Abstract
The need for a safe and reliable presumptive test for law enforcement, first responders, and laboratory personnel is critical in the era of dangerous synthetic opioids and other novel psychoactive substances. Obtaining drug identification information without handling bulk powder is one way of accomplishing this task. This work evaluates whether trace residue on the exterior of drug packaging presents a potential source for presumptive testing. Utilizing a wipe-based approach, the outside of the packaging of nearly 200 case exhibits were sampled and analyzed by thermal desorption direct analysis in real time mass spectrometry (TD-DART-MS). While residue on the law enforcement (outer) packaging was a poor indicator of the contents (less than 50% accurate), the exterior of the drug (inner) packaging was shown to be an excellent indicator of its contents (92% accurate).
Quantitative analysis of the wipes, using liquid chromatography mass spectrometry (LC–MS/MS) showed that typical masses of residue on the exterior of packaging ranged from single to tens of micrograms – enough for detection by a number of trace detection tools. These initial results demonstrate that wipe-based trace sampling approaches present a promising, reliable, and safe method for presumptive testing by law enforcement, first responders, or laboratory personnel.
Keywords: Drug evidence, Opioids, DART-MS, Presumptive testing, Trace detection
1. Introduction
The ever-escalating danger of exposure to synthetic opioids and other novel psychoactive substances (NPSs) has forced law enforcement agencies to restrict the ability for officers to conduct presumptive testing in the field. This restriction puts pressure on forensic laboratories to provide rush analyses of cases in lieu of presumptive tests. The main driver for reducing, or eliminating, field testing is the inherent risk of handling and sampling bulk powders [1]. Approaches that eliminate the need to handle bulk powders, therefore, have the potential to restore field testing capabilities to law enforcement while also providing an additional tool for other first responders and laboratory personnel who need a rapid preliminary identification of an unknown material for health and safety purposes or for triaging of items entering the laboratory.
Presumptive testing completed without handling bulk material has two possible avenues: (1) utilizing spectroscopy-based techniques (e.g., Raman spectroscopy [2–4], Fourier transform infrared (FTIR) spectroscopy [5,6]) that can penetrate packaging material or (2) utilizing trace detection tools (e.g., ion mobility spectrometry (IMS) [7–11], lateral flow immunoassays (LFIs) [12], portable mass spectrometry (MS) [13–15]) to detect trace residue on the exterior of packaging. While the first approach, using spectroscopy-based techniques, has been demonstrated in the past, there are some limitations. These techniques typically perform well with relatively pure samples (greater than 10% mass fraction [16]) and require transparent or semi-transparent packaging. Since a large portion of drug evidence that is seized is in clear packaging the second limitation is not a substantial drawback. The inability to detect low weight percent components in a mixture, however, represents a significant challenge, especially for synthetic opioids, which are commonly present at well under 10% mass fraction [17].
Trace detection technologies, especially those that utilize a wipe-based collection approach, rely on particulate residue from the contents to be present on the exterior of the packaging. This allows for sampling of any type of packaging (transparent or opaque). These techniques also have excellent sensitivity (typically nanogram or lower limits of detection [7,18]). However, wipe-based collection recovers all residue off a surface (e.g., dirt, plasticizers), not only the drug residue, meaning detection needs to occur in the presence of a complex matrix.
To understand whether wipe-based trace detection approaches would be successful in this application, several questions must be investigated. First, whether a recoverable residue exists on the exterior of the packaging needs to be established because if no residue exists, trace detection would not be possible. Second, if a trace residue exists, the agreement between what is recovered by the wipe and the contents of the packaging needs to be established. Third, the amount (mass) of residue on the exterior of the packaging needs to be quantified to determine if there is enough material present for the instrument to detect. This work aims to address these questions. Wipes of the packaging of case exhibits were collected and analyzed using thermal desorption direct analysis in real time mass spectrometry (TD-DART-MS) and a subset further quantified using liquid chromatography mass spectrometry (LC–MS/MS). TD-DART-MS [18] is a well-suited tool for this type of trace detection as it allows for direct wipe introduction with excellent sensitivity and compound identification capabilities. Comparison of the TD-DART-MS spectra from wipes of drug packaging to extracts of their contents was used to evaluate the ability to detect trace residues and understand how well those residues predicted the contents of the packaging. LC–MS/MS was employed to gain quantitative measurements of the mass of residue present on the packaging. The work presented here provides a foundation for the ability of trace detection to be considered as a presumptive testing tool for law enforcement, first responders, or laboratory personnel.
2. Materials and methods
2.1. Sampling collection and preparation
Samples were collected from actual evidence submissions received at either the Maryland State Police Forensic Sciences Division or the Vermont Forensic Laboratory. Prior to an exhibit being analyzed for casework, wipes were taken of the exterior of outer and inner packaging to collect any available trace residue. Outer packaging was defined as the packaging the submitting officer put the item, or items, of evidence into and was typically a heat sealed or taped polyester evidence bag. The inner packaging was defined as the piece of evidence (e.g., glassine envelope, plastic bag, pill bottle) that was collected from the scene and contained the material to be analyzed. In cases with multiple items or submissions, the overall case packaging (e.g., a large K-Pak, paper bag, or box containing multiple exhibits) was not sampled. The type of material (e.g., plastic, paper, glassine, etc.) of the outer and inner packaging was noted in addition to the physical form of the suspected drugs (e.g., powder, pills, plant material, etc.).
Both the outer and inner packaging were sampled using a meta-aramid wipe (DSA Detection, North Andover, MA, USA) in a unidirectional manner using a firm force (between 5 N and 7 N). While the collection efficiencies of dry wipes are lower than wet wipes, dry wipes were used to avoid the possibility of disrupting or dissolving important markings or signatures on the packaging. The entire surface area of the exterior of the packaging was sampled using a single wipe and stored, individually, in manila envelopes. To ensure the residue on the exterior did not result from the opening of evidence within the laboratory, all samples were taken prior to opening of the respective packaging. Additionally, to evaluate whether the residue collected off the exterior of the packaging matched the contents, a single drop (approximately 5 μL) of an alcoholic extract of the pill, powder, etc. was placed on a separate wipe and stored in its own envelope. The alcoholic extract was used by the drug chemists for their gas chromatography mass spectrometry (GC–MS) analysis. Wipes were shipped from the participating forensic laboratories to a single laboratory for analysis.
Once the wipes were received from the forensic laboratories, they were logged and prepared for analysis TD-DART-MS and, if necessary, LC–MS/MS. TD-DART-MS was used as a non-targeted trace screening tool to determine how well the identity of drug compounds present in the evidence matched the trace residue recovered off the outer and inner packaging. When necessary, reference back to the gas chromatography mass spectrometry (GC–MS) data collected at the forensic laboratories was used to help determine the identity of the contents. LC–MS/MS was used to obtain quantitative information on the amount of residue that was present on the exterior of the packaging.
Fig. 1 shows a schematic of the workflow of the entire collection and analysis process. Wipes from the outer packaging were expected to have minimal trace residue and therefore the entire wipe was analyzed by TD-DART-MS. The wipe containing the alcoholic extract was also only analyzed using TD-DART-MS since it was solely used to correlate the contents of the item to the compounds recovered off the packaging. Wipes from the inner packaging were cut in half, lengthwise, with one half being analyzed by TD-DART-MS and the other half extracted to acquire quantitative information with LC–MS/MS.
Fig. 1.
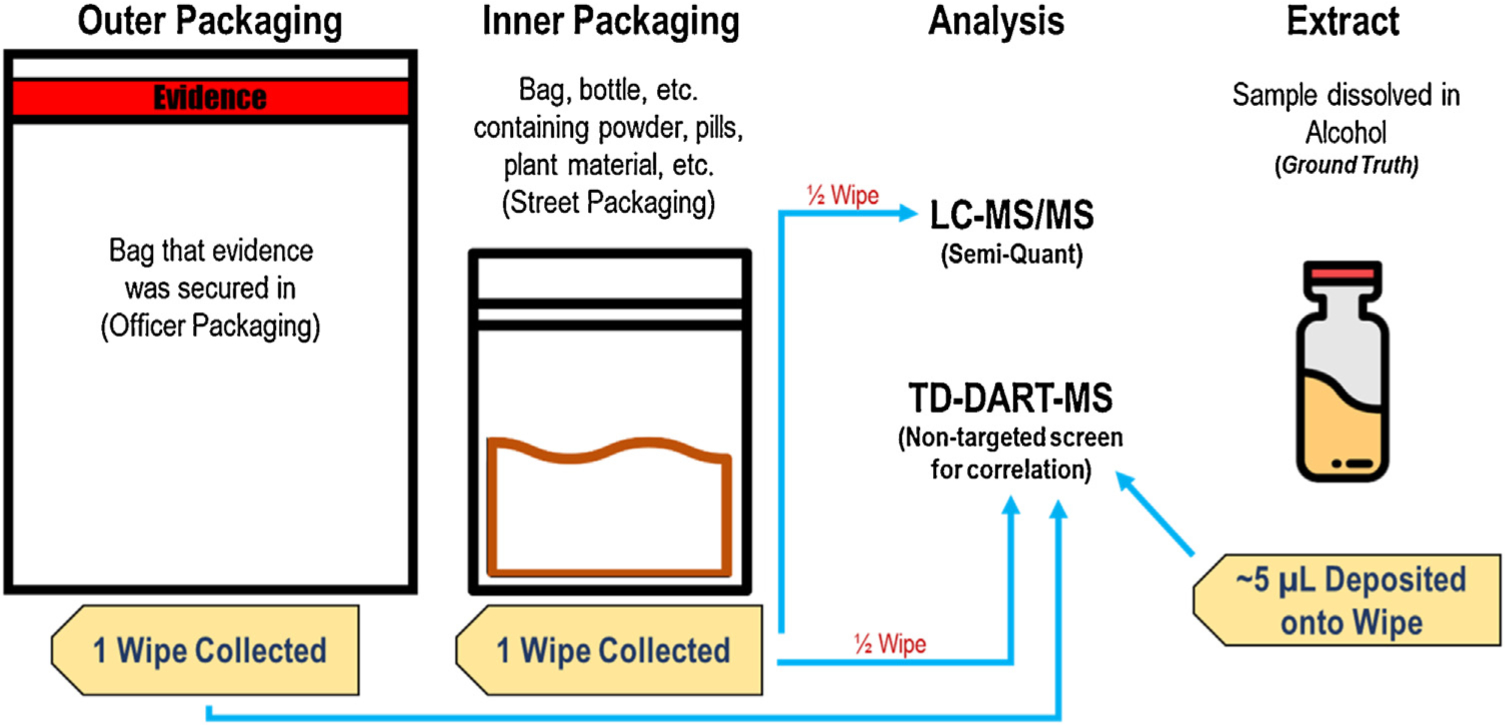
Schematic of how samples were collected and how each wipe was analyzed.
2.2. TD-DART-MS screening
TD-DART-MS, which is described in detail elsewhere [18], was used for the non-targeted screening of trace residues. It is ideally suited for this type of analysis because it allows for the direct introduction of wipes into the thermal desorber without any sample preparation or pre-treatment. The TD-DART-MS system used included an IonSense DART-SVP ion source (Saugus, MA, USA) coupled with an in-house built thermal desorption unit mounted on a JEOLJMS-T100LP mass spectrometer (JEOL USA, Peabody, MA, USA). The DART source was operated in positive ionization mode, with a grid voltage of +100 V, a 400 °C gas temperature, and zero-air nitrogen as the DART standby and ionization gas. The thermal desorber was operated at 265 °C and connected to a Vapur interface that pulled the sample vapor and DART gas towards the inlet of the mass spectrometer at a rate of 4 L min−1. The mass spectrometer was also operated in positive ionization mode with an orifice 1 voltage of +20 V, a ring voltage of +5 V, an orifice 2 voltage of +5V, an orifice temperature of 100 °C, and a Peaks voltage of +800 V. Mass spectra were collected at 2 scans s−1 across the range of m/z 80 to m/z 600. The resulting mass spectra, which were background subtracted from the spectra of a blank wipe, were searched against an in-house list of over 600 drugs of abuse and excipients using Mass Mountaineer (Fineview, NY, USA). Search parameters included a minimum absolute peak height of 2% and a mass agreement of ±0.005 Da.
2.3. LC–MS/MS quantitation
It was realized early on in the study that the wipes from the inner packaging contained sufficient amounts of material for the TD-DART-MS analysis. To gain more information from the sample set, inner packaging wipes were cut in half lengthwise, and one half was used for quantitative analysis by LC–MS/MS. Extraction of the halved wipes was completed by placing them into 2 mL vials and adding 500 μL of methanol containing a suite of deuterated internal standards (cocaine-d3, fentanyl-d5, heroin-d9, metham-phetamine-d5, and THC-d9). The capped vial was vortexed at 3000 rpm for 30 s after which the extract was transferred to a second 2 mL glass vial, containing an insert, for analysis. More information on the extraction process is provided elsewhere [19].
Quantitative analysis was completed using a Thermo Ulti-Mate3000 liquid chromatography system (Waltham, MA, USA) coupled to a Sciex 4000 QTrap mass spectrometer (Framingham, MA, USA). Specific run parameters are detailed elsewhere [19]. A panel of 11 drugs was used for LC–MS/MS analysis and included: cocaine, cyclopropyl fentanyl, fentanyl, furanyl fentanyl, heroin, ketamine, levamisole, 3,4-methylenedioxymethamphetamine (MDMA), methamphetamine, Δ9-tetrahydrocannabinol (THC), and U-47700. While this panel did not cover all drugs identified by TD-DART-MS in this study, it did provide a sufficient number to gain insight into the magnitude of the amount of material present on the inner packaging.
3. Results & discussion
3.1. Sample breakdown
For this study, a total of 191 different items were sampled, resulting in a total of 191 inner packaging wipes and extract wipes. Because multiple items were occasionally packaged in the same outer package, only 144 outer packaging wipes were collected. Of the 144 pieces of outer packaging sampled, 98% (141 out of 144) were plastic bags (e.g., K-Pak), and the remaining 2% (3 out of 144) were paper bags. The interior packaging, which contained the suspected drug evidence, was broken into five different categories: plastic bags, glassine envelopes, heat sealed foil bags, bottles (pharmaceutical pill bottles or glass bottles), and paper. The evidence itself was differentiated as either: powder, plant material, pills, residue, liquid, or food. Table 1 provides a breakdown of both the inner packaging and the type of evidence that was sampled. Of the 191 samples that were analyzed, 28 (≈15%) were found by the casework GC–MS analysis and TD-DART-MS analysis of the extract to not contain any drugs – providing a set of known negative samples.
Table 1.
Make-up of the inner packaging and type of evidence that was sampled.
| Inner Packaging | |||||||
|---|---|---|---|---|---|---|---|
| Plastic | Glassine | Bottle | Foil | Paper | Total | ||
| Evidence Type | Powder | 100 | 53 | 10 | 4 | 2 | 169 |
| Residue | 2 | 1 | 0 | 0 | 4 | 7 | |
| Pill | 4 | 0 | 2 | 0 | 0 | 6 | |
| Plant | 2 | 0 | 0 | 4 | 0 | 6 | |
| Liquid | 2 | 0 | 0 | 0 | 0 | 2 | |
| Food | 0 | 0 | 0 | 1 | 0 | 1 | |
| Total: | 110 | 54 | 12 | 9 | 6 | 191 | |
3.2. Outer packaging results
A total of 144 outer packaging wipes were collected and their TD-DART-MS responses were compared to those from the extract wipes to evaluate (1) whether detectable levels of residue existed on the outer packaging and (2) whether the residue on the outer packaging was indicative of the contents. Table 2 presents a summary of the agreement between the outer packaging and the contents (extract). In total, 54% (78 out of 144) of the outer packaging wipes were found to have no detectable drug residue present while at least one drug was detected on 46% (66 out of 144) of the samples. All but one of the wipes from samples that did not contain drugs had no detectable drug signature. However, of the samples where the extract did contain one or more drugs, 62 outer packaging wipes were found to have no detectable drug present. The make-up of drugs that were not seen on the outer packaging varied greatly, and no trend for the type of drug or type of material (e.g., pills, powders, etc.) that led to false negatives was observed. The high level of false negatives (43%) was not unexpected for the outer packaging as these bags were provided by law enforcement officers to store evidence in and likely spent little to no time in the environment where the evidence was seized. Additionally, this packaging likely did not come into direct contact with the powders, pills, etc. and therefore the probability of particulate transfer was low.
Table 2.
Results of agreement between the outer packaging and the extract for the 144 exhibits analyzed.
| Outer Packaging | Extract | Percent Occurrence | Result Type |
|---|---|---|---|
| Drug Detected | Same Drug Detected | 32% (n = 46) | True Positive |
| Drug Detected | No Drug Detected | 1% (n = 1) | False Positive |
| Drug Detected | Different Drug Detected | 13% (n = 19) | False Positive |
| No Drug Detected | Drug Detected | 43% (n = 62) | False Negative |
| No Drug Detected | No Drug Detected | 11% (n = 16) | True Negative |
| Overall Accuracy: | 43% |
Of the 66 outer packaging wipes that did have detectable drug residue, roughly 70% (46 out of 66) were found to have at least one of the drugs present in the extract also on the outer packaging. This led to 32% of the samples producing a true positive result, and an overall accuracy (including true negatives) of 43%. The true positive samples spanned the classes and types of drugs identified in the study and therefore was considered to largely be a true representation of contamination from either the inner packaging, the inner packaging contents, or the environment where the evidence was collected. The remaining 20 samples, which represented the instances of false positive detection, represented two possibilities. In 19 of the 20 instances the outer packaging contained a different drug than was present in the extract whereas in the remaining instance there was no drug present in the extract. The recovery of cocaine off the outer packaging which was not present in the extract was the most frequent cause of a false positive identification, accounting for 19 out of 20 of the instances. The prevalence of cocaine on the outer packaging was thought to be caused by the prevalence of that drug in forensic laboratories [19], police stations [20,21], and/or the general environment [22–25]. With an accuracy of only 43%, the residue on the outer packaging was not a reliable predictor of its contents. Fig. 2 provides an example of TD-DART-MS spectra for a true positive, false positive, and false negative sample.
Fig. 2.
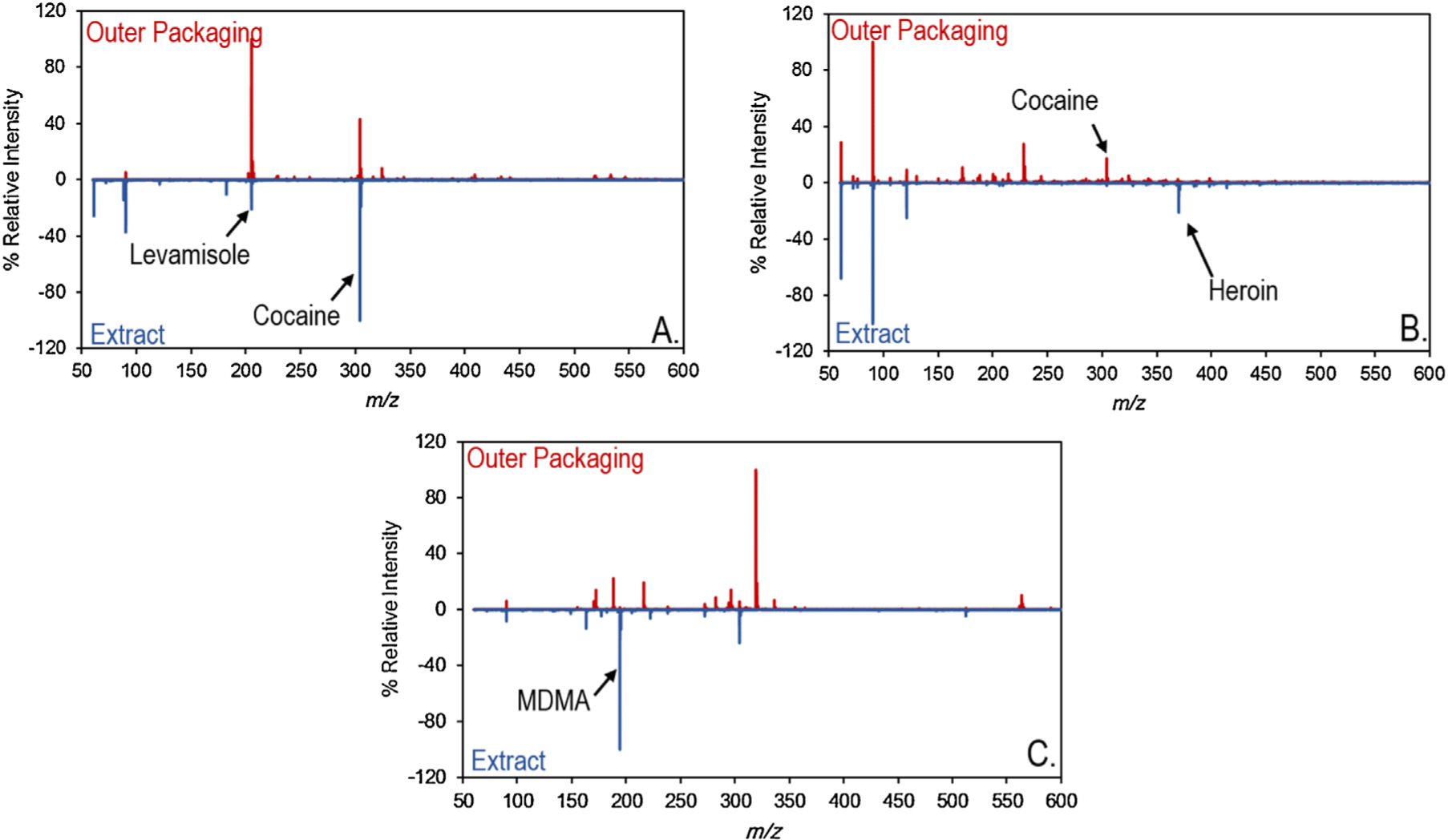
TD-DART-MS mass spectral comparison of (A.) a true positive, (B.) a false positive, and (C.) a false negative (C.) example of outer bag wipes.
3.3. Inner packaging results
Because of the direct contact with the evidence (e.g., powders, pills, etc.), the residue on the inner packaging was expected to be a better predictor, or presumptive tool, to identify the contents inside the packaging. While the residue on the inner packaging might come from particulate of the evidence itself when the container was opened or closed; a second possibility for contamination could also have occurred from the packaging being present in the environment where the contents were cut or used. In this instance, particulate from the environment might transfer to the packaging. The agreement between the inner packaging wipes and the extract, shown in Table 3, presented the cumulative results of the 191 inner packaging samples and highlights the expected increased agreement compared to the outer packaging wipes. In 151 of the 191 items that were sampled, a true positive detection – defined as at least one of the drugs present in the extract being detected on the exterior of the inner packaging – was achieved. This definition was used as the technique was being evaluated as presumptive, not confirmatory – which would require detection of all drugs present in the samples. Additionally, 25 of the 28 known negative samples had no detectable residue on the exterior of the bag, leading to an accurate identification 92% of the time (176 out of 191) and a true positive rate of 95%. Fig. 3 highlights an example of how well the TD-DART-MS spectra of the inner packaging wipe and the extract wipe agreed.
Table 3.
Results of agreement between the inner packaging and the extract for the 191 exhibits analyzed.
| Inner Packaging | Extract | Percent Occurrence | Result Type |
|---|---|---|---|
| Drug Detected | Same Drug Detected | 79% (n = 151) | True Positive |
| Drug Detected | No Drug Detected | 1.5% (n = 3) | False Positive |
| Drug Detected | Different Drug Detected | 2.5% (n = 5) | False Positive |
| No Drug Detected | Drug Detected | 4% (n = 7) | False Negative |
| No Drug Detected | No Drug Detected | 13% (n = 25) | True Negative |
| Overall Accuracy: | 92% |
Fig. 3.
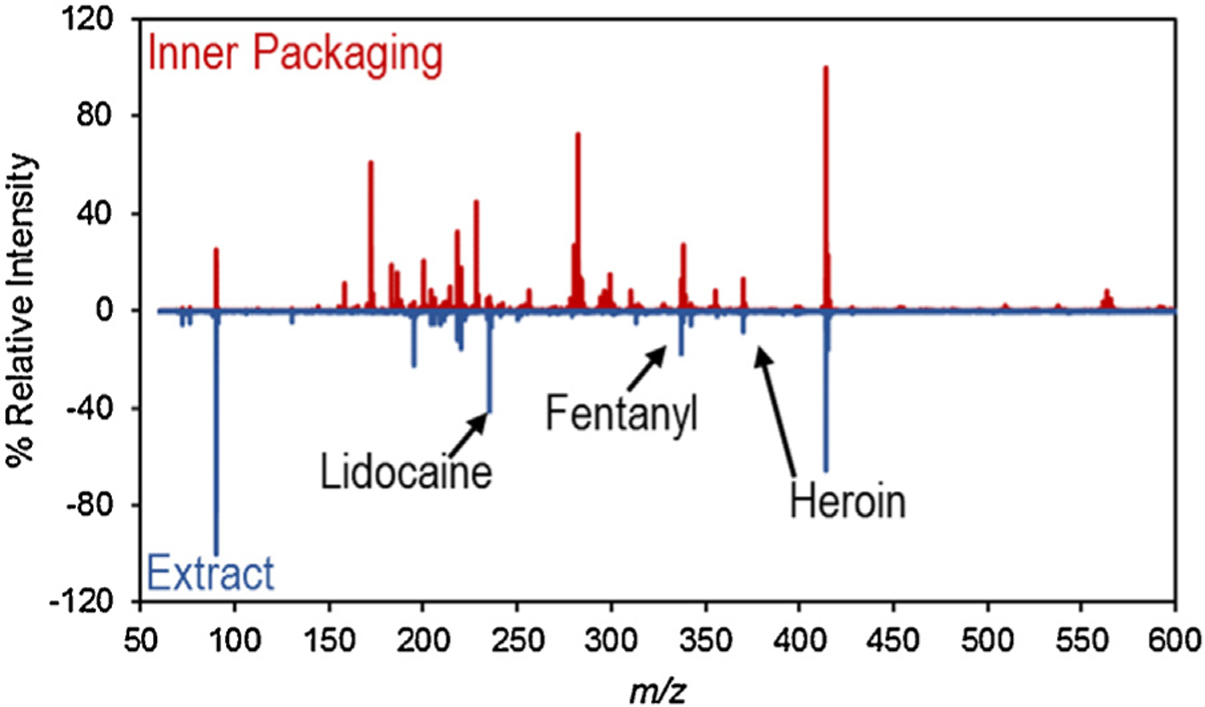
TD-DART-MS spectral comparison of an inner packaging sample containing lidocaine, fentanyl, and heroin.
Depending on the application of a screening technique, false positive or false negatives may be more problematic. If a screening tool is being used for triaging samples to identify ones that contain opioids, so they can be handled differently, a false positive is more desirable than a false negative as it errs on the side of safety. However, if a screening tool is being used for presumptive testing in the field, a false positive is more detrimental than a false negative as it may lead to unnecessary detainment of a suspect. For the sample set investigated here, the percentage of false positives and false negatives were both 4%.
In terms of instances that lead to a false positive, there were three samples where a drug was detected on the inner packaging, but no drug was present in the item. Cocaine was found on the inner packaging of two of the samples while 3-methoxy-PCP was detected on the third. In an attempt to understand how these three false positive cases occurred, it was determined that the likely cause was contamination from another item in the case which contained that drug. In all three instances, the drug that was detected on the item that lead to the false positive was present in another item in the case. The remaining false positive samples (5 instances where a different drug was detected on the inner packaging than what was inside) were also able to be attributed to instances where the presence of the drug detected on the packaging was likely caused by contamination from a different item in the case. Three of these five samples were heat-sealed foil bags containing plant material sprayed with synthetic cannabinoids. Considering the various pathways for how a residue can make it onto the exterior of the bag (e.g., aerosolization of particulate in pouring samples into the container) it would be difficult for particulate from this type of material to make it to the exterior of the packaging. A similar instance occurred in one of the other items where pharmaceutical grade pills were present, but their signature was not detectable on the exterior of the inner packaging.
Out of the 191 samples, there were seven instances that resulted in a false negative – where no residue was detected on the exterior of the packaging, yet drugs were present. For two of these seven instances, the cause was likely due to lack of residue transfer because the item was either plant material in a heat-sealed foil bag or a pharmaceutical grade pill. The remaining five samples all came from a case where a large quantity of dextromethorphan, a cutting agent, was recovered. For these samples, the amount of dextromethorphan recovered off the packaging was high enough to suppress the response of the actual drug(s) residue present in the sample. However, this was another example of an instance where the environment with which similar items were collected led to transfer of particulate across packaging.
The idea of environmental contamination being transferred onto the inner packaging was further highlighted in a set of items sampled from another case. In this case, items were collected from a home where pure carfentanil was previously seized. A second seizure from the home, the items of which were sampled as part of this study, consisted of several plastic bags containing white powder which were initially presumed to be carfentanil. Analysis of the exhibits showed that they did not contain carfentanil but instead contained cyclopropyl fentanyl. Sampling of the exterior of the items from this case, shown in Fig. 4, produced a signature not only for cyclopropyl fentanyl but also for carfentanil. A strong signal for both compounds was obtained on TD-DART-MS, indicating a relatively high level of residue on the exterior of the bag. For another item in the case, which contained cyclopropyl fentanyl and fentanyl, the peak identification threshold was lowered to 1% and produced additional hits for three other fentanyl analogs on the packaging. The presence of these additional compounds at low levels likely provides a signature of the various fentanyl analogs the suspect possessed over time. These low intensity peaks may have significant intelligence implications that could be utilized by law enforcement. Research is currently underway to evaluate whether this type of low-level signature may be a good predictor of analytes that were present in the environment in the past.
Fig. 4.
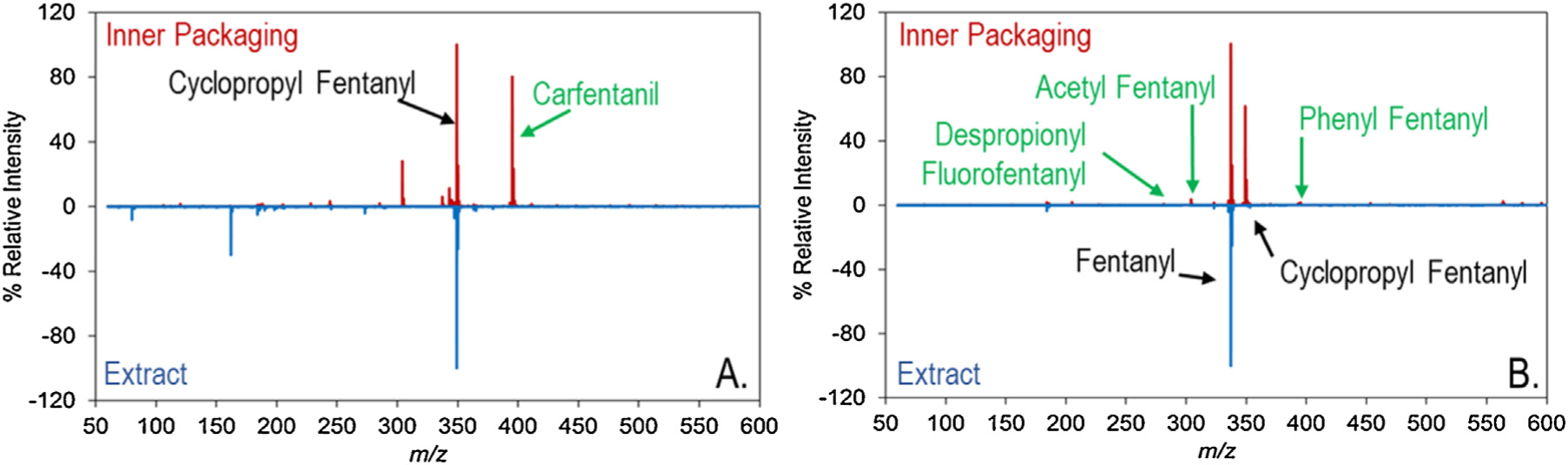
Example of how the residue on the inner packaging could be used for intelligence purposes. Both spectra (A. and B.) are wipes of inner packages that came from a location where other seizures recovered carfentanil. Compounds in green are those that were found on the packaging, but not in the extract.
While the trace residue on the exterior of the inner packaging provides an excellent indication of what is present inside the bag, depending on the application the identification of the actual drug may not be necessary. In some instances, the goal may simply be to identify if the package contains on opioid, so that it can be triaged in a different manner than other items. If this approach is desired, a simple yes or no for the presence of an opioid is all that is required. The sample set analyzed in this work provided an accuracy of 100% in correctly identifying the presence or absence of an opioid inside the item.
3.4. Quantitative analysis
While the main driver of this research was to evaluate the ability to correctly identify the compounds inside packaging based on the exterior trace residue, it was realized that quantitative estimations could also be obtained – providing an understanding of the magnitude of trace residue present on the exterior of the evidence. The quantitative analysis, which was completed on one half of the wipe was identical to that described elsewhere [19] and allowed for the measurement of 11 of the drugs that were detected in the screening portion of the study. The results of the quantitative analysis, for instances where the exterior residue matched the inner packaging, are presented in Table 4.
Table 4.
Quantitative results of the inner packaging wipes. Uncertainties represent the uncertainty in the measurement. [19] The values reported here assume that the residue was equally distributed across the wipe and the collection efficiency of the meta-aramid wipe was approximately 33%.
| Drug | Average Amount Recovered (μg) | Median Amount Recovered (μg) | Minimum Amount Recovered (μg) | Maximum Amount Recovered (μg) | Number of Samples |
|---|---|---|---|---|---|
| Cocaine | 6.45 (±0.74) | 1.92 (±0.22) | 0.03 (±0.003) | 46.62 (±5.31) | 37 |
| Cyclopropyl Fentanyl | 0.69 (±0.08) | - | - | - | 1 |
| Fentanyl | 5.90 (±0.70) | 2.64 (±0.31) | 0.09 (±0.01) | 34.92 (±4.16) | 43 |
| Furanyl Fentanyl | 7.32 (±0.90) | - | - | - | 1 |
| Heroin | 197.02 (±13.99) | 39.39 (±2.80) | 1.02 (±0.07) | 2430.00 (±172.53) | 42 |
| Ketamine | 11.28 (±1.35) | 8.93 (±1.07) | 0.15 (±0.02) | 36.75 (±4.41) | 8 |
| Levamisole | 4.32 (±0.43) | 3.00 (±0.30) | 2.34 (±0.23) | 9.75 (±0.98) | 5 |
| MDMA | 22.03 (±2.51) | 11.70 (±1.33) | 0.33 (±0.04) | 106.80 (±12.18) | 10 |
| Methamphetamine | 20.57 (±2.80) | 14.01 (±1.91) | 0.15 (±0.02) | 54.09 (±7.36) | 4 |
| THC | 6.87 (±0.69) | - | - | - | 1 |
| U-47700 | 1.05 (±0.07) | 1.05 (±0.07) | 0.81 (±0.06) | 1.29 (±0.09) | 2 |
For nearly all drugs, shown in Table 4, an average of single to tens of micrograms of residue was present on the packaging. Approximately 82% of the inner packages had greater than 1 μg of residue on the exterior and all but two samples had greater than 0.1 μg. For the major drugs – cocaine, fentanyl, heroin, and methamphetamine – packages containing greater than 10 mg of residue were common, and in three instances, greater than 1 mg of heroin was present. Individual values for the quantitative analysis can be found in the Supplemental Information. Given that trace detection technology sensitivities are typically at or below 100 ng wipe−1 [7,10,12,13,18], detection of residue should be possible using techniques other than TD-DART-MS (to include technologies such as IMS or LFI).
There were also instances when drugs were detected on the exterior of the inner packaging that were not present inside. For the wipes that were analyzed using this panel, quantitative numbers were only obtained for heroin and cocaine. In one instance, approximately 30 ng of heroin was found on the exterior of the inner packaging that did not contain that drug. There were 16 instances were cocaine was present at quantifiable amounts on the exterior of the packaging but was not present in the contents, with levels ranging 30 ng to 42 μg. The majority of these false cocaine samples (13 out of 16), had less than 1 μg present, with tens to hundreds of nanograms most frequently recovered.
4. Conclusions
While the residue on the exterior of the packaging containing drug evidence showed excellent correlation to its content, outer layers of packaging (those that the officer uses to store the evidence) were poor predictors of the contents. This was not unexpected as this level of packaging never encounters the actual material (e.g., powders, pills, etc.) and is not present in a contaminated environment for a long enough period to accumulate substantial residue levels. The accuracy was less than 50% with false negatives more prevalent than false positives.
The trace residue on the exterior of packaging containing drugs presents a potentially viable tool for presumptive testing without the need for interacting with bulk powders. With nearly 200 items sampled, the ability to identify either the absence of drugs or the presence of at least one drug in sample was over 92%. False detections seemed to be driven by one of two issues. In cases where one item was a pure drug, such as cocaine, or a cutting agent, such as dextromethorphan, residue from that item was detected on other items, which could suppress the detection of residue from those items – likely because of competitive ionization. Also, poor detection of compounds sprayed onto plant material and stored in heat-sealed foil bags was observed, due to limited possibility for trace particulate to be transferred to the exterior packaging. If this approach was to be used only for the detection of synthetic opioids – such as triaging cases that enter a laboratory – an accuracy of 100% was achieved.
Quantitative analysis showed that the mass of residue that exists on the exterior of the packaging ranges from tens of nanograms to milligrams, with single to tens of micrograms most commonly observed. This amount of mass should be sufficient for recovery and detection of drugs with a number of trace detection technologies (e.g., IMS, LFIs, DART-MS, portable MS) not just TD-DART-MS. This is encouraging as it means that techniques more portable and less costly then TD-DART-MS could be utilized for fieldable applications, providing this capability to law enforcement and first responders in addition to laboratory personnel. For false positive cases, where drugs were detected on the exterior of the packaging, mass loadings were lower (single to hundreds of nanograms), indicating a possibility for signal thresholding to increase the true detection rate.
Current efforts are focused on expanding the population of samples to capture more samples and obtain a more diverse sample set. The impact of signal thresholding based on mass loading is also being explored as is the implementation of additional trace detection technologies such as IMS. The ability to obtain intelligence information for instances where items were seized from a suspected dealer location (or wipes from a dealer location) are also being investigated to evaluate whether the trace signatures obtained can provide important information as to the types of drugs that have been present in that environment. This initial work shows the promise of using trace residues as a reliable presumptive test in most cases.
Supplementary Material
Footnotes
Publisher's Disclaimer: Disclaimer
Publisher's Disclaimer: Certain commercial products are identified in order to adequately specify the procedure; this does not imply endorsement or recommendation by NIST, nor does it imply that such products are necessarily the best available for the purpose.
Publisher's Disclaimer: Certain commercial products are identified in order to adequately specify the procedure; this does not imply endorsement or recommendation by Maryland State Police, nor does it imply that such products are necessarily the best available for the purpose.
Publisher's Disclaimer: Certain commercial products are identified in order to adequately specify the procedure; this does not imply endorsement or recommendation by Vermont Forensic Laboratory, nor does it imply that such products are necessarily the best available for the purpose.
Appendix A. Supplementary data
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.forsciint.2019.109939.
References
- [1].DEA Warning to Police and Public: Fentanyl Exposure Kills, n.d. https://www.dea.gov/press-releases/2016/06/10/dea-warning-police-and-public-fentanyl-exposure-kills (Accessed 2 April 2019).
- [2].Penido C.A.F. de O., Pacheco MTT, Lednev IK, Silveira L, Raman spectroscopy in forensic analysis: identification of cocaine and other illegal drugs of abuse, J. Raman Spectrosc 47 (2016) 28–38, doi: 10.1002/jrs.4864. [DOI] [Google Scholar]
- [3].de Veij M, Deneckere A, Vandenabeele P, de Kaste D, Moens L, Detection of counterfeit Viagra® with Raman spectroscopy, J. Pharm. Biomed. Anal 46 (2008) 303–309, doi: 10.1016/j.jpba.2007.10.021. [DOI] [PubMed] [Google Scholar]
- [4].Olds WJ, Jaatinen E, Fredericks P, Cletus B, Panayiotou H, Izake EL, Spatially offset Raman spectroscopy (SORS) for the analysis and detection of packaged pharmaceuticals and concealed drugs, Forensic Sci. Int 212 (2011) 69–77, doi: 10.1016/j.forsciint.2011.05.016. [DOI] [PubMed] [Google Scholar]
- [5].Materazzi S, Gregori A, Ripani L, Apriceno A, Risoluti R, Cocaine profiling: implementation of a predictive model by ATR-FTIR coupled with chemo-metrics in forensic chemistry, Talanta 166 (2017) 328–335, doi: 10.1016/j.talanta.2017.01.045. [DOI] [PubMed] [Google Scholar]
- [6].Coelho Neto J, Rapid detection of NBOME’s and other NPS on blotter papers by direct ATR-FTIR spectrometry, Forensic Sci. Int 252 (2015) 87–92, doi: 10.1016/j.forsciint.2015.04.025. [DOI] [PubMed] [Google Scholar]
- [7].Sisco E, Verkouteren J, Staymates J, Lawrence J, Rapid detection of fentanyl, fentanyl analogues, and opioids for on-site or laboratory based drug seizure screening using thermal desorption DART-MS and ion mobility spectrometry, Forensic Chem. 4 (2017) 108–115, doi: 10.1016/j.forc.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Keller T, Miki A, Regenscheit P, Dirnhofer R, Schneider A, Tsuchihashi H, Detection of designer drugs in human hair by ion mobility spectrometry (IMS), Forensic Sci. Int 94 (1998) 55–63, doi: 10.1016/S0379-0738(98)00051-6. [DOI] [PubMed] [Google Scholar]
- [9].Tam M, Maheux CR, Lalonde S, Binette M-J, Unleashing the power from commercial off-the-shelf ion mobility spectrometer, Int. J. Ion Mobil. Spectrom 22 (2019) 11–20, doi: 10.1007/s12127-019-00245-z. [DOI] [Google Scholar]
- [10].Zaknoun H, Binette M-J, Tam M, Analyzing fentanyl and fentanyl analogues by ion mobility spectrometry, Int. J. Ion Mobil. Spectrom 22 (2019) 1–10, doi: 10.1007/s12127-019-00244-0. [DOI] [Google Scholar]
- [11].Armenta S, Garrigues S, de la Guardia M, Brassier J, Alcalà M, Blanco M, Perez-Alfonso C, Galipienso N, Detection and characterization of emerging psychoactive substances by ion mobility spectrometry, Drug Test. Analysis 7 (2015) 280–289, doi: 10.1002/dta.1678. [DOI] [PubMed] [Google Scholar]
- [12].Angelini Daniel J., Biggs Tracey D., Maughan Michele N., Feasel Michael G., Sisco Edward, Sekowski Jennifer W., Evaluation of a lateral flow immunoassay for the detection of the synthetic opioid fentanyl, Forensic Sci. Int (n.d.) Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Lawton ZE, Traub A, Fatigante WL, Mancias J, O’Leary AE, Hall SE, Wieland JR, Oberacher H, Gizzi MC, Mulligan CC, Analytical validation of a portable mass spectrometer featuring interchangeable, ambient ionization sources for high throughput forensic evidence screening, J. Am. Soc. Mass Spectrom 28 (2017) 1048–1059, doi: 10.1007/s13361-016-1562-2. [DOI] [PubMed] [Google Scholar]
- [14].Leary PE, Dobson GS, Reffner JA, Development and applications of portable gas chromatography–mass spectrometry for emergency responders, the military, and law-enforcement organizations, Appl. Spectrosc 70 (2016) 888–896. [DOI] [PubMed] [Google Scholar]
- [15].Bruno AM, Cleary SR, O’Leary AE, Gizzi MC, Mulligan CC, Balancing the utility and legality of implementing portable mass spectrometers coupled with ambient ionization in routine law enforcement activities, Anal. Methods 9 (2017) 5015–5022, doi: 10.1039/C7AY00972K. [DOI] [Google Scholar]
- [16].Ryder AG, O’Connor GM, Glynn TJ, Quantitative analysis of cocaine in solid mixtures using Raman spectroscopy and chemometric methods, J. Raman Spectrosc 31 (2000) 221–227, doi:. [DOI] [Google Scholar]
- [17].National Drug Threat Assessment, Drug Enforcement Agency, (2018).
- [18].Sisco E, Forbes TP, Staymates ME, Gillen G, Rapid analysis of trace drugs and metabolites using a thermal desorption DART-MS configuration, Anal. Methods 8 (2016) 6494–6499, doi: 10.1039/C6AY01851C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Sisco E, Najarro M, Burns A, A snapshot of drug background levels on surfaces in a forensic laboratory, Forensic Chem. 11 (2018) 47–57, doi: 10.1016/j.forc.2018.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Doran GS, Deans R, De Filippis C, Kostakis C, Howitt JA, The presence of licit and illicit drugs in police stations and their implications for workplace drug testing, Forensic Sci. Int 278 (2017) 125–136, doi: 10.1016/j.forsciint.2017.06.034. [DOI] [PubMed] [Google Scholar]
- [21].Doran GS, Deans RM, Filippis CD, Kostakis C, Howitt JA, Quantification of licit and illicit drugs on typical police station work surfaces using LC-MS/MS, Anal. Methods 9 (2017) 198–210, doi: 10.1039/C6AY02668K. [DOI] [Google Scholar]
- [22].Angelo Cecinato, Catia Balducci, Detection of cocaine in the airborne particles of the Italian cities Rome and Taranto, J. Sep. Sci 30 (2007) 1930–1935, doi: 10.1002/jssc.200700039. [DOI] [PubMed] [Google Scholar]
- [23].Cecinato A, Balducci C, Nervegna G, Occurrence of cocaine in the air of the World’s cities: an emerging problem? A new tool to investigate the social incidence of drugs?, Sci. Total Environ 407 (2009) 1683–1690, doi: 10.1016/j.scitotenv.2008.11.004. [DOI] [PubMed] [Google Scholar]
- [24].Hudson JC, Analysis of currency for cocaine contamination, Can. Soc. Forensic Sci. J 22 (1989) 203–218, doi: 10.1080/00085030.1989.10757434. [DOI] [Google Scholar]
- [25].Smith FP, McGrath KR, Cocaine surface contamination and the medico-legal implications of its transfer, Egypt. J. Forensic Sci 1 (2011) 1–4, doi: 10.1016/j.ejfs.2011.04.002. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


