Abstract
Venovenous extracorporeal membrane oxygenation (ECMO) has emerged as an important tool in the treatment of acute respiratory distress syndrome (ARDS). The creation of portable ECMO circuits and pumps has supported the development of interfacility ECMO programs. Prior studies have demonstrated that ECMO transport is safe; however, long-term outcomes for these patients remain unknown. Retrospective analysis of our 5-year experience identified 58 patients transported on ECMO and 82 patients cannulated at our institution. When short-term (30 days) and long-term (1 year) outcomes were compared between these cohorts, there was no statistically significant difference in survival (P = 0.44 and 0.49). There were no deaths related to transport, and the rate of ECMO-related complications was similar between the groups. With established patient safety and similar long-term survival, ECMO transport is a feasible solution to provide access to ECMO for all communities.
Keywords: Acute respiratory distress syndrome, ECMO outcomes, ECMO transport, extracorporeal membrane oxygenation
Management of acute respiratory distress syndrome (ARDS) has dramatically evolved over the past two decades. Initial utilization of extracorporeal membrane oxygenation (ECMO) for ARDS yielded poor outcomes, with overall survival of 10%.1 With improvements in ECMO circuits and increased physician expertise, venovenous ECMO has demonstrated a survival benefit in patients with ARDS compared to traditional ventilation strategies alone.2–7 Further advancements in the circuit and pump size have led to interfacility transport of critically ill patients on ECMO.8–12 Experienced centers have established that ECMO transport is safe and can be performed with minimal transport-related complications.8–12 It remains unclear, however, whether these transported patients have long-term outcomes similar to those of patients cannulated at the primary ECMO center. We report our institutional experience with venovenous ECMO transport in patients with refractory respiratory failure.
METHODS
Our ECMO program was established in 2012. One year later, our mobile ECMO team was created and consisted of a cardiothoracic surgeon, perfusionist, and advanced paramedic. Referrals were discussed with the on-call cardiothoracic surgeon; ECMO was considered for patients with reversible causes of respiratory failure or lung transplant candidates with ARDS. Decisions regarding initiating ECMO followed Extracorporeal Life Support Organization guidelines for respiratory failure.13,14 Patients accepted for transfer were classified as either stable or unstable for transport. Patients who were too unstable for transport were cannulated at the referring hospital by our mobile ECMO team. Stable patients were transferred on mechanical ventilation, and cannulation was performed upon arrival to our institution if deemed appropriate. Patients were transported by ambulance, helicopter, or fixed-wing plane.
All patients requiring ECMO were treated in our cardiothoracic intensive care unit and managed by a multidisciplinary team including a surgical intensivist, perfusionist, cardiothoracic surgeon, and specialized nurse practitioners. Anticoagulation, ventilator settings, and transfusion parameters followed Extracorporeal Life Support Organization guidelines.13,14 The decision to separate from ECMO was based on improved gas exchange, pulmonary compliance, and radiographic appearance. Most patients were decannulated bedside in the intensive care unit.
Our institutional review board approved a retrospective review of the ECMO database from June 2012 to April 2017 for patients requiring ECMO for refractory respiratory failure. Patients were excluded from analysis if conversion to venoarterial ECMO support was performed during their hospitalization. Information on demographics and ECMO-related complications was prospectively collected. Short- and long-term survival was verified by directly contacting patients by phone or by outpatient follow-up documentation in our electronic medical record. Patients without direct contact were searched in the Social Security Death Index database. This database has been validated as an accurate indicator of death outcomes for patients in the USA who have been lost to follow-up.15
Descriptive statistics on patient demographics and clinical measures were summarized by mean and standard deviation or median and interquartile range (IQR) for continuous variables. Categorical variables were summarized by percentage. Student’s t test, chi-square test, and Fisher’s exact test were used to compare these characteristics by ECMO group for continuous and categorical data, respectively. Kaplan-Meier estimation was used to generate survival curves, and log-rank tests were used to compare survival by ECMO group. Data were analyzed in SAS 9.4 and R version 3.5.0 statistical software, with two-tailed P values <0.05 considered statistically significant.
RESULTS
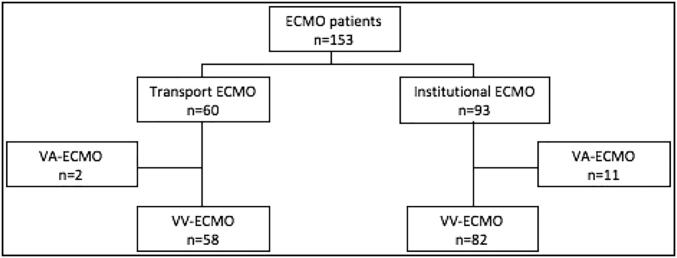
During the study period, 153 patients required venovenous ECMO for respiratory failure secondary to ARDS (Figure 1). Ninety-three patients were from our institution and 60 patients were transported on ECMO. Thirteen patients were excluded from analysis due to venovenous-to-venoarterial ECMO conversion during their hospitalization, 11 within the institutional group and 2 within the transport group. No patients were excluded for incomplete data.
Figure 1.
Study flowchart. ECMO indicates extracorporeal membrane oxygenation.
Demographic and clinical characteristics of the study population are displayed in Table 1. There were no significant differences between the institutional and ECMO transport cohorts for patient age, gender, or race. Surrogate markers for severity of respiratory failure assessed included the ratio of arterial oxygen partial pressure to fractional inspired oxygen (PaO2/FiO2) at the time of cannulation, Respiratory ECMO Survival Prediction score, and Acute Physiology and Chronic Health Evaluation III score (Table 1). The etiologies of respiratory failure and ECMO cannulation strategies are listed in Table 1. Within the institutional group, there was a similar distribution of the cannulation strategies. None of the transported patients was initially cannulated with the Avalon Bi-Caval Dual Lumen at the referring facility. Three patients were converted to the Avalon cannula during their ECMO run, two within the transport cohort and one within the institutional cohort.
Table 1.
Demographic and clinical characteristics of patients receiving ECMO
| Variable | ECMO group |
P value | ||
|---|---|---|---|---|
| All (n = 140) | Institutional (n = 82) | Transport (n = 58) | ||
| Age (years) | 46.8 ± 14.5 | 47.9 ± 14.1 | 45.1 ± 15.0 | 0.26 |
| Men | 89 (64%) | 51 (62%) | 38 (66%) | 0.68 |
| Race | 0.09 | |||
| Asian | 3 (2%) | 0 (0%) | 3 (5%) | |
| Black | 22 (16%) | 14 (17%) | 8 (14%) | |
| Hispanic | 13 (9%) | 4 (5%) | 9 (16%) | |
| White | 78 (56%) | 40 (49%) | 38 (66%) | |
| Not reported | 24 (17%) | 24 (30%) | 0 (0%) | |
| PaO2/FiO2 prior to ECMO (mean) | 77.7 ± 52.6 | 87.1 ± 63.5 | 64.5 ± 26.7 | 0.02 |
| RESP score (median) | 1.0 (−2 to 3) | 0 (−2 to 2) | 2.0 (−1 to 4) | 0.004 |
| APACHE III score (median) | 66.0 (51–79) | 68.5 (54–84) | 61.5 (49–76) | 0.02 |
| Vent days prior to ECMO | 0.45 | |||
| 0–2 | 74 (53%) | 47 (57%) | 27 (47%) | |
| 3–7 | 41 (30%) | 22 (27%) | 19 (33%) | |
| >7 | 25 (18%) | 13 (16%) | 12 (21%) | |
| Etiology of ARDS | <0.0001 | |||
| Acute graft dysfunction | 11 (8%) | 11 (13%) | 0 (0%) | 0.0027 |
| Aspiration | 11 (8%) | 2 (2%) | 9 (16%) | 0.008 |
| Influenza | 26 (19%) | 10 (12%) | 16 (28%) | 0.02 |
| Pneumonia | 30 (21%) | 14 (17%) | 16 (28%) | 0.15 |
| Postsurgical | 19 (14%) | 14 (15%) | 5 (9%) | 0.21 |
| Airway | 9 (6%) | 9 (11%) | 0 (0%) | 0.01 |
| Trauma | 10 (7%) | 8 (10%) | 2 (3%) | 0.19 |
| Bridge to transplant | 13 (9%) | 10 (12%) | 3 (5%) | 0.24 |
| Other | 11 (8%) | 4 (5%) | 7 (12%) | 0.20 |
| ECMO cannulation site | 0.0004 | |||
| Femoral–femoral | 33 (24%) | 15 (18%) | 18 (32%) | 0.07 |
| Femoral–internal jugular | 39 (28%) | 22 (27%) | 17 (30%) | 0.70 |
| Femoral–subclavian | 46 (33%) | 24 (30%) | 22 (39%) | 0.25 |
| Avalon Bi-Caval Dual Lumen | 21 (15%) | 21 (26%) | 0 (0%) | <0.0001 |
APACHE indicates Acute Physiology and Chronic Health Evaluation; ARDS, acute respiratory distress syndrome; ECMO, extracorporeal membrane oxygenation; PaO2/FiO2, ratio of arterial oxygen partial pressure to fractional inspired oxygen; RESP, Respiratory ECMO Survival Prediction.
The duration of mechanical ventilation prior to ECMO cannulation was not significantly different between the two groups (P = 0.45). Overall, there was a trend toward early initiation of ECMO, with more than half of all patients cannulated within 48 hours of intubation. Fewer patients were initiated on ECMO after 7 days of mechanical ventilator support in both the transport and institutional groups.
Transported patients were referred from 27 different facilities within five surrounding states; the median distance traveled on ECMO was 28.8 miles (IQR = 17.5–35.7), and mean transport distance was 70 miles (±119 miles). The longest distance traveled on ECMO in our series was 636 miles. Most patients were transported by ambulance; only 22% were transferred by helicopter or fixed-wing plane (Table 2). There were two complications related to transportation. One involved failure of the portable ECMO pump, requiring manual circuit operation until arrival to our institution. The other complication was an ECMO cannula displacement in a morbidly obese patient. The patient was supported with increased ventilator support and manual compression of the cannula site until recannulation was possible. There were no deaths from transport-related complications.
Table 2.
Transport characteristics for 58 patients transferred for ECMO
| Variable | N (%) |
|---|---|
| Transport distance (miles) | |
| 0–10 | 2 (4%) |
| 10–25 | 23 (40%) |
| 25–50 | 23 (40%) |
| 50–100 | 0 (0%) |
| 100+ | 9 (16%) |
| Mode of transport | |
| Air (fixed-wing plane or helicopter) | 13 (22%) |
| Ground | 45 (78%) |
ECMO indicates extracorporeal membrane oxygenation.
Secondary endpoints included length of stay, ECMO duration, and complications; these are displayed in Table 3. No statistically significant difference was seen between the two groups in either intensive care unit length of stay (P = 0.27) or hospital length of stay (P = 0.47). The duration of ECMO support was different between the cohorts; median extracorporeal life support run was 5 days (IQR = 3–10) for institutional patients and 10 days (IQR = 6–14) for transported patients (P < 0.0001). ECMO-related complications such as neurologic injury, cannula site infection, and limb ischemia were similar between the groups (Table 3).
Table 3.
Outcomes of patients receiving ECMO
| Variable | ECMO group |
P value | ||
|---|---|---|---|---|
| All (n = 140) | Institutional (n = 82) | Transport (n = 58) | ||
| ICU length of stay (days) | 18 (11–27) | 17 (8–26) | 19.5 (13–32) | 0.27 |
| Hospital length of stay (days) | 21 (14–35.5) | 21 (13–33) | 22 (14–37) | 0.47 |
| Duration of ECMO (days) | 7 (3–13) | 5 (3–10) | 10 (6–14) | <0.0001 |
| ECMO-related complications | ||||
| Brain injury | 7 (5%) | 4 (5%) | 3 (5%) | 0.55 |
| Acute renal failure | 52 (37%) | 30 (37%) | 22 (38%) | 0.87 |
| Dialysis on ECMO | 51 (36%) | 29 (35%) | 22 (38%) | 0.76 |
| ECMO cannula site infection | 5 (4%) | 2 (2%) | 3 (5%) | 0.64 |
| Limb ischemia | 4 (3%) | 2 (2%) | 2 (3%) | 0.99 |
ECMO indicates extracorporeal membrane oxygenation; ICU, intensive care unit.
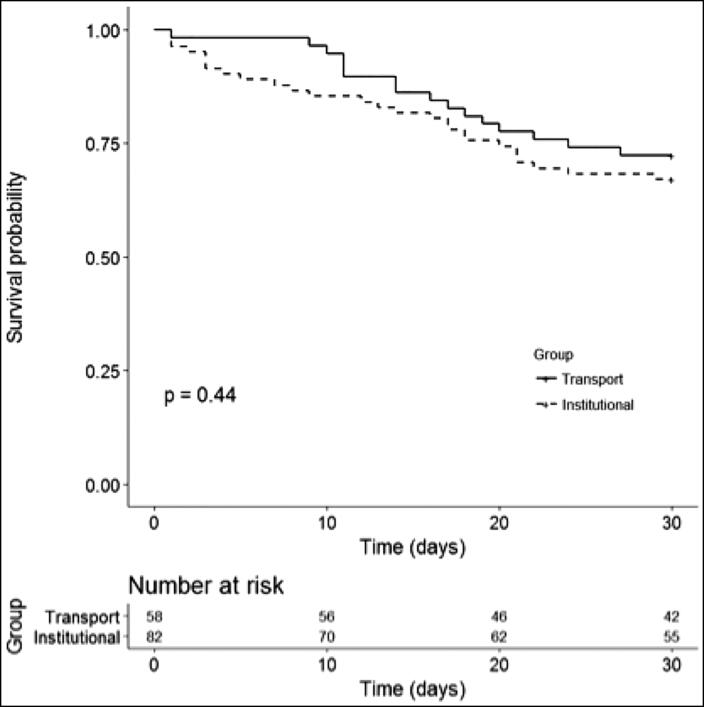
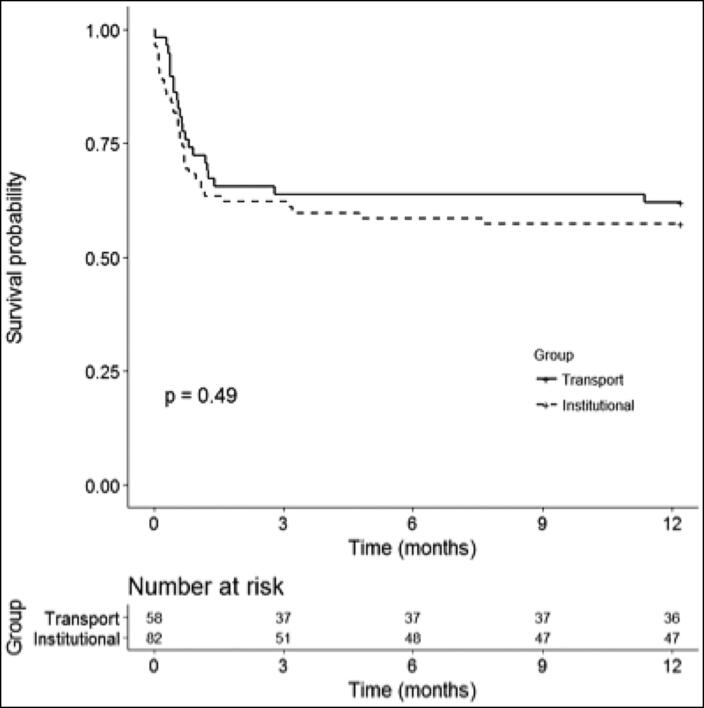
Primary endpoints included short-term (30 days) and long-term (1 year) survival (Figures 2 and 3). Short-term outcomes were similar between the institutional and transport ECMO patients (67% vs 72% survival, P = 0.44) At 1 year, survival decreased to 57% in the institutional cohort and 62% in the transport cohort; no statistically significant difference was observed (P = 0.49). Most of the observed deaths in our series occurred within the first 60 days.
Figure 2.
Short-term outcomes (30-day survival) in the transport vs institutional group.
Figure 3.
Long-term outcomes (1-year survival) in the transport vs institutional group.
DISCUSSION
Venovenous ECMO continues to be a valuable tool in the treatment of refractory respiratory failure.1–12 With the expansion of existing ECMO programs to include ECMO transport, access to this life-saving technology has improved substantially.16 Patient safety has been established by large-volume ECMO centers, and few transport-related complications have been reported.8–12 Limitations of these initial studies were the focus on short-term outcomes only and the lack of a control group for comparison. Recently, Bréchot et al investigated the intensive care unit and 30-day mortality of patients cannulated in the field and at their ECMO center.17 The main shortcoming of their study was the short distance traveled (9.3 miles) for patient transport.17 They reported no significant difference in patient outcomes between the two groups, although long-term outcomes were not addressed.17 These findings support our conclusion that ECMO transport does not impact patient survival.
No published studies have investigated the long-term outcomes for patients transported on ECMO. Our series is the first to establish the 1-year survival of ECMO transport patients and confirm that their outcomes are similar to those of patients cannulated at our center (62% vs 57%; P = 0.49). We believe that long-term survival is a reflection of successful pulmonary recovery. We hypothesize that once decannulated, all patients should have similar survival regardless of their location of cannulation and severity of disease at presentation. Most deaths in our series occurred within the first 60 days of ECMO. The majority of the early deaths involved withdrawal of ECMO support due to failure to show clinical signs of improvement. Late deaths in our series were unrelated to ECMO and were related to readmissions for infections/septic shock or gastrointestinal bleeding.
Despite similar lengths of stay, ECMO-related complications, and survival, the duration of ECMO was significantly different between the two cohorts (Table 3). Patients are only separated from ECMO when they demonstrate pulmonary recovery and therefore the duration of ECMO support is a reflection of the severity of ARDS. Other established surrogate markers of respiratory failure such as PaO2/FiO2 ratio, Acute Physiology and Chronic Health Evaluation score, and Respiratory ECMO Survival Prediction score provide a static assessment at the time of cannulation and are not true markers of ongoing pathophysiology. Duration of ECMO support is a continuous measurement that represents the dynamic nature of ARDS recovery. We believe that ECMO duration can be influenced by the timing of cannulation. Patients cannulated early experience less ventilator-induced lung injury as a secondary insult and therefore may have accelerated pulmonary recovery.18
There was a tendency in both the institutional and transport ECMO groups to have early initiation of ECMO, with 57% and 47% of patients cannulated in the first 48 hours, respectively (Table 1). The transport cohort did have a higher proportion of patients cannulated in 2 to 7 days and >7 days, likely reflecting delay in referral, potentially from trials of alternative ventilation strategies such as prone positioning or high-frequency oscillatory ventilation. These differences, however, did not reach statistical significance (P = 0.45). Our prior research established that cannulation timing can impact patient survival in influenza-induced ARDS.18 Patient survival decreased from 80% when cannulated within 48 hours to 60% (3–6 days) and 16.7% (>7 days) in the later cannulation cohorts. Currently, our group favors early cannulation when feasible for any etiology of ARDS, not just influenza associated.
The etiology of ARDS was notably different between the cohorts (Table 1). There were more lung transplant patients in the institutional group. These patients were either bridge to transplant candidates or developed primary graft dysfunction after transplantation. Of the 13 transplant candidates placed on ECMO, only 4 were successfully bridged to transplantation, leading to a high mortality within this subset of patients. Another notable group within the institutional cohort was patients cannulated electively as part of the surgical plan (e.g., complex airway resections and therapeutic whole-lung lavage) who had no comparable transport cohort. Alternatively, in the transport group, there was an observed preference toward patients with influenza, aspiration, and bacterial pneumonia. This likely reflects referral patterns from the surrounding community or perhaps selection bias from the accepting physician.
Our patient series is from a single institution and a relatively young ECMO center, which limits the ability to generalize our findings. The etiology/indications for ECMO varied between the two cohorts, but this reflects referral patterns from within the institution and surrounding community. All accepted transfers are screened by the on-call surgeon, and a second surgeon confirms the indication for ECMO prior to dispatching our mobile ECMO team. The second surgeon is designed to minimize selection bias, but because these patients do utilize a large amount of financial and personnel resources, it is possible that bias persists. All demographic characteristics, complications, and survival to discharge were recorded in the ECMO database prospectively; comorbidities were not captured. Conversely, the 30-day and 1-year survival data were collected retrospectively and were therefore more susceptible to bias.
As interest in ECMO grows and centers become more experienced, we anticipate that more institutions will integrate interfacility ECMO transport into their programs. Now with data that demonstrate a long-term survival benefit for patients transported on ECMO, we advocate for the centralization of ECMO care by adopting a “hub and spoke” model. By aggregating resources and experience to large ECMO centers with teams that can safely transfer patients from the surrounding community, we can continue to improve patient survival.
ACKNOWLEDGMENTS
The authors thank the members of our cardiothoracic intensive care unit, including nurses, perfusionists, and intensivists of North Texas Critical Care, for their assistance in caring for these patients.
References
- 1.Zapol WM, Snider MT, Hill DJ, et al. Extracorporeal membrane oxygenation in severe acute respiratory failure: a randomized prospective study. JAMA. 1979;242:2193–2196. doi: 10.1001/jama.1979.03300200023016. [DOI] [PubMed] [Google Scholar]
- 2.Peek GJ, Mugford M, Tiruvoipati R, et al. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomized controlled trial. Lancet. 2009;374:1351–1363. doi: 10.1016/S0140-6736(09)61069-2. [DOI] [PubMed] [Google Scholar]
- 3.Brodie D, Bacchetta M. Extracorporeal membrane oxygenation for ARDS in adults. N Engl J Med. 2011;365:1905–1914. doi: 10.1056/NEJMct1103720. [DOI] [PubMed] [Google Scholar]
- 4.Davies A, Jones D, Bailey M, et al. Extracorporeal membrane oxygenation for 2009 influenza A(H1N1) acute respiratory distress syndrome. JAMA. 2009;302:1888–1895. [DOI] [PubMed] [Google Scholar]
- 5.Peek GJ, Moore HM, Moore N, et al. Extracorporeal membrane oxygenation for adult respiratory failure. Chest. 1997;112:759–764. doi: 10.1378/chest.112.3.759. [DOI] [PubMed] [Google Scholar]
- 6.Squiers JJ, Lima B, DiMaio JM. Contemporary extracorporeal membrane oxygenation therapy in adults: fundamental principles and systematic review of the evidence. J Thorac Cardiovasc Surg. 2016;152:20–32. doi: 10.1016/j.jtcvs.2016.02.067. [DOI] [PubMed] [Google Scholar]
- 7.Hemmila MR, Rowe SA, Boules TN, et al. Extracorporeal life support for severe acute respiratory distress syndrome in adults. Ann Surg. 2004;240:595–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Foley DS, Pranikoff T, Younger JG, et al. A review of 100 patients transported on extracorporeal life support. ASAIO J. 2002;48:612–619. doi: 10.1097/00002480-200211000-00007. [DOI] [PubMed] [Google Scholar]
- 9.Bryner B, Cooley E, Copenhaver W, et al. Two decades’ experience with interfacility transport on extracorporeal membrane oxygenation. Ann Thorac Surg. 2014;98:1363–1370. doi: 10.1016/j.athoracsur.2014.06.025. [DOI] [PubMed] [Google Scholar]
- 10.Biscotti M, Agerstrand C, Abrams D, et al. One hundred transports on extracorporeal support to an extracorporeal membrane oxygenation center. Ann Thorac Surg. 2015;100:34–40. doi: 10.1016/j.athoracsur.2015.02.037. [DOI] [PubMed] [Google Scholar]
- 11.Javidfar J, Brodie D, Takayama H, et al. Safe transport of critically ill adult patients on extracorporeal membrane oxygenation support to a regional extracorporeal membrane oxygenation center. ASAIO J. 2011;57:421–425. doi: 10.1097/MAT.0b013e3182238b55. [DOI] [PubMed] [Google Scholar]
- 12.Philipp A, Arlt M, Amann M, et al. First experience with the ultra compact mobile extracorporeal membrane oxygenation system Cardiohelp in interhospital transport. Interactive Cardiovasc Thorac Surg. 2011;12:978–981. doi: 10.1510/icvts.2010.264630. [DOI] [PubMed] [Google Scholar]
- 13.Extracorporeal Life Support Organization . Guidelines for respiratory adult failure. https://www.elso.org/Portals/0/IGD/Archive/FileManager/989d4d4d14cusersshyerdocumentselsoguidelinesforadultrespiratoryfailure1.3.pdf. Published December 2013. Accessed September 23, 2018.
- 14.Extracorporeal Life Support Organization . H1N1 specific supplements to the ELSO general guidelines. https://www.elso.org/Portals/0/Files/elso%20h1n1%20specific%20guidelines.pdf. Published November 2009. Accessed September 23, 2018.
- 15.Quinn J, Kramer N, McDermott D. Validation of the Social Security Death Index (SSDI): an important readily available outcomes database for researchers. West J Emerg Med. 2008;9:6–8. [PMC free article] [PubMed] [Google Scholar]
- 16.Extracorporeal Life Support Organization . Registry statistics. https://www.elso.org/Registry/Statistics.aspx. Accessed September 23, 2018.
- 17.Bréchot N, Mastroianni C, Schmidt M, et al. Retrieval of severe acute respiratory failure patients on extracorporeal membrane oxygenation: any impact on their outcomes? J Thorac Cardiovasc Surg. 2018;155:1621–1629. doi: 10.1016/j.jtcvs.2017.10.084. [DOI] [PubMed] [Google Scholar]
- 18.Steimer DA, Hernandez O, Mason DP, et al. Timing of ECMO initiation impacts survival in influenza-associated ARDS. Thorac Cardiovasc Surg. 2019;67:212–215. [DOI] [PubMed] [Google Scholar]