Abstract
Objective
Oral healthcare professionals are frequently confronted with patients using drugs on a daily basis. These drugs can cause taste disorders as adverse effect. The literature that discusses drug‐induced taste disorders is fragmented. This article aims to support oral healthcare professionals in their decision making whether a taste disorder can be due to use of drugs by providing a comprehensive overview of drugs with taste disorders as an adverse effect.
Materials and methods
The national drug information database for Dutch pharmacists, based on scientific drug information, guidelines, and summaries of product characteristics, was analyzed for drug‐induced taste disorders. “MedDRA classification” and “Anatomic Therapeutical Chemical codes” were used to categorize the results.
Results
Of the 1,645 drugs registered in the database, 282 (17%) were documented with “dysgeusia” and 61 (3.7%) with “hypogeusia.” Drug‐induced taste disorders are reported in all drug categories, but predominantly in “antineoplastic and immunomodulating agents,” “antiinfectives for systemic use,” and “nervous system.” In ~45%, “dry mouth” coincided as adverse effect with taste disorders.
Conclusion
Healthcare professionals are frequently confronted with drugs reported to cause taste disorders. This article provides an overview of these drugs to support clinicians in their awareness, diagnosis, and treatment of drug‐induced taste disorders.
Keywords: drug‐induced taste disorders, drugs adverse effects, dysgeusia, hypogeusia, oral adverse effects
1. INTRODUCTION
The global consumption of drugs to treat acute and chronic diseases continues to increase (WHO, 2011). Inevitably, healthcare professionals are frequently confronted with patients using one or more drugs on a daily basis. These drugs can cause adverse effects in the oral region such as xerostomia, hyposalivation, mucositis, and taste disorders.
Due to the large number of different drugs available and their wide range of adverse effects, it is difficult and time‐consuming for healthcare professionals to take all the potential consequences into account during their daily practice. To support oral healthcare professionals in their decision making, the journal of Oral Diseases will publish a series of articles discussing the most frequent adverse effects of drugs in the oral region. The first paper in this series discusses drug‐induced taste disorders (DITD).
Fark, Hummel, Hahner, Nin, and Hummel (2013) divided taste disorders into quantitative taste disorders and qualitative taste disorders. Quantitative taste disorders include hypergeusia (an abnormally heightened sense of taste), normogeusia (a normal sense of taste), hypogeusia (an abnormally lowered sense of taste), and ageusia (a lacking sense of taste). Qualitative taste disorders are dysgeusia (a distortion in sense taste) and phantogeusia (a taste perception without a stimulus) (Fark et al., 2013). Although disturbances in taste seem harmless, they can interfere with a patients’ social behavior by avoiding dinners or lead to a change in diet which can, among others, cause weight loss, nutrient deficiencies, or overweight due to excessive use of salt and sugar to compensate bad flavors (Noel, Sugrue, & Dando, 2017). As such, taste disorders can lead to a significant reduction in the quality of life (Ponticelli et al., 2017). Therefore, it is important that oral healthcare professionals are aware of the possible causes and treatment modalities of taste disorders. Adverse effects of drugs account for 9%–22% of the taste disorders (Fark et al., 2013; Hamada, Endo, & Tomita, 2002). This article aims to support oral healthcare professionals in their decision making whether a taste disorder can be due to use of drugs by providing a comprehensive overview of drugs documented with taste disorders as an adverse effect.
2. MATERIALS AND METHODS
2.1. Data source
The Informatorium Medicamentorum (IM) of the Royal Dutch Pharmacists Association (KNMP) is the leading national drug information database and reference work for pharmacists in the Netherlands. This database is based on scientific drug information, guidelines, and summaries of product characteristics (SmPCs) (KNMP, 2019). The IM is updated every 2 weeks with the latest available information from scientific publications, warnings of authorities, and SmPCs of the European Medicines Agency and Medicines Evaluation Board in the Netherlands.
The IM was last searched on August 1, 2018, and all data regarding adverse effects available that time were included in this study. Of each drug, the category “side effects” from the IM was searched for taste disorders and synonyms (e.g., dysgeusia).
The following characteristics of drugs causing DITD were registered: generic name of the drug, term of the adverse effect, incidence of the adverse effect, and Anatomic Therapeutical Chemical (ATC) codes of the drug. The ATC classification was developed by the World Health Organization and categorizes all active substances in drugs according to a hierarchy with five levels. It serves as a tool for exchanging data on drug use on a national and international level (WHO, 2003). It is worth noting that one active substance can be used in different drugs with different treatment goals. Therefore, it is possible that one active substance (e.g., miconazole) has several ATC codes (Figure 1).
Figure 1.
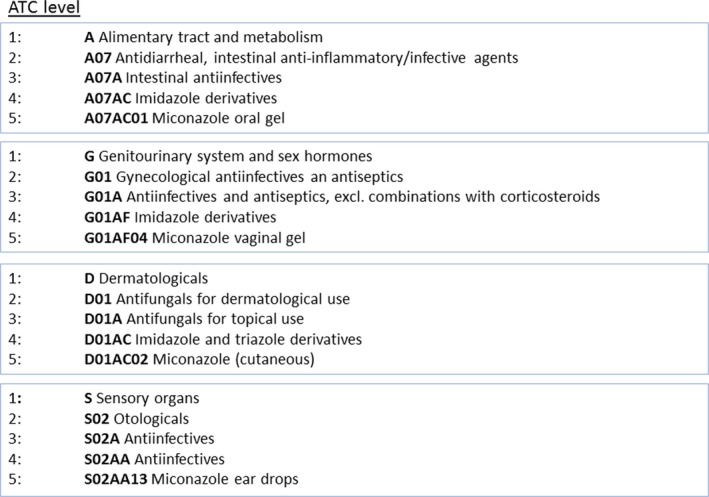
Hierarchy of ATC levels for miconazole [Colour figure can be viewed at http://wileyonlinelibrary.com]
Originally, the terms used to describe one adverse effect (e.g., taste disorders) in the SmPCs varied between drugs and throughout the years. In order to create a standardized structured database, the MedDRA classification was manually applied after the selection of drugs causing DITD. The MedDRA classification is developed by the International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human and endeavors to standardize all international medical terminology, including terms for adverse effects (Meddra, 2019). The MedDRA classification is a hierarchical system that distinguishes five levels in the categorization of medical terminology. The most specific level is the “Lowest Level Term (LLT)” and the next level is called the “Preferred Term (PT).” Each LLT is directly linked to only one PT. Each PT is linked to at least one LLT (itself) and sometimes several synonyms of the LLT. In Figure 2, the PT “Hypogeusia” is presented with its LLTs. After the selection of drugs related to DITD from the IM, the adverse effect terms were first matched in accordance with the support document (Meddra, 2018), with the most applicable LLT in Dutch. Terms were then translated into English by using the LLT codes and the English version of MedDRA. The English LLT was automatically matched with the English PT level according to the MedDRA hierarchy.
Figure 2.
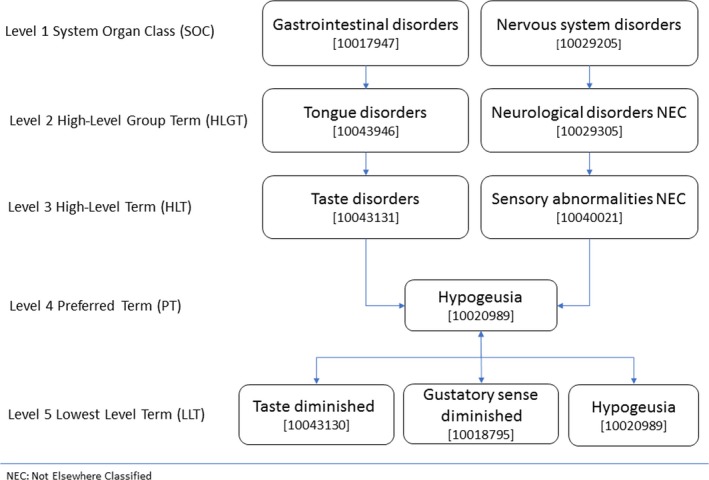
Hierarchy of “Hypogeusia” in MedDRA [Colour figure can be viewed at http://wileyonlinelibrary.com]
Microsoft® Excel (version 16.16.1) was used to create the database with the acquired information on DITD and to perform descriptive statistics.
3. RESULTS
In total, 1,645 drugs (active substances) were registered in the IM. Each drug can cause multiple adverse effects resulting in approximately 65,000 unique combinations between a drug and an adverse effect in the IM. Of these 65,000 combinations, 2,335 (3.5%) were defined by the authors as relevant for the oral healthcare provider and 343 (0.5%) concerned taste disorders. Of the 1,645 drugs, 314 (19%) could cause DITD. As IM discriminates different administration forms per drug, the number of drugs (314) and number of combinations (343) causing taste disorders differ. For example, “Budesonide,” which can be administered rectally, nasally, and by inhalation, is registered three times with dysgeusia as a potential adverse effect with three different incidences. Table 1 presents the different LLTs and PTs used in the IM for taste disorders and the number drugs which can potentially cause them. Taste disturbance as an adverse effect was reported in all level 1 categories of the ATC classification (Table 2).
Table 1.
LLTs and PT for taste disorders in IM analysis
| Adverse effect term | No. of drugs |
|---|---|
| Dysgeusia (PT) | 282 |
| Dysgeusia (LLT) | 15 |
| Taste bitter (LLT) | 9 |
| Taste disturbance (LLT) | 245 |
| Taste garlic (LLT) | 1 |
| Taste metallic (LLT) | 12 |
| Hypogeusia (PT) | 61 |
| Hypogeusia (LLT) | 61 |
| Total | 343 |
Table 2.
Number of drugs causing dysgeusia or hypogeusia per ATC level 1 category
| ATC level 1 category | Dysgeusia (%) | Hypogeusia (%) | Total |
|---|---|---|---|
| Alimentary tract and metabolism | 24 (8.5) | 2 (3.1) | 26 |
| Antiinfectives for systemic use | 44(15.6) | 7 (11.0) | 51 |
| Antineoplastic and immunomodulating agents | 53 (18.8) | 22 (39.0) | 75 |
| Antiparasitic products, insecticides, and repellents | 5 (1.7) | ‐ | 5 |
| Blood and blood forming organs | 13 (4.6) | 1 (1.4) | 14 |
| Cardiovascular system | 23 (8.1) | 5 (7.8) | 28 |
| Dermatologicals | 13 (4.6) | 2 (3.2) | 15 |
| Genitourinary system and sex hormones | 5 (1.7) | 3 (4.7) | 8 |
| Musculoskeletal system | 12 (4.3) | 2 (3.1) | 14 |
| Nervous system | 39 (13.8) | 12 (19.0) | 51 |
| Respiratory system | 16 (5.7) | ‐ | 16 |
| Sensory organs | 10 (3.5) | 1 (1.5) | 10 |
| Systemic hormonal preparations, excl. | 7 (2.5) | 2 (3.1) | 9 |
| Various | 18 (6.3) | 2 (3.1) | 20 |
| Total | 282 | 61 | 343 |
“Normogeusia,” “hypergeusia,” “ageusia,” and “phantogeusia” were not reported in the IM.
3.1. Dysgeusia
Dysgeusia (PT) as an adverse effect was reported 282 times (17.1% of 1,645 drugs) (Table 1). The drug categories “antineoplastic and immunomodulating agents” (18.8%), “antiinfectives for systemic use” (15.6%), and “nervous system” (13.8%) account for almost half of the drug‐induced dysgeusia (Table 2). Hypergeusia, ageusia, and phantogeusia were not reported.
Table 3 presents a selection of the drugs that could cause dysgeusia (PT) and comprises only the category “Alimentary tract and metabolism.” The frequencies of the adverse effect and whether a drug also causes the adverse effects “parosmia,” “anosmia,” “dry mouth,” or “hyposalivation” are presented as well, since these adverse effects are closely related to taste disorders. In some drugs, dysgeusia is only caused when the drug is administered through a specific route or under certain circumstances. The full table of all the 282 drugs causing dysgeusia is presented online as supplementary data (Table S1).
Table 3.
Drug‐induced dysgeusia (PT) in level 1 ATC category: alimentary tract and metabolism
| ATC level 1 | ATC level 3 | Generic name | ATC Code | LLT MedDRA | Frequency | Specific type of administration | Coinciding adverse effects |
|---|---|---|---|---|---|---|---|
| ALIMENTARY TRACT AND METABOLISM | Antiemetics and antinauseants | Aprepitant | A04AD12 | Taste disturbance | Frequency not known | – | D |
| Rolapitant | A04AD14 | Taste disturbance | Uncommon (0.1%–1%) | – | – | ||
| Antipropulsives | Loperamide | A07DA03 | Taste disturbance | Frequency not known | – | D | |
| Blood glucose‐lowering drugs Excl. insulins | Exenatide | A10BJ01 A10BJ01 | Taste disturbance | Uncommon (0.1%–1%) | – | – | |
| Glimepiride | A10BB12 | Taste disturbance | Frequency not known | – | – | ||
| Liraglutide | A10BJ02 | Taste disturbance | Common (1%–10%) | – | D | ||
| Metformin | A10BA02 | Taste disturbance | Common (1%–10%) | – | – | ||
| Drugs for peptic ulcer and gastroesophageal reflux disease (GORD) | Esomeprazole | A02BC05 | Taste disturbance | Frequency not known | After intravenous administration | D | |
| Famotidine | A02BA03 | Taste disturbance | Uncommon (0.1%–1%) | – | D | ||
| Lansoprazole | A02BC03 | Taste disturbance | Frequency not known | – | D | ||
| Rabeprazole | A02BC04 | Taste disturbance | Frequency not known | – | D | ||
| Intestinal antiinfectives | Fidaxomicin | A07AA12 | Taste disturbance | Uncommon (0.1%–1%) | – | D | |
| Miconazole | A07AC01 D01AC02 G01AF04 S02AA13 | Dysgeusia | Common (1%–10%) | After oral administration | D | ||
| Miconazole | A07AC01 D01AC02 G01AF04 S02AA13 | Taste disturbance | Uncommon (0.1%–1%) | After oral administration | D | ||
| Intestinal anti‐inflammatory agents | Budesonide | A07EA06 R01AD05 R03BA02 | Taste disturbance | Uncommon (0.1%–1%) | After rectal administration | D,P | |
| Budesonide | A07EA06 R01AD05 R03BA02 | Taste disturbance | Common (1%–10%) | After inhalation | D,P | ||
| Budesonide | A07EA06 R01AD05 R03BA02 | Taste disturbance | Frequency not known | After nasal administration | D,P | ||
| Cromoglicic acid | A07EB01 R01AC01 R03BC01 S01GX01 | Dysgeusia | Uncommon (0.1%–1%) | – | |||
| Sulfasalazine | A07EC01 | Taste disturbance | Common (1%–10%) | – | A | ||
| Other alimentary tract and metabolism products | Agalsidase alfa | A16AB03 | Taste disturbance | Common (1%–10%) | – | A | |
| Sodium phenylbutyrate | A16AX03 | Taste disturbance | Common (1%–10%) | – | – | ||
| Stomatological preparations | Chlorhexidine | A01AB03 B05CA02 D08AC02 D09AA12 S01AX09 | Taste disturbance | Rare or very rare (<0.1%) | – | – | |
| Triamcinolone | A01AC01 D07AB09 H02AB08 R01AD11 S01BA05 S02BA | Taste disturbance | Rare or very rare (<0.1%) | After nasal administration | – | ||
| Hydrogen peroxide | A01AB02 | Dysgeusia | Frequency not known | – | – |
Abbreviations: A, anosmia; ATC, Anatomic Therapeutical Chemical; D, dry mouth; LLT, lowest level term; P, parosmia
In these 282 drugs, the frequency of dysgeusia was “very common” in 7.1%, “common” in 31.2%, “uncommon” in 32.7%, and “rare or very rare” in 9.9% of the drugs. In 19.1% of the drugs, the “frequency was not known,” which means that in the IM, the frequency could not be estimated based on the available data.
Dysgeusia coincided in 114/282 drugs (40.4%) with “dry mouth” as an adverse effect, in 5/282 drugs (1.7%) with “anosmia,” in 2/282 drugs (0.7%) with “parosmia,” in 6/282 drugs (2.1%) with “dry mouth and anosmia,” and in 3/282 drugs (1.0%) with “dry mouth and parosmia.” None of these drugs were reported to cause “hyposalivation.”
Supplementary online Tables S2 and S3 present drugs that cause a bitter taste (LLT) or metallic taste (LLT), respectively. Disulfiram (N07BB01), a drug used to treat patients with alcohol abuses, was the only drug reported to cause a garlic taste (LLT).
3.2. Hypogeusia
Drug‐induced hypogeusia was reported in 61 drugs (3.7% of 1,645). Hypogeusia was predominantly reported in the drug categories “Antineoplastic and immunomodulating agents” (39.0%) and “Nervous system” (19%). Hypogeusia did not occur in the drug categories “Respiratory system” and “Antiparasitic products, insecticides and repellents” (Table 2). Table 4 presents all drugs in the IM that are reported to cause hypogeusia. In these 61 drugs, the frequency of hypogeusia was “very common” in 9.5%, “common” in 31.7%, “uncommon” in 25.4%, and “rare or very rare” in 15.9% of the drugs. In 17.5% of the drugs, the “frequency was not known.” Hypogeusia coincided in 28/61 drugs (45.9%) with “dry mouth,” in 1/61 drugs (1.6%) with “anosmia,” and in 2/61 drugs (3.2%) with “dry mouth/anosmia.” None of these drugs were reported to cause “hyposalivation.”
Table 4.
Drug‐induced hypogeusia (PT) in all ATC level 1 categories
| ATC level 1 | ATC level 3 | Generic name | ATC Code | LLT MedDRA | Frequency | Specific type of administration | Coinciding adverse effects |
|---|---|---|---|---|---|---|---|
| ALIMENTARY TRACT AND METABOLISM | Belladonna and derivatives, plain | Atropine | A03BA01 S01FA01 | Hypogeusia | Frequency not known | – | D |
| Intestinal antiinfectives | Colistin | A07AA10 J01XB01 | Hypogeusia | Rare or very rare (<0.1%) | After inhalation | – | |
| ANTIINFECTIVES FOR SYSTEMIC USE | Antimycotics for systemic use | Micafungin | J02AX05 | Hypogeusia | Uncommon (0.1%–1%) | – | – |
| Direct‐acting antivirals | Darunavir | J05AE10 | Hypogeusia | Frequency not known | – | D | |
| Drugs for treatment of tuberculosis | Rifabutin | J04AB04 | Hypogeusia | Rare or very rare (<0.1%) | – | – | |
| Macrolides, lincosamides, and streptogramins | Claritromycine | J01FA09 | Hypogeusia | Rare or very rare (<0.1%) | – | D | |
| Other antibacterials | Methenamine | J01XX05 | Hypogeusia | Rare or very rare (<0.1%) | – | – | |
| Quinolone antibacterials | Levofloxacin | J01MA12 | Hypogeusia | Rare or very rare (<0.1%) | After oral and intravenous administration | – | |
| Ofloxacin | J01MA01 S01AE01 S02AA16 | Hypogeusia | Rare or very rare (<0.1%) | After oral administration | D,A | ||
| ANTINEOPLASTIC AND IMMUNOMODULATING AGENTS | Antimetabolites | Capecitabine | L01BC06 | Hypogeusia | Common (1%–10%) | – | D |
| Tegafur | L01BC03 | Hypogeusia | Common (1%–10%) | – | D | ||
| Hormone antagonists and related agents | Anastrozole | L02BG03 | Hypogeusia | Common (1%–10%) | – | – | |
| Immunostimulants | Aldesleukin | L03AC01 | Hypogeusia | Common (1%–10%) | – | – | |
| Other antineoplastic agents | Afatinib | L01XE13 | Hypogeusia | Common (1%–10%) | – | – | |
| Axitinib | L01XE17 | Hypogeusia | Very common (>10%) | – | – | ||
| Bosutinib | L01XE14 | Hypogeusia | Common (1%–10%) | – | – | ||
| Cabozantinib | L01XE26 | Hypogeusia | Common (1%–10%) | – | – | ||
| Cisplatin | L01XA01 | Hypogeusia | Frequency not known | – | – | ||
| Crizotinib | L01XE16 | Hypogeusia | Very common (>10%) | – | – | ||
| Dasatinib | L01XE06 | Hypogeusia | Common (1%–10%) | – | – | ||
| Everolimus | L01XE10 L04AA18 | Hypogeusia | Common (1%–10%) | In case of oncologic treatment | D | ||
| Necitumumab | L01XC22 | Hypogeusia | Common (1%–10%) | – | – | ||
| Nilotinib | L01XE08 | Hypogeusia | Common (1%–10%) | – | – | ||
| Palbociclib | L01XE33 | Hypogeusia | Common (1%–10%) | – | – | ||
| Panobinostat | L01XX42 | Hypogeusia | Common (1%–10%) | – | D | ||
| Sorafenib | L01XE05 | Hypogeusia | Common (1%–10%) | – | D | ||
| Temsirolimus | L01XE09 | Hypogeusia | Common (1%–10%) | – | – | ||
| Trastuzumab | L01XC03 | Hypogeusia | Very common (>10%) | – | D | ||
| Trastuzumab emtansine | L01XC14 | Hypogeusia | Common (1%–10%) | – | D | ||
| Vandetanib | L01XE12 | Hypogeusia | Common (1%–10%) | – | D | ||
| Vismodegib | L01XX43 | Hypogeusia | Common (1%–10%) | – | – | ||
| BLOOD AND BLOOD FORMING ORGANS | Iron, parenteral preparations | Ferric carboxymaltose | B03AC | Hypogeusia | Uncommon (0.1%–1%) | – | – |
| CARDIOVASCULAR SYSTEM | Ace inhibitors, plain | Captopril | C09AA01 | Hypogeusia | Common (1%–10%) | – | D |
| Enalapril | C09AA02 | Hypogeusia | Frequency not known | – | D | ||
| Ramipril | C09AA05 | Hypogeusia | Uncommon (0.1%–1%) | – | D,A | ||
| Beta‐blocking agents | Esmolol | C07AB09 | Hypogeusia | Uncommon (0.1%–1%) | – | D | |
| Lipid‐modifying agents, plain | Atorvastatin | C10AA05 | Hypogeusia | Uncommon (0.1%–1%) | – | – | |
| DERMATO LOGICALS | Antifungals for topical use | Terbinafine | D01AE15 D01BA02 | Hypogeusia | Uncommon (0.1%–1%) | – | – |
| Other dermatological preparations | Tacrolimus | D11AH01 L04AD02 S01XA | Hypogeusia | Frequency not known | After intravenous administration | – | |
| GENITOURINARY SYSTEM AND SEX HORMONES | Hormonal contraceptives for systemic use | Ulipristal | G03AD02 G03XB02 | Hypogeusia | Frequency not known | When used as emergency anticonceptive | D |
| Other urologicals, Incl. antispasmodics | Solifenacin | G04BD08 | Hypogeusia | Uncommon (0.1%–1%) | – | D | |
| Tiopronine | G04BX16 | Hypogeusia | Uncommon (0.1%–1%) | – | – | ||
| MUSCULO SKELETAL SYSTEM | Muscle relaxants, centrally acting agents | Baclofen | M03BX01 | Hypogeusia | Uncommon (0.1%–1%) | – | D |
| Specific antirheumatic agents | Penicillamine | M01CC01 | Hypogeusia | Common (1%–10%) | – | – | |
| NERVOUS SYSTEM | Anesthetics, local | Articaine | N01BB08 | Hypogeusia | Frequency not known | – | – |
| Cocaine | N01BC01 S01HA01 | Hypogeusia | Frequency not known | – | A | ||
| Mepivacaine | N01BB03 | Hypogeusia | Frequency not known | – | – | ||
| Antidepressants | Duloxetine | N06AX21 | Hypogeusia | Uncommon (0.1%–1%) | – | D | |
| Maprotiline | N06AA21 | Hypogeusia | Frequency not known | – | D | ||
| Antiepileptics | Pregabalin | N03AX16 | Hypogeusia | Uncommon (0.1%–1%) | – | D | |
| Antimigraine preparations | Rizatriptan | N02CC04 | Hypogeusia | Uncommon (0.1%–1%) | – | D | |
| Antipsychotics | Paliperidone | N05AX13 | Hypogeusia | Uncommon (0.1%–1%) | – | D | |
| Dopaminergic agents | Opicapone | N04BX04 | Hypogeusia | Uncommon (0.1%–1%) | – | D | |
| Drugs used in addictive disorders | Varenicline | N07BA03 | Hypogeusia | Frequency not known | – | D | |
| Opioids | Hydromorphone | N02AA03 | Hypogeusia | Uncommon (0.1%–1%) | After oral administration | D | |
| Psychostimulants, agents used for ADHD and nootropics | Dexamfetamine | N06BA02 | Hypogeusia | Rare or very rare (<0.1%) | – | D | |
| SENSORY ORGANS | Antiglaucoma preparations and miotics | Brinzolamide | S01EC04 | Hypogeusia | Rare or very rare (<0.1%) | After systemic administration | D |
| SYSTEMIC HORMONAL PREPARATIONS, EXCL. | Antithyroid preparations | Carbimazole | H03BB01 | Hypogeusia | Frequency not known | – | – |
| Propylthiouracil | H03BA02 | Hypogeusia | Rare or very rare (<0.1%) | – | – | ||
| VARIOUS | Allergens | Grass pollen | V01AA02 V01AA | Hypogeusia | Rare or very rare (<0.1%) | After subcutaneous administration | D |
| Magnetic resonance imaging contrast media | Gadoteric acid | V08CA02 | Hypogeusia | Uncommon (0.1%–1%) | After intravenous administration | – |
Abbreviations: A, anosmia, ATC, Anatomic Therapeutical Chemical; D, dry mouth; LLT, lowest level term.
4. DISCUSSION
In total, 20% (343/1,645) of the drugs used in the Netherlands has been reported to potentially cause DITD (dysgeusia and hypogeusia). DITD was reported in all ATC level 1 categories, suggesting that all healthcare professionals may frequently encounter the adverse effects of these drugs. Healthcare professionals that treat patients using antineoplastic drugs are most likely to be confronted with DITD. Despite the recorded percentage of our search, the exact incidence of DITD is unclear due to a lack of systematic well controlled clinical trials (Schiffman, 2018).
To the best of our knowledge, this study is the first comprehensive overview of DITD based on the analysis of a national drug information database which includes adverse effects. The available literature that discusses DITD is fragmented, since previous articles usually report on a specific type of patients with DITD (e.g., cancer) (de Coo & Haan, 2016; Okada et al., 2016; Tuccori et al., 2011), specific drug categories causing DITD (e.g., cardiovascular drugs) (Che, Li, Fang, Reis, & Wang, 2018; van der Werf, Rovithi, Langius, de van der Schueren, & Verheul, 2017) or summarize the literature instead of providing an overall analysis of what registered drugs are linked to DITD (Mortazavi, Shafiei, Sadr, & Safiaghdam, 2018; Schiffman, 2018; Wang, Glendinning, Grushka, Hummel, & Mansfield, 2017). In addition, the ATC classification is not always applied, making it difficult to compare the results of the various studies.
Our data source contains predominantly PT level terms. Although this is in accordance with the MedDRA guidelines, it is likely that specific LLT terms like “bitter taste” and “metallic taste” might therefore be underreported compared to previous studies which do not use the MedDRA. It also has to be mentioned that the terms and incidences used in the database (e.g., "dysgeusia", "hypoguesia") are based on patient‐reported adverse effects during pharmacological developing studies or postmarketing studies. This subjective reporting by patients might lead to a reporting bias or inaccuracy in terminology. The difference between objective and subjective adverse effects measuring is a common point of discussion when reporting on adverse effects and one without a clear solution. When considering taste disorders, there is no commonly used test available for objectifying taste disorders, which makes it impossible to report solely objective data. In order to make future studies on oral adverse effects more comparable, it is recommended that the MedDRA terminology and hierarchy and, if available, objective tests are used during data collection and describing the results. Homogenous reporting of results, on for instance incidences, will lead to clinically more applicable data.
Due to differences in local and regional laws and regulations on drug admission, registered drugs differ per country. Thus, there will be drugs that are reported in the current study that are not available in some countries and reverse. However, with regard to the European countries, most of the reported drugs will be available in all countries. By applying the ATC and MedDRA classification, the data are internationally applicable and could serve as a guidance for future reports on DITD.
The exact mechanisms underlying DITD are still unclear and may vary between individuals. Individual variations may be caused by polypharmacy (drug interactions), dosage differences, and patient‐specific variables (e.g., genetics, age, and medical conditions) (Schiffman, 2018). Schiffman (2018) describes several presumed mechanisms behind DITD. Some drugs have sensory properties that cause a bitter or metallic taste. These drugs interact with the taste buds: (a) after oral application, (b) by diffusion into the saliva after absorption in the gut or intravenous administration, or (c) by accumulation in the taste buds when used chronically. The latter might explain why DITD can occur months or years after the initial usage (e.g., lithium carbonate). Other drugs distort taste and smell signals for sweet or salt, causing a bitter or sour taste perception of food and beverages. The garlic‐like taste caused by disulfiram is due to exhalation of carbon disulfide. Drug–drug interactions can lead to elevated blood plasma levels beyond therapeutic concentrations and therefore cause DITD, which particularly could occur in polypharmacy patients.
Saliva could also play a role in the underlying mechanism of DITD. Saliva protects the external environment of the taste receptor cells and acts as a solvent and transportation medium for taste substances (Matsuo, 2000). Many drugs are known to cause quantitative or qualitative changes in saliva (Wolff et al., 2017). Almost 45% of the drugs known to potentially cause DITD coincided with dry mouth as an adverse effect, suggesting that there is at least some correlation. However, the exact correlation is difficult to assess since both MedDRA and the data that underlie the IM do not clearly discriminate between subjective “xerostomia” and objective “hyposalivation.” The term “dry mouth” is presumably used for both.
A healthcare professional confronted with a patient with DITD should assess which drug, or drug combination, is presumably responsible for the DITD. This can be done by comparing the temporal onset of DITD with the alterations in the drug usage (e.g., dosage, new drugs). However, as stated before, it is possible that DITD occurs months or years after the initial usage, complicating the assessment of a temporal relationship. Another possibility is to consult pharmaceutical databases and overviews like the approach used in the present study.
Cessation of the drug responsible for DITD will most likely result in a decrease and eventually even recovery of DITD, but this (partial) recovery could take months. If cessation and alterations are not possible, other treatment modalities could be considered to relieve the symptoms. The evidence behind these modalities is scarce and based on research on taste disorders with other causes than DITD. Proposed treatment modalities include improving oral hygiene, suppletion of zinc, stimulation food flavors, saliva substitutes, and administration of alpha lipoic acid (Briggs, 2009; Femiano, Scully, & Gombos, 2002; Kumbargere Nagraj et al., 2017; Schiffman, 2018).
5. CONCLUSION
Healthcare professionals are frequently confronted with drugs that are documented with DITD. The exact incidences of DITD remain unclear. This overview supports clinicians in their awareness, diagnosis, and possible treatment of DITD, and could serve as a reference for future research reporting on DITD.
CONFLICT OF INTEREST
None.
AUTHOR CONTRIBUTION
All authors contributed to some extent to the current paper. WR was responisble for the study design, data collection, data analysis and drafting of the paper. YA,AH,KC all supported the data analysis and drafting the current paper. JL, AV supported the drafting of the current paper. FR guided the process from study desing to drafting te current paper.
Supporting information
ACKNOWLEDGEMENT
The authors would like to acknowledge the Royal Dutch Pharmacists Association for providing access to IM.
Rademacher WMH, Aziz Y, Hielema A, et al. Oral adverse effects of drugs: Taste disorders. Oral Dis. 2020;26:213–223. 10.1111/odi.13199
REFERENCES
- Briggs, E. R. (2009). Taste disturbances related to medication use. The Consultant Pharmacist, 24(7), 538–543. 10.4140/TCP.n.2009.538 [DOI] [PubMed] [Google Scholar]
- Che, X. , Li, Y. , Fang, Y. , Reis, C. , & Wang, H. (2018). Antiarrhythmic drug‐induced smell and taste disturbances: A case report and literature review. Medicine (Baltimore), 97(29), e11112 10.1097/md.0000000000011112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Coo, I. F. , & Haan, J. (2016). Long lasting impairment of taste and smell as side effect of lithium carbonate in a cluster headache patient. Headache, 56(7), 1201–1203. 10.1111/head.12872 [DOI] [PubMed] [Google Scholar]
- Fark, T. , Hummel, C. , Hahner, A. , Nin, T. , & Hummel, T. (2013). Characteristics of taste disorders. European Archives of Oto‐Rhino‐Laryngology, 270(6), 1855–1860. 10.1007/s00405-012-2310-2 [DOI] [PubMed] [Google Scholar]
- Femiano, F. , Scully, C. , & Gombos, F. (2002). Idiopathic dysgeusia; An open trial of alpha lipoic acid (ALA) therapy. International Journal of Oral and Maxillofacial Surgery, 31(6), 625–628. 10.1054/ijom.2002.0276 [DOI] [PubMed] [Google Scholar]
- Hamada, N. , Endo, S. , & Tomita, H. (2002). Characteristics of 2278 patients visiting the Nihon University Hospital Taste Clinic over a 10‐year period with special reference to age and sex distributions. Acta Oto‐Laryngologica, 546, 7–15. 10.1080/00016480260046373 [DOI] [PubMed] [Google Scholar]
- KNMP (2019). Kennisbank. Retrieved from https://www.knmp.nl/producten/knmp-kennisbank. [Google Scholar]
- Kumbargere Nagraj, S. , George, R. P. , Shetty, N. , Levenson, D. , Ferraiolo, D. M. , & Shrestha, A. (2017). Interventions for managing taste disturbances. Cochrane Database Systematic Review, 12, CD010470 10.1002/14651858.CD010470.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuo, R. (2000). Role of saliva in the maintenance of taste sensitivity. Critical Reviews in Oral Biology and Medicine, 11(2), 216–229. 10.1177/10454411000110020501 [DOI] [PubMed] [Google Scholar]
- Meddra (2018). MedDRA® TERM SELECTION: POINTS TO CONSIDER. Retrieved from https://www.meddra.org/sites/default/files/guidance/file/000240_termselptc_r4_16_sep2018.pdf. [Google Scholar]
- Meddra (2019). Vision of meddra. Retrieved from https://www.meddra.org/about-meddra/vision. [Google Scholar]
- Mortazavi, H. , Shafiei, S. , Sadr, S. , & Safiaghdam, H. (2018). Drug‐related dysgeusia: A systematic review. Oral Health Prev Dent, 16(6), 499–507. 10.3290/j.ohpd.a41655 [DOI] [PubMed] [Google Scholar]
- Noel, C. A. , Sugrue, M. , & Dando, R. (2017). Participants with pharmacologically impaired taste function seek out more intense, higher calorie stimuli. Appetite, 117, 74–81. 10.1016/j.appet.2017.06.006 [DOI] [PubMed] [Google Scholar]
- Okada, N. , Hanafusa, T. , Abe, S. , Sato, C. , Nakamura, T. , Teraoka, K. , … Ishizawa, K. (2016). Evaluation of the risk factors associated with high‐dose chemotherapy‐induced dysgeusia in patients undergoing autologous hematopoietic stem cell transplantation: Possible usefulness of cryotherapy in dysgeusia prevention. Supportive Care in Cancer, 24(9), 3979–3985. 10.1007/s00520-016-3244-9 [DOI] [PubMed] [Google Scholar]
- Ponticelli, E. , Clari, M. , Frigerio, S. , De Clemente, A. , Bergese, I. , Scavino, E. , … Sacerdote, C. (2017). Dysgeusia and health‐related quality of life of cancer patients receiving chemotherapy: A cross‐sectional study. European Journal of Cancer Care, 26(2), e12633 10.1111/ecc.12633 [DOI] [PubMed] [Google Scholar]
- Schiffman, S. S. (2018). Influence of medications on taste and smell. World Journal of Otorhinolaryngology ‐ Head and Neck Surgery, 4(1), 84–91. 10.1016/j.wjorl.2018.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuccori, M. , Lapi, F. , Testi, A. , Ruggiero, E. , Moretti, U. , Vannacci, A. , … Blandizzi, C. (2011). Drug‐induced taste and smell alterations: A case/non‐case evaluation of an Italian database of spontaneous adverse drug reaction reporting. Drug Safety, 34(10), 849–859. 10.2165/11593120-000000000-00000 [DOI] [PubMed] [Google Scholar]
- van der Werf, A. , Rovithi, M. , Langius, J. , de van der Schueren, M. , & Verheul, H. (2017). Insight in taste alterations during treatment with protein kinase inhibitors. European Journal of Cancer, 86, 125–134. 10.1016/j.ejca.2017.09.006 [DOI] [PubMed] [Google Scholar]
- Wang, T. , Glendinning, J. , Grushka, M. , Hummel, T. , & Mansfield, K. (2017). From the cover: Drug‐induced taste disorders in clinical practice and preclinical safety evaluation. Toxicological Sciences, 156(2), 315–324. 10.1093/toxsci/kfw263 [DOI] [PubMed] [Google Scholar]
- WHO (2003). The Anatomical Therapeutic Chemical Classification System with Defined Daily Doses (ATC/DDD). Retrieved from http://www.who.int/classifications/atcddd/en/. [Google Scholar]
- WHO (2011). THE WORLD MEDICINES SITUATION 2011. Retrieved from http://apps.who.int/medicinedocs/en/m/abstract/Js20035en/. [Google Scholar]
- Wolff, A. , Joshi, R. K. , Ekström, J. , Aframian, D. , Pedersen, A. M. L. , Proctor, G. , … Dawes, C. (2017). A Guide to Medications Inducing Salivary Gland Dysfunction, Xerostomia, and Subjective Sialorrhea: A Systematic Review Sponsored by the World Workshop on Oral Medicine VI. Drugs in R & D, 17(1), 1–28. 10.1007/s40268-016-0153-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


