Abstract
Background
This study aimed to investigate the expression of microRNA-639 (miR-639) in tumor tissue from patients with hepatocellular carcinoma (HCC) and its effects on patient outcome, to identify the targets for miR-639 using bioinformatics and luciferase reporter analysis, and the effects of miR-639 in human HCC cells in vitro to identify the molecular pathways involved.
Material/Methods
Expression levels of miR-639 were compared in tumor tissue and adjacent normal liver tissue from 50 patients with HCC, and Kaplan-Meier curves identified the association with overall survival (OS). miR-639 expression was measured in HCC cells cultured in vitro using quantitative reverse transcription-polymerase chain reaction (RT-qPCR) and Western blot. HCC cells were studied using the MTT assay, the colony formation assay, and the transwell assay. Bioinformatics and luciferase reporter analysis identified the role of the histone acetyltransferase gene, KAT7, in HCC.
Results
The expression of miR-639 was significantly reduced in HCC tissues compared with normal adjacent liver tissues, and inhibited cell proliferation and epithelial-mesenchymal transition (EMT) of HCC cells. Bioinformatics and luciferase reporter analysis showed that miR-639 directly targeted KAT7 and inhibit its expression. KAT7 expression promoted cell proliferation, and migration of human HCC cells in vitro, and miR-639 inhibited cell proliferation and EMT by down-regulating the KAT7/Wnt/β-catenin signaling pathway.
Conclusions
miR-639 was down-regulated in HCC tumor tissue, and inhibited proliferation and migration of HCC cells by the down-regulation of KAT7/Wnt/β-catenin signaling and was associated with reduced OS. These findings supported the potential role of miR-639 as a tumor suppressor.
MeSH Keywords: Carcinoma, Hepatocellular; Epithelial-Mesenchymal Transition; MicroRNAs; Wnt Signaling Pathway
Background
Worldwide, hepatocellular carcinoma (HCC) is a common malignancy that is associated with a high mortality rate [1]. Although there have been recent advances in the diagnosis and management of HCC, patient prognosis and survival remain poor [2,3]. Also, the recurrence rate for HCC is high following surgery [4]. Therefore, there remains a need to identify new diagnostic biomarkers and therapeutic targets for HCC.
Recent studies have shown that microRNAs (miRNAs) are involved in the pathogenesis and progression of HCC [5–7]. The miRNAs are a family of short, single-stranded, noncoding RNAs that are between 19–22 nucleotides (nt) in length, which have key roles in many cell processes and regulate the expression of downstream messenger RNAs (mRNAs) by targeting the 3′ untranslated region (3′-UTR) to prevent translation or to accelerate the degradation of target mRNAs [8,9]. Previous studies have shown that dysregulation of miRNAs has an important role in pathological processes, including carcinogenesis and cancer metastasis [10–12]. For example, microRNA-639 (miR-639) has been shown to promotes cell proliferation, cell migration, and results in cell cycle arrest in breast cancer and thyroid cancer cells [13,14]. Also, miR-639 has been shown to regulate transforming growth factor β (TGF-β) and inhibit epithelial-mesenchymal transition (EMT) in oral squamous cancer cells by targeting FOXC1 [15]. However, the roles and mechanisms for miR-639 in other malignant tumors, including HCC, remain poorly understood.
Therefore, this study aimed to compare the expression of miR-639 in HCC tissues with adjacent normal liver tissue and its effects on patient outcome, to use bioinformatics and luciferase reporter analysis to identify the targets for miR-639 in HCC, and to study the effects of miR-639 in human HCC cells in vitro to identify the signaling pathways involved.
Material and Methods
Tissue samples and cell lines
Fifty paired tumor tissue samples of histologically-confirmed hepatocellular carcinoma (HCC) and adjacent normal liver tissue samples were obtained from patients diagnosed by the Tianjin First Center Hospital. The Ethics Committee of the Tianjin First Center Hospital approved the study. All the samples were obtained and analyzed with prior written and informed consent from the patients.
The human HCC cell lines, SMMC-7721, Huh7, HepG2, and HepG2.2.15, and the normal human hepatic cell line LO2, were cultivated in RPMI 1640 or Dulbecco’s modified Eagle’s medium (DMEM) medium (HyClone, Logan, UT, USA) supplemented with 10% fetal bovine serum (FBS) (AusGeneX, Brisbane, Australia) and penicillin-streptomycin solution consisting of 100 U/ml of penicillin and 100 g/ml streptomycin in 0.9% saline as a sterile and filtered solution. The cells were cultured in a humidified chamber with 5% CO2, and at 37°C.
RNA extraction and quantitative reverse transcription-polymerase chain reaction (RT-qPCR)
Total RNA from the fresh HCC tumor tissues, normal liver tissues, or the cultured cells was extracted with TRIzol reagent (Invitrogen, Carlsbad, CA, USA), according to the manufacturer’s instructions. To detect the microRNA (miRNA) expression, the microRNA (miRNA) First Strand cDNA Synthesis kit, using the stem-loop method was used (Sangon Biotech., Shanghai, China). The extracted miRNA underwent RT-qPCR using a miRNA-specific TaqMan Kit for miR-639 (ID 001583) (Thermo Fisher Scientific, Waltham, MA, USA) to quantify the levels of miR-639, based on the manufacturer’s instructions, and normalized by U6 snRNA (ID 001973) (Thermo Fisher Scientific, Waltham, MA, USA).
To measure the levels of mRNA, first-strand complementary DNA (cDNA) was generated using the M-MuLV First Strand cDNA Synthesis Kit (Sangon Biotech., Shanghai, China), and RT-qPCR was performed using SGExcel FastSYBR Mixture (with ROX) (Proteogenix, Portland, OR, USA), based on the manufacturer’s instructions. β-actin was selected as an internal control to normalize the transcript levels of the RNAs. The RT-qPCR primers used were as follows: Vimentin, forward: 5′-AGTCCACTGAGTACCGGAGAC-3′;
Vimentin, reverse: 5′-CATTTCACGCATCTGGCGTTC-3′;
E-cadherin, forward: 5′-AAAGGCCCATTTCCTAAAAACCT-3′;
E-cadherin, reverse: 5′-TGCGTTCTCTATCCAGAGGCT-3′;
KAT7, forward: 5′-TCTCCGCTACCTGCATAATTTTCAAGGC-3′;
KAT7, reverse: 5′-TTGGAGTTGGACCTTTTGGCCTCTTTGG-3′;
β-actin, forward: 5′-CGTGACATTAAGGAGAAGCTG-3′; and
The 2−ΔΔCt method was performed to analyze the data.
Plasmid construction
To construct KAT7 3′UTR luciferase reporter plasmids, included top, 5′-CTAGCCCTGTCATTCCGAGCCAGCGAACT-3′, and bottom, 5′-CTAGAGTTCGTGGCTCGGAATGACAGGG-3′. The strands of KAT7 3′UTR were annealed and inserted into the NheI and XbaI restriction sites of the pmirGLO dual-luciferase miRNA target vector (Promega), generating vector pmirGLO/Luc-KAT7 3′UTR. In addition, the mutant top (5′-CTAGCCCTGTCATTCCGAGCACGATGACT-3′) and mutant bottom (5′-CTAGAGTCATCGTGCTCGGAATGACAGGG-3′) strands of KAT7 3′UTR were annealed and inserted into the NheI and XbaI restriction sites of pmirGLO dual-luciferase miRNA target vector (Promega, Madison, WI, USA), and generated the vector pmirGLO/Luc-KAT7 3′UTR mut. Construction of the plasmid pcDNA3-KAT7 for KAT7 overexpression, and the whole sequences of KAT7 were obtained from the KAT7 cDNA using the PCR primers:
KAT7, forward: 5′-CGGAAGCTTGCCACCATGCCGCGAAGG AAGAGG-3′; and
KAT7, reverse: 5′-CTTTCTAGATTAAGTG CCCTTGGGAGGGGTCCA-3′).
The whole sequences of KAT7 were inserted into the HindIII and XbaI restriction sites of pcDNA3.1. To construct the KAT7 knockdown plasmid, shRNA sequences of shRNA-KAT7 sense (5′-GATCCGCTCAAATACTGGAAGGGAAACTCGAGTTT CCCTTCCAGTATTTGAGCTTTTTGA-3′) and antisense (5′-AGCTTCAA AAAGCTCAAATACTGGAAGGGAAACTCGAGTTTCCCTTC CAGTATTTGAGCG-3′) oligonucleotides were annealed and inserted into the BamHI and HindIII of pSilencer 2.1-U6 Neo vector (Thermo Fisher Scientific, Waltham, MA, USA), generated by pshR-KAT7 plasmids.
Cell transfection
Transfection of miRNAs or related plasmids was performed using Lipofectamine RNAiMAX transfection (Thermo Fisher Scientific, Waltham, MA, USA), according to the manufacturer’s protocol.
MTT assay
Related miRNAs or plasmids were transfected into the HCC cell lines, Huh7 and HepG2, and cultured for 24 h. Then, 3×103 transfected cells were transferred into 96-well plates and cultured for 24 h, 48 h, and 96 h. The MTT assay was performed to evaluate the cell viability at a wavelength of 570 nm using a Quant Universal microplate spectrophotometer (BioTek, Winooski, VT, USA).
Colony formation assay
Huh7 and HepG2 cells were transfected with related miRNAs or plasmids and cultured for 24 h, and 3×102 cells were grown in the 12-well plates and cultured for two weeks. Crystal violet was used to stain the colonies, as previously described [18].
Transwell migration assay
The transwell migration assay used 3×104 Huh7 cells and 5×104 HepG2 cells, which were seeded on the upper chamber of the 8 μm pore size insert (BD Biosciences, Franklin Lakes, NJ, USA) after transfection for 24 h in serum-free culture medium. The transwell invasion assay used 5×104 Huh7 cells and 8×104 HepG2 cells, which were seeded on the upper chamber of the inserts coated with 40 μl Matrigel (2 μg/μl) (BD Biosciences, Franklin Lakes, NJ, USA) in serum-free culture medium. The bottom of the inserts contained 700 μl of DMEM containing 20% FBS. After 24 h of incubation for the migration assay, and 48 h of incubation for the invasion assay, the cells were stained with crystal violet, and the cells were counted and photographed by microscopy.
Luciferase assay
HCC cells were co-transfected with pmir/Luc-KAT7 3′UTR or pmir/Luc-KAT7 3′UTR mut plasmids with miR-NC, miR-639 mimics, ASO-NC and ASO-miR-639 and cultured for 48 h. Lysates were collected and measured using the Dual-Luciferase Reporter (DLR™) assay system (Promega, Madison, WI, USA), according to the manufacturer’s instructions. The activity of Renilla luciferase was quantified according to the activity of firefly luciferase. The relative luciferase intensity equals to the ratio of Renilla and firefly.
Top-Flash/Fop-Flash luciferase reporter assay
To detect the activation of Wnt pathway, the indicated plasmids were transfected into HCC cells with the TCF signaling reporter Top-Flash (TCF reporter plasmid) and the negative control Fop-Flash (mutant, inactive TCF binding site). Then, HCC cells were transfected with either pFop-Flash or pTop-Flash plasmid and the internal control plasmid pSV40-Renilla for 48 h. The activity of the firefly luciferase was quantified according to the activity of Renilla luciferase (Relative luciferase intensity=firefly intensity/Renilla intensity).
Western blot
Proteins were extracted with RIPA buffer and resolved using 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). The proteins in the SDS-PAGE gel were transferred onto polyvinylidene fluoride (PVDF) membranes (BioRad, Berkeley, CA, USA). Proteins were detected by using primary antibodies to human E-cadherin (1: 500) (Bioworld Technology, Louis Park, MN, USA), vimentin (1: 1000) (Bioworld Technology, Louis Park, MN, USA), KAT7 (1: 500) (Abcam, Cambridge, MA, USA), Wnt (1: 500) (Abcam, Cambridge, MA, USA), β-catenin (1: 500) (Abcam, Cambridge, MA, USA), H2A.X (1: 500) (Abcam, Cambridge, MA, USA), and GAPDH (1: 2000) (Proteintech, Chicago, IL, USA). Peroxidase-conjugated secondary antibody (1: 2000) (Abcam, Cambridge, MA, USA) was added to the PVDF membranes and visualized using a Tanon chemiluminescence System (Tanon Science & Technology Co., Ltd, Shanghai, China). Western blot bands were quantified using the Tanon Gel Image System (Tanon Science & Technology Co., Ltd, Shanghai, China).
Immunohistochemistry (IHC) staining analysis
Immunohistochemistry for KAT7 and β-catenin was performed according to the methods previously reported [19].
Statistical analysis
The experiments were performed in triplicate. The overall survival (OS) of patients with HCC was analyzed using Kaplan-Meier plots (http://kmplot.com/analysis/) [20]. Data were analyzed using Student’s t-test and the chi-squared χ2 test and Graph Pad Prism version 6 (GraphPad Software, La Jolla, CA, USA). A P-value <0.05 was considered to be statistically significant.
Results
Down-regulation of microRNA-639 (miR-639) expression was associated with reduced overall survival (OS) in patients with hepatocellular carcinoma (HCC)
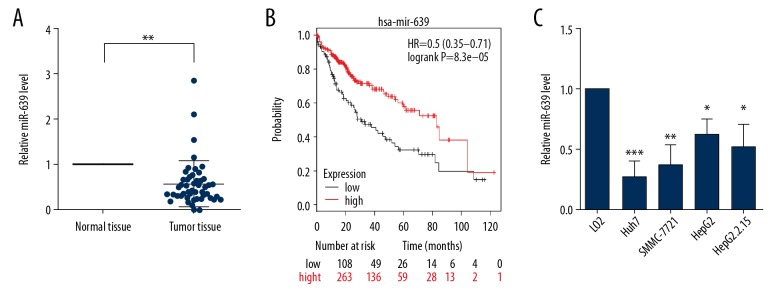
MicroRNA-639 (miR-639) expression levels in human HCC tumor tissues and normal liver tissues were measured by quantitative reverse transcription-polymerase chain reaction (RT-qPCR). In the fifty-paired tissue specimens, the level of miR-639 was significantly down-regulated in HCC tumor tissues compared with normal liver tissues (Figure 1A). Correlation analysis showed that significant increased expression of miR-639 was significantly associated with reduced TNM stage, tumor size, and lymph node metastasis, but there were no differences between patient gender, age, and histological grade of HCC (Table 1). Also, patients with HCC and low expression levels of miR-639 had a significantly reduced overall survival (OS), according to the Kaplan-Meier analysis [20] (Figure 1B). The expression of miR-639 was significantly reduced in Huh7, HepG2, HepG2.2.15, and SMMC-7721 human hepatocellular carcinoma cells compared with LO2 normal human liver cells (Figure 1C). These findings indicated that miR-639 might act as a tumor suppressor in HCC.
Figure 1.
Down-regulation of microRNA-639 (miR-639) was found in hepatocellular carcinoma (HCC) tissues and cells. (A) The level of miR-639 in fifty paired HCC tumor tissues and adjacent human normal liver tissues. (B) The prognosis of patients with HCC who had high expression and low expression of miR-639 analyzed by Kaplan-Meier curves. (C) The level of miR-639 in a normal human liver cell line, LO2, and human HCC cell lines, SMMC-7721, Huh7, HepG2, and HepG2.2.15. Studies were performed in triplicate. (* p<0.05; ** p<0.01; *** p<0.001).
Table 1.
Clinicopathological characteristics of the 50 patients with hepatocellular carcinoma (HCC) and levels of microRNA-639 (miR-639) expression.
| Variables | n=50 | microRNA-639 (miR-639) expression | p-Value | |
|---|---|---|---|---|
| Low (n) | High (n) | |||
| Age (years) | ||||
| ≥45 | 28 | 23 | 5 | p=0.905 |
| <45 | 22 | 19 | 3 | |
| Gender | ||||
| Male | 25 | 20 | 5 | p=0.44 |
| Female | 25 | 22 | 3 | |
| Tumor size | ||||
| ≥5 cm | 32 | 30 | 2 | p=0.035* |
| <5 cm | 18 | 12 | 6 | |
| TNM stage | ||||
| I–II | 37 | 34 | 3 | p=0.033* |
| III–IV | 13 | 8 | 5 | |
| LNM | ||||
| No | 30 | 29 | 1 | p=0.009** |
| Yes | 20 | 13 | 7 | |
χ2 test.
p<0.05;
p<0.01.
TNM – tumor node metastasis; LNM – lymph node metastasis.
The effects of miR-639 in cell proliferation, migration, and expression of markers of epithelial-mesenchymal transition (EMT) in two human HCC cell lines, Huh7 and HepG2
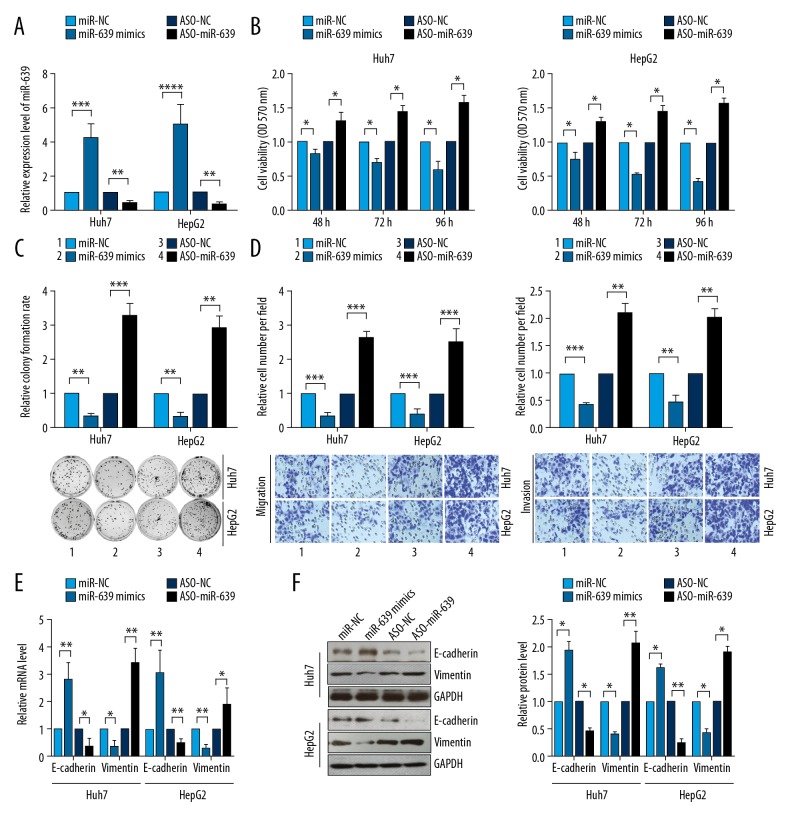
The levels of miR-639 expression were detected in two human HCC cell lines, Huh7 and HepG2, that had been transfected with miR-639 mimics or miR-639 inhibitor (ASO-miR-639). The results showed that miR-639 was significantly upregulated in HCC cells transfected with miR-639 mimics. However, miR-639 expression was significantly reduced when the cells were transfected with miR-639 inhibitor (Figure 2A). The upregulation of miR-639 resulted in reduced cell viability and proliferation, and inhibition of miR-639 increased HCC cell viability and proliferation (Figure 2B, 2C). The transwell migration assay showed that HCC cell migration was reduced when cells were transfected with miR-639 mimics. However, the down-regulation of miR-639 expression using the miR-639 inhibitor promoted cell migration in the two human HCC cell lines (Figure 2D). HCC cells transfected with miR-639 mimics showed that the mRNA and protein levels of E-cadherin were increased. However, the down-regulation of miR-639 significantly inhibited the mRNA and protein levels of vimentin (Figure 2E, 2F). These findings indicated that miR-639 inhibited the cell proliferation and EMT of HCC cells and acted as a tumor suppressor.
Figure 2.
microRNA-639 (miR-639) inhibited cell proliferation, cell migration, colony formation, and epithelial-mesenchymal transition (EMT) in human hepatocellular carcinoma (HCC) cell lines, Huh7 and HepG2. (A) miR-639 expression was detected using quantitative reverse transcription-polymerase chain reaction (RT-qPCR) in two human HCC cell lines, Huh7 and HepG2. (B) Cell viabilities of HCC cells were analyzed using the MTT assay. (C) Colony formation of HCC cells was assessed by using the colony formation assay. (D) Transwell migration and invasion of human HCC cell lines, Huh7 and HepG2, were studied using transwell assays. (E) Quantitative reverse transcription-polymerase chain reaction (RT-qPCR) was performed to evaluate the transcription levels of EMT markers. (F) Western blot was used to detect the expression levels of EMT markers. Studies were performed in triplicate. (* p<0.05; ** p<0.01; *** p<0.001; **** p<0.0001).
miR-639 inhibited KAT7 expression by targeting the 3′UTR
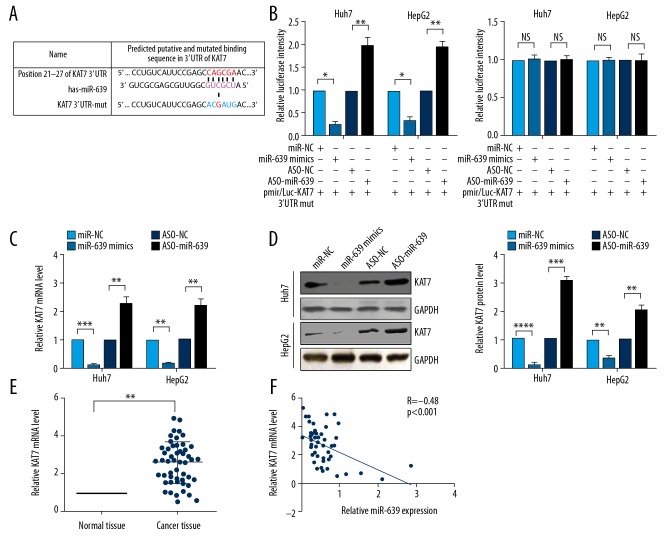
Regulation of the expression level of targets by miRNA usually occurs through the 3′UTR sequence [21]. Therefore, in this study, the target genes of miR-639 were predicted using TargetScan 7.1, and KAT7 was selected to study further (Figure 3A). To confirm that KAT7 was directly targeted by miR-639, the target relationship between miR-639 and KAT7 3′UTR was determined by the luciferase reporter assay. The findings showed that transfection with miR-639 reduced the luciferase fluorescence intensity, and inhibition of the expression of miR-639 by ASO-miR-639 increased the fluorescence intensity (Figure 3B). However, neither overexpression nor inhibition of miR-639 had a significant effect on the fluorescence intensity when the binding site of KAT7 3′UTR was mutated (Figure 3B). The transcriptional and translational levels of KAT7 were measured by quantitative reverse transcription-polymerase chain reaction (RT-qPCR) and Western blot. Expression of miR-639 reduced both the mRNA and protein expression levels of KAT7 and inhibition of miR-639 resulted in increased expression of mRNA and protein levels of KAT7 (Figure 3C, 3D). Also, the transcriptional level of KAT7 was significantly increased in HCC tumor tissues compared with normal liver tissues (Figure 3E). A negative correlation was found between the level of miR-639 and KAT7 (Figure 3F). These findings indicated that KAT7 was a target of miR-639 in HCC.
Figure 3.
microRNA-639 (miR-639) directly targeted KAT7 in human hepatocellular carcinoma (HCC). (A) The targets of miR-639 were predicted using TargetScan 7.1. KAT7 mRNA 3′UTR or the mutational 3′UTR of KAT7 mRNA are shown. (B) Luciferase experiments showed that miR-639 bound the 3′UTR of KAT7 mRNA. (C) The transcription levels of KAT7 and (D) the translation levels of KAT7 were evaluated by quantitative reverse transcription-polymerase chain reaction (RT-qPCR) and Western blot. (E) The mRNA level of KAT7 in HCC tissues was evaluated using RT-qPCR. (F) A negative correlation is shown between miR-639 expression and the KAT7 mRNA level. Studies were performed in triplicate. (* p<0.05; ** p<0.01; *** p<0.001; **** p<0.0001; NS – not significant).
KAT7 promoted cell proliferation and EMT in HCC
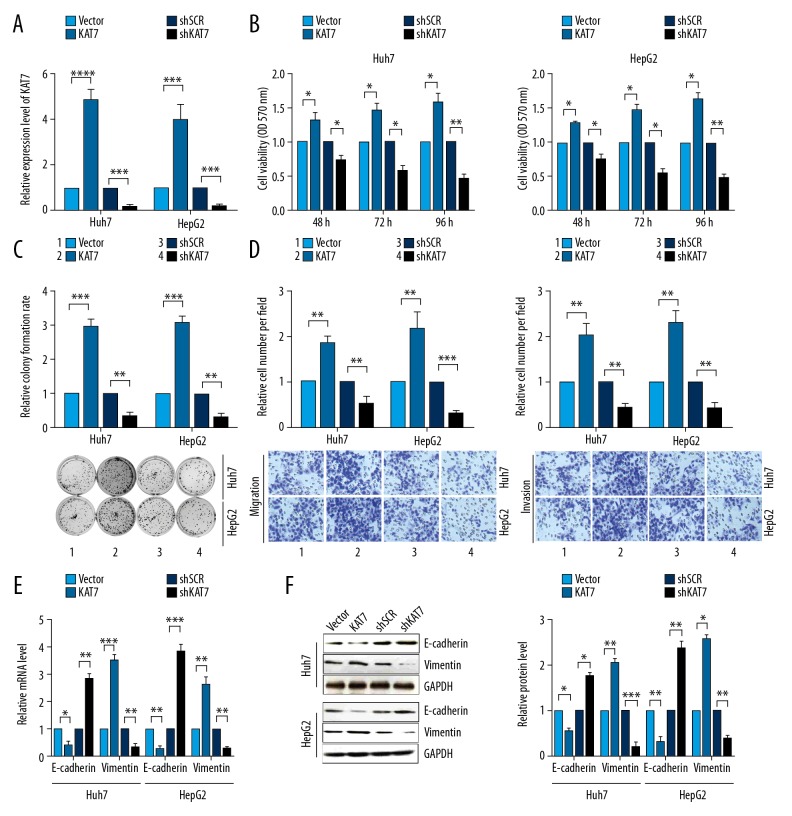
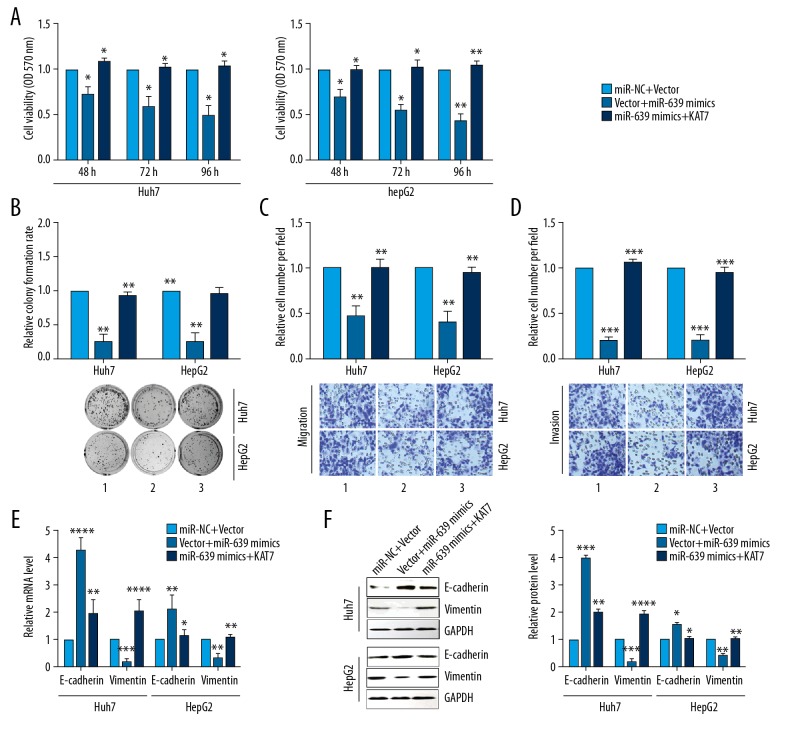
To further study the function of KAT7, the mRNA level of KAT7 in HCC cells was first detected after transfection with pcDNA3-KAT7 or pshR-KAT7 plasmids. The findings showed that the expression of mRNA of KAT7 was significantly increased after KAT7 overexpression, whereas the mRNA of KAT7 was significantly reduced after knockdown of KAT7 (Figure 4A). Overexpression of KAT7 significantly increased cell viability and colony formation of human HCC cells, and down-regulation KAT7 expression significantly reduced cell viabilities and colony formation (Figure 4B, 4C). HCC cells showed high migration capacity with the upregulation of KAT7, and the migration and invasion abilities for HCC cells were reduced when KAT7 was down-regulated (Figure 4D). Increased expression of KAT7 reduced E-cadherin expression but increased the expression of vimentin. Knockdown KAT7 significantly increased levels of transcription and translation of E-cadherin, but vimentin levels were significantly reduced (Figure 4E, 4F). Overexpression of KAT7 partly abolished the tumorigenic phenotypes and the transcription and translation levels of the EMT markers, E-cadherin and vimentin, caused by miR-639 (Figure 5A–5F). These findings indicated that KAT7 acted as an oncogene and promoted cell proliferation and EMT of HCC cells.
Figure 4.
KAT7 enhanced cell proliferation, cell migration, colony formation, and epithelial-mesenchymal transition (EMT) in human hepatocellular carcinoma (HCC) cell lines, Huh7 and HepG2. (A) KAT7 mRNA expression was detected using quantitative reverse transcription-polymerase chain reaction (RT-qPCR) in human HCC cell lines, Huh7 and HepG2. (B) The MTT assay evaluated the cell viabilities of HCC cells. (C) The colony formation assay was performed to assess colony formation in human HCC cell lines. (D) Transwell migration and invasion experiments were performed to evaluate human HCC cell lines. (E) RT-qPCR evaluated the mRNA levels of EMT markers for E-cadherin and vimentin. (F) Western blot was used to evaluate the expression levels of EMT markers, E-cadherin and vimentin. Studies were performed in triplicate. (* p<0.05; ** p<0.01; *** p<0.001; **** p<0.0001).
Figure 5.
Over-expression of KAT7 reduced the tumor suppressor effect of microRNA-639 (miR-639) on cell proliferation, cell migration, colony formation, and epithelial-mesenchymal transition (EMT) in human hepatocellular carcinoma (HCC) cell lines, Huh7 and HepG2. Over-expression of KAT7 reduced the tumor suppressor effect of miR-639 on cell viability (A), colony formation (B), transwell migration (C), and transwell invasion (D) in human HCC cell lines, Huh7 and HepG2. (E, F) Quantitative reverse transcription-polymerase chain reaction (RT-qPCR) and Western blot evaluated mRNA and protein levels of the EMT markers, E-cadherin and vimentin. Studies were performed in triplicate. (* P<0.05; ** P<0.01; *** P<0.001; **** P<0.0001).
miR-639 inhibited the Wnt/β-catenin pathway by down-regulating KAT expression in HCC
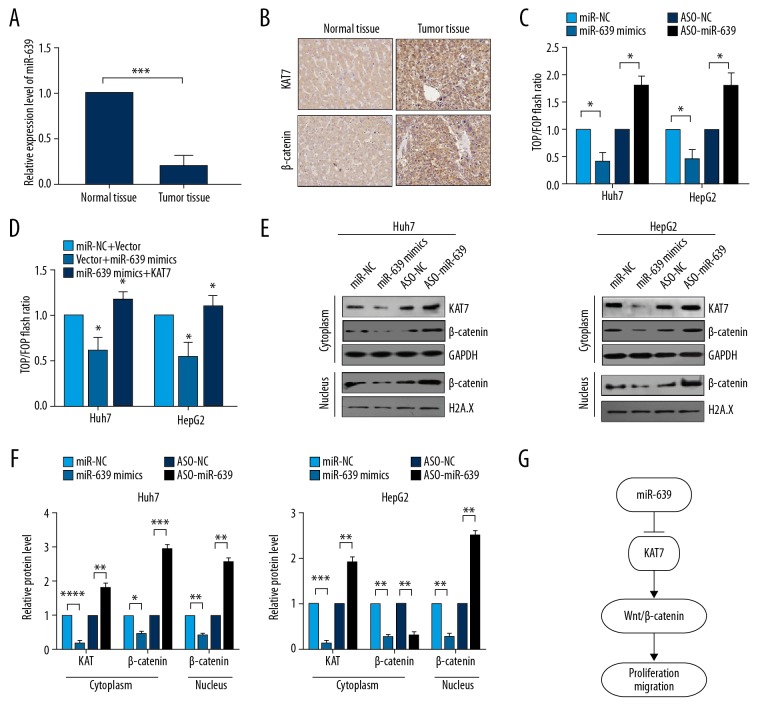
Tissue samples of HCC with low expression of miR-639 were used to investigate the expression levels of KAT7 and β-catenin (Figure 6A). Immunohistochemistry showed that KAT7 and β-catenin were both highly expressed in HCC tissues (Figure 6B), and the expression level of miR-639 was negatively associated with the expression of KAT7 and β-catenin. The reporter plasmids, pTop-Flash and pFop-Flash, were used to determine whether the Wnt/β-catenin signaling pathway was activated via miR-639. The results showed that the level of miR-639 was negatively associated with the activation of the Wnt/β-catenin pathway in HCC cells (Figure 6C). Also, the pTop-Flash/pFop-Flash reporter assay showed that co-expression of KAT7 and miR-639 partly recovered the transcriptional activity of Wnt/β-catenin pathway compared with the expression of miR-639 (Figure 6D). Also, inhibition of miR-639 enhanced the translocation of β-catenin into the nucleus from the cytoplasm, when measured by Western blot of nuclear and cytoplasmic extracts (Figure 6E, 6F). These findings showed that miR-639 inhibited cell proliferation and EMT by targeting KAT7 and down-regulating KAT7 expression via the Wnt/β-catenin pathway in HCC cells (Figure 6G).
Figure 6.
microRNA-639 (miR-639) down-regulated the Wnt/β-catenin pathway in human hepatocellular carcinoma (HCC) tissues and cell lines. (A) Quantitative reverse transcription-polymerase chain reaction (RT-qPCR) was used to analyze the level of miR-639 in- paired HCC tumor tissue and adjacent human normal liver tissue. (B) Immunohistochemistry was used to evaluate the expression of KAT7 and β-catenin in HCC tissue that showed reduced expression of miR-639. (C, D) pTop-Flash/pFop-Flash reporter vectors were used to evaluate the transcriptional activity of the Wnt/β-catenin signaling pathway. (E, F) Western blot analyzed the translation level of KAT7 and b-catenin. (G) The proposed mechanism of miR-639 inhibition of cell proliferation and migration of HCC cells by inhibition of the KAT7/Wnt/β-catenin signaling pathway. Studies were performed in triplicate. (* p<0.05; ** p<0.01; *** p<0.001; **** p<0.0001).
Discussion
Recent studies have shown that microRNAs (miRNAs) may have important biological roles in cell proliferation, cell differentiation, cell apoptosis, and tumor metastasis [26–28]. Altered expression of miRNAs is associated with the development and progression of several types of cancer, including hepatocellular carcinoma (HCC) [29–31]. Therefore, miRNAs were potential molecular biomarkers for cancer diagnosis and may be novel therapeutic targets in human cancer [32]. However, currently, the role of many miRNAs remains unclear.
In the present study, microRNA-639 (miR-639) was significantly down-regulated in HCC tumor tissue samples when compared with normal liver tissues. Down-regulation of miR-639 expression was associated with important clinical and pathologic characteristics of patients with HCC, including TNM stage, tumor size, and lymph node metastasis. Patients with HCC with low expression levels of miR-639 had a significantly reduced overall survival (OS), and miR-639 was also down-regulated in HCC cells. Also, the study of miR-639 in human HCC cells showed that the upregulation of miR-639 inhibited cell proliferation and epithelial-mesenchymal transition (EMT) in HCC cells. The findings from the present study indicated that miR-639 had a role as a tumor suppressor in HCC. These findings are supported by previous studies that have investigated the role of miR-639 in other human tumor types. Previous studies have shown that miR-639 plays an oncogenic role in several cancers. For example, miR-639 has been shown to promote cancer progression in breast cancer and thyroid cancer cells [13,14]. However, miR-639 has also been shown to inhibit EMT in human oral squamous carcinoma cancer cells [15]. The findings from the present study supported that miR-639 was a tumor suppressor in human HCC.
KAT7 has been shown to promote cell proliferation by activating the Wnt/β-catenin pathway in bladder cancer [17]. KAT7, also known as histone acetyltransferase binding to ORC1 (HBO1) or MOZ, YBF2/SAS3, SAS2 and TIP60 protein 2 (MYST2), is a member of the MYST family of histone acetyltransferases that was first identified as a regulator of DNA replication [22]. Recent studies have shown that KAT7 was upregulated in several human cancers and that overexpression of KAT7 promoted tumor invasion [23–25]. However, the role of KAT7 in HCC remained unclear. In the present study, the use of bioinformatics analysis and the luciferase reporter assay showed that that miR-639 directly targeted KAT7. Although the role of KAT7 as an oncogene has previously been demonstrated in other tumors [18–20], the role of KAT7 in HCC was first investigated in HCC in the present study. The findings demonstrated that KAT7 acted as an oncogene by promoting the proliferation and migration of human HCC cells in vitro.
Currently, several previous studies have shown that miRNAs regulate the Wnt/β-catenin pathway in human malignancy. For example, the miR-621/TRIM29 axis was reported to inhibit Wnt/β-catenin signaling pathway in bladder cancer [33]. Yao et al. showed that miR-3194-3p inhibited the Wnt/β-catenin pathway by targeting BCL9 in HCC [34]. Liu et al. demonstrated that miR-504 suppressed the Wnt/β-catenin pathway by directly targeting the FZD7 gene in glioblastoma [35]. A recently reported study showed that KAT7 promoted cell proliferation by activating the Wnt/β-catenin pathway in bladder cancer [17]. The findings from this study showed that miR-639 was associated with the activation of the Wnt/β-catenin pathway and was mediated by KAT7.
Conclusions
The aims of this study were to compare the expression of microRNA-639 (miR-639) in human hepatocellular carcinoma (HCC) tissues with adjacent normal liver tissue and its effects on patient outcome, to use bioinformatics and luciferase reporter analysis to identify the targets for miR-639 in HCC, and to study the effects of miR-639 in human HCC cells in vitro to identify the signaling pathways involved. The findings showed that miR-639 was down-regulated in HCC tumor tissue, miR-639 inhibited the proliferation and migration of HCC cells by the down-regulation of the KAT7/Wnt/β-catenin signaling, and miR-639 was associated with reduced overall survival (OS) in patients with HCC. These findings supported the potential role of miR-639 as a tumor suppressor in HCC.
Footnotes
Source of support: Departmental sources
Conflict of interest
None.
References
- 1.Siegel R, KDM, Jemal A. Cancer Statistics, 2017. Cancer J Clin. 2017;67(1):7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 2.Maluccio M, Covey A. Recent progress in understanding, diagnosing, and treating hepatocellular carcinoma. Cancer J Clin. 2012;62(6):394–99. doi: 10.3322/caac.21161. [DOI] [PubMed] [Google Scholar]
- 3.Borel F, Konstantinova P, Jansen PL. Diagnostic and therapeutic potential of miRNA signatures in patients with hepatocellular carcinoma. J Hepatol. 2012;56(6):1371–83. doi: 10.1016/j.jhep.2011.11.026. [DOI] [PubMed] [Google Scholar]
- 4.Morise Z, Kawabe N, Tomishige H, et al. Recent advances in the surgical treatment of hepatocellular carcinoma. World J Gastroenterol. 2014;20(39):14381–92. doi: 10.3748/wjg.v20.i39.14381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Budhu A, Jia HL, Forgues M, et al. Identification of metastasis-related microRNAs in hepatocellular carcinoma. Hepatology. 2008;47(3):897–907. doi: 10.1002/hep.22160. [DOI] [PubMed] [Google Scholar]
- 6.Negrini M, Gramantieri L, Sabbioni S, et al. microRNA involvement in hepatocellular carcinoma. Anticancer Agents Med Chem. 2011;11(6):500–21. doi: 10.2174/187152011796011037. [DOI] [PubMed] [Google Scholar]
- 7.Li W, Xie L, He X, et al. Diagnostic and prognostic implications of microRNAs in human hepatocellular carcinoma. Int J Cancer. 2008;123(7):1616–22. doi: 10.1002/ijc.23693. [DOI] [PubMed] [Google Scholar]
- 8.Sevignani C, Calin GA, Siracusa LD, et al. Mammalian microRNAs: A small world for fine-tuning gene expression. Mamm Genome. 2006;17(3):189–202. doi: 10.1007/s00335-005-0066-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bartel DP. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell. 2004;116(2):281–97. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 10.Shenouda SK, Alahari SK. MicroRNA function in cancer: Oncogene or a tumor suppressor? Cancer Metastasis Rev. 2009;28(3–4):369–78. doi: 10.1007/s10555-009-9188-5. [DOI] [PubMed] [Google Scholar]
- 11.Zhu Z, Zhang X, Wang G, et al. Role of microRNAs in hepatocellular carcinoma. Hepat Mon. 2014;14(8):e18672. doi: 10.5812/hepatmon.18672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cui SY, Wang R, Chen LB. MicroRNAs: Key players of taxane resistance and their therapeutic potential in human cancers. J Cell Mol Med. 2013;17(10):1207–17. doi: 10.1111/jcmm.12131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li L, Qiu X, Lv P, et al. miR-639 promotes the proliferation and invasion of breast cancer cell in vitro. Cancer Cell Int. 2014;14(1):39. doi: 10.1186/1475-2867-14-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lei ST, Shen F, Chen JW, et al. MiR-639 promoted cell proliferation and cell cycle in human thyroid cancer by suppressing CDKN1A expression. Biomed Pharmacother. 2016;84:1834–40. doi: 10.1016/j.biopha.2016.10.087. [DOI] [PubMed] [Google Scholar]
- 15.Lin Z, Sun L, Chen W, et al. miR-639 regulates transforming growth factor beta-induced epithelial-mesenchymal transition in human tongue cancer cells by targeting FOXC1. Cancer Sci. 2014;105(10):1288–98. doi: 10.1111/cas.12499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu W, Wang S, Sun Q, et al. DCLK1 promotes epithelial-mesenchymal transition via the PI3K/Akt/NF-κB pathway in colorectal cancer. Int J Cancer. 2017;142(10):2068–79. doi: 10.1002/ijc.31232. [DOI] [PubMed] [Google Scholar]
- 17.Chen Z, Zhou L, Wang L, et al. HBO1 promotes cell proliferation in bladder cancer via activation of Wnt/β-catenin signaling. Mol Carcinog. 2017;57(1):12–21. doi: 10.1002/mc.22715. [DOI] [PubMed] [Google Scholar]
- 18.Qiu HJ, Lu XH, Yang SS, et al. MiR-769 promoted cell proliferation in human melanoma by suppressing GSK3B expression. Biomed Pharmacother. 2016;82:117–23. doi: 10.1016/j.biopha.2016.04.052. [DOI] [PubMed] [Google Scholar]
- 19.Jin N, Wang Y, Liu L, et al. Dysregulation of the renin-angiotensin system and cardiometabolic status in mice fed a long-term high-fat diet. Med Sci Monit. 2019;25:6605–14. doi: 10.12659/MSM.914877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nagy A, Lanczky A, Menyhart O, et al. Validation of miRNA prognostic power in hepatocellular carcinoma using expression data of independent datasets. Sci Rep. 2018;8(1):9227. doi: 10.1038/s41598-018-27521-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005;120(1):15–20. doi: 10.1016/j.cell.2004.12.035. [DOI] [PubMed] [Google Scholar]
- 22.Sapountzi V, Côté J. MYST-family histone acetyltransferases: Beyond chromatin. Cell Mol Life Sci. 2011;68(7):1147–56. doi: 10.1007/s00018-010-0599-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Avvakumov N, Côté J. The MYST family of histone acetyltransferases and their intimate links to cancer. Oncogene. 2007;26(37):5395–407. doi: 10.1038/sj.onc.1210608. [DOI] [PubMed] [Google Scholar]
- 24.Iizuka M, Susa T, Takahashi Y, et al. Histone acetyltransferase HBO1 destabilizes estrogen receptor α by ubiquitination and modulates proliferation of breast cancers. Cancer Sci. 2013;104(12):1647–55. doi: 10.1111/cas.12303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Voss AK, Thomas T. MYST family histone acetyltransferases take center stage in stem cells and development. Bioessays. 2009;31(10):1050–61. doi: 10.1002/bies.200900051. [DOI] [PubMed] [Google Scholar]
- 26.Kumar MS, Lu J, Mercer KL, et al. Impaired microRNA processing enhances cellular transformation and tumorigenesis. Nat Genet. 2007;39(5):673–77. doi: 10.1038/ng2003. [DOI] [PubMed] [Google Scholar]
- 27.Lu J, Getz G, Miska EA, et al. MicroRNA expression profiles classify human cancers. Nature. 2009;435(7043):834–38. doi: 10.1038/nature03702. [DOI] [PubMed] [Google Scholar]
- 28.Ozen M, Creighton CJ, Ozdemir M, et al. Widespread deregulation of microRNA expression in human prostate cancer. Oncogene. 2008;27(12):1788–93. doi: 10.1038/sj.onc.1210809. [DOI] [PubMed] [Google Scholar]
- 29.Sun Q, Zhang J, Cao W, et al. Dysregulated miR-363 affects head and neck cancer invasion and metastasis by targeting podoplanin. Int J Biochem Cell Biol. 2013;45(3):513–20. doi: 10.1016/j.biocel.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 30.Meng F, Henson R, Wehbe-Janek H, et al. MicroRNA-21 regulates expression of the PTEN tumor suppressor gene in human hepatocellular cancer. Gastroenterology. 2007;133(2):647–58. doi: 10.1053/j.gastro.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang X, Liu S, Hu T, et al. Up-regulated microRNA-143 transcribed by nuclear factor kappa B enhances hepatocarcinoma metastasis by repressing fibronectin expression. Hepatology. 2009;50(2):490–99. doi: 10.1002/hep.23008. [DOI] [PubMed] [Google Scholar]
- 32.Shi M, Xie D, Gaod Y, et al. Targeting miRNAs for pancreatic cancer therapy. Curr Pharm Des. 2014;20(33):5279–86. doi: 10.2174/1381612820666140128210443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tian H, Wang X, Lu J, et al. MicroRNA-621 inhibits cell proliferation and metastasis in bladder cancer by suppressing Wnt/beta-catenin signaling. Chem Biol Interact. 2019;308:244–51. doi: 10.1016/j.cbi.2019.05.042. [DOI] [PubMed] [Google Scholar]
- 34.Yao B, Li Y, Wang L, et al. MicroRNA-3194-3p inhibits metastasis and epithelial-mesenchymal transition of hepatocellular carcinoma by decreasing Wnt/beta-catenin signaling through targeting BCL9. Artif Cells Nanomed Biotechnol. 2019;47(1):3885–95. doi: 10.1080/21691401.2019.1670190. [DOI] [PubMed] [Google Scholar]
- 35.Liu Q, Guan Y, Li Z, et al. miR-504 suppresses mesenchymal phenotype of glioblastoma by directly targeting the FZD7-mediated Wnt-beta-catenin pathway. J Exp Clin Cancer Res. 2019;38(1):35. doi: 10.1186/s13046-019-1370-1. [DOI] [PMC free article] [PubMed] [Google Scholar]