Abstract
Regenerative stem cell‐based therapies for bronchopulmonary dysplasia (BPD), the most common preterm birth complication, demonstrate promise in animals. Failure to objectively appraise available preclinical data and identify knowledge gaps could jeopardize clinical translation. We performed a systematic review and network meta‐analysis (NMA) of preclinical studies testing cell‐based therapies in experimental neonatal lung injury. Fifty‐three studies assessing 15 different cell‐based therapies were identified: 35 studied the effects of mesenchymal stromal cells (MSCs) almost exclusively in hyperoxic rodent models of BPD. Exploratory NMAs, for select outcomes, suggest that MSCs are the most effective therapy. Although a broad range of promising cell‐based therapies has been assessed, few head‐to‐head comparisons and unclear risk of bias exists. Successful clinical translation of cell‐based therapies demands robust preclinical experimental design with appropriately blinded, randomized, and statistically powered studies, based on biological plausibility for a given cell product, in standardized models and endpoints with transparent reporting.
Keywords: animal model, bronchopulmonary dysplasia, cell‐based therapy, lung injury, network meta‐analysis, preclinical, preterm birth, stem cells, systematic review, translation

Significance statement.
Bronchopulmonary dysplasia (BPD), a chronic lung disease that follows ventilator and oxygen therapy for acute respiratory failure after premature birth, is the most frequent complication of extreme prematurity. BPD is associated with long‐term respiratory and neurological consequences reaching into adulthood and currently lacks effective therapy. Regenerative cell‐based therapy, demonstrating therapeutic benefit in experimental neonatal lung injury, has now spawned clinical trials. Yet there has been no systematic review to assess the extent of current evidence regarding safety and efficacy of cell‐based therapies in preclinical BPD and identify gaps that could jeopardize successful clinical translation.
1. INTRODUCTION
Preterm birth complications have now surpassed infectious diseases as a leading cause of death in children below the age of 5 years.1, 2 Bronchopulmonary dysplasia (BPD), a chronic lung disease defined as oxygen requirement at 36 weeks' postmenstrual age, is the most common sequela of preterm birth affecting up to 40% of survivors.3, 4 BPD is strongly associated with late death or disability, and adds considerable economic burden to the health care system.5, 6 BPD is a multifactorial disease: prematurity, perinatal lung inflammation, growth restriction, mechanical ventilation, and oxygenation7 lead to interrupted lung alveolar and vascular growth, often complicated by pulmonary hypertension.8 None of the existing therapies have made any perceptible impact in reducing the burden of BPD among survivors.9
Stem cells are unique in their capacity to self‐renew and differentiate into specialized cell types thereby promoting organogenesis, tissue regeneration, maintenance, and repair.10 Therefore, regenerative cell‐based therapy for BPD has received singular interest, with manifold preclinical studies testing assorted cell products for their feasibility, safety, and efficacy.11 Although the fundamental mechanism of action of many of these therapies continues to be unraveled, mesenchymal stromal cells (MSCs), by virtue of their pleotropic effects, a postulated paracrine‐mediated action and apparent safety, have been extensively studied and already spawned numerous clinical trials (NCT03378063, NCT02443961, NCT01207869, NCT02381366, NCT01297205, NCT01828957, NCT01897987, NCT01632475, NCT03392467, and NCT02023788). Over the past 5 years, additional candidate cell therapies have proliferated, often without clear biological plausibility. The plethora of cell therapies warranted a systematic review to assess the extent of current evidence regarding preclinical safety and efficacy of available cell‐based therapies in experimental BPD.
Systematic reviews can help assess the methodological quality of existing studies and assess their totality of findings, both of which can help inform the design of new experiments, provide evidence‐based choice of animal models, and facilitate evidence‐based translation from bench to bedside.12 A meta‐analysis, while being more robust than single animal studies, provides a comprehensive knowledge of treatment or efficacy by quantitatively combining study outcome data across experiments, thereby often resulting in more reliable conclusions 13, 14 In many cases, new knowledge can be obtained by evaluation of heterogeneity between studies, which may affect the design of future animal or clinical experiments.15 Failing to consider weaknesses of past evidence has historically resulted in failure of translation of promising therapies to the clinic. 16, 17 Undeterred by the widespread heterogeneity in preclinical studies, stem cell therapy in cardiac repair advanced to clinical trials more than a decade ago, where it was found to be safe yet with inconsistent or conflicting benefit and today it is far from being an approved tool in the management of adult cardiac disease and is not much closer to becoming a clinical reality.18, 19 Even more telling is the translation failure in preclinical stroke research, given that of the over 500 interventions deemed beneficial only one was found to be effective in a clinical trial.20
The purpose of this systematic scoping review was to draw an evidence map of existing primary studies of cell‐based therapies for BPD that have been studied in the preclinical setting, an approach that will further the research community's understanding and awareness regarding the current distribution of evidence in the field of interest.21, 22
2. METHODS
This mapping exercise was carried out to address the following broadly framed research question of interest: “In controlled preclinical studies of BPD, do cell‐based therapies reduce the severity of lung injury?” The protocol, developed using the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA)‐P checklist,23 was prospectively registered and is available on the Collaborative Approach to Meta‐Analysis and Review of Animal Data from Experimental Studies website (CAMARADES).24 We followed the PRISMA guidelines25 in preparing this manuscript.
Please refer to the Online Supplement for detailed description of methods including search strategy, process of study selection, study eligibility criteria, data collection, risk of bias assessment, evidence summaries, exploratory meta‐analyses, post hoc amendments to exploratory network meta‐analysis (NMA), and details of the NMA model.
3. RESULTS
3.1. Extent of literature identified
A flow diagram of the study selection process is presented in Figure 1. A total of 2464 unique abstracts remained for screening following removal of duplicates across databases. Based on relevancy screening, a total of 87 remained for the full text evaluation. A total of 53 of these articles were retained for final inclusion;26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78 reasons for exclusion at full text screening are provided in Figure 1. Supplemental Table S1 presents an overview of the set of included studies, the characteristics of which are described in the subsequent sections below.
Figure 1.
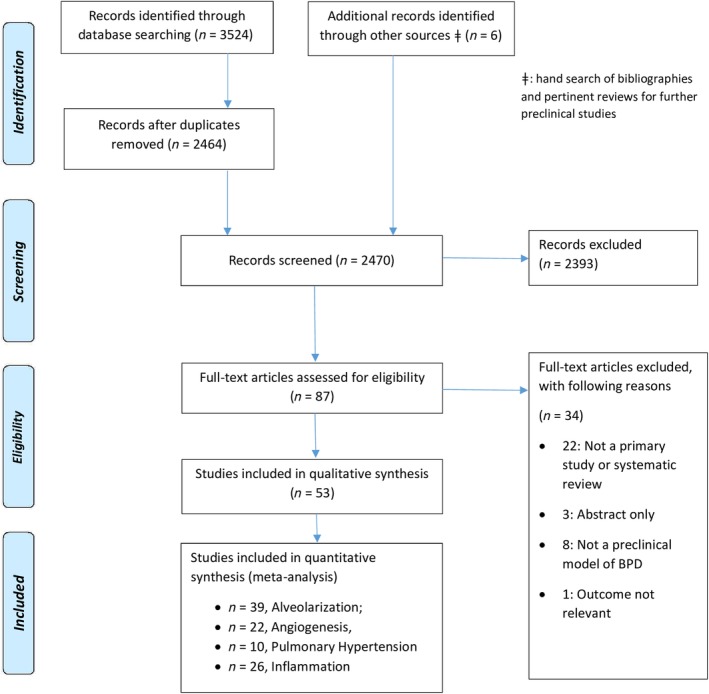
PRISMA 2009 flow diagram. “Other sources” refer to hand search of bibliographies of included studies and pertinent reviews for further preclinical studies
3.2. Overview of study characteristics and study populations
The majority of included studies were published during the last decade, with a median year of publication of 2013 (range 2007‐2017) (Supplemental Table S1). Although often unreported, the median sample size among studies (where available) was 56 (range 15‐166). Rodents were used to model BPD in nearly all studies, with the lamb model being the solitary exception.36, 45, 64 Induction of the BPD model, for the most part, was by hyperoxia where the O2 concentrations ranged from 60% to 95% and the duration of exposure ranged from 3.5 to 28 days. Alternate models included bleomycin,60 prenatal LPS in combination with postnatal hyperoxia,29 prenatal LPS,64 mechanical ventilation (2‐12 hours),36 and hypoxia followed by hyperoxia.47
3.3. Overview of interventions assessed
The range of cell therapy interventions identified across studies is outlined in Table 1. “Cell‐containing” therapies represented the majority of the interventions tested in preclinical BPD. These included MSC (n = 35 studies),26, 27, 28, 29, 32, 34, 35, 37, 39, 40, 42, 43, 48, 49, 51, 52, 53, 55, 56, 57, 58, 59, 61, 62, 63, 66, 68, 69, 70, 72, 73, 75, 76, 77, 78 human amniotic epithelial cells (hAEC, n = 4),36, 64, 65, 74 mononuclear CD34 + (n = 4),27, 30, 46, 47 endothelial colony forming cells (ECFC, n = 3),46, 64, 70 endothelial progenitor cells (EPCs, n = 3),31, 41, 71 bone marrow derived angiogenic cells (BMDAC, n = 1),71 bone marrow derived (BM) ckit+ cells (n = 1),50 cord blood CD34 + (n = 1),44 human amniotic fluid stem cells (hAFSC, n = 1),33 human‐induced pluripotent stem cells (hiPSC)‐derived lung progenitor cells (LPC, n = 1),53 hiPSC‐derived alveolar epithelial cells (AEC, n = 1),53 and undifferentiated hiPSC (n = 1).53 In contrast, fewer “Cell‐free” therapies were investigated, and these included MSC‐derived conditioned media (CdM, n = 6),34, 35, 48, 49, 56, 66 ECFC‐Conditioned media (CdM, n = 2),38, 60 or MSC‐derived exosomes (n = 1).67 The control groups were most often treated with saline (n = 32)26, 27, 28, 29, 30, 31, 32, 34, 37, 40, 44, 46, 47, 49, 51, 52, 56, 57, 58, 59, 61, 62, 64, 65, 68, 69, 70, 71, 72, 74, 75, 76, 77 or with a control cell/ cell‐free media (n = 12).33, 35, 46, 48, 49, 50, 53, 60, 63, 66, 67, 78 Rarely, the control would receive no treatment (sham control) (n = 2).36, 39
Table 1.
Interventions for bronchopulmonary dysplasia (BPD) investigated in preclinical studies
| Intervention category/class | # studies (with references) |
|---|---|
| Cell‐based therapy | |
| 1. Mesenchymal stromal cell (MSC) | 3526, 27, 28, 29, 32, 34, 35, 37, 39, 40, 42, 43, 48, 49, 51, 52, 53, 55, 56, 57, 58, 59, 61, 62, 63, 66, 68, 69, 70, 72, 73, 75, 76, 77, 78 |
| 2. Human amnion epithelial cells (hAEC) | 436, 64, 65, 74 |
| 3. Mononuclear CD34+ | 427, 30, 46, 47 |
| 4. Endothelial colony forming cells (ECFC) | 346, 64, 70 |
| 5. Endothelial progenitor cells (EPCs) | 331, 41, 71 |
| 6. Bone marrow derived angiogenic cells (BMDAC) | 171 |
| 7. Human amniotic fluid stem cells (hAFSC) | 133 |
| 8. Cord blood (CB) CD34+ | 144 |
| 9. Bone marrow (BM) derived ckit+ cells | 150 |
| 10. Undifferentiated human‐induced pluripotent stem cells (hiPSC) | 153 |
| 11. hiPSC‐derived LPSCs | 153 |
| 12. hiPSC‐derived AECs | 153 |
| Cell free therapy | |
| 1. MSC conditioned media | 634, 35, 48, 49, 56, 66 |
| 2. MSC exosome | 167 |
| 3. ECFC‐conditioned media | 238, 60 |
3.4. Overview of outcomes
The range of clinical outcomes assessed across the set of included studies is outlined in Supplemental Table S2. We systematically collected a listing of all outcome measures reported from studies meeting our eligibility criteria to establish an understanding of the information available from these studies. For the purposes of the current review, overviews and syntheses were focused on four measures: alveolarization, lung angiogenesis, pulmonary hypertension, and lung inflammation.
Alveolarization was examined as an outcome in 47 of the 53 studies.26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 46, 47, 48, 49, 50, 51, 52, 53, 55, 56, 57, 58, 60, 62, 63, 64, 65, 66, 67, 69, 71, 72, 73, 74, 75, 76, 77, 78 Lung angiogenesis was investigated as an outcome in 24 primary studies,26, 27, 29, 31, 35, 38, 39, 41, 42, 43, 48, 51, 52, 54, 55, 56, 60, 63, 67, 70, 71, 73, 74, 75 pulmonary hypertension in 13 studies,35, 38, 48, 49, 50, 51, 52, 56, 60, 63, 66, 67, 74 and lung inflammation was tested using 32 biomarkers of inflammation (among which we focused on IL‐1a, IL‐1b, IL‐6, TNF‐α, TGF‐β1, or their mRNA, macrophages, and neutrophils).26, 27, 28, 29, 36, 37, 39, 42, 45, 49, 51, 52, 55, 56, 57, 58, 59, 61, 62, 64, 65, 69, 70, 72, 73, 74, 75, 76, 77, 78
3.5. Risk of bias evaluations
The risk of bias assessment from the 53 studies is presented in Table 2A (individual studies) and Table 2B (overall). Although 34 of the studies (66%, Table 2B) reported randomizing animals to treatment, none described the method of randomization (ie, sequence generation). None of the included studies described their methods of allocation concealment. Blinding was reported rarely and inconsistently depending on where in the experimental process the blinding occurred (Table 2B), with blinding of outcome assessment being the most frequent (51%, Table 2B). Although only six studies (11%) reported that no animals were lost due to dropouts (attrition bias), 100% reported the outcome in both the methods and results of the study. Two studies (4%) reported how the sample size was calculated, whereas the remaining studies were unclear (Table 2B). The source of funding was always reported in sufficient detail to assess risk of bias. In total, 35 (66%) studies reported a nonindustry source of funding that was a low risk of bias, with 14 (27%) reporting industry funding that presented a risk of bias (Table 2B). Finally, 31 (59%) studies reported no conflicts of interest, 8 (15%) reported a conflict, and 14 (26%) were rated as unclear for not reporting whether conflicts of interest existed or not (Table 2B).
Table 2A.
Risk of bias

|
Table 2B.
Risk of bias

|
3.6. Summary of authors' conclusions regarding interventions
Supplemental Table S1 presents the individual categorizations of author interpretations drawn, whereas Supplemental Table S4 provides an overview of these interpretations categorized by the strength of conclusion. In considering findings observed across all outcome measures, the appraisal of the effects of cell‐based therapies, overall, concluded benefit. “Beneficial” therapies included MSC,26, 27, 28, 29, 34, 35, 37, 39, 40, 42, 43, 48, 49, 51, 52, 53, 55, 56, 57, 58, 59, 61, 62, 63, 66, 68, 69, 70, 72, 73, 75, 76, 77, 78 ECFC,38 EPC,31, 41 BMDAC,71 hAEC,36, 74 hAFSC,33 hiPSC‐derived LPC,53 and hiPSC‐derived AEC.53 Different from this were “probably beneficial” therapies which included ECFC,60 hAEC,60, 64, 65 CB CD 34+,44 Mononuclear CD 34+,46, 47 and BM Ckit+.50 Furthermore, two studies with Mononuclear cells (MNC) (different animal species, route, hyperoxia duration and the dose of MNCs),27, 30 one study with MSCs (intranasal route)32 and one with embryonic EPC,71 concluded that these therapies were not effective. Conversely, four therapies, EPC Cultured (aberrant tissue formation in lungs and inflammation),31 miPSC (cystic teratomas),53 mESC (fibrosarcomas),53 and hiPSC (lung teratomas and perivascular infiltration of the lungs, liver, heart, and kidneys)53 were found to be harmful, although the translational potential of these therapies remains circumspect, as the rationale behind the use of these cells in the primary studies remains unclear. Although these negative effects were anticipated with iPSCs and ESC, the finding with cultured EPCs was unexpected and warrants further studies.
Following the criteria of interventions for NMA described in the Methods section, a total of 39, 22, 10, and 26 studies remained for each of the outcomes of alveolarization,27, 28, 29, 30, 31, 32, 35, 37, 38, 39, 40, 41, 42, 43, 44, 46, 48, 49, 51, 52, 53, 55, 56, 57, 58, 60, 62, 63, 65, 66, 69, 71, 72, 73, 74, 75, 76, 77, 78 lung angiogenesis,26, 27, 29, 31, 35, 38, 39, 41, 42, 43, 48, 51, 52, 54, 55, 56, 60, 63, 70, 71, 73, 75 pulmonary hypertension,35, 38, 48, 49, 51, 52, 56, 60, 63, 66 and inflammation (seven pro‐inflammatory markers: IL‐1α, IL‐1β, IL‐6, TNF‐α, TGF‐β1, or their mRNA, and macrophages, neutrophils),26, 27, 28, 29, 37, 39, 43, 45, 51, 52, 55, 56, 57, 58, 59, 61, 62, 64, 65, 69, 72, 74, 75, 76, 77, 78 respectively. Network diagrams describing the evidence base for each of the four outcomes are presented in Figure 2.
Figure 2.
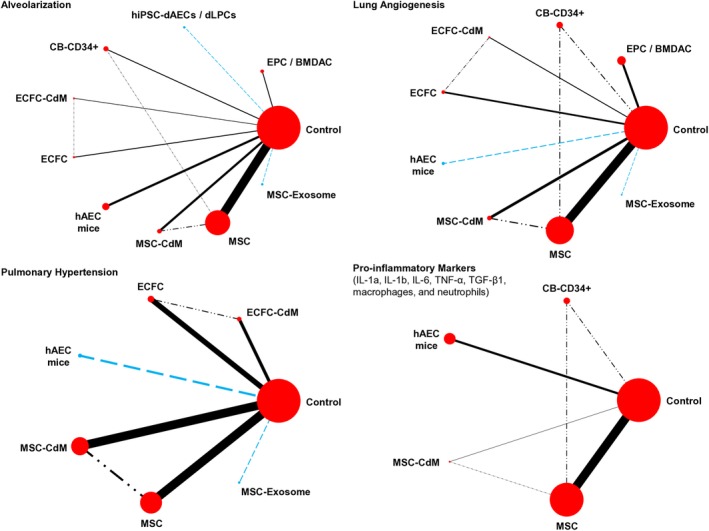
Network diagrams underlying exploratory network meta‐analyses (NMAs). The blue‐colored dashed lines represent comparisons between interventions which were assessed in a single study vs bronchopulmonary dysplasia (BPD)‐only control. The dash‐dotted lines represent comparisons that were assessed in a single study but involved intervention(s) other than control. Nodes are proportionately sized to reflect the numbers of animals studied with each intervention. Edge width reflects the number of experiments or metrics for each comparison
Figure 3 presents forest plots of findings for all four outcomes with estimated standardized mean differences (SMDs) of interventions compared with BPD‐only control, whereas Supplemental Table S5 and Online Appendix 4 present a summary of secondary measures (including SUCRA and treatment rankings). Online Appendix 5 presents the league tables of posterior median pairwise SMDs with 95% credible intervals in the lower triangle of the league tables, and the pairwise probabilities that an intervention is better than another in the upper triangle.
Figure 3.

Forest plots of the estimated standardized mean differences (SMDs) compared to bronchopulmonary dysplasia (BPD)‐only control for each outcome. The SMDs (with 95% credible intervals) were estimated from the random‐effects consistency model. A, Alveolarization; B, lung angiogenesis; C, pulmonary hypertension; D, lung inflammation: pro‐inflammatory markers
4. DISCUSSION
Our exploratory NMAs represent the first and most expansive synthesis of data on cell‐based therapies in preclinical BPD. We found 15 distinct cell‐derived therapies, largely tested in the hyperoxic rodent model of BPD. MSCs appear as the most effective cell‐based therapy for all the four clinically relevant outcomes of alveolarization, lung angiogenesis, pulmonary hypertension, and lung inflammation. The second‐ranked therapy was contingent on the outcome: MSC‐CdM for alveolarization and lung angiogenesis, ECFC for pulmonary hypertension, and CB CD34+ for lung inflammation. Although a broad range of promising cell‐based therapies has been assessed, few head‐to‐head comparisons and unclear risk of bias exist.
4.1. External validity and implications for preclinical research
4.1.1. Replicative or duplicative research: When too much is too little and small is not enough
MSCs and MSC‐derived conditioned media made up for the biggest share (84%) of cell‐based therapies investigated in experimental BPD over the last decade, from 2007 to as recent as 2017. Conspicuously, this therapy was investigated solely in the hyperoxic rodent model of BPD. Although a well‐established model, other animal models do exist.80, 81, 88 Replicability and reproducibility is key in preclinical research, as single experiments must be repeated (replicability) and research hypotheses constantly reexamined by independent observers to confirm and corroborate one another's findings (reproducibility), thereby conferring confidence to conclusions drawn from them. We found that 34 of 35 studies (97.1%) on MSCs concluded a beneficial therapeutic effect in ameliorating neonatal lung injury in the select hyperoxic rodent model, and that this research finding is well endorsed in this model. Therefore, one could argue, repeating confirmatory experiments in the same model no longer adds to the knowledge base and, in fact, risks becoming duplicative. This duplication is intrinsically wasteful and should be guarded against.91, 92 One means by which to avoid such waste is by investigating this promising therapy in diverse and larger animal models such as the fetal lamb model80 or the nonhuman primate model,88 recreating the clinical setting of preterm birth and respiratory failure demanding extended invasive mechanical ventilation with oxygen‐rich gas and reproducing the evolving pathophysiology of human neonatal BPD. Such a complementary approach, an important attribute in research, could potentially fast track (or reconsider) the successful translation of such therapies into the clinic while addressing regulatory concerns.79 MSCs have advanced to Phases I and II clinical trials without undergoing testing in larger animal models.
Alternate therapies to the widely used MSCs have been investigated, although these are few and far between. EPCs were found to be beneficial in two out of three studies, whereas ECFC (one of two) and hAEC (two of four) were found to be beneficial in half the studies they were investigated in Supplemental Table S4. Of note is the spread of ECFC and hAEC in both “beneficial” and “probably beneficial” categories. This could be attributed to the use of a diverse induction (Bleomycin60) or animal model (fetal lamb64). Authors investigating mononuclear cells speculated on their probable benefit, whereas others determined that these cells had no effect (Supplemental Table S4). At this juncture, the unambiguous benefits of these alternate therapies require further compelling evidence as they await further replication and continuing complementary research. Refreshingly, hAEC were explored not only in the ubiquitous rodent model, but also in a larger animal model (fetal lamb)36, 64 with the use of diverse modes of injury induction.
4.1.2. Isolated exploratory research: A research waste?
In contrast, we identified a number of isolated therapies that were investigated in single exploratory experiments (BMDAC, hAFSC, and BM‐derived Ckit+).33, 50, 71 The rationale for testing these novel therapies ranged from speculative role for a bone marrow‐derived cell population in the maintenance of adult lung structure exposed to hyperoxia (BMDAC), likeness to MSC (hAFSC) or promise demonstrated in adult cardiac repair (Ckit+), although the research integrity of the latter has been recently brought into question.93 Exploratory studies are precisely articulated hypothesis‐generating research based on sound scientific rationale and may involve demonstrating a dose‐response relationship. These studies are further tested in confirmatory studies which are akin to clinical trials in terms to rigor and quality, confirming or rejecting the hypothesis in terms of safety and efficacy.79 Despite being described as beneficial in studies dating back to between 2010 and 2015, the findings for these exploratory interventions have not been reproduced or replicated to date. Robust, reproducible research is the cornerstone on which scientific discoveries are made. Irreproducibility remains a challenge in preclinical research when upward of 50% findings fail replication resulting in over $28 billion US/y in preclinical research waste.89 Reasons for irreproducibility could be observer bias or confirmation bias. Other opportunities for improvement include: following through with promising cell products, use of bona fide controls, validation of reagents, and the use of appropriate statistical tests.82
4.1.3. Interstudy standardization of experimental design
Poorly connected network
Since head‐to‐head preclinical trials ascertaining the comparative efficacy of every single cell‐based intervention may not be feasible, NMA, which allows for the synthesis of direct and indirect evidence, is a useful alternative.90 Although MSCs were found to be the most heavily investigated cell therapy in our mapping of the evidence base, remarkably, there were only two direct comparisons between this preferred therapy and alternate therapies, such as MSC‐Cdm and CB‐CD34+. The reliance on extensive indirect comparisons in our meta‐analysis resulted in poorly connected networks, as illustrated by the network diagram for all four outcomes (Figure 2). Findings from meta‐analyses based upon such networks may be less reliable than those from networks wherein most therapies have been directly compared.83 For this reason, there is a need to rethink current experimental modeling strategies and promote collaboration and reciprocity between independent research teams in designing experiments which directly compare, where indicated, some of these interventions. Remaining evidence gaps could potentially be filled by a NMA, thereby allowing evidence‐based choice of therapies which are not only the most efficacious in the animal model but also have the highest chance of translational success.
Standardization of metrics measuring identical outcomes
We observed an array of measurements being used to assess identical outcomes. For instance, 32 different biomarkers were used across 31 included primary studies to assess lung inflammation alone. Furthermore, many of these biomarkers were measured using assorted techniques (eg, mRNA vs protein), precluding interstudy comparison, synthesis, and subsequent translatability to clinic trials. Although exploratory studies may justify the use of novel measurements of common outcomes, there is a need to collaboratively develop an essential minimal set of measurements for outcomes in exploratory and confirmatory studies and promote minimal a priori reporting standards for these outcome data which are not only coherent but also verifiable and comparable.84 Specifically, investigation of lung morphometry by adopting state of the art and unbiased stereological methods and guidelines will improve the validity and uniformity of structural assessment in lung biology.85
Preclinical alliance for successful translation
Given the preceding contentious issues, there is a need to promote a preclinical network where independent laboratories with expertise in well‐endorsed animal models can transparently share standardized models and protocols: collaborate on multicenter, randomized, blinded, and well‐powered preclinical studies with a priori outcomes, sample size, and exclusion criteria.86
4.2. Implications for clinical trial design and successful translation
Please see Online Supplement for discussion on candidate therapy for clinical trials manufacturing challenges, trial design, optimal route of administration, dose, and dose frequency.
4.3. Strengths and limitations
The strengths of our systematic review include a rigorous peer‐reviewed search strategy and use of international guidance and standards to conduct our systematic review and NMA (Online Appendix 8).87 However, our review was limited by the fact that a large number of published data were available only in the form of figures and not in an easily extractable numerical form. Almost all the data were extracted from the figures in the published article using an open source program that can work with a variety of plot types and images. Minor distortion of data is possible, but all groups would be equally affected. Also, incorrectly reported SDs/SEMs could have potentially impacted SMD estimates. Additionally, to facilitate data synthesis, we made several post hoc variations (Online Appendix 9). Furthermore, the risk of bias assessment for the primary studies was hampered by poor reporting quality of important domains such as randomization, blinding, and sample size calculation. Finally, high prevalence of publication bias in animal research and bloated effect sizes causing potentially biased conclusions is a cause for concern.94
5. CONCLUSION
In this systematic review incorporating exploratory NMAs on cell‐based therapies in preclinical BPD, MSCs appeared as the most efficacious among all interventions, although exclusively investigated in the rodent hyperoxic model of BPD. Numerous limitations point toward the need for more robust experimental design and reporting in preclinical studies including due consideration of the biological plausibility for a given cell product, use of standardized models and endpoints appropriately powered for statistics, blinding, and randomization. Adoption of these considerations may enhance the successful translation of cell‐based therapies into the clinic.
CONFLICT OF INTEREST
B.H. has previously received honoraria from Cornerstone Research Group for the provision of methodologic advice related to the conduct of systematic reviews and meta‐analysis. The other authors indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
S.A.: conception and design, collection and/or assembly of data, manuscript writing, final approval of manuscript; W.C.: collection and/or assembly of data, manuscript writing, final approval of manuscript, design, data analysis and interpretation; M.T.A.: collection and/or assembly of data, final approval of manuscript, design; M.L.C., S.M.C.L.: collection and/or assembly of data, final approval of manuscript; B.H.: final approval of manuscript, critical revisions to the manuscript; B.T.: conception and design, manuscript writing, final approval of manuscript, financial support.
Supporting information
Appendix S1: Supporting Information
Supplemental Table 1: Characteristics of included studies
Supplemental Table 2: Outcomes Assessed in Pre‐Clinical Studies
Supplemental Table 3: Approach to Categorizing Authors' Conclusions
Supplemental Table 4: Rating of Authors' Conclusions approximately Intervention Effectiveness
Supplemental Table 5: Mean SUCRA value and mean rank of each intervention assessed in two or more studies, for each outcome in descending order of mean SUCRA. Larger values of the mean SUCRA or the smaller values of the mean rank suggest better treatments. SUCRA: The Surface Under the Cumulative RAnking curve, which value represents the probabilities for each intervention to be among the n‐best options.
Supplemental Table 6: Optimal Route of administration, Dose and Dose frequency
ACKNOWLEDGMENTS
We thank Margaret Sampson, MLIS, PhD, AHIP (Children's Hospital of Eastern Ontario) for developing the electronic search strategies and Raymond Daniel for building the Reference Manager database and deduplicating references.
Augustine S, Cheng W, Avey MT, et al. Are all stem cells equal? Systematic review, evidence map, and meta‐analyses of preclinical stem cell‐based therapies for bronchopulmonary dysplasia. STEM CELLS Transl Med. 2020;9:158–168. 10.1002/sctm.19-0193
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- 1. Lau C, Ambalavanan N, Chakraborty H, Wingate MS, Carlo WA. Extremely low birth weight and infant mortality rates in the United States. Pediatrics. 2013;131:855‐860. [DOI] [PubMed] [Google Scholar]
- 2. Lawn JE, Kinney M. Preterm birth: now the leading cause of child death worldwide. Sci Transl Med. 2014;6:263ed21. [DOI] [PubMed] [Google Scholar]
- 3. Baraldi E, Filippone M. Chronic lung disease after premature birth. N Engl J Med. 2007;357:1946‐1955. [DOI] [PubMed] [Google Scholar]
- 4. Stoll BJ, Hansen NI, Bell EF, et al. Trends in care practices, morbidity, and mortality of extremely preterm Neonates, 1993‐2012. JAMA. 2015;314:1039‐1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Schmidt B, Roberts RS, Davis PG, et al. Prediction of late death or disability at age 5 years using a count of 3 neonatal morbidities in very low birth weight infants. J Pediatr. 2015;167:982‐986.e2. [DOI] [PubMed] [Google Scholar]
- 6. Johnson TJ, Patel AL, Jegier BJ, Engstrom JL, Meier PP. Cost of morbidities in very low birth weight infants. J Pediatr. 2013;162:243‐249.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Demauro SB, Dysart K, Kirpalani H. Stopping the swinging pendulum of postnatal corticosteroid use. J Pediatr. 2014;164:9‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Thebaud B, Abman SH, Thébaud B, Abman SH, Thebaud B, Abman SH. Bronchopulmonary dysplasia: where have all the vessels gone? Roles of angiogenic growth factors in chronic lung disease. Am Crit Care Med. 2007;175:978‐985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. McEvoy CT, Jain L, Schmidt B, Abman S, Bancalari E, Aschner JL. Bronchopulmonary dysplasia: NHLBI workshop on the primary prevention of chronic lung diseases. Ann Am Thorac Soc. 2014;11:S146‐S153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. JMW S. Origin of stem cells in organogenesis. Science. 2008;322:1498‐1501. [DOI] [PubMed] [Google Scholar]
- 11. Mobius MA, Thebaud B, Thébaud B. Stem cells and their mediators ‐ next generation therapy for bronchopulmonary dysplasia. Front Med. 2015;2:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. de Vries RBM, Wever KE, Avey MT, Stephens ML, Sena ES, Leenaars M. The usefulness of systematic reviews of animal experiments for the design of preclinical and clinical studies. ILAR J. 2014;55:427‐437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Egger M, Davey‐Smith G, Altman D, eds. Systematic Reviews in Health Care: Meta‐Analysis in Context. John Wiley & Sons, Hoboken, New Jersey, United States; 2001. [Google Scholar]
- 14. Hooijmans CR, IntHout J, Ritskes‐Hoitinga M, Rovers MM. Meta‐analyses of animal studies: an introduction of a valuable instrument to further improve healthcare. ILAR J. 2014;55:418‐426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Augustine S, Avey MT, Harrison B, et al. Mesenchymal stromal cell therapy in bronchopulmonary dysplasia: systematic review and meta‐analysis of preclinical studies. Stem Cells Translational Medicine. 2017;6:2079‐2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Contopoulos‐Ioannidis DG, Ntzani EE, Ioannidis JPA. Translation of highly promising basic science research into clinical applications. Am J Med. 2003;114:477‐484. [DOI] [PubMed] [Google Scholar]
- 17. Sena ES, Currie GL, McCann SK, Macleod MR, Howells DW. Systematic reviews and meta‐analysis of preclinical studies: why perform them and how to appraise them critically. J Cereb Blood Flow Metab. 2014;34:737‐742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mathur A, Fernández‐Avilés F, Dimmeler S, et al. The consensus of the Task Force of the European Society of Cardiology concerning the clinical investigation of the use of autologous adult stem cells for the treatment of acute myocardial infarction and heart failure: update 2016. Eur Heart J. 2017;38:2930‐2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nguyen PK, Rhee J‐W, Wu JC. Adult stem cell therapy and heart failure, 2000 to 2016. JAMA Cardiol. 2016;1:831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. O'Collins VE, Macleod MR, Donnan GA, Horky LL, Van Der Worp BH, Howells DW. 1,026 experimental treatments in acute stroke. Ann Neurol. 2006;59:467‐477. [DOI] [PubMed] [Google Scholar]
- 21. Hetrick SE, Parker AG, Callahan P, Purcell R. Evidence mapping: illustrating an emerging methodology to improve evidence‐based practice in youth mental health. J Eval Clin Pract. 2010;16:1025‐1030. [DOI] [PubMed] [Google Scholar]
- 22. Arksey H, O'Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol. 2005;8:19‐32. [Google Scholar]
- 23. Shamseer L, Moher D, Clarke M, et al. Preferred reporting items for systematic review and meta‐analysis protocols (PRISMA‐P) 2015: elaboration and explanation. BMJ. 2015;349:g7647. [DOI] [PubMed] [Google Scholar]
- 24. Augustine S, Avey MT, Moher D, Bernard, T . Comparative therapeutic potential of all cell‐based therapies in experimental Bronchopulmonary dysplasia: systematic review and network meta‐analysis. 2017. http://www.dcn.ed.ac.uk/camarades/research.html
- 25. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group . Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. Ann Intern Med. 2009;151:264‐269. [DOI] [PubMed] [Google Scholar]
- 26. Ahn SY, Chang YS, Kim SY, et al. Long‐term (Postnatal day 70) outcome and safety of intratracheal transplantation of human umbilical cord blood‐derived mesenchymal stem cells in neonatal hyperoxic lung injury. Yonsei Med J. 2013;54:416‐424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ahn SY, Chang YS, Sung DK, et al. Cell type‐dependent variation in paracrine potency determines therapeutic efficacy against neonatal hyperoxic lung injury. Cytotherapy. 2015;17:1025‐1035. [DOI] [PubMed] [Google Scholar]
- 28. Chen C‐M, Chou H‐C, Lin W, Tseng C. Surfactant effects on the viability and function of human mesenchymal stem cells: in vitro and in vivo assessment. Stem Cell Res Ther. 2017;8:180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chou HC, Li YT, Chen CM. Human mesenchymal stem cells attenuate experimental bronchopulmonary dysplasia induced by perinatal inflammation and hyperoxia. Am J Transl Res. 2016;8:342‐353. [PMC free article] [PubMed] [Google Scholar]
- 30. De Paepe ME, Mao Q, Chu S, et al. Long‐term outcome of human cord blood‐derived hematopoietic progenitor cells in murine lungs. Exp Lung Res. 2013;39:59‐69. [DOI] [PubMed] [Google Scholar]
- 31. Firsova AB, Bird AD, Abebe D, Ng J, Mollard R, Cole TJ. Fresh noncultured endothelial progenitor cells improve neonatal lung hyperoxia‐induced alveolar injury. Stem Cells Translational Medicine. 2017;6:2094‐2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fritzell JA, Mao Q, Gundavarapu S, et al. Fate and effects of adult bone marrow cells in lungs of normoxic and hyperoxic newborn mice. Am J Respir Cell Mol Biol. 2009;40:575‐587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Grisafi D, Pozzobon M, Dedja A, et al. Human amniotic fluid stem cells protect rat lungs exposed to moderate hyperoxia. Pediatr Pulmonol. 2013;48:1070‐1080. [DOI] [PubMed] [Google Scholar]
- 34. Gülasi S, Necat Ş, Mustafa Y, et al. Mesenchymal stem cell treatment in hyperoxia‐induced lung injury in newborn rats. Pediatr Int. 2016;58:206‐213. [DOI] [PubMed] [Google Scholar]
- 35. Hansmann G, Fernandez‐Gonzalez A, Aslam M, et al. Mesenchymal stem cell‐mediated reversal of bronchopulmonary dysplasia and associated pulmonary hypertension. Pulm Circ. 2012;2:170‐181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hodges RJ, Jenkin G, Hooper SB, et al. Human amnion epithelial cells reduce ventilation‐induced preterm lung injury in fetal sheep. Am J Obstet Gynecol. 2012;206:448.e8‐448.e15. [DOI] [PubMed] [Google Scholar]
- 37. Hou C, Peng D, Gao L, et al. Human umbilical cord‐derived mesenchymal stem cells protect from hyperoxic lung injury by ameliorating aberrant elastin remodeling in the lung of O2‐exposed newborn rat. Biochem Biophys Res Commun. 2017;495:1972‐1979. [DOI] [PubMed] [Google Scholar]
- 38. Alphonse RS, Vadivel A, Fung M, et al. Existence, functional impairment, and lung repair potential of endothelial colony‐forming cells in oxygen‐induced arrested alveolar growth. Circulation. 2014;129:2144‐2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kim YE, Park WS, Sung DK, et al. Intratracheal transplantation of mesenchymal stem cells simultaneously attenuates both lung and brain injuries in hyperoxic newborn rats. Pediatr Res. 2016;80:415‐424. [DOI] [PubMed] [Google Scholar]
- 40. Liu L, Mao Q, Chu S, et al. Intranasal versus intraperitoneal delivery of human umbilical cord tissue‐derived cultured mesenchymal stromal cells in a murine model of neonatal lung injury. Am J Pathol. 2014;184:3344‐3358. [DOI] [PubMed] [Google Scholar]
- 41. Lu A, Sun B, Qian L. Combined iNO and endothelial progenitor cells improve lung alveolar and vascular structure in neonatal rats exposed to prolonged hyperoxia. Pediatr Res. 2015;77:784‐792. [DOI] [PubMed] [Google Scholar]
- 42. Luan Y, Ding W, Ju Z‐YY, Zhang Z‐HH, Zhang X, Kong F. Bone marrow‐derived mesenchymal stem cells protect against lung injury in a mouse model of bronchopulmonary dysplasia. Mol Med Rep. 2015;11:1945‐1950. [DOI] [PubMed] [Google Scholar]
- 43. Luan Y, Zhang L, Chao S, et al. Mesenchymal stem cells in combination with erythropoietin repair hyperoxia‐induced alveoli dysplasia injury in neonatal mice via inhibition of TGF‐β1 signaling. Oncotarget. 2016;7:47082‐47094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mao QF, Chu SR, Ghanta S, Padbury JF, De Paepe ME. Ex vivo expanded human cord blood‐derived hematopoietic progenitor cells induce lung growth and alveolarization in injured newborn lungs. Respir Res. 2013;14:1‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Melville JM, McDonald CA, Bischof RJ, et al. Human amnion epithelial cells modulate the inflammatory response to ventilation in preterm lambs. PLoS One. 2017;12:1‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Mills DR, Mao Q, Chu S, et al. Effects of human umbilical cord blood mononuclear cells on respiratory system mechanics in a murine model of neonatal lung injury. Exp Lung Res. 2017;43:66‐81. [DOI] [PubMed] [Google Scholar]
- 47. Monz D, Tutdibi E, Mildau C, et al. Human umbilical cord blood mononuclear cells in a double‐hit model of bronchopulmonary dysplasia in neonatal mice. PLoS One. 2013;8:e74740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Pierro M, Ionescu L, Montemurro T, et al. Short‐term, long‐term and paracrine effect of human umbilical cord‐derived stem cells in lung injury prevention and repair in experimental bronchopulmonary dysplasia. Thorax. 2013;68:475‐484. [DOI] [PubMed] [Google Scholar]
- 49. Aslam M, Baveja R, Liang OD, et al. Bone marrow stromal cells attenuate lung injury in a murine model of neonatal chronic lung disease. Online data supplement. Am J Respir Crit Care Med. 2009;180:1122‐1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ramachandran S, Suguihara C, Drummond S, et al. Bone marrow‐derived c‐kit(+) cells attenuate neonatal hyperoxia‐induced lung injury. Cell Transplant. 2015;24:85‐95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Reiter J, Drummond S, Sammour I, et al. Stromal derived factor‐1 mediates the lung regenerative effects of mesenchymal stem cells in a rodent model of bronchopulmonary dysplasia. Respir Res. 2017;18:1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sammour I, Somashekar S, Huang J, et al. The effect of gender on mesenchymal stem cell (MSC) efficacy in neonatal hyperoxia‐induced lung injury. PLoS One. 2016;11:1‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Shafa M, Ionescu LI, Vadivel A, et al. Human induced pluripotent stem cell–derived lung progenitor and alveolar epithelial cells attenuate hyperoxia‐induced lung injury. Cytotherapy. 2018;20:108‐125. [DOI] [PubMed] [Google Scholar]
- 54. Solomon I, O'Reilly M, Ionescu L, et al. Functional differences between placental micro‐ and macrovascular endothelial colony‐forming cells. Stem Cells Translational Medicine. 2016;5:291‐300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Sung DK, Chang YS, Ahn SY, et al. Optimal route for human umbilical cord blood‐derived mesenchymal stem cell transplantation to protect against neonatal hyperoxic lung injury: gene expression profiles and histopathology. PLoS One. 2015;10:1‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Sutsko RP, Young KC, Ribeiro A, et al. Long‐term reparative effects of mesenchymal stem cell therapy following neonatal hyperoxia‐induced lung injury. Pediatr Res. 2013;73:46‐53. [DOI] [PubMed] [Google Scholar]
- 57. Tian ZF, Du J, Wang B, Hong XY, Feng ZC. Intravenous infusion of rat bone marrow derived mesenchymal stem cells ameliorates hyeroxia‐induced lung injury in neonatal rats. J South Med Univ. 2007;27:1692‐1695. [PubMed] [Google Scholar]
- 58. Tian ZF, Li YH, Fu XM, Feng ZC, Du J. Marrow‐derived mesenchymal stem cells protect the lung injury caused by exposure to high oxygen: experiment with rats. Natl Med J China. 2008;88:2715‐2718. [PubMed] [Google Scholar]
- 59. Tian ZF, Li YH, Ji P, Zhao S, Cheng HP. Mesenchymal stem cells protects hyperoxia‐induced lung injury in newborn rats via inhibiting receptor for advanced glycation end‐products/nuclear factor kappa B signaling. Exp Biol Med. 2013;238:242‐247. [DOI] [PubMed] [Google Scholar]
- 60. Baker CD, Seedorf GJ, Wisniewski BL, et al. Endothelial colony‐forming cell conditioned media promote angiogenesis in vitro and prevent pulmonary hypertension in experimental bronchopulmonary dysplasia. AJP Lung Cell Mol Physiol. 2013;305:L73‐L81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Tian ZF, Ji P, Li Y, Zhao S, Wang X. Influence of human mesenchymal stem cells on hyperoxia‐exposed newborn rats by RAGE‐NF‐kappaB signaling in lung. Chinese J Pediatr. 2012;50:356‐360. [PubMed] [Google Scholar]
- 62. Tian ZF, Du J, Fu XM, Wang B, Hong XY, Feng ZC. Influence of human bone marrow‐derived mesenchymal stem cells on the lung of newborn rats damaged by hyperoxia. Chinese J Pediatr. 2008;46:4‐8. [PubMed] [Google Scholar]
- 63. van Haaften T, Byrne R, Bonnet S, et al. Airway delivery of mesenchymal stem cells prevents arrested alveolar growth in neonatal lung injury in rats. Am J Respir Crit Care Med. 2009;180:1131‐1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Vosdoganes P, Hodges RJ, Lim R, et al. Human amnion epithelial cells as a treatment for inflammation‐induced fetal lung injury in sheep. Am J Obstet Gynecol. 2011;205:156.e26‐156.e33. [DOI] [PubMed] [Google Scholar]
- 65. Vosdoganes P, Lim R, Koulaeva E, et al. Human amnion epithelial cells modulate hyperoxia‐induced neonatallung injury in mice. Cytotherapy. 2013;15:1021‐1029. [DOI] [PubMed] [Google Scholar]
- 66. Waszak P, Alphonse R, Vadivel A, et al. Preconditioning enhances the paracrine effect of mesenchymal stem cells in preventing oxygen‐induced neonatal lung injury in rats. Stem Cells Dev. 2012;21:2789‐2797. [DOI] [PubMed] [Google Scholar]
- 67. Willis GR, Fernandez‐Gonzalez A, Anastas J, et al. Mesenchymal stromal cell exosomes ameliorate experimental bronchopulmonary dysplasia and restore lung function through macrophage immunomodulation. Am J Respir Crit Care Med. 2018;197:104‐116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Yao L, Liu CJ, Luo Q, et al. Protection against hyperoxia‐induced lung fibrosis by KGF‐induced MSCs mobilization in neonatal rats. Pediatr Transplant. 2013;17:676‐682. [DOI] [PubMed] [Google Scholar]
- 69. Zhang H, Fang J, Su H, et al. Bone marrow mesenchymal stem cells attenuate lung inflammation of hyperoxic newborn rats. Pediatr Transplant. 2012;16:589‐598. [DOI] [PubMed] [Google Scholar]
- 70. Zhang H, Fang J, Wu Y, Mai Y, Lai W, Su H. Mesenchymal stem cells protect against neonatal rat hyperoxic lung injury. Expert Opin Biol Ther. 2013;13:817‐829. [DOI] [PubMed] [Google Scholar]
- 71. Balasubramaniam V, Ryan SL, Seedorf GJ, et al. Bone marrow‐derived angiogenic cells restore lung alveolar and vascular structure after neonatal hyperoxia in infant mice. Am J Physiol Lung Cell Mol Physiol. 2010;298:L315‐L323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Zhang X, Wang H, Shi Y, et al. Role of bone marrow‐derived mesenchymal stem cells in the prevention of hyperoxia‐induced lung injury in newborn mice. Cell Biol Int. 2012;36:589‐594. [DOI] [PubMed] [Google Scholar]
- 73. Zhang ZH, Pan YY, Jing RS, et al. Protective effects of BMSCs in combination with erythropoietin in bronchopulmonary dysplasia‐induced lung injury. Mol Med Rep. 2016;14:1302‐1308. [DOI] [PubMed] [Google Scholar]
- 74. Zhu D, Tan J, Maleken AS, et al. Human amnion cells reverse acute and chronic pulmonary damage in experimental neonatal lung injury. Stem Cell Res Ther. 2017;8:1‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Chang YS, Ahn SY, Jeon HB, et al. Critical role of vascular endothelial growth factor secreted by mesenchymal stem cells in hyperoxic lung injury. Am J Respir Cell Mol Biol. 2014;51:391‐399. [DOI] [PubMed] [Google Scholar]
- 76. Chang YS, Choi SJ, Ahn SY, et al. Timing of umbilical cord blood derived mesenchymal stem cells transplantation determines therapeutic efficacy in the neonatal hyperoxic lung injury. PLoS One. 2013;8:e52419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Chang YS, Choi SJ, Sung DK, et al. Intratracheal transplantation of human umbilical cord blood derived mesenchymal stem cells dose‐dependently attenuates hyperoxia‐induced lung injury in neonatal rats. Cell Transplant. 2011;12:1‐32. [DOI] [PubMed] [Google Scholar]
- 78. Chang YS, Oh W, Choi SJ, et al. Human umbilical cord blood‐derived mesenchymal stem cells attenuate hyperoxia‐induced lung injury in neonatal rats. Cell Transplant. 2009;18:869‐886. [DOI] [PubMed] [Google Scholar]
- 79. Kimmelman J, Mogil JS, Dirnagl U. Distinguishing between exploratory and confirmatory preclinical research will improve translation. PLoS Biol. 2014;12:12‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Albertine KH. Utility of large‐animal models of BPD: chronically ventilated preterm lambs. Am J Physiol Cell Mol Physiol. 2015;308:L983‐L1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. D'Angio CT, Ryan RM, Angio CTD, Ryan RM. Animal models of bronchopulmonary dysplasia. The preterm and term rabbit models. Am J Physiol Lung Cell Mol Physiol. 2014;307:L959‐L969. [DOI] [PubMed] [Google Scholar]
- 82. Begley CG, Ioannidis JPA. Reproducibility in science: improving the standard for basic and preclinical research. Circ Res. 2015;116:116‐126. [DOI] [PubMed] [Google Scholar]
- 83. Tonin FS, Rotta I, Mendes AM, Pontarolo R. Network meta‐analysis: a technique to gather evidence from direct and indirect comparisons. Pharm Pract. 2017;15:1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Taylor CF, Field D, Sansone S, et al. NIH Public Access. Bioinformatics. 2009;26:889‐896. [Google Scholar]
- 85. Hsia CCW, Hyde DM, Ochs M, Weibel ER. An official research policy statement of the American Thoracic Society/European Respiratory Society: standards for quantitative assessment of lung structure. Am J Respir Crit Care Med. 2010;181:394‐418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Chamuleau SAJ, van der Naald M, Climent AM, et al. Translational research in cardiovascular repair. Circ Res. 2018;122:310‐318. [DOI] [PubMed] [Google Scholar]
- 87. Hutton B, Salanti G, Caldwell DM, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta‐analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162:777. [DOI] [PubMed] [Google Scholar]
- 88. Yoder BA, Coalson JJ. Animal models of bronchopulmonary dysplasia. The preterm baboon models. Am J Physiol Lung Cell Mol Physiol. 2014;307:L970‐L977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Freedman LP, Cockburn IM, Simcoe TS. The economics of reproducibility in preclinical research. PLoS Biol. 2015;13:1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Jansen JP, Fleurence R, Devine B, et al. Interpreting indirect treatment comparisons and network meta‐analysis for health‐care decision making: report of the ISPOR task force on indirect treatment comparisons good research practices: part 1. Value Heal. 2011;14:417‐428. [DOI] [PubMed] [Google Scholar]
- 91. Ioannidis JPA, Greenland S, Hlatky MA, et al. Increasing value and reducing waste in research design, conduct, and analysis. Lancet. 2014;383:166‐175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Moher D, Shamseer L, Cobey KD, et al. Stop this waste of people, animals and money. Nat News. 2017;549:23‐25. [DOI] [PubMed] [Google Scholar]
- 93. Drazen JM. Retraction: Kajstura J et al. Evidence for human lung stem cells. N Engl J Med 2011;364:1795‐806. N Engl J Med. 2018;379:1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Sena ES, van der Worp HB, Bath PM, Howells DW, Macleod MR. Publication bias in reports of animal stroke studies leads to major overstatement of efficacy. PLoS Biol. 2010;8:e1000344. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: Supporting Information
Supplemental Table 1: Characteristics of included studies
Supplemental Table 2: Outcomes Assessed in Pre‐Clinical Studies
Supplemental Table 3: Approach to Categorizing Authors' Conclusions
Supplemental Table 4: Rating of Authors' Conclusions approximately Intervention Effectiveness
Supplemental Table 5: Mean SUCRA value and mean rank of each intervention assessed in two or more studies, for each outcome in descending order of mean SUCRA. Larger values of the mean SUCRA or the smaller values of the mean rank suggest better treatments. SUCRA: The Surface Under the Cumulative RAnking curve, which value represents the probabilities for each intervention to be among the n‐best options.
Supplemental Table 6: Optimal Route of administration, Dose and Dose frequency
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


