Abstract
In the present study, we employ a longitudinal design and a generalizability framework to examine the sources of variance in the diurnal rhythm of salivary α-amylase (sAA). The sample consisted of 122 first-year law students (55% male, mean age = 23.9 years), who collected five saliva samples on each of three consecutive days at each of five data collection waves. In total, over 6900 saliva samples were collected, which allowed us to examine the properties of diurnal variation in sAA in great detail. Systematic individual differences accounted for 15–29% of the variability in the awakening response and diurnal slope, and for 59–65% of the variation in overall daily levels (i.e., diurnal mean, area under the curve with respect to ground [AUCg]). Although less than 1% of the variation was due to differences between waves and between days, the generalizability analyses revealed that between 16 and 18% of the variance in the diurnal mean, slope and AUCg is due to person by wave interactions, indicating that individuals vary in their biological sensitivity to environmental influences. In sum, this study documents sufficient stability and variation in diurnal sAA to warrant future studies on the origins and consequences of alterations in the diurnal rhythm of sAA worthwhile, and proposes guidelines on obtaining reliable measures.
Keywords: salivary alpha-amylase, reliability, stability, sensitivity to context, diurnal, individual differences
Introduction
Within the past decade, there has been a significant increase in studies employing salivary α-amylase (sAA) as a surrogate marker of activity of the autonomic nervous system (ANS) in response to stress (Granger et al., 2007; Nater and Rohleder, 2009). Because saliva sampling is minimally invasive and can be performed in community- and home-based settings, developmental and psychological sciences embraced sAA as a measure in studies on the effects of stress and adversity on psychological adjustment and physical health. However, significant gaps in our knowledge remain regarding the nature, validity and reliability of sAA as a marker of ANS activity. The current study employs a longitudinal design (five data collection waves) and the diurnal collection of saliva samples on three days at each wave. Using a generalizability theory framework, we examine the reliability of several diurnal measures, and how much variation in the diurnal profile of sAA is due to systematic and stable individual differences, situational influences, person by situation interactions, and measurement error.
Salivary α-amylase is a digestive enzyme produced by the salivary glands. The secretion of sAA into oral fluids is largely controlled by the sympathetic nervous system (SNS), the fast acting component of the biological stress response that results in the release of catecholamines into the bloodstream (Chrousos and Gold, 1992). Stimulation of α- and β-adrenergic receptors in the salivary glands leads to the secretion of sAA into oral fluids (Nater and Rohleder, 2009), although concurrent activity of the parasympathetic branch of the ANS may augment the effects of the SNS (Proctor and Carpenter, 2007). Similar to other measures of the ANS, levels of sAA rise in response to physical and psychological stress, peak within 5–10 min after the onset of the stressor and quickly return to pre-task baseline (for reviews see Granger et al., 2007; Nater and Rohleder, 2009). This stress-related increase can be inhibited by administration of β-adrenergic agonists (Van Steegeren et al., 2006).
Salivary α-amylase follows a characteristic secretion pattern across the day: levels of sAA decrease in the first 30 minutes after awakening, which is followed by a steady increase throughout the day (e.g., Adam et al., 2011; Nater et al., 2007). Several studies have reported that individuals experiencing (chronic) stress or with a stress-related disease such as PTSD show alterations in their diurnal profile of sAA secretion. Some of these alterations concern the awakening response (Thoma et al., in press); others involve the change across the day (e.g., Rohleder et al., 2009; Strahler et al., 2010) or overall, daily levels of sAA (Nater et al., 2007). For example, in a longitudinal study on caregivers for patients who were being treated for a brain tumor, Rohleder and colleagues (2009) demonstrated that caregivers’ diurnal sAA rhythm became flatter and less pronounced during the course of treatment, indicating that caring for a family member with cancer is associated with significant distress.
Several studies have also suggested that sAA levels are relatively stable across time and conditions (e.g., El-Sheikh et al., 2008; Granger et al., 2006). For example, Wolf and colleagues (2008) collected saliva samples from children on two days, and noted that sAA levels showed a much higher stability than cortisol with correlations ranging between .48 and .65. Similarly, a large part of the variance in sAA levels in adults across the day could be explained by differences between rather than within individuals (Nater et al., 2007), and a recent behavioral genetic study showed that at least part of the variance in sAA is due to stable, heritable individual differences (Out et al., 2010).
However, a few recent reports have raised important concerns related to the validity and reliability of sAA as a measure of ANS activation. More specifically, measured levels of sAA appear to be influenced by salivary flow rate (Beltzer et al., 2010), specific instructions for sample collection (e.g., chewing on cotton-based devices; Bosch et al., 2011), and sAA levels differ depending on which type of oral fluid is collected (Harmon et al., 2008). For example, Harmon and colleagues showed that the diurnal profile is flatter and less pronounced when saliva is collected from the submandibular and sublingual gland areas.
Advancing our understanding of the determinants of individual differences in sAA has become a major focus of developmental, psychological and health sciences interested in the impact of stress on physical health and overall adjustment. Before this effort can advance, it is critical that we have a more comprehensive understanding of the nature of the variation in sAA. In the present study, we employ a longitudinal design and a generalizability theory framework to examine the diurnal rhythm of sAA in law students. Participants provided saliva samples collected on three consecutive days at each of five data collection waves varying in the amount of stress students experienced.
Variance component decomposition methods are used to determine the amount of variation around the diurnal profile of sAA that can be attributed to 1) systematic variation between persons, waves and days, 2) interactions between these components, 3) idiosyncratic responses/measurement error. Thus, if the diurnal profile of sAA is sensitive to environmental influences and stress (“state variance”), then differences between waves and days would explain a substantial amount of variance in diurnal sAA. In contrast, if sAA is as stable as suggested by a few studies (“trait variance”), then the variance in diurnal sAA would largely be explained by systematic individual differences. If however, methodological issues related to sample collection and salivary flow rate would obscure any meaningful variation in sAA, then the variance components for individuals, waves and days would be low, and for measurement error, high.
Estimates for each of these variance components are then used to calculate different forms of reliability described by Cranford and colleagues (2006), which may inform future cross-sectional and longitudinal studies on the number of days of saliva collection needed to achieve reliable measures of the diurnal profile of sAA. Finally, we address how many samples should be collected within one day to accurately and reliably assess the diurnal change.
Method
Sample
Over a period of five years, all members of the incoming law school class received recruitment packets during the summer before starting law school. If interested in the study, potential participants were instructed to return a signed informed consent and a questionnaire on their physical and mental health. Questions related to physical health focused on whether they had an infectious, autoimmune, blood or venereal disease; a form of cancer or tumor; an immune deficiency; or any other disease. They were also asked whether they took prescription medications (other than birth control pills) or had surgery involving anesthesia in the last three months. Questions related to mental health focused on hospitalization for a psychiatric problem, psychiatric medications taken in the last three months, or experiencing severe psychological distress in the last three months that interfered with work or other daily activities for two weeks or more. Participants were excluded if any of these conditions was true for them. Other inclusion criteria were related to their age (between 18 and 40 years) and substance use (no more than 2 drinks of alcohol every day, no use of recreational/intravenous drugs).
The total sample included 122 first-year students who completed questionnaires and collected saliva samples at five data collection waves. The sample was 55% male and 90% white, with the remainder African-American (7%), Asian American (1%) and multiple races (2%). Approximately one-quarter of the sample was married (23%). Mean age was 23.9 years (SD = 2.9, range = 21 – 37). Mean Law School Admissions Test score was 158.8 (SD = 4.4), which is representative of the law school as a whole (Segerstrom & Sephton, 2010). The study was approved by the Institutional Review Board for the Protection of Human Research Participants at the University of Kentucky.
Procedure
Participants were assessed at five data collection waves: August, at the beginning of the semester; October, mid-semester; December, during final exams; January, at the beginning of the semester when most first-semester grades were available; and February, after all grades were available and interviews for summer internships were starting. At each wave, participants collected in total 15 saliva samples over three consecutive days. They were instructed to collect saliva upon awakening, 30 minutes after waking, 12:00 pm, 5:00 pm, and 9:00 pm. Participants recorded the actual time of collection on the sample tube. They received $50 for their participation at each time point.
Measures
Salivary α-amylase.
Saliva samples were collected using Salivettes. Participants were instructed to place the swab on the tongue, between the cheek and lower teeth, or to move it around in the mouth, until the swab felt saturated. They were asked not to smoke, brush their teeth, use mouthwash, eat or drink anything 30 min prior to collecting saliva samples. In addition, participants were instructed to refrigerate samples at their earliest convenience; samples were then temporarily stored at −20°C until transported on dry ice to the analysis lab. All samples were stored frozen at –80°C until assayed for sAA. On the day of testing, all samples were centrifuged at 3000 rpm for 15 min to remove mucins. Samples were assayed using a commercially available kinetic reaction assay (Salimetrics, State College, PA). The assay employs a chromagenic substrate, 2-chloro-p-nitrophenol, linked to maltotriose. The enzymatic action of sAA on this substrate yields 2-chloro-p-nitrophenol, which can be spectrophotometrically measured at 405 nm using a standard laboratory plate reader. The amount of sAA activity is directly proportional to the increase (over a 2-min period) in absorbance at 405 nm. Results are computed in U/mL of sAA using the formula: [Absorbance difference per minute × total assay volume (328 ml) × dilution factor (200)]/ [millimolar absorptivity of 2-chloro-p-nitrophenol (12.9) × sample volume (.008 ml) × light path (.97)]. Intra- and interassay coefficients of variation were less than 10% and 15% (Chard, 1990).
Statistical approach
There were 539 waves of data collected out of a total possible 610 waves of data (122 participants * 5 waves; 88%). The majority of the sample participated in all 5 waves (68%); 17% completed 4 waves; 6%, 3 waves; 7%, 2 waves, and 2%, 1 wave. For each wave, there were between 282 and 305 days of saliva collected. Complete data for sAA at the five time points across the day were available for 85–95% of the days for wave 1, 78–80-% of the days for wave 2, 81–85% of the days for wave 3, 78–88% of the days for wave 4, and 67–78% of the days for wave 5. Primary reasons for missing data were missing samples, insufficient specimen volume (samples were first tested for salivary cortisol) and undetectable levels.
Data processing.
In total, 6939 saliva samples were analyzed for levels of sAA. Two values were higher than 1000 U/ml and excluded from the analyses. Data were also excluded if the reported collection time was greater than four SDs from the mean for that particular sample (37 values, or 0.5% of all samples). For the remaining 12:00 pm, 5:00 pm and 9:00 pm samples, between 81% and 85% of the samples were collected within a +1/−1 hr time frame. For 84 days (6% of the total number of days), the second sample was not collected between 15 and 45 minutes after the first (waking) sample; these days were therefore not included in the analyses of the awakening response. Finally, missing time values were imputed through the expectation- maximization (EM) algorithm for the estimation of missing values in SPSS, using the other time values within each wave. Waking and 30 min post-waking time values were only imputed if the subject had at least one valid waking or 30 min post-waking value during the current wave (5 out of 173 missing time values were not imputed as a result).
For each day, we calculated the awakening response, the diurnal slope, the diurnal mean and the area under the curve with respect to ground (AUCg). The awakening response was calculated by subtracting sAA levels for the waking sample from those for the 30 min post- waking sample. The slope of the diurnal change in sAA occurring from waking to the evening sample was calculated by fitting a linear regression line for each day, which predicted the sAA values from time since waking. For AUCg, as a measure of total sAA secretion during the day, we calculated the total area under the curve from the sAA measures in U/ml on the y-axis and the time between the sAA measures on the x-axis (Pruessner et al., 2003).
To investigate the number of samples per day that are needed to accurately characterize the diurnal slope, we calculated this measure using different combinations of samples, with the waking sample always included as the anchor (following Kraemer et al., 2006; see Table 1 for the different combinations). The waking sample instead of the 30-minute post-waking sample was selected as the anchor in order to avoid any effects of the awakening response on the diurnal slope. We compared the distributions of each of the diurnal slopes to the one based on four time points (waking, 12:00 am, 5:00 pm, 9:00 pm) and calculated Spearman rank correlations. For AUCg, similar analyses were conducted, with the waking and 30 min post- waking samples included in every computation so that the awakening response was always included, as well as at least one additional sample. All diurnal measures were calculated using the untransformed, raw sAA measures; log transformations were applied to the summary variables in order to correct for the skewed distributions.
Table 1.
Distributions of each combination of two-, three- and four-point diurnal slopes and Spearman rank correlations coefficients of each combination with the four-point diurnal slope
| Waking | 12:00 pm | 5:00 pm | 9:00 pm | 25th percentile | Median | 75th percentile | Mean Spearman rank correlation (range)a |
|---|---|---|---|---|---|---|---|
| X | X | X | X | −0.20 | 1.02 | 3.46 | |
| X | X | X | −0.05 | 1.71 | 5.34 | .67 (.48 - .80) | |
| X | X | X | −0.38 | 0.77 | 3.01 | .94 (.91 - .98) | |
| X | X | X | −0.08 | 1.24 | 3.82 | .94 (.86 - .97) | |
| X | X | −0.67 | 2.62 | 9.68 | .31 (.11 - .49) | ||
| X | X | −0.09 | 1.69 | 5.39 | .66 (.44 - .79) | ||
| X | X | −0.23 | 0.95 | 3.29 | .91 (.83 - 96) |
Note. For the distributions, N = 1202–1331 days.
Spearman rank correlations were calculated for each of the 15 collection days, n ranged between 59 and 93 individuals.
Generalizability analyses.
Generalizability theory (Brennan, 2001; Shavelson & Webb, 1991) is an extension of classical reliability theory, with the capacity to estimate multiple sources of variance (called facets) in a single analysis. For the current study, the first source of variability is referred to as the object of measurement, and arises from systematic differences between individuals in their diurnal sAA rhythm across all waves and days. The next two sources of variability arise from differences in the diurnal profile between waves and between days. Interactions between person and wave, person and day, and between wave and day may also contribute to the observed variance in diurnal rhythm: the effects of waves or days may be different across individuals, and the difference in diurnal sAA between days may be larger or smaller for some waves than for others. The final source of variance is the residual that includes idiosyncratic differences (person by wave by day interactions) and measurement error (the two are statistically indistinguishable).
A generalizability (G) study uses variance estimates from ANOVA or similar models to partition variance among these seven different sources. In the present study, the completely crossed and random facets of the G study were persons, waves, and days. SPSS VARCOMP was used to estimate the magnitude of each facet and of each interaction between facets, using the ANOVA procedure, which is considered to be a suitable and practical procedure for unbalanced designs (Brennan, 2001). The percentage of variance attributable to each facet and each interaction between facets was calculated by dividing each coefficient by the total.
Reliability estimation.
Variance estimates from the G study were used to estimate the four kinds of reliability described by Cranford and colleagues (2006) which are relevant for cross-sectional studies (1st and 2nd estimates) and for longitudinal studies (3rd and 4th estimates). The first concerns the reliability of diurnal sAA measurements assessed at a single, fixed wave. This estimate reflects the ability of the diurnal measure to differentiate between persons measured at a single, fixed wave, and thus under similar circumstances. The second estimate focuses on the reliability of the diurnal rhythm assessed at a single, random wave. This coefficient reflects the capacity of the diurnal measure to discriminate between individuals who were assessed at different timepoints, and thus under different circumstances. The third reliability coefficient focuses on the precision of the measurement of change in diurnal sAA. This estimate reflects whether the diurnal measure can reliably assess the change in diurnal sAA within individuals across measurement occasions. Finally, the fourth estimate focuses on relatively stable individual differences in diurnal sAA. This coefficient reflects the ability of sAA to detect differences between people that are present across measurement occasions.
Results
Diurnal measures of sAA
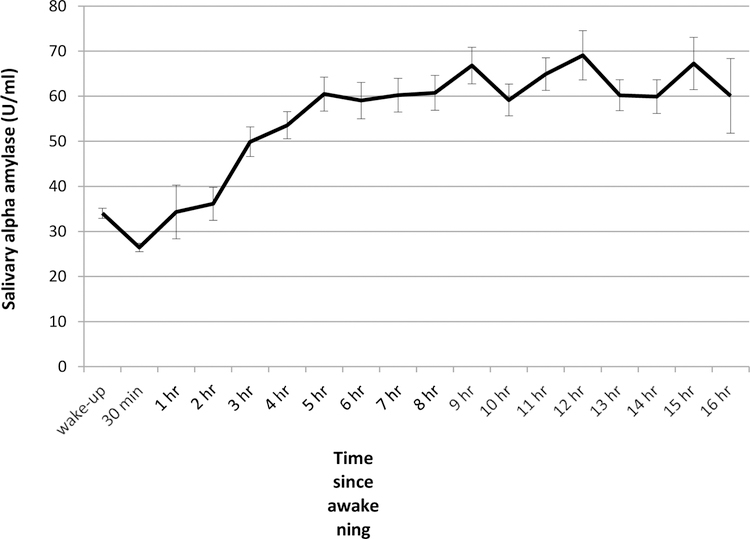
Figure 1 shows the levels of sAA at awakening and 30 minutes after, and for each hour since awakening, averaged across individuals, days and waves (the variability in waking time as well as some additional variability in the collection times allowed us to calculate mean sAA levels for every hour after awakening). There was a small decrease in levels of sAA occurring in the 30 minutes after awakening, followed by a sharp increase until 5 hours post-waking after which levels of sAA remained relatively stable.
Figure 1.
Diurnal course of salivary α-amylase (M ± SE) averaged across waves and days (based on original time points, not including the imputed time values).
In Table 1, the distributions and Spearman rank correlations are presented for the diurnal slope, calculated using different combinations of time points, with the waking sample as the anchor. Correlations were calculated separately for each of the 15 collection days. Results indicated that as long as the evening sample was included in the computation, each combination of time points resulted in a strong correlation with the diurnal slope based on all four time points, even when the slope was calculated using only the waking and evening sample (mean Spearman r = .91 – .94, p’s < .01, n = 59–93). Similar analyses were conducted for the AUCg, which was calculated using different combinations of time points. The morning samples were always included in order to capture the magnitude of the awakening response. Spearman rank correlations indicated that each of the AUCg variables was strongly associated with the AUCg based on all five time points (mean Spearman rho = .82 – .97, p’s < .01, n = 54–92).
Therefore, the diurnal slope was computed when the waking and evening samples were valid; when sAA levels were available for other time points, they were also included in the computation. The AUCg as well as the diurnal mean were calculated when both morning samples and at least one other sample were valid. In order to correct for the skewed distribution, we applied a log transformation to the diurnal slope, mean and AUCg, which resulted in normal distributions. Descriptive statistics for the raw, untransformed diurnal measures are provided in Table 2.
Table 2.
Descriptive statistics for the (untransformed) diurnal measures of sAA (U/ml)
| Diurnal measure | N | M | Median | SD |
|---|---|---|---|---|
| Awakening response | 1265 | −8.08 | −2.60 | 40.96 |
| Diurnal slope | 1331 | 2.15 | 0.97 | 5.12 |
| Diurnal mean | 1261 | 48.10 | 37.18 | 41.24 |
| AUCg | 1342 | 2530484.07 | 1919943.93 | 2294341.41 |
Variance component estimation
Generalizability analyses were conducted to investigate the amount of variance in the diurnal course of sAA that can be attributed to systematic differences between persons, waves, and days; interactions between these components; and idiosyncratic differences and measurement error. Results from these analyses can be found in Table 3. For the awakening response and diurnal slope, 28.5% and 15.0% of the variance was accounted for by the component ‘persons’, suggesting that individuals differed systematically in the magnitude of their awakening response and in the diurnal change in sAA. For the diurnal mean and the AUCg, these estimates were substantially higher, indicating that 65.2% and 59.2% of the variance in these measures could be attributed to differences between individuals.
Table 3.
Results from generalizability analyses of the sAA awakening response, diurnal slope, diurnal mean and AUCg
| Variance component | Variance due to: | Awakening response | Diurnal slope (log) | Diurnal mean (log) | AUCg (log) |
|---|---|---|---|---|---|
| Person | Differences between people | 28.5% | 15.0% | 65.2% | 59.2% |
| Wave | Differences between waves | 0.3% | 0.2% | 0.4% | 0.3% |
| Day | Differences between days | 0.2% | 0.5% | 0%a | 0.1% |
| Person * wave | Effects of wave differing across people | 5.9% | 17.2% | 15.9% | 17.7% |
| Person * day | Effects of day differing across people | 0.8% | 0%a | 0.6% | 0.4% |
| Wave * day | Effects of day differing across waves | 0%a | 0%a | 0.1% | 0% |
| Person * Wave * Day, error | Idiosyncratic differences and error | 64.3% | 67.1% | 17.8% | 22.3% |
Small negative variance set at 0.
For all of the diurnal measures, the variance component estimates for wave and day were small, suggesting that across all individuals, there were no systematic differences in the diurnal rhythm between waves and between days. Although there was no effect of wave per se, there were meaningful amounts of variance in the diurnal measures that could be attributed to individual differences in the effects of wave. Thus, the effects of wave on the diurnal rhythm of sAA was dependent on the individual. The variance components estimates furthermore show no evidence that the effects of day differed across people or across waves, and especially for the awakening response and the diurnal slope, a large portion of the variance was accounted for by idiosyncratic differences, which also includes measurement error.1
Reliability estimation
In Table 4 and 5, the estimates for the four forms of reliability of the diurnal measures are displayed. The results from the present design, with three days of data collection for each of the five waves, are in bold. The tables also present hypothetical reliabilities if data were collected at 7, 10, 14 and 21 days (Table 4) and at one, three and five days/waves (Table 5).
Table 4.
Results of reliability estimation for the sAA diurnal measures
| Awakening response | Diurnal slope (log) | Diurnal mean (log) | AUCg (log) | ||
|---|---|---|---|---|---|
|
Between people, same single wave | |||||
| Number of days | 3 | .57 | .40 | .92 | .89 |
| 7 | .76 | .61 | .96 | .95 | |
| 14 | .86 | .76 | .98 | .97 | |
| 21 | .90 | .82 | .99 | .98 | |
|
Between people, different single waves | |||||
| Number of days | 3 | .51 | .27 | .75 | .70 |
| 7 | .65 | .36 | .78 | .74 | |
| 14 | .73 | .40 | .79 | .75 | |
| 21 | .75 | .42 | .79 | .76 | |
|
Within people, change across waves | |||||
| Number of days | 3 | .22 | .44 | .73 | .70 |
| 7 | .39 | .64 | .86 | .85 | |
| 14 | .56 | .78 | .93 | .92 | |
| 21 | .66 | .84 | .95 | .94 | |
Note. Estimates in bold are for the design of the current study. Hypothetical reliabilities are presented for 7, 14, and 21 days.
Table 5.
Reliability estimation (between people, across all waves) for the sAA awakening response and diurnal slope
| Awakening response | Diurnal slope (log) | |||||
|---|---|---|---|---|---|---|
| Number of waves | 1 | 3 | 5 | 1 | 3 | 5 |
| # Days | ||||||
| 1 | .31 | .58 | .69 | .18 | .40 | .53 |
| 3 | .57 | .80 | .87 | .40 | .67 | .77 |
| 5 | .69 | .87 | .92 | .53 | .77 | .85 |
| Diurnal mean (log) | AUCg (log) | |||||
| Number of waves | 1 | 3 | 5 | 1 | 3 | 5 |
| # Days | ||||||
| 1 | .79 | .92 | .95 | .73 | .89 | .93 |
| 3 | .92 | .97 | .98 | .89 | .96 | .98 |
| 5 | .95 | .98 | .99 | .93 | .98 | .99 |
Note. Estimates in bold are for the design of the current study. Hypothetical reliabilities are presented for 1, 3 and 5 waves, and for 1, 3 and 5 days per wave.
For the awakening response and diurnal slope, assessed for all individuals at the same wave, the reliability estimates are considerably lower than for the diurnal mean and AUCg (see Table 4). Using a liberal value of adequate reliability (.60), the estimate for the awakening response approaches satisfactory reliability with at least three days of data collection, whereas seven days of saliva sampling are necessary to detect reliable individual differences in the diurnal slope. In other words, at least three and seven days of saliva sampling are necessary to reliably estimate the awakening response and diurnal slope when individuals are measured at substantively similar timepoints. In contrast, the reliability estimates were high for the diurnal mean (.92) and the AUCg (.88). Thus, three days of saliva collection are sufficient to reliably discriminate between individuals in their mean levels of sAA and in the total secretion of sAA across the day.
As can be expected, reliability estimates are lower when different persons were to be measured at different waves (see Table 4). For the diurnal mean and AUCg, reliability values are satisfactory when saliva is collected for three days (.75 and .70 respectively). In contrast, for the awakening response at least seven days of data collection is required whereas 21 days of saliva collection is not even sufficient to detect reliable individual differences in the diurnal slope. Thus, when participants are measured at substantively different time points, three days of data collection are sufficient for the diurnal mean and AUCg, seven days of data collection is required for the awakening response, and for the diurnal slope more than 21 days of saliva sampling is needed.
Similarly, reliability estimates for individual differences in the change of the diurnal rhythm across waves were adequate for the diurnal mean (.73) and the AUCg (.70), but lower for the diurnal slope (.44) and especially low for the awakening response (.22). At least seven days of data collection are needed to reliably assess changes in the diurnal slope across waves; for the awakening response, more than 14 days of saliva collection is necessary to achieve adequate reliability.
Finally, as displayed in Table 5, reliability estimates for the diurnal measures averaged across a fixed number of days and waves are clearly excellent for the present design, with three days of data collection for each of the five waves (.77 – .98). For the awakening response, between three and five days of data collection would be sufficient to reliably discriminate between individuals; for the diurnal slope at least three days of saliva collection for three waves is necessary; and for the diurnal mean and AUCg a single day of saliva collection is sufficient to detect reliable individual differences.
Discussion
In the current study, we examined the sources of variance in diurnal sAA for a large sample of first year law students who collected in total over 6900 saliva samples over the course of six months. To our knowledge, this is the most extensive study on sAA, and this provided us with a unique opportunity to examine the basic, “psychometric” characteristics of diurnal sAA. Generalizability analyses showed that systematic and stable individual differences accounted for a substantial amount of variance in the diurnal rhythm. There was also evidence for “person by wave interactions”, indicating that individuals differed in their sensitivity to context. With regard to an optimal research design for assessing diurnal sAA, the current study demonstrates that the collection of a morning (waking) and an evening sample is sufficient to accurately characterize the diurnal slope, whereas three samples are sufficient to measure the total secretion of sAA. The reliability estimates for the awakening response and diurnal slope indicated that multiple days of saliva sampling are required to reliably measure individual differences as well as the change over time. In contrast, reliability estimates were excellent for the diurnal mean and the AUC, which require only one to three days of saliva sampling per wave.
Consistent with previous studies on the stability of sAA across time and conditions (e.g., Granger et al., 2006; Nater et al., 2007; Out et al., 2010), the generalizability analyses provide further evidence for systematic and stable differences between individuals in their diurnal sAA over the course of six months. Approximately 15% and 29% of the variance in the diurnal slope and awakening response respectively could be accounted for by differences between people, and these estimates were higher for the diurnal mean (65%) and the AUC (59%). To our knowledge, this is one of the first studies on the long-term stability in sAA levels and diurnal profile in adults. Rohleder and colleagues (2009) demonstrated for a sample of young women that the diurnal slope was moderately stable across a period of two years (r = .45). Similarly, basal levels of sAA were stable for substantial time periods in infants, with coefficients ranging between .38 and .54 for infants assessed at 7, 15 and 24 months (Fortunato et al., 2009). Interestingly, infant cortisol levels were not significantly correlated across this time period (see also Wolf et al., 2008). Similarly, saliva samples collected for the current sample of law students were also assayed for cortisol (Segerstrom & Sephton, unpublished observations), and analyses revealed that systematic differences between people accounted for a smaller percentage of variance in diurnal cortisol compared to sAA, especially with regard to the diurnal mean.
These stable individual differences in the diurnal rhythm of sAA are noteworthy given the saliva collection procedure. Samples were collected for the purposes of cortisol analyses, and before any information was available on how collection duration, location and saliva flow affect measured levels of sAA. Recent studies have shown that these methodological factors can introduce substantial measurement error across sampling occasions (e.g., Beltzer et al., 2010; Bosch et al., 2011; Harmon et al., 2008). Participants were also not screened for oral health problems, which could influence levels of sAA (e.g., Marcotte & Lavoie, 1998; Rai et al., 2011; Scannapieco et al., 1993). Indeed, idiosyncratic differences and measurement error accounted for a large part of the variation in the awakening response (64%) and the diurnal slope (67%), and although the two sources are statistically indistinguishable, it is likely that the lack of standardization of the saliva collection procedure contributed to these high percentages. Estimates for individual differences were indeed higher for the diurnal mean and the AUC – the measures in which the amount of error variation was reduced by calculating the average or total secretion of sAA. Yet, it is noteworthy that the diurnal measures of sAA are as reliable and stable as for cortisol, in spite of the lack of standardization. It is to be expected that the stability and reliability of diurnal sAA will be even higher when more attention is given to the collection procedure and when oral health problems and salivary flow rate are controlled for.
Interestingly, less than 1% of the variation in any of the diurnal measures was due to differences between measurement occasions, suggesting that differences in situational factors between waves and days did not affect the diurnal profile for all individuals in the same way. This is surprising, as sAA was assessed during time periods that varied in the amount of stress (Roach et al., 2010). One explanation could be that the diurnal profile of sAA is relatively robust against momentary and moderate levels of stress, in contrast to momentary levels of sAA, which are highly sensitive to acute physical and psychological stress (e.g., Granger et al., 2007; Nater and Rohleder, 2009). Significant alterations in the diurnal rhythm in sAA may be more likely to occur in response to chronic stress or repeated stressful experiences, as has been shown for caregivers of patients with cancer (Rohleder et al., 2009), competitive ballroom dancers (Strahler et al., 2010) and war refugees (Thoma et al., in press). Nater and colleagues (2007) similarly reported that momentary stress, as assessed in hourly intervals, were not associated with differences in the diurnal course of sAA, whereas chronic stress was related to higher daily sAA levels.
An alternative explanation for the lack of situational influences on the diurnal rhythm focuses on variation between individuals in their biological sensitivity to context (Boyce & Ellis, 2005; Ellis et al., 2011). Indeed, the generalizability analyses revealed that between 16 and 18% of the variance in the diurnal mean, AUC and the diurnal slope is due to person by wave interactions, indicating that environmental changes and demands affect the diurnal profile of sAA, but those effects depend on the individual. It is now widely acknowledged that individuals vary in whether and how much they are affected by the environment, as described in the transactional/dual-risk model (Sameroff, 1983), the diathesis-stress model (Gottesman & Shields, 1967; Monroe & Simons, 1991) and in more recent models such as the differential susceptibility theory (Belsky, 2005; Ellis et al., 2011). The longitudinal design of our study and the generalizability analyses allowed us to actually separate the variance in diurnal sAA due to effects of wave that are similar for all people and effects of wave that are different across individuals, and thereby demonstrate that main effects of context are negligible when individual differences in sensitivity to context are taken into account.
A growing number of studies indicate that ANS activity/regulation is one of the endophenotypic markers that moderate the effect of both positive and negative environmental influences on physical health and psychological adjustment (for reviews see Belsky & Pluess, 2009; Boyce & Ellis, 2005; Ellis et al., 2011). The current study suggests that sAA and its diurnal rhythm may be such an endophenotypic marker reflecting individual differences in sensitivity to context, which opens up exciting new avenues for future studies. What are the neurobiological and developmental mechanisms that explain individual differences in the activation threshold and response magnitude of sAA? Which outcomes in terms of behavior, development, health or psychopathology are moderated by individual differences in diurnal sAA? For these studies, it is imperative to study changes in sAA in response to ecologically valid and developmentally salient contexts, which are meaningful for the specific sample being studied. Such “provocation ecologies” (Granger et al., in press) are most likely to elicit meaningful variation in physiological regulation, and thereby maximize the probability of finding significant relationships with the outcomes of interest.
The second aim of the current study is to inform future cross-sectional and longitudinal studies on a number of design issues: how many samples are needed to calculate the diurnal slope and how many days of sampling are minimally necessary to reliably assess diurnal sAA. Based on the results for these analyses, the following preliminary set of guidelines is proposed. First, only two time points are sufficient to adequately measure the change in sAA over the course of the day, with one sample collected after awakening and the second sample obtained at the end of the day. For the AUC, samples collected at waking and 30 min post-waking and a third sample collected in the (after)noon/evening are sufficient to capture the total secretion of sAA over the course of the day. Secondly, for cross-sectional studies interested in the awakening response and diurnal slope, the diurnal rhythm should be assessed on multiple days and if possible, under similar circumstances for all participants. For mean levels of sAA and the AUC, one day of sampling is sufficient to achieve reliable estimates. The reliability for the awakening response can furthermore be enhanced by increasing participants’ compliance with the saliva sampling timing. Finally, also for longitudinal studies interested in dynamic processes and change in diurnal sAA within persons over time, mean levels of sAA and AUC are the most reliable measures.
Thus, the current study suggests that for purposes of optimizing the reliability of the diurnal sAA measures, the number of samples collected within one day is less important than the number of days of sampling, at least with regard to the awakening response and diurnal slope. In this context, it is striking that few studies have been conducted on the diurnal rhythm of sAA, and all of these studies focused on including as many saliva samples as possible within a day (ranging between 3 and 15 samples), but only for one, two and occasionally three days. In addition, with a few exceptions (Harmon et al., 2008; Karibe et al., 2011), absorbent materials were used but saliva collection was not standardized in terms of stimulation, collection duration and location, and salivary flow rate was not controlled for. This lack of standardization and too few days of sampling may therefore account for some of the divergent findings that have been reported so far on the correlates of alterations in the diurnal rhythm.
In sum, our study provides evidence for stability in diurnal sAA across time and for individual differences in the sensitivity of the diurnal rhythm to environmental changes and demands. It should be noted that the results of the generalizability and reliability analyses are specific to the variance in diurnal sAA found in this relatively healthy sample of students. The size of the variance components may be different in clinical samples or high-risk samples consisting of individuals exposed to (chronic) stress and adversity (e.g., abuse, neglect, poverty). However, even for this sample and even with less optimal saliva collection procedures, our study provides evidence that diurnal sAA is as reliable and stable as salivary cortisol, captures individual differences in the susceptibility to environmental influences, and overall, confirms the value of sAA as a minimally invasive biomarker for future studies on the effects of stress on physical health and psychological adjustment.
Footnotes
To investigate whether part of the idiosyncratic/error variance in the awakening response can be reduced by excluding the days when the second sample was not collected close to 30 min after the first sample, we repeated the generalizability analysis using a more stringent criterion. We included only the days when the second sample was collected between 25 and 35 min after the waking sample (N = 987 days). The size of the variance components for person, wave, day, person by wave and wave by day did not change substantially (difference ranged between 0% and 0.7%). However, the error component decreased from 64.3% to 53.6% and the amount of variance that could be attributed to the person by day interaction increased from 0.8% to 10.3%. Thus, part of the error variance can be reduced by controlling participants’ compliance with the collection instructions, which is especially important for studies on individual differences in susceptibility to environmental changes and demands.
References
- Adam EK, Till Hoyt L, Granger DA, 2011. Diurnal alpha amylase in adolescents: Associations with puberty and momentary mood states. Biol. Psychol 88, 170–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belsky J, 2005. Differential susceptibility to rearing influences: an evolutionary hypothesis and some evidence In Ellis B & Bjorklund D. (Eds.), Origins of the social mind: evolutionary psychology and child development (pp.139–163). New York: Guilford Press. [Google Scholar]
- Belsky J, Pluess M, 2009. Beyond diathesis stress: differential susceptibility to environmental influences. Psychol. Bull 135, 885–908. [DOI] [PubMed] [Google Scholar]
- Beltzer EK, Fortunato CK, Guaderrama MM, Peckins MK, Garramone BM, Granger DA, 2010. Salivary flow and alpha amylase: collection technique, duration and oral fluid type. Physiol. Beh 10, 289–296. [DOI] [PubMed] [Google Scholar]
- Bosch JA, Veerman ECI, De Geus EJ, Proctor GB, 2011. Αlpha-amylase as a reliable and convenient measure of sympathetic activity: don’t start salivating just yet! Psychoneuroendocrinology 36, 449–453. [DOI] [PubMed] [Google Scholar]
- Boyce WT, Ellis BJ, 2005. Biological sensitivity to context: I. An evolutionary-developmental theory of the origins and functions of stress reactivity. Dev. Psychopathol 17, 271–301. [DOI] [PubMed] [Google Scholar]
- Brennan RL, 2001. Generalizability theory New York: Springer-Verlag. [Google Scholar]
- Chard T, 1990. An introduction to radioimmunoassay and related techniques Amsterdam: Elsevier. [Google Scholar]
- Chrousos GP, Gold PW, 1992. The concepts of stress and stress system disorders. JAMA 267, 1244–1252. [PubMed] [Google Scholar]
- Cranford JA, Shrout PE, Iida M, Rafaeli E, Yip T, Bolger N, 2006. A procedure for evaluating sensitivity to within-person change: can mood measures in diary studies detect change reliably? Pers. Soc. Psychol. Bull 32, 917–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis BJ, Boyce WT, Belsky J, Bakermans-Kranenburg MJ, Van IJzendoorn MH, 2011. Differential susceptibility to the environment: an evolutionary-neurodevelopmental theory. Dev. Psychopathol 23, 7–28. [DOI] [PubMed] [Google Scholar]
- El-Sheikh M, Erath SA, Buckhalt JA, Granger DA, Milze J, 2008. Cortisol and children’s adjustment: the moderating role of sympathetic nervous system activity. J. Abnorm. Child Psychol 36, 601–611. [DOI] [PubMed] [Google Scholar]
- Fortunato CK, Granger DA, Blair C, Kivlighan KK, Stifter CA, the FLP Investigators, 2009. Salivary alpha-amylase, affective behaviors, and emotion regulation: developmental differences across the early childhood years. Paper presented at the meeting of the Society for Research in Child Development, Denver, CO. [Google Scholar]
- Gottesman II, Shields J, 1967. A polygenic theory of schizophrenia. Proc. Natl. Acad. Sci. USA 58, 199–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granger DA, Fortunato CK, Beltzer E, Virag M, Bright M, Out D, in press Salivary bioscience and research on adolescence: an integrated perspective Journal of Adolescence [DOI] [PubMed] [Google Scholar]
- Granger DA, Kivlighan KT, Blair C, El-Sheikh M, Mize J, Lisonbee JA, Buckhalt JA, Stroud LR, Handwerger K, Schwartz EB, 2006. Integrating the measurement of salivary alpha-amylase into studies of child health, development and social relationship. J. Soc. Pers. Relat 23, 267–290. [Google Scholar]
- Granger DA, Kivlighan KT, El-Sheikh M, Gordis EB, Stroud LR, 2007. Salivary α-amylase in biobehavioral research. Ann. N.Y. Acad. Sc 1098, 122–144. [DOI] [PubMed] [Google Scholar]
- Harmon AG, Towe-Goodman NR, Fortunato CK, Granger DA, 2008. Differences in saliva collection location and disparities in baseline and diurnal rhythms of alpha-amylase: a preliminary note of caution. Horm. Beh 54, 592–596. [DOI] [PubMed] [Google Scholar]
- Karibe H, Aoyagi K, Koda A, Kawakami T, 2011. Characteristics of the salivary alpha amylase level in resting sublingual saliva as an index of psychological stress. Stress Health 27, 282–288. [Google Scholar]
- Kraemer HC, Giese-Davis J, Yutsis M, O’Hara R, Neri E, Gallagher-Thompson D, Taylor B, Spiegel D, 2006. Design decisions to optimize reliability of daytime cortisol slopes in an older population. Am. J. Geriatr. Psychiatry 14, 325–333. [DOI] [PubMed] [Google Scholar]
- Marcotte H, & Lavoie MC, 1998. Oral microbial ecology and the role of salivary immunoglobulin A. Microbiol. Mol. Biol. Rev 62, 71–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monroe SM, Simons AD, 1991. Diathesis-stress theories in the context of life stress research: implications for the depressive disorders. Psychol. Bull 110, 406–425. [DOI] [PubMed] [Google Scholar]
- Nater UM, Rohleder N, 2009. Salivary alpha-amylase as a non-invasive biomarker for the sympathetic nervous system: current state of research. Psychoneuroendocrinology 34, 486–496. [DOI] [PubMed] [Google Scholar]
- Nater UM, Rohleder N, Schlotz W, Ehlert U, Kirschbaum C, 2007. Determinants of the diurnal course of salivary alpha-amylase. Psychoneuroendocrinology 32, 392–401. [DOI] [PubMed] [Google Scholar]
- Out D, Bakermans-Kranenburg MJ, Granger DA, Cobbaert CM, Van IJzendoorn MH, 2011. State and trait variance in salivary α-amylase: a behavioral genetic study. Biol. Psychol 88, 147–154. [DOI] [PubMed] [Google Scholar]
- Proctor GB, Carpenter GH, 2007. Regulation of salivary gland function by autonomic nerves. Auton. Neurosci 133, 3–18. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Kirschbaum C, Meinlschmid G, Hellhammer DH, 2003. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology 28, 916–931. [DOI] [PubMed] [Google Scholar]
- Rai B, Kaur J, Anand SC, Jacobs R, 2011. Salivary stress markers, stress and periodontitis: a pilot study. J. Periodontol 82, 287–292. [DOI] [PubMed] [Google Scholar]
- Roach AR, Salt CE, Segerstrom SC, 2010. Generalizability of repetitive thought: Examining stability of thought content and process. Cognit. Ther. Res 34, 144–158. [Google Scholar]
- Rohleder N, Jungmann F, Kirschbaum C, Miller GE, 2009. Stability of salivary alpha-amylase diurnal profiles. Paper presented at the annual meeting of the Society for Psychophysiological Research, Berlin, Germany. [Google Scholar]
- Rohleder N, Marin TJ, Ma R, Miller GE, 2009. Biologic cost of caring for a cancer patient: dysregulation of pro- and anti-inflammatory signaling pathways. J. Clin. Oncol 18, 2909–2915. [DOI] [PubMed] [Google Scholar]
- Sameroff AJ, 1983. Developmental systems: contexts and evolution In Mussen P. (Ed.), Handbook of Child Psychology (Vol 1, pp. 237–294). New York: Wiley. [Google Scholar]
- Scannapieco FA, Torres G, Levine MJ, 1993. Salivary alpha-amylase: role in dental plaque and caries formation. Crit. Rev. Oral Biol. Med 4, 301–307. [DOI] [PubMed] [Google Scholar]
- Segerstrom SC, Sephton SE, 2010. Optimistic expectancies and cell-mediated immunity: The role of positive affect. Psychol. Sci 21, 448–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shavelson RJ, Webb NM, 1991. Generalizability theory: a primer London, UK: Sage Publications. [Google Scholar]
- Strahler J, Berndt C, Kirschbaum C, Rohleder N, 2010. Aging diurnal rhythms and chronic stress: distinct alteration of diurnal rhythmicity of salivary α-amylase and cortisol. Biol. Psychol 84, 248–256. [DOI] [PubMed] [Google Scholar]
- Thoma MV, Joksimovic L, Kirschbaum C, Wolf JM, Rohleder N, in press Altered salivary alpha-amylase awakening response in Bosnian War refugees with posttraumatic stress disorder. Psychoneuroendocrinology [DOI] [PubMed]
- Van Stegeren A, Rohleder N, Everaerd W, Wolf OT, 2006. Salivary alpha amylase as a marker for adrenergic activity during stress: effect of betablockade. Psychoneuroendocrinology 31, 137–141. [DOI] [PubMed] [Google Scholar]
- Wolf JM, Nicholls E, Chen E, 2008. Chronic stress, salivary cortisol, and alpha-amylase in children with asthma and healthy children. Biol. Psychol 78, 20–28. [DOI] [PubMed] [Google Scholar]