Abstract
Particulate matter (PM) inhalation exposure affects exhaled CO2 concentration. Such exhaled CO2 refers to ventilation and perfusion of the cardiorespiratory system, the analysis of which is painless, non-invasive and simple to perform. This study examined the effect of prallethrin and d-phenothrin inhalation exposure on exhaled CO2 in mice using a simple method. Prallethrin and d-phenothrin were administered in male mice (Mus musculus) in a series of repeated inhalation exposures of lower and higher doses for 60 days. The lower dose was a mixture of 0.000141 mg/L prallethrin and 0.104 mg/L d-phenothrin, while the higher dose was a mixture of 0.00141 mg/L prallethrin and 1.04 mg/L d-phenothrin. The lower dose was based on a NOAEL value of prallethrin and d-phenothrin of 28 days exposure, while the higher one was ten times of the lower dose concentration. CO2 concentration was measured by means of the passage through NaOH 0.1 N, titrated by HCl 0.1 N. PMs were generated by the process of producing bubbles, inserted into the chamber containing mice. Mice were divided into four groups, namely: negative control (NC), positive control (PC), and lower- and higher-dose treatment groups, with three replicates for each group. Statistical difference analyses were observed in body weight and exhaled CO2 concentration between negative control and treatment groups, nevertheless, they did not differ significantly between the control and the treatment (lower and higher dose) groups. This study suggests that exhaled CO2 and body weight are not specific biomarkers to observe PMs inhalation exposure with respect to prallethrin and d-phenothrin mixtures.
Keywords: Prallethrin, d-Phenothrin, Pyrethroid, Particulate matter, Exhaled CO2, Inhalation exposure
Introduction
Prallethrin and d-phenothrin are compounds of the pyrethroid family and are common ingredients of mosquito repellents (mats, spray). They are found in many developing countries. They are neurotoxic in mammals, with sodium channels being their well-established target site. Pyrethroid exposure affects various parameters such as those associated with biochemistry (plasma alanine aminotransferase (ALT), aspartate aminotransferase (AST) activities, and the levels of creatinine, total protein and RNA contents in the liver and kidney tissues) of albino rats [1]. The prallethrin account cis-isomer (13–32% of dose) and trans-isomer (45–62% of dose) are excreted in urine, faeces and less than 0.1% as exhaled CO2. d-Phenothrin-isomer excretions are found in urine (85% and 66% of dose) and faeces (15% and 28% of dose) over 6 days of exposure, nevertheless, it have not been reported as exhaled CO2 [2]. The prallethrin and d-phenothrin mixture exposure affect on exhaled CO2 has not been previously studied.
During inhalation exposure, pyrethroids penetrate the body as a particulate. PMs are obtained from spraying mechanisms, nebulization and bubble making. Epidemiological studies suggest that there is a significant relationship between particulate exposure and subsequent health problems [3, 4]. Impaired health disorders may include disturbances in the respiratory tract [5], cardiovascular system [6, 7] and may even increase mortality [8, 9].
Breath testing analyses in exhaled breath are found that normal breath is complex in terms of gas composition. Factors influencing breath composition variations include physical condition, general health of the subject, food intake, environmental influences and lifestyle [10, 11]. Exhaled breath testing has many advantages compared to other methods because it is non-invasive, painlessness and simple to perform [11, 12]. It is highly beneficial given the limitations of measuring devices and methods of exhaled breath composition in laboratory animal testing. Exhaled breath includes a very small number of inorganic compounds such as NO, O2, CO2, volatile organic compounds (hydrocarbons, alcohols, ketones, aldehydes, esters) and non-volatile substances such as isoprostanes, cytokines, leukotrienes and hydrogen peroxide [13]. Exhaled CO2 is a sensitive indicator not only for ventilation efficiency but also for pulmonary perfusion and cardiac output, and can also be used to estimate the metabolic rate and nutritional requirements [14].
In a previous study of inhalation exposure methods, the effects of some xenobiotic exposures to CO2 were investigated [14–16]. Kim and Ghanayem [17] study the effect of trichloroethylene at varying doses to exhaled CO2 in two strains of mice. A significant decrease in exhaled CO2 is observed prior to and after treatment in both genotypes, with knockout mice being lower than the wild type mice at the same dose. In this study, we examine the effects of prallethrin and d-phenothrin PMs inhalation exposure on exhaled CO2 in mice using a simple method—breath testing.
Materials and methods
Chemicals and reagents
Prallethrin and d-phenothrin were purchased from Sigma Aldrich with catalogue number of prallethrin 32,917 and d-phenothrin 36,193. In this research, two doses of prallethrin and d-phenothrin mixtures comprised the lower and higher doses. The lower dose was a mixture of 0.0001 mg/L prallethrin and 0.104 mg/l d-phenothrin, while the higher dose was a mixture of 0.001 mg/l prallethrin and 1.04 mg/l d-phenothrin. The lower dose was based on the NOAEL value of prallethrin and d-phenothrin of 28 days exposure [18], while the higher one was ten times larger. These active ingredients were diluted two times to obtain the preferred concentration. NaOH 0.1 N was produced by means of dissolving 4 g NaOH in distilled water, standardized using KHC8H4O4, whereas HCl 0.1 N was obtained from diluted 8.3 ml pure HCl in distilled water, standardized using Na2CO3 0.05 N. Both reagent standardizations referred to standard methods for the examination of waste and wastewater 2310B [19].
Mechanism of inhalation exposure
Mice were inhalation exposed to prallethrin and d-phenothrin, which were dissolved in acetonitrile then diluted two times in distilled water [20]. The solution was volatilized using a diffuser, in which the air supply was derived from an air pump (RC-Q6) obtained from Adam Aquarium manufacturing, discharged at 4 L/min into the solution [21]. The aerosol formed was inserted into the whole body exposure chamber which contained three mice. Mice were exposed for 4 h a day [18] during 60 days observation with a mixture of prallethrin and d-phenothrin, which was executed at temperatures of 31.5 °C ± 0.5 °C and RH of 94% ± 2.5%, and was performed in a chamber as a continuous system.
Animal husbandry and maintenance
Six-week-old male BALB/c mice were obtained from the Airlangga University pharmacology laboratory and acclimatized for 14 days. The room was maintained at 30.3 °C ± 1.4 °C with a relative humidity (RH) of 63.08% ± 2. 87% and light:dark cycles of 12:12 h. The mice were housed together with 12 mice in stainless steel wire mesh cages (W 350 mm × L 400 mm × H 180 mm), ad libitum provided for tap water and a commercial diet from PT. Charoen Pokphand Indonesia. After acclimatization, three mice were placed into a chamber for exposure to a prallethrin and d-phenothrin mixture for 4 h a day during 60 days. The study was approved by the Animal Care and Use Committee (ACUC) of Veterinary Faculty of Airlangga University with certificate number 716-KE.
Experimental groups
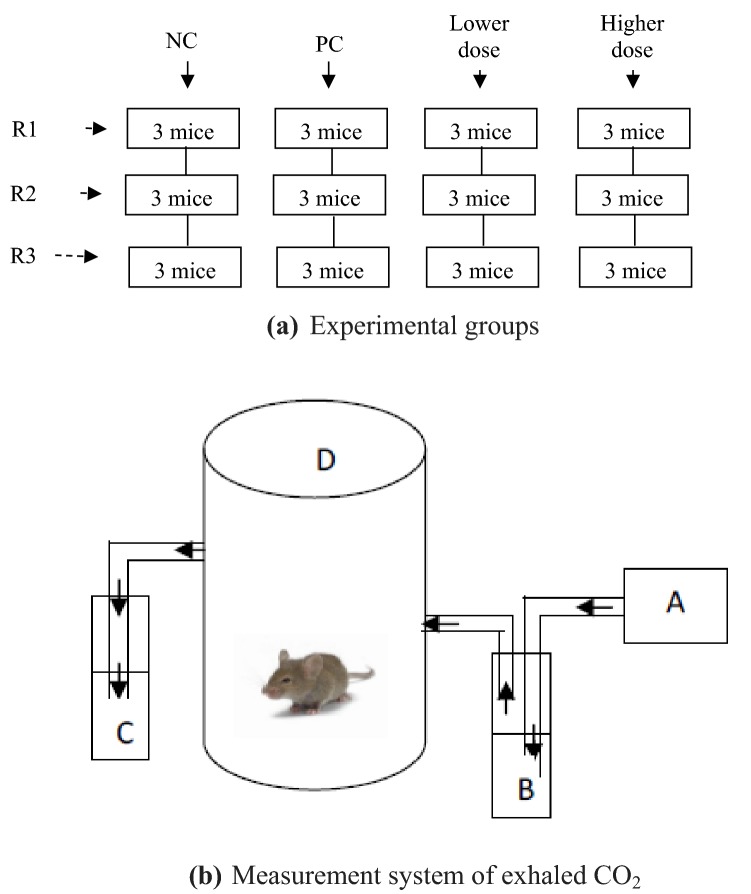
Mice were divided into four groups, namely: negative control, positive control and treatment (lower and higher dose) groups (Fig. 1). The negative control groups were the mice without any treatment, positive control groups were mice with solvent exposure of acetonitrile (1667 × 10−3 mL/L), whereas lower-dose treatment groups were mice exposed to a mixture of 0.0001 mg/L prallethrin and 0.104 mg/l d-phenothrin exposures and higher-dose treatment groups were mice exposed to a mixture of 0.001 mg/l prallethrin and 1.04 mg/l d-phenothrin. There were three replicates (R) for each group.
Fig. 1.
Illustration of a experimental groups and b the measurements of exhaled CO2. PC positive control group, R replicate, Lower dose groups of 0.0001 mg/L prallethrin and 0.104 mg/L d-phenothrin exposures; Higher dose groups of 0.001 mg/L prallethrin and 1.04 mg/L d-phenothrin exposures. A: air supplier (mini compressor); B: 20 mL NaOH solution 0.1 N; C: 20 mL NaOH solution 0.1 N; D: a chamber containing mice
General observation
Mice were observed daily to determine their general symptoms and mortality from starting treatment of 60 days.
Measurement of body weight
The weight gain was monitored every day using a digital counting scale device of Sartorius ENTRIS 4202-1S from Sartorius manufacturing to observe the health condition of mice prior to the treatment and to observe the treatment effect on mice.
Measurement of particulate matters
PMs measurements were conducted at the third hour of exposures using an Aerocet 531S Particle Mass Profiler and Counter from Met One Instruments Inc. manufacturing, which employed factory calibrated. It was executed in the breathing zone of mice of 7 cm in height from the bottom of the chamber to represent inhaled PMs.
The standard solutions of analytical validation method were provided in three doses (A, B and C). The mixture was obtained in the concentration ratio of prallethrin and d-phenothrin of 1:1. Dose B contained a mixture of prallethrin and d-phenothrin based on NOAEL value, dose A was 10% of dose B, whereas dose C was ten times larger of dose B. The analytical validation data of PM concentration was executed by comparing the PM concentration of two measurement devices, a High Volume Air Sampler from Sole Agent HI-Q Environmental Products Company manufacturing and an Aerocet 531S Particle Mass Profiler and Counter from Met One Instruments Inc. manufacturing. The PM in size of 2.5 μm selected in the present study to test the performance of the methodology adopted [22], according to the criteria of linearity, precision and accuracy. The results of each measurement were compared.
Measurement of exhaled CO2
Exhaled CO2 measurements refer to the Redgrave et al. [23] method. CO2 from the ambient supplied using a mini compressor was trapped in 20 mL NaOH 0.1 N; subsequently, air without CO2 passed through an inlet of the chamber containing mice (Fig. 1). Mice were deployed in the secured chamber to collect exhaled CO2 prior to and after treatment. The air was removed from the chamber by passing it through a solution of 20 mL NaOH 0.1 N to capture exhaled CO2 in mice. The final aqueous solution of NaOH containing dissolved CO2 was titrated by HCl 0.1 N solution with phenolphthalein 0.1 N as a color indicator. The design of the CO2 measurement system was depicted in Fig. 1. This measurement was performed on days 0, 20, 40 and 60 during 60 days’ observations to examine the effect of exposure duration to exhaled CO2 of every mice (all chambers and all replicates).
The analytical validation method of exhaled CO2 was conducted by comparing the developed method in the present study with spectrophotometric techniques by Genesys 30 Visible Spectrophotometer from Thermo Fisher Scientific manufacturing to determine linearity, limit of detection (LOD) and limit of quantification [22, 24]. The standard solutions were prepared in three concentration of NaOH, such as 0.025 N, 0.05 N and 0.2 N. In addition, the tests were performed with certified and standardized reference material and factory calibrated instruments which ensured the traceability of the results.
Statistical analysis
All statistical analysis was conducted using SPSS 21.0. The homogeneity of variance test was carried out using a Levene test, while normality was determined with a Kolmogorov–Smirnov test. One-way ANOVAs were conducted to determine differences between groups and p values < 0.05 was considered to be statistically significant [6].
Results
General observations
Mice were observed to present no general symptoms of sensitivity (in the eyes, skin, face). No mortality occurred in mice as well as during acclimatization and treatment phases in all groups, and subsequently, there were tremor appearances in 2 of 3 of higher dose group mice during the final 20 days.
Monitoring body weight
Figure 2 illustrates that the mean body weight of negative control increased significantly, with the largest weight on the 40th day even up to 45.4 g ± 3.6 g, subsequently on the 60th day this reduced to 37.1 g ± 4.7 g, whereas weight gain patterns of positive control and treatment groups (lower and higher dose) were similar. There were no significant increases in body weight on days 20, 40 and 60, even likely to be constant. The influence of PMs exposures (with respect to all diameter particles) against body weight was statistically significantly different in body weight between negative and positive controls and lower-and higher-dose treatment groups (p value = 0.021); however, there were no significant differences between positive control and treatment (lower and higher dose) groups (p value = 0.402).
Fig. 2.
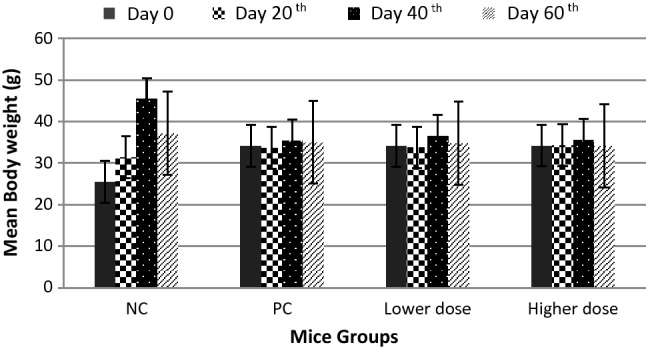
Mean body weight of mice. Data are presented as mean ± standard deviation (SD). NC negative control group, PC positive control group, Lower dose groups of 0.0001 mg/L prallethrin and 0.104 mg/L d-phenothrin exposures, Higher dose groups of 0.001 mg/L prallethrin and 1.04 mg/L d-phenothrin exposures
Monitoring particulate matter
PMs were measured in the respiratory zone of mice at days 20, 40 and 60 (Table 1). PMs generated from the process of bubble producing on average were small in size, with the highest concentration for the size of 0.3 µm at days 20, 40 and 60. 10 µm particles were not observed for either measurement. PMs concentration (particles/ft3) of the higher dose was significantly different compared with the lower dose, with a statistically significant difference in mean PMs concentration (p value = 0.000). However, there were no statistically significant differences between mean PMs concentrations in all replicate chambers of the same dose (p value = 0.000).
Table 1.
Particulate matters concentration in mice breathing zone
| Data | Lower Dose | Higher Dose | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0.3 µm | 0.5 µm | 1 µm | 5 µm | 10 µm | 0.3 µm | 0.5 µm | 1 µm | 5 µm | 10 µm | |
| Particulate matter (PM) concentration—day 20th (104 particles/ft3) | ||||||||||
| Upper | 6283.2 | 782.5 | 104.5 | 6711 | 0 | 9603.5 | 1228.9 | 289 | 23.4 | 0.1 |
| Lower | 4196.7 | 481.4 | 67.8 | 0.6 | 0 | 9357.3 | 1252.4 | 401.8 | 29.1 | 0.1 |
| Mean | 4918.1 | 590.0 | 78.5 | 0.6 | 0 | 9480.4 | 1240.6 | 345.4 | 26.3 | 0.1 |
| SD | 1182.9 | 167.2 | 22.6 | 0.1 | 0 | 174.1 | 16.6 | 79.7 | 4.0 | 0 |
| Particulate matter (PM) concentration—day 40th (104 particles/ft3) | ||||||||||
| Upper | 9999.9* | 9999.9* | 3159.8 | 2.5 | 0 | 9999.9* | 9999.9* | 3796.5 | 7.5 | 0 |
| Lower | 9999.9* | 9999.9* | 2554.0 | 4.0 | 0 | 9999.9* | 9999.9* | 2478.8 | 4.2 | 0 |
| Mean | 9999.9* | 9999.9* | 2776.9 | 3.1 | 0 | 9999.9* | 9999.9* | 3117.8 | 5.8 | 0 |
| SD | 0 | 0 | 333.0 | 0.8 | 0 | 0 | 0 | 659.8 | 1.7 | 0 |
| Particulate matter (PM) concentration—day 60th (104 particles/ft3) | ||||||||||
| Upper | 9999.9* | 3125.7 | 462.3 | 0.8 | 0 | 8370.8 | 1222.1 | 97.5 | 0.4 | 0 |
| Lower | 9999.9* | 3185.3 | 455.3 | 0.5 | 0 | 6711.3 | 811.0 | 72.9 | 0.6 | 0 |
| Mean | 9999.9* | 3155.5 | 458.8 | 0.7 | 0 | 7727.6 | 980.4 | 77.7 | 0.4 | 0 |
| SD | 0 | 42.1 | 4.9 | 0.2 | 0 | 890.4 | 214.9 | 17.8 | 0.1 | 0 |
*Concentration limit: 0–3000,000 particles per cubic feet
The results of one-way ANOVA of analytical validation data illustrates that PM concentration in size of 2.5 μm had a significant difference in number concentration between the three of standard solutions (p value = 0.000). The curve was quite linear in the concentrations evaluated. The relative errors determining the accuracy of PM measurement varied from 0.8 to 10%, whereas the relative standard deviations observed in the analysis of repeatability and intermediate precision were 0.5–10%.
Monitoring exhaled CO2 concentration
Exhaled CO2 of mice measurements were performed on days 0, 20, 40 and 60 (Fig. 3). In the negative control group, decreased exhaled CO2 concentration was observed on day 20, but was not noted for positive control and treatment (lower and higher dose) groups, where such concentrations tended to be constant. There were no statistically significant differences in exhaled CO2 concentrations between groups (p value = 0.354). Therefore, the concentration of exhaled CO2 between negative and positive control and treatment (lower and higher dose) groups on all day was relatively similar.
Fig. 3.
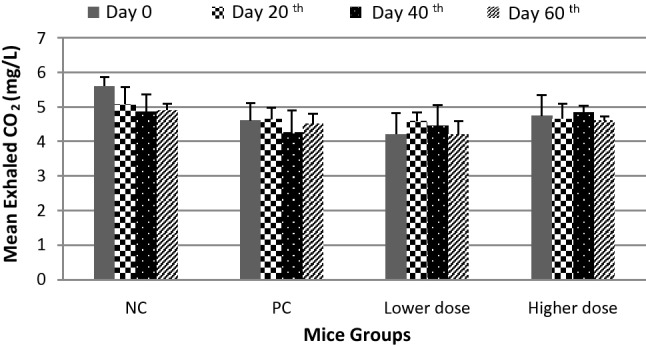
Mean exhaled CO2 in mice. Data are presented as mean ± standard deviation (SD). NC, negative control group; PC, positive control group; Lower dose, groups of 0.0001 mg/L prallethrin and 0.104 mg/L d-phenothrin exposures; Higher dose, groups of 0.001 mg/L prallethrin and 1.04 mg/L d-phenothrin exposures
The result of one-way ANOVA test of the analytical validation data of exhaled CO2 concentration illustrates a significant difference (p value = 0.02) in the three standard solutions and the curve illustrates quite linear as well. The detection limits examined in spectrophotometric result were close values can be observed in the developed method in the present study according to the relative error (ER) value (12–18%).
Both of the PM and exhaled CO2 concentration illustrates linearity based on ANOVA one way significance values and the curve pattern. The validation method of PM measurement found that the accuracy and the precision were satisfactory due to the value was below the maximum recommended (20%) [24]. The detection limits of exhaled CO2 in relation to the ER value were below the maximum recommended (20%) as well [24]. Therefore, the developed method in the present study was reliable to determine PM and the exhaled CO2 concentration.
Discussion
General observations
The absence of sensitization and mortality indicated that the compounds which affected mice in acclimatization and treatment stages could still be tolerated by the mice, even though there was a significant RH difference between in the cage and in the chamber. RH participated in an important role of mice susceptibility against infection. High level of RH encouraged bacteria and ammonia proliferation in the cage [25] which made mice more vulnerable to infection. High level of RH prevented desiccation of urine and faeces, resulting in a proliferation of urease-positive bacteria and subsequent production of ammonia [26].
The chamber condition planted to be clean by means of rinsed the chamber with running water, thus there was no urine and feces in the chamber when used the next day. It could be traced to inhibit the bacteria proliferation that could give rising to mice infection and to inhibit ammonia producing as well. Thus, the results finding the absence of sensitization and mortality in all mice of the negative control and treatment (lower and higher dose) groups were reasonable. Ammonia produced by the bacteria which producing urease or bedding containing heat-labile urease-activating enzymes that converted urea in urine and faeces to ammonia [27]. Ammonia contained a potential irritating mucus membrane of eyes, skin and respiratory tract [26]. It could cause changes including a reduction in the number of cilia on respiratory epithelial cells, hyperplasia of respiratory epithelial cells, as well as formation of glandular crypts in respiratory and olfactory epithelium [27]. These changes reduced the efficacy of respiratory tract defenced mechanisms, rendering mice more vulnerable to pathogens.
Tremor syndrome appeared in the higher dose group mice and was suspected due to prallethrin and d-phenothrin mixture exposures of type I pyrethroid compounds. Type I pyrethroid compound was generally considered to produce the tremor syndrome (TS) of intoxication and type II compound was thought to produce the choreoathetosis with salivation syndrome (CS) [28].
Monitoring body weight
The body weight gain by age indicates healthy mice with excellent growth and development at the acclimatization stage (data not shown). This result provided evidence that the mice were physically healthy prior to treatment being executed. Body weight was a growth indicator that was a result of many factors’ interaction. At the time of acclimatization, factors affecting weight included the social environment of mice including caging, aggressiveness, contact with the rat in the animal room [29–33], husbandry [34], new environment, duration and mode of transportation, noise, vibration and reduction of food and drink availability [35]. When treatment was conducted, interactions among factors that were affected were increasingly complex due to the process and materials carried out for treatment which also affected weight. Thus, the results finding no significant differences between the negative control and treatment (lower and higher dose) groups were reasonable.
The solvent acetonitrile which was used to dissolve prallethrin and d-phenothrin mixture also influence weight because it inhibited metabolism by reducing cellular oxygen [36]. This phenomenon might explain the lack of statistically significant differences between positive control and treatment (lower and higher dose) groups.
Monitoring particulate matter
PMs resulting from the bubble producing process comprised suspended solids along with liquids in the air (gas) as a medium, referred to as an aerosol [37, 38]. The PM patterns in the breathing zone for both doses illustrate the polydispersed distribution by the concentration of the largest particulate (particles/ft3) of diameter 0.3 µm, followed by 0.5 µm, 1 µm, and 5 µm, while particulates of 10 µm diameter were not formed at all. The same pattern occurred during all sample collection days for days 20, 40 and 60.
Owen et al. [39] investigated health effects due to indoor PMs associated with diameter and mass particles. The most important aspect considered related to deposition particles in the lungs was particle diameter. Particles of an aerodynamic diameter range > 30 µm had low probability to penetrate nasal passages. Phalen et al. [40] studied particles with an aerodynamic diameter of 5–10 µm deposited in the nose and pharyngeal passages favor rapid and sharp air flow.
Decreased air velocity and directional changes were observed on the tracheal bronchiolar region and thus the particles deposited in this region were 1–5 µm in size. Particles smaller than 1 µm were distributed through the alveolar segments of the respiratory tract, resulting in increasingly fewer particles reaching the alveolar as a consequence of a decrease in air velocity approaching zero. Gravity did not affect very small particles (< 1 µm diameter), thus, they deposited on the alveolar walls mostly by diffusion. When the particle size was reduced from micrometer to nanometer range, increasing toxicity appears due to the increase in particle surface area [41], while interactions between particles and cells depended on where particles were deposited and their solubility. Table 1 illustrates that the process of bubble manufacture generated the largest percentage of PMs in the diameter range of 0.3 µm (± 73.6%), followed by 0.5 µm (± 23%), 1 µm (± 3,4%), 5 µm (± 0%) and 10 µm (± 0%); therefore, the particles were disposed toward the alveolar segments of the respiratory tract and then diffused to the cardiovascular system.
Monitoring exhaled CO2 concentration
The concentration of exhaled CO2 was measured as an indicator of the capability of mice pulmonary ventilation. Siobal [14] observed that it was a sensitive indicator which not only demonstrated ventilation efficiency but also lung perfusion and heart output because it was excreted by the cardiorespiratory system. It also could be employed to estimate the metabolic rate and nutritional needs in critical conditions. The statistical analysis demonstrated that there were no significant differences between groups. These result simply that a mixture of prallethrin and d-phenothrin did not affect lung ventilation and perfusion in mice and thus did not influence exhaled CO2 concentration significantly; even the solvent acetonitrile, which was used in the positive control and treatment (lower and higher dose) groups, impacted exhaled CO2.
The inhalation of prallethrin and d-phenothrin mixture might result in complex metabolisms inside the mouse body, including hydrolysis, oxidations, conjugative carboxylic acid and disposition of intermediate components in tissues. The complex processes of prallethrin and d-phenothrin metabolism produced a significant amount of carboxylic acid group, that further converted into coA group as a raw material for intracellular respiration. This could result in the increasing of exhaled CO2. The decreasing of exhaled CO2 could be an indication of a mixture or intermediate compounds disposition in the mouse body. The disposition of mixture or intermediate compounds might result in several diseases in which the compounds located in.
Important chemical and toxicological aspects of pyrethroid toxicity were related to its cis- and trans-stereochemical configurations, particularly associated with the replacement of hydrogen atoms (substituents). Pyrethroids with a trans-configuration illustrate faster hydrolysis by esterase, thus, they were less toxic to mammals compared with those of cis-configuration. The pyrethroid metabolites were generally found in the urine of humans and other animals [42, 43]. Prallethrin released a very small amount of cis-isomer (13–32% of dose) and trans-isomer (45–62% of dose) in urine, faeces and less than 0.1% as exhaled CO2. d-Phenothrin-isomer excretions are found in urine (85% and 66% of dose) and faeces (15% and 28% of dose) over 6 days, nevertheless, it have not been reported as an exhaled CO2 [2].
Acetonitrile used as a solvent for the active ingredients in this research was inorganic cyanide, which reacted with Fe3+ of cytochrome oxidase in mitochondria and ensured cellular respiration by oxygen reduction [36]. Formaldehyde and formic acid have been postulated as being products of acetonitrile metabolism [44]. This postulation might explain the statistical results of the relationship between PMs and exhaled CO2 concentrations in positive control and treatment (lower and higher dose) groups, with acetonitrile having an important role to secure cellular respiration by oxygen reduction, and thus, affecting exhaled CO2 concentration [36].
Conclusion
Prallethrin and d-phenothrin are members of the pyrethroid family and were analyzed as particulate matters (PMs) with respect to inhalation exposure. PMs play a role in exacerbating human health, while pyrethroids are neurotoxic and thought to affect body weight and exhaled CO2 concentration. In this study, PMs were generated from the process of producing bubbles, with polydispersed distribution by concentration, and with the largest PMs being of 0.3 μm diameter, while exhaled CO2 concentration was measured using a simple breath test. There were no significant differences between groups of negative control, positive control and treatment (lower and higher dose) groups for both parameters (body weight and exhaled CO2). Body weight is a parameter resulting from the several factors that affect it, and it is increasingly complex when the treatment is carried out, as well as exhaled CO2 concentration. Prallethrin and d-phenothrin contain trans-isomers which are less toxic to mammals and their metabolites are yet to be observed being excreted through inhalation. In addition, acetonitrile which was used as a solvent in this work, has a role in cellular oxygen reduction, and thus affects exhaled CO2 concentration. This research suggests that body weight and exhaled CO2 concentration are not specific biomarkers of prallethrin and d-phenothrin mixtures with respect to inhalation exposure, due to complex interactions among many factors.
Acknowledgements
This work is supported by a Research Grants Doctoral Dissertation, Ministry of Research, Technology and Higher Education of the Republic of Indonesia 2018, the agreement letter number 1678.2/PL19/LT/2018.
Compliance with ethical standards
Conflict of interest
The authors declare that there are no conflicts of interest.
Contributor Information
Indri Santiasih, Email: indri.santiasih@ppns.ac.id.
Harmin Sulistiyaning Titah, Email: harmin_st@its.ac.id.
Joni Hermana, Email: hermana@its.ac.id.
References
- 1.Abd-Elhady HK, Abou-Elghar GE. Abamectin induced biochemical and histopathological changes in The Albino Rat, Rattus Norvegicus. J Plant Prot Res. 2013;53:263–270. [Google Scholar]
- 2.Copping LG. Metabolic pathways of agrochemicals: part two -insecticides and fungicides, eds-in-chief T Roberts and D Hutson, Royal Society of Chemistry, Cambridge, 1999, 1475 pp, price UK, 225 ISBN 085404 499 X. Pest Manag Sci. 2000;56:103–104. [Google Scholar]
- 3.Park JH, Kwon J-T, Arassh M-T, Hwang S-K, Chang S-H, Lim HT, Cho H-S, Cho M-H. Inhalation toxicity of particulate matters doped with arsenic induced genotoxicity and altered akt signaling pathway in lungs of mice. Toxicol Res. 2010;26:261–266. doi: 10.5487/TR.2010.26.4.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee J-S, Shin J-H, Lee J-O, Lee K-M, Kim J-H, Choi B-S. Levels of exhaled breath condensate pH and fractional exhaled nitric oxide in retired coal miners. Toxicol Res. 2010;26:329–337. doi: 10.5487/TR.2010.26.4.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim JW, Park S, Lim CW, Lee K, Kim B. The role of air pollutants in initiating liver disease. Toxicol Res. 2014;30:65–70. doi: 10.5487/TR.2014.30.2.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim C-W, Go R-E, Choi K-C. Treatment of BG-1 ovarian cancer cells expressing estrogen receptors with lambda-cyhalothrin and cypermethrin caused a partial estrogenicity via an estrogen receptor-dependent pathway. Toxicol Res. 2015;31:331–337. doi: 10.5487/TR.2015.31.4.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Franck U, Odeh S, Wiedensohler A, Wehner B, Herbarth O. The effect of particle size on cardiovascular disorders—the smaller the worse. Sci Total Environ. 2011;409(20):4217–4221. doi: 10.1016/j.scitotenv.2011.05.049. [DOI] [PubMed] [Google Scholar]
- 8.Beelen R, Raaschou-Nielsen O, Stafoggia M, Andersen ZJ, Weinmayr G, Hoffmann B, Wolf K, Samoli E, Fischer P, Nieuwenhuijsen M, Vineis P, Xun WW, Katsouyanni K, Dimakopoulou K, Oudin A, Forsberg B, Modig L, Havulinna AS, Lanki T, Turunen A, Oftedal B, Nystad W, Nafstad P, De Faire U, Pedersen NL, Östenson C-G, Fratiglioni L, Penell J, Korek M, Pershagen G, Eriksen KT, Overvad K, Ellermann T, Eeftens M, Peeters PH, Meliefste K, Wang M, Bueno-de-Mesquita B, Sugiri D, Krämer U, Heinrich J, de Hoogh K, Key T, Peters A, Hampel R, Concin H, Nagel G, Ineichen A, Schaffner E, Probst-Hensch N, Künzli N, Schindler C, Schikowski T, Adam M, Phuleria H, Vilier A, Clavel-Chapelon F, Declercq C, Grioni S, Krogh V, Tsai M-Y, Ricceri F, Sacerdote C, Galassi C, Migliore E, Ranzi A, Cesaroni G, Badaloni C, Forastiere F, Tamayo I, Amiano P, Dorronsoro M, Katsoulis M, Trichopoulou A, Brunekreef B, Hoek G. Effects of long-term exposure to air pollution on natural-cause mortality: an analysis of 22 European cohorts within the multicentre ESCAPE project. Lancet. 2013;383(9919):785–795. doi: 10.1016/S0140-6736(13)62158-3. [DOI] [PubMed] [Google Scholar]
- 9.Mar TF, Norris GA, Koenig JQ, Larson TV. Associations between air pollution and mortality in Phoenix, 1995–1997. Environ Health Perspect. 2000;108:347–353. doi: 10.1289/ehp.00108347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Amann A, Poupart G, Telser S, Ledochowski M, Schmid A, Mechtcheriakov S. Applications of breath gas analysis in medicine. Int J Mass Spectrom. 2004;239:227–233. [Google Scholar]
- 11.Libardoni M, Stevens PT, Waite JH, Sacks R. Analysis of human breath samples with a multi-bed sorption trap and comprehensive two-dimensional gas chromatography (GC × GC) J Chromatogr B. 2006;842:13–21. doi: 10.1016/j.jchromb.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 12.Prado C, Marín P, Periago JF. Application of solid-phase microextraction and gas chromatography–mass spectrometry to the determination of volatile organic compounds in end-exhaled breath samples. J Chromatogr A. 2003;1011:125–134. doi: 10.1016/s0021-9673(03)01103-8. [DOI] [PubMed] [Google Scholar]
- 13.Montuschi P, Nightingale JA, Kharitonov SA, Barnes PJ. Ozone-induced increase in exhaled 8-isoprostane in healthy subjects is resistant to inhaled budesonide. Free Radic Biol Med. 2002;33:1403–1408. doi: 10.1016/s0891-5849(02)01084-5. [DOI] [PubMed] [Google Scholar]
- 14.Siobal MS. Monitoring exhaled carbon dioxide. Respir Care. 2016;61(10):1397–1416. doi: 10.4187/respcare.04919. [DOI] [PubMed] [Google Scholar]
- 15.Kivimagi I, Kuusik A, Ploomi A, Metspalu L, Jogar K, Williams IH, Sibul I, Hiiesaar K, Luik A, Mand M. Gas exchange patterns in Platynus assimilis (Coleoptera: Carabidae): respiratory failure induced by a pyrethroid. Eur J Entomol. 2013;110:47–54. [Google Scholar]
- 16.Kullmann T, Barta I, Lázár Z, Szili B, Barát E, Valyon M, Kollai M, Horváth I. Exhaled breath condensate pH standardised for CO2 partial pressure. Eur Respir J. 2007;29:496–501. doi: 10.1183/09031936.00084006. [DOI] [PubMed] [Google Scholar]
- 17.Kim D, Ghanayem BI. Comparative metabolism and disposition of trichloroethylene in Cyp2e1−/− and wild-type mice. Drug Metab Dispos. 2006;34(12):2020–2027. doi: 10.1124/dmd.106.010538. [DOI] [PubMed] [Google Scholar]
- 18.WHO . WHO specifications for pesticides used in public health. Washington: WHO; 2017. [Google Scholar]
- 19.Rice E, Baird R, Eaton A, Clesceri L. Standard methods for the examination of water and wastewater. Washington: American Water Works Association; 1905. [Google Scholar]
- 20.Godin SJ, Scollon EJ, Hughes MF, Potter PM, DeVito MJ, Ross MK. Species differences in the in vitro metabolism of deltamethrin and esfenvalerate: differential oxidative and hydrolytic metabolism by humans and rats. Drug Metab Dispos. 2006;34:1764–1771. doi: 10.1124/dmd.106.010058. [DOI] [PubMed] [Google Scholar]
- 21.US EPA O (1998) Series 870-health effects test guidelines OPPTS 870.1300 acute inhalation toxicity
- 22.Ventura LMB, Amaral BS, Wanderley KB, Godoy JM, Gioda A. Validation method to determine metals in atmospheric particulate matter by inductively coupled plasma optical emission spectrometry. J Braz Chem Soc. 2014;25:1571–1582. [Google Scholar]
- 23.Redgrave TG, Martins IJ, Mortimer B-C. Measurement of expired carbon dioxide to assess the metabolism of remnant lipoproteins. J Lipid Res. 1995;36:2670–2675. [PubMed] [Google Scholar]
- 24.US Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER), and Center for Veterinary Medicine (CVM) Bioanalytical method validation guidance for industry. Washington: Biopharmaceutics; 2018. [Google Scholar]
- 25.Reeb-Whitaker CK, Paigen B, Beamer WG, Bronson RT, Churchill GA, Schweitzer IB, Myers DD. The impact of reduced frequency of cage changes on the health of mice housed in ventilated cages. Lab Anim. 2001;35:58–73. doi: 10.1258/0023677011911381. [DOI] [PubMed] [Google Scholar]
- 26.Perkins SE, Lipman NS. Evaluation of microenvironmental conditions and noise generation in three individually ventilated rodent caging systems and static isolator cages. Contemp Top Lab Anim Sci. 1996;35:61–65. [PubMed] [Google Scholar]
- 27.Baker DG, Lipman NS, Anderson L, Otto G, Pritchett-Corning K, Whary M (2015) Factors that can influence animal research. In: Anderson L, Otto G, Pritchett-Corning K, Whary M (eds) Laboratory animal medicine. Elsevier, pp 1441–1496
- 28.Lawrence LJ, Casida JE. Pyrethroid toxicology: mouse intracerebral structure-toxicity relationships. Pestic Biochem Physiol. 1982;18:9–14. [Google Scholar]
- 29.Emond M, Faubert S, Perkins M. Social conflict reduction program for male mice. Contemp Top Lab Anim Sci. 2003;42(5):24–26. [PubMed] [Google Scholar]
- 30.D’Arbe M, Einstein R, Lavidis NA. Stressful animal housing conditions and their potential effect on sympathetic neurotransmission in mice. Am J Physiol Integr Comp Physiol. 2002;282:R1422–R1428. doi: 10.1152/ajpregu.00805.2000. [DOI] [PubMed] [Google Scholar]
- 31.Foltz C, Carbone L, DeLong D, Rollin BE, Van Loo P, Whitaker J, Wolff A. Considerations for determining optimal mouse caging density. Lab Anim (NY) 2007;36:40–49. doi: 10.1038/laban1107-40. [DOI] [PubMed] [Google Scholar]
- 32.Baer H. Long-term isolation stress and its effects on drug response in rodents. Lab Anim Sci. 1971;21:341–349. [PubMed] [Google Scholar]
- 33.Brain P. What does individual housing mean to a mouse? Life Sci. 1975;16:187–200. doi: 10.1016/0024-3205(75)90017-x. [DOI] [PubMed] [Google Scholar]
- 34.Kramer K, van de Weerd H, Mulder A, Van Heijningen C, Baumans V, Remie R, Voss H-P, van Zutphen BFM. Effect of conditioning on the increase of heart rate and body temperature provoked by handling in the mouse. Altern Lab Anim. 2004;32:177–181. doi: 10.1177/026119290403201s29. [DOI] [PubMed] [Google Scholar]
- 35.Obernier JA, Baldwin RL. Establishing an appropriate period of acclimatization following transportation of laboratory animals. ILAR J. 2006;47:364–369. doi: 10.1093/ilar.47.4.364. [DOI] [PubMed] [Google Scholar]
- 36.Albaum HG, Tepperman J, Bodansky O. The in vivo inactivation by cyanide of brain cytochrome oxidase and its effect on glycolysis and on the high energy phosphorus compounds in brain. J Biol Chem. 1946;164:45–51. [PubMed] [Google Scholar]
- 37.Vallero DA. Fundamentals of air pollution. 4. Amsterdam: Elsevier; 2007. pp. 1–15. [Google Scholar]
- 38.Kulkarni P, Baron PA, Willeke K. Aerosol measurement. Hoboken: Wiley; 2011. Introduction to aerosol characterization; pp. 1–13. [Google Scholar]
- 39.Owen M, Ensor D, Sparks L. Airborne particle sizes and sources found in indoor air. Atmos Environ Part A Gen Top. 1992;26:2149–2162. [Google Scholar]
- 40.Phalen RF, Hinds WC, John W, Lioy PJ, Lippmann M, McCawley MA, Raabe OG, Soderholm SC, Stuart BO. Rationale and recommendations for particle size-selective sampling in the workplace. Appl Ind Hyg. 1986;1:3–14. [Google Scholar]
- 41.Santiasih I, Hermana J. A review: the physicochemical characteristics of indoor particulate matters in relation to human health. ARPN J Eng Appl Sci. 2017;12:1813–1821. [Google Scholar]
- 42.Cole LM, Ruzo LO, Wood EJ, Casida JE. Pyrethroid metabolism: comparative fate in rats of tralomethrin, tralocythrin, deltamethrin, and (1R, alpha S)-cis-cypermethrin. J Agric Food Chem. 1982;30:631–636. doi: 10.1021/jf00112a003. [DOI] [PubMed] [Google Scholar]
- 43.Heudorf U, Angerer J. Metabolites of pyrethroid insecticides in urine specimens: current exposure in an urban population in Germany. Environ Health Perspect. 2001;109:213–217. doi: 10.1289/ehp.01109213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ahmed AE, Loh JP, Ghanayem B, Hussein GI. Studies on the mechanism of acetonitrile toxicity. I: whole body autoradiographic distribution and macromolecular interaction of 2-14C-acetonitrile in mice. Pharmacol Toxicol. 1992;70:322–330. doi: 10.1111/j.1600-0773.1992.tb00481.x. [DOI] [PubMed] [Google Scholar]