Abstract
Background
Deficits in auditory event-related potentials (ERPs), brain responses to stimuli indexing different cognitive processes, have been demonstrated widely in chronic schizophrenia (SZ) patients though much less is known about these responses across the early course of psychosis. The present study examined multiple ERP components in first episode psychosis (FEP) patients longitudinally and investigated the relationships between ERPs, psychosocial functioning, and clinical features over time.
Methods
N1, P2, P3a, and P3b ERPs were elicited using a three-stimulus (novelty) auditory oddball paradigm. FEP patients included SZ-spectrum and psychotic bipolar disorder (BD) diagnoses. Data were collected from 41 patients at baseline, 20 patients at 12-month follow-up, 14 at 24-month follow-up, and 29 healthy control subjects.
Results
N1 and P2 ERPs were intact across the early stages of psychosis. Baseline P2 was significantly larger in BD than SZ patients. Reduced P3a and P3b ERPs were found in patients followed longitudinally and are stable over time. ERPs tracked distinct aspects of symptomology and medication, though specific associations were inconsistent across time. Baseline P3a amplitude predicted later psychosocial functioning. The pattern of correlations between ERP components in patients differed from controls.
Discussion
Baseline P3a ERP, and PANSS general score were significant and independent predictors of later MCAS functioning at 12-month. Overall, individuals with worse functioning and greater symptomology produced smaller amplitudes. Our results highlight the heterogeneity within the FEP population. Correlation patterns among ERPs are similar between patients and controls. P3a and P3b amplitudes appear to link with higher-order cognitive and psychosocial functioning.
Keywords: Event related potentials, Longitudinal, First episode psychosis, Psychosocial functioning
1. Introduction
Auditory event-related potentials (ERPs) elicited during discrimination tasks (e.g., oddball) consist of several components. The auditory P3b ERP is elicited in response to attended target stimuli, reflects top-down cognitive processes of effortful attention and immediate memory, and is typically largest over parietal regions (Comerchero and Polich, 1999; Ford et al., 1994; Polich and Criado, 2006; Polich and Kok, 1995). The P3b is one of the most studied ERPs in schizophrenia (SZ) and it is well established that individuals with chronic SZ show a robust reduction in amplitude (Bramon et al., 2004; Jeon and Polich, 2001). The preceding P3 component, the P3a, elicited typically by novel or unexpected stimuli, is thought to represent an automatic reorienting or shifting of attention and is largest in frontal regions (Comerchero and Polich, 1999; Ford et al., 1994; Polich and Criado, 2006; Squires et al., 1975). P3a and P3b generation stems from frontal and temporal/parietal activations. Available evidence suggests that P3a is related to frontal focal attention and working memory mediated in part by dopaminergic activity and by glutamate neurotransmitter function (Dien et al., 2003; Hall et al., 2015; Knight et al., 1989), and that P3b is generated when subsequent attentional resources activations promote memory operations in temporal-parietal areas mediated by norepinephrine inputs (Polich, 2007; Polich and Criado, 2006).
P3b ERP is both a trait and state biomarker. Two meta-analyses of P3b in auditory oddball paradigms found reduced amplitude between patients with SZ and controls with a large pooled standardized effect size of 0.85 and 0.89 respectively (Bramon et al., 2005; Polich and Jeon, 2003). Psychopathology severity and antipsychotic medications were unrelated to P3 amplitude effect size (Polich and Jeon, 2003). Patients with first episode psychosis (FEP) and individuals at ultra high risk for psychosis also show amplitude reductions (Brown et al., 2002; de Wilde et al., 2008; del Re et al., 2015; Devrim-Üçok et al., 2006; Hirayasu et al., 1998; Kruiper et al., 2018; Morales-Muñoz et al., 2017; Renoult et al., 2007; Salisbury et al., 1998; Sumich et al., 2006; Umbricht et al., 2006; van Tricht et al., 2011). A meta-analysis of P3b found a reduction of amplitude in unaffected relatives of patients as compared to controls with a moderate pooled standardized effect size of 0.61 (Bramon et al., 2005). These meta-analyses and reports support P3b deficits as a trait biomarker. On the other hand, a meta-analysis of P3b in drug-naive SZ patients (early stage) found no significant reduction in the amplitude. This result supports P3b being a state dependent biomarker (Chen et al., 2014) which may be associated with disease chronicity/progression in patients with schizophrenia (Mathalon et al., 2000).
Several studies found reduced P3a amplitude in chronic SZ (Devrim-Üçok et al., 2006; Hamilton et al., 2018; Jahshan et al., 2012a; Kirihara et al., 2009; Mathalon et al., 2000; Pfefferbaum et al., 1989; Rissling et al., 2012) as well as in FEP patients (del Re et al., 2015; Hermens et al., 2010; Jahshan et al., 2012a; Kaur et al., 2011; Kruiper et al., 2018; Morales-Muñoz et al., 2017), suggesting that P3a deficit is already present at an early stage. The N1 and P2 ERPs reflect perceptual information registration and processing (Dien et al., 2004; Picton and Hillyard, 1974). Studies have generally found impairments in these ERPs in chronic SZ (Baribeau-Braun et al., 1983; Brown et al., 2000; Foxe et al., 2011; McCarley et al., 1991; O’Donnell et al., 1994; Roth et al., 1981; Salisbury et al., 2010; Shenton et al., 1989; Turetsky et al., 2009) but not always (Egan et al., 1994). In FEP patients, some studies found deficits in N1 and P2 (Brown et al., 2002; del Re et al., 2015; Foxe et al., 2011; Morales-Muñoz et al., 2017; Salisbury et al., 2010; Sumich et al., 2006) though there are also inconsistent findings (del Re et al., 2015; Morales-Muñoz et al., 2017; Valkonen-Korhonen et al., 2003). There is still debate regarding trait versus state dependence of the P3a, N1, and P2 ERPs (Ahveninen et al., 2006; Ethridge et al., 2015; Frangou et al., 1997; Karoumi et al., 2000; Michie et al., 2002; Rosburg et al., 2008; van Tricht et al., 2011), whether deficits are indicators of a trait or if they wax and wane with symptom severity or disease chronicity. Longitudinal studies of FEP patients could address this issue by tracking changes in the ERP components over time and clinical aspects simultaneously.
Longitudinal studies of FEP patients are rare. Devrim-Üçok et al. (2016) reported that FEP patients showed a consistent impairment in P3b amplitude at baseline and at a six year follow-up timepoint compared to control subjects. Kaur et al. (2013) reported a trend level impairment in P3a amplitude in FEP patients at baseline and that amplitudes did not significantly change at follow-up 12–30 months later. To our knowledge, no study has examined both components together in FEP patients so it is unclear if this pattern of longitudinally impaired P3a and P3b amplitudes occurs simultaneously within the same subject or if deficits in each component vary based on patient samples.
In addition, the P3a component has been documented to be associated with psychosocial functioning in non-psychiatric individuals (Light et al., 2007), chronic SZ patients (Light et al., 2015), and in FEP patients (Hermens et al., 2010). However, the relationship between P3a and psychosocial functioning is inconsistent in the literature. P3a was not correlated with Global Assessment of Functioning (GAF) in recent onset SZ (Jahshan et al., 2012a) nor predicted chronic SZ patients’ role functioning status (Hamilton et al., 2018). Clinical heterogeneity and disease chronicity/progression are likely contributors to the inconsistency. FEP patients frequently present with a mixture of affective and psychotic symptoms, making it difficult to make a definitive diagnosis. About a third of patients receive diagnoses of affective psychosis at the end of two years and about two thirds receive a diagnosis of SZ (Keshavan and Schooler, 1992). Studies examining the relationship between P3b and functioning are rare. Hamilton et al. (2018) report no association between role functioning and P3b in chronic SZ. To our knowledge, no study has examined the correlation between functioning and P3b in FEP patients. The vast majority of correlational studies between ERP components and psychosocial functioning have been cross-sectional, which only gives a snapshot of the relationship and does not provide predictive value as longitudinal studies can. One longitudinal study of FEP patients found no significant associations between baseline P3a amplitude and psychosocial functioning at follow-up (Kaur et al., 2013).
The aims of this study were to examine multiple ERP components in FEP patients longitudinally and to investigate relationships between each ERP component and psychosocial functioning, clinical features, and medication. By employing a longitudinal study design, one can track changes in the same individuals which provides critical insight into the early pathology of the disorder. We hypothesized that the P3a and P3b ERPs would be impaired in FEP patients at baseline and the deficit would be stable over time, reflecting primarily trait biomarkers whereas N1 and P2 ERPs would be intact at baseline but impaired at follow-up, reflecting illness related deficits. In addition, we hypothesized P3a and P3b would be significantly associated with psychosocial functioning in FEP patients at baseline and follow-up.
2. Methods and materials
2.1. Subjects
The sample consisted of 47 first episode psychosis (FEP) patients and 29 healthy control (HC) subjects. Patients consisted of 20 schizophrenia (SZ) spectrum diagnoses and 27 bipolar disorder (BD) with psychotic features, as assessed by the Structured Clinical Interview for DSM-IV (SCID) and review of medical records at their initial assessment. Patients were clinically stable and were recruited from outpatient clinics, inpatient hospital units, flyers posted at McLean Hospital, and physician referrals. Study inclusion criteria were: 1) age between 18 and 45 years; 2) fluency in English; 3) IQ>70; 4) patients with FEP diagnosed with SZ, schizoaffective disorder, schizophreniform disorder, psychotic disorder NOS, psychotic depression, or psychotic bipolar disorder. Exclusion criteria consisted of: 1) diagnosed neurological disorder such as seizures or Parkinson’s; 2) brain injury including stroke or serious head injury resulting in loss of consciousness; 4) hearing impairments, blindness, or deafness; 5) electroconvulsive therapy within the past 6 months; 6) outside the age range of 18–45 years. HC subjects were recruited from the Partners Research Portal and subject to the same exclusion criteria plus the following: no current or past history of psychotic or affective disorders, no substance abuse or previous chronic dependence, and no first-degree relative with a history of psychosis or bipolar disorder. The study was approved by McLean Hospital Institutional Review Board. All subjects provided written informed consent after receiving a complete description of the study.
2.2. Clinical and functioning assessments
Clinical and functioning measures included the Montgomery-Asberg Depression Rating Scale (MADRS; Montgomery and Asberg, 1979), Young Mania Rating Scale (YMRS; Young et al., 1978), the Positive and Negative Syndrome Scale (PANSS) subscales for Positive, Negative, and General symptoms (Kay et al., 1987), the Multnomah Community Ability Scale (MCAS; Hendryx et al., 2001), and the Global Assessment of Functioning (GAF) Scale (Axis V of DSM-IV). Medication information was collected at each assessment time point. Antipsychotics included first- and second-generation antipsychotic medications and were converted into chlorpromazine (CPZ) equivalents based on the recommendations of Baldessarini (2013).
2.3. Electrophysiological recordings and processing
The electroencephalogram (EEG) was recorded continuously using the BioSemi Active Two system (BioSemi Inc., Amsterdam, Netherlands) at a digitization rate of 512 Hz, with a bandpass of DC–104 Hz, and a Common Mode Sense (CMS) as the reference (PO2 site) using either an 18- or 64-channel electrode cap. EOG electrodes were placed below and at the outer canthi of the left eye. A three-stimulus (novelty) auditory oddball paradigm was used to elicit N1, P2, P3a, and P3b ERPs. Stimuli consisted of 400 binaural, 80 dB tones of 50 ms duration presented to the subjects through foam insert earphones. Twelve percent of the stimuli were target tones (1500 Hz), 12% infrequent “novel” sounds (a bird call or a water drop), and 76% standard tones (1000 Hz), with an inter-stimulus interval varying between 1.8 and 2.2 s. Participants were instructed to click the mouse in response to target tones only; a correct response was defined as occurring between 100 and 900 ms after the tone. A two-tone auditory oddball paradigm with 20% target and 80% standard tones (without novelty stimuli) was used with eight participants where N1, P2, and P3b (but not P3a) were elicited. Responses did not differ by paradigms so data were pooled for analyses.
Data were processed using BrainVision Analyzer 2 (Brain Products GmbH, Munich, Germany). Signals were re-referenced to an average of the mastoids and bandpass filtered between 0.01 and 20 Hz using a zero phase shift Butterworth filter, with an additional 8.5 Hz lowpass filter for calculating P3a and P3b. Data were segmented by stimulus marker from −100 to 1000 ms, with the responses to standard tones used to calculate N1 and P2, responses to novel sounds used for P3a, and correct responses to target tones used for P3b. Segments were baseline corrected using −100 to 0 ms pre-stimulus time and eye-blink corrected using established measures (Gratton et al., 1983). Artifact rejection for individual channels was performed and a given segment was rejected if the voltage gradient exceeded 50 μV/ms, amplitude was ± 100 μV, or the signal was flat (< 0.5 μV for>100 ms). Segments were averaged across stimulus markers and the maximum (or minimum for N1) amplitude peak was chosen between 50 and 200 ms for N1, 150–300 ms for P2, 250–450 ms for P3a, and 280–650 ms for P3b. Peak markers were checked for accuracy and verified that the earlier component preceded the later (N1 < P2 < P3a < P3b).
Forty-one FEP patients had baseline ERP recordings, of those, data from 41 individuals were included in N1 and P2 analyses, 35 for P3a, and 39 for P3b. The slight variations are due to technical issues where 6 subjects had two-tone oddball recordings (missing P3a) and one subject had very few (n=8) correct target responses and were excluded from P3b analyses. Twenty FEP patients were recorded at the 12-month timepoint and 14 at 24 months. Of the other 22 individuals for whom only baseline recordings were performed, 11 are still within their one-year follow-up timepoint, two were excluded from follow-up due to involvement in a cognitive treatment study, and nine were lost to follow-up.
2.4. Statistical analyses
Statistical analyses were carried out using STATA 14 (StataCorp, College Station, Texas). Peak amplitudes and latencies were analyzed using the Cz electrode site for N1, P2, and P3a, and Pz site for P3b. ERP latencies did not differ between groups and were dropped from analyses (see Supplementary Table 1). Comparisons of control versus patient demographics and BD versus SZ demographics and clinical features were performed using two-tailed t-tests and Chi-squared tests. Longitudinal changes within the same patients were assessed using paired t-tests. Linear regression analysis with standard errors was used for comparisons between groups (controls versus patients; SZ versus BD) across time (baseline, 12 months, and 24 months), controlling for age and sex. The “cluster” command in STATA was used, which allows for non-independent observation (i.e., follow-up timepoints). Separate regression analyses were performed for each ERP variable. Forward selection stepwise regression models were used to assess the relationships among clinical features, medication, and functional measures on each of the patient ERPs at baseline and 12 months. Variables examined included (in this order): age, sex, education, diagnosis (SZ or BD), scores of PANSS positive, negative, and general, MADRS total score, YMRS total score, GAF, MCAS total score, and CPZ equivalents dose. For predictors of functional outcome at 12 months (GAF and MCAS), two stepwise regression models (one for GAF and one for MCAS) were performed, including (in this order) the four ERP amplitudes, age, sex, education, PANSS positive, negative, and general, MADRS total score, and YMRS total score as potential predictors. Correlations between baseline ERP variables were calculated using Pearson’s pairwise correlations separately for control subjects and patients.
3. Results
3.1. Descriptive statistics
Demographic characteristics of subjects are presented in Table 1. Compared to patients, HCs consisted of more female participants (χ2=7.67, p=0.006) and had higher education (t=2.54, p=0.01). There were significantly more smokers in the patient group compared to controls (χ2=9.13, p=0.003). SZ and BD patients did not differ on any demographics, substance use, or medication dose, with the exception of Lithium, which was lower in the SZ group than BD (t=−2.14, p=0.04) (Table 2). Compared to BD, the SZ group had significantly lower baseline GAF (t=−3.15, p=0.003) and MCAS (t=−2.35, p=0.02) scores, and higher PANSS negative (t=2.39, p=0.02) and PANSS general (t=3.02, p=0.004) scores (Table 2). Trials with incorrect button presses were excluded from analysis, and there were no group differences in response accuracy for the target tones (p=0.91) or the number of accepted trials for the novel tones (p=0.09).
Table 1.
Controls versus first episode psychosis (FEP) patient demographics for all subjects.
| Controls (n = 29) | FEP Patients (n = 47) | Statistic (df) | p value | |
|---|---|---|---|---|
| Age, years | 22.9 (3.2) | 22.8 (3.3) | t(74) = 0.07 | p = 0.95 |
| Female, N (%) | 18 (62.1) | 14 (29.8) | χ2(1) = 7.67 | p = 0.006 |
| Education, years | 15.5 (1.5) | 14.7 (1.3) | t(74) = 2.54 | p = 0.01 |
| Cannabis use, N (%) | 7 (24.1) | 18 (43.9) | χ2(1) = 2.89 | p = 0.09 |
| Smokers, N (%) | 0 (0) | 10 (31.3) | χ2(1) = 9.13 | p = 0.003 |
| Alcohol use, N (%) | 26 (89.7) | 32 (76.2) | χ2(1) = 2.08 | p = 0.15 |
Note: standard deviations in parentheses unless otherwise specified.
Significant differences are highlighted in bold.
Table 2.
Demographics and clinical variables between Schizophrenia (SZ) versus bipolar disorder (BD) patients.
| SZ patients (n = 20) | BD patients (n = 27) | Statistic (df) | p value | |
|---|---|---|---|---|
| Age, years | 23.0 (4.0) | 22.7 (2.8) | t(45) = 0.34 | p = 0.74 |
| Female, N (%) | 5 (25.0) | 9 (33.3) | χ2(1) = 0.38 | p = 0.54 |
| Education, years | 14.7 (1.3) | 14.7 (1.4) | t(45) = 0.08 | p = 0.93 |
| Cannabis use, N (%) | 6 (31.6) | 12 (54.5) | χ2(1) = 2.18 | p = 0.14 |
| Smokers, N (%) | 4 (28.6) | 6 (33.3) | χ2(1) = 0.08 | p = 0.77 |
| Alcohol use, N (%) | 15 (79.0) | 17 (73.9) | χ2(1) = 0.15 | p = 0.70 |
| CPZ equivalents, mg/d | 230.3 (246.1) | 202.2 (221.6) | t(42) = 0.40 | p = 0.69 |
| Lithium, mg/d | 244.7.5 (461.5) | 609.6 (628.3) | t(43) = −2.14 | p = 0.04 |
| PANSS positive | 15.4 (6.7) | 12.1 (6.1) | t(45) = 1.72 | p = 0.09 |
| PANSS negative | 14.4 (6.6) | 10.8 (3.6) | t(45) = 2.39 | p = 0.02 |
| PANSS general | 31.4 (6.1) | 25.9 (6.1) | t(45) = 3.02 | p = 0.004 |
| MADRS total | 11.9 (8.6) | 9.1 (6.3) | t(45) = 1.24 | p = 0.22 |
| YMRS total | 8.0 (7.6) | 5.3 (6.5) | t(44) = 1.31 | p = 0.20 |
| GAF | 51.8 (14.2) | 65.2 (13.7) | t(42) = −3.15 | p = 0.003 |
| MCAS total | 44.4 (6.2) | 48.3 (5.0) | t(44) = −2.35 | p = 0.02 |
Note: standard deviations in parentheses unless otherwise specified.
Significant differences are highlighted in bold.
3.2. N1 and P2 ERP components
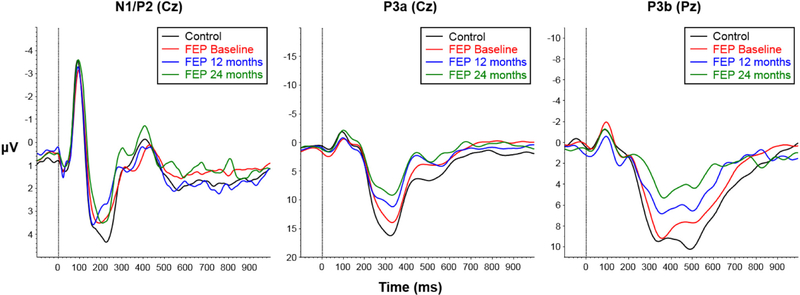
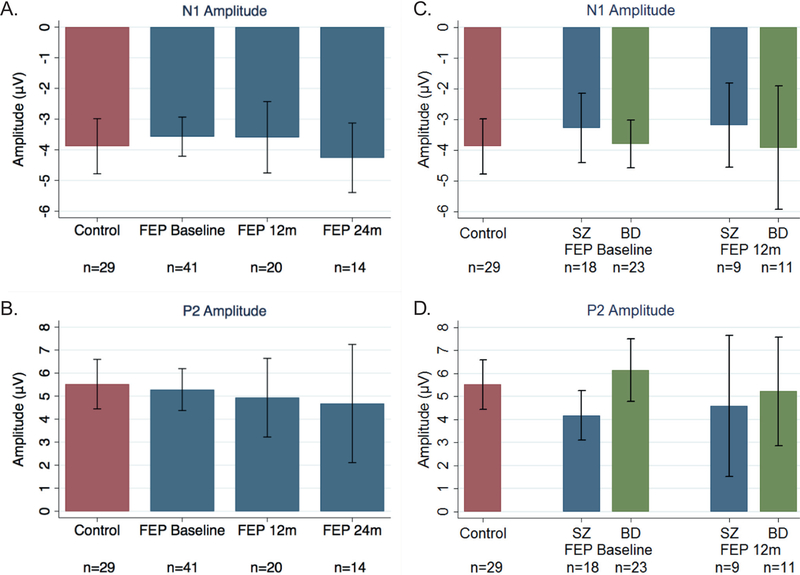
No differences in N1 or P2 amplitudes were found between HCs and patients at any of the timepoints (N1 baseline: F(3, 37)=0.56, p=0.64, R2=0.04; N1 longitudinal: F(5, 75)=1.69, p=0.15, R2=0.06; P2 baseline: F(3, 37)=2.33, p=0.09, R2=0.16; P2 longitudinal: F(5, 75)=1.32, p=0.26, R2=0.04) (Fig. 1, left). Within the patients, no changes in baseline N1 (Fig. 2a) or P2 (Fig. 2b) amplitudes were found at either follow-up timepoints. However, at baseline, BD patients had significantly larger P2 amplitudes (M=6.15, SD=0.65) compared to SZ patients (M=4.18, SD=0.50; t=2.16 p=0.04) (Fig. 2d); the difference in N1 amplitude between the two groups was not significant (SZ: M=–3.29, SD=2.27; BD: M=–3.79, SD=1.81; t=−0.56, p=0.59) (Fig. 2c). Longitudinally, amplitudes in patients were similar from baseline to 12 months for both N1 (paired t=0.64, p=0.54) and P2 (paired t=0.02, p=0.98) (Fig. 2a & b).
Fig. 1.
Grand average waveforms for control subjects (black) and first episode psychosis (FEP) patients at baseline (red), 12 months (blue), and 24 months (green). Left: N1 and P2 recorded at Cz in response to standard stimuli. Middle: P3a recorded at Cz in response to infrequent novel stimuli. Right: P3b recorded at Pz in response to infrequent target stimuli. Dotted vertical line at 0 ms marks stimulus presentation. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Fig. 2.
Average amplitudes for N1 (top) and P2 (bottom). A) N1 amplitude for control subjects (red) and first episode psychosis (FEP) patients (blue) at baseline, 12 months, and 24 months. B) Same as A but for P2 amplitude. C) N1 amplitude for control subjects (red), schizophrenia (SZ) patients (blue), and bipolar disorder (BD) patients (green) at baseline and 12 months. D) Same as C but for P2 amplitude. (Note: vertical bars represent standard deviations.) (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.3. N1 and P2 ERPs in relationships with clinical features and functioning
Results of stepwise regression analyses were presented in Supplementary Material (Table S2). At baseline, PANSS negative score (β =0.13 (SE=0.05), partial r=0.46, p=0.02), sex (β=1.4 (SE=0.60), p=0.04 partial r=0.36) and CPZ equivalents (β=0.003 (SE=0.001), p=0.03 partial r=0.38) were significant predictors of baseline N1 amplitude such that greater negative symptoms, male gender, and higher dose of CPZ predicted smaller (i.e., more positive) N1 magnitude. At the 12-month follow-up MADRS score predicted greater N1 response (β=−0.15 (SE=0.07), p=0.04, partial r=−0.50) such that lower depressive symptoms predicted greater N1 amplitude. Baseline N1 amplitude was not predictive of either MCAS or GAF functioning measures at the 12-month follow-up.
At baseline, diagnosis (β=1.16 (SE=0.48), p=0.02, partial r=0.39), PANSS negative score (β=−0.18 (SE=0.09), p=0.049, partial r=−0.31), and CPZ equivalents (β=−0.004 (SE=0.002), p=0.048, partial r=−0.35) were significant predictors of baseline P2 amplitude, such that a SZ diagnosis, more severe negative symptoms, and higher CPZ equivalents were correlated with smaller P2 amplitudes (Table S2). At the 12-month follow-up, P2 amplitude was not associated with clinical or functioning measures.
3.4. P3a ERP component
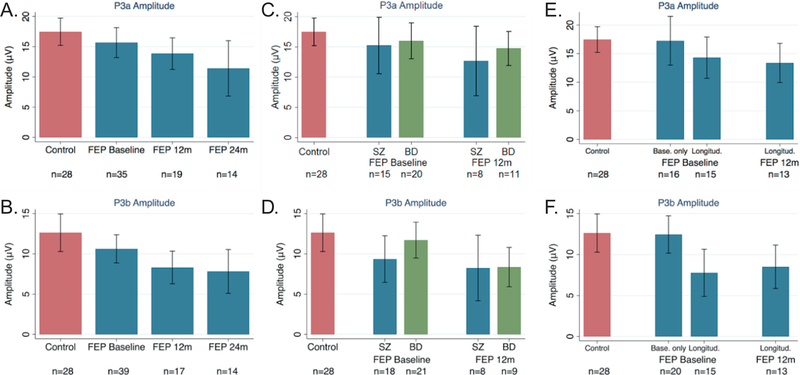
At baseline, patients versus HCs showed no deficits in P3a amplitude (F(3, 31)=0.18, p=0.91, R2=0.02), however, significant group differences emerged at 12 months (β=−3.97 (SE=1.56), p=0.01) and 24 months (β=−6.64 (SE=2.28), p=0.005, full model: F(5, 69)=2.60, p=0.03, R2=0.10) (Fig. 3a). Comparing patients with baseline data only (n=16) versus longitudinal follow-up patients (n=15), those with baseline data only had intact P3a amplitudes and those with follow-up data showed reduced P3a amplitudes at baseline (full model: F(4, 41)=1.61, p=0.19, R2=0.15); β=−3.64 (SE=1.84), p=0.05) and at the 12-month follow-up timepoint (β=−4.11 (SE=1.72), p=0.02) (Fig. 3e). Longitudinally, p3a amplitude reduction in follow-up patients did not change over time from baseline to 12 months (paired t=−0.24, p=0.82) (Fig. 3e). The two diagnostic groups did not differ in P3a amplitude at any time point (Fig. 3c).
Fig. 3.
Average amplitudes for P3a (top) and P3b (bottom). A) P3a amplitude for control subjects (red) and first episode psychosis (FEP) patients (blue) at baseline, 12 months, and 24 months. B) Same as A but for P3b amplitude. C) P3a amplitude for control subjects (red), schizophrenia (SZ) patients (blue), and bipolar disorder (BD) patients (green) at baseline and 12 months. D) Same as C but for P3b amplitude. E) P3a amplitude for control subjects (red) and FEP patients (blue) at baseline and 12 months for those with baseline only recordings (Base. only) and those with longitudinal recordings (Longitud.). F) Same as E but for P3b amplitude. (Note: vertical bars represent standard deviations.) (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
P3a ERP in relationship with clinical features and functioning. At baseline, P3a amplitude was not associated with any clinical or functioning measures (Table S2). At 12 months, PANSS positive score was a predictor of P3a amplitude (β=−0.96 (SE=0.27), p=0.003, partial r=−0.68,), such that more severe positive symptoms predicted smaller amplitudes. No significant associations were found between P3a ERP and functional measures at the follow-up time point (Table S2).
3.5. P3b ERP component
Patients and HCs did not significantly differ in P3b amplitude at baseline (F(3, 35)=1.75, p=0.17, R2=0.13), though significant group differences were found at 12 months (β=−3.87 (SE=1.66), p=0.02) and 24 months (β=−4.30 (SE=1.82), p=0.02; full model: F(5, 74)=2.67, p=0.03, R2=0.10)) (Fig. 3b). Comparing patients with baseline data only (n=20) versus longitudinal follow-up patients (n=15), patients with baseline data only had an intact P3b amplitude (M=12.46, SD=4.88) compared to controls (M=12.63, SD=6.02), whereas patients with follow-up data had a significantly reduced P3b amplitude at baseline (M=7.77, SD=5.21; (β=−4.58 (SE=1.83), p=0.03,) and 12 months (M=8.51, SD=4.39; β=−3.84 (SE=2.08), p=0.04; Full model: F(4,40)=2.05, p=0.11, R2=0.16) (Fig. 3f). Longitudinally, P3b amplitude reduction in follow-up patients did not change over time from baseline to 12 months (paired t=0.65, p=0.53) (Fig. 3f). The two diagnostic groups did not differ in P3b at any time point (Fig. 3d).
3.6. P3b ERP in relationship with clinical features and functioning
At baseline, no significant associations were found between P3b and clinical or functional variables (F(1, 32)=1.99, p=0.17). Whereas at the 12 month follow-up, P3b was significantly associated with age (β=1.07 (SE=0.25), p=0.002, partial r=0.81), CPZ dose (β=−0.01 (SE=0.004), p=0.01, partial r=−0.66), MADRS score (β=0.30 (SE=0.09), p=0.007, partial r=0.75), and MCAS score (β=0.32 (SE=0.15), p=0.06, partial r=0.72), where order age, higher CPZ dose, greater depressive symptoms, and higher functioning were associated with a larger amplitude, respectively (Table S2).
3.7. Predictors of functional outcome
Results of regression models predicting later functioning (GAF or MCAS) at 12-month from baseline demographics, clinical and ERP variables were presented in Supplementary Material (Table S3). Baseline P3a ERP and PANSS general score were significant and independent predictors of later MCAS functioning at 12-month (F(3, 11)=7.23, p=0.006, R2=0.66), such that greater P3a amplitude at baseline predicted higher functioning (β=0.32 (SE=0.16), p=0.06, partial r=0.57) and more severe symptoms at baseline predicted lower functioning (β=−0.43 (SE=0.15), p=0.01, partial r=−0.70) a year later. Similarly, baseline P3a ERP was a significant predictor of later GAF functioning at 12-months (F(3, 11)=2.77, p=0.09, R2=0.43), such that greater P3a amplitude at baseline predicted higher functioning (β=1.08 (SE=0.4), p=0.04, partial r=0.57) a year later. Baseline N1 or P2 amplitude was not predictive of either MCAS or GAF functioning measures at the 12-month follow-up.
3.8. Correlations of ERP components
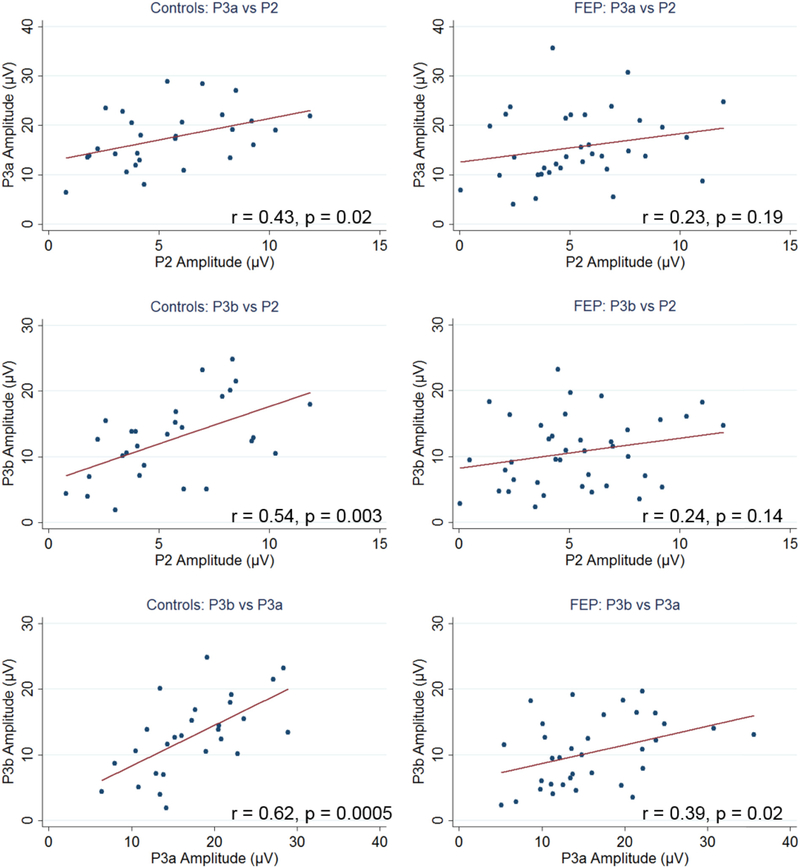
In the whole sample, significant positive correlations were found between P2 and P3a amplitudes (r=0.30, p=0.02), P2 and P3b amplitudes (r=0.33, p=0.01), and between P3a and P3b (r=0.42, p=0.001). Correlation patterns among ERPs are similar between patients and controls (Fig. 4).
Fig. 4.
Correlations between P2, P3a, and P3b amplitudes for control subjects (left) and first episode psychosis (FEP) patients (right).
Note: blue dots represent individual subjects and red lines mark the best linear fit. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
4. Discussion
In this study we aimed to examine longitudinal aspects of event-related brain responses in FEP patients and the relation to functional and clinical correlates. The N1 and P2 components reflect stimulus registration and processing at early stages of information processing (Dien et al., 2004; Picton and Hillyard, 1974). In the present study, no significant deficits in the N1 or P2 responses were observed at baseline or over time. Rather, N1 amplitude was remarkably intact (Fig. 1, left) which corroborates some findings in FEP patients (Morales-Muñoz et al., 2017; Valkonen-Korhonen et al., 2003) but is inconsistent with others (Brown et al., 2002; del Re et al., 2015; Foxe et al., 2011; Salisbury et al., 2010; Sumich et al., 2006). Similarly, we did not find deficits in P2 amplitude overall, which agrees with del Re et al. (2015) but not others (Morales-Muñoz et al., 2016; Salisbury et al., 2010). Our study sample included patients with both SZ and affective psychosis diagnoses whereas previous studies reporting N1 and P2 deficits focused on specifically SZ patients only, which could explain this discrepancy. Our results showed that patients with a BD diagnosis had a significantly larger baseline P2 amplitude than those with a SZ diagnosis (Fig. 2d); the N1 response was also larger though not significantly (Fig. 2c). This corroborates data from O’Donnell et al. (2004) showing reduced N1 and P2 amplitude in chronic SZ but not BD patients (approximately half with psychotic symptoms).
N1 was more reduced for those having more severe negative and depressive symptoms, suggesting N1 ERP is a state dependent biomarker tracking depressive symptom severity. P2 amplitude was greater for those experiencing fewer negative symptoms and taking lower doses of CPZ equivalents independent of diagnosis. Patients with a diagnosis of SZ had more impaired P2 amplitude than those with a BD diagnosis, suggesting that P2 amplitude might distinguish between the two diagnostic categories and that P2 ERP also is a state dependent biomarker being modulated by symptom severity and antipsychotic medication. Neither N1 nor P2 ERP was associated with functional measures or predictive of functioning a year later indicating that they are not sensitive biomarkers for functioning related clinical studies.
The present study examined cross-sectional aspects and the longitudinal course of P3a and P3b ERPs simultaneously in FEP. Crosssectional results revealed that both P3a and P3b amplitudes in patients were impaired at 12- and 24-month follow-up timepoints, but not at baseline (Fig. 3a & b). These results, in part, agree with Mathalon et al. (2000) that P3a and P3b tracked illness duration longitudinally or across different stages of illnesses (Jahshan et al., 2012a). However, when examining data longitudinally, we found that patients already showed impairment at baseline and this deficit was stable over time, similar to previous reports (del Re et al., 2015; Hermens et al., 2010; Jahshan et al., 2012a; Kruiper et al., 2018; Morales-Muñoz et al., 2017). A similar pattern of results was found with P3a amplitude, suggesting P3a deficits are already present at a first episode, substantiating other reports (Brown et al., 2002; de Wilde et al., 2008; del Re et al., 2015; Demiralp et al., 2002; Devrim-Üçok et al., 2016, 2006; Hirayasu et al., 1998; Kruiper et al., 2018; Morales-Muñoz et al., 2017; Renoult et al., 2007; Salisbury et al., 1998; Sumich et al., 2006; Umbricht et al., 2006). In addition, our results suggest patients with FEP are heterogeneous and there appears to be two subgroups: those with intact (i.e., our baseline data only sample) and those with impaired (i.e., our follow-up data sample) P3 responses. However, we did not find any single significant demographic or clinical difference between the two subgroups, although there were more BD than SZ diagnosis in the baseline data only sample (59% vs. 40%, p=0.25). In addition, the baseline only group tended to have higher GAF (M=60.0, SD=14.7 vs. M=56.7, SD=16.2) and less severe symptoms (PANSS total: M=54.0, SD=16.9 vs. M=58.1, SD=16.1; YMRS: M=5.9, SD=5.4 vs. M=6.9, SD=8.5; MADRS: M=9.5, SD=7.1 vs. M=12.2, SD=8.8), which in general mediated larger P3a and P3b responses. Due to small sample size, statistical comparisons between these two subgroups were not significant. We will examine the two subgroups in the future with a larger sample. Half of the baseline recording only group is still actively enrolled in the study but not yet at 12-month timepoint, suggesting the difference cannot be attributed simply to features unique to study dropouts.
BD patients had higher functioning and fewer symptoms than SZ patients, but largely similar ERP responses, with the exception of P2 amplitude. Hermens et al. (2010) and Kaur et al. (2011) found no diagnostic group differences in P3a amplitudes in FEP patients, consistent with our findings. However, Salisbury et al. (1998) found reduced P3b amplitudes only in patients with a SZ diagnosis but not affective psychosis. These results again suggest heterogeneity within the FEP population and partial overlapping etiology between SZ and BD diagnoses.
Our results showed that in general, greater symptom severity attenuated N1, P2, and P3a ERP responses in FEP patients, consistent with reports of chronic and FEP patients (Boutros et al., 1997; Rissling et al., 2013; Sumich et al., 2006; Turetsky et al., 2009). Importantly, our results showed that baseline P3a amplitudes and PANSS general score were significant and independent predictors of later MCAS functioning at 12-months, such that greater P3a amplitudes at baseline predicted higher real-life functioning as measured by both MCAS and GAF and more severe general pathological symptoms at baseline predicted lower functioning one year later. This result provides supporting evidence that both P3a amplitude and PANSS general are useful functioning-linked biomarker in outcome studies, and is consistent with cross-sectional studies (Hermens et al., 2010; Light et al., 2015, 2007). Furthermore, our results add to the literature that P3a amplitude provides predictive value of functioning status in FEP patients. One longitudinal study of FEP patients found no significant associations between baseline P3a amplitude and psychosocial functioning at follow-up (Kaur et al., 2013). This study used the Social and Occupational Functioning Assessment Scale (SOFAS) to assess functioning. SOFAS is highly correlated with the GAF and is rated as an overall impression of occupation and functioning performance. MCAS, on the other hand, scores several different axes of functionality independently (e.g., independent living, meaningful activity, social activity and relationships, management of money), measuring functioning of people with mental illness living in the community. Baseline PANSS general was significant in predicting later functioning using MCAS as an outcome but non-significant in the model using GAF, suggesting MCAS might be a more sensitive tool in capturing a wider range of community functioning than GAF. In our study we found P3a and P3b amplitudes predicted both MCAS and GAF functioning a year later but only P3a was statistically significant, likely due to shared covaiance between them (r=0.42). When P3a was removed in the stepwise regression, P3b amplitude became a significant predictor of MCAS functioning (patial correlations R=0.61, p=0.03), suggesting that, with a bigger sample size, P3b likely also provide predictive value of functioning status.
Our results showed that antipsychotic medications mediate P2 ERP responses at baseline but not at follow-up. This result is consistent with some studies finding medication effects on ERPs (Ethridge et al., 2015; Pfefferbaum et al., 1989). However, medication effects on N1 and P2 ERPs reported in the literature tend to be mixed with many reporting no effects (del Re et al., 2015; Hirayasu et al., 1998; Jahshan et al., 2012b; Qiu et al., 2014; Salisbury et al., 2010) or detrimental effects (Pfefferbaum et al., 1989; Salisbury et al., 2010; Turetsky et al., 2009). Given that FEP patients were prescribed different types and combinations of medications, much larger samples are needed to properly evaluate effects of medications over time.
The overall correlation patterns among ERPs are similar between patients and controls. Significant positive correlations were found between P2 and P3a amplitudes (r=0.30), P2 and P3b amplitudes (r=0.33), and between P3a and P3b (r=0.42). However, there are subtle differences in terms of the pattern of correlations between ERP components in HCs and in FEP patients (Fig. 4). In controls, the amplitudes of P2, P3a, and P3b were all significantly and positively correlated. However, in patients only a weak correlation between P3a and P3b was found. In chronic SZ, similar patterns have been reported (Mathalon et al., 2000; Shenton et al., 1989). This subtle difference in patients indicates a “noisier” brain in patients than healthy individuals, reflecting, to some extent, weakened underlying neuronal connections and/or circuits.
This study has a number of limitations. First, sample size of individuals with follow-up data is relatively small. Of the 22 individuals for whom only baseline recordings were performed, half are still within their one-year follow-up timepoint. We therefore are unable to comprehensively address our hypotheses until all participants are completed the study at all timepoints.
In conclusion, the present study found that N1 and P2 ERPs were intact during early stages of psychotic illness, though SZ patients showed impairment in P2 when compared to BD patients. P3a and P3b ERPs were impaired in FEP patients who had been followed longitudinally, independent of diagnosis. Also, greater P3a and P3b amplitudes at baseline predicted higher real-life functioning and more severe general pathological symptoms at baseline predicted lower functioning one year later.
Supplementary Material
Acknowledgments
Funding
This work was supported by the National Institute of Mental Health [R01MH109687] to MHH and [K24MH104449] to DO.
Footnotes
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijpsycho.2019.05.007.
References
- Ahveninen J, Jääskeläinen IP, Osipova D, Huttunen MO, Ilmoniemi RJ, Kaprio J, Lönnqvist J, Manninen M, Pakarinen S, Therman S, Näätänen R, Cannon TD, 2006. Inherited auditory-cortical dysfunction in twin pairs discordant for schizophrenia. Biol. Psychiatry 60, 612–620. 10.1016/j.biopsych.2006.04.015. [DOI] [PubMed] [Google Scholar]
- Baldessarini RJ, 2013. Chemotherapy in Psychiatry. Springer, New York, NY: 10.1007/978-1-4614-3710-9. [DOI] [Google Scholar]
- Baribeau-Braun J, Picton T, Gosselin J, 1983. Schizophrenia: a neurophysiological evaluation of abnormal information processing. Science (80-.) 219, 874–876. 10.1126/science.6823555. [DOI] [PubMed] [Google Scholar]
- Boutros NN, Nasrallah H, Leighty R, Torello M, Tueting P, Olson S, 1997. Auditory evoked potentials, clinical vs. research applications. Psychiatry Res. 69, 183–195. 10.1016/S0165-1781(96)02919-8. [DOI] [PubMed] [Google Scholar]
- Bramon E, Rabe-Hesketh S, Sham P, Murray RM, Frangou S, 2004. Meta-analysis of the P300 and P50 waveforms in schizophrenia. Schizophr. Res 70, 315–329. 10.1016/j.schres.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Bramon E, McDonald C, Croft RJ, Landau S, Filbey F, Gruzelier JH, Sham PC, Frangou S, Murray RM, 2005. Is the P300 wave an endophenotype for schizophrenia? A meta-analysis and a family study. Neuroimage 27, 960–968. 10.1016/j.neuroimage.2005.05.022. [DOI] [PubMed] [Google Scholar]
- Brown K, Gordon E, Williams L, Bahramali H, Harris A, Gray J, Gonsalvez C, Meares R, 2000. Misattribution of sensory input reflected in dysfunctional target:non-target ERPs in schizophrenia. Psychol. Med 30, 1443–1449. [DOI] [PubMed] [Google Scholar]
- Brown KJ, Gonsalvez CJ, Harris AWF, Williams LM, Gordon E, 2002. Target and non-target ERP disturbances in first episode vs. chronic schizophrenia. Clin. Neurophysiol 113, 1754–1763. 10.1016/S1388-2457(02)00290-0. [DOI] [PubMed] [Google Scholar]
- Chen KC, Lee IH, Yang YK, Landau S, Chang WH, Chen PS, Lu RB, David AS, Bramon E, 2014. P300 waveform and dopamine transporter availability: a controlled EEG and SPECT study in medication-naive patients with schizophrenia and a meta-analysis. Psychol. Med 44, 2151–2162. 10.1017/S0033291713002808. [DOI] [PubMed] [Google Scholar]
- Comerchero MD, Polich J, 1999. P3a and P3b from typical visual and auditory stimuli. Clin. Neurphysiology 110, 24–30. [DOI] [PubMed] [Google Scholar]
- Demiralp T, Üçok A, Devrim M, Isoglu-Alkaç Ü, Tecer A, Polich J, 2002. N2 and P3 components of event-related potential in first-episode schizophrenic patients: scalp topography, medication, and latency effects. Psychiatry Res. 111, 167–179. 10.1016/S0165-1781(02)00133-6. [DOI] [PubMed] [Google Scholar]
- Devrim-Üçok M, Keskin-Ergen Y, Üçok A, 2006. Novelty P3 and P3b in first-episode schizophrenia and chronic schizophrenia. Prog. Neuro-Psychopharmacology Biol. Psychiatry 30, 1426–1434. 10.1016/j.pnpbp.2006.05.019. [DOI] [PubMed] [Google Scholar]
- Devrim-Üçok M, Keskin-Ergen Y, Üçok A, 2016. Lack of progressive reduction in P3 amplitude after the first–episode of schizophrenia: a 6-year follow-up study. Psychiatry Res. 243, 303–311. 10.1016/j.psychres.2016.02.065. [DOI] [PubMed] [Google Scholar]
- Dien J, Spencer KM, Donchin E, 2003. Localization of the event-related potential novelty response as defined by principal components analysis. Cogn. Brain Res. 17, 637–650. 10.1016/S0926-6410(03)00188-5. [DOI] [PubMed] [Google Scholar]
- Dien J, Spencer KM, Donchin E, 2004. Parsing the late positive complex: mental chronometry and the ERP components that inhabit the neighborhood of the P300. Psychophysiology 41, 665–678. 10.1111/j.1469-8986.2004.00193.x. [DOI] [PubMed] [Google Scholar]
- Egan MF, Duncan CC, Suddath RL, Kirch DG, Mirsky AF, Wyatt RJ, 1994. Event-related potential abnormalities correlate with structural brain alterations and clinical features in patients with chronic schizophrenia. Schizophr. Res 11, 259–271. 10.1016/0920-9964(94)90020-5. [DOI] [PubMed] [Google Scholar]
- Ethridge LE, Hamm JP, Pearlson GD, Tamminga CA, Sweeney JA, Keshavan MS, Clementz BA, 2015. Event-related potential and time-frequency endophenotypes for schizophrenia and psychotic bipolar disorder. Biol. Psychiatry 77, 127–136. 10.1016/j.biopsych.2014.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford JM, Sullivan EV, Marsh L, White PM, Lim KO, Pfefferbaum A, 1994. The relationship between P300 amplitude and regional gray matter volumes depends upon the attentional system engaged. Electroencephalogr. Clin. Neurophysiol 90, 214–228. [DOI] [PubMed] [Google Scholar]
- Foxe JJ, Yeap S, Snyder AC, Kelly SP, Thakore JH, Molholm S, 2011. The N1 auditory evoked potential component as an endophenotype for schizophrenia: highdensity electrical mapping in clinically unaffected first-degree relatives, first-episode, and chronic schizophrenia patients. Eur. Arch. Psychiatry Clin. Neurosci 261, 331–339. 10.1007/s00406-010-0176-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frangou S, Sharma T, Alarcon G, Sigmudsson T, Takei N, Binnie C, Murray RM, 1997. The Maudsley Family Study, II: endogenous event-related potentials in familial schizophrenia. Schizophr. Res 23, 45–53. 10.1016/S0920-9964(96)00089-8. [DOI] [PubMed] [Google Scholar]
- Gratton G, Coles MGH, Donchin E, 1983. A new method for off-line removal of ocular artifact. Electroencephalogr. Clin. Neurophysiol 55, 468–484. 10.1016/0013-4694(83)90135-9. [DOI] [PubMed] [Google Scholar]
- Hall MH, Jensen JE, Du F, Smoller JW, O’Connor L, Spencer KM, Öngür D, 2015. Frontal P3 event-related potential is related to brain glutamine/glutamate ratio measured in vivo. Neuroimage 111, 186–191. 10.1016/j.neuroimage.2015.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton HK, Perez VB, Ford JM, Roach BJ, Jaeger J, Mathalon DH, 2018. Mismatch negativity but not P300 is associated with functional disability in schizophrenia. Schizophr. Bull 44, 492–504. 10.1093/schbul/sbx104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendryx M, Dyck DG, McBride D, Whitbeck J, 2001. A test of the reliability and validity of the Multnomah community ability scale. Community Ment. Health J 37, 157–168. 10.1023/A:1002713816110. [DOI] [PubMed] [Google Scholar]
- Hermens DF, Ward PB, Hodge MAR, Kaur M, Naismith SL, Hickie IB, 2010. Impaired MMN/P3a complex in first-episode psychosis: cognitive and psychosocial associations. Prog. Neuro-Psychopharmacology Biol. Psychiatry 34, 822–829. 10.1016/j.pnpbp.2010.03.019. [DOI] [PubMed] [Google Scholar]
- Hirayasu Y, Asato N, Ohta H, Hokama H, Arakaki H, Ogura C, 1998. Abnormalities of auditory event-related potentials in schizophrenia prior to treatment. Biol. Psychiatry 43, 244–253. 10.1016/S0006-3223(97)00275-8. [DOI] [PubMed] [Google Scholar]
- Jahshan C, Cadenhead KS, Rissling AJ, Kirihara K, Braff DL, Light GA, 2012a. Automatic sensory information processing abnormalities across the illness course of schizophrenia. Psychol. Med 42, 85–97. 10.1017/S0033291711001061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahshan C, Wynn JK, Mathis KI, Altshuler LL, Glahn DC, Green MF, 2012b. Cross-diagnostic comparison of duration mismatch negativity and P3a in bipolar disorder and schizophrenia. Bipolar Disord. 14, 239–248. 10.1111/j.1399-5618.2012.01008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon YW, Polich J, 2001. P300 asymmetry in schizophrenia: a meta-analysis. Psychiatry Res. 104, 61–74. 10.1016/S0165-1781(01)00297-9. [DOI] [PubMed] [Google Scholar]
- Karoumi B, Laurent A, Rosenfeld F, Rochet T, Brunon AM, Dalery J, D’Amato T, Saoud M, 2000. Alteration of event related potentials in siblings discordant for schizophrenia. Schizophr. Res 41, 325–334. 10.1016/S09209964(99)00062-6. [DOI] [PubMed] [Google Scholar]
- Kaur M, Battisti RA, Ward PB, Ahmed A, Hickie IB, Hermens DF, 2011. MMN/ P3a deficits in first episode psychosis: comparing schizophrenia-spectrum and affective-spectrum subgroups. Schizophr. Res 130, 203–209. 10.1016/j.schres.2011.03.025. [DOI] [PubMed] [Google Scholar]
- Kaur M, Lagopoulos J, Lee RSC, Ward PB, Naismith SL, Hickie IB, Hermens DF, 2013. Longitudinal associations between mismatch negativity and disability in early schizophrenia- and affective-spectrum disorders. Prog. NeuroPsychopharmacology Biol. Psychiatry 46, 161–169. 10.1016/j.pnpbp.2013.07.002. [DOI] [PubMed] [Google Scholar]
- Kay SR, Fiszbein A, Opler LA, 1987. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr. Bull 13, 261–276. 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- Keshavan MS, Schooler NR, 1992. First-episode studies in schizophrenia: criteria and characterization. Schizophr. Bull 18, 491–513. doi:1411336. [DOI] [PubMed] [Google Scholar]
- Kirihara K, Araki T, Uetsuki M, Yamasue H, Hata A, Rogers MA, Iwanami A, Kasai K, 2009. Association study between auditory P3a/P3b event-related potentials and thought disorder in schizophrenia. Brain Imaging Behav 3, 277–283. 10.1007/s11682-009-9069-0. [DOI] [PubMed] [Google Scholar]
- Knight RT, Scabini D, Woods DL, Clayworth CC, 1989. Contributions of temporalparietal junction to the human auditory P3. Brain Res. 502, 109–116. 10.1016/0006-8993(89)90466-6. [DOI] [PubMed] [Google Scholar]
- Kruiper C, Fagerlund B, Nielsen MØ, Düring S, Jensen MH, Ebdrup BH, Glenthøj BY, Oranje B, 2018. Associations between P3a and P3b amplitudes and cognition in antipsychotic-naïve first-episode schizophrenia patients. Psychol. Med. 1–8. 10.1017/S0033291718001575. [DOI] [PubMed] [Google Scholar]
- Light GA, Swerdlow NR, Braff DL, 2007. Preattentive sensory processing as indexed by the MMN and P3a brain responses is associated with cognitive and psychosocial functioning in healthy adults. J. Cogn. Neurosci 19, 1624–1632. 10.1162/jocn.2007.19.10.1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Light GA, Swerdlow NR, Thomas ML, Calkins ME, Green MF, Greenwood TA, Gur RE, Gur RC, Lazzeroni LC, Nuechterlein KH, Pela M, Radant AD, Seidman LJ, Sharp RF, Siever LJ, Silverman JM, Sprock J, Stone WS, Sugar CA, Tsuang DW, Tsuang MT, Braff DL, Turetsky BI, 2015. Validation of mismatch negativity and P3a for use in multi-site studies of schizophrenia: characterization of demographic, clinical, cognitive, and functional correlates in COGS-2. Schizophr. Res 163, 63–72. 10.1016/j.schres.2014.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathalon DH, Ford JM, Pfefferbaum A, 2000. Trait and state aspects of p300 amplitude reduction in schizophrenia: a retrospective longitudinal study. Biol. Psychiatry 47, 434–449. 10.1016/S0006-3223(99)00277-2. [DOI] [PubMed] [Google Scholar]
- McCarley RW, Faux SF, Shenton ME, Nestor PG, Adams J, 1991. Event-related potentials in schizophrenia: their biological and clinical correlates and new model of schizophrenic pathophysiology. Schizophr. Res 4, 209–231. 10.1016/0920-9964(91)90034-O. [DOI] [PubMed] [Google Scholar]
- Michie PT, Innes-Brown H, Todd J, Jablensky AV, 2002. Duration mismatch negativity in biological relatives of patients with schizophrenia spectrum disorders. Biol. Psychiatry 52, 749–758. 10.1016/S0006-3223(02)01379-3. [DOI] [PubMed] [Google Scholar]
- Montgomery SA, Asberg M, 1979. A new depression scale designed to be sensitive to change. Br. J. Psychiatry 134, 382–389. 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- Morales-Muñoz I, Jurado-Barba R, Fernández-Guinea S, Rodríguez-Jiménez R, Jiménez-Arriero MÁ, Criado JR, Rubio G, 2016. Sensory gating deficits in first-episode psychosis. J. Nerv. Ment. Dis. 204, 877–884. 10.1097/NMD.0000000000000572. [DOI] [PubMed] [Google Scholar]
- Morales-Muñoz I, Jurado-Barba R, Fernández-Guinea S, Álvarez-Alonso MJ, Rodríguez-Jiménez R, Jiménez-Arriero MA, Rubio G, 2017. Cognitive impairments in patients with first episode psychosis: the relationship between neurophysiological and neuropsychological assessments. J. Clin. Neurosci 36, 80–87. 10.1016/j.jocn.2016.10.023. [DOI] [PubMed] [Google Scholar]
- O’Donnell BF, Hokama H, McCarley RW, Smith RS, Salisbury DF, Mondrow E, Nestor PG, Shenton ME, 1994. Auditory ERPs to non-target stimuli in schizophrenia: relationship to probability, task-demands, and target ERPs. Int. J. Psychophysiol 17, 219–231. 10.1016/0167-8760(94)90065-5. [DOI] [PubMed] [Google Scholar]
- O’Donnell BF, Vohs JL, Hetrick WP, Carroll CA, Shekhar A, 2004. Auditory event-related potential abnormalities in bipolar disorder and schizophrenia. Int. J. Psychophysiol 53, 45–55. 10.1016/j.ijpsycho.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Ford JM, White PM, Roth WT, 1989. P3 in schizophrenia is affected by stimulus modality, response requirements, medication status, and negative symptoms. Arch. Gen. Psychiatry 46, 1035–1044. [DOI] [PubMed] [Google Scholar]
- Picton TW, Hillyard SA, 1974. Human auditory evoked potentials. II. Effects of attention. Electroencephalogr. Clin. Neurophysiol 36, 191–199. 10.1016/0013-4694(74)90156-4. [DOI] [PubMed] [Google Scholar]
- Polich J, 2007. Updating P300: an integrative theory of P3a and P3b. Clin. Neurophysiol 118, 2128–2148. 10.1016/j.clinph.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polich J, Criado JR, 2006. Neuropsychology and neuropharmacology of P3a and P3b. Int. J. Psychophysiol 60, 172–185. 10.1016/j.ijpsycho.2005.12.012. [DOI] [PubMed] [Google Scholar]
- Polich J, Jeon Y-W, 2003. Meta-analysis of P300 and schizophrenia: patients, paradigms, and practical implications. Psychophysiology 40, 684–701. [DOI] [PubMed] [Google Scholar]
- Polich J, Kok A, 1995. Cognitive and biological determinants of P300: an integrative review. Biol. Psychol 41, 103–146. 10.1016/0301-0511(95)05130-9. [DOI] [PubMed] [Google Scholar]
- Qiu Y, Tang Y, Chan RCK, Sun X, He J, 2014. P300 aberration in first-episode schizophrenia patients: a meta-analysis. PLoS One 9, e97794 10.1371/journal.pone.0097794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Re EC, Spencer KM, Oribe N, Mesholam-Gately RI, Goldstein J, Shenton ME, Petryshen T, Seidman LJ, McCarley RW, Niznikiewicz MA, 2015. Clinical high risk and first episode schizophrenia: auditory event-related potentials. Psychiatry Res. 231, 126–133. 10.1016/j.pscychresns.2014.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renoult L, Prévost M, Brodeur M, Lionnet C, Joober R, Malla A, Debruille JB, 2007. P300 asymmetry and positive symptom severity: a study in the early stage of a first episode of psychosis. Schizophr. Res 93, 366–373. 10.1016/j.schres.2007.03.024. [DOI] [PubMed] [Google Scholar]
- Rissling AJ, Braff DL, Swerdlow NR, Hellemann G, Rassovsky Y, Sprock J, Pela M, Light GA, 2012. Disentangling early sensory information processing deficits in schizophrenia. Clin. Neurophysiol. 123, 1942–1949. 10.1016/j.clinph.2012.02.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rissling AJ, Park SH, Young JW, Rissling MB, Sugar CA, Sprock J, Mathias DJ, Pela M, Sharp RF, Braff DL, Light GA, 2013. Demand and modality of directed attention modulate “pre-attentive” sensory processes in schizophrenia patients and nonpsychiatric controls. Schizophr. Res. 146, 326–335. 10.1016/j.schres.2013.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosburg T, Boutros NN, Ford JM, 2008. Reduced auditory evoked potential component N100 in schizophrenia - a critical review. Psychiatry Res. 161, 259–274. 10.1016/j.psychres.2008.03.017. [DOI] [PubMed] [Google Scholar]
- Roth WT, Pfefferbaum A, Kelly AF, Berger PA, Kopell BS, 1981. Auditory event-related potentials in schizophrenia and depression. Psychiatry Res. 4, 199–212. 10.1016/0165-1781(81)90023-8. [DOI] [PubMed] [Google Scholar]
- Salisbury DF, Shenton ME, Sherwood AR, Fischer IA, Yurgelun-Todd DA, Tohen M, McCarley RW, 1998. First-episode schizophrenic psychosis differs from first-episode affective psychosis and controls in P300 amplitude over left temporal lobe. Arch. Gen. Psychiatry 55, 173–180. 10.1001/archpsyc.55.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salisbury DF, Collins KC, McCarley RW, 2010. Reductions in the N1 and P2 auditory event-related potentials in first-hospitalized and chronic schizophrenia. Schizophr. Bull 36, 991–1000. 10.1093/schbul/sbp003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenton ME, Faux SF, McCarley RW, Ballinger R, Coleman M, Duffy FH, 1989. Clinical correlations of auditory P200 topography and left temporo-central deficits in schizophrenia: a preliminary study. J. Psychiatr. Res. 23, 13–34. 10.1016/0022-3956(89)90014-9. [DOI] [PubMed] [Google Scholar]
- Squires NK, Squires KC, Hillyard SA, 1975. Two varieties of long-latency positive waves evoked by unpredictable auditory stimuli in man. Electroencephalogr. Clin. Neurophysiol. 38, 387–401. [DOI] [PubMed] [Google Scholar]
- Sumich A, Harris A, Flynn G, Whitford T, Tunstall N, Kumari V, Brammer M, Gordon E, Williams LM, 2006. Event-related potential correlates of depression, insight and negative symptoms in males with recent-onset psychosis. Clin. Neurophysiol 117, 1715–1727. 10.1016/j.clinph.2006.04.017. [DOI] [PubMed] [Google Scholar]
- van Tricht MJ, Nieman DH, Koelman JHTM, Bour LJ, van der Meer JN, van Amelsvoort TA, Linszen DH, de Haan L, 2011. Auditory ERP components before and after transition to a first psychotic episode. Biol. Psychol 87, 350–357. 10.1016/j.biopsycho.2011.04.005. [DOI] [PubMed] [Google Scholar]
- Turetsky BI, Bilker WB, Siegel SJ, Kohler CG, Gur RE, 2009. Profile of auditory information-processing deficits in schizophrenia. Psychiatry Res. 165, 27–37. 10.1016/j.psychres.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umbricht D, Bates JA, Lieberman JA, Kane JM, Javitt DC, 2006. Electrophysiological indices of automatic and controlled auditory information processing in first-episode, recent-onset and chronic schizophrenia. Biol. Psychiatry 59, 762–772. 10.1016/j.biopsych.2005.08.030. [DOI] [PubMed] [Google Scholar]
- Valkonen-Korhonen M, Purhonen M, Tarkka IM, Sipilä P, Partanen J, Karhu J, Lehtonen J, 2003. Altered auditory processing in acutely psychotic never-medicated first-episode patients. Cogn. Brain Res. 17, 747–758. 10.1016/S09266410(03)00199-X. [DOI] [PubMed] [Google Scholar]
- de Wilde OM, Bour LJ, Dingemans PM, Koelman JHTM, Boerée T, Linszen DH, 2008. P300 deficits are present in young first-episode patients with schizophrenia and not in their healthy young siblings. Clin. Neurophysiol. 119, 2721–2726. 10.1016/j.clinph.2008.08.024. [DOI] [PubMed] [Google Scholar]
- Young RC, Biggs JT, Ziegler VE, Meyer DA, 1978. A rating scale for mania: reliability, validity and sensitivity. Br. J. Psychiatry 133, 429–435. 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.