Abstract
Statins, which reduce LDL-cholesterol by inhibition of 3-hydroxy-3-methylglutaryl–coenzyme A reductase, are among the most widely prescribed drugs. Skeletal myopathy is a known statin-induced adverse effect associated with mitochondrial changes. We hypothesized that similar effects would occur in cardiac myocytes in a lipophilicity-dependent manner between 2 common statins: atorvastatin (lipophilic) and pravastatin (hydrophilic). Neonatal cardiac ventricular myocytes were treated with atorvastatin and pravastatin for 48 h. Both statins induced endoplasmic reticular (ER) stress, but only atorvastatin inhibited ERK1/2T202/Y204, AktSer473, and mammalian target of rapamycin signaling; reduced protein abundance of caveolin-1, dystrophin, epidermal growth factor receptor, and insulin receptor-β; decreased Ras homolog gene family member A activation; and induced apoptosis. In cardiomyocyte-equivalent HL-1 cells, atorvastatin, but not pravastatin, reduced mitochondrial oxygen consumption. When male mice underwent atorvastatin and pravastatin administration per os for up to 7 mo, only long-term atorvastatin, but not pravastatin, induced elevated serum creatine kinase; swollen, misaligned, size-variable, and disconnected cardiac mitochondria; alteration of ER structure; repression of mitochondria- and endoplasmic reticulum–related genes; and a 21% increase in mortality in cardiac-specific vinculin-knockout mice during the first 2 months of administration. To our knowledge, we are the first to demonstrate in vivo that long-term atorvastatin administration alters cardiac ultrastructure, a finding with important clinical implications.—Godoy, J. C., Niesman, I. R., Busija, A. R., Kassan, A., Schilling, J. M., Schwarz, A., Alvarez, E. A., Dalton, N. D., Drummond, J. C., Roth, D. M., Kararigas, G., Patel, H. H., Zemljic-Harpf, A. E. Atorvastatin, but not pravastatin, inhibits cardiac Akt/mTOR signaling and disturbs mitochondrial ultrastructure in cardiac myocytes.
Keywords: Statins, statin-induced myopathy, cardiomyocytes, oxygen consumption rate, heart failure
Statins are used to lower LDL-cholesterol (LDL-C) serum levels in patients for the prevention and treatment of cardiovascular disease and are among the most widely prescribed drug classes in the world. A meta-analysis of 26 randomized controlled trials, including 170,000 individuals, showed that more intensive LDL-C lowering further reduces the occurrence of heart attack, revascularization, and ischemic stroke, when compared to standard statin regimens (1). Statins are life-saving drugs leading to reduction in cardiovascular events and death in patients with cardiovascular disease (2). Plaque stabilization is especially important in patients with acute myocardial infarction (3–5). Thus, it has become standard practice to initiate statin therapy immediately after acute coronary syndromes, regardless of lipid level. Full implementation of the new 2013 American College of Cardiology/American Heart Association guidelines for the prevention of cardiovascular disease would result in statin use by ∼56 million Americans (6, 7).
Statins are 3-hydroxy-3-methylglutaryl–coenzyme A (HMG-CoA) reductase inhibitors, which act to limit mevalonic acid synthesis (8, 9). HMG-CoA reductase is ubiquitously expressed in mammalian cells and found in the membrane of the endoplasmic reticulum (ER) (10, 11). First generation/class I statins are fungal metabolites (mevastatin, pravastatin, simvastatin, and lovastatin). Synthetic second-generation/class II statins (atorvastatin, cerivastatin, fluvastatin, pitavastatin, and rosuvastatin) have greater binding affinity to HMG-CoA reductase. On the basis of their chemical nature, statins are further divided into lipophilic (cerivastatin, lovastatin, simvastatin, pitavastatin, fluvastatin, and atorvastatin) and hydrophilic (pravastatin, rosuvastatin) types. Based on the degree of highest octanol–water coefficient (lipophilicity), statins can be ordered as cerivastatin > simvastatin > lovastatin > atorvastatin > fluvastatin > pitavastatin > pravastatin > rosuvastatin (12, 13). The most lipophilic synthetic statin cerivastatin (Baycol; Bayer A.G., Leverkeusen, Germany) was withdrawn from the market because of the occurrence of rhabdomyolysis (14). Currently, 5 lipophilic and 2 hydrophilic statins are used clinically.
Irrespective of these benefits, like all drugs, statins harbor potential adverse effects. The most common statin adverse effects are elevated liver enzymes and muscle-related symptoms, ranging from muscle weakness, pain, cramps, and exercise intolerance to rare cases of rhabdomyolysis (15–17). Skeletal muscle biopsies from patients with statin myopathy show defects in mitochondrial structure/function (18–22), and impaired mitochondrial oxidative phosphorylation has been demonstrated in patients receiving statins (21, 22). Mitochondrial dysfunction has been postulated to be a principal cause of statin-induced myopathy (23–25).
Whether long-term statin treatment may alter cardiac muscle is currently unknown. However, given that similar oxidative phosphorylation pathways are relevant in skeletal and cardiac muscle and that skeletal myopathy is the most common statin side effect, we hypothesized that statins also exert adverse effects on cardiac muscle. We further surmised that, for reasons of greater intracellular access, the effects on cardiac muscle would be more evident with a lipophilic statin than with a hydrophilic agent. Thus, our study aimed to investigate the potential effects of 2 chemically different statins (atorvastatin and pravastatin) on cardiac myocytes using in vitro (signaling and mitochondrial function) and in vivo [lipid profile, cardiac systolic function, heart failure (HF) survival, and ultrastructure] approaches.
MATERIALS AND METHODS
Animals
All mice were housed in the 10,700 ft2 Veterinary Medical Unit of the Veterans Affairs San Diego Healthcare System (VASDHS). The VASDHS program for animal care is fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care, International (AAALAC), and holds an approved U.S. National Institutes of Health Assurance and U.S. Department of Agriculture license. It is staffed with a veterinarian, who is a diplomate of the American College of Laboratory Animals, and a veterinary technician, both of whom are on site 5 d/wk. Veterinary support was on call for evenings, and weekends and emergency care were available on call 24 h/d, 7 d/wk. Consultation with the veterinarian was part of protocol preparation and implementation. Animals were observed daily by the veterinary technician and housed in individually ventilated microisolator cages (4 animals/cage) and changed according to Veterinary Medical Unit standard operating procedures. Mice had free access to food, water, and environmental enrichment.
Cardiac myocyte isolation, culture, and statin treatment
The experimental procedures were conducted in accordance with the Guide for the Care and Use of Laboratory Animals (National Academy of Science, Washington, DC, USA) and approved by the VASDHS Institutional Animal Care and Use Committee. Mice were housed in an AAALAC accredited facility under temperature, humidity, and light cycle–controlled conditions (12-h dark–light with lights on at 6 am) with access to food and water ad libitum. Neonatal mouse ventricular myocytes (NMVMs) were isolated from 0- to 2-d-old mouse hearts. Confluent and beating primary myocytes were used as described by Zemljic-Harpf et al. (26). Briefly, hearts were excised, atria were removed, and small ventricular tissue pieces were digested overnight in 50 ml of 0.5 mg/ml trypsin-HBSS at 4°C. On the following day, NMVMs were isolated, cultured for 1 wk, and treated with atorvastatin, pravastatin, or vehicle (10% DMSO in H2O) for 48 h, based on previous reports (27). Protein expression was normalized to tubulin expression on the same membrane. One week after plating, only confluent and beating cultures of NMVMs were used. Statins (atorvastatin/PZ0001 and pravastatin-P4498) were purchased from MilliporeSigma (Burlington, MA, USA). Cells were treated with atorvastatin or pravastatin at 0.1–10 μM or vehicle (10% DMSO in H2O) diluted into the medium for up to 48 h.
HL-1 cardiomyocyte culture
HL-1 cells were kindly provided by Dr. William C. Claycomb (deceased) (University Health Sciences Center, New Orleans, LA, USA) (28). HL-1 cells were cultured on gelatin (0.02%)/fibronectin (10 μg/ml)–coated plates (Corning, Corning, NY, USA) and were maintained at 37°C and 5% CO2 in Claycomb medium (MilliporeSigma) supplemented with 10% (v/v) fetal bovine serum, 2 mM glutamine, 0.1 mM norepinephrine, 100 U/ml penicillin, and 100 μg/ml streptomycin.
Bioenergetic analysis of HL1 cells
Assessment of mitochondrial function was performed with an XF24 seahorse flux analyzer (Seahorse Biosciences, North Billerica, MA, USA). HL-1 cells were seeded at a density of 45,000 cells/well in a gelatin precoated 24-well seahorse plate and left to adhere for 24 h in Claycomb medium. The cells were then incubated for 48 h with either atorvastatin or pravastatin at a final concentration of 10 μM in DMSO. Control cells were incubated with 0.05% DMSO. After incubation, the medium was replaced with XF medium without fetal bovine serum and placed in 37°C without CO2. Oxygen consumption rate (OCR) measurements were performed, followed by a sequential addition oligomycin, carbonyl cyanide-4(trifluoromethoxy)phenylhydrazone (FCCP), rotenone, and antimycin A for final assay concentrations of 1, 0.5, 1, and 1 μM, respectively. Experimental treatments were performed on 8 wells of each plate, as technical replicates, and each experiment had at least 3 biologic replicates. OCR was normalized for the amount of protein in each well.
Long-term statin administration in mice
Wild-type (WT) and cardiac-specific vinculin-knockout (cVclKO) mice were housed as previously described. The animals were genotyped as described by Zemljic-Harpf et al. (29). Because of breeding difficulties, cVclKO mice were fed a breeder chow (8626; Harlan Sprague Dawley Industries, Indianapolis, IN, USA) and maintained on a mixed genetic background of SV129/Black Swiss and C57BL/6. Statin treatment was initiated in 5-wk-old male mice. Atorvastatin (Lipitor; Pfizer, New York, NY, USA) and pravastatin (Pravachol; Bristol Meyer Squibb, New Yok, NY, USA) were purchased at the VASDHS pharmacy. Drugs were crushed and dissolved in 10% EtOH and water. The vehicle (placebo) was 10% EtOH in water. Animals received atorvastatin (5 mg/kg), pravastatin (5 mg/kg), or vehicle once in the late afternoon by oral gavage (feeding tube; Braintree Scientific, Inc., Braintree, MA, USA) for up to 7 mo. Five weeks after initiation of statin therapy, peripheral blood was collected (serum collection tubes, 365967 Microtainer; BD Bioscience, Franklin Lakes, NJ, USA) according to blood collection guidelines. Blood was allowed to coagulate for 30–60 min, followed by 30 min centrifugation at 3000 rpm. Clear serum was transferred into a new tube and fresh frozen in aliquots for further analysis. A lipid panel [direct HDL-cholesterol (C), direct LDL-C, total cholesterol, and triglycerides], a liver panel (albumin, glutathione, aspartate transaminase, and alanine transaminase), and creatine kinase (CK) were analyzed (Idexx Laboratories, West Sacramento, CA, USA).
Immunoblot analysis, Ras homolog gene family, member A activity, and cytotoxicity assays
For immunoblot analysis, cultured myocytes were lysed in buffer containing 50 mM Tris, 150 mM NaCl, 1 mM EDTA, 1% deoxycholic acid, 0.2% SDS, and 1% NP-40 (pH 7.5) for 20 min on ice, then scraped, needle homogenized, and sonicated. Equal protein amounts (5 μg for structural protein analysis, 25–50 μg for signaling proteins) of whole-cell lysate were used for immunoblot analysis. Lactate dehydrogenase (LDH) release into the culture medium was measured according to the manufacturer’s instructions (Cytotoxicity Detection Kit Plus; Roche Diagnostics, GmbH, Mannheim, Germany). A rhotekin-rhodopsin [Ras homolog gene family (Rho)-binding domain (RBD)] bead pulldown was used based on the manufacturer’s recommendation (Rho Activation Assay Biochem Kit; Cytoskeleton, Inc., Denver, CO, USA). The glutathione S-transferase (GST)–RBD assay uses a fusion protein of glutathione-S-transfsrase and the Rho binding domain of the Rho effector protein Rhotekin, which has specificity for Rho, member A (Rho A). Immunoblot analysis with a Rho-specific antibody determined the amount of activated Rho. More detailed information is included in the Supplemental Data.
Echocardiography
Male cVclKO and littermate control mice received atorvastatin, pravastatin, or vehicle. M-mode and Doppler echocardiography was performed in 5-wk-old mice before the initiation of stain therapy (baseline) and at consecutive 2-wk intervals throughout the statin treatment, as described by Zemljic-Harpf et al. (29). Male 5- to 40-wk-old mice were anesthetized with isoflurane via nosecone (for induction, 4% for 30 s, maintained at 0.5–1.5% while oxygen was delivered at 1 L/min to achieve heart rates between 550–600 bpm). A Philips Sonos 5500 Ultrasound machine (Philips, Amsterdam, The Netherlands) was used to obtain short- and long-axis views of the left ventricle by slight angulation and rotation of the transducer. Two-dimensional targeted M-mode measurements were taken at the level of the largest left ventricle diameter from short-axis views. M-mode measurements of left ventricle end diastolic dimension (EDD) and end systolic dimension (ESD) were made from original tracings by using the leading-edge convention of the American Society of Echocardiography and by using the steepest echoes. EDD was taken at the onset of the QRS complex, and the ESD was measured at the peak of the posterior wall motion. Aortic ejection time was measured in long-axis views. Echocardiography was repeated at 2-wk intervals for 18 wk. Control mice underwent a final echo analysis after 7 mo of treatment prior to euthanasia. Investigators blinded to genotype and treatment of the animal measured echocardiographic images. The sonographer was blinded to the genotype and treatment regimen at all time points. Echo data were analyzed for all time points (until 18 wk) by a baseline-adjusted, repeated-measures 3-way ANOVA with the factors genotype, treatment, and time. We found a significant effect of genotype by time for percentage of fractional shortening (%FS) and velocity of circumferential fiber shortening (VCF), as well as a time × treatment and a time × treatment × genotype effect for left ventricular posterior wall thickness at end-diastole (LVPWd; P < 0.05). Because many cVclKO+A mice died, we had too few animals at the later time points (14–18 wk) to perform normality tests. Therefore, we repeated the analysis only until the 12-wk time point. We found a significant effect of time×genotype for the measurements %FS and VCF (P < 0.05). In addition, a time ×‴ genotype × treatment effect was noted for %FS (P < 0.05). No effects were found for LVPWd, suggesting that the later time points with too few animals caused the effect found when all data were analyzed. Because WT mice did not show decreased function over time, we followed up by analyzing isolated time points in only our cVclKO mice with a 1-way ANOVA and Tukey’s honest significant difference post hoc comparison.
Ultrastructural analysis
Four animals per group were transcardially perfused with 4% paraformaldehyde and 1.5% glutaraldehyde in 0.1 M cacodylate buffer while under deep pentobarbital anesthesia. Samples were postfixed in 1% OsO4 in 0.1 M cacodylate buffer, en bloc stained with uranyl acetate, dehydrated through a standard ethanol series, and embedded in longitudinal orientation LX-112 (Ladd Research, Williston, VT, USA). Blocks were polymerized 48 h at 60°C. Seventy nanometer sections were cut and mounted on Cu grids and stained in uranyl acetate and lead citrate before transmission electron microscopic (TEM; Jeol 1200 EX-II; Akishima, Japan; or Tecnai 12; Philips) examination by observers blinded to treatment regimen.
Hybridization and microarray profiling
Seven months after treatment started, total RNA was isolated from control hearts (left ventricular free wall; n = 3–5/group) using the RNeasy Fibrous Tissue Kit (74704; Qiagen, Germantown, MD, USA), according to the manufacturer’s protocol. The RNA quality and quantity were established with a 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA, USA). Microarray data have been deposited in the Gene Expression Omnibus database (National Center for Biotechnology Information, Bethesda, MD, USA; https://www.ncbi.nlm.nih.gov/geo/; Accession No. GSE102666).
Microarray data analysis
The computational and statistical analyses of the microarray data were carried out with the R v.2.14.2 software (https://www.r-project.org/) and Bioconductor packages (https://www.bioconductor.org/). After correction, expression data were normalized and log2 transformed with the median polish algorithm of robust multiarray average. The quality of the data was assessed with the Bioconductor arrayQualityMetrics package. To detect differences in probe set expression between conditions, a moderated linear model was applied by using the limma package. To extract biologically useful information, we generated gene lists that selected candidates with an uncorrected value of P < 0.01.
Statistical analysis
All values are expressed as means ± sem. In each figure legend, we mention the specific analysis between multiple groups by 1-, 2-, or 3-way ANOVA, with values of P < 0.05 considered statistically significant (Prism 7; GraphPad, La Jolla, CA, USA; IBM-SPSS-Statistics v.24; IBM, Armonk, NY, USA). To analyze echocardiographic data, a 3-way ANOVA for genotype, treatment, and time was performed with IBM-SPSS Statistics v.24. Because of the low number of animals in the cVclKO group at 16 and 18 wk after treatment resulting from deaths, 2- and 1-way ANOVAs within WT animals only were used as indicated, with Tukey’s post hoc comparison.
RESULTS
Atorvastatin and pravastatin have distinct effects on prosurvival signaling in cardiac myocytes
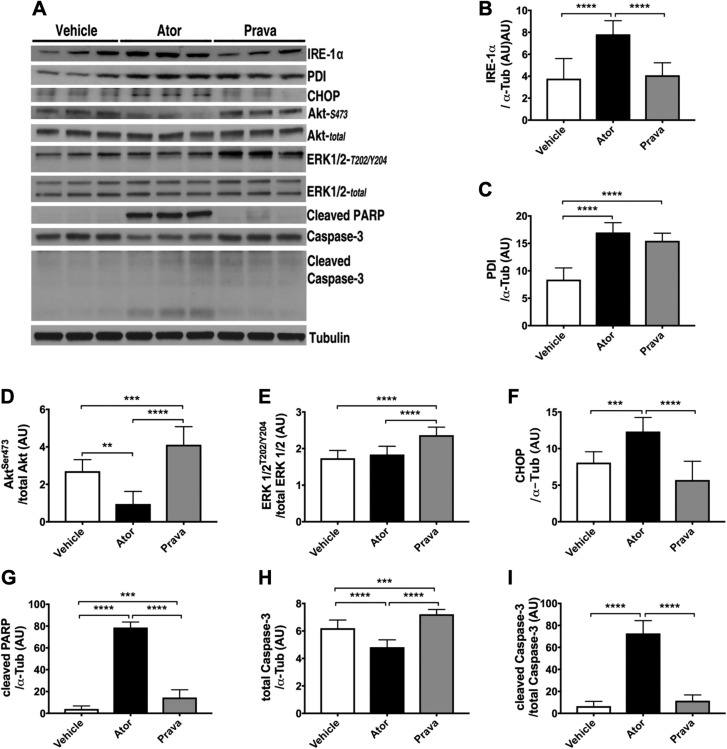
In the heart, proteins are folded and corrected within the ER by a pool of molecular chaperone proteins, including protein disulfide isomerase (PDI), the serine/threonine kinase inositol-requiring enzyme (IRE)-1α, and C/EBP homologous protein (CHOP) (30). Because HMG-CoA reductase is localized in the ER membrane (11) and inhibited by statins, we investigated statin’s effects on ER stress, ERK1/2, and Akt signaling. In NMVMs, only atorvastatin increased IRE-1α, but both statins increased PDI protein levels (Fig. 1A–C). Atorvastatin administration decreased, whereas pravastatin increased protein kinase B (Akt)S473 protein expression, compared with vehicle-treated cells (Fig. 1A, D). Pravastatin increased ERK1/2 activation, without changing total ERK1/2 protein levels, when compared with vehicle or atorvastatin (Fig. 1A, E). Atorvastatin, but not pravastatin or vehicle, increased CHOP protein levels, and cleavage of poly(ADP-ribose) polymerase and caspase-3 in cardiac myocytes, indicating initiation of apoptotic signal transduction (Fig. 1).
Figure 1.
Atorvastatin, but not pravastatin, induced unresolved ER stress, inhibited Akt, and induced apoptosis in cardiac myocytes. NMVMs were incubated with either atorvastatin (Ator) or pravastatin (Prava) for 48 h. A) Immunoblot analysis of total cell lysates. B) Ator increased the expression of IRE-1α. C) Both statins increased the protein expression of PDI, indicative of ER stress. D) Ator reduced Akt survival signaling, whereas Prava increased the activation of AktS473. E) Prava increased the expression of ERK-1/2t202/Y204. F–I) Ator increased the expression of CHOP (F), cleaved poly(ADP-ribose) polymerase (G), and cleaved caspase-3 (H, I). B–I) Densitometric analysis of representative immunoblot bands (n = 3 wells/treatment, each experiment repeated 3 times). Means ± sem. **P < 0.01, ***P < 0.001, ****P < 0.0001 (1-way ANOVA with Tukey’s post hoc comparison).
Atorvastatin and pravastatin have different effects on the regulation of mTOR signaling
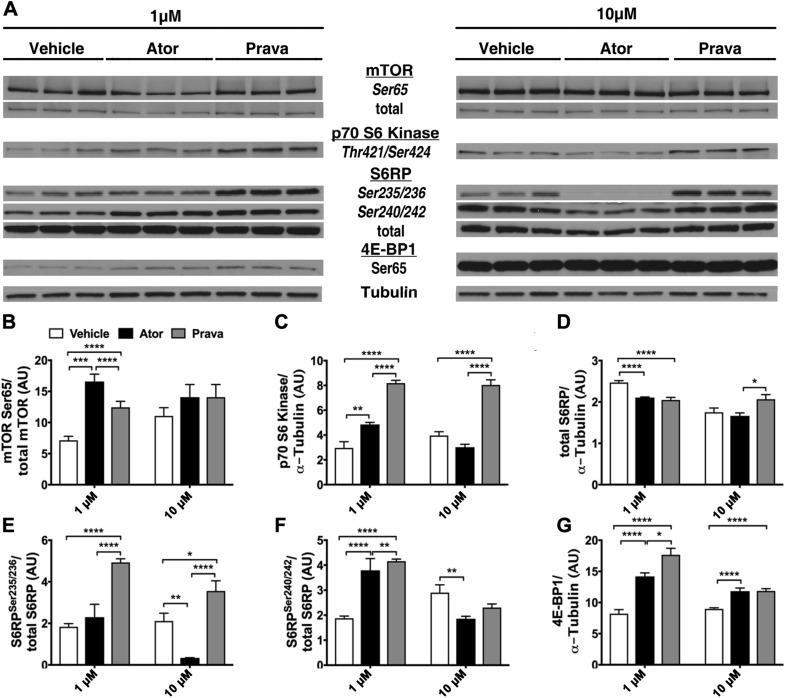
The mTOR signaling pathway is a crucial regulator of protein synthesis and cellular metabolism and is localized to the ER and ribosomes (31). Because the ER stress response is an energy-conserving, antigrowth process that regulates protein translation and mTOR activation, we investigated statin’s effects on mTOR signaling in cardiac myocytes (31). In cardiac myocytes treated with 1 μM statin concentrations, only pravastatin increased p70s S6Thr421/Ser424 and S6RPSer235/236 protein levels (Fig. 2A–C). However, at 10 μM concentration, atorvastatin reduced p70 S6Thr421/ser424 kinase (Fig. 2A, C) and S6RPSer240/242 (Fig. 2A, F) levels and abolished S6RPSer235/236 (Fig. 2A, E), whereas pravastatin increased p70 S6Thr421/ser424, S6RPSer235/236, and S6RPSer240/242 levels (Fig. 2A, E, F). Both statins increased 4E-BP1protein expression (Fig. 2A, G). mTOR protein expression did not change in the 3 groups (Fig. 2A, B). These data suggest that, when compared to vehicle, atorvastatin inhibits and pravastatin activates mTOR signaling in cardiac myocytes.
Figure 2.
Atorvastatin (Ator), but not pravastatin (Prava), inhibited mTOR activation in cardiac myocytes. NMVMs were incubated with either 1 or 10 μM Ator, Prava, or vehicle for 48 h. A) Total lysates underwent immunoblot analysis. B) Both statins did not alter total or activated mTORSer65 protein expression. C–F) The downstream targets of activated mTOR, p70 S6 (C, D), and S6 RP (E, F) were up-regulated in Prava-treated cells but decreased in Ator-treated cells. G) Both statins increased 4E-BP1Ser65 expression. Tubulin was used as loading control. B–F) Densitometric analysis of representative immunoblot bands (n = 3 wells/treatment, each experiment repeated 3 times). Means ± sem. *P < 0.05, *P < 0.01, *P < 0.001, *P < 0.0001 (1-way ANOVA with Tukey’s post hoc comparison).
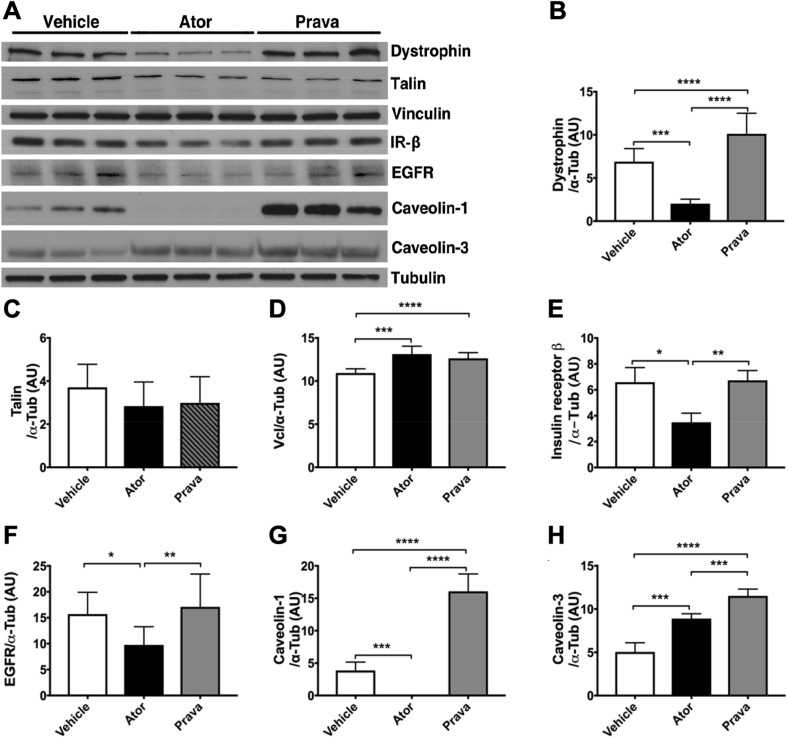
Because we detected reduced mTOR activation with administration of atorvastatin, but not pravastatin, we next investigated structural protein abundance in statin-treated cardiac myocytes. Post-translational modification of proteins often depends on prenylation or N-linked glycosylation of membrane receptors, which can be impaired as a result of inhibition of the mevalonate pathway (32, 33). Our data show that atorvastatin reduced membrane-associated expression of dystrophin (Fig. 3A, B) and caveolin-1 (Fig. 3A, G), but pravastatin increased their expression. Both statins increased caveolin-3 and vinculin protein levels (Fig. 3A, D, H), but only atorvastatin reduced the protein expression of insulin receptor-beta (IR-β) and epidermal growth factor receptor (EGFR) in cardiac myocytes (Fig. 3A, E, F).
Figure 3.
Atorvastatin (Ator), but not pravastatin (Prava), inhibited structural protein expression in cardiac myocytes. A) NMVMs were incubated with either Ator or Prava for 48 h, and total cell lysates were subjected to immunoblot analysis. B) Ator decreased, but Prava increased dystrophin protein expression. C–H) Both statins did not change talin (C) protein expression, but increased vinculin (Vcl; D) and caveolin-3 (H) protein expression. Ator decreased protein expression of IR-β (E), EGFR (F), and caveolin-1 (G). Prava did not alter the protein expression of IR-EGFR, but increased caveolin-1 expression. B–H) Densitometric analysis of representative immunoblot bands (n = 3 wells/ treatment, each experiment repeated 3 times). Means ± sem. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 (1-way ANOVA with Tukey’s post hoc comparison).
Atorvastatin inhibited RhoA activation to a greater degree than pravastatin
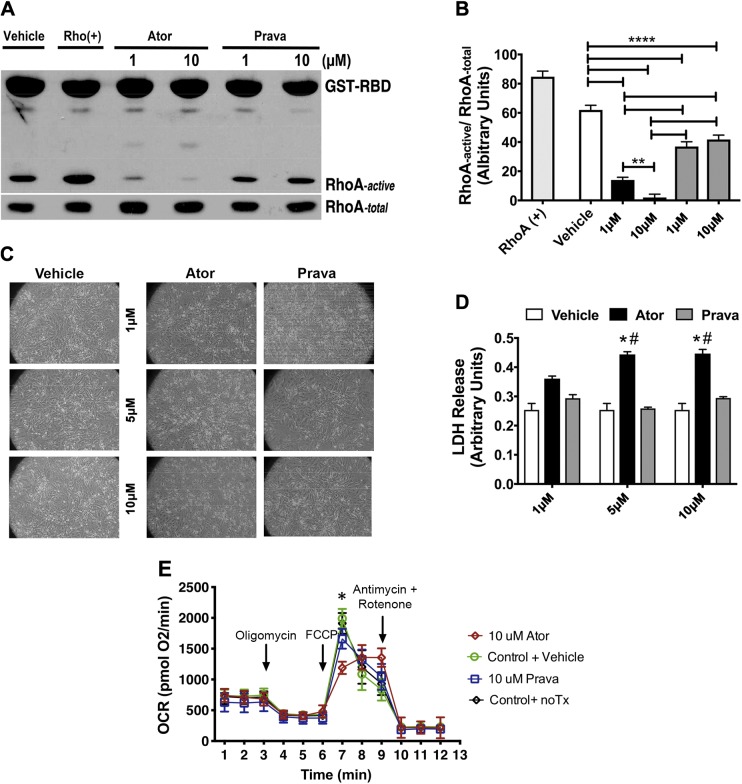
Statins have been shown to block the development of cardiac hypertrophy, and the inhibition of Rho and Rac pathways was suggested as an underlying mechanism (34). Inhibition of small GTPases and Rho kinase by statins, presumably as a result of decreased prenylation, lead to inhibition of vascular smooth muscle cell proliferation and increased endothelial NO production (35). We investigated the role of atorvastatin and pravastatin on RhoA kinase activation in cultured cardiac myocytes and found that 1 and 10 μM concentrations of atorvastatin inhibited RhoA activation to a greater extent than pravastatin did, and 10 μM atorvastatin abolished RhoA activation (Fig. 4A, B). After atorvastatin administration, cardiac myocytes appeared smaller when compared to pravastatin- and vehicle-treated cells (Fig. 4C). We further found that increasing atorvastatin concentrations elevated LDH production, but pravastatin-treated cells showed LDH release comparable to that in vehicle-treated cells (Fig. 4D).
Figure 4.
Atorvastatin (Ator) and pravastatin (Prava) treatment inhibited RhoA kinase activity in cardiac myocytes, but only Ator increased LDH release and reduced oxygen consumption rate. Cardiac myocytes were treated with vehicle, RhoA activator (RhoA+), Ator, or Prava at 1 and 10 μM. A) After 48 h, total RhoA and activated RhoA expression was assessed by pull-down with GST-RBD. B) Densitometric analysis of RhoA pulldown assay. **P < 0.001, ****P < 0.0001 (1-way ANOVA with Tukey’s post hoc comparison). C) Representative images of NMVMs 48 h after treatment with, Ator, Prava, or vehicle at 1, 5, and 10 μM for 48 h. D) LDH release was measured in the culture medium 48 h after Ator, Prava, or vehicle treatment (n = 3 wells/treatment, each experiment repeated 3 times). Means ± sem. *P < 0.0001, Ator vs. Vehicle; #P < 0.0001, Ator vs. Prava (2-way ANOVA, with Tukey’s post hoc comparison). E) Bioenergetic analysis of HL-1 cells treated with vehicle, Ator, or Prava at 10 μM for 48 h. The OCR was measured after addition of oligomycin, FCCP, rotenone, and antimycin A 45,000 cells/well, 8 wells/plate, and repeated 3 times). Means ± sem. *P < 0.05 (2-way ANOVA).
Atorvastatin and pravastatin have different effects on the OCR of HL-1 cardiomyocytes
We wanted to further examine the effect of atorvastatin and pravastatin on HL-1 cardiomyocyte mitochondrial function by measuring the oxygen consumption rates (OCRs) with the Metabolic Flux Analyzer XF24 (Seahorse Biosciences). Initially, cardiomyocytes incubated with atorvastatin showed similar basal respiratory rates compared with the cells treated with pravastatin. Addition of oligomycin to inhibit ATP synthesis did not show a difference between groups. Furthermore, we wanted to assess the maximum OCR between the different groups. To do so, we added the mitochondrial uncoupler FCCP. After the addition of FCCP, cardiomyocytes treated with pravastatin showed a significantly higher respiration rate than cells treated with atorvastatin (Fig. 4E). Moreover, rotenone and antimycin A were added to inhibit electron flow. These results clearly show that cardiomyocytes treated with atorvastatin have altered mitochondrial function compared to the cardiomyocytes treated with pravastatin.
Atorvastatin administration decreased LDL-C and increased serum CK levels
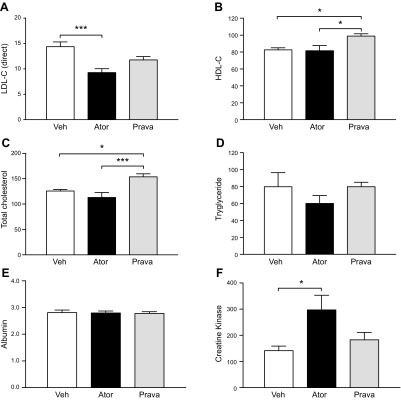
As described earlier, mice received atorvastatin, pravastatin (both statins at 5 mg/kg/d), or vehicle daily via oral gavage. Five weeks after the start of treatment, we performed serum analysis to assess lipid profile and potential muscle injury. Atorvastatin and pravastatin decreased direct, nonfasting LDL-C by 36 and 18%, respectively, compared with vehicle (Fig. 5A). HDL-C levels were increased in pravastatin-treated animals compared with those that received atorvastatin or vehicle (Fig. 5B). Because of the increased HDL-C, pravastatin-treated mice also showed greater serum total cholesterol levels than atorvastatin and vehicle-treated animals (Fig. 5C). Neither statin had an impact on serum triglyceride levels (Fig. 5D). Serum albumin levels did not differ in the 3 groups (Fig. 5E). Atorvastatin was associated with greater CK levels than pravastatin or vehicle (Fig. 5F). In summary, LDL-C reduction vs. vehicle was 36 and 18% (the latter not significant), for atorvastatin and pravastatin, respectively; but pravastatin administration increased HDL-C, and therefore total cholesterol. Only atorvastatin increased CK levels.
Figure 5.
Atorvastatin (Ator) and pravastatin (Prava) treatment reduced LDL-C, but only Ator increased CK serum levels. Five weeks after statin treatment (5 mg/kg/d orally), peripheral blood was collected for serological analysis. A) Direct LDL-C (in mg/dl, Veh 14.38 ± 0.92, Ator 9.25 ± 0.77, and Prava 11.78 ± 0.64; n = 8–9 each). Means ± sem. ***P < 0.0004, Veh vs. Ator (1-way ANOVA with Tukey post hoc comparison). B) HDL-C (mg/dl: Veh 82.63 ± 2.44, Ator 81.50 ± 6.27, and Prava 95.33 ± 3.3, n = 8–9 each). Means ± sem. *P < 0.025, Veh vs. Prava; *P < 0.016 Ator vs. Prava. C) Total-cholesterol (mg/dl: Veh 125.8 ± 2.9, Ator 112.9 ± 10.06, and Prava 153.8 ± 5.77; n = 8–9 each). Means ± sem. *P < 0.02, Veh vs. Prava; ***P < 0.001, Ator vs. Prava. D) Triglycerides (mg/dl: Veh 79.88 ± 16.5, Ator 60.13 ± 9.29, and Prava 79.89 ± 5.23; n = 8–9 each). Means ± sem. E) Albumin (g/dl: Veh 2.8 ± 0.1, Ator 2.7 ± 0.07, and Prava 2.77 ± 0.07. Means ± sem. F) CK (IU/L: Veh 132.3 ± 14.8, Ator 388.0 ± 69.43, and Prava 182.7 ± 28.45). Means ± sem, Veh vs. Ator, P < 0.01). Veh, vehicle. Data (n = 8–9/group) were analyzed with 1-way ANOVA followed by Tukey’s post hoc multiple comparison, and values of P < 0.05 were considered significant.
Long-term atorvastatin or pravastatin administration in cardiomyopathic mice did not significantly mitigate the decline in systolic cardiac function
Studies have demonstrated that atorvastatin and pravastatin administration (both at 5 mg/kg/d) ameliorated cardiac hypertrophy after pressure-overload and prevented the development of HF in WT mice (36, 37). On this basis, we investigated the effects of atorvastatin and pravastatin on the survival of mice susceptible to early sudden death or idiopathic dilated cardiomyopathy because of cardiac myocyte–specific inactivation of the Vcl gene (cVclKO) (29). We have shown that cVclKO mice die prematurely, either because of ventricular tachycardia, or, that if mice survive this vulnerable phase, they develop dilated cardiomyopathy (29). The role of statin therapy in genetic forms of dilated cardiomyopathy is currently unknown.
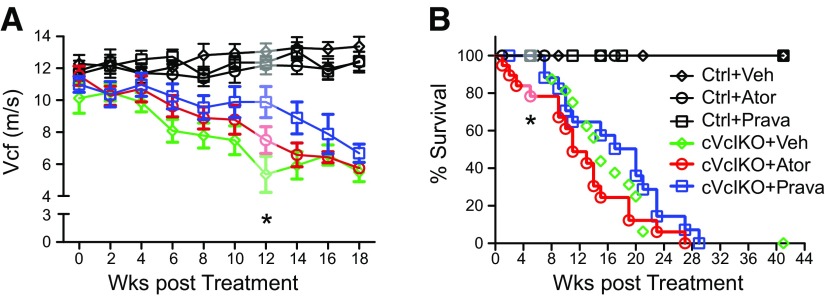
Systolic cardiac function was similar in all Ctrl treatment groups (Ctrl+vehicle, Ctrl+atorvastatin, Ctrl+pravastatin), and statin administration did not result in improved systolic cardiac function in cVclKO mice (cVclKO+vehicle, cVclKO+atorvastatin, and cVclKo+pravastatin) during the first 10 wk of statin administration. Cardiac function declined in parallel in all cVclKO treatment groups over time, when compared to control treatment groups (Fig. 6A and Supplemental Fig. 1A). However, after 12 wk, thinning of the LVPWd was reduced in cVclKO mice treated with pravastatin when compared with that in cVclKO mice treated with vehicle (Supplemental Fig. 1B). Twelve weeks after treatment, pravastatin administration was also associated with improved cardiac function (VCF and %FS, cVclKO+vehicle vs. cVclKO+pravastatin; P < 0.05) in cVclKO mice when compared to vehicle-treated cVclKO mice (Fig. 6A and Supplemental Fig. 1A). However, this difference was no longer evident after 14, 16, or 18 wk of statin administration.
Figure 6.
Atorvastatin (Ator) treatment did not improve systolic cardiac function but increased mortality in cVclKO mice. Echocardiographic analysis was performed in 5-wk-old cVclKO and control animals at baseline (0 wk), followed by 2-wk intervals after the initiation of statin. A) VCF: echocardiogram data were analyzed for all time points (until 18 wk) by a baseline adjusted RM 3-way ANOVA with the factors genotype, treatment, and time. Improved cardiac function (VCF) for pravastatin (Prava)-treated cVclKO mice was noted at the 12-wk timepoint (gray shading, P < 0.05) when compared to cVclKO+Vehicle (Veh). B) Kaplan-Meier survival analysis. After 6 wk survival of cVclKO+Ator was reduced when compared to cVclKO+Veh and cVclKO+Prava (gray shading, 4 deaths of 19 VclKO+A animals, 0 deaths of 16 cVclKO+Veh, and 0 deaths of 18 cVclKO+Prava; P < 0.05). After 16 wk, survival of cVclKO+Ator animals was reduced when compared to cVclKO+Veh and cVclKO+Prava (13 deaths of 19 VclKO+Ator, 9 deaths of 16 cVclKO+Veh, and 7 deaths of 18 cVclKO+Prava). Survival analysis at 44 wk did not show any significant change in cVclKO survival (Ctrl+Veh, n = 28; Ctrl+Ator, n = 21; Ctrl+Prava, n = 29; cVclKO+Veh, n = 16; cVclKO+Ator, n = 19; and cVclKO+Prava, n = 18; Gehan-Breslow-Wilcoxon test, cVclKO+Veh vs. cVclKO+Prava, P = 0.7499; cVclKO+Veh vs. cVclKO+Ator, P = 0.1165; and cVclKO+Ator vs. cVclKO+Prava, P = 0.086).
Chronic atorvastatin administration increased sudden death in mice susceptible to HF and arrhythmias, whereas cardiac function was preserved
Chronic inflammation is present in the failing myocardium, and because statins show anti-inflammatory properties (36–38), we hypothesized that statin treatment would improve survival in cVclKO mice, but Kaplan-Meier survival analysis for the entire 44-wk study period did not show a significant change in overall cVclKO survival (Fig. 6B). However, analysis at shorter intervals, suggested accelerated mortality in animals that received atorvastatin. After 6 wk, survival of cVclKO+atorvastatin was reduced when compared to cVclKO+vehicle and cVclKO+pravastatin (4 deaths of 19 VclKO+atorvastatin, 0 deaths of 16 cVclKO+vehicle, and 0 deaths of 18 cVclKO+pravastatin, cVclKO+atorvastatin vs. cVclKO+vehicle or cVclKO+pravastatin). Postmortem analysis of mice that died prematurely (<8 wk) revealed morphologic “stone hearts,” with cardiac arrest occurring during systole, and normal heart size with lack of ventricular dilatation and fluid retention. Because of the survival study design and the postmortem decay (delay between the death and tissue collection) cardiac tissue from deceased cVclKO mice could not be processed for biochemical or ultrastructural analysis.
Long-term atorvastatin treatment altered cardiac ultrastructure in mice
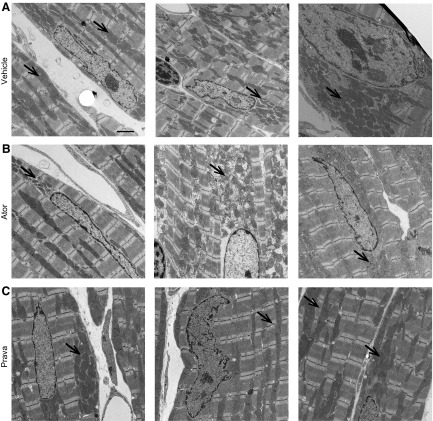
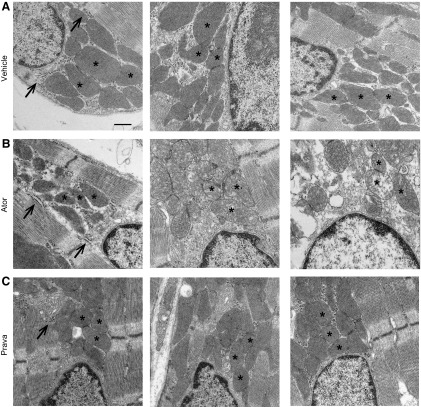
Muscle biopsies of patients with statin-induced skeletal myopathy have shown evidence of mitochondrial dysfunction, abnormally increased lipid stores and ragged red fibers, which are all findings similar to those in patients with mitochondrial myopathy (18, 20). Our previous study demonstrated that swollen and misaligned mitochondria are present in cVclKO mice at 6 wk of age, a time point before the onset of cardiac dysfunction (29). Because all cVclKO mice in the present survival study died spontaneously, we performed a final echocardiographic analysis followed by ultrastructural analysis on littermate controls that underwent the same treatment regimen for 7 mo. We did not observe any differences in cardiac systolic function in the 3 groups (Supplemental Fig. 2), but TEM analysis of perfusion-fixed cardiac muscle revealed distinct between-group differences. In longitudinal sections of normal hearts, mitochondria tend to run in rows parallel to contractile fibers and are connected through intermitochondrial junctions. Specifically, in atorvastatin-treated control mice, but not in pravastatin- or vehicle-treated animals, the mitochondria were misaligned, with scattered mitochondrial organization, swollen cristae, and lack of mitochondrial electron-dense contact sites (Figs. 7 and 8). Because we observed ER stress responses in statin-treated cardiac myocytes in vitro, we assessed cardiac ER structure in all groups. In atorvastatin-treated hearts, the rough ER lumen appeared enlarged, with lack of ER–mitochondria connections (Fig. 8). These changes were not seen in pravastatin- and vehicle-treated animals.
Figure 7.
Long-term atorvastatin (Ator) treatment altered mitochondrial ultrastructure. Healthy WT mice treated with vehicle (A), atorvastatin (Ator) (B), or pravastatin (Prava) (C) for 7 mo were perfusion fixed and assessed by TEM. Ultrastructural analysis revealed altered mitochondrial size and shape and a scattered arrangement in Ator-treated hearts (B, arrow), vs. clustered mitochondria in Prava- and Vehicle-treated controls (A, C, arrows) (n = 4 hearts/group, 2 blocks analyzed, 15 areas scanned/heart block at 2900 × each). Scale bar, 2 μm.
Figure 8.
Long-term atorvastatin (Ator) treatment induced enlarged rough ER lumen and reduced electron-dense mitochondrial contact sites. Healthy WT mice treated with vehicle (Veh) (A), atorvastatin (Ator) (B), or pravastatin (Prava) (C) for 7 mo were perfusion fixed and assessed by TEM. Arrows: rough ER membrane structures decorated with ribosomes; asterisks: mitochondria, with or without electron-dense contact sites. Enlarged ER lumen, lack of electron-dense contact sites, and swollen cristae formation were seen only in Ator-treated hearts (n = 4 hearts/group, 15 areas scanned/heart at 11,000 × each). Scale bar, 500 nm.
Different effects of atorvastatin and pravastatin on the cardiac transcriptome
To further investigate the cardiac effects of long-term atorvastatin and pravastatin treatment, we performed genome-wide expression profiling of left ventricular tissue of WT mice that received atorvastatin, pravastatin, or vehicle. We examined those genes with an unadjusted P < 0.01, which included 415 genes with altered regulation in response to atorvastatin and 320 genes altered in response to pravastatin. Unsupervised hierarchical clustering of the expression profiles for the identified genes revealed distinct statin-specific gene expression profiles, as verified by the separate clustering of the RNA sources based on the treatment annotation. We found that expression of ER-related (anterior gradient 2), mitochondria-related (slowmo homolog 1, leucine rich repeat kinase, NRD convertase 1), GPCR-related genes [GPCR family C, group 5, member C (Gprc5c)], and regulatory associated protein of mTOR complex-1 (Rptor1) were suppressed by atorvastatin (Table 1). By contrast, expression of cytoskeletal/sarcomeric/myofibrillar–related genes [nebulin; regulatory myosin, light polypeptide-7 = myosin light chain (MLC)-2a; and myosin, light polypeptide 4 = MLC-1a] were increased by pravastatin. Our comparative transcriptomic analysis revealed that pravastatin and atorvastatin led to distinctly different patterns of transcriptional regulation, which may in turn explain the different ultrastructural phenotypes that we observed.
TABLE 1.
Effects of long-term atorvastatin and pravastatin administration on cardiac gene expression
| Atorvastatin | Pravastatin | ||||
|---|---|---|---|---|---|
| Gene name | Gene symbol | Fold change P | Fold change P | ||
| Anterior gradient 2 (Xenopus laevis) | Agr2 | 0.65 | <0.008 | ||
| Slowmo homolog 1 (Drosophila) | Simo1 | 0.69 | <0.01 | ||
| Leucine-rich repeat kinase 1 | Lrrk1 | 0.69 | <0.01 | 0.58 | <0.001 |
| Nardilysin, N-arginine dibasic convertase, NRD convertase 1 | Nrd1 | 0.72 | <0.01 | ||
| GPCR, family C, group 5C | Gprc5c | 0.74 | <0.008 | ||
| Regulatory associated protein of mTOR complex1 | Rptor | 0.69 | <0.006 | ||
| Myosin, light polypeptide 7, regulatory | Myl7 | 23.17 | <0.004 | ||
| Myosin, light polypeptide 4 | Myl4 | 7.34 | <0.009 | ||
| Nebulin | Neb | 2.13 | <0.004 | ||
| Troponin T3, skeletal, fast | Tnnt3 | 1.70 | <0.009 | ||
| Erythrocyte protein band 4.1-like 4b | Epb4.1l4b | 1.54 | <0.01 | ||
Atorvastatin reduced the expression of Arg2 (0.7-fold), Simo1 (0.7-fold), Lrrk1 (0.7-fold), Nrd1 (0.7-fold), Gprc5c (0.7-fold), and Rptor (0.7-fold), but did not alter the transcriptome expression of Myl7, Myl4, Neb, Tnnt, and Epb4.1I4b. Pravastatin also reduced Lrrk1 (0.58-fold), did not alter Arg2, Simo1, Nrd1, Gprc5c, and Rptor, but increased the expression of Myl7 (23.2-fold), Myl4 (7.3-fold), Neb (2.1-fold), Tnnt3 (1.7-fold), and Epb4.1I4b (1.5-fold). The left ventricular free wall was used for RNA extraction (n = 3–5/group). To extract biologically useful information, gene lists were generated selecting candidates with an uncorrected P < 0.01. Not statistically significant where no values are indicated.
DISCUSSION
In our present investigation, to our knowledge, we are the first to show the adverse effects of atorvastatin, but not pravastatin, on cardiac muscle integrity, with assessment of multiple complementary in vitro and in vivo endpoints. Mechanistically, we found that the 2 statins induced differential effects on the activation of mTOR, structural protein expression, and cell survival signaling in cardiac myocytes. In the intact heart we report qualitative differences in mitochondrial ultrastructure and transcriptional regulation between these 2 statins, with disruption of cardiac cytoarchitecture in atorvastatin- but not in pravastatin-treated animals. The demonstration of distinct cellular mechanisms between atorvastatin and pravastatin in the heart is novel and has significant clinical implications.
Because of differences in lipophilicity, we had anticipated that effects of the 2 statins would differ. We had expected qualitatively similar effects, but with differing magnitude, (i.e., atorvastatin > pravastatin), rather than the distinctly different patterns of effect that we found. Whereas the common clinical purpose of administration of statins is reduction of LDL-C, statins also have effects on many other biologic pathways (39, 40), and our data suggest that pleiotropic effects vary from statin to statin. These novel and unexpected findings provide important insights into the mechanism by which atorvastatin and pravastatin have distinct cellular effects.
Although the precise cause of statin-induced myopathy is undefined, there is considerable evidence that a statin-induced impairment of oxidative phosphorylation within mitochondria is involved (21–25). Muscle biopsies of patients with statin myopathy show mitochondrial changes, similar to patients with mitochondrial myopathies (18–20). Coenzyme-Q10 plays an important role in the mitochondrial electron transport chain (41), and lovastatin treatment reduces CoQ10 levels in patients, thereby affecting energy metabolism in muscle cells (42). Simvastatin reduces citrate synthase activity in human skeletal muscle thereby impairing exercise capacity (21). It also causes a decrease in mitochondrial respiration in C2C12 myotubes (43). The lipophilic statins cerivastatin, atorvastatin, simvastatin, and fluvastatin, but not the hydrophilic pravastatin, induce mitochondrial swelling, cytochrome c release, and DNA fragmentation in skeletal muscle cells (44). Because mitochondrial density, ATP consumption rate, and constant energy demand are the greatest in cardiac tissue, we hypothesized that cardiac muscle is also vulnerable to potent HMG-CoA inhibition by statins.
It has been reported that certain statins increase the risk of new-onset type 2 diabetes mellitus (45, 46). It has also been shown that atorvastatin, but not pravastatin and rosuvastatin, inhibit insulin-induced glucose uptake in primary cultured rat cardiomyocytes (47). Jiang et al. (47) report reduced IR substrate with atorvastatin, but not with pravastatin or rosuvastatin treatment, in primary rat neonatal cardiomyocytes. These data conform with our data in primary mouse neonatal cardiomyocytes, showing reduced protein expression of IR-β (Fig. 3A, E) with atorvastatin, but not with pravastatin. Oral glucose tolerance test results in patients with diabetes suggest that pravastatin has a favorable effect on pancreatic β-cell function when compared to atorvastatin (48). More recently, Chen et al. (49) showed in mouse pancreatic NIT-1 cells that atorvastatin increased intracellular reactive oxygen species and induced cell death, whereas pravastatin did not. Because of these reports, we conclude that alterations in cardiomyocyte-related insulin signaling also contributed to our findings.
In atorvastatin-treated hearts we observed that mitochondria were physically disconnected, variable in size, and randomly localized. It has been shown experimentally that mitochondrial abnormalities in the heart can be detected early and may be the first sign of metabolic alterations, often preceding cardiac dysfunction (50, 51). AMPK plays an important role in mitochondrial homeostasis and is considered to be an energy sensor when ATP levels drop. Mikus et al. (21) have reported reduced ATP levels in skeletal muscle of patients taking simvastatin, but levels of AMPK activation were not assessed. Recently, it was shown that simvastatin increases AMPK phosphorylation and reduces angiogenesis in breast cancer (52). Atorvastatin has also been reported to increase AMPK activation and eNOS activation in mesenchymal stem cells and prevents hypoxia-induced cell damage (53). Pravastatin has been shown to activate AMPK in endothelial cells, thereby preventing avascular necrosis (54). Because AMPK-α2 was shown to regulate p70S6K signaling in the heart (55) and we report different effects of atorvastatin and pravastatin on p70S6K signaling in cardiac myocytes, further studies are under way to investigate mitochondrial homeostasis after administration of different statins. It is important to show that the mTOR partner, Rptor, also known as an AMPK target, is reduced in atorvastatin-treated hearts (Table 1). These reports show a clear involvement of statin-induced AMPK signaling changes, but it is currently unclear how these metabolic processes are regulated in the heart under physiologic conditions.
Besides energy production, mitochondria serve as high-capacity sinks for calcium, thus allowing buffering of cytoplasmic calcium (56). It has been shown that simvastatin reduces peroxisome proliferator-activated receptor gamma coactivator-1α mRNA and mitochondrial DNA copy number in murine skeletal muscle while lowering voluntary physical activity (57). The 2 lipophilic statins, cerivastatin and simvastatin, have been shown to cause massive Ca2+ release from the sarcoplasmic reticulum (SR) in cultured skeletal myoblasts (58), as well as in skeletal muscle fibers (59). The lipophilic statin lovastatin decreased sarcolemmal Na+/K+ ATPase density and pump current in skeletal and cardiac muscle resulting in increased intracellular Ca2+ (60). Statins were shown to decrease Ca2+ ATPase activity thereby increasing sarcoplasmic Ca2+ (61, 62). In rats with type 1 diabetes, a 7 d administration of atorvastatin or simvastatin, but not of pravastatin, improved cardiac systolic function (63), and the researchers speculated that short-term lipophilic statin treatment increased the contractile capacity in these diabetic hearts. Because it has been shown that calcium flux from the ER to the mitochondria is an important regulator for mitochondrial function (64, 65), we speculate that weakened ER–mitochondrial contact sites disturbed cellular calcium flux in cVclKO mice. The different effects of lipophilic and hydrophilic statin administration on calcium signaling in the intact heart should be investigated further.
We also investigated effects of long-term statin treatment on global gene expression in hearts of WT mice and found that atorvastatin repressed genes important for mitochondrial integrity (slowmo homolog-1), and ER function (anterior gradient-2), whereas pravastatin induced genes essential for the sarcomeric cytoskeleton (MLC-7, MLC-4, and nebulin). Pravastatin caused a 23-fold increase in myosin, light polypeptide-7 and a 2.1-fold increase in nebulin mRNA expression. It has been shown that short-term (1 mo) treatment with different statins administered through animal chow confers divergent effects on gene expression in the heart (66). Our genome-wide analysis after long-term (7 mo) daily per os statin administration is in agreement with these data, as we report that the lipophilic atorvastatin and the hydrophilic pravastatin exerted differential agent-specific effects on transcriptional regulation. These differential effects may result in distinctly different effects of atorvastatin and pravastatin on cardiac ultrastructure.
By controlling the initiation of translation mTOR activates the global ribosomal machinery. mTOR inhibitors suppress cell proliferation and beneficially control plaque growth, inflammation, and plaque rupture (67). Lovastatin inhibits mTOR in smooth muscle cells and prevents intimal hyperplasia (68, 69). By contrast, pravastatin induces rat endothelial cell proliferation and migration by rapid activation of PI3K/Akt signaling (70). Those in vitro data obtained in endothelial cells are consistent with our findings that pravastatin increased Akt/mTOR/p70 S6 signaling in cardiomyocytes. mTOR inhibitors suppress cell proliferation and control plaque growth and rupture (67). These findings may explain the excellent antiproliferative/anti-inflammatory, plaque-stabilizing effect of atorvastatin. However, in addition to the plaque-stabilizing effects of mTOR inhibition, cardiac mTOR regulates physiologic heart function and myocyte survival (71). Therefore, our results raise the question of how long-term mTOR inhibition, by statin administration, will affect cardiomyocyte integrity.
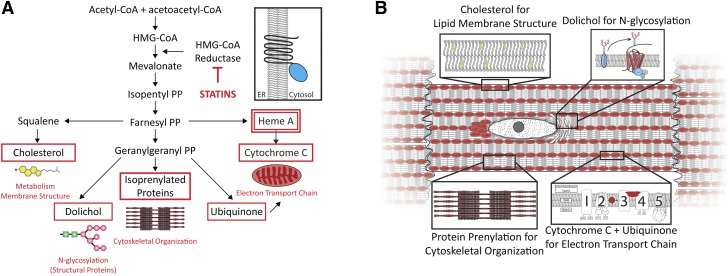
We also considered the possibility that the lipophilic nature of atorvastatin, and therefore its greater potential to penetrate cell membranes, is the basis for its ability to affect intracellular Akt/mTOR signaling. At the same dosage, however, pravastatin profoundly increased mTOR signaling and structural protein expression, albeit with effects opposite those of atorvastatin. These findings indicate that both statins pass the myocyte membrane but result in different cellular responses. Because statins inhibit all downstream pathways for which mevalonate is a precursor (39) we suggest that multiple pathways are affected in cardiac myocytes (Fig. 9A). Because of the high cardiac energy demand and constant mechanical stress, statins may affect membranes, the ER, cytoskeletal organization, and mitochondrial morphology (Fig. 9B).
Figure 9.
Molecular pathways affected by HMG-CoA reductase inhibition: potential effects on cardiac muscle. A) HMG-CoA reductase is an ER membrane-bound protein, and multiple cellular pathways may be affected by HMG-CoA inhibition in striated muscle including: 1) cholesterol synthesis dependent lipid composition of biologic membranes (lipid rafts, caveolin); 2) dolichol synthesis and N-linked glycosylation of structural proteins (e.g., EGFR and IR-β); 3) isoprenoid synthesis and protein prenylation/farnesylation (Rap, Ras, RhoA); 4) ubiquinone production (enzyme in the electron transport system); and 5) heme A and cytochrome c synthesis. B) Ultrastructural compartments (sarcolemma, ER, mitochondria, and sarcolemma) in cardiac myocytes that may be affected by potent HMG-CoA reductase inhibition.
We suggest explanations for the opposing effects on prosurvival signaling and transcriptome regulation between atorvastatin and pravastatin: 1) because of major pharmacokinetic differences between atorvastatin (t½14 h) and pravastatin (t½ 1.8 h), we think it possible that the short half-life of pravastatin, with only intermittent inhibition of HMG-CoA reductase, causes feedback upregulation of HMG-CoA reductase activity, compared to continuous HMG-CoA reductase inhibition with atorvastatin; and 2) because lipophilic statins pass cellular membranes easier than hydrophilic ones they may target mitochondrial structure. Because HMG-CoA is ER localized, our data suggest that atorvastatin and pravastatin have agent-specific cellular effects on ER function and stress and thereby on mitochondrial integrity.
The common clinical purpose of administration of all statins is reduction of LDL-C, but statins have effects on many other biologic pathways, and these effects may reasonably be expected to vary from statin to statin. In hypercholesterolemic humans, only pravastatin, not atorvastatin, leads to a reduction in the atherogenic lipoprotein-associated phospholipase A2 (72). Lovastatin, but not pravastatin, increased mortality in cardiomyopathic hamsters (73). The lipophilic simvastatin induced a shift from the fast myosin heavy chain IIb to the slower myosin heavy chain isoform IIa/x and simultaneously caused impaired skeletal muscle performance in rats (74), and pravastatin, but not simvastatin, improved survival and neurofunctional outcome after cardiac arrest in mice (75). Consistent with the notion of statin-specific pleiotropic effects are reports that several statins have been associated with the onset of type 2 diabetes but not pitavastatin (76) or pravastatin (45, 46).
We observed that atorvastatin induced ER stress and subsequent apoptosis. Pravastatin did not induce ER stress–induced apoptosis in vitro or swollen ER lumen in vivo. Cardiac myocytes harbor an extensive ER membrane system spanning the perinuclear ER localization to the SR adjacent to transverse (T) tubules. The importance of SR function during cardiac excitation–relaxation coupling is well known, but little is known about the function of the cardiac perinuclear ER and SR in terms of membrane protein synthesis and quality control. Unresolved ER stress affects post-translational modification, inhibits protein synthesis, and activates programmed cell death (30). The lipophilic simvastatin has been reported to induce ER stress and apoptosis in human atrial fibroblasts (77).
In the United States, HF is a major healthcare burden, and it is estimated that the prevalence of HF will increase by 46% in the next 2 decades, affecting both sexes and all racial and ethnic subgroups (78).
Although it is known that low LDL-C levels correlate with higher HF mortality (79–83), the specific mechanisms are undefined (80, 84, 85). The T-tubule structure in cardiac myocytes is enriched in cholesterol. It was recently shown by Zhu et al. (86) that cholesterol depletion disturbs T-tubule structure and Ca2+ handling in cardiac myocytes. Identification of a definitive molecular mechanism of atorvastatin’s effect on cardiac ultrastructure in vivo requires further investigation (87).
One study limitation was that normolipidemic animals were used. However, individuals without cardiovascular disease receive statins for primary prevention, even if LDL-C levels are within the normal range, and our model is entirely relevant to those circumstances. We used dosages of 5 mg/kg per day for both drugs based on previous reports of improved cardiac remodeling and function (36, 37). The relative LDL-C–lowering efficacy differs between atorvastatin and pravastatin. Therefore, this study did not use equipotent LDL-C–reducing doses. Greater pravastatin dosages did not cause any cytotoxicity in vitro; future in vivo studies using equi-LDL–lowering doses will address this question.
The strength of our study resides in the long-term (7 mo) comparison of 2 statins administered by oral gavage to mimic the pharmacokinetics of a single daily dose administered per os. Given a normal lifespan of ∼2.5 yr (28–36 mo) for a mouse and ∼80 yr in a human (88), our treatment temporally mimicked an ∼20-yr human treatment regimen. Previous investigations have studied short-term statin administration, either by gavage or via chow. Neither corresponds to the pattern of long-term use in humans, and chow administration is not comparable to the pharmacokinetics of a single daily statin administration. We studied sex- and age-matched animals free of any comorbidities and drug–drug interactions. Therefore, the in vivo effects on cardiac myocyte ultrastructure and gene expression could have been caused only by the statin.
Although a multitude of previous investigations have examined the effects of statins, with the observations assumed to represent class effects, the present investigation offers evidence that, beyond kinetic parameters, not all statins are created equal and that bioequivalence should not be assumed. To our knowledge, we are the first to report an ultrastructural change in whole heart after long-term atorvastatin, but not after pravastatin, administration. Our findings lead us to conclude that statin-induced adverse muscle effects occur in cardiac as well as skeletal muscle. Various statins may have distinct cellular mechanisms that regulate differential cellular responses, with pravastatin inducing protective signaling that may be beneficial to the failing heart. Observational studies in patients with reduced ejection fraction reported no benefit of statin treatment (89). More clinical studies are needed to confirm the clinical relevance of our observations. A better understanding of the many molecular pathways that contribute to pleiotropic statin effects is necessary and will have implications in the decision-making process as to which statin to use in specific patient populations.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
ACKNOWLEDGMENTS
The authors thank Drs. Marilyn Farquhar (University of California, San Diego), Velia Fowler, and Malcom Wood (both from Scripps Research, La Jolla, CA, USA) for helpful discussions regarding the TEM analysis; Ying Jones (University of California, San Diego) for excellent technical assistance in preparation of tissues for electron microscopic analysis; Drs. Joan Heller Brown and Sunny Y. Xiang (both from the University of California, San Diego) for providing the GST plasmid; the Veterans Affairs/Veterans Medical Research Foundation (VA/VMRF) Microarray and Next Generation Sequencing (NGS) Core (San Diego, CA, USA) for microarray profiling; and Dr. Benedict Lucchesi (University of Michigan, Ann Arbor, MI, USA) for valuable scientific discussions and encouragement to pursue this project. The authors declare no conflicts of interest. This work was funded by the U.S. National Institutes of Health HL107200, HL091071 (National Heart, Lung, and Blood Institute), and AG052722 (National Institute on Aging) (to H.H.P.), and by American Heart Association Beginning Grant-in-Aid (BGIA) 2260359 (to A.E.Z.-H.).
Glossary
- Akt
protein kinase B
- CHOP
C/EBP homologous protein
- CK
creatine kinase
- cVclKO
cardiac-specific vinculin-knockout mice
- EGFR
epidermal growth factor receptor
- ER
endoplasmic reticulum
- ERK
extracellular signal–regulated kinases
- FCCP
carbonyl cyanide-4(trifluoromethoxy)phenylhydrazone
- FS
fractional shortening
- GST
glutathione S-transferase
- HDL-C
high-density lipoprotein cholesterol
- HF
heart failure
- HMG-CoA
3-hydroxy-3-methylglutaryl–coenzyme A reductase
- IR
insulin receptor
- IRE
inositol-requiring enzyme
- LDH
lactate dehydrogenase
- LDL-C
low-density lipoprotein cholesterol
- LVPWd
left ventricular posterior wall thickness at end-diastole
- MLC
myosin light chain
- mTOR
mammalian target of rapamycin
- NMVM
neonatal mouse ventricular myocytes
- OCR
oxygen consumption rate
- PDI
protein disulfate-isomerase
- RBD
Rho binding domain
- Rho
Ras homolog gene family
- Rptor
regulatory-associated protein of mTOR complex
- SR
sarcoplasmic reticulum
- TEM
transmission electron microscopy
- T tubule
transverse tubule
- VCF
velocity of circumferential fiber shortening
- WT
wild type
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
J. C. Godoy and A. E. Zemljic-Harpf designed the research; J. C. Godoy, I. R. Niesman, Adam Kassan, A. Schwarz, N. D. Dalton, and A. E. Zemljic-Harpf performed the experiments; J. C. Godoy, J. M. Schilling, G. Kararigas, and A. E. Zemljic-Harpf analyzed the data; J. C. Drummond, D. M. Roth, G. Kararigas, H. H. Patel, and A. E. Zemljic-Harpf wrote the manuscript; A. E. Zemljic-Harpf designed and A. R. Busija generated the diagram and illustration in Fig. 9; and all authors approved the final draft.
REFERENCES
- 1.Cholesterol Treatment Trialists’ (CTT) Collaboration (2010) Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet 376, 1670–1681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jarcho J. A., Keaney J. F., Jr (2015) Proof that lower is better: LDL cholesterol and improve-it. N. Engl. J. Med. 372, 2448–2450 [DOI] [PubMed] [Google Scholar]
- 3.Teshima Y., Yufu K., Akioka H., Iwao T., Anan F., Nakagawa M., Yonemochi H., Takahashi N., Hara M., Saikawa T. (2009) Early atorvastatin therapy improves cardiac function in patients with acute myocardial infarction. J. Cardiol. 53, 58–64 [DOI] [PubMed] [Google Scholar]
- 4.Kadota S., Matsuda M., Izuhara M., Baba O., Moriwaki S., Shioji K., Takeuchi Y., Uegaito T. (2008) Long-term effects of early statin therapy for patients with acute myocardial infarction treated with stent implantation. J. Cardiol. 51, 171–178 [DOI] [PubMed] [Google Scholar]
- 5.Zheng G., Chen J., Lin C., Huang X., Lin J. (2015) Effect of statin therapy on fibrous cap thickness in coronary plaques using optical coherence tomography: a systematic review and meta-analysis. J. Interv. Cardiol. 28, 514–522 [DOI] [PubMed] [Google Scholar]
- 6.Pencina M. J., Navar-Boggan A. M., D’Agostino R. B., Sr., Williams K., Neely B., Sniderman A. D., Peterson E. D. (2014) Application of new cholesterol guidelines to a population-based sample. N. Engl. J. Med. 370, 1422–1431 [DOI] [PubMed] [Google Scholar]
- 7.American College of Cardiology/American Heart Association Task Force on Practice Guidelines (2014) 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice guidelines Circulation 129, S1–S45; correction: S46–S48 [DOI] [PubMed] [Google Scholar]
- 8.Endo A. (1992) The discovery and development of HMG-CoA reductase inhibitors. J. Lipid Res. 33, 1569–1582 [PubMed] [Google Scholar]
- 9.Istvan E. S., Deisenhofer J. (2001) Structural mechanism for statin inhibition of HMG-CoA reductase. Science 292, 1160–1164 [DOI] [PubMed] [Google Scholar]
- 10.Goldstein J. L., Brown M. S. (1990) Regulation of the mevalonate pathway. Nature 343, 425–430 [DOI] [PubMed] [Google Scholar]
- 11.Roitelman J., Olender E. H., Bar-Nun S., Dunn W. A., Jr., Simoni R. D. (1992) Immunological evidence for eight spans in the membrane domain of 3-hydroxy-3-methylglutaryl coenzyme A reductase: implications for enzyme degradation in the endoplasmic reticulum. J. Cell Biol. 117, 959–973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sarr F. S., André C., Guillaume Y. C. (2008) Statins (HMG-coenzyme A reductase inhibitors): biomimetic membrane binding mechanism investigated by molecular chromatography. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 868, 20–27 [DOI] [PubMed] [Google Scholar]
- 13.American Heart Association Clinical Pharmacology Committee of the Council on Clinical Cardiology; Council on Hypertension; Council on Quality of Care and Outcomes Research; and Council on Functional Genomics and Translational Biology (2016) Recommendations for management of clinically significant drug-drug interactions with statins and select agents used in patients with cardiovascular disease: a scientific statement from the American Heart Association. Circulation 134, e468–e495 [DOI] [PubMed] [Google Scholar]
- 14.Staffa J. A., Chang J., Green L. (2002) Cerivastatin and reports of fatal rhabdomyolysis. N. Engl. J. Med. 346, 539–540 [DOI] [PubMed] [Google Scholar]
- 15.Omar M. A., Wilson J. P., Cox T. S. (2001) Rhabdomyolysis and HMG-CoA reductase inhibitors. Ann. Pharmacother. 35, 1096–1107 [DOI] [PubMed] [Google Scholar]
- 16.Omar M. A., Wilson J. P. (2002) FDA adverse event reports on statin-associated rhabdomyolysis. Ann. Pharmacother. 36, 288–295 [DOI] [PubMed] [Google Scholar]
- 17.Thompson P. D., Panza G., Zaleski A., Taylor B. (2016) Statin-associated side effects. J. Am. Coll. Cardiol. 67, 2395–2410 [DOI] [PubMed] [Google Scholar]
- 18.Scripps Mercy Clinical Research Center (2002) Statin-associated myopathy with normal creatine kinase levels. Ann. Intern. Med. 137, 581–585 [DOI] [PubMed] [Google Scholar]
- 19.Gambelli S., Dotti M. T., Malandrini A., Mondelli M., Stromillo M. L., Gaudiano C., Federico A. (2004) Mitochondrial alterations in muscle biopsies of patients on statin therapy. J. Submicrosc. Cytol. Pathol. 36, 85–89 [PubMed] [Google Scholar]
- 20.Giordano N., Senesi M., Mattii G., Battisti E., Villanova M., Gennari C. (1997) Polymyositis associated with simvastatin. Lancet 349, 1600–1601 [DOI] [PubMed] [Google Scholar]
- 21.Mikus C. R., Boyle L. J., Borengasser S. J., Oberlin D. J., Naples S. P., Fletcher J., Meers G. M., Ruebel M., Laughlin M. H., Dellsperger K. C., Fadel P. J., Thyfault J. P. (2013) Simvastatin impairs exercise training adaptations. J. Am. Coll. Cardiol. 62, 709–714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Allard N. A. E., Schirris T. J. J., Verheggen R. J., Russel F. G. M., Rodenburg R. J., Smeitink J. A. M., Thompson P. D., Hopman M. T. E., Timmers S. (2018) Statins affect skeletal muscle performance: evidence for disturbances in energy metabolism. J. Clin. Endocrinol. Metab. 103, 75–84 [DOI] [PubMed] [Google Scholar]
- 23.Larsen S., Stride N., Hey-Mogensen M., Hansen C. N., Bang L. E., Bundgaard H., Nielsen L. B., Helge J. W., Dela F. (2013) Simvastatin effects on skeletal muscle: relation to decreased mitochondrial function and glucose intolerance. J. Am. Coll. Cardiol. 61, 44–53 [DOI] [PubMed] [Google Scholar]
- 24.Apostolopoulou M., Corsini A., Roden M. (2015) The role of mitochondria in statin-induced myopathy. Eur. J. Clin. Invest. 45, 745–754 [DOI] [PubMed] [Google Scholar]
- 25.Golomb B. A., Evans M. A. (2008) Statin adverse effects : a review of the literature and evidence for a mitochondrial mechanism. Am. J. Cardiovasc. Drugs 8, 373–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zemljic-Harpf A. E., Godoy J. C., Platoshyn O., Asfaw E. K., Busija A. R., Domenighetti A. A., Ross R. S. (2014) Vinculin directly binds zonula occludens-1 and is essential for stabilizing connexin-43-containing gap junctions in cardiac myocytes. J. Cell Sci. 127, 1104–1116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kobayashi M., Chisaki I., Narumi K., Hidaka K., Kagawa T., Itagaki S., Hirano T., Iseki K. (2008) Association between risk of myopathy and cholesterol-lowering effect: a comparison of all statins. Life Sci. 82, 969–975 [DOI] [PubMed] [Google Scholar]
- 28.Claycomb W. C., Lanson N. A., Jr., Stallworth B. S., Egeland D. B., Delcarpio J. B., Bahinski A., Izzo N. J., Jr (1998) HL-1 cells: a cardiac muscle cell line that contracts and retains phenotypic characteristics of the adult cardiomyocyte. Proc. Natl. Acad. Sci. USA 95, 2979–2984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zemljic-Harpf A. E., Miller J. C., Henderson S. A., Wright A. T., Manso A. M., Elsherif L., Dalton N. D., Thor A. K., Perkins G. A., McCulloch A. D., Ross R. S. (2007) Cardiac-myocyte-specific excision of the vinculin gene disrupts cellular junctions, causing sudden death or dilated cardiomyopathy. Mol. Cell. Biol. 27, 7522–7537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Glembotski C. C. (2008) The role of the unfolded protein response in the heart. J. Mol. Cell. Cardiol. 44, 453–459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Appenzeller-Herzog C., Hall M. N. (2012) Bidirectional crosstalk between endoplasmic reticulum stress and mTOR signaling. Trends Cell Biol. 22, 274–282 [DOI] [PubMed] [Google Scholar]
- 32.Aebi M. (2013) N-linked protein glycosylation in the ER. Biochim. Biophys. Acta 1833, 2430–2437 [DOI] [PubMed] [Google Scholar]
- 33.Johnson T. E., Zhang X., Bleicher K. B., Dysart G., Loughlin A. F., Schaefer W. H., Umbenhauer D. R. (2004) Statins induce apoptosis in rat and human myotube cultures by inhibiting protein geranylgeranylation but not ubiquinone. Toxicol. Appl. Pharmacol. 200, 237–250 [DOI] [PubMed] [Google Scholar]
- 34.Laufs U., Kilter H., Konkol C., Wassmann S., Böhm M., Nickenig G. (2002) Impact of HMG CoA reductase inhibition on small GTPases in the heart. Cardiovasc. Res. 53, 911–920 [DOI] [PubMed] [Google Scholar]
- 35.Oesterle A., Laufs U., Liao J. K. (2017) Pleiotropic effects of statins on the cardiovascular system. Circ. Res. 120, 229–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liao Y., Zhao H., Ogai A., Kato H., Asakura M., Kim J., Asanuma H., Minamino T., Takashima S., Kitakaze M. (2008) Atorvastatin slows the progression of cardiac remodeling in mice with pressure overload and inhibits epidermal growth factor receptor activation. Hypertens. Res. 31, 335–344 [DOI] [PubMed] [Google Scholar]
- 37.Zhao H., Liao Y., Minamino T., Asano Y., Asakura M., Kim J., Asanuma H., Takashima S., Hori M., Kitakaze M. (2008) Inhibition of cardiac remodeling by pravastatin is associated with amelioration of endoplasmic reticulum stress. Hypertens. Res. 31, 1977–1987 [DOI] [PubMed] [Google Scholar]
- 38.Hasegawa H., Yamamoto R., Takano H., Mizukami M., Asakawa M., Nagai T., Komuro I. (2003) 3-Hydroxy-3-methylglutaryl coenzyme A reductase inhibitors prevent the development of cardiac hypertrophy and heart failure in rats. J. Mol. Cell. Cardiol. 35, 953–960 [DOI] [PubMed] [Google Scholar]
- 39.Zhou Q., Liao J. K. (2010) Pleiotropic effects of statins: basic research and clinical perspectives. Circ. J. 74, 818–826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Davignon J. (2004) Beneficial cardiovascular pleiotropic effects of statins. Circulation 109(23 Suppl 1), III39–III43 [DOI] [PubMed] [Google Scholar]
- 41.Crane F. L. (2007) Discovery of ubiquinone (coenzyme Q) and an overview of function. Mitochondrion 7(Suppl), S2–S7 [DOI] [PubMed] [Google Scholar]
- 42.Folkers K., Langsjoen P., Willis R., Richardson P., Xia L. J., Ye C. Q., Tamagawa H. (1990) Lovastatin decreases coenzyme Q levels in humans. Proc. Natl. Acad. Sci. USA 87, 8931–8934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mullen P. J., Zahno A., Lindinger P., Maseneni S., Felser A., Krähenbühl S., Brecht K. (2011) Susceptibility to simvastatin-induced toxicity is partly determined by mitochondrial respiration and phosphorylation state of Akt. Biochim. Biophys. Acta 1813, 2079–2087 [DOI] [PubMed] [Google Scholar]
- 44.Kaufmann P., Török M., Zahno A., Waldhauser K. M., Brecht K., Krähenbühl S. (2006) Toxicity of statins on rat skeletal muscle mitochondria. Cell. Mol. Life Sci. 63, 2415–2425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sattar N., Preiss D., Murray H. M., Welsh P., Buckley B. M., de Craen A. J., Seshasai S. R., McMurray J. J., Freeman D. J., Jukema J. W., Macfarlane P. W., Packard C. J., Stott D. J., Westendorp R. G., Shepherd J., Davis B. R., Pressel S. L., Marchioli R., Marfisi R. M., Maggioni A. P., Tavazzi L., Tognoni G., Kjekshus J., Pedersen T. R., Cook T. J., Gotto A. M., Clearfield M. B., Downs J. R., Nakamura H., Ohashi Y., Mizuno K., Ray K. K., Ford I. (2010) Statins and risk of incident diabetes: a collaborative meta-analysis of randomised statin trials. Lancet 375, 735–742 [DOI] [PubMed] [Google Scholar]
- 46.Brault M., Ray J., Gomez Y. H., Mantzoros C. S., Daskalopoulou S. S. (2014) Statin treatment and new-onset diabetes: a review of proposed mechanisms. Metabolism 63, 735–745 [DOI] [PubMed] [Google Scholar]
- 47.Jiang Z., Yu B., Li Y. (2016) Effect of three statins on glucose uptake of cardiomyocytes and its mechanism. Med. Sci. Monit. 22, 2825–2830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mita T., Watada H., Nakayama S., Abe M., Ogihara T., Shimizu T., Uchino H., Hirose T., Kawamori R. (2007) Preferable effect of pravastatin compared to atorvastatin on beta cell function in Japanese early-state type 2 diabetes with hypercholesterolemia. Endocr. J. 54, 441–447 [DOI] [PubMed] [Google Scholar]
- 49.Chen Y. H., Chen Y. C., Liu C. S., Hsieh M. C. (2016) The different effects of atorvastatin and pravastatin on cell death and PARP activity in pancreatic NIT-1 cells. J. Diabetes Res. 2016, 1828071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Milner D. J., Mavroidis M., Weisleder N., Capetanaki Y. (2000) Desmin cytoskeleton linked to muscle mitochondrial distribution and respiratory function. J. Cell Biol. 150, 1283–1298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zemljic-Harpf A. E., Ponrartana S., Avalos R. T., Jordan M. C., Roos K. P., Dalton N. D., Phan V. Q., Adamson E. D., Ross R. S. (2004) Heterozygous inactivation of the vinculin gene predisposes to stress-induced cardiomyopathy. Am. J. Pathol. 165, 1033–1044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang J. C., Li X. X., Sun X., Li G. Y., Sun J. L., Ye Y. P., Cong L. L., Li W. M., Lu S. Y., Feng J., Liu P. J. (2018) Activation of AMPK by simvastatin inhibited breast tumor angiogenesis via impeding HIF-1α-induced pro-angiogenic factor. Cancer Sci. 109, 1627–1637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dong Q., Yang Y., Song L., Qian H., Xu Z. (2011) Atorvastatin prevents mesenchymal stem cells from hypoxia and serum-free injury through activating AMP-activated protein kinase. Int. J. Cardiol. 153, 311–316 [DOI] [PubMed] [Google Scholar]
- 54.Liao Y., Zhang P., Yuan B., Li L., Bao S. (2018) Pravastatin protects against avascular necrosis of femoral head via autophagy. Front. Physiol. 9, 307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Demeulder B., Zarrinpashneh E., Ginion A., Viollet B., Hue L., Rider M. H., Vanoverschelde J. L., Beauloye C., Horman S., Bertrand L. (2013) Differential regulation of eEF2 and p70S6K by AMPKalpha2 in heart. Biochim. Biophys. Acta 1832, 780–790 [DOI] [PubMed] [Google Scholar]
- 56.Rizzuto R., De Stefani D., Raffaello A., Mammucari C. (2012) Mitochondria as sensors and regulators of calcium signalling. Nat. Rev. Mol. Cell Biol. 13, 566–578 [DOI] [PubMed] [Google Scholar]
- 57.Chung H. R., Vakil M., Munroe M., Parikh A., Meador B. M., Wu P. T., Jeong J. H., Woods J. A., Wilund K. R., Boppart M. D. (2016) The impact of exercise on statin-associated skeletal muscle myopathy. PLoS One 11, e0168065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nakahara K., Yada T., Kuriyama M., Osame M. (1994) Cytosolic Ca2+ increase and cell damage in L6 rat myoblasts by HMG-CoA reductase inhibitors. Biochem. Biophys. Res. Commun. 202, 1579–1585 [DOI] [PubMed] [Google Scholar]
- 59.Inoue R., Tanabe M., Kono K., Maruyama K., Ikemoto T., Endo M. (2003) Ca2+-releasing effect of cerivastatin on the sarcoplasmic reticulum of mouse and rat skeletal muscle fibers. J. Pharmacol. Sci. 93, 279–288 [DOI] [PubMed] [Google Scholar]
- 60.Gray D. F., Bundgaard H., Hansen P. S., Buhagiar K. A., Mihailidou A. S., Jessup W., Kjeldsen K., Rasmussen H. H. (2000) HMG CoA reductase inhibition reduces sarcolemmal Na(+)-K(+) pump density. Cardiovasc. Res. 47, 329–335 [DOI] [PubMed] [Google Scholar]
- 61.Baker S. K., Tarnopolsky M. A. (2001) Statin myopathies: pathophysiologic and clinical perspectives. Clin. Invest. Med. 24, 258–272 [PubMed] [Google Scholar]
- 62.Liantonio A., Giannuzzi V., Cippone V., Camerino G. M., Pierno S., Camerino D. C. (2007) Fluvastatin and atorvastatin affect calcium homeostasis of rat skeletal muscle fibers in vivo and in vitro by impairing the sarcoplasmic reticulum/mitochondria Ca2+-release system. J. Pharmacol. Exp. Ther. 321, 626–634 [DOI] [PubMed] [Google Scholar]
- 63.Crespo M. J., Cruz N., Quidgley J., Torres H., Hernandez C., Casiano H., Rivera K. (2014) Daily administration of atorvastatin and simvastatin for one week improves cardiac function in type 1 diabetic rats. Pharmacology 93, 84–91 [DOI] [PubMed] [Google Scholar]
- 64.Cárdenas C., Miller R. A., Smith I., Bui T., Molgó J., Müller M., Vais H., Cheung K. H., Yang J., Parker I., Thompson C. B., Birnbaum M. J., Hallows K. R., Foskett J. K. (2010) Essential regulation of cell bioenergetics by constitutive InsP3 receptor Ca2+ transfer to mitochondria. Cell 142, 270–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Krols M., Bultynck G., Janssens S. (2016) ER–mitochondria contact sites: a new regulator of cellular calcium flux comes into play. J. Cell Biol. 214, 367–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kumazaki M., Ando H., Ushijima K., Fujimura A. (2013) Comparative effects of statins on murine cardiac gene expression profiles in normal mice. Eur. J. Pharmacol. 707, 71–77 [DOI] [PubMed] [Google Scholar]
- 67.Martinet W., De Loof H., De Meyer G. R. (2014) mTOR inhibition: a promising strategy for stabilization of atherosclerotic plaques. Atherosclerosis 233, 601–607 [DOI] [PubMed] [Google Scholar]
- 68.Martin K. A., Rzucidlo E. M., Merenick B. L., Fingar D. C., Brown D. J., Wagner R. J., Powell R. J. (2004) The mTOR/p70 S6K1 pathway regulates vascular smooth muscle cell differentiation. Am. J. Physiol. Cell Physiol. 286, C507–C517 [DOI] [PubMed] [Google Scholar]
- 69.Wagner R. J., Martin K. A., Powell R. J., Rzucidlo E. M. (2010) Lovastatin induces VSMC differentiation through inhibition of Rheb and mTOR. Am. J. Physiol. Cell Physiol. 299, C119–C127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nakao T., Shiota M., Tatemoto Y., Izumi Y., Iwao H. (2007) Pravastatin induces rat aortic endothelial cell proliferation and migration via activation of PI3K/Akt/mTOR/p70 S6 kinase signaling. J. Pharmacol. Sci. 105, 334–341 [DOI] [PubMed] [Google Scholar]
- 71.Zhang D., Contu R., Latronico M. V., Zhang J., Rizzi R., Catalucci D., Miyamoto S., Huang K., Ceci M., Gu Y., Dalton N. D., Peterson K. L., Guan K. L., Brown J. H., Chen J., Sonenberg N., Condorelli G. (2010) MTORC1 regulates cardiac function and myocyte survival through 4E-BP1 inhibition in mice. J. Clin. Invest. 120, 2805–2816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ky B., Burke A., Tsimikas S., Wolfe M. L., Tadesse M. G., Szapary P. O., Witztum J. L., FitzGerald G. A., Rader D. J. (2008) The influence of pravastatin and atorvastatin on markers of oxidative stress in hypercholesterolemic humans. J. Am. Coll. Cardiol. 51, 1653–1662 [DOI] [PubMed] [Google Scholar]
- 73.März W., Siekmeier R., Müller H. M., Wieland H., Gross W., Olbrich H. G. (2000) Effects of lovastatin and pravastatin on the survival of hamsters with inherited cardiomyopathy. J. Cardiovasc. Pharmacol. Ther. 5, 275–279 [DOI] [PubMed] [Google Scholar]
- 74.Trapani L., Melli L., Segatto M., Trezza V., Campolongo P., Jozwiak A., Swiezewska E., Pucillo L. P., Moreno S., Fanelli F., Linari M., Pallottini V. (2011) Effects of myosin heavy chain (MHC) plasticity induced by HMGCoA-reductase inhibition on skeletal muscle functions. FASEB J. 25, 4037–4047 [DOI] [PubMed] [Google Scholar]
- 75.Bergt S., Grub A., Wagner S., Engelke H., Nöldge-Schomburg G., Vollmar B., Roesner J. P., Wagner N.-M. (2017) Pravastatin but not simvastatin improves survival and neurofunctional outcome after cardiac arrest and cardiopulmonary resuscitation. JACC Basic Transl. Sci. 2, 149–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Vallejo-Vaz A. J., Kondapally Seshasai S. R., Kurogi K., Michishita I., Nozue T., Sugiyama S., Tsimikas S., Yoshida H., Ray K. K. (2015) Effect of pitavastatin on glucose, HbA1c and incident diabetes: a meta-analysis of randomized controlled clinical trials in individuals without diabetes. Atherosclerosis 241, 409–418 [DOI] [PubMed] [Google Scholar]
- 77.Ghavami S., Yeganeh B., Stelmack G. L., Kashani H. H., Sharma P., Cunnington R., Rattan S., Bathe K., Klonisch T., Dixon I. M., Freed D. H., Halayko A. J. (2012) Apoptosis, autophagy and ER stress in mevalonate cascade inhibition-induced cell death of human atrial fibroblasts. Cell Death Dis. 3, e330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.American Heart Association Advocacy Coordinating Committee ; Council on Arteriosclerosis, Thrombosis and Vascular Biology ; Council on Cardiovascular Radiology and Intervention ; Council on Clinical Cardiology ; Council on Epidemiology and Prevention ; Stroke Council (2013) Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ. Heart Fail. 6, 606–619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Greene S. J., Vaduganathan M., Lupi L., Ambrosy A. P., Mentz R. J., Konstam M. A., Nodari S., Subacius H. P., Fonarow G. C., Bonow R. O., Gheorghiade M.; EVEREST Trial Investigators (2013) Prognostic significance of serum total cholesterol and triglyceride levels in patients hospitalized for heart failure with reduced ejection fraction (from the EVEREST Trial). Am. J. Cardiol. 111, 574–581 [DOI] [PubMed] [Google Scholar]
- 80.Vaduganathan M., Greene S. J., Ambrosy A. P., Gheorghiade M. (2014) Lipids, statins, and clinical outcomes in heart failure: rethinking the data. Heart Fail. Rev. 19, 695–698 [DOI] [PubMed] [Google Scholar]
- 81.Horwich T. B., Hamilton M. A., Maclellan W. R., Fonarow G. C. (2002) Low serum total cholesterol is associated with marked increase in mortality in advanced heart failure. J. Card. Fail. 8, 216–224 [DOI] [PubMed] [Google Scholar]
- 82.Horwich T. B., Hernandez A. F., Dai D., Yancy C. W., Fonarow G. C. (2008) Cholesterol levels and in-hospital mortality in patients with acute decompensated heart failure. Am. Heart J. 156, 1170–1176 [DOI] [PubMed] [Google Scholar]
- 83.Güder G., Frantz S., Bauersachs J., Allolio B., Wanner C., Koller M. T., Ertl G., Angermann C. E., Störk S. (2009) Reverse epidemiology in systolic and nonsystolic heart failure: cumulative prognostic benefit of classical cardiovascular risk factors. Circ. Heart Fail. 2, 563–571 [DOI] [PubMed] [Google Scholar]
- 84.Tousoulis D., Oikonomou E., Siasos G., Stefanadis C. (2014) Statins in heart failure: with preserved and reduced ejection fraction. An update. Pharmacol. Ther. 141, 79–91 [DOI] [PubMed] [Google Scholar]
- 85.Yancy C. W., Jessup M., Bozkurt B., Butler J., Casey D. E., Jr., Colvin M. M., Drazner M. H., Filippatos G. S., Fonarow G. C., Givertz M. M., Hollenberg S. M., Lindenfeld J., Masoudi F. A., McBride P. E., Peterson P. N., Stevenson L. W., Westlake C. (2017) 2017 ACC/AHA/HFSA focused. Update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. 136, e137–e161 [DOI] [PubMed] [Google Scholar]
- 86.Zhu Y., Zhang C., Chen B., Chen R., Guo A., Hong J., Song L. S. (2016) Cholesterol is required for maintaining T-tubule integrity and intercellular connections at intercalated discs in cardiomyocytes. J. Mol. Cell. Cardiol. 97, 204–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bakakos A., Oikonomou E., Vogiatzi G., Siasos G., Tsalamandris S., Antonopoulos A., Mourouzis C., Fountoulakis P., Vavuranakis M., Tousoulis D. (2017) Statins and left ventricular function. [E-pub ahead of print] Curr. Pharm. Des. doi: 10.2174/1381612823666170926125754 [DOI] [PubMed] [Google Scholar]
- 88.Fox J. G. (2007) The Mouse in Biomedical Research, Academic Press, Cambridge, MA, USA [Google Scholar]
- 89.CHART-2 Investigators (2015) Prognostic impact of statin use in patients with heart failure and preserved ejection fraction. Circ. J. 79, 574–582 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.