Abstract
This study used a longitudinal design to examine the development of mismatch responses (MMRs) to Mandarin lexical tones, an index of neural speech discriminative responses, in late talkers and typical controls at 3, 5, and 6 years of age. Lexical tones are phonetic suprasegments that distinguish the lexical meanings of syllables in tonal languages. The 2 year-old late talkers were later divided into persistent language delay and late bloomer groups according to their performance on standardized language tests at 4 years. Results showed that children with persistent language delay demonstrated more positive mismatch responses than the typical controls at 3 years of age. At the age of 5, no group difference were found in the amplitude of MMRs, but the maturation of MMRs could be observed in the change of topography, with more prominent negative response in the frontal sites only in the typical group. Correlations were found between the index of MMRs at 3 years and children’s language performance outcome at 6 years. Our results indicate that the development of fine-grained tone representations is delayed in late-talking children between 3 and 5 years and may be one of the underlying mechanisms which associated with later language performance.
Keywords: Speech perception, Mismatch responses, ERP, Late-talking, Lexical tone
1. Introduction
Within the field of a typical language development, the terms “late talkers” (LTs), “children with expressive language delay,” and “children with late language emergence” refer to toddlers who developmentally lag behind their typically developing peers in certain aspects of language, including vocabulary, phonology, and syntax (Kelly, 1998, Rescorla, 1989, Thal, 2000). Although these children possess limited expressive vocabulary and/or receptive language, no cognitive, neurological, socioemotional, or sensory deficits are present. LTs have been defined by a variety of criteria, but studies reported that delayed expressive vocabulary is the most robust measure (Desmarais et al., 2008, Moyle et al., 2011, Tsybina and Eriks-Brophy, 2007).
A subset of LTs might be identified as having specific language impairment (SLI) by school age, exhibiting deficits in various language domains such as semantics, syntax, and discourse in the presence of normal nonverbal cognitive abilities (Paul, 1993; Roos and Ellis Weismer, 2008). Although some LTs turn out to be language impaired, studies have indicated that most LTs scored within the normal range on vocabulary measures by the age of 3 years and in the normal range of grammar and conversational skills by school age (Ellis and Thal, 2008, Domsch et al., 2012, Fischel et al., 1989, Rice et al., 2008, Whitehouse et al., 2011). Nevertheless, although these earlier delayed children (called “late bloomers” or “resolved late talkers”) may appear to catch up, they continue to show significantly weaker language skills compared with their typically developing counterparts (Rescorla et al., 2002). Thus, the late language emergence may indicate vulnerability for slow language acquisition and that children who catch up can be considered less impaired than children who are later diagnosed with SLI.
Many risk factors may lead to disrupted language development (Bishop et al., 2003, Rice, 2012, Zubrick et al., 2007). One predictor drawing attention is early receptive language skill. Evidence suggests that the receptive language status of a late-talking child is predictive of expressive language outcomes (Ellis Weismer et al., 1994; Rescorla and Schwartz, 1990). The prospective longitudinal studies monitoring the prerequisite abilities that pertain to language learning might improve the understanding of why some children who are initially delayed in language development turn out to be language impaired and others do not.
Speech perception is one of the prerequisites for infants to develop language. Before producing their first words, infants exhibit sophisticated speech perception skills. Infants distinguish speech sounds of both native and foreign languages before 6 months of age (Eimas et al., 1971, Jusczyk, 1977, Polka and Werker, 1994), but rapidly develop language-specific perception by improving accuracy to discriminate phonemic categories consistent with their native language and increasing difficulty in perceiving nonnative speech sounds around the first birthday (Werker and Tees, 2002). The development of speech perception abilities continues in early childhood for children speaking different languages (Arai et al., 2008, Boets et al., 2007; Hazan and Barrett, 2000; Liu et al., 2013, Vance et al., 2009).
Fine-grained speech perception abilities are essential to language development, as demonstrated in behavioral and event-related potential (ERP) studies. Infants’ ERP responses when processing vowels predicted their language status at 5 years of age and their reading performance at 8 years (Molfese et al., 1999; Molfese, 2000). A positive correlation has also been observed between 6-month-old infants’ perceptual abilities and later expressive vocabulary and syntactic complexity at 24 months (Kuhl et al., 2005, Tsao et al., 2004).
Early speech discrimination sensitivity are also found to correlated with preschool- and school-aged children’s performance in standardized vocabulary and receptive language tests (Vance et al., 2009, Burnham, 2003). Höhle et al., (2006) used a preferential-looking paradigm that related sensitivity to mispronunciations at 19 months to language abilities at 30 months to reveal the relationship between poor perceptual performance and language development in at-risk toddlers. These findings suggest that children with reduced speech perception abilities may be less able to process acoustic or linguistic information and meet more difficulty in developing language.
When assessing speech perception ability in young children, researchers have focused on using auditory ERPs as a relatively objective electrophysiological measure of the brain’s responses to distinct speech features. One ERP component is mismatch negativity (MMN), which is elicited by a passive auditory oddball paradigm containing rare (deviant) stimuli presented in a sequence of frequent (standard) stimuli. MMN is quantified by subtracting the average standard stimulus waveform from the average deviant waveform and usually peaks between 100 and 250 ms after stimulus change onset. It is used as an index of speech perception sensitivity because its amplitude is associated with performance in discriminating between-sound differences (Bradlow et al., 1999, Kraus et al., 1996, Näätänen and Winkler, 1999) and children have exhibited an enhanced MMN amplitude in discriminating speech sounds after short-term training (Cheour et al., 2002, Kraus et al., 1995).
Whereas a typical frontocentral MMN was evident in adults, a frontal positive mismatch response (p-MMR) was observed in infants and young children with less mature speech discrimination responses (Ahmmed et al., 2008, Friederici et al., 2002, Morr et al., 2002, Shafer et al., 2010). Friederici et al. (2002) examined the mismatch responses of 2-month-old infants to syllables varying in vowel duration (short/ba/ vs. long/ba:/) and discovered that the p-MMR peaked at approximately 400 ms, particularly for the long syllable.
A second negativity that has been elicited from deviant stimuli in passive auditory oddball experiments is late discriminative negativity (LDN). This negative component appears after MMN and peaks at approximately 400–430 ms in response to changes in speech stimuli for both children and young adults (Korpilahti et al., 1995, Čeponienė et al., 2003, Čeponienė et al., 2004, Kraus et al., 1993). Compared with MMN, LDN was stably observed in children aged 2–3 years exposed to changes in the fundamental frequency, duration, intensity, and source in complex tones pattern (Putkinen et al., 2012).
On the basis of a combination of the mismatch responses (MMR) patterns from age groups and two speech contrasts with different discriminative difficulty, Liu et al. (2014) demonstrated that the emergence of mismatch responses (MMN, p-MMR, and LDN) may indicate the neural discriminative processing of speech features. At the beginning stage of speech discrimination development, an enhancement in p-MMR might reflect involuntary attention orientation, when children fail to analyze the acoustic difference between two speech stimuli and regard the deviant sound as a novel stimulus. When entering the advanced level, children begin to process the sound structures, as reflected by the emergence of LDN and its latency change. Finally, children develop the automatic processing to discriminate between the subtle acoustic differences in their speech inventory that adults possess. The study has clearly established the utility of using the converging patterns of MMR measures as research tools to understand the nature of developmental disorders involving speech and language impairments, particularly in situations where behavioral responses cannot be readily elicited.
For Mandarin-speaking children, the ability to discriminate lexical tones is critical for language development. A Mandarin syllable may be composed of four possible elements. Tone and vowels serve as the compulsory units, and consonants are optional units occurring in either the initial or final position. Mandarin Chinese has four tones: T1 ([ma1] means “mother”), T2 ([ma2] means “hemp”), T3 ([ma3] means “horse”), and T4 ([ma4] means “scold”). T1 through T4 can be described phonetically as high level, high rising, low falling and rising, and high falling, respectively. Among the four tones, T2 is acoustically similar to T3; the two tones only differ in the turning time point of the pitch contour. Studies have confirmed that T2 and T3 constitute the most confusing tone pair for children and second language learners (Wong et al., 2005, Chandrasekaran et al., 2007, Tsao, 2008).
Behavioral and ERP studies have demonstrated that lexical tone perception abilities develop quickly in infancy and continue to be fine-tuned in childhood. Cheng et al. (2013) used a two-deviant MMN paradigm and demonstrated that the T1/T3 pair elicited a left frontal-distributed p-MMR in newborns, whereas it elicited an adult-like MMN in 6-month-old infants. However, the T2/T3 pair did not elicit any MMRs in newborns and elicited a p-MMR in 6-month-old infants only when they were awake. The p-MMR switched to the adult-like MMN as the infants grew, but the trajectory of the polarity transition depended on the degree of deviance. Lee et al. (2012) also reported that the T1/T3 pair elicited adult-like MMNs between 150 and 300 ms in 4-, 5-, and 6-year-old children, but T2/T3 elicited only p-MMRs in the 5- and 6-year-old groups. Liu et al. (2013) used AX phonetic discrimination tasks to explore the developmental changes of speech discrimination abilities in Mandarin-speaking children of preschool and school age. Results indicated that accuracy in discriminating the T2/T3 pair increased with age, reaching nearly 90% at 8 years. In addition, the regression model showed that lexical tone discrimination sensitivity contributed to the variance of PPVT scores, suggesting that lexical tone perception plays an essential role in word comprehension development in Mandarin-speaking children.
Compared with a control group, preschool and school-aged children with SLI showed deficits in a lexical tone (/i2/–/i3/) discrimination task, and lexical tone discrimination sensitivity had additional predictive power regarding their overall language comprehension ability after adjustment for existing vocabulary ability (Chen, 2012) or the consonant and pure tone frequency discrimination sensitivities (Chen and Liu, 2010). Moreover, Lu and Tsao (2014) demonstrated that the poor lexical tone perception ability of Mandarin-speaking late-talking children, and it was associated with word-learning efficiency at 2 years of age, suggesting that the fragile lexical tone representation could adversely affect the word-learning efficiency in LTs.
As of this writing, MMRs have not been used to study Mandarin lexical tone discrimination in late-talking children, but results from children with reading difficulties might provide some insights. Meng et al. (2005) tested MMN responses in children with and without reading difficulties aged 8–13 years. The speech stimuli contrasts were initial consonants (/da/–/ga/), rhyming parts (/dan/–/dai/), and lexical tones (/ba1/–/ba2/). Syllables deviating in initial consonants and vowels resulted in MMN differences between the two groups, but no group differences in lexical tone condition were observed. Nevertheless, because of the lack of original waveform and amplitude measures for the typically developing group in the study by Meng et al., interpreting the development patterns based on the results is difficult.
Zhang et al. (2012) used the /pa2/–/pa4/ continuum to examine the categorical perceptions of Mandarin lexical tone in school children with or without reading difficulties. The results indicated that the age-matched control group demonstrated significantly enhanced MMN amplitudes in response to the across-category deviants compared with the within-category deviants, whereas the children with reading difficulties did not exhibit such effects. The enhanced MMN elicited using across-category tonal contrast indicates that 10-year-old typically developing Mandarin-speaking children have formed phonological representations of lexical tones similar to those of adults.
In summary, lexical tones manifest lexical meanings in Mandarin syllables, and previous studies have provided evidence that lexical tone discrimination ability is essential to language learning in Mandarin-speaking children. Children’s use of fine-grained cues to discriminate between lexical tone contrasts is a developing process in childhood; therefore, examining the process of discriminating lexical tones in late-talking children and testing the relationship between tonal perception abilities and later language outcomes could clarify possible causes of language delay.
This study used a longitudinal design to examine the development of MMRs, an index of neural discriminative responses, in late-talking children and typical language development (TLD) controls at 3, 5, and 6 years. The LTs identified at 2 years of age were further classified into persistent language delay (PLD) and late bloomer (LB) groups at 4 years of age according to their performance on standardized language tests. By comparing the MMR patterns among groups at each age level and testing the correlations between MMRs and language outcome measures at 6 years of age, it was hypothesized that LTs have less mature brain responses to lexical tones at young ages, and this deficiency may hinder language development in PLD children.
2. Methods
2.1. Participants
The participants in the current study were a subset of children who participated in an ongoing longitudinal study on the language and cognitive development of late-talking children. All children began participating in the longitudinal study at ages between 2 and 2.5 years, and were follow-up assessed for language-related tasks at 3, 4, 5, and 6 years. The children were administered the ERP experiments at 3, 5, and 6 years of age to index the developmental neural response to speech discrimination. The present study focused specifically on the development of neural discriminative responses to Mandarin lexical tone contrast between the ages of 3 and 6 years, and the relationships between neural discriminative response development and language proficiency.
To recruit children with TLD, announcements were used to locate parents of 2 and 2.5 year-old children. Children with language delays were recruited from local pediatric and parenting websites. Children’s language abilities were measured using the Mandarin Chinese version of the MacArthur–Bates Communicative Development Inventories, Toddler Form (MCDI-T; Liu and Tsao, 2010) and the subtests of standardized receptive and productive language test, the Comprehensive Developmental Inventory for Infants and Toddlers (CDIIT; Wang et al., 2002). Twenty children were identified as LTs by scoring at or below the 16th percentile for total productive vocabulary on the MCDI-T or the productive language subtest in the CDIIT. Children in the TLD control group (n = 15) scored above the 35th percentile in all language tests. Children with results between these two classifications were not included in the analysis, because the aim was to construct two clearly defined groups. All children exhibited normal hearing and none had a history of cognitive, emotional, or social disorders or delays, according to parental reports and their medical records.
At 4 years of age, the late-talking children were divided into the PLD and LB groups according to their performance on standardized language tests. The children’s overall language abilities were assessed using the Child Language Disorder Scale-Revised (CLDS-R; Lin et al., 2008). The expressive language subtest evaluated the children’s expressive vocabulary, syntactic accuracy, and story retelling abilities. Children with scores below the 25th percentile on the expressive language subtest of the CLDS-R were placed in the PLD group. Children who scored higher than the 25th percentile on the expressive language subtest were placed in the LB group. Ten children were placed in the PLD group, and 10 were categorized as LBs. All children exhibited normal nonverbal IQ, as indicated by scores higher than 85 on the Test of Nonverbal Intelligence, Third Edition (Wu et al., 2006).
Table 1 lists the language and IQ measures of the participants. The PLD and LB groups achieved significantly lower scores than those of the TLD group at 2–2.5 years on the productive vocabulary and complexity scales in the MCDI (F(2,32) = 21.0, 17.1, ps < 0.001, ηp2 = 0.567, 0.516) and the receptive and productive subtests in the CDIIT (F(2,32) = 16.9, 51.1, ps < 0.001, ηp2 = 0.513, 0.762). The PLD group scored lower than the LB group did only on the comprehensive subtest in the CDIIT (p = 0.035). At 4 years, the PLD group performed significantly lower in the receptive and expressive language subtests than did the other two groups (F(2,32) = 25.4, 41.3, p < 0.001, ηp2 = 0.614, 0.721). There were no significant differences in nonverbal IQ among the three groups (F(2,32) = 1.81, p = 0.179, ηp2 = 0.102).
Table 1.
Mean scoresa (and standard deviations) on standardized language and IQ measures among the three participant groups.
| Persistent Language Delay (n = 10) | Late-Bloomer (n = 10) |
Typical Controls (n = 15) |
|
|---|---|---|---|
| 2 to 2.5 years old | |||
| MCDI-T, Total productive vocabulary | 83.7 (130.5) | 184.8 (129.4) | 440.9 (158.2) |
| MCDI-T, Complexity score | 5.1 (12.8) | 11.5 (10.2) | 34.1 (14.9) |
| CDIIT, Receptive subtest | 94.9 (18.2) | 110.4 (12.4) | 126.1 (9.3) |
| CDIIT, Productive subtest | 74.4 (9.2) | 82.1 (6.0) | 111.1 (11.7) |
| 4 years old | |||
| CLDS-R, Receptiveb | 44.2 (8.0) | 56.7 (3.2) | 58.7 (3.7) |
| CLDS-R, Expressivec | 39.0 (5.0) | 52.1 (3.8) | 54.1 (3.9) |
| TONI-3 | 112.7 (8.9) | 113.4 (6.5) | 118.3 (8.6) |
Notes. MCDI-T = Mandarin-Chinese version of the McArthur Communicative Development Inventories (Toddler Form; Liu and Tsao, 2010); CDIIT = Comprehensive Developmental Inventory for Infants and Toddlers (Wang et al., 2002); CLDS-R = Child Language Disorder Scale-Revised (preschool versions: Lin et al., 2008); TONI–3 = Test of Noverbal Intelligence, third edition (Wu et al., 2006).
Raw score in MCDI; standard score in CDIIT and TONI-3; t-score in CLDS.
Receptive language subtest.
Expressive language subtest.
The research protocol was reviewed and approved by the Institutional Review Board at National Taiwan Normal University. Informed consent forms were signed by the parents of the child participants during the first visit.
2.2. ERPs
The Mandarin lexical tone pair (/i2/–/i3/) was used in previous behavioral and ERP studies examining the development of speech discrimination abilities in children (Liu et al., 2013, Liu et al., 2014). The two stimuli were from a lexical tone continuum and were acoustically identical except for pitch contour, reflected in the variation in fundamental frequency (F0). The acoustic features of F0 in/i2/were as follows: onset = 219 Hz, turning point (at 34% of the syllable duration) = 195 Hz, and endpoint = 245 Hz. Those of/i3/were as follows: onset = 216 Hz, turning point (at 71% of the syllable duration) = 156 Hz, and endpoint = 209 Hz. The duration of each syllable was 270 ms. The formant frequencies of the steady-state vowel/i/were 290, 2815, 3945, and 4973 Hz, and the bandwidths were 100, 220, 115, and 239 Hz, respectively. The speech stimuli were synthesized using Praat, and their RMS amplitude was normalized.
Speech stimuli were presented over loudspeakers at 70 dBA. The ratio of standard (/i2/) to deviant (/i3/) stimuli was 8:1, and the total number of stimuli was 1080. The presentation of stimuli was in a pseudorandomized order with at least three successive standards between deviants. The interstimulus interval was 430 ms. The purpose of using shorter ISI is to shorten the EEG measurement time for preschoolers. It was similar to other MMN studies using Mandarin lexical tone stimuli (500–700 ms, Lee et al., 2012, Meng et al., 2005, Xi et al., 2010, Luo et al., 2006), and no ISI effect on the amplitude of MMN within the range of 350–700 ms was reported (Čeponienė et al., 1998). The children were seated in a comfortable chair in a sound-attenuated electrically shielded room and were instructed to watch silent self-selected movies, ignore the sound stimuli, and sit as quietly as possible. Parents and a trained research assistant accompanied the child in the room and comforted the child when necessary. Breaks were given when requested by the participant.
2.3. ERP data recording, preprocessing, and analysis
EEG signals were recorded at 500-Hz sampling rate by using a 32-channel cap (Quik-Caps; Neuroscan Labs, El Paso, TX) embedded with sintered Ag/AgCl electrodes, referenced to the left and right mastoids. Linked pairs of electrooculogram (EOG) electrodes recorded horizontal (electrodes on the outer canthi) and vertical (electrodes above and below the left eye) eye movements. Electrode impedances were maintained below 10 KΩ for all electrodes during the recording. The continuous EEG signal was amplified from 0.05 to 70 Hz by using a band-pass filter and the SynAmps2 (Neuroscan, Inc.) amplifier and low-pass-filtered by 30 Hz offline (12 dB/octave). Eye-blink correction of the raw EEG data was implemented using the linear regression function provided by NeuroScan software (Semlitsch et al., 1986). The continuous EEG data were epoched from −100 to 600 ms and time-locked to the onset of the auditory stimuli. Each trial was baseline-corrected to the average voltage of the 100 ms prestimulus interval. Any epoch containing a voltage variation that exceeded ±150 μV at HEOG and any other electrodes was considered an artifact and was rejected.
Average ERPs were calculated separately for standard and deviant stimuli for individual participants. The standard trials that immediately followed the deviant trials were excluded from averaging. The difference waveforms (i.e., MMRs) were created by subtracting the standard waveform from the deviant waveform for each participant. The mean amplitudes of the standard and deviant waveforms for each participant were calculated for two time intervals: 185–335 ms and 335–535 ms. These two time windows were determined by visual inspection of the data and the following two rationales: (1) The starting point of the first time window was established according to the conventional definition of MMR (i.e., 100 ms after the onset of the acoustic difference between the standard and deviant sounds); (2) The results from previous study of Liu et al. (2014) using the same pair of lexical tone stimuli and 50-ms successive time window analysis to investigate the developmental trend of MMRs found that adults showed a typical MMN in the 185–335 ms interval, and the children groups only showed negative response in the interval later than 335 ms. Thus, the two time intervals were chosen to indicate different developmental levels of process to the speech stimuli.
Statistical analyses were performed at six scalp electrodes (F3, Fz, F4, C3, Cz, and C4) according to previous studies suggesting that MMN is the most prominent in frontocentral locations (Lee et al., 2012, Liu et al., 2014, Shafer et al., 2010). Because this study focused on different MMR patterns among three subject groups within developmental stages, separate ANOVAs with the mean amplitude as the dependent variable were used for statistical comparisons for each age stage. For each age stage, repeated-measure ANOVAs with stimulus type (standard/deviant), time (185–335 ms/335–535 ms), and channel (F3/Fz/F4/C3/Cz/C4) as the within-subject factors and group (PLD/LB/TLD) as the between-subjects factor were conducted. The Greenhouse-Geisser adjustment was applied to all ANOVAs to correct for violations of sphericity associated with repeated measures. In cases where there was a significant interaction related to group, step-down analyses using simpler repeated-measure ANOVAs and Bonferroni post hoc tests were conducted. Only significant differences (p < 0.05) are reported.
2.4. Language outcome measures at 6 years of age
To examine the relationship between the children’s brain speech discrimination responses and language performance outcomes, a series of standardized language tests was administered at 6 years of age. The children’s overall language performance and oral receptive vocabulary were assessed using the school child version of the CLDS-R (Lin et al., 2009) and Peabody Picture Vocabulary Test-Revised (Lu and Liu, 1998), respectively. The children’s performance on oral language syntax were tested using the Oral Language Syntax Ability Diagnostic Test for preschool and first- and second-grade children (Yang et al., 2005). Pearson correlations between MMRs and the children’s language measures at 6 years were calculated to examine the relationship between neural speech discrimination responses and language performance outcomes.
3. Results
3.1. MMR results
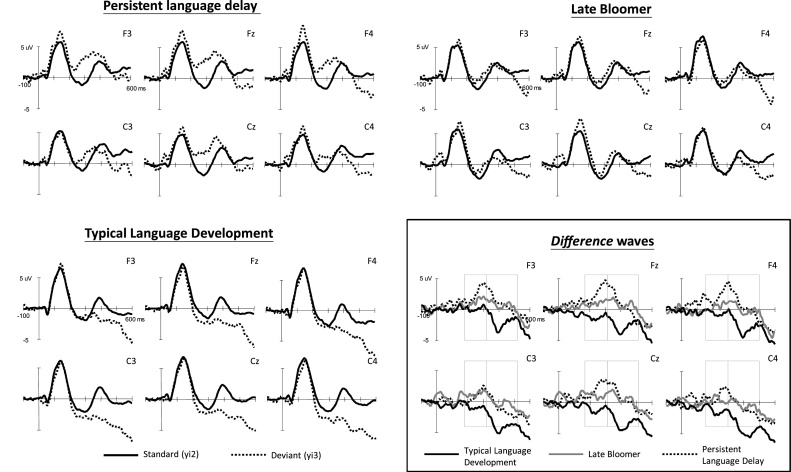
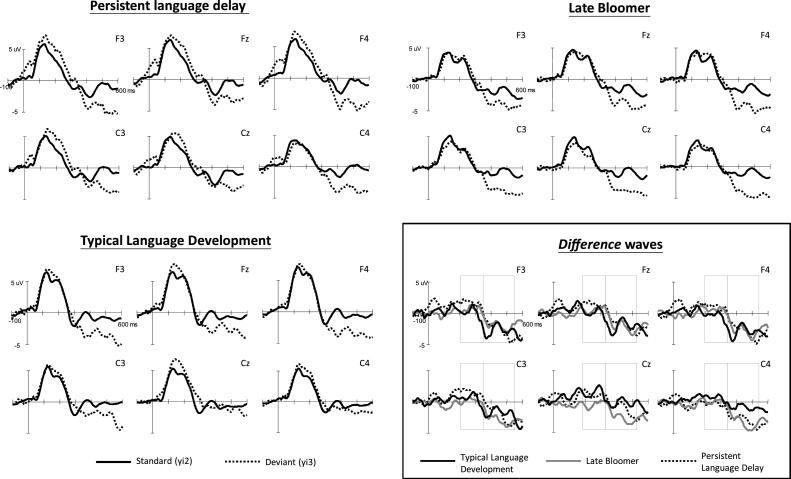
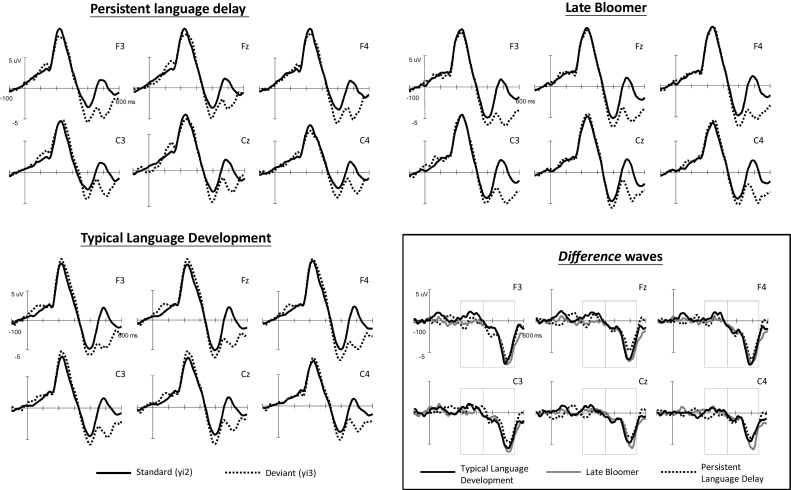
The average ERPs for the standard and deviant stimuli at the six selected electrodes as well as the difference waveforms among the participant groups at the three age stages (3, 5, and 6 years old) are displayed in Fig. 1, Fig. 2, Fig. 3 , respectively. The data are presented for each age because this study focused on the developmental differences among the three age groups.
Fig. 1.
Grand average waveforms of the standard and deviant stimuli for the three participant groups at 3 years of age and difference waveforms among groups.
Fig. 2.
Grand average waveforms of the standard and deviant stimuli for the three participant groups at 5 years of age and difference waveforms among groups.
Fig. 3.
Grand average waveforms of the standard and deviant stimuli for the three participant groups at 6 years of age and difference waveforms among groups.
3.1.1. Three years of age
At the 3 years of age, no significant main effects were discovered. Significant two-way interactions existed between stimulus and group (F(2,31) = 3.94, p = 0.030, ηp2 = 0.203), stimulus and time (F(1,31) = 15.2, p < 0.001, ηp2 = 0.329), stimulus and channel (F(5,155) = 4.38, p = 0.007, ηp2 = 0.124), and time and channel (F(5,155) = 2.81, p = 0.035, ηp2 = 0.083). Because significant interactions related to group existed, additional analyses were performed for each time window to examine the group effect. Both late-talking groups showed positive MMRs. Only the TLD group demonstrated the typical negative MMRs. Results from one-way ANOVA revealed significant group differences in the mean amplitude of MMRs between 185 and 335 ms in the Fz (F(2,32) = 4.46, p = 0.020, ηp2 = 0.218) and Cz (F(2,32) = 5.38, p = 0.010, ηp2 = 0.252) channels. The post hoc test showed significant differences between the PLD and TLD groups in these two midline channels (ps = 0.018, 0.010). For the 335–535 ms interval, there was a significant group difference in the Cz (F(2,32) = 4.51, p = 0.019, ηp2 = 0.220) channel, again resulting from the difference between the PLD and TLD groups.
3.1.2. Five years of age
At the 5 years of age, the results revealed significant main effects for stimulus (F(1,32) = 4.22, p = 0.048, ηp2 = 0.116) and time (F(1,32) = 21.05, p < 0.001, ηp2 = 0.397. Two-way interactions between stimulus and time (F(1,32) = 59.12, p < 0.001, ηp2 = 0.649), and time and channel (F(5,160) = 12.62, p < 0.001, ηp2 = 0.283) were significant. Three-way interactions were observed among stimulus, channel, and group (F(10,160) = 3.11, p = 0.003, ηp2 = 0.163) and stimulus, time, and channel (F(5,160) = 5.59, p = 0.001, ηp2 = 0.149). To examine the three-way interaction among stimulus, channel, and group, the six electrodes were categorized on the basis of hemisphere (left/mid/right) and site (frontal/central). Separate ANOVAs on the difference waves were tested for each time window. The results indicated significant site-by-group interactions in the 185–335 ms (F(2,32) = 7.21, p = 0.003, ηp2 = 0.310) and 335–535 ms (F(2,32) = 7.80, p = 0.002, ηp2 = 0.328) intervals.
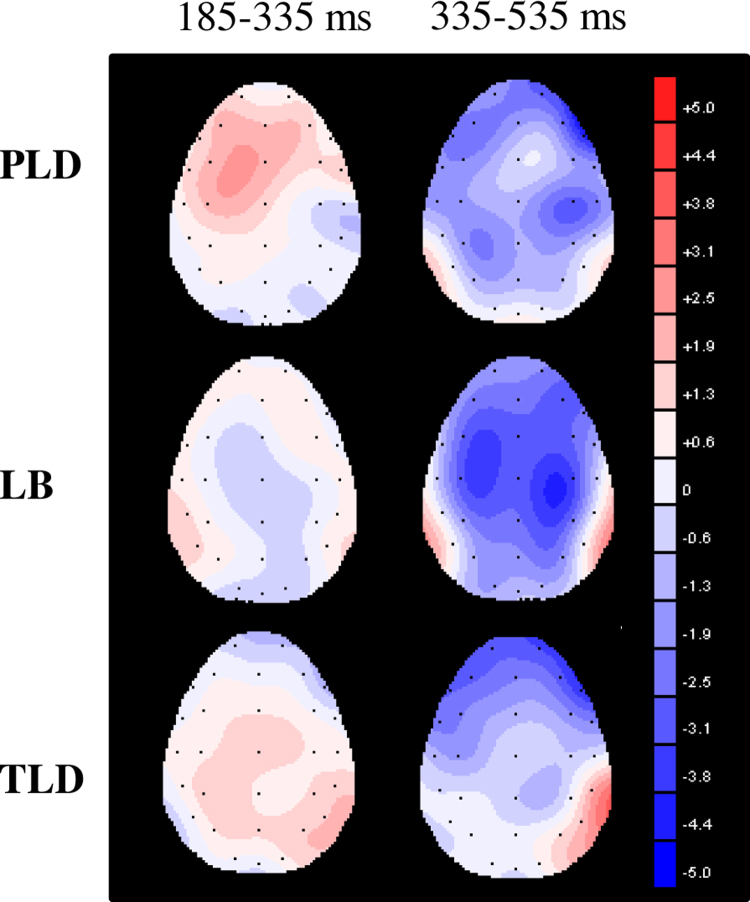
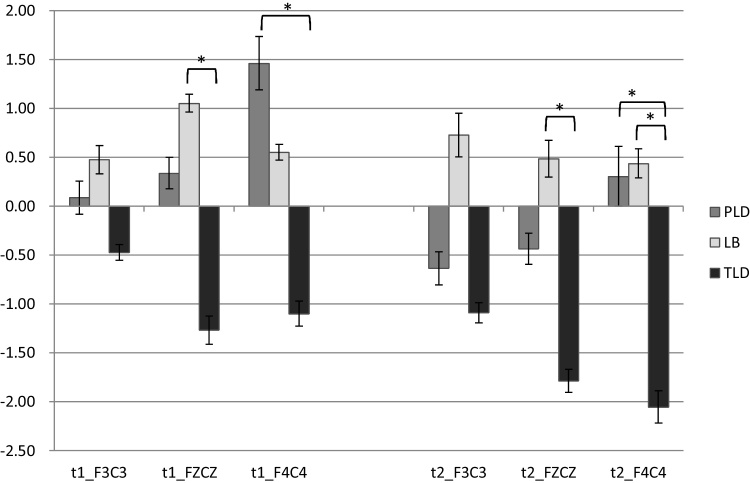
To specify site effect patterns among groups, the site effect was quantified by calculating the difference in MMR between the frontal and central sites (i.e., F3–C3, Fz–Cz, F4–C4). The results showed significant group differences in the site effect at the midline (Fz–Cz) in the 185–335 ms (F(2,32) = 5.86, p = 0.007, ηp2 = 0.268) and 335–535 ms (F(2,32) = 5.34, p = 0.010, ηp2 = 0.250) intervals. Significant group differences in the site effect were also present at the right hemisphere (F4–C4) in the 185–335 ms (F(2,32) = 5.42, p = 0.009, ηp2 = 0.253) and 335–535 ms (F(2,32) = 4.31, p = 0.022, ηp2 = 0.212) intervals. Fig. 4 demonstrated the potential distribution of MMRs over the entire scalp at 5 years old, and the site effects among groups at the mid-frontal areas were depicted by Fig. 5. Post hoc comparisons revealed that, compared with the two late-talking groups, the TLD group demonstrated a higher proportion of negative MMRs in frontal sites compared with central sites.
Fig. 4.
The potential distributions of MMRs over the entire scalp by group at 5-year-old.
Fig. 5.
The site by group interaction at mid-frontal areas in MMRs at 5 years of age. (t1: 185–335 ms, t2: 335–535 ms, significant level at <0.05, error bar = 1 s.e.).
3.1.3. Six years of age
At the 6 years of age, significant main effects of stimulus (F(1,32) = 8.54, p = 0.006, ηp2 = 0.211) and time (F(1,32) = 189.72, p < 0.001, ηp2 = 0.856) were discovered. In addition, significant two-way interactions between stimulus and time (F(1,32) = 54.73, p < 0.001, ηp2 = 0.631) and between time and channel (F(5,160) = 30.62, p < 0.001, ηp2 = 0.489) were observed. A three-way interaction among stimulus, time, and channel was also significant (F(5,160) = 9.24, p < 0.001, ηp2 = 0.224). Because no significant interactions related to group existed, no further analysis was performed.
3.2. Correlations between early MMRs and language performance outcomes
We examined the relationship between children’s MMR responses and their language performance outcomes at 6 years of age. Table 2 shows the language measure scores for the three participant groups. The PLD group achieved significantly lower scores than those of the other two groups on overall language abilities on the CLDS-R (F(2,32) = 24.9, p < 0.001, ηp2 = 0.609).The PLD group also showed lower scores than those of the TLD group on the OLSDT (F(2,32) = 13.0, p < 0.001, ηp2 = 0.447). Regarding oral vocabulary, both the PLD and LB groups demonstrated lower standard scores on the PPVT compared with the TLD group (F(2,32) = 7.15, p = 0.003, ηp2 = 0.447). The results demonstrate that the PLD group had persistent language development difficulties. Although the LB group caught up to the TLD group in overall language performance, the LB group still possessed weaker skills in specific language domains, such as vocabulary, than those of the TLD group.
Table 2.
Mean scoresa (and standard deviations) on standardized language measures among the three participant groups at 6 years of age.
| Persistent Language Delay (n = 10) | Late-Bloomer (n = 10) |
Typical Controls (n = 15) |
|
|---|---|---|---|
| CLDS-R | 57.7 (6.2) | 68.9 (3.7) | 72.5 (5.3) |
| PPVT-R | 115.0 (9.9) | 117.6 (11.9) | 132.2 (14.1) |
| OLSDT | 50.4 (3.9) | 56.2 (1.9) | 59.4 (5.6) |
Notes. CLDS-R = Child Language Disorder Scale-Revised (school versions: Lin et al., 2009); PPVT = Peabody Picture Vocabulary Test (Lu and Liu, 1998); OLSDT = Oral Language Syntax ability Diagnostic Test (Yang et al., 2005).
Raw score in CLDS-R; standard score in PPVT; t-score in OLSDT.
As group differences in MMR amplitude were found at channel Fz and Cz at 3 years of age, we conducted correlational analysis on these two representative channels. The mean MMR amplitudes in the 185–335 ms interval in the Fz and Cz channels (r = –.515, −0.420, ps < 0.05) and the mean MMR amplitudes in the 335–535 ms interval in these two channels (r = –.483,–.479, ps < 0.05) were significantly correlated with raw CLDS-R score at 6 years. For oral comprehension vocabulary, the mean MMR amplitudes in the 185–335 ms and the 335–535 ms intervals in the Cz channel were significantly correlated with the PPVT standard scores at 6 years (r = –.437, −0.437, ps < 0.05). For syntactic ability, the mean MMR amplitudes in the 185–335 ms interval in the Fz and Cz channels (r = –.549, −0.438, ps < 0.05) and the mean MMR amplitudes in the 335–535 ms interval in these two channels (r = –.447,–.460, ps < 0.05) were significantly correlated with OLSDT standard scores at 6 years. Table 3 summarizes the correlation results. Significant correlations existed between the mean MMR amplitudes at 3 years of age and various language scores at 6 years.
Table 3.
Correlations between the MMRs of 3 year-old children and language outcome measures at 6 years of agea.
| 185–335 ms |
335–535 ms |
|||
|---|---|---|---|---|
| Fz | Cz | Fz | Cz | |
| CLDS-R | −0.515** | −0.420* | −0.483* | −0.479 |
| PPVT-R | −0.437* | −0.437* | ||
| OLSDT | −0.549** | −0.438* | −0.447* | −0.460* |
Note. CLDS-R = Child Language Disorder Scale-Revised (school versions: Lin et al., 2009); PPVT = Peabody Picture Vocabulary Test (Lu and Liu, 1998); OLSDT = Oral Language Syntax ability Diagnostic Test (Yang et al., 2005).
Raw score in CLDS-R; standard score in PPVT; t-score in OLSDT.
Correlation is significant at the 0.05 level (2-tailed).
Correlation is significant at the 0.01 level (2-tailed).
4. Discussion
This study focused on examining the development of MMRs, an index of neural discriminative responses, in late-talking children and TLD controls at 3, 5, and 6 years of age. The LTs were divided into PLD and LB groups according to their performance on standardized language tests at 4 years. In addition to our primary focus on MMR pattern differences between the three groups, we conducted correlational analyses to explore the relationships between brain activation patterns and language performance outcomes at 6 years. These analyses indicated that the development of fine-grained lexical tone representation is delayed in late-talking children between 3 and 5 years and that the brain responses that distinguish typical children and LTs are associated with individual performance on language tests at later ages. These findings suggest that the development of robust lexical tone representations can affect language performance.
In 3-year-olds, children with normal language development did not show the typical MMN in the early time window associated with the difference in acoustic features between the synthetic standard and deviant stimuli. Adults showed MMN responses to the (/i2/–/i3/) speech contrast in the 185–335 ms interval (Liu et al., 2014). Our results indicate that the ability to identify specific speech features of Mandarin lexical tones is not fully developed in children aged 3 years. However, these children already demonstrated an LDN-like response in the 335–535 ms interval in frontocentral channels, suggesting that children aged 3 years analyze the phonological structures of tone stimuli but do not do so automatically yet. The results showed that children with PLD demonstrated p-MMRs in the 185–335 ms interval in all six channels and the mean amplitude differed significantly from that of the TLD group in the Fz and Cz channels. In addition, the emerging LDN response in the 335–535 ms interval was evident in the PLD group, but the amplitude was significantly more positive than that of the TLD group.
We also examined whether the LBs’ lexical tone discrimination abilities indicate a developmental delay. Group comparisons demonstrated no evidence to support that proposition. Because there is a substantial variation in data regarding children, this result might be due to the small sample size (10 children in the LB group). Although the group differences were not significant, a positive response was observed in the 185–335 ms interval in the LB group. Because p-MMR and reduced-amplitude LDN are generally thought to reflect immature (i.e., poorer) representations of the phonetic categories within a speaker’s native language, our results demonstrate that Mandarin-speaking LBs are less able to distinguish the subtle pitch contour differences between T2 and T3.
At the age of 5 years, all three groups exhibited more negative responses to the deviant stimuli in the 335–535 ms interval, and there was no group difference in the LDN mean amplitude. This suggests that the lexical tone perception abilities of late-talking children continue to develop with age, growing closer to the ability level of the TLD group. Nevertheless, we observed less mature MMRs in LTs in the change of topography. The results showed a site-by-group interaction in the 185–335 ms and 335–535 ms intervals. The differences between the frontal and central MMRs (i.e., Fz–Cz, F4–C4) were negative in the two time intervals for the TLD group. In the two late-talking groups, the MMRs were more positive than those of the TLD group at the frontal sites in the mid and right channels in the 185–335 ms and 335–535 ms intervals.
Studies using the MMN paradigm to investigate the categorical perception of Mandarin lexical tone in native Chinese adults reported that across-category deviants elicited higher MMN than within-category deviants in the left frontal site (Xi et al., 2010), but children with dyslexia did not show this pattern (Zhang et al., 2012). Furthermore, when infants’ neural responses to Finnish consonant-vowel syllables were tested, the brain responses to/ga/at approximately 600 ms in at-risk newborns had a slower polarity shift from the major positive peak toward the later negative deflection; this pattern was the clearest at the right hemisphere (Guttorm et al., 2001). A subsequent longitudinal study showed that children who exhibit at-risk processing patterns at birth achieve lower receptive language skill scores between the ages of 2.5 and 5 years and have lower word/nonword reading accuracy and fluency measures in the first grade than children who exhibit normative responses do (Guttorm et al., 2005). Corresponding to the previous study, the rightward site differences in our study indicated that LTs still possess a less sophisticated speech perception ability at 5 years of age.
Grossheinrich et al., (2010) compared MMN responses to frequency change in pure tones (1000 Hz vs. 1200 Hz) under two interstimulus (ISI) conditions to investigate whether a reduced duration of auditory sensory memory is found in late talking children. Their results showed that MMN mean amplitude was reduced only for the ISI of 2000 ms in former late talking children both with and without persistent language deficits. Comparing the results of Grossheinrich et al. (2010) and our study, while their LT participants exhibit a MMN comparable to those of controls for shorter ISI conditions (500 ms), our results demonstrated the group differences in MMR amplitude at 3 years old and topographic distribution at 5 years old. One of the reasons for the discrepancy might be resulted by the participants’ age (4 years and 7 months); the other possibility would be that LTs only have deficits in discriminating speech stimuli and have preserved ability to discriminate nonspeech (pure tone) frequency changes. Future studies directly compare MMRs to speech and nonspeech stimuli would clarify this issue.
Several studies have suggested that the MMR elicited in infants is predictive of later language outcomes (Leppänen et al., 2002, van Leeuwen et al., 2006, van Zuijen et al., 2013). Our correlation analysis extended the predictive role of MMRs to later language outcome measures in the preschool period. The mean MMR amplitude at 3 years of age in the frontocentral channels were significantly correlated with vocabulary, syntax, and overall language performance on standardized tests at 6 years.
Comparing with previous behavioral studies, MacRoy-Higgins et al. (2013) examined the causal relationship between phonology and lexical acquisition in 24-month-old children who either were LTs or had TLD. The toddlers were taught 12 novel words differing in phonotactic probability (high vs. low) by using focused stimulation procedures over the course of 10 training sessions. After the training sessions, a preferential-looking paradigm was used to test word retention through comprehension, production, and toddlers’ ability to detect mispronunciations of the newly learned words. The results showed that LTs did not differentiate between words containing high and those containing low phonotactic probability. The authors concluded that the underlying impairment in late-talking toddlers could be an early inability to detect regularities in the phonological system of the language that they are exposed to, which in turn inhibits their ability to store the phonological forms required to acquire lexical items. The correlation results in our study confirmed that the sophisticated speech discrimination ability at the early age might not only be a supporting mechanism for establishing stable phonological representation but also be essential for language development in children with late expressive language.
Finally, the behavioral data in our study confirmed that although the LBs’ scores on the language test were similar to the TLD scores at 4 years of age, the LBs still lagged behind in language performance when tested at 6 years. However, all three groups showed similar MMR patterns and no significant differences were found at 6 years, suggesting that children’s abilities to use the dominant pitch contour cue to discriminate between T2 and T3 reached a similar developmental level.
Of particular relevance here is the notion of “illusory recovery” (Scarborough and Dobrich, 1990), which suggests that although LTs may catch up in some language areas, their underlying language impairment results in weaknesses in other aspects of language learning later in their development. Another factor accounting for the similar MMR patterns among groups may be the stimuli used in our study. The standard/i2/and deviant/i3/are both real Mandarin Chinese words that appear with high frequency (7688 and 2412 per million, respectively). Although word frequency seems to be a relatively insignificant factor driving MMN enhancement (Pulvermuller et al., 2001, Pulvermuller et al., 2004), the unexpected delayed latency of MMRs at 6 years of age suggests that children might also process lexical meanings of the two stimuli instead of processing the acoustic-phonetic components, which could lead to the cross-group similarities that we observed. Future studies using pseudowords with pitch contours similar to lexical tones could clarify this concern.
Identifying the specific underlying neural features of language learning systems and documenting the features longitudinally should facilitate elucidating differences at the behavioral level. Our study, which provided this type of evidence by targeting the speech perception ability, identified a difference in developmental progression between LTs and their TLD peers and described the relationship between early speech perception ability and later language performance. A history of late talking might be a risk factor but is not the underlying cause of later language impairment. The disruption of the mechanisms underlying language learning, such as speech perception ability, may be a target in searching for the risk factors of language impairment.
To summarize, our longitudinal MMR pattern results suggest that late-talking children may have less sophisticated speech perception abilities at the early developmental stage in which language acquisition begins. Our longitudinal data provide supporting evidence showing that targeting early perception may have a lasting and beneficial result on learning higher-order language abilities. Future intervention studies are required to provide direct and complimentary data for the causal relationship when assess the long-term effects of speech perception ability in the language development of late-talking children.
References
- Ahmmed A.U., Clarke E.M., Adams C. Mismatch negativity and frequency representational width in children with specific language impairment. Dev. Med. Child Neurol. 2008;50(12):938–944. doi: 10.1111/j.1469-8749.2008.03093.x. [DOI] [PubMed] [Google Scholar]
- Arai T., Ohki E., Iitaka K. Perception of long vowels in Japanese by Children. Acoust. Sci. Technol. 2008;29(1):106–109. [Google Scholar]
- Bishop D.V., Price T.S., Dale P.S., Plomin R. Outcomes of early language delay II. Etiology of transient and persistent language difficulties. J. Speech Lang. Hear. Res. 2003;46(3):561–575. doi: 10.1044/1092-4388(2003/045). [DOI] [PubMed] [Google Scholar]
- Boets B., Ghesquière P., van Wieringen A., Wouters J. Speech perception in preschoolers at family risk for dyslexia: relations with low-level auditory processing and phonological ability. Brain Lang. 2007;101(1):19–30. doi: 10.1016/j.bandl.2006.06.009. [DOI] [PubMed] [Google Scholar]
- Bradlow A.R., Kraus N., Nicol T.G., McGee T.J., Cunningham J., Zecker S.G., Carrell T.D. Effects of lengthened formant transition duration on discrimination and neural representation of synthetic CV syllables by normal and learning-disabled children. J. Acoust. Soc. Am. 1999;106(4):2086–2096. doi: 10.1121/1.427953. [DOI] [PubMed] [Google Scholar]
- Burnham D. Language specific speech perception and the onset of reading. Read. Writ.: An Interdiscipli. J. 2003;16(6):573–609. [Google Scholar]
- Čeponienė R., Cheour M., Näätänen R. Interstimulus interval and auditory event-related potentials in children: evidence for multiple generators. Electroencephalogr. Clin. Neurol. 1998;108:345–354. doi: 10.1016/s0168-5597(97)00081-6. [DOI] [PubMed] [Google Scholar]
- Čeponienė R., Lepistö T., Alku P., Aro H., Näätänen R. Event-related potential indices of auditory vowel processing in 3-year-old children. Clin. Neurophysiol. 2003;114:652–661. doi: 10.1016/s1388-2457(02)00436-4. [DOI] [PubMed] [Google Scholar]
- Čeponienė R., Lepistö T., Soininen M., Aronen E., Alku P., Näätänen R. Event-related potentials associated with sound discrimination versus novelty detection in children. Psychophysiology. 2004;41:130–141. doi: 10.1111/j.1469-8986.2003.00138.x. [DOI] [PubMed] [Google Scholar]
- Chandrasekaran B., Krishnan A., Gandour J.T. Mismatch negativity to pitch contours is influenced by language experience. Brain Res. 2007;1128:148–156. doi: 10.1016/j.brainres.2006.10.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L.Y., Liu H.M. Auditory processing in school-aged children with specific language impairments. Bull. Spec. Educ. 2010;35(1):1–18. [Google Scholar]
- Chen Y.C. Unpublished Doctoral Dissertation. National Taiwan Normal University; Taipei, Taiwan: 2012. Novel word learning and phonological processing abilities in preschool children with specific language impairment. [Google Scholar]
- Cheng Y.Y., Wu H.C., Tzeng Y.L., Yang M.T., Zhao L.L., Lee C.Y. The development of mismatch responses to Mandarin lexical tones in early infancy. Dev. Neuropsychol. 2013;38(5):281–300. doi: 10.1080/87565641.2013.799672. [DOI] [PubMed] [Google Scholar]
- Cheour M., Shestakova A., Alku P., Ceponiene R., Näätänen R. Mismatch negativity shows that 3–6-year-old children can learn to discriminate non-native speech sounds within two months. Neurosci. Lett. 2002;325(3):187–190. doi: 10.1016/s0304-3940(02)00269-0. [DOI] [PubMed] [Google Scholar]
- Desmarais C., Sylvestre A., Meyer F., Bairati I., Rouleau N. Systematic review of the literature on characteristics of late-talking toddlers. Int. J. Lang. Commun. Disord. 2008;43:361–389. doi: 10.1080/13682820701546854. [DOI] [PubMed] [Google Scholar]
- Domsch C., Richels C., Saldana M., Coleman C., Wimberly C., Maxwell L. Narrative skill and syntactic complexity in school-age children with and without late language emergence. Int. J. Lang. Commun. Disord. 2012;47:197–207. doi: 10.1111/j.1460-6984.2011.00095.x. [DOI] [PubMed] [Google Scholar]
- Eimas P.D., Siqueland E.R., Jusczyk P., Vigorito J. Speech perception in infants. Science. 1971;171:303–306. doi: 10.1126/science.171.3968.303. [DOI] [PubMed] [Google Scholar]
- Ellis Weismer S., Murray-Branch J., Miller J. A prospective longitudinal study of language development in late talkers. J. Speech Hear. Res. 1994;37:852–867. doi: 10.1044/jshr.3704.852. [DOI] [PubMed] [Google Scholar]
- Ellis E.M., Thal D.J. Early language delay and risk for language impairment. SIG 1 Perspect. Lang. Learn. Educ. 2008;15(3):93–100. [Google Scholar]
- Fischel J.E., Whitehurst G.J., Caulfield M.B., DeBaryshe B. Language growth in children with expressive language delay. Pediatrics. 1989;83(2):218–227. [PubMed] [Google Scholar]
- Friederici A.D., Friedrich M., Weber C. Neural manifestation of cognitive and precognitive mismatch detection in early infancy. Neuroreport. 2002;13:1251–1254. doi: 10.1097/00001756-200207190-00006. [DOI] [PubMed] [Google Scholar]
- Grossheinrich N., Kademann S., Bruder J., Bartling J., Von Suchodoletz W. Auditory sensory memory and language abilities in former late talkers: a mismatch negativity study. Psychophysiology. 2010;47(5):822–830. doi: 10.1111/j.1469-8986.2010.00996.x. [DOI] [PubMed] [Google Scholar]
- Guttorm T.K., Leppänen P.H.T., Richardson U., Lyytinen H. Event-related potentials and consonant differentiation in newborns with familial risk for dyslexia. J. Learn. Disabil. 2001;34(6):534–544. doi: 10.1177/002221940103400606. [DOI] [PubMed] [Google Scholar]
- Guttorm T.K., Leppänen P.H., Poikkeus A.M., Eklund K.M., Lyytinen P., Lyytinen H. Brain event-related potentials (ERPs) measured at birth predict later language development in children with and without familial risk for dyslexia. Cortex. 2005;41(3):291–303. doi: 10.1016/s0010-9452(08)70267-3. [DOI] [PubMed] [Google Scholar]
- Hazan V., Barrett S. The development of phonemic categorization in children aged 6–12. J. Phonetics. 2000;28(4):377–396. [Google Scholar]
- Höhle B., van de Vijver R., Weissenborn J. Word processing at 19 months and its relation to language performance at 30 months: a retrospective analysis of data from German learning children. Int. J. Speech-Lang. Pathol. 2006;8(4):356–363. [Google Scholar]
- Jusczyk P.W. Perception of syllable-final stop consonants by 2-month-old infants. Percept. Psychophys. 1977;21(5):450–454. [Google Scholar]
- Kelly D.J. A clinical synthesis of the late talker literature implications for service delivery. Lang. Speech Hear. Serv. Schools. 1998;29(2):76–84. doi: 10.1044/0161-1461.2902.76. [DOI] [PubMed] [Google Scholar]
- Korpilahti P., Lang H., Aaltonen O. Is there a late-latency mismatch negativity (MMN) component? Electroencephalogr. Clin. Neurophysiol. 1995;95(4):P96. doi: 10.1016/0013-4694(94)90189-9. [DOI] [PubMed] [Google Scholar]
- Kraus N., McGee T., Carrell T., Sharma A., Micco A., Nichol T. Speech-evoked cortical potentials in children. J. Am. Acad. Audiol. 1993;4:238–248. [PubMed] [Google Scholar]
- Kraus N., McGee T., Carrell T.D., King C., Tremblay K., Nicol T. Central auditory system plasticity associated with speech discrimination training. J. Cogn. Neurosci. 1995;7(1):25–32. doi: 10.1162/jocn.1995.7.1.25. [DOI] [PubMed] [Google Scholar]
- Kraus N., McGee T.J., Carrell T.D., Zecker S.G., Nicol T.G., Koch D.B. Auditory neurophysiologic responses and discrimination deficits in children with learning problems. Science. 1996;273(5277):971–973. doi: 10.1126/science.273.5277.971. [DOI] [PubMed] [Google Scholar]
- Kuhl P.K., Conboy B.T., Padden D., Nelson T., Pruitt J. Early speech perception and later language development: implications for the critical period. Lang. Learn. Dev. 2005;1(3–4):237–264. [Google Scholar]
- Lee C.Y., Yen H.L., Yeh P.W., Lin W.H., Cheng Y.Y., Tzeng Y.L., Wu H.C. Mismatch responses to lexical tone, initial consonant, and vowel in Mandarin-speaking preschoolers. Neuropsychologia. 2012;50(14):3228–3239. doi: 10.1016/j.neuropsychologia.2012.08.025. [DOI] [PubMed] [Google Scholar]
- Leppänen P.H., Richardson U., Pihko E., Eklund K.M., Guttorm T.K., Aro M., Lyytinen H. Brain responses to changes in speech sound durations differ between infants with and without familial risk for dyslexia. Dev. Neuropsychol. 2002;22(1):407–422. doi: 10.1207/S15326942dn2201_4. [DOI] [PubMed] [Google Scholar]
- Lin B.G., Huang Y.C., Huang G.C., Hsuan C.H. Ministry of Education; Taipei, Taiwan: 2008. Child Language Disorder Scale-Revised (Preschool Version) [Google Scholar]
- Lin B.G., Huang Y.C., Huang G.C., Hsuan C.H. Ministry of Education; Taipei, Taiwan: 2009. Child Language Disorder Scale-Revised (School Version) [Google Scholar]
- Liu H.M., Tsao F.M. The standardization and application of Mandarin-Chinese communicative developmental inventory for infants and toddlers. Formos. J. Ment. Health. 2010;23:503–534. [Google Scholar]
- Liu H.M., Tsao F.M., Chang C.C., Hsu L.L. The development of speech discrimination in preschool and school-aged children: association with word comprehension. Bull. Educ. Psychol. 2013;45:221–240. in Chinese. [Google Scholar]
- Liu H.M., Chen Y., Tsao F.M. Developmental changes in mismatch responses to mandarin consonants and lexical tones from early to middle childhood. PLoS One. 2014;9(4):e95587. doi: 10.1371/journal.pone.0095587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L., Liu H.S. Psychological Publishing; Taipei, Taiwan: 1998. The Peabody Picture Vocabulary Test: Revised in Chinese. [Google Scholar]
- Lu H.H., Tsao F.M. Lexical-tone perception and word learning abilities in two-year-old late-talking children. Chin. J. Psychol. 2014;56(4):415–435. [Google Scholar]
- Luo H., Ni J.T., Li Z.H., Li X.O., Zhang D.R., Zeng F.G., Chen L. Opposite patterns of hemisphere dominance for early auditory processing of lexical tones and consonants. Proc. Natl. Acad. Sci. U. S. A. 2006;103:19558–19563. doi: 10.1073/pnas.0607065104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacRoy-Higgins M., Schwartz R.G., Shafer V.L., Marton K. Influence of phonotactic probability/neighborhood density on lexical learning in late talkers International. J. Lang. Commun. Disord. 2013;2:188–199. doi: 10.1111/j.1460-6984.2012.00198.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng X., Sai X., Wang C., Wang J., Sha S., Zhou X. Auditory and speech processing and reading development in Chinese school children: behavioural and ERP evidence. Dyslexia. 2005;11:292–310. doi: 10.1002/dys.309. [DOI] [PubMed] [Google Scholar]
- Molfese D.L., Molfese V.J., Espy K.A. The predictive use of event-related potentials in language development and the treatment of language disorders. Dev. Neuropsychol. 1999;16:373–377. [Google Scholar]
- Molfese D.L. Predicting dyslexia at 8 years of age using neonatal brain responses. Brain Lang. 2000;72:238–245. doi: 10.1006/brln.2000.2287. [DOI] [PubMed] [Google Scholar]
- Morr M.L., Shafer V.L., Kreuzer J.A., Kurtzberg D. Maturation of mismatch negativity in typically developing infants and preschool children. Ear Hear. 2002;23(2):118–136. doi: 10.1097/00003446-200204000-00005. [DOI] [PubMed] [Google Scholar]
- Moyle J., Stokes S.F., Klee T. Early language delay and specific language impairment. Dev. Disabil. Res. Rev. 2011;17:160–169. doi: 10.1002/ddrr.1110. [DOI] [PubMed] [Google Scholar]
- Näätänen R., Winkler I. The concept of auditory stimulus representation in cognitive neuroscience. Psychol. Bull. 1999;125(6):826. doi: 10.1037/0033-2909.125.6.826. [DOI] [PubMed] [Google Scholar]
- Paul R. Outcomes of early expressive language delay. J. Childhood Commun. Disord. 1993;15:7–14. [Google Scholar]
- Polka L., Werker J.F. Developmental changes in perception of nonnative vowel contrasts. J. Exp. Psychol. Hum. Percept. Perform. 1994;20(2):421–435. doi: 10.1037//0096-1523.20.2.421. [DOI] [PubMed] [Google Scholar]
- Pulvermuller F., Kujala T., Shtyrov Y., Simola J., Tiitinen H., Alku P., Alho K., Martinkauppi S., Ilmoniemi R.J., Näätanen R. Memory traces for words as revealed by the mismatch negativity. Neuroimage. 2001;14:607–616. doi: 10.1006/nimg.2001.0864. [DOI] [PubMed] [Google Scholar]
- Pulvermuller F., Shtyrov Y., Kujala T., Näätanen R. Word-specific cortical activity as revealed by the mismatch negativity. Psychophysiology. 2004;41:106–112. doi: 10.1111/j.1469-8986.2003.00135.x. [DOI] [PubMed] [Google Scholar]
- Putkinen V., Niinikuru R., Lipsanen J., Tervaniemi M., Huotilainen M. Fast measurement of auditory event-related potential profiles in 2–3-year-olds. Dev. Neuropsychol. 2012;37(1):51–75. doi: 10.1080/87565641.2011.615873. [DOI] [PubMed] [Google Scholar]
- Rescorla L., Schwartz K. Outcome of toddlers with specific expressive language delay. Appl. Psycholinguist. 1990;11:393–407. [Google Scholar]
- Rescorla L. The language development survey a screening tool for delayed language in toddlers. J. Speech Hear. Disord. 1989;54(4):587–599. doi: 10.1044/jshd.5404.587. [DOI] [PubMed] [Google Scholar]
- Rescorla L., Dahlsgaard K., Roberts J. Language and reading outcomes to age 9 in late-talking toddlers. J. Speech Lang. Hear. Res. 2002;45(2):360–371. doi: 10.1044/1092-4388(2002/028). [DOI] [PubMed] [Google Scholar]
- Rice M.L., Taylor C.L., Zubrick S.R. Language outcomes of 7-year-old children with or without a history of late language emergence at 24 months. J. Speech Lang. Hear. Res. 2008;51:394–407. doi: 10.1044/1092-4388(2008/029). [DOI] [PubMed] [Google Scholar]
- Rice M.L. Toward epigenetic and gene regulation models of specific language impairment: looking for links among growth, genes, and impairments. J. Neurodev. Disord. 2012;4:4–27. doi: 10.1186/1866-1955-4-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos E.M., Ellis Weismer S. Language outcomes of late talking toddlers at preschool and beyond. SIG 1 Perspect. Lang. Learn. Educ. 2008;15(3):119–126. doi: 10.1044/lle15.3.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarborough H.S., Dobrich W. Development of children with early language delay. J. Speech Lang. Hear. Res. 1990;33(1):70–83. doi: 10.1044/jshr.3301.70. [DOI] [PubMed] [Google Scholar]
- Semlitsch H.V., Anderer P., Schuster P., Presslich O.A. Solution for reliable and valid reduction of ocular artifacts applied to the P300 ERP. Psychophysiology. 1986;23:695–703. doi: 10.1111/j.1469-8986.1986.tb00696.x. [DOI] [PubMed] [Google Scholar]
- Shafer V.L., Yu Y.H., Datta H. Maturation of speech discrimination in 4-to 7-yr-old children as indexed by event-related potential mismatch responses. Ear Hear. 2010;31(6):735–745. doi: 10.1097/AUD.0b013e3181e5d1a7. [DOI] [PubMed] [Google Scholar]
- Thal D. San Diego State University Press; San Diego CA: 2000. Late Talking Toddlers: Are They at Risk? [Google Scholar]
- Tsao F.-M., Liu H.-M., Kuhl P.K. Speech perception in infancy predicts language development in the second year of life: a longitudinal study. Child Dev. 2004;75:1067–1084. doi: 10.1111/j.1467-8624.2004.00726.x. [DOI] [PubMed] [Google Scholar]
- Tsao F.M. The effect of acoustical similarity on lexical-tone perception of one-year-old Mandarin learning infants. Chin. J. Psychol. 2008;50:111–124. [Google Scholar]
- Tsybina I., Eriks-Brophy A. Issues in research on children with early language delay. Contemp. Issues Commun. Sci. Disord. 2007;34:118–133. [Google Scholar]
- van Leeuwen T., Been P., Kuijpers C., Zwarts F., Maassen B., van der Leij A. Mismatch response is absent in 2-month-old infants at risk for dyslexia. Neuroreport. 2006;17(4):351–355. doi: 10.1097/01.wnr.0000203624.02082.2d. [DOI] [PubMed] [Google Scholar]
- van Zuijen T.L., Plakas A., Maassen B.A., Maurits N.M., Leij A. Infant ERPs separate children at risk of dyslexia who become good readers from those who become poor readers. Dev. Sci. 2013;16(4):554–563. doi: 10.1111/desc.12049. [DOI] [PubMed] [Google Scholar]
- Vance M., Rosen S., Coleman M. Assessing speech perception in young children and relationships with language skills. Int. J. Audiol. 2009;48:708–717. doi: 10.1080/14992020902930550. [DOI] [PubMed] [Google Scholar]
- Wang T.M., Su C.W., Liao H.F.H., Lin L.Y., Tsou K.S., Lin S.H. Ministry of Education; Taipei, Taiwan: 2002. Comprehensive Developmental Inventory for Infants and Toddlers. [Google Scholar]
- Werker J.F., Tees R.C. Cross-language speech perception: evidence for perceptual reorganization during the first year of life. Infant Behav. Dev. 2002;25(1):121–133. [Google Scholar]
- Whitehouse A.J.O., Robinson M., Zubrick S.R. Late talking and the risk for psychosocial problems during childhood and adolescence. Pediatrics. 2011;128:324–332. doi: 10.1542/peds.2010-2782. [DOI] [PubMed] [Google Scholar]
- Wong P., Schwartz R.G., Jenkins J.J. Perception and production of lexical tones by 3-yearold, Mandarin-speaking children. J. Speech Lang. Hear. Res. 2005;48:1065–1079. doi: 10.1044/1092-4388(2005/074). [DOI] [PubMed] [Google Scholar]
- Wu W.D., Hu S.T., Tsai C.C., Wang J.D., Lin H.T., Kuo C.C. Third ed. Psychological Publishing; Taipei, Taiwan: 2006. Test of Nonverbal Intelligence. [Google Scholar]
- Xi J., Zhang L., Shu H., Zhang Y., Li P. Categorical perception of lexical tones in Chinese revealed by mismatch negativity. Neuroscience. 2010;170:223–231. doi: 10.1016/j.neuroscience.2010.06.077. [DOI] [PubMed] [Google Scholar]
- Yang K.T., Chang S.H., Li S.Y. Ministry of Education; Taipei. Taiwan: 2005. Oral Language Syntax Ability Diagnostic Test. [Google Scholar]
- Zhang Y., Zhang L., Shu H., Xi J., Wu H., Zhang Y. Universality of categorical perception deficit in developmental dyslexia: an investigation of Mandarin Chinese tones. J. Child Psychol. Psychiatry. 2012;53:874–882. doi: 10.1111/j.1469-7610.2012.02528.x. [DOI] [PubMed] [Google Scholar]
- Zubrick S.R., Taylor K.L., Rice M.L., Slegers D.W. Late language emergence at 24 months: an epidemiological study of prevalence, predictors, and covariates. J. Speech Lang. Hear. Res. 2007;50:1562–1592. doi: 10.1044/1092-4388(2007/106). [DOI] [PMC free article] [PubMed] [Google Scholar]