Abstract
Increasing evidence supports the important role of H2S in renal physiology and the pathogenesis of kidney injury. Whether H2S regulates water metabolism in the kidney and the potential mechanism are still unknown. The present study was conducted to determine the role of H2S in urine concentration. Inhibition of both cystathionine-γ-lyase (CSE) and cystathionine-β-synthase (CBS), 2 major enzymes for endogenous H2S production, with propargylglycine (PPG) and amino-oxyacetate (AOAA), respectively, caused increased urine output and reduced urine osmolality in mice that was associated with decreased expression of aquaporin (AQP)-2 in the renal inner medulla. Mice treated with both PPG and AOAA developed a urine concentration defect in response to dehydration that was accompanied by reduced AQP-2 protein expression. Inhibition of CSE alone was associated with a mild decrease in AQP-2 protein level in the renal medulla of heterozygous CBS mice. GYY4137, a slow H2S donor, markedly improved urine concentration and prevented the down-regulation of renal AQP-2 protein expression in mice with lithium-induced nephrogenic diabetes insipidus (NDI). GYY4137 significantly increased cAMP levels in cell lysates prepared from inner medullary collecting duct (IMCD) suspensions. AQP-2 protein expression was also upregulated, but was significantly inhibited by the adenyl cyclase inhibitor MDL12330A or the PKA inhibitor H89, but not the vasopressin 2 receptor (V2R) antagonist tolvaptan. Inhibition of endogenous H2S production impaired urine concentration in mice, whereas an exogenous H2S donor improved urine concentration in lithium-induced NDI by increasing AQP-2 expression in the collecting duct principal cells. H2S upregulated AQP-2 protein expression, probably via the cAMP-PKA pathway.—Luo, R., Hu, S., Liu, Q., Han, M., Wang, F., Qiu, M., Li, S., Li, X., Yang, T., Fu, X., Wang, W., Li, C. Hydrogen sulfide upregulates renal AQP-2 protein expression and promotes urine concentration.
Keywords: kidney, water channel, gasotransmitter, cAMP
Gasotransmitters are important physiologic molecules. The gasotransmitter H2S is highly lipophilic, which allows it to enter cells freely across the plasma membrane, where it becomes biologically active (1). H2S is a key mediator in physiology and diseases, including inflammation, vascular function, and obesity. In addition to being present as free H2S, this gasotransmitter is produced by enzymes and released from cellular stores (2). The endogenous production of H2S is primarily catalyzed by 2 enzymes in the transsulfuration pathway: cystathionine-β-synthase (CBS) and cystathionine-γ-lyase (CSE), which catalyze H2S biogenesis by the condensation of cysteine and homocysteine and the desulfhydration of cysteine, respectively (3–6). CBS catalyzes homocysteine derived from methionine to produce cystathionine, which is converted into l-cysteine by CSE. l-Cysteine can be used as a substrate by both CBS and CSE to generate H2S. CSE can also catalyze H2S production by the conversion of homocysteine into homolanthionine (4, 5), CBS is critical in the development of neonatal mice. Moribund CBS knockout (cbs−/−) mice exhibit severe fibrosis and neutrophil invasion. The majority of cbs−/− animals (>90%) die of liver failure between 2 and 4 wk of age (7, 8).
Propargylglycine (PPG) is an antibiotic that irreversibly inhibits CSE activity and H2S production. Amino-oxyacetic acid (AOAA) is a nonspecific inhibitor of CBS (9). H2S production is completely abolished by inhibiting both CBS and CSE. However, single inhibition of CBS or CSE induced a slight decrease in H2S production (10). GYY4137, a newly synthesized H2S donor, slowly (over hours) releases low concentrations of H2S in aqueous solution under physiologic conditions and may effectively mimic the time course of H2S release in vivo (11).
The kidney is one of the major organs that regulate endogenous H2S levels. Both CBS and CSE are expressed in the cortex and may play dominant roles in H2S generation in the kidney (5, 12). Emerging evidence has revealed a complex role for H2S in renal physiology and the pathogenesis of kidney injury. In an intrarenal arterial infusion rat model, renal H2S concentrations correlated with increased renal blood flow (RBF), glomerular filtration rate (GFR), urinary excretion, natriuresis, and kaliuresis. The possible mechanisms of the renal actions in this acute study (2 h) was believed to include the induction of vasodilation and inhibition of the tubular Na,K-ATPase and the Na+-K+-2Cl− cotransporter (NKCC-2). However, the effect of H2S on these 2 cotransporters was not studied directly (10). The involvement of H2S in renal water handling was not examined.
Arginine-vasopressin (AVP)–regulated water channel aquaporin-2 (AQP-2) in the collecting duct principal cells is a critical determinant of urine concentration in the kidney. It is well established that the cAMP-PKA signaling pathway is involved in AQP-2 trafficking and expression on the apical membrane (13). PKA-induced phosphorylation of the cAMP-responsive element binding protein (CREB) stimulates transcription from the AQP-2 promoter via the cAMP-responsive element (14). All these events render the apical membrane highly permeable to water, which is a key step in urine concentration. The altered expression of AQP-2 protein has been implicated in several types of acquired water balance disorders (15, 16). Several studies have demonstrated an important role for H2S in the cAMP-PKA signaling pathway in different cell types. H2S increases intracellular Ca2+ concentrations by activating the cAMP-PKA pathway (17). This effect is attenuated by PKA inhibition (18).
In the current study, we sought to investigate whether H2S regulates renal water handling and the potential molecular mechanism by using H2S donors and specific inhibitors of CBS and CSE in mice.
MATERIALS AND METHODS
Reagents
For semiquantitative immunoblot and immunohistochemical analyses, previously characterized affinity-purified polyclonal antibodies to AQP-2 were used (19). Anti-CBS (sc-67154) was purchased from Santa Cruz Biotechnology (Dallas, TX, USA) and anti-CSE (ab151769) was purchased from Abcam (Cambridge, United Kingdom). Anti-V2R was purchased from Abcam (ab109326), anti-phosphorylated CREB at Ser133 (9198) and anti-CREB (9197) was bought from Cell Signaling Technology (Danvers, MA, USA). The horseradish peroxidase–conjugated secondary antibodies were obtained from Agilent Technologies (Santa Clara, CA, USA). H89, MDL12330A, AOAA, PPG, and NaHS were purchased from MilliporeSigma (Burlington, MA, USA). GYY4137 was purchased from Santa Cruz Biotechnology. Tolvaptan was obtained from MedchemExpress (Shanghai, China).
Animals and treatments
Animal procedures were approved by the Animal Care and Use Committee of Sun Yat-sen University. The cbs+/− (B6.129P2-Cbstm1Unc/J, stock number 002853) and wild-type mice were purchased from The Jackson Laboratory (Bar Harbor, ME, USA). The mouse colony was propagated at the Sun Yat-sen University. Genotypes were identified by PCR. All the animals at 3–4 mo of age were maintained on a 12/12 h light–dark cycle at 24°C and received food and water ad libitum before the experiments.
All of the male mice were maintained in metabolic cages during experimentation. Their daily water and food intakes were monitored. For protocols I–IV, 2 cohorts of animals were used. Urine samples for clearance studies were collected over 24-h periods. On the day of euthanasia, all mice were anesthetized with pentobarbital, and both kidneys were removed from each animal and prepared for protein, mRNA measurement, or histologic analysis.
Protocol I
The C57BL/6 mice were assigned to 4 groups (control, AOAA, PPG, and AOAA+PPG; n = 6–8/group). Mice in the AOAA group received 10 mg/kg AOAA/d in saline. Mice in the PPG group received 30 mg/kg PPG/d in saline. Mice in the AOAA+PPG group received both AOAA (10 mg/kg/d in saline) and PPG (30 mg/kg/d in saline). The control group was given saline. All treatments were administered by intraperitoneal injection for 5 d.
Protocol II
The C57BL/6 mice were assigned to 3 groups: control, water-deprived (WD), and AOAA+PPG+WD (n = 6/group). The WD group was deprived of water for 36 h and had free access to the chow diet. The mice in this group were intraperitoneally injected with saline. The AOAA+PPG+WD group was given AOAA (10 mg/kg/d in saline) and PPG (30 mg/kg/d in saline) by intraperitoneal injection for 5 d followed by WD. The control group was injected intraperitoneally with saline alone and given free access to water.
Protocol III
Wild-type (cbs+/+) and CBS heterozygous (cbs+/−) mice were assigned to 4 groups: cbs+/+, cbs+/++PPG, cbs+/−, and cbs+/−+PPG (n = 5–7/group). The cbs+/++PPG and cbs+/−+PPG groups were intraperitoneally injected with PPG (30 mg/kg/d in saline) for 5 d. The cbs+/+ and cbs+/− control groups were intraperitoneally injected with saline alone.
Protocol IV
The C57BL/6 mice were assigned to 4 groups: control, GYY, lithium treatment, and lithium+GYY. The control and GYY groups (n = 9 per group) were fed a standard diet for 7 d. The lithium and lithium+GYY groups (n = 11–12/group) were fed a standard diet supplemented with LiCL (40 mmol/kg of dry food) for 4 d and 20 mmol/kg of LiCl in dry food for 3 additional days. In both the GYY treatment groups, mice were intraperitoneally injected with GYY4137 (50 mg/kg/d in saline) for 7 d. The control and lithium only groups were intraperitoneally injected with saline alone. To examine the effect of GYY4137 on AQP-2 in inner medullary collecting duct (IMCD) suspensions prepared from lithium-treated rats, Wistar rats were treated with LiCl in the diet, as previously described.
IMCD tubule suspension and primary cell culture
Rat IMCD suspensions were prepared as previously described by Wang et al. (19) with slight modification.
Protocol V
The IMCD suspensions were incubated with either NaHS (100 μM) for 5, 10, or 30 min, or GYY4137 (10−5 M) for 0.5, 1, or 2 h.
Protocol VI
The IMCD suspensions were pretreated with MDL12330A (10−7 M), or H89 (10−5 M) or left untreated for 30 min, and then incubated with NaHS (100 μM) or a vehicle for 5 min.
Protocol VII
The IMCD suspensions were pretreated with tolvaptan (10−9 M), MDL12330A (10−7 M) or H89 (10−5 M) or left untreated for 30 min and then incubated with GYY4137 (10−5 M) or a vehicle for 1 h.
Protocol VIII
The IMCD suspensions were treated with or without forskolin (20 μM) and GYY4137 (10−5 M) or left untreated for 0.5, 1, and 2 h.
Upon completion of the incubation, the protein was isolated by using RIPA buffer containing a proteinase cocktail, and the samples were used for immunoblot analysis as described by Wang et al. (19). All in vitro experiments were repeated 3 times.
Primary rat IMCD cells were cultured as described by Wang et al. (19). The samples were incubated with GYY4137 (10−5 M) for 0.5, 1, and 2 h. Immunofluorescence was performed to examine AQP-2 expression and trafficking in IMCD cells.
Blood and urine chemistry
Urine was collected and clearance studies were performed during 24-h periods. At the end of each protocol, blood samples were collected into heparinized tubes for the determination of serum creatinine and osmolality when the mice were euthanized. The osmolality of urine and serum was determined by freezing-point depression (OM 806; Omometer, Loser, Germany). The serum and urine concentrations of creatinine were determined with an EIA kit, used according to the manufacturer’s instructions (BioAssay System, Hayward, CA, USA).
Western blot analysis and immunohistochemistry
Western blot analysis and immunohistochemistry were performed, as previously described (19). The Western blot analysis membranes were visualized with Pierce ECL (Thermo Fisher Scientific, Waltham, MA, USA) and processed for signal detection by using the 5200 Luminescent Imaging Workstation (Tanon, Shanghai, China).
RNA extraction and real-time quantitative PCR
Total RNA was extracted from kidneys according to the manufacturer’s instructions for Trizol reagent (Thermo Fisher Scientific). Total RNA (500 ng) was used for reverse transcription using the PrimeScript RT Reagent Kit Perfect Real Time Kit (Takara Bio, Kusatsu, Japan). cDNA was used for real-time quantitative PCR analysis with SYBR Premix Ex Taq (Perfect Real Time; Takara Bio). Target mRNA was determined using the comparative cycle threshold method of relative quantitation. The calibrator sample was selected from PBS-treated tissue, and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as an internal control. Primer sequences used are provided in Table 1.
TABLE 1.
Mouse primer sequences for RT-PCR
| Target gene | Primer sequence, 5′–3′ | |
|---|---|---|
| Forward | Reverse | |
| AQP-2 | GGACCTGGCTGTCAATGCTC | GCGGGCTGGATTCATGGAG |
| V2R | GCTGTGGCTCTGTTTCAAGTG | CCAGGATCATGTAGGAAGAGGC |
| GAPDH | TGACCTCAACTACATGGTCTACA | CTTCCCATTCTCGGCCTTG |
| MCP-1 | TTAAAAACCTGGATCGGAACCAA | GCATTAGCTTCAGATTTACGGGT |
| KIM-1 | ACATATCGTGGAATCACAACGAC | ACTGCTCTTCTGATAGGTGACA |
Immunofluorescence staining
Immunofluorescence staining was performed as described by Li et al. (20). In brief, filters were detached from the holders, and cells were fixed with methanol for 2 min at −20°C, and permeabilized with PBS-Triton 0.3% for 25 min at room temperature and then vacuum permeabilization buffer. Filters were incubated with the rabbit polyclonal anti-AQP-2 antibody (dilution 1:50) overnight at 4°C. Samples were incubated with a goat anti-rabbit IgG antibody (dilution 1:250) for 90 min at room temperature. The cells were examined by the DMI4000B fluorescence microscope (Leica Microsystems, Wetzlar, Germany).
Measurement of cAMP
cAMP concentrations in IMCD cell lysate were determined by an ELISA, according to the guidelines from the manufacturer (Mlbio, Shanghai, China).
Measurement of H2S concentration
H2S concentrations in the medium or organ homogenate were measured as described by Kang et al. (21). In brief, tissues were homogenized in ice-cold 100 mM potassium phosphate buffer (pH 7.4). Tissues homogenates (75 μl) were mixed with 100 μl distilled water and 300 μl 10% trichloroacetic acid. The reaction was stopped by the addition of 150 μl of 1% zinc acetate. Then, N,N-dimethyl-p-phenylenediamine sulfate (20 μM; 133 μl) in 7.2 M HCI and FeCI3 (30 μM; 133 μl) in 1.2 M HCI were added. After 15 min of incubation, the absorbance of the resulting solution (670 nm) was measured with a 96-well microplate reader. The calibration curve of NaHS (3.125–250) was used to calculate H2S concentration in each sample. The same procedure was applied to measure the medium H2S level. The results of tissues homogenates are expressed as nanomoles of H2S formed per milligram of tissue.
Statistical analysis
Results are presented as means ± se. Data were analyzed by a 1-way ANOVA and Student-Newman-Keuls test for multiple comparisons. Statistical significance was accepted at the P < 0.05 level. Values represent means ± se of 3 independent sets of experiments in cell culture studies.
RESULTS
Protocol I: cotreatment of mice with CSE and CBS inhibitors was associated with decreased AQP-2 protein expression in the inner medulla
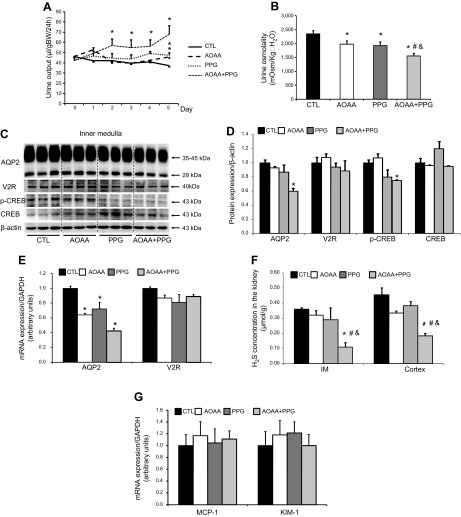
Both CSE and CBS play important roles in H2S production in the kidney. Therefore, we examined whether reduced H2S production due to the inhibition of CSE, CBS, or both enzymes by specific inhibitors (PPG and AOAA, respectively) affects renal water handling and AQP-2 expression. Treatment with a combination of PPG (30 mg/kg/d) (22) and AOAA (10 mg/kg/d) progressively increased urine output starting on d 2 (Fig. 1A). On d 5, urine output was 62 ± 8 μl/g body weight (BW)/d for the combination treatment group compared to 37 ± 3 μl/g BW/d for the control (Fig. 1A). In contrast, the urine osmolality was markedly reduced (1613 ± 100 mOsm/kg/H2O for the combination vs. 2334 ± 125 mOsm/kg/H2O for the control; Fig. 1B). Serum sodium levels were slightly lower in mice with AOAA and PPG cotreatment than in controls, and urinary sodium excretion was mildly increased in these mice (Table 2). Also, creatinine clearance was unchanged in AOAA, PPG, or combination-treatment groups when compared with controls (Table 2).
Figure 1.
Inhibition of both CBS and CSE caused increased urine output, reduced urine osmolality, and decreased AQP-2 protein expression in mice. A, B) Urine output (A) and urine osmolality (B) in mice treated with AOAA (10 mg/kg/d), PPG (30 mg/kg/d), or their combination. C) Representative semiquantitative immunoblots of AQP-2, V2R, phosphorylated-CREB, and CREB in the kidney inner medulla of mice treated with AOAA, PPG, or their combination. D) The corresponding densitometric analyses of the protein expression levels normalized by β-actin. E) AQP-2 and V2R mRNA levels in the inner medulla of mice treated with AOAA, PPG, or their combination. F) The H2S concentration in the inner medulla and cortex and outer medulla of the kidneys from mice treated with AOAA, PPG, or their combination. G) mRNA levels of MCP-1 and KIM-1 in the cortex of the kidney from mice treated with AOAA, PPG, or their combination (n = 6–8/group). *P < 0.05 vs. controls, #P < 0.05 vs. mice treated with AOAA, &P < 0.05 vs. mice treated with PPG.
TABLE 2.
Functional data for protocol I
| Treatment | CTL | AOAA | PPG | AOAA+PPG |
|---|---|---|---|---|
| Posm (mOsm/kg · H2O) | 301 ± 4 | 301 ± 3 | 304 ± 4 | 303 ± 3 |
| PNa (mM) | 152.8 ± 0.9 | 151.3 ± 0.6 | 151.8 ± 1.3 | 148.5 ± 1.4* |
| CCr (μl/min/g) | 2.7 ± 0.7 | 3.0 ± 0.5 | 2.2 ± 0.2 | 3.1 ± 0.3 |
| UNa×UO (nmol/24 h) | 94 ± 11 | 104 ± 8 | 109 ± 12 | 143 ± 12* |
Values are means ± se. N = 4 for all groups. CTL, control group; Posm, plasma osmolality; PNa, plasma sodium; Ccr, creatinine clearance; UNa×UO, rate of urinary sodium excretion. *P < 0.05, vs. CTL.
In the inner medulla, the PPG+AOAA combination treatment reduced the AQP-2 protein level by 40% (Fig. 1C, D). The p-CREB protein level was also significantly reduced after this treatment (Fig. 1C, D). Consistent with those data, AQP-2 mRNA levels were decreased by the combination treatment (Fig. 1E), suggesting transcriptional regulation mediated by H2S. Immunohistochemistry showed AQP-2 staining in both the intracellular compartments and apical plasma membrane of the collecting duct principal cells in the kidney of mice, no distribution differences of AQP-2 were found among groups (Fig. 2C). The protein (Fig. 1C) and mRNA (Fig. 1E) levels of V2R in the inner medulla were unchanged in the mouse kidney, regardless of treatment, suggesting that the regulation of AQP-2 protein expression by H2S is independent of the vasopressin signaling pathways. In addition, the inner medullary H2S concentration was dramatically decreased by ∼70% after inhibition of both CSE and CBS (Fig. 1F).
Figure 2.
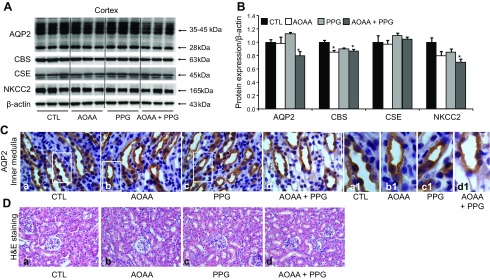
Inhibition of both CBS and CSE was associated with decreased cortical and outer medullary AQP-2 and NKCC-2 protein expression in mice. A) Representative semiquantitative immunoblots of AQP-2, CBS, CSE, and NKCC-2 in the kidney cortex and outer medulla of mice treated with AOAA, PPG, or their combination. B) The corresponding densitometric analyses of protein expression levels normalized to β-actin (n = 6–8/group). *P < 0.05 vs. controls. C) Immunohistochemistry showed no different intracellular distributions of AQP-2 in the collecting duct principal cells of mice among groups. Strong staining of AQP-2 was shown in both the apical plasma membrane and intracellular compartments of the IMCD principal cells. Original magnification, ×1000. D) Hematoxylin and eosin (H&E) staining showed no kidney injury in mice treated with AOAA, PPG, or their combination vs. controls. Original magnification, ×400.
In the cortex, the combination treatment was associated with decreased AQP-2 protein expression (Fig. 2A, B). The pattern of AQP-2 staining in the principal cells of the cortical collecting ducts was similar to that of the inner medulla (Fig. 2C). Treatment with PPG only did not affect the levels of CBS or CSE. AOAA or cotreatment with both inhibitors slightly reduced CBS expression (Fig. 2A, B). Similar to the inner medulla, the H2S concentration in this part of the kidney was markedly decreased in response to PPG and AOAA cotreatment (Fig. 1F). NKCC-2, an important sodium cotransporter in the thick ascending limbs that is involved in the buildup of high medullary osmolality and urine concentration, was also downregulated in the kidney cortex and outer medulla of mice after cotreatment with PPG and AOAA (66 ± 9 vs. 100 ± 7% in the control; P < 0.05; Fig. 2A, B). These data indicate that H2S may regulate AQP-2 and NKCC-2 expression and play a role in urine concentration.
H2S can serve as an agent that ameliorates kidney injury in some disease states. To examine whether depletion of H2S concentration in the kidney by both PPG and AOAA causes broad kidney injury and thus downregulates protein expression including AQP-2, mRNA levels of an inflammatory factor MCP-1 and the tubular injury marker KIM-1 were measured in the cortex. No differences in mRNA levels are found among the groups (Fig. 1G). H&E staining showed similar renal morphology in 4 groups (Fig. 2D). These data indicate that depletion of H2S by both PPG and AOAA does not cause kidney injury.
Protocol II: mice cotreated with CSE and CBS inhibitors had a concentrating defect in response to dehydration
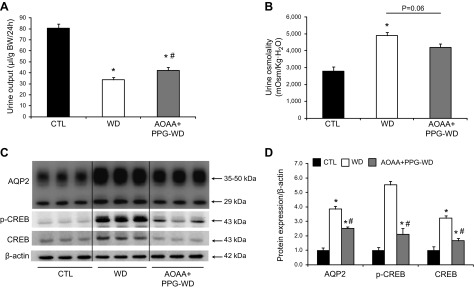
Inhibition of both CSE and CBS resulted in reduced H2S production in the kidney, which was associated with increased urine output and decreased AQP-2 protein expression. Therefore, we examined whether mice cotreated with PPG and AOAA developed a urinary concentration defect. After 36 h of water deprivation, mice showed a dramatic reduction in urine output and an increased urine osmolality (Fig. 3). Compared with the dehydrated control mice, dehydrated mice cotreated with PPG and AOAA showed an ∼25% increase in urine output and a 20% reduction in urine osmolality (Fig. 3A, B). These data suggest a mild concentration defect associated with the inhibition of H2S production. Consistent with the changes in urine concentration, there was reduced AQP-2 and p-CREB protein expression in the inner medulla of mice cotreated with PPG+AOAA (Fig. 3C, D).
Figure 3.
Inhibition of both CBS and CSE in mice with impaired urine concentration in response to dehydration. A, B) Increased urine output (A) and reduced urine osmolality (B) in mice treated with both AOAA and PPG compared to the corresponding controls after dehydration. C) Representative semiquantitative immunoblots of AQP-2, phosphorylated-CREB, and CREB in the inner medulla of kidneys from mice after dehydration. D) The corresponding densitometric analyses of protein expression levels normalized to β-actin. CTL, control group; WD, water deprivation group; AOAA+PPG-WD, water deprivation with AOAA+PPG treatment group (n = 6/group). *P < 0.05 vs. control mice; #P < 0.05 vs. WD mice.
Protocol III: CSE inhibitor treatment was associated with mildly decreased AQP-2 protein expression in the inner medulla of cbs+/− mice
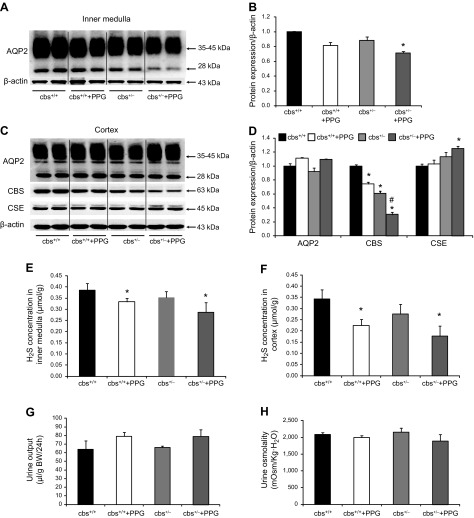
We examined whether the inhibition of CSE with PPG was associated with reduced renal AQP-2 protein expression in cbs+/− mice (cbs−/− mice do not survive >5 wk). There was no significant difference in renal inner medullary AQP-2 expression between cbs+/+ and cbs+/− mice (Fig. 4A, B). PPG caused a slight decrease in AQP-2 protein levels in the inner medulla in both cbs+/+ and cbs+/− (71 ± 2 vs. 89 ± 5%) for cbs+/− control mice. These changes were associated with a mild increase in urine output and a slight decrease in urine osmolality which did not reach statistical significance (Fig. 4G, H). PPG treatment resulted in ∼20 and 25% decreases in the H2S concentration in the inner medulla of cbs+/+ and cbs+/− mice, respectively (Fig. 4E). These findings may indicate that PPG at the current dose could not completely inhibit CSE activity in the cbs+/− mice. CSE expression may have been upregulated to compensate for the reduction in the CBS protein level in the cortex. There was no difference in the AQP-2 protein expression in the cortex and outer medulla between any of the treatment groups (Fig. 4C, D). Compared with the untreated cbs+/+ mice, the CBS protein levels decreased in cbs+/+ mice treated with PPG and cbs+/− mice. The lowest CBS protein level was observed in cbs+/− mice treated with PPG (30 ± 3 vs. 61 ± 3% in cbs+/− mice and 75 ± 2% in cbs+/+ treated with PPG; Fig. 4C, D). In contrast, the CSE protein level was significantly increased in cbs+/− mice treated with PPG (125 ± 4 vs. 113 ± 7% in cbs+/− mice; Fig. 4C, D), probably because of a compensatory response after the inhibition of CSE with PPG in these mice. Compared to cbs+/+ mice, the H2S concentration in the cortex was reduced in both cbs+/+ and cbs+/− mice treated with PPG (Fig. 4F).
Figure 4.
Inhibition of CSE by PPG was associated with mildly decreased renal medullary AQP-2 protein expression in cbs+/− mice. A) Representative semiquantitative AQP-2 immunoblots for the inner medulla of kidneys from cbs+/+ and cbs+/− mice treated with PPG or left untreated (30 mg/kg/d). B) The corresponding densitometric analyses of AQP-2 protein expression levels normalized to β-actin. C) Representative immunoblots of AQP-2, CBS, and CSE protein in the cortex and outer medulla of kidneys from cbs+/+ and cbs+/− mice treated or not with PPG (30 mg/kg/d). D) The corresponding densitometric analyses of protein expression levels normalized to β-actin. E, F) H2S concentration in the inner medulla and cortex and outer medulla of kidneys from cbs+/+ and cbs+/− mice treated or not with PPG. G, H) Urine output and urine osmolality on d 5 for cbs+/+ and cbs+/− mice (n = 5–6/group) treated or not with PPG. *P < 0.05 vs. cbs+/+ mice, #P < 0.05 vs. cbs+/− mice (n = 5–7/group).
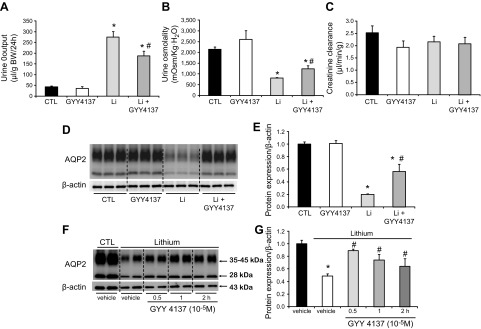
Protocol IV: H2S donor GYY4137 improved polyuria and increased AQP-2 protein expression in lithium-treated mice
Lithium-induced NDI is associated with reduced renal AQP-2 expression. Therefore, we investigated whether exogenous H2S improves the urine concentration defect and promotes AQP-2 expression in lithium-induced NDI. Lithium administration was associated with a progressive increase in urine output during the experimental period. After 1 wk of lithium treatment, the urine volume increased 6.5-fold, and urine osmolality decreased by 63% (Fig. 5A, B). GYY4137 treatment significantly decreased the urine volume by 32% (Fig. 5A) and increased the urine osmolality by 1.5-fold (Fig. 5B) in the lithium-treated mice. There was no statistically significant difference in urine output and urine osmolality between the untreated control mice and the controls treated with GYY4137 (Fig. 5A, B). Creatinine clearance showed slight, insignificant decreases in the 3 groups when compared to the controls (Fig. 5C). As expected, AQP-2 protein expression decreased dramatically in the inner medulla of the kidney from mice treated with lithium compared to the controls (20 ± 2 vs. 100 ± 4% for the control, P < 0.05; Fig. 5D, E). The GYY4137 treatment caused a marked increase in the AQP-2 protein level in the renal inner medulla of lithium-treated mice (57 ± 11%; Fig. 5D, E), which was consistent with increased urine osmolality, likely indicating an improvement in urine concentration. In the ex vivo study, the AQP-2 protein level decreased dramatically in IMCD cells prepared from lithium-treated rats (49 ± 4% vs. control). However, the levels markedly increased at 0.5, 1, and 2 h after GYY4137 treatment (Fig. 5F, G). These results suggest that H2S improves urine concentration in lithium-induced NDI by upregulating AQP-2 expression in the inner medulla.
Figure 5.
The H2S donor GYY4137 improved urine concentration and prevented the reduction of AQP-2 protein levels in the inner medulla of kidneys from lithium-treated mice. A–C) Urine output (A), urine osmolality (B), and creatinine clearance (C) were examined on d 7 for lithium-treated mice with or without GYY4137 treatment. D, E) Representative semiquantitative AQP-2 immunoblots and the corresponding densitometric analyses of protein expression levels normalized to β-actin (n = 5 in controls and GYY groups; n = 7 in Li and Li+GYY groups). The experiment was repeated 3 times. CTL, control group; GYY4137, GYY4137 treatment group; Li, lithium treatment group; Li+GYY4137, lithium plus GYY4137 treatment group. A, B, E) *P < 0.05 vs. controls, #P < 0.05 vs. lithium-treated mice. F, G) GYY4137 increased AQP-2 protein expression in IMCD cell suspensions prepared from lithium-treated rats. *P < 0.05 vs. control-treated with vehicle, #P < 0.05 vs. lithium treatment.
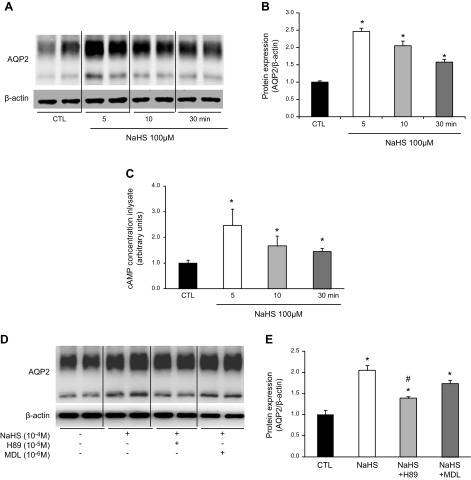
Protocols V–VIII: NaHS and GYY4137 increased AQP-2 protein expression in rat IMCD cells via the cAMP-PKA signaling pathway
To examine the potential signaling pathways mediating AQP-2 upregulation by H2S, fast (NaHS) and slow (GYY4137) H2S donors were used to treat rat IMCD suspensions or primarily cultured IMCD cells. In protocol V, NaHS treatment caused a rapid increase in AQP-2 protein expression in the IMCD cells after 5 min (246 ± 10 vs. 100 ± 4% for the control, P < 0.05; Fig. 6A, B). This increase in the AQP-2 protein level was further observed at 10 and 30 min after NaHS treatment, but not at 1 h (data not shown). Increased AQP-2 protein expression was associated with an elevated cAMP concentration in the cell lysates (Fig. 6C). To further examine the role of the cAMP in the regulation of AQP-2 protein expression by NaHS, rat IMCD suspensions were pretreated with either PKA inhibitor H89 or adenylyl cyclase (AC) inhibitor MDL12330A for 30 min before incubation, with or without NaHS (protocol VI). At 5 min after NaHS treatment, PKA inhibitor H89, but not MDL12330A, suppressed upregulated AQP-2 protein expression induced by NaHS (Fig. 6D, E).
Figure 6.
H2S donors NaHS rapidly increased AQP-2 protein expression in IMCD suspensions. A fast H2S donor (NaHS) promoted AQP-2 protein expression in IMCD suspensions within minutes. A, B) Representative immunoblots (A) and the corresponding densitometric analyses of protein expression levels normalized to β-actin (B). C) NaHS increased cAMP levels in IMCD cell lysate. D, E) In IMCD suspensions, H89, but not MDL-12330A, inhibited increased AQP-2 expression induced by NaHS in 5 min. *P < 0.05 vs. controls, #P < 0.05 vs. NaHS.
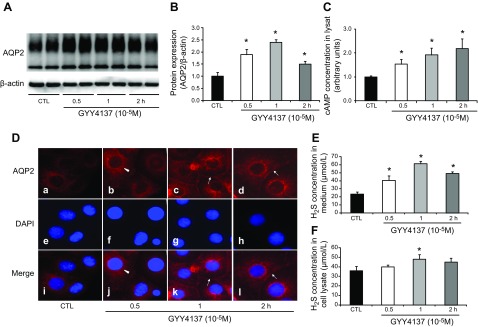
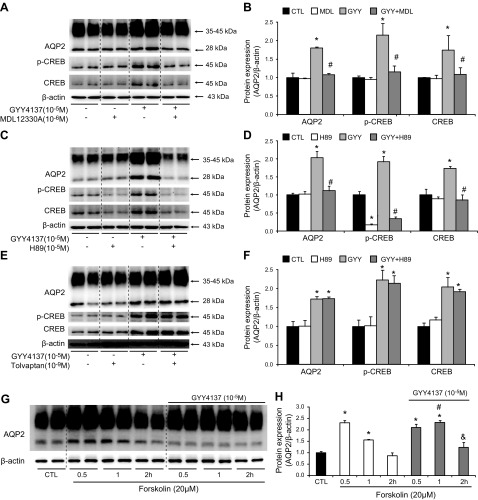
The increased AQP-2 protein level that was induced by GYY4137 was observed at 0.5, 1, and 2 h after treatment (190, 240, and 150% of the control, respectively Fig. 7A, B) and was also associated with increased cAMP levels (Fig. 7C). Immunofluorescence demonstrated that AQP-2 staining was dispersed throughout the cytoplasm with minimum apical staining in the primary cultured IMCD cells. Treatment with GYY4137 for 0.5, 1, and 2 h caused a marked increase in AQP-2 staining intensity in both the plasma membrane and intracellular compartments (Fig. 7D). These data suggest that H2S increases AQP-2 protein expression and trafficking in the IMCD principal cells. The H2S concentration in both the cell culture medium and cell lysates was significantly increased after GYY4137 treatment (Fig. 7E, F). The increased AQP-2 protein expression induced by GYY4137 in IMCD cells was blocked by either MDL12330A (112 ± 13% in GYY+MDL12330A vs. 265 ± 18% in GYY, P < 0.05; Fig. 8A, B) or H89 (85 ± 12% in GYY+H89 vs. 221 ± 6% in GYY; Fig. 8C, D), but not by the V2R antagonist tolvaptan (Fig. 8E, F) (protocol VII). Moreover, GYY4137 treatment was associated with increased p-CREB protein expression, which was also blocked by MDL12330A or H89 (Fig. 8A–D) but not tolvaptan (Fig. 8E, F). Taken together, these data indicate that GYY4137 likely induces AQP-2 expression in IMCD cells via the cAMP-PKA pathway, independent of V2R activation.
Figure 7.
H2S donors GYY4137 increased AQP-2 protein expression and intracellular trafficking in IMCD suspensions or primarily cultured IMCD cells. A slow H2S donor (GYY4137) promoted AQP-2 protein expression in IMCD suspensions within hours. A, B) Representative immunoblots (A) and the corresponding densitometric analyses of protein expression levels normalized to β-actin (B). C) GYY4137 increased cAMP levels in IMCD cell lysate. D) In primary cultured IMCD cells, GYY4137 increased intracellular AQP-2 protein expression (arrowheads; a–d) from 0.5 h (b, j) and promoted AQP-2 membrane localization (arrows; b-d) at 1 h (c, k) and 2 h (d, l) compared with controls (a, i). Nuclei are labeled with DAPI (e–h). E, F) H2S concentration in culture medium and in cell lysates after GYY4137 treatment. *P < 0.05 vs. control.
Figure 8.
H2S donor GYY4137 increased AQP-2 protein expression by activating the cAMP-PKA signaling pathway in IMCD suspensions. A–F) MDL-12330A (A, B) and H89 (C, D), but not tolvaptan (E, F), inhibited the AQP-2 expression and phosphorylation of CREB induced by GYY4137 in IMCD cell suspensions. G, H) GYY4137 potentiated the effect of forskolin on AQP-2 protein expression. Representative immunoblots (A, C, E, G) and the corresponding densitometric analyses of protein expression levels normalized to β-actin (B, D, F, H). *P < 0.05 vs. controls, #P < 0.05 vs. GYY4137 treatment group (A–F); *P < 0.05 vs. controls, #P < 0.05 vs. forskolin treatment at 1 h, &P < 0.05 vs. forskolin treatment at 2 h (G, H).
Intracellular cAMP concentrations could be the result of either enhanced cAMP production by AC or inhibition of cAMP degradation by phosphodiesterase (PDE). To discriminate between AC- and PDE-mediated contributions to AQP-2 regulation, rat IMCD suspensions were stimulated with maximally effective concentrations of forskolin (an AC activator). If H2S potentiates the effect of forskolin, H2S likely prevents cAMP degradation by PDE (protocol VIII). Treatment with forskolin for 0.5 h increased the AQP-2 protein level. However, AQP-2 protein expression was reduced 1 and 2 h after forskolin treatment (Fig. 8G, H), which suggests a reduction in the intracellular cAMP levels due to degradation. When treatment consisted of both forskolin and GYY4137, AQP-2 protein expression was not reduced 1 and 2 h after treatment (Fig. 8G, H), suggesting that H2S causes an increase in cAMP levels by inhibiting the endogenous PDE in the IMCD cells.
DISCUSSION
Both CBS and CSE are critical for H2S generation in the kidney (5, 12). Our data showed that the suppression of the endogenous H2S production by the inhibition of CBS and CSE in mice caused increased urine output, decreased urine osmolality, and impaired urine concentration in response to water deprivation. The compromised capacity of the kidneys to handle water metabolism after the inhibition of H2S production was associated with the reduced expression of both AQP-2 and NKCC-2, which play critical roles in urine concentration in the kidney collecting ducts and the thick ascending limbs. The reduced AQP-2 expression was accompanied by decreased p-CREB levels. Our data also demonstrated that GYY4137, a slow H2S donor, markedly attenuated polyuria, improved urine concentration, and increased the inner medullary AQP-2 expression in mice with lithium-induced NDI. These data support the concept that H2S specifically upregulates AQP-2 expression and therefore is involved in renal water regulation and urine concentration.
Increased urine output and reduced AQP-2 protein expression appear to be associated with an inhibitory level of endogenous H2S production. In PPG and AOAA cotreated mice (protocol I) in which CBS was expected to be inhibited, the H2S concentration in the inner medulla was reduced by nearly 70% and associated with an ∼40% decrease in the AQP-2 protein level and 1-fold increase in urine volume. Although the same dose of PPG was used to treat cbs+/− mice (protocol III) that had a moderately lower renal CBS protein level, the H2S concentration in the inner medulla only showed a minor reduction, which was associated with a mild decrease in AQP-2 expression. The lower dose of PPG than that used in the current study has shown an inhibitory effect on H2S production in animal studies (9, 22, 23); however, a higher dose of PPG was reported to be toxic to renal proximal tubular cells (24). It is likely that PPG at current dose incompletely inhibited the CSE in the cbs+/− mice, given that the CSE activity may be increased to compensate for the reduction of the CBS protein level to maintain H2S homeostasis in the kidney. Therefore, mice cotreated with PPG+AOAA had a lower H2S concentration in the inner medulla than was observed in cbs+/− mice treated with PPG alone, which was associated with further downregulation of AQP-2 and increased urine volume. These findings suggest that H2S regulates renal water metabolism by modulating AQP-2 protein expression. Alternatively, it suggests a compensatory effect between CBS and CSE on renal regulation. A deficiency in 1 enzyme’s activity could be compensated by the other to maintain the endogenous H2S level (25).
Our data demonstrated a direct effect of H2S on AQP-2 protein expression and intracellular trafficking. The cAMP-PKA pathway is the principal mediator of AQP-2 trafficking and transcription (26). Increased levels of intracellular cAMP cause the activation of PKA, phosphorylation of AQP-2, and the subsequent redistribution of AQP-2 to the plasma membrane. In addition, PKA-induced phosphorylation of CREB stimulates AQP-2 transcription via the cAMP-responsive element (14). In the rat IMCD suspensions and primary cultured IMCD cells, fast (NaHS) and slow (GYY4137) H2S donors increased cAMP production in the cell lysates and increased AQP-2 protein expression. In particular, GYY4137 caused enhanced apical staining of AQP-2, which is indicative of membrane trafficking from the intracellular compartments. The observed upregulation of AQP-2 expression was associated with increased p-CREB expression after GYY4137 treatment. The increased AQP-2 expression was markedly inhibited by AC and PKA inhibitors, but not by the V2R antagonist tolvaptan. These data suggest that the H2S-induced increase in AQP-2 expression occurs by the direct activation of the cAMP-PKA pathway. However, by using several next generation sequencing techniques, a recent study reported that there were no CREB binding sites within 390 kb of the AQP-2 gene in mouse collecting duct cells, indicating that any roles of CREB in transcriptional regulation of AQP-2 are likely to be indirect. Apparently, more studies are therefore warranted to uncover the role of CREB in AQP-2 regulation (27).
In addition, an increase in the intracellular cAMP concentration could result from the inhibition of cAMP degradation by PDE. Thus, H2S may stimulate AC or inhibit PDE; both would result in accumulation of intracellular cAMP (28). Indeed, GYY4137 potentiated the effect of forskolin on AQP-2 expression in the current study. In summary, our data demonstrate that H2S likely increases cAMP production by stimulating AC and inhibiting the degradation of intracellular cAMP in rat IMCD cells (Fig. 8).
The finding that NaHS rapidly regulates AQP-2 expression in IMCD cells is surprising. The mechanism underlying this remains unknown. H2S is reported to increase the phosphorylation of diverse kinases, such as AKT, p38, and ERK in a short time (10 min) (29), which have been shown to be important for AQP-2 regulation (30, 31). Nedvetsky et al. (30) nicely demonstrated that cAMP elevation induces a rapid increase in AQP-2 protein abundance in IMCD cells in a short time, independent of accelerated transcription and de novo protein synthesis. The rise of intracellular cAMP leads to depolyubiquitination of AQP-2 and prevents its proteasomal degradation, likely through decreasing the phosphorylation of p38-MAPK (30). Therefore, H2S-induced elevated intracellular cAMP may both promote AQP-2 expression through PKA and prevent AQP-2 degradation.
Vasopressin is the key hormone that regulates AQP-2 protein expression. In the current study, vasopressin levels were not measured in mice with inhibition of H2S. Even the best assays are unable to quantify vasopressin in the low range of physiologic values (32). Thus, AVP measurement remains complex and challenging (33). The V2R mRNA and protein levels in the kidneys of the mice were unchanged in response to both CBS and CSE inhibition, whereas H2S directly increased cAMP levels and induced AQP-2 protein expression in IMCD cells. These findings likely suggest a vasopressin-independent pathway by which H2S regulates AQP-2 protein expression (Fig. 9).
Figure 9.
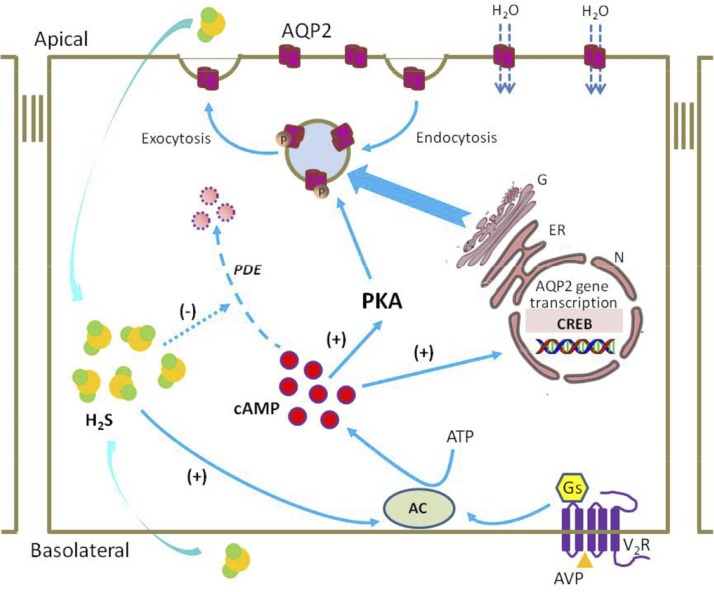
A potential role for H2S in AQP-2 protein expression and trafficking in collecting duct principal cells. The H2S produced by kidney epithelial cells diffuses into principal cells and activates AC, which causes an increase in intracellular cAMP levels, activation of PKA, and subsequent phosphorylation of AQP-2. This event results in the redistribution of AQP-2 from the intracellular vesicles to the apical membrane. cAMP may also increase AQP-2 expression by activating transcription factors (e.g., CREB) that stimulate the transcription of AQP-2 at the promoter. Driven by the transcellular osmotic gradient, water enters the principal cells through AQP-2 and passes through the basolateral plasma membrane into the blood. H2S may also inhibit PDE activity, preventing the degradation of cAMP. N, nuclear; ER, endoplasmic reticulum; G, Golgi apparatus; Gs, G-protein subunit.
H2S can serve as an agent that ameliorates kidney injury in some disease states. There is a possibility that depletion of H2S concentration in the kidney causes broad kidney injury and thus downregulates many proteins including AQP-2. Our data showed that inhibition of renal H2S production was not associated with inflammatory responses and tubular injuries, as normal renal histology, unchanged creatinine clearance and MCP-1/KIM-1 mRNA levels in the cortex were observed (protocol I). Together with ex vivo studies (protocols V–VIII), our data support the concept that H2S directly regulates AQP-2 protein expression in the collecting duct principal cells. However, as the protective effect of H2S in the kidney injuries has been widely recognized, an indirect role of H2S in regulating AQPs and other transporters cannot be excluded.
The effect of H2S on renal water and sodium reabsorption and excretion is not fully understood. In an early acute servocontrol study in rats (10), renal H2S concentrations that were induced by intrarenal infusion of NaHS correlated with increased RBF, GFR, urinary volume, natriuresis, and kaliuresis and were attenuated by CBS and CSE inhibition. In that study, the mechanism was ascribed to the inhibition of NKCC-2 and Na,K-ATPase by H2S, although the effect of H2S on these cotransporters was not studied directly (10). In the present study, we showed slightly increased urinary sodium excretion in mice with inhibition of H2S production, which was associated with mild downregulation of NKCC-2 protein. NKCC-2 activity and expression have only been confirmed in the medullary and cortical thick ascending limb and in macula densa cells. AVP markedly up-regulates NKCC-2 protein expression (34–36). The cAMP pathway was recognized as a major regulatory pathway affecting the membrane expression and transport activity of NKCC-2, which is stimulatory (37, 38). H2S-induced intracellular cAMP may be involved in NKCC-2 protein expression. The reason for the discrepancy regarding the role of H2S in urinary sodium excretion is unknown. However, the diuresis and natriuresis induced by NaHS that was observed in acute studies (10) may be attributable to its vasorelaxation effects, which may increase RBF and GFR and promote sodium and water excretion. Indeed, mice deficient in H2S production display a diminished endothelium-dependent vasorelaxation (39, 40). In the current study, the H2S concentration in the inner medulla was almost the same as in the cortex. The oxidation of H2S in the mitochondria is O2 dependent (41), and H2S has been posited to act as an O2 sensor in the kidney, especially in the inner medulla (5, 42). The availability of O2 in the medulla of the kidney is low compared to that in the cortical region. This hypoxia may impair H2S metabolism and increase its concentration in the inner medulla. It is, therefore, possible that the vasodilatory effects of H2S results in a high medullary perfusion that washes out the corticomedullary osmotic gradient and causes diuresis and natriuresis (10, 43). More studies are needed to clarify the role of H2S on the regulation of sodium reabsorption and excretion in the kidney.
In summary, in this study, H2S directly upregulated AQP-2 protein expression and was involved in fluid metabolism in the kidney. Inhibition of endogenous H2S production was associated with impaired urine concentration and reduced AQP-2 protein expression. H2S-induced AQP-2 protein expression in IMCD cells most likely occurs via the activation of the cAMP-PKA pathway. More studies are needed to gain understanding of the physiologic significance of H2S in the kidney and its role in maintaining homeostasis.
ACKNOWLEDGMENTS
This work was supported by Natural Science Foundation of China Grants 81570635, 81670646, and 81370822, and Natural Science Foundation of Guangdong Province Grants 2014A020212623, 2014A030313168, and 2016A020215034. The authors declare no conflicts of interest.
Glossary
- AC
adenylyl cyclase
- AOAA
amino-oxyacetic acid
- AQP
aquaporin
- AVP
arginine-vasopressin
- BW
body weight
- CBS
cystathionine-β-synthase
- CREB
cAMP-responsive element binding protein
- CSE
cystathionine-γ-lyase
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- GFR
glomerular filtration rate
- IMCD
inner medullary collecting duct
- KIM
kidney injury molecular
- MCP-1
monocyte chemotactic protein 1
- NDI
nephrogenic diabetes insipidus
- NKCC-2
Na+-K+-2Cl−cotransporter
- PDE
phosphodiesterase
- PPG
propargylglycine
- RBF
renal blood flow
- V2R
vasopressin type 2 receptor
- WD
water deprived
AUTHOR CONTRIBUTIONS
R. Luo, S. Hu, Q. Liu, M. Han, F. Wang, M. Qiu, S. Li, and X. Li performed the experiments; R. Luo, S. Hu, Q. Liu, W. Wang, and C. Li analyzed the data; R. Luo, S. Hu, W. Wang, and C. Li interpreted results of the experiments; R. Luo, S. Hu, and C. Li prepared the figures; R. Luo, W. Wang, and C. Li drafted the manuscript; R. Luo, T. Yang, X. Fu, W. Wang, and C. Li edited and revised the manuscript; and W. Wang and C. Li approved the final version of the manuscript.
REFERENCES
- 1.Mathai J. C., Missner A., Kügler P., Saparov S. M., Zeidel M. L., Lee J. K., Pohl P. (2009) No facilitator required for membrane transport of hydrogen sulfide. Proc. Natl. Acad. Sci. USA 106, 16633–16638 10.1073/pnas.0902952106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ishigami M., Hiraki K., Umemura K., Ogasawara Y., Ishii K., Kimura H. (2009) A source of hydrogen sulfide and a mechanism of its release in the brain. Antioxid. Redox Signal. 11, 205–214 10.1089/ars.2008.2132 [DOI] [PubMed] [Google Scholar]
- 3.Chiku T., Padovani D., Zhu W., Singh S., Vitvitsky V., Banerjee R. (2009) H2S biogenesis by human cystathionine gamma-lyase leads to the novel sulfur metabolites lanthionine and homolanthionine and is responsive to the grade of hyperhomocysteinemia. J. Biol. Chem. 284, 11601–11612 10.1074/jbc.M808026200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feliers D., Lee H. J., Kasinath B. S. (2016) Hydrogen sulfide in renal physiology and disease. Antioxid. Redox Signal. 25, 720–731 10.1089/ars.2015.6596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cao X ., Bian J. S. (2016) The role of hydrogen sulfide in renal system. Front. Pharmacol. 7, 385 10.3389/fphar.2016.00385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Singh S., Padovani D., Leslie R. A., Chiku T., Banerjee R. (2009) Relative contributions of cystathionine beta-synthase and gamma-cystathionase to H2S biogenesis via alternative trans-sulfuration reactions. J. Biol. Chem. 284, 22457–22466 10.1074/jbc.M109.010868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Watanabe M., Osada J., Aratani Y., Kluckman K., Reddick R., Malinow M. R., Maeda N. (1995) Mice deficient in cystathionine beta-synthase: animal models for mild and severe homocyst(e)inemia. Proc. Natl. Acad. Sci. USA 92, 1585–1589 10.1073/pnas.92.5.1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maclean K. N., Sikora J., Kožich V., Jiang H., Greiner L. S., Kraus E., Krijt J., Crnic L. S., Allen R. H., Stabler S. P., Elleder M., Kraus J. P. (2010) Cystathionine beta-synthase null homocystinuric mice fail to exhibit altered hemostasis or lowering of plasma homocysteine in response to betaine treatment. Mol. Genet. Metab. 101, 163–171 10.1016/j.ymgme.2010.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang R. (2012) Physiological implications of hydrogen sulfide: a whiff exploration that blossomed. Physiol. Rev. 92, 791–896 10.1152/physrev.00017.2011 [DOI] [PubMed] [Google Scholar]
- 10.Xia M., Chen L., Muh R. W., Li P. L., Li N. (2009) Production and actions of hydrogen sulfide, a novel gaseous bioactive substance, in the kidneys. J. Pharmacol. Exp. Ther. 329, 1056–1062 10.1124/jpet.108.149963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li L., Whiteman M., Guan Y. Y., Neo K. L., Cheng Y., Lee S. W., Zhao Y., Baskar R., Tan C. H., Moore P. K. (2008) Characterization of a novel, water-soluble hydrogen sulfide-releasing molecule (GYY4137): new insights into the biology of hydrogen sulfide. Circulation 117, 2351–2360 10.1161/CIRCULATIONAHA.107.753467 [DOI] [PubMed] [Google Scholar]
- 12.Bos E. M., Wang R., Snijder P. M., Boersema M., Damman J., Fu M., Moser J., Hillebrands J. L., Ploeg R. J., Yang G., Leuvenink H. G., van Goor H. (2013) Cystathionine γ-lyase protects against renal ischemia/reperfusion by modulating oxidative stress. J. Am. Soc. Nephrol. 24, 759–770 10.1681/ASN.2012030268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Knepper M. A., Kwon T. H., Nielsen S. (2015) Molecular physiology of water balance. N. Engl. J. Med. 372, 1349–1358 10.1056/NEJMra1404726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matsumura Y., Uchida S., Rai T., Sasaki S., Marumo F. (1997) Transcriptional regulation of aquaporin-2 water channel gene by cAMP. J. Am. Soc. Nephrol. 8, 861–867 [DOI] [PubMed] [Google Scholar]
- 15.Li C., Wang W., Summer S. N., Cadnapaphornchai M. A., Falk S., Umenishi F., Schrier R. W. (2006) Hyperosmolality in vivo upregulates aquaporin 2 water channel and Na-K-2Cl co-transporter in Brattleboro rats. J. Am. Soc. Nephrol. 17, 1657–1664 10.1681/ASN.2005121381 [DOI] [PubMed] [Google Scholar]
- 16.Li C., Wang W., Summer S. N., Westfall T. D., Brooks D. P., Falk S., Schrier R. W. (2008) Molecular mechanisms of antidiuretic effect of oxytocin. J. Am. Soc. Nephrol. 19, 225–232 10.1681/ASN.2007010029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kwong R. W., Perry S. F. (2015) Hydrogen sulfide promotes calcium uptake in larval zebrafish. Am. J. Physiol. Cell Physiol. 309, C60–C69 10.1152/ajpcell.00053.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee S. W., Hu Y. S., Hu L. F., Lu Q., Dawe G. S., Moore P. K., Wong P. T., Bian J. S. (2006) Hydrogen sulphide regulates calcium homeostasis in microglial cells. Glia 54, 116–124 10.1002/glia.20362 [DOI] [PubMed] [Google Scholar]
- 19.Wang W., Luo R., Lin Y., Wang F., Zheng P., Levi M., Yang T., Li C. (2015) Aliskiren restores renal AQP2 expression during unilateral ureteral obstruction by inhibiting the inflammasome. Am. J. Physiol. Renal Physiol. 308, F910–F922 10.1152/ajprenal.00649.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li C., Wang W., Rivard C. J., Lanaspa M. A., Summer S., Schrier R. W. (2011) Molecular mechanisms of angiotensin II stimulation on aquaporin-2 expression and trafficking. Am. J. Physiol. Renal Physiol. 300, F1255–F1261 10.1152/ajprenal.00469.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kang K., Zhao M., Jiang H., Tan G., Pan S., Sun X. (2009) Role of hydrogen sulfide in hepatic ischemia-reperfusion-induced injury in rats. Liver Transpl. 15, 1306–1314 10.1002/lt.21810 [DOI] [PubMed] [Google Scholar]
- 22.Jin S., Tan B., Teng X., Meng R., Jiao X., Tian D., Xiao L., Xue H., Guo Q., Duan X., Wu Y. (2017) Diurnal fluctuations in plasma hydrogen sulfide of the mice. Front. Pharmacol. 8, 682 10.3389/fphar.2017.00682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Triguero A., Barber T., García C., Puertes I. R., Sastre J., Viña J. R. (1997) Liver intracellular L-cysteine concentration is maintained after inhibition of the trans-sulfuration pathway by propargylglycine in rats. Br. J. Nutr. 78, 823–831 10.1079/BJN19970198 [DOI] [PubMed] [Google Scholar]
- 24.Konno R., Ikeda M., Yamaguchi K., Ueda Y., Niwa A. (2000) Nephrotoxicity of D-proparglyglycine in mice. Arch. Toxicol. 74, 473–479 10.1007/s002040000156 [DOI] [PubMed] [Google Scholar]
- 25.Roy A., Khan A. H., Islam M. T., Prieto M. C., Majid D. S. (2012) Interdependency of cystathione γ-lyase and cystathione β-synthase in hydrogen sulfide-induced blood pressure regulation in rats. Am. J. Hypertens. 25, 74–81 10.1038/ajh.2011.149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Knepper M. A., Kwon T. H., Nielsen S. (2015) Molecular physiology of water balance. N. Engl. J. Med. 373, 196 10.1056/NEJMc1505505 [DOI] [PubMed] [Google Scholar]
- 27.Jung H. J., Raghuram V., Lee J. W., Knepper M. A. (2018) Genome-wide mapping of DNA accessibility and binding sites for CREB and C/EBPβ in vasopressin-sensitive collecting duct cells. J. Am. Soc. Nephrol. 29, 1490–1500 10.1681/ASN.2017050545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Perniss A., Preiss K., Nier M., Althaus M. (2017) Hydrogen sulfide stimulates CFTR in Xenopus oocytes by activation of the cAMP/PKA signalling axis [Published correction in Sci. Rep. 2018;8:4420]. Sci. Rep. 7, 3517 10.1038/s41598-017-03742-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Altaany Z., Yang G., Wang R. (2013) Crosstalk between hydrogen sulfide and nitric oxide in endothelial cells. J. Cell. Mol. Med. 17, 879–888 10.1111/jcmm.12077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nedvetsky P. I., Tabor V., Tamma G., Beulshausen S., Skroblin P., Kirschner A., Mutig K., Boltzen M., Petrucci O., Vossenkämper A., Wiesner B., Bachmann S., Rosenthal W., Klussmann E. (2010) Reciprocal regulation of aquaporin-2 abundance and degradation by protein kinase A and p38-MAP kinase. J. Am. Soc. Nephrol. 21, 1645–1656 10.1681/ASN.2009111190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim H. Y., Choi H. J., Lim J. S., Park E. J., Jung H. J., Lee Y. J., Kim S. Y., Kwon T. H. (2011) Emerging role of Akt substrate protein AS160 in the regulation of AQP2 translocation. Am. J. Physiol. Renal Physiol. 301, F151–F161 10.1152/ajprenal.00519.2010 [DOI] [PubMed] [Google Scholar]
- 32.Bankir L., Bichet D. G., Morgenthaler N. G. (2017) Vasopressin: physiology, assessment and osmosensation. J. Intern. Med. 282, 284–297 10.1111/joim.12645 [DOI] [PubMed] [Google Scholar]
- 33.Christ-Crain M., Fenske W. (2016) Copeptin in the diagnosis of vasopressin-dependent disorders of fluid homeostasis. Nat. Rev. Endocrinol. 12, 168–176 10.1038/nrendo.2015.224 [DOI] [PubMed] [Google Scholar]
- 34.Igarashi P., Whyte D. A., Li K., Nagami G. T. (1996) Cloning and kidney cell-specific activity of the promoter of the murine renal Na-K-C1 cotransporter gene. J. Biol. Chem. 271, 9666–9674 10.1074/jbc.271.16.9666 [DOI] [PubMed] [Google Scholar]
- 35.Kim G. H., Ecelbarger C. A., Mitchell C., Packer R. K., Wade J. B., Knepper M. A. (1999) Vasopressin increases Na-K-2Cl cotransporter expression in thick ascending limb of Henle’s loop. Am. J. Physiol. 276, F96–F103 [DOI] [PubMed] [Google Scholar]
- 36.Work J., Galla J. H., Booker B. B., Schafer J. A., Luke R. G. (1985) Effect of ADH on chloride reabsorption in the loop of Henle of the Brattleboro rat. Am. J. Physiol. 249, F698–F703 [DOI] [PubMed] [Google Scholar]
- 37.Castrop H., Schießl I. M. (2014) Physiology and pathophysiology of the renal Na-K-2Cl cotransporter (NKCC2). Am. J. Physiol. Renal Physiol. 307, F991–F1002 10.1152/ajprenal.00432.2014 [DOI] [PubMed] [Google Scholar]
- 38.Ortiz P. A. (2006) cAMP increases surface expression of NKCC2 in rat thick ascending limbs: role of VAMP. Am. J. Physiol. Renal Physiol. 290, F608–F616 10.1152/ajprenal.00248.2005 [DOI] [PubMed] [Google Scholar]
- 39.Yang G., Wu L., Bryan S., Khaper N., Mani S., Wang R. (2010) Cystathionine gamma-lyase deficiency and overproliferation of smooth muscle cells. Cardiovasc. Res. 86, 487–495 10.1093/cvr/cvp420 [DOI] [PubMed] [Google Scholar]
- 40.Tang G., Yang G., Jiang B., Ju Y., Wu L., Wang R. (2013) H2S is an endothelium-derived hyperpolarizing factor. Antioxid. Redox Signal. 19, 1634–1646 10.1089/ars.2012.4805 [DOI] [PubMed] [Google Scholar]
- 41.Olson K. R., Healy M. J., Qin Z., Skovgaard N., Vulesevic B., Duff D. W., Whitfield N. L., Yang G., Wang R., Perry S. F. (2008) Hydrogen sulfide as an oxygen sensor in trout gill chemoreceptors. Am. J. Physiol. Regul. Integr. Comp. Physiol. 295, R669–R680 10.1152/ajpregu.00807.2007 [DOI] [PubMed] [Google Scholar]
- 42.Koning A. M., Frenay A. R., Leuvenink H. G., van Goor H. (2015) Hydrogen sulfide in renal physiology, disease and transplantation: the smell of renal protection. Nitric Oxide 46, 37–49 10.1016/j.niox.2015.01.005 [DOI] [PubMed] [Google Scholar]
- 43.Ge S. N., Zhao M. M., Wu D. D., Chen Y., Wang Y., Zhu J. H., Cai W. J., Zhu Y. Z., Zhu Y. C. (2014) Hydrogen sulfide targets EGFR Cys797/Cys798 residues to induce Na(+)/K(+)-ATPase endocytosis and inhibition in renal tubular epithelial cells and increase sodium excretion in chronic salt-loaded rats. Antioxid. Redox Signal. 21, 2061–2082 10.1089/ars.2013.5304 [DOI] [PMC free article] [PubMed] [Google Scholar]