Abstract
Background:
Timely removal from activity after concussion symptoms remains problematic despite heightened awareness. Previous studies indicated potential adverse effects of continuing to participate in physical activity immediately after sustaining a concussion.
Hypothesis/Purpose:
The purpose was to determine the effect of timing of removal from play after concussion on clinical outcomes. It was hypothesized that immediate removal from activity after sport-related concussion (SRC) would be associated with less time missed from sport, a shorter symptomatic period, and better outcomes on acute clinical measures.
Study Design:
Cohort study; Level of evidence, 3.
Methods:
Data were reported from the National Collegiate Athletic Association and Department of Defense Grand Alliance: Concussion Awareness, Research, and Education (CARE) Consortium. Participants with 506 diagnosed SRCs from 18 sports and 25 institutions and military service academies were analyzed and classified as either immediate removal from activity (I-RFA) or delayed removal from activity (D-RFA). Outcomes of interest included time missed from sport attributed to their SRC, symptom duration, and clinical assessment scores.
Results:
There were 322 participants (63.6%) characterized as D-RFA. I-RFA status was associated with significantly less time missed from sport (R2 change = .022–.024, P < .001 to P = .001) and shorter symptom duration (R2 change = .044–.046, P < .001 [all imputations]) while controlling for other SRC recovery modifiers. These athletes missed approximately 3 fewer days from sport participation. I-RFA athletes had significantly less severe acute SRC symptoms and were at lower risk of recovery taking ≥14 days (relative risk = .614, P < .001, small-medium effect size) and ≥21 days (relative risk = .534, P = .010, small effect size).
Conclusion:
I-RFA is a protective factor associated with less severe acute symptoms and shorter recovery after SRC. Conveying this message to athletes, coaches, and others involved in the care of athletes may promote timely injury reporting.
Keywords: brain injury, concussion reporting, CARE Consortium, mTBI
Recognition and reporting of sport-related concussion (SRC) have improved in the past 2 decades, owing to media attention, improvements in injury detection and diagnosis, changing attitudes toward injury reporting in sports culture, and the implementation of systematic protocols for concussion management.18,21,26 Athlete-specific studies showed that symptoms commonly subside within approximately 7 to 14 days after injury.30–32
Current SRC management guidelines recommend that athletes who are suspected of sustaining a concussion be immediately removed from play and medically evaluated.35 However, as many as 50% of athletes either fail to disclose their injuries or are delayed in being removed from athletic participation.1,33 This is most often due to the difficulty in determining the seriousness of their symptoms, poor awareness and education about concussion, a desire to remain in play, and concerns about coaches’ reactions.4,7,33,45 A recent preliminary study of collegiate athletes demonstrated that athletes who were not immediately removed from practice or game play after an SRC took approximately 5 days longer to return to play (RTP), a finding that may motivate athletes, coaches, and sports institutions to take timely SRC reporting seriously.1 This study used retrospective medical chart review at a single collegiate institution and did not account for acute injury factors, such as loss or alteration of consciousness. It also did not include any clinical measures, such as acute symptom, cognitive, or balance examinations.
Three similar studies examined adolescent athletes. Two reported that athletes who continued to play took twice as long to recover and had more substantial cognitive impairment and symptoms than those who were immediately removed.12,20 Another study investigated the effect of sustaining an additional head impact sufficient to worsen already existing symptoms and found that an additional impact, not simply continuation of play, lengthened recovery and resulted in more severe symptoms.46 These studies involved young cohorts, emanated from specialized concussion referral clinics, were retrospective, or lacked sufficient sample sizes to conduct more in-depth analyses of covariates. The purpose of the present study was to prospectively examine removal from athletic activity and its effect on sport time lost because of SRC and performance on acute clinical measures in a large sample of collegiate athletes. In so doing, we hoped to provide empirical evidence of recovery benefit in immediate removal of athletes from play after SRC.
METHODS
Study Design and Setting
Data were obtained from the National Collegiate Athletic Association and Department of Defense Concussion Assessment, Research, and Education (CARE) Consortium. The CARE Consortium has prospectively collected SRC-related assessment data on student-athletes since 2014 and currently consists of 30 universities, including 4 US military service academies across the country. Student-athletes at participating institutions complete annual preseason baseline test batteries, which are repeated in the event of a suspected concussion. The battery includes, at a minimum, an assessment of concussion-like symptoms, cognition, and postural stability. They also complete a clinical reporting form describing sport participation history, academic history, personal and family medical history, and socioeconomic status. Clinicians complete a postinjury clinical reporting form describing concussion-related factors, such as timing, mechanism of injury, player reporting characteristics (eg, immediate or delayed reporting, presence of delayed symptom onset), and acute indicators of injury severity (eg, observed mental status and level of consciousness) (see the Appendix, available in the online version of this article). A detailed description of the CARE Consortium methods is provided elsewhere.5 Institutional review board approval was obtained by the lead study site, and the Human Research Protection Office of the US Department of Defense further reviewed and approved the CARE Consortium study protocol. Individual sites also obtained local institutional review board and subsequent Human Research Protection Office approval.
Participants
The initial data set contained information from 815 SRCs occurring between August 2014 and September 2016. Our final analyses included 506 SRCs sustained across 18 sports at 22 institutions: 62.1% of initial data set, 311 men (61.5%), 193 women (38.2%), 2 unknown (0.3%). Figure 1 describes exclusion criteria and the process of case removal. Athletes with >1 concussion (“repeat concussions”) had only their initial concussion included so that each participant contributed equally to the data set.
Figure 1.
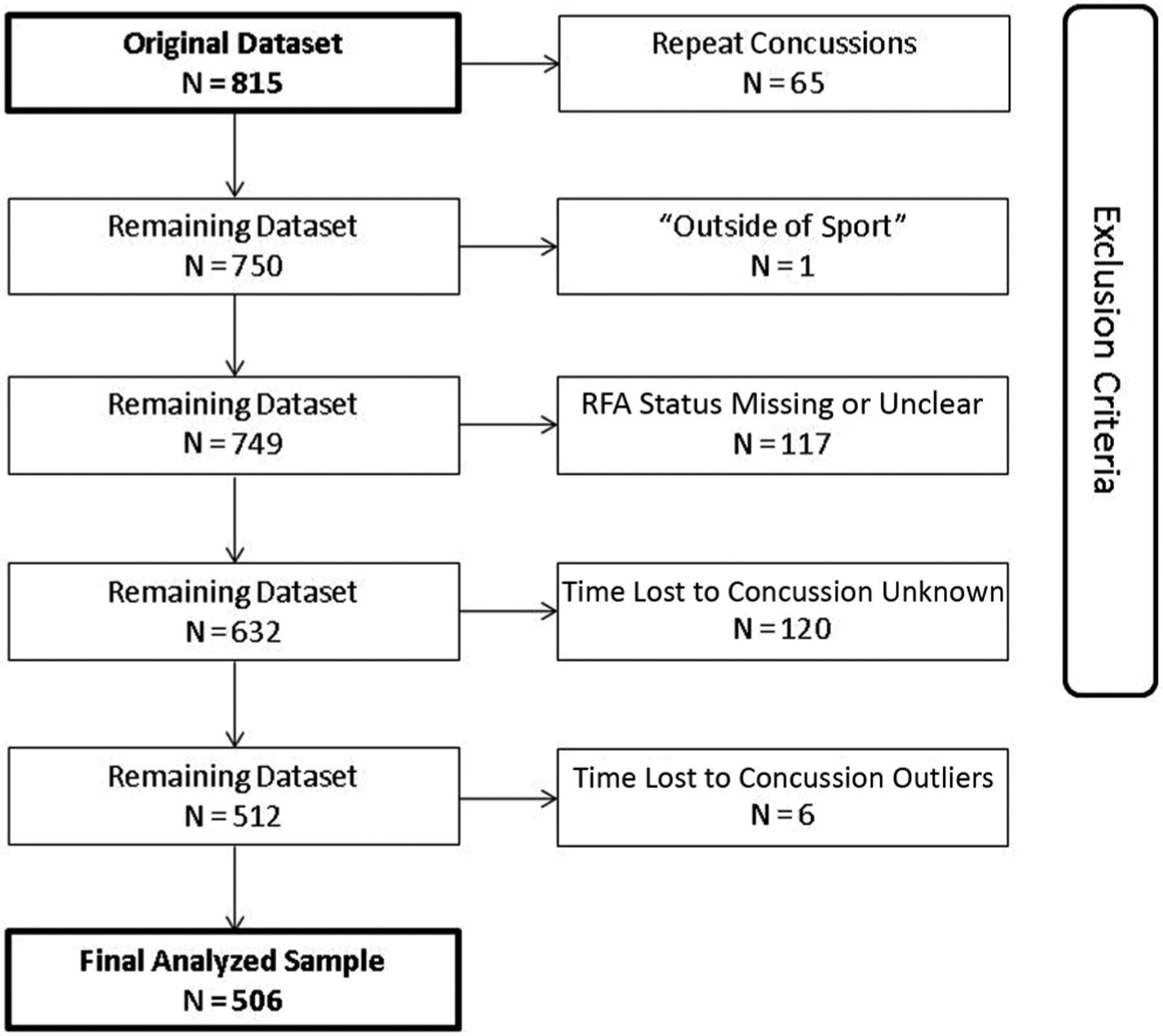
Process of case removal from the initial sport-related concussion data set before analyses and subsequent outlier removal (time lost to concussion >3 SD above sample mean). RFA, removal from activity.
Measures
Our primary independent variable was removal from activity (RFA) status, coded as immediate RFA (I-RFA) or delayed RFA (D-RFA). We ascertained RFA status via 2 yes/no questions completed by clinicians on the postinjury clinical reporting form: “Did the athlete immediately report the injury?” and “Was the athlete immediately removed from play?” Table 1 describes the RFA classification algorithm and associated sample size based on the clinician responses to these 2 questions. A “yes” response to both questions classified participants as I-RFA (ie, removal from play coincident with concussion-causing impact). A response of “no” to either of the 2 questions warranted classification as D-RFA (ie, removal from play noncoincident with concussion-causing impact). For example, if a clinician responded to “Did the athlete immediately report the injury?” with “no” but responded to “Was the athlete immediately removed from play?” with “yes,” this was interpreted as a scenario where the athlete delayed reporting the injury and thus continued participating in athletic activity but was then removed from activity immediately after eventual evaluation/diagnosis. Cases of missing data for question 1 or 2 with a corresponding “yes” response to the other question were excluded from analyses, since it was unclear if the athlete continued participating, as were cases of missing data for both questions (n = 117).
TABLE 1.
Classification of Athletes Into I-RFA (n = 184) or D-RFA (n = 322) and Associated Sample Sizesa
| “Was the Athlete Immediately Removed From Play?” | “Did the Athlete Immediately Report the Injury?” | ||
|---|---|---|---|
| Yes | No | NA | |
| Yes | I-RFA (n = 184) | D-RFA (n = 34) | — |
| No | D-RFA (n = 39) | D-RFA (n = 217) | D-RFA (n = 13) |
| NA | — | D-RFA (n = 19) | — |
Based on 2 clinician-completed questions regarding removal from play after sport-related concussion. D-RFA, delayed removal from activity; I-RFA, immediate removal from activity; NA, not applicable.
The primary outcome variable was total time lost attributed to SRC (measured in days), beginning on the clinician-reported date and time of concussion and ending on the date on which the athlete received clearance for unrestricted RTP. This represents the combined duration of the time from injury until graduated RTP was begun plus the time required for completion of a graduated RTP protocol through unrestricted RTP for noncollision sport athletes and clearance for full contact participation for collision sport athletes, which corresponds to stage 5 of most standard RTP protocols.34,35 Cases of missing data (n = 120) were excluded, and so were statistical outliers (n = 6, time lost to concussion >3 SD above sample mean). Differences in clinician-indicated duration of symptoms were analyzed in a subset of 429 student-athletes with these data available. This approximately reflects the elapsed time between point of injury and initiation of the RTP protocol, although clinicians may commonly begin early stages of the protocol if an athlete reports only minor symptoms.
We considered covariates that, per previous literature, may lengthen recovery time. These included noninjury-related factors10,19,22,36,39,43,49 and injury-related factors22,25,27,28,35,36 obtained from baseline and postinjury clinical reporting forms. Table 2 describes the sample characteristics. Age was not included as a covariate, given the homogeneity of the sample (mean ± SD, 19.6 ± 1.3 years) and the lack of difference between RFA groups (t500 = .997, P = .313).
TABLE 2.
Sample Demographics and Prevalence of Covariates Stratified by RFA Groupa
| Factor | RFA Group, n (% of Group) | Total, n (% of Sample) | χ2 | P Value | Missing, n (%) | |
|---|---|---|---|---|---|---|
| Immediate (n = 184) | Delayed (n = 322) | |||||
| Noninjury related | ||||||
| Sex | 0.011 | .918 | 2 (0.3) | |||
| Men | 113 (61.4) | 198 (61.5) | 311 (61.5) | |||
| Women | 71 (38.6) | 122 (38.5) | 193 (38.5) | |||
| LD or ADHD | 27 (14.7) | 43 (13.4) | 70 (13.8) | 0.171 | .679 | 0 (0.0) |
| Psych | 8 (4.3) | 24 (7.5) | 32 (6.3) | 1.906 | .167 | 0 (0.0) |
| Migraines | 23 (12.8) | 30 (9.6) | 53 (10.8) | 1.290 | .256 | 13 (2.6) |
| Concussion historyb | 3.919 | .270 | 11 (2.2) | |||
| 0 | 85 (47.0) | 174 (55.4) | 259 (52.3) | |||
| 1 | 71 (39.2) | 97 (30.9) | 168 (33.9) | |||
| 2 | 18 (9.9) | 31 (9.9) | 49 (9.9) | |||
| 3+ | 7 (3.9) | 12 (3.8) | 19 (3.8) | |||
| Baseline, mean ± SD | ||||||
| SCAT3 | 4.7 ± 8.0 | 5.5 ± 8.8 | t = 0.979 | .328 | 25 (4.9) | |
| BSI-18 | 2.4 ± 4.2 | 3.0 ± 4.9 | t = 1.39 | .165 | 20 (4.0) | |
| Injury related | ||||||
| LOC | 16 (8.7) | 9 (2.8) | 25 (5.0) | 8.566 | .003c | 5 (1.0) |
| AOC | 113 (62.8) | 128 (40.5) | 241 (48.6) | 22.771 | <.001c | 9 (1.8) |
| Amnesia | 30 (16.3) | 42 (13.0) | 72 (14.2) | 1.020 | .312 | 0 (0.0) |
| Sportd | 1 (0.2) | |||||
| Football | 56 (33.5) | 111 (66.5) | 167 (33.0) | |||
| Soccer | 39 (50.0) | 39 (50.0) | 78 (15.4) | |||
| Basketball | 22 (34.9) | 41 (65.1) | 63 (12.5) | |||
| Lacrosse | 11 (28.2) | 28 (71.8) | 39 (7.7) | |||
| Water polo | 8 (34.8) | 15 (65.2) | 23 (4.5) | |||
| Volleyball | 7 (35.0) | 13 (65.0) | 20 (4.0) | |||
| Gymnastics | 6 (31.6) | 13 (68.2) | 19 (3.8) | |||
| Ice hockey | 3 (23.1) | 10 (76.9) | 13 (2.6) | |||
| Othere | 32 (38.6) | 51 (61.4) | 83 (16.4) | |||
ADHD, attention-deficit/hyperactivity disorder; AOC, alteration of consciousness; BSI-18, Brief Symptom Inventory (18 items); LD, learning disability; LOC, loss of consciousness; Psych, diagnosed depression or other psychiatric diagnosis; RFA, removal from activity; SCAT3, Sport Concussion Assessment Tool–Third Edition symptom severity score.
Concussion history remained a continuous variable within the linear regression analyses (range, 0–6).
P < .05.
Percentages within the RFA group columns represent the percentage of athletes within that sport who had either immediate or delayed RFA (eg, 33.5% of the football participants in the study had immediate removal, and 66.5% had delayed removal). The percentage in the last column represents the proportion of the overall sample per sport (eg, football participants represented 33.0% of the overall sample).
Other sports: swimming (n = 13), softball (n = 13), wrestling (n = 12), baseball (n = 11), diving (n = 8), field hockey (n = 7), rowing/crew (n = 7), cross-country/track (n = 6), tennis (n = 4), and cheerleading (n = 2).
Data were unavailable for evaluating whether any athletes sustained additional head impacts while continuing to participate after SRC versus just continued exertion. We attempted to address the differentiation by comparing groups of D-RFA athletes based on assumed risk of exposure with additional head impacts within their sport. Football athletes (n = 111) were compared with a group of athletes from sports with lower risk of head impacts (n = 65): basketball (n = 41), swimming (n = 11), cross-country/track (n = 3), baseball (n = 5), rowing/crew (n = 4), and tennis (n = 1).
Secondary outcome variables included clinical concussion measures obtained at 2 acute time points after injury, 0 to 6 hours (time 1) and 24 to 48 hours (time 2), in a subset of the initial sample with complete data (see Table 2 for associated sample sizes). Measures included the Sport Concussion Assessment Tool–Third Edition (SCAT3)36 total number of symptoms and symptom severity score, Brief Symptom Inventory 18 (BSI-18; time 2)9 total score, the Standardized Assessment of Concussion34 (SAC) total score, and Balance Error Scoring System40 (BESS) total score. Acute symptom severity has been associated with recovery duration.22 This factor was not included in the regression model, because <50% of the participants had these data available. Participants with missing symptom data were disproportionately classified as D-RFA (χ2 = 20.597, df = 506, P < .001) and significantly differed on time missed from sport (t504 = 22.239, P = .026) and duration of symptom expression (t427 = 2.993, P = .003). Therefore, we compared RFA groups on acute symptom severity separately with the understanding that these RFA groups may not be representative of the larger sample.
A phenomenon of interest is that of delayed symptom onset after SRC, a factor potentially related to delayed symptom reporting or removal. Thus, in a final analysis, we evaluated our primary outcome variables of time missed from sport and symptom duration in D-RFA and I-RFA student-athletes with and without reported delayed symptom onset.
Statistical Methods
Analyses were performed with SPSS (v 22; IBM). All outcome variables were evaluated for normality. Several outcome variables were significantly skewed and thus transformed before analyses such that the z(skew) statistic was P > .05.
A 3-step hierarchical regression was used to evaluate the unique contribution of RFA status to variance in time lost because of SRC and symptom duration, controlling for noninjury- and injury-related factors (Table 2). All covariates remained in the model regardless of significance at each step.22,35 The same method was used for the analysis based on collision sport participation within the D-RFA group. We examined associations among covariates (noninjury and injury related) and RFA status with chi-square tests and independent samples t tests.
For our secondary aims, we used multivariate analyses of variance to evaluate the effect of RFA group on acute symptom scores (SCAT3 symptom total and severity) at time 1 and time 2 (SCAT3 symptom total and severity and BSI-18). Analyses of variance evaluated main effects and interactions associated with time missed from sport and symptom duration with 2 fixed factors: RFA status and presence/absence of delayed symptom onset. SAC and BESS performances at time 1 and time 2 were compared with independent samples t tests. A priori significance was set at P < .05 for all analyses and Bonferroni-adjusted for multiple comparisons.
RESULTS
After outlier removal, 506 patients were eligible for analyses. The included cases did not differ statistically from excluded cases on predictor or outcome variables. We imputed missing data related to noninjury- and injury-related factors (see Table 2) to preserve the original sample size.14 Twenty imputations were performed. The ranges of values obtained across the 20 imputations are reported when pooled statistics are not provided.
Table 3 provides descriptive statistics for all outcome variables stratified by RFA group. Groups did not differ statistically on any noninjury predictors (P > .05 for all analyses). Athletes with loss of consciousness (LOC) (χ2 = 8.57, df = 501, ϕ = −0.131, small effect size) or alteration of consciousness (χ2 = 22.77, df = 496, ϕ = −0.214, small-medium effect size) at the time of injury were significantly more likely to be in the I-RFA group.
TABLE 3.
Unadjusted Descriptive Statistics of All Outcome Variablesa
| Clinical Outcome | n (% D-RFA) | RFA Group, Mean ± SD | Test Statistic | P Value | Effect Size, d | |
|---|---|---|---|---|---|---|
| Immediate | Delayed | |||||
| Time lost to concussion, d | 506 (63.6) | 12.0 ± 8.2 | 14.7 ± 9.8 | β = 0.154–0.161 | .001b | |
| Duration of symptoms, d | 429 (63.6) | 4.8 ± 3.7 | 6.8 ± 5.2 | β = 0.217–0.223 | <.001b | |
| Time 1 (0–6 h) | ||||||
| SCAT3 symptom total | 318 (51.6) | 10.1 ± 4.9 | 11.8 ± 5.4 | 5.080c | .003d | .330 |
| SCAT3 symptom severity | 26.3 ± 19.3 | 31.5 ± 20.9 | .024d | .259 | ||
| SAC | 323 (51.7) | 26.3 ± 2.9 | 26.1 ± 3.0 | 0.765e | .445 | .068 |
| BESS | 246 (48.4) | 16.2 ± 8.3 | 16.9 ± 9.0 | −0.505e | .614 | .081 |
| Time 2 (24–48 h) | ||||||
| SCAT3 symptom total | 232 (53.9) | 8.4 ± 5.8 | 10.6 ± 6.4 | 4.319c | .009d | .360 |
| SCAT3 symptom severity | 19.3 ± 20.2 | 25.6 ± 22.7 | .027d | .293 | ||
| BSI-18 | 4.3 ± 5.7 | 7.1 ± 8.0 | .002d | .403 | ||
| SAC | 248 (52.8) | 26.9 ± 2.5 | 26.4 ± 2.4 | 1.393e | .165 | .204 |
| BESS | 236 (53.4) | 12.9 ± 6.6 | 14.7 ± 8.1 | −1.860e | .064 | .244 |
BESS, Balance Error Scoring System; BSI-18, Brief Symptom Inventory (18 items); D-RFA, delayed removal from activity; RFA, removal from activity; SAC, Standardized Assessment of Concussion; SCAT3, Sport Concussion Assessment Tool–Third Edition.
Significance value corresponds to the unique association of the RFA group and the associated clinical outcome, controlling for all other predictors in the final regression model.
Multivariate analysis of variance (Pillai trace F statistic) with the RFA group as the fixed factor and with clinical symptom assessments for each time point as the dependent variables.
Significance value corresponds to univariate group comparison.
Independent samples t test.
Effect of RFA Status on Recovery Times
Neither noninjury- nor injury-related factors significantly predicted time lost because of SRC (model 1: R2 = 0.012–0.021, F7,498 = 0.900–1.522, P = .157–.506; model 2: R2 change = 0.004 [all imputations], P = .518–.613, F10,495 = 0.821–1.274, P = .242–.609). None of the individual model 1 or model 2 predictors significantly predicted time lost attributed to SRC (P > .05 for all factors). Results were similar when symptom duration was used as the outcome variable.
RFA status significantly improved upon model 2 (model 3: R2 change = 0.022–0.024, P < .001 to P = .001), and model 3 significantly predicted time lost because of SRC (F11,494 = 1.865–1.294, P = .010–.042). Controlling for all other factors in the model, RFA status was the only factor to significantly predict time lost attributed to SRC (B = 2.95 [pooled], β = 0.154–0.161, P < .001 to P = .001, small effect size). Immediate removal from athletic activity resulted in approximately 3 fewer days lost because of SRC than that seen in D-RFA participants. Similarly, RFA status significantly improved upon model 2 in predicting duration of symptom expression (R2 change = 0.044–0.046, P < .001 [all imputations]). Controlling for all other factors in the model, RFA status was the only factor to significantly predict symptom duration (B = 2.02 [pooled], β = 0.217–0.223, P < .001 [all imputations], small effect size).
Group differences in recovery trajectories are shown in Figure 2. We evaluated the relative risk (RR) of I-RFA participants missing ≥14 days and ≥21 days from sport participation. I-RFA athletes had a 39% lower likelihood of taking ≥14 days (χ2 = 12.663, df = 2, 506, P < .001, ϕ = 0.158, RR = .614, small-medium effect size) and 47% lower likelihood of taking ≥21 days (χ2 = 6.620, df = 2, 506, P = .010, ϕ = 0.121, RR = 0.534, small effect size) to achieve full RTP clearance than D-RFA athletes.
Figure 2.
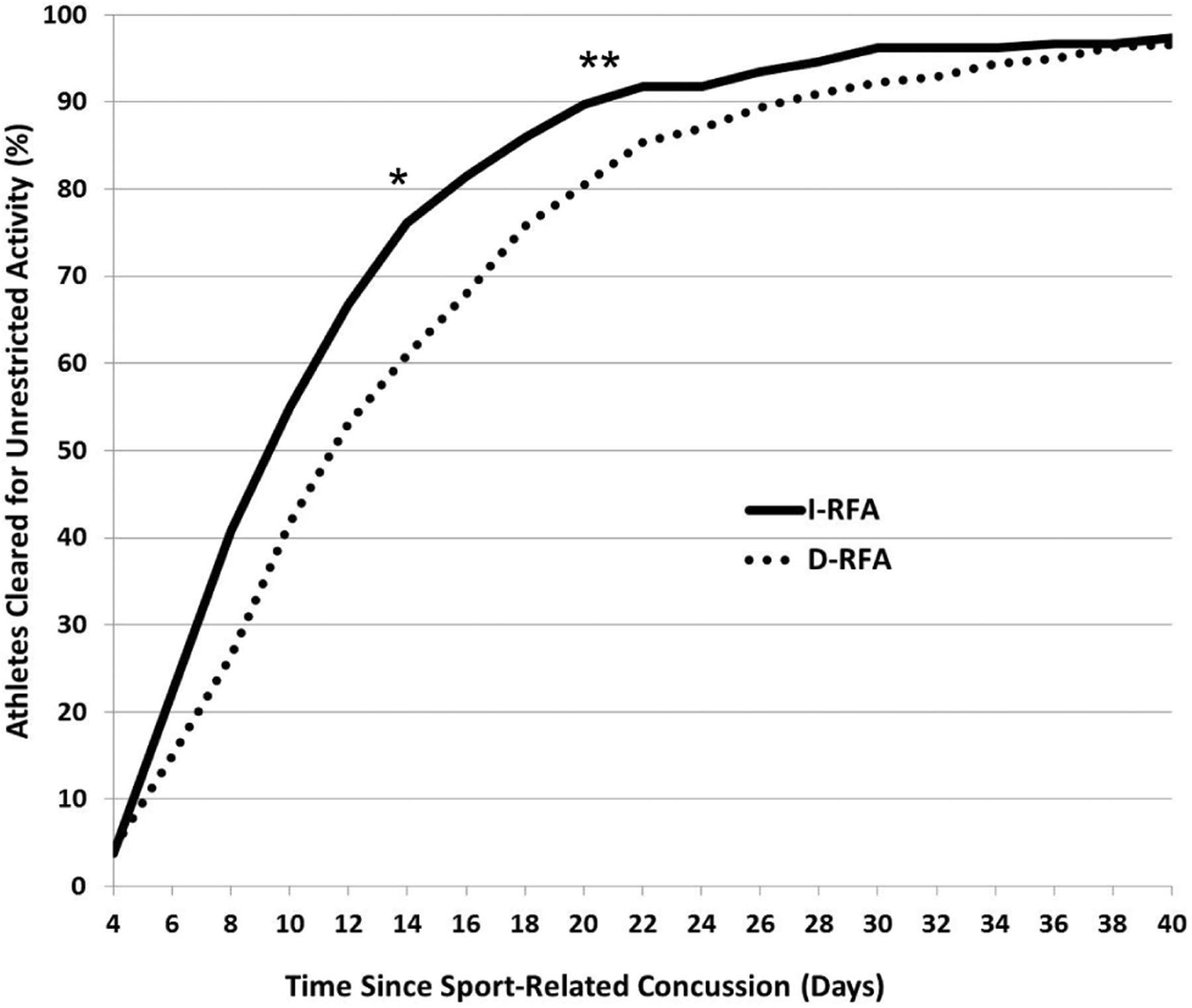
Recovery trajectories of the percentage of athletes achieving full medical clearance based on the number of days after a sport-related concussion: I-RFA vs D-RFA. *I-RFA group at 39% lower likelihood of missing ≥14 days (relative risk = 0.614, P < .001). **I-RFA at 47% lower likelihood of missing ≥21 days (relative risk = 0.534, P = .010). D-RFA, delayed removal from activity; I-RFA, immediate removal from activity.
Football participation was not associated with longer time lost because of SRC when compared with participation in sports with low risk of repeat head impacts (P > .05 for all regression statistics). Results were similar when the higher-risk sport group was broadened to include soccer and hockey student-athletes.
Effect of RFA Status on Acute Symptoms and Clinical Measures
At time 1, there was a significant omnibus effect of RFA group on SCAT3 symptom evaluation outcome scores (Pillai trace F2,315 = 5.080, P = .007, partial η2 = 0.031). I-RFA student-athletes reported significantly fewer SCAT3 symptoms (P = .003, Cohen d = 0.330, small-medium effect size) and significantly lower SCAT3 symptom severity (P = .024, Cohen d = 0.259, small-medium effect size). Groups did not differ on SAC or BESS scores at time 1. BSI-18 was not performed at time 1.
At time 2, there was also a significant omnibus effect of RFA group on acute symptom scores (Pillai trace F3,228 = 4.319, P = .006, partial η2 = .054). I-RFA student-athletes reported significantly fewer SCAT3 symptoms (P = .009, Cohen d = 0.360, small-medium effect size), significantly lower SCAT3 symptom severity (P = .027, Cohen d = 0.293, small-medium effect size), and significantly lower BSI-18 total score (P = .002, Cohen d = 0.403, small-medium effect size). Groups did not differ on SAC or BESS scores at time 2.
Effect of Delayed Symptom Onset
A significantly higher proportion of D-RFA student-athletes reported delayed symptom onset (χ2 = 54.089, df = 497, P < .001, φ = 0.330, moderate effect size). Of 318 D-RFA student-athletes analyzed, 153 (48.1%) had delayed symptom onset, as opposed to just 27 of 179 (15.1%) I-RFA student-athletes with available data. For time missed from sport and duration of symptom expression, analyses of variance revealed a main effect of RFA group only (F1,493 = 6.916, P = .009; F1,417 = 6.984, P = .009, respectively). There was no main effect of delayed symptom onset and no interaction effect of delayed symptom onset and RFA group on recovery outcomes (P > .05 for all effects).
DISCUSSION
Student-athletes immediately removed from athletic activity after an SRC had significantly less time lost—approximately 3 days—from sport when compared with athletes with D-RFA. They also experienced approximately 2 days’ shorter duration of symptoms. The current study extended previous studies by controlling for several noninjury- and injury-related factors previously shown to moderate recovery from brain injury.† None of these other factors predicted time lost in our sample. Immediate removal from athletic activity was associated with a 39% lower likelihood of requiring ≥14 days and 47% lower likelihood of requiring ≥21 days to achieve full medical clearance for sport. Our secondary analyses also indicated that student-athletes with immediate removal had significantly fewer and less severe symptoms (SCAT3 and BSI-18) reported 0 to 6 and 24 to 48 hours after SRC. A recent systematic review concluded that acute symptom severity is the most consistent predictor of SRC clinical recovery.22 This factor was not included in our overall regression model owing to missing data in over half of our sample, which disproportionately affected the D-RFA group as its time of reporting was frequently outside the acute period. However, these exploratory findings indicate a potential relationship between timing of removal from athletic participation and acute symptoms, both of which have growing support as prognostic factors.
Integration With Existing Literature
Our findings in this large multisite sample build on the results of recent investigations on the effects of continuing to play after SRC. In a retrospective chart review of 97 collegiate athletes, Asken and colleagues1 reported that athletes who were immediately removed from play took approximately 5 fewer days to return to sport versus those with delayed removal from athletic activity (6.8 vs 12.3 days). In a prospective study of 64 adolescent athletes, Elbin and colleagues12 reported that athletes who were immediately removed recovered 22 days sooner than those with delayed removal. The size of this difference may reflect the younger age of the sample, since younger age is a factor that is associated with a longer recovery time. Elbin et al12 also reported that athletes with immediate removal performed better on a computerized neurocognitive measure and had less severe symptoms. We found similar effects on symptom severity despite different measurement time points (1–7 days and 8–30 days after injury vs 0–6 hours and 24–48 hours in the present study). While our results did not support findings of cognitive score disparities, the current study used only a brief cognitive screening measure. Terwilliger et al46 reported an effect of additional head impacts sustained shortly after SRC. This was indirectly evaluated in the present study by comparing football student-athletes with D-RFA and student-athletes from sports with little to no risk of repetitive head impacts, but there was no effect. Importantly, Terwilliger et al46 specifically identified individuals who reported sustaining a head impact that worsened already-existing SRC symptoms before removal from play, which may explain the discrepant findings. Additionally, participants from the Terwilliger et al46 and Elbin et al12 studies were recruited from a specialty clinic and may not represent concussed athletes who did not receive specialty referral.
Clinical and preclinical research offers plausible explanations for our results and supports a “window of vulnerability” after concussion.24 Several animal studies reported adverse outcomes after physical exertion immediately after brain injury believed to result from heightened neuroinflammation and excitotoxicity.15,16 Such findings could be attributable to other physiologic factors that compound the immediate effects of SRC, including elevated systemic blood pressure, heart rate, and/or body temperature.41,48 Data suggest that prolonged rest may also increase risk of protracted recovery,8,13,44,47 while properly timed physical activity could improve outcomes via mechanisms such as promoting upregulation of proteins important for neural repair and homeostatic restoration.17,38,42 Consensus guidelines recommend a 24- to 48-hour period of relative rest immediately after SRC.35 Future studies would do well to retrospectively quantify a dose-response effect for the amount of time that an athlete continues participating after a concussion and to measure adverse outcomes such as recovery time and functional impairment. This could also be validated prospectively through animal models. Other factors should also be considered, such as the intensity and nature of physical exertion and whether additional head impacts are incurred.45
Educational and Clinical Considerations
Our data indicate that 63.6% (322 of 506) of athletes included in the sample had D-RFA after SRC. This percentage may overestimate the true incidence of this problem, since 117 cases were excluded because of missing data for RFA determination. Regardless, the rate in the current study is higher than that from Asken and colleagues,1 who reported that approximately half (51.5%) of their retrospectively identified sample fit this description. There are many reasons why an athlete may not be immediately removed from participation after a concussion, so these high rates of delayed removal likely do not simply reflect athletes who willfully withheld symptoms or clinicians’ non-compliance with concussion management regulations. For example, we found a high proportion of individuals with delayed symptom onset within the D-RFA group, possibly explaining why some student-athletes may continue participating after an SRC. If symptoms are not present immediately after a head impact, athletes may have difficulty associating later symptom development with a specific impact. However, the data showed that even athletes with delayed symptom onset who were removed from activity immediately upon recognition of symptoms and diagnosis had shorter symptom duration and time missed from sport than did athletes with delayed removal. Data suggest that removal at the point of symptom onset, even if delayed after the injurious impact, gives the best chance of avoiding prolonged recovery. The current findings underscore the importance of educating athletes, coaches, and medical personnel about symptom reporting and identification immediately after SRC. In so doing, we may help reduce the chance that an athlete is removed from athletic and academic participation for extended periods, potentially reducing or avoiding adverse outcomes such as depression, stress, or anxiety.29,37
In the current study, there was a disproportionate prevalence of LOC and alteration of consciousness in the I-RFA group. This is expected since these are overt signs of injury that are easily identified by medical personnel, resulting in immediate removal. Although prolonged LOC may reflect more serious brain injuries, the role of brief LOC in predicting severity of SRC is less clear.6,22,27,28,36 In our study, LOC and alteration of consciousness were less prevalent in the D-RFA group, yet the D-RFA student-athletes missed more time than did their I-RFA counterparts. This further argues that delayed removal per se is a risk factor for prolonged recovery time.
Limitations
A primary limitation of the present study pertains to missing data. The original data set contained 815 participants with a diagnosed concussion, but only 506 had the requisite predictor variable data (sufficient for determining RFA status) and outcome data (time missed from sport excluding outliers). The preceding analyses and results are predicated upon the assumption that the 506 participants were representative of the original 815, and we have no clear reason to suspect that data were not missing at random. However, missing data may systematically influence findings either in support of or in opposition to the null hypothesis, generally increasing uncertainty of results. Prior studies reporting similar results raise confidence in the present findings, but future research efforts should prioritize data completeness to ensure representativeness of the sample.
This study relied on clinician report and athlete self-report for many of the predictors used in our analyses. Particularly for athletes with longer participation in athletic activity after SRC, clinicians may have had to estimate the date and time of the injury. Athletes who continued participation may include those who attempted to play through symptoms but then reported their symptoms after they did not resolve. In contrast, athletes who initially hid their symptoms but may have spontaneously recovered and never reported them would not be included within the data set. Similarly, athletes who immediately stopped participating after SRC may hide the fact that they had actually begun experiencing symptoms before reporting their injury to medical staff. Clinician interpretation of how to define/record timing of symptom report and timing of removal may result in inconsistent group classification. For example, 27 student-athletes were classified as “immediate removal” but also indicated delayed symptom onset. Conceivably, the clinician thought a classification of “immediately reported” was warranted if the student-athlete reported the injury as soon as she or he experienced symptoms, even if the SRC-causing mechanism occurred earlier in the event. Another scenario is that of a clinician’s monitoring or evaluating an asymptomatic athlete after observation of a significant impact as a precautionary measure, and the athlete then develops symptoms while being monitored. In this case, it may not be considered “immediate report,” but the athlete had still ceased physical activity immediately after getting concussed. Other clinicians may define this differently, and future studies should refine this classification procedure to yield more homogeneous samples. Last, considering a diagnostic certainty rating as reported retrospectively by the clinician may be beneficial in future research since the nonspecificity of concussion symptoms2,3,23 and/or delayed symptom onset could introduce clinical uncertainty regarding symptom attribution and contribute to further heterogeneity within the study sample.
CONCLUSION
Immediate removal from athletic participation after SRC may optimize recovery (shorter symptom duration and less total time missed from sport) and result in less severe acute symptoms in collegiate athletes. Removal from sport should be considered among the many factors traditionally considered important predictors of recovery, including pre-existing psychiatric disorder, prior SRC, and LOC at time of injury.
Athletes should engage medical staffs and report symptoms immediately if they are experiencing concussion-like symptoms, to RTP faster and to mitigate potentially negative effects of physical exertion or additional head impacts within this “window of vulnerability.”
Supplementary Material
ACKNOWLEDGMENT
The authors thank all site-specific primary investigators within the CARE Consortium for their efforts in overseeing data collection and quality at their respective institutions.
Opinions, interpretations, conclusions, and recommendations are those of the authors and are not necessarily endorsed by the Department of Defense (Defense Health Program funds).
One or more of the authors has declared the following potential conflict of interest or source of funding: This publication was supported in part by the Grand Alliance Concussion Assessment, Research, and Education Consortium, which receives funding from the National Collegiate Athletic Association and the Department of Defense. The US Army Medical Research Acquisition Activity is the awarding and administering acquisition office. This work was supported by the Office of the Assistant Secretary of Defense for Health Affairs through the Psychological Health and Traumatic Brain Injury Program (award W81XWH-14-2-0151). The study was also supported by National Center for Advancing Translational Sciences (UL1 RR029890) and Veterans Health Administration Office of Research and Development (1 I21 RX001730; R.M.B.).
Footnotes
REFERENCES
- 1.Asken BM, McCrea MA, Clugston JR, Snyder AR, Houck ZM, Bauer RM. “Playing through it”: delayed reporting and removal from athletic activity after concussion predicts prolonged recovery. J Athl Train. 2016;51(4):329–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Asken BM, Snyder AR, Clugston JR, Gaynor LS, Sullan MJ, Bauer RM. Concussion-like symptom reporting in non-concussed collegiate athletes [published online December 1, 2017]. Arch Clin Neuropsychol. doi: 10.1093/arclin/acx018 [DOI] [PubMed] [Google Scholar]
- 3.Asken BM, Snyder AR, Smith MS, Zaremski JL, Bauer RM. Concussion-like symptom reporting in non-concussed adolescent athletes. Clin Neuropsychol. 2017;31(1):138–153. [DOI] [PubMed] [Google Scholar]
- 4.Baugh CM, Kroshus E, Daneshvar DH, Stern RA. Perceived coach support and concussion symptom-reporting: differences between freshmen and non-freshmen college football players. J Law Med Ethics. 2014;42(3):314–322. [DOI] [PubMed] [Google Scholar]
- 5.Broglio SP, McCrea MA, McAllister T, et al. A national study of the effects of concussion in collegiate athletes and US military service academy members: the NCAA-DoD Concussion Assessment, Research, and Education (CARE) Consortium structure and methods. Sports Med. 2017;47(7):1437–1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cantu RC. Posttraumatic retrograde and anterograde amnesia: pathophysiology and implications in grading and safe return to play. J Athl Train. 2001;36(3):244. [PMC free article] [PubMed] [Google Scholar]
- 7.Chrisman SP, Quitiquit C, Rivara FP. Qualitative study of barriers to concussive symptom reporting in high school athletics. J Adolesc Health. 2013;52(3):330–335, e333. [DOI] [PubMed] [Google Scholar]
- 8.Collins MW, Kontos AP, Okonkwo DO, et al. Statements of agreement from the Targeted Evaluation and Active Management (TEAM) approaches to treating concussion meeting held in Pittsburgh, October 15–16, 2015. Neurosurgery. 2016;79(6):912–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Derogatis LR. BSI 18, Brief Symptom Inventory 18: Administration, Scoring and Procedures Manual. Bloomington, MN: NCS Pearson Inc; 2001. [Google Scholar]
- 10.Dvorak J, McCrory P, Kirkendall DT. Head injuries in the female football player: incidence, mechanisms, risk factors and management. Br J Sports Med. 2007;41(suppl 1):I44–I46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eisenberg MA, Andrea J, Meehan W, Mannix R. Time interval between concussions and symptom duration. Pediatrics. 2013; 132(1):1–10. [DOI] [PubMed] [Google Scholar]
- 12.Elbin R, Sufrinko A, Schatz P, et al. Removal from play after concussion and recovery time. Pediatrics. 2016;138(3):e20160910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gibson S, Nigrovic LE, O’Brien M, Meehan WP 3rd. The effect of recommending cognitive rest on recovery from sport-related concussion. Brain Inj. 2013;27(7–8):839–842. [DOI] [PubMed] [Google Scholar]
- 14.Graham JW, Olchowski AE, Gilreath TD. How many imputations are really needed? Some practical clarifications of multiple imputation theory. Prev Sci. 2007;8(3):206–213. [DOI] [PubMed] [Google Scholar]
- 15.Griesbach GS. Exercise after traumatic brain injury: is it a double-edged sword? PM R. 2011;3(6)(suppl 1):S64–S72. [DOI] [PubMed] [Google Scholar]
- 16.Griesbach GS, Gomez-Pinilla F, Hovda DA. The upregulation of plasticity-related proteins following TBI is disrupted with acute voluntary exercise. Brain Res. 2004;1016(2):154–162. [DOI] [PubMed] [Google Scholar]
- 17.Griesbach GS, Hovda D, Molteni R, Wu A, Gomez-Pinilla F. Voluntary exercise following traumatic brain injury: brain-derived neurotrophic factor upregulation and recovery of function. Neuroscience. 2004;125(1):129–139. [DOI] [PubMed] [Google Scholar]
- 18.Guerriero RM, Proctor MR, Mannix R, Meehan WP. Epidemiology, trends, assessment and management of sport-related concussion in United States high schools. Curr Opin Pediatr. 2012;24(6):696–701. [DOI] [PubMed] [Google Scholar]
- 19.Guskiewicz KM, McCrea M, Marshall SW, et al. Cumulative effects associated with recurrent concussion in collegiate football players: the NCAA Concussion Study. JAMA. 2003;290(19):2549–2555. [DOI] [PubMed] [Google Scholar]
- 20.Heyer GL, Schaffer CE, Rose SC, Young JA, McNally KA, Fischer AN. Specific factors influence postconcussion symptom duration among youth referred to a sports concussion clinic. J Pediatr. 2016;174:33–38, e32. [DOI] [PubMed] [Google Scholar]
- 21.Hootman JM, Dick R, Agel J. Epidemiology of collegiate injuries for 15 sports: summary and recommendations for injury prevention initiatives. J Athl Train. 2007;42(2):311–319. [PMC free article] [PubMed] [Google Scholar]
- 22.Iverson GL, Gardner AJ, Terry DP, et al. Predictors of clinical recovery from concussion: a systematic review. Br J Sports Med. 2017;51(12):941–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iverson GL, Silverberg ND, Mannix R, et al. Factors associated with concussion-like symptom reporting in high school athletes. JAMA Pediatr. 2015;169(12):1132–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kamins J, Bigler E, Covassin T, et al. What is the physiological time to recovery after concussion? A systematic review. Br J Sports Med. 2017;51(12):935–940. [DOI] [PubMed] [Google Scholar]
- 25.Leininger BE, Gramling SE, Farrell AD, Kreutzer JS, Peck EA. Neuropsychological deficits in symptomatic minor head injury patients after concussion and mild concussion. J Neurol Neurosurg Psychiatry. 1990;53(4):293–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lincoln AE, Caswell SV, Almquist JL, Dunn RE, Norris JB, Hinton RY. Trends in concussion incidence in high school sports: a prospective 11-year study. Am J Sports Med. 2011;39(5):958–963. [DOI] [PubMed] [Google Scholar]
- 27.Lovell MR, Collins MW, Iverson GL, et al. Recovery from mild concussion in high school athletes. J Neurosurg. 2003;98(2):296–301. [DOI] [PubMed] [Google Scholar]
- 28.Lovell MR, Iverson GL, Collins MW, McKeag D, Maroon JC. Does loss of consciousness predict neuropsychological decrements after concussion? Clin J Sport Med. 1999;9(4):193–198. [DOI] [PubMed] [Google Scholar]
- 29.Mainwaring LM, Bisschop SM, Green RE, et al. Emotional reaction of varsity athletes to sport-related concussion. J Sport Exerc Psychol. 2004;26(1):119–135. [Google Scholar]
- 30.McClincy MP, Lovell MR, Pardini J, Collins MW, Spore MK. Recovery from sports concussion in high school and collegiate athletes. Brain Inj. 2006;20(1):33–39. [DOI] [PubMed] [Google Scholar]
- 31.McCrea M, Guskiewicz K, Randolph C, et al. Incidence, clinical course, and predictors of prolonged recovery time following sport-related concussion in high school and college athletes. J Int Neuropsychol Soc. 2013;19(1):22–33. [DOI] [PubMed] [Google Scholar]
- 32.McCrea M, Guskiewicz KM, Marshall SW, et al. Acute effects and recovery time following concussion in collegiate football players: the NCAA Concussion Study. JAMA. 2003;290(19):2556–2563. [DOI] [PubMed] [Google Scholar]
- 33.McCrea M, Hammeke T, Olsen G, Leo P, Guskiewicz K. Unreported concussion in high school football players: implications for prevention. Clin J Sports Med. 2004;14(1):13–17. [DOI] [PubMed] [Google Scholar]
- 34.McCrea M, Kelly JP, Randolph C, et al. Standardized assessment of concussion (SAC): on-site mental status evaluation of the athlete. J Head Trauma Rehabil. 1998;13(2):27–35. [DOI] [PubMed] [Google Scholar]
- 35.McCrory P, Meeuwisse W, Dvorak J, et al. Consensus statement on concussion in sport–the 5th International Conference on Concussion in Sport held in Berlin, October 2016 [published online April 26, 2017]. Br J Sports Med. doi: 10.1136/bjsports-2017-097699 [DOI] [PubMed] [Google Scholar]
- 36.McCrory P, Meeuwisse WH, Aubry M, et al. Consensus statement on concussion in sport: the 4th International Conference on Concussion in Sport held in Zurich, November 2012. Br J Sports Med. 2013;47(5):250–258. [DOI] [PubMed] [Google Scholar]
- 37.Meares S, Shores EA, Taylor AJ, et al. The prospective course of postconcussion syndrome: the role of mild traumatic brain injury. Neuropsychology. 2011;25(4):454. [DOI] [PubMed] [Google Scholar]
- 38.Mychasiuk R, Hehar H, Ma I, Candy S, Esser MJ. Reducing the time interval between concussion and voluntary exercise restores motor impairment, short-term memory, and alterations to gene expression. Eur J Neurosci. 2016;44(7):2407–2417. [DOI] [PubMed] [Google Scholar]
- 39.Nelson LD, Tarima S, LaRoche AA, et al. Preinjury somatization symptoms contribute to clinical recovery after sport-related concussion. Neurology. 2016;86(20):1856–1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Riemann BL, Guskiewicz KM. Effects of mild head injury on postural stability as measured through clinical balance testing. J Athl Train. 2000;35(1):19. [PMC free article] [PubMed] [Google Scholar]
- 41.Sakurai A, Atkins CM, Alonso OF, Bramlett HM, Dietrich WD. Mild hyperthermia worsens the neuropathological damage associated with mild traumatic brain injury in rats. J Neurotrauma. 2012;29(2): 313–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schneider KJ, Leddy JJ, Guskiewicz KM, et al. Rest and treatment/rehabilitation following sport-related concussion: a systematic review. Br J Sports Med. 2017;51(12):930–934. [DOI] [PubMed] [Google Scholar]
- 43.Silverberg ND, Gardner AJ, Brubacher JR, Panenka WJ, Li JJ, Iverson GL. Systematic review of multivariable prognostic models for mild traumatic brain injury. J Neurotrauma. 2015;32(8):517–526. [DOI] [PubMed] [Google Scholar]
- 44.Silverberg ND, Iverson GL. Is rest after concussion “the best medicine?” Recommendations for activity resumption following concussion in athletes, civilians, and military service members. J Head Trauma Rehabil. 2013;28(4):250–259. [DOI] [PubMed] [Google Scholar]
- 45.Sye G, Sullivan SJ, McCrory P. High school rugby players’ understanding of concussion and return to play guidelines. Br J Sports Med. 2006;40(12):1003–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Terwilliger VK, Pratson L, Vaughan CG, Gioia GA. Additional post-concussion impact exposure may affect recovery in adolescent athletes. J Neurotrauma. 2016;33(8):761–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thomas DG, Apps JN, Hoffmann RG, McCrea M, Hammeke T. Benefits of strict rest after acute concussion: a randomized controlled trial. Pediatrics. 2015;135:239–245. [DOI] [PubMed] [Google Scholar]
- 48.Titus DJ, Furones C, Atkins CM, Dietrich WD. Emergence of cognitive deficits after mild traumatic brain injury due to hyperthermia. Exp Neurol. 2015;263:254–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wäljas M, Iverson GL, Lange RT, et al. A prospective biopsychosocial study of the persistent post-concussion symptoms following mild traumatic brain injury. J Neurotrauma. 2015;32(8):534–547. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


