Abstract
Objective
A large‐scale, double‐blind trial (SP0993; NCT01243177) demonstrated that lacosamide was noninferior to controlled‐release carbamazepine (carbamazepine‐CR) in terms of efficacy, and well tolerated as first‐line monotherapy in patients (≥16 years of age) with newly diagnosed epilepsy. We report primary safety outcomes from the double‐blind extension of the noninferiority trial (SP0994; NCT01465997) and post hoc analyses of pooled long‐term safety and efficacy data from both trials.
Methods
Patients were randomized 1:1 to lacosamide or carbamazepine‐CR. Doses were escalated (lacosamide: 200/400/600 mg/d; carbamazepine‐CR: 400/800/1200 mg/d) based on seizure control. Eligible patients continued randomized treatment in the extension. Primary outcomes of the extension were treatment‐emergent adverse events (TEAEs), serious TEAEs, and discontinuations due to TEAEs. Post hoc analyses of data from combined trials included 12‐ and 24‐month seizure freedom and TEAEs by number of comorbid conditions.
Results
A total of 886 patients were treated in the initial trial and 548 in the extension; 211 of 279 patients (75.6%) on lacosamide and 180/269 (66.9%) on carbamazepine‐CR completed the extension. In the extension, 181 patients (64.9%) on lacosamide and 182 (67.7%) on carbamazepine‐CR reported TEAEs; in both groups, nasopharyngitis, headache, and dizziness were most common. Serious TEAEs were reported by 32 patients (11.5%) on lacosamide and 22 (8.2%) on carbamazepine‐CR; 12 (4.3%) and 21 (7.8%) discontinued due to TEAEs. In the combined trials (median exposure: lacosamide 630 days; carbamazepine‐CR 589 days), Kaplan‐Meier estimated proportions of patients with 12‐ and 24‐month seizure freedom from first dose were 50.8% (95% confidence interval 46.2%‐55.4%) and 47.0% (42.2%‐51.7%) on lacosamide, and 54.9% (50.3%‐59.6%) and 50.9% (46.0%‐55.7%) on carbamazepine‐CR. Incidences of drug‐related TEAEs and discontinuations due to TEAEs increased by number of comorbid conditions and were lower in patients on lacosamide.
Significance
Long‐term (median ~2 years) lacosamide monotherapy was efficacious and generally well tolerated in adults with newly diagnosed epilepsy. Seizure freedom rates were similar with lacosamide and carbamazepine‐CR.
Keywords: antiepileptic drug, comorbidity, lacosamide monotherapy, tolerability
Key Points.
Long‐term outcomes from a double‐blind noninferiority trial and double‐blind extension trial, including analyses of tolerability by number of comorbidities
In the extension trial, fewer patients treated with lacosamide than with carbamazepine‐CR had any treatment‐emergent adverse events (TEAEs) considered drug‐related by the investigator or discontinued due to TEAEs
In the combined trials, treatment retention was high, and Kaplan‐Meier estimated 12‐ and 24‐month seizure freedom was similar on lacosamide and carbamazepine‐CR
Drug‐related TEAEs and discontinuations due to TEAEs increased with a higher number of comorbidities; and were lower in patients on lacosamide than in patients on carbamazepine‐CR
Long‐term lacosamide monotherapy was efficacious and well tolerated over a median of ~2 years of treatment in adult patients with newly diagnosed epilepsy
1. INTRODUCTION
Antiepileptic drug (AED) monotherapy is the preferred option for initial treatment of epilepsy in newly diagnosed patients because of better tolerability than polytherapy and a reduced potential for drug‐drug interactions.1 Lacosamide is a third‐generation AED, which is approved as adjunctive and monotherapy for patients (≥4 years of age) with focal (partial‐onset) seizures in the European Union (EU), the United States, and other countries.2, 3 Efficacy and safety of lacosamide as adjunctive therapy were established in three randomized, double‐blind, placebo‐controlled trials.4, 5, 6 In the corresponding long‐term open‐label extension trials, adjunctive lacosamide was generally well tolerated and efficacy was maintained.7, 8, 9
Monotherapy approval of lacosamide in the EU was based on the results of a large‐scale, double‐blind trial, which demonstrated that lacosamide was noninferior to controlled‐release carbamazepine (carbamazepine‐CR) and was well tolerated as first‐line monotherapy in patients with newly diagnosed epilepsy.10 A double‐blind extension of the noninferiority trial was performed to obtain data on the long‐term safety of lacosamide and carbamazepine‐CR monotherapy, and to allow eligible patients to continue to receive their randomized treatment while maintaining blinding until database lock of the initial trial. We report primary safety data from the extension trial, in addition to post hoc analyses that explored clinically relevant long‐term safety and efficacy outcomes during the combined double‐blind period in both trials. Long‐term safety outcomes included an analysis of tolerability by number of comorbid conditions.
2. METHODS
2.1. Double‐blind noninferiority trial
SP0993 (ClinicalTrials.gov: NCT01243177) was a phase 3, randomized, double‐blind, noninferiority trial conducted between April 2011 and August 2015.10 Patients 16 years of age or older were enrolled if they had newly or recently diagnosed epilepsy with unprovoked focal seizures (simple partial, complex partial, or partial evolving to secondarily generalized with clear focal origin, according to the International Classification of Epileptic Seizures, 1981)11 or generalized tonic‐clonic seizures (without clear focal or generalized onset). Patients were required to have at least two unprovoked seizures in the previous 12 months, with at least one in the previous 3 months. Patients were excluded if they had any medical or psychiatric condition, which, in the opinion of the investigator, could have jeopardized their health or compromised their ability to participate in the trial.10
Patients were randomized 1:1 to lacosamide or carbamazepine‐CR. Following uptitration and stabilization at the first target dose (lacosamide: 200 mg/d; carbamazepine‐CR: 400 mg/d), patients entered a 6‐month assessment period (Figure S1). If a seizure occurred, the dose was escalated to the second target dose (lacosamide: 400 mg/d; carbamazepine‐CR: 800 mg/d) and the patient started a new 6‐month assessment period. If a further seizure occurred, the dose was escalated to the third target dose (lacosamide: 600 mg/d; carbamazepine‐CR: 1200 mg/d). If seizures were not controlled at the third target dose, the patient was withdrawn from the trial.
Patients who remained seizure‐free during the 6‐month assessment period continued into a 6‐month maintenance period on their last assessed dose. During the assessment and maintenance periods, patients who escalated to the second or third target dose could undergo one dose reduction (100 mg/d lacosamide or 200 mg/d carbamazepine‐CR) if they were unable to tolerate the increased dose. These patients could not be returned to the higher target dose or have further uptitration in case a new seizure occurred.
2.2. Double‐blind extension trial
Eligible patients from the initial noninferiority trial could continue on their randomized treatment in a phase 3, multicenter, double‐blind, double‐dummy, extension trial (SP0994; NCT01465997), conducted between May 2012 and January 2017 at 149 sites in 29 countries in Europe, North America, and the Asia Pacific. Patients were eligible for the extension trial if they had remained seizure‐free and completed the 6‐month maintenance period of the initial trial, or had experienced one or more seizures during the 6‐month maintenance period while on the first or second target dose. A small number of patients (<2%) transferred to the extension trial as result of a protocol amendment aimed to define the completion date of the initial trial. Patients were excluded if they had experienced a seizure at the third target dose during the initial trial, had met a withdrawal criterion for the initial trial, were experiencing an ongoing serious adverse event, or were receiving any investigational drugs or experimental devices in addition to lacosamide or carbamazepine‐CR.
Patients could enter the extension trial at one of the target dose levels of the initial trial or at a reduced dose (Figure S1). Doses were escalated in case of a seizure. Visits occurred approximately every 13 weeks. Patients requiring a higher target dose returned to the clinic for additional visits. If a patient was entered at a reduced dose and required escalation in the extension trial, the patient was moved to the next higher full target dose. Patients who experienced a seizure at the highest permitted dose level were discontinued. One dose reduction (lacosamide 100 mg/d or carbamazepine‐CR 200 mg/d) was allowed for tolerability reasons during the combined trials.
Following the database lock and unblinding of the initial trial, the extension trial was unblinded and closed for all patients. Patients who were receiving lacosamide had access to open‐label follow‐up treatment with lacosamide until monotherapy approval.
Both trials were conducted in accordance with Good Clinical Practice, the Declaration of Helsinki, and local laws. A national, regional, or independent ethics committee or institutional review board reviewed the trial protocol, amendments, and patient informed consent. Throughout both trials, patients were required to keep a daily diary recording their seizure activity.
2.3. Outcomes
2.3.1. Prospective analyses of data from the double‐blind extension trial
Data were analyzed for the safety set (SS) of all randomized patients who took at least one dose of trial medication. The primary safety outcomes were treatment‐emergent adverse events (TEAEs) reported spontaneously by the patient and/or caregiver or observed by the investigator, serious TEAEs, and patient discontinuations due to TEAEs. TEAEs were defined as adverse events that started (or whose intensity worsened) on or after the date of first dose of trial medication in the extension trial and within 30 days following the date of last trial medication administration. TEAEs were coded using the Medical Dictionary for Regulatory Activities (MedDRA) version 16.1. Descriptive analyses were performed for all safety assessments.
2.3.2. Post hoc analyses of pooled data from the combined trials
To provide an overview of the long‐term benefit and risk of the first monotherapy in newly and recently diagnosed patients with epilepsy, data were pooled from the combined double‐blind period in the initial trial and extension trial. Analyses were performed for the time from first dose of trial medication in the initial trial up to the time of unblinding of the extension trial. Data are reported for the SS and full analysis set (FAS), which were both defined as all randomized patients who took at least one dose of trial medication.
All exploratory efficacy assessments were based on diary data. Time to discontinuation due to lack of efficacy and/or TEAEs were estimated using Kaplan‐Meier methods. Efficacy outcomes were 12 and 24 months of seizure freedom from the first dose of trial medication, patients with a 6‐ or 12‐month seizure‐free interval at any point during the combined double‐blind period, and dose at onset of the first 12‐ or 24‐month seizure‐free interval. Results for Kaplan‐Meier estimated seizure freedom were adjusted for the number of seizures (two or fewer, or more than two) in the 3 months before screening in the initial trial. Tolerability outcomes included an analysis of TEAEs by number of ongoing comorbid conditions (MedDRA, version 16.1) at the Screening Visit (ie, none, one to two, three or more).
3. RESULTS
3.1. Prospective analyses of data from the double‐blind extension trial
A total of 886 patients (lacosamide, 444; carbamazepine‐CR, 442) were treated in the initial trial, of whom 549 were enrolled and 548 (SS: lacosamide, 279; carbamazepine‐CR, 269) were treated in the extension. Two hundred eleven of 279 patients (75.6%) on lacosamide and 180/269 (66.9%) on carbamazepine‐CR completed the extension trial. The most common reasons for discontinuation (≥5% of patients in either group) were withdrawn consent (lacosamide, 24 [8.6%]; carbamazepine‐CR, 19 [7.1%]) and adverse events (11 [3.9%]; 15 [5.6%]).
Baseline demographics were comparable between treatment groups (Table 1). In the extension trial, the median duration of exposure to trial medication was slightly longer in the lacosamide group (603.0 days; 489.2 patient‐years) than the carbamazepine‐CR group (549.0 days; 437.1 patient‐years). Two hundred sixty patients (93.2%) on lacosamide and 250 patients (92.9%) on carbamazepine‐CR had a duration of exposure of >182 days (6 months).
Table 1.
Baseline demographics and epilepsy characteristics (SS)
| Double‐blind extension trial only | Combined trials | |||
|---|---|---|---|---|
| Lacosamide (N = 279) | Carbamazepine‐CR (N = 269) | Lacosamide (N = 444) | Carbamazepine‐CR (N = 442) | |
| Patient demographics | ||||
| Age, mean (SD), y | 43.2 (17.2)a | 42.7 (16.7)a | 41.9 (17.9)b | 41.8 (17.2)b |
| ≤18 y, n (%) | 8 (2.9) | 8 (3.0) | 27 (6.1) | 19 (4.3) |
|
>18 to <65 y, n (%) |
230 (82.4) | 225 (83.6) | 355 (80.0) | 366 (82.8) |
| ≥65 y, n (%) | 41 (14.7) | 36 (13.4) | 62 (14.0) | 57 (12.9) |
| Female, n (%) | 125 (44.8) | 125 (46.5) | 201 (45.3) | 210 (47.5) |
| Body mass index, mean (SD), kg/m2 | 25.19 (4.63)a, c | 25.65 (4.71)a, d | 25.10 (4.88)b, e | 25.70 (5.29)b, e |
| History of epileptic seizures | ||||
| Age at diagnosis, mean (SD), y | 41.7 (17.1) | 41.2 (17.0) | 41.6 (17.8) | 41.5 (17.4) |
| Median (Q1, Q3), y | 40.0 (26.0, 54.0) | 41.0 (27.0, 54.0) | 39.0 (25.0, 55.0) | 41.0 (26.0, 55.0) |
| Time since diagnosis, mean (SD), y | 1.45 (2.29)a | 1.47 (1.80)a | 0.25 (1.81)b | 0.26 (1.41)b |
| Median (Q1, Q3), y | 1.16 (1.13, 1.40) | 1.17 (1.13, 1.39) | 0.08 (0.05, 0.13) | 0.07 (0.05, 0.13) |
| Number of seizures in past year,f median (Q1, Q3) | 4.0 (2.0, 12.0)g | 3.0 (2.0, 9.0)h | 4.0 (2.0, 12.0)i | 4.0 (2.0, 10.0)j |
| Number of seizures in past 3 mo,f median (Q1, Q3) | 2.0 (1.0, 6.0) | 2.0 (2.0, 5.0) | 3.0 (1.0, 6.0) | 2.0 (2.0, 5.0)c |
| 0, n (%) | 0 | 1 (0.4) | 0 | 1 (0.2) |
| 1, n (%) | 87 (31.2) | 63 (23.4) | 122 (27.5) | 104 (23.5) |
| 2, n (%) | 59 (21.1) | 91 (33.8) | 99 (22.3) | 130 (29.4) |
| 3‐5, n (%) | 63 (22.6) | 51 (19.0) | 105 (23.6) | 96 (21.7) |
| ≥6, n (%) | 70 (25.1) | 63 (23.4) | 118 (26.6) | 110 (24.9) |
| Unknown | 0 | 0 | 0 | 1 (0.2) |
| Classification of seizures in the 1 y before screening in the initial trial,k n (%) | ||||
| Focal seizures (partial‐onset) | 255 (91.4) | 241 (89.6) | 403 (90.8) | 402 (91.0) |
| Focal aware (simple partial) | 67 (24.0) | 71 (26.4) | 119 (26.8) | 142 (32.1) |
| Focal impaired awareness (complex partial) | 133 (47.7) | 127 (47.2) | 210 (47.3) | 206 (46.6) |
| Focal to bilateral tonic‐clonic (partial evolving to secondarily generalized) | 164 (58.8) | 164 (61.0) | 252 (56.8) | 261 (59.0) |
| Generalized seizures | ||||
| Myoclonic | 0 | 0 | 0 | 1 (0.2) |
| Unknownl | 27 (9.7) | 29 (10.8) | 47 (10.6) | 41 (9.3) |
Abbreviations: SD, standard deviation; SS, safety set.
At entry into the extension trial.
At entry into the initial trial.
n = 272.
n = 267.
n = 441.
Before Visit 1 in the initial trial;
n = 275;
n = 266.
n = 433.
n = 431.
Patients could have more than one response in a classification level and/or category; seizure types are listed per the International League Against Epilepsy (ILAE) 2017 classification,23 with the older terminology11 provided in parentheses.
Patients had generalized tonic‐clonic seizures with unclassified seizure onset.
During the extension trial, TEAEs were reported by 181 patients (64.9%) on lacosamide and 182 (67.7%) on carbamazepine‐CR (Table 2). Nasopharyngitis, headache, and dizziness were the most common TEAEs in both treatment groups. Fewer patients on lacosamide (43 [15.4%]) than carbamazepine‐CR (54 [20.1%]) had TEAEs that were considered to be drug‐related (as assessed by the investigator). The only drug‐related TEAEs reported by ≥2% of patients were increased γ‐glutamyltransferase (6 [2.2%]) and hypercholesterolemia (7 [2.6%]) in the carbamazepine‐CR group. Most TEAEs were mild or moderate in intensity; 21 patients (7.5%) on lacosamide and 20 patients (7.4%) on carbamazepine‐CR had a severe TEAE.
Table 2.
TEAEs during the treatment period in the extension trial (SS)
| Lacosamide (n = 279) | Carbamazepine‐CR (n = 269) | |
|---|---|---|
| Any TEAEs | 181 (64.9) | 182 (67.7) |
| Drug‐related TEAEsa | 43 (15.4) | 54 (20.1) |
| Discontinuations due to TEAEs | 12 (4.3) | 21 (7.8) |
| Serious TEAEs | 32 (11.5) | 22 (8.2) |
| Severe TEAEs | 21 (7.5) | 20 (7.4) |
| Deaths | 1 (0.4) | 0 |
| TEAEsb reported by ≥3% of patients in any treatment group, n (%) | ||
| Nasopharyngitis | 20 (7.2) | 16 (5.9) |
| Headache | 17 (6.1) | 15 (5.6) |
| Dizziness | 12 (4.3) | 17 (6.3) |
| Hypercholesterolemia | 10 (3.6) | 13 (4.8) |
| Back pain | 10 (3.6) | 7 (2.6) |
| Hypertension | 9 (3.2) | 4 (1.5) |
| Nausea | 9 (3.2) | 1 (0.4) |
| GGT increased | 7 (2.5) | 11 (4.1) |
| Upper respiratory tract infection | 3 (1.1) | 9 (3.3) |
Abbreviations: GGT, γ‐glutamyltransferase; MedDRA, Medical Dictionary for Regulatory Activities; TEAE, treatment‐emergent adverse event.
As assessed by the investigator.
Preferred Term (MedDRA, version 16.1).
Fewer patients on lacosamide (12 [4.3%]) than carbamazepine‐CR (21 [7.8%]) discontinued the extension trial due to TEAEs (Table 2). No TEAEs led to discontinuation in more than one patient on lacosamide. TEAEs leading to discontinuation in at least two patients on carbamazepine‐CR were pregnancy on contraceptive (4 [1.5%]), increased γ‐glutamyltransferase (2 [0.7%]), suicidal ideation, and suicide attempt (2 patients each [0.7%]). One patient (68 years; 440 days on 200 mg/d lacosamide) died because of acute renal failure and adenocarcinoma; neither event was considered to be related to lacosamide by the investigator.
Serious TEAEs with onset during the treatment period of the extension trial were reported by 32 patients (11.5%) on lacosamide and 22 (8.2%) on carbamazepine‐CR (Table 2). Angina pectoris and transient ischemic attack were the only serious TEAEs reported by more than one patient on lacosamide (two patients each [0.7%]). Serious TEAEs reported by more than one patient on carbamazepine‐CR were gastroenteritis (three patients [1.1%]), pregnancy on contraceptive, suicidal ideation, and suicide attempt (two patients each [0.7%]). Serious TEAEs were considered drug‐related by the investigator in two patients on lacosamide (hepatic enzyme increased, status epilepticus, one patient each) and five patients on carbamazepine‐CR (toxicity to various agents, suicidal ideation, and suicide attempt in one patient; atrioventricular block second degree, hyponatremia, convulsion, pregnancy on contraceptive, one patient each).
Five patients (1.8%) on lacosamide and three patients (1.1%) on carbamazepine‐CR had cardiac‐related TEAEs. Of these, two events in patients on lacosamide (atrial fibrillation and bradycardia) were serious; both were mild or moderate in intensity and considered not related to trial medication. Two serious cardiac TEAEs were reported in patients on carbamazepine‐CR: second‐degree atrioventricular block was moderate in intensity, was considered related to trial medication, and led to discontinuation; atrial fibrillation was moderate in intensity and considered not related to trial medication.
Three patients (1.1%) on lacosamide and four patients (1.5%) on carbamazepine‐CR reported TEAEs related to suicidality. These included suicidal ideation (lacosamide, three [1.1%]; carbamazepine‐CR, four [1.5%]), suicide attempt (carbamazepine‐CR, two [0.7%]), suicidal behavior (lacosamide, one [0.4%]), and intentional overdose (carbamazepine‐CR, one [0.4%]). One patient on lacosamide had two serious TEAEs related to suicidality (suicidal ideation and suicidal behavior); both were moderate in intensity and were not considered to be related to trial medication. Two patients in the carbamazepine‐CR group had five serious TEAEs related to suicidality that were severe in intensity; one patient had suicidal ideation, intentional overdose, and suicide attempt, all of which were not considered to be related to trial medication; one patient had suicidal ideation and suicide attempt, both of which were considered to be related to trial medication by the investigator. Two events related to suicidality in the lacosamide group and five events in the carbamazepine‐CR group led to discontinuation.
Five patients (1.8%) on lacosamide and two patients (0.7%) on carbamazepine‐CR reported TEAEs of syncope. One event in the lacosamide group was severe in intensity and considered not related to the trial medication by the investigator. None of the events led to discontinuation. One patient (0.4%) on lacosamide and two patients (0.7%) on carbamazepine‐CR reported TEAEs of rash. No events of drug‐induced hepatotoxicity were reported during the extension trial.
3.2. Post hoc analyses of pooled data from the combined trials
Of 886 patients treated in the combined trials, 451 (lacosamide, 227/444 [51.1%]; carbamazepine‐CR, 224/442 [50.7%]) were ongoing at the time of unblinding (Figure 1). At baseline of the initial trial, 245 patients (27.7%) had no comorbid conditions, 305 patients (34.4%) had one to two comorbid conditions, and 336 patients (37.9%) had three or more comorbid conditions. The most common comorbid conditions in all patients were hypertension (lacosamide, 20.3%; carbamazepine‐CR, 24.2%) and hypercholesterolemia (lacosamide, 7.4%; carbamazepine‐CR, 9.7%) (Table S1).
Figure 1.

Patient disposition in the combined trials until unblinding. FAS, full analysis set; SS, safety set. a548 patients were treated in the extension trial (lacosamide, 279 patients; carbamazepine‐CR, 269 patients)
Baseline characteristics were similar in the comorbid condition subgroups, although patients with a higher number of comorbid conditions were generally older (mean age: no conditions, 31.2 years; one to two conditions, 38.8 years; three or more conditions, 52.4 years) and were taking higher numbers of concomitant non‐AED medications at entry into the initial trial (Table S2). Within the subgroups, baseline characteristics were generally consistent between patients in the lacosamide and carbamazepine‐CR groups. The subgroup of patients with three or more comorbid conditions included a higher proportion of male patients in the lacosamide than carbamazepine‐CR group (53.9% vs 44.4%).
In the combined double‐blind period, the median duration of exposure from the time of first dose of trial medication was slightly longer in the lacosamide group (630.0 days; 744.2 patient‐years) than in the carbamazepine‐CR group (589.0 days; 708.3 patient‐years). In both treatment groups, the median duration of exposure was slightly shorter in patients with more comorbid conditions (lacosamide: no conditions, 681.0 days; one to two conditions, 599.0 days; three or more conditions, 585.0 days; carbamazepine‐CR: no conditions, 606.0 days; one to two conditions, 611.0 days; three or more conditions, 540.0 days). The majority of patients (lacosamide, 285 [64.2%]; carbamazepine‐CR, 294 [66.5%]) remained on the lowest target dose level throughout both trials. The proportions of patients reaching each dose level were similar in subgroups of patients with no comorbid conditions, and in those with one to two or at least three comorbid conditions (Table S3).
For patients who discontinued due to lack of efficacy or adverse events, the observed median time to discontinuation was 191.0 days for patients on lacosamide (n = 89) and 115.0 days for patients on carbamazepine‐CR (n = 103). The median time to discontinuation was longer on lacosamide than carbamazepine‐CR for patients who discontinued due to adverse events (lacosamide: n = 59, 112.0 days; carbamazepine‐CR: n = 84, 57.5 days) and for patients who discontinued due to lack of efficacy (lacosamide: n = 30, 342.0 days; carbamazepine‐CR: n = 19, 252.0 days). Based on Kaplan‐Meier analyses of discontinuation due to lack of efficacy or adverse events, the estimated proportions of patients remaining on treatment at 12 and 24 months were 84.4% and 79.5%, respectively, with lacosamide, and 80.6% and 74.5%, respectively, with carbamazepine‐CR (Figure 2). The Kaplan‐Meier estimated proportion of patients who did not discontinue due to adverse events at 12 and 24 months was higher in patients with no comorbid conditions than in patients with three or more comorbid conditions (Figure S2).
Figure 2.
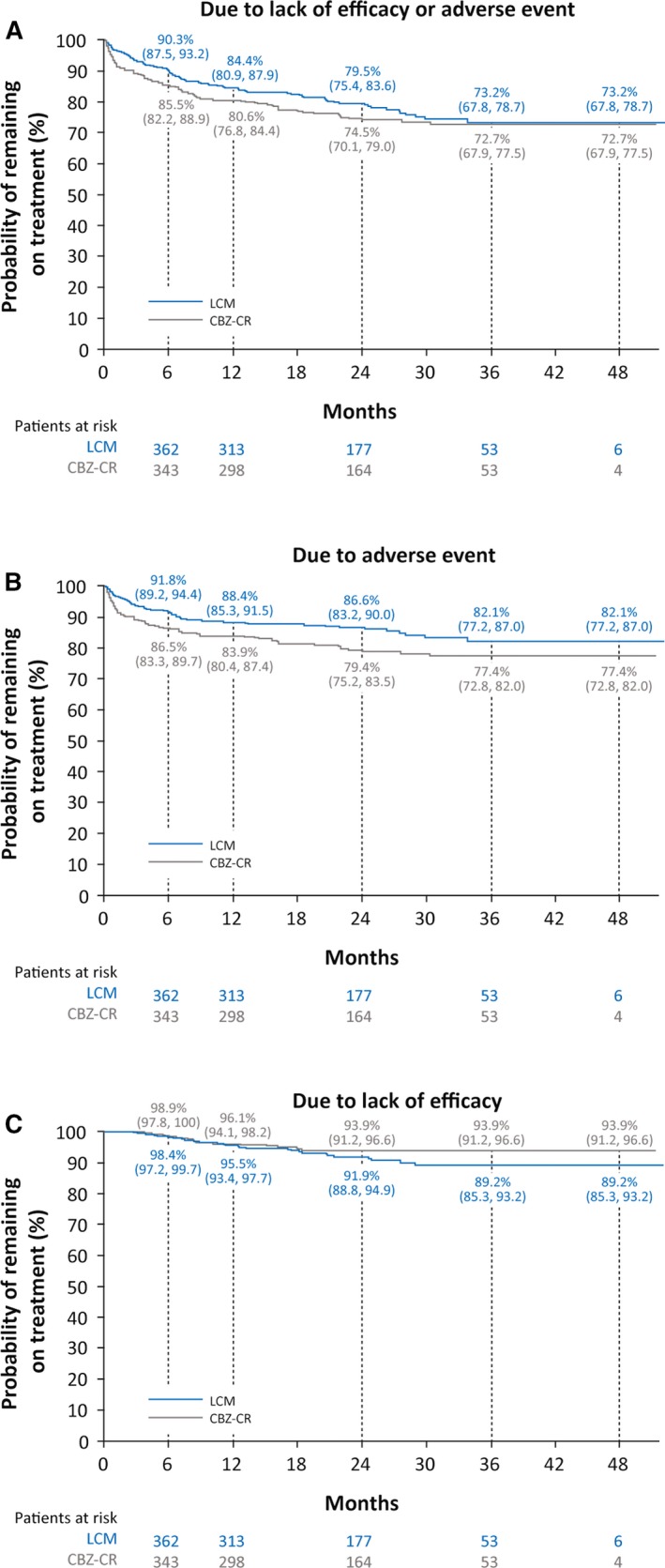
Time to discontinuation due to lack of efficacy or adverse event (A), due to adverse event (B), or due to lack of efficacy (C) during the combined double‐blind period (FAS). CBZ‐CR, controlled‐release carbamazepine; FAS, full analysis set; LCM, lacosamide
The observed proportions of patients with a 6‐ or 12‐month seizure‐free interval at any point during the combined double‐blind period were 75.7% (336/444) and 64.4% (286/444), respectively, in the lacosamide group, and 71.9% (318/442) and 62.7% (277/442), respectively, in the carbamazepine‐CR group (Figure 3). The majority of patients with 6‐ and 12‐month seizure‐free intervals achieved their first seizure‐free interval on the lowest target dose level for lacosamide and carbamazepine‐CR.
Figure 3.
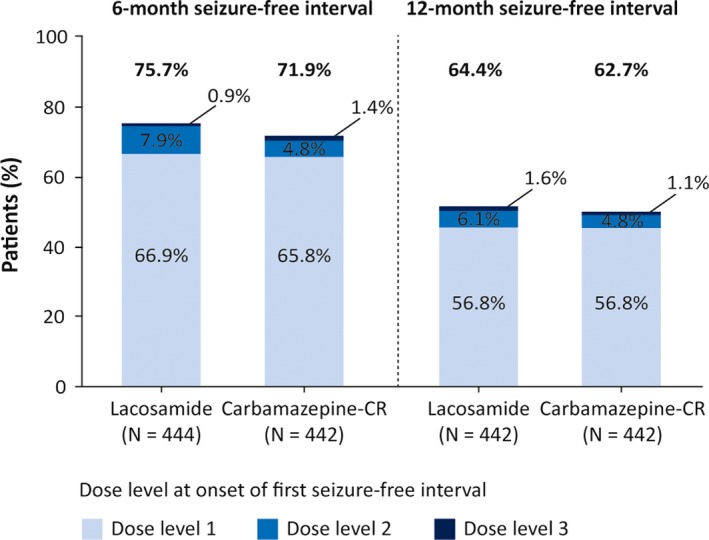
Patients with at least one 6‐ and 12‐mo seizure‐free interval up to unblinding (FAS). Lacosamide: dose level 1, 200 mg/d; dose level 2, 400 mg/d; dose level 3, 600 mg/d; carbamazepine‐CR: dose level 1, 400 mg/d; dose level 2, 800 mg/d; dose level 3, 1200 mg/d. FAS, full analysis set
The observed proportions of patients who completed 12 and 24 months of treatment from the date of first dose and remained seizure‐free were 41.4% (184/444) and 23.6% (105/444) on lacosamide, and 42.8% (189/442) and 21.5% (95/442) on carbamazepine‐CR. The Kaplan‐Meier estimated proportions of patients who were seizure‐free for 12 and 24 months from the date of first dose were 50.8% and 47.0% on lacosamide, and 54.9% and 50.9% on carbamazepine‐CR, respectively (Figure S3).
In the combined trials, TEAEs were reported by 355 patients (80.0%) on lacosamide and 368 (83.3%) on carbamazepine‐CR (Table 3). Headache and dizziness were the most common TEAEs (≥10%) with lacosamide, and headache, dizziness, and fatigue were the most common TEAEs with carbamazepine‐CR. Fewer patients on lacosamide (181 [40.8%]) than carbamazepine‐CR (222 [50.2%]) reported TEAEs that were considered by the investigator to be related to trial medication. In both treatment groups, most TEAEs were mild or moderate in intensity; 47 patients (10.6%) on lacosamide and 57 patients (12.9%) on carbamazepine‐CR reported severe TEAEs. Serious TEAEs were reported by 52 (11.7%) patients on lacosamide and 58 (13.1%) on carbamazepine‐CR. Fewer patients on lacosamide (58 [13.1%]) than carbamazepine‐CR (84 [19.0%]) discontinued due to TEAEs.
Table 3.
TEAEs during the combined double‐blind period (SS)
| Overall population | Number of comorbid conditions | |||||||
|---|---|---|---|---|---|---|---|---|
| No comorbid conditions | One to two comorbid conditions | Three or more comorbid conditions | ||||||
| LCM (N = 444) | CBZ‐CR (N = 442) | LCM (n = 122) | CBZ‐CR (n = 123) | LCM (n = 157) | CBZ‐CR (n = 148) | LCM (n = 165) | CBZ‐CR (n = 171) | |
| Any TEAEs | 355 (80.0) | 368 (83.3) | 85 (69.7) | 85 (69.1) | 126 (85.1) | 142 (86.1) | 157 (91.8) | |
| Drug‐related TEAEsa | 181 (40.8) | 222 (50.2) | 35 (28.7) | 47 (38.2) | 67 (42.7) | 75 (50.7) | 79 (47.9) | 100 (58.5) |
| Discontinuations due to TEAEs | 58 (13.1) | 84 (19.0) | 11 (9.0) | 17 (13.8) | 20 (12.7) | 26 (17.6) | 27 (16.4) | 41 (24.0) |
| Serious TEAEs | 52 (11.7) | 58 (13.1) | 3 (2.5) | 10 (8.1) | 20 (12.7) | 15 (10.1) | 29 (17.6) | 33 (19.3) |
| Severe TEAEs | 47 (10.6) | 57 (12.9) | 7 (5.7) | 11 (8.9) | 15 (9.6) | 15 (10.1) | 25 (15.2) | 31 (18.1) |
| Deaths | 2 (0.5) | 1 (0.2) | 0 | 0 | 1 (0.6) | 0 | 1 (0.6) | 1 (0.6) |
| TEAEsb reported by ≥5% of patients in any treatment group in the overall population, n (%) | ||||||||
| Headache | 67 (15.1) | 61 (13.8) | 17 (13.9) | 12 (9.8) | 26 (16.6) | 23 (15.5) | 24 (14.5) | 26 (15.2) |
| Dizziness | 56 (12.6) | 49 (11.1) | 14 (11.5) | 7 (5.7) | 19 (12.1) | 14 (9.5) | 23 (13.9) | 28 (16.4) |
| Fatigue | 36 (8.1) | 48 (10.9) | 5 (4.1) | 8 (6.5) | 12 (7.6) | 14 (9.5) | 19 (11.5) | 26 (15.2) |
| Nasopharyngitis | 36 (8.1) | 35 (7.9) | 9 (7.4) | 9 (7.3) | 8 (5.1) | 14 (9.5) | 19 (11.5) | 12 (7.0) |
| Nausea | 30 (6.8) | 23 (5.2) | 6 (4.9) | 4 (3.3) | 9 (5.7) | 7 (4.7) | 15 (9.1) | 12 (7.0) |
| Somnolence | 27 (6.1) | 43 (9.7) | 8 (6.6) | 11 (8.9) | 13 (8.3) | 12 (8.1) | 6 (3.6) | 20 (11.7) |
| Hypercholesterolemia | 18 (4.1) | 26 (5.9) | 5 (4.1) | 6 (4.9) | 8 (5.1) | 9 (6.1) | 5 (3.0) | 11 (6.4) |
| GGT increased | 13 (2.9) | 45 (10.2) | 3 (2.5) | 11 (8.9) | 4 (2.5) | 11 (7.4) | 6 (3.6) | 23 (13.5) |
| Drug‐related TEAEsb reported by ≥5% of patients in any treatment group in the overall population, n (%) | ||||||||
| Dizziness | 37 (8.3) | 22 (5.0) | 7 (5.7) | 2 (1.6) | 15 (9.6) | 10 (6.8) | 15 (9.1) | 10 (5.8) |
| Fatigue | 25 (5.6) | 32 (7.2) | 3 (2.5) | 4 (3.3) | 9 (5.7) | 13 (8.8) | 13 (7.9) | 15 (8.8) |
| Somnolence | 22 (5.0) | 40 (9.0) | 7 (5.7) | 11 (8.9) | 11 (7.0) | 10 (6.8) | 4 (2.4) | 19 (11.1) |
| Headache | 18 (4.1) | 23 (5.2) | 1 (0.8) | 4 (3.3) | 9 (5.7) | 9 (6.1) | 8 (4.8) | 10 (5.8) |
| GGT increased | 7 (1.6) | 33 (7.5) | 1 (0.8) | 7 (5.7) | 2 (1.3) | 9 (6.1) | 4 (2.4) | 17 (9.9) |
| TEAEsb leading to discontinuation in ≥1% of patients in any treatment group in the overall population, n (%) | ||||||||
| Dizziness | 6 (1.4) | 2 (0.5) | 2 (1.6) | 0 | 2 (1.3) | 0 | 2 (1.2) | 2 (1.2) |
| Rash | 4 (0.9) | 7 (1.6) | 0 | 2 (1.6) | 3 (1.9) | 2 (1.4) | 1 (0.6) | 3 (1.8) |
| AST increased | 3 (0.7) | 5 (1.1) | 1 (0.8) | 1 (0.8) | 1 (0.6) | 1 (0.7) | 1 (0.6) | 3 (1.8) |
| GGT increased | 2 (0.5) | 8 (1.8) | 1 (0.8) | 2 (1.6) | 1 (0.6) | 0 | 0 | 6 (3.5) |
| ALT increased | 2 (0.5) | 5 (1.1) | 1 (0.8) | 2 (1.6) | 0 | 0 | 1 (0.6) | 3 (1.8) |
| Somnolence | 1 (0.2) | 6 (1.4) | 0 | 1 (0.8) | 0 | 0 | 1 (0.6) | 5 (2.9) |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; CBZ‐CR, controlled‐release carbamazepine; GGT, γ‐glutamyltransferase; LCM, lacosamide; MedDRA, Medical Dictionary for Regulatory Activities; SS, safety set; TEAE, treatment‐emergent adverse event.
As assessed by the investigator.
Preferred Term (MedDRA, version 16.1).
The incidences of TEAEs, drug‐related TEAEs, and discontinuations due to TEAEs increased with a higher number of comorbid conditions (Table 3). The incidences of drug‐related TEAEs and discontinuations due to TEAEs were lower in patients on lacosamide than on carbamazepine‐CR. The incidences of fatigue (lacosamide, carbamazepine‐CR), headache (carbamazepine‐CR), and dizziness (carbamazepine‐CR) increased (≥5%) as the number of comorbid conditions increased.
4. DISCUSSION
In a long‐term extension (SP0994) of a randomized, double‐blind, noninferiority trial (SP0993), lacosamide monotherapy was generally well tolerated in adult patients with newly diagnosed epilepsy. The proportions of patients reporting any TEAEs were similar between the lacosamide and carbamazepine‐CR treatment groups (64.9% and 67.7%, respectively). The observed TEAEs with lacosamide were consistent with its known safety profile, and no new safety signals were identified. Post hoc analyses of data from the combined double‐blind period of the initial trial and long‐term extension showed that lacosamide monotherapy was efficacious, with a favorable tolerability profile over a median of ~2 years of treatment.
Several second‐ and third‐generation AEDs have been directly compared to carbamazepine‐CR as initial monotherapy for patients with newly diagnosed epilepsy.10, 12, 13, 14 These trials showed that levetiracetam, zonisamide, lacosamide, and eslicarbazepine were noninferior to carbamazepine‐CR, and provided class I evidence of efficacy in adults with focal epilepsy that led to approval of these drugs as monotherapy options in Europe.10, 12, 13, 14 A recent network meta‐analysis of these noninferiority trials compared the efficacy and tolerability of these AED monotherapies and found no statistical differences in the 6‐ and 12‐month seizure freedom and incidence of TEAEs between levetiracetam, zonisamide, lacosamide, eslicarbazepine, and carbamazepine‐CR.15 However, lacosamide was associated with a significantly lower incidence of discontinuations due to TEAEs than carbamazepine‐CR.15
In the lacosamide extension trial, most patients on lacosamide and carbamazepine‐CR remained at the lowest dose level, similar to what was observed in the initial trial.10 The most commonly reported TEAEs with lacosamide were nasopharyngitis, headache, and dizziness, in line with those reported previously.4, 5, 6, 10, 16 The overall proportions of patients on lacosamide reporting any TEAEs, drug‐related TEAEs, and TEAEs leading to discontinuation were lower in the extension trial compared with the initial trial (percent differences: −9.0%, −21.8%, and −6.3%, respectively) despite a longer treatment duration, although the proportions of patients with serious and severe TEAEs were similar in both trials (less than 5% difference).
The most common individual TEAEs with lacosamide in the initial trial were headache (14%) and dizziness (12%); the incidences of both were notably lower in the extension trial (headache, 6.1%; dizziness, 4.3%).10 This may be because TEAEs are generally more common at the initiation of AED treatment and during titration.17, 18, 19 Furthermore, patients who did not respond at the highest dose level or those who experienced TEAEs leading to discontinuation during the initial double‐blind trial were not included in the extension. Four patients on lacosamide and seven patients on carbamazepine‐CR discontinued due to rash in the initial double‐blind trial. Few TEAEs of rash were reported in the extension trial (one patient on lacosamide and two patients on carbamazepine‐CR). The overall incidence of TEAEs was similar with lacosamide and carbamazepine‐CR in the extension trial (64.9% and 67.7%), and between the test drug and carbamazepine‐CR in the double‐blind long‐term extensions of the levetiracetam (38.0% and 38.4%) and zonisamide (52.6% and 46.2%) noninferiority trials.20, 21, 22 In the combined double‐blind period of both lacosamide trials, the incidence of any TEAEs was similar in patients on lacosamide and carbamazepine‐CR (80.0% and 83.3%); however, fewer patients on lacosamide than on carbamazepine‐CR experienced drug‐related TEAEs (40.8% vs 50.2%) or discontinued due to TEAEs (13.1% vs 19.0%).
Post hoc analyses of data from the combined double‐blind period of both trials showed high treatment retention in patients on lacosamide and on carbamazepine‐CR. The Kaplan‐Meier estimated proportions of patients with 12 months (lacosamide, 50.8%; carbamazepine‐CR, 54.9%) and 24 months of seizure freedom (47.0%; 50.9%) from the date of first dose were similar in both treatment groups. These data cannot be compared directly with the Kaplan‐Meier estimated 12‐month seizure‐freedom rates reported in the initial trial (lacosamide, 78%; carbamazepine‐CR, 83%), due to differences in the analyses.10 In the initial trial, seizure freedom was evaluated following patient stabilization at the last assessed dose, rather than from the date of first dose.10 Analyses of seizure‐free intervals during the combined trials showed that a similar proportion of patients on lacosamide and carbamazepine‐CR had a 6‐ (75.7% and 71.9%) or 12‐month (64.4% and 62.7%) seizure‐free interval at any point during double‐blind treatment. The majority of patients who attained seizure freedom for at least 6 or 12 months did so while at the lowest dose level in both treatment groups.
Because lacosamide is not enzyme‐inducing and has a low potential for drug‐drug interactions,2, 3 it may be a suitable long‐term treatment option for patients with comorbidities taking concomitant medications. As expected, patients with a higher number of comorbid conditions were older and were taking higher numbers of concomitant medications. Post hoc analyses of tolerability by number of comorbid conditions suggested an increased overall incidence of TEAEs in patients with a higher number of comorbid conditions (no/one to two/three or more comorbid conditions; lacosamide, 69.7%/81.5%/86.1%; carbamazepine‐CR, 69.1%/85.1%/91.8%). The incidences of drug‐related TEAEs (lacosamide, 28.7%/42.7%/47.9%; carbamazepine‐CR, 38.2%/50.7%/58.5%) and discontinuations due to TEAEs (9.0%/12.7%/16.4%; 13.8%/17.6%/24.0%) also increased in patients with a higher number of comorbid conditions, and were consistently lower in patients on lacosamide than on carbamazepine‐CR. These findings were supported by the Kaplan‐Meier estimated proportion of patients who did not discontinue due to adverse events during 12 and 24 months of treatment. These data suggest that lacosamide may be a suitable option for first‐line monotherapy in patients with higher comorbidity burdens.
A limitation of this analysis was that MedDRA was used for the coding of comorbid conditions, and age‐related physiologic conditions such as menopause and postmenopause were therefore coded as comorbid conditions. Furthermore, per the trial eligibility criteria, patients were excluded from the initial trial if they had medical/psychiatric conditions, which, in the opinion of the investigator, could have jeopardized their health or compromised their ability to participate. As such, the enrolled patients may have had relatively mild comorbid conditions.
The results of the extension trial and post hoc analysis demonstrated that long‐term treatment with lacosamide was well tolerated as first‐line monotherapy in adult patients with newly or recently diagnosed epilepsy and efficacy was maintained.
CONFLICT OF INTERESTS
Elinor Ben‐Menachem has served as a paid consultant for Eisai, Sandoz, and UCB Pharma; has received research grants from Eisai, GW Pharmaceuticals, SK Life Science, and UCB Pharma; and is the Editor‐in‐Chief of Acta Neurologica Scandinavica. Hans‐Peter Grebe received advisory board honoraria from Eisai. Kiyohito Terada has received speaker's fees from Daiichi‐Sankyo, Eisai, Otsuka Pharmaceutical, and UCB Pharma. Lori Jensen, Ting Li, Marc De Backer, Björn Steiniger‐Brach, Teresa Gasalla, and Melissa Brock are employees of UCB Pharma. Victor Biton has served as a paid consultant for Avigen, Eisai, GlaxoSmithKline, Icagen, Jazz Pharmaceuticals, Lundbeck, Merck, Ortho‐McNeil, Pfizer, UCB Pharma, Upsher‐Smith Laboratories, and Valeant Pharmaceuticals. We confirm that we have read the journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Supporting information
ACKNOWLEDGMENTS
This trial was funded by UCB Pharma. The authors thank the patients and their caregivers, the clinical project team, and the investigators and their teams who contributed to these trials (co‐investigator appendix). The authors acknowledge Ying Zhang, MS (UCB Pharma, Raleigh, NC, USA) and Svetlana Dimova, MD, PhD (UCB Pharma, Brussels, Belgium) for support with the design of post hoc analyses and data interpretation. Writing and editorial assistance was provided by Michaela Fuchs, PhD, CMPP (Evidence Scientific Solutions, Horsham, UK) and was funded by UCB Pharma. Publication management was provided by Barbara Pelgrims, PhD (UCB Pharma, Brussels, Belgium). Underlying data from this manuscript may be requested by qualified researchers 6 months after product approval in the United States and/or EU, or global development is discontinued, and 18 months after trial completion. Investigators may request access to anonymized individual patient data and redacted study documents, which may include: raw datasets, analysis‐ready datasets, study protocol, blank case report form, annotated case report form, statistical analysis plan, dataset specifications, and clinical study report. Before use of the data, proposals need to be approved by an independent review panel at http://www.clinicalstudydatarequest.com and a signed data sharing agreement will need to be executed. All documents are available in English only, for a prespecified time, typically 12 months, on a password‐protected portal.
Ben‐Menachem E, Grebe HP, Terada K, et al. Long‐term safety and efficacy of lacosamide and controlled‐release carbamazepine monotherapy in patients with newly diagnosed epilepsy. Epilepsia. 2019;60:2437–2447. 10.1111/epi.16381
REFERENCES
- 1. St Louis EK, Rosenfeld WE, Bramley T. Antiepileptic drug monotherapy: the initial approach in epilepsy management. Curr Neuropharmacol. 2009;7:77–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Vimpat® (lacosamide) Summary of Product Characteristics. Brussels, Belgium: UCB Pharma, SA; 2018. Available at: https://www.ema.europa.eu/documents/product-information/vimpat-epar-product-information_en.pdf. Accessed June 10, 2019. [Google Scholar]
- 3. Vimpat® (lacosamide) C‐V Prescribing Information. Smyrna, GA: UCB, Inc; 2019. Available at: https://www.vimpat.com/vimpat-prescribing-information.pdf. Accessed June 10, 2019. [Google Scholar]
- 4. Ben‐Menachem E, Biton V, Jatuzis D, Abou‐Khalil B, Doty P, Rudd GD. Efficacy and safety of oral lacosamide as adjunctive therapy in adults with partial‐onset seizures. Epilepsia. 2007;48:1308–17. [DOI] [PubMed] [Google Scholar]
- 5. Halász P, Kälviäinen R, Mazurkiewicz‐Beldzińska M, Rosenow F, Doty P, Hebert D, et al. Adjunctive lacosamide for partial‐onset seizures: efficacy and safety results from a randomized controlled trial. Epilepsia. 2009;50:443–53. [DOI] [PubMed] [Google Scholar]
- 6. Chung S, Sperling MR, Biton V, Krauss G, Hebert D, Rudd GD, et al. Lacosamide as adjunctive therapy for partial‐onset seizures: a randomized controlled trial. Epilepsia. 2010;51:958–67. [DOI] [PubMed] [Google Scholar]
- 7. Husain A, Chung S, Faught E, Isojärvi J, McShea C, Doty P. Long‐term safety and efficacy in patients with uncontrolled partial‐onset seizures treated with adjunctive lacosamide: results from a Phase III open‐label extension trial. Epilepsia. 2012;53:521–8. [DOI] [PubMed] [Google Scholar]
- 8. Rosenfeld W, Fountain NB, Kaubrys G, Ben-Menachem E, McShea C, Isojärvi J, et al. Safety and efficacy of adjunctive lacosamide among patients with partial‐onset seizures in a long‐term open‐label extension trial of up to 8 years. Epilepsy Behav. 2014;41:164–70. [DOI] [PubMed] [Google Scholar]
- 9. Rosenow F, Kelemen A, Ben‐Menachem E, McShea C, Isojärvi J, Doty P, et al. Long‐term adjunctive lacosamide treatment in patients with partial‐onset seizures. Acta Neurol Scand. 2016;133:136–44. [DOI] [PubMed] [Google Scholar]
- 10. Baulac M, Rosenow F, Toledo M, Terada K, Li T, De Backer M, et al. Efficacy, safety, and tolerability of lacosamide monotherapy versus controlled‐release carbamazepine in patients with newly diagnosed epilepsy: a phase 3, randomised, double‐blind, non‐inferiority trial. Lancet Neurol. 2017;16:43–54. [DOI] [PubMed] [Google Scholar]
- 11. Commission on Classification and Terminology of the International League Against Epilepsy . Proposal for revised clinical and electroencephalographic classification of epileptic seizures. Epilepsia. 1981;22:489–501. [DOI] [PubMed] [Google Scholar]
- 12. Brodie MJ, Perucca E, Ryvlin P, Ben‐Menachem E, Meencke HJ. Levetiracetam Monotherapy Study Group . Comparison of levetiracetam and controlled‐release carbamazepine in newly diagnosed epilepsy. Neurology. 2007;68:402–8. [DOI] [PubMed] [Google Scholar]
- 13. Baulac M, Brodie MJ, Patten A, Segieth J, Giorgi L. Efficacy and tolerability of zonisamide versus controlled‐release carbamazepine for newly diagnosed partial epilepsy: a phase 3, randomised, double‐blind, non‐inferiority trial. Lancet Neurol. 2012;11:579–88. [DOI] [PubMed] [Google Scholar]
- 14. Trinka E, Ben‐Menachem E, Kowacs PA, Elger C, Keller B, Löffler K, et al. Efficacy and safety of eslicarbazepine acetate versus controlled‐release carbamazepine monotherapy in newly diagnosed epilepsy: A phase III double‐blind, randomized, parallel‐group, multicenter study. Epilepsia. 2018;59:479–91. [DOI] [PubMed] [Google Scholar]
- 15. Lattanzi S, Zaccara G, Giovannelli F, Grillo E, Nardone R, Silvestrini M, et al. Antiepileptic monotherapy in newly diagnosed focal epilepsy. A network meta‐analysis. Acta Neurol Scand. 2019;139:33–41. [DOI] [PubMed] [Google Scholar]
- 16. Wechsler RT, Li G, French J, O'Brien TJ, D'Cruz O, Williams P, et al. Conversion to lacosamide monotherapy in the treatment of focal epilepsy: results from a historical‐controlled, multicenter, double‐blind study. Epilepsia. 2014;55:1088–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Biton V, Gil‐Nagel A, Isojärvi J, Doty P, Hebert D, Fountain NB. Safety and tolerability of lacosamide as adjunctive therapy for adults with partial‐onset seizures: analysis of data pooled from three randomized, double‐blind, placebo‐controlled clinical trials. Epilepsy Behav. 2015;52:119–27. [DOI] [PubMed] [Google Scholar]
- 18. Zadeh WW, Escartin A, Byrnes W, Tennigkeit F, Borghs S, Li T, et al. Efficacy and safety of lacosamide as first add‐on or later adjunctive treatment for uncontrolled partial‐onset seizures: a multicentre open‐label trial. Seizure. 2015;31:72–9. [DOI] [PubMed] [Google Scholar]
- 19. Cramer JA, Mintzer S, Wheless J, Mattson RH. Adverse effects of antiepileptic drugs: a brief overview of important issues. Expert Rev Neurother. 2010;10:885–91. [DOI] [PubMed] [Google Scholar]
- 20.ClinicalTrials.gov. Identifier NCT0015078. Monotherapy with levetiracetam or carbamazepine in patients suffering from epilepsy. Bethesda, MD: National Library of Medicine; Available at: https://clinicaltrials.gov/ct2/show/NCT00150787. Accessed June 10, 2019. [Google Scholar]
- 21. Clinical Study Summary RXCE06E1672. UCB, Inc; 2006. Available at: https://www.ucb.com/website/_up/ucb_com_patients/documents/N01093_CSS_20070726.pdf. Accessed June 10, 2019.
- 22. Baulac M, Patten A, Giorgi L. Long‐term safety and efficacy of zonisamide versus carbamazepine monotherapy for treatment of partial seizures in adults with newly diagnosed epilepsy: results of a phase III, randomized, double‐blind study. Epilepsia. 2014;55:1534–43. [DOI] [PubMed] [Google Scholar]
- 23. Fisher RS, Cross JH, French JA, Higurashi N, Hirsch E, Jansen FE, et al. Operational classification of seizure types by the International League Against Epilepsy: Position Paper of the ILAE Commission for Classification and Terminology. Epilepsia. 2017;58:522–30. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


