8 | −0.0843 | 0.8 |
| Alkaline phosphatase (130–525) | 186.0 (140.0–240.0) | −0.1376 | 0.2 | −0.3881 | <0.01 |
| Platelets (180–440) | 211.0 (154.0–248.0) | −0.3853 | <0.01* | −0.1889 | 0.1 |
| Albumin (37–56) | 45.0 (41.0–47.0) | 0.0609 | 0.6 | −0.0271 | 0.8 |
| Fontan pressure, mm Hg | 13.0 (12.0–14.0) | 0.3241 | 0.3 | 0.5825 | 0.04 |
| PVR, mm Hg·min/L | 1.4 (0.9–1.8) | −0.5819 | 0.2 | −0.1009 | 0.8 |
ALT indicates alanine aminotransferase; APRI, aspartate transaminase to platelet ratio index; AST, aspartate aminotransferase; GGT, gamma‐glutamyl transferase; IQR, interquartile range; PVR, pulmonary vascular resistance.
Table 4.
Association Between the Median Liver Stiffness and the Presence or Absence of Findings on Abdominal Ultrasound
| Ultrasound Parameter | Median Liver Stiffness, kPa | z Statistic | P Value | |||
|---|---|---|---|---|---|---|
| Present | Absent | |||||
| No. | Median (IQR) Liver Stiffness, KPa | No. | Median (IQR) Liver Stiffness, KPa | |||
| Hepatomegaly | 16 | 16.6 (14.7–18.6) | 35 | 16.0 (12.2–21.0) | ||
| Splenomegaly | 15 | 18.5 (15.0–23.8) | 36 | 15.5 (11.6–17.2) | −2.22 | 0.03 |
| Ascites | 6 | 16.9 (10.6–18.5) | 45 | 16.0 (13.3–20.0) | −0.13 | 0.9 |
| Abnormal hepatic echotexture | 27 | 16.0 (13.1–20.0) | 24 | 16.1 (12.7–19.3) | −0.12 | 0.9 |
| Nodules | 8 | 17.4 (14.7–21.0) | 43 | 15.8 (12.2–18.6) | −0.89 | 0.4 |
IQR indicates interquartile range.
Table 5.
Association Between the Presence of Splenomegaly and Laboratory Measurements of Liver Health
| Investigation | Splenomegaly | No Splenomegaly | z Statistic | P Value | ||
|---|---|---|---|---|---|---|
| No. | Median (IQR) | No. | Median (IQR) | |||
| APRI (<0.7) | 15 | 0.4 (0.4–0.6) | 36 | 0.4 (0.4–0.6) | −0.796 | 0.4 |
| ALT (10–45) | 20 | 37.5 (30.0–42.0) | 42 | 38.0 (30.0–44.0) | 0.226 | 0.8 |
| AST (15–40) | 15 | 36.0 (32.0–41.0) | 41 | 40.0 (35.0–52.0) | 2.398 | 0.02 |
| GGT (12–33) | 18 | 57.0 (35.0–85.0) | 39 | 42.0 (29.0–58.0) | −1.700 | 0.09 |
| Bilirubin (2–17) | 18 | 9.5 (5.0–12.0) | 29 | 9.0 (7.0–11.0) | −0.099 | 0.9 |
| Alkaline phosphatase (130–525) | 20 | 170.0 (154–206) | 39 | 217.0 (182–237) | 2.402 | 0.02 |
| Platelets (180–440) | 19 | 164.0 (137–235) | 39 | 220.0 (194–266) | 1.939 | 0.05 |
| Albumin (37–56) | 20 | 43.0 (38.5–47.5) | 37 | 46.0 (43.0–48.0) | 1.703 | 0.09 |
ALT indicates alanine aminotransferase; APRI, aspartate transaminase to platelet ratio index; AST, aspartate aminotransferase; GGT, gamma‐glutamyl transferase; IQR, interquartile range.
Liver biopsies were performed in 17 patients. One biopsy was complicated by bleeding requiring a 24‐hour admission for observation. Analysis of each biopsy using the scoring scheme proposed by Schwartz et al18 demonstrated mild to moderate abnormal pathologic findings in all patients who underwent biopsy, but no severe changes were noted. Thirteen of 17 (76%) patients showed mild to moderate (score 1–2/4) portal fibrosis. All (17/17; 100%) patients showed mild to moderate sinusoidal fibrosis (score 1–2/4), and 16 of 17 (94%) patients showed mild to moderate sinusoidal dilatation (score 1–2/3). Mild to moderate portal inflammation (score 1–2/3) was also identified in 13 of 17 (76%) patients. No patients in this series showed severe portal fibrosis (score 3–4/4), severe sinusoidal fibrosis (score 3–4/4), severe portal inflammation (score 3/3), or severe sinusoidal dilatation (score 3/3).
The pathological scores for each patient who had a liver biopsy using the scoring criteria are displayed in Table S4 along with the liver stiffness, age at Fontan, time from Fontan, and systemic ventricle(s) for each patient. A total of 11 patients had both TE and biopsy within a 6‐month period. The median liver stiffness, PVR, and APRI were not significantly different between patients with mild versus moderate liver pathology and this finding was consistent for each of the 4 biopsy scoring systems (Tables S5 through S7).
Discussion
In our systematic evaluation of children and adolescents with Fontan physiology, we found that there was liver disease present based on pathologic evaluation. Liver biopsy remains the gold standard for evaluating liver disease. In this cohort, comprising patients who underwent liver biopsy ≈10 years post‐Fontan, mild to moderate histopathologic changes were present in all patients, but no severe disease (eg, cardiac cirrhosis) was identified. While it is known that there is a significant burden of liver disease in young adults with Fontan physiology,7 our study demonstrates that liver pathology begins in the pediatric age cohort. Furthermore, mild to moderate portal‐based fibrosis, typically a manifestation of inflammatory injury to the portal tract, was found in nearly all patients who underwent biopsy, consistent with findings of earlier studies.5, 17, 18 This suggests that institution of liver surveillance throughout childhood and adolescence is warranted.
Hemodynamic data demonstrated that there was not an association between the extent of liver pathology and PVR. This is relevant with respect to the growing interest in the investigation of pulmonary vasodilator therapy to minimize the sequelae of the Fontan circulation.27 Given our results, it is possible that interventions to reduce PVR may not be the only therapeutic target that can potentially modify the natural history of Fontan‐associated liver disease.
We were particularly interested in the use of TE as a method of noninvasive assessment of liver health. We found that liver stiffness is directly correlated with the duration from the Fontan operation but does not correlate with histopathologic findings at a single time point. Based on this result, it is reasonable to infer that TE may be a useful method to monitor evolving liver stiffness over time in patients with Fontan physiology, but serial analysis of TE in this population will be required to address this hypothesis. Furthermore, the serial trend should be compared with liver biopsy results as the serial data may be a better reflection of evolving liver pathology. The absence of severe fibrosis and the limited variability in the age of patients who underwent biopsy is a limitation in our study that makes a significant relationship between TE and liver pathology difficult to establish.
Liver stiffness measurement with TE is a rapid and noninvasive method of assessing for liver fibrosis. Similarly, APRI is a minimally invasive, easily accessible, and inexpensive test. These characteristics make these tests attractive modalities for liver health surveillance in pediatric patients with Fontan physiology. However, in our cohort, neither the APRI nor the TE measured at a single time point correlated with the severity of histopathologic changes. Given our small sample size and the possibility for type 2 error, the utility of these investigations as markers of liver disease based on a single measurement in patients with Fontan physiology requires further investigation in a larger cohort of patients.
Although single measurements with TE may not coincide with the extent of liver pathology, the trend in TE values in individual patients over time may be informative. It has previously been shown that TE increases acutely following the Fontan completion in association with hepatic congestion secondary to an acute rise in the inferior vena cava pressure.14, 23 Changes that occur subsequent to the initial accommodation to Fontan physiology could potentially reflect evolving liver pathology or changes in Fontan physiology (such as increased PVR and increased venous pressures). We found that liver stiffness is positively correlated with time from Fontan and patient age. This supports the idea that the change in an individual patients’ liver stiffness over time may represent a combination of both preexisting hepatic congestion and evolving liver pathology. The lack of association between both the Fontan pressure and PVR with TE suggests that the hepatic changes occurring in the patients in our cohort may represent evolving hepatic pathology rather than an interval increase in hepatic congestion secondary to an elevated PVR. Serial evaluation of TE and liver pathology in future research may clarify these concepts. Furthermore, we have demonstrated that there is an association between increasing liver stiffness and established biochemical markers of liver function, including platelet count and APRI. This supports the notion that interval increases in liver stiffness may be an indicator of evolving liver disease but will require further investigation to confirm.
Abdominal ultrasound demonstrated a significant correlation between splenomegaly and TE. This may act as a surrogate marker for evolving portal hypertension. We are unable to define a clear threshold for when splenomegaly can be inferred from a single TE measurement based on cross‐sectional data. Further prospective study will be required to explore this association. Furthermore, a low platelet count, low AST, and low alkaline phosphatase were also correlated with the presence of splenomegaly in our population. These investigations may also serve as an early indicator of splenomegaly and potential portal hypertension. There were a variety of other abnormal findings on ultrasound that included abnormal hepatic echotexture, liver surface nodularity, and hepatomegaly, but none of these findings were associated with measurements of liver stiffness by TE.
Despite the growing evidence for liver disease in patients with Fontan physiology, a noninvasive method to accurately identify and monitor liver pathology remains elusive, with liver biopsy as the gold standard for definitive diagnosis.4, 5, 6, 15 Furthermore, there are no validated measures to risk‐stratify pediatric patients with Fontan physiology with respect to the evolution of liver disease. This study demonstrates a significant presence of liver disease in children and adolescent patients with Fontan physiology based on biopsy data. Given that mild to moderate pathologic features were universally found within our subset of patients with Fontan physiology who underwent biopsy in adolescence, we conclude that early liver disease is ubiquitous among patients with Fontan physiology before transitioning to adult care. Pediatric Fontan‐associated liver disease is often clinically silent and, hence, mandates a standardized investigative approach to facilitate early identification of liver dysfunction. An evidence‐based surveillance protocol has yet to be established in this regard; however, our data indicate that there may be utility in laboratory markers of liver function and abdominal ultrasound as a means of screening for evolving liver dysfunction and portal hypertension. Abnormalities with screening should prompt referral to a hepatology specialist, and optimization of Fontan hemodynamics. Liver biopsies performed in our cohort confirmed the ubiquity of liver pathology in pediatric patients with Fontan physiology. Liver biopsy is presently the only way to confirm the presence and extent of liver pathology in this population. Noninvasive liver health evaluation would be ideal in this population to identify patients at an early stage of disease and be able to monitor changes serially to facilitate timely intervention.
Measurement of liver stiffness by TE shows promise as a noninvasive investigation to evaluate the evolution of liver disease, and this should prompt more prospective evaluation to determine the utility of this test as a method of liver health surveillance. Given the association between TE and splenomegaly, further prospective study may allow the determination of a threshold value that infers evolving portal hypertension. Our current understanding of Fontan‐associated liver disease should also encourage pediatric practitioners to counsel families about liver health and promote preventative measures such as routine hepatitis vaccination, and recommendations regarding diet, exercise, and alcohol consumption.
Study Limitations
The limitations of this study include a small sample size of patients with Fontan physiology who underwent liver biopsy with concurrent hemodynamic assessment due to the single‐center design. The transjugular approach for liver biopsy is also a limitation since it only samples a small proportion of the liver adjacent to the inferior vena cava. Despite this limitation, this route for biopsy was chosen to reduce the risk of bleeding and take advantage of being able to combine a transjugular liver biopsy with a cardiac catheterization in a single procedure. In some patients, we were unable to coordinate all investigations in the Fontan pathway at each clinic assessment because of the outreach model that is used to serve the large geographic area of British Columbia; however, by having a research team member travel to these clinics we were able to include children from across the entire province. Furthermore, given that catheterization and biopsy were performed as per clinical protocol at ≈10 years post‐Fontan, the age range of patients within this subset is limited and therefore only represents the distribution of liver pathology over a limited demographic within the pediatric population. Significant correlations with noninvasive investigations are therefore difficult to establish.
Conclusions
Liver disease in patients with Fontan physiology begins during childhood and is universally present. The spectrum of liver disease in our cohort ranges from mild to moderate in severity and is only reliably detected by liver biopsy. Abdominal ultrasound TE and APRI measurements do not correlate with the severity of liver pathology but show potential as noninvasive measures of change over time that will require further prospective analysis to determine how they may be used to refine clinical care. Until a noninvasive study of liver pathology has been validated, liver biopsy will continue to be the gold standard for identifying and staging liver disease in children with Fontan physiology. Noninvasive measures of liver health including biochemical markers and ultrasound should be used to guide consultation from specialists in hepatology and consideration of liver biopsy to define the extent of liver disease.
Sources of Funding
This work was supported by the Rare Disease Foundation Microgrant Program, Vancouver, BC.
Disclosures
None.
Supporting information
Table S1. BC Children's Hospital Liver Health Surveillance Pathway for Patients With Fontan
Table S2. System Used to Stage Liver Fibrosis and Grade Portal Inflammation in All Transjugular Liver Biopsy Specimens
Table S3. Association Between the Median Liver Stiffness and the Type of Systemic Ventricle
Table S4. Histological Scores and Demographics for Each Patient Who Underwent Liver Biopsy
Table S5. Association Between the Median Liver Stiffness and the Severity of Liver Pathology
Table S6. Association Between the Median APRI and the Degree of Severity of Liver Pathology
Table S7. Association Between the Median PVR and the Severity of Liver Pathology
(J Am Heart Assoc. 2020;9:e012529 DOI: 10.1161/JAHA.119.012529.)
References
- 1. Rychik J. Forty years of the Fontan operation: a failed strategy. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. 2010;13:96–100. [DOI] [PubMed] [Google Scholar]
- 2. de Leval MR, Deanfield JE. Four decades of Fontan palliation. Nat Rev Cardiol. 2010;7:520–527. [DOI] [PubMed] [Google Scholar]
- 3. Rychik J. Protein‐losing enteropathy after Fontan operation. Congenit Heart Dis. 2007;2:288–300. [DOI] [PubMed] [Google Scholar]
- 4. Rychik J. The relentless effects of the Fontan paradox. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. 2016;19:37–43. [DOI] [PubMed] [Google Scholar]
- 5. Surrey LF, Russo P, Rychik J, Goldberg DJ, Dodds K, O'Byrne ML, Glatz AC, Rand EB, Lin HC. Prevalence and characterization of fibrosis in surveillance liver biopsies of patients with Fontan circulation. Hum Pathol. 2016;57:106–115. [DOI] [PubMed] [Google Scholar]
- 6. Rychik J, Veldtman G, Rand E, Russo P, Rome JJ, Krok K, Goldberg DJ, Cahill AM, Wells RG. The precarious state of the liver after a Fontan operation: summary of a multidisciplinary symposium. Pediatr Cardiol. 2012;33:1001–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Krishna K, Pundi KN, Kamath PS, Cetta F, Li Z, Poterucha JT, Driscoll DJ, Johnson JN. Liver disease in patients after the Fontan operation. Am J Cardiol. 2015;117:456–460. [DOI] [PubMed] [Google Scholar]
- 8. Asrani SK, Asrani NS, Freese DK, Phillips SD, Warnes CA, Heimbach J, Kamath PS. Congenital heart disease and the liver. Hepatology. 2012;56:1160–1169. [DOI] [PubMed] [Google Scholar]
- 9. Wu FM, Kogon B, Earing MG, Aboulhosn JA, Broberg CS, John AS, Harmon A, Sainani NI, Hill AJ, Odze RD, Johncilla ME, Ukomadu C, Gauvreau K, Valente M, Landzberg MJ. Liver health in adults with Fontan circulation: a multicenter cross‐sectional study. J Thorac Cardiovasc Surg. 2016;153:1–10. [DOI] [PubMed] [Google Scholar]
- 10. Wu FM, Ukomadu C, Odze RD. Liver disease in the patient with Fontan circulation. Congenit Heart Dis. 2011;6:190–201. [DOI] [PubMed] [Google Scholar]
- 11. Camposilvan S, Milanesi O, Stellin G, Pettenazzo A, Zancan L, D'Antiga L. Liver and cardiac function in the long term after Fontan operation. Ann Thorac Surg. 2008;86:177–182. [DOI] [PubMed] [Google Scholar]
- 12. Kiesewetter CH, Sheron N, Vettukattill JJ, Hacking N, Stedman B, Millward‐Sadler H, Haw M, Cope R, Salmon AP, Sivaprakasam MC, Kendall T, Keeton BR, Iredale JP, Veldtman GR. Hepatic changes in the failing Fontan circulation. Heart. 2007;93:579–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ghaferi AA, Hutchins GM. Progression of liver pathology in patients undergoing the Fontan procedure: chronic passive congestion, cardiac cirrhosis, hepatic adenoma, and hepatocellular carcinoma. J Thorac Cardiovasc Surg. 2005;129:1348–1352. [DOI] [PubMed] [Google Scholar]
- 14. DiPaola FW, Schumacher KR, Goldberg CS, Friedland‐Little J, Parameswaran A, Dillman JR. Effect of Fontan operation on liver stiffness in children with single ventricle physiology. Eur Radiol. 2016;27:2434–2442. [DOI] [PubMed] [Google Scholar]
- 15. Wu F, Opotowsky A, Raza R, Harney S, Ukomadu C, Landzberg M, Valente A, Breitbart R, Singh M, Gauvreau K. Transient elastography may identify Fontan patients with unfavorable hemodynamics and advanced hepatic fibrosis. Congenit Heart Dis. 2014;9:438–447. [DOI] [PubMed] [Google Scholar]
- 16. Furukawa T, Akimoto K, Ohtsuki M, Sato K, Suzuki M, Takahashi K, Kishiro M, Shimizu T, Kawasaki S. Non‐invasive assessment of liver fibrosis in patients after the Fontan operation. Pediatr Int. 2011;53:980–984. [DOI] [PubMed] [Google Scholar]
- 17. Schwartz M, Sullivan L, Cohen M, Russo P, John A, Guo R, Guttenberg M. Hepatic pathology may develop before the Fontan operation in children with functional single ventricle: an autopsy study. J Thorac Cardiovasc Surg. 2012;143:904–909. [DOI] [PubMed] [Google Scholar]
- 18. Schwartz M, Sullivan L, Glatz A, Rand E, Russo P, Goldberg D, Rome J. Portal and sinusoidal fibrosis are common on liver biopsy after Fontan surgery. Pediatr Cardiol. 2013;34:135–142. [DOI] [PubMed] [Google Scholar]
- 19. Kutty SS, Peng Q, Danford DA, Fletcher SE, Perry D, Talmon GA, Scott C, Kugler JD, Duncan KF, Quiros‐Tejeira RE, Kutty S. Increased hepatic stiffness as consequence of high hepatic afterload in the Fontan circulation: a vascular Doppler and elastography study. Hepatology. 2014;59:251–260. [DOI] [PubMed] [Google Scholar]
- 20. Narkewicz MR, Sondheimer HM, Ziegler JW, Otanni Y, Lorts A, Shaffer EM, Horgan JG, Sokol RJ. Hepatic dysfunction following the Fontan procedure. J Pediatr Gastroenterol Nutr. 2003;36:352–357. [DOI] [PubMed] [Google Scholar]
- 21. Chen B, Schreiber RA, Human D, Potts JE, Guttman OR. Assessment of liver stiffness in pediatric post‐Fontan patients using transient elastography. Hepatology. 2013;58:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gnanappa GK, Celermajer DS, Sholler GF, Gentles T, Winlaw D, d'Udekem Y, Ayer J. The long‐term management of children and adults with a Fontan circulation: a systematic review and survey of current practice in Australia and New Zealand. Pediatr Cardiol. 2016;38:56–69. [DOI] [PubMed] [Google Scholar]
- 23. Deorsola L, Aidala E, Cascarano MT, Valori A, Agnoletti G, Napoleone CP. Liver stiffness modifications shortly after total cavopulmonary connection. Interact Cardiovasc Thorac Surg. 2016;23:513–518. [DOI] [PubMed] [Google Scholar]
- 24. Lin ZH, Xin YN, Dong QJ, Wang Q, Jiang XJ, Zhan SH, Sun Y, Xuan SY. Performance of the aspartate aminotransferase‐to‐platelet ratio index for the staging of hepatitis C‐related fibrosis: an updated meta‐analysis. Hepatology. 2011;53:726–736. [DOI] [PubMed] [Google Scholar]
- 25. Rathgeber SL, Harris KC. Fontan‐associated liver disease: evidence for early surveillance of liver health in pediatric Fontan patients. Can J Cardiol. 2019;35:217–220. [DOI] [PubMed] [Google Scholar]
- 26. Seckeler MD, Hirsch R, Beekman RH, Goldstein BH. A new predictive equation for oxygen consumption in children and adults with congenital and acquired heart disease. Heart. 2015;101:517–524. [DOI] [PubMed] [Google Scholar]
- 27. Snarr BS, Paridon SM, Rychik J, Goldberg DJ. Pulmonary vasodilator therapy in the failing Fontan circulation: rationale and efficacy. Cardiol Young. 2015;25:1489–1492. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. BC Children's Hospital Liver Health Surveillance Pathway for Patients With Fontan
Table S2. System Used to Stage Liver Fibrosis and Grade Portal Inflammation in All Transjugular Liver Biopsy Specimens
Table S3. Association Between the Median Liver Stiffness and the Type of Systemic Ventricle
Table S4. Histological Scores and Demographics for Each Patient Who Underwent Liver Biopsy
Table S5. Association Between the Median Liver Stiffness and the Severity of Liver Pathology
Table S6. Association Between the Median APRI and the Degree of Severity of Liver Pathology
Table S7. Association Between the Median PVR and the Severity of Liver Pathology


