Abstract
Background
Defects in the renal fatty acid β‐oxidation pathway have been implicated in the development of renal fibrosis. Our group has developed a therapeutic vaccine targeting PCSK9 (proprotein convertase subtilisin/kexin type 9), named PCSK9Qβ‐003. In this study, we investigated the potential effectiveness of the PCSK9Qβ‐003 vaccine on hypercholesterolemia with renal fibrosis.
Methods and Results
The low‐density lipoprotein receptor+/− male mice fed with a high‐cholesterol (1%) Western diet were randomly assigned into 4 groups: the sham group (or the control group), the phosphate‐buffered saline group, the Qβ virus‐like particles group and the PCSK9Qβ‐003 vaccine group. Mice of the PCSK9Qβ‐003 group were injected with the PCSK9Qβ‐003 vaccine (100 μg/time) every 2 or 4 weeks. The mice were administered with either unilateral ureteral obstruction for 2 weeks or N‐nitro‐l‐arginine methyl ester (50 mg/kg per day) for 6 weeks to establish a renal fibrosis model. Compared with the other 3 groups, the PCSK9Qβ‐003 vaccine obviously decreased total cholesterol and low‐density lipoprotein cholesterol in low‐density lipoprotein receptor+/− mice with hypercholesterolemia. Compared with the phosphate‐buffered saline and Qβ virus‐like particles groups, the PCSK9Qβ‐003 vaccine improved hepatic steatosis and renal function. Histology analysis showed that the PCSK9Qβ‐003 vaccine significantly ameliorated renal lipid accumulation and renal fibrosis. Moreover, the PCSK9Qβ‐003 vaccine obviously upregulated the expression of low‐density lipoprotein receptor, very‐low‐density lipoprotein receptor, sterol‐regulatory element binding protein 2, and fatty acid β‐oxidation–related factors, and ameliorated renal fibrosis‐related molecules both in the unilateral ureteral obstruction and N‐nitro‐l‐arginine methyl ester models.
Conclusions
This study suggested that the PCSK9Qβ‐003 vaccine improved renal lipid accumulation and renal fibrosis by regulating fatty acid β‐oxidation, which may provide a promising method for treating hypercholesterolemia with renal fibrosis.
Keywords: fatty acid β‐oxidation, hyperlipidemia, proprotein convertase subtilisin/kexin type 9, renal fibrosis, vaccine
Subject Categories: Fibrosis, Lipids and Cholesterol, Nephrology and Kidney
Clinical Perspective
What Is New?
This is the first report showing that the PCSK9 (proprotein convertase subtilisin/kexin type 9) Qβ‐003 vaccine significantly improved renal lipid accumulation and attenuated the development of renal fibrosis induced in low‐density lipoprotein receptor +/− mice with hypercholesterolemia administered with unilateral ureteral obstruction and N‐nitro‐l‐arginine methyl ester by regulating the fatty acid β‐oxidation pathway.
What Are the Clinical Implications?
It is remarkable that immunization of the PCSK9 Qβ‐003 vaccine significantly improved renal lipid accumulation and attenuated the development of renal fibrosis, and has some potentially superior advantages, including the ability to stably decrease lipid levels, improvement of patient compliance, and low cost.
Accordingly, the PCSK9Qβ‐003 vaccine may provide a promising avenue for treating hypercholesterolemia with renal fibrosis.
Introduction
Renal fibrosis is a common pathological process during the progression of chronic kidney disease (CKD) to end‐stage renal disease.1 CKD has exerted a great burden on public health worldwide. According to the World Health Organization estimate, CKD accounted for 1.5% of deaths worldwide in 2012.2 Patients with CKD have an increased prevalence of hyperlipidemia and an increased risk of cardiovascular disease.3 Hyperlipidemia is known to escalate the progression of glomerular injury. Unfortunately, little evidence exists to guide the optimal treatment of dyslipidemia with CKD. Statins are the most common pharmacological intervention to treat dyslipidemia with CKD. However, statins are associated with skeletal muscle complaints, ranging from mild serum creatine kinase elevations and myalgia to severe muscle weakness, muscle cramps, myositis, and rhabdomyolysis.4, 5 Moreover, previous studies indicated that statins showed limited effectiveness in reducing dyslipidemia in nephrotic syndrome.6, 7, 8, 9 Therefore, current therapies are insufficient, which necessitates the search for new therapeutic strategies in hyperlipidemia with CKD.
The relationship between lipid disturbances and renal diseases has been studied for several decades. It is well recognized that when the balance of renal lipid uptake, synthesis, oxidation, and outflow is disrupted, lipids will undergo excessive oxidation and be sequestrated as lipid droplets, generating toxic metabolites that could cause nephrotoxicity in diverse renal diseases.10, 11 Fatty acid β‐oxidation (FAO) is one of the major pathways for ATP production in the normal kidney.12 Defects in the FAO pathway have been implicated in the development of renal fibrosis. Serial studies indicated that improving FAO may offer a new approach for the treatment and prevention of kidney fibrosis.12, 13
PCSK9 (proprotein convertase subtilisin/kexin type 9) is a hepatic enzymatic protein that negatively regulates low density lipoprotein receptor (LDLR). Plasma PCSK9 binds to the extracellular domain of LDLR, and then mediates internalization and degradation of LDLR, which results in the increase of low‐density lipoprotein cholesterol (LDL‐C) level. To date, the most advanced approach for PCSK9 inhibition is monoclonal antibody (mAb), which has become one of the most promising therapeutic targets for hypercholesterolemia and could reduce incidences of cardiovascular events in clinical trials.14 Also, PCSK9 inhibitor was well tolerated in dyslipidemia with CKD. Of note, in rats with experimental CKD, serum PCSK9 level was significantly increased, and liver LDLR was decreased, accompanied by higher cholesterol.10 In patients with CKD, there was a significantly direct correlation between increased PCSK9 and high total cholesterol (TC) and LDL‐C in plasma.15 Additionally, PCSK9 level was elevated in patients with CKD and negatively correlated with estimated glomerular filtration rate.16 An increased level of PCSK9 in patients with nephrotic syndrome was directly correlated with proteinuria.15, 16, 17 Genetic ablation of podocytes and nephrotoxic serum administration resulted in hypercholesterolemia and increased level of PCSK9 in mice.16, 17 Recently, successful treatment of resistant nephrotic syndrome with PCSK9 mAb has been described.18 Moreover, studies showed that selective endothelin‐A antagonism decreased proteinuria and improved lipid profiles in optimally managed patients with CKD through a reduction in PCSK9 level.19, 20 These findings suggested that increased PCSK9 may play a significant role in CKD, and aggravate renal dysfunction by worsening lipid disturbances. Therefore, possible benefits of PCSK9 inhibitor in hyperlipidemia with CKD need to be investigated.
Peroxisome proliferator‐activated receptors (PPARs) and PPAR gamma coactivator‐1a (PGC1) are the key transcription factors that regulate the expression of proteins involved in fatty acid uptake and oxidation in various types of tissues and organs.21, 22, 23 Apart from SREBP2 (Sterol‐regulatory element binding protein 2), PPAR is another transcription factor that regulate PCSK9 transcription.24 Considering the significant role of PCSK9 in lipid metabolism, we hypothesized that PCSK9 may participate in the regulation of FAO pathway. PCSK9 mAb is expensive and faces important shortcomings including frequent administration, anti‐mAb antibodies production, and high doses. In contrast, active vaccination could overcome these shortcomings.25, 26 Previously, our group has developed a therapeutic vaccine targeting PCSK9 named PCSK9Qβ‐003.27 In this study, we investigated the potential effectiveness of the PCSK9Qβ‐003 vaccine on renal fibrosis by regulating FAO.
Materials and Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Peptide Synthesis and Vaccine Preparation
The PCSK9‐003 epitope peptide (F‐A‐Q‐S‐I‐P‐W‐N) derived from human PCSK9 was synthesized by GL Ltd. (Shanghai, China). The purity was above 98% and was determined using high‐performance liquid chromatography and mass spectrometry. The peptide was conjugated to the Qβ virus‐like particles using Sulfo‐SMCC crosslinker (Thermo Fisher Scientific, Waltham, MA, USA) to produce the PCSK9Qβ‐003 vaccine. The conjugation rate of PCSK9Qβ‐003 vaccine was analyzed on an SDS‐PAGE gel. The vaccine concentration was determined using Bradford protein assay kit (Thermo Fisher Scientific, Waltham, MA, USA).
Animals
LDLR−/− mice (B6/JNju‐Ldlrem1Cd82/Nju) were purchased from the Nanjing BioMedical Research Institute of Nanjing University (Nanjing, China). Wild‐type C57BL/6 mice were from Beijing HFK Bio‐Technology Co., LTD. Mice were maintained in the Animal Center of Tongji Medical College of Huazhong University of Science and Technology under a specific pathogen‐free condition (Permit Number, No. 00261727). LDLR+/− mice were bred using F1 from wild‐type and LDLR−/− mice. All interventions and animal caring procedures were approved by the Animal Care and Use Committee of Union Hospital of Huazhong University of Science and Technology. LDLR+/− mice were fed a high‐cholesterol (1%) Western diet containing 21% fat from 4 weeks old to the end of the experiment.
Animals Experiment
Unilateral ureteral obstruction model
The male LDLR+/− mice aged 10 weeks were randomly divided into the following 4 groups (n=6 per group), namely the sham group, the phosphate‐buffered saline (PBS) group, the Qβ virus‐like particles (VLP) group, and the PCSK9Qβ‐003 vaccine group. Mice of the PCSK9Qβ‐003 group were subcutaneously immunized with 100 μg of PCSK9Qβ‐003 vaccine at weeks 10, 12, 14, and 18. Mice of other groups were administered with an equal volume of PBS or VLP, respectively. The specific peptide antibody titers were detected by ELISA. At week 20, the mice weighing 27 to 29 g were used to establish a unilateral ureteral obstruction (UUO) model. After general anesthesia (1% sodium pentobarbital, 0.5 μg/g, intraperitoneal injection), complete UUO was carried out by double ligating the left ureter through 4‐0 silk following the left abdominal incision. The ureters of the sham group were exposed but not ligated. Mice were weighed each week. Systolic blood pressure was determined using the tail‐cuff method (BP‐2010A, Softron, Tokyo, Japan) every 2 weeks. Mice were euthanized at week 22. The ligated kidney was immediately frozen and saved for histology analysis. The detailed proceeding is shown in Figure 1A.
Figure 1.
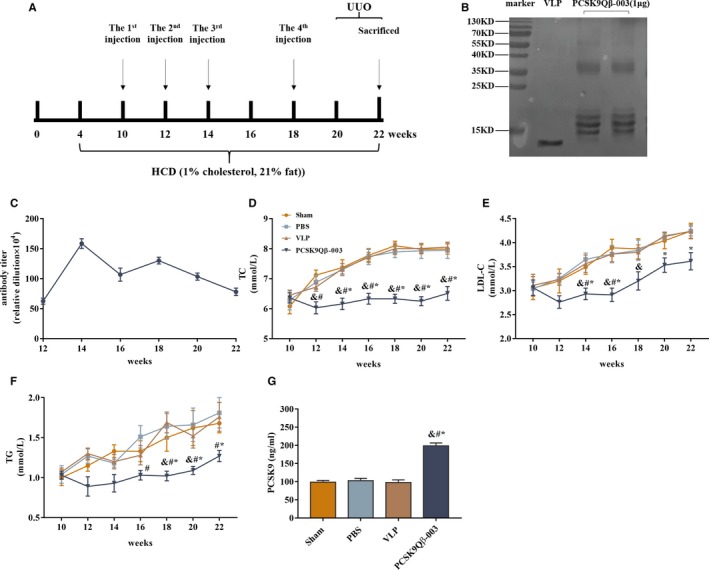
PCSK9Qβ‐003 vaccine decreased serum TC and LDL‐C in the UUO model. A, Process of animal experiments in the LDLR +/− mice+UUO. B, The vaccine was analyzed on a SDS‐PAGE gel. The figure showed the PCSK9 peptide conjugated to the Qβ virus‐like particles. C, The LDLR +/− mice were immunized subcutaneously, and the anti‐PCSK9Qβ‐003 titers were measured by ELISA. D through F, TC, LDLC and TG was decreased in the PCSK9Qβ‐003 vaccine group compared with the other groups. G, The total PCSK9 level was significantly elevated in the PCSK9Qβ‐003 vaccine group compared with the other groups. n=6 per group. Data are expressed as means±SEM. & P<0.05 vs the Sham group; # P<0.05 vs the PBS group; *P<0.05 vs the Qβ virus‐like particles group. LDL‐C indicates low‐density lipoprotein cholesterol; PBS, the phosphate‐buffered saline group; PCSK9, proprotein convertase subtilisin/kexin type 9; PCSK9Qβ‐003, the PCSK9Qβ‐003 vaccine group; Sham, the sham group; TC, total cholesterol; TG, triglyceride; UUO, unilateral ureteral obstruction; VLP, the Qβ virus‐like particles group.
N‐nitro‐l‐arginine methyl ester–induced renal injury
Male LDLR+/− mice aged 10 weeks were randomly divided into the following 4 groups (n=6 per group), namely, the control group, the PBS group, the VLP group and the PCSK9Qβ‐003 vaccine group. Mice of the PCSK9Qβ‐003 group were subcutaneously immunized with 100 μg of PCSK9Qβ‐003 vaccine at weeks 10, 12, 14, 18, and 22. Mice of other groups were administered with an equal volume of PBS or VLP, respectively. The specific peptide antibody titers were detected by ELISA. The PBS, VLP, and PCSK9Qβ‐003 groups were fed with N‐nitro‐l‐arginine methyl ester (L‐NAME; 50 mg/kg per day) in drinking water from weeks 22 to 28. Mice were weighed each week. Systolic blood pressure was determined using the tail‐cuff method every 2 weeks. The detailed proceeding is shown in Figure S1A.
Biochemical measurement
Before the measurement, all mice were fasted for 12 hours. The sera were separated by centrifugation at a rate of 1000g for 10 minutes at room temperature. The sera lipids including TC, triglyceride (TG) and LDL‐C were measured using biochemical kits (Najing Jianchen Bioengineering Institue, Najing, China). The serum creatinine and blood urea nitrogen were also measured using biochemical kits (Najing Jianchen Bioengineering Institue, Najing, China). Urine samples were collected by using metabolic cages for 24 hours, and the supernatant was used for examination of the urinary protein.
Serum total PCSK9 quantification
Serum total PCSK9 level was tested by a mouse PCSK9 ELISA kit (R&D Systems, Minneapolis, MN, USA) comparing experimental sera samples to an internal standard curve according to manufacturer's protocol.
Histology and immunostaining
The fresh liver and kidney tissues were immediately fixed in 4% paraformaldehyde overnight and then cut into optimal sections for Oil Red O staining. The other parts were embedded in paraffin for hematoxylin‐eosin, Masson's trichrome, and periodic acid‐Schiff (PAS) staining.
Real‐time quantitative polymerase chain reaction analysis
Total RNA from liver and kidney was extracted using RNAiso Plus (Takara, Shiga, Japan) following the manufacturer's protocol. The expression of associated genes was assessed by real‐time quantitative polymerase chain reaction with a Step One Real‐Time PCR machine (Applied Biosystems, Foster City, CA, USA) using TB Green Premix Ex Taq (Takara, Shiga, Japan). Primers are shown in Table S1.
Western blotting
Liver or kidney samples (20‐mg tissues of equivalent location) were homogenized by electric homogenizer (Thundersci, Shanghai, China) in ice‐cold protein extraction buffer (Pierce, Dallas, TX, USA) containing a protease inhibitor cocktail (Roche, Basel, Switzerland). Then the homogenates were centrifuged at 15 294g for 15 minutes at 4°C. Protein concentrations were confirmed via the BCA assay kit (Pierce, Dallas, TX, USA). Equivalent amounts of the extracted protein were electrophoresed on 10% SDS polyacrylamide gels and then electro‐transferred onto polyvinylidene fluoride membranes (Roche Applied Science, Penzberg, Germany). After being blocked with 5% skim milk, the membranes were incubated with appropriate primary antibodies at 4°C overnight, followed by incubation with an horseradish peroxidase‐conjugated secondary antibody. The antigens were bound by the primary antibodies as follows: anti‐SREBP2 (Abcam, ab30682), anti‐LDLR (Abcam, ab52818), anti‐PPARα (Arigo, ARG55240), anti–peroxisomal acyl‐coenzyme A oxidase 1 (ACOX1; Abcam, ab184032), anti‐pSmad3 (CST, 9520S), anti‐Smad3 (CST, 9523S), and anti–transforming growth factor (TGF)‐β (Abcam, ab6179965). The specific bands were detected using Super ECL reagent (Thermo Fisher Scientific, Waltham, MA, USA). The protein levels were determined by densitometry analysis using the Image Lab 3.0 software (Bio‐Rad Laboratories, Hercules, CA, USA).
Statistical Analysis
Data are expressed as mean±SEM. One‐way analysis of variance using Bonferroni's method (for comparison of >2 groups) was used for the statistical analyses. The calculations were performed using Prism 7.0 (GraphPad Software, La Jolla, CA, USA). P<0.05 was considered as significant.
Results
Animal Characteristics
In comparison with the control animals, the 2 batches of mice in the PCSK9Qβ‐003 group had no difference in body weight and kidney weight (Table). Compared with the sham group, the ratio of kidney weight/body weight in the PBS and VLP groups was increased, but no difference was observed between the PCSK9Qβ‐003 and sham groups in LDLR+/− mice intervened by UUO. In mice intervened by L‐NAME, the ratio of kidney weight/body weight had no obvious difference among all groups. Moreover, all groups had similar systolic blood pressure and heart rate. No evidence of skin damages and mortality was noted in the vaccine‐treated animals.
Table 1.
Systemic Parameters of Mice at the End of Study
| Parameters | Sham/Con | PBS | VLP | PCSK9Qβ‐003 |
|---|---|---|---|---|
| Mice intervened by UUO | ||||
| Body weight, g | 29.35±0.80 | 28.88±0.73 | 28.98±0.93 | 29.06±0.71 |
| Kidney weight, mg | 309±6 | 365±13 | 365±8 | 335±13 |
| KW/BW, mg/g | 10.53±0.13 | 12.65±0.16a | 12.66±0.44a | 11.53±0.31 |
| Heart rate, bpm | 653±14 | 657±11 | 622±19 | 642±27 |
| SBP, mm Hg | 132±3 | 131±2 | 134±3 | 133±3 |
| Mice intervened by L‐NAME | ||||
| Body weight, g | 29.08±0.73 | 28.63±0.82 | 28.88±1.03 | 28.79±0.81 |
| Kidney weight, mg | 318±12 | 308±5 | 304±17 | 337±9 |
| KW/BW, mg/g | 10.96±0.52 | 10.78±0.37 | 10.86±0.47 | 10.95±1.42 |
| Heart rate, bpm | 587±18 | 572±20 | 569±22 | 582±20 |
| SBP, mm Hg | 130±3 | 138±2 | 135±3 | 137±4 |
Data are expressed as means±SEM. Con indicates the control group; KW/BW, kidney weight‐to‐body weight ratio; L‐NAME, N‐nitro‐l‐arginine methyl ester; PBS, the phosphate‐buffered saline group; PCSK9Qβ‐003, the PCSK9Qβ‐003 vaccine group; SBP, systolic blood pressure; Sham, the sham group; UUO, unilateral ureteral obstruction; VLP, the Qβ virus‐like particles group.
P<0.05 vs the control group or the sham group.
PCSK9Qβ‐003 Vaccine Decreased TC and LDL‐C
Hypercholesterolemia was induced in male LDLR+/– mice fed with high‐cholesterol Western diet, which presented changes with steadily increased blood lipid level (Figure S1B through S1D). The conjugated PCSK9Qβ‐003 vaccine manifested that 1 monomer of VLP coupled with 1 to 4 PCSK9 epitopes (Figure 1B). To confirm the effect of the PCSK9Qβ‐003 vaccine on hypercholesterolemia, the vaccine was used to vaccinate male LDLR+/− mice with hypercholesterolemia. After the second injection of the vaccine, the PCSK9Qβ‐003 specific antibody titer was 1:400 000 to 1:900 000 and rose after the third injection, then peaked at week 14 (1:1 500 000) and gradually decreased thereafter (Figure 1C, Figure S1E). Except the PCSK9Qβ‐003 group, the other 3 groups had similar serum lipids level in the UUO model. Compared with the sham group, obvious decline of TC (6.33±0.14 versus 8.10±0.15 mmol/L, 21.8% decline; P<0.001), LDL‐C (2.91±0.14 versus 3.89±0.18 mmol/L, 25.2% decline; P=0.002) and TG (1.02±0.06 versus 1.50±0.17 mmol/L, 32.0% decline; P=0.033) following PCSK9Qβ‐003 vaccination was observed in LDLR+/− mice after the third injection (Figure 1D through 1F). These results are in agreement with that of the L‐NAME model (Figure S1F through S1H). To evaluate serum PCSK9 level, total PCSK9 level was measured at the end of the experiment. Total PCSK9 level was significantly elevated in mice vaccinated with the PCSK9Qβ‐003 vaccine compared with the other groups (Figure 1G, Figure S1I).
PCSK9Qβ‐003 Vaccine Reduced Hepatic Steatosis
To investigate the liver lipid composition, we performed lipid analyses on livers from high‐cholesterol Western diet –fed male mice. As shown, the PCSK9Qβ‐003 group had almost no steatosis, whereas other groups had severe steatosis and large lipid droplet (Figure 2A). Oil Red O staining showed that PCSK9Qβ‐003 markedly reduced the formation of fatty liver (Figure 2B). The hepatic TG content in the PCSK9Qβ‐003 group was significantly decreased compared with that in other groups; however, the hepatic TC only had a descending trend (Figure 2C and 2D). These results were basically consistent with the mice induced by L‐NAME (Figure S2A through S2D).
Figure 2.
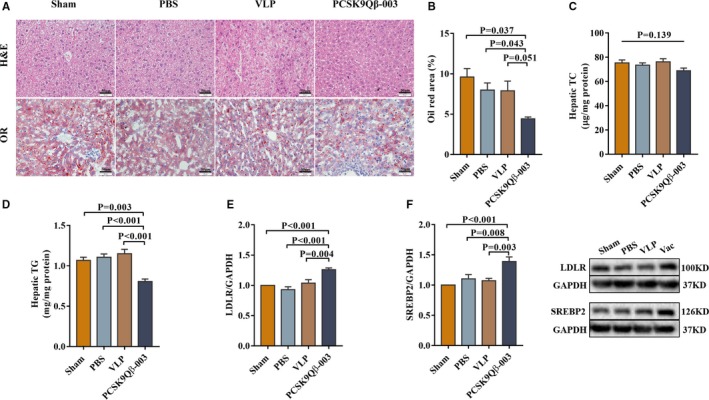
PCSK9Qβ‐003 vaccine reduced hepatic steatosis in the UUO model. A, Histology of the livers stained with hematoxylin‐eosin (H&E) and Oil Red O (bar=50 μm). B, Representative Oil Red O–stained liver sections show that PCSK9Qβ‐003 markedly reduced the formation of fatty liver. C and D, Hepatic TC and TG contents. E and F, Compared with the other groups, the protein expression level of LDLR and SREBP2 in liver was increased obviously in the PCSK9Qβ‐003 vaccine group. n=6 per group. Data are expressed as means±SEM. H&E indicates hematoxylin‐eosin; LDLR, low‐density lipoprotein receptor; OR, Oil Red O; PBS, the phosphate‐buffered saline group; Sham, the sham group; SREBP2, sterol regulatory element‐binding protein 2; TC, total cholesterol; TG, triglyceride; Vac, the PCSK9Qβ‐003 vaccine group; VLP, the Qβ virus‐like particles group.
Next, transcription factors and target genes associated with the development of hepatic steatosis were investigated. The PCSK9Qβ‐003 vaccine increased the expression level of LDLR, very‐low‐density lipoprotein receptor (VLDLR), and SREBP2 in the liver in both UUO and L‐NAME models (Figures 2E, 2F, and 3A through 3C). In addition, the PCSK9Qβ‐003 vaccine remarkably upregulated the mRNA levels of FAO‐related factors, including PPARα, PGC1, ACOX1, carnitine palmitoyl transferase alpha, and lipoprotein lipase (LPL) (Figure 3D and 3E). Moreover, the PCSK9Qβ‐003 vaccine upregulated the level of transporting factors ATP binding cassette transporter A1 (ABCA1) and ATP binding cassette subfamily G member 1 (ABCG1), while downregulated acetyl‐CoA acetyltransferase 1 (ACAT1) (Figure 3F). In addition, PCSK9Qβ‐003 administration obviously reduced the level of hepatic fibrosis including TGF‐β, collagen alpha‐1 and collagen alpha‐3 (Figure 3G).
Figure 3.
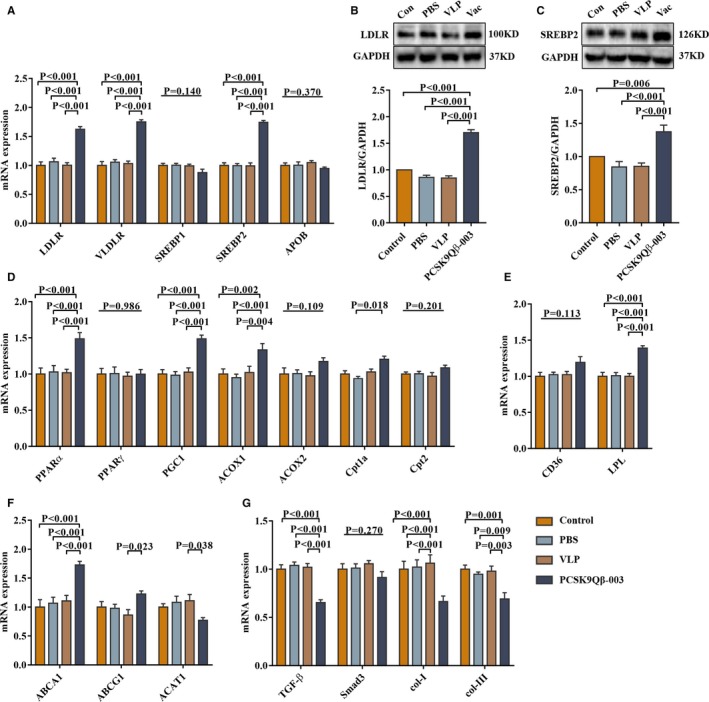
PCSK9Qβ‐003 vaccine remarkably improved the levels of transcription factors and target genes associated with the development of hepatic steatosis. A through C, PCSK9Qβ‐003 increased the expression level of LDLR, VLDLR, and SREBP2. D and E, PCSK9Qβ‐003 remarkably upregulated the mRNA levels of fatty acid oxidation related factors. F, PCSK9Qβ‐003 up‐regulated the level of transporting factors ABCA1 and ABCG1. G, PCSK9Qβ‐003 administration obviously reduced the level of hepatic fibrosis–related factors. n=6 per group. Data are expressed as means±SEM. ABCA1 indicates ATP binding cassette transporter A1; ABCG1, ATP binding cassette subfamily G member 1; ACAT1, acetyl‐CoA acetyltransferase 1; ACOX1, acyl‐CoA oxidase 1; ACOX2, acyl‐CoA oxidase 2; APOB, apolipoprotein B; CD36, cluster of differentiation 36; col‐I, collagen alpha‐1; col‐III, collagen alpha‐3; Con, the Control group; Cpt1a, carnitine palmitoyl transferase 1α; Cpt2, carnitine palmitoyl transferase 2; LDLR, low‐density lipoprotein receptor; LPL, lipoprotein lipase; PBS, the phosphate‐buffered saline group; PGC‐1, PPARγ coactivator‐1a; PPARα, peroxisome proliferator‐activated receptor α; PPARγ, peroxisome proliferator‐activated receptor γ; SREBP1, sterol regulatory element–binding protein 1; SREBP2, sterol regulatory element–binding protein 2; TGF‐β, transforming growth factor‐β; Vac, the PCSK9Qβ‐003 vaccine group; VLDLR, very‐low‐density lipoprotein receptor; VLP, the Qβ virus‐like particles group.
PCSK9Qβ‐003 Vaccine Ameliorated Renal Lipid Deposition and Renal Fibrosis Induced by UUO
We further investigated the effect of the PCSK9Qβ‐003 vaccine on renal pathology induced by UUO. Compared with the PBS and VLP groups, hematoxylin and eosin staining showed that the PCSK9Qβ‐003 vaccine significantly ameliorated tubular dilatation, edema, inflammatory cell infiltration, and interstitial fibrosis of kidney (Figure 4A). Oil Red O staining showed that Oil Red O–positive regions mostly located in the renal tubule was significantly decreased in the PCSK9Qβ‐003 group (Figure 4A and 4B). Masson's staining showed all UUO mice exhibited large areas of renal interstitial fibrosis; however, the degree of interstitial fibrosis was remarkably reduced by PCSK9Qβ‐003 treatment in contrast to the PBS and VLP groups (Figure 4A and 4C). PAS analysis showed that the PAS‐positive area of the glomerulus was obviously reduced in the PCSK9Qβ‐003 group compared with other UUO groups (Figure 4A and 4D).
Figure 4.
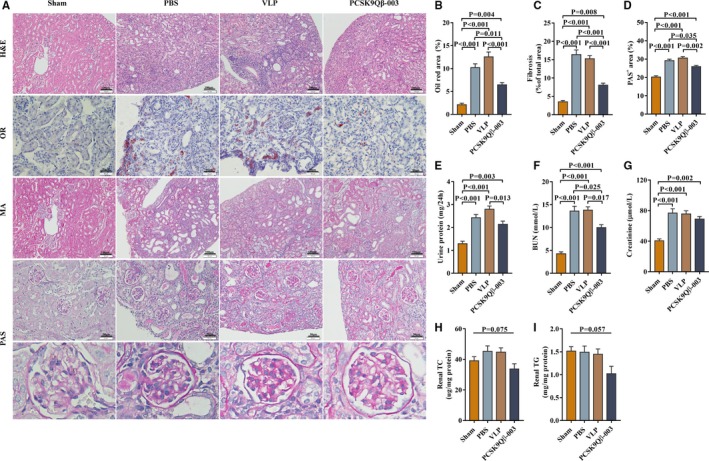
PCSK9Qβ‐003 vaccine ameliorated renal lipid deposition and renal fibrosis induced by UUO. A, Histology of the livers stained with H&E, Oil Red O, Masson (bar=100 μm) and PAS (bar=50 μm). B, Representative Oil Red O–stained renal sections, showed that PCSK9Qβ‐003 markedly reduced the renal lipid accumulation. C, Interstitial fibrosis was significantly ameliorated in the PCSK9Qβ‐003 group compared with the other 3 groups. D, PAS analysis showed that GME was reduced by in PCSK9Qβ‐003 group. E through G, The PCSK9Qβ‐003 vaccine significantly reduced the 24‐hour urine protein and blood urea nitrogen of mice compared with the PBS and VLP groups. The creatinine level had a tendency to decrease in the PCSK9Qβ‐003 group (H and I). Renal TC and TG contents. n=6 per group. Data are expressed as means±SEM. BUN indicates blood urea nitrogen; H&E, hematoxylin and eosin; MA, Masson's trichrome; OR, Oil Red O; PAS, periodic acid‐Schiff; PBS, the phosphate‐buffered saline group; PCSK9Qβ‐003, the PCSK9Qβ‐003 vaccine group; Sham, the sham group; TC, total cholesterol; TG, triglyceride; VLP, the Qβ virus‐like particles group.
Renal function was determined in the UUO model. Results showed, compared with the VLP group, the PCSK9Qβ‐003 vaccine significantly reduced the level of 24‐hour urine protein (Figure 4E). All of the mice intervened by UUO had a higher level of blood urea nitrogen and creatinine. Compared with other UUO groups, the blood urea nitrogen level was notably decreased after PCSK9Qβ‐003 treatment; however, the creatinine level only had a tendency to decrease (Figure 4F and 4G). In addition, lipid analysis showed that the renal TC and TG contents in the PCSK9Qβ‐003 group tended to be decreased than that in other 3 groups (Figure 4H and 4I).
PCSK9Qβ‐003 Vaccine Upregulated FAO‐Related Factors and Ameliorated Renal Fibrosis‐Related Molecules in the UUO Model
FAO‐related transcription factors and target molecules associated with the development of renal fibrosis were investigated. Compared with the other 3 groups, the PCSK9Qβ‐003 vaccine increased the expression of LDLR, VLDLR, and SREBP2 (Figure 5A and 5E). And, the PCSK9Qβ‐003 vaccine remarkably upregulated the levels of fatty acid metabolism–related factors, including PPARα, PPARγ, PGC1, ACOX1, CD36, and LPL, in contrast with the other 3 groups (Figure 5B, 5C, 5F, and 5G). In addition, compared with the PBS and VLP groups, the PCSK9Qβ‐003 vaccine administration obviously reduced the level of TGF‐β and pSmad3, which indicated the antifibrosis effect of the PCSK9Qβ‐003 vaccine (Figure 5D, 5H, and 5I).
Figure 5.
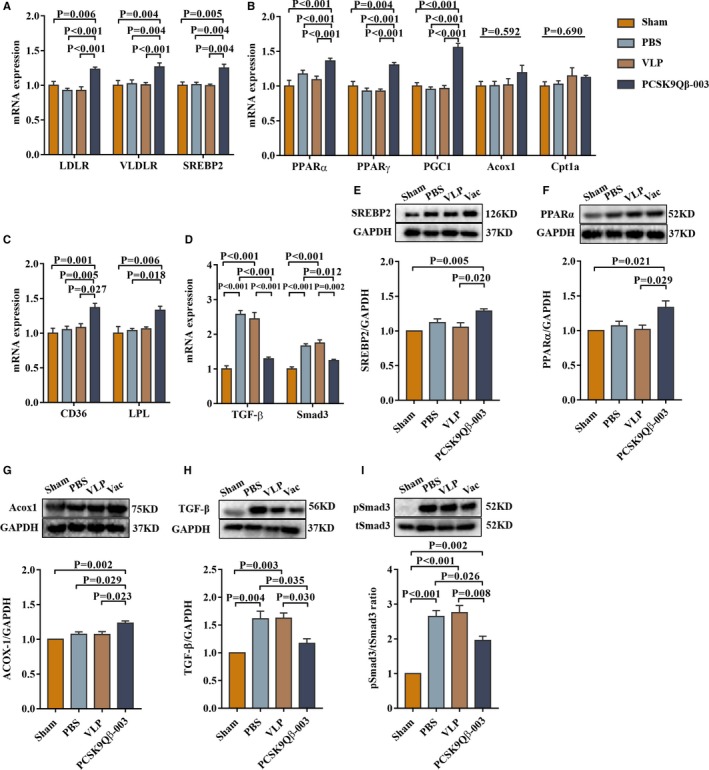
PCSK9Qβ‐003 vaccine upregulated FAO‐related factors and ameliorated renal fibrosis–related molecules in the UUO model. A, PCSK9Qβ‐003 increased the expression level of LDLR, VLDLR, and SREBP2. B and C, PCSK9Qβ‐003 remarkably upregulated the levels of fatty acid metabolism–related factors. D, The PCSK9Qβ‐003 vaccine obviously reduced the level of TGF‐β and Smad3. E through I, PCSK9Qβ‐003 treatment improved the protein expression of SREBP2, PPARα, ACOX1, TGF‐β, and pSmad3. n=6 per group. Data are expressed as means±SEM. ACOX1 indicates acyl‐CoA oxidase 1; CD36, cluster of differentiation 36; Cpt1a, carnitine palmitoyl transferase 1α; LDLR, low‐density lipoprotein receptor; LPL, lipoprotein lipase; PBS, the phosphate‐buffered saline group; PGC‐1, PPARγ coactivator‐1a; PPARα, peroxisome proliferator‐activated receptor α; PPARγ, peroxisome proliferator‐activated receptor γ; Sham, the sham group; SREBP2, sterol regulatory element–binding protein 2; TGF‐β, transforming growth factor‐β; Vac, the PCSK9Qβ‐003 vaccine group; VLDLR, very‐low‐density lipoprotein receptor; VLP, the Qβ virus‐like particles group.
PCSK9Qβ‐003 Vaccine Improved Renal Lipid Accumulation and Renal Fibrosis Induced by L‐NAME
Renal pathology was also investigated in the renal fibrosis model induced by L‐NAME. Compared with the PBS and VLP groups, Oil Red O staining showed that the Oil Red O–positive region mostly located in the renal tubule, and was significantly decreased in the PCSK9Qβ‐003 group (Figure 6A and 6B). Masson's staining showed that the degree of interstitial fibrosis was remarkably reduced by PCSK9Qβ‐003 treatment in contrast with the PBS and VLP groups (Figure 6A and 6C). PAS analysis showed that the PAS‐positive area of the glomerulus was obviously reduced in the PCSK9Qβ‐003 group compared with other L‐NAME groups (Figure 6A and 6D). Renal function detection showed that, compared with the PBS and VLP groups, the PCSK9Qβ‐003 vaccine not only significantly reduced the level of 24‐hour urine protein at weeks 22 and 24 (Figure 6E) but also notably decreased the level of blood urea nitrogen and creatinine (Figure 6F and 6G). All of these results indicated the positive effect of the PCSK9Qβ‐003 vaccine on renal protection.
Figure 6.
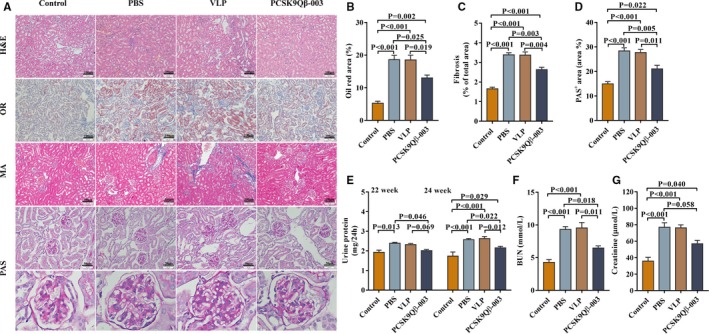
PCSK9Qβ‐003 vaccine improved renal lipid accumulation and renal fibrosis induced by L‐NAME. A, H&E, Oil Red O, Masson (bar=100 μm) and PAS (bar=50 μm). B, Representative Oil Red O–stained renal sections, showed that PCSK9Qβ‐003 markedly reduced the tubule epithelial lipid accumulation. C, Interstitial fibrosis was significantly ameliorated in the PCSK9Qβ‐003 group compared with the other 3 groups. D, PAS analysis showed that GME was reduced in PCSK9Qβ‐003 group. E through G, The PCSK9Qβ‐003 vaccine significantly reduced the 24‐hour urine protein, BUN and creatinine compared with PBS and VLP groups. n=6 per group. Data are expressed as means±SEM. BUN indicates blood urea nitrogen; Con, the control group; H&E, hematoxylin and eosin; MA, Masson's trichrome; OR, Oil Red O; PAS, periodic acid‐Schiff; PBS, the phosphate‐buffered saline group; PCSK9Qβ‐003, the PCSK9Qβ‐003 vaccine group; VLP, the Qβ virus‐like particles group.
PCSK9Qβ‐003 Vaccine Upregulated FAO‐Related Factors and Ameliorated Renal Fibrosis–Related Molecules in the L‐NAME Model
FAO‐related transcription factors and target molecules associated with the development of renal fibrosis were also investigated in the L‐NAME renal fibrosis model. Compared with the other 3 groups, the PCSK9Qβ‐003 vaccine increased the expression level of LDLR, VLDLR, and SREBP2 (Figure 7A). In addition, the PCSK9Qβ‐003 vaccine remarkably upregulated the levels of fatty acid metabolism–related factors, including PPARα, PPARγ, PGC1, ACOX1, CD36, and LPL (Figure 7B, 7C, 7E, and 7F). Moreover, compared with the PBS and VLP groups, the PCSK9Qβ‐003 vaccine administration obviously reduced the level of TGF‐β but only tended to decrease the level of pSmad3 (Figure 7D, 7G, and 7H).
Figure 7.
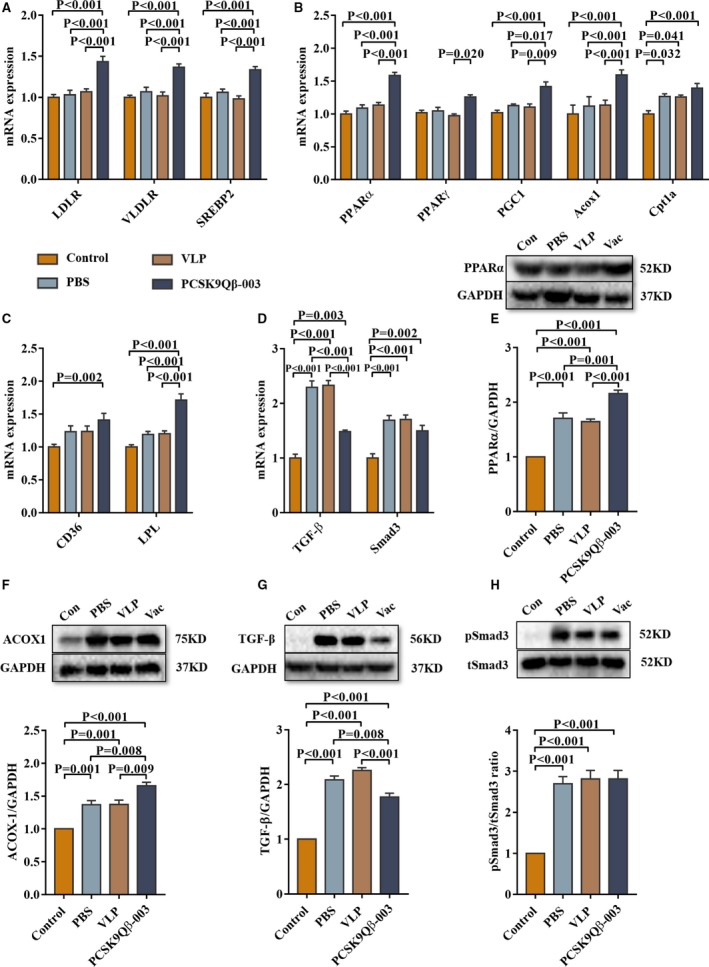
PCSK9Qβ‐003 vaccine upregulated FAO‐related factors and ameliorated renal fibrosis–related molecules in the L‐NAME model. A, PCSK9Qβ‐003 increased the expression level of LDLR, VLDLR, and SREBP2. B and C, PCSK9Qβ‐003 remarkably upregulated the levels of fatty acid metabolism–related factors. D, The PCSK9Qβ‐003 vaccine obviously reduced the level of TGF‐β and Smad3. E through H, PCSK9Qβ‐003 treatment improved the protein expression of PPARα, ACOX1, TGF‐β and pSmad3. n=6 per group. Data are expressed as means±SEM. ACOX1 indicates acyl‐CoA oxidase 1; CD36, cluster of differentiation 36; Con, the control group; Cpt1a, carnitine palmitoyl transferase 1α; LDLR, low‐density lipoprotein receptor; LPL, lipoprotein lipase; PBS, the phosphate‐buffered saline group; PGC‐1, PPARγ coactivator‐1a; PPARα, peroxisome proliferator–activated receptor α; PPARγ, peroxisome proliferator‐activated receptor γ; SREBP2, sterol regulatory element‐binding protein 2; TGF‐β, transforming growth factor‐β; Vac, the PCSK9Qβ‐003 vaccine group; VLDLR, very‐low‐density lipoprotein receptor; VLP, the Qβ virus‐like particles group.
Discussion
In the present study, we demonstrated that the PCSK9Qβ‐003 vaccine significantly decreased serum TC and LDL‐C, improved renal lipid accumulation, and attenuated the development of renal fibrosis induced by UUO and L‐NAME in LDLR+/− mice with hypercholesterolemia for the first time. Moreover, the PCSK9Qβ‐003 vaccine obviously upregulated FAO‐related factors and ameliorated renal fibrosis‐related genes both in the UUO and L‐NAME models.
Our results showed that total PCSK9 level was significantly elevated in mice vaccinated with the PCSK9Qβ‐003 vaccine compared with the other 3 groups. The results were consistent with previous studies. Clinical trials demonstrated that anti‐PCSK9 antibodies engaged PCSK9 in vivo and formed an immune complex, which could actually raise plasma total PCSK9 level.28 However, the level of free PCSK9 after treatment of PCSK9 inhibitors is decreased. This result also has been reported in our previous study, which showed that free PCSK9 level was substantially decreased in the PCSK9Qβ‐003 vaccine group, similar to anti‐PCSK9 mAbs.27 That is, the increase of total PCSK9 level after immunization with the PCSK9Qβ‐003 vaccine suggested the effectiveness of the vaccine.
Clinical observations indicated a potential association between lipid levels and CKD development.29 Higher intracellular lipid accumulation has been proposed to have a key role in the development of fibrosis by inducing lipotoxicity.12 PCSK9 might aggravate dyslipidemia and exacerbate renal dysfunction in patients with CKD. Conversely, the effect of PCSK9 inhibitors on renal function and fibrosis is worth exploring.30, 31 Significantly, in addition to the decrease of LDL‐C level, the PCSK9Qβ‐003 vaccine further ameliorated renal lipid accumulation and renal fibrosis, and then improved renal function in the LDLR+/− UUO and L‐NAME mice. This was a novel and important finding for PCSK9 inhibitors.
Defects in the FAO pathway have received substantial attention in the context of acute and CKD. Defective FAO in tubule epithelial cells has been associated with ATP depletion, cell death, dedifferentiation, and lipid accumulation, which were the phenotypes of renal fibrosis.12 It has been shown that improvement of FAO could protect mice from tubulointerstitial fibrosis. The PPARs and PGC1 are the key transcription factors that regulate the expression of proteins involved in fatty acid uptake and oxidation.22, 32, 33 Metabolism of fatty acids requires that they be transported into the mitochondria, which is mediated by carnitine palmitoyl transferase alpha, an enzyme could conjugate fatty acids with carnitine.34 In our study, the results showed that the PCSK9Qβ‐003 vaccine remarkably improved FAO‐associated molecules expression, such as PPARα, PGC1, ACOX1, and CD36 but significantly inhibited TGF‐β expression and Smad3 phosphorylation. These data suggested that the PCSK9Qβ‐003 may ameliorate renal fibrosis by regulating the FAO pathway.
PCSK9 was reported to affect the activity of other receptors beyond LDLR, such as CD36, VLDLR, apolipoprotein E receptors, and LDLR‐related protein 1.35, 36 Our results showed that the PCSK9Qβ‐003 vaccine significantly increased the expression of LDLR and VLDLR. Thus, in addition to kidney protection, PCSK9 inhibitors may have other pleiotropic effects that need to be clarified in the future studies. It is noteworthy that PCSK9 is also produced by the kidneys, and PCSK9 seems to reduce the expression of the epithelial sodium channel in renal tubules known to markedly affect sodium reabsorption.36 Theoretically, PCSK9 inhibitors might be associated with increased sodium reabsorption and hypertension, but this is not the case in experimental modes, in individuals with PCSK9 loss‐of‐function mutations or after the administration of monoclonal antibodies.34, 37 In our study, coincident results showed that, compared with other groups, the systolic blood pressure had no significant difference in PCSK9Qβ‐003 treatment.
Given the efficacy of PCSK9 mAbs in treating dyslipidemia and the potential link between acquired LDLR deficiency and the pathogenesis of CKD,38 future trials exploring the use of PCSK9 inhibitors in treating dyslipidemia with CKD are warranted. Compared with traditional chemical drugs, the PCSK9Qβ‐003 vaccine has some potentially superior advantages, including the ability to stably decrease lipid levels, improvement of patient compliance, and the low cost, which may provide a promising method for treating hypercholesterolemia with renal fibrosis.
Sources of Funding
This work was supported by the Major Research Plan of the National Natural Science Foundation of China (No. 91439207) and the National Natural Science Foundation of China (No. 81900459, No. 81770366, No. 81670461, No. 81500388).
Disclosures
None.
Supporting information
Table S1. Detail Information of Primers
Figure S1. PCSK9Qβ‐003 vaccine decreased plasma TC in mice intervened by L‐NAME.
Figure S2. PCSK9Qβ‐003 vaccine decreased hepatic steatosis in mice induced by L‐NAME.
(J Am Heart Assoc. 2020;9:e014358 DOI: 10.1161/JAHA.119.014358.)
Contributor Information
Yuhua Liao, Email: liaoyh27@163.com.
Zhihua Qiu, Email: qiu_zhihua512@163.com.
References
- 1. Gewin LS. Renal fibrosis: primacy of the proximal tubule. Matrix Biol. 2018;68:248–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Webster AC, Nagler EV, Morton RL, Masson P. Chronic kidney disease. Lancet. 2017;389:1238–1252. [DOI] [PubMed] [Google Scholar]
- 3. Culleton BF, Larson MG, Wilson PW, Evans JC, Parfrey PS, Levy D. Cardiovascular disease and mortality in a community‐based cohort with mild renal insufficiency. Kidney Int. 1999;56:2214–2219. [DOI] [PubMed] [Google Scholar]
- 4. Kato K, Onodera K, Iwasaki Y, Matsuda M, Kawakami T, Higuchi M, Kato K, Kato Y, Taniguchi M, Furukawa H. Pravastatin‐induced rhabdomyolysis and purpura fulminans in a patient with chronic renal failure. Int J Surg Case Rep. 2015;8:84–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Thompson PD, Clarkson P, Karas RH. Statin‐associated myopathy. JAMA. 2003;289:1681–1690. [DOI] [PubMed] [Google Scholar]
- 6. Sharma M, Nand N, Aggarwal HK, Nand D. Evaluation of effects of lovastatin on hypercholesterolaemia and renal functions in nephrotic syndrome. J Indian Acad Clin Med. 2004;5:143–146. [Google Scholar]
- 7. Gheith OA, Sobh MAK, Mohamed KES, El‐Baz MA, El‐Husseini F, Gazarin SS, Ahmed HA, Rasem MW, Amer GM. Impact of treatment of dyslipidemia on renal function, fat deposits and scarring in patients with persistent nephrotic syndrome. Nephron. 2002;91:612–619. [DOI] [PubMed] [Google Scholar]
- 8. Olbricht CJ, Wanner C, Thiery J, Basten A. Simvastatin in nephrotic syndrome. Kidney Int. 1999;56:S113–S116. [DOI] [PubMed] [Google Scholar]
- 9. Kong X, Yuan H, Fan J, Li Z, Wu T, Jiang L. Lipid‐lowering agents for nephrotic syndrome. Cochrane Database Syst Rev. 2013;12:CD005425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sucajtys‐Szulc E, Szolkiewicz M, Swierczynski J, Rutkowski B. Up‐regulation of liver Pcsk9 gene expression as a possible cause of hypercholesterolemia in experimental chronic renal failure. Mol Cell Biochem. 2016;411:281–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Khan S, Abu Jawdeh BG, Goel M, Schilling WP, Parker MD, Puchowicz MA, Yadav SP, Harris RC, El‐Meanawy A, Hoshi M, Shinlapawittayatorn K, Deschênes I, Ficker E, Schelling JR. Lipotoxic disruption of NHE1 interaction with PI (4, 5) P2 expedites proximal tubule apoptosis. J Clin Invest. 2014;124:1057–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kang HM, Ahn SH, Choi P, Ko YA, Han SH, Chinga F, Park AS, Tao J, Sharma K, Pullman J, Bottinger EP, Goldberg IJ, Susztak K. Defective fatty acid oxidation in renal tubular epithelial cells has a key role in kidney fibrosis development. Nat Med. 2015;21:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chung KW, Lee EK, Lee MK, Oh GT, Yu BP, Chung HY. Impairment of PPARαand the fatty acid oxidation pathway aggravates renal fibrosis during aging. J Am Soc Nephrol. 2018;29:1223–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kazi DS, Moran AE, Coxson PG, Penko J, Ollendorf DA, Pearson SD, Tice JA, Guzman D, Bibbins‐Domingo K. Cost‐effectiveness of PCSK9 inhibitor therapy in patients with heterozygous familial hypercholesterolemia or atherosclerotic cardiovascular disease. JAMA. 2016;316:743–753. [DOI] [PubMed] [Google Scholar]
- 15. Haas ME, Levenson AE, Sun X, Liao WH, Rutkowski JM, de Ferranti SD, Schumacher VA, Scherer PE, Salant DJ, Biddinger SB. The role of proprotein convertase subtilisin/kexin type 9 in nephrotic syndrome‐associated hypercholesterolemia. Circulation. 2016;134:61–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pavlakou P, Liberopoulos E, Dounousi E, Elisaf M. PCSK9 in chronic kidney disease. Int Urol Nephrol. 2017;49:1015–1024. [DOI] [PubMed] [Google Scholar]
- 17. Morris AW. Nephrotic syndrome: PCSK9: a target for hypercholesterolaemia in nephrotic syndrome. Nat Rev Nephrol. 2016;12:510. [DOI] [PubMed] [Google Scholar]
- 18. Awanami Y, Fukuda M, Nonaka Y, Takashima T, Matsumoto K, Yamasaki M, Miyazono M, Ikeda Y. Successful treatment of a patient with refractory nephrotic syndrome with PCSK9 inhibitors: a case report. BMC Nephrol. 2017;18:221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Farrah TE, Anand A, Gallacher PJ, Kimmitt R, Carter E, Dear JW, Mills NL, Webb DJ, Dhaun N. Endothelin receptor antagonism improves lipid profiles and lowers PCSK9 (proprotein convertase subtilisin/kexin type 9) in patients with chronic kidney disease. Hypertension. 2019;74:323–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dhaun N, MacIntyre IM, Kerr D, Melville V, Johnston NR, Haughie S, Goddard J, Webb DJ. Selective endothelin‐A receptor antagonism reduces proteinuria, blood pressure, and arterial stiffness in chronic proteinuric kidney disease. Hypertension. 2011;57:772–779. [DOI] [PubMed] [Google Scholar]
- 21. Tran M, Tam D, Bardia A, Bhasin M, Rowe GC, Kher A, Zsengeller ZK, Akhavan‐Sharif MR, Khankin EV, Saintgeniez M, David S, Burstein D, Karumanchi SA, Stillman IE, Arany Z, Parikh SM. PGC‐1alpha promotes recovery after acute kidney injury during systemic inflammation in mice. J Clin Invest. 2011;121:4003–4014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Proctor G, Jiang T, Iwahashi M, Wang Z, Li J, Levi M. Regulation of renal fatty acid and cholesterol metabolism, inflammation, and fibrosis in Akita and OVE26 mice with type 1 diabetes. Diabetes. 2006;55:2502–2509. [DOI] [PubMed] [Google Scholar]
- 23. Chau BN, Xin C, Hartner J, Ren S, Castano AP, Linn G, Li J, Tran PT, Kaimal V, Huang X, Chang AN, Li S, Kalra A, Grafals M, Portilla D, MacKenna DA, Orkin SH, Duffield JS. MicroRNA‐21 promotes fibrosis of the kidney by silencing metabolic pathways. Sci Transl Med. 2012;4:121ra118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Duan Y, Chen Y, Hu W, Li X, Yang X, Zhou X, Yin Z, Kong D, Yao Z, Hajjar DP, Liu L, Liu Q, Han J. Peroxisome proliferator‐activated receptor γ activation by ligands and dephosphorylation induces proprotein convertase subtilisin kexin type 9 and low density lipoprotein receptor expression. J Biol Chem. 2012;287:23667–23677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bachmann MF, Dyer MR. Therapeutic vaccination for chronic diseases: a new class of drugs in sight. Nat Rev Drug Discov. 2004;3:81. [DOI] [PubMed] [Google Scholar]
- 26. Chackerian B, Remaley A. Vaccine strategies for lowering LDL by immunization against proprotein convertase subtilisin/kexin type 9. Curr Opin Lipidol. 2016;27:345–350. [DOI] [PubMed] [Google Scholar]
- 27. Pan Y, Zhou Y, Wu H, Chen X, Hu X, Zhang H, Zhou Z, Qiu Z, Liao Y. A therapeutic peptide vaccine against PCSK9. Sci Rep. 2017;7:12534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhang L, McCabe T, Condra JH, Ni YG, Peterson LB, Wang W, Strack AM, Wang F, Pandit S, Hammond H, Wood D, Lewis D, Rosa R, Mendoza V, Cumiskey AM, Johns DG, Hansen BC, Shen X, Geoghagen N, Jensen K, Zhu L, Wietecha K, Wisniewski D, Huang L, Zhao JZ, Ernst R, Hampton R, Haytko P, Ansbro F, Chilewski S, Chin J, Mitnaul LJ, Pellacani A, Sparrow CP, An Z, Strohl W, Hubbard B, Plump AS, Blom D, Sitlani A. An anti‐PCSK9 antibody reduces LDL‐cholesterol on top of a statin and suppresses hepatocyte SREBP regulated genes. Int J Biol Sci. 2012;8:310–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Baragetti A, Grejtakova D, Casula M, Olmastroni E, Jotti GS, Norata GD, Catapano AL, Bellosta S. Proprotein convertase subtilisin‐kexin type‐9 (PCSK9) and triglyceride‐rich lipoprotein metabolism: facts and gaps. Pharmacol Res. 2018;130:1–11. [DOI] [PubMed] [Google Scholar]
- 30. Busuioc RM, Covic A, Kanbay M, Banach M, Burlacu A, Mircescu G. Protein convertase subtilisin/kexin type 9 biology in nephrotic syndrome: implications for use as therapy. Nephrol Dial Transplant. 2019;gfz108 DOI: 10.1093/ndt/gfz108. [DOI] [PubMed] [Google Scholar]
- 31. Kwakernaak AJ, Lambert G, Slagman MCJ, Waanders F, Laverman GD, Petrides F, Dikkeschei BD, Navis G, Dullaart RP. Proprotein convertase subtilisin–kexin type 9 is elevated in proteinuric subjects: relationship with lipoprotein response to antiproteinuric treatment. Atherosclerosis. 2013;226:459–465. [DOI] [PubMed] [Google Scholar]
- 32. Roubtsova A, Munkonda MN, Awan Z, Marcinkiewicz J, Chamberland A, Lazure C, Cianflone K, Seidah NG, Prat A. Circulating proprotein convertase subtilisin/kexin 9 (PCSK9) regulates VLDLR protein and triglyceride accumulation in visceral adipose tissue. Arterioscler Thromb Vasc Biol. 2011;31:785–791. [DOI] [PubMed] [Google Scholar]
- 33. Tran MT, Zsengeller ZK, Berg AH, Khankin EV, Bhasin MK, Kim W, Clish CB, Stillman IE, Karumanchi SA, Rhee EP, Parikh SM. PGC1α drives NAD biosynthesis linking oxidative metabolism to renal protection. Nature. 2016;531:528–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Berger JM, Vaillant N, Le May C, Calderon C, Brégeon J, Prieur X, Hadchouel J, Loirand G, Cariou B. PCSK9‐deficiency does not alter blood pressure and sodium balance in mouse models of hypertension. Atherosclerosis. 2015;239:252–259. [DOI] [PubMed] [Google Scholar]
- 35. Levy E, Ben Djoudi Ouadda A, Spahis S, Sane AT, Garofalo C, Grenier É, Emonnot L, Yara S, Couture P, Beaulieu JF, Ménard D, Seidah NG, Elchebly M. PCSK9 plays a significant role in cholesterol homeostasis and lipid transport in intestinal epithelial cells. Atherosclerosis. 2013;227:297–306. [DOI] [PubMed] [Google Scholar]
- 36. Sharotri V, Collier DM, Olson DR, Zhou R, Snyder PM. Regulation of epithelial sodium channel trafficking by proprotein convertase subtilisin/kexin type 9 (PCSK9). J Biol Chem. 2012;287:19266–19274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chapman MJ, Ginsberg HN, Amarenco P, Andreotti F, Borén J, Catapano AL, Descamps OS, Fisher E, Kovanen PT, Kuivenhoven JA, Lesnik P, Masana L, Nordestgaard BG, Ray KK, Reiner Z, Taskinen MR, Tokgözoglu L, Tybjærg‐Hansen A, Watts GF; European Atherosclerosis Society Consensus Panel . Triglyceride‐rich lipoproteins and high‐density lipoprotein cholesterol in patients at high risk of cardiovascular disease: evidence and guidance for management. Eur Heart J. 2011;32:1345–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Toth PP, Dwyer JP, Cannon CP, Colhoun HM, Rader DJ, Upadhyay A, Louie MJ, Koren A, Letierce A, Mandel J, Banach M. Efficacy and safety of lipid lowering by alirocumab in chronic kidney disease. Kidney Int. 2018;93:1397–1408. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Detail Information of Primers
Figure S1. PCSK9Qβ‐003 vaccine decreased plasma TC in mice intervened by L‐NAME.
Figure S2. PCSK9Qβ‐003 vaccine decreased hepatic steatosis in mice induced by L‐NAME.


