Abstract
Background
The contemporary impact of glycemic control on patients with diabetes mellitus at high cardiovascular risk remains unclear. We evaluated the utility of hemoglobin A1c (HbA1c) as a marker of risk on the composite end point of cardiovascular death, nonfatal myocardial infarction, stroke, hospitalization for unstable angina, and coronary revascularization in an optimally treated population with diabetes mellitus and established coronary artery disease enrolled in the ACCELERATE (Assessment of Clinical Effects of Cholesteryl Ester Transfer Protein Inhibition With Evacetrapib in Patients at a High Risk for Vascular Outcomes) trial.
Methods and Results
We included all patients with established diabetes mellitus and measured HbA1c (N=8145) and estimated Kaplan‐Meier (KM) events rates, stratified by increasing baseline HbA1c levels censored at 30 months. We then performed a multivariable regression for the primary end point. Increasing baseline HbA1c was strongly associated with the occurrence of the primary end point (KM estimate, 12.6–18.2; P<0.001). Increasing baseline HbA1c was also associated with the triple end point of death, nonfatal myocardial infarction, and stroke (KM estimate, 7.8–11.3; P=0.003) as well as the individual end points of nonfatal myocardial infarction (KM estimate, 3.1–7.0; P<0.001), hospitalization for unstable angina (KM estimate, 1.8–5.0; P=0.003), and revascularization (KM estimate, 7.3–11.1; P=0.001), although not stroke (KM estimate, 1.4–2.4; P=0.45). The rates of cardiovascular mortality (KM estimate, 2.6–4.3; P=0.21) and all‐cause mortality (KM estimate, 4.8–5.9; P=0.21) were similar regardless of baseline HbA1c levels. When adjusting for relevant baseline characteristics, baseline HbA1c was an independent predictor for the primary end point (hazard ratio, 1.06; 95% CI, 1.02–1.11; P=0.003).
Conclusions
Glycemic control, as measured by HbA1c, remains strongly and independently associated with cardiovascular outcomes in high‐risk patients with diabetes mellitus on statin therapy.
Clinical Trial Registration
URL: https://www.clinicaltrials.gov. Unique identifier: NCT01687998.
Keywords: hemoglobin A1c, major adverse cardiovascular events, risk stratification
Subject Categories: Biomarkers; Cardiovascular Disease; Diabetes, Type 2; Secondary Prevention; Metabolic Syndrome; Vascular Disease
Clinical Perspective
What Is New?
Although recent trials have suggested that strict glucose regimens may not be the optimal treatment to reduce cardiovascular risk for patients with diabetes mellitus, our study demonstrates that hemoglobin A1c remains an independent predictor for future cardiovascular events in a contemporary population of optimally treated patients with diabetes mellitus.
There has been concern for a J‐shaped relationship between glycemic control and mortality, with increased death observed at both the highest and lowest levels of hemoglobin A1c. A similar phenomenon was not noted in our study.
What Are the Clinical Implications?
Given the recent availability of new antiglycemic agents that improve cardiovascular outcomes, hemoglobin A1c measurements remain a guiding tool to identify, initiate, monitor, and modify these beneficial treatments.
Introduction
Patients with diabetes mellitus (DM) carry a significant burden of future cardiovascular morbidity and mortality.1, 2 Hemoglobin A1c (HbA1c) is a standard‐of‐care biomarker that correlates with long‐term glycemic control and risk for complications, and it is used for both the diagnosis and the management of patients with DM.3, 4, 5 The seminal UKPDS (UK Prospective Diabetes Study) in patients with type 2 DM established a strong association between observed HbA1c levels and the risk of macrovascular and microvascular events.6 In patients with DM, optimal control of low‐density lipoprotein cholesterol (LDL‐C) and blood pressure, cessation of smoking, appropriate revascularization, and the use of potent antiplatelet therapy have all been shown to mitigate residual risk in a secondary prevention setting.7 In contrast, the mere reduction of HbA1c with stringent glycemic control using predominantly sulfonylurea‐based pharmacotherapy has not been associated with macrovascular benefits.8
The recent approval of pharmacologic agents that are proved to improve glycemic control while simultaneously reducing cardiovascular risk has added significantly to our therapeutic armamentarium.9 The utility of HbA1c as a marker of risk in an optimally treated contemporary population with type 2 DM at high cardiovascular risk remains unclear. The ACCELERATE (Assessment of Clinical Effects of Cholesteryl Ester Transfer Protein Inhibition With Evacetrapib in Patients at a High Risk for Vascular Outcomes) trial was a randomized, double‐blinded, placebo‐controlled trial investigating the use of evacetrapib, a CETP (cholesteryl ester transfer protein) inhibitor, in patients with high‐risk vascular disease, including those with recent acute coronary syndrome, peripheral arterial disease, cerebrovascular disease, and DM with known history of coronary artery disease.10 We performed a subanalysis examining the relationship between baseline HbA1c and future cardiovascular events among participants with DM enrolled in the ACCELERATE trial.
Methods
The authors declare that all supporting data are available within the article. The study protocol was approved by the Cleveland Clinic Foundation institutional review board; need for informed consent was waived.
The trial design of the ACCELERATE trial has previously been described.11 Briefly, 12 092 patients with high‐risk vascular disease, as described above, were randomized in a 1:1 manner to receive the CETP inhibitor evacetrapib, 130 mg, versus placebo. The trial was event driven, with a primary end point of major adverse cardiovascular events, which included cardiovascular death, nonfatal myocardial infarction (MI), stroke, coronary revascularization, or hospitalization for unstable angina. A secondary triple end point, including the composite of cardiovascular death, nonfatal MI, and stroke, as well as individual end points of all‐cause mortality, cardiovascular death, nonfatal MI, stroke, coronary revascularization, and hospitalization for unstable angina were also assessed. Because of clinical futility for the primary composite end point, the trial was terminated prematurely after a mean follow‐up of 30 months.10 Follow‐up of participants for clinical events was near complete, with an end‐of‐study visit completed in 98.8% of trial participants. Clinical events were identified and prospectively adjudicated by an independent clinical end points committee blinded to treatment assignment using prespecified standardized definitions.
HbA1c levels were measured in participants at study initiation using the Bio‐Rad Variant II and Variant II Turbo high‐performance liquid chromatography method. Both assays are certified by the National Glycohemoglobin Standardization Program and are traceable to the DCCT (Diabetes Control and Complications Trial) reference method and values. Patients with DM were defined as those receiving treatment with an oral or parenteral antiglycemic agent and/or insulin or being managed by diet alone as a result of a preexisting diagnosis of DM. A new diagnosis of DM was based on fasting plasma glucose measurements ≥126 mg/dL, 2‐hour plasma glucose ≥200 mg/dL during an oral glucose tolerance test, or HbA1c levels ≥6.5%.
Baseline patient characteristics, medications, and laboratory parameters were collected as per protocol. LDL‐C levels were directly measured using the Roche LDL‐C Plus second‐generation assay. Mean±SD or median with interquartile range was reported for continuous variables, and frequency with percentages was reported for categorical variables. Kaplan‐Meier (KM) estimates were calculated for the risk of end point with increasing HbA1c by 0.5% increments. An ANOVA or χ2 test of trend with 1 df was performed to compare baseline characteristics and clinical end points across HbA1c groups. A multivariable Cox proportional hazard model was created to assess for risk of the primary composite end point with HbA1c as a continuous variable after adjusting for relevant baseline characteristics. Last, a sensitivity analysis considering mortality as a competing risk was performed. Hazard ratios with 95% CIs are reported. KM curves illustrate the cumulative incidence of major adverse cardiovascular events, the secondary triple end point, and all‐cause mortality by the baseline HbA1c groups. P≤0.05 was considered statistically significant. Statistical analyses were performed using SAS, version 9.4 (SAS Institute Inc, Cary, NC), and figures were created in SigmaPlot, version 11.0 (Systat Software, Inc, San Jose, CA).
Results
Among 12 092 patients enrolled in the ACCELERATE trial, 8236 had DM, of which 8145 had a baseline HbA1c analyzed. Baseline characteristics, lipid parameters, and medical therapy for patients with DM are presented in Table 1. Statistically significant trends for age, sex, body mass index, current smoking, and presence of peripheral artery disease and congestive heart failure were noted with increasing levels of baseline HbA1c. Baseline LDL‐C, triglycerides, apolipoprotein B, and high‐sensitivity CRP (C‐reactive protein) were significantly greater, whereas high‐density lipoprotein cholesterol and apolipoprotein A1 were significantly less, with increasing baseline HbA1c. Patients with increasing baseline HbA1c were significantly more likely to be treated with a high‐intensity statin and antiglycemic medications, particularly sulfonylureas and insulin.
Table 1.
Baseline Characteristics of Patients With DM in the ACCELERATE Trial, Stratified by HbA1c
| Characteristic | Total (N=8145) | HbA1c, % | P Value | |||||
|---|---|---|---|---|---|---|---|---|
| <6.0 (N=1418) | 6.0–<6.5 (N=1620) | 6.5–<7.0 (N=1554) | 7.0–<7.5 (N=1111) | 7.5–<8.0 (N=792) | ≥8.0 (N=1650) | |||
| Age, y | 65.5±8.7 | 65.5±8.6 | 66.7±8.6 | 66.6±8.3 | 65.9±8.6 | 65.4±8.4 | 63.0±9.0 | <0.001 |
| Men, n (%) | 6218 (76.3) | 1131 (79.8) | 1268 (78.3) | 1173 (75.5) | 835 (75.2) | 605 (76.4) | 1206 (73.1) | <0.001 |
| White, n (%) | 6354 (78.4) | 1180 (83.5) | 1260 (78.3) | 1214 (78.5) | 875 (79.1) | 623 (79.4) | 1202 (73.2) | <0.001 |
| Body mass index, kg/m2 | 31.1±5.9 | 30.4±5.4 | 30.6±6.1 | 31.1±5.9 | 31.1±6.1 | 31.3±5.5 | 32.2±6.1 | <0.001 |
| Recent acute coronary syndrome, n (%) | 1269 (15.6) | 250 (17.6) | 249 (15.4) | 212 (13.6) | 159 (14.3) | 127 (16.0) | 272 (16.5) | 0.77 |
| Cerebral vascular disease, n (%) | 1735 (21.3) | 299 (21.1) | 342 (21.1) | 350 (22.5) | 250 (22.5) | 176 (22.2) | 318 (19.3) | 0.33 |
| Peripheral artery disease, n (%) | 1354 (16.6) | 212 (15.0) | 238 (14.7) | 261 (16.8) | 189 (17.0) | 144 (18.2) | 310 (18.8) | <0.001 |
| Coronary artery disease, n (%) | 7594 (93.2) | 1327 (93.6) | 1518 (93.7) | 1447 (93.1) | 1046 (94.1) | 736 (92.9) | 1520 (92.1) | 0.09 |
| Hypertension, n (%) | 7474 (91.8) | 1313 (92.6) | 1491 (92.0) | 1417 (91.2) | 1027 (92.4) | 732 (92.4) | 1494 (90.5) | 0.11 |
| Current smoker, n (%) | 1168 (14.3) | 160 (11.3) | 225 (13.9) | 234 (15.1) | 158 (14.2) | 123 (15.5) | 268 (16.2) | <0.001 |
| Prior myocardial infarction, n (%) | 4551 (59.9) | 832 (62.7) | 900 (59.2) | 833 (57.5) | 602 (57.5) | 441 (59.9) | 943 (62.0) | 0.92 |
| Prior percutaneous coronary intervention, n (%) | 5358 (70.5) | 927 (69.9) | 1088 (71.7) | 1003 (69.3) | 737 (70.5) | 512 (69.6) | 1091 (71.8) | 0.55 |
| Prior coronary artery bypass grafting, n (%) | 2518 (33.1) | 428 (32.2) | 501 (33.0) | 490 (33.8) | 359 (34.3) | 265 (36.0) | 475 (31.3) | 0.94 |
| Congestive heart failure, n (%) | 1249 (15.3) | 194 (13.7) | 208 (12.8) | 221 (14.2) | 207 (18.6) | 129 (16.3) | 290 (17.6) | <0.001 |
| Chronic obstructive pulmonary disease, n (%) | 883 (10.8) | 157 (11.1) | 177 (10.9) | 172 (11.1) | 116 (10.4) | 91 (11.5) | 170 (10.3) | 0.56 |
| Renal impairment, n (%) | 858 (10.5) | 113 (8.0) | 170 (10.5) | 186 (12.0) | 134 (12.1) | 93 (11.7) | 162 (9.8) | 0.13 |
| Baseline laboratory values | ||||||||
| Low‐density lipoprotein cholesterol, mg/dL | 80.0±27.4 | 78.7±27.2 | 79.8±26.2 | 79.3±27.1 | 79.3±25.2 | 78.5±27.1 | 83.3±30.3 | <0.001 |
| High‐density lipoprotein cholesterol, mg/dL | 44.2±11.3 | 45.7±11.7 | 45.2±11.3 | 45.1±11.5 | 43.9±11.3 | 42.5±10.5 | 42.2±10.8 | <0.001 |
| Triglyceride, mg/dL | 136 (99–186) | 121 (91–164) | 130 (97–177) | 134 (99–182) | 140 (102–188) | 144 (106–195) | 153 (111–218) | <0.001 |
| Apolipoprotein A‐I, mg/dL | 137.4±25.0 | 139.6±26.5 | 138.7±24.2 | 138.7±24.6 | 137.2±25.3 | 133.7±24.1 | 134.8±24.9 | <0.001 |
| Apolipoprotein B, mg/dL | 78.3±21.6 | 75.8±21.2 | 76.9±20.3 | 77.2±20.9 | 78.6±20.4 | 77.6±20.9 | 83.0±23.9 | <0.001 |
| Lipoprotein(a), nmol/L | 27 (10–100) | 29 (11–99) | 28 (11–104) | 29 (10–99) | 26 (10–105) | 25 (11–100) | 25 (9–90) | 0.33 |
| High‐sensitivity CRP, mg/L | 1.54 (0.76–3.42) | 1.22 (0.63–2.61) | 1.29 (0.66–2.91) | 1.52 (0.75–3.17) | 1.55 (0.76–3.41) | 1.68 (0.86–4.16) | 2.16 (1.01–4.76) | <0.001 |
| Medications, n (%) | ||||||||
| Statin | 7831 (96.1) | 1362 (96.1) | 1551 (95.7) | 1496 (96.3) | 1073 (96.6) | 761 (96.1) | 1588 (96.2) | 0.56 |
| High‐dose statin | 3427 (42.6) | 561 (40.0) | 675 (42.3) | 657 (42.8) | 459 (41.8) | 350 (45.0) | 725 (44.5) | 0.012 |
| Angiotensin‐converting enzyme inhibitor/angiotensin‐II receptor blocker | 6549 (80.4) | 1145 (80.7) | 1296 (80.0) | 1244 (80.1) | 875 (78.8) | 647 (81.7) | 1342 (81.3) | 0.48 |
| Aspirin | 6801 (83.5) | 1203 (84.8) | 1359 (83.9) | 1295 (83.3) | 916 (82.4) | 648 (81.8) | 1380 (83.6) | 0.20 |
| Antiglycemic medications, n (%) | ||||||||
| Any medication | 7182 (88.2) | 1083 (76.4) | 1326 (81.9) | 1390 (89.4) | 1037 (93.3) | 759 (95.8) | 1587 (96.2) | <0.001 |
| Insulin | 2391 (29.4) | 104 (7.3) | 208 (12.8) | 367 (23.6) | 387 (34.8) | 350 (44.2) | 975 (59.1) | <0.001 |
| Oral medication | 6313 (77.5) | 1032 (72.8) | 1258 (77.7) | 1270 (81.7) | 902 (81.2) | 627 (79.2) | 1224 (74.2) | 0.61 |
| Biguanide | 4894 (60.1) | 796 (56.1) | 985 (60.8) | 993 (63.9) | 689 (62.0) | 486 (61.4) | 945 (57.3) | 0.97 |
| Sulfonylurea | 2385 (29.3) | 263 (18.5) | 391 (24.1) | 437 (28.1) | 411 (37.0) | 312 (39.4) | 571 (34.6) | <0.001 |
| α‐Glucosidase inhibitor | 320 (3.9) | 30 (2.1) | 76 (4.7) | 61 (3.9) | 56 (5.0) | 34 (4.3) | 63 (3.8) | 0.09 |
| Thiazolidinedione | 296 (3.6) | 42 (3.0) | 61 (3.8) | 63 (4.1) | 43 (3.9) | 31 (3.9) | 56 (3.4) | 0.68 |
| Dipeptidyl peptidase‐4 inhibitor | 981 (12.0) | 116 (8.2) | 162 (10.0) | 200 (12.9) | 180 (16.2) | 109 (13.8) | 214 (13.0) | <0.001 |
| Other oral medication | 396 (4.9) | 39 (2.8) | 59 (3.6) | 65 (4.2) | 75 (6.8) | 51 (6.4) | 107 (6.5) | <0.001 |
Data are given as mean±SD or median (interquartile range), unless otherwise indicated. ACCELERATE indicates Assessment of Clinical Effects of Cholesteryl Ester Transfer Protein Inhibition With Evacetrapib in Patients at a High Risk for Vascular Outcomes; CRP, C‐reactive protein; DM, diabetes mellitus; HbA1c, hemoglobin A1c.
Clinical outcomes among patients with DM, stratified by HbA1c levels at study baseline, are presented in Table 2. Increasing baseline HbA1c levels were strongly associated with the occurrence of the primary composite end point (KM estimate, 12.6–18.2; P<0.001; Figure). Increasing baseline HbA1c levels were also associated with the triple end point of cardiovascular death, nonfatal MI, and stroke (KM estimate, 7.8–11.3; P=0.003) as well as the individual end points of nonfatal MI (KM estimate, 3.1–7.0; P<0.001), hospitalization for unstable angina (KM estimate, 1.8–5.0; P=0.003), and need for coronary revascularization (KM estimate, 7.3–11.1; P=0.001), although not for nonfatal stroke (KM estimate, 1.4–2.4; P=0.45). The observed rates of cardiovascular mortality (KM estimate, 2.6–4.3; P=0.21) and all‐cause mortality (KM estimate, 4.8–5.9; P=0.21) were similar regardless of baseline HbA1c levels. The competing risk of death did not alter conclusions on the individual end points of nonfatal MI, stroke, coronary revascularization, or hospitalization for unstable angina. When adjusting for significant baseline characteristics in a multivariable model, baseline HbA1c was an independent predictor for the primary composite end point censored at 915 days (hazard ratio, 1.06; 95% CI, 1.02–1.11; P=0.003) (Table 3).
Table 2.
Kaplan‐Meier Estimates for Risk of MACEs by Baseline HbA1c in Patients With DM in the ACCELERATE Trial
| End Point | HbA1c, % | P Value | |||||
|---|---|---|---|---|---|---|---|
| <6.0 (N=1418) | 6.0–<6.5 (N=1620) | 6.5–<7.0 N=1554) | 7.0–<7.5 (N=1111) | 7.5–<8.0 (N=792) | ≥8.0 (N=1650) | ||
| MACE | 168 (12.6) | 212 (14.5) | 201 (14.0) | 163 (16.1) | 117 (16.3) | 278 (18.2) | <0.001 |
| Cardiovascular death/MI/stroke | 101 (7.8) | 130 (8.9) | 111 (7.8) | 83 (7.9) | 58 (8.0) | 172 (11.3) | 0.003 |
| Cardiovascular death | 45 (3.4) | 41 (3.0) | 42 (2.9) | 36 (3.6) | 18 (2.6) | 63 (4.3) | 0.21 |
| All‐cause mortality | 63 (4.8) | 69 (5.0) | 71 (4.9) | 50 (5.0) | 35 (5.1) | 88 (5.9) | 0.21 |
| Nonfatal MI | 41 (3.1) | 84 (5.6) | 58 (4.1) | 46 (4.3) | 35 (5.0) | 106 (7.0) | <0.001 |
| Stroke | 27 (2.3) | 22 (1.5) | 27 (2.0) | 14 (1.4) | 13 (1.8) | 35 (2.4) | 0.45 |
| Revascularization | 96 (7.3) | 142 (9.6) | 125 (8.9) | 99 (10.3) | 75 (10.4) | 168 (11.1) | 0.001 |
| Hospitalization for unstable angina | 31 (2.3) | 25 (1.8) | 40 (2.8) | 46 (5.0) | 23 (3.1) | 52 (3.4) | 0.003 |
Data are given as number of patients (Kaplan‐Meier estimate). MACEs include cardiovascular death, nonfatal MI, cerebrovascular accident, hospitalization for unstable angina, and revascularization. ACCELERATE indicates Assessment of Clinical Effects of Cholesteryl Ester Transfer Protein Inhibition With Evacetrapib in Patients at a High Risk for Vascular Outcomes; DM, diabetes mellitus; HbA1c, hemoglobin A1c; MACE, major adverse cardiovascular event; MI, myocardial infarction.
Figure 1.
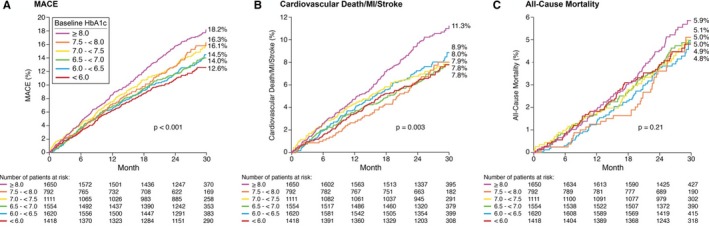
Kaplan–Meier estimates of major adverse cardiovascular events (MACEs), the triple end point (cardiovascular death, myocardial infarction [MI], and stroke), and all‐cause mortality by baseline hemoglobin A1c (HbA1c) among patients with diabetes mellitus enrolled in the ACCELERATE (Assessment of Clinical Effects of Cholesteryl Ester Transfer Protein Inhibition With Evacetrapib in Patients at a High Risk for Vascular Outcomes) trial.
Table 3.
Multivariable Model for the Primary Composite End Point by Increasing HbA1c
| Variable | HR (95% CI) | P Value |
|---|---|---|
| Baseline HbA1c (%) | 1.06 (1.02–1.11) | 0.003 |
| Congestive heart failure | 1.62 (1.40–1.86) | <0.001 |
| Recent acute coronary syndrome | 1.56 (1.35–1.81) | <0.001 |
| Baseline systolic blood pressure, mm Hga | 1.13 (1.08–1.17) | <0.001 |
| Baseline diastolic blood pressure, mm Hga | 0.86 (0.80–0.92) | <0.001 |
| Peripheral artery disease | 1.26 (1.08–1.46) | 0.003 |
| Renal impairment | 1.28 (1.08–1.52) | 0.004 |
| Hypertension | 1.46 (1.13–1.90) | 0.005 |
| Baseline apolipoprotein B (mg/dL)a | 1.03 (1.01–1.06) | 0.012 |
| Current smoker | 1.17 (1.00–1.38) | 0.053 |
| History of stroke | 1.21 (0.99–1.48) | 0.057 |
HbA1c indicates hemoglobin A1c; HR, hazard ratio.
Other variables considered, but not significant, in the final model include age, sex, body mass index, prior myocardial infarction, baseline laboratory values (low‐density lipoprotein cholesterol, high‐density lipoprotein cholesterol, triglycerides, and apolipoprotein A‐I), and baseline use of antiglycemic medication and high‐intensity statin.
HR and 95% CI are given per 10‐unit increase.
Discussion
Historically, HbA1c levels in patients with type 2 DM have been strongly predictive of risk for future complications.6, 12 In the UKPDS, a 1% increase in HbA1c was associated with a 14% heightened risk for MI and an even greater 37% increased risk for microvascular complications.6 These observations in the UKPDS were made on a background of suboptimal blood pressure control, inadequate antiplatelet treatment, and suboptimal lipid management in a prestatin era. However, trials that targeted intensive over moderate HbA1c goals failed to favorably modify clinical outcomes, suggesting that strict glucose regimens may not be the optimal treatment to reduce vascular risk for patients with DM.13, 14, 15, 16 This has led to uncertainty about the utility of HbA1c in risk stratifying patients with DM and known cardiovascular disease.
The current analysis from a rigorously performed randomized clinical trial identifies glycemic control, as measured by HbA1c levels, in patients with DM at high cardiovascular risk to remain an independent predictor for the future development of cardiovascular events in contemporary clinical practice, despite statin use and near optimal LDL‐C levels at baseline. Moreover, our results suggest that although improved glycemic control may have diminishing returns in reduction of hard clinical end points, such as mortality and nonfatal stroke, among those with HbA1c <8.0%, as demonstrated in the ACCORD (Action to Control Cardiovascular Risk in Diabetes)14 and ADVANCE (Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Release Controlled Evaluation) trials,16 it remains strongly predictive of softer but clinically important end points, such as coronary revascularization and hospitalization for unstable angina. Last, there has been concern for a J‐shaped relationship between HbA1c levels and mortality, with increased death observed at both the highest and lowest levels of HbA1c, the latter attributed to hypoglycemia in the setting of stringent blood sugar control.13, 17 Although limited by short duration of follow‐up, a similar phenomenon was not noted in our study and observed rates of cardiovascular as well as all‐cause mortality were not influenced by HbA1c levels.
There are multiple roles for biomarkers in a clinical setting, including diagnosis, risk stratification, disease progression, and therapeutic response. The utility of any biomarker is highest when it can both identify future risk and result in actionable intervention that can translate to improved clinical outcomes. In light of previous randomized studies targeting improved glycemic control, HbA1c has been presumed to have clinical utility only in risk stratification but not as a target for treatment that could improve outcomes. However, the availability of new antiglycemic agents proved to improve cardiovascular outcomes despite modest reductions in HbA1c has altered this paradigm.9 Results from these cardiovascular outcome trials have confirmed the importance of the specific agent used to improve glycemic control over mere biochemical improvement alone. Although it remains unclear if the benefit of these agents is mediated by attenuation of hyperglycemia, HbA1c measurements remain a guiding tool to identify, initiate, monitor, and modify these beneficial treatments. The practical lessons arising from our observations are self‐evident. HbA1c levels remain strongly predictive of future cardiovascular events in patients with DM, despite optimal lipid, blood pressure, and preventive strategies and despite recent outcomes data that may shift the focus away from glycemic control. Awareness of the HbA1c levels will enable clinicians to recognize residual risk and initiate or substitute proven medications that improve downstream cardiovascular outcomes in this vulnerable population.
Despite its strengths, our analysis has several limitations. Given the post hoc nature of the analysis, it is vulnerable to confounding arising from both unmeasured and unaccounted variables. Some important variables, like duration of DM and changes in glycemic control over time, were not captured in our data set. Although the association between HbA1c and the primary composite end point appeared qualitatively linear across all groups and remained significant after multivariable adjustment, the relationship between HbA1c and the triple end point was likely driven by nonfatal MI, with no association noted between HbA1c and cardiovascular death or stroke. However, in the context of a chronic disease, like DM, the overall duration of follow‐up was relatively small and it may be reasonable to expect that the relationship between HbA1c and adverse cardiovascular outcomes would accentuate over time and among those with HbA1c >8.0%. Further study with longer duration of follow‐up should be performed. In clinical trials, exposure to evacetrapib as well as other CETP inhibitors has been shown to improve glycemic profile. No overall benefits or harm with evacetrapib was noted in the ACCELERATE trial, leading to our decision to evaluate the entire population with DM regardless of treatment assignment. In a sensitivity analysis restricted to the control arm, the association between increasing HbA1c levels and adverse cardiovascular outcomes persisted. Last, data on the use of novel antiglycemic agents, particularly those that influence cardiovascular outcomes, were not obtained.
Conclusions
In a contemporary population of patients with DM and established coronary artery disease on optimum medical therapy, HbA1c was found to be an independent predictor for major adverse cardiac events in the ACCELERATE trial. No associated increase in mortality with decreasing HbA1c levels was noted.
Sources of Funding
The trial was sponsored by Eli Lilly and was coordinated by the Cleveland Clinic Coordinating Center for Clinical Research (C5Research) and Covance (Princeton, NJ).
Disclosures
None.
(J Am Heart Assoc. 2020;9:e014328 DOI: 10.1161/JAHA.119.014328.)
References
- 1. Rao Kondapally Seshasai S, Kaptoge S, Thompson A, Di Angelantonio E, Gao P, Sarwar N, Whincup PH, Mukamal KJ, Gillum RF, Holme I, Njolstad I, Fletcher A, Nilsson P, Lewington S, Collins R, Gudnason V, Thompson SG, Sattar N, Selvin E, Hu FB, Danesh J; Emerging Risk Factors Collaboration . Diabetes mellitus, fasting glucose, and risk of cause‐specific death. N Engl J Med. 2011;364:829–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Emerging Risk Factors Collaboration , Sarwar N, Gao P, Seshasai SR, Gobin R, Kaptoge S, Di Angelantonio E, Ingelsson E, Lawlor DA, Selvin E, Stampfer M, Stehouwer CD, Lewington S, Pennells L, Thompson A, Sattar N, White IR, Ray KK, Danesh J. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta‐analysis of 102 prospective studies. Lancet. 2010;375:2215–2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gillett MJ. International Expert Committee report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care. 2009;32:1327–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nathan DM, Kuenen J, Borg R, Zheng H, Schoenfeld D, Heine RJ; A1c‐Derived Average Glucose Study Group . Translating the A1C assay into estimated average glucose values. Diabetes Care. 2008;31:1473–1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. American Diabetes Association . Diagnosis and classification of diabetes mellitus. Diabetes Care. 2010;33(suppl 1):S62–S69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. UK Prospective Diabetes Study (UKPDS) Group. Intensive blood‐glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352:837–853. [PubMed] [Google Scholar]
- 7. Ryden L, Grant PJ, Anker SD, Berne C, Cosentino F, Danchin N, Deaton C, Escaned J, Hammes HP, Huikuri H, Marre M, Marx N, Mellbin L, Ostergren J, Patrono C, Seferovic P, Uva MS, Taskinen MR, Tendera M, Tuomilehto J, Valensi P, Zamorano JL; ESC Committee for Practice Guidelines . ESC guidelines on diabetes, pre‐diabetes, and cardiovascular diseases developed in collaboration with the EASD: the Task Force on diabetes, pre‐diabetes, and cardiovascular diseases of the European Society of Cardiology (ESC) and developed in collaboration with the European Association for the Study of Diabetes (EASD). Eur Heart J. 2013;34:3035–3087. [DOI] [PubMed] [Google Scholar]
- 8. Gore MO, McGuire DK. A test in context: hemoglobin A1c and cardiovascular disease. J Am Coll Cardiol. 2016;68:2479–2486. [DOI] [PubMed] [Google Scholar]
- 9. Ahmed HM, Khraishah H, Cho L. Cardioprotective anti‐hyperglycaemic medications: a review of clinical trials. Eur Heart J. 2018;39:2368–2375. [DOI] [PubMed] [Google Scholar]
- 10. Lincoff AM, Nicholls SJ, Riesmeyer JS, Barter PJ, Brewer HB, Fox KAA, Gibson CM, Granger C, Menon V, Montalescot G, Rader D, Tall AR, McErlean E, Wolski K, Ruotolo G, Vangerow B, Weerakkody G, Goodman SG, Conde D, McGuire DK, Nicolau JC, Leiva‐Pons JL, Pesant Y, Li W, Kandath D, Kouz S, Tahirkheli N, Mason D, Nissen SE; ACCELERATE Investigators . Evacetrapib and cardiovascular outcomes in high‐risk vascular disease. N Engl J Med. 2017;376:1933–1942. [DOI] [PubMed] [Google Scholar]
- 11. Nicholls SJ, Lincoff AM, Barter PJ, Brewer HB, Fox KA, Gibson CM, Granger C, Menon V, Montalescot G, Rader D, Tall AR, McErlean E, Riesmeyer J, Vangerow B, Ruotolo G, Weerakkody GJ, Nissen SE. Assessment of the clinical effects of cholesteryl ester transfer protein inhibition with evacetrapib in patients at high‐risk for vascular outcomes: rationale and design of the ACCERLATE trial. Am Heart J. 2015;170:1061–1069. [DOI] [PubMed] [Google Scholar]
- 12. Kirk JK, D'Agostino RB Jr, Bell RA, Passmore LV, Bonds DE, Karter AJ, Narayan KM. Disparities in HbA1c levels between African‐American and non‐Hispanic white adults with diabetes: a meta‐analysis. Diabetes Care. 2006;29:2130–2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Currie CJ, Peters JR, Tynan A, Evans M, Heine RJ, Bracco OL, Zagar T, Poole CD. Survival as a function of HbA(1c) in people with type 2 diabetes: a retrospective cohort study. Lancet. 2010;375:481–489. [DOI] [PubMed] [Google Scholar]
- 14. ADVANCE Collaborative Group , Patel A, MacMahon S, Chalmers J, Neal B, Billot L, Woodward M, Marre M, Cooper M, Glasziou P, Grobbee D, Hamet P, Harrap S, Heller S, Liu L, Mancia G, Mogensen CE, Pan C, Poulter N, Rodgers A, Williams B, Bompoint S, de Galan BE, Josh R, Travert F. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358:2560–2572. [DOI] [PubMed] [Google Scholar]
- 15. Duckworth W, Abraira C, Moritz T, Reda D, Emanuele N, Reaven PD, Zieve FJ, Marks J, Davis SN, Hayward R, Warren SR, Goldman S, McCarren M, Vitek ME, Henderson WG, Huang GD; VADT Investigators . Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360:129–139. [DOI] [PubMed] [Google Scholar]
- 16. The ACCORD Study Group , Gerstein HC, Miller ME, Genuth S, Ismail‐Beigi F, Buse JB, Goff DC Jr, Probstfield JL, Cushman WC, Ginsberg HN, Bigger JT, Grimm RH Jr, Byington RP, Rosenberg YD, Friedewald WT. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358:2545–2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Arnold LW, Wang Z. The HbA1c and all‐cause mortality relationship in patients with type 2 diabetes is J‐shaped: a meta‐analysis of observational studies. Rev Diabet Stud. 2014;11:138–152. [DOI] [PMC free article] [PubMed] [Google Scholar]


