Abstract
Emerging evidence supports that cancer incidence is increased in patients with cardiovascular (CV) disease and heart failure (HF), and patients with HF frequently die from cancer. Recently, data have been generated showing that circulating factors in relation to HF promote tumour growth and development in murine models, providing proof that a causal relationship exists between both diseases. Several common pathophysiological mechanisms linking HF to cancer exist, and include inflammation, neuro‐hormonal activation, oxidative stress and a dysfunctional immune system. These shared mechanisms, in combination with risk factors, in concert may explain why patients with HF are prone to develop cancer. Investigating the new insights linking HF with cancer is rapidly becoming an exciting new field of research, and we herein review the most recent data. Besides insights in mechanisms, we call for clinical awareness, that is essential to optimize treatment strategies of patients having developed cancer with a history of HF. Finally, ongoing and future trials should strive for comprehensive phenotyping of both CV and cancer end points, to allow optimal usefulness of data, and to better describe and understand common characteristics of these two lethal diseases.
Keywords: Cardio‐oncology, Circulating factors, Heart failure, Cancer
Introduction
Cardiovascular (CV) disease, especially heart failure (HF), and cancer are the two major causes of death worldwide. HF‐related mortality rates in Europe were estimated to be 23.6% for acute HF and 6.4% for chronic HF, whereas the rates for the combined endpoint of mortality or HF hospitalisation within 1 year were 36% for acute HF and 14.5% for chronic HF.1 At the same time, more than one quarter of all people in Europe die of cancer, and cancer accounts for an even higher share (∼30%) of deaths among men than among women (∼25%).2
Thanks to better prevention, smoking ban, dietary measures but also improvement in treatment, with combinatorial medication and early percutaneous cardiac intervention, HF mortality has declined in recent decades but at the same time, cancer‐related mortality appears to have risen.3 Historically, in epidemiological studies, mortality was often categorised as being either due to CV disease, non‐CV disease (e.g. cancer), or ‘other’. Yet, a sizeable proportion of patients will develop both HF and cancer. Indeed, the field of cardio‐oncology has attracted increasing interest, as cancer survivors might develop HF as a result of chemotherapy, radiotherapy and immunotherapy, often in combination.4, 5 As a result, it is now advocated that in cancer patients, their HF risk factors must be assessed (preferably at baseline, i.e. before cardio‐toxic treatment), and also that such patients should be monitored long term for development of HF and be treated aggressively, if needed.6, 7
In recent years, epidemiological studies have reported that the reverse may also be true, i.e. that the incidence of cancer in patients with HF is elevated.8, 9, 10, 11, 12 Identification of those HF patients at particular risk for cancer will be a scientific goal for the coming years. Furthermore, recently, a neutral study regarding the latter association has been published.13 In order to provide the reader with a summary of published results, we summarize the current available data in Table 1. These data show that cancer development and related mortality are substantial in patients with HF. However, no attention or (CV disease‐related) guidelines or recommendations currently exist with regard to how best identify or treat cancer in patients with prevalent CV disease. Furthermore, these studies only point to an association between HF and cancer, but the pathophysiological mechanisms underlying this association, i.e. the mechanisms involved, have remained unclear.
Table 1.
Overview of clinical studies addressing the incidence of cancer in patients with heart failure (primary outcome)
| Study | Design | Population | Patients, n | Follow‐up period (years) | Primary outcome | Secondary outcomes |
|---|---|---|---|---|---|---|
| Hasin et al.8 | Case‐control | General population with or without HF |
Total: 1192 HF: 596 Cancer: 102 |
7.7 ± 6.4 | HR 1.68, 95% CI 1.13–2.50 adjusted for BMI, smoking, and co‐morbidities | Incident cancer increased the risk of death: HR 1.56, 95% CI 1.22–1.99 adjusted for age, sex, index year, and co‐morbidities |
| Hasin et al.9 | Prospective cohort study | Post‐MI with or without HF |
Total: 1081 HF: 228 Cancer: 28 |
4.9 ± 3.0 | HR 1.71, 95% CI 1.07–2.73 adjusted for age, sex, and Charlson co‐morbidity index | Incident cancer increased the risk of death: HR 3.91, 95% CI 1.88–8.12 |
| Rinde et al.10 | Prospective population‐based study | Patients with either a MI or not |
Total: 28 763 MI: 1747 Cancer: 146 |
15.7 | HR 1.46, 95% CI 1.21–1.77 adjusted for age, sex, BMI, systolic blood pressure, diabetes mellitus, HDL cholesterol, smoking, physical activity and education level |
Increased cancer incidence highest during the first 6 months post‐MI: HR 2.15, 95% CI 1.29–3.58 3 years post‐MI risk of incidence cancer: HR 1.60, 95% CI 1.27–2.03 |
| Banke et al.11 | Prospective study |
HF subjects (LVEF < 45%) compared to the general population |
Total: 4 949 968 HF: 9307 Cancer: 975 |
4.5 ± 2.3 | IRR 1.24, 95% CI 1.15–1.33 adjusted for age, sex |
After 180 days: IRR 1.17, 95% CI 1.08–1.27 After 365 days: IRR 1.14, 95% CI 1.05–1.24 |
| Berton et al.12 | Prospective study | Post‐MI |
Total: 589 MI: 589 Cancer: 99 |
17 | IR 17.8 cases/1000 person‐years | Incident cancer increased the risk of death: HR 1.8, 95% CI 1.1–2.9 |
| Selvaraj et al.13 | Pooled RCTs |
PHS I: control vs. low‐dose aspirin and β‐carotene PHS II: control vs. vitamin supplementation Self‐reported HF, no data on cardiac function |
Total: 28 341 HF: 1420 Cancer: 177 |
19.9 [25th–75th percentile: 11.0–26.8] | HR 1.02, 95% CI 0.84–1.25 adjusted for enrolment group, race, cigarette smoking (never, former, current), alcohol use, aspirin use, family history of cancer, cirrhosis, proton pump inhibitor or H2 blocker use, and sun exposure | No increased risk of cancer death: HR 1.16, 95% CI 0.82–1.65 adjusted for the same model as the primary outcome |
BMI, body mass index; CI, confidence interval; HDL, high‐density lipoprotein; HF, heart failure; HR, hazard ratio; IRR, incidence rate ratio; LVEF, left ventricular ejection fraction; MI, myocardial infarction; PHS, Physicians' Health Study; RCT, randomised clinical trial.
In the present review, we will discuss this possible relation between HF and cancer. We will try to dissect the potential mechanisms to explain this phenomenon, including circulating factors but also other mechanisms such as inflammation, neuro‐hormonal activation, oxidative stress, and the influence of CV risk factors and of common CV medication. Furthermore, we will discuss existing controversies and knowledge gaps.
Epidemiology of incident cancer in heart failure
Heart failure: a disease of co‐morbidities
Heart failure is the most deadly disease in the CV disease domain, characterised by abnormal cardiac structure and/or function, with typical signs and symptoms and a negative impact on quality of life.14 The prognosis of patients with HF is poor, with a 5‐year and 10‐year survival rate of only ∼50% and ∼25%, respectively. This accounts for both patients with HF with reduced (HFrEF) and preserved (HFpEF) ejection fraction.15, 16
Historically, cardiologists assumed that most patients with HF will die either from HF itself or from other CV causes, especially in HFrEF.17 However, many patients with HF currently die from non‐CV causes.18, 19 In fact, depending on age and aetiology, non‐CV mortality rates range between 20% and 50%. Figure 1 displays the mode of non‐CV deaths of three recent large randomised clinical trials in HFpEF and HFrEF, which reveal that 34–39% of all non‐CV deaths were related to cancer. Of note, mortality was much more often due to cancer than for instance renal function, stroke, infection/sepsis, or chronic obstructive pulmonary disease,20 which all are co‐morbidities that have been studied in detail, and are well accepted as relevant prognostic factors in the setting of HF. Clearly, the adjudication of cancer endpoints and cancer‐related deaths in CV endpoint trials may have been limited and not fully correct. Therefore, in future CV and HF trials, we call for multidisciplinary adjudication committees, where non‐CV death is reported and adjudicated in more detail.
Figure 1.
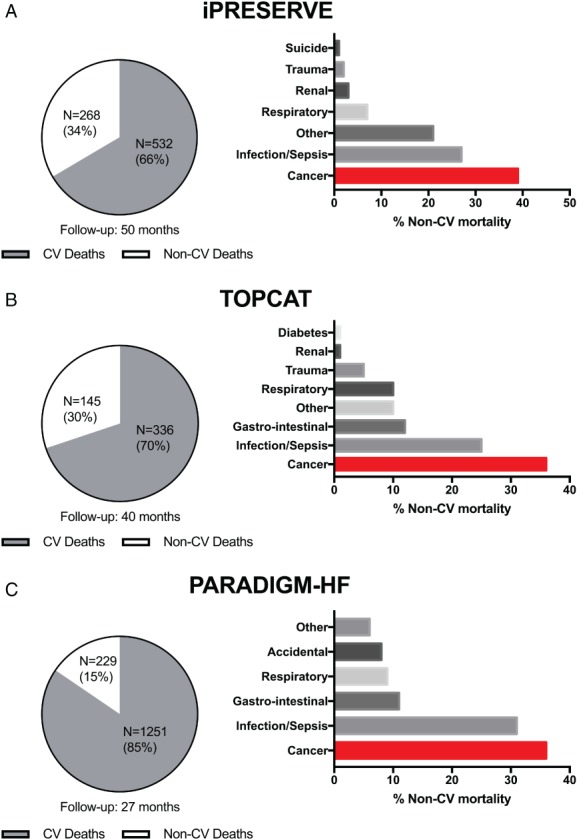
Non‐cardiovascular (CV) death accounted for ∼30% in the iPRESERVE and TOPCAT trials, enrolling patients with heart failure with preserved ejection fraction (HFpEF), and for 15% in the PARADIGM‐HF trials, enrolling patients with heart failure with reduced ejection fraction (HFrEF). In all trials, cancer was the dominant cause of non‐CV death, accounting for 35–40% of non‐CV deaths. Data shown for the: (A) Irbesartan in Heart Failure With Preserved Systolic Function (iPRESERVE); (B) Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist (TOPCAT); and (C) Prospective Comparison of ARNI with an ACE‐Inhibitor to Determine Impact on Global Mortality and Morbidity in Heart Failure (PARADIGM‐HF).
As described, it has been acknowledged that HF and cancer may coincide. However, the literature has almost exclusively focused on HF as a consequence of cancer and its related therapies, such as chemotherapy and radiation.
Heart failure and cancer development
One of the first studies examining the association between HF and the development of cancer investigated 961 subjects with HF incidence and compared them to 961 matched controls. In order to explore the incidence of cancer among HF patients, all patients with a prior cancer diagnosis were excluded. During an average follow‐up of 7.7 ± 6.4 years, HF patients had a 60% higher risk of developing cancer [hazard ratio (HR) 1.60, 95% confidence interval (CI) 1.14–2.26]. This elevated risk was maintained following adjustments for body mass index, Charlson co‐morbidity index and smoking. No statistically significant difference in sex and age interaction was observed. A time‐dependent interaction was found, with an increased risk of cancer in a later time period, suggestive that cancer development had not yet begun at the time of HF diagnosis, but may be provoked by new‐onset HF. Cancer incidence in HF patients resulted in an elevated risk of death (HR 1.68, 95% CI 1.33–2.14).8 A post‐hoc study of the same cohort, which only included those who developed HF following a myocardial infarction (MI), revealed that post‐MI HF was also associated with an increased risk for cancer. Twelve per cent of the individuals developed cancer compared to 8% who did not develop HF after MI during a follow‐up period of 4.9 ± 3.0 years (HR 2.16, 95% CI 1.39–3.35).9 In a large general cohort (> 28 000 subjects), in which cancer incidence and MI were reported, an increased cancer incidence was also observed in those who had a prior MI. This effect was more pronounced among women than men.10
A second study used data from the Danish National Register to evaluate the risk of HF with respect to new‐onset cancer among over 9000 subjects with HF but without a prior cancer diagnosis. In comparison to the general population, the incidence rate of cancer increased in those with HF (HR 1.24, 95% CI 1.15–1.33). Importantly, this observation persisted in the first year following the diagnosis of HF.11 This finding was recently confirmed in a non‐Caucasian population: a large retrospective study of over 5000 Japanese HF patients concluded that cancer incidence occurred four times more often than in controls.21
In order to shed further light on the interaction between CV disease and incidence of cancer, another study reported on abnormal wall motion during stress echocardiography as a proxy for CV disease. It was demonstrated that in addition to CV mortality (HR 1.18, 95% CI 1.03–1.35), abnormal stress echocardiography also predicted new‐onset cancer (HR 1.19, 95% CI 1.16–1.73).22
In contrast, a recently published post‐hoc analysis of the Physicians' Health Studies I and II did not confirm the association between HF and all‐cancer incidence or site‐specific cancer incidence.13 This study differed in that it only included males, and more importantly, HF was primarily recorded through self‐reporting instead of clinical or echocardiographic findings, as has been discussed previously.23
Most of the studies described above focus on HF after MI, and therefore would largely include HFrEF patients. Other studies do not mention the subtype of HF, and only report that HF was present based on the Framingham criteria or was self‐reported. Therefore, we can only speculate that the effects observed are based upon ischaemic HF, and studies on non‐ischaemic HF, including in particular HFpEF, are clearly needed.
Clearly, the presence of a second lethal disease in the setting of HF has strong prognostic implications. Indeed, in HF patients, an increasing number of non‐cardiac co‐morbidities is associated with a higher risk for all‐cause hospital admissions (P < 0.001).24 These co‐morbidities, including cancer, had similar impacts on mortality in patients with HFpEF compared with those with HFrEF.24 A recent position statement from the Heart Failure Association called for greater attention to cancer incidence in patients with prevalent HF because physicians are faced with multiple dilemmas.25 Patients with HF diagnosed with cancer have higher mortality rates after any type of cancer treatment. These patients, often receiving cardiotoxic drugs, should be under strict surveillance. Physicians should strive to optimize HF therapy before anti‐cancer treatment of any sort is initiated. On the other hand, cancer patients receiving therapy might endure complications either due to cancer or cancer therapy including hypotension, electrolyte depletion and acutely worsening renal function caused by vomiting and/or diarrhoea. All these factors influence current HF therapy. Treatment dose might be reduced or treatment completely stopped. A close collaboration between cardiologists and oncologists is fundamental to improve the management of these patients, with both specialists understanding the benefits of therapy for HF and cancer, and the risks of withholding or sub‐optimally treating either or both diseases.25 Recent evidence highlights a direct interaction between HF and cancer development, as discussed below.
Shared risk factors between heart failure and cancer
Hypertension, obesity, smoking, diabetes and poor lifestyle are all associated with both CV disease and cancer incidence.26, 27, 28, 29 Table 2 summarises the meta‐analysed HRs of shared risk factors for HF and cancer incidence. The associations suggest that modifications of risk factors, usually with the aim of lowering the risk of CV disease, might help reduce susceptibility to cancer as well. We recently published a review dedicated to shared risk factors between HF and cancer.30 Therefore, we only briefly touch upon important risk factors in this paragraph.
Table 2.
Hazard ratio (95% confidence interval) of different risk factors in association with either heart failure or cancer incidence26, 27, 28, 29
| Risk factor | Incidence of heart failurea | Incidence of cancer |
|---|---|---|
| BMIb, per kg/m2 | 1.03 (1.01–1.06) | 1.08 (1.06–1.10) |
| Smoking | 1.84 (1.46–2.32) | 1.68 (1.65–1.72) |
| Diabetes mellitus | 1.41 (1.12–1.79) | 1.10 (1.03–1.18) |
| Hypertension | 1.65 (1.33–2.06) | 1.03 (0.98–1.09) |
| Heart rateb | 1.02 (1.01–1.03) | 1.09 (1.01–1.18)c |
BMI, body mass index.
Study population (Health ABC, PREDICTOR, PROSPER).
Hazard ratios are expressed per 1‐unit increase in continuous risk factors.
Per 10 b.p.m. increase.
Hypertension
Hypertension is a silent yet extremely prevalent risk factor that represents a significant contributor to CV disease,31 especially for HF.32 In men, a 10 mmHg increment was related to an increased risk of cancer incidence (HR 1.07, 95% CI 1.04–1.09). Further, a 10 mmHg increment in blood pressure both in men and women was associated with cancer‐related mortality (HR 1.12, 95% CI 1.08–1.15 and HR 1.06, 95% CI 1.02–1.11, respectively).33 Mechanistically, the interaction between hypertension and cancer does not appear very straightforward. Vascular endothelial growth factor might play a role due to the promotion of vascular formation, a key phenomenon in tumorigenesis.34, 35 The effects of anti‐hypertensive medication related to cancer incidence are discussed in further detail below.
Obesity
Obesity is also connected to both HF and cancer. Today it is estimated that one out of five types of cancer is related to obesity.36 Obesity is associated with a chronic pro‐inflammatory state, which could cause DNA damage and increase the likelihood of malignant mutations and cancer incidence.37
Furthermore, fatty tissue is thought to function as a large endocrine organ that produces large amounts of oestrogen, rendering women more vulnerable to hormone‐driven cancers including ovarian and breast cancer.38 In addition to hormones, numerous adipokines are secreted by adipose tissue, resulting in a very complex and deleterious secretome.39 Several studies are investigating the effects of epicardial fat.40 These factors can either stimulate or inhibit cell growth. One of the best studied adipokines is leptin, which has cell‐proliferative effects.41 This effect contrasts with another adipokine, adiponectin, which is less abundant in obese people than in those of average weight, and is reported to have anti‐proliferative effects.41
In addition, obese subjects have increased insulin levels and insulin‐like growth factor (IGF)‐1. This often precedes the development of type 2 diabetes. High levels of IGF‐1 have been associated with the development of cancer.42 Subjects who have lower weight gain during adulthood experience a reduced risk of colon cancer.43
Evidence that weight loss might reduce cancer risk has also been derived from bariatric surgery cohorts: obese people who have bariatric surgery appear to have a lower risk of obesity‐related cancers compared to obese people who did not have bariatric surgery.44 Akin to cancer, the relationship between obesity and HF is complex. Obese people do have a higher risk of developing HF, but seem to have a survival advantage, which is called the ‘obesity paradox’45 and is also being investigated in patients with cancer.46
Smoking and air pollution
Smoking sets off multiple damaging mechanisms, one key component of which is nicotine, which has also been implicated in the pathogenesis of both CV disease and cancer.47, 48 It is associated with the development of atherosclerotic disease and acute MI.49 Given the overwhelming evidence that smoking causes cancer and CV disease, it is highly likely that smoking‐related cancer is also prevalent in patients with CV disease.50 Among post‐MI patients, exposure to nitrogen oxides, a proxy measure for traffic‐related air pollution, was associated with increased cancer incidence (HR 1.06, 95% CI 0.96–1.18) and cancer mortality (HR 1.08, 95% CI 0.93–1.26).51
Diabetes mellitus
Given the current availability of strict glycaemic control and high‐quality insulin therapy, diabetes mellitus (DM) rarely leads to severe cardiac dysfunction on its own.52 The insulin‐cancer hypothesis postulates a central role played by elevated levels of IGF, which promote cell proliferation.53 Meta‐analyses have indicated an increased risk of colorectal cancer, prostate cancer and premenopausal breast cancer associated with high serum levels of IGF.54 A large Italian registry (over 400 000 subjects) compared those with and without DM. During a follow‐up of 10 years, the cancer incidence rate ratio was 1.22 (95% CI 1.15–1.29). This risk already existed 2 years after the DM diagnosis.55
Healthy lifestyle
Lifestyle modification is a powerful tool in combatting CV disease, but reducing CV risk factors may also reduce cancer incidence. In a study including over 13 000 participants and 17–19 years of follow‐up, healthy behaviours such as smoking cessation, increased physical activity, weight loss, healthy diet, reductions to total cholesterol and blood pressure and adequate blood sugar control were shown to facilitate a lower incidence of CV disease as well as a lower incidence of cancer.56 These findings supplement the growing literature that cancer may be amenable to improvements regarding CV risk factors and the treatment of CV disease.57 Indeed, participants who met their personal targets regarding six of these goals demonstrated a 51% lower risk of cancer incidence than controls who did not alter their lifestyle.
Another large, recent prospective cohort study of over 400 000 subjects showed that chronic disease burden was independently associated with cancer incidence. A chronic risk score was assembled, comprising CV disease, diabetes, chronic kidney disease, pulmonary disease and gouty arthritis, all of which were individually associated with incident cancer or cancer death risk.29 However, the accumulative score of chronic diseases was most strongly associated (in a dose–response manner) with an increased risk of cancer incidence and cancer death. The same study reported that increased physical activity was associated with a 40% reduction in cancer incidence and cancer‐related death.58
Pathophysiology: circulating factors
In HF, numerous proteins are secreted by the heart and affected organs, which has sparked interest in their use as biomarkers to facilitate diagnosis, to risk stratify patients or to target treatments. However, a substantial number of these circulating factors are in fact biologically active proteins that may exert effects on peripheral organs, including tumours.59, 60 Examples of known pro‐cancerous factors that are elevated in HF and are secreted are tumour necrosis factor‐α, interleukin (IL)‐6, IL‐1 and vascular endothelial growth factor.61 However, whether the heart itself behaves as a true endocrine organ remains unclear.
Recent work from our group has demonstrated direct and causal proof that these circulating factors may play a role, sharing new insights regarding the relationship between HF and cancer incidence in a translational study.62 The first level of evidence was provided by studying if HF in a model susceptible to cancer would provoke tumour growth. It was demonstrated that post‐MI HF in a murine model of genetic pre‐cancerous colon adenomas (APCmin mice) resulted in enhanced tumour growth. Compared to sham‐operated mice, mice with HF demonstrated an increased amount and size of tumours, which resulted in a two‐fold to three‐fold increase in tumour load in the intestine. Excess tumour progression was correlated with markers of cardiac remodelling, such as left ventricular ejection fraction and myocardial fibrosis. To further extend and validate these findings, a second model without haemodynamic impairment was investigated and again the presence of a failing heart accelerated tumour growth. It was postulated that cardiac‐derived proteins might exert exocrine effects on tumour cells (Figure 2).63
Figure 2.
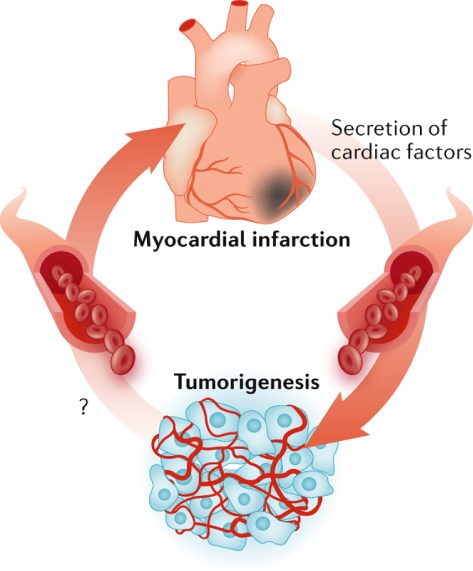
Hypothesis of the interplay between cardiac‐derived proteins of a failing heart and cancer, and vice versa. Reproduced with permission from Richards.63
Using existing proteomic databases, several candidate proteins that are demonstrated as to be secreted by the failing heart into the blood stream, and that have proven effects on colon tissue, were identified and further investigated in vitro. One of the factors – alpha 1‐antichymotrypsin (SERPINA3/ACT) – dose‐dependently accelerated tumour growth via phosphorylation of Akt and rpS6 in vitro.
Corroborating evidence was obtained by mapping new‐onset cancer in a community‐based cohort study with 8592 subjects, and a mean follow‐up of 12 years. During this period, 1132 subjects (13.1%) were diagnosed with cancer, 132 (11.7%) with colorectal cancer. At baseline, a large panel of biomarkers was measured. Strikingly, N‐terminal pro‐B‐type natriuretic peptide (NT‐proBNP), known for its established diagnostic and prognostic performance in HF,64 independently predicted new‐onset cancer.62 When patients were stratified based upon their NT‐proBNP levels at baseline, patients with the highest tertile of NT‐proBNP clearly demonstrated a higher incidence of all‐cause cancer (Figure 3) and also for colon cancer. These data suggest that cardiac production and the secretion of certain (bio)markers not only signal myocardial damage, but also affect distant tumour growth, possibly via exocrine effects.
Figure 3.
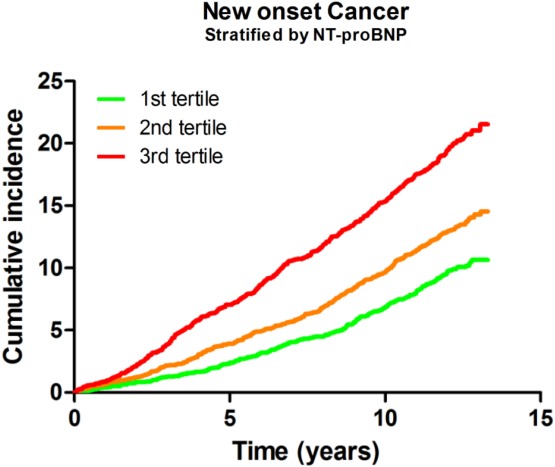
Cumulative incidence of cancer according to N‐terminal pro‐B‐type natriuretic peptide (NT‐proBNP) tertiles. Reproduced with permission from Meijers et al.62
In an accompanying editorial it was discussed that these results will stimulate further delineation of the connections between HF and cancer.65 In addition, in the same issue of the Journal, this line of research was further emphasized by a review focused on possible underlying mechanisms like inflammation and neuro‐hormonal activation by Bertero and colleagues.66 These new data provide some first evidence that the condition of HF per se may lead to an environment of increased susceptibility to cancer development or growth.
Other pathophysiological mechanisms link heart failure and cancer
Besides the above mentioned direct link between HF and cancer, chronic activation of several systemic systems may – at least in part – explain the interplay between the heart and the function or dysfunction of peripheral organs, including cancer. In this paragraph we discuss the role of neuro‐hormonal activation, oxidative stress, inflammation and the immune system.
Neuro‐hormonal activation
Activation of the renin–angiotensin–aldosterone system (RAAS) is one of the central compensatory homeostatic responses in HF. RAAS activation initially aims to uphold blood pressure and cardiac output by vasoconstriction and cardiac hypertrophy; however, chronic activation triggers detrimental effects to the heart, kidneys and blood vessels.67 Besides a systemic RAAS, most target organs are equipped with a local RAAS with a differential expression of RAAS hormones and receptors in the heart, blood vessels and kidneys, as well as in different forms of cancer.68 For instance, the increased expression of angiotensin II receptor type 1 (AT1R) in cancer biology is associated with more advanced tumours and a worse prognosis.69 On the other hand, the modulation of the RAAS may affect tumour growth, although data are inconsistent. Overall, the angiotensin II (AngII)/AT1R axis is deemed to enhance tumour growth, whereas AngII/AT2R signalling exerts the opposite effect.68 The measurement of RAAS hormones plays no role in daily HF care, but RAAS inhibition, e.g. by angiotensin‐converting enzyme inhibitors (ACEi) or angiotensin II receptor blockers (ARBs), is the cornerstone of treatment. These therapies will be discussed in the following paragraphs.
Oxidative stress
One of the major contributors of oxidative stress is the reactive oxygen species (ROS) family of molecules. ROS plays an important role in both HF and cancer.70, 71 It is known that the heart is a high energy consumer that primarily relies on mitochondrial oxidative phosphorylation, which also plays an essential role in cancer progression.72 Experimental research provides evidence that dietary fibre supplementation has beneficial effects on oxidative stress in cardiac tissue73 and reduces the incidence of cancer.74 Further, increased glycolysis occurs in HF, and glucose oxidation is impaired, resulting in the production of lactate. One of the characteristics of cancer is altered mitochondrial oxidative metabolism, resulting in increased glycolysis. This is associated with cells that rapidly proliferate, such as tumour cells. Key players and modulators in this process include pyruvate dehydrogenase (PDH) and PDH kinases (PDK). PDH limits the rate of glucose oxidation and is responsible for the mitochondrial decarboxylation of pyruvate to acetyl coenzyme A.75 PDK can phosphorylate and inhibit PDH. In HF, PDK is up‐regulated and PDH is phosphorylated and inhibited.75 A similar up‐regulation of PDK and inhibition of PDH also occurs in tumour cells.76 Dichloro‐acetate, a PDK inhibitor that results in enhanced PDH activity, has demonstrated in HF studies to lower ischaemic injury and improved cardiac function,77 while also reducing cancer development.76 Although these observations are intriguing, there is no convincing proof for causation and additional research will be required to provide robust evidence if targeting this pathway might benefit both HF and cancer development.
Inflammation
Inflammation is closely related to HF, although the degree of inflammation is generally regarded as low intensive.78 Numerous pro‐inflammatory cytokines are elevated in HF, especially during the progression of the disease, supporting the hypothesis that inflammation contributes to HF development.79 Enhanced inflammation in HF for example leads to bone marrow dysfunction.80 However, no direct proof exists that the release of pro‐inflammatory cytokines or certain inflammatory cells from the heart affect tumour cells or tumours. Nevertheless, it is plausible that cardiac‐derived inflammatory factors can exert downstream effects due to the well‐described role they play in tumour growth.81 Furthermore, the study of Meijers et al.62 also investigated inflammatory factors such as high‐sensitivity C‐reactive protein and mid‐regional pro‐adrenomedullin and demonstrated that these were predictive of new‐onset cancer.
Supportive evidence that inflammation is at the crossroads of CV disease and cancer was provided by the recently published CANTOS trial. In this phase III trial, the IL‐1β blocker canakinumab was investigated in patients who had experienced MI, with the aim to test the hypothesis that IL‐1β inhibition attenuated future coronary events. The treatment with canakinumab resulted in a 25% reduction in major adverse CV events in comparison to placebo (HR 0.75, 95% CI 0.66–0.85).82 Yet strikingly, the same intervention, significantly and dose‐dependently, reduced lung cancer incidence and lung cancer mortality [highest dose (300 mg) HR 0.33, 95% CI 0.18–0.59; P < 0.0001 and HR 0.23, 95% CI 0.10–0.54; P = 0.0002, respectively].83 Thus, this study links CV disease to cancer, positioning inflammation as a central player.
Immune system
Dysfunction of the immune system is linked to the development of both cancer and HF.84, 85 Influx of different immune cells is a necessity in the early response to injury, in an effort to limit and repair initial damage, but chronic activation seems to exert adverse effects.86 A complete overview of the immune system (dys)function and HF has recently been published by the Working Group on Myocardial Function of the European Society of Cardiology.87 The aetiology of HF varies, and therefore most likely also the pathophysiological mechanisms that influence the immune activation.
For example, in post‐MI patients, within hours neutrophils are the first to invade the heart and start a pro‐inflammatory phase. The next proliferative phase is characterised by infiltration of macrophages, resolution of dead tissue, and the beginning of scar formation (days 3–30). This is followed by the remodelling phase, during which the inflammatory response should decrease and the non‐infarcted myocardium undergoes pathophysiological changes. Cytokines and innate immune receptors regulate the invasion of inflammatory cells after MI. On the other hand, in HFpEF, more driven by obesity, hypertension, diabetes, and metabolic syndrome, other elements of the immune system may come into play. This phenotype is characterised by cardiac hypertrophy and fibrosis. Cardiac macrophages are involved in inducing cardiomyocyte death and interstitial fibrosis in the HFpEF heart. Depletion of cardiac macrophages reduced hypertrophic cardiomyocyte growth in hypertension. Nevertheless, the exact working mechanism is unknown.
In cancer, an inadequate immune system is correlated with cancer development and the occurrence of metastases.88 Several aspects and elements of the immune system may be viable targets for therapy. In fact, currently, the use of immune therapy in cancer is a booming business, and spectacular results have been observed for immune checkpoint inhibitors.89 With the more wide use of these drugs, however, it has emerged their use may be limited by life‐threatening cases of myocarditis, which furthermore suggest that interference with the tumour immune system may go at the expense of the well‐regulated myocardial immune system.90 With the ever‐expanding use of immunotherapy in cancer there is a clear need for registry data to monitor unexpected and rare myocardial complications of such therapies.91
Thus, essential systemic regulatory systems such as inflammation, neuro‐hormonal, oxidative stress and the immune system share pathophysiological mechanisms between HF and cancer. In line with this, several co‐morbidities, particularly chronic inflammatory diseases, have been linked to both HF and cancer incidence.92, 93 These will be discussed in the next paragraph.
Cardiovascular drug: inducer or repressor of incidence cancer?
Causality is difficult to prove given the complexity of the intertwining pathways and the lack of targeted intervention trials. In the event that specific (CV) drugs counteract (CV) risk factors that are also associated with cancer development, or when they attenuate left ventricular remodelling, one could hypothesise that such treatments should reduce cancer onset as well, in line with the hypothesis that CV risk factors and HF may cause cancer. However, since CV drugs generally are aimed to reduce CV‐related mortality, one could also anticipate that cancer‐related mortality might seemingly increase, due to competing risks (less HF‐related death might in part be associated with more cancer‐related death). So, a priori hypothesizing what effect CV drugs might have for cancer‐related death, in either way, is troublesome.
To date, three large meta‐analyses have been published that investigate the effects of anti‐hypertensive drugs in light of new‐onset cancer. In a large meta‐analysis in which all classes of anti‐hypertensive drugs were studied, it was observed that a 5.0–10.0% relative increase in the risk of cancer or cancer‐related death existed in those using ARBs, ACEi, β‐blockers, diuretics and calcium channel blockers (CCBs).94 However, two other meta‐analyses that were published subsequently did not confirm these data.95, 96 ARB use and the risk of cancer in type 2 DM demonstrated a negative association for losartan [odds ratio (OR) 0.78, 95% CI 0.63–0.97] but a positive association for candesartan (OR 1.79, 95% CI 1.05–3.06) and telmisartan (OR 1.54, 95% CI 0.97–2.43) with the overall occurrence of cancer. Currently, no mechanism can explain these observations.97 Clearly, the data for anti‐hypertensive agents are not straightforward and the importance of hypertension for cancer development has yet to be fully established.
Another established treatment in preventing adverse events in patients with CV disease is aspirin. Recently it was observed that low‐dose aspirin in a low–medium CV risk population did not result in a lower cancer incidence.98 Aspirin as several working mechanisms, for example in CV disease, leads to antiplatelet effects, whereas in cancer it works via cyclooxygenase‐dependent and cyclooxygenase‐independent mechanisms.99 It has been demonstrated that using low‐dose aspirin compared with no aspirin was associated with a markedly higher sensitivity for detecting advanced colorectal neoplasms.100
Lastly, statins are not associated with reduced cancer incidence but the evidence is inconclusive.101
Gaps in evidence and future suggestions
Awareness of the cardio‐oncology field is growing, but several questions remain unanswered and additional pre‐clinical and clinical studies are dearly needed.
At present, few pre‐clinical models of CV disease are studied in the setting of cancer development, which could help to unravel the emerging relationship in a scientific, systematic, and temporal manner. We lack data regarding specific circulating factors or pathophysiological mechanisms linked to specific forms of cancer. In clinical trials, both the cardiologist and oncologist, but especially the multi‐morbid patient, would be served with precise phenotyping of both CV as cancer history, risk factors, and treatment regimens, and to precisely define CV and cancer endpoints, to more effectively study the interplay between the diseases. We thus advocate to more systematically record cancer‐related endpoints in CV trials, and CV endpoints in oncology trials.
In addition to these research aspects, clinical awareness and expertise are essential for the optimal treatment of patients having developed cancer with a history of CV disease, or who develop CV disease with a history of cancer. With the growing prevalence of CV disease in western society, the number of these patients will further increase. We postulate that certain windows of opportunity exist within HF management programmes wherein early detection of cancer might be possible. Every hospital visit, either at the outpatient clinic or an admission via the emergency department, could lead to detection of cancer during the physical, biochemical or radiological examination. Furthermore, anticoagulant therapy is commonly prescribed by cardiologists [e.g. for concomitant atrial fibrillation, coronary artery disease, or prosthetic (valve) material], and this may cause small tumours and polyps to bleed and, as such, provide a higher sensitivity for certain tests. Shared risk factors and pathophysiological mechanisms should prompt general practitioners and specialists to be aware of a possible concomitance of both diseases.30 And finally, within clinical trials, both CV and cancer endpoints should ideally be adjudicated by a multidisciplinary team of experts, so that valid data in both domains can be collected and analysed. All these factors will help to create awareness of cancer detection in patients with HF. We summarised these topics in Table 3.
Table 3.
Factors that may explain (early) cancer detection in patients with heart failure
| Heart failure management events | Detection of cancer by |
|---|---|
| Heart failure‐related hospital visits (outpatient and/or admission) | Physical, biochemical and radiological examinations |
| Use of oral anticoagulants | Blood loss from tumour prone to bleeding; more sensitive oncological testing |
| Cardiovascular risk factor |
Shared risk factors – relevance for cancer detection Regular visits to cardiologist and/or general practitioner |
| Shared pathophysiological mechanisms | Knowledge exchange and research may improve cancer awareness in heart failure patients among cardiologists and oncologists; to date unclear if this will translate into better patient management |
| Trial inclusion and execution | Cardiovascular and cancer endpoints; state‐of‐the‐art endpoint adjudication |
Conclusion
Cancer is a frequent co‐morbidity in patients with HF, and existing data suggest that a pathophysiological connection exists between the two. Circulating factors directly link both diseases, and shared pathophysiological mechanisms may explain the interactions, but the relation is very complex. Comprehensive and detailed studies will be needed to elucidate the connection between CV disease in general, HF and cancer. A new research agenda will provide insights, proposals for surveillance strategies, and are eagerly awaited.
Funding
Dr. de Boer is supported by the Netherlands Heart Foundation (CVON DOSIS, grant 2014‐40, CVON SHE‐PREDICTS‐HF, grant 2017‐21, and CVON RED‐CVD, grant 2017‐11); by the Innovational Research Incentives Scheme programme of the Netherlands Organization for Scientific Research (NWO VIDI, grant 917.13.350), and the European Research Council (ERC CoG 818715, SECRETE‐HF). Dr. Meijers is supported by the Netherlands Heart Foundation (CVON–Talent program 2017). Prof. van der Meer is supported by the European Research Council (ERC STg 715732, STOP‐HF). Prof. van Veldhuisen is an Established Investigator of the Netherlands Heart Foundation (grant D97.017).
Conflict of interest: none declared.
References
- 1. Crespo‐Leiro MG, Anker SD, Maggioni AP, Coats AJ, Filippatos G, Ruschitzka F, Ferrari R, Piepoli MF, Delgado Jimenez JF, Metra M, Fonseca C, Hradec J, Amir O, Logeart D, Dahlström U, Merkely B, Drozdz J, Goncalvesova E, Hassanein M, Chioncel O, Lainscak M, Seferovic PM, Tousoulis D, Kavoliuniene A, Fruhwald F, Fazlibegovic E, Temizhan A, Gatzov P, Erglis A, Laroche C, Mebazaa A; Heart Failure Association (HFA) of the European Society of Cardiology (ESC). European Society of Cardiology Heart Failure Long‐Term Registry (ESC‐HF‐LT): 1‐year follow‐up outcomes and differences across regions. Eur J Heart Fail 2016;18:613–625. [DOI] [PubMed] [Google Scholar]
- 2. Ferlay J, Colombet M, Soerjomataram I, Dyba T, Randi G, Bettio M, Gavin A, Visser O, Bray F. Cancer incidence and mortality patterns in Europe: estimates for 40 countries and 25 major cancers in 2018. Eur J Cancer 2018;103:356–387. [DOI] [PubMed] [Google Scholar]
- 3. Weir HK, Anderson RN, Coleman King SM, Soman A, Thompson TD, Hong Y, Moller B, Leadbetter S. Heart disease and cancer deaths ‐ trends and projections in the United States, 1969–2020. Prev Chronic Dis 2016;13:160211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Curigliano G, Cardinale D, Suter T, Plataniotis G, De Azambuja E, Sandri MT, Criscitiello C, Goldhirsch A, Cipolla C, Roila F. Cardiovascular toxicity induced by chemotherapy, targeted agents and radiotherapy: ESMO clinical practice guidelines. Ann Oncol 2012;23Suppl 7:vii155–vii166. [DOI] [PubMed] [Google Scholar]
- 5. Zamorano JL, Lancellotti P, Rodriguez Muñoz D, Aboyans V, Asteggiano R, Galderisi M, Habib G, Lenihan DJ, Lip GY, Lyon AR, Lopez Fernandez T, Mohty D, Piepoli MF, Tamargo J, Torbicki A, Suter TM. 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: The Task Force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology. Eur Heart J 2016;37:2768–2801. [DOI] [PubMed] [Google Scholar]
- 6. Cardinale D, Colombo A, Bacchiani G, Tedeschi I, Meroni CA, Veglia F, Civelli M, Lamantia G, Colombo N, Curigliano G, Fiorentini C, Cipolla CM. Early detection of anthracycline cardiotoxicity and improvement with heart failure therapy. Circulation 2015;131:1981–1988. [DOI] [PubMed] [Google Scholar]
- 7. Herrmann J, Lerman A, Sandhu N, Villarraga H, Mulvagh S, Kohli M. Evaluation and management of patients with heart disease and cancer: cardio‐oncology. Mayo Clin Proc 2014;89:1287–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hasin T, Gerber Y, McNallan SM, Weston SA, Kushwaha SS, Nelson TJ, Cerhan JR, Roger VL. Patients with heart failure have an increased risk of incident cancer. J Am Coll Cardiol 2013;62:881–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hasin T, Gerber Y, Weston SA, Jiang R, Killian JM, Manemann SM, Cerhan JR, Roger VL. Heart failure after myocardial infarction is associated with increased risk of cancer. J Am Coll Cardiol 2016;68:265–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rinde LB, Småbrekke B, Hald EM, Brodin EE, Njølstad I, Mathiesen EB, Løchen ML, Wilsgaard T, Brækkan SK, Vik A, Hansen JB. Myocardial infarction and future risk of cancer in the general population – the Tromsø Study. Eur J Epidemiol 2017;32:193–201. [DOI] [PubMed] [Google Scholar]
- 11. Banke A, Schou M, Videbaek L, Moller JE, Torp‐Pedersen C, Gustafsson F, Dahl JS, Kober L, Hildebrandt PR, Gislason GH. Incidence of cancer in patients with chronic heart failure: a long‐term follow‐up study. Eur J Heart Fail 2016;18:260–266. [DOI] [PubMed] [Google Scholar]
- 12. Berton G, Cordiano R, Cavuto F, Bagato F, Segafredo B, Pasquinucci M. Neoplastic disease after acute coronary syndrome: incidence, duration, and features: the ABC‐4* study on heart disease. J Cardiovasc Med (Hagerstown) 2018;19:546–553. [DOI] [PubMed] [Google Scholar]
- 13. Selvaraj S, Bhatt DL, Claggett B, Djoussé L, Shah SJ, Chen J, Imran TF, Qazi S, Sesso HD, Gaziano JM, Schrag D. Lack of association between heart failure and incident cancer. J Am Coll Cardiol 2018;71:1501–1510. [DOI] [PubMed] [Google Scholar]
- 14. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, González‐Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GM, Ruilope LM, Ruschitzka F, Rutten FH, v dan er Meer P . 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2016;18:891–975. [DOI] [PubMed] [Google Scholar]
- 15. Savarese G, Lund LH. Global public health burden of heart failure. Card Fail Rev 2017;3:7–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lam CS, Gamble GD, Ling LH, Sim D, Leong KT, Yeo PS, Ong HY, Jaufeerally F, Ng TP, Cameron VA, Poppe K, Lund M, Devlin G, Troughton R, Richards AM, Doughty RN. Mortality associated with heart failure with preserved vs. reduced ejection fraction in a prospective international multi‐ethnic cohort study. Eur Heart J 2018;39:1770–1780. [DOI] [PubMed] [Google Scholar]
- 17. Lee DS, Gona P, Albano I, Larson MG, Benjamin EJ, Levy D, Kannel WB, Vasan RS. A systematic assessment of causes of death after heart failure onset in the community: impact of age at death, time period, and left ventricular systolic dysfunction. Circ Heart Fail 2011;4:36–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lund LH, Donal E, Oger E, Hage C, Persson H, Haugen‐Löfman I, Ennezat PV, Sportouch‐Dukhan C, Drouet E, Daubert JC, Linde C. Association between cardiovascular vs. non‐cardiovascular co‐morbidities and outcomes in heart failure with preserved ejection fraction. Eur J Heart Fail 2014;16:992–1001. [DOI] [PubMed] [Google Scholar]
- 19. Tribouilloy C, Rusinaru D, Mahjoub H, Soulière V, Lévy F, Peltier M, Slama M, Massy Z. Prognosis of heart failure with preserved ejection fraction: a 5 year prospective population‐based study. Eur Heart J 2008;29:339–347. [DOI] [PubMed] [Google Scholar]
- 20. Vaduganathan M, Patel RB, Michel A, Shah SJ, Senni M, Gheorghiade M, Butler J. Mode of death in heart failure with preserved ejection fraction. J Am Coll Cardiol 2017;69:556–569. [DOI] [PubMed] [Google Scholar]
- 21. Sakamoto M, Hasegawa T, Asakura M, Kanzaki H, Takahama H, Amaki M, Mochizuki N, Anzai T, Hamasaki T, Kitakaze M. Does the pathophysiology of heart failure prime the incidence of cancer? Hypertens Res 2017;40:831–836. [DOI] [PubMed] [Google Scholar]
- 22. Carpeggiani C, Landi P, Michelassi C, Andreassi MG, Sicari R, Picano E. Stress echocardiography positivity predicts cancer death. J Am Heart Assoc 2017;6:e007104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Boffetta P, Malhotra J. Impact of heart failure on cancer incidence: a complicated question. J Am Coll Cardiol 2018;71:1511–1512. [DOI] [PubMed] [Google Scholar]
- 24. Ather S, Chan W, Bozkurt B, Aguilar D, Ramasubbu K, Zachariah AA, Wehrens XHT, Deswal A. Impact of noncardiac comorbidities on morbidity and mortality in a predominantly male population with heart failure and preserved versus reduced ejection fraction. J Am Coll Cardiol 2012;59:998–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ameri P, Canepa M, Anker MS, Belenkov Y, Bergler‐Klein J, Cohen‐Solal A, Farmakis D, López‐Fernández T, Lainscak M, Pudil R, Ruschitska F, Seferovic P, Filippatos G, Coats A, Suter T, Von Haehling S, Ciardiello F, de Boer RA, Lyon AR, Tocchetti CG; Heart Failure Association Cardio‐Oncology Study Group of the European Society of Cardiology. Cancer diagnosis in patients with heart failure: epidemiology, clinical implications and gaps in knowledge. Eur J Heart Fail 2018;20:879–887. [DOI] [PubMed] [Google Scholar]
- 26. Jacobs L, Efremov L, Ferreira JP, Thijs L, Yang WY, Zhang ZY, Latini R, Masson S, Agabiti N, Sever P, Delles C, Sattar N, Butler J, Cleland JG, Kuznetsova T, Staessen JA, Zannad F; Heart ‘OMics’ in AGEing (HOMAGE) Investigators. Risk for incident heart failure: a subject‐level meta‐analysis from the Heart ‘OMics’ in AGEing (HOMAGE) study. J Am Heart Assoc 2017;6:e005231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bayliss EA, Reifler LM, Zeng C, McQuillan DB, Ellis JL, Steiner JF. Competing risks of cancer mortality and cardiovascular events in individuals with multimorbidity. J Comorb 2014;4:29–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Akinyemiju T, Wiener H, Pisu M. Cancer‐related risk factors and incidence of major cancers by race, gender and region; analysis of the NIH‐AARP diet and health study. BMC Cancer 2017;17:597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tu H, Wen CP, Tsai SP, Chow WH, Wen C, Ye Y, Zhao H, Tsai MK, Huang M, Dinney CP, Tsao CK, Wu X. Cancer risk associated with chronic diseases and disease markers: prospective cohort study. BMJ 2018;360:k134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Meijers WC, de Boer RA. Common risk factors for heart failure and cancer. Cardiovasc Res 2019;115:844–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lawes CM, Vander Hoorn S, Rodgers A; International Society of Hypertension.Global burden of blood‐pressure‐related disease, 2001. Lancet 2008;371:1513–1518. [DOI] [PubMed] [Google Scholar]
- 32. Drazner MH. The progression of hypertensive heart disease. Circulation 2011;123:327–334. [DOI] [PubMed] [Google Scholar]
- 33. Stocks T, Van Hemelrijck M, Manjer J, Bjorge T, Ulmer H, Hallmans G, Lindkvist B, Selmer R, Nagel G, Tretli S, Concin H, Engeland A, Jonsson H, Stattin P. Blood pressure and risk of cancer incidence and mortality in the Metabolic Syndrome and Cancer Project. Hypertension 2012;59:802–810. [DOI] [PubMed] [Google Scholar]
- 34. Ferrara N. VEGF and the quest for tumour angiogenesis factors. Nat Rev Cancer 2002;2:795–803. [DOI] [PubMed] [Google Scholar]
- 35. Nishida N, Yano H, Nishida T, Kamura T, Kojiro M. Angiogenesis in cancer. Vasc Health Risk Manag 2006;2:213–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wolin KY, Carson K, Colditz GA. Obesity and cancer. Oncologist 2010;15:556–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gregor MF, Hotamisligil GS. Inflammatory mechanisms in obesity. Annu Rev Immunol 2011;29:415–445. [DOI] [PubMed] [Google Scholar]
- 38. Gérard C, Brown KA. Obesity and breast cancer – role of estrogens and the molecular underpinnings of aromatase regulation in breast adipose tissue. Mol Cell Endocrinol 2018;466:15–30. [DOI] [PubMed] [Google Scholar]
- 39. Scherer PE. Adipose tissue: from lipid storage compartment to endocrine organ. Diabetes 2006;55:1537–1545. [DOI] [PubMed] [Google Scholar]
- 40. Woerden G, Gorter TM, Westenbrink BD, Willems TP, van Veldhuisen DJ, Rienstra M. Epicardial fat in heart failure patients with mid‐range and preserved ejection fraction. Eur J Heart Fail 2018;20:1559–1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. VanSaun MN. Molecular pathways: adiponectin and leptin signaling in cancer. Clin Cancer Res 2013;19:1926–1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Gallagher EJ, LeRoith D. Obesity and diabetes: the increased risk of cancer and cancer‐related mortality. Physiol Rev 2015;95:727–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Keum N, Greenwood DC, Lee DH, Kim R, Aune D, Ju W, Hu FB, Giovannucci EL. Adult weight gain and adiposity‐related cancers: a dose‐response meta‐analysis of prospective observational studies. J Natl Cancer Inst 2015;107:1–14. [DOI] [PubMed] [Google Scholar]
- 44. Tee MC, Cao Y, Warnock GL, Hu FB, Chavarro JE. Effect of bariatric surgery on oncologic outcomes: a systematic review and meta‐analysis. Surg Endosc 2013;27:4449–4456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Horwich TB, Fonarow GC, Hamilton MA, MacLellan WR, Woo MA, Tillisch JH. The relationship between obesity and mortality in patients with heart failure. J Am Coll Cardiol 2001;38:789–795. [DOI] [PubMed] [Google Scholar]
- 46. Kasi PM, Zafar SY, Grothey A. Is obesity an advantage in patients with colorectal cancer? Expert Rev Gastroenterol Hepatol 2015;9:1339–1342. [DOI] [PubMed] [Google Scholar]
- 47. Sanner T, Grimsrud TK. Nicotine: carcinogenicity and effects on response to cancer treatment – a review. Front Oncol 2015;5:196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ambrose JA, Barua RS. The pathophysiology of cigarette smoking and cardiovascular disease: an update. J Am Coll Cardiol 2004;43:1731–1737. [DOI] [PubMed] [Google Scholar]
- 49. Morris PB, Ference BA, Jahangir E, Feldman DN, Ryan JJ, Bahrami H, El‐Chami MF, Bhakta S, Winchester DE, Al‐Mallah MH, Sanchez Shields M, Deedwania P, Mehta LS, Phan BA, Benowitz NL. Cardiovascular effects of exposure to cigarette smoke and electronic cigarettes: clinical perspectives from the Prevention of Cardiovascular Disease Section Leadership Council and Early Career Councils of the American College of Cardiology. J Am Coll Cardiol 2015;66:1378–1391. [DOI] [PubMed] [Google Scholar]
- 50. van Kruijsdijk RC, van der Graaf Y, Peeters PH, Visseren FLJ. Cancer risk in patients with manifest vascular disease: effects of smoking, obesity, and metabolic syndrome. Cancer Epidemiol Biomarkers Prev 2013;22:1267–1277. [DOI] [PubMed] [Google Scholar]
- 51. Cohen G, Levy I, Yuval, Kark JD, Levin N, Broday DM, Steinberg DM, Gerber Y. Long‐term exposure to traffic‐related air pollution and cancer among survivors of myocardial infarction: a 20‐year follow‐up study. Eur J Prev Cardiol 2017;24:92–102. [DOI] [PubMed] [Google Scholar]
- 52. Konduracka E, Gackowski A, Rostoff P, Galicka‐Latala D, Frasik W, Piwowarska W. Diabetes‐specific cardiomyopathy in type 1 diabetes mellitus: no evidence for its occurrence in the era of intensive insulin therapy. Eur Heart J 2007;28:2465–2471. [DOI] [PubMed] [Google Scholar]
- 53. Renehan AG, Frystyk J, Flyvbjerg A. Obesity and cancer risk: the role of the insulin–IGF axis. Trends Endocrinol Metab 2006;17:328–336. [DOI] [PubMed] [Google Scholar]
- 54. Basen‐Engquist K, Chang M. Obesity and cancer risk: recent review and evidence. Curr Oncol Rep 2011;13:71–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ballotari P, Vicentini M, Manicardi V, Gallo M, Chiatamone Ranieri S, Greci M, Giorgi Rossi P. Diabetes and risk of cancer incidence: results from a population‐based cohort study in northern Italy. BMC Cancer 2017;17:703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Rasmussen‐Torvik LJ, Shay CM, Abramson JG, Friedrich CA, Nettleton JA, Prizment AE, Folsom AR. Ideal cardiovascular health is inversely associated with incident cancer: the Atherosclerosis Risk in Communities study. Circulation 2013;127:1270–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Opie LH, Lopaschuk GD. What is good for the circulation also lessens cancer risk. Eur Heart J 2015;36:1157–1162. [DOI] [PubMed] [Google Scholar]
- 58. Kushi LH, Doyle C, McCullough M, Rock CL, Demark‐Wahnefried W, Bandera EV, Gapstur S, Patel AV, Andrews K, Gansler T; American Cancer Society 2010 Nutrition and Physical Activity Guidelines Advisory Comittee. American Cancer Society Guidelines on nutrition and physical activity for cancer prevention: reducing the risk of cancer with healthy food choices and physical activity. CA Cancer J Clin 2012;62:30–67. [DOI] [PubMed] [Google Scholar]
- 59. Braunwald E. Biomarkers in heart failure management. N Engl J Med 2008;358:2148–2159. [DOI] [PubMed] [Google Scholar]
- 60. Dewey CM, Spitler KM, Ponce JM, Hall DD, Grueter CE. Cardiac‐secreted factors as peripheral metabolic regulators and potential disease biomarkers. J Am Heart Assoc 2016;5:e003101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Arab S, Gramolini AO, Ping P, Kislinger T, Stanley B, van Eyk J, Ouzounian M, MacLennan DH, Emili A, Liu PP. Cardiovascular proteomics: tools to develop novel biomarkers and potential applications. J Am Coll Cardiol 2006;48:1733–1741. [DOI] [PubMed] [Google Scholar]
- 62. Meijers WC, Maglione M, Bakker SJL, Oberhuber R, Kieneker LM, de Jong S, Haubner BJ, Nagengast WB, Lyon AR, van der Vegt B, van Veldhuisen DJ, Westenbrink BD, van der Meer P, Silljé HH, de Boer RA. The failing heart stimulates tumor growth by circulating factors. Circulation 2018;138:678–691. [DOI] [PubMed] [Google Scholar]
- 63. Richards AM. Can heart failure cause cancer? Nat Rev Cardiol 2019;16:7–8. [DOI] [PubMed] [Google Scholar]
- 64. de Boer RA, Daniels LB, Maisel AS, Januzzi JL. State of the art: newer biomarkers in heart failure. Eur J Heart Fail 2015;17:559–569. [DOI] [PubMed] [Google Scholar]
- 65. Kitsis RN, Riquelme JA, Lavandero S. Heart disease and cancer. Circulation 2018;138:692–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Bertero E, Canepa M, Maack C, Ameri P. Linking heart failure to cancer ‐ background evidence and research perspectives. Circulation 2018;138:735–742. [DOI] [PubMed] [Google Scholar]
- 67. Hartupee J, Mann DL. Neurohormonal activation in heart failure with reduced ejection fraction. Nat Rev Cardiol 2017;14:30–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. George AJ, Thomas WG, Hannan RD. The renin‐angiotensin system and cancer: old dog, new tricks. Nat Rev Cancer 2010;10:745–759. [DOI] [PubMed] [Google Scholar]
- 69. Ino K, Shibata K, Kajiyama H, Yamamoto E, Nagasaka T, Nawa A, Nomura S, Kikkawa F. Angiotensin II type 1 receptor expression in ovarian cancer and its correlation with tumour angiogenesis and patient survival. Br J Cancer 2006;94:552–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Reuter S, Gupta SC, Chaturvedi MM, Aggarwal BB. Oxidative stress, inflammation, and cancer: how are they linked? Free Radic Biol Med 2010;49:1603–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Takimoto E, Kass DA. Role of oxidative stress in cardiac hypertrophy and remodeling. Hypertension 2007;49:241–248. [DOI] [PubMed] [Google Scholar]
- 72. Maiuri MC, Kroemer G. Essential role for oxidative phosphorylation in cancer progression. Cell Metab 2015;21:11–12. [DOI] [PubMed] [Google Scholar]
- 73. Diniz YS, Cicogna AC, Padovani CR, Silva MD, Faine LA, Galhardi CM, Rodrigues HG, Novelli EL. Dietary restriction and fibre supplementation: oxidative stress and metabolic shifting for cardiac health. Can J Physiol Pharmacol 2003;81:1042–1048. [DOI] [PubMed] [Google Scholar]
- 74. Aune D, Chan DS, Greenwood DC, Vieira AR, Navarro Rosenblatt DA, Vieira R, Norat T. Dietary fiber and breast cancer risk: a systematic review and meta‐analysis of prospective studies. Ann Oncol 2012;23:1394–1402. [DOI] [PubMed] [Google Scholar]
- 75. Lopaschuk GD, Ussher JR, Folmes CD, Jaswal JS, Stanley WC. Myocardial fatty acid metabolism in health and disease. Physiol Rev 2010;90:207–258. [DOI] [PubMed] [Google Scholar]
- 76. Bonnet S, Archer SL, Allalunis‐Turner J, Haromy A, Beaulieu C, Thompson R, Lee CT, Lopaschuk GD, Puttagunta L, Bonnet S, Harry G, Hashimoto K, Porter CJ, Andrade MA, Thebaud B, Michelakis ED. A mitochondria‐K+channel axis is suppressed in cancer and its normalization promotes apoptosis and inhibits cancer growth. Cancer Cell 2007;11:37–51. [DOI] [PubMed] [Google Scholar]
- 77. Ussher JR, Wang W, Gandhi M, Keung W, Samokhvalov V, Oka T, Wagg CS, Jaswal JS, Harris RA, Clanachan AS, Dyck JRB, Lopaschuk GD. Stimulation of glucose oxidation protects against acute myocardial infarction and reperfusion injury. Cardiovasc Res 2012;94:359–369. [DOI] [PubMed] [Google Scholar]
- 78. Anker SD, von Haehling S. Inflammatory mediators in chronic heart failure: an overview. Heart 2004;90:464–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Mann DL. Innate immunity and the failing heart: the cytokine hypothesis revisited. Circ Res 2015;116:1254–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Westenbrink BD, Voors AA, de Boer RA, Schuringa JJ, Klinkenberg T, van der Harst P, Vellenga E, van Veldhuisen DJ, van Gilst WH. Bone marrow dysfunction in chronic heart failure patients. Eur J Heart Fail 2010;12:676–684. [DOI] [PubMed] [Google Scholar]
- 81. Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell 2010;140:883–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Ridker PM, MacFadyen JG, Everett BM, Libby P, Thuren T, Glynn RJ. Relationship of C‐reactive protein reduction to cardiovascular event reduction following treatment with canakinumab: a secondary analysis from the CANTOS randomised controlled trial. Lancet 2018;391:319–328. [DOI] [PubMed] [Google Scholar]
- 83. Ridker PM, MacFadyen JG, Thuren T, Everett BM, Libby P, Glynn RJ; CANTOS Trial Group. Effect of interleukin‐1β inhibition with canakinumab on incident lung cancer in patients with atherosclerosis: exploratory results from a randomised, double‐blind, placebo‐controlled trial. Lancet 2017;390:1833–1842. [DOI] [PubMed] [Google Scholar]
- 84. Swann JB, Smyth MJ. Immune surveillance of tumors. J Clin Invest 2007;117:1137–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Zhang Y, Bauersachs J, Langer HF. Immune mechanisms in heart failure. Eur J Heart Fail 2017;19:1379–1389. [DOI] [PubMed] [Google Scholar]
- 86. Epelman S, Liu PP, Mann DL. Role of innate and adaptive immune mechanisms in cardiac injury and repair. Nat Rev Immunol 2015;15:117–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Frantz S, Falcao‐Pires I, Balligand JL, Bauersachs J, Brutsaert D, Ciccarelli M, Dawson D, de Windt LJ, Giacca M, Hamdani N, Hilfiker‐Kleiner D, Hirsch E, Leite‐Moreira A, Mayr M, Thum T, Tocchetti CG, van der Velden J, Varricchi G, Heymans S. The innate immune system in chronic cardiomyopathy: a European Society of Cardiology (ESC) scientific statement from the Working Group on Myocardial Function of the ESC. Eur J Heart Fail 2018;20:445–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Pandya PH, Murray ME, Pollok KE, Renbarger JL. The immune system in cancer pathogenesis: potential therapeutic approaches. J Immunol Res 2016;2016:4273943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 2012;12:252–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Moslehi JJ, Salem JE, Sosman JA, Lebrun‐Vignes B, Johnson DB. Increased reporting of fatal immune checkpoint inhibitor‐associated myocarditis. Lancet 2018;391:933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Moslehi JJ. The link between cancer and cardiovascular disease. http://www.cardioonc.org (19 June 2019).
- 92. Colotta F, Allavena P, Sica A, Garlanda C, Mantovani A. Cancer‐related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis 2009;30:1073–1081. [DOI] [PubMed] [Google Scholar]
- 93. Burkard T, Pfister O, Rickli H, Follath F, Hack D, Zaker R, Pittl U, Handschin R, Pfisterer M, Brunner‐La Rocca HP. Prognostic impact of systemic inflammatory diseases in elderly patients with congestive heart failure. QJM 2014;107:131–138. [DOI] [PubMed] [Google Scholar]
- 94. Sipahi I, Debanne SM, Rowland DY, Simon DI, Fang JC. Angiotensin‐receptor blockade and risk of cancer: meta‐analysis of randomised controlled trials. Lancet Oncol 2010;11:627–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. ARB Trialists Collaboration . Effects of telmisartan, irbesartan, valsartan, candesartan, and losartan on cancers in 15 trials enrolling 138,769 individuals. J Hypertens 2011;29:623–635. [DOI] [PubMed] [Google Scholar]
- 96. Bangalore S, Kumar S, Kjeldsen SE, Makani H, Grossman E, Wetterslev J, Gupta AK, Sever PS, Gluud C, Messerli FH. Antihypertensive drugs and risk of cancer: network meta‐analyses and trial sequential analyses of 324,168 participants from randomised trials. Lancet Oncol 2011;12:65–82. [DOI] [PubMed] [Google Scholar]
- 97. Chang CH, Lin JW, Wu LC, Lai MS. Angiotensin receptor blockade and risk of cancer in type 2 diabetes mellitus: a nationwide case‐control study. J Clin Oncol 2011;29:3001–3007. [DOI] [PubMed] [Google Scholar]
- 98. Gaziano JM, Brotons C, Coppolecchia R, Cricelli C, Darius H, Gorelick PB, Howard G, Pearson TA, Rothwell PM, Ruilope LM, Tendera M, Tognoni G; ARRIVE Executive Committee. Use of aspirin to reduce risk of initial vascular events in patients at moderate risk of cardiovascular disease (ARRIVE): a randomised, double‐blind, placebo‐controlled trial. Lancet 2018;392:1036–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Thorat MA, Cuzick J. Role of aspirin in cancer prevention. Curr Oncol Rep 2013;15:533–540. [DOI] [PubMed] [Google Scholar]
- 100. Brenner H, Tao S, Haug U. Low‐dose aspirin use and performance of immunochemical fecal occult blood tests. JAMA 2010;304:2513–2520. [DOI] [PubMed] [Google Scholar]
- 101. Kuoppala J, Lamminpää A, Pukkala E. Statins and cancer: a systematic review and meta‐analysis. Eur J Cancer 2008;44:2122–2132. [DOI] [PubMed] [Google Scholar]


