Abstract
Thromboembolectomy is often guided with fluoroscopy. For intracardiac and great vessel thromboemboli, transesophageal echocardiography (TEE) can assess these thrombi, guide precise suction catheter placement, prevent intracardiac injury, and serve as a hemodynamic monitor. TEE can also be used to assess blood flow and thrombotic material reduction following embolectomy. TEE is a low-risk, high-value, real-time imaging modality that facilitates thromboembolectomy and increases patient safety.
Keywords: Chronic thrombus, suction-directed catheter, thromboembolism, transesophageal echocardiography
Venous thromboembolic disease afflicts >900,000 Americans annually. Pulmonary embolism and acute thromboembolic disease of the great vessels and heart itself can be treated with open surgical embolectomy, but incur tremendous perioperative risk.1 A new directed suction catheter device may be employed to perform percutaneous thromboembolectomy with a lower mortality rate.2 Successful employment of this rigid embolectomy catheter requires precise, direct imaging to target the thromboembolism, while simultaneously avoiding iatrogenic injury. We describe the therapeutic use of such a catheter with real-time transesophageal echocardiography (TEE) guidance.
CASE PRESENTATION
A 37-year-old woman with a history of superior vena cava thrombus presented to the emergency department after experiencing a syncopal event. A computed tomography scan showed interval increase in the size of the thrombus. Therapeutic anticoagulation with heparin was initiated. Mechanical thromboembolectomy was planned, utilizing a suction catheter system in series with extracorporeal circulatory support.
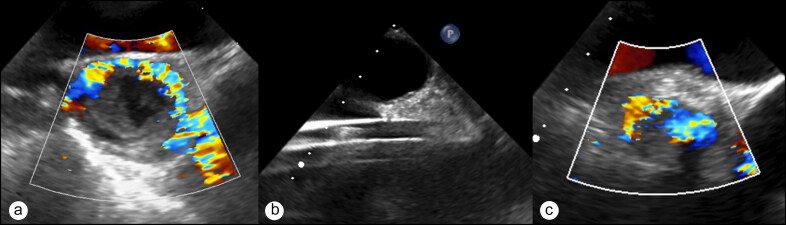
In the operating room, a radial arterial catheter and a left internal jugular vein introducer sheath were placed. General anesthesia was induced with ketamine and sevoflurane, preserving spontaneous ventilation. A three-dimensional TEE probe (X8-2t xMATRIX Array, EPIQ 7, Philips Medical, Cambridge, MA) was placed, confirming atriocaval thrombus. Superior vena cava flow was documented with the use of color-flow Doppler. Via a 26 French sheath in the right common femoral vein, a directed suction catheter (AngioVac Cannula, Angiodynamics, Latham, NY) was advanced into the right atrium over a guidewire utilizing fluoroscopic and TEE guidance. An 18 French reinfusion cannula was placed in the left common femoral vein. Heparin was administered with a target activated clotting time of >300 seconds. Mechanical thrombectomy was then performed with aspiration of both acute and chronic thrombus from the superior vena cava. TEE monitoring was utilized to precisely engage the mobile thrombus in the right atrium, avoid the atrial wall and septum, and assess the thrombus burden in the right atrium and superior vena cava. TEE demonstrated improved patency of the superior vena cava, although there was still some residual thrombus. The patient was taken to the intensive care unit and discharged on postoperative day 2 without complications (Figure 1).
Figure 1.
Mid-esophageal ascending aortic short-axis echocardiography views demonstrating (a) thrombus obstructing flow within the superior vena cava; (b) the highly echogenic thrombectomy catheter, seen as linear echogenicities across the screen; and (c) improved flow by color flow Doppler after embolectomy.
DISCUSSION
Directed suction catheter embolectomy with a bypass system can be a safe, feasible alternative to invasive surgery for eliminating large thrombotic volume without compromising hemodynamic stability.2,3 Basman reported >80% successful evacuation of iliocaval and intracardiac thrombi and 44% of pulmonary artery thrombi using this approach.4 Although catheter embolectomy is safer than open surgery, vein perforation, arrhythmia, distal embolization of thrombi, damage to intracardiac structures, and air embolism have all been reported.5
Fluoroscopy is limited by mobile units with inferior image quality, poor visualization of thrombi and atrial anatomy, and risks of ionizing radiation and contrast administration (anaphylaxis and renal failure). TEE, however, provides real-time images of the heart, vessels, cannulas, and thrombi. This facilitates precise catheter and wire movements. Thrombi susceptible to embolism are more readily identified and are therefore prioritized by the proceduralist. In the event thromboembolism does occur, TEE is an excellent tool for monitoring cardiovascular and hemodynamic effects and guiding hemodynamic management. In our patient, TEE identified areas of chronic thrombosis that were not amenable to thrombectomy. Utilizing TEE, chronic thrombi appear echogenic, calcified, nonmobile, and adherent to adjacent structures. One center reported a success rate of 33% with chronic thrombus vs 83% with acute thrombus,6 with thrombi located in the right heart, pulmonary system, or iliocaval vessels. (In that series, TEE was used for 5 patients, but its beneficial role was not specifically studied.) Furthermore, TEE reduces patient and operating room personnel exposure to ionizing radiation and risk of contrast administration.
Damage to intracardiac structures may result from aggressive suction and cannula repositioning. In this case, the catheter was advanced inadvertently towards and abutted the interatrial septum many times, which likely would not have been identified without TEE guidance. Catastrophic structural damage to the right ventricle and pulmonary arteries using the directed suction catheter has led to patient death.7 Real-time TEE guidance may prevent iatrogenic cardiac and vascular injury by stopping advancement of a misdirected catheter. Quantification of thrombotic burden reduction may also be demonstrated via TEE with color-flow Doppler.
Directed suction catheter devices are primarily indicated for the removal of acute, soft emboli. Prior studies have found that directed suction catheter use has successfully treated iliocaval thrombosis that was resistant to thrombolytics, secondary congenital thrombophilias, and secondary to inferior vena cava filters, indicating suction catheters’ potential to treat chronic venous thrombosis.8,9 Given the technical challenge of aspirating hard thrombus, TEE real-time guidance may be even more valuable in patients with chronic lesions by facilitating precise positioning of the suction catheter.
During percutaneous thromboembolectomy, intraoperative TEE provides diagnostic information and therapeutic interventional guidance and aids in management of cannulas and hemodynamic assessment. Further studies are needed to delineate the full role of TEE in managing removal of chronic thrombotic material with directed suction catheters, but as a low-risk, real-time imaging technique, its use may not only improve procedure success rates but also simultaneously reduce risk.
References
- 1.Tak T, Karturi S, Sharma U, et al. Acute pulmonary embolism: contemporary approach to diagnosis, risk-stratification, and management. Int J Angiol. 2019;28:100–111. doi: 10.1055/s-0039-1692636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Salsamendi J, Doshi M, Bhatia S, et al. Single center experience with the AngioVac aspiration system. Cardiovasc Intervent Radiol. 2015;38(4):998–1004. doi: 10.1007/s00270-015-1152-x. [DOI] [PubMed] [Google Scholar]
- 3.Donaldson CW, Baker JN, Narayan RL, et al. Thrombectomy using suction filtration and veno-venous bypass: single center experience with a novel device. Cathet Cardiovasc Intervent. 2015;86(2):E81–E87. doi: 10.1002/ccd.25583. [DOI] [PubMed] [Google Scholar]
- 4.Basman C, Rashid U, Parmar YJ, et al. The role of percutaneous vacuum assisted thrombectomy for intracardiac and intravascular pathology. J Card Surg. 2018;33(10):666–672. doi: 10.1111/jocs.13806. [DOI] [PubMed] [Google Scholar]
- 5.Enezate TH, Kumar A, Aggarwal K, et al. Non-surgical extraction of right atrial mass by AngioVac aspiration device under fluoroscopic and transesophageal echocardiographic guidance. Cardiovasc Diagn Ther. 2017;7(3):331–335. doi: 10.21037/cdt.2016.09.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.D’Ayala M, Worku B, Gulkarov I, et al. Factors associated with successful thrombus extraction with the AngioVac device: an institutional experience. Ann Vasc Surg. 2017; 38:242–247. doi: 10.1016/j.avsg.2016.04.015. [DOI] [PubMed] [Google Scholar]
- 7.Al-Hakim R, Park J, Bansal A, et al. Early experience with AngioVac aspiration in the pulmonary arteries. J Vasc Interv Radiol. 2016;27(5):730–734. doi: 10.1016/j.jvir.2016.01.012. [DOI] [PubMed] [Google Scholar]
- 8.Smith SJ, Behrens G, Sewall LE, et al. Vacuum-assisted thrombectomy device (AngioVac) in the management of symptomatic iliocaval thrombosis. Vasc Interv Radiol. 2014;25(3):425–430. doi: 10.1016/j.jvir.2013.11.017. [DOI] [PubMed] [Google Scholar]
- 9.Jabaar AA, Jenkins S. The role of vacuum assisted thrombectomy (AngioVac) in treating chronic venous thromboembolic disease. Systematic review and a single center's experience. Cardiovasc Revasc Med. 2018;19(7 Pt A):799–804. doi: 10.1016/j.carrev.2018.02.005. [DOI] [PubMed] [Google Scholar]