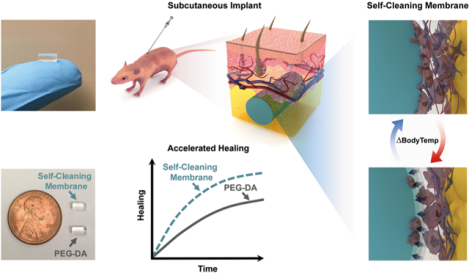
Abstract
Long-term, subcutaneously implanted continuous glucose biosensors have the potential to improve diabetes management and reduce associated complications. However, the innate foreign body reaction (FBR) both alters the local glucose concentrations in the surrounding tissues and compromises glucose diffusion to the biosensor due to the recruitment of high-metabolizing inflammatory cells and the formation of a dense, collagenous fibrous capsule. Minimizing the FBR has mainly focused on “passively antifouling” materials that reduce initial cellular attachment, including poly(ethylene glycol) (PEG). Instead, the membrane reported herein utilizes an “actively antifouling” or “self-cleaning” mechanism to inhibit cellular attachment through continuous, cyclic deswelling/reswelling in response to normal temperature fluctuations of the subcutaneous tissue. This thermoresponsive double network (DN) membrane is based on N-isopropylacrylamide (NIPAAm) and 2-acrylamido-2-methylpropane sulfonic acid (AMPS) (75:25 and 100:0 NIPAAm:AMPS in the 1st and 2nd networks, respectively; “DN-25%”). The extent of the FBR reaction of a subcutaneously implanted DN-25% cylindrical membrane was evaluated in rodents in parallel with a PEG-diacrylate (PEG-DA) hydrogel as an established benchmark biocompatible control. Notably, the DN-25% implants were more than 25× stronger and tougher than the PEG-DA implants while maintaining a modulus near that of subcutaneous tissue. From examining the FBR at 7, 30 and 90 days after implantation, the thermoresponsive DN-25% implants demonstrated a rapid healing response and a minimal fibrous capsule (~20–25 μm), similar to the PEG-DA implants. Thus, the dynamic self-cleaning mechanism of the DN-25% membranes represents a new approach to limit the FBR while achieving the durability necessary for long-term implantable glucose biosensors.
Keywords: Biocompatibility, Thermoresponsive, Hydrogel, PNIPAAm, Double network
Graphical Abstract
1. Introduction
Current commercial continuous glucose monitors (CGMs) are limited to ~7–14 days of use before replacement of the transcutaneous electrode is required due to a combination of biofouling, fluctuations in local analyte levels via metabolic reactions and irritation/infection at the implant site [1–4]. The first fully subcutaneous CGM, using fluorescent-based glucose detection, has recently received FDA approval but must be replaced after three months or less [5]. To extend the lifetime of transcutaneous and subcutaneous biosensors, it is essential to minimize the innate inflammatory response and foreign body reaction (FBR) while also maintaining requisite mechanical properties for long-term durability. Broadly, the normal progression seen immediately after implantation includes the recruitment of proteins and cells to the implant site during acute inflammation (days to weeks) which resolves through the encapsulation of the foreign material with dense, avascular collagenous tissue that may be 30 to >200-μm-thick [6–8] (weeks to months) [9, 10]. Thicker fibrous capsules can significantly inhibit analyte diffusion to the biosensor, ultimately diminishing its function [1, 8, 11]. Furthermore, the local glucose concentrations are influenced by high-metabolizing inflammatory cells, primarily macrophages, which are recruited during acute inflammation and persist throughout the FBR [1, 3, 12]. Therefore, a method to reduce the extent of fibrous encapsulation and accelerate the clearance of inflammatory cells could significantly improve the longevity of implantable glucose biosensors.
To overcome these challenges, most prior efforts have focused on “passively antifouling” membranes that use a hydration layer to minimize initial protein and cell adhesion, including hydrogel coatings (i.e. PEG [13, 14] and PHEMA [15, 16]), surface functionalization [17] and biomimetic coatings [18, 19]. However, in vivo results have been inconsistent, possibly due to the inability to prevent non-specific protein adhesion and thus cellular adhesion long-term as well as their lack of durability (i.e. coating degradation or de-lamination) [20]. Alternatively, porous materials have been utilized to encourage ingrowth of the surrounding tissue and to promote neovascularization to decrease the lag time of interstitial tissue analytes to the biosensor [20–22]. Although these porous implants have shown promising results for increasing the rate of transport of small molecules to biosensors, the continued presence of macrophages and other high-metabolizing cells could potentially impact glucose sensor functionality [21].
While multiple studies utilized passively antifouling materials to minimize the FBR around subcutaneous implants [13–19], none to our knowledge have explored a dynamic “self-cleaning” strategy. Thus, we have reported self-cleaning membranes based on thermoresponsive poly (N-isopropylacrylamide) (PNIPAAm) hydrogels whose cyclical deswelling/reswelling at temperatures above and below, respectively, their volume phase transition temperature (VPTT) may minimize biofouling and the FBR of subcutaneously implanted glucose biosensors [23, 24]. Moreover, we anticipate that such membranes could be used to contain a liquid glucose sensing assay [25, 26] to form an electronics-free, subcutaneously implantable glucose biosensor that could be used with a wearable, optical detection method. In our previous study [23], we demonstrated the ability of a cylindrical thermoresponsive, nanocomposite membrane to minimize the fibrous capsule formation when implanted into the subcutaneous tissue of a rodent model. These double network nanocomposite (DNNC) hydrogels were composed of two asymmetrically crosslinked, PNIPAAm-co-poly(N-vinylpyrrolidone) [P(NIPAAm-co-NVP)] networks with polysiloxane nanoparticles embedded within the 1st network to enhance swelling kinetics. By precisely tuning the DNNC membrane’s VPTT [onset (Tonset) ~36.5 °C, maximum (Tmax) ~39.5 °C], dynamic self-cleaning based on cyclic deswelling and reswelling would be triggered by natural body temperature fluctuations of rats (Trat ~37–38 °C [27–29]). This resulted in a favorably thin fibrous capsule (~30 μm) and an increase in local vascularization compared to a stiff PEG-DA control (i.e. prepared from low molecular weight PEG-DA, 575 g/mol, 100%) [23]. However, this DNNC membrane also displayed low mechanical strength (~0.4 MPa) [30] which could potentially lead to poor long-term durability. In our later studies, to enhance membrane mechanical properties for improved biosensor longevity, an electrostatic comonomer was incorporated into the 1st network of a PNIPAAm-based DN hydrogel [31]. Denoted as DN-25%, these membranes were composed of a tightly crosslinked 1st network of NIPAAm copolymerized with 2-acrylamido-2-methylpropane sulfonic acid (AMPS) at a wt% ratio of 75:25 (NIPAAm:AMPS) and a loosely crosslinked 2nd network of NIPAAm copolymerized with NVP to tune the VPTT [24]. These in vitro studies on DN-25% planar hydrogels confirmed the simultaneous enhancement of mechanical strength and hydration due to the incorporation of the negatively charged AMPS comonomer as well as cytocompatibility and successful thermally-driven release of cultured fibroblasts [24, 31].
Herein, we sought to examine the effect of a DN-25% membrane implant on the FBR and fibrous capsule formation when implanted in a subcutaneous rodent model experiencing normal local body temperature fluctuations (i.e. without an external transdermal heating device). As for our earlier study [23], the DN-25% VPTT was precisely tuned (Tonset ~36.5 °C; Tmax of ~41 °C) such that, throughout the cyclical deswelling/reswelling in response to body temperature fluctuations, the membrane would change in diameter ~20–25 μm (~1% of total diameter) while primarily remaining in a hydrated, swollen state, thereby maximizing the potential for glucose diffusion. A conventional PEG-DA hydrogel (3.4 kDa, 10 wt%) was implanted in parallel to provide a benchmark tissue response to a passively antifouling hydrogel with well-established biocompatibility. Since both the DN-25% and PEG-DA hydrogels displayed moduli similar to the native subcutaneous and dermis tissues (E ~0.01–0.25 MPa) [32–34], adverse contributions due to shear stresses were avoided. Both hydrogels were fabricated as small, cylindrical membranes (~2.5 × 5 mm, diameter × length), representing a plausible geometry for a membrane-coated glucose biosensor that permits implantation via injection to minimize local injury. The mechanical integrity, including the modulus, strength and toughness, of both DN-25% and PEG-DA implants were evaluated prior to implantation. To examine overall biocompatibility, both types of implants were injected subcutaneously via trocar needle into the dorsal of CD® Hairless rats (N = 33, male, ~8 weeks old, Charles River Laboratories). Histological analysis was performed at three well-established time points of 7, 30 and 90 days post-implantation to determine the intensity of the FBR and extent of fibrous encapsulation during the acute inflammatory response (7 days), early-to-mid stage healing (30 days) and late-stage healing (90 days). Primarily, the rate of progression from initial inflammation to complete healing as well as tissue organization were evaluated to assess the potential of the self-cleaning DN-25% as a long-term, subcutaneously implantable glucose biosensor membrane.
2. Experimental section
2.1. Materials
N-isopropylacrylamide (NIPAAm, 97%), 2-acrylamido-2-methylpropane sulfonic acid (AMPS, 97%), 1-vinyl-2-pyrrolidinone (NVP), N,N′-methylenebisacrylamide crosslinker (BIS, 99%), acryloyl chloride, triethylamine (Et3N), K2CO3, MgSO4, poly(ethylene glycol) (PEG; PEG-3400, MW = 3000–3700 g/mol per manufacturers specifications) and 2,2-di-methyl-2-phenyl-acetophenone (DMAP) were obtained from Sigma Aldrich. 1-[4-(2-Hydroxyethoxy)-phenyl]-2-hydroxy-2-methyl-1-propane-1-one (Irgacure® 2959) was purchased from BASF. Ethyl ether anhydrous was acquired from Fisher Scientific. For hydrogel fabrication, deionized water (DI) with a resistance of 18 MΩ·cm (Cascada LS MK2, Pall) was used. Phosphate-buffered saline (PBS, 1×, pH 7.4, without calcium and magnesium) was obtained from Corning®.
2.2. Preparation of thermoresponsive DN hydrogels (“DN-25%”)
The thermoresponsive DN hydrogels were formed through a sequential, two step UV-cure process. The 1st network precursor solution consisted of NIPAAm monomer (0.75 g), AMPS monomer (0.25 g, 75:25 wt% NIPAAm:AMPS), BIS crosslinker (0.04 g), Irgacure-2959 photoinitiator (0.08 g) and DI water (7.0 mL). The 2nd network precursor solution was formed by combining NIPAAm (6.0 g), NVP (0.96 g), BIS (0.012 g), Irgacure 2959 (0.24 g), and DI H2O (21.0 mL). The thermoresponsive DN hydrogels are denoted as “DN-25%” where 25% equals the wt% of AMPS in the 1st network’s NIPAAm:AMPS wt% ratio. Cylindrical hydrogels (~2.5 × 5 mm, diameter × length) were prepared by pipetting the 1st network precursor solution into a cylindrical glass mold (inside diameter = ~1 mm, length = 10 mm) and sealing the open ends with Parafilm®. The mold was immersed in an ice water bath and exposed for 30 min to longwave UV light. Cylindrical hydrogels were removed from their molds, rinsed with DI, and soaked in a Petri dish containing DI for 2 days at RT with daily water changes. The resulting single network (SN) cylindrical hydrogels were then transferred into a Petri dish containing the 2nd network precursor solution for 48 h at 2 °C. Next, the cylindrical hydrogel was wrapped in Saran wrap, submerged in an ice water bath, exposed for 10 min to longwave UV light, and soaked in DI as above. The final swollen diameter was ~2.5 mm and a clean razor blade was lastly used to trim ends to achieve a cylindrical length of 5 mm.
2.3. Preparation of non-thermoresponsive PEG-DA hydrogels (“PEG-DA”)
The conventional PEG-DA hydrogels were formed through a one-step UV-cure process. The precursor solution consisted of PEG-DA (0.1 g, 3.4 k g/mol, synthesized as previously reported) [35, 36], 30 wt% DMAP in NVP (10 μL) and DI water (1.0 mL). The PEG-DA hydrogels are denoted as “PEG-DA”. Cylindrical hydrogels (~2.5 × 5 mm, diameter × length) were prepared by pipetting the 1st network precursor solution into a cylindrical glass mold (inside diameter = ~2.4 mm, length = 10 mm) and sealing the open ends with Parafilm®. The mold was exposed for 2 min at RT to longwave UV light. Cylindrical hydrogels were removed from their molds, rinsed with DI, and soaked in a Petri dish containing DI for 2 days at RT with daily water changes. The final swollen diameter was ~2.5 mm and a clean razor blade was used to trim ends to achieve a cylindrical length of 5 mm.
2.4. Mechanical testing of hydrogel implants
The mechanical properties of the DN-25% and PEG-DA hydrogel cylindrical rods were analyzed with an Instron 3340 at RT under static compression at a rate of 1 mm min−1 until fracture. The as-prepared implants were sliced into cylindrical cross-sections (~2.5 × 2 mm, diameter×length) and tested in replicate (N = 4). The elastic compressive modulus (E) was obtained from the slope of the linear portion of the stress–strain curve from 0% to 10% strain. The ultimate compressive strength (σf) and the % strain at break (εf) were defined, respectfully, as the stress and strain values at the point of fracture. Finally, the toughness was obtained from the integration of the stress-strain curve. GraphpadPrism was used to analyze statistical significance (p < 0.05) between the DN-25% and PEG-DA implants through student’s t tests using Welch’s correction.
2.5. Implantation
For each time point (7, 30 and 90 days), each animal (N = 11) were implanted with one thermoresponsive DN-25% and one conventional PEG-DA hydrogels (~2.5 × 5 mm, diameter × length). Prior to implantation, all cylindrical hydrogels were sterilized by soaking in 70% ethanol for 45 min, then transferring into sterile PBS for three consecutive 30 min washes, followed by two overnight soaks in fresh PBS. Using isoflurane by inhalation, animals were anesthetized and anesthesia depth was tested by foot pinch reaction. Sterile trocar needles (10 G; inner diameter = ~2.7 mm, Innovative Research of America) were utilized to inject one DN-25% and one PEG-DA cylindrical hydrogel into the subcutaneous tissue (2–3 mm in depth) in the dorsal side of CD® Hairless rats (N = 33, male, ~8 weeks old, Charles River Laboratories). For animals designated for the 90 day studies, a cellophane tape debridement method followed by a small incision was utilized prior to injection via trocar needle. The CD® Hairless rats were chosen to avoid shaving of the implant site that commonly results in undesirable skin irritation. Male rats were selected for their well-established body temperature fluctuations, ranging from ~37.0 to 38.0 °C [27, 28]. Following implantation, the injection site was closed with surgical adhesive (3 M Vetbond™ Tissue Adhesive, No. 1469SB). Material composition and dorsal placement were recorded for each rat/implant. Portable temperature monitors (RC-5 USB Temperature Data Logger, Elitech®) recorded the temperature of the room every 5 min in three different locations. IACUC Approval: NIH guidelines for the care and use of laboratory animals (NIH Publication #85–23 Rev. 1985) have been observed. All animal investigations conducted were approved by the Texas A&M University Institutional Animal Care and Use Committee and fell under the Animal Use Protocol #2015–0287.
2.6. Histological evaluation
At 7, 30 or 90 days post-implantation, the designated 11 animals were euthanized by CO2 asphyxiation, photographed, evaluated for gross changes and immediately fixed in 10% neutral buffered formalin for two weeks. Implants and their surrounding tissue were removed and processed for histology by serial dehydration, paraffin embedding, sectioning, and staining (hematoxylin and eosin (H&E), Masson’s trichrome). All morphometric analysis was performed in the Cardiovascular Pathology Laboratory (CVP) in the College of Veterinary Medicine & Biomedical Sciences at Texas A&M University by a board certified pathologist (Dr Fred Clubb, DVM) that remained blinded throughout the study. Each tissue cross-section was scanned to enable consistent viewing at 100× using OlyVIA Olympus slide-viewing software during analysis to provide a standard field of view. To quantify the cell types and fibrotic tissue present in the capsule surrounding the hydrogel cylinders, the tissue cross-sections were divided into 4 sectors (Fig. S1). To examine cellular response, 100 cells per sector were identified manually by established morphometric parameters as neutrophils, eosinophils, lymphocytes, erythrophagocytosis, hemosiderin-laden macrophages, macrophages, fibroblasts, fibrocytes or multinucleated giant cells (MNGCs). The percentages of each cell type were calculated for each sector and averaged, giving an average cellular presence around each implant type per animal. These values were then averaged over all animals for each implant type to get the final reported percentages at each time point. Additionally, in each sector the presence of capillaries, fibrin, loose collagen and dense collagen was recorded as (−) indicating little to no presence, (±) indicating low presence or (+) indicating high presence. Finally, a healing score was assigned to each sector utilizing a criteria (Table S1) previously established in the CVP lab [23] as a rating of overall healing in each region.
Fibrous capsule thickness was measured at 8 distinct locations, 12:00 corresponding to the most superior point of the capsule nearest the dermis and 6:00 corresponding to the most inferior point of the capsule (Fig. S1). The depth of the implant (2–3 mm, Fig. S2) was determined from the outermost edge of the dermis to the interface of the implant with the tissue at the 12:00 position. Furthermore, the diameter of each hydrogel cylinder was measured from the 12:00 to 6:00 position (perpendicular to the dermis) and the 3:00 to 9:00 position (parallel to the dermis). All capsule measurements were performed blinded to avoid potential bias. GraphpadPrism was used to analyze statistical significance (p < 0.05) between the DN-25% and PEG-DA implants through multiple t tests or one-way ANOVA (Sidak’s multiple comparisons test) for all histological analyses.
3. Results and discussion
3.1. Mechanical properties
The mechanical properties of a membrane for an implantable glucose biosensor are of critical importance to performance and longevity. A narrow, cylindrical geometry affords simple implantation via injection and also minimizes injury to the surrounding tissue. Thus, the membrane must withstand the forces of initial implantation as well as those of everyday activities which may vary depending on implant location. For instance, the wrist represents a potentially desirable location of a subcutaneous glucose biosensor as it could be coupled with a wearable “watchlike” detection device. While many variables may contribute to the impact forces experienced by a subcutaneously implanted biosensor, we hypothesized that a compressive strength of >1 MPa could ensure integrity during most common activities. For example, the force of impact due to a short fall onto outstretched hands has been reported to produce a stress of up to ~0.8 MPa on the palm [37]. To evaluate the compressive strength of the implants, the cylindrical membrane implants were prepared then cut cross-sectionally into discs (~2.5 mm × ~1 mm, diameter×thickness) and compressed until fracture. Notably, the DN-25% membranes exhibited high strength (3.34 MPa) and toughness (446.8 kJ m−3), greater than 25× that of the PEG-DA membranes (0.13 MPa and 14.8 kJ m−3, respectively) (Fig. 1). This enhancement in strength and toughness compared to a conventional hydrogel is expected to provide improved durability. However, it is also important that the membrane stiffness does not greatly exceed that of the local subcutaneous and dermis tissues (E ~0.01–0.25 MPa) [32–34] in order to minimize local shear stress that can lead to a more severe FBR [38]. As such, studies have demonstrated that decreasing the modulus of hydrogel implants can improve the FBR and even reduce fibrous capsule formation [39]. In our study, both types of membranes displayed moduli near that of the surrounding tissue, with DN-25% having a modulus (~0.50 MPa) ~2× higher than that of PEG-DA (~0.22 MPa) (Fig. 1c). Further, these moduli values are much lower than that of transcutaneous metallic CGM electrodes (~GPa). Thus, the DN-25% membrane maintains an elastic modulus in the sub-MPa range, relatively close to that of the surrounding subcutaneous tissue, while providing a significantly higher strength and toughness than most standard hydrogels with established biocompatibilities, including the PEG-DA implants.
Fig. 1.
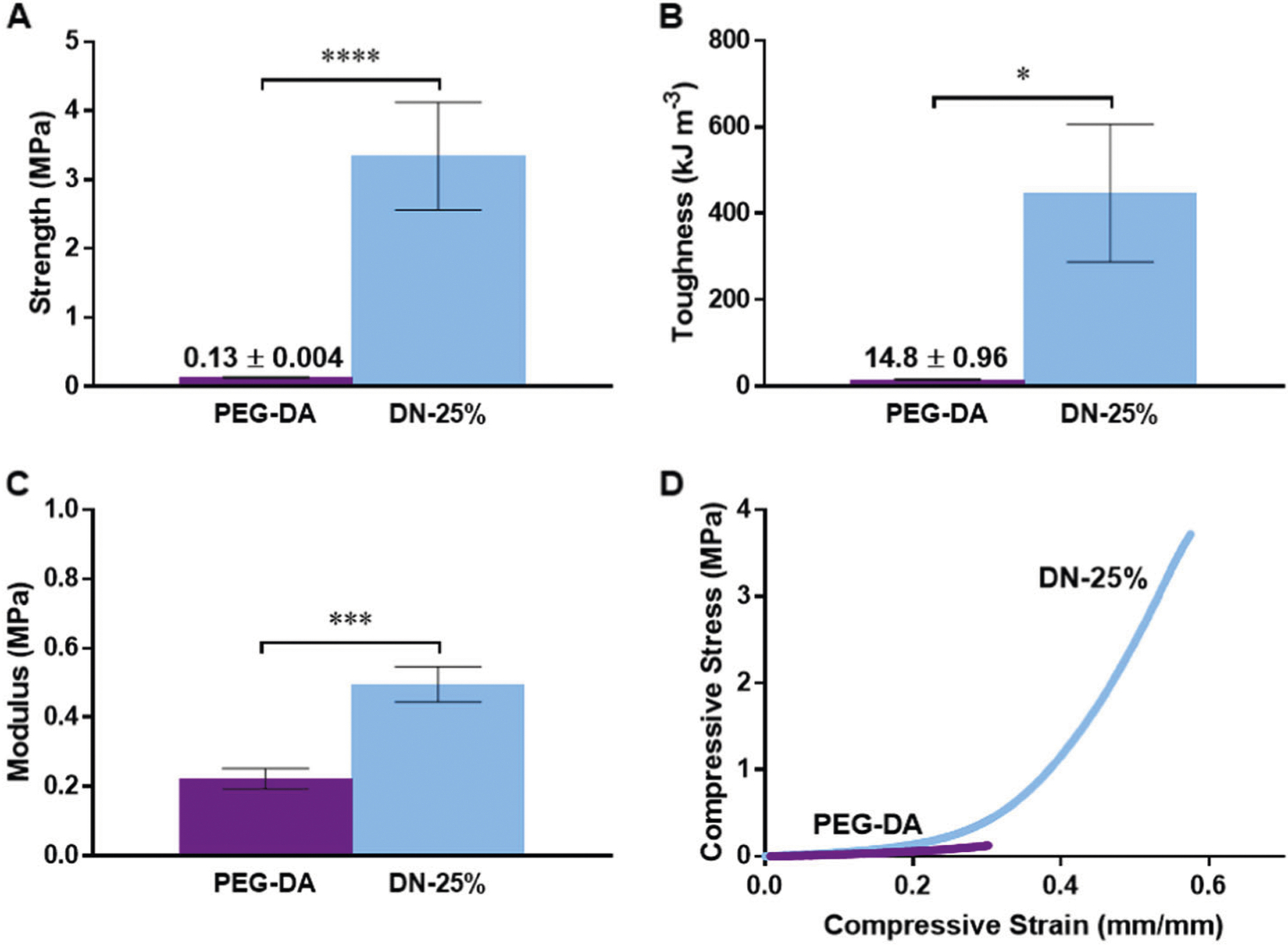
Compressive mechanical properties of thermoresponsive DN-25% and conventional PEG-DA implants, including a strength, b toughness, c elastic modulus and d a representative stress vs. strain curve, where * indicates a significant difference of p < 0.05, *** indicates a significant difference of p < 0.001 and **** indicates a significant difference of p < 0.0001
3.2. Implantation
Implantation of the 7 & 30 day time points resulted in small amounts of keratin debris within the subcutaneous tissue that was pulled in alongside the trocar needle during insertion. Due to its inflammatory nature, this keratin was thought to have produced the observed localized pyogranulomas surrounding the debris. These keratin pyogranulomas were shown to be independent of capsular fibrosis, capsular inflammation and gel composition at both 7 and 30 days. To avoid this in later studies (i.e. the 90 day time point), a cellophane tape debridement method was used to remove any loose keratin on the dermis before making a small incision to allow for easy insertion of the trocar needle into the dorsal subcutaneous tissue of the rats. No keratin debris was observed at the 90 day time points. The observed depth of implantation at each time point was not significantly different between implant types and their diameters remained similar to the initial implant diameters (~2.5 mm) across all time points (Fig. S2).
3.3. Cellular response
The cell types present in the capsular tissue surrounding the hydrogel implants are a major indicator for the overall implant biocompatibility and the stage of healing. For a biocompatible implant, a standard wound healing response (Fig. 2) will inevitably occur due to acute injury caused during implantation [40–42]. Any cellular presence other than what is recruited in this natural response would suggest that the implant could be intensifying or prolonging the normal inflammatory reaction. Typically, at 7 days, it is expected that most neutrophils will have been cleared and macrophages and fibroblasts as well as potentially low numbers of lymphocytes will have been recruited to the area. For a biocompatible implant, as acute inflammation transitions into the early stages of healing at 30 days, macrophages and few lymphocytes will still be present and the ratio of inactive fibrocytes to active fibroblasts will begin to increase as the healing process of fibrosis begins. Progressing into the later stages of healing at 90 days, some macrophages and fibroblasts may remain and the presence of fibrocytes will increase to form a mature fibrous capsule. Herein, this expected cellular response to injury was used as the basis to evaluate the response in the tissue surrounding the DN-25%. Moreover, results were compared to that of the PEG-DA membrane as it was anticipated to produce a benchmark, biocompatible response. As described below, the quantification of each major cell type, including neutrophils, macrophages, fibroblasts and fibrocytes, was utilized to evaluate the overall progression towards resolution of the capsular tissue adjacent to the thermoresponsive implant surfaces.
Fig. 2.
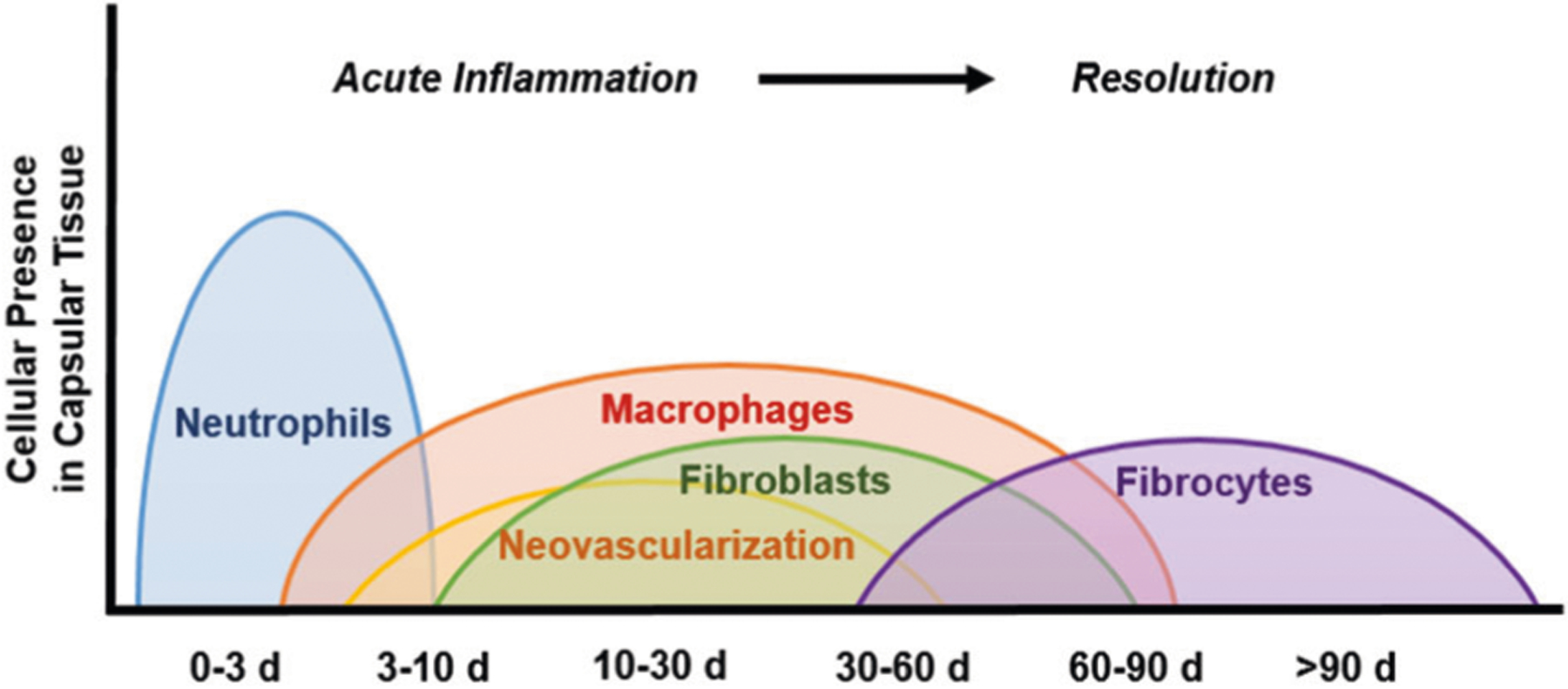
Schematic timeline showing the typical changes in cellular presence in the tissue surrounding a biocompatible implant during the progression of acute inflammation and healing after injury (i.e. implantation)
At 7 days, we found <5% neutrophils around the thermoresponsive DN-25% cylindrical membranes and no presence of neutrophils at both 30 and 90 days (Fig. 3). This indicated normal resolution of acute inflammation. Furthermore, if a more chronic inflammatory response had ensued, an increase in lymphocytes and plasma cells would have been observed [40]. Low percentages of lymphocytes (<5%) were observed around the DN-25% implants at 7 and 30 days, dropping to <1% by 90 days. Plasma cells and other inflammatory cells, such as eosinophils, were also absent at all time points in the capsular tissue surrounding the implants. As expected, a similarly mild response was seen around the PEG-DA implants. Therefore, the DN-25% implants did not elicit observable chronic inflammation but rather a normal wound healing response.
Fig. 3.
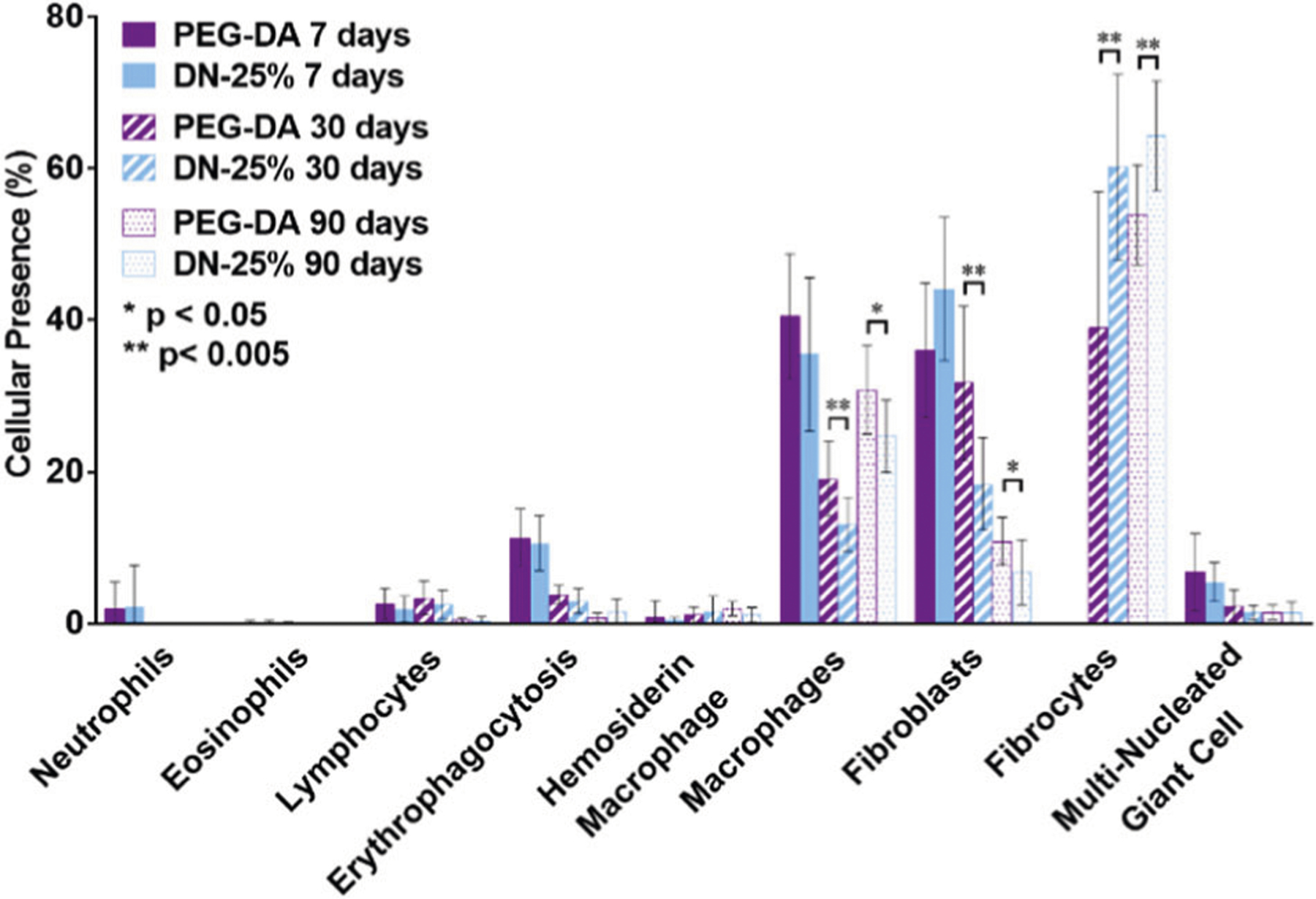
Graphical analysis displaying the cellular response surrounding the thermoresponsive DN-25% and conventional PEG-DA implants through the presence of various cell types after 7 days (solid), 30 days (striped) and 90 days (dots), where * indicates a significant difference of p < 0.05 and ** indicates a significant difference of p < 0.01
Additionally, macrophages play a major role in inflammation as well as healing. These cells are recruited early to the site of injury and remain present until the later stages of the FBR. Macrophages can interfere with local glucose concentration measurements due to their high metabolism of interstitial glucose. Thus, minimizing these inflammatory cells surrounding the implant is expected to optimize the accuracy of an implantable glucose biosensor. As expected, the largest percentage of macrophages was present at 7 days, decreasing at the later time points (Fig. 3). The reduction of macrophages surrounding the DN-25% implants after 30 days demonstrated progression from acute inflammation towards resolution. This was similarly observed in the normal healing response elicited by the PEG-DA implants. The apparent increase in macrophage presence around all implants from 30 to 90 days may be due to the qualitatively lower overall cellular density observed at 90 days. Notably, versus the PEG-DA implants with well-established biocompatibility, a lower percentage of macrophages were seen surrounding the DN-25% implants at both 30 and 90 days. Thus, the self-cleaning DN-25% membrane could potentially provide a more effective resolution of inflammation than conventional, passively antifouling hydrogels, such as PEG-DA membranes.
The amount of erythrophagocytosis and hemosiderin laden macrophages, macrophages involved in the engulfing and digestion, respectively, of red blood cells (RBCs) from injured capillaries, were also analyzed. The decrease in erythrophagocytosis from 7 days to 30 days (Fig. 3) indicates healing of the capillaries and clearance of any remaining RBCs from the area. The merging of several macrophages into a multinucleated giant cell (MNGC) that tries to engulf the material is a key indicator of an active FBR [40]. However, MNGCs were scarcely present around the thermoresponsive DN-25% implants (<2% at the 30 and 90 day time points) and showed no significant differences from the PEG-DA implants, signifying negligible aggravated inflammation as well as little activation of a FBR.
Lastly, the presence of fibroblasts and fibrocytes was observed over these three time points. In normal resolution, fibroblasts will be recruited to the site of injury shortly after macrophages during the early stages of healing to synthesize fibrous matrix proteins such as collagen. As healing progresses, the presence of activated fibroblasts declines as the number of inactive fibrocytes increases to aid in the later stages of healing and fibrosis [42, 43]. At 7 days, the DN-25% implants showed an abundance of fibroblasts while no fibrocytes were present (Fig. 3). By 30 days, comparatively more fibrocytes were present than fibroblasts around the DN-25% implants. This shift from predominantly fibroblasts to more fibrocytes was expected for normal healing and was similarly seen surrounding the benchmark PEG-DA implants. Notably, a greater amount of fibrocytes was observed around the thermoresponsive DN-25% implants (~60%) than the PEG-DA implants (~40%) at 30 days which could indicate that the self-cleaning hydrogels exhibited a more advanced healing response. This trend continued after 90 days; however, differences were less pronounced as resolution neared completion, with fibrocytes accounting for ~65% of all cells surrounding the DN-25% implants and ~55% of the cells around the PEG-DA implants (Fig. 3). Generally, a more rapid healing response would be beneficial for an implantable biosensor to reduce fluctuations in the local physiologic environment. Overall, by examining the cellular presence of multiple key cell types of the host response, the DN-25% membrane showed the potential to elicit milder inflammation as well as an accelerated healing response when compared to a benchmark biocompatible PEG-DA membrane at both the 30 and 90 day time points.
3.4. Fibrous capsule formation
To evaluate the extent of fibrous capsule formation around the implants, the thickness of the fibrous capsule was measured over eight locations around the cross-section of the membranes and averaged over all animals (Fig. S1 & S3). At each time point, no significant differences in thickness were seen between the DN-25% and the highly biocompatible PEG-DA implants. For both, the average capsule thickness was extraordinarily thin, never exceeding 25 μm, even after 90 days (Fig. 4a, b). As reported in our previous study, a rigid PEG-DA cylindrical implant (i.e. prepared from low molecular weight PEG-DA, 575 g/mol, 100%) produced a greater capsule thickness of ~45 μm at just 30 days and that of a nanocomposite thermoresponsive membrane was ~30 μm [23]. Such a capsule thickness of ~30 μm is consistently reported as the thinnest typically seen in most previous literature [6–8]. The fibrous capsule tissue, due to its dense, avascular nature, could inhibit the rate of glucose diffusion into an enclosed subcutaneous biosensor, with diffusion lag primarily dependent on capsule thickness. Reichert et al. estimated the diffusion rate of glucose (Deff) through fibrous capsule tissue to be ~1.87 × 10−6cm2 s−1 [44], which is comparable to the diffusion coefficient of the DN-25% membrane (~1.99 × 10−6cm2 s−1) [24]. The observed capsule thickness (~25 μm) is relatively insignificant compared to the estimated biosensor membrane wall thickness (~800–1000 μm). Thus, no major inhibition of glucose is predicted to be observed due to the remarkably thin fibrous capsule surrounding these implants.
Fig. 4.
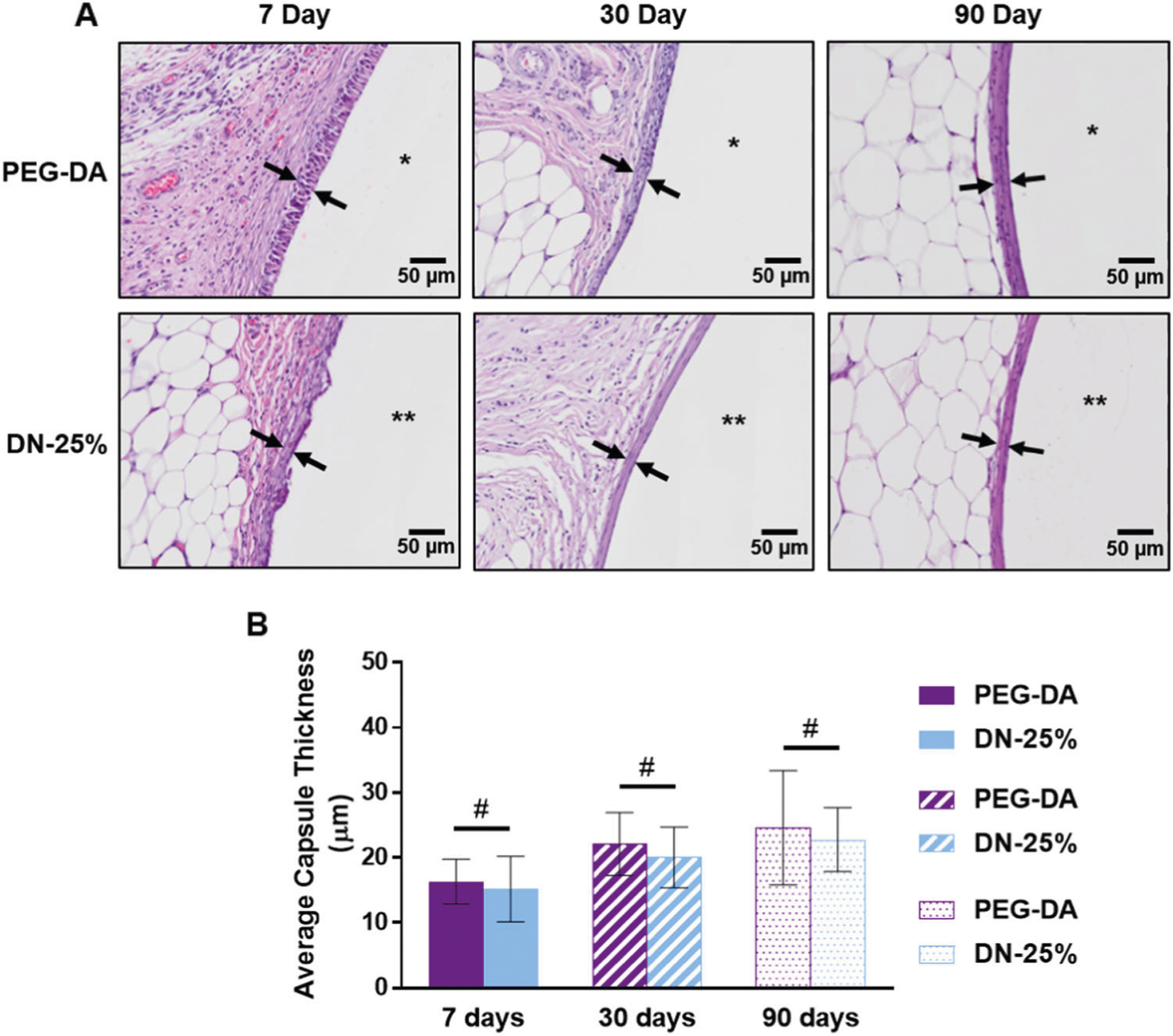
Evaluation of fibrous capsule formation around hydrogel implants. a H & E stained images representing capsules formed around PEG-DA (top, *) and DN-25% (bottom, **) implants at 7, 30 and 90 days. Differences in cellular organization near the implants seen only at the 7 day time point. b Average fibrous capsule thickness (n = 11) at 7, 30 and 90 days showing statistical similarity (#p > 0.05) between materials at each time point
While there were no significant differences in fibrous capsule thickness between the DN-25% and PEG-DA implants, a distinction was seen in the cellular organization within the tissue capsule at 7 days. Since fibrosis occurs in the later stages of the FBR (weeks rather than days) [40], the tissue capsule around the implants at 7 days was primarily an unorganized cellular layer without significant fibrous tissue as expected. However, the innermost cells surrounding the PEG-DA implant appeared to be more organized than those near the DN-25% implant (Fig. 4a). We hypothesize that the cyclical deswelling/reswelling of the thermoresponsive DN-25% surface resulted in a less organized tissue capsule initially. However, this trend did not continue after 30 and 90 days as fibrosis progressed at which both implant types resulted in a highly organized fibrous capsule that was easily distinguishable from the surrounding native tissue (Fig. 4a, Fig. S4).
To further analyze the composition of the fibrous capsules, the presence of other extracellular matrix (ECM) components was examined, including fibrin and collagen as well as neovascularization. Immediately after implantation, a fibrous scaffolding will begin to develop by the polymerization of fibrinogen into fibrin to aid in healing [11]. Fibrin was observed around the DN-25% implants at 7 days and dissipated after 30 and 90 days as expected (Fig. S5A & Fig. S5C). Subsequently, activated fibroblasts arriving to the implant site will produce collagen, which was seen as early as 7 days. Initially, the collagen forms a loose structure that overtime densifies with healing [40]. This densification was observed starting at 30 days (Fig. S5B & Fig. S5C) and continued to increase at 90 days (Fig. S5C), showing appropriate healing was able to occur around the thermoresponsive DN-25% implants, similar to the benchmark PEG-DA implants. Moreover, this trend matched the timeline of the cellular response with the highest fibroblast presence at 7 days correlating to a majority of loose collagen and the increase in ratio of fibrocytes to fibroblasts at 30 and 90 days correlating to an increasing presence of dense collagen as fibrosis progresses. Additionally, neovascularization will mainly occur in the early stages of healing and, as the healing proceeds, the amount of vascularization will recede back to the normal levels of the subcutaneous tissue [11]. This expected trend was observed for both DN-25% and PEG-DA implants, where at 7 days a much higher vascularization was present compared to at 30 and 90 days (Fig. S5C). Moreover, as the capsule densified at 30 and 90 days, the tissue was observed to be more avascular nearest the membrane surface around both types of implants (Fig. S5C). This low vascular presence confirms the need to minimize the fibrous capsule to avoid inhibition of glucose diffusion.
3.5. Healing
Finally, an overall healing score (Table S1), previously established by the CVP lab [23], was evaluated independently of the aforementioned data as a more general, semi-quantitative analysis of the healing stage at each time point. At 7 days, the DN-25% implants scored between a 1 and 2, similar to the PEG-DA implants (Fig. 5), indicating both were undergoing early-stage healing defined by the presence of both fibrin and loose collagen as well as the following dominant cellular components: macrophages, fibroblasts, neutrophils and lymphocytes. No major differences in overall healing were observed between the DN-25% implants and the benchmark control at this early time point, possibly due to the acute inflammatory response to injury masking any material-specific differences. However, at 30 days the thermoresponsive DN-25% implants showed slightly more advanced healing (score ~5) over the biocompatible PEG-DA implants (score ~4). This superior score was mainly due to an increase in dense collagen compared to loose fibrous tissue and a greater presence of fibrocytes compared to fibroblasts surrounding the DN-25% implants. Notably, a score of 4 corresponded to a normal healing response seen between days 30–60, whereas a score of 5 represents a mid-stage healing to healed response typically not seen until day 60–90. This indicates that the DN-25% membrane showed an enhancement over the expected timeframe for a normal healing response as well as the benchmark biocompatible PEG-DA implants. At 90 days, both the DN-25% and PEG-DA implants reached a nearly healed state, each receiving a similar score of ~5. As expected, at this time point we saw mainly dense collagen with small amounts of loose collagen and mostly fibrocytes along with some remaining macrophages and fibroblasts. Overall, these temporal healing scores agreed well with the qualitative results for fibrous tissue (Fig. S5C) and quantitative results for cellular presence (Fig. 3), further confirming the improvement in healing rate seen around the self-cleaning DN-25% implants over that of the conventional, passively antifouling PEG-DA implants. Thus, this improved healing score is also attributed to the cyclical deswelling/reswelling of the self-cleaning membrane.
Fig. 5.
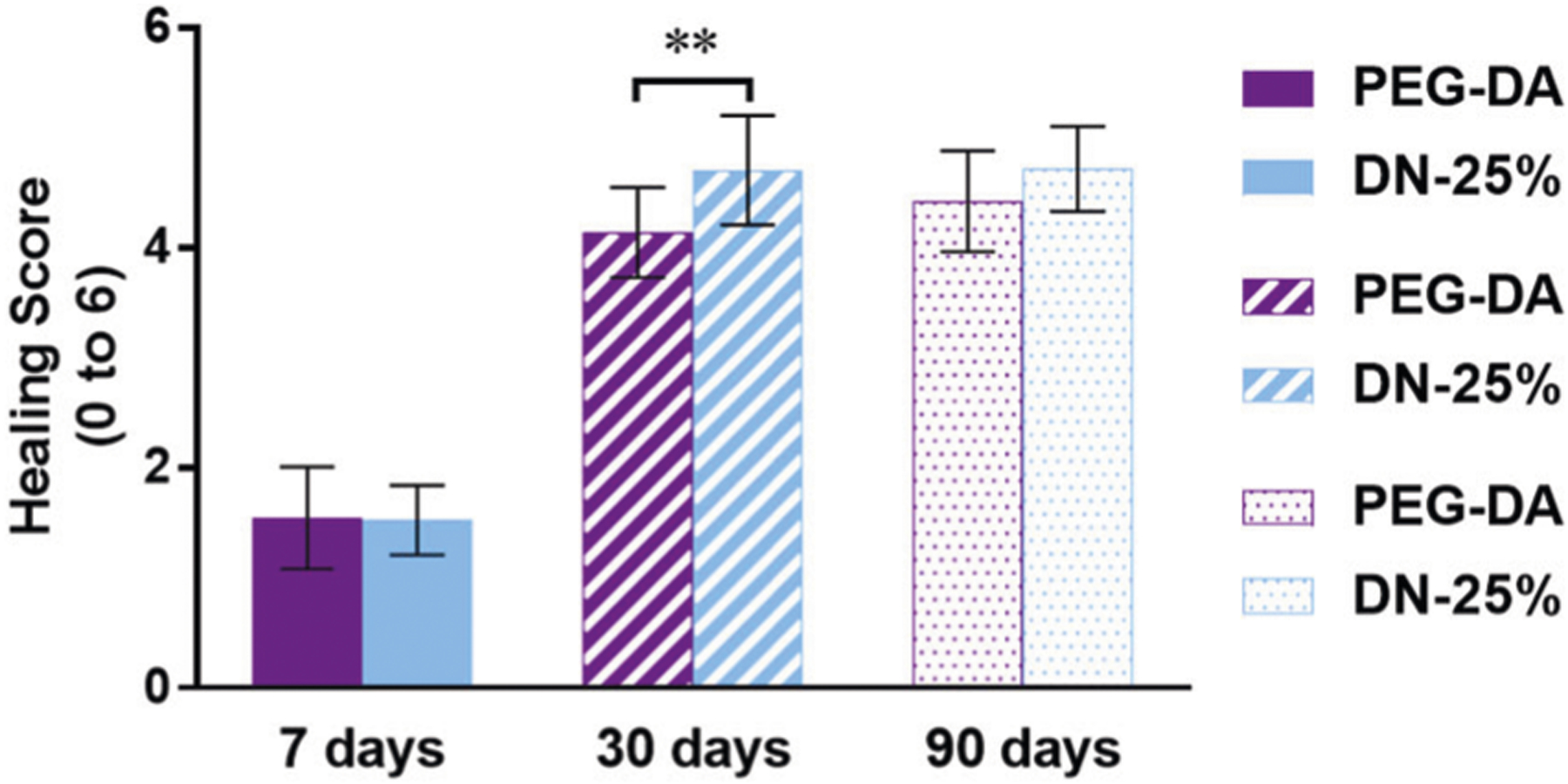
Average healing score given to both implant types after 7, 30 and 90 days with a score = 6 indicating fully healed (scores defined in Table S1), where ** indicates a significant difference of p < 0.01
4. Conclusions
Towards improving the longevity of subcutaneous glucose biosensors, we have developed a self-cleaning thermoresponsive hydrogel with robust mechanical properties and an ability to minimize the FBR through cyclical deswelling/reswelling stimulated by normal fluctuations in body temperature. Designated as DN-25%, this membrane is composed of a tightly crosslinked 1st network of NIPAAm copolymerized with an electrostatic AMPS comonomer at a wt% ratio of 75:25 (NIPAAm:AMPS) and a loosely crosslinked 2nd network of NIPAAm copolymerized with NVP to precisely tune the VPTT. Due to their electrostatic nature and double network structure, DN-25% cylindrical implants (~2.5 × 5 mm, diameter × length) achieved substantially higher strength (>25×) and toughness (>30×) than the conventional PEG-DA hydrogel implants (i.e. a benchmark biocompatible control) while maintaining the same order of stiffness (i.e. modulus) as both PEG-DA and the surrounding subcutaneous tissue. Although the DN-25% membranes exhibited a slightly higher modulus compared to PEG-DA membranes, the severity of the FBR was not increased. At 30 and 90 days post-implantation into the subcutaneous tissue of rats, the DN-25% implants showed milder inflammation as well as an accelerated healing response versus the well-established biocompatible PEG-DA implants. Notably, at these time points, a significantly lower number of macrophages and a higher ratio of fibrocytes to fibroblasts were observed surrounding the DN-25% implants. Thus, the self-cleaning DN-25% membranes demonstrated the potential to promote more effective resolution of inflammation than current passively antifouling membranes, such as PEG-DA implants. By 90 days, an extremely thin fibrous capsule of only ~20–25 μm formed around the DN-25% implants, similar to that of the PEG-DA implants. This unique combination of a reduction in highly-metabolizing macrophages and the thin surrounding capsule is predicted to better maintain glucose diffusion through the DN-25% membrane. In summary, this self-cleaning membrane provides an opportunity to improve subcutaneous glucose biosensor longevity due to its robust mechanical properties and ability to minimize the FBR.
Supplementary Material
Acknowledgements
Funding from the NIH/NIDDK (1R01DK095101-01A1) is gratefully acknowledged. This work was supported, in part, by funding from the National Science Foundation Engineering Research Center for Precise Advanced Technologies and Health Systems for Underserved Populations (PATHS-UP) (Award No. 1648451) and funding from the Robert J. Kleberg, Jr. and Helen C. Kleberg Foundation. A.K.M. thanks the NSF Graduate Research Fellowship Program (NSF GRFP M1703014).
Footnotes
Conflict of interest The authors declare that they have no conflict of interest.
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information The online version of this article (https://doi.org/10.1007/s10856-019-6282-2) contains supplementary material, which is available to authorized users.
References
- 1.Frost M, Meyerhoff ME. In vivo chemical sensors: tackling biocompatibility. Anal Chem. 2006;78:7370–77. 10.1021/ac069475k. [DOI] [PubMed] [Google Scholar]
- 2.Wisniewski N, Moussy F, Reichert WM. Characterization of implantable biosensor membrane biofouling. Fresenius’ J Anal Chem. 2000;366:611–21. 10.1007/s002160051556. [DOI] [PubMed] [Google Scholar]
- 3.Rebrin K, Fischer U, Hahn von Dorsche H, von Woetke T, Abel P, Brunstein E. Subcutaneous glucose monitoring by means of electrochemical sensors: fiction or reality? J Biomed Eng. 1992;14:33–40. 10.1016/0141-5425(92)90033-H. [DOI] [PubMed] [Google Scholar]
- 4.Nichols SP, Koh A, Storm WL, Shin JH, Schoenfisch MH. Biocompatible materials for continuous glucose monitoring devices. Chem Rev. 2013;113:2528–49. 10.1021/cr300387j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caccomo S FDA approves first continuous glucose monitoring system with a fully implantable glucose sensor and compatible mobile app for adults with diabetes. U.S. Food & Drug Administration; 2018. https://www.fda.gov/news-events/press-announcements/fda-approves-first-continuous-glucose-monitoring-system-fully-implantable-glucosesensor-and. Accessed 21 Jun 2019. [Google Scholar]
- 6.Ratner BD, Bryant SJ. Biomaterials: where we have been and where we are going. Annu Rev Biomed Eng. 2004;6:41–75. 10.1146/annurev.bioeng.6.040803.140027. [DOI] [PubMed] [Google Scholar]
- 7.Wang C, Yu B, Knudsen B, Harmon J, Moussy F, Moussy Y. Synthesis and performance of novel hydrogels coatings for implantable glucose sensors. Biomacromolecules. 2008;9:561–7. 10.1021/bm701102y. [DOI] [PubMed] [Google Scholar]
- 8.Novak MT, Yuan F, Reichert WM. Modeling the relative impact of capsular tissue effects on implanted glucose sensor time lag and signal attenuation. Anal Bioanal Chem. 2010;398:1695–1705. 10.1007/s00216-010-4097-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gerritsen M, Jansen JA, Lutterman JA. Performance of subcutaneously implanted glucose sensors for continuous monitoring. Neth J Med. 1999;54:167–79. 10.1016/S0300-2977(99)00006-6. [DOI] [PubMed] [Google Scholar]
- 10.Anderson JM, Rodriguez A, Chang DT. Foreign body reaction to biomaterials. Semin Immunol. 2008;20:86–100. 10.1016/j.smim.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Onuki Y, Bhardwaj U, Papadimitrakopoulos F, Burgess DJ. A review of the biocompatibility of implantable devices: current challenges to overcome foreign body response. J Diabetes Sci Technol. 2008;2:1003–15. 10.1177/193229680800200610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Daley JM, Shearer JD, Mastrofrancesco B, Caldwell MD. Glucose metabolism in injured tissue: a longitudinal study. Surgery. 1990;107:187–92. [PubMed] [Google Scholar]
- 13.Quinn CAP, Connor RE, Heller A. Biocompatible, glucose-permeable hydrogel for in situ coating of implantable biosensors. Biomaterials. 1997;18:1665–70. 10.1016/S0142-9612(97)00125-7. [DOI] [PubMed] [Google Scholar]
- 14.Espadas-Torre C, Meyerhoff ME. Thrombogenic properties of untreated and poly(ethylene oxide)-modified polymeric matrixes useful for preparing intraarterial ion-selective electrodes. Anal Chem. 1995;67:3108–3114. 10.1021/ac00114a003. [DOI] [PubMed] [Google Scholar]
- 15.Quinn CP, Pathak CP, Heller A, Hubbell JA. Photo-crosslinked copolymers of 2-hydroxyethyl methacrylate, poly(ethylene glycol) tetra-acrylate and ethylene dimethacrylate for improving biocompatibility of biosensors. Biomaterials. 1995;16:389–96. 10.1016/0142-9612(95)98856-9. [DOI] [PubMed] [Google Scholar]
- 16.Yu B, Wang C, Ju YM, West L, Harmon J, Moussy Y, et al. Use of hydrogel coating to improve the performance of implanted glucose sensors. Biosens Bioelectron. 2008;23:1278–84. 10.1016/j.bios.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 17.Ratner BD. Surface modification of polymers: chemical, biological and surface analytical challenges. Biosens Bioelectron. 1995;10:797–804. 10.1016/0956-5663(95)99218-A. [DOI] [PubMed] [Google Scholar]
- 18.Nishida K, Sakakida M, Ichinose K, Uemura T, Uehara M, Kajiwara K, et al. Development of a ferrocene-mediated needle-type glucose sensor covered with newly designed biocompatible membrane, 2-methacryloyloxyethyl phosphorylcholine-co-n-butyl methacrylate. Med Prog Technol. 1995;21:91–103. [PubMed] [Google Scholar]
- 19.Lewis AL. Phosphorylcholine-based polymers and their use in the prevention of biofouling. Colloids Surf B Biointerfaces. 2000;18:261–75. 10.1016/S0927-7765(99)00152-6. [DOI] [PubMed] [Google Scholar]
- 20.Bryers JD, Giachelli CM, Ratner BD. Engineering biomaterials to integrate and heal: the biocompatibility paradigm shifts. Biotechnol Bioeng. 2012;109:1898–1911. 10.1002/bit.24559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sharkawy AA, Klitzman B, Truskey GA, Reichert WM. Engineering the tissue which encapsulates subcutaneous implants. III. Effective tissue response times. J Biomed Mater Res. 1998;40:598–605. [DOI] [PubMed] [Google Scholar]
- 22.Brauker JH, Carr-Brendel VE, Martinson LA, Crudele J, Johnston WD, Johnson RC. Neovascularization of synthetic membranes directed by membrane microarchitecture. J Biomed Mater Res. 1995;29:1517–24. 10.1002/jbm.820291208. [DOI] [PubMed] [Google Scholar]
- 23.Abraham AA, Means AK, Clubb FJ, Fei R, Locke AK, Gacasan EG, et al. Foreign body reaction to a subcutaneously implanted self-cleaning, thermoresponsive hydrogel membrane for glucose biosensors. ACS Biomater Sci Eng. 2018;4:4104–4111. 10.1021/acsbiomaterials.8b01061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fei R, Means AK, Abraham AA, Locke AK, Coté GL, Grunlan MA. Self-cleaning, thermoresponsive P(NIPAAm-co-AMPS) double network membranes for implanted glucose biosensors. Macromol Mater Eng. 2016;301:935–43. 10.1002/mame.201600044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cummins BM, Li M, Locke AK, Birch DJS, Vigh G, Coté GL. Overcoming the aggregation problem: a new type of fluorescent ligand for ConA-based glucose sensing. Biosens Bioelectron. 2015;63:53–60. https://doi.org/10.1016Zj.bios.2014.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Locke AK, Means AK, Dong P, Nichols TJ, Coté GL, Grunlan MA. A layer-by-layer approach to retain a fluorescent glucose sensing assay within the cavity of a hydrogel membrane. ACS Appl Bio Mater. 2018. 10.1021/acsabm.8b00267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bolles RC, Duncan PM. Daily course of activity and subcutaneous body temperature in hungry and thirsty rats. Physiol Behav. 1969;4:87–9. 10.1016/0031-9384(69)90018-3. [DOI] [Google Scholar]
- 28.Shido O, Sakurada S, Kohda W, Nagasaka T. Day–night changes of body temperature and feeding activity in heat-acclimated rats. Physiol Behav. 1994;55:935–9. 10.1016/0031-9384(94)90082-5. [DOI] [PubMed] [Google Scholar]
- 29.Kort WJ, Hekking-Weijma JM, Tenkate MT, Sorm V, VanStrik R. A microchip implant system as a method to determine body temperature of terminally ill rats and mice. Lab Anim. 1998;32:260–9. 10.1258/002367798780559329. [DOI] [PubMed] [Google Scholar]
- 30.Fei R, George JT, Park J, Grunlan MA. Thermoresponsive nanocomposite double network hydrogels. Soft Matter. 2012;8:481–87. 10.1039/C1SM06105D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fei R, George JT, Park J, Means AK, Grunlan MA. Ultra-strong thermoresponsive double network hydrogels. Soft Matter. 2013;9:2912–19. 10.1039/C3SM27226E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pailler-Mattei C, Bec S, Zahouani H. In vivo measurements of the elastic mechanical properties of human skin by indentation tests. Med Eng Phys. 2008;30:599–606. 10.1016/j.medengphy.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 33.Liang X, Boppart SA. Biomechanical properties of in vivo human skin from dynamic optical coherence elastography. IEEE Trans Biomed Eng. 2010;57:953–59. 10.1109/TBME.2009.2033464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li C, Guan G, Reif R, Huang Z, Wang RK. Determining elastic properties of skin by measuring surface waves from an impulse mechanical stimulus using phase-sensitive optical coherence tomography. J R Soc Interface. 2012;9:831–41. 10.1098/rsif.2011.0583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gacasan EG, Sehnert RM, Ehrhardt DA, Grunlan MA. Templated, macroporous PEG-DA hydrogels and their potential utility as tissue engineering scaffolds. Macromol Mater Eng. 2017;302:1600512 10.1002/mame.201600512. [DOI] [Google Scholar]
- 36.Hou Y, Schoener CA, Regan KR, Munoz-Pinto D, Hahn MS, Grunlan MA. Photo-cross-linked PDMSstar-PEG hydrogels: synthesis, characterization, and potential application for tissue engineering scaffolds. Biomacromolecules. 2010;11:648–56. 10.1021/bm9012293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Choi WJ, Robinovitch SN. Pressure distribution over the palm region during forward falls on the outstretched hands. J Biomech. 2011;44:532–39. 10.1016/j.jbiomech.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Helton KL, Ratner BD, Wisniewski NA. Biomechanics of the sensor-tissue interface—effects of motion, pressure, and design on sensor performance and foreign body response—part II: examples and application. J Diabetes Sci Technol. 2011;5:647–56. 10.1177/193229681100500318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Blakney AK, Swartzlander MD, Bryant SJ. The effects of substrate stiffness on the in vitro activation of macrophages and in vivo host response to poly(ethylene glycol)-based hydrogels. J Biomed Mater Res A. 2012;100:1375–86. 10.1002/jbm.a.34104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Anderson JM. Biological responses to materials. Annu Rev Mater Res. 2001;31:81–110. 10.1146/annurev.matsci.31.1.81. [DOI] [Google Scholar]
- 41.Witte MB, Barbul A. General principles of wound healing. Surg Clin North Am. 1997;77:509–28. 10.1016/S0039-6109(05)70566-1. [DOI] [PubMed] [Google Scholar]
- 42.Metz CN. Fibrocytes: a unique cell population implicated in wound healing. Cell Mol Life Sci. 2003;60:1342–50. 10.1007/s00018-003-2328-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bucala R, Spiegel LA, Chesney J, Hogan M, Cerami A. Circulating fibrocytes define a new leukocyte subpopulation that mediates tissue repair. Mol Med. 1994;1:71–81. [PMC free article] [PubMed] [Google Scholar]
- 44.Adam SA, Bruce KATG, Monty RW. Engineering the tissue which encapsulates subcutaneous implants. I. Diffusion properties. J Biomed Mater Res. 1997;37:401–12. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


