Abstract
Sodium valproate (VPA) has analgesic effects in clinical and experimental studies, but the mechanisms are still unclear. The present study examined the effects of VPA on stress-induced somatic hyperalgesia and visceral hypersensitivity and the role of 5-HT2C receptors in the spinal cord. Repeated 3 day forced swim (FS) significantly reduced the thermal withdrawal latency and mechanical withdrawal threshold, and increased the magnitude of the visceromotor response to colorectal distention compared to the baseline values in rats. The somatic hyperalgesia and visceral hypersensitivity were accompanied by significant down-regulation of 5-HT2C receptor expression in the L4-L5 and L6-S1 dorsal spinal cord. Intraperitoneal administration of VPA (300 mg/kg) before each FS and 1 day post FS prevented the development of somatic hyperalgesia and visceral hypersensitivity induced by FS stress, as well as down-regulation of 5-HT2C receptors in the spinal cord. The reversal of somatic hyperalgesia and visceral hypersensitivity by VPA in FS rats was blocked by intrathecal administration of the selective 5-HT2C receptor antagonist RS-102221 (30 μg/10 μL) 30 min after each VPA injection. The results suggest that VPA attenuates FS-induced somatic hyperalgesia and visceral hypersensitivity by restoring down-regulated function of 5-HT2C receptors in the spinal cord.
Keywords: Sodium valproate, Serotonin, Forced swim, Fibromyalgia, Visceromotor response, Irritable bowel syndrome
1. Introduction
Pain is an unpleasant sensory and emotional experience. The treatment of pain is often difficult, especial chronic pain. Many drugs now used to treat chronic pain were originally prescribed for other neurological disorders. Sodium valproate (VPA) has multiple biological effects (Ximenes JCM, 2012), and is widely used as an anti-epileptic drug. However, VPA has also been reported to have analgesic properties in many preclinical and clinical studies. VPA could alleviate chronic headache (Yancey et al., 2014), migraine (Shahien et al., 2011), endometriosis pain (Liu et al., 2012), neuropathic pain (Dosenovic et al., 2017), and inflammatory pain (Bai et al., 2010). Previous studies showed that VPA affected neurotransmitters such as norepinephrine, dopamine, γ-amino butyric acid (GABA) and serotonin (5-hydroxytryptamine, 5-HT) (Hamed and Abdellah, 2017; Kammerer et al., 2011; Wilson et al., 2014). In a rat model of post-traumatic stress disorder (PTSD), VPA increases fear extinction by increasing 5-HT in the hippocampus and prefrontal cortex (Wilson et al., 2014). The anti-depressant mechanism of VPA is partly related to elevated 5-HT, and the reuse of 5-HT in the vesicles of presynaptic nerve endings may also contribute to this action (Qiu et al., 2014). However, whether 5-HT is involved in the analgesic of VPA is poorly understood.
Stress can induce either analgesia (Butler and Finn, 2009) or hyperalgesia (Jennings et al., 2014), depending on the intensity and duration of the stressor. Acute stress is often analgesic (Butler and Finn, 2009), whereas chronic stress induces hyperalgesia (Jennings et al., 2014; Wang et al., 2013). Three day forced swim (FS), as a subchronic stressor, induces somatic hyperalgesia of the rat hindpaw, alongside increased neuronal activity in the spinal cord (Guevara et al., 2015; Quintero et al., 2011). FS-induced hyperalgesia results from decreased GABA release, increased glutamate release and NMDA receptor activation in the spinal cord (Quintero et al., 2011). In addition, other neurotransmitter systems that are involved in both the stress response and pain transmission may also be implicated in FS-induced hypersensitivity. One possibility is a deficit in central serotonergic transmission, such as in the raphe magnus and the spinal dorsal horn (Quintero et al., 2000). Systemic administration of the serotonin-norepinephrine reuptake inhibitor (SNRI) duloxetine, the serotonin-selective reuptake inhibitors (SSRI) clomipramine and fluoxetine or the serotonin precursor tryptophan prevented stress-induced hyperalgesia evoked by thermal and chemical stimuli (Nyland et al., 2015; Quintero et al., 2000). However, the mechanisms underlying stress-induced pain have not been completely elucidated.
5-HT is an important modulator of nociceptive transmission (Ossipov et al., 2010). The modulation of 5-HT on pain is complex as 5-HT may have inhibitory or excitatory effects in descending 5-HTergic pathways projecting into the spinal cord depending on the receptor subtypes (Bardin, 2011; Cortes-Altamirano et al., 2018). As one of seven 5-HT receptor subfamilies, 5-HT2 receptors are widely distributed in the spinal cord (Fonseca et al., 2001). This distribution suggests that this receptor may play a role in pain processing at the spinal level. 5-HT2 receptors can be divided into three subtypes including 5-HT2A, 5-HT2B, and 5-HT2C (Bombardi, 2014). The role of 5-HT2C receptors in the spinal cord is controversial. Some studies indicate that activation of 5-HT2C receptors increases anti-nociception and attenuates muscular hyperalgesia (Baptista et al., 2012; Ogino et al., 2013; Tavares et al., 2018). In contrast, others have reported that stimulation of spinal 5-HT2C receptors facilitates nociceptive transmission (Cervantes-Duran et al., 2016).
Although the SNRI, SSRI or 5-HT precursor tryptophan decreased hyperalgesia in the 3 day FS stress model (Nyland et al., 2015; Quintero et al., 2000), the related 5-HT receptor subtype is unclear. Since stress-induced hyperalgesia in rats may involve serotonin signaling (Nyland et al., 2015; Quintero et al., 2000), and 5-HT2C receptors in the spinal cord have been reported to be associated with the regulation of pain (Cervantes-Duran et al., 2016; Nakai et al., 2010), we hypothesized that VPA reduced FS-evoked hyperalgesia in rats through regulating spinal 5-HT2C receptors. To test this hypothesis, we used the subchronic FS stress-induced pain model to evaluate the potential anti-hyperalgesic effect of VPA. In addition, the possible implication of the 5-HT2C receptors was assessed by intrathecal administration of the 5-HT2C receptor antagonist RS-102221 and the expression of 5-HT2C receptor in the spinal cord of the rats.
2. Materials and methods
2.1. Experimental protocol and groups
The experimental protocol is shown in Fig. 1. Experimental protocols were approved by the Institutional Animal Care and Use Committees of Xi’an Jiaotong University (NO. 2016–006), China and University of Maryland, Baltimore, USA (NO. 1217013). A total of 44 female Sprague-Dawley rats weighing 225–250 g were obtained from Xi’an Jiaotong University Laboratory Animal Center (Xi’an, Shaanxi, China) for somatic pain experiments and 30 rats for visceromotor response (VMR) experiments were from Envigo (Frederick, MD, USA). Pain syndromes, e.g. fibromyalgia syndrome (FMS) or irritable bowel syndrome (IBS), are often associated with stress in human, and these diseases are more prevalent in women (Fischer et al., 2016; Papaefthymiou et al., 2019). In addition, FS stress induced visceral hypersensitivity only lasting a few days in male rats which farther shorter than female rats (Ji et al. 2018). Therefore, female rats were selected in the present study. All experiments except the VMR experiments were conducted in Xi’an Jiaotong University College of Stomatology. The VMR experiments were conducted in University of Maryland School of Dentistry. All animals were treated in accordance with the guidelines for pain research in conscious animals recommended by the International Association for the Study of Pain (Zimmermann, 1983). All animals were housed in standard laboratory condition (22 ± 1℃; 12 h light/dark, lights on at 7 am) with free access to food and water. Rat cages were plastic boxes (46 × 35 × 21 cm) with metal tops and sawdust as bedding, and three to four rats in a cage except single rat in a cage after EMG surgery.
Fig. 1.
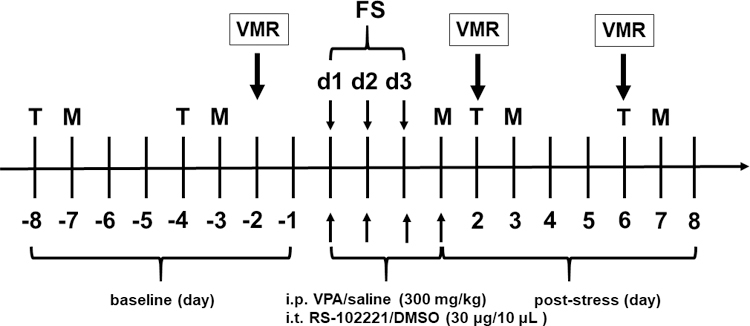
Schematic experimental protocol. Rats were treated with VPA or vehicle (saline, i.p.) for 4 days (30 min before each FS and 1 day after FS). Rats swam for 10 min on the first day and 20 min on the next 2 days. The behavioral tests for thermal withdrawal latency and mechanical withdrawal threshold were assessed before (baseline) and after FS. d, day; T, Thermal withdrawal latency; M, mechanical withdrawal threshold; FS, forced swim; i.p., intraperitoneal; i.t., intrathecal injection. Visceromotor response (VMR) experiments were conducted in a separate group.
Previous studies have indicated 3 day FS induced somatic hyperalgesia (Nyland et al., 2015; Quintero et al., 2000) and visceral hypersensitivity (Cao et al., 2016; Ji et al., 2018; Traub et al., 2014). In the present study, the behavioral tests were assessed before (baseline) and up to 4 weeks after the last FS. The thermal withdrawal latency and mechanical withdrawal threshold were performed on the 2nd and 3rd days after FS and then every 4 days, respectively. Visceral sensitivity was assessed by recording the VMR to colorectal distention. The behavioral examiners were blinded and all behavioral experiments were carried out at the same time in a quiet room between 09:00 and 12:00 h.
Rats were randomly assigned to treatment groups. In the first set of experiments, rats were restrained in acrylic tubes and intraperitoneally injected (i.p.) with 300 mg/kg VPA (Sigma; St Louis, MO, USA) 30 min before each swim session and one day after the last FS. Thirty min is a time to allow peak plasma VPA level to be reached after intraperitoneal injection (Winkler et al., 2005). The dose of VPA was chosen based on the previous studies (Dobashi et al., 2010; Winkler et al., 2005) and our preliminary studies. Vehicle treated rats were injected with saline.
In the second set of experiments, in order to elucidate the role of 5-HT2C receptors in the spinal cord during stress-induced hyperalgesia, the 5-HT2C receptor antagonist RS-102221 (30 μg/10 μL, Tocris Bioscience, Bristol, UK) was intrathecally injected (i.t.) 30 min after VPA injection and 30 min before each FS. Because of its low solubility in saline, RS-102221 was dissolved in 20% dimethylsulfoxide (DMSO). The control group received an equal volume of 20% DMSO.
2.2. Forced swim
Rats were subjected to subchronic stress induced by a 3 day FS paradigm (10 min on 1st day and 20 min for next 2 days) by placing them in the plexiglass cylinder (diameter 30 cm, height 50 cm) that contained 20 cm height of water at 24–26°C (Imbe and Kimura, 2015; Quintero et al., 2000; Traub et al., 2014). The FS cylinders were filled with fresh water for each rat. After swimming sessions, rats were carefully dried with a heater fan and returned to home cages.
2.3. Intrathecal injection
Rats were anesthetized with isoflurane (5% induction reduced to 2%–3%, Zoetis, Parsippany, NJ, USA) and drugs were administered through a lumbar puncture between the L4 and L5 spinal processes using a 25-gauge stainless steel needle attached to a glass microsyringe (Zhao et al., 2018). When the needle was successfully inserted into the subarachnoid space, which was judged by a sudden lateral movement of the tail, 10 μL of drug was slowly injected in 1 min.
2.4. Thermal withdrawal latency
Thermal hyperalgesia was assessed by measuring the withdrawal latency of the right hindpaw from a radiant heat source. A rat was placed in a transparent plexiglass chamber and acclimated for 30 min. An infrared (IR) beam was applied from underneath the glass floor and focused on the plantar surface of right hindpaw, and the paw withdrawal latency was recorded. A 20 s cutoff was used to avoid excessive tissue injury (Li et al., 2019). Thermal withdrawal latency was performed before (baseline), the 2nd day after FS and then every 4 days. Thermal stimuli were delivered three times to right hindpaw, with an interval of 5–8 min. The average of three tests was used as the thermal withdrawal latency.
2.5. Mechanical withdrawal threshold
The mechanical sensitivity of the hindpaw was measured with von Frey filaments and was performed before (baseline), the 1st and 3rd day after FS and then every 4 days. The animals were placed in the plexiglass test cages equipped with a metal mesh floor. A habituation period of 30 min was allowed before the test. The von Frey filaments were applied to the plantar surface of the left hindpaw and the withdrawal threshold was evaluated by applying force ranging from 0.41 to 26 g (4–255 mN). The cutoff force was 26 g. Six values were measured by up-down paradigm (Li et al., 2019), a 5 min interval was set between tests, and the mechanical pain threshold was calculated using the threshold calculation software (JFlashDixon Calcultor, University of Arizona, USA).
2.6. Visceromotor response
Visceral sensitivity was measured by the VMR to colorectal distention as previously described (Cao et al., 2016; Traub et al., 2014; Zhao et al., 2018). Rats were anesthetized with isoflurane (5% induction reduced to 2%–3%, Zoetis, Parsippany, NJ, USA) and electromyogram (EMG) electrodes made from Teflon-coated 40 gauge stainless steel wire (Cooner Wire Company, Chatsworth, CA, USA) were stitched into the ventrolateral abdominal wall. The electrode leads were tunneled subcutaneously and exteriorized at the back of the neck. Rats were injected subcutaneously with buprenorphine (0.03 mg/kg, Reckitt Benckiser Healthcare UK Ltd, Hull, UK) as analgesic drug twice for 2 days after EMG surgery. Following 6 days for recovery, rats were loosely restrained in the acrylic tubes (diameter of 6.5 cm and length of 22.5 cm) 1 h for 4 days to acclimate to the testing conditions. Rats were fasted (in a cage equipped with a metal mesh floor and water was available ad libitum) for 20–24 h prior to recording the VMR. On the day of testing, rats were briefly sedated with isoflurane and a 5–6 cm balloon attached to Tygon tubing was placed into descending colon and rectum through the anus. The distal end of the balloon was at least 1 cm proximal to the external anal sphincter and the tubing was taped to the tail. Rats were loosely restrained in the acrylic tubes and given 30 min to recover. The EMG signals were recorded with a CED 1401 and analyzed using Spike 2 for Windows software (Cambridge Electronic Design, Cambridge, UK).
The VMR to colorectal distention (five 20 s distentions of 60 mmHg with 3 min interstimulus intervals) was recorded by inflating the distention balloon with air under computer control (B482CM-1 Valve controller, University of Iowa, Iowa City, IA). The responses of the five distentions for each trial were averaged and the final value calculated as the average of the 3 trials. The baseline VMR was recorded two days before the first day of FS. The response to FS was recorded at 2 and 6 days after the last FS. The recorded EMG signal was rectified in Spike 2 and the area under the curve (AUC) was calculated. The response for each distention stimulus was the AUC of the EMG for the 20 s before distention (background) subtracted from the AUC during the 20 s distention.
2.7. Western blot
Rats were deeply anesthetized with isoflurane (5%, Zoetis, Parsippany, NJ, USA) and euthanized by decapitation. The spinal cord was removed by pressure ejection with ice cold saline as described previously (Cao et al., 2016; Cao et al., 2015; Zhao et al., 2018). The spinal cord segments of L4-L5 and L6-S1 were excised, rapidly frozen on dry ice and stored at −80°C until use. The dorsal spinal cord was cut off, homogenized in RIPA buffer (1% NP-40, 1% Sodium deoxycholate, 0.1% SDS) and protease inhibitor cocktail (Boster, AR1182, Wuhan, China). The tissues were sonicated on ice using three 10 s bursts with a 10 s cooling period between each burst, and were incubated on ice for 2 h. The samples were centrifuged at 10,000 g for 15 min at 4°C. Protein concentrations in the supernatant were measured by using the bicinchoninic acid (BCA, Boster, AR0146, Wuhan, China) method. Protein samples (20 μg) fractionated per lane on 4–12% SDS-PAGE gel (Boster, AR0138, Wuhan, China) by electrophoresis and transferred onto PVDF membranes (Millipore, IPVH00010, Darmstadt, Germany). Membranes were blocked with 5% nonfat milk for 2 h, and then were incubated with anti-rabbit 5-HT2C receptor (1:3000, Abcam, ab133570, Cambridge, UK) and anti-rabbit GAPDH (1:4000, Boster, BA2913, Wuhan, China) primary antibodies at 4°C overnight. After washing with TBST, the membranes were incubated for 2 h in TBST containing the appropriate secondary antibody (1:4000, Boster, BA1054, Wuhan, China). The antigen-antibody complexes were visualized by enhanced chemiluminescence (Thermo Scientific, Waltham, MA, USA). The immunoreactive band densities were analyzed using Image J 18.0 software. The 5-HT2C protein levels were first performed by dividing the intensity of the 5-HT2C receptor bands by the intensity of the GAPDH bands, after which the signal was calculated as a percentage relative to the control groups.
2.8. Data analysis
All data are presented as mean ± SD. Statistical analyses were performed using GraphPad Prism 7 software. One-way repeated measures analysis of variance (ANOVA) followed by Dunnett post hoc test was used for comparisons of means of the protein expression of 5-HT2C receptor. Two-way ANOVA followed by Sidak post hoc test was used for thermal withdrawal latency, mechanical withdrawal threshold and VMR data. P < 0.05 was considered significant.
3. Results
3.1. Behavior tests
3.1.1. VPA inhibited thermal hyperalgesia induced by FS
VPA inhibited thermal hyperalgesia induced by FS (Two way ANOVA, F6,66 = 3.55, P = 0.0042 for interaction; F6,66 = 2.60, P = 0.0252 for time factor; F1,11 = 4.49, P = 0.0576 for group factor, Fig. 2). The thermal withdrawal latency significantly decreased on day 2 and day 6 post FS compared to baseline in the vehicle group (P = 0.0002 and 0.0098, respectively, n = 7). However, pre-treatment of VPA blocked the decrease of thermal withdrawal latency post FS (P > 0.05, n = 6). In addition, the thermal withdrawal latency was significantly lower on day 2 and day 6 post FS in the saline + FS group compared to VPA + FS group (Sidak post-hoc test: P = 0.0021 and 0.0090, respectively), indicating that administration of VPA prevented the development of thermal hyperalgesia induced by FS.
Fig. 2.
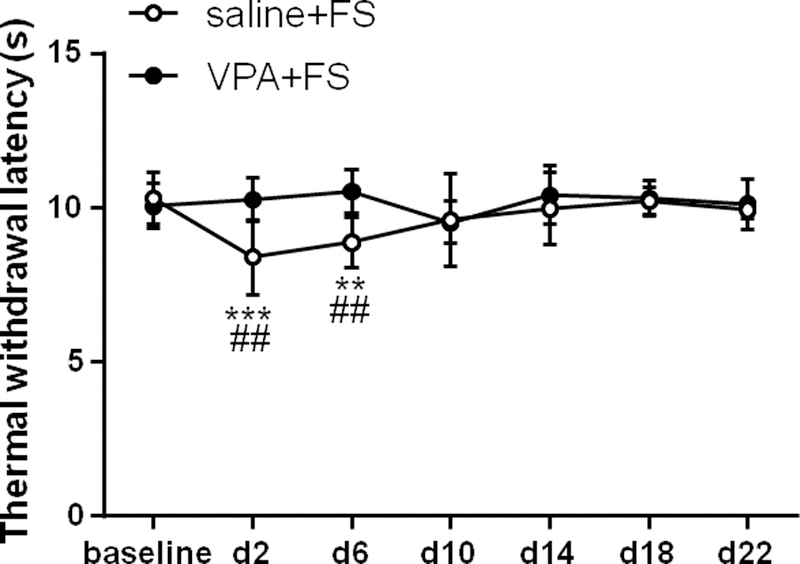
VPA inhibited thermal hyperalgesia induced by FS stress. Stress decreased the thermal withdrawal latency for 6 days in saline treated rats compared to baseline, and VPA blocked the decrease in the thermal withdrawal latency. **, *** P < 0.01, 0.001 vs baseline, respectively. The thermal withdrawal latency significantly decreased at day 2 and day 6 post FS stress in saline treated rats compared to VPA treated. ## P < 0.01 vs the VPA +FS group at the same time point.
3.1.2. VPA inhibited mechanical hyperalgesia induced by FS
VPA inhibited mechanical hyperalgesia induced by FS (Two way ANOVA, F8,88 = 3.21, P = 0.0030 for interaction; F8,88 = 6.60, P < 0.0001 for time factor; F1,11 = 5.15, P = 0.0443 for group factor, Fig. 3). The mechanical withdrawal threshold decreased for 19 days post FS compared to baseline in the vehicle group (P < 0.0001 for day 1, 3, 7, 11, 15 and P = 0.0009 for day 19 post FS, n = 7), indicating that mechanical hyperalgesia was induced by FS. Preventive i.p. administration of VPA blocked the decrease of mechanical withdrawal threshold (P > 0.05, n = 6). In addition, the mechanical withdrawal threshold was significantly lower on day 3 and day 11 post FS in the saline + FS group compared to VPA + FS group (Sidak post-hoc test: P = 0.0226 and 0.0474, respectively). These data suggest that the administration of VPA prevented the development of FS-induced mechanical hyperalgesia.
Fig. 3.
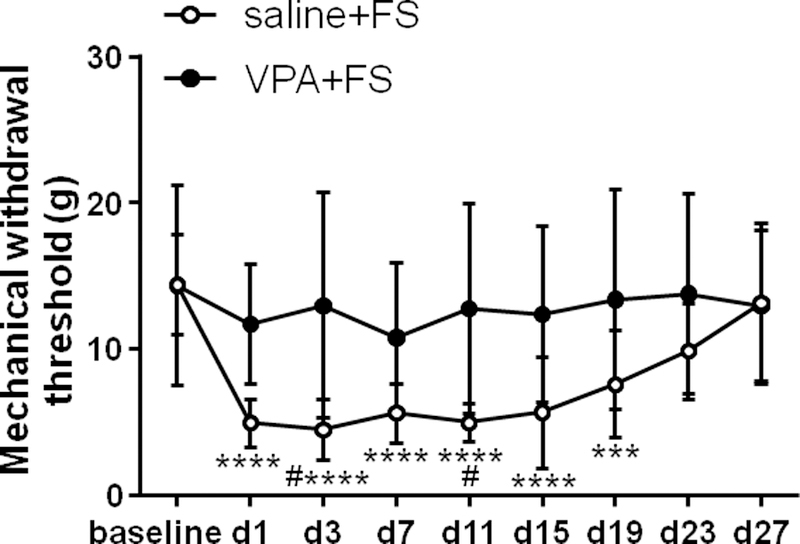
VPA inhibited mechanical hyperalgesia induced by FS stress. Stress decreased the mechanical withdrawal threshold for 19 days in saline treated rats compared to baseline, and VPA blocked the decrease in the mechanical withdrawal threshold. ***, **** P < 0.001, 0.0001 vs baseline, respectively. The mechanical withdrawal threshold significantly decreased at day 3 and day 11 post stress in saline treated rats compared to VPA treated. # P < 0.05 vs the VPA +FS group at the same time point.
3.1.3. VPA attenuated visceral hypersensitivity induced by FS
In the saline + FS group, the magnitude of the VMR significantly increased on day 2 post FS compared to baseline (Two way ANOVA, F1,13 = 3.94, P = 0.0686 for interaction; F1,13 = 2.23, P = 0.1597 for time factor; F1,13 = 8.19, P = 0.0133 for group factor; Sidak post-hoc test: P = 0.0482 for VMR on day 2 post FS vs baseline, n = 8, Fig. 4). However, VPA pre-treatment significantly blocked the development of visceral hypersensitivity induced by FS stress, and there was no significant difference in the magnitude of the VMR between day 2 post FS and baseline (P = 0.9327, n = 7, Fig. 4).
Fig. 4.
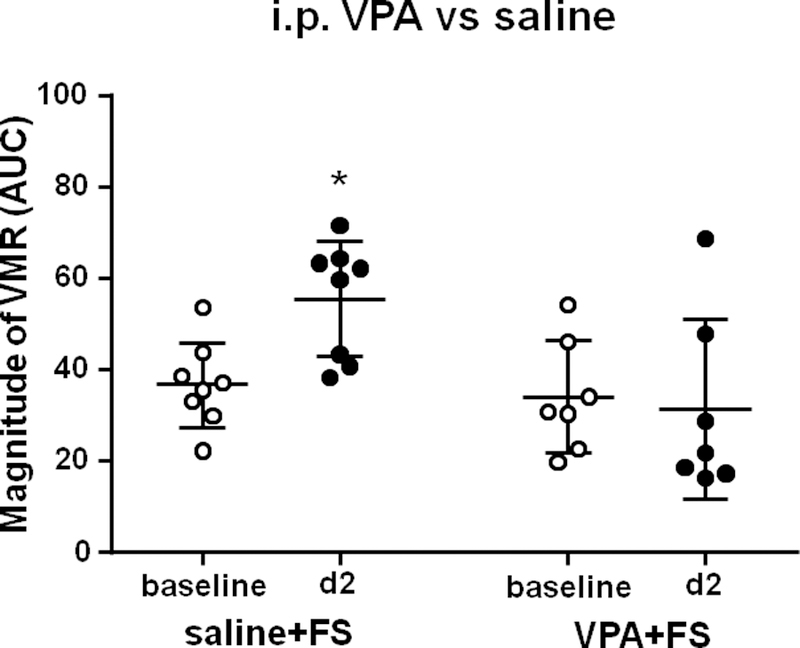
VPA inhibited FS-induced visceral hypersensitivity. FS significantly increased the magnitude of VMR to colorectal distention in the saline + FS group. Administration of VPA before each FS significantly inhibited the increase in the magnitude of VMR to colorectal distention. * P < 0.05 vs baseline.
3.2. VPA blocked the decrease of 5-HT2C receptor expression in the spinal cord induced by FS
5-HT2C receptor expression in the L4-L5 dorsal horn of the spinal cord was significantly down-regulated post FS compared to the saline control group and i.p. administration of VPA blocked the down-regulation of 5-HT2C receptor expression induced by FS (F2,11 = 25.68, P < 0.0001, n = 4 – 5 per group; Dunnett post-hoc test: the saline + FS group vs saline control group, P = 0.0001; the VPA + FS group vs saline + FS group, P = 0.0094, Fig. 5A). The 5-HT2C receptor expression in the L6-S1 dorsal horn of the spinal cord was significantly down-regulated post FS compared to the saline control group, and i.p. administration of VPA inhibited the down-regulation of 5-HT2C receptor expression induced by FS (F2,12 = 4.78, P = 0.0297, n = 5 per group; Dunnett post-hoc test: the saline + FS group vs saline control group, P = 0.0286; the VPA + FS group vs saline + FS group, P = 0.0486, Fig. 5B).
Fig. 5.
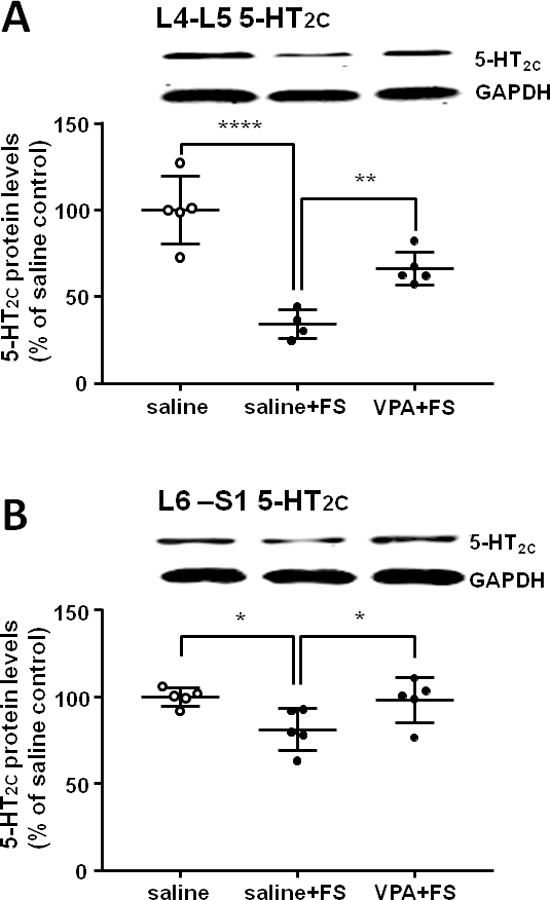
VPA reversed the decrease of 5-HT2C receptor expression in the L4–5 (A) and L6-S1 (B) segments of the spinal dorsal horn induced by FS stress. *, **, **** P < 0.05, 0.01, 0.0001, respectively.
3.3. 5-HT2C receptor antagonist RS-102221 blocked anti-nociceptive effect of VPA
3.3.1. 5-HT2C receptor antagonist RS-102221 blocked the anti-nociceptive effect of VPA on stress-induced thermal hyperalgesia
5-HT2C receptor antagonist RS-102221 blocked the anti-nociceptive effect of VPA on stress-induced thermal hyperalgesia (Two way ANOVA, F5,65 = 10.91, P < 0.0001 for interaction; F5,65 = 6.06, P = 0.0001 for time factor; F1,13 = 26.35, P = 0.0002 for group factor, Fig. 6). I.t. administration of DMSO (vehicle of RS-102221) did not significantly change the thermal withdrawal latency in the VPA + DMSO + FS group (P > 0.05, n = 8). However, in the VPA + RS-102221 + FS group, the thermal withdrawal latency was significantly reduced at day 2 (P < 0.0001, n = 7) and day 6 (P = 0.0158) post FS compared to baseline. In addition, the thermal withdrawal latency was significantly lower on day 2 and day 6 post FS in the VPA + RS-102221 + FS group compared to VPA + DMSO + FS group (Sidak post-hoc test: P < 0.0001 for day 2 and P = 0.0316 for day 6 post FS). These results indicate that i.t. pretreatment with 5-HT2C receptor antagonist RS-102221 blocked the anti-nociceptive effect of VPA stress-induced thermal hyperalgesia.
Fig. 6.
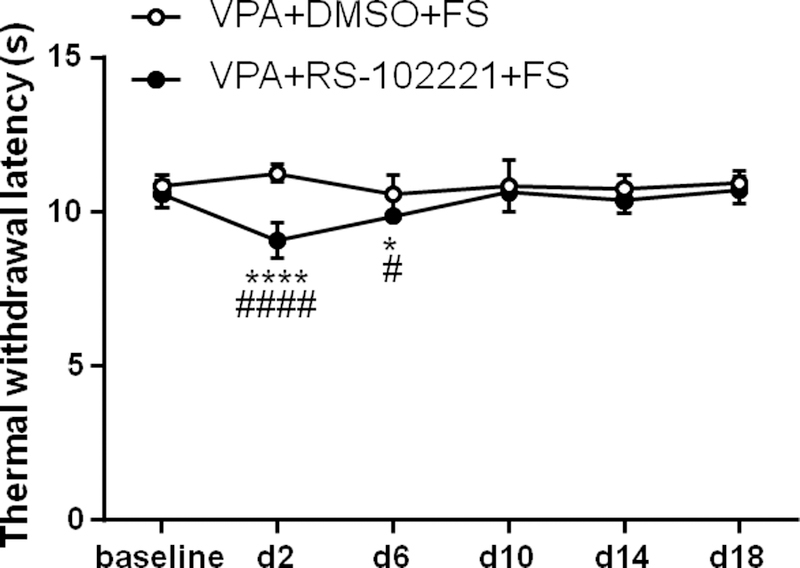
Intrathecal injection of 5-HT2C receptor antagonist RS-102221 blocked anti-nociceptive effect of VPA on thermal hyperalgesia. Intrathecal administration of DMSO (vehicle of RS-102221) and systemic administration of VPA did not change the thermal withdrawal latency evoked by FS. 5-HT2C receptor antagonist RS-102221 blocked the anti-nociceptive effect of VPA. *, **** P < 0.05, 0.0001 vs baseline, respectively. The thermal withdrawal latency significantly decreased at day 2 and day 6 post stress in VPA + RS-102221 + FS group compared to the VPA + DMSO + FS group. #, #### P < 0.05, 0.0001 vs the VPA + DMSO + FS group at the same time point, respectively.
3.3.2. 5-HT2C receptor antagonist RS-102221 blocked anti-nociceptive effect of VPA on stress-induced mechanical hyperalgesia
5-HT2C receptor antagonist RS-102221 blocked anti-nociceptive effect of VPA on stress-induced mechanical hyperalgesia (Two way ANOVA, F6,78 = 7.61, P < 0.0001 for interaction; F6,78 = 7.28, P < 0.0001 for time factor; F1,13 = 5.22, P = 0.0399 for group factor, Fig. 7). I.t. administration of DMSO did not change the mechanical withdrawal threshold compared to baseline in the VPA + DMSO + FS group (P > 0.05, n = 9). However, in the VPA + RS-102221 + FS group, the mechanical withdrawal threshold was significantly reduced for 15 days post FS compared to baseline (P < 0.0001 for all time points till 15 day post FS, n = 6). In addition, the mechanical withdrawal threshold was significantly lower on day 3, 7, 11, 15 post FS in the VPA + RS-102221 + FS group compared to VPA + DMSO + FS group (P = 0.0047, 0.0483, 0.0244, 0.0281, respectively). These results indicate that i.t. pretreatment with 5-HT2C receptor antagonist RS-102221 blocked the anti-nociceptive effect of VPA on stress-induced mechanical hyperalgesia.
Fig. 7.
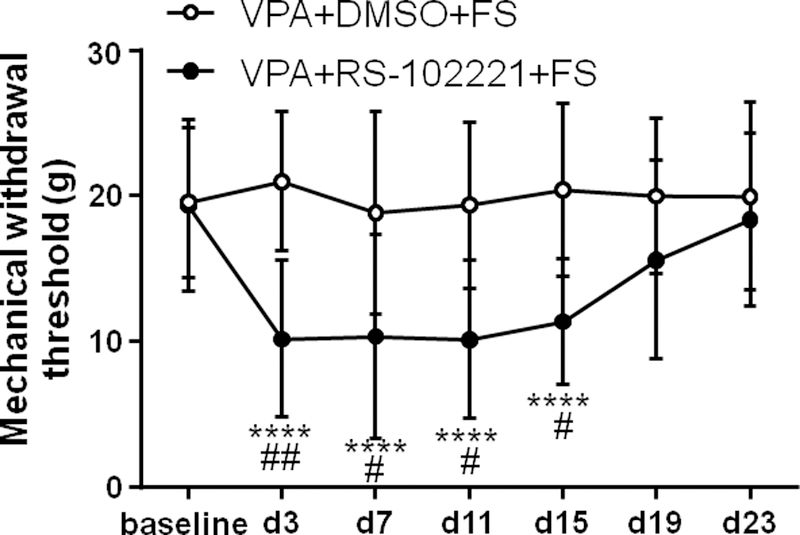
Intrathecal injection of 5-HT2C receptor antagonist RS-102221 blocked anti-nociceptive effect of VPA on mechanical hyperalgesia. Intrathecal administration of DMSO (vehicle of RS-102221) and systemic administration of VPA did not change mechanical withdrawal threshold post FS. 5-HT2C receptor antagonist RS-102221 blocked the anti-nociceptive effect of VPA. **** P < 0.0001 vs baseline. The mechanical withdrawal threshold significantly decreased at day 3, 7, 11 and 15 post FS in the VPA + RS-102221 + FS group compared to VPA + DMSO + FS group. #, ## P < 0.05, 0.01 vs the VPA + DMSO + FS group at the same time point, respectively.
3.3.3. 5-HT2C receptor antagonist RS-102221 blocked anti-nociceptive effect of VPA on stress-induced visceral hypersensitivity
To investigate the role of 5-HT2C receptor in the spinal cord in the inhibition of VPA on FS-induced visceral hypersensitivity, the 5-HT2C receptor antagonist RS-102221 was spinally administered to VPA-treated FS rats. Following VPA pretreatment on FS stress rats, i.t. injection of DMSO had no effect on the magnitude of the VMR compared to the baseline. In contrast, i.t. RS-102221 blocked the inhibitory effect of VPA on stress-induced visceral hypersensitivity at day 2 after FS, but the effect vanished at day 6 after FS (Two way ANOVA, F2,26 = 5.65, P = 0.0091 for interaction; F2,26 = 2.19, P = 0.1318 for time factor; F1,13 = 0.77, P = 0.3965 for group factor; Sidak post-hoc test: P = 0.9933 and 0.4908 for day 2 and day 6 post FS respectively compared to baseline in the VPA + DMSO + FS group, n = 7; P = 0.0080 and 0.9916 for day 2 and day 6 post FS respectively compared to baseline in the VPA + RS-102221 + FS group, n = 8, Fig. 8). These data suggest that systemic administration of VPA inhibits FS-induced visceral hypersensitivity through up-regulation of 5-HT2C receptor function in the spinal cord.
Fig. 8.
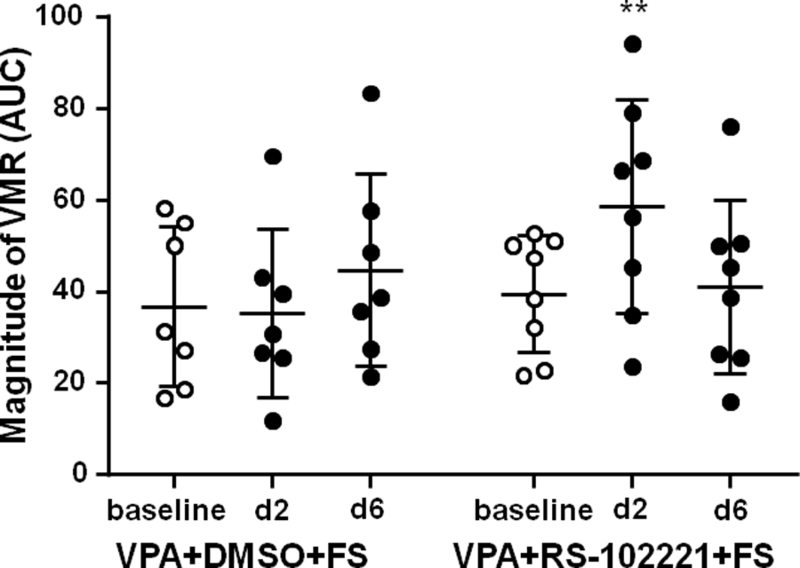
Intrathecal administration of 5-HT2C receptor antagonist RS-102221 blocked VPA’s inhibition on visceral hypersensitivity. Intrathecal administration of DMSO (vehicle of RS-102221) and systemic administration of VPA did not change the magnitude of VMR to colorectal distention post FS. 5-HT2C receptor antagonist RS-102221 blocked the anti-hypersensitivity effect of VPA at day 2 post FS, but the effect vanished at day 6 post FS. ** P < 0.01 vs baseline.
4. Discussion
In the present study, using the FS stress model in rats, we investigated the effects of VPA on stress-induced hyperalgesia. Systemic administration of VPA inhibited the somatic hyperalgesia and visceral hypersensitivity, and restored expression of 5-HT2C receptor in the spinal cord after stress. Moreover, the inhibitory effect of VPA on somatic hyperalgesia and visceral hypersensitivity was blocked by i.t. administration of the 5-HT2C receptor antagonist RS-102221. These results suggest that increasing spinal 5-HT2C receptor expression contributes to the inhibitory effect of VPA on stress-induced hypersensitivity.
4.1. Analgesic activity of VPA
The present study found that VPA attenuated somatic hyperalgesia induced by stress, in partial agreement with previous reports. For example, systemic administration of VPA reduced neuropathic pain induced tactile allodynia (Winkler et al., 2005) and repeated oral administration of VPA blocked the development of hyperalgesia after spinal nerve ligation (SNL) in rats (Hobo et al., 2011; Yoshizumi et al., 2013). VPA also inhibited inflammatory hyperalgesia induced by formalin or complete Freund’s adjuvant (CFA) in mice (Bai et al., 2010; Ximenes et al., 2013). I.p. injection of VPA attenuated nitroglycerin-induced trigeminovascular activation and scratching behavior in an animal model of migraine (Li et al., 2016). Given VPA could decrease somatic hyperalgesia, we further investigated whether VPA attenuated stress-induced visceral hypersensitivity. Our data suggest that systemic administration of VPA reduced visceral hypersensitivity evoked by stress which was similar to the anti-nociceptive effects of VPA on visceral hypersensitivity induced by experimental endometriosis in rats (Liu et al., 2012). In humans, conclusions from clinical studies were equivocal. Some studies suggested that VPA possessed significant analgesic properties on migraine (Shahien et al., 2011), chronic headache (Yancey et al., 2014), neuropathic pain (Dosenovic et al., 2017), adenomyosis (Xishi et al., 2010), endometriosis (He et al., 2010), and postherpetic neuralgia (Dosenovic et al., 2017). On the contrary, other studies reported that VPA had no analgesic effect on chronic central pain following spinal cord injury (Drewes et al., 1994) and postoperative pain (Martin et al., 1988).
4.2. 5-HT2C receptor and analgesia
In the present study, somatic hyperalgesia and visceral hypersensitivity induced by FS stress were attenuated by intraperitoneal administration of VPA, and VPA evoked analgesia was, at least partially, mediated by 5-HT2C receptors since the effect was blocked by the selective 5-HT2C receptor antagonist RS-102221. To date, most studies suggested that the 5-HT2C receptor had an anti-nociceptive/analgesic role in pain transmission. Pretreatment with the 5-HT2C receptor antagonist mesulergine reversed the anti-nociception induced by i.t. injection of 5-HT (Bardin et al., 2000). I.t. administration of the selective 5HT2C receptor agonist mCPP (1-(3-chlorophenyl) piperazine dihydrochloride) or TFMPP (m - trifluoromethylphenyl - piperazine HCl) in acute thermal stimulation models in rats and mice produced anti-nociception (Sawynok and Reid, 1994) and this effect was abolished by mesulergine (Chojnacka-Wojcik et al., 1994). In an inflammatory pain model, i.t. administration of 5-HT or the 5-HT2C receptor agonist MK212 (6-Chloro-2-(1-piperazinyl) pyrazine hydrochloride) suppressed both phases of behaviors produced by 5% formalin, and the anti-nociception was blocked by the 5-HT2C receptor antagonist D - MC (N – ormethylclozapine / 8 – Chloro – 11 - (1 - piperazinyl) - 5H – dibenzo [b,e] [1,4] diazepine) (Jeong et al., 2004). In neuropathic pain models, 5-HT2C receptor agonists had marked anti-allodynic effects in SNL (Obata et al., 2007; Obata et al., 2004) and in chronic constriction injury to the infraorbital nerve (Nakai et al., 2010). These anti-allodynic effects of 5-HT2C receptor agonists were diminished by pretreatment with the 5-HT2C receptor antagonist RS-102221. Recently, in a rat model of fibromyalgia, systemic administration of the 5-HT2C receptor agonist lorcaserin significantly relieved chronic pain symptoms, and intraperitoneal injection of the 5-HT2C receptor antagonist SB242804 significantly inhibited the anti-nociceptive effect of lorcaserin (Ogino et al., 2013).
The inhibitory role of 5-HT2C receptors in modulating spinal nociceptive transmission is supported by several electrophysiological studies. 5-HT mediated inhibition of spinal wide dynamic range (WDR) neurons to C-fiber input was reversed by the 5-HT2C receptor antagonist RS-102221, and the 5-HT2C receptor agonist MK212 also inhibited the C-fiber responses of WDR neurons (Liu et al., 2007). Spinal administration with the selective 5-HT2C receptor agonist WAY-161503 significantly depressed evoked field potentials in the spinal dorsal horn by C-fiber input in both SNL and sham surgery animals (Aira et al., 2010). Another study of substantia gelatinosa (SG) neurons in rat spinal cord slices demonstrated that 5-HT modulated inhibitory transmission in the SG by activation of 5-HT2A and 5-HT2C receptor subtypes located predominantly at inhibitory interneuron terminals, and a 5-HT2C receptor agonist WAY-161503 mimicked the 5-HT effect and this effect was blocked by a 5-HT2C receptor antagonist N-desmethylclozapine (Xie et al., 2012).
In contrast, other studies suggest 5-HT2C receptors evoke pronociceptive effects in the spinal cord. In a rapid eye movement (REM) sleep deprivation-induced hypersensitivity rat model, i.t. administration of the selective 5-HT2C receptor antagonist RS-102221 attenuated mechanical hypersensitivity both in REM sleep-deprived rats and controls (Wei et al., 2008). I.t. injection of the 5-HT2 receptor agonist DOI (2,5-methoxy-4-iodoamphetamine) increased formalin-induced secondary allodynia and hyperalgesia, and RS-102221 prevented and reversed formalin-induced secondary allodynia and hyperalgesia (Cervantes-Duran et al., 2016). The inconsistent studies on the role of 5-HT2C receptor on nociceptive processing need further investigation.
4.3. 5-HT2C receptor expression in the spinal cord
Using Western blot, we analyzed the protein expression of 5-HT2C receptor in the spinal cord following stress. Compared to naïve animals, stress decreased the expression of 5-HT2C receptor which was restored following treatment with VPA. Considered the expression of 5-HT2C receptor combined with the above behavioral data, it indicates that 5-HT2C receptors are anti-nociceptive. These results suggest stress induces somatic hyperalgesia and visceral hypersensitivity by down-regulating 5-HT2C receptors in the spinal cord, and the anti-nociceptive effect of VPA is partially due to the reverse of the decrease of spinal 5-HT2C receptor expression post FS. Our results are consistent with the previous study that 5-HT2C receptor protein expression in the L4-L6 dorsal root ganglia decreased in inflammatory pain induced by formalin (Cervantes-Duran et al., 2016). In contrast, in neuropathic pain induced by oxaliplatin (an anti-neoplastic drug), although different regulation direction in four important areas of the nociceptive system of the rat, such as spinal cord, rostral ventral medulla, midbrain periaqueductal gray and amygdala, ex vivo analysis revealed that 5-HT2C receptor mRNA expression and protein levels in the lumbar spinal cord were up-regulated after oxaliplatin administration (Baptista-de-Souza et al., 2014). The discrepancy may be partly due to the different pain models, site examined, time examined, and experimental protocols.
In the present study, after administration of VPA, the 5-HT2C receptor protein was up-regulated, indirectly suggesting that the mechanism underlying VPA’s analgesic effect is likely to be 5-HT2C receptor activation. Moreover, i.t. administration of the 5-HT2C receptor antagonist RS-102221 inhibited the anti-nociceptive effects of VPA, which directly clarifies the involvement of 5-HT2C receptor. Taken together, it appears that down-regulation of 5-HT2C receptor at least partially is responsible for FS-induced hyperalgesia, and analgesia by VPA requires spinal 5-HT2C receptor activation.
4.4. Mechanisms of VPA’s analgesia
In the present study, whether the inhibition of VPA in FS-induced somatic hyperalgesia and visceral hypersensitivity is obtained via direct effects on the spinal cord circuitry, or via top-down modulation arising from supraspinal sites needs further investigation (Jennings et al., 2014), but we may outline some possible mechanisms. Previously, VPA delivered intrathecally primarily targeted the spinal cord and dorsal root ganglions (Bai et al., 2010). Given the fact that VPA can cross the blood-brain barrier (Eikel et al., 2006), systemic administration of VPA can act on both the peripheral and central nervous system, but the function of VPA in the central nervous system seems to be the most likely explanation.
Psychological stress, such as FS stress, neonatal maternal separation and social defeat, exacerbates nociceptive processing at the level of the spinal cord (Jennings et al., 2014; Quintero et al., 2011; Ren et al., 2007; Rivat et al., 2010). Our results indicate that FS stress induces hyperalgesia and decreases 5-HT2C receptor expression in the spinal dorsal horn. VPA prevented the development of hyperalgesia and reversed the down-regulation of 5-HT2C receptor expression in the spinal cord. Previous studies suggest that central serotonergic transmission contributes to stress-induced hyperalgesia (Nyland et al., 2015; Quintero et al., 2000). Based on others and our results, the serotoninergic neurotransmitter network in the spinal cord and brain should be considered in exploration and interpretation of analgesic effects of VPA.
Serotoninergic neurons in the central nervous system are confined in the raphe nuclei of the brainstem, which project to the majority of the brain and spinal cord (Millan, 2002; Yoshimura and Furue, 2006). In this regard, alterations in central 5-HT neuronal activity may cause pain transmission changes at the spinal cord level. 5-HT2C receptors are involved in the descending inhibitory system and stress-induced down-regulation of 5-HT2C receptor expression may indicate the impairment of the descending inhibitory system evoked by stress. On the other hand, VPA had effects on the serotonin system in the brain, which could increase 5-HT levels by single i.p. administration (Biggs et al., 1992) or 30 days oral administration (Wilson et al., 2014), and increased 5-hydroxy-indoleacetic acid (5-HIAA) by repeated twice i.p. administration (Mitsikostas et al., 1993). Therefore, VPA may attenuate hyperalgesia through activation of a descending serotonergic system to the spinal cord acting on 5-HT2C receptors in the spinal dorsal horn.
Stress has a significant impact on multiple neural networks in the body to produce the overall pain experience. Based on the results reported here, we speculated that FS-induced hyperalgesia results in the activation of certain brain sites which modulate the activation of descending serotonergic pathways within the spinal cord in a process mediated in part by 5-HT2C receptor. Since VPA has multiple biological effects (Ximenes JCM, 2012), one single mechanism may not underlie VPA’s anti-hyperalgesia after FS stress. For example, as a histone deacetylase inhibitor, VPA inhibited somatic hyperalgesia in mice (Bai et al., 2010), and the inhibition of VPA on visceral hypersensitivity in the present study is similar to the effects of another histone deacetylase inhibitor (suberoylanilide hydroxamic acid, SAHA) on stress-induced visceral hypersenstitivity (Cao et al., 2016), therefore the involvement of epigenetic mechanisms of VPA on pain modulation cannot be excluded. However, this mechanism needs further investigation.
In conclusion, we demonstrated that VPA inhibited somatic hyperalgesia and visceral hypersensitivity following repeated FS stress by restoring down-regulated function of 5-HT2C receptor in the spinal cord, suggesting that this anti-hyperalgesia effect is involved in activation of 5-HT2C receptor in the spinal cord. Given VPA is clinically used frequently, the results from the present study suggest that this compound may have potential clinical utility for the treatment of patients with stress-induced hyperalgesia such as fibromyalgia and irritable bowel syndrome.
Acknowledgements:
This work was supported by National Natural Science Foundation of China (81971049, 81671097), partially by the open funds of the State Key Laboratory of Medical Neurobiology of China and National Institutes of Health grant R01 NR015472, USA.
Footnotes
Disclosures:
The authors have no conflicts of interest to declare.
References
- Aira Z, Buesa I, Salgueiro M, Bilbao J, Aguilera L, Zimmermann M, Azkue JJ, 2010. Subtype-specific changes in 5-HT receptor-mediated modulation of C fibre-evoked spinal field potentials are triggered by peripheral nerve injury. Neuroscience 168, 831–841. [DOI] [PubMed] [Google Scholar]
- Bai G, Wei D, Zou S, Ren K, Dubner R, 2010. Inhibition of class II histone deacetylases in the spinal cord attenuates inflammatory hyperalgesia. Mol Pain 6, 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baptista-de-Souza D, Di Cesare Mannelli L, Zanardelli M, Micheli L, Nunes-de-Souza RL, Canto-de-Souza A, Ghelardini C, 2014. Serotonergic modulation in neuropathy induced by oxaliplatin: effect on the 5HT2C receptor. Eur J Pharmacol 735, 141–149. [DOI] [PubMed] [Google Scholar]
- Baptista D, Nunes-de-Souza RL, Canto-de-Souza A, 2012. Activation of 5-HT(2C) receptors in the dorsal periaqueductal gray increases antinociception in mice exposed to the elevated plus-maze. Behav Brain Res 235, 42–47. [DOI] [PubMed] [Google Scholar]
- Bardin L, 2011. The complex role of serotonin and 5-HT receptors in chronic pain. Behav Pharmacol 22, 390–404. [DOI] [PubMed] [Google Scholar]
- Bardin L, Lavarenne J, Eschalier A, 2000. Serotonin receptor subtypes involved in the spinal antinociceptive effect of 5-HT in rats. Pain 86, 11–18. [DOI] [PubMed] [Google Scholar]
- Biggs CS, Pearce BR, Fowler LJ, Whitton PS, 1992. Regional effects of sodium valproate on extracellular concentrations of 5-hydroxytryptamine, dopamine, and their metabolites in the rat brain: an in vivo microdialysis study. J Neurochem 59, 1702–1708. [DOI] [PubMed] [Google Scholar]
- Bombardi C, 2014. Neuronal localization of the 5-HT2 receptor family in the amygdaloid complex. Front Pharmacol 5, 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler RK, Finn DP, 2009. Stress-induced analgesia. Prog Neurobiol 88, 184–202. [DOI] [PubMed] [Google Scholar]
- Cao DY, Bai G, Ji Y, Karpowicz JM, Traub RJ, 2016. EXPRESS: Histone hyperacetylation modulates spinal type II metabotropic glutamate receptor alleviating stress-induced visceral hypersensitivity in female rats. Mol Pain 12, 1744806916660722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao DY, Bai G, Ji Y, Traub RJ, 2015. Epigenetic upregulation of metabotropic glutamate receptor 2 in the spinal cord attenuates oestrogen-induced visceral hypersensitivity. Gut 64, 1913–1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervantes-Duran C, Vidal-Cantu GC, Godinez-Chaparro B, Granados-Soto V, 2016. Role of spinal 5-HT2 receptors subtypes in formalin-induced long-lasting hypersensitivity. Pharmacol Rep 68, 434–442. [DOI] [PubMed] [Google Scholar]
- Chojnacka-Wojcik E, Klodzinska A, Deren-Wesolek A, 1994. Involvement of 5-HT2C receptors in the m-CPP-induced antinociception in mice. Pol J Pharmacol 46, 423–428. [PubMed] [Google Scholar]
- Cortes-Altamirano JL, Olmos-Hernandez A, Jaime HB, Carrillo-Mora P, Bandala C, Reyes-Long S, Alfaro-Rodriguez A, 2018. Review: 5-HT1, 5-HT2, 5-HT3 and 5-HT7 receptors and their role in the modulation of pain response in the central nervous system. Curr Neuropharmacol 16, 210–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobashi T, Tanabe S, Jin H, Nishino T, Aoe T, 2010. Valproate attenuates the development of morphine antinociceptive tolerance. Neurosci Lett 485, 125–128. [DOI] [PubMed] [Google Scholar]
- Dosenovic S, Jelicic Kadic A, Miljanovic M, Biocic M, Boric K, Cavar M, Markovina N, Vucic K, Puljak L, 2017. Interventions for neuropathic pain: an overview of systematic reviews. Anesth Analg 125, 643–652. [DOI] [PubMed] [Google Scholar]
- Drewes AM, Andreasen A, Poulsen LH, 1994. Valproate for treatment of chronic central pain after spinal cord injury. A double-blind cross-over study. Paraplegia 32, 565–569. [DOI] [PubMed] [Google Scholar]
- Eikel D, Hoffmann K, Zoll K, Lampen A, Nau H, 2006. S-2-pentyl-4-pentynoic hydroxamic acid and its metabolite s-2-pentyl-4-pentynoic acid in the NMRI-exencephaly-mouse model: pharmacokinetic profiles, teratogenic effects, and histone deacetylase inhibition abilities of further valproic acid hydroxamates and amides. Drug Metab Dispos 34, 612–620. [DOI] [PubMed] [Google Scholar]
- Fischer S, Doerr JM, Strahler J, Mewes R, Thieme K, Nater UM, 2016. Stress exacerbates pain in the everyday lives of women with fibromyalgia syndrome--The role of cortisol and alpha-amylase. Psychoneuroendocrinology 63, 68–77. [DOI] [PubMed] [Google Scholar]
- Fonseca MI, Ni YG, Dunning DD, Miledi R, 2001. Distribution of serotonin 2A, 2C and 3 receptor mRNA in spinal cord and medulla oblongata. Brain Res Mol Brain Res 89, 11–19. [DOI] [PubMed] [Google Scholar]
- Guevara C, Fernandez AC, Cardenas R, Suarez-Roca H, 2015. Reduction of spinal PGE2 concentrations prevents swim stress-induced thermal hyperalgesia. Neurosci Lett 591, 110–114. [DOI] [PubMed] [Google Scholar]
- Hamed SA, Abdellah MM, 2017. The relationship between valproate induced tremors and circulating neurotransmitters: a preliminary study. Int J Neurosci 127, 236–242. [DOI] [PubMed] [Google Scholar]
- He W, Liu X, Zhang Y, Guo SW, 2010. Generalized hyperalgesia in women with endometriosis and its resolution following a successful surgery. Reprod Sci 17, 1099–1111. [DOI] [PubMed] [Google Scholar]
- Hobo S, Eisenach JC, Hayashida K, 2011. Up-regulation of spinal glutamate transporters contributes to anti-hypersensitive effects of valproate in rats after peripheral nerve injury. Neurosci Lett 502, 52–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imbe H, Kimura A, 2015. Repeated forced swim stress prior to complete Freund’s adjuvant injection enhances mechanical hyperalgesia and attenuates the expression of pCREB and DeltaFosB and the acetylation of histone H3 in the insular cortex of rat. Neuroscience 301, 12–25. [DOI] [PubMed] [Google Scholar]
- Jennings EM, Okine BN, Roche M, Finn DP, 2014. Stress-induced hyperalgesia. Prog Neurobiol 121, 1–18. [DOI] [PubMed] [Google Scholar]
- Jeong CY, Choi JI, Yoon MH, 2004. Roles of serotonin receptor subtypes for the antinociception of 5-HT in the spinal cord of rats. Eur J Pharmacol 502, 205–211. [DOI] [PubMed] [Google Scholar]
- Ji Y, Hu B, Li J, Traub RJ, 2018. Opposing roles of estradiol and testosterone on stress-induced visceral hypersensitivity in rats. J Pain 19, 764–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kammerer M, Rassner MP, Freiman TM, Feuerstein TJ, 2011. Effects of antiepileptic drugs on GABA release from rat and human neocortical synaptosomes. Naunyn Schmiedebergs Arch Pharmacol 384, 47–57. [DOI] [PubMed] [Google Scholar]
- Li R, Liu Y, Chen N, Zhang Y, Song G, Zhang Z, 2016. Valproate attenuates nitroglycerin-induced trigeminovascular activation by preserving mitochondrial function in a rat model of migraine. Med Sci Monit 22, 3229–3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li ZL, Xue Y, Tao ZY, Du WZ, Jiang YG, Cao D, 2019. [Express] Spinal 5-HT3 receptor contributes to somatic hyperalgesia induced by sub-chronic stress. Mol Pain, 1744806919859723. [DOI] [PMC free article] [PubMed]
- Liu FY, Xing GG, Qu XX, Xu IS, Han JS, Wan Y, 2007. Roles of 5-hydroxytryptamine (5-HT) receptor subtypes in the inhibitory effects of 5-HT on C-fiber responses of spinal wide dynamic range neurons in rats. J Pharmacol Exp Ther 321, 1046–1053. [DOI] [PubMed] [Google Scholar]
- Liu M, Liu X, Zhang Y, Guo SW, 2012. Valproic acid and progestin inhibit lesion growth and reduce hyperalgesia in experimentally induced endometriosis in rats. Reprod Sci 19, 360–373. [DOI] [PubMed] [Google Scholar]
- Martin C, Martin A, Rud C, Valli M, 1988. Comparative study of sodium valproate and ketoprofen in the treatment of postoperative pain. Ann Fr Anesth Reanim 7, 387–392. [DOI] [PubMed] [Google Scholar]
- Millan MJ, 2002. Descending control of pain. Prog Neurobiol 66, 355–474. [DOI] [PubMed] [Google Scholar]
- Mitsikostas D, Sfikakis A, Papadopoulou-Daifoti Z, Varonos D, 1993. The effects of valproate in brain monoamines of juvenile rats after stress. Prog Neuropsychopharmacol Biol Psychiatry 17, 295–310. [DOI] [PubMed] [Google Scholar]
- Nakai K, Nakae A, Oba S, Mashimo T, Ueda K, 2010. 5-HT2C receptor agonists attenuate pain-related behaviour in a rat model of trigeminal neuropathic pain. Eur J Pain 14, 999–1006. [DOI] [PubMed] [Google Scholar]
- Nyland JE, McLean SA, Averitt DL, 2015. Prior stress exposure increases pain behaviors in a rat model of full thickness thermal injury. Burns 41, 1796–1804. [DOI] [PubMed] [Google Scholar]
- Obata H, Ito N, Sasaki M, Saito S, Goto F, 2007. Possible involvement of spinal noradrenergic mechanisms in the antiallodynic effect of intrathecally administered 5-HT2C receptor agonists in the rats with peripheral nerve injury. Eur J Pharmacol 567, 89–94. [DOI] [PubMed] [Google Scholar]
- Obata H, Saito S, Sakurazawa S, Sasaki M, Usui T, Goto F, 2004. Antiallodynic effects of intrathecally administered 5-HT(2C) receptor agonists in rats with nerve injury. Pain 108, 163–169. [DOI] [PubMed] [Google Scholar]
- Ogino S, Nagakura Y, Tsukamoto M, Watabiki T, Ozawa T, Oe T, Shimizu Y, Ito H, 2013. Systemic administration of 5-HT(2C) receptor agonists attenuates muscular hyperalgesia in reserpine-induced myalgia model. Pharmacol Biochem Behav 108, 8–15. [DOI] [PubMed] [Google Scholar]
- Ossipov MH, Dussor GO, Porreca F, 2010. Central modulation of pain. J Clin Invest 120, 3779–3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papaefthymiou A, Doulberis M, Kountouras J, Kolokytha C, Galanopoulos M, Liatsos C, Kyriakos N, Giakoumis M, Papadomichelakis M, Polyzos SA, Kotronis G, Katsinelos P, 2019. Impact of occupational stress on irritable bowel syndrome pathophysiology and potential management in active duty noncombat Greek military personnel: a multicenter prospective survey. Eur J Gastroenterol Hepatol 31, 954–963. [DOI] [PubMed] [Google Scholar]
- Qiu HM, Yang JX, Jiang XH, Fei HZ, Liu D, Hu XY, Zhou QX, 2014. Upregulating serotonin transporter expression and downregulating monoamine oxidase-A and indoleamine 2, 3-dioxygenase expression involved in the antidepressant effect of sodium valproate in a rat model. Neuroreport 25, 1338–1343. [DOI] [PubMed] [Google Scholar]
- Quintero L, Cardenas R, Suarez-Roca H, 2011. Stress-induced hyperalgesia is associated with a reduced and delayed GABA inhibitory control that enhances post-synaptic NMDA receptor activation in the spinal cord. Pain 152, 1909–1922. [DOI] [PubMed] [Google Scholar]
- Quintero L, Moreno M, Avila C, Arcaya J, Maixner W, Suarez-Roca H, 2000. Long-lasting delayed hyperalgesia after subchronic swim stress. Pharmacol Biochem Behav 67, 449–458. [DOI] [PubMed] [Google Scholar]
- Ren TH, Wu J, Yew D, Ziea E, Lao L, Leung WK, Berman B, Hu PJ, Sung JJ, 2007. Effects of neonatal maternal separation on neurochemical and sensory response to colonic distension in a rat model of irritable bowel syndrome. Am J Physiol Gastrointest Liver Physiol 292, G849–856. [DOI] [PubMed] [Google Scholar]
- Rivat C, Becker C, Blugeot A, Zeau B, Mauborgne A, Pohl M, Benoliel JJ, 2010. Chronic stress induces transient spinal neuroinflammation, triggering sensory hypersensitivity and long-lasting anxiety-induced hyperalgesia. Pain 150, 358–368. [DOI] [PubMed] [Google Scholar]
- Sawynok J, Reid A, 1994. Spinal supersensitivity to 5-HT1, 5-HT2 and 5-HT3 receptor agonists following 5,7-dihydroxytryptamine. Eur J Pharmacol 264, 249–257. [DOI] [PubMed] [Google Scholar]
- Shahien R, Saleh SA, Bowirrat A, 2011. Intravenous sodium valproate aborts migraine headaches rapidly. Acta Neurol Scand 123, 257–265. [DOI] [PubMed] [Google Scholar]
- Tavares LRR, Baptista-de-Souza D, Canto-de-Souza A, 2018. Activation of 5-HT2C (but not 5-HT1A) receptors in the amygdala enhances fear-induced antinociception: Blockade with local 5-HT2C antagonist or systemic fluoxetine. Neuropharmacology 135, 376–385. [DOI] [PubMed] [Google Scholar]
- Traub RJ, Cao DY, Karpowicz J, Pandya S, Ji Y, Dorsey SG, Dessem D, 2014. A clinically relevant animal model of temporomandibular disorder and irritable bowel syndrome comorbidity. J Pain 15, 956–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Ocampo MA, Pang RD, Bota M, Bradesi S, Mayer EA, Holschneider DP, 2013. Alterations in prefrontal-limbic functional activation and connectivity in chronic stress-induced visceral hyperalgesia. Plos One 8, e59138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei H, Ma A, Wang YX, Pertovaara A, 2008. Role of spinal 5-HT receptors in cutaneous hypersensitivity induced by REM sleep deprivation. Pharmacol Res 57, 469–475. [DOI] [PubMed] [Google Scholar]
- Wilson CB, McLaughlin LD, Ebenezer PJ, Nair AR, Francis J, 2014. Valproic acid effects in the hippocampus and prefrontal cortex in an animal model of post-traumatic stress disorder. Behav Brain Res 268, 72–80. [DOI] [PubMed] [Google Scholar]
- Winkler I, Blotnik S, Shimshoni J, Yagen B, Devor M, Bialer M, 2005. Efficacy of antiepileptic isomers of valproic acid and valpromide in a rat model of neuropathic pain. Br J Pharmacol 146, 198–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie DJ, Uta D, Feng PY, Wakita M, Shin MC, Furue H, Yoshimura M, 2012. Identification of 5-HT receptor subtypes enhancing inhibitory transmission in the rat spinal dorsal horn in vitro. Mol Pain 8, 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ximenes JC, de Oliveira Goncalves D, Siqueira RM, Neves KR, Santos Cerqueira G, Correia AO, Felix FH, Leal LK, de Castro Brito GA, da Graca Naffah-Mazzacorati M, Viana GS, 2013. Valproic acid: an anticonvulsant drug with potent antinociceptive and anti-inflammatory properties. Naunyn Schmiedebergs Arch Pharmacol 386, 575–587. [DOI] [PubMed] [Google Scholar]
- Ximenes JCM L-VE, Naffah-Mazzacoratti MG, Viana GSB, 2012. Valproic acid, a drug with multiple molecular targets related to its potential neuroprotective action. Neurosci Med 3, 107–123. [Google Scholar]
- Xishi L, Lei Y, Guo SW, 2010. Valproic acid as a therapy for adenomyosis: a comparative case series. Reprod Sci 17, 904–912. [DOI] [PubMed] [Google Scholar]
- Yancey JR, Sheridan R, Koren KG, 2014. Chronic daily headache: diagnosis and management. Am Fam Physician 89, 642–648. [PubMed] [Google Scholar]
- Yoshimura M, Furue H, 2006. Mechanisms for the anti-nociceptive actions of the descending noradrenergic and serotonergic systems in the spinal cord. J Pharmacol Sci 101, 107–117. [DOI] [PubMed] [Google Scholar]
- Yoshizumi M, Eisenach JC, Hayashida K, 2013. Valproate prevents dysregulation of spinal glutamate and reduces the development of hypersensitivity in rats after peripheral nerve injury. J Pain 14, 1485–1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao YJ, Li JH, Hu B, Wang Y, Chang XF, Traub RJ, Cao DY, 2018. Extracellular signal-regulated kinase activation in the spinal cord contributes to visceral hypersensitivity induced by craniofacial injury followed by stress. Neurogastroenterol Motil 30, e13161. [DOI] [PubMed] [Google Scholar]
- Zimmermann M, 1983. Ethical guidelines for investigations of experimental pain in conscious animals. Pain 16, 109–110. [DOI] [PubMed] [Google Scholar]


