Abstract
On the basis of the previous lead N-4-t-butylbenzyl 2-(3-fluoro-4-methylsulfonylaminophenyl) propanamide (3) as a potent TRPV1 antagonist, structure–activity relationships for the B (propanamide part) and C-region (4-t-butylbenzyl part) have been investigated for rTRPV1 in CHO cells. The B-region was modified with dimethyl, cyclopropyl and reverse amides and then the C-region was replaced with 4-substituted phenyl, aryl alkyl and diaryl alkyl derivatives. Among them, compound 50 showed high binding affinity with Ki = 21.5 nM, which was twofold more potent than 3 and compound 54 exhibited potent antagonism with Ki(ant) = 8.0 nM comparable to 3.
Keywords: TRPV1 antagonists, Analgesic, Capsaicin, Resiniferatoxin
1. Introduction
The transient receptor potential V1 (TRPV1) receptor1 is a molecular integrator of nociceptive stimuli, functioning as a non-selective cation channel with high Ca2+ permeability. The receptor, located predominantly in primary sensory neurons, is activated by protons,2 heat,3 inflammatory mediators such as anandamide4 and lipoxygenase products,5 and vanilloids such as capsaicin (CAP)6 and resiniferatoxin (RTX).7 Its activation leads to an increase in intracellular Ca2+, which in turn causes excitation of the primary sensory neurons and ultimately the central perception of pain. Since TRPV1 antagonists inhibit the transmission of nociceptive signaling from the periphery to the CNS as well as block other pathological states associated with this receptor, they have been promising drug candidates as novel analgesic and antiinflammatory agents, particularly for chronic pain and inflammatory hyper-algesia.8 The clinical development and therapeutic potentials of TRPV1 antagonists has been extensively reviewed.9–13
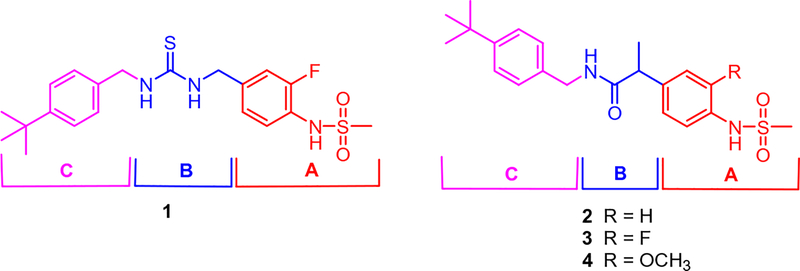
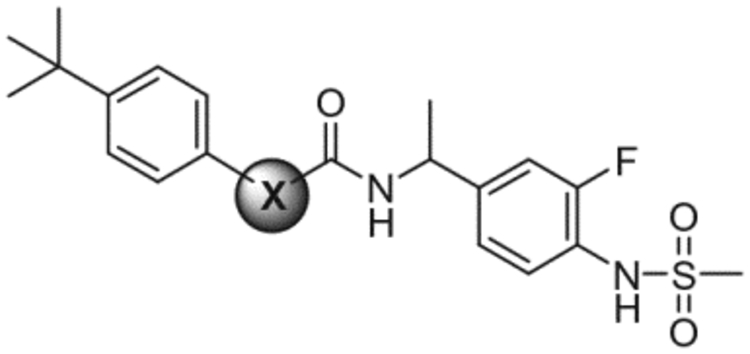
Previously, we have reported a prototype antagonist, N-(4-t-butylbenzyl)-N′−4-(3-fluoro-4-methylsulfonylaminobenzyl) thiourea (1),14–17 in rat TRPV1/CHO (Fig. 1). The thiourea showed potent antagonism not only for capsaicin stimulation of rTRPV1 but also for stimulation by temperature and pH.14,16 Further optimization in the B-region (thiourea part) provided the propanamide antagonist 3, which exhibited higher binding affinity and more potent antagonism for both rTRPV1 and hTRPV1 in CHO cells compared to 1 (Fig. 1).18 Its stereospecific activity was demonstrated with marked selectivity for the (S)-configuration (S-3 vs. R-3); whereas the (S)-isomer was ca. twofold more potent than the racemate 3, the (R)-isomer was 30- to 40-fold weaker. A docking study of S-3 isomer with our hTRPV1 homology model identified crucial hydrogen bonds between the ligand and the receptor contributing to its stereospecific potency. A further advantage of the propanamide antagonists over the thiourea antagonists, such as 1, is that the propanamide antagonists avoid the potential toxicity associated with the thiourea functionality.
Figure 1.
As a continuation of our effort to optimize the 4-methylsulfonamide TRPV1 antagonists, we reported the structure–activity relationships for the A-region (4-methylsulfonylaminophenyl part) in a series of N-4-t-butylbenzyl 2-(4-methylsulfonylaminophenyl) propanamides as TRPV1 antagonists.18 In this paper, we have investigated the structure–activity relationships of the B (propanamide part) and C-region (4-t-butylbenzyl part) in a series of 2-(3-fluoro-4-methylsulfonylaminophenyl) propanamide TRPV1 antagonists.
2. Result and discussion
2.1. Chemistry
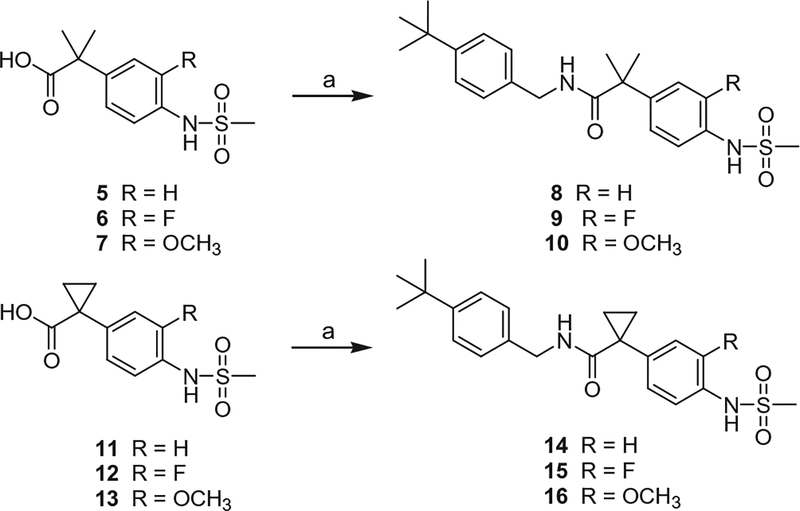
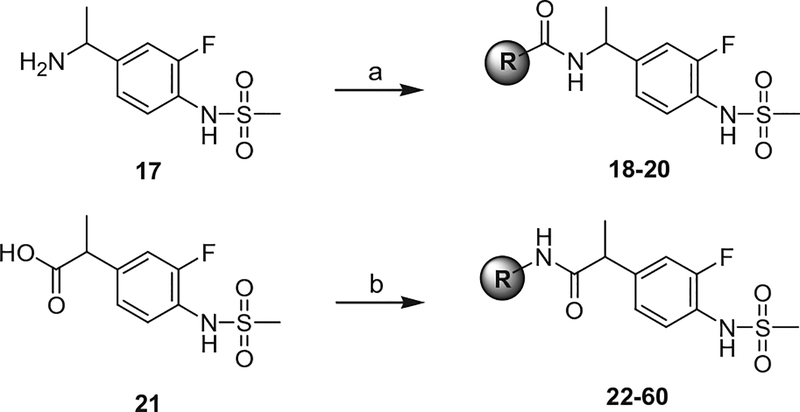
The dimethyl and cyclopropyl amide analogues of 2–4 were synthesized from the corresponding acids, 5–7 and 11–13,19 by the coupling with 4-t-butylbenzyl amine to provide 8–10 and 14–16, respectively (Scheme 1). The reverse amide analogues were prepared by the coupling of α-methyl benzylamine 1720 with the corresponding acids to give the final compounds 18–20 (Scheme 2). The syntheses of 2-(3-fluoro-4-methylsulfonylaminophenyl) propanamide analogues 22–60 were conducted by following the previous report.18
Scheme 1.
Syntheses of dimethyl and cyclopropyl amide analogues. Reagents and conditions: (a) R-NH2, EDC, CH2Cl2.
Scheme 2.
Syntheses of reverse amide and propanamide analogues. Reagents and conditions: (a) R-CO2H, EDC, CH2Cl2; (b) R-NH2, EDC, CH2Cl2.
2.2. Biological activity
The binding affinities and potencies as agonists/antagonists of the synthesized TRPV1 ligands were assessed in vitro by a competitive binding assay with [3H]RTX and by a functional 45Ca2+ uptake assay using rat TRPV1 heterologously expressed in Chinese hamster ovary (CHO) cells, as previously described.16 The results are summarized in Tables 1–4, together with the potencies of the previously reported parent antagonists 2–4.18
Table 1.
SAR-1 of B-region
 | |||||
|---|---|---|---|---|---|
| X | R | Kia (nM) binding affinity | EC50b (nM) agonism | Kia (nM) antagonism | |
| 2 | >CHCH3 | H | 106 | NE | 17.5 |
| 3 | >CHCH3 | F | 46.2 | NE | 7.6 |
| 4 | >CHCH3 | OCH3 | 540 | NE | 232 |
| 8 | >C(CH3)2 | H | 430 (±120) | NE | 2550 (±760) |
| 9 | >C(CH3)2 | F | 1070 (±330) | NE | 467 (±86) |
| 10 | >C(CH3)2 | OCH3 | 414 (±55) | NE | 707 (±81) |
| 14 | >C(CH2)2 | H | 610 (±130) | NE | 1770 (±140) |
| 15 | >C(CH2)2 | F | NE | NE | WE |
| 16 | >C(CH2)2 | OCH3 | 750 (±130) | NE | 2250 (±610) |
Values represent mean ± SEM from 3 or more experiments.
NE, no effect. WE, weak effect (quantitation of no effect/fractional agonism/antagonism is from 1 to 3 experiments).
Table 4.
SAR-2 of C-region
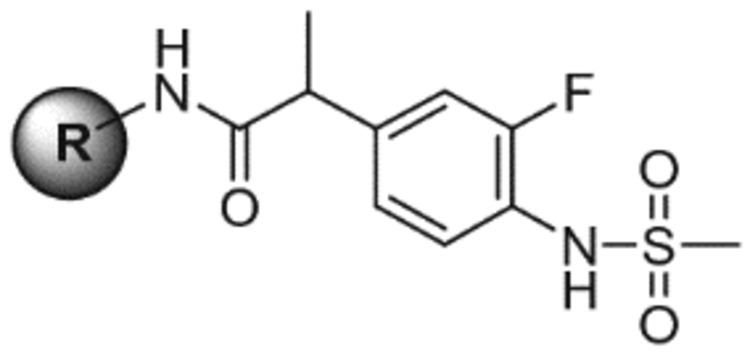 | ||||
|---|---|---|---|---|
| R | Kia (nM) binding affinityc | EC50a (nM) agonismc | Kia (nM) antagonismc | |
| 47 | 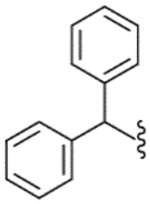 |
5900 (±1300) | NE | (58%)b |
| 48 | 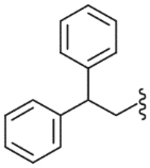 |
1680 (±640) | NE | 2490 (±880) |
| 49 | 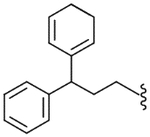 |
130 (±21) | NE | 36.6 (±7.2) |
| 50 | 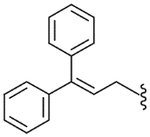 |
21.5 (±5.1) | NE | 14.2 (±4.0) |
| 51 | 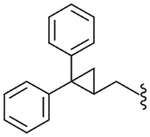 |
188 (±35) | NE | 148 (±51) |
| 52 | 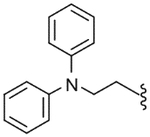 |
115 (±25) | NE | 47 (±24) |
| 53 | 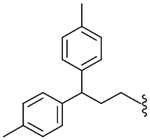 |
96 (±21) | NE | 32.1 (±3.7) |
| 54 | 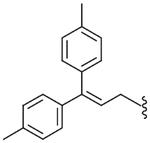 |
30.9 (±5.5) | NE | 8.0 (±1.9) |
| 55 | 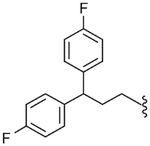 |
79 (±34) | NE | 24.8 (±0.99) |
| 56 | 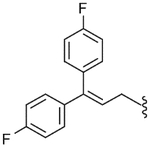 |
43.3 (±8.1) | NE | 29.2 (±5.4) |
| 57 | 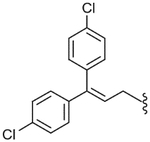 |
29.6 (±7.0) | NE | 24.5 (±6.3) |
| 58 |  |
121 (±48) | NE | 31 (±11) |
| 59 |  |
141 (±44) | NE | 122 (±11) |
| 60 |  |
117 (±31) | NE | 163 (±7.6) |
Values represent mean ± SEM from 3 or more experiments.
NE, no effect. WE, weak effect (quantitation of no effect/fractional agonism/antagonism is from 1 to 3 experiments).
Only fractional antagonism.
For the modification of the propanamide B-region, the two α,α′-disubstituted analogues, the dimethyl (8–10) and cyclopropyl amides (14–16), have been investigated as TRPV1 ligands (Table 1). They all showed a dramatic loss in receptor activity compared to the corresponding α-methyl amides (propanamide) (2–4) regardless of the 3-substituents, indicating that an α-methyl in the B-region might constitute a principal pharmacophore by making a stereospecific interaction with the hydrophobic pocket of the receptor, as modeled previously.19 As another B-region modification, the reversed amides, N-acyl α-methyl benzylamine (18–20), were explored (Table 2). Compound 18, a reverse amide of 3, exhibited a marked loss of activity compared to 3. However, its one-carbon elongation analogue (19) improved the receptor activity and conformational restriction (20) provided further enhancement in activity, achieving a level only 2–3-fold less potent than parent 3.
Table 2.
SAR-2 of B-region
 | ||||
|---|---|---|---|---|
| X | Kia (nM) binding affinity | EC50 (nM)b agonism | Kia (nM) antagonism | |
| 3 | 46.2 | NE | 7.6 | |
| 18 | CH2 | 2100 (±500) | NE | 2220 (±400) |
| 19 | CH2CH2 | 380 (±100) | NE | 104 (±24) |
| 20 | CH=CH(E) | 105 (±14) | NE | 23.9 (±4.6) |
Values represent mean ± SEM from 3 or more experiments.
NE, no effect. WE, weak effect (quantitation of no effect/fractional agonism/antagonism is from 1 to 3 experiments).
Previously, the structure–activity relations for the A-region in a series of 2-(4-methylsulfonylaminophenyl) propanamides were evaluated with the 4-t-butylbenzyl group as a fixed C-region. These studies identified the 2-(3-fluoro-4-methylsulfonylamino)phenyl propanamide moiety as the best A/B-regions with high binding affinity and potent antagonism. We therefore explored the extensive structure–activity relationships of the C-region with the above fixed A/B-region. The aryl alkyl and diaryl alkyl analogues of the C-region were represented in Tables 3 and 4, respectively.
Table 3.
SAR-1 of C-region
 | ||||
|---|---|---|---|---|
| R | Kia (nM) binding affinity | EC50b (nM) agonism | Kia (nM) antagonism | |
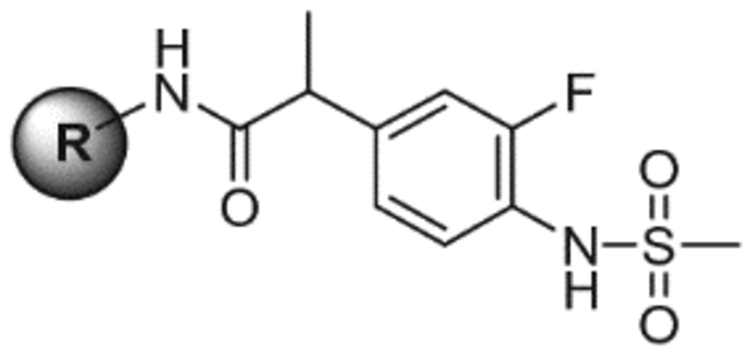
| 3 | 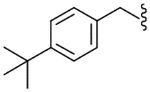 |
46.2 | NE | 7.6 |
| 22 | 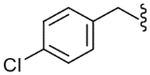 |
1060 (±220) | NE | 367 (±23) |
| 23 | 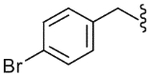 |
412 (±24) | NE | 250 (±70) |
| 24 | 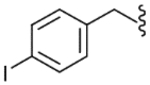 |
502 (±84) | NE | 107 (±38) |
| 25 | 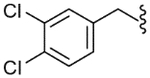 |
200 (±52) | NE | 115 (±43) |
| 26 | 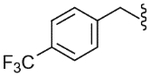 |
660 (±170) | NE | 274 (±90) |
| 27 | 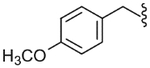 |
7800 (±1100) | NE | 5350 (±810) |
| 28 | 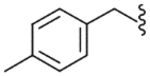 |
3100 (±570) | NE | 930 (±160) |
| 29 | 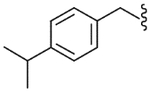 |
127 (±23) | NE | 144 (±28) |
| 30 | 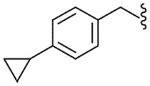 |
411 (±92) | NE | 55.5 (±9.1) |
| 31 | 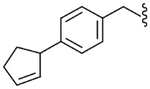 |
162 (±31) | NE | 15 (±1.1) |
| 32 | 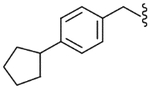 |
93 (±23) | NE | 20.8 (±2.5) |
| 33 | 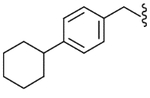 |
138 (±49) | NE | 43 (±17) |
| 34 | 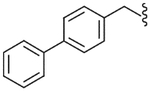 |
1750 (±480) | NE | 261 (±69) |
| 35 | 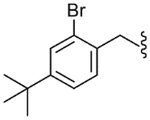 |
47 (±11) | NE | 12.7 (±3.4) |
| 36 | 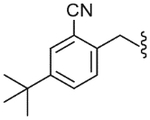 |
44.0 (±5.5) | NE | 40 (±15) |
| 37 | 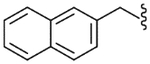 |
960 (±110) | NE | 240 (±95) |
| 38 | 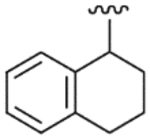 |
WE | NE | NE |
| 39 | 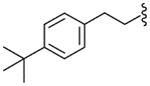 |
554 (±64) | NE | 43 (±11) |
| 40 | 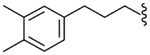 |
413 (±53) | NE | 97 (±43) |
| 41 | 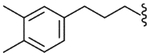 |
950 (±140) | NE | 204 (±28) |
| 42 | 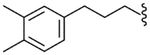 |
257 (±47) | NE | 33.7 (±6.4) |
| 43 | 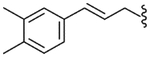 |
277 (±64) | NE | 153 (±20) |
| 44 | 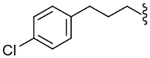 |
470 (±140) | NE | 135 (±40) |
| 45 | 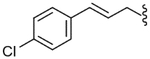 |
897 (±63) | NE | 384 (±78) |
| 46 | 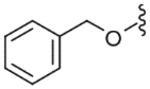 |
9400 (±1400) | NE | (34%)c |
Values represent mean ± SEM from 3 or more experiments.
NE, no effect. WE, weak effect (quantitation of no effect/fractional agonism/antagonism is from 1 to 3 experiments).
Only fractional antagonism.
The SAR of the C-region was started from the modification at the 4-position of the 4-t-butyl benzyl group. First, halogen groups, including 4-chloro (22), 4-bromo (23), 4-iodo (24) and 3,4-dichloro (25), were introduced as electron-withdrawing and lipophilic groups to replace the 4-t-butyl group of lead compound 3. Although their receptor activities improved as the size of the halogen increased, the activity of the 4-iodo analogue (24) was still 10-fold less potent than that of the parent 3. The 4-trifluoromethyl analogue (26) showed similar potency compared to the 4-chloro and 4-bromo analogues, reflecting their similar physicochemical properties. The 4-methoxy analogue (27), which placed an electron-donating group in the 4-position, exhibited a dramatic reduction in receptor potency. Next, various alkyl groups were incorporated into the 4-position. The activity increased with the size of 4-alkyl group up to the cyclopentyl group (32) but was then reduced in the cyclohexyl analogue (33). Interestingly, the 4-phenyl analogue (34) led to a dramatic loss in activity. The analysis indicated that the t-butyl group at the 4-position was best for potency, among the compounds examined, probably due to an optimal fit to the hydrophobic pocket.19 Incorporation of the 2-bromo (35) and 2-cyano (36) groups into the 4-t-butylbenzyl did not affect the potency of the parent compound (3), suggesting that the 2-substituent was directed away from the binding pocket. Bicyclic analogues, naphthalene (37) and tetrahydronaphthalene (38), were found to be weak antagonists. One-carbon elongation of 3 to give 39 led a modest reduction in potency. The 3,4-dimethylphenylpropyl group, found in the previous clinical candidate DA-5018, was used as the C-region to provide 40. Although it was found to be ca. 10-fold less potent than 3, the stereospecific potency of the α-methyl group could be further confirmed with its two chiral compounds (41, 42), in which the (S)-isomer (42) proved to be the active isomer with ca. twofold more potency than the racemate 40. Conformational restriction of 40 to give 43 yielded little improvement in receptor potency. The 4-chlorophenylpropyl analogues, 44 and 45, showed similar potencies compared to the corresponding 3,4-dimethylphenyl analogues, 40 and 43, respectively. The benzyloxy analogue (46) was found to a weak partial antagonist.
Next, a series of diphenylalkyl groups (47–60) as a C-region were investigated (Table 4).
The diphenylmethyl analogue (47) showed very weak binding affinity and partial antagonism. However, activity was enhanced as the number of carbons in the linker increased. The diphenylpropyl analogue (49) exhibited good binding affinity and antagonism. The modification of the propyl linker provided the three derivatives 50–52; whereas the diphenylpropenyl analogue (50) showed several-fold enhanced potency, the diphenylcyclopropylmethyl (51) and the diphenylaminoethyl (52) analogues displayed a modest reduction in potency compared to 49. The analysis indicated that the flat environment rather than a conformational restriction in the C-region was favorable for binding to the receptor. Compound 50 was found to have high affinity and potent antagonism with Ki = 21.5 nM and Ki(ant) = 14.2 nM, which was twofold more potent in binding affinity and twofold less potent in antagonism compared to lead 3. For further optimization from 49, 4,4′-disubstituted diphenylpropyl (53, 55 and 58) and diphenylpropenyl (54, 56 and 57) analogues were explored, indicating that the substitutions did not affect the activity. They exhibited similar activities compared to the corresponding unsubstituted analogues, 49 and 50. In particular, the (4,4′-dimethyl)diphenylpropenyl analogue (54) had potent antagonism with Ki(ant) = 8.0 nM. The two diphenylcycloheptane analogues (59, 60), as further conformationally constrained analogues of 50, were prepared. However, they showed a 5–10-fold reduction in binding affinity and antagonism compared to 50.
3. Conclusion
In order to further optimize the TRPV1 antagonism from the previous lead, N-4-t-butylbenzyl 2-(3-fluoro-4-methylsulfonylaminophenyl) propanamide (3), the structure activity relationships for the B and C-region have been investigated in detail. In the B-region, the propanamide was modified by dimethyl, cyclopropyl and reverse amides and in the C-region the 4-t-butylbenzyl was replaced with 4-substituted phenyl, aryl alkyl and diaryl alkyl derivatives. Among the compounds in the series, the diphenylpropenyl analogue (50) showed high binding affinity with Ki = 21.5 nM, which was twofold more potent than the lead 3, and the (4,4′-dimethyl)diphenylpropenyl (54) analogue exhibited potent antagonism with Ki(ant) = 8.0 nM comparable to 3 for rTRPV1 in the CHO cells.
4. Experimental
4.1. General
All chemical reagents were commercially available. Melting points were determined on a melting point Buchi B-540 apparatus and are uncorrected. Silica gel column chromatography was performed on Silica Gel 60, 230–400 mesh, Merck. Proton NMR spectra were recorded on a JEOL JNM-LA 300 at 300 MHz and Bruker Analytik, DE/AVANCE Digital 400 at 400 MHz. Chemical shifts are reported in ppm units with Me4Si as a reference standard. Mass spectra were recorded on a VG Trio-2 GC–MS. Combustion analyses were performed on an EA 1110 Automatic Elemental Analyzer, CE Instruments.
4.2. Generel procedure for coupling
A mixture of acid (10 mmol), amine (12 mmol) and 1-(3-dimethylaminopropyl)-3-ethyl-carbodiimide hydrochloride (12 mmol) in CH2Cl2 (20 mL) was stirred for 12 h at room temperature. The reaction mixture was filtered off and the filtrate was concentrated. The residue was purified by flash column chromatography on silica gel using EtOAc–hexanes as eluant.
4.2.1. N-(4-tert-Butylbenzyl)-2-[4-(methylsulfonylamino) phenyl]-2-methylpropionamide (8)
Yield 92%, white solid, mp = 141–143 °C, 1H NMR (CDCl3) δ 7.36 (d, 2H, J = 8.5 Hz, Ar), 7.31 (d, 2H, J = 7.9 Hz, Ar), 7.18 (d, 2H, J = 8.5 Hz, Ar), 7.07 (d, 1H, J = 7.9 Hz, Ar), 6.40 (br s, 1H, NHSO2), 5.46 (br t, 1H, NH), 4.36 (d, 1H, J = 5.7 Hz, ArCH2NH), 3.00 (s, 3H, SO2CH3), 1.59 (s, 3H, CH3), 1.55 (s, 3H, CH3), 1.30 (s, 9H, C(CH3)3); IR (KBr) 2964, 1644, 1513, 1333, 1154 cm−1; MS (FAB) m/z 403 (MH+); Anal. Calcd for C22H30N2O3S: C, 65.64; H, 7.51; N, 6.96. Found: C, 65.84; H, 7.49; N, 6.93.
4.2.2. N-(4-tert-Butylbenzyl)-2-[3-fluoro-4-(methylsulfonylamino)phenyl]-2-methylpropionamide (9)
Yield 74%, white solid, mp = 48–51 °C, 1H NMR (CDCl3) δ 7.53 (t, 1H, J = 8.2 Hz, H-5), 7.33 (d, 2H, Ar), 7.14–7.2 (m, 2H, H-2 and H-6), 7.09 (d, 2H, Ar), 6.45 (br s, 1H, NHSO2), 5.50 (br t, 1H, NH), 4.37 (d, 1H, J = 5.5 Hz, ArCH2NH), 3.03 (s, 3H, SO2CH3), 1.58 (s, 3H, CH3), 1.55 (s, 3H, CH3), 1.30 (s, 9H, C(CH3)3); IR (KBr) 2964, 1648, 1512, 1333, 1272, 1158, 1121 cm−1; MS (FAB) m/z 421 (MH+); Anal. Calcd for C22H29FN2O3S: C, 62.83; H, 6.95; N, 6.66. Found: C, 62.60; H, 6.93; N, 6.64.
4.2.3. N-(4-tert-Butylbenzyl)-2-[3-methoxy-4-(methylsulfonylamino)phenyl]-2-methylpropionamide (10)
Yield 93%, white solid, mp = 54–56 °C, 1H NMR (CDCl3) δ 7.46 (d, 1H, J = 8.2 Hz, H-5), 7.30 (d, 2H, Ar), 7.09 (d, 2H, Ar), 6.98 (dd, 1H, J = 1.8, 8.2 Hz, H-6), 6.83 (d, 1H, J = 1.8 Hz, H-2), 6.77 (br s, 1H, NHSO2), 5.55 (br t, 1H, NH), 4.36 (d, 1H, J = 5.7 Hz, ArCH2NH), 3.80 (s, 3H, OCH3), 2.94 (s, 3H, SO2CH3), 1.59 (s, 6H, 2 × CH3), 1.29 (s, 9H, C(CH3)3); IR (KBr) 3348, 2964, 1652, 1513, 1461, 1395, 1335, 1267, 1157, 1127, 1032 cm−1; MS (FAB) m/z 433 (MH+); Anal. Calcd for C23H32N2O4S: C, 63.86; H, 7.46; N, 6.48. Found: C, 63.60; H, 7.43; N, 6.45.
4.2.4. N-(4-tert-Butylbenzyl)-1-[4-(methylsulfonylamino) phenyl]cyclopropanecarboxamide (14)
Yield 90%, white solid, mp = 200–203 °C, 1H NMR (DMSO-d6) δ 9.75 (br s, 1H, NH), 4.15 (d, 2H, J = 6 Hz, ArCH2NH), 2.98 (s, 3H, SO2CH3), 1.32 (dd, 2H, CH2CCH2), 1.25 (s, 9H, C(CH3)3), 0.94 (dd, 2H, CH2CCH2); IR (KBr) 2961, 1644, 1515, 1331, 1224, 1154 cm−1; MS (FAB) m/z 401(MH+); Anal. Calcd for C22H28N2O3S: C, 65.97; H, 7.05; N, 6.99. Found: C, 66.21; H, 7.08; N, 6.96.
4.2.5. N-(4-tert-Butylbenzyl)-1-[3-fluoro-4-(methylsulfonylamino)phenyl]cyclopropanecarboxamide (15)
Yield 75%, white solid, mp = 56–58 °C, 1H NMR (CDCl3) δ 7.50 (br s, 1H, NHSO2), 7.32 (br d, 2H, Ar), 7.05–7.2 (m, 5H, Ar), 5.84 (br t, 1H, NH), 4.36 (d, 2H, J = 5.7 Hz, ArCH2NH), 3.33 (s, 3H, SO2CH3), 1.67 (dd, 2H, CH2CCH2), 1.29 (s, 9H, C(CH3)3), 1.15 (dd, 2H, CH2CCH2); IR (KBr) 2961, 1640, 1520, 1469, 1324, 1263, 1149 cm−1; MS (FAB) m/z 441 ([M+Na]+); Anal. Calcd for C22H27FN2O3S: C, 63.13; H, 6.50; N, 6.69. Found: C, 62.82; H, 6.47; N, 6.66.
4.2.6. N-(4-tert-Butylbenzyl)-1-[3-methoxy-4-(methylsulfonylamino)phenyl]cyclopropanecarboxamide (16)
Yield 82%, white solid, mp = 78–80 °C, 1H NMR (CDCl3) δ 7.48 (d, 1H, J = 8.1 Hz, H-5), 7.31 (br d, 2H, Ar), 7.09 (br d, 2H, Ar), 7.03 (dd, 1H, J = 1.8, 8.1 Hz, H-6), 6.94 (d, 1H, J = 1.8 Hz, H-2), 6.80 (br s, 1H, NHSO2), 5.67 (br t, 1H, NH), 4.36 (d, 2H, J = 5.85 Hz, ArCH2NH), 3.86 (s, 3H, OCH3), 2.97 (s, 3H, SO2CH3), 1.65 (dd, 2H, CH2CCH2), 1.29 (s, 9H, C(CH3)3), 1.06 (dd, 2H, CH2CCH2); IR (KBr) 2961, 1652, 1513, 1338, 1246, 1157, 1125, 1032 cm−1; MS (FAB) m/z 431(MH+), 453 ([M+Na]+); Anal. Calcd for C23H30N2O4S: C, 64.16; H, 7.02; N, 6.51. Found: C, 63.91; H, 7.00; N, 6.48.
4.2.7. N-{1-[3-Fluoro-4-(methylsulfonylamino)phenyl]ethyl}−3-(4-tert-butylphenyl)acetamide (18)
Yield 36%, white solid, mp = 134–136 °C, 1H NMR (CDCl3) δ 7.48 (t, 1H, J = 8.8 Hz, H-5), 7.39 (br d, 2H, J = 8.3 Hz, Ar), 7.18 (br d, 2H, J = 8.3 Hz, Ar), 6.92–7.02 (m, 2H, Ar), 6.44 (br s, 1H, NHSO2), 5.58 (d, 1H, J = 7.8 Hz, NH), 5.06 (m, 1H, CHCH3), 3.56 (s, 2H, ArCH2CO), 3.00 (s, 3H, SO2CH3), 1.37 (d, 3H, J = 7 Hz, CHCH3), 1.33 (s, 9H, C(CH3)3); IR (KBr) 3279, 2963, 1648, 1513, 1331, 1156 cm−1; MS (FAB) m/z 407 (MH+); Anal. Calcd for C21H27FN2O3S: C, 62.05; H, 6.69; N, 6.89. Found: C, 61.85; H, 6.67; N, 6.86.
4.2.8. N-{1-[3-Fluoro-4-(methylsulfonylamino)phenyl]ethyl}−3-(4-tert-butylphenyl) propanamide (19)
Yield 29%, white solid, mp = 152–154 °C, 1H NMR (CDCl3) δ 7.44 (t, 1H, J = 8 Hz, H-5), 7.31 (br d, 2H, J = 8.3 Hz, Ar), 7.11 (br d, 2H, J = 8.3 Hz, Ar), 6.95–7.02 (m, 2H, Ar), 6.82 (br s, 1H, NHSO2), 5.72 (d, 1H, J = 7.1 Hz, NH), 5.02 (m, 1H, CHCH3), 3.00 (s, 3H, SO2CH3), 2.93 (t, 2H, J = 7.1 Hz, CH2Ar), 2.50 (m, 2H, CH2CO), 1.34 (d, 3H, J = 7 Hz, CHCH3), 1.30 (s, 9H, C(CH3)3); IR (KBr) 3274, 2963, 1647, 1513, 1452, 1332, 1157 cm−1; MS (FAB) m/z 421 (MH+); Anal. Calcd for C22H29FN2O3S: C, 62.83; H, 6.95; N, 6.66. Found: C, 62.62; H, 6.93; N, 6.64.
4.2.9. N-{1-[3-Fluoro-4-(methylsulfonylamino)phenyl]ethyl}−3-(4-tert-butylphenyl)-2-propenamide (20)
Yield 67%, white solid, mp = 154–156 °C, 1H NMR (CDCl3) δ 7.62 (d, 1H, J = 15.5 Hz, CH=), 7.52 (t, 1H, J = 8 Hz, H-5), 7.41 (dd, 4H, Ar), 7.12–7.18 (m, 2H, Ar), 6.54 (br s, 1H, NHSO2), 6.37 (d, 1H, J = 15.5 Hz, =CH), 5.88 (d, 1H, J = 7.1 Hz, NH), 5.21 (m, 1H, CHCH3), 3.02 (s, 3H, SO2CH3), 1.53 (d, 3H, J = 7 Hz, CHCH3), 1.32 (s, 9H, C(CH3)3); IR (KBr) 3274, 2964, 1657, 1616, 1514, 1331, 1221, 1156 cm−1; MS (FAB) m/z 419 (MH+); Anal. Calcd for C22H27FN2O3S: C, 63.13; H, 6.50; N, 6.69. Found: C, 63.23; H, 6.52; N, 6.67.
4.2.10. N-(4-Chlorobenzyl)-2-[3-fluoro-4-(methylsulfonylamino)phenyl]propionamide (22)
Yield 98%, white solid, mp = 129–130 °C, 1H NMR (CDCl3) δ 7.53 (t, 1H, J = 8.3 Hz, H-5), 7.25–7.3 (m, 2H), 7.06–7.2 (m, 4H, Ar), 6.44 (br s, 1H, NHSO2), 5.67 (br t, 1H, NHCO), 4.37 (ddd of AB, 2H, ArCH2NH), 3.53 (q, 1H, J = 7.1 Hz, CH3CH), 3.03 (s, 3H, SO2CH3), 1.52 (d, 3H, J = 7.1 Hz, CH3CH); IR (KBr) 3295, 1652, 1539, 1496, 1330, 1235, 1156, 1092 cm−1; MS (FAB) m/z 385 [M-H]+; Anal. Calcd for C17H18ClFN2O3S: C, 53.05; H, 4.71; N, 7.28. Found: C, 52.87; H, 4.70; N, 7.25.
4.2.11. N-(4-Bromo-benzyl)-2-(3-fluoro-4-methanesulfonylamino-phenyl)-propionamide (23)
Yield 60%, white solid, mp = 172–174 °C, 1H NMR (CD3OD) δ 7.38–7.44 (m, 3H, Ar-H), 7.07–7.21 (m, 4H, Ar-H), 4.23 (s, 2H, ArCH2NH), 3.64 (q, 1H, J = 7.2 Hz, CH3CH), 2.96 (s, 3H, SO2CH3), 1.44 (d, 3H, J = 7.2 Hz, CHCH3); IR (KBr) 3741, 3274, 1650, 1512, 1330, 1155, 973, 757, 616 cm−1; MS (FAB) m/z 431 [M-H]+; Anal. Calcd for C17H18BrFN2O3S: C, 47.56; H, 4.23; N, 6.53. Found: C, 47.43; H, 4.22; N, 6.51.
4.2.12. 2-(3-Fluoro-4-methanesulfonylamino-phenyl)-N-(4-iodo-benzyl)-propionamide (24)
Yield 60%, white solid, mp = 79–81 °C, 1H NMR (CDCl3) δ 7.62 (d, 2H, J = 8.4 Hz, Ar-H), 7.51 (dd,1H, J = 8.1,1.8 Hz, Ar-H), 7.16 (dd, 1H, J = 8.1, 1.8 Hz, Ar-H), 7.08 (d, 1H, J = 8.1 Hz, Ar-H), 6.94 (d, 2H, J = 8.4 Hz, Ar-H), 6.52 (br s, 1H, NHSO2), 5.73 (br t, 1H, NHCO), 4.33 (d, 2H, J = 5.7 Hz, ArCH2NH), 3.52 (q, 1H, J = 7.2 Hz, CH3CH), 3.02 (s, 3H, SOCH3), 1.51 (d, 3H, J = 7.2 Hz, CHCH3); IR (KBr) 3739, 3293, 2922, 1642, 1511, 1332, 1153, 789 cm−1; MS (FAB) m/z 477 [M-H]+; Anal. Calcd for C17H18FIN2O3S: C, 42.87; H, 3.81; N, 5.88. Found: C, 42.66; H, 3.83; N, 5.86.
4.2.13. N-(3,4-Dichlorobenzyl)-2-[3-fluoro-4-(methylsulfonylamino)phenyl]propionamide (25)
Yield 76%, white solid, mp = 130–133 °C, 1H NMR (CDCl3) δ 7.53 (t, 1H, J = 8.3 Hz, H-5), 7.36 (d, 1H, Ar), 7.23 (d, 1H, Ar), 7.16 (dd, 1H, Ar), 7.10 (br d, 1H, Ar), 7.02 (dd, 1H, Ar), 6.51 (br s, 1H, NHSO2), 5.76 (br t, 1H, NHCO), 4.36 (d of AB, 2H, ArCH2NH), 3.54 (q, 1H, J = 7.1 Hz, CH3CH), 3.03 (s, 3H, SO2CH3), 1.52 (d, 3H, J = 7.1 Hz, CH3CH); IR (KBr) 3738, 1647, 1512, 1459, 1325, 1153 cm−1; MS (FAB) m/z 419 (MH+); Anal. Calcd for C17H17Cl2FN2O3S: C, 48.70; H, 4.09; N, 6.68. Found: C, 48.87; H, 4.11; N, 6.66.
4.2.14. N-(4-Trifluoromethylbenzyl)-2-[3-fluoro-4-(methylsulfonylamino)phenyl]propionamide (26)
Yield 81%, white solid, mp = 150–152 °C, 1H NMR (CDCl3) δ 7.5–7.6 (m, 3H, Ar), 7.26 (d, 2H, Ar), 7.05–7.2 (m, 2H, Ar), 5.86 (br t, 1H, NHCO), 4.46 (ddd of AB, 2H, ArCH2NH) 3.56 (q, 1H, J = 7.1 Hz, CH3CH), 3.02 (s, 3H, SO2CH3), 1.52 (d, 3H, J = 7.1 Hz, CH3CH); IR (KBr) 3291, 1653, 1512, 1421, 1327, 1159, 1119, 1067, 1019 cm−1; MS (FAB) m/z 419 (MH+); Anal. Calcd for C18H18F4N2O3S: C, 51.67; H, 4.34; N, 6.70. Found: C, 51.87; H, 4.32; N, 6.68.
4.2.15. N-(4-Methoxybenzyl)-2-[3-fluoro-4-(methylsulfonylamino)phenyl]propionamide (27)
Yield 96%, white solid, mp = 138 °C, 1H NMR (CDCl3) δ 7.48 (t, 1H, J = 8.3 Hz, H-5), 7.05–7.2 (m, 4H, Ar), 6.82 (d, 2H, Ar), 6.69 (br s, 1H, NHSO2), 5.80 (br t, 1H, NHCO), 4.33 (ddd of AB, 2H, ArCH2NH), 3.78 (s, 3H, OCH3), 3.52 (q, 1H, J = 7.1 Hz, CH3CH), 3.01 (s, 3H, SO2CH3), 1.51 (d, 3H, J = 7.1 Hz, CH3CH); IR (KBr) 3315, 3206, 1642, 1510, 1455, 1322, 1246, 1155, 1033 cm−1; MS (FAB) m/z 381 (MH+); Anal. Calcd for C18H21FN2O4S: C, 56.83; H, 5.56; N, 7.36. Found: C, 56.61; H, 5.54; N, 7.33.
4.2.16. N-(4-Methylbenzyl)-2-[3-fluoro-4-(methylsulfonylamino)phenyl]propionamide (28)
Yield 96%, white solid, mp = 166 °C, 1H NMR (CDCl3) δ 7.51 (t, 1H, J = 8.3 Hz, H-5), 7.05–7.2 (m, 6H, Ar), 6.50 (br s, 1H, NHSO2), 5.66 (br t, 1H, NHCO), 4.36 (ddd of AB, 2H, ArCH2NH), 3.51 (q, 1H, J = 7.1 Hz, CH3CH), 3.02 (s, 3H, SO2CH3), 2.32 (s, 3H, CH3), 1.52 (d, 3H, J = 7.1 Hz, CH3CH); IR (KBr) 3307, 3212, 1637, 1537, 1445, 1326, 1154 cm−1; MS (FAB) m/z 365 (MH+); Anal. Calcd for C18H21FN2O3S: C, 59.32; H, 5.81; N, 7.69. Found: C, 59.07; H, 5.79; N, 7.67.
4.2.17. N-(4-Isopropylbenzyl)-2-[3-fluoro-4-(methylsulfonylamino)phenyl]propionamide (29)
Yield 69%, white solid, mp = 137–139 °C, 1H NMR (CDCl3) δ 7.49 (t, 1H, J = 8.3 Hz, H-5), 7.05–7.2 (m, 6H, Ar), 6.70 (br s, 1H, NHSO2), 5.80 (br t, 1H, NHCO), 4.36 (ddd of AB, 2H, ArCH2NH), 3.52 (q, 1H, J = 7.1 Hz, CH3CH), 3.00 (s, 3H, SO2CH3), 2.88 (m, 2H, CH(CH3)2), 1.51 (d, 3H, J = 7.1 Hz, CH3CH), 1.22 (d, 6H, CH(CH3)2); IR (KBr) 3364, 2957, 1664, 1509, 1456, 1333, 1219, 1155 cm−1; MS (FAB) m/z 393 (MH+); Anal. Calcd for C20H25FN2O3S: C, 61.20; H, 6.42; N, 7.14. Found: C, 61.43; H, 6.40; N, 7.16.
4.2.18. N-(4-Cyclopropyl-benzyl)-2-(3-fluoro-4-methanesulfonylamino-phenyl)-propionamide (30)
Yield 71%, white solid, mp = 115–117 °C, 1H NMR (DMSO-d6) δ 9.54 (s, 1H, NHCO), 8.46 (t, 1H, J = 5.7 Hz, Ar-H), 7.12–7.34 (m, 4H, Ar-H), 6.95–7.04 (m, 3H, Ar-H), 4. 17 (d, 2H, J = 5.7 Hz, ArCH2NH), 3.67 (q, 1H, J = 7.2 Hz, CH3CH), 3.00 (s, 3H, SO2CH3), 1.86 (m, 1H, cyclo-H), 1.33 (d, 3H, J = 7.2 Hz, CHCH3), 0.89 (m, 2H, cyclo-H), 0.60 (m, 2H, cyclo-H); IR (KBr) 3289, 1650, 1600, 1512, 1331, 1156, 973 cm−1; MS (FAB) m/z 391 (MH+); Anal. Calcd for C20H23FN2O3S: C, 61.52; H, 5.94; N, 7.17. Found: C, 61.78; H, 5.92; N, 7.15.
4.2.19. N-(4-Cyclopent-2-enyl-benzyl)-2-(3-fluoro-4-methanesulfonylamino-phenyl)-propionamide (31)
Yield 68%, white solid, mp = 125–127 °C, 1H NMR (CDCl3) δ 7.51 (t, 1H, J = 8.25 Hz, Ar), 7.12 (m, 6H, Ar), 6.45 (br s, 1H, NH), 5.93 (m, 1H, CH2CH2), 5.73 (m, 1H, CH2CH2), 5.64 (br s, 1H, NH), 4.39 (ddd, 2H, ArCH2NH), 3.86 (m, 1H, cyclopentene), 3.50 (q, 1H, J = 7.14 Hz, CHCH3), 3.02 (s, 1H, SO2CH3), 2.43 (m, 2H, cyclopentene), 1.68 (m, 2H, cyclopentene), 1.52 (d, 3H, J = 7.14 Hz, CHCH3); IR (KBr) 3740, 3294, 1649, 1512, 1455, 1333, 1157, 973, 757, 616 cm−1; MS (FAB) m/z 417 (MH+); Anal. Calcd for C22H25FN2O3S: C, 63.44; H, 6.05; N, 6.73. Found: C, 63.24; H, 6.03; N, 6.71.
4.2.20. N-(4-Cyclopentyl-benzyl)-2-(3-fluoro-4-methanesulfonylamino-phenyl)-propionamide (32)
Yield 74%, white solid, mp = 121–123 °C, 1H NMR (CDCl3) δ 7.51 (t, 1H, J = 8.32 Hz, Ar), 7.14 (m, 6H, Ar), 6.50 (br s, 1H, NH), 5.66 (br t, 1H, NH), 4.38 (ddd, 2H, ArCH2), 3.50 (q, 1H, J = 7.14 Hz, CHCH3), 3.02 (s, 1H, SO2CH3), 2.96 (m, 1H, cyclopentyl), 2.04 (m, 2H, cyclopentyl), 1.79 (m, 4H, cyclopentyl), 1.68 (m, 4H, cyclopentyl), 1.57 (d, 3H, J = 7.14 Hz, CHCH3); IR (KBr) 3438, 3227, 2951, 1640, 1547, 1509, 1332, 1157, 1116, 983, 774 cm−1; MS (FAB) m/z 419 (MH+); Anal. Calcd for C22H27FN2O3S: C, 63.13; H, 6.50; N, 6.69. Found: C, 63.39; H, 6.52; N, 6.67.
4.2.21. N-(4-Cyclohexyl-benzyl)-2-(3-fluoro-4-methanesulfonylamino-phenyl)-propionamide (33)
Yield 61%, white solid, mp = 72–74 °C, 1H NMR (CDCl3) δ 7.51 (dd, 1H, J = 8.1, 1.8 Hz, Ar-H), 7.08–7.20 (m, 6H, Ar-H), 5.63 (br t, 1H, NHCO), 4.38 (qd, 2H, J = 14.4, 5.4 Hz, ArCH2NH), 3.50 (q, 1H, J = 7.2 Hz, CH3CH), 3.02 (s, 3H, SO2CH3), 2.47 (m, 1H, cyclo-H), 1.72–1.84 (m, 4H, cyclo-H), 1.52 (d, 3H, J = 7.2 Hz, CHCH3), 1.26–1.41 (m, 6H, cyclo-H); IR (KBr) 3290, 2925, 2851, 1650, 1511, 1449, 1332, 1157, 973 cm−1; MS (FAB) m/z 433 (MH+); Anal. Calcd for C23H29FN2O3S: C, 63.86; H, 6.76; N, 6.48. Found: C, 63.63; H, 6.78; N, 6.47.
4.2.22. N-(4-Biphenylmethyl)-2-[3-fluoro-4-(methylsulfonylamino)phenyl]propionamide (34)
Yield 78%, white solid, mp = 155–157 °C, 1H NMR (CDCl3) δ 7.1–7.58 (m, 12H, Ar), 6.45 (br s, 1H, NHSO2), 5.71 (br t, 1H, NHCO), 4.45 (ddd, 2H, ArCH2NH), 3.55 (q, 1H, J = 7.1 Hz, CH3CH), 3.01 (s, 3H, SO2CH3), 1.54 (d, 3H, J = 7.1 Hz, CH3CH); IR (KBr) 3430, 1647, 1530, 1405, 1324, 1152 cm−1; MS (FAB) m/z 427 (MH+); Anal. Calcd for C23H23FN2O3S: C, 64.77; H, 5.44; N, 6.57. Found: C, 64.51; H, 5.43; N, 6.53.
4.2.23. N-(2-Bromo-4-tert-butyl-benzyl)-2-(3-fluoro-4-methanesulfonylamino-phenyl)-propionamide (35)
Yield 71%, white solid, mp = 138–140 °C, 1H NMR (CDCl3) δ 7.51–7.46 (m, 2H, Ar-H), 7.28–7.06 (m, 4H, Ar-H), 6.71 (br s, 1H, NHSO2), 6.00 (br s, 1H, NHCO), 4.42 (m, 2H, ArCH2NH), 3.54 (q, 1H, J = 7.14 Hz, CH3CH), 3.02 (s, 3H, SO2CH3), 1.49 (d, 3H, J = 7.14 Hz, CH3CH), 1.28 (s, 9H, C(CH3)3); IR (KBr) 3315, 2931, 1649, 1510, 1421, 1327, 1159, 1110, 927 cm−1; MS (FAB) m/z 486 (MH+); Anal. Calcd for C21H26BrFN2O3S: C, 51.96; H, 5.40; N, 5.77. Found: C, 51.76; H, 5.43; N, 5.74.
4.2.24. N-(4-tert-Butyl-2-cyano-benzyl)-2-(3-fluoro-4-methanesulfonylamino-phenyl)-propionamide (36)
Yield 68%, white solid, mp = 129–131 °C, 1H NMR (CDCl3) δ 7.51 (m, 2H, Ar-H), 7.24 (m, 2H, Ar-H), 7.13 (dd, 1H, J = 11.37, 2.01 Hz, Ar-H), 7.07 (d, 1H, J = 7.43 Hz, Ar-H), 6.50 (br s, 1H, NHSO2), 5.88 (br s, 1H, NHCO), 4.42 (m, 2H, ArCH2NH), 3.52 (q, 1H, J = 7.14 Hz, CH3CH), 3.02 (s, 3H, SO2CH3), 1.50 (d, 3H, J = 7.14 Hz, CH3CH), 1.29 (s, 9H, C(CH3)3); IR (KBr) 3421, 2951, 2928, 2250, 1650, 1512, 1449, 1333, 1200, 1120, 981 cm−1; MS (FAB) m/z 432 (MH+); Anal. Calcd for C22H26FN3O3S: C, 61.23; H, 6.07; N, 9.74. Found: C, 61.00; H, 6.05; N, 9.72.
4.2.25. N-(1-Naphthylmethyl)-2-[3-fluoro-4-(methylsulfonylamino)phenyl]propionamide (37)
Yield 79%, white solid, mp = 159–161 °C, 1H NMR (CDCl3) δ 7.75–7.9 (m, 3H, Ar-H), 7.3–7.5 (m, 5H, Ar-H), 7.16 (dd, 1H, Ar-H), 7.04 (br d, 1H, Ar-H), 6.52 (br s, 1H, NHSO2), 5.69 (br t, 1H, NHCO), 4.86 (ddd, 2H, ArCH2NH), 3.49 (q, 1H, J = 7.1 Hz, CH3CH), 2.96 (s, 3H, SO2CH3), 1.51 (d, 3H, J = 7.1 Hz, CH3CH); IR (KBr) 3292, 1648, 1511, 1453, 1329, 1156 cm−1; MS (FAB) m/z 401 (MH+); Anal. Calcd for C21H21FN2O3S: C, 62.98; H, 5.29; N, 7.00. Found: C, 62.79; H, 5.27; N, 6.98.
4.2.26. N-(1,2,3,4-Tetrahydro-1-naphthalenyl)-2-[3-fluoro-4-(methylsulfonylamino)phenyl]propionamide (38)
Yield 73%, white solid, mp = 116–117 °C, 1H NMR (CDCl3) δ 7.51 (m, 1H, Ar-H), 6.8–7.2 (m, 6H, Ar-H), 6.53 (br s, 1H, NHSO2), 5.62 (br d, 1H, NHCO), 5.15 (m, 1H, CHNH), 3.51 (q, 1H, J = 7.14 Hz, CH3CH), 3.00 (s, 3H, SO2CH3), 2.75 (m, 2H, cyclo-H), 1.7–1.9 (m, 4H, cyclo-H), 1.53 (d, 3H, J = 7.14 Hz, CH3CH); IR (KBr) 3430, 3235, 1657, 1511, 1452, 1325, 1153 cm−1; MS (FAB) m/z 391 (MH+); Anal. Calcd for C20H23FN2O3S: C, 61.52; H, 5.94; N, 7.17. Found: C, 61.32; H, 5.91; N, 7.15.
4.2.27. N-[2-(4-t-Butylphenyl)ethyl]-2-[3-fluoro-4-(methylsulfonylamino)phenyl]propionamide (39)
Yield 64%, white solid, mp = 124–126 °C, 1H NMR (CDCl3) δ 7.50 (t, 1H, J = 8.3 Hz, H-5), 7.29 (br d, 2H, Ar-H), 6.95–7.15 (m, 4H, Ar-H), 6.52 (br s, 1H, NHSO2), 5.41 (br t, 1H, NHCO), 3.47 (m, 3H, CH2NH and CH3CH), 3.03 (s, 3H, SO2CH3), 2.72 (t, 2H, J = 6.8 Hz, CH2Ar), 1.47 (d, 3H, J = 7.3 Hz, CH3CH), 1.31 (s, 9H, C(CH3)3); IR (KBr) 3286, 2961, 1734, 1650, 1512, 1455, 1334, 1265, 1157 cm−1; MS (FAB) m/z 421 (MH+); Anal. Calcd for C22H29FN2O3S: C, 62.83; H, 6.95; N, 6.66. Found: C, 62.99; H, 6.98; N, 6.64.
4.2.28. N-[3-(3,4-Dimethylphenyl)propyl]-2-[3-fluoro-4-(methylsulfonylamino)phenyl]propionamide (40)
Yield 95%, white solid, mp = 128–130 °C, 1H NMR (CDCl3) δ 7.50 (t, 1H, J = 8.3 Hz, H-5), 7.13 (dd, 1H, J = 1.95,11.2 Hz, H-2), 7.0–7.07 (m, 2H, Ar-H), 6.83–6.92 (m, 2H, Ar-H), 6.57 (br s, 1H, NHSO2), 3.41 (q, 1H, J = 7.1 Hz, CHMe), 3.2–3.3 (m, 2H, CH2NH), 3.01 (s, 3H, SO2CH3), 2.51 (t, 2H, J = 7.6 Hz, CH2Ar), 2.22 (s, 6H, 2 × CH3), 1.7–1.8 (m, 2H, CH2), 1.45 (d, 3H, J = 7.1 Hz, CHCH3); IR (KBr) 3294, 2931, 1648, 1509, 1449, 1331, 1157 cm−1; MS (FAB) m/z 407 (MH+)+); Anal. Calcd for C21H27FN2O3S: C, 62.05; H, 6.69; N, 6.89. Found: C, 62.25; H, 6.67; N, 6.86.
4.2.29. N-[3-(3,4-Dimethylphenyl)propyl]-(2R)-2-[3-fluoro-4-(methylsulfonylamino)phenyl]propionamide (41)
Yield 96%, white solid, mp = 128–130 °C, 1H NMR (CDCl3) δ 7.50 (t, 1H, J = 8.3 Hz, H-5), 7.13 (dd, 1H, J = 1.95, 11.2 Hz, H-2), 7.0–7.07 (m, 2H, Ar-H), 6.83–6.92 (m, 2H, Ar-H), 6.57 (br s, 1H, NHSO2), 3.41 (q, 1H, J = 7.1 Hz, CHMe), 3.2–3.3 (m, 2H, CH2NH), 3.01 (s, 3H, SO2CH3), 2.51 (t, 2H, J = 7.6 Hz, CH2Ar), 2.22 (s, 6H, 2 × CH3), 1.7–1.8 (m, 2H, CH2), 1.45 (d, 3H, J = 7.1 Hz, CHCH3); IR (KBr) 3294, 2931, 1648, 1509, 1449, 1331, 1157 cm−1; MS (FAB) m/z 407 (MH+); [α] −4.23 (c 0.25, CHCl3); Anal. Calcd for C21H27FN2O3S: C, 62.05; H, 6.69; N, 6.89. Found: C, 62.31; H, 6.72; N, 6.90.
4.2.30. N-[3-(3,4-Dimethylphenyl)propyl]-(2S)-2-[3-fluoro-4-(methylsulfonylamino)phenyl]propionamide (42)
Yield 95%, white solid, mp = 128–130 °C, 1H NMR (CDCl3) δ 7.50 (t, 1H, J = 8.3 Hz, H-5), 7.13 (dd, 1H, J = 1.95, 11.2 Hz, H-2), 7.0–7.07 (m, 2H, Ar-H), 6.83–6.92 (m, 2H, Ar-H), 6.57 (br s, 1H, NHSO2), 3.41 (q, 1H, J = 7.1 Hz, CHMe), 3.2–3.3 (m, 2H, CH2NH), 3.01 (s, 3H, SO2CH3), 2.51 (t, 2H, J = 7.6 Hz, CH2Ar), 2.22 (s, 6H, 2 × CH3), 1.7–1.8 (m, 2H, CH2), 1.45 (d, 3H, J = 7.1 Hz, CHCH3); IR (KBr) 3294, 2931, 1648, 1509, 1449, 1331, 1157 cm−1; MS (FAB) m/z 407 (MH+); [α] +4.34 (c 0.25, CHCl3); Anal. Calcd for C21H27FN2O3S: C, 62.05; H, 6.69; N, 6.89. Found: C, 61.93; H, 6.71; N, 6.86.
4.2.31. N-[3-(3,4-Dimethylphenyl)-2-propenyl]-2-[3-fluoro-4-(methylsulfonylamino)phenyl]propionamide (43)
Yield 78%, white solid, mp = 144–146 °C, 1H NMR (CDCl3) δ 7.52 (t, 1H, J = 8.3 Hz, H-5), 7.0–7.2 (m, 5H, Ar-H), 6.58 (br s, 1H, NHSO2), 6.37 (d, 1H, J = 15.8 Hz, ArCH=CH), 6.06 (dt, 1H, J = 6.2, 15.8 Hz, ArCH=CH), 5.57 (br t, 1H, NHCO), 3.9–4.02 (m, 2H, CH2NH), 3.53 (q, 1H, J = 7.14 Hz, CHMe), 3.01 (s, 3H, SO2CH3), 2.24 (s, 6H, 2 × CH3), 1.52 (d, 3H, J = 7.14Hz, CH3); IR (KBr) 3280, 1642, 1509, 1331, 1157 cm−1; MS (FAB) m/z 405 (MH+); Anal. Calcd for C21H25FN2O3S: C, 62.35; H, 6.23; N, 6.93. Found: C, 62.57; H, 6.21; N, 6.90.
4.2.32. N-[3-(4-Chloro-phenyl)-propyl]-2-(3-fluoro-4-methanesulfonylamino-phenyl)-propionamide (44)
Yield 70%, white solid, mp = 141–143 °C, 1H NMR (CDCl3) δ 7.51 (t, 1H, J = 8.3 Hz, H-5), 7.02–7.25 (m, 6H, Ar-H), 6.52 (br s, 1H, NHSO2), 5.38 (br t, 1H, NHCO), 3.44 (q, 1H, J = 7.14 Hz, CH3CH), 3.24 (ddd, 2H, CH2NH), 3.02 (s, 3H, SO2CH3), 2.55 (t, 2H, J = 7.5 Hz, ArCH2), 1.76 (m, 2H, ArCH2CH2), 1.47 (d, 3H, J = 7.14 Hz, CH3CH); IR (KBr) 3430, 2934, 1644, 1548, 1509, 1445, 1405, 1325, 1154, 1116 cm−1; MS (FAB) m/z 413 (MH+); Anal. Calcd for C19H22ClFN2O3S: C, 55.27; H, 5.37; N, 6.78. Found: C, 55.10; H, 5.39; N, 6.80.
4.2.33. N-[3-(4-Chloro-phenyl)-allyl]-2-(3-fluoro-4-methanesulfonylamino-phenyl)-propionamide (45)
Yield 72%, white solid, mp = 151–153 °C, 1H NMR (CDCl3) δ 7.52 (t, 1H, J = 8.3 Hz, H-5), 7.08–7.3 (m, 6H, Ar), 6.60 (br s, 1H, NHSO2), 6.37 (d, 1H, J = 15.8 Hz, ArCH=CH), 6.10 (dt, 1H, J = 6.2, 15.8 Hz, ArCH=CH), 5.61 (br t, 1H, NHCO), 3.9–4.1 (m, 2H, CH2NH), 3.54 (q, 1H, J = 7.1 Hz, CHMe), 3.02 (s, 3H, SO2CH3), 1.52 (d, 3H, J = 7.1 Hz, CH3); IR (KBr) 3430, 3318, 3216, 1644, 1543, 1508, 1407, 1326, 1156, 1092 cm−1; MS (EI) m/z 410 (M+); Anal. Calcd for C19H20ClFN2O3S: C, 55.54; H, 4.91; N, 6.82. Found: C, 55.77; H, 4.93; N, 6.80.
4.2.34. N-Benzyloxy-2-[3-fluoro-4-(methylsulfonylamino) phenyl]propionamide (46)
Yield 76%, white solid, mp = 182–183 °C, 1H NMR (CDCl3) δ 7.94 (s, 1H, ONH), 7.49 (t, 1H, J = 8.3 Hz, H-5), 7.25–7.35 (m, 5H, Ph), 7.12 (dd, 1H, J = 2, 11.2 Hz, H-2), 7.02 (dd, 1H, J = 2, 8.2 Hz, H-6), 6.52 (br s, 1H, NHSO2), 4.87 (s, 2H, PhCH2O), 3.35 (q, 1H, J = 7.1 Hz, CHCH3), 3.02 (s, 3H, SO2CH3), 1.46 (d, 3H, J = 7.1 Hz, CHCH3); IR (KBr) 3235, 1671, 1511, 1455, 1331, 1156 cm−1; MS (FAB) m/z 367 (MH+); Anal. Calcd for C17H19FN2O4S: C, 55.73; H, 5.23; N, 7.65. Found: C, 55.94; H, 5.21; N, 7.64.
4.2.35. N-Benzhydryl-2-[3-fluoro-4-(methylsulfonylamino)phenyl]propionamide (47)
Yield 79%, white solid, mp = 160–161 °C, 1H NMR (CDCl3) δ 7.51 (t, 1H, J = 8.3 Hz, H-5), 7.0–7.4 (m, 10H, Ar), 6.20 (d, 1H, Ph2CH), 6.04 (br t, 1H, NHCO), 3.58 (q, 1H, J = 7.1 Hz, CHCH3), 3.00 (s, 3H, SO2CH3), 1.52 (d, 3H, J = 7.1 Hz, CHCH3); IR (KBr) 3374, 3089, 1657, 1588, 1513, 1445, 1323, 1156 cm−1; MS (FAB) m/z 427 (MH+); Anal. Calcd for C23H23FN2O3S: C, 64.77; H, 5.44; N, 6.57. Found: C, 64.95; H, 5.42; N, 6.59.
4.2.36. N-(2,2-Diphenylethyl)-2-[3-fluoro-4-(methylsulfonylamino)phenyl]propionamide (48)
Yield 64%, white solid, mp = 129 °C, 1H NMR (CDCl3) δ 7.42 (t, 1H, J = 8.3 Hz, H-5), 7.1–7.3 (m, 10H, Ar), 6.95 (dd, 1H, H-6), 6.87 (d, 1H, H-2), 6.50 (br s, 1H, NHSO2), 5.28 (br t, 1H, NHCO), 4.12 (t, 1H, Ph2CH), 3.75–3.95 (m, 2H, CH2NH), 3.37 (q, 1H, J = 7.1 Hz, CHCH3), 3.01 (s, 3H, SO2CH3), 1.40 (d, 3H, J = 7.1 Hz, CHCH3); IR (KBr) 3374, 3027, 1656, 1508, 1451, 1330, 1158, 1116 cm−1; MS (FAB) m/z 441 (MH+); Anal. Calcd for C24H25FN2O3S: C, 65.43; H, 5.72; N, 6.36. Found: C, 65.19; H, 5.74; N, 6.38.
4.2.37. N-(3,3-Diphenylpropyl)-2-[3-fluoro-4-(methylsulfonylamino)phenyl]propionamide (49)
Yield 76%, white solid, mp = 66–68 °C, 1H NMR (CDCl3) δ 7.51 (t, 1H, J = 8.3 Hz, H-5), 7.0–7.3 (m, 12H, Ar-H), 6.45 (br s, 1H, NHSO2), 5.27 (br t, 1H, NHCO), 3.85 (t, 1H, J = 7.8 Hz, Ph2CH), 3.34 (q, 1H, J = 7.1 Hz, CH3CH), 3.21 (ddd, 2H, CH2NH), 3.01 (s, 3H, SO2CH3), 2.24 (dd, 2H, CHCH2), 1.43 (d, 3H, J = 7.1 Hz, CH3CH); IR (KBr) 3290, 1649, 1510, 1450, 1331, 1156 cm−1; MS (FAB) m/z 455 (MH+); Anal. Calcd for C25H27FN2O3S: C, 66.06; H, 5.99; N, 6.16. Found: C, 66.29; H, 5.97; N, 6.13.
4.2.38. N-(3,3-Diphenyl-2-propenyl)-2-[3-fluoro-4-(methylsulfonylamino)phenyl]propionamide (50)
Yield 78%, white solid, mp = 155–157 °C, 1H NMR (CDCl3) δ 7.52 (t, 1H, J = 8.3 Hz, H-5), 7.05–7.4 (m, 12H, Ar), 6.50 (br s, 1H, NHSO2), 6.00 (t, 1H, J = 7.0 Hz, >C=CH), 5.44 (br t, 1H, NHCO), 3.85–4.0 (m, 2H, CH2NH), 3.46 (q, 1H, J = 7.1 Hz, CHCH3), 3.01 (s, 3H, SO2CH3), 1.48 (d, 3H, J = 7.1 Hz, CHCH3); IR (KBr) 3736, 1647, 1512, 1333, 1157 cm−1; MS (EI) m/z 452 (M+); Anal. Calcd for C25H25FN2O3S: C, 55.73; H, 5.23; N, 7.65. Found: C, 55.61; H, 5.22; N, 7.64.
4.2.39. N-(2,2-Diphenyl-cyclopropylmethyl)-2-(3-fluoro-4-methanesulfonylamino-phenyl)-propionamide (51)
Yield 83%, white solid, mp = 166–168 °C, 1H NMR (CDCl3) δ 7.55 (m, 1H, Ar-H), 7.24–7.06 (m, 12H, Ar-H), 6.52 (s, 1H, SO2NH), 5.30 (s, 1H, NHCO), 3.49 (m, 4H, Ar2CH, CH2NH and COCH), 2.95 (s, 3H, SO2CH3), 1.95 (m, 1H), 1.50 (d, 3H, J = 4.95 Hz), 1.34 (m, 1H, cyclo-H), 1.16 (m, 1H, cyclo-H); IR (KBr) 3290, 2983, 1649, 1510, 1450, 1331, 1156, 1045, 974 cm−1; MS (FAB) m/z 467 (MH+); Anal. Calcd for C26H27FN2O3S: C, 66.93; H, 5.83; N, 6.00. Found: C, 66.68; H, 5.81; N, 6.03.
4.2.40. N-(2-Diphenylamino-ethyl)-2-(3-fluoro-4-methanesulfonylamino-phenyl)-propionamide (52)
Yield 87%, white solid, mp = 156–158 °C, 1H NMR (CDCl3) δ 7.47 (t, 1H, J = 8.23 Hz, Ar), 7.25 (m, 4H, Ar), 7.07 (d, 1H, J = 11.16 Hz, Ar), 6.95 (m, 7H, Ar), 6.49 (s, 1H, Ar), 5.58 (br t, 1H, NH), 3.86 (q, 2H, CH2NH), 3.46 (q, 2H, Ar2NCH2), 3.37 (q, 1H, CHCH3), 2.99 (s, 3H, SO2CH3), 1.44 (d, 3H, J = 7.14Hz, CHCH3); IR (KBr) 3296, 1651, 1589, 1498, 1332, 1157, 1116, 972, 909, 752, 697 cm−1; MS (FAB) m/z 455 (MH+); Anal. Calcd for C24H26FN3O3S: C, 63.28; H, 5.75; N, 9.22. Found: C, 63.57; H, 5.73; N, 9.20.
4.2.41. N-(3,3-Di-p-tolyl-propyl)-2-(3-fluoro-4-methansulfonylamino-phenyl)-propionamide (53)
Yield 90%, white solid, mp = 150–152 °C, 1H NMR (CDCl3) δ 7.51 (t, 1H, J = 8.40 Hz, Ar-H), 7.13–7.01 (m, 10H, Ar), 6.45 (br s, 1H, NHSO2), 5.25 (br t, 1H, NHCO), 3.33 (q, 1H, J = 7.14 Hz, CHCH3), 3.20 (m, 2H, CH2NH), 3.01 (s, 3H, SO2CH3), 2.28 (s, 6H, (Ar-CH3)2), 2.18 (q, 2H, J = 7.50 Hz, Ar-CH2), 1.42 (d, 3H, J = 7.14 Hz, CHCH3); IR (KBr) 3292, 2928, 1649, 1511, 1448, 1332, 1158, 1118, 972 cm−1; MS (EI) m/z 483 (M+); Anal. Calcd for C27H31FN2O3S: C, 67.19; H, 6.47; N, 5.80. Found: C, 67.37; H, 6.45; N, 5.81.
4.2.42. N-[3,3-Di(4-methylphenyl)-2-propenyl]-2-[3-fluoro-4-(methylsulfonylamino)phenyl]propionamide (54)
Yield 72%, white solid, mp = 163–165 °C, 1H NMR (CDCl3) δ 7.49 (t, 1H, J = 8.3 Hz, H-5), 6.95–7.2 (m, 10H, Ar-H), 5.93 (t, 1H, J = 7.0 Hz, >C=CH), 5.56 (br t, 1H, NHCO), 3.8–4.0 (m, 2H, CH2NH), 3.47 (q, 1H, J = 7.1 Hz, CHCH3), 3.00 (s, 3H, SO2CH3), 2.34 (d, 6H, 2 × CH3), 1.47 (d, 3H, J = 7.1 Hz, CHCH3); IR (KBr) 3279, 1648, 1511, 1451, 1332, 1158, 1116 cm−1; MS (FAB) m/z 481 (MH+); Anal. Calcd for C27H29FN2O3S: C, 67.48; H, 6.08; N, 5.83. Found: C, 67.19; H, 6.05; N, 5.80.
4.2.43. N-[3,3-Bis-(4-fluoro-phenyl)-propyl]-2-(3-fluoro-4-methansulfonylamino-phenyl)-propionamide (55)
Yield 93%, white solid, mp = 62–64 °C, 1H NMR (CDCl3) δ 7.52 (t, 1H, J = 8.43 Hz, Ar-H), 7.14–6.93 (m, 10H, Ar-H), 6.46 (br s, 1H, NHSO2), 5.32 (s, 1H, NHCO), 3.82 (t, 1H, J = 7.71 Hz, Ar-CH), 3.40 (q, 1H, J = 7.14 Hz, CHCH3), 3.18 (m, 2H, CH2NH), 3.02 (s, 3H, SO2CH3), 2.17 (q, 2H, J = 7.50 Hz, CH–CH2), 1.45 (d, 3H, J = 7.14 Hz, CHCH3); IR (KBr) 3291, 1650, 1508, 1451, 1334, 1223, 1158, 970 cm−1; MS (EI) m/z 491 (M+); Anal. Calcd for C25H25F3N2O3S: C, 61.21; H, 5.14; N, 5.71. Found: C, 61.48; H, 5.15; N, 5.70.
4.2.44. N-[3,3-Di(4-fluorophenyl)-2-propenyl]-2-[3-fluoro-4-(methylsulfonylamino)phenyl]propionamide (56)
Yield 78%, white solid, mp = 57–60 °C, 1H NMR (CDCl3) δ 7.49 (t, 1H, J = 8.3 Hz, H-5), 6.9–7.2 (m, 10H, Ar), 6.72 (br s, 1H, NHSO2), 5.92 (t, 1H, J = 7.0 Hz, >C=CH), 5.58 (br s, 1H, NHCO), 3.8–4.0 (m, 2H, CH2NH), 3.48 (q, 1H, J = 7.1 Hz, CHCH3), 3.02 (s, 3H, SO2CH3), 1.48 (d, 3H, J = 7.1 Hz, CHCH3); IR (KBr) 3280, 1650, 1599, 1509, 1332, 1225, 1157 cm−1; MS (FAB) m/z 489 (MH+); Anal. Calcd for C25H23F3N2O3S: C, 61.46; H, 4.75; N, 5.73. Found: C, 61.66; H, 4.73; N, 5.71.
4.2.45. N-[3,3-Bis-(4-chloro-phenyl)-allyl]-2-(3-fluoro-4-methanesulfonylamino-phenyl)-propionamide (57)
Yield 73%, white solid, mp = 72–74 °C, 1H NMR (CDCl3) δ 7.50 (t, 1H, J = 8.23 Hz, Ar), 7.34 (d, 2H, J = 8.61 Hz, Ar), 7.24 (d, 2H, J = 12.99 Hz, Ar), 7.15 (dd, 1H, J = 11.37 Hz, Ar), 7.10–7.02 (m, 5H, Ar), 6.60 (br s, 1H, NH), 5.97 (t, 1H, J = 6.87 Hz, CCHCH2), 5.54 (br t, 1H, NH), 3.89 (m, 2H, CH2NH), 3.47 (q, 1H, J = 7.14 Hz, CHCH3), 3.02 (s, 3H, SO2CH3), 1.48 (d, 3H, J = 7.14 Hz, CHCH3); IR (KBr) 3293, 1649, 1512, 1332, 1157, 1092, 973, 831, 757 cm−1; MS (FAB) m/z 521 (MH+); Anal. Calcd for C25H23Cl2FN2O3S: C, 57.59; H, 4.45; N, 5.37. Found: C, 57.32; H, 4.43; N, 5.35.
4.2.46. 2-(3-Fluoro-4-methanesulfonylamino-phenyl)-N-[3-(4-fluoro-phenyl)-3-p-tolyl-propyl]-propionamide (58)
Yield 72%, white solid, mp = 63–65 °C, 1H NMR (CDCl3) δ 7.51 (t, 1H, Ar), 7.15–7.02 (m, 8H, Ar), 6.94 (t, 1H, Ar), 6.46 (br, 1H, NH), 5.28 (br t, 1H, NH), 3.78 (td, 1H, ArCHAr), 3.37 (q, 1H, J = 7.14 Hz, CHCH3), 3.18 (m, 2H, CH2NH), 3.01 (s, 3H, SO2CH3), 2.29 (s, 3H, ArCH3), 2.17 (q, 2H, Ar2CHCH2), 1.44 (d, 3H, J = 7.14 Hz, CHCH3); IR (KBr), 1649, 1509, 1454, 1332, 1222, 1157, 972, 819, 758 cm−1; MS (FAB) m/z 487 (M+); Anal. Calcd for C26H28F2N2O3S: C, 64.18; H, 5.80; N, 5.76. Found: C, 64.39; H, 5.82; N, 5.75.
4.2.47. N-[2-(10,11-Dihydro-5H-dibenzo[a,d]cyclohepten-5-yliden)ethyl]-2-[3-fluoro-4-(methylsulfonylamino) phenyl]propionamide (59)
Yield 76%, white solid, mp = 67–69 °C, 1H NMR (CDCl3) δ 7.50 (t, 1H, J = 8.3 Hz, H-5), 7.05–7.25 (m, 10H, Ar), 6.49 (br s, 1H, NHSO2), 5.80 (t, 1H, >C=CH), 5.40 (br t, 1H, NHCO), 4.13 (m, 1H, CH2NH), 3.71 (m, 1H, CH2NH), 3.43 (q, 1H, J = 7.1 Hz, CHCH3), 3.2–3.4 (m, 4H, PhCH2CH2Ph), 3.01 (s, 3H, SO2CH3), 1.46 (d, 3H, J = 7.1 Hz, CHCH3); IR (KBr) 3294, 2927, 1649, 1511, 1453, 1332, 1156 cm−1; MS (FAB) m/z 479 (MH+); Anal. Calcd for C27H27FN2O3S: C, 67.76; H, 5.69; N, 5.85. Found: C, 67.54; H, 5.70; N, 5.82.
4.2.48. N-[2-Dibenzo[a,d]cyclohepten-5-ylidene-ethyl]-2-(3-fluoro-4-methylsulfonylamino-phenyl)-propionamide (60)
Yield 91%, white solid, mp = 70–72 °C, 1H NMR (CDCl3) δ 7.48 (m, 1H, Ar-H), 7.33–7.26 (m, 8H, Ar-H), 7.16–7.00 (m, 3H, Ar-H), 6.85 (s, 2H, Ar-H and NHSO2), 5.50 (m, 1H, >C=CH), 5.34 (br t, 1H, NHCO), 4.30 (m, 1H, CH2NH), 3.55 (m, 1H, CH2NH), 3.39 (q, 1H, J = 6.78 Hz, CHCH3), 3.00 (s, 3H, SO2CH3), 1.44 (d, 3H, J = 7.14 Hz, CHCH3); IR (KBr) 3450, 2951, 2938, 1650, 1511, 1448, 1332, 1160 cm−1; MS (FAB) m/z 477 (MH+); Anal. Calcd for C27H25FN2O3S: C, 68.05; H, 5.29; N, 5.88. Found: C, 68.31; H, 5.27; N, 5.86.
Acknowledgments
This research was supported by Research Funding from Digital-biotech, Grants R11-2007-107-02001-0 from the National Research Foundation of Korea (NRF), and the Intramural Research Program of the National Institutes of Health, Center for Cancer Research, National Cancer Institute (Z1A BC 005270). We thank multiple additional fellows for technical assistance.
References and notes
- 1.Szallasi A; Blumberg PM Pharmacol. Rev 1999, 51,159. [PubMed] [Google Scholar]
- 2.Tominaga M; Caterina MJ; Malmberg AB; Rosen TA; Gilbert H; Skinner K; Raumann BE; Basbaum AI; Julius D Neuron 1998, 21, 531. [DOI] [PubMed] [Google Scholar]
- 3.Caterina MJ; Schumacher MA; Tominaga M; Rosen TA; Levine JD; Julius D Nature 1997, 389, 816. [DOI] [PubMed] [Google Scholar]
- 4.Zygmunt PM; Petersson J; Andersson DA; Chuang H-H; Sorgard M; Di Marzo V; Julius D; Hogestatt ED Nature 1999, 400, 452. [DOI] [PubMed] [Google Scholar]
- 5.Hwang SW; Cho H; Kwak J; Lee SY; Kang CJ;Jung J; Cho S; Min KH; Suh YG; Kim D; Oh U Proc. Natl. Acad. Sci. U.S.A. 2000, 97, 6155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Walpole CSJ; Wrigglesworth R Capsaicin in the Study of Pain; Academic Press, 1993. pp 63. [Google Scholar]
- 7.Appendino G; Szallasi A Life Sci. 1997, 60, 681. [DOI] [PubMed] [Google Scholar]
- 8.Szallasi A; Cruz F; Geppetti P Trends Mol. Med 2006, 12, 545. [DOI] [PubMed] [Google Scholar]
- 9.Voight EA; Kort ME Exp. Opin. Ther. Pat 2010, 20, 1. [DOI] [PubMed] [Google Scholar]
- 10.Lazar J; Gharat L; Khairathkar-Joshi N; Blumberg PM; Szallasi A Exp. Opin. Drug Discov. 2009, 4, 159. [DOI] [PubMed] [Google Scholar]
- 11.Gunthorpe MJ; Chizh BA Drug Discovery Today 2009, 14, 56. [DOI] [PubMed] [Google Scholar]
- 12.Wong GY; Gavva NR Brain Res. Rev 2009, 60, 267. [DOI] [PubMed] [Google Scholar]
- 13.Kym PR; Kort ME; Hutchins CW Biochem. Pharmacol 2009, 78, 211. [DOI] [PubMed] [Google Scholar]
- 14.Min KH; Suh Y-G; Park M-K; Park H-G; Park Y-H; Kim H-D; Oh U; Blumberg PM; Lee J Mol. Pharm 2002, 62, 947 [published erratum appears in Mol. Pharmacol. 2003, 63, 958]. [Google Scholar]
- 15.Wang Y; Toth A; Tran R; Szabo T; Welter JD; Blumberg PM; Lee J; Kang S-U; Lim J-O; Lee J Mol. Pharm 2003, 64, 325. [DOI] [PubMed] [Google Scholar]
- 16.Lee J; Lee J; Kang M; Shin M-Y; Kim J-M; Kang S-U; Lim J-O; Choi H-K; Suh Y-G; Park H-G; Oh U; Kim H-D; Park Y-H; Ha H-J; Kim Y-H; Toth A; Wang Y; Tran R; Pearce LV; Lundberg DJ; Blumberg PM J. Med. Chem 2003, 46, 3116. [DOI] [PubMed] [Google Scholar]
- 17.Suh Y-G; Lee Y-S; Min K-H; Park O-H; Kim J-K; Seung H-S; Seo S-Y; Lee B-Y; Nam Y-H; Lee K-O; Kim H-D; Park H-G; Lee J; Oh U; Lim J-O; Kang S-U; Kil M-J; Koo J-Y; Shin SS; Joo Y-H; Kim JK; Jeong Y-S; Kim S-Y; Park Y-H J. Med. Chem 2005, 48, 5823. [DOI] [PubMed] [Google Scholar]
- [18].Kim YS; Kil M-J; Kang S-U; Ryu H; Kim MS; Cho Y; Bhondwe RS; Thorat SA; Sun W; Liu K; Lee JH; Choi S; Pearce LV; Pavlyukovets VA; Morgan MA; Tran R; Lazar J; Blumberg PM; Lee J. Bioorg. Med. Chem 2012, 20, 215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ryu H; Jin M-K; Kang S-U; Kim SY; Kang DW; Lee J; Pearce LV; Pavlyukovets VA; Morgan MA; Tran R; Toth A; Lundberg DJ; Blumberg PM J. Med. Chem 2008, 51, 57. [DOI] [PubMed] [Google Scholar]
- 20.Chung J-U; Kim SY; Lim J-O; Choi H-K; Kang S-U; Yoon H-S; Ryu H; Kang DW; Lee J; Kang B; Choi S; Toth A; Pearce LV; Pavlyukovets VA; Lundberg DJ; Blumberg PM Bioorg. Med. Chem 2007, 15, 6043. [DOI] [PubMed] [Google Scholar]