Abstract
Background
Approximately 15% of community-prescribed antibiotics are used in treating urinary tract infections (UTIs). Increase in antibiotic resistance necessitates considering alternatives.
Aim
To assess the impact of increased fluid intake in individuals at risk for UTIs, for impact on UTI recurrence (primary outcome), antimicrobial use, and UTI symptoms (secondary outcomes).
Design and setting
A systematic review.
Method
The authors searched PubMed, Cochrane CENTRAL, EMBASE, two trial registries, and conducted forward and backward citation searches of included studies in January 2019. Randomised controlled trials of individuals at risk for UTIs were included; comparisons with antimicrobials were excluded. Different time-points (≤6 months and 12 months) were compared for the primary outcome. Risk of bias was assessed using Cochrane Risk of Bias tool. Meta-analyses were undertaken where ≥3 studies reported the same outcome.
Results
Eight studies were included; seven were meta-analysed. There was a statistically non-significant reduction in the number of patients with any UTI recurrence in the increased fluid intake group compared with control after 12 months (odds ratio [OR] 0.39, 95% confidence interval [CI] = 0.15 to 1.03, P = 0.06); reduction was significant at ≤6 months (OR 0.13, 95% CI = 0.07 to 0.25, P<0.001). Excluding studies with low volume of fluid (<200 ml) significantly favoured increased fluid intake (OR 0.25, 95% CI = 0.11 to 0.59, P = 0.001). Increased fluid intake reduced the overall rate of all recurrent UTIs (rate ratio [RR] 0.46, 95% CI = 0.40 to 0.54, P<0.001); there was no difference in antimicrobial use (OR 0.52, 95% CI = 0.25 to 1.07, P = 0.08). Paucity of data precluded meta-analysing symptoms.
Conclusion
Given the minimal potential for harm, patients with recurrent UTIs could be advised to drink more fluids to reduce recurrent UTIs. Further research is warranted to establish the optimal volume and type of increased fluid.
Keywords: antibacterial agents; drinking; drug resistance, microbial; fluid therapy; systematic review; urinary tract infections
INTRODUCTION
Urinary tract infections (UTIs) are very common, with 50–60% of females experiencing a UTI at least once in their lifetime,1 and approximately 15% of all community-prescribed antibiotics are used to treat UTIs. They impose significant clinical and financial burdens. In the US, for example, uncomplicated UTIs are responsible for >7 million physician visits annually, and the cost of the antibiotics alone is approximately 1.6 billion USD every year.2 The widespread increase in antibiotic resistance means that antibiotic use should be reduced.3
There is increasing interest in effective non-antibiotic alternative treatments for infections. Candidates for alternative treatments of UTIs include non-steroidal anti-inflammatory drugs (NSAIDs),4,5 and drinking more fluids. The rationale for the latter derives from the observation that dehydration appears to increase the risk of UTI. Both animal models and observational studies suggest better hydration may reduce risk, though the mechanisms are unclear.6
A recent 12-month randomised controlled trial (RCT)7 assessed the impact of increasing usual fluid intake by an additional 1.5 L of water daily (in the water group), and comparing it with no additional fluid intake (control group) in 140 females with recurrent UTIs. The trial showed that drinking an extra 1.5 L of water daily reduced recurrent UTIs. The present authors searched for, but did not find, systematic reviews examining the impact of increased fluids on recurrent UTIs; one non-systematic review that focused on the mechanisms by which low fluid intake may impact UTIs was identified.8 Therefore, the present systematic review was undertaken to test whether the finding of the RCT was supported by other trials.
METHOD
Inclusion criteria
According to an a priori protocol, the authors included RCTs of individuals at risk for UTIs (as defined by each individual trial’s inclusion criteria), of any age and sex, who were ambulatory, that is, non-catheterised. RCTs of interventions involving increased fluids, for example, water, D-mannose dissolved in fluid, or juice, were included. RCTs were excluded if the controls used antimicrobials, or cranberry in non-liquid form (tablet, powder, supplement, or fruit).
The primary outcome was UTIs, and secondary outcomes were antimicrobial use, and UTI symptoms, for example, burning, dysuria, urgency, frequency, and nocturia.
Searches to identify studies
A search strategy was developed by conducting a word frequency analysis on an initial set of seven potentially relevant articles using the Systematic Review Accelerator (SRA) — Word Frequency Analyser9 to determine key terms. These were expanded to create an initial search in PubMed using a combination of keywords and subject terms (MeSH terms), for example, ‘Urinary Tract Infections’ AND ‘Prevention’ AND ‘Intervention’ AND ‘Recurrence’ AND ‘Randomized controlled trial’ NOT ‘Catheters’ NOT ‘Cranberry’. This was combined with a ‘PICO (participants, interventions, comparisons, and outcomes) in title’ screening technique, which was modified for use in this search.10
How this fits in
| GPs often advise patients with urinary tract infections (UTIs) to drink more fluids but until now there has been no systematic evaluation of the evidence to support this advice. A meta-analysis of seven studies suggests that increased fluids decrease the number of patients with UTIs (significantly at ≤6 months, non-significantly at 12 months) and the overall rate of recurrent UTIs. Given the minimal potential for harm, patients with recurrent UTIs could be advised to drink more fluids to reduce UTIs but evidence is needed to establish the optimal volume of additional fluid intake and type of fluid. |
The search strategy was converted using the Polyglot Search Translator (PST)11 to rerun in Cochrane CENTRAL and EMBASE (see Supplementary Box S1 for details of the search strategy). All database searches were run on 21 January 2019. No language or date restrictions were applied. However, publications that were published in full, or as abstract only, for example, conference abstracts, were only included if there was a corresponding clinical trial registry record containing additional information.
The database search was converted to search for ongoing trials in two clinical trial registries: ClinicalTrials.gov and the World Health Organization’s International Clinical Trials Registry Platform (ICTRP) on 24 January 2019.
A backwards and forwards citation search of the included studies was also undertaken using the Scopus database on 24 January 2019.
Study selection and screening
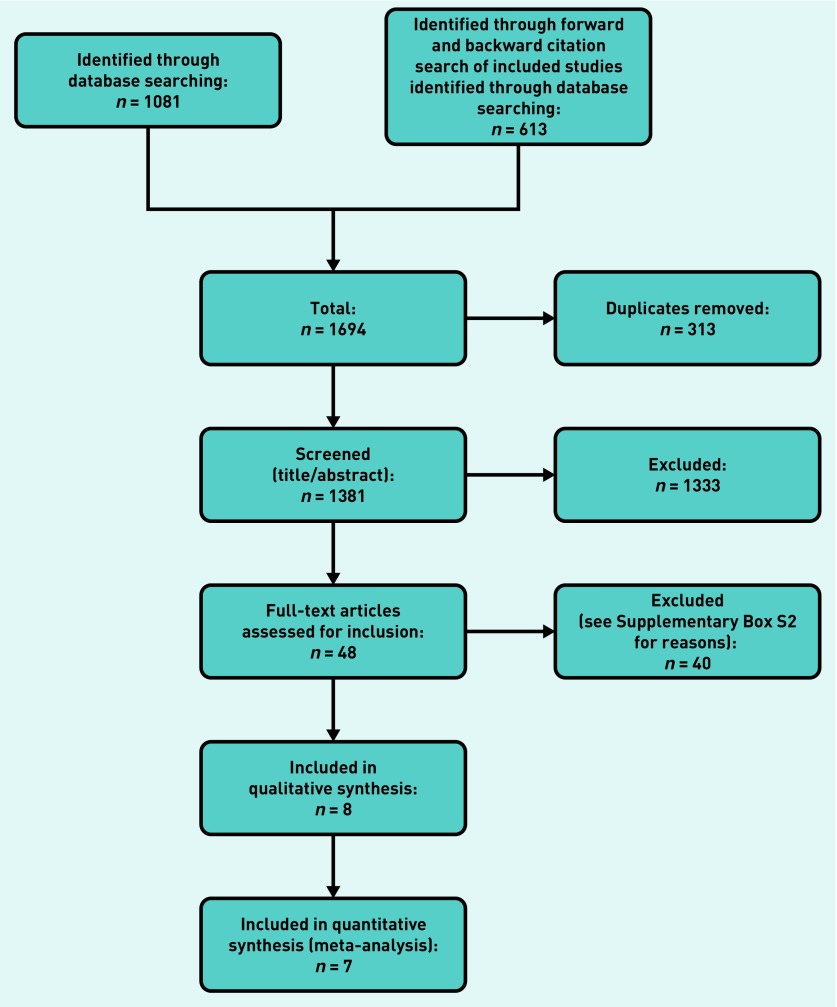
The identified citations were first screened in RobotSearch, an automated screening tool, to identify RCTs.12 Two authors then independently screened the remaining titles and abstracts for inclusion against the inclusion criteria. One author retrieved full texts and then a further two authors screened the full texts for inclusion. Any disagreements were resolved by discussion, or reference to a third author. The selection process was recorded in sufficient detail to complete a Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram (Figure 1) and a list of excluded (full-text) studies with reasons for exclusion (see Supplementary Box S2 for list of excluded studies).
Figure 1.
PRISMA study flow diagram.
PRISMA = preferred reporting items for systematic reviews and meta-analysis.
Data extraction
A data extraction form for study characteristics and outcome data was used, which was piloted on two studies in the review. Two authors extracted the following data from the included studies:
methods: study authors, location, design, duration;
participants: N, age (mean or median; range), sex, number of previous UTI episodes;
interventions and comparators: type of fluid, dose, volume, frequency of intake, for example, per day, duration; and
outcomes: primary and secondary outcomes.
Assessment of risk of bias in included studies
Two authors independently assessed the risk of bias for each included study using the criteria outlined in the Cochrane Handbook,13 assisted by RobotReviewer, an automated tool for assessing the risk of bias.14 All disagreements were resolved by discussion or by referring to a third author. The following domains were assessed:
random sequence generation;
allocation concealment;
blinding of participants and personnel;
blinding of outcome assessment;
incomplete outcome data;
selective outcome reporting; and
other bias (focusing on potential biases owing to funding or conflict of interest).
Each potential source of bias was graded as low, high, or unclear, and each judgement was supported by a quote from the relevant trial.
Measurement of effect and data synthesis
Review Manager 5 was used to calculate the treatment effect. Odds ratios (ORs) or rate ratios (RRs) for dichotomous outcomes were used: OR for results reporting the number of patients with an event, and RR for results reporting the number of events only. Meta-analyses were only undertaken when meaningful (when ≥3 studies or comparisons reported the same outcome); anticipating considerable heterogeneity, the authors used a random effects model.
The patient was used as the unit of analysis, where possible. Investigators or study sponsors were contacted to provide missing data in two cases: to clarify the units reported15 and the content of educational training.16
Assessment of heterogeneity and reporting biases
The authors used the I2 statistic to measure heterogeneity among the included trials. A funnel plot was not created as <10 trials were included.
Subgroup and sensitivity analyses
The authors had planned to compare interventions focused on drinking more alone compared to drinking more plus any other intervention, but there were insufficient data for the analysis. Different time-points (≤6 months and 12 months) were compared for the primary outcome and number of patients with UTIs. A sensitivity analysis by including versus excluding studies at high risk of bias was planned; however, owing to a low number of included studies it was not conducted.
Patient and public involvement
Neither patients nor the public were directly involved in the conduct or writing of this review.
RESULTS
The search identified 1081 publications, supplemented with 613 references from forward and backward citation searches, totalling 1694 articles. Removing duplicates left 1381, which were screened by title and abstract, excluding 1333, to leave 48 that were screened in full text, excluding a further 40 (see Supplementary Box S2 for details of excluded studies). A total of eight RCTs that met the inclusion criteria were included and seven were meta-analysed (Figure 1).
All of the included trials took place in Europe, with an exception of one study in the US.17 The trials ranged in size from 60 to 236 participants. Nearly all trials included 100% females, with the exception of a crossover trial that took place in nursing homes that included 68% females.18
Of the eight included trials, one trial was not meta-analysed. The trial was a four-arm trial, comparing the intake of: 4 oz of cranberry juice, 8 oz of cranberry juice, 4 oz of placebo, and 8 oz of placebo. The trial reported its results for combined cranberry groups (8 oz and 4 oz amalgamated), and for the combined placebo groups (8 oz and 4 oz amalgamated); it did not report data on the impact of increased fluid intake.17
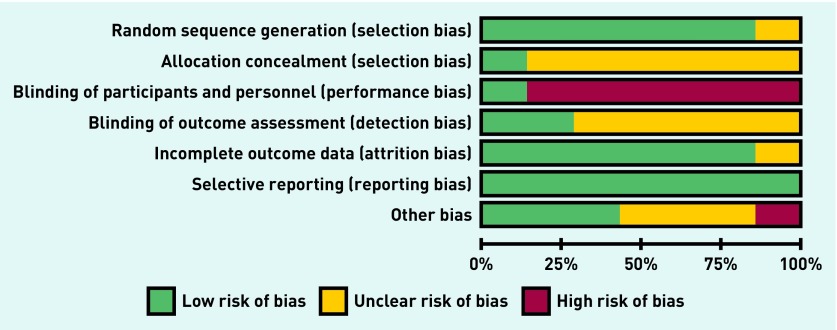
Overall, the seven meta-analysable studies were mostly of low or unclear risk of bias. However, six had a high risk of bias from inadequate blinding of participants and personnel (Figure 2), one from inadequate blinding of outcome data, and another bias from potential conflict of interest: a bottled-water company was involved in funding the trial and its conduct (Table 1).
Figure 2.
Risk of bias graph: authors’ judgements about each risk of bias item presented as percentages across all included studies.
Table 1.
Characteristics of included studies
| Study lead author, year, country | RCT type | Participants randomised, n; mean age, years; female,% | Participants analysed (ITT), n | Mean UTIs in past 12 months, n | Intervention volume; frequency; duration (additives) | Comparator volume; frequency; duration (additives) | Duration of follow-up, months |
|---|---|---|---|---|---|---|---|
| De Leo, 2017, Italy15 | Two arm, parallel | 150; 48; 100 | Intervention: 100 Comparator: 50 |
8.6 | 250 ml;a daily during first 10 days of the month; 3 months | n/a; 3 months | 3 |
| Ferrara, 2009, Italy21 | Three arm, parallel | 84; 7.5; 100 | Intervention: 28 Comparator 1: 27 Comparator 2: 29 |
>1b | 250 ml;c daily; 6 months | Comparator 1: 100 ml;d 5 days a month; 6 months Comparator 2: not reported; 6 months |
6 |
| Handeland, 2014, Norway18 | Two arm, crossover, cluster RCT | 236; 85; 67.8 | Intervention: 110 Comparator: 126 |
0.2e | 233 ml;f,g daily; 3 months then 156 ml;h daily; 3 months |
89 ml;h daily; 3 months then 78 ml;g daily; 3 months |
6 |
| Hooton, 2018, Bulgaria7 | Two arm, parallel | 140; 35.7; 100 | Intervention: 70 Comparator: 70 |
3.3 | 1500 ml;i daily; 12 months | No intervention; 12 months |
12 |
| Kontiokari, 2001, Finland19 | Three arm, parallel | 150; 30; 100 | Intervention: 50 Comparator 1: 50j Comparator 2: 50 |
6 | 250 ml;c daily; 6 months | Comparator 1: 100 ml drink;d 5 days a month; 12 months Comparator 2: no intervention (control) |
12 |
| Kranjčec, 2014, Croatia20 | Three arm, parallelk | 205;l 49; 100 | Intervention: 103 Comparator: 102 |
2m | 200 ml water;n daily; 6 months | No intervention; 6 months | 6 |
| Temiz, 2018, Turkey16 | Three arm, parallel | 60; 64; 31.7 | Intervention: 20 Comparator 1: 20 Comparator 2: 20 |
68.3%o | Recommended 2–3 L of water a day (which translates to 1–2 L above baseline, given estimates of baseline consumption of approx. 1.1 L (Hooton 2018)7 | Comparator 1: One capsule;p twice a day; 3 months Comparator 2: no intervention; 3 months |
3 |
| Stapleton, 2012, US (not meta-analysed as groups amalgamated in analyses)17 | Four arm, parallel | 186; 25; 100 | Intervention 1: 63 Intervention 2: 62 Comparator 1: 31 Comparator 2: 30 |
1.95 | Intervention 1:q,r, 4 oz (∼120 ml); daily; 6 months Intervention 2: 8 oz (∼240 ml); daily; 6 months |
Comparator 1:s 4 oz (∼120 ml); daily, 6 months Comparator 2: 8 oz (∼240 ml); daily; 6 months |
6 |
One glass of water with one Kistinox Forte sachet containing cranberry, propolis extract, and D-mannose.
Inclusion criteria specified that history of >1 UTI required.
Cranberry juice contained 7.5 g of cranberry concentrate and 1.7 g of lingonberry concentrate in 50 ml of water; the families were allowed to add 200 ml of unsweetened water to the 50 ml of concentrate.
Lactobacillus GG drink containing 4 × 10^7 CFU of Lactobacillus GG/100 ml.
Three-month baseline period.
All four arms were offered 300 ml fluid (chokeberry juice versus placebo) but consumed varying amounts; fluid volume consumed reported.
Placebo drink matching the black chokeberry juice as closely as possible in terms of colour, taste.
Chokeberry juice from concentrate.
In addition to usual water consumption.
One omitted due to antibiotic prophylaxis.
Antibiotic arm (nitrofurantoin) excluded from analysis.
In total, 308 participants when antibiotic arm included.
Median episodes reported in past 6 months.
With 2 g D-mannose.
Percentage of participants with UTI history; patients were included in study if they had undergone ileal conduit diversion.
One capsule contains 400 mg cranberry with 18% proanthocyanidins (9 mg).
Juice contained 27% cranberry juice and sucralose (Splenda).
Study reported all outcomes as intervention (4 oz and 8 oz cranberry juice) versus comparator (4 oz and 8 oz placebo drink).
Placebo drink of similar colour and taste to intervention but did not contain cranberry juice. ITT = intention to treat. n/a = not applicable. RCT = randomised controlled trial. UTI = urinary tract infection.
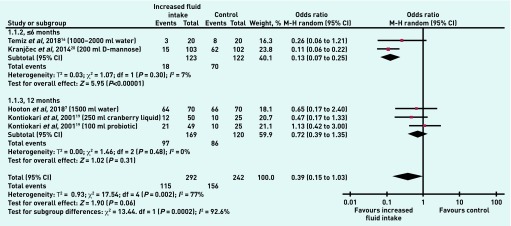
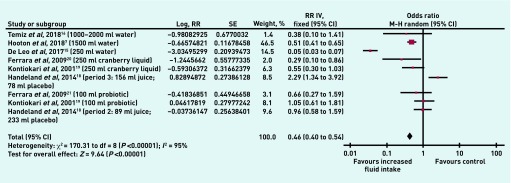
The primary outcome was the number of people with UTIs at ≤6 or 12 months (set a priori and documented in the protocol). Heterogeneity was high; I2 = 77% (P = 0.002). A difference between increased fluid intake and control was found, with an OR of 0.39 (95% confidence interval [CI] = 0.15 to 1.03, P = 0.06). Data were sufficient to subgroup the studies by those reporting the outcome at ≤6 months, and those reporting the outcome at 12 months. In the subgroup of RCTs reporting this outcome at ≤6 months there were two relevant trials with low heterogeneity (I2 = 7%, OR 0.13, 95% CI = 0.07 to 0.25, P<0.001); at 12 months there were two trials (three comparisons) with 289 participants, and no heterogeneity (OR 0.72, 95% CI = 0.39 to 1.35, P = 0.31). The test for subgroup differences was statistically significant (P<0.001) (Figure 3).
Figure 3.
Number of patients with UTIs in increased fluid intake versus control group (all volumes).
CI = confidence interval. M–H = Mantel-Haenszel.
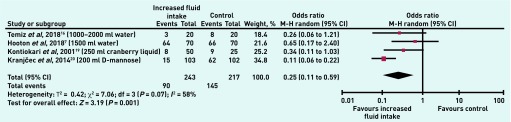
Overall heterogeneity was explored by excluding the arm of one trial in which intervention participants drank less than a single glass (200 ml).19 This left four studies with a total of 460 participants: important levels of heterogeneity remained (I2 = 58%, P = 0.07), but there was a larger difference between increased fluid intake and control (OR 0.25, 95% CI = 0.11 to 0.59, P = 0.001) (Figure 4).
Figure 4.
Number of patients with UTIs (events) in increased fluid intake versus control group (studies with increased fluid intake ≥200 ml only).
CI = confidence interval. M–H = Mantel-Haenszel.
Six RCTs, some with multiple arms, reported the total number of events, that is, UTIs, in each group. They had important levels of heterogeneity (I2 = 95%, P<0.001); increased fluids reduced the rate of UTIs compared with controls, with an RR of 0.46 (95% CI = 0.40 to 0.54, P<0.001) (Figure 5).
Figure 5.
Number of UTIs (events) in increased fluid intake versus control group (all volumes).
CI = confidence interval. IV = inverse variance. M-H = Mantel–Haenszel. RR = rate ratio. SE = standard error.
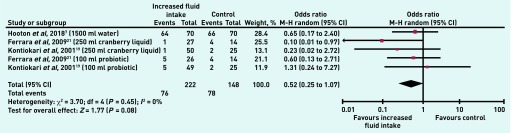
A secondary outcome was antimicrobial use: three trials, with five comparisons, reported this (370 participants). There was no clear difference between the two treatments (OR 0.52, 95% CI = 0.25 to 1.07, P = 0.08) and no heterogeneity (Figure 6).
Figure 6.
Number of patients with antimicrobial use in increased fluid intake versus control group.
CI = confidence interval. M-H = Mantel–Haenszel.
It was not possible to meta-analyse the secondary outcome, UTI symptoms, owing to paucity of data and heterogeneity in reporting.
DISCUSSION
Summary
The seven meta-analysed RCTs suggest that increased fluid intake leads to a statistically significant reduction in the number of people with recurrent UTIs at ≤6 months, but not a statistically significant reduction overall at 12 months. There was also a significant decrease in the total number of UTIs. There was a comparable reduction, not statistically significant, from the subset of RCTs measuring antimicrobial use; however, these results should be interpreted with caution because of the considerable clinical and statistical heterogeneity.
Strengths and limitations
There are several limitations to this review. First, the impact of increased fluids is confounded by other components, for example, educational components in some of the studies; in the study by Temiz et al,16 patients in the intervention group received a brochure with information about UTIs and how to prevent them. Second, incomplete reporting of both the intervention details, and numerical results, limited the authors’ ability to incorporate all results of all studies in the analysis, despite helpful clarifications from several authors. Third, for the RR analysis (Figure 5), a statistical assumption was made that the risk of an event was constant across participants and over time: if this assumption is false then clustering effects might increase the variance and widen the confidence limits, though it is unlikely to render the results non-significant, as the upper confidence limit for the RR was 0.54. The study populations varied, with some studies including participants with current UTI symptoms,19 and others excluding them;7 the number of prior UTI episodes among included participants also varied considerably, from >121 to ≥3.7 Finally, one difference between the protocol and the systematic review is reported: the authors had initially intended to exclude studies including cranberry juice comparison, as the impact of cranberry intake in various forms has been systematically reviewed previously.22 However, as studies including increased intake of other juices were included in the present study, the PICO was amended to include studies of increased cranberry juice intake as well, which resulted in inclusion of one study (whose data were not meta-analysable),17 and an inclusion of the cranberry study arm in two previously included three-armed trials, both of which compared cranberry juice with probiotics (in fluid) and with control.19,21
Comparison with existing literature
Any previous systematic reviews on increased fluids in recurrent UTI could not be identified; however, the present findings are consistent with the results of the RCTs that found that drinking >1 L/day reduced recurrence of UTI.7,16 The other six RCTs used considerably less additional fluid, often little more than ∼200 ml/day.15,17–21 The reporting of extra fluid drunk was insufficient to allow a reliable examination of a dose–response relationship, though removing the trial with the lowest fluid doses (100 ml/day) strengthened the effect size.
Implications for research and practice
Given the minimal potential for harm of increased fluid intake, this review suggests considering clinically adopting its results and advising patients with recurrent UTIs to drink more to reduce recurrent UTIs. However, further research is warranted to confirm the results, and examine the optimal dose, volume of fluid, and the influence of different types of fluid. Future research on the effects of substances such as cranberry, chokeberry, and D-mannose needs to consider the impact of drinking more, which should be matched in at least one control group, which few trials have done.
Acknowledgments
The authors would like to thank Zeynep Temiz and Ikbal Cavdar for providing them with information and copies of educational materials used in their study; and Valentina Cappelli and Vincenzo De Leo for additional information about their study.
Funding
The present systematic review was conducted as part of the work of the Centre of Research Excellence in Minimising Antibiotic Resistance in the Community (CRE-MARC), funded by the National Health and Medical Research Council (NHMRC), Australia (grant reference number: GNT1153299). The funder had no involvement in this systematic review.
Ethical approval
Ethical approval was not required for this study.
Provenance
Freely submitted; externally peer reviewed.
Competing interests
The authors have declared no competing interests.
Discuss this article
Contribute and read comments about this article: bjgp.org/letters
REFERENCES
- 1.Rahn DD. Urinary tract infections: contemporary management. Urol Nurs. 2008;28(5):333–341. [PubMed] [Google Scholar]
- 2.Foxman B. Epidemiology of urinary tract infections: incidence, morbidity, and economic costs. Am J Med. 2002;113(Suppl 1A):5S–13S. doi: 10.1016/s0002-9343(02)01054-9. [DOI] [PubMed] [Google Scholar]
- 3.Mazzulli T. Resistance trends in urinary tract pathogens and impact on management. J Urol. 2002;168(4 Pt 2):1720–1722. doi: 10.1097/01.ju.0000028385.10311.c9. [DOI] [PubMed] [Google Scholar]
- 4.Bleidorn J, Gágyor I, Kochen MM, et al. Symptomatic treatment (ibuprofen) or antibiotics (ciprofloxacin) for uncomplicated urinary tract infection? — results of a randomized controlled pilot trial. BMC Med. 2010;8:30. doi: 10.1186/1741-7015-8-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gágyor I, Bleidorn J, Kochen MM, et al. Ibuprofen versus fosfomycin for uncomplicated urinary tract infection in women: randomised controlled trial. BMJ. 2015;351:h6544. doi: 10.1136/bmj.h6544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beetz R. Mild dehydration: a risk factor of urinary tract infection? Eur J Clin Nutr. 2003;57(Suppl 2):S52–S58. doi: 10.1038/sj.ejcn.1601902. [DOI] [PubMed] [Google Scholar]
- 7.Hooton TM, Vecchio M, Iroz A, et al. Effect of increased daily water intake in premenopausal women with recurrent urinary tract infections: a randomized clinical trial. JAMA Intern Med. 2018;178(11):1509–1515. doi: 10.1001/jamainternmed.2018.4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lotan Y, Daudon M, Bruyère F, et al. Impact of fluid intake in the prevention of urinary system diseases: a brief review. Curr Opin Nephrol Hypertens. 2013;22(Suppl 1):S1–S10. doi: 10.1097/MNH.0b013e328360a268. [DOI] [PubMed] [Google Scholar]
- 9.Institute for Evidence-Based Healthcare. Word frequency analyser. 2019. http://sr-accelerator.com/#/help/wordfreq (accessed 3 Jan 2020).
- 10.Rathbone J, Albarqouni L, Bakhit M, et al. Expediting citation screening using PICo-based title-only screening for identifying studies in scoping searches and rapid reviews. Syst Rev. 2017;6(1):233. doi: 10.1186/s13643-017-0629-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Institute for Evidence-Based Healthcare. Systematic review accelerator: polyglot search. http://crebp-sra.com/#/polyglot (accessed 19 Dec 2019)
- 12.Marshall IJ, Noel-Storr A, Kuiper J, et al. Machine learning for identifying randomized controlled trials: an evaluation and practitioner’s guide. Res Synth Methods. 2018;9(4):602–614. doi: 10.1002/jrsm.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Higghins JPT, Green SE, editors. Cochrane handbook for systematic reviews of interventions: version 5.1.0. 2011. https://training.cochrane.org/handbook (accessed 19 Dec 2019)
- 14.Marshall IJ, Kuiper J, Wallace BC. RobotReviewer: evaluation of a system for automatically assessing bias in clinical trials. J Am Med Inform Assoc. 2016;23(1):193–201. doi: 10.1093/jamia/ocv044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Leo V, Cappelli V, Massaro MG, et al. Evaluation of the effects of a natural dietary supplement with cranberry, Noxamicina(R) and D-mannose in recurrent urinary infections in perimenopausal women. (In Italian) Minerva Ginecol. 2017;69(4):336–341. doi: 10.23736/S0026-4784.17.04074-6. [DOI] [PubMed] [Google Scholar]
- 16.Temiz Z, Cavdar I. The effects of training and the use of cranberry capsule in preventing urinary tract infections after urostomy. Complement Ther Clin Pract. 2018;31:111–117. doi: 10.1016/j.ctcp.2018.01.017. [DOI] [PubMed] [Google Scholar]
- 17.Stapleton AE, Dziura J, Hooton TM, et al. Recurrent urinary tract infection and urinary Escherichia coli in women ingesting cranberry juice daily: a randomized controlled trial. Mayo Clin Proc. 2012;87(2):143–150. doi: 10.1016/j.mayocp.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Handeland M, Grude N, Torp T, Slimestad R. Black chokeberry juice (Aronia melanocarpa) reduces incidences of urinary tract infection among nursing home residents in the long term — a pilot study. Nutr Res. 2014;34(6):518–525. doi: 10.1016/j.nutres.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 19.Kontiokari T, Sundqvist K, Nuutinen M, et al. Randomised trial of cranberry-lingonberry juice and Lactobacillus GG drink for the prevention of urinary tract infections in women. BMJ. 2001;322(7302):1571. doi: 10.1136/bmj.322.7302.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kranjčec B, Papeš D, Altarac S. D-mannose powder for prophylaxis of recurrent urinary tract infections in women: a randomized clinical trial. World J Urol. 2014;32(1):79–84. doi: 10.1007/s00345-013-1091-6. [DOI] [PubMed] [Google Scholar]
- 21.Ferrara P, Romaniello L, Vitelli O, et al. Cranberry juice for the prevention of recurrent urinary tract infections: a randomized controlled trial in children. Scand J Urol Nephrol. 2009;43(5):369–372. doi: 10.3109/00365590902936698. [DOI] [PubMed] [Google Scholar]
- 22.Jepson RG, Williams G, Craig JC. Cranberries for preventing urinary tract infections. Cochrane Database Syst Rev. 2012;(10):CD001321. doi: 10.1002/14651858.CD001321.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]